Abstract
Background
Influenza is an acute respiratory illness caused by influenza A and B viruses. Complications may occur, especially among children and the elderly.
Objectives
To assess the effectiveness and safety of amantadine and rimantadine in preventing, treating and shortening the duration of influenza A in children and the elderly.
Search methods
We searched CENTRAL (2014, Issue 9), MEDLINE (1966 to September week 4, 2014) and EMBASE (1980 to October 2014).
Selection criteria
Randomised controlled trials (RCTs) or quasi‐RCTs comparing amantadine and/or rimantadine with no intervention, placebo, other antivirals or different doses or schedules of amantadine or rimantadine in children and the elderly with influenza A.
Data collection and analysis
Two review authors independently assessed the search results. We extracted and analysed data using the standard Cochrane methodology.
Main results
We identified 12 studies (2494 participants: 1586 children and 908 elderly) comparing amantadine and rimantadine with placebo, paracetamol (one trial: 69 children) or zanamivir (two trials: 545 elderly) to treat influenza A.
Amantadine was effective in preventing influenza A in children (773 participants, risk ratio (RR) 0.11; 95% confidence interval (CI) 0.04 to 0.30). The assumed risk of influenza A in the control group was 10 per 100. The corresponding risk in the rimantadine group was one per 100 (95% CI 0 to 3). Nevertheless, the quality of the evidence was low and the safety of the drug was not well established.
For treatment, rimantadine was beneficial in abating fever on day three of treatment in children: one selected study with low risk of bias, moderate evidence quality and 69 participants (RR 0.36; 95% CI 0.14 to 0.91). The assumed risk was 38 per 100. The corresponding risk in the rimantadine group was 14 per 100 (95% CI 5 to 34).
Rimantadine did not show any prophylactic effect in the elderly. The quality of evidence was very low: 103 participants (RR 0.45; 95% CI 0.14 to 1.41). The assumed risk was 17 per 100. The corresponding risk in the rimantadine group was 7 per 100 (95% CI 2 to 23).
There was no evidence of adverse effects caused by treatment with amantadine or rimantadine.
We found no studies assessing amantadine in the elderly.
Authors' conclusions
The quality of the evidence combined with a lack of knowledge about the safety of amantadine and the limited benefits of rimantadine, do not indicate that amantadine and rimantadine compared to control (placebo or paracetamol) could be useful in preventing, treating and shortening the duration of influenza A in children and the elderly.
Plain language summary
Amantadine and rimantadine to prevent and treat influenza A in children and the elderly
Review question
As recommended by the World Health Organization (WHO), oseltamivir (Tamiflu) is currently used for people with influenza A. In previous pandemics, the virus was susceptible to amantadine and rimantadine. If they are safe and the circulating strain proves to be susceptible to these drugs, they could be an alternative for managing influenza. We therefore wanted to answer the question of whether or not amantadine and rimantadine can prevent and treat influenza A in children and the elderly.
Background
Influenza A is a respiratory infection causing cough, runny nose and fever. Most symptoms pass without treatment within three to seven days. However, hospitalisation, pneumonia and even death are rare complications of the illness, especially among children and the elderly. Pandemics are also a cause for concern.
Key results and quality of the evidence
We identified 12 trials (2494 participants: 1586 children and 908 elderly). We looked for trials that compared amantadine or rimantadine with no intervention, placebos or control drugs in children and the elderly. The most recent searches were completed in October 2014. We looked at several outcomes, including influenza A, fever duration, cough, headache, nausea/vomiting, dizziness and stimulation/insomnia.
Although amantadine was effective in preventing influenza A in children, it would be necessary to use it in up to 17 children over a period of 14 to 18 weeks to prevent one case of influenza A. Furthermore, the safety of the drug was not well established. The quality of the evidence was low.
The effectiveness of both antivirals was limited to a benefit from rimantadine in the reduction of fever by day three of treatment in children. The quality of the evidence was moderate. This benefit does not seem to justify a recommendation for using rimantadine to treat all children with influenza A.
Rimantadine did not show a prophylactic (preventative) effect in the elderly. The quality of evidence was very low.
Conclusion
The quality of the evidence combined with a lack of knowledge about the safety of amantadine and the limited benefits of rimantadine, do not indicate that amantadine and rimantadine compared to control (placebo or paracetamol) could be useful in preventing, treating and shortening the duration of influenza A in children and the elderly.
