Abstract
Coronavirus disease 2019 is an emerging disease with a rapid increase in cases and deaths since its first identification in Wuhan, China, in December 2019. Limited data are available about coronavirus disease 2019 during pregnancy; however, information on illnesses associated with other highly pathogenic coronaviruses (ie, severe acute respiratory syndrome and the Middle East respiratory syndrome) might provide insights into coronavirus disease 2019’s effects during pregnancy. Coronaviruses cause illness ranging in severity from the common cold to severe respiratory illness and death. Currently the primary epidemiologic risk factors for coronavirus disease 2019 include travel from mainland China (especially Hubei Province) or close contact with infected individuals within 14 days of symptom onset. Data suggest an incubation period of ∼5 days (range, 2–14 days). Average age of hospitalized patients has been 49–56 years, with a third to half with an underlying illness. Children have been rarely reported. Men were more frequent among hospitalized cases (54–73%). Frequent manifestations include fever, cough, myalgia, headache, and diarrhea. Abnormal testing includes abnormalities on chest radiographic imaging, lymphopenia, leukopenia, and thrombocytopenia. Initial reports suggest that acute respiratory distress syndrome develops in 17–29% of hospitalized patients. Overall case fatality rate appears to be ∼1%; however, early data may overestimate this rate. In 2 reports describing 18 pregnancies with coronavirus disease 2019, all were infected in the third trimester, and clinical findings were similar to those in nonpregnant adults. Fetal distress and preterm delivery were seen in some cases. All but 2 pregnancies were cesarean deliveries and no evidence of in utero transmission was seen.
Data on severe acute respiratory syndrome and Middle East respiratory syndrome in pregnancy are sparse. For severe acute respiratory syndrome, the largest series of 12 pregnancies had a case-fatality rate of 25%. Complications included acute respiratory distress syndrome in 4, disseminated intravascular coagulopathy in 3, renal failure in 3, secondary bacterial pneumonia in 2, and sepsis in 2 patients. Mechanical ventilation was 3 times more likely among pregnant compared with nonpregnant women. Among 7 first-trimester infections, 4 ended in spontaneous abortion. Four of 5 women with severe acute respiratory syndrome after 24 weeks’ gestation delivered preterm. For Middle East respiratory syndrome, there were 13 case reports in pregnant women, of which 2 were asymptomatic, identified as part of a contact investigation; 3 patients (23%) died. Two pregnancies ended in fetal demise and 2 were born preterm. No evidence of in utero transmission was seen in severe acute respiratory syndrome or Middle East respiratory syndrome. Currently no coronavirus-specific treatments have been approved by the US Food and Drug Administration. Because coronavirus disease 2019 might increase the risk for pregnancy complications, management should optimally be in a health care facility with close maternal and fetal monitoring. Principles of management of coronavirus disease 2019 in pregnancy include early isolation, aggressive infection control procedures, oxygen therapy, avoidance of fluid overload, consideration of empiric antibiotics (secondary to bacterial infection risk), laboratory testing for the virus and coinfection, fetal and uterine contraction monitoring, early mechanical ventilation for progressive respiratory failure, individualized delivery planning, and a team-based approach with multispecialty consultations. Information on coronavirus disease 2019 is increasing rapidly. Clinicians should continue to follow the Centers for Disease Control and Prevention website to stay up to date with the latest information (https://www.cdc.gov/coronavirus/2019-nCoV/hcp/index.html).
Key words: fetal death, fetus, maternal death, Middle East respiratory syndrome, newborn, novel coronavirus, 2019 novel coronavirus, perinatal infection, pneumonia, pregnancy, preterm birth, severe acute respiratory syndrome, severe acute respiratory syndrome coronavirus 2, vertical transmission
Click Video under article title in Contents at ajog.org
Emerging infections have been shown to have an important impact on pregnant women and their fetuses,1 with the increased risk of complications in pregnant women with the 2009 pandemic H1N1 influenza virus2 and the severe fetal effects of Zika virus as recent examples.3 , 4 The emergence of a coronavirus not previously seen in humans, first reported in Wuhan, China, on Dec. 31, 2019, has attracted much interest throughout the world. Since then, the number of reported cases has increased rapidly, with more than 51,800 laboratory-confirmed cases and 1600 deaths as of Feb. 16, 2020.
Glossary of terms.
-
•
2019-nCoV: 2019-novel coronavirus (previous name for COVID-19 and SARS-CoV-2).
-
•
Basic reproduction number: estimate of number of individuals who will become infected from a single person in a population in which all individuals are susceptible.
-
•
CDC: US Centers for Disease Control and Prevention.
-
•
COVID-19: coronavirus disease 2019 (previously called 2019 novel coronavirus [2019-nCoV]; illness caused by SARS-CoV-2.
-
•
MERS: Middle East respiratory syndrome.
-
•
MERS-CoV: Middle East respiratory syndrome coronavirus, virus that causes Middle East respiratory syndrome (MERS).
-
•
N95 respirator: respiratory protective device that removes at least 95% of very small (0.3 μm) test particles; also called N95 filtering facepiece respirator.
-
•
SARS: severe acute respiratory syndrome.
-
•
SARS-CoV: severe acute respiratory syndrome coronavirus, virus that caused severe acute respiratory syndrome (SARS).
-
•
SARS-CoV-2: severe acute respiratory syndrome coronavirus 2 virus (current name of the novel coronavirus, according to the International Committee on Taxonomy of Viruses), virus that causes COVID-19.
-
•
WHO: World Health Organization.
