Abstract
Background
Data on the relationship between the clinical and microbiological features of community-acquired pneumonia (CAP) and its computed tomography (CT) findings are limited. The aim of the present study was to investigate the clinic-microbiological features of patients with CAP presenting with ground-glass opacity (GGO) and centrilobular nodules or tree-in-bud pattern on CT images.
Methods
Patients with CAP who underwent a CT scan at presentation were retrospectively classified using CT findings into consolidation, GGO and bronchiolitis groups. These 3 groups were compared in terms of clinical parameters and microbiological data.
Results
A total of 40 patients (2.4%) were allocated to the bronchiolitis group and 46 (2.8%) to the GGO group. The most common pathogen in the bronchiolitis group was Mycoplasma pneumoniae, which was significantly more frequently isolated in this group. The bronchiolitis group was characterized by a higher percentage of cough, a lower percentage of chest pain and lower blood levels of inflammatory markers. Common pathogens in the GGO group were not significantly different from those in the other 2 groups. Unlike that observed in the consolidation group, complicated parapneumonic effusion or empyema was not observed in the bronchiolitis or GGO group. Outcome variables were similar in the 3 groups.
Conclusions
The bronchiolitis group was characterized by a higher frequency of M. pneumoniae and a less severe form of CAP. The GGO and consolidation groups was similar with respect to causative microorganisms and the clinical features of CAP. No patient in the bronchiolitis or GGO group exhibited complicated parapneumonic effusion or empyema.
Key Indexing Terms: Bronchiolitis, Community-acquired pneumonia, Computed tomography, Mycoplasma pneumoniae
Introduction
Community-acquired pneumonia (CAP) remains an important cause of morbidity and mortality worldwide, despite the use of an antibiotic armamentarium and the availability of vaccines.1 Therefore, early diagnosis and appropriate antimicrobial treatment are critical steps in the management of CAP. Chest radiography is usually used in combination with a constellation of compatible symptoms or signs, such as cough and sputum production to confirm the diagnosis of pneumonia.2, 3 Computed tomography (CT) is useful when the plain chest radiograph does not reveal findings that explain the patient’s clinical presentation.4 CT images can provide more detailed information regarding lung parenchyma,1 suggest specific causative agents,5 rule out noninfectious pneumonia5 and detect other underlying conditions.4 Thus, CT provides additional benefits for the diagnosis of CAP and aids its typing.2 In particular, when performed early, CT can affect the diagnosis and clinical management of CAP in the emergency department.4 Accordingly, the use of CT in patients with suspected CAP can be expected to increase in real world practice.
A variety of microbial agents, including typical and atypical pathogens, can cause CAP. A considerable number of studies on the CT findings of CAP triggered by different pathogens, including Streptococcus pneumoniae,6, 7, 8 Klebsiella pneumoniae,9, 10 Mycoplasma pneumoniae,11, 12, 13, 14 Chlamydia pneumoniae 14, 15 and Legionella pneumophila,16, 17 have been published. Patients with CAP attributed to a specific pathogen present with more than 1 CT pattern, although 1 of these patterns predominates.5 CAP has been classically divided into 3 patterns based on CT findings, that is, consolidation-predominant, peribronchial nodules-predominant and ground-glass opacity (GGO)-predominant.5 However, data on relationships between the clinical and microbiologic features of CAP and CT findings are scarce. In CAP, pure or nearly pure GGO lesions are uncommon regardless of causative pathogens,16, 18 and a CT finding multiple centrilobular nodules is also rare.19 We hypothesized that the clinical manifestations of patients with CAP are related to CT findings, and thus, we compared the clinical features and microbiologic data of CAP patients classified based on CT findings.
Materials and Methods
Study Design
We retrospectively enrolled consecutive patients with CAP admitted to and treated at the Respiratory Department of Kyungpook National University Hospital (KNUH), a tertiary referral center, in Daegu, South Korea between January 2011 and December 2016.20 Baseline patient characteristics were recorded at admission, although not all patients underwent the same laboratory tests. Pneumonia was diagnosed using the following criteria: (1) a new radiographic infiltrate, (2) 1 or more symptoms or signs consistent with pneumonia (cough, sputum, dyspnea, fever or pleuritic chest pain) and (3) the exclusion of other causes.21 Patients with hospital-acquired pneumonia,22 healthcare-associated pneumonia,22 an active thoracic malignancy or taking immunosuppressants or steroids (>15 mg/day of prednisone for > 14 days) were excluded. Patients without an available chest CT scan at presentation were also excluded.
The CT findings of CAP were classified into 3 categories: bronchiolitis, GGO and consolidation. These 3 groups were compared in terms of clinical characteristics and microbiologic data. The study was approved by the Institutional Review Board of KNUH, which waived the requirement for written informed patient consent because of the retrospective nature of the study.