Summary of findings
for the main comparison.
| Amantadine compared with placebo for prevention and treatment of influenza A in children | ||||||
|
Patient or population: children with no influenza A infection (prevention) or with influenza A infection (treatment) Settings: all Intervention: amantadine Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Amantadine | |||||
|
Cases of influenza A during prophylaxis (follow‐up:14 to 18 weeks) |
Medium risk population | RR 0.11 (0.04 to 0.3) | 773 (2) | ⊕⊕⊝⊝ low1,2 | ||
| 10 per 100 | 1 per 100 (0 to 3) | |||||
|
Fever after initiation of treatment (follow‐up: 3 days) |
Medium risk population | RR 0.37 (0.08 to 1.75) | 104 (2) | ⊕⊕⊝⊝ low3,4 | ||
| 23 per 100 | 9 per 100 (2 to 40) | |||||
| Cough after initiation of treatment | See comment | See comment | Not estimable | 0 (0) |
See comment | No selected trial |
|
Dizziness (follow‐up: 7 days) |
Medium risk population | RR 6.63 (0.32 to 137.33) | 599 (2) | ⊕⊝⊝⊝ very low3,4 | ||
| 0 per 100 | 0 per 100 (0 to 0) | |||||
|
Nausea/vomiting (follow‐up: 7 days) |
Medium risk population | RR 0.54 (0.15 to 2) | 599 (2) | ⊕⊝⊝⊝ very low3,4,5 | ||
| 13 per 100 | 7 per 100 (2 to 27) | |||||
|
Stimulation/insomnia (follow‐up: 7 days) |
Medium risk population | RR 0.46 (0.12 to 1.74) | 599 (2) | ⊕⊕⊝⊝ low3,4 | ||
| 3 per 100 | 7 per 100 (2 to 27) | |||||
| CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
*The basis for the assumed risk (e.g. median control group risk across studies) was calculated on the basis of control event rate. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
1Allocation concealment not used or unclear.
2Sparse data.
3Allocation concealment unclear.
4Sparse data, confidence intervals do not rule out potential for null effect or harm.
5High heterogeneity unexplained.
2.
| Rimantadine compared with placebo for prevention and treatment of influenza A in children | ||||||
|
Patient or population: children with no influenza A infection (prevention) or with influenza A infection (treatment) Settings: any Intervention: rimantadine Comparison: control (placebo or acetaminophen) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Rimantadine | |||||
| Cases of influenza A during prophylaxis (follow‐up: 1 to 35 days) | Medium risk population | RR 0.49 (0.21 to 1.15) | 178 (3) | ⊕⊕⊝⊝ low1,2 | ||
| 24 per 100 | 12 per 100 (5 to28) | |||||
|
Fever after initiation of treatment (follow‐up: 3 days) |
Medium risk population | RR 0.36 (0.14 to 0.91) | 69 (1) | ⊕⊕⊕⊝ moderate2 | ||
| 38 per 100 | 14 per 100 (5 to 34) | |||||
|
Cough after initiation of treatment (follow‐up: 7 days) |
Medium risk population | RR 0.83 (0.63 to 1.1) | 69 (1) | ⊕⊕⊕⊝ moderate2 | ||
| 81 per 100 | 67 per 100 (51 to 89) | |||||
|
Dizziness (follow‐up: 35 days) |
Medium risk population | RR 3.21 (0.14 to 75.68) | 56 (1) | ⊕⊝⊝⊝ very low1,2 | ||
| 0 per 100 | 0 per 100 (0 to 0) | |||||
|
Nausea/vomiting (follow‐up: 7 to 35 days) |
Medium risk population | RR 0.96 (0.1 to 9.01) | 125 (2) | ⊕⊕⊝⊝ low2 | ||
| 2 per 100 | 2 per 100 (0 to 15) | |||||
| Stimulation/insomnia | See comment | See comment | Not estimable | 0 (0) | See comment | No selected trial |
| CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
*The basis for the assumed risk (e.g. median control group risk across studies) was calculated on the basis of control event rate. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
1Allocation concealment unclear.
