Abstract
Objective
Ribavirin and corticosteroids were used widely as front-line treatments for severe acute respiratory syndrome; however, previous evaluations were inconclusive. We assessed the effectiveness of ribavirin and corticosteroids as the initial treatment for severe acute respiratory syndrome using propensity score analysis.
Methods
We analyzed data on 1755 patients in Hong Kong and 191 patients in Toronto with severe acute respiratory syndrome using a generalized propensity score approach.
Results
The adjusted excess case fatality ratios of patients with severe acute respiratory syndrome receiving the combined therapy of ribavirin and corticosteroids within 2 days of admission, compared with those receiving neither treatment within 2 days of admission, were 3.8% (95% confidence interval, −1.5 to 9.2) in Hong Kong and 2.1% (95% confidence interval, −44.3 to 48.5) in Toronto.
Conclusions
Our results add strength to the hypothesis that the combination of ribavirin and corticosteroids has no therapeutic benefit when given early during severe acute respiratory syndrome infection. Further studies may investigate the effects of these treatments later in disease course.
Keywords: Corticosteroids, Effectiveness, Propensity score, Ribavirin, Severe acute respiratory syndrome
The severe acute respiratory syndrome outbreak in 2002 and 2003 infected more than 8000 people in 29 territories with an overall worldwide case fatality ratio of 9.6%.1 Hong Kong and Toronto bore a significant proportion of the global case burden, together accounting for 25% of all “probable cases.”1 Although there have been only sporadic human infections with the severe acute respiratory syndrome coronavirus since the first global outbreak,2 the possibility of widespread reemergence cannot be ruled out given the continued trading and consumption of palm civets, a likely animal reservoir.3, 4 Meanwhile, severe acute respiratory syndrome-like coronaviruses are endemic in bats in Hong Kong and southern China.5, 6
Clinical Significance.
-
•
Previous evaluations on the effectiveness of ribavirin and corticosteroids in treating severe acute respiratory syndrome were inconclusive.
-
•
On the basis of a large dataset with 25% of global severe acute respiratory syndrome cases and properly adjusted for treatment selection bias, the combination of ribavirin and corticosteroids was found to have no significant beneficial effect in the initial treatment of patients with severe acute respiratory syndrome.
Ribavirin has broad activity against many DNA and RNA viruses.7 Corticosteroids can be protective against extensive inflammatory lung damage.8, 9 Therefore, these 2 drugs were recommended for use as front-line treatments during the outbreak, although not without controversy.10 Evaluation of the effectiveness of these 2 drugs in the treatment of severe acute respiratory syndrome has consisted largely of uncontrolled studies and small hospital cohorts. A recent meta-analysis confirmed that there are few reliable data on which to base future treatment decisions in severe acute respiratory syndrome, largely because of the lack of adjustment for important confounding variables in evaluative studies published to date.11 Although randomized controlled trials would provide more conclusive evidence, they cannot be conducted unless large future outbreaks occur,12 and in the meantime it is critical that current recommendations, both for the treatment of patients and for the identification of those agents that should be prioritized for testing in future trials, are based on the best available data.
We analyzed data on patients with severe acute respiratory syndrome from Hong Kong and Toronto to assess and compare the treatment efficacies, as measured by mortality reduction, of early administration of ribavirin alone, corticosteroids alone, or both in combination. We used propensity score analysis to adjust for potential confounding effects, an approach that has been proposed as the most valid analytic strategy for observational data in the absence of a randomized design13 and has been used to evaluate drug effectiveness in many observational studies.14, 15, 16, 17, 18 In addition to providing important evidence-based guidance for future human cases of severe acute respiratory syndrome, our work may serve as a template for the evaluation of treatment efficacies for other emerging infectious diseases.
Materials and Methods
Data Sources
We analyzed an integrated database containing clinical and epidemiologic details on all 1755 patients in Hong Kong reported to have “probable severe acute respiratory syndrome” according to the World Health Organization definition,19 derived from the Hong Kong Hospital Authority Electronic Severe Acute Respiratory Syndrome system and the Hong Kong Department of Health's Master list.19, 20 For the Toronto data, the Canadian Severe Acute Respiratory Syndrome Research Network conducted a retrospective cohort study of all probable severe acute respiratory syndrome cases among adults (age ≥ 16 years) admitted to a hospital during the outbreak.20, 21 Medical charts were reviewed by trained research staff, and clinical, radiographic, and laboratory data were double entered into a database.
Key patient characteristics at hospital admission that were extracted from these 2 sources included gender, age, occupation, preexisting comorbid conditions, calendar date of symptom onset, delay between symptom onset and admission, lactate dehydrogenase level, chest radiograph findings, neutrophil count, platelet count, lymphocyte count, and oxygen saturation. We classified patients into 4 treatment groups based on whether they had started a course of ribavirin, corticosteroids, both ribavirin and corticosteroids, or neither drug within 2 days of admission to hospital.
We included all patients in Hong Kong. In Toronto the outbreak occurred in 2 waves (first wave: March 2 to April 21; second wave: April 22 to June 10),22 and ribavirin was not used at all during the second wave because of clinical experience in the first wave.12 In this analysis we require that each patient had the opportunity to be treated with ribavirin or corticosteroids, and therefore we excluded the 94 patients in Toronto who were admitted to hospital during the second wave. We excluded the 12 patients (0.6%) in Hong Kong and 2 patients (1%) in Toronto who died within 2 days of admission.
Statistical Analysis
In randomized controlled trials, the randomization process should on average balance patient characteristics across treatment groups, and comparison of crude event rates across treatment groups should be unconfounded,23 although adjustment for potential confounders may improve precision.24 However, in situations in which treatment assignment is not random, but may depend on patients' presenting characteristics and clinical course, failure to appropriately adjust for such factors may lead to misleading findings. Standardization or stratification can be carried out for each characteristic but is typically not feasible when there are a large number of potential confounders. Regression adjustment is often used provided there are sufficient events; a simple rule of thumb is 20 events (eg, deaths) per potential confounder.25
An alternative approach is the use of propensity score analysis.13 Under this approach, a model is specified to explain the assignment of treatments in terms of patient characteristics. The predicted probabilities, or “propensity scores” from this model are in turn incorporated in a successive model to assess the association between the treatment and the outcome of interest. In the latter model, the estimate of the treatment effect should reflect adjustment for differences in the observed patient characteristics between treatment groups.