Radiologic Data
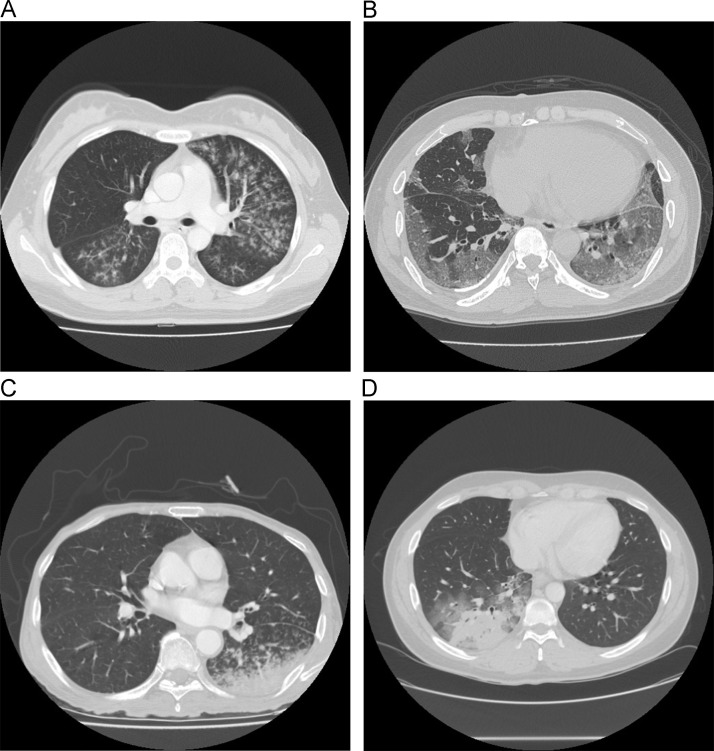
Two chest radiologists (J.K.L. and K.M.S.) reviewed the chest CT scans of patients with CAP and classified them into the 3 groups ( Figure). In the bronchiolitis group, chest CT indicated centrilobular nodules or tree-in-bud pattern in most lesions with no or minimal GGO or consolidation (Figure).23 In the GGO group, chest CT indicated focal or diffuse GGO with no or minimal centrilobular nodules, tree-in-bud pattern or consolidation. In the consolidation group, chest CT indicated consolidation with or without variable extents of the CT features of bronchiolitis or GGO.
FIGURE.
Representative computed tomography images of community-acquired pneumonia. Computed tomography (CT) findings in the bronchiolitis group (A) indicate centrilobular nodules or tree-in-bud pattern in most lesions with no or minimal ground glass opacity (GGO) or consolidation. CT findings in the GGO group (B) indicate focal or diffuse GGO with no or minimal centrilobular nodules, tree-in-bud pattern or consolidation. CT findings in the consolidation group show consolidation with or without variable extents of bronchiolitis or GGO (C-D).
Data Collection
Two chest physicians (H.S. and S.I.C.) reviewed medical records. Resident physicians initially recorded baseline data, which were confirmed by attending chest physicians. Demographic data included age, sex, smoking history and alcohol consumption. Heavy drinking was defined as the consumption of 7 or more drinks (>60 g of alcohol) on 1 occasion for men, and 5 or more drinks (>40 g of alcohol) on 1 occasion for women at least twice a week. We reviewed symptoms, vital signs, comorbidities, pneumonia severity indices,24 CURB-65 scores,25 Eastern Cooperative Oncology Group (ECOG) performance statuses26 and retrospectively calculated Charlson comorbidity indices.27 Use of mechanical ventilation, corticosteroid treatment, vasopressor infusion and pleural drainage with percutaneous catheters or chest tubes were checked. Outcome variables included length of hospital stay, 30-day mortality, in-hospital mortality and clinical success. Treatment success was defined as improvements in clinical symptoms or signs and radiologic findings. Laboratory data included complete blood count, erythrocyte sedimentation rate, liver function testing, C-reactive protein (CRP), procalcitonin, N-terminal of prohormone brain natriuretic peptide, blood urea nitrogen, creatinine, sodium, lactate dehydrogenase, lactate and arterial blood gas analysis.
Microbiological Data
The criteria for a causative pathogen were as follows21: a microorganism isolated from blood or pleural fluid; positive urinary antigen test for S. pneumoniae or L. pneumophila serogroup 1 (BinaxNOW S. pneumoniae and Legionella urinary antigen cards, Alere, Scarborough, ME); a culture of bacteria from a sputum sample (>25 neutrophils and <10 squamous epithelial cells per low-power field) collected within 24 hours of admission plus a compatible Gram-stain finding; identification of M. pneumoniae based on a positive immunoglobulin M (IgM) result or a 4-fold increase in immunoglobulin G (IgG) levels in convalescent versus initial blood samples by chemiluminescence immunoassay (LIAISON, DiaSorin, Saluggia, Italy) or positivity for M. pneumoniae in a sputum by polymerase chain reaction (PCR) (AmpliSens Mycoplasma pneumoniae-FEP PCR, Central Research Institute for Epidemiology, Moscow, Russia); the presence of C. pneumoniae as determined by a positive IgM or a 4-fold increase in IgG levels by microimmunofluorescence (an in-house method) or by enzyme-linked immunosorbent assay (Diesse Diagnostica Senese, Monteriggioni, Italy); positivity for a respiratory virus (adenovirus, influenza [types A and B], parainfluenza virus [types 1, 2, 3 and 4], rhinovirus, respiratory syncytial virus [types A and B], bocavirus, metapneumovirus, coronavirus [229E, NL63 and OC43] or enterovirus) in a throat or nasopharyngeal swab as determined by multiplex PCR (Anyplex RV16 detection, Seegene, Seoul, Korea) and the identification of influenza A or B antigen in a throat swab by rapid chromatographic immunoassay (SD BIOLINE Influenza Antigen test, Standard Diagnostics, Yongin, Korea or BD Veritor System for Rapid Detection of FluA+B, BD Diagnostics, Sparks, MD).
Statistical Analysis
Data were expressed as medians (interquartile ranges) for continuous variables or as numbers and percentages for categorical variables. Continuous variables were compared using the Kruskal-Wallis test, and Dunn’s test was used as a post-hoc test. Categorical variables were compared using the chi-square test among groups and Bonferroni’s correction was used as a post-hoc follow-up test. The kappa statistic was used to measure interinterpreter agreement regarding CT findings. IBM SPSS Statistics for Windows version 22.0 (IBM Corp., Armonk, NY) was used for the statistical analysis. P < 0.05 was considered statistically significant.