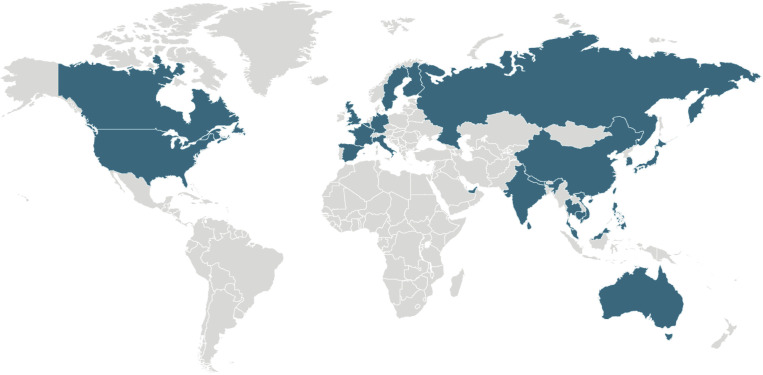
In addition to China, cases have spread to 25 other countries (Figure 1 ) including 15 cases in the United States. Initial outbreak data from China show a near exponential growth of reported cases.5 Reported numbers are likely underestimates of the true numbers because milder cases are less likely to be reported.
Figure 1.
Global map of confirmed COVID-19 cases
Global map of confirmed COVID-19 cases (as of Feb. 14, 2020) (from https://www.cdc.gov/coronavirus/2019-ncov/locations-confirmed-cases.html).
COVID-19, coronavirus disease 2019.
Rasmussen. 2019 novel coronavirus and pregnancy. Am J Obstet Gynecol 2020.
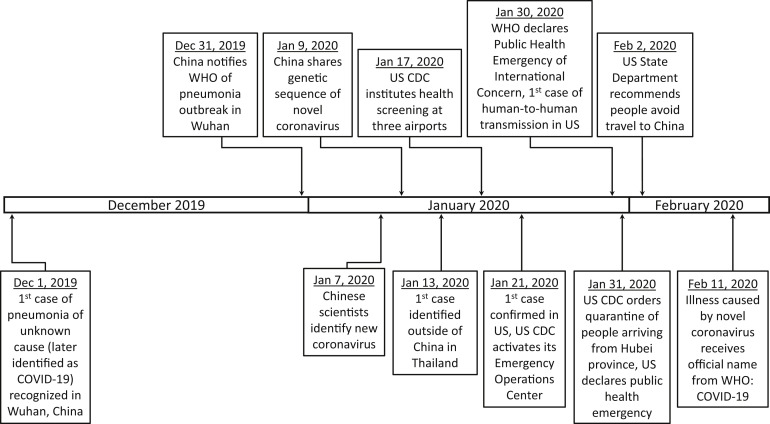
On Jan. 30, 2020, the World Health Organization declared the outbreak as a public health emergency of international concern; on Jan. 31, 2020, the United States declared a public health emergency, and the Centers for Disease Control and Prevention issued a federal quarantine for 195 Americans who traveled from Wuhan, China, its first federal quarantine in more than 50 years.
On Feb. 11, the new coronavirus disease (previously referred to as 2019 novel coronavirus (2019-nCoV)) received an official name from the World Health Organization (WHO), Coronavirus Disease 19 (COVID-19) (Figure 2 ).6 The International Committee on Taxonomy of Viruses has proposed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as the name of the virus that causes COVID-19.7
Figure 2.
Timeline showing key events in the COVID-19 outbreak
Timeline showing key events in the COVID-19 outbreak, Dec. 1, 2019, through Feb. 15, 2020.
CDC, Centers for Disease Control and Prevention; COVID-19, coronavirus disease 2019; US, United States; WHO, World Health Organization.
Rasmussen. 2019 novel coronavirus and pregnancy. Am J Obstet Gynecol 2020.
Coronaviruses are single-stranded RNA, nonsegmented, enveloped viruses, which cause illness ranging in severity from the common cold to severe and fatal illness. The term coronavirus derives from the Latin word corona, which means crown or halo; that designation arises from the appearance of coronavirus virions viewed by electron microscopy, in which the virus particles display a crown-like fringe typically referred to as spikes (Figure 3 ).
Figure 3.
Illustration of the causative virion for COVID-19
Reproduced, with permission, from the Centers for Disease Control and Prevention/Alissa Eckert, MS (obtained from the CDC’s Public Health Image Library; https://phil.cdc.gov/Details.aspx?pid=23312).
COVID-19, coronavirus disease 2019.
Rasmussen. 2019 novel coronavirus and pregnancy. Am J Obstet Gynecol 2020.
In the past 2 decades, 2 other coronaviruses that cause severe respiratory illness in humans have emerged: severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV). With the emergence of SARS-CoV-2, a third coronavirus that can cause severe respiratory illness has been identified. In a short period of time, this novel coronavirus has caused more cases of illness than were reported for MERS and SARS combined.
Here we summarize what is currently known about COVID-19 and what this means for practicing obstetricians and their pregnant patients. Because so little is currently known about COVID-19 in pregnancy, we also review available information on the effects of SARS and MERS during pregnancy to inform care of pregnant women with COVID-19 until additional data on pregnant women and their fetuses become available.
SARS and its effects on pregnant women
Severe acute respiratory syndrome (SARS) is caused by the SARS-CoV. Reports of the emergence of SARS-CoV appeared in February 2003, with the first cases observed in Guangdong Province in China. The virus spread to nearly 30 countries throughout the world, resulting in more than 8000 cases and 770 deaths.8
The outbreak was brought under control after public health control measures to reduce contact with infected persons were put into place, and no cases have been seen since 2004. Manifestations of SARS consist of fever, chills, headache, malaise, and myalgia. Diarrhea was seen in some patients. Pneumonia was nearly always seen in patients diagnosed with SARS, with mechanical ventilation being required in 10–20% of cases. Case fatality rate was estimated at 9–10% (Table ).
Table.