To study the relative effects on mortality of the 4 treatment groups, namely, neither drug, ribavirin alone, corticosteroids alone, or both drugs, we used a generalized propensity score approach that can allow for more than 2 treatment choices.26 We estimated the propensity scores using a multinomial regression model adjusting for the key patient characteristics at admission as described above and 3 other factors, including calendar date of onset, onset-to-admission delay, and admitting hospital. Because some prognostic factors were only available for a subset of approximately 30% of the patients in Hong Kong and 60% of the patients in Toronto,20 we used multiple imputation to make best use of all available data in the propensity score model.27, 28, 29, 30 Ten datasets were constructed on the basis of all clinical, demographic, and outcome variables with imputed values based on a generalized additive model and predictive mean matching (function aregImpute in R library Hmisc). We used chi-square tests to evaluate whether the propensity scores were able to balance observed covariates across treatment groups. We then weighted the observed individual outcome by the inverse estimated propensity scores to infer adjusted case fatality ratios in each treatment arm.26 We used case-resampling bootstrap with 1000 repetitions in each imputed dataset to estimate 95% confidence intervals (CIs) for the case fatality ratios.31
For the primary analysis we focused on treatment choice within 2 days of hospital admission, because early treatment decisions were most likely based on patient characteristics at hospital admission as opposed to later treatment decisions that also would have been affected by the effects of initial treatments and the subsequent course of illness. We carried out sensitivity analyses to examine the effectiveness of treatment assignments within 1 to 5 days of admission. Further technical details of the methods are described in the Appendix. All analyses were conducted in R version 2.3.1.32
This study received ethics approval from the institutional review boards of the University of Hong Kong/Hospital Authority Hong Kong West Cluster, Hong Kong; McMaster University, Hamilton, Ontario; the University of Toronto, Toronto; and all Toronto-area hospitals where data were collected.
Results
Table 1 summarizes the patient characteristics in Hong Kong and Toronto, by treatment within 2 days of admission. More detailed comparison of the characteristics is given in Appendix Table 1. There were statistically significant differences (P < .001) in all demographic variables across the 4 treatment groups in Hong Kong, whereas clinical variables were relatively more balanced across groups. For patients in Toronto, the distribution of characteristics was more balanced across treatment groups, especially in demographic variables, but there were greater differences in delay between symptom onset and admission, lactate dehydrogenase level, and neutrophil count (P ≤ .05). In Hong Kong and Toronto, 301 (17.3%) and 25 (12.6%) deaths occurred in total, respectively. The crude case fatality ratios were higher in the patients untreated with ribavirin or corticosteroids within 2 days of admission (23.3% and 20.0%, respectively) compared with those treated with ribavirin and corticosteroids within 2 days of admission (12.6% and 12.8%, respectively). The crude combined treatment effect of ribavirin and corticosteroids in Hong Kong and Toronto, in terms of absolute risk reduction, was 10.7% and 7.2%, respectively.
Table 1.
Characteristics at Hospital Admission of All Patients with Probable Severe Acute Respiratory Syndrome in Hong Kong and Patients Infected in the First Phase of the Toronto Epidemica (Onset before April 22, 2003)
| Characteristic | Hong Kong (n = 1743) |
Toronto (n = 191) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neither Treatment | (%) | Ribavirin Only | (%) | Corticosteroids Only | (%) | Ribavirin and Corticosteroids | (%) | Neither Treatment | (%) | Ribavirin Only | (%) | Ribavirin and Corticosteroids | (%) | |
| Men | 379 | (50.5) | 85 | (42.1) | 23 | (45.1) | 286 | (38.7) | 16 | (35.6) | 40 | (37.4) | 18 | (46.2) |
| Age ≤ 39 y | 297 | (39.5) | 123 | (60.9) | 19 | (37.3) | 403 | (54.5) | 18 | (40.0) | 43 | (40.2) | 14 | (35.9) |
| Age 40-49 y | 124 | (16.5) | 36 | (17.8) | 6 | (11.8) | 172 | (23.3) | 9 | (20.0) | 26 | (24.3) | 9 | (23.1) |
| Age 50-59 y | 73 | (9.7) | 15 | (7.4) | 7 | (13.7) | 93 | (12.6) | 7 | (15.6) | 16 | (15.0) | 7 | (17.9) |
| Age 60-69 y | 70 | (9.3) | 14 | (6.9) | 8 | (15.7) | 30 | (4.1) | 4 | (8.9) | 13 | (12.1) | 5 | (12.8) |
| Age > 69 y | 187 | (24.9) | 14 | (6.9) | 11 | (21.6) | 41 | (5.5) | 7 | (15.6) | 9 | (8.4) | 4 | (10.3) |
| Health care worker | 120 | (16.0) | 72 | (35.6) | 7 | (13.7) | 205 | (27.7) | 14 | (31.1) | 48 | (44.9) | 16 | (41.0) |
| With preexisting comorbid conditions | 220 | (29.3) | 28 | (13.9) | 18 | (35.3) | 90 | (12.2) | 11 | (24.4) | 18 | (16.8) | 5 | (12.8) |
| Data on comorbid conditions missing | 2 | (0.3) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Died | 175 | (23.3) | 18 | (8.9) | 15 | (29.4) | 93 | (12.6) | 9 | (20.0) | 10 | (9.3) | 5 | (12.8) |
Only 3 patients were treated with corticosteroids in Toronto and were excluded.
In Hong Kong, younger patients, health care workers, those with a longer delay between symptom onset and admission, and those with earlier calendar date of symptom onset were more likely to be treated with both ribavirin and corticosteroids (Appendix Table 2). In Toronto, patients with younger age, longer delay between symptom onset and admission, high lactate dehydrogenase level, or hazy chest radiograph were more likely to be treated with both ribavirin and corticosteroids. After adjustment for propensity scores, balance was achieved among all patient characteristics (test for heterogeneity across treatment groups not explained by propensity score, P > .24 for all covariates in Hong Kong; P > .10 for all covariates in Toronto).
Table 2 shows the estimated case fatality ratio by treatment group in Hong Kong and Toronto based on the generalized propensity score weighting. By adjusting for patient characteristics, the model predicted that the overall case fatality ratio would have been highest (19.2%) if all patients in Hong Kong had been treated with both ribavirin and corticosteroids within 2 days of admission, whereas it would have been lowest (15.4%) if no Hong Kong patients had been treated with ribavirin or with corticosteroids within 2 days of admission. The difference in case fatality ratios was 3.8% (95% CI, −1.5 to 9.2, P = .16), which suggests at most no effectiveness of combined therapy with ribavirin and corticosteroids, and is inconsistent with even a mildly beneficial effect of these treatments. The results for Toronto were generally consistent albeit with wide confidence bounds because of the relatively small sample size (Table 2), and the adjusted excess case fatality ratios for ribavirin and corticosteroids versus neither treatment within 2 days of admission was 2.1% (95% CI, −44.3 to 48.5, P = .93).
Table 2.