Results
Enrolled Patients
Initially, 1,708 patients with CAP were identified. Of these, 12 patients without available CT scans and 31 patients with no identifiable parenchymal lesion due to parapneumonic effusion were excluded. Consequently, 1,665 patients were included in the study. For patients admitted between 2015 and 2016 (n = 531), 2 radiologists (K.M.S. and J.K.L.) independently reviewed the CT findings and classified them as consolidation, bronchiolitis or GGO by consensus (the kappa statistic was 0.943). For the remaining patients, 1 of the 2 radiologists determined CT findings. Of the 1,665 patients, 40 (2.4%) were included in the bronchiolitis group, 46 (2.8%) in the GGO group and 1,579 (94.8%) in the consolidation group. Clinical and microbiologic variables of these 3 groups were compared.
Clinical Characteristics of the Bronchiolitis, GGO and Consolidation Groups
Clinical parameters are presented in Table 1. The percentage of patients with cough differed significantly among the groups, and the percentage was significantly greater in the bronchiolitis group than in the consolidation group (40 [100.0%] versus 1,368 [86.6%], P = 0.013). The frequency of chest pain significantly differed among groups: none of the 40 patients in the bronchiolitis group presented with chest pain, whereas chest pain was present in a quarter of the 1,579 patients in the consolidation group (Bonferroni’s corrected P < 0.001). The percentage of pleural effusion was significantly lower in the bronchiolitis (Bonferroni’s corrected P < 0.001) and GGO (Bonferroni’s corrected P = 0.001) groups than in the consolidation group. Complicated parapneumonic effusion or empyema was not observed in the bronchiolitis and GGO groups, whereas it was observed in 145 patients (9.2%) in the consolidation group. Along the same lines, no patient in the bronchiolitis and GGO groups underwent pleural drainage, whereas 172 patients (11.0%) in the consolidation group did. Corticosteroids were significantly more frequently administered in the GGO group than in the consolidation group (19 [41.3%] versus 353 [22.4%], Bonferroni’s corrected P = 0.003) or bronchiolitis group (19 [41.3%] versus 6 [15.0%], Bonferroni’s corrected P = 0.007). Regarding outcome variables, 30-day mortality, in-hospital mortality, treatment success and length of hospital stay were similar in the 3 groups.
TABLE 1.
Baseline characteristics of the patients.
| Characteristics | Consolidation (n = 1,579) | Bronchiolitis (n = 40) | GGO (n = 46) | P Value |
|---|---|---|---|---|
| Age, years | 69 (56-77) | 64 (39-74) | 66 (53-76) | 0.080 |
| Male | 1,053 (66.7) | 25 (62.5) | 31 (67.4) | 0.852 |
| Smoking | ||||
| Ever-smoker | 896 (56.9) | 20 (50.0) | 21 (45.7) | 0.225 |
| Pack-years | 10 (0-33) | 2.5 (0-20) | 0 (0-30) | 0.121 |
| Heavy drinking | 282 (17.9) | 2 (5.0) | 5 (10.9) | 0.053 |
| Charlson comorbidity index | 0 (0-1) | 0 (0-1) | 0 (0-1) | 0.148 |
| ECOG 3-4 | 217 (13.7) | 6 (15.0) | 2 (4.3) | 0.178 |
| Systolic blood pressure, mmHg | 130 (112-147) | 129 (104-142) | 128 (102-139) | 0.476 |
| Pulse rate/minute | 96 (83-109) | 92 (82-113) | 99 (83-114) | 0.652 |
| Respiratory rate/minute | 20 (19-22) | 19 (18-20) | 20 (20-24) | 0.004a |
| Symptoms | ||||
| Duration of symptom, days | 5 (3-7) | 6 (3-7) | 5 (2-8) | 0.580 |
| Cough | 1,368 (86.6) | 40 (100.0) | 44 (95.7) | 0.010b |
| Sputum production | 1,132 (71.7) | 35 (87.5) | 33 (71.7) | 0.089 |
| Dyspnea | 884 (56.0) | 20 (50.0) | 31 (67.4) | 0.224 |
| Fever | 1,025 (64.9) | 23 (57.5) | 30 (65.2) | 0.624 |
| Altered mental status | 84 (5.3) | 1 (2.5) | 0 (0.0) | 0.253 |
| Hemoptysis | 130 (8.2) | 2 (5.0) | 4 (8.7) | 0.859 |
| Chest pain | 389 (24.6) | 0 (0.0) | 6 (13.0) | <0.001c |
| CURB-65 | 1 (0-2) | 1 (0-2) | 1 (0-2) | 0.182 |
| CURB-65, 3-5 | 152 (9.6) | 1 (2.5) | 2 (4.3) | 0.199 |
| PSI class | 3 (2-4) | 2 (1-4) | 3 (2-4) | 0.099 |
| PSI class 4-5 | 550 (34.8) | 12 (30.0) | 13 (28.3) | 0.542 |
| Pleural effusion | 643 (40.7) | 3 (7.5) | 10 (21.7) | <0.001d,e |
| Complicated parapneumonic effusion or empyema | 145 (9.2) | 0 (0.0) | 0 (0.0) | 0.006 |
| Pleural drainage | 173 (11.0) | 0 (0.0) | 0 (0.0) | 0.001f |
| Mechanical ventilation | 95 (6.0) | 0 (0.0) | 3 (6.5) | 0.300 |
| Corticosteroids | 353 (22.4) | 6 (15.0) | 19 (41.3) | 0.005g,h |
| Vasopressor infusion | 142 (9.0) | 1 (2.5) | 3 (6.5) | 0.380 |
| 30-day mortality | 121 (7.7) | 2 (5.0) | 4 (8.7) | 0.850 |
| In-hospital mortality | 105 (6.6) | 1 (2.5) | 3 (6.5) | 0.730 |
| Length of hospital stay, days | 9 (6-13) | 7 (6-10) | 9 (6-14) | 0.138 |
| Treatment success | 1,428/1,551 (92.1) | 36/39 (92.3) | 42/45 (93.3) | >0.999 |
Data are presented as median (interquartile range) or n (%).