2Sparse data and confidence intervals do not rule out the potential for no effect or harm
3.
| Amantadine compared with placebo for prevention and treatment of influenza A in the elderly | ||||||
|
Patient or population: elderly people with no influenza A infection (prevention) or with influenza A infection (treatment) Settings: any Intervention: amantadine Comparison: control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Amantadine | |||||
| Cases of influenza A during prophylaxis | See comment | Not estimable | 0 (0) | See comment | No selected trial | |
| Fever after initiation of treatment | See comment | Not estimable | 0 (0) | See comment | No selected trial | |
| Cough after initiation of treatment | See comment | Not estimable | 0 (0) | See comment | No selected trial | |
| Dizziness | See comment | Not estimable | 0 (0) | See comment | No selected trial | |
| Nausea | See comment | Not estimable | 0 (0) | See comment | No selected trial | |
| Vomiting | See comment | Not estimable | 0 (0) | See comment | No selected trial | |
| Stimulation/insomnia | See comment | Not estimable | 0 (0) | See comment | No selected trial | |
4.
|
Patient or population: elderly people with no influenza A infection (prevention) or with influenza A infection (treatment) Settings: any Intervention: rimantadine Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Rimantadine | |||||
| Cases of influenza A during prophylaxis | Medium risk population | RR 0.45 (0.14 to 1.41) | 103 (2) | ⊕⊝⊝⊝ very low1,2 | ||
| 17per 100 | 7 per 100 (2 to 23) | |||||
| Fever after initiation of treatment | See comment | 0 (0) | See comment | See comment | No selected trial | |
| Cough after initiation of treatment | See comment | 0 (0) | See comment | See comment | No selected trial | |
|
Dizziness (follow‐up: 12 weeks) |
Medium risk population | |||||
| 12 per 100 | 11 per 100 (2 to 70) | RR 0.94 (0.15 to 5.97) | 35 (1) |
⊕⊕⊝⊝ low2,3 | ||
|
Nausea (follow‐up: 8 to 12 weeks) |
Medium risk population | RR 1.99 (0.45 to 8.75) | 233 (2) | ⊕⊝⊝⊝ very low1,2,4 | ||
| 8 per 100 | 15 per 100 (3 to 66) | |||||
|
Vomiting (follow‐up: 8 to 12 weeks) |
Medium risk population | RR 0.99 (0.38 to 2.6) | 233 (2) | ⊕⊕⊝⊝ low1,2 | ||
| 7 per 100 | 7 per 100 (3 to 17) | |||||
| Stimulation/insomnia (follow‐up: 8 to 12 weeks) | Medium risk population | RR 1.61 (0.43 to 6.02) | 233 (2) | ⊕⊕⊝⊝ low1,2 | ||
| 7 per 100 | 11 per 100 (3 to 40) | |||||
| CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
*The basis for the assumed risk (e.g. median control group risk across studies) was calculated on the basis of control event rate. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
1Allocation concealment unclear and 1 study had high withdrawal rate.
2Sparse data and confidence interval do not rule out no effect or harm.
3Allocation concealment unclear
4High heterogeneity unexplained.
Background
Description of the condition
Influenza is an acute and usually self limiting respiratory illness caused by influenza A and B viruses, which are members of the Orthomyxoviridae family (Nicholson 1992). Influenza may cause annual epidemics and intermittent pandemics (Sasaki 2011). Typically, seasonal influenza occurs most frequently during autumn and winter in temperate regions, but in some tropical countries it may occur throughout the year with one or two peaks during rainy seasons (Monto 2008; Yang 2010).
Although the natural transmission of the influenza virus predominantly occurs via aerosols dispersed by coughing or sneezing, it is also transmitted by nasal secretions and contact with contaminated surfaces. While all respiratory viruses, including influenza, use the nose as the entry channel, they can also enter through the tear ducts, draining into patients' sinuses and airways (Bitko 2007). The virus particles are deactivated by the ultraviolet rays in sunlight and common disinfectants such as soap (Barik 2012).
The illness is characterised by an abrupt onset of symptoms. These symptoms include headache, fever, general aches, weakness and myalgia, accompanied by respiratory tract signs, particularly cough and sore throat. However, a wide spectrum of clinical presentations may occur, ranging from a mild, febrile upper respiratory illness, to severe prostration and respiratory and systemic signs and symptoms.
The most common complication that occurs during outbreaks of influenza is pneumonia (both viral and bacterial). A number of extra‐pulmonary complications may also occur. These include Reye's syndrome in children (most commonly between two and 16 years of age), myocarditis, pericarditis and central nervous system (CNS) diseases. Again these include encephalitis, transverse myelitis and Guillain‐Barré syndrome (Barik 2012; Wiselka 1994).