Case Fatality Ratios and Associated 95% Confidence Intervals in Hong Kong and Toronto by Treatment Received within 2 Days of Hospital Admission, Estimated by Generalized Propensity Score Analysis
| Treatment | Hong Kong |
Toronto |
||||
|---|---|---|---|---|---|---|
| Case Fatality Ratioa (%) | 95% CI | P Valueb | Case Fatality Ratioa (%) | 95% CI | P Valueb | |
| Neither ribavirin nor corticosteroids | 15.4 | (13.2-17.6) | — | 16.6 | (0.0-44.9) | — |
| Ribavirin | 17.0 | (6.2-27.8) | .77 | 13.4 | (0.0-33.0) | .85 |
| Corticosteroids | 18.9 | (4.6-33.1) | .64 | — | — | — |
| Both | 19.2 | (14.2-24.3) | .16 | 18.7 | (0.0-54.7) | .93 |
CI = confidence interval.
Each case fatality ratio is the predicted case fatality ratio had all severe acute respiratory syndrome cases been assigned to the corresponding treatment group.
P value for a difference in case fatality ratio compared with no treatment.
In sensitivity analyses, Figures 1 and 2 show the estimated case fatality ratios in Hong Kong and Toronto, respectively, for 5 separate analyses in which patients were grouped by the treatment they had received within days of hospital admission for d = 1, 2, … , 5. For Hong Kong, the estimated case fatality ratios were consistent with the main results in Table 2 and generally stable across the different cutoffs, except for the small numbers of patients receiving corticosteroids only. Appendix Table 3 shows the estimated adjusted odds ratio of case fatality ratios among treatments, based on conventional multinomial logistic regression models on 10 imputed datasets. For Hong Kong, the estimated odds ratio is consistent with the main results in Table 2, where patients treated with both ribavirin and corticosteroids had a higher case fatality ratio and the difference was marginally statistically significant. For Toronto, the results were not statistically significant with wide CIs.
Figure 1.
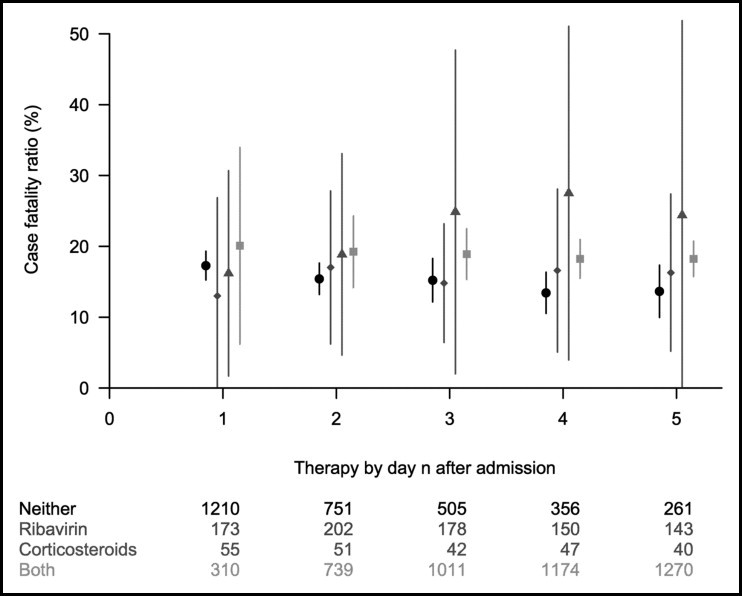
Estimated case fatality ratios and associated 95% CIs for alternative treatment strategies in Hong Kong (circle: neither treatment; diamond: ribavirin only; triangle: corticosteroids only; square: both treatments) within 1 to 5 days of hospital admission of all patients with probable severe acute respiratory syndrome, using generalized propensity score analysis.
Figure 2.
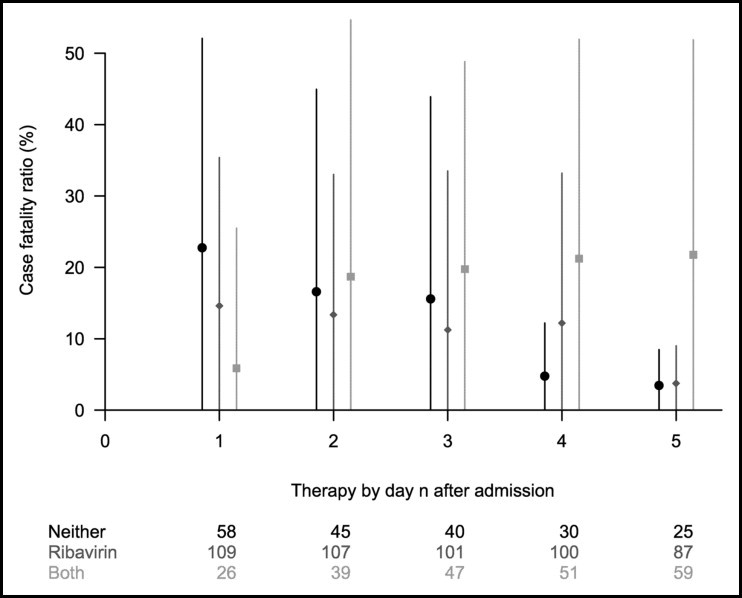
Estimated case fatality ratios and the associated 95% CIs under alternative treatment strategies in Toronto (circle: neither treatment; diamond: ribavirin only; triangle: corticosteroids only; square: both treatments) within 1 to 5 days of hospital admission of all patients with probable severe acute respiratory syndrome, using generalized propensity score analysis.
Discussion
During the 2003 severe acute respiratory syndrome outbreaks in Hong Kong and Toronto, ribavirin and corticosteroids were widely used, although no controlled studies of their effectiveness were performed because of the novelty of the causative agent. This study used propensity score methods to estimate the effectiveness of ribavirin and corticosteroids for severe acute respiratory syndrome early in the course of disease, adjusting for differences in patient characteristics between treatment arms.11 For example, the most significant factors associated with severe acute respiratory syndrome fatality in Hong Kong are age more than 60 years old, preexisting comorbid conditions, and non-health care worker status,19, 33 all of which were more commonly present for patients not receiving ribavirin or corticosteroids (Table 1). The high crude case fatality ratio for patients receiving neither treatment was likely because of this case mix that was fully adjusted in our propensity score analysis.12
We did not find any evidence that early treatment with both ribavirin and corticosteroids was beneficial to patients in general, which is consistent with previous studies.34, 35, 36, 37 By observing that there were higher case fatality ratios for the ever treated patients but lower case fatality ratios in patients treated within 2 days on hospital admission, it is possible that treatment decisions made later during hospitalization depended on whether patients' health status was improving or deteriorating rather than baseline clinical characteristics. Without detailed data on clinical progression, which we do not have, we cannot assess the potential benefits of therapies later in the clinical course and in particular as rescue treatment. It is always easier to act with the benefit of hindsight, and our results should not be taken as a criticism of those early prescribing decision but rather as an evaluation of treatment strategies based on information that has since become available. This also points to the need for common data-collection protocols and specification of a standardized dataset (or minimum dataset), which may facilitate integration of data during the initial period of epidemics and allow more comprehensive retrospective analyses after epidemics.