CURB-65, a 6 point score, 1 point for each of confusion, urea 47 mmol/L, respiratory rate ≥30/minute, low systolic (<90 mm Hg) or diastolic (≤60 mm Hg) blood pressure, and age ≥65 years: ECOG, Eastern Cooperative Oncology Group performance status; GGO, ground glass opacity; PSI, pneumonia severity index.
Post-hoc analysis.
Bronchiolitis vs. GGO, P = 0.003 (Dunn’s test).
Bronchiolitis vs. consolidation, Bonferroni’s corrected P = 0.013.
Bronchiolitis vs. consolidation, Bonferroni’s corrected P < 0.001.
Bronchiolitis vs consolidation, Bonferroni’s corrected P < 0.001.
GGO vs. consolidation, Bonferroni’s corrected P = 0.010.
GGO vs. consolidation, Bonferroni’s corrected P = 0.012.
GGO vs. consolidation, Bonferroni’s corrected P = 0.003.
GGO vs. bronchiolitis, Bonferroni’s corrected P = 0.007.
Laboratory Findings of Patients in the Bronchiolitis, GGO and Consolidation Groups
Blood levels of CRP, procalcitonin, albumin, total protein and sodium were significantly different in the 3 groups ( Table 2). CRP (9.3 mg/dL [3.7-14.0 mg/dL] versus 12.9 mg/dL [6.6-21.0 mg/dL], P = 0.009) and procalcitonin (0.11 ng/mL [0.06-0.42 ng/mL] versus 0.35 ng/mL [0.10-1.79 ng/mL], P = 0.006) were significantly lower in the bronchiolitis group than in the consolidation group, and the bronchiolitis group had significantly higher blood levels of albumin (3.5 g/dL [3.4-4.0 g/dL] versus 3.3 g/dL [2.9-3.8 g/dL], P = 0.004) and total protein (6.8 g/dL [6.4-7.4 g/dL] versus 6.4 g/dL [5.9-6.9 g/dL], P < 0.001) than the consolidation group.
TABLE 2.
Blood laboratory findings of the patients.
| Parameters | Consolidation | n | Bronchiolitis | n | GGO | n | P-value |
|---|---|---|---|---|---|---|---|
| WBC count, /μL | 10,710 (7,680-15,220) | 1,579 | 10,400 (6,955-13,783) | 40 | 10,385 (7,735-15,350) | 46 | 0.782 |
| ESR, mm/hour | 45 (28-64) | 1,572 | 40 (24-61) | 40 | 39 (16-56) | 46 | 0.183 |
| C-reactive protein, mg/dL | 12.9 (6.6-21.0) | 1,575 | 9.3 (3.7-14.0) | 40 | 12.0 (6.1-16.9) | 46 | 0.011a |
| Procalcitonin, ng/mL | 0.35 (0.10-1.79) | 1,479 | 0.11 (0.06-0.42) | 37 | 0.21 (0.10-0.90) | 40 | 0.007b |
| NT-proBNP, pg/mL | 432.0 (139.0-1,589.8) | 1,480 | 401.0 (53.0-998.0) | 35 | 365.1 (89.8-1,045.0) | 44 | 0.287 |
| Troponin I, ng/mL | 0.015 (0.015-0.030) | 1,304 | 0.015 (0.015-0.050) | 28 | 0.015 (0.015-0.019) | 42 | 0.272 |
| Hemoglobin, g/dL | 12.5 (11.2-13.6) | 1,579 | 13.3 (11.0-14.3) | 40 | 13.0 (11.4-14.5) | 46 | 0.067 |
| Platelet, 103/µL | 235 (176-318) | 1,579 | 235 (177-294) | 40 | 243 (192-307) | 46 | 0.799 |
| Albumin, g/dL | 3.3 (2.9-3.8) | 1,579 | 3.5 (3.4-4.0) | 40 | 3.5 (3.0-3.8) | 46 | 0.002c |
| Total protein, g/dL | 6.4 (5.9-6.9) | 1,579 | 6.8 (6.4-7.4) | 40 | 6.5 (5.9-6.9) | 46 | < 0.001d,e |
| Total bilirubin, mg/dL | 0.60 (0.39-0.90) | 1,578 | 0.51 (0.37-0.70) | 40 | 0.61 (0.42-0.98) | 46 | 0.169 |
| AST, U/L | 25 (18-39) | 1,578 | 24 (16-38) | 40 | 31 (21-50) | 46 | 0.064 |
| ALT, U/L | 20 (13-32) | 1,578 | 18 (12-33) | 40 | 23 (17-32) | 46 | 0.215 |
| ALP, U/L | 77 (62-105) | 1,575 | 77 (60-93) | 40 | 72 (58-103) | 46 | 0.371 |
| BUN, mg/dL | 15.8 (11.1-23.8) | 1,579 | 15.5 (10.7-33.3) | 40 | 15.1 (12.3-20.3) | 46 | 0.969 |
| Creatinine, mg/dL | 0.90 (0.70-1.21) | 1,579 | 0.88 (0.69-1.27) | 40 | 0.92 (0.74-1.16) | 46 | 0.899 |
| Sodium, mmol/L | 136 (134-139) | 1,579 | 139 (135-141) | 40 | 136 (134-138) | 46 | 0.014f |
| PaO2/FiO2, mmHg | 347.1 (282.5-412.4) | 1,400 | 356.7 (304.8-404.1) | 36 | 326.7 (228.0-420.9) | 44 | 0.374 |
| PaCO2, mmHg | 28.8 (26.0-32.3) | 1,400 | 29.6 (27.5-33.9) | 37 | 28.9 (26.0-33.1) | 46 | 0.485 |
| LDH, U/L | 380 (289-479) | 1,094 | 355 (296-416) | 27 | 422 (333-587) | 35 | 0.056 |
| Lactate, mmol/L | 2.0 (1.5-2.8) | 970 | 1.8 (1.4-2.7) | 21 | 2.3 (1.6-2.7) | 33 | 0.544 |
Data are presented as median (interquartile range).