An interesting and clinically relevant aspect of pandemic and epidemic influenza that sets it apart from seasonal influenza is the induction of the so‐called cytokine storm, consisting of interleukin‐6, tumour necrosis factor and interferon‐g. Together, these proinflammatory cytokines cause systemic inflammatory response syndrome, leading to multi‐organ failure that includes airway destruction, vascular endothelial damage and plasma leakage (Barik 2012; Cheung 2002)
Description of the intervention
Nowadays there are two main measures for the treatment and prophylaxis of influenza viruses: immunisation using influenza vaccines directly isolated from influenza A and B viruses and antiviral agents (Demicheli 2000; Noah 2013). Vaccination is the primary strategy for the prevention of influenza (Antanova 2012; Hsu 2012). Nevertheless, there are a number of likely scenarios for which effective antiviral agents would be of utmost importance. For example, the available evidence on the safety, efficacy or effectiveness of influenza vaccines for people aged 65 years or older is of poor quality (Jefferson 2010; Thomas 2011). Vaccination among the elderly may not be as effective as their immune systems are less responsive (Sasaki 2011). Influenza vaccines are efficacious in children older than two but little evidence is available for children under two (Demicheli 2012). During any influenza season, antigenic drift in the virus may occur after formulation of the year's vaccine. The vaccine can therefore be less protective and outbreaks can more easily occur in high‐risk populations. In the course of a pandemic, vaccine supplies would be inadequate. Moreover, vaccine production by current methods cannot be carried out with the speed required to halt the progress of a new strain of influenza virus. Therefore, it is likely that vaccines would not be available for those infected by the first wave of the virus (Hayden 2004). Additionally, in a study published in 2013, the author stated that vaccination‐only strategies were not cost‐effective for any pandemic scenario, saving few lives and incurring substantial vaccination costs (Kelso 2013). Vaccination, coupled with long duration social distancing, antiviral treatment and antiviral prophylaxis, was considered to be cost‐effective for moderate and extreme pandemics, as it can save lives while simultaneously reducing the total pandemic cost (Kelso 2013). Antiviral agents therefore form an important part of a rational approach to influenza management (Kelso 2013; Moscona 2005).
Antiviral drugs for influenza are currently divided into two classes: M2 ion channel inhibitors and neuraminidase inhibitors. The first class includes amantadine and rimantadine and the latter zanamivir, oseltamivir, laninamivir (approved in Japan) and peramivir (approved in Japan and Korea) (Barik 2012). M2 ion channel inhibitors affect ion channel activity through the cell membrane and are reported to be effective by interfering with the replication cycle of type A viruses (but not type B). The neuraminidase inhibitors interfere with the release of influenza virus progeny from infected host cells and are effective against influenza A and B (Moscona 2005). Both drug classes have shown partial effectiveness for the prevention and treatment of influenza A viruses, although neuraminidase inhibitors are less likely to promote the development of drug‐resistant influenza (Moscona 2005).
Resistance to M2 inhibitors remained low until 2003 (Bright 2005; Ziegler 1999). An epidemiological study into resistance to amantadine carried out from 1991 to 1995 described a frequency of 1% (16/2017) of resistant variants among H1N1 and H3N2 viruses (Ziegler 1999). However, there was a subsequently a dramatic increase in strains of influenza A (H3N2) with a specific mutation (Ser31Asn). An increase in resistance to amantadine was showed in communities located in Asia and North America (Bright 2005; Bright 2006). This resistance in 70% to 90% of strains occurred despite the absence of sustained selective drug pressure (Bright 2005; Bright 2006).
During the 2005 to 2006 season, 16% of H1N1 and 91% of H3N2 viruses were resistant around the world. Although the estimate for the proportion of resistance in H1N1 viruses was very low, an analysis conducted in China showed that the frequency of resistant H1N1 viruses had greatly increased from 28% (8/29) in the 2004 to 2005 season to 72% (33/46) in the 2005 to 2006 season. Similar studies were conducted in other countries in the 2005 to 2006 season. The following frequencies of resistance were obtained: 45% (13/29) in Europe, 24% (4/17) in Taiwan and 33% (1/3) in Canada (Deyde 2007).
A global pandemic emerged in 2009, caused by a new influenza A strain (H1N1) (WHO 2010a). All influenza A (H1N1) viruses tested in WHO Collaborating Centres to date have been shown to be resistant to amantadine and rimantadine (WHO 2011; WHO 2012).