Early in the severe acute respiratory syndrome epidemic when the causative agent was still unknown, ribavirin, along with other broad-spectrum antibiotics, was used in Hong Kong and Toronto because of its in vitro activity against a broad spectrum of viral agents. On this basis, it was thought that ribavirin had the potential to suppress acute viral replication in the early phase of severe acute respiratory syndrome.38, 39 Early treatment with ribavirin was considered important because the viral load in patients with severe acute respiratory syndrome peaked at day 10 after symptom onset,9 and the peak level was independently associated with mortality.40 However, the in vivo inhibitory effect of ribavirin at clinically achievable doses remains controversial, significant adverse events are associated with ribavirin,37, 41 and our findings suggest that it is unlikely that ribavirin had any significant beneficial effect on patient outcome.
Corticosteroids were used to reduce pulmonary damage because of the immune-mediated inflammatory responses that usually started in the second week.9, 42 However, early corticosteroids treatment during the stage of viral replication may have suppressed the immune response and allowed a higher peak viral level.43, 44 The clinical effectiveness of corticosteroid therapy has not been established for severe acute respiratory syndrome45, 46 or acute respiratory distress syndrome,47 and corticosteroids use in severe acute respiratory syndrome was associated with aspergillus superinfection, avascular necrosis, reduced bone mineral density, and other long-term adverse outcomes.48, 49, 50
Limitations
A limitation of our analysis is that a propensity score model cannot adjust for unmeasured confounding variables and that the unconfoundedness assumption of propensity score models is inherently untestable. However, given that our analysis included and balanced the most important variables that have been identified as predictive of survival,20, 34, 51 the best effort was made to control for potential confounders. Our data on treatment assignments are limited to information on the first date of ribavirin use and the first date of corticosteroid use; therefore, we have been unable to investigate alternative strategies of treatment administration, for example, whether corticosteroids were pulsed or not or variations in dosage or duration of treatment. We do not have data on other treatments, such as lopinavir/ritonavir, which was associated with improved clinical outcomes in a small uncontrolled study,52 or traditional Chinese medicine.53 The main analyses were based on treatment choice within 2 days of admission; however, results were consistent when treatment within 1, 3, 4, or 5 days of admission was considered. A further limitation is the presence of substantial proportions of missing data on some clinical variables. We implemented multiple imputation to use all available data in the analysis, which is considered the gold standard approach,54 and the estimated treatment effects were consistent with those estimated in a complete case analysis (data not shown). Another limitation is that outcomes other than death were not considered; common side effects such as avascular necrosis due to corticosteroid therapy might have a significant impact on long-term quality of life, but such outcomes were not considered in this study.48, 49 A more complete comparison between treatments should consider both nonfatal adverse drug effects and quality of life. A complete dataset including disease progression and all clinical events (eg, ventilation) was not available. Finally, our analyses were based on patients with probable severe acute respiratory syndrome according to the World Health Organization definition.55 We adopted this definition rather than laboratory confirmation because the latter definition may be potentially biased toward including more survivors, particularly in the early cases.19
Conclusions
On the basis of a large sample from 2 epicenters, our results add strength to the hypothesis that the combination of ribavirin and corticosteroids has no significant beneficial effect in the treatment of severe acute respiratory syndrome. In the absence of further evidence, clinicians should not use ribavirin and corticosteroids to treat severe acute respiratory syndrome because to the best of our knowledge, they provide no benefit in terms of survival. Investigators should consider other promising therapies in the event of another outbreak of severe acute respiratory syndrome, and ideally protocols would be in place to evaluate alternative treatments in randomized trials.
Acknowledgments
We thank colleagues in the Department of Health and Hospital Authority involved with the public health control of and clinical care associated with the severe acute respiratory syndrome epidemic and for data collection and processing.
Footnotes
Funding: This work was supported in part through a research grant from the Research Fund for the Control of Infectious Diseases of the Food and Health Bureau of the Hong Kong Special Administrative Region Government (grant no. HKU-AA-018) and by the Canadian Institutes for Health Research.
Conflict of Interest: None of the authors have any conflicts of interest associated with the work presented in this manuscript.
Authorship: All authors had access to the data and played a role in writing this manuscript.
Appendix
For the primary analysis that compares all 4 treatment choices within the first 2 days after admission, we applied a multivariable multinomial regression model to estimate the propensity scores for each of the 4 treatment alternatives, including variables that were likely to be influential in early treatment decisions. The latter included the following known prognostic factors: age, gender, health care worker, presence of comorbid condition, hospital, delay in admission, onset date, lactate dehydrogenase level, chest radiograph, oxygen saturation on room air, neutrophil count, platelets, and lymphocyte count,20 and 3 other factors that may have been potentially important in treatment assignment, including date of onset, onset-to-admission delay, and hospital. Because some prognostic factors were only available for a subset of up to approximately 30% of the patients in Hong Kong and 60% of the patients in Toronto,20 we used multiple imputation to facilitate inclusion of these factors in the propensity score model.27 The resulting propensity scores were then used to weigh the case fatality ratios in each treatment group, allowing estimation of the mean causal effect,26 that is, for each treatment group we estimated the overall case fatality ratios had all patients been assigned to that particular treatment. Let p it be the estimated propensity score for patient i with treatment t, D i be 1 for a death outcome for patient i, and I be an indicator function; then the propensity score-adjusted case fatality ratio for each treatment t is given by:
Appendix Table 1.