Comparison of characteristics of SARS, MERS, and COVID-19a
| Characteristics | SARS | MERS | COVID-19 |
|---|---|---|---|
| First patients reported | Guangdong, China, November 2002 | Zarga, Jordan, April 2012, and Jeddah, Saudi Arabia, June 2012 | Wuhan, China, December 2019 |
| Virus | SARS-CoV | MERS-CoV | SARS-CoV-2 |
| Type of coronavirus | Betacoronavirus | Betacoronavirus | Betacoronavirus |
| Host cell receptor | Angiotensin converting enzyme 2 | Dipeptidyl peptidase 4 | Structural analysis suggests angiotensin converting enzyme 2 receptor52 |
| Sequence similarity | Reference | 79% to SARS-CoV, 50% to MERS-CoV35 | |
| Animal hosts | Bats (natural reservoir), masked palm civet and raccoon dogs may be intermediate hosts | Bats (natural reservoir), dromedary camel (intermediate host) | Bats, animals sold at the seafood market in Wuhan might represent an intermediate host35 |
| Incubation period | |||
| Mean (95% CI, d) | 4.6 (3.8–5.8) | 5.2 (1.9–14.7) | 5.2 days (95% confidence interval [CI], 4.1–7.0); 95th percentile of the distribution was 12.5 days33 |
| Range, d | 2–14 | 2–13 | 2–14 |
| Time from illness onset until hospitalization | 2–8 days | 0–16 days | 12.5 days (mean) (95% CI, 10.3–14.8), onset before Jan. 1 9.1 days (mean); 95% CI, 8.6–9.7 (onset Jan. 1–11)33 |
| Basic reproduction number (R0)b | 2–3 | <1 | 2.2 (95% CI, 1.4–3.9)33 |
| Patient characteristics | |||
| Adults | 93% | 98% | Nearly all reported patients are adults |
| Children | 5–7% | 2% | Children have been infrequently reported (<1% of cases)39 |
| Age range, y | 1–91 | 1–94 | 10–89 y |
| Average age, y | Mean, 39.9 | Median, 50 | 59 years (median)33 |
| Sex ratio (M:F) | 43%:57% | 64.5%:35.5% | 56%:44%33 |
| Mortality | |||
| Case fatality rate overall | 9.6% | 35–40% | Initial estimate is 1%38 |
| Clinical manifestations | From hospitalized patients32,36,37 | ||
| Fever | 99–100% | 98% | 83–100% |
| Cough | 62–100% | 83% | 59–82% |
| Myalgia | 45–61% | 32% | 11–35% |
| Headache | 20–56% | 11% | 7–8% |
| Diarrhea | 20–25% | 26% | 2–10% |
| Laboratory findings | |||
| Radiographic abnormalities on chest imaging | 94–100% | 90–100% | 100% |
| Leukopenia | 25–35% | 14% | 9–25% |
| Lymphopenia | 65–85% | 32% | 35–70% |
| Thrombocytopenia | 40–45% | 36% | 5–12% |
COVID-19, coronavirus disease 2019; MERS, Middle East respiratory syndrome; MERS-CoV, Middle East respiratory syndrome coronavirus; SARS, severe acute respiratory syndrome; SARS-CoV, severe acute respiratory syndrome coronavirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Rasmussen. 2019 novel coronavirus and pregnancy. Am J Obstet Gynecol 2020.
Modified from Rasmussen et al23
Basic reproduction number, defined as average number of people who will become infected from a single infected person.
The natural reservoir for SARS-CoV is believed to be bats; however, some evidence supported civet cats or raccoon dogs as possible intermediate sources of these illnesses.8 SARS is transmitted by close person-to-person contact through contact of the mucus membranes of the respiratory tract with respiratory droplets formed when an infected person coughs or sneezes. Fecal-oral transmission and transmission via fomites have also been reported.8 Airborne spread because of inhalation of small particle aerosols may also be possible.
Transmission in health care settings was frequently seen during the 2003 outbreak, with superspreading (when a single patient transmits infection to a disproportionate number of contacts) reported.9 The incubation period was estimated at a mean of 4.6 days, with a range of 2–14 days. Transmission appeared to occur most often during the second week of illness when viral excretion is highest; there is no evidence that a person with SARS is contagious before symptom onset.
The largest case series of pregnant women with SARS was from the 2003 outbreak in Hong Kong, in which 12 pregnant women were identified.10 The case-fatality rate was 25% (3 deaths). Clinical and laboratory findings were similar to those seen in the nonpregnant population. Pneumonia on chest radiograph or computed tomography was seen in all patients. Major medical complications included adult respiratory distress syndrome in 4, disseminated intravascular coagulopathy (DIC) in 3, renal failure in 3, secondary bacterial pneumonia in 2, and sepsis in 2 patients.
Pregnancy outcomes varied by trimester of presentation.10 Among the 7 women who became ill in the first trimester, 4 had a spontaneous abortion, 2 had pregnancy terminations for social reasons after recovery from SARS, and 1 delivered a full-term healthy infant. Among the 5 women who presented after 24 weeks’ gestation, 4 delivered preterm. Three women delivered by cesarean delivery because of deteriorating maternal condition from their SARS illness at 26, 28, and 32 weeks’ gestation.11 These babies had birthweights appropriate for gestational age. Two of the infants had respiratory distress syndrome requiring surfactant (born at 26 and 28 weeks’ gestation), with one later developing bronchopulmonary dysplasia.
Gastrointestinal complications were observed in 2 infants, including a jejunal perforation in an infant delivered at 26 weeks and necrotizing enterocolitis with ileal perforation in an infant delivered at 28 weeks’ gestation. Whether these gastrointestinal complications were related to complications from SARS or its treatment or whether they were secondary to preterm delivery is unknown.11 The two infants who were delivered after their mothers’ recovery from SARS had intrauterine growth restriction. No clinical, radiologic, or laboratory evidence for transmission from mother to fetus was observed, despite laboratory testing of different specimens.12 , 13
A matched case-control study14 compared 10 of the 12 pregnant women noted in the previous text (2 were excluded because they were unable to be matched) with 40 nonpregnant women with SARS. Women were matched on sex, age, timing of contracting SARS, health care worker status, underlying illness, and whether the woman resided in a housing area in which there was a large outbreak.
Pregnancy appeared to have no effect on clinical symptoms or time to presentation after symptom onset. However, complications and adverse outcomes were more common among pregnant women: women who were pregnant had a longer hospital stay, were statistically significantly more likely to develop renal failure, sepsis, and DIC, and were more likely to require intensive care unit admission. Forty percent of pregnant women required mechanical ventilation, compared with 13% of nonpregnant patients (P = .07). Pregnant women were also significantly more likely to die (P = .01).