When an avian influenza A (H7N9) virus was detected as the cause of human infections in China, its susceptibility to antiviral drugs was assessed. The outbreak viruses carried the established adamantine resistance marker. Once again neuraminidase inhibitors remained the only licensed treatment option (Li 2014).
Influenza A resistance to amantadine and rimantadine has been frequently reported over the last few years and, as such, it may seem unnecessary to continue testing sensitivity to these drugs. However, patterns of sensitivity and resistance of influenza viruses to antiviral drugs may change over time and so we consider it necessary to continue monitoring sensitivity and resistance.
How the intervention might work
The use of amantadine and rimantadine for the treatment and prevention of influenza A in adults has already been the topic of a review (Jefferson 2006b). The results of that review confirmed that amantadine and rimantadine had a comparable efficacy and effectiveness in the treatment of influenza A in healthy adults, although their effectiveness in interrupting transmission was probably low. As previous pandemics proved to be susceptible to this class of drugs, it seems reasonable to review the evidence for amantadine and rimantadine for treating and preventing influenza A in children and the elderly (Hayden 2006b).
Why it is important to do this review
Although the disease occurs in all age groups (Pineda Solas 2006), the risks of complications, hospitalisations and deaths from influenza are higher among three groups of people: 1) persons older than 65 years; 2) young children; and 3) persons of any age who have medical conditions that place them at increased risk. Rates of infection are highest amongst children and they are also one of the most important links for transmission (Dolin 2005).
Pandemics occur when influenza spreads globally, infecting 20% to 40% of the world's population in one year. This results in as many as 10 million deaths (WHO 2003). They usually arise in China, where pigs, ducks and humans live in close proximity to each other, and spread westward to the rest of Asia, Europe and the Americas (Bonn 1997). In the past 110 years there have been five pandemics caused by different influenza A viral subtypes. The Spanish influenza pandemic (1918 to 1919) is considered to have caused an estimated 40 million deaths worldwide. Most years, typical influenza epidemics infect 5% to 20% of the population and result in anywhere between 250,000 and 500,000 deaths, according to the World Health Organization (WHO), although other estimates accounting for deaths due to complications of influenza are as high as 1 million to 1.5 million.
In 2009, a new influenza A strain (H1N1) caused a global pandemic. According to the WHO, as of 24 January 2010, more than 214 countries and overseas territories had reported laboratory‐confirmed cases of pandemic influenza H1N1, resulting in at least 18,449 deaths (WHO 2010a).
In an earlier version of a Cochrane review in adults, the review authors stated that neuraminidase inhibitors were effective in reducing symptoms and complications, however there are now doubts about their effectiveness against complications (Jefferson 2014).
In a Cochrane review published in 2007, the review authors concluded that oseltamivir may be considered for the treatment of children aged one to 12 years with influenza infection (Matheson 2007). This antiviral is likely to shorten the duration of symptoms, hasten the return to normal activities and reduce the incidence of secondary complications. Nevertheless, the review authors also concluded that more data were needed to clarify the benefits of neuraminidase inhibitors for the treatment of influenza in asthmatic children (including addressing the potential confounder of prior vaccination).
Nowadays, neuraminidase inhibitors are used as a prescription drug for patients suffering from influenza on the recommendation of the WHO (WHO 2010b). Governments have spent billions of dollars stockpiling neuraminidase inhibitors as a public health measure (WHO 2010b). In previous pandemics, the influenza A virus was susceptible to amantadine and rimantadine. Therefore, these antivirals could be a less expensive alternative in the management of influenza if the circulating strain proves to be susceptible to amantadine and rimantadine (Hayden 2006b). However, we should emphasise the resistance patterns of the pandemic viruses in 2009. All influenza A (H1N1) viruses tested in WHO Collaborating Centres to date were sensitive to zanamivir and all were resistant to amantadine and rimantadine (WHO 2011).
These facts reinforce the importance of conducting and maintaining reviews of a variety of treatments, especially less expensive ones, for the treatment and prevention of influenza.
Objectives
To assess the effectiveness and safety of amantadine and rimantadine in preventing, treating and shortening the duration of influenza A in children and the elderly.