AST, aspartate aminotransferase; ALT, alanine transaminase; ALP, alkaline phosphatase; BUN, blood urea nitrogen; ESR, erythrocyte sedimentation rate; NT-proBNP, N-terminal of prohormone brain natriuretic peptide; LDH, lactate dehydrogenase; WBC, white blood cell.
Post-hoc analysis.
Bronchiolitis vs. consolidation, P = 0.009 (Dunn’s test).
Bronchiolitis vs. consolidation, P = 0.006 (Dunn’s test).
Bronchiolitis vs. consolidation, P = 0.004 (Dunn’s test).
Bronchiolitis vs. consolidation, P < 0.001 (Dunn’s test).
Bronchiolitis vs. GGO, P = 0.029 (Dunn’s test).
Bronchiolitis vs. consolidation, P = 0.010 (Dunn’s test).
Microbiologic Data of Patients in the Bronchiolitis, GGO and Consolidation Groups
Microbiologic data are summarized in Table 3. Eight hundred and fifty-nine potential pathogens were identified in 715 patients (42.9%): 800 pathogens in 670 patients (42.4%) of the consolidation group, 37 pathogens in 27 patients (67.5%) of the bronchiolitis group and 22 pathogens in 18 patients (39.1%) of the GGO group, respectively. The prevalence of Hemophilus influenzae was significantly different among the 3 groups, but not between any 2 groups. The prevalence of M. pneumoniae was also significantly different among the 3 groups, the bronchiolitis group had significantly higher prevalence than the consolidation group (16 [43.2%] versus 142 [17.8%], Bonferroni’s corrected P < 0.001) or GGO group (16 [43.2%] versus 7 [31.8%], Bonferroni’s corrected P = 0.001). In the bronchiolitis group, the most common pathogen was M. pneumoniae (16 [43.2%]), followed by S. pneumoniae (6 [16.2%]), C. pneumoniae (6 [16.2%]) and K. pneumoniae (4 [10.8%]). The most common pathogens in the GGO group were M. pneumoniae (7 [31.8%]) and C. pneumoniae (6 [27.2%]), followed by S. pneumoniae (4 [18.2%]) and K. pneumoniae (3 [13.6%]).
TABLE 3.
Microbiologic diagnosis.
| Consolidation (n = 800) | Bronchiolitis (n = 37) | GGO (n = 22) | P Value | |
|---|---|---|---|---|
| Streptococcus pneumoniae | 168 (21.0) | 6 (16.2) | 4 (18.2) | 0.618 |
| Streptococcus milleri group | 12 (1.5) | 0 (0) | 0 (0) | >0.999 |
| S. constellatus | 6 | 0 | 0 | |
| S. intermedius | 5 | 0 | 0 | |
| S. anginosus | 2 | 0 | 0 | |
| Other viridans streptococci | 13 (1.6) | 0 (0) | 0 (0) | >0.999 |
| S. mitis/oralis | 7 | 0 | 0 | |
| S. sanguis | 2 | 0 | 0 | |
| S. gordonii | 2 | 0 | 0 | |
| S. salivarius | 2 | 0 | 0 | |
| Other streptococci species | 5 (0.6) | 0 | 0 | >0.999 |
| S. agalactiae | 2 | 0 | 0 | |
| Not specified | 3 | 0 | 0 | |
| Staphylococcus aureus | 38 (4.8) | 1 (2.7) | 1 (4.5) | >0.999 |
| Methicillin-susceptible | 29 | 1 | 1 | |
| Methicillin-resistant | 9 | 0 | 0 | |
| Klebsiella pneumoniae | 106 (13.3) | 4 (10.8) | 3 (13.6) | 0.616 |
| Pseudomonas aeruginosa | 23 (2.9) | 1 (2.7) | 0 (0) | 0.526 |
| Acinetobacter baumannii | 13 (1.6) | 0 (0) | 0 (0) | >0.999 |
| Enterococcus faecium | 2 (0.3) | 0 (0) | 0 (0) | >0.999 |
| Enterococcus faecalis | 3 (0.4) | 0 (0) | 0 (0) | >0.999 |
| Escherichia coli | 10 (1.2) | 1 (2.7) | 0 (0) | 0.263 |
| Stenotrophomonas maltophilia | 4 (0.5) | 0 (0) | 0 (0) | >0.999 |
| Hemophilus influenzae | 8 (1.0) | 2 (5.4) | 0 (0) | 0.029 |
| Serratia marcescens | 3 (0.4) | 0 (0) | 0 (0) | >0.999 |
| Proteus mirabilis | 2 (0.3) | 0 | 0 | >0.999 |
| Morganella morganii | 1 (0.1) | 0 | 0 | 0.973 |
| Mycoplasma pneumoniae | 142 (17.8) | 16 (43.2) | 7 (31.8) | <0.001a,b |
| Chlamydia pneumoniae | 212 (26.5) | 6 (16.2) | 6 (27.2) | 0.956 |
| Legionella pneumophila | 9 (1.1) | 0 (0) | 0 (0) | >0.999 |
| Virus | 26 (3.2) | 0 (0) | 1 (4.5) | 0.764 |
| Influenza A | 15 | 0 | 1 | |
| Influenza B | 2 | 0 | 0 | |
| Metapneumovirus | 2 | 0 | 0 | |
| Rhinovirus | 2 | 0 | 0 | |
| Adenovirus | 2 | 0 | 0 | |
| Respiratory syncytial virus-A | 2 | 0 | 0 | |
| Coronavirus OC43 | 2 | 0 | 0 |
Data are presented as n (%).