Characteristics at Hospital Admission of all Patients with Probable Severe Acute Respiratory Syndrome in Hong Kong and Patients Infected in the First Phase of the Toronto Epidemica (Onset before April 22, 2003)
| Characteristic | Hong Kong |
Toronto |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neither Treatment | (%) | Ribavirin Only | (%) | Corticosteroids Only | (%) | Ribavirin and Corticosteroids | (%) | Neither Treatment | (%) | Ribavirin Only | (%) | Ribavirin and Corticosteroids | (%) | |
| Gender | ||||||||||||||
| Women | 372 | (49.5) | 117 | (57.9) | 28 | (54.9) | 453 | (61.3) | 29 | (64.4) | 67 | (62.6) | 21 | (53.8) |
| Men | 379 | (50.5) | 85 | (42.1) | 23 | (45.1) | 286 | (38.7) | 16 | (35.6) | 40 | (37.4) | 18 | (46.2) |
| Age, y | ||||||||||||||
| ≤39 | 297 | (39.5) | 123 | (60.9) | 19 | (37.3) | 403 | (54.5) | 18 | (40.0) | 43 | (40.2) | 14 | (35.9) |
| 40-49 | 124 | (16.5) | 36 | (17.8) | 6 | (11.8) | 172 | (23.3) | 9 | (20.0) | 26 | (24.3) | 9 | (23.1) |
| 50-59 | 73 | (9.7) | 15 | (7.4) | 7 | (13.7) | 93 | (12.6) | 7 | (15.6) | 16 | (15.0) | 7 | (17.9) |
| 60-69 | 70 | (9.3) | 14 | (6.9) | 8 | (15.7) | 30 | (4.1) | 4 | (8.9) | 13 | (12.1) | 5 | (12.8) |
| >69 | 187 | (24.9) | 14 | (6.9) | 11 | (21.6) | 41 | (5.5) | 7 | (15.6) | 9 | (8.4) | 4 | (10.3) |
| Health care worker | ||||||||||||||
| Yes | 120 | (16.0) | 72 | (35.6) | 7 | (13.7) | 205 | (27.7) | 14 | (31.1) | 48 | (44.9) | 16 | (41.0) |
| No | 631 | (84.0) | 130 | (64.4) | 44 | (86.3) | 534 | (72.3) | 31 | (68.9) | 59 | (55.1) | 23 | (59.0) |
| Preexisting comorbid conditions | ||||||||||||||
| Yes | 220 | (29.3) | 28 | (13.9) | 18 | (35.3) | 90 | (12.2) | 11 | (24.4) | 18 | (16.8) | 5 | (12.8) |
| No | 529 | (70.4) | 174 | (86.1) | 33 | (64.7) | 649 | (87.8) | 34 | (75.6) | 89 | (83.2) | 34 | (87.2) |
| Data missing | 2 | (0.3) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Delay in admission, d | ||||||||||||||
| ≤0 | 229 | (30.5) | 26 | (12.9) | 19 | (37.3) | 60 | (8.1) | 19 | (42.2) | 6 | (5.6) | 1 | (2.6) |
| 1-2 | 190 | (25.3) | 79 | (39.1) | 10 | (19.6) | 233 | (31.5) | 11 | (24.4) | 18 | (16.8) | 10 | (25.6) |
| 3-4 | 189 | (25.2) | 64 | (31.7) | 10 | (19.6) | 214 | (29.0) | 8 | (17.8) | 30 | (28.0) | 11 | (28.2) |
| ≥5 | 143 | (19.0) | 33 | (16.3) | 12 | (23.5) | 232 | (31.4) | 7 | (15.6) | 53 | (49.5) | 17 | (43.6) |
| Symptom onset date | ||||||||||||||
| February 15 to March 23 | 238 | (31.7) | 25 | (12.4) | 16 | (31.4) | 161 | (21.8) | — | — | — | |||
| March 24 to March 29 | 126 | (16.8) | 27 | (13.4) | 10 | (19.6) | 296 | (40.1) | — | — | — | |||
| March 30 to April 9 | 171 | (22.8) | 39 | (19.3) | 11 | (21.6) | 186 | (25.2) | — | — | — | |||
| April 9 to May 31 | 216 | (28.8) | 111 | (55.0) | 14 | (27.5) | 96 | (13.0) | — | — | — | |||
| Lactate dehydrogenase levelb | ||||||||||||||
| Greater than upper limit of normal | 299 | (39.8) | 85 | (42.1) | 22 | (43.1) | 329 | (44.5) | 6 | (13.3) | 46 | (43.0) | 14 | (35.9) |
| Normal | 311 | (41.4) | 105 | (52.0) | 20 | (39.2) | 345 | (46.7) | 20 | (44.4) | 31 | (29.0) | 15 | (38.5) |
| Data missing | 141 | (18.8) | 12 | (5.9) | 9 | (17.6) | 65 | (8.8) | 19 | (42.2) | 30 | (28.0) | 10 | (25.6) |
| Chest radiograph | ||||||||||||||
| Normal | 44 | (5.9) | 26 | (12.9) | 4 | (7.8) | 82 | (11.1) | 5 | (11.1) | 22 | (20.6) | 15 | (38.5) |
| Haziness | 63 | (8.4) | 20 | (9.9) | 2 | (3.9) | 164 | (22.2) | — | — | — | |||
| Pneumonic consolidation | 53 | (7.1) | 7 | (3.5) | 5 | (9.8) | 66 | (8.9) | 28 | (62.2) | 68 | (63.6) | 19 | (48.7) |
| Data missing | 591 | (78.7) | 149 | (73.8) | 40 | (78.4) | 427 | (57.8) | 12 | (26.7) | 17 | (15.9) | 5 | (12.8) |
| Neutrophil countc | ||||||||||||||
| Less than lower limit of normal | 10 | (1.3) | 5 | (2.5) | 0 | (0.0) | 16 | (2.2) | 1 | (2.2) | 12 | (11.2) | 6 | (15.4) |
| Normal | 99 | (13.2) | 34 | (16.8) | 7 | (13.7) | 245 | (33.2) | 31 | (68.9) | 79 | (73.8) | 28 | (71.8) |
| Greater than upper limit of normal | 14 | (1.9) | 2 | (1.0) | 1 | (2.0) | 20 | (2.7) | 6 | (13.3) | 9 | (8.4) | 5 | (12.8) |
| Data missing | 628 | (83.6) | 161 | (79.7) | 43 | (84.3) | 458 | (62.0) | 7 | (15.6) | 7 | (6.5) | 0 | (0.0) |
| Platelet countd | ||||||||||||||
| Less than lower limit of normal | 42 | (5.6) | 11 | (5.4) | 5 | (9.8) | 98 | (13.3) | 7 | (15.6) | 22 | (20.6) | 13 | (33.3) |
| Normal | 85 | (11.3) | 37 | (18.3) | 3 | (5.9) | 195 | (26.4) | 32 | (71.1) | 83 | (77.6) | 25 | (64.1) |
| Data missing | 624 | (83.1) | 154 | (76.2) | 43 | (84.3) | 446 | (60.4) | 6 | (13.3) | 2 | (1.9) | 1 | (2.6) |
| Lymphocyte counte | ||||||||||||||
| Less than lower limit of normal | 87 | (11.6) | 27 | (13.4) | 7 | (13.7) | 214 | (29.0) | 32 | (71.1) | 88 | (82.2) | 33 | (84.6) |
| Normal | 21 | (2.8) | 7 | (3.5) | 1 | (2.0) | 30 | (4.1) | 3 | (6.7) | 10 | (9.3) | 4 | (10.3) |
| Data missing | 643 | (85.6) | 168 | (83.2) | 43 | (84.3) | 495 | (67.0) | 10 | (22.2) | 9 | (8.4) | 2 | (5.1) |
| Oxygen saturation | ||||||||||||||
| <95% | 8 | (1.1) | 0 | (0.0) | 2 | (3.9) | 9 | (1.2) | 13 | (28.9) | 40 | (37.4) | 13 | (33.3) |
| ≥95% | 130 | (17.3) | 52 | (25.7) | 8 | (15.7) | 295 | (39.9) | 29 | (64.4) | 62 | (57.9) | 25 | (64.1) |
| Data missing | 613 | (81.6) | 150 | (74.3) | 41 | (80.4) | 435 | (58.9) | 4 | (6.7) | 5 | (4.7) | 1 | (2.6) |
| Outcome | ||||||||||||||
| Death | 175 | (23.3) | 18 | (8.9) | 15 | (29.4) | 93 | (12.6) | 9 | (20.0) | 10 | (9.3) | 5 | (12.8) |
| Recovery | 576 | (76.7) | 184 | (91.1) | 36 | (70.6) | 646 | (87.4) | 36 | (80.0) | 97 | (90.7) | 34 | (87.2) |
Only 3 patients were treated with corticosteroids only in Toronto and were excluded.