We tested the following hypotheses in comparisons between groups intended for amantadine or rimantadine prophylaxis or treatment compared with control groups:
there is no difference in the number of cases of influenza A or in the duration of influenza symptoms; and
there is no difference in the number of adverse effects.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs comparing amantadine or rimantadine, or both, with placebo, control drugs, different doses or schedules of amantadine or rimantadine, or both, or no intervention, in children and the elderly.
Types of participants
We included studies where at least 75% of the population was up to 19 years of age, or 65 years of age or older. We also included trials with a wider age range where data by age subgroups were available.
Types of interventions
Comparisons of amantadine or rimantadine, or both, to placebo, control drugs, other antivirals, no interventions or different doses of amantadine or rimantadine, or both, as prophylaxis and/or treatment for influenza A.
Types of outcome measures
Primary outcomes
Response to treatment (measured as cases on the specified day of treatment): fever on day three of treatment, cough on day seven of treatment, malaise on day six of treatment and conjunctivitis and eye symptoms on day five of treatment.
Cases of influenza, studied in all prophylaxis comparisons, including those in which two antivirals (rimantadine and zanamivir) (Gravenstein 2005; Schilling 1998), and two different doses of rimantadine were compared (Monto 1995).
Cases of side effects in children: diarrhoea, exanthema, malaise, muscular limb pain, headache, dyspnoea, dizziness, stimulation/insomnia, nausea, vomiting, arrhythmia, gastrointestinal (GI) symptoms, CNS symptoms, change in behaviour, hyperactivity and tinnitus.
Cases of side effects in the elderly: headache, dizziness, stimulation/insomnia, nausea, vomiting, anxiety, confusion, fatigue, depression, impaired concentration, loss of appetite, rash or allergic reaction, seizures or clonic twitching, dry mouth, insomnia or sleeplessness, body weakness and debility.
We used dichotomous outcomes for all the comparisons.
Secondary outcomes
The following outcomes appeared in the protocol but in the end we did not consider them in the analysis, as they were not reported in the included trials: patients' well‐being, admission to hospital, general practitioner (GP) visits and other drugs used. We could not analyse deaths. Although cited by Monto 1995, they were included among other causes of withdrawal.
Search methods for identification of studies
Electronic searches
For this 2014 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL 2014, Issue 9) (accessed 7 October 2014), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (June 2011 to September week 4, 2014) and EMBASE (June 2011 to October 2014).
The search strategy for MEDLINE and CENTRAL is described in Appendix 1. See Appendix 2 for the EMBASE search strategy. We imposed no language or publication restrictions. We used the same search strategy for our previous update in 2011, searching the Cochrane Central Register of Controlled Trials (CENTRAL 2011, Issue 2), MEDLINE (July 2007 to June week 3, 2011) and EMBASE.com (July 2007 to June 2011). Details of the review's initial search are in Appendix 3.
Searching other resources
We searched the trials registries WHO International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov for completed and ongoing trials (latest search 7 October 2014). We screened bibliographies of retrieved articles and reviews in order to identify further trials. We contacted pharmaceutical companies and researchers active in the field for unpublished trials.
Data collection and analysis
Selection of studies
Two review authors (MG and MS) independently applied the selection criteria to all retrieved articles and extracted data using a data extraction form, specifically designed for this review. We resolved disagreements by consensus. We appointed one review author (AC) as arbitrator when necessary.
We entered extracted data into RevMan 2012. Combination of data was dependent on population characteristics and outcomes studied.
Data extraction and management
Two review authors (MG, MS) independently read the retrieved trials and applied the selection criteria. We independently extracted and reviewed data using the data collection form previously developed for this review. Two review authors (MG, MS) resolved disagreements on the quality of the trials by consensus. We appointed a third author (AC) as arbitrator if necessary.
We emailed the authors of primary studies when the complete information sought was not available in study reports. We obtained authors' contact details from the study reports, other recent publications, university directories or by searching the world wide web. We recorded the following data.
Setting: hospital, emergency, offices or clinics, primary health care, nursing homes, communities, prisons, military personnel, nursery or day care.
Participants: criteria for patients to join the trial, age, gender, diagnostic criteria and co‐morbid conditions.
Interventions: placebo, other than amantadine and rimantadine antiviral controls, comparing different doses or schedules of amantadine and/or rimantadine or no intervention.
Outcome measures: global symptom improvements, relief, death, cases of influenza, malaise, fever, nausea, arthralgia, rash, headache, systemic and serious side effects, well‐being, admission to hospital, GP visits, other drugs used, cough, coryza, sore throat, hoarseness, vomiting, abdominal pain, insomnia, irritability, behaviour changes and anorexia.