Post-hoc analysis.
Bronchiolitis vs. consolidation, Bonferroni’s corrected P <0.001.
Bronchiolitis vs. GGO, Bonferroni’s corrected P = 0.010.
Initial Antimicrobial Treatment
Initially used antibiotics are summarized and compared between the groups in Table 4. Third-generation cephalosporin- or ampicillin-sulbactam-based regimen was most commonly used in each group. There were no significant differences in antibiotic regimens between the groups, except fluoroquinolone with or without aminoglycoside. The higher frequency of fluoroquinolone with or without aminoglycoside in the bronchiolitis group was not statistically significant in post-hoc analysis, as compared with the consolidation group (Bonferroni’s corrected P = 0.042).
TABLE 4.
Initial antibiotic treatment.
| Antibiotic regimen | Consolidation (n = 1,579) | Bronchiolitis (n = 40) | GGO (n = 46) | P Value |
|---|---|---|---|---|
| Third-generation cephalosporin or ampicillin-sulbactam with or without macrolide, fluoroquinolone or clindamycin | 1,327 (84.0) | 36 (90.0) | 39 (84.8) | 0.590 |
| Fluoroquinolone with or without aminoglycoside | 50 (3.2) | 4 (10.0) | 3 (6.5) | 0.029 |
| Antipseudomonal beta-lactam with or without macrolide, fluoroquinolone or aminoglycoside | 138 (8.7) | 0 (0.0) | 4 (8.7) | 0.120 |
| Carbapenem with or without macrolide, fluoroquinolone or aminoglycoside | 15 (0.9) | 0 (0.0) | 0 (0.0) | >0.999 |
| Antipeudomonal beta-lactam or carbapenem plus glycopeptide with or without macrolide, fluoroquinolone or aminoglycoside | 38 (2.4) | 0 (0.0) | 0 (0.0) | 0.711 |
| Othersa | 11 (0.7) | 0 (0.0) | 0 (0.0) | >0.999 |
Data are presented as n (%).
Others include macrolide alone (n = 1), glycopeptide plus clindamycin (n = 1), fluoroquinolone plus clindamycin plus aminoglycoside (n = 1), antipseudomonal beta-lactam plus clindamycin (n = 1), third-generation cephalosporin plus glycopeptide (n = 1), carbapenem plus aminoglycoside plus macrolide (n = 1), fluoroquinolone plus glycopeptide (n = 2), macrolide plus glycopeptide (n = 1) and third-generation cephalosporin plus aminoglycoside (n = 2).
Discussion
This study confirms that predominantly bronchiolitis or GGO pattern on CT images is uncommon in patients with CAP. In the bronchiolitis group, the most common pathogen was M. pneumoniae, followed by S. pneumoniae and C. pneumoniae, and M. pneumoniae was significantly more common than in the consolidation group. As compared with the consolidation group, the bronchiolitis group was characterized by a higher percentage of cough, lower rates of chest pain and pleural effusion and the absence of complicated parapneumonic effusion or empyema and pleural drainage. Furthermore, the bronchiolitis group was associated with lower blood levels of inflammatory markers and higher albumin and total protein levels in blood. The common causative pathogens in the GGO group were M. pneumoniae, C. pneumoniae, S. pneumoniae and K. pneumoniae and these were not significantly different from those observed in the other 2 groups. As observed for the bronchiolitis group, complicated parapneumonic effusion or empyema was not observed and no patient underwent pleural drainage in the GGO group. Outcome variables, such as 30-day mortality, were not significantly different among the 3 groups.
Centrilobular nodules on CT correspond pathologically to cellular infiltration in bronchioles with exudates or granulation tissue in bronchiolar lumens, whereas consolidation reflects neutrophils and exudates in alveolar lumen.28 A previous study showed the microbiological features of CAP patients with a tree-in-bud pattern share similarities with those of pneumonia in the general population.29 In another study, M. pneumoniae infection was frequently associated with a bronchiolitis pattern.30 M. pneumoniae has an affinity for airway cilia and bronchioles and causes peribronchial and perivascular infiltration of mononuclear cells and edematous and ulcerative lesions in bronchial walls.28 In the present study, more than 40% of cases in the bronchiolitis group were caused by M. pneumoniae, which concurs with the findings of a previous study, in which M. pneumoniae was determined to be the most common pathogen of diffuse acute infectious bronchiolitis in adults.19 Interestingly, common CAP pathogens, such as C. pneumoniae, S. pneumoniae and K. pneumoniae, also cause a bronchiolitis pattern. The mechanisms responsible for centrilobular nodules or tree-in-bud pattern in some patients and consolidation or GGO in others can be explained by the up- or down-regulations of host cell-mediated immunity, respectively.28
In a previous study, the rate of diffuse bronchiolitis was reported to be 1.2% among community-acquired lower respiratory tract infections.19 The difference between this rate and the value found in the present study for the bronchiolitis pattern is explained by the different definitions used. In the present study, bronchiolitis was defined as either the presence of centrilobular nodules or a tree-in-bud pattern in most lesions with or without GGO or consolidation in the present study, whereas, in the previous study, diffuse acute infectious bronchiolitis was defined as multiple centrilobular nodules in 4 or more lobes.19 However, no previous study has addressed the clinical manifestations of the bronchiolitis or bronchiolitis-predominant CAP. As expected based on published findings,19, 30 our bronchiolitis group exhibited a clinically less severe form of CAP characterized by lower levels of inflammatory markers and the absence of complicated parapneumonic effusion or empyema.