We identified 5 reports of additional cases of SARS during pregnancy treated in Hong Kong (n = 2), the United States (n = 2), and Canada (n = 1).15, 16, 17, 18, 19 Two of the 5 women required mechanical ventilation, 1 required hemodialysis for acute renal failure, and 1 had seizures and positive cerebrospinal fluid for SARS-CoV, suggestive of a central nervous system infection. All patients recovered from their illness. In 1 case, the pregnancy was terminated at the mother’s request; the remaining pregnancies ended in liveborn infants (2 at term and 2 preterm). Testing of neonatal specimens for SARS-CoV RNA was negative.
Several hospitals in Toronto and Hong Kong reported measures instituted on obstetrics services during the SARS outbreak to decrease transmission to pregnant women, their families, community members, and health care workers.20 , 21 For example, all hospital staff, patients, and visitors were screened for symptoms at the hospital entrance and wore N95 respirators. Visitors were limited to 1 per patient on labor and delivery, with no visitors allowed in the postpartum ward.
Postpartum stays were reduced in length with a postpartum nurse home visit added. Postpartum patients were asked to observe a 10 day home quarantine. Health care workers were asked to observe a work quarantine in which they were asked to go directly from home to work and vice versa to minimize interaction in the community. Obstetric services considered to be nonessential such as routine ultrasound and prenatal diagnosis were suspended. Although the impact of these interventions was not evaluated, there may be some relevant lessons learned from these experiences during SARS that could help inform the approach to COVID-19.
MERS and its effects on pregnant women
Middle East Respiratory Syndrome (MERS) is a respiratory illness caused by MERS-CoV. The illness was first identified in Saudi Arabia in 2012, with spread to other countries in the Arabian peninsula and eventually to countries outside the Arabian peninsula, including the United States.22 , 23 The largest outbreak outside the Arabian Peninsula was in the Republic of Korea in 2015.
Nearly 2500 cases of MERS-CoV illness and more than 860 deaths have been reported with continuing reports into the present. The manifestations of MERS include severe respiratory illness characterized by fever, cough, and shortness of breath. Some patients also have diarrhea. The case fatality rate is estimated to be 35–40%.
Patients who developed MERS were more likely to be older (median age is 50 years) with about two thirds of patients being male. Patients with MERS were also more likely to have an underlying illness. Some patients with MERS-CoV infection have been asymptomatic (identified through contact investigations).
The mean incubation period is 5.2 days, with a range of 2–13 days. As with SARS, MERS is mainly spread person to person through close contact, with transmission in health care settings, and superspreading events have been observed. However, since 2016, the number of cases of MERS-CoV has been dramatically reduced after public health efforts to prevent MERS-CoV transmission were put into place.24
Information on MERS among pregnant women is limited. We identified reports of 13 cases of pregnant women with MERS from several countries, including Saudi Arabia (n = 8), Korea (n = 2), Jordan (n = 1), United Arab Emirates (n = 1), and Philippines (n = 1).13 , 25, 26, 27, 28, 29, 30, 31 Two women were asymptomatic, identified as part of a contact investigation. Among the 11 symptomatic women, manifestations were similar to those seen in nonpregnant patients with MERS.
Seven of 13 patients were admitted to an intensive care unit for respiratory deterioration or acute respiratory distress syndrome, 5 required ventilator support, 3 died, and 8 recovered. Among the 3 deaths, the mothers died 8–25 days after delivery. Both babies born to asymptomatic women were born healthy at term; among those who were symptomatic, there was 1 intrauterine fetal demise, 1 stillbirth, 1 baby delivered at 25 weeks who died 4 hours after birth, 2 healthy preterm infants, and 5 healthy term infants (infant status was not mentioned for 1).
Coronavirus disease 2019 (COVID-19)
Clinical, epidemiologic, and viral characteristics
Respiratory illness caused by a novel coronavirus (now referred to as SARS-CoV-2) was first noted in December 2019 in Wuhan, Hubei Province, China. The WHO China Country office was notified of an outbreak of pneumonia of unknown etiology on Dec. 31, 2019 (Figure 2). Between Dec. 31, 2019, and Jan. 3, 2020, 44 cases were reported to the WHO. On Jan. 7, 2020, Chinese authorities identified a novel coronavirus as the cause. The virus has quickly spread first through Wuhan and subsequently to other areas of China and other countries in the world (Figure 1).
Early data suggested an association between the Huanan Seafood Wholesale Market and COVID-19 with 27 of 41 cases in 1 report32 and 26 of 47 in another report33 with epidemiologic links to the market, leading to closure of the market on Jan. 1, 2020. Given that the earliest case reported (illness onset on Dec. 1, 2019)32 did not have exposure to the market raises the possibility that the initial emergence into humans occurred elsewhere. However, sampling of the market’s environment supports the market’s importance in early transmission of the virus. Later cases were much less likely to have visited the market, supporting the role of person-to-person transmission in later cases.
The SARS-CoV-2 is a betacoronavirus similar to SARS-CoV and MERS-CoV (Table). Sequencing data show that the SARS-CoV-2 is most closely related to coronaviruses found in bats, with more than 85% nucleotide identity with a bat SARS-like CoV.34 , 35 The virus has 79% nucleotide identity to SARS-CoV and about 50% to MERS-CoV.35
Bats appear to be the natural reservoirs of both SARS-CoV and MERS-CoV. The emergence of these viruses in humans has been attributed to host switching: the virus jumped from an intermediary host species (eg, civet cats for SARS-CoV and dromedary camels for MERS-CoV) to humans. An intermediary host species is thought to be likely for SARS-CoV-2,35 although it has been yet to be identified. Sequence data show a high degree (>99.98%) of similarity of the virus among different patients, suggesting a recent emergence in humans.
Clinical manifestations of COVID-19 are similar to those with SARS and MERS (Table). Studies of hospitalized patients with COVID-19 show that patients commonly develop severe pneumonia, with 23–32% admitted to the intensive care unit and 17–29% of cases progressing to acute respiratory distress syndrome. 32 , 36 , 37 Among hospitalized patients, 4–15% have died.32 , 36 , 37 Overall case fatality ratio estimates (including asymptomatic and symptomatic infections) appear to be in the range of 1% (95% confidence interval, 0.5–4%),38 although these estimates should be considered preliminary.