Adverse effects: dry mouth, drowsiness/fatigue, constipation, urinary retention, sweating, headache, diarrhoea, palpitations, irritability, blurred vision, dizziness/light headedness and nausea/vomiting and any other systemic and serious side effects.
Assessment of risk of bias in included studies
Two review authors (MG, MS) independently screened trial quality. We resolved disagreements by discussion. We appointed a third author (AC) to act as arbitrator when necessary. We used the criteria recommended by the Cochrane Handbook for Systematic Reviews of Interventions to assess the risk of bias (Higgins 2011). We developed a form and populated it to assess the risk of bias, based on a Cochrane review (Ahovuo‐Saloranta 2014). We indicated if the risk of bias was low, high , or even unclear, indicating either a lack of information or uncertainty over the potential for bias.
1. Sequence generation: was the method used to generate the allocation sequence appropriate to produce comparable groups? We considered that the risk of bias was low if the authors described a random component in the sequence generation process (for example, a random number table, a computerised random number table, coin tossing, shuffling cards or envelopes, throwing dice, drawing of lots). If there was no or insufficient information about the sequence generation process, we marked this domain 'unclear'. We considered that there was a high risk of bias if the sequence was generated by: 1) odds and evens or date of birth; 2) some rule based on date (or day) of admission; 3) some rule based on hospital or clinic record number.
2. Allocation sequence concealment: was the method used to conceal the allocation sequence appropriate to prevent the allocation being known in advance of, or during, enrolment? We marked this domain 'low risk' of bias if the trial authors described adequate concealment (for example, by means of either central allocation, sequentially numbered drug containers of identical appearance, or sequentially numbered, opaque, sealed envelopes) and 'high risk' of bias if: 1) inadequate concealment was documented; 2) allocation concealment was not used (for example, using either an open random allocation schedule, assignment envelopes without appropriate safeguards, alternation or rotation, date of birth or case record number). We marked this domain 'unclear' if: 1) insufficient information about allocation concealment was provided; 2) the information was unclearly reported.
3. Blinding of participants and personnel: were adequate measures used to blind study participants and personnel from knowing which intervention a participant received? We marked this domain 'low risk' of blinding if the RCT authors stated that: 1) there was no blinding; 2) there was incomplete blinding but the review authors judged that the outcome was not likely to be influenced by said incomplete blinding; 3) blinding of participants and key study personnel was ensured and it is unlikely that the blinding could have been broken. We marked this domain 'high risk' of bias, if the RCT authors described: 1) no blinding; 2) incomplete blinding and the outcome was likely to be influenced by said incomplete blinding; 3) blinding of key study participants and personnel but it was likely that the blinding could have been broken. We marked this domain 'unclear' if there was insufficient information or if the study did not address this outcome.
4. Blinding of outcome assessment: were adequate measures used to blind outcome assessors from knowing which intervention a participant received? We marked this domain 'low risk' of bias if there was: 1) no blinding of outcome assessment but the review authors judged that the outcome measurement was not likely to be influenced by lack of blinding; 2) blinding of outcome assessors was ensured and it is unlikely that the blinding could have been broken. We marked this domain 'high risk' of bias, if: 1) no blinding of outcome assessment was stated and the outcome measurement was likely to be influenced by lack of blinding; 2) there was blinding of outcome assessors but it was likely that the blinding could have been broken. We marked this domain 'unclear' if there was insufficient information or if the study did not address this outcome.
5. Incomplete outcome data describes how complete the data were for the clinical outcomes. Were dropout rates and reasons for withdrawals reported? Were missing data imputed appropriately? We marked this domain 'low risk' of bias if the RCT authors stated that: 1) there were no missing outcome data; 2) the reasons for missing outcome data were unlikely to be related to true outcome: 3) missing outcome data balanced out across intervention groups, with similar reasons for missing data across said groups; 4) the proportion of missing outcomes compared with the observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate; 5) missing data were imputed using appropriate methods. We marked this domain 'high risk' of bias, representing a high risk of attrition bias, if: 1) the reason for missing outcome data was likely to be related to true outcome, with either an imbalance in numbers or reasons for missing data across intervention groups; 2) the proportion of missing outcomes compared with the observed event risk was enough to induce clinically relevant bias in the intervention effect estimate; 3) 'as‐treated' analysis was done with substantial departure of the intervention received from that assigned at randomisation; 4) there was potentially inappropriate application of simple imputation. 'unclear risk of bias' was the expected classification of studies in which there was insufficient reporting of attrition/exclusions to permit the classification of 'low risk' or 'high risk' (e.g. number of randomised patients not stated, no reasons for missing data provided), or if the study did not address this outcome.