Information regarding the incidence of the GGO pattern in CAP patients is lacking. The pathophysiology of GGO includes incomplete alveolar filling by inflammatory cells or exudate, pulmonary edema secondary to infection leaving air in alveoli or interstitial infiltrates of inflammatory cells.5 The resolving stage of lobar pneumonia can also exhibit a GGO appearance as alveolar aeration is restored. In previous studies, the rate of GGO in CAP was found to depend on the pathogen, that is, 19% for viral infections,31 17% for legionella pneumonia16 and 4% in pneumococcal pneumonia.8 However, in the present study, the prevalence of causative agents in the GGO and consolidation group were similar. In part, this difference might have arisen only because the CAP patients underwent throat or nasopharyngeal swab testing for respiratory viruses. A GGO pattern on CT is clinically associated with the more frequent use of systemic corticosteroids and the lack of complicated parapneumonic effusion or empyema and pleural drainage. In the present study, the use of corticosteroids was determined by attending physicians, and the reasons why they were administered could not be ascertained. However, we speculate corticosteroids were administered to patients with a GGO pattern because the possibility of a noninfectious condition, such as cryptogenic organizing pneumonia, was not excluded.
As noted earlier, the bronchiolitis and GGO groups had their own clinical features. On the contrary, initial antimicrobial treatment in the bronchiolitis or GGO group was not significantly different from the consolidation group. This discrepancy can be explained by the fact that because this study was retrospective, antibiotic therapy was determined by treating physician′s judgement and the CAP guidelines.3 Consequently, third-generation cephalosporin- or ampicillin-sulbactam–based antibiotic therapy was most commonly used in the present study. Given that M. pneumoniae was the most common pathogen in the bronchiolitis group, fluoroquinolone with or without aminoglycoside tended to be more frequently administered in the bronchiolitis group than the consolidation group.
The present study has several limitations. First, it was retrospectively conducted in a single institution, which suggests the possibility of selection bias. As described in a previous study,21 emergency physicians at our institution that encountered patients with suspected pneumonia tended to confirm the presence of pneumonia and transfer them from the emergency department to the internal medicine department. For this reason, most, though not all, CAP patients underwent a CT scan, although it is usually not necessary for the diagnosis of CAP. Second, some tests for causative pathogens were not performed in all CAP patients, only a proportion of patients underwent evaluation for respiratory viruses. Third, because of the retrospective design of the study, therapeutic decision-making, including the selection of antibiotics, was determined by attending physicians. Finally, the numbers of patients in the bronchiolitis and GGO groups were too small to allow us to reach definitive conclusions. Thus, we suggest a large-scale prospective study be performed to confirm our findings.
Conclusions
In conclusion, the bronchiolitis group was found to be associated with a higher M. pneumoniae frequency, a less severe form of CAP, and the absence of complicated parapneumonic effusion or empyema. On the other hand, the GGO group was similar to the consolidation group in terms of causative microorganisms, severity and prognosis, but unlike the consolidation group, was not found to be associated with complicated parapneumonic effusion or empyema. However, bronchiolitis and GGO groups were similar to the consolidation group in terms of 30-day or in-hospital mortality and length of hospital stay.
Author Contribution
H.S. and S.I.C. conceived and designed the analysis, H.S, S.I.C., K.M.S., J.K.L., S.S.Y., S.Y.L, J.L., C.H.K. and J.Y.P. collected the data, H.S, S.I.C., K.M.S. and J.K.L. contributed data or analysis tools, H.S. and S.I.C. performed the analysis, H.S. and S.I.C. wrote the paper. All authors read and approved the manuscript.
Footnotes
The authors have no financial or other conflicts of interest to disclose.