Average age of hospitalized patients was 49–56 years, with 32–51% having an underlying illness. Most patients (54–73%) were men. Children with COVID-19 appear to be rarely identified, with only 28 children reported as of Jan. 30, 2020 (<1% of total), and most of those identified had mild symptoms.39 No pregnant women were reported in any of these initial cohorts. Common manifestations among hospitalized patients were fever (83–100%), cough (59–82%), myalgia (11–35%), headache (7–8%), and diarrhea (2–10%). All patients had abnormalities on radiographic imaging of the chest.
Person-to-person transmission of SARS-CoV-2 is thought to be similar to transmission of influenza and other respiratory pathogens; respiratory droplets are formed when an infected person coughs or sneezes and these droplets are inhaled by close contacts, generally within 6 feet. It is unclear whether infection can be transmitted from fomites. Fecal-oral transmission might be possible, given that SARS-CoV-2 has been identified in stool specimens40 and SARS-CoV might have been transmitted in this manner.41
The basic reproduction number, R0 (the average number of people who will become infected from a single infected person in a population in which all persons are susceptible) is affected by factors such as the duration of infectivity, the transmissibility of the pathogen, and the number of susceptible contacts. Measles, which is highly infective, has an R0 of 12–18, while 2009 H1N1 influenza and SARS have an R0 of 1.2–1.6 and 2–5, respectively.42 Current estimates of R0 for SARS-CoV-2 places it at 2.2 (95% confidence interval, 1.4–3.9)33 As with SARS and MERS, nosocomial transmission is playing a key role in transmission, presumed to be responsible for infection of 29% of affected health professionals and 12% of hospitalized patients in a recent study.37
Implications of COVID-19 for pregnant women
In the midst of a rapidly evolving outbreak that could have significant effects on our public health and medical infrastructure, the unique needs of pregnant women should be included in preparedness and response plans. In previous outbreaks, clinicians have at times been reluctant to treat or vaccinate pregnant women because of concerns for fetal safety.43 It is critical that pregnant women not be denied potentially life-saving interventions in the context of a serious infectious disease threat unless there is a compelling reason to exclude them. As with all decisions regarding treatment during pregnancy, carefully weighing of the benefits of interventions for the mother and fetus with potential risks is necessary. As surveillance systems for cases of COVID-19 are established, it is essential that information on pregnancy status, as well as maternal and fetal outcomes, be collected and reported.
Susceptibility to and severity of COVID-19 in pregnancy
Although data are limited, there is no evidence from other severe coronavirus infections (SARS or MERS) that pregnant women are more susceptible to infection with coronavirus. Thus far, in this outbreak of novel coronavirus infection, more men have been affected than women.32 , 33 , 36 , 37 This observed gender difference could be due to differences in reporting, susceptibility, exposure, or recognition and diagnosis of infection. There are no data to inform whether pregnancy increases susceptibility to COVID-19.
Previous data on SARS and MERS suggest that clinical findings during pregnancy can range from no symptoms to severe disease and death. The most common symptoms of COVID-19 are fever and cough, with more than 80% of hospitalized patients presenting with these symptoms.36
In a recent study by Chen et al,44 9 women diagnosed with COVID-19 during the third trimester of pregnancy were reported. In this small series, clinical presentation was similar to that seen in nonpregnant adults, with fever in 7, cough in 4, myalgia in 3, and sore throat and malaise each in 2 women. Five had lymphopenia. All had pneumonia, but none required mechanical ventilation, and none died. All women had a cesarean delivery, and Apgar scores were 8–9 at 1 minute and 9–10 at 5 minutes.
In a second series of 9 pregnancies with 10 infants (1 set of twins) reported by Zhu et al.,45 symptom onset was before delivery (1–6 days) in 4, on the day of delivery in 2, and after delivery (1–3 days) in 3 cases. Clinical presentation of COVID-19 was similar to that seen in nonpregnant patients. Among the 9 pregnancies, intrauterine fetal distress was noted in 6, 7 were cesarean deliveries, and 6 infants were born preterm. Based on these limited reports and the available data from other respiratory pathogens such as SARS and influenza, it is unknown whether pregnant women with COVID-19 will experience more severe disease.
Travel guidance for pregnant women
Travel recommendations have been instituted to limit exposure to persons in the United States. All persons, including pregnant women, should not travel to China. On Feb. 2, 2020, the US State Department upgraded their travel advisory to level 4, the highest level of travel advisory. Obstetric providers should obtain a detailed travel history for all patients and should specifically ask about travel in the past 14 days to areas experiencing widespread transmission of SARS-CoV-2. Currently this is limited to China, but this situation is rapidly evolving and obstetricians should stay alert to the global situation by consulting the Centers for Disease Control and Prevention website and following media coverage.
Vaccination in pregnancy
There is currently no vaccine to prevent COVID-19. Since posting of a SARS-CoV-2 virus genetic sequence online on Jan. 10, 2020, multiple organizations, including the National Institutes of Health, have been working to rapidly develop a COVID-19 vaccine. Development of this vaccine builds on and benefits from work on SARS and MERS vaccines.46 However, it is not known how quickly a safe and effective vaccine may be readily available.
Infection control measures and diagnostic testing
All patients, including pregnant women, should be evaluated for fever and signs and symptoms of a respiratory infection. Ideally, screening procedures begin before arrival on a labor and delivery unit or prenatal care clinic. For example, when scheduling appointments, patients should be instructed what to do if they have respiratory symptoms on the day of their appointment, or if a patient calls triage prior to presentation, respiratory signs and symptoms should be assessed over the telephone.
Those patients with respiratory symptoms should be separated from other waiting patients and a facemask should be placed on them. Patients who meet criteria for a person under investigation (Box 1 ) should be immediately placed in an airborne infection isolation room (single-patient rooms at negative pressure). Once in isolation, the patient’s facemask may be removed. Health care personnel should adhere to standard, contact, and airborne precautions. Infection control personnel and local/state health departments should be notified immediately; local/state health departments can help to arrange testing of relevant specimens (upper and lower respiratory specimens and serum are currently recommended; other specimens [stool and urine] may also be sent).