Lactate dehydrogenase level (upper limit of normal: > 225 U/L).
Neutrophil count (lower limit of normal = 2.0 × 109 cells/L; upper limit of normal = 7.5 × 109 cells/L).
Platelet count (lower limit of normal = 150 × 109 cells/L).
Lymphocyte count (lower limit of normal = 1.5 × 109 cells/L).
Appendix Table 2.
Factors Associated with Treatment Assignment of Probable Severe Acute Respiratory Syndrome Cases in Hong Kong and in the First Phase of the Epidemica (Onset before April 22, 2003) in Toronto, with Associated Confidence Intervals for the Estimated Odds Ratiob in the Propensity Score Multinomial Regression Model, Based on 10 Imputed Datasets
| Characteristic | Hong Kong |
Toronto |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ribavirin Only |
Corticosteroids Only |
Ribavirin and Corticosteroids |
Ribavirin Only |
Ribavirin and Corticosteroids |
||||||
| OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | |
| Interceptc | 0.04 | (0.01-0.17) | 0.07 | (0.01-0.69) | 0.18 | (0.07-0.44) | 0.2 | (0.02-1.30) | 0 | (0.00-0.24) |
| Gender | ||||||||||
| Women | 1 | 1 | 1 | 1 | 1 | |||||
| Men | 0.84 | (0.59-1.2) | 0.72 | (0.39-1.32) | 0.72 | (0.57-0.92) | 0.9 | (0.34-2.25) | 1.4 | (0.45-4.56) |
| Age, y | ||||||||||
| ≤39 | 1 | 1 | 1 | 1 | 1 | |||||
| 40-49 | 0.63 | (0.39-1.01) | 0.74 | (0.28-1.95) | 1.12 | (0.82-1.53) | 0.5 | (0.15-1.65) | 0.5 | (0.13-2.14) |
| 50-59 | 0.45 | (0.23-0.87) | 1.33 | (0.50-3.54) | 1.2 | (0.81-1.78) | 0.7 | (0.19-2.76) | 0.9 | (0.18-4.73) |
| 60-69 | 0.6 | (0.29-1.25) | 1.54 | (0.55-4.31) | 0.5 | (0.30-0.85) | 1.2 | (0.24-5.96) | 1 | (0.14-6.51) |
| >69 | 0.21 | (0.10-0.45) | 0.65 | (0.22-1.88) | 0.38 | (0.23-0.62) | 0.8 | (0.13-5.32) | 1.1 | (0.11-10.9) |
| Health care worker | ||||||||||
| No | 1 | 1 | 1 | 1 | 1 | |||||
| Yes | 1.67 | (1.02-2.74) | 0.95 | (0.34-2.66) | 2.45 | (1.73-3.48) | 2.6 | (0.95-7.12) | 2.1 | (0.63-6.87) |
| Preexisting comorbid conditions | ||||||||||
| No | 1 | 1 | 1 | 1 | 1 | |||||
| Yes | 0.79 | (0.44-1.40) | 1.37 | (0.61-3.07) | 1.03 | (0.71-1.51) | 1.6 | (0.36-7.51) | 1.1 | (0.18-6.90) |
| Delay in admission, d | ||||||||||
| ≤0 | 1 | 1 | 1 | 1 | 1 | |||||
| 1-2 | 2.99 | (1.72-5.19) | 0.55 | (0.23-1.33) | 2.59 | (1.74-3.86) | 8.5 | (1.77-40.4) | 22 | (1.64-286) |
| 3-4 | 3.22 | (1.81-5.73) | 0.56 | (0.23-1.38) | 2.65 | (1.76-3.99) | 15 | (3.22-66.9) | 29 | (2.26-372) |
| ≥5 | 3.08 | (1.61-5.87) | 0.97 | (0.39-2.42) | 4.96 | (3.22-7.64) | 25 | (5.21-123.1) | 37 | (2.84-493) |
| Symptom onset date | ||||||||||
| February 15 to March 23 | 1 | 1 | 1 | — | — | |||||
| March 24 to March 29 | 2.68 | (1.36-5.27) | 1.19 | (0.47-2.99) | 3.52 | (2.48-5.00) | — | — | ||
| March 30 to April 9 | 4.22 | (2.28-7.79) | 1.17 | (0.47-2.93) | 2.66 | (1.86-3.81) | — | — | ||
| April 9 to May 31 | 16.36 | (9.17-29.2) | 1.33 | (0.52-3.4) | 2.12 | (1.42-3.16) | — | — | ||
| Lactate dehydrogenase leveld | ||||||||||
| Normal | 1 | 1 | 1 | 1 | 1 | |||||
| High | 1.5 | (0.97-2.3) | 1.11 | (0.56-2.19) | 1.44 | (1.06-1.94) | 4.9 | (1.58-14.9) | 4.3 | (1.09-16.9) |
| Chest radiograph | ||||||||||
| Normal | 1 | 1 | 1 | 1 | 1 | |||||
| Haziness | 0.87 | (0.45-1.66) | 0.73 | (0.23-2.33) | 0.97 | (0.66-1.42) | — | — | ||
| Pneumonic consolidation | 0.81 | (0.44-1.50) | 0.81 | (0.28-2.34) | 0.88 | (0.59-1.30) | 1.1 | (0.35-3.39) | 4 | (1.09-14.4) |
| Neutrophil counte | ||||||||||
| Less than lower limit of normal | 1 | 1 | 1 | 1 | 1 | |||||
| Normal | 0.86 | (0.34-2.16) | 1.08 | (0.17-6.83) | 1.24 | (0.62-2.48) | 0.4 | (0.08-2.02) | 0.9 | (0.13-5.56) |
| Greater than upper limit of normal | 0.74 | (0.18-3.02) | 0.87 | (0.06-12.2) | 1.25 | (0.53-2.96) | 2.8 | (0.38-20.0) | 6.4 | (0.72-57.2) |
| Platelet countf | ||||||||||
| Normal | 1 | 1 | 1 | 1 | 1 | |||||
| Less than lower limit of normal | 0.92 | (0.57-1.49) | 1.32 | (0.52-3.37) | 1.04 | (0.74-1.46) | 0.7 | (0.22-2.01) | 1.5 | (0.40-5.32) |
| Lymphocyte count | ||||||||||
| Normal | 1 | 1 | 1 | 1 | 1 | |||||
| Less than lower limit of normal | 0.98 | (0.49-1.95) | 1.28 | (0.44-3.69) | 1.18 | (0.79-1.77) | 0.8 | (0.18-4.02) | 0.8 | (0.12-5.10) |
| Oxygen saturation | ||||||||||
| ≥95% | 1 | 1 | 1 | 1 | 1 | |||||
| <95% | 0.76 | (0.20-2.9) | 1.25 | (0.31-5.12) | 0.99 | (0.50-1.95) | 1.6 | (0.55-4.87) | 1.4 | (0.37-5.27) |
OR = odds ratio; CI = confidence interval.