References
- 1.Prina E., Ranzani O.T., Torres A. Community-acquired pneumonia. Lancet. 2015;386:1097–1108. doi: 10.1016/S0140-6736(15)60733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Syrjala H., Broas M., Suramo I. High-resolution computed tomography for the diagnosis of community-acquired pneumonia. Clin Infect Dis. 1998;27:358–363. doi: 10.1086/514675. [DOI] [PubMed] [Google Scholar]
- 3.Mandell L.A., Wunderink R.G., Anzueto A. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claessens Y.E., Debray M.P., Tubach F. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med. 2015;192:974–982. doi: 10.1164/rccm.201501-0017OC. [DOI] [PubMed] [Google Scholar]
- 5.Nambu A., Ozawa K., Kobayashi N. Imaging of community-acquired pneumonia: roles of imaging examinations, imaging diagnosis of specific pathogens and discrimination from noninfectious diseases. World J Radiol. 2014;6:779–793. doi: 10.4329/wjr.v6.i10.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haroon A., Higa F., Fujita J. Pulmonary computed tomography findings in 39 cases of Streptococcus pneumoniae pneumonia. Intern Med. 2012;51:3343–3349. doi: 10.2169/internalmedicine.51.7326. [DOI] [PubMed] [Google Scholar]
- 7.Okada F., Ando Y., Matsushita S. Thin-section CT findings of patients with acute Streptococcus pneumoniae pneumonia with and without concurrent infection. Br J Radiol. 2012;85:e357–e364. doi: 10.1259/bjr/18544730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yagihashi K., Kurihara Y., Fujikawa A. Correlations between computed tomography findings and clinical manifestations of Streptococcus pneumoniae pneumonia. Jpn J Radiol. 2011;29:423–428. doi: 10.1007/s11604-011-0574-x. [DOI] [PubMed] [Google Scholar]
- 9.Okada F., Ando Y., Honda K. Clinical and pulmonary thin-section CT findings in acute Klebsiella pneumoniae pneumonia. Eur Radiol. 2009;19:809–815. doi: 10.1007/s00330-008-1233-1. [DOI] [PubMed] [Google Scholar]
- 10.Okada F., Ando Y., Honda K. Acute Klebsiella pneumoniae pneumonia alone and with concurrent infection: comparison of clinical and thin-section CT findings. Br J Radiol. 2010;83:854–860. doi: 10.1259/bjr/28999734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee I., Kim T.S., Yoon H.K. Mycoplasma pneumoniae pneumonia: CT features in 16 patients. Eur Radiol. 2006;16:719–725. doi: 10.1007/s00330-005-0026-z. [DOI] [PubMed] [Google Scholar]
- 12.Miyashita N., Sugiu T., Kawai Y. Radiographic features of Mycoplasma pneumoniae pneumonia: differential diagnosis and performance timing. BMC Med Imaging. 2009;9:7. doi: 10.1186/1471-2342-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nei T., Yamano Y., Sakai F. Mycoplasma pneumoniae pneumonia: differential diagnosis by computerized tomography. Intern Med. 2007;46:1083–1087. doi: 10.2169/internalmedicine.46.6460. [DOI] [PubMed] [Google Scholar]
- 14.Okada F., Ando Y., Wakisaka M. Chlamydia pneumoniae pneumonia and Mycoplasma pneumoniae pneumonia: comparison of clinical findings and CT findings. J Comput Assist Tomogr. 2005;29:626–632. doi: 10.1097/01.rct.0000167809.89352.93. [DOI] [PubMed] [Google Scholar]
- 15.Nambu A., Saito A., Araki T. Chlamydia pneumoniae: comparison with findings of Mycoplasma pneumoniae and Streptococcus pneumoniae at thin-section CT. Radiology. 2006;238:330–338. doi: 10.1148/radiol.2381040088. [DOI] [PubMed] [Google Scholar]
- 16.Kim K.W., Goo J.M., Lee H.J. Chest computed tomographic findings and clinical features of legionella pneumonia. J Comput Assist Tomogr. 2007;31:950–955. doi: 10.1097/RCT.0b013e31804b211d. [DOI] [PubMed] [Google Scholar]
- 17.Sakai F., Tokuda H., Goto H. Computed tomographic features of Legionella pneumophila pneumonia in 38 cases. J Comput Assist Tomogr. 2007;31:125–131. doi: 10.1097/01.rct.0000233129.06056.65. [DOI] [PubMed] [Google Scholar]
- 18.Park C.K., Kwon H., Park J.Y. Thin-section computed tomography findings in 104 immunocompetent patients with adenovirus pneumonia. Acta Radiol. 2017;58:937–943. doi: 10.1177/0284185116681039. [DOI] [PubMed] [Google Scholar]
- 19.Ryu K., Takayanagi N., Ishiguro T. Etiology and outcome of diffuse acute infectious bronchiolitis in adults. Ann Am Thorac Soc. 2015;12:1781–1787. doi: 10.1513/AnnalsATS.201507-473OC. [DOI] [PubMed] [Google Scholar]
- 20.Lee S.Y., Cha S.I., Seo H. Multimarker prognostication for hospitalized patients with community-acquired pneumonia. Intern Med. 2016;55:887–893. doi: 10.2169/internalmedicine.55.5764. [DOI] [PubMed] [Google Scholar]
- 21.Seo H., Cha S.I., Shin K.M. Clinical relevance of necrotizing change in patients with community-acquired pneumonia. Respirology. 2017;22:551–558. doi: 10.1111/resp.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niederman M.S., Bonten M.J., Chastre J. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 23.Pipavath S.J., Lynch D.A., Cool C. Radiologic and pathologic features of bronchiolitis. AJR Am J Roentgenol. 2005;185:354–363. doi: 10.2214/ajr.185.2.01850354. [DOI] [PubMed] [Google Scholar]
- 24.Fine M.J., Auble T.E., Yealy D.M. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 25.Lim W.S., van der Eerden M.M., Laing R. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oken M.M., Creech R.H., Tormey D.C. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 27.Charlson M.E., Pompei P., Ales K.L. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka H. Correlation between radiological and pathological findings in patients with Mycoplasma pneumoniae pneumonia. Front Microbiol. 2016;7:695. doi: 10.3389/fmicb.2016.00695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimon G., Yonit W.W., Gabriel I. The tree-in-bud pattern on chest CT: radiologic and microbiologic correlation. Lung. 2015;193:823–829. doi: 10.1007/s00408-015-9759-x. [DOI] [PubMed] [Google Scholar]
- 30.Cha S.I., Shin K.M., Kim M. Mycoplasma pneumoniae bronchiolitis in adults: clinicoradiologic features and clinical course. Scand J Infect Dis. 2009;41:515–519. doi: 10.1080/00365540902942840. [DOI] [PubMed] [Google Scholar]
- 31.Shiley K.T., Lautenbach E., Lee I. The use of antimicrobial agents after diagnosis of viral respiratory tract infections in hospitalized adults: antibiotics or anxiolytics? Infect Control Hosp Epidemiol. 2010;31:1177–1183. doi: 10.1086/656596. [DOI] [PMC free article] [PubMed] [Google Scholar]