Box 1.
Criteria to guide evaluation of persons under investigation for COVID-19
| Clinical features | AND | Epidemiologic risk |
|---|---|---|
| Fevera or signs/symptoms of lower respiratory illness (eg, cough or shortness of breath) | AND | Any person, including health care workers, who has had close contactb with a laboratory-confirmed COVID-19 patient within 14 days of symptom onset |
| Fevera and signs/symptoms of a lower respiratory illness (eg, cough or shortness of breath) | AND | A history of travel from Hubei Province, China, within 14 days of symptom onset |
| Fevera and signs/symptoms of a lower respiratory illness (eg, cough or shortness of breath) requiring hospitalization | AND | A history of travel from mainland China within 14 days of symptom onset |
The criteria are intended to serve as guidance for evaluation. Patients should be evaluated and discussed with public health departments on a case-by-case basis if their clinical presentation or exposure history is equivocal (eg, uncertain travel or exposure) (see https://www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html for updates).
COVID-19, coronavirus disease 2019.
Rasmussen. 2019 novel coronavirus and pregnancy. Am J Obstet Gynecol 2020.
Fever may be subjective or confirmed
Close contact is defined as follows: (1) being within ∼6 feet (2 m) of a COVID-19 case for a prolonged period of time while not wearing recommended personal protective equipment (eg, gowns, gloves, NIOSH-certified disposable N95 respirator, eye protection); close contact can occur while caring for, living with, visiting, or sharing a health care waiting area or room with a COVID-19 case; OR (2) having direct contact with infectious secretions of a COVID-19 case (eg, being coughed on) while not wearing recommended personal protective equipment.
Management of COVID-19 in pregnancy
General principles regarding management of COVID-10 during pregnancy include early isolation, aggressive infection control procedures, testing for SARS-CoV-2 and coinfection, oxygen therapy as needed, avoidance of fluid overload, empiric antibiotics (because of secondary bacterial infection risk), fetal and uterine contraction monitoring, early mechanical ventilation for progressive respiratory failure, individualized delivery planning, and a team-based approach with multispecialty consultations (Box 2 ).
Box 2.
|
AIIR, airborne infection isolation room; CDC, Centers for Disease Control and Prevention; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Rasmussen. 2019 novel coronavirus and pregnancy. Am J Obstet Gynecol 2020.
All guidance should be considered subject to revision as additional data on pregnant women with COVID-19 become available.
Team-based management is recommended for pregnancies managed in a health care facility and should include a determination of the optimal clinical unit on which to provide care. Ability to provide surveillance for early detection of a worsening maternal course of illness, as well as an ability to monitor for evidence of obstetric complications (eg, preterm labor or fetal compromise), are needed.
Changes in fetal heart rate pattern may be an early indicator of maternal respiratory deterioration. Based on experience with SARS and MERS, severe respiratory failure might occur in pregnant women, and in the most severe cases, mechanical ventilation might not be sufficient to support adequate oxygenation. If that occurs, limited literature suggests a potential role of extracorporeal membrane oxygenation in pregnancy; use should be considered only in centers that have experience with this technique.47 Whether delivery provides benefit to a critically ill mother is unknown; decisions regarding delivery should consider the gestational age of the fetus and should be made in conjunction with the neonatologist.48
There are currently no antiviral medications approved by the US Food and Drug Administration for treatment of COVID-19, although broad-spectrum antivirals used in animal models of MERS are being evaluated for activity against SARS-CoV-2.46 Corticosteroids for the treatment of coronavirus-associated pneumonia should be avoided unless other indications are present because they were not shown to be beneficial in MERS and could lead to delayed MERS-CoV clearance.49 Therefore, decisions about the use of corticosteroids for fetal lung maturity should be made in consultation with infectious disease specialists and maternal-fetal medicine consultants. All guidance should be considered subject to revision as additional data on pregnant women with COVID-19 become available.
Care of infants born to mothers with COVID-19
Although the limited experience with newborn evaluations after delivery with SARS and MERS has not identified cases of maternal-to-fetal transmission, reports have appeared in the media of a 30 hour infant who was diagnosed with COVID-19, suggesting the possibility of in utero transmission.50 However, insufficient information is included in media reports to rule out perinatal or postnatal modes of transmission.
Data from the recent case series published by Chen et al44 and Zhu et al45 of 18 women (19 infants) infected in the third trimester of pregnancy with SARS-CoV-2 identified no laboratory evidence of vertical transmission. Testing of amniotic fluid, cord blood, and neonatal throat swab samples was negative for SARS-CoV-2 in the 6 patients reported by Chen et al.44
In the report by Zhu et al.,45 some infants were symptomatic (shortness of breath in 6, cyanosis in 3, gastric bleeding in 2, and 1 baby died of multiple organ failure and DIC); however, throat swab testing of all infants was negative for SARS-CoV-2 , suggesting that these neonatal complications might not be related intrauterine transmission. Thus, at this time, it is unknown whether SARS-CoV-2 can be transmitted from mother-to-fetus.
Given the current lack of information, it seems reasonable to assume that a newborn born to a mother with COVID-19 at delivery could possibly be infected, either in utero or perinatally, and thus should be placed in isolation to avoid exposure to other newborns. Although the ideal setting for a healthy infant is within a healthy mother’s room, temporary separation of an ill mother and her infant, as was recommended during pandemic H1N1,51 seems prudent.
Whether COVID-19 can be transmitted through breastmilk is unknown. We are aware of a single report of SARS-CoV testing of breastmilk in a mother who had recovered from SARS and no viral RNA was detected; however, the specimen was collected ∼130 days after illness onset.15 SARS-CoV antibodies were seen in breastmilk of that patient15 but not in another patient who was infected at 7 weeks’ gestation with breastmilk tested at postpartum days 12 and 30.16 Breastmilk was tested for SARS-CoV-2 in 6 of the mothers reported by Chen et al44; all specimens were negative.