Only 3 patients were treated with corticosteroids only in Toronto and were excluded.
OR represents the relative odds of the corresponding treatment choice versus no treatment, relative to the reference group. An OR of 0.84 for men under “ribavirin only” means that men has 16% less odds than women to be treated with ribavirin only instead of no treatment.
Intercept represents the baseline relative odds of the corresponding treatment choice versus no treatment.
Lactate dehydrogenase level (high: > 231 U/L).
Neutrophil count (lower limit of normal = 2.0 × 109 cells/L; upper limit of normal = 7.5 × 109 cells/L).
Platelet count (lower limit of normal = 150 × 109 cells/L).
Appendix Table 3.
Odds Ratios and Associated 95% Confidence Intervals in Hong Kong and Toronto by Treatment Received within 2 Days of Hospital Admission, Estimated by Multinomial Logistic Regression Models
| Treatment | Hong Kong |
Toronto |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Valuea | OR | 95% CI | P Valuea | |
| Neither ribavirin nor corticosteroids | Reference | — | — | Reference | — | — |
| Ribavirin | 0.76 | (0.35-1.62) | .43 | 0.42 | (0.06-2.98) | .35 |
| Corticosteroids | 1.34 | (0.52-3.45) | .49 | — | — | — |
| Both | 1.52 | (0.94-2.47) | .08 | 0.54 | (0.06-4.65) | .53 |
OR = odds ratio; CI = confidence interval.
P value for the odds of death relative to no treatment, adjusted for other demographic and clinical covariates.
References
- 1.World Health Organization Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. http://www.who.int/csr/sars/country/table2004_04_21/en/index.html Accessed 19 June 2008.
- 2.World Health Organization Situation updates - SARS. www.who.int/csr/don/archive/disease/severe_acute_respiratory_syndrome/en/index.html Accessed June 19 2008.
- 3.CHINAdaily Civet cats found at restaurants again. http://www.chinadaily.com.cn/china/2007-02/14/content_808890.htm Accessed June 19, 2008.
- 4.Wang M., Yan M., Xu H. SARS-CoV infection in a restaurant from palm civet. Emerg Infect Dis. 2005;11:1860–1865. doi: 10.3201/eid1112.041293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu D.K., Poon L.L., Chan K.H. Coronaviruses in bent-winged bats (Miniopterus spp.) J Gen Virol. 2006;87(Pt 9):2461–2466. doi: 10.1099/vir.0.82203-0. [DOI] [PubMed] [Google Scholar]
- 6.Li W., Shi Z., Yu M. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 7.Crotty S., Maag D., Arnold J.J. The broad-spectrum antiviral ribonucleoside ribavirin is an RNA virus mutagen. Nat Med. 2000;6:1375–1379. doi: 10.1038/82191. [DOI] [PubMed] [Google Scholar]
- 8.Nicholls J.M., Poon L.L., Lee K.C. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:1773–1778. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peiris J.S., Chu C.M., Cheng V.C. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cyranoski D. Critics slam treatment for SARS as ineffective and perhaps dangerous. Nature. 2003;423:4. doi: 10.1038/423004a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller M.P., McGeer A., Straus S.E. Clinical trials and novel pathogens: lessons learned from SARS. Emerg Infect Dis. 2004;10:389–394. doi: 10.3201/eid1003.030702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenbaum P.R., Rubin D.B. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 14.Madaras-Kelly K.J., Remington R.E., Oliphant C.M. Efficacy of oral beta-lactam versus non-beta-lactam treatment of uncomplicated cellulitis. Am J Med. 2008;121:419–425. doi: 10.1016/j.amjmed.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 15.Mangano D.T., Tudor I.C., Dietzel C. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354:353–365. doi: 10.1056/NEJMoa051379. [DOI] [PubMed] [Google Scholar]
- 16.Reeves M.J., Gargano J.W., Luo Z. Effect of pretreatment with statins on ischemic stroke outcomes. Stroke. 2008;39:1779–1785. doi: 10.1161/STROKEAHA.107.501700. [DOI] [PubMed] [Google Scholar]
- 17.Seshadri R., Feldman B.M., Ilowite N. The role of aggressive corticosteroid therapy in patients with juvenile dermatomyositis: a propensity score analysis. Arthritis Rheum. 2008;59:989–995. doi: 10.1002/art.23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaya F.T., Blume S.W., Blanchette C.M. Selective cyclooxygenase-2 inhibition and cardiovascular effects: an observational study of a Medicaid population. Arch Intern Med. 2005;165:181–186. doi: 10.1001/archinte.165.2.181. [DOI] [PubMed] [Google Scholar]
- 19.Leung G.M., Hedley A.J., Ho L.M. The epidemiology of severe acute respiratory syndrome in the 2003 Hong Kong epidemic: an analysis of all 1755 patients. Ann Intern Med. 2004;141:662–673. doi: 10.7326/0003-4819-141-9-200411020-00006. [DOI] [PubMed] [Google Scholar]
- 20.Cowling B.J., Muller M.P., Wong I.O. Clinical prognostic rules for severe acute respiratory syndrome in low- and high-resource settings. Arch Intern Med. 2006;166:1505–1511. doi: 10.1001/archinte.166.14.1505. [DOI] [PubMed] [Google Scholar]
- 21.Muller M.P., Richardson S.E., McGeer A. Early diagnosis of SARS: lessons from the Toronto SARS outbreak. Eur J Clin Microbiol Infect Dis. 2006;25:230–237. doi: 10.1007/s10096-006-0127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svoboda T., Henry B., Shulman L. Public health measures to control the spread of the severe acute respiratory syndrome during the outbreak in Toronto. N Engl J Med. 2004;350:2352–2361. doi: 10.1056/NEJMoa032111. [DOI] [PubMed] [Google Scholar]
- 23.Pocock S. Wiley; Chichester: 1983. Clinical Trials: A Practical Approach. [Google Scholar]
- 24.Hernandez A.V., Steyerberg E.W., Habbema J.D. Covariate adjustment in randomized controlled trials with dichotomous outcomes increases statistical power and reduces sample size requirements. J Clin Epidemiol. 2004;57:454–460. doi: 10.1016/j.jclinepi.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Peduzzi P., Concato J., Kemper E. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 26.Imbens G.W. The role of the propensity score in estimating dose-response functions. Biometrika. 2000;87:706–710. [Google Scholar]