We completed a 'Risk of bias' table for each included study (see 'Risk of bias' tables in the Characteristics of included studies table).
Measures of treatment effect
We calculated risk ratios (RRs) and 95% confidence intervals (CI) for each study as all the outcomes studied were dichotomous. We tested for heterogeneity for each outcome.
Unit of analysis issues
In the Gravenstein 2005 trial, the author stated that the study was conducted over three winter seasons and that some participants were randomised more than once. Taking into account that influenza was the outcome of interest and that in each season different influenza viruses emerge, participants that had acquired the infection in one of the seasons could not be considered to be immunologically resistant to influenza in the next season. Consequently, we decided to include all participants described by the trial authors, as this does not seem to produce bias.
In the Crawford 1988 and Clover 1991 studies, eligible family members were randomly assigned as a block to study rimantadine in the prevention of influenza. For the purpose of this review, we selected the children as the subgroup of interest. It could be expected that children from families in the intervention group could be more protected from influenza than children in the control group. Nevertheless, no effect was shown in either of the three trials selected for this comparison (Clover 1986; Clover 1991; Crawford 1988).
Dealing with missing data
We contacted the trial authors to request missing data when data were not clearly provided. We analysed the available data, taking into account the relatively small number and randomness of missing data.
Assessment of heterogeneity
We stored the data extracted from primary studies in the Review Manager software (RevMan 2012). All the outcomes we studied were dichotomous. We determined whether there were sufficiently homogeneous data to combine when there were two or more selected studies for a given comparison. We grouped the previously selected articles according to the characteristics of interventions, outcomes and populations studied. We had to take into account that pooled studies may still differ from each other even though the initial application of this filter was supposed to reduce the possibility of heterogeneity.
We initially inspected forest plots generated by RevMan 2012 to evaluate the possibility of heterogeneity between studies. We applied the Cochrane test for homogeneity. With this aim we set a P value of 0.1 as the limit for considering the existence of heterogeneity (CCI 2006). We also applied the I2 statistic to quantify heterogeneity among the trials and to verify the impact on the meta‐analysis, considering that some clinical and methodological diversity always occurs in a meta‐analysis. We considered values above 50% to be representative of significant heterogeneity (Higgins 2011), and we explored the causes. We used the subgroup analysis of participants or a subgroup analysis of the studies selected for each comparison when the heterogeneity was relevant to the outcome of the meta‐analysis.
Assessment of reporting biases
We considered assessment of reporting biases to be at risk because of the small number of studies selected for each comparison. Nevertheless, we relied on extensive research and carefully examined the references of the studies found in the search results to avoid reporting biases. We analysed all trials that met the inclusion criteria, independently of the journal's impact factor, the year of publication, the language in which the article was written and the origin of both author and publication. The use of these criteria can be confirmed by checking the lists of included and excluded studies.
Data synthesis
We used the risk ratio (RR) and respective 95% confidence interval (CI) as a summary measure to combine data. We calculated the necessary number of patients to be treated for an individual to benefit from treatment with respect to an outcome (number needed to treat to benefit (NNTB)) and its 95% CI, when a statistical difference was found. We estimated the occurrence of an event in the population, or absolute risk (baseline risk) based on the rate of event occurrence in controls (control group rate (CGR)) for this calculation.
We used the random‐effects model to calculate the summary measure, with the assumption that although the articles could have addressed somewhat different issues, they could be viewed as a family of studies on similar questions. We considered that the articles were a random sample of all studies that addressed the questions we were interested in. Therefore, even considering the possibility of failure of the statistical tests of homogeneity, the combination of similar studies would still be a reasonable procedure. Although it is impossible to state if the articles were really a random sample of all research on an issue, this model is more realistic and less prone to overestimate accuracy (Fletcher 2006).
Subgroup analysis and investigation of heterogeneity
We pre‐specified some subgroup analyses to investigate heterogeneity. We planned to take into account the drugs used for control and treatment, their doses and the previous use of anti‐influenza vaccine(