Until additional data are available, mothers who intend to breastfeed and are well enough to express breastmilk should be encouraged to do so; breastfeeding can be instituted after she is no longer considered infectious. No data are available to guide length of separation and will need to be decided on a case-by-case basis after discussion between infection control experts and neonatologists.
Conclusions
The COVID-19 outbreak is rapidly increasing in the number of cases, deaths, and countries affected. Much is unknown about the virus and its effects, including its modes of transmission, the basic reproduction number, risk factors for illness, and case fatality rate. Although cases are primarily in China, it is highly likely that there will be additional global spread of the virus.
At the present time, limited data are available on pregnant women with COVID-19 on which to base recommendations for pregnancy-specific care; however, early reports and lessons from SARS, MERS, and other respiratory infections suggest that pregnant women could have a severe clinical course. Surveillance systems for cases of COVID-19 need to include information on pregnancy status as well as maternal and fetal outcomes.
It is important to be vigilant about the spread of the disease and be able to provide rapid implementation of outbreak control and management measures once the virus reaches a community. Standard interventions to manage any severe respiratory infection is the foundation of care for any pregnant woman with COVID-19 and should be implemented aggressively in a team-based care model.
Footnotes
The authors report no conflict of interest.
Supplementary Data
References
- 1.Rasmussen S.A., Hayes E.B. Public health approach to emerging infections among pregnant women. Am J Public Health. 2005;95:1942–1944. doi: 10.2105/AJPH.2004.054957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siston A.M., Rasmussen S.A., Honein M.A. Pandemic 2009 influenza A (H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303:1517–1525. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore C.A., Staples J.E., Dobyns W.B. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr. 2017;171:288–295. doi: 10.1001/jamapediatrics.2016.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasmussen S.A., Jamieson D.J., Honein M.A., Petersen L.R. Zika vrus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374:1981–1987. doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- 5.Zhao S, Lin Q, Ran J, et al. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis 2020;92:214–7. [DOI] [PMC free article] [PubMed]
- 6.World Health Organization Coronavirus disease (COVID-19) outbreak. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Available at:
- 7.Gorbalenya A.E., Baker S.C., Baric R.S. Severe acute respiratory syndrome-related coronavirus: the species and its viruses—a statement of the Coronavirus Study Group. https://www.biorxiv.org/content/10.1101/2020.02.07.937862v1.full.pdf Available at:
- 8.Hui D.S.C., Zumla A. Severe acute respiratory syndrome: Historical, epidemiologic, and clinical features. Infect Dis Clin North Am. 2019;33:869–889. doi: 10.1016/j.idc.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong G., Liu W., Liu Y., Zhou B., Bi Y., Gao G.F. MERS, SARS, and Ebola: the role of super-spreaders in infectious disease. Cell Host Microbe. 2015;18:398–401. doi: 10.1016/j.chom.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong S.F., Chow K.M., Leung T.N. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191:292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shek C.C., Ng P.C., Fung G.P. Infants born to mothers with severe acute respiratory syndrome. Pediatrics. 2003;112:e254. doi: 10.1542/peds.112.4.e254. [DOI] [PubMed] [Google Scholar]
- 12.Ng P.C., Leung C.W., Chiu W.K., Wong S.F., Hon E.K. SARS in newborns and children. Biol Neonate. 2004;85:293–298. doi: 10.1159/000078174. [DOI] [PubMed] [Google Scholar]
- 13.Park M.H., Kim H.R., Choi D.H., Sung J.H., Kim J.H. Emergency cesarean section in an epidemic of the middle east respiratory syndrome: a case report. Korean J Anesthesiol. 2016;69:287–291. doi: 10.4097/kjae.2016.69.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lam C.M., Wong S.F., Leung T.N. A case-controlled study comparing clinical course and outcomes of pregnant and non-pregnant women with severe acute respiratory syndrome. BJOG. 2004;111:771–774. doi: 10.1111/j.1471-0528.2004.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robertson C.A., Lowther S.A., Birch T. SARS and pregnancy: a case report. Emerg Infect Dis. 2004;10:345–348. doi: 10.3201/eid1002.030736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stockman L.J., Lowther S.A., Coy K., Saw J., Parashar U.D. SARS during pregnancy, United States. Emerg Infect Dis. 2004;10:1689–1690. doi: 10.3201/eid1009.040244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yudin M.H., Steele D.M., Sgro M.D., Read S.E., Kopplin P., Gough K.A. Severe acute respiratory syndrome in pregnancy. Obstet Gynecol. 2005;105:124–127. doi: 10.1097/01.AOG.0000151598.49129.de. [DOI] [PubMed] [Google Scholar]
- 18.Jiang X., Gao X., Zheng H. Specific immunoglobulin g antibody detected in umbilical blood and amniotic fluid from a pregnant woman infected by the coronavirus associated with severe acute respiratory syndrome. Clin Diagn Lab Immunol. 2004;11:1182–1184. doi: 10.1128/CDLI.11.6.1182-1184.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lau K.K., Yu W.C., Chu C.M., Lau S.T., Sheng B., Yuen K.Y. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis. 2004;10:342–344. doi: 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haines C.J., Chu Y.W., Chung T.K. The effect of Severe Acute Respiratory Syndrome on a hospital obstetrics and gynaecology service. BJOG. 2003;110:643–645. doi: 10.1016/S1470-0328(03)03007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owolabi T., Kwolek S. Managing obstetrical patients during severe acute respiratory syndrome outbreak. J Obstet Gynaecol Can. 2004;26:35–41. doi: 10.1016/S1701-2163(16)30694-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bialek S.R., Allen D., Alvarado-Ramy F. First confirmed cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infection in the United States, updated information on the epidemiology of MERS-CoV infection, and guidance for the public, clinicians, and public health authorities, May 2014. MMWR Morb Mortal Wkly Rep. 2014;63:431–436. [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmussen S.A., Watson A.K., Swerdlow D.L. Middle East respiratory syndrome (MERS) Microbiol Spectr. 2016;4 doi: 10.1128/microbiolspec.EI10-0020-2016. [DOI] [PubMed] [Google Scholar]