- 27.Qu Y., Lipkovich I. Propensity score estimation with missing values using a multiple imputation missingness pattern (MIMP) approach. Stat Med. 2009;28:1402–1414. doi: 10.1002/sim.3549. [DOI] [PubMed] [Google Scholar]
- 28.Hayes J.R., Groner J.I. Using multiple imputation and propensity scores to test the effect of car seats and seat belt usage on injury severity from trauma registry data. J Pediatr Surg. 2008;43:924–927. doi: 10.1016/j.jpedsurg.2007.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rutten-van Mölken M.P., van Nooten F.E., Lindemann M. A 1-year prospective cost-effectiveness analysis of roflumilast for the treatment of patients with severe chronic obstructive pulmonary disease. PharmacoEcon. 2007;25:695–711. doi: 10.2165/00019053-200725080-00007. [DOI] [PubMed] [Google Scholar]
- 30.Hill J.L., Waldfogel J., Brooks-Gunn J., Han W.J. Maternal employment and child development: a fresh look using newer methods. Dev Psychol. 2005;41:833–850. doi: 10.1037/0012-1649.41.6.833. [DOI] [PubMed] [Google Scholar]
- 31.Tu W., Zhou X. A bootstrap confidence interval procedure for the treatment effect using propensity score subclassification. Health Serv Outcomes Res Methodol. 2003;3:135–147. [Google Scholar]
- 32.R Development Core Team R: A Language and Environment for Statistical Computing. http://www.R-project.org Accessed June 19, 2008.
- 33.Lau EHY, Hsiung CA, Cowling BJ, et al. A comparative epidemiologic analysis of SARS in Hong Kong, Beijing and Taiwan, China. Bull World Health Organ. In press. [DOI] [PMC free article] [PubMed]
- 34.Booth C.M., Matukas L.M., Tomlinson G.A. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 35.Sung J.J., Wu A., Joynt G.M. Severe acute respiratory syndrome: report of treatment and outcome after a major outbreak. Thorax. 2004;59:414–420. doi: 10.1136/thx.2003.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsui P.T., Kwok M.L., Yuen H., Lai S.T. Severe acute respiratory syndrome: clinical outcome and prognostic correlates. Emerg Infect Dis. 2003;9:1064–1069. doi: 10.3201/eid0909.030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller M.P., Dresser L., Raboud J. Adverse events associated with high-dose ribavirin: evidence from the Toronto outbreak of severe acute respiratory syndrome. Pharmacotherapy. 2007;27:494–503. doi: 10.1592/phco.27.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee N., Hui D., Wu A. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 39.Poutanen S.M., Low D.E., Henry B. Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;348:1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- 40.Cheng V.C., Hung I.F., Tang B.S. Viral replication in the nasopharynx is associated with diarrhea in patients with severe acute respiratory syndrome. Clin Infect Dis. 2004;38:467–475. doi: 10.1086/382681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koren G., King S., Knowles S., Phillips E. Ribavirin in the treatment of SARS: a new trick for an old drug? CMAJ. 2003;168:1289–1292. [PMC free article] [PubMed] [Google Scholar]
- 42.Wong C.K., Lam C.W., Wu A.K. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee N., Allen Chan K.C., Hui D.S. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304–309. doi: 10.1016/j.jcv.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peiris J.S.M., Guan Y., Poon L.L.M., Cheng V.C.C. Severe acute respiratory syndrome (SARS) In: Scheld W.M., Hooper D.C., Hughes J.M., editors. Emerging Infections. ASM Press; Washington: 2007. [Google Scholar]
- 45.Peiris J.S., Yuen K.Y., Osterhaus A.D., Stohr K. The severe acute respiratory syndrome. N Engl J Med. 2003;349:2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- 46.Peter J.V., John P., Graham P.L. Corticosteroids in the prevention and treatment of acute respiratory distress syndrome (ARDS) in adults: meta-analysis. BMJ. 2008;336:1006–1009. doi: 10.1136/bmj.39537.939039.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lew T.W., Kwek T.K., Tai D. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290:374–380. doi: 10.1001/jama.290.3.374. [DOI] [PubMed] [Google Scholar]
- 48.Hong N., Du X.K. Avascular necrosis of bone in severe acute respiratory syndrome. Clin Radiol. 2004;59:602–608. doi: 10.1016/j.crad.2003.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lau E.M., Chan F.W., Hui D.S. Reduced bone mineral density in male Severe Acute Respiratory Syndrome (SARS) patients in Hong Kong. Bone. 2005;37:420–424. doi: 10.1016/j.bone.2005.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Auyeung T.W., Lee J.S., Lai W.K. The use of corticosteroid as treatment in SARS was associated with adverse outcomes: a retrospective cohort study. J Infect. 2005;51:98–102. doi: 10.1016/j.jinf.2004.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J.T., Sheng W.H., Fang C.T. Clinical manifestations, laboratory findings, and treatment outcomes of SARS patients. Emerg Infect Dis. 2004;10:818–824. doi: 10.3201/eid1005.030640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan K.S., Lai S.T., Chu C.M. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med J. 2003;9:399–406. [PubMed] [Google Scholar]
- 53.Jia W., Gao W. Is traditional Chinese medicine useful in the treatment of SARS? Phytother Res. 2003;17:840–841. doi: 10.1002/ptr.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donders A.R., van der Heijden G.J., Stijnen T., Moons K.G. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59:1087–1091. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 55.World Health Organization Case Definitions for Surveillance of Severe Acute Respiratory Syndrome (SARS) http://www.who.int/csr/sars/casedefinition/en/ Accessed June 19, 2008.