- 24.Donnelly C.A., Malik M.R., Elkholy A., Cauchemez S., Van Kerkhove M.D. Worldwide reduction in MERS cases and deaths since 2016. Emerg Infect Dis. 2019;25:1758–1760. doi: 10.3201/eid2509.190143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alfaraj S.H., Al-Tawfiq J.A., Memish Z.A. Middle East respiratory syndrome coronavirus (MERS-CoV) infection during pregnancy: report of two cases and review of the literature. J Microbiol Immunol Infect. 2019;52:501–503. doi: 10.1016/j.jmii.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alserehi H., Wali G., Alshukairi A., Alraddadi B. Impact of Middle East respiratory syndrome coronavirus (MERS-CoV) on pregnancy and perinatal outcome. BMC Infect Dis. 2016;16:105. doi: 10.1186/s12879-016-1437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Assiri A., Abedi G.R., Al Masri M., Bin Saeed A., Gerber S.I., Watson J.T. Middle East respiratory syndrome coronavirus infection during pregnancy: a report of 5 cases from Saudi Arabia. Clin Infect Dis. 2016;63:951–953. doi: 10.1093/cid/ciw412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malik A., El Masry K.M., Ravi M., Sayed F. Middle East respiratory syndrome coronavirus during pregnancy, Abu Dhabi, United Arab Emirates, 2013. Emerg Infect Dis. 2016;22:515–517. doi: 10.3201/eid2203.151049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Payne D.C., Iblan I., Alqasrawi S. Stillbirth during infection with Middle East respiratory syndrome coronavirus. J Infect Dis. 2014;209:1870–1872. doi: 10.1093/infdis/jiu068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Racelis S., de los Reyes V.C., Sucaldito M.N., Deveraturda I., Roca J.B., Tayag E. Contact tracing the first Middle East respiratory syndrome case in the Philippines, February 2015. Western Pac Surveill Response J. 2015;6:3–7. doi: 10.5365/WPSAR.2015.6.2.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeong S.Y., Sung S.I., Sung J.H. MERS-CoV infection in a pregnant woman in Korea. J Korean Med Sci. 2017;32:1717–1720. doi: 10.3346/jkms.2017.32.10.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed]
- 33.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020;382:1199–207. [DOI] [PMC free article] [PubMed]
- 34.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–33. [DOI] [PMC free article] [PubMed]
- 35.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020;395:565–74. [DOI] [PMC free article] [PubMed]
- 36.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–13. [DOI] [PMC free article] [PubMed]
- 37.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA, in press. [DOI] [PMC free article] [PubMed]
- 38.Dorigatti I., Okell L., Cori A. Report 4: severity of 2019-novel coronavirus (nCoV). WHO Collaborating Centre for Infectious Disease Modelling, MRC Centre for Global Infectious Disease Analysis, Imperial College London. https://www.imperial.ac.uk/media/imperial-college/medicine/sph/ide/gida-fellowships/Imperial-College-2019-nCoV-severity-10-02-2020.pdf Available at:
- 39.Shen KL, Yang YH. Diagnosis and treatment of 2019 novel coronavirus infection in children: a pressing issue. World J Pediatr, in press. [DOI] [PMC free article] [PubMed]
- 40.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020;382:929–36. [DOI] [PMC free article] [PubMed]
- 41.Yu I.T., Li Y., Wong T.W. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med. 2004;350:1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- 42.Chen J. Pathogenicity and transmissibility of 2019-nCoV—a quick overview and comparison with other emerging viruses. Microbes Infect 2020;22:69–71. [DOI] [PMC free article] [PubMed]
- 43.Haddad L.B., Jamieson D.J., Rasmussen S.A. Pregnant women and the Ebola crisis. N Engl J Med. 2018;379:2492–2493. doi: 10.1056/NEJMp1814020. [DOI] [PubMed] [Google Scholar]
- 44.Chen H., Guo J., Wang C. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020 doi: 10.1016/S0140-6736(20)30360-3. published online Feb. 12, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr 2020;9:51–60. [DOI] [PMC free article] [PubMed]
- 46.Paules CI, Marston HD, Fauci AS. Coronavirus infections—more than just the common cold. JAMA, in press. [DOI] [PubMed]
- 47.Pacheco L.D., Saade G.R., Hankins G.D.V. Extracorporeal membrane oxygenation (ECMO) during pregnancy and postpartum. Semin Perinatol. 2018;42:21–25. doi: 10.1053/j.semperi.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 48.Lapinsky S.E. Management of acute respiratory failure in pregnancy. Semin Respir Crit Care Med. 2017;38:201–207. doi: 10.1055/s-0037-1600909. [DOI] [PubMed] [Google Scholar]
- 49.Arabi Y.M., Mandourah Y., Al-Hameed F. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 50.D'Amore R. Can coronavirus pass from mother to baby? Maybe, but experts need more research. Global news. https://globalnews.ca/news/6515302/coronavirus-mother-baby-transmission/ Posted Feb. 7 Available at: 2020. Accessed Feb. 10, 2020.
- 51.Rasmussen S.A., Kissin D.M., Yeung L.F. Preparing for influenza after 2009 H1N1: special considerations for pregnant women and newborns. Am J Obstet Gynecol. 2011;204:S13–S20. doi: 10.1016/j.ajog.2011.01.048. [DOI] [PubMed] [Google Scholar]
- 52.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J Virol 2020 Mar 17;94(7). [DOI] [PMC free article] [PubMed]
- 53.Centers for Disease Control and Prevention Interim infection prevention and control recommendations for patients with confirmed 2019 novel coronavirus (2019-nCoV) or patients under investigation for 2019-nCoV in healthcare settings. Centers for Disease Control and Prevention. Updated Feb. 3, 2020. https://www.cdc.gov/coronavirus/2019-nCoV/hcp/infection-control.html Available at:
- 54.Centers for Disease Control and Prevention Interim guidance for implementing home care of people not requiring hospitalization for 2019 novel coronavirus (2019-nCoV). Updated Jan. 31, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-home-care.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fguidance-home-care.html Available at:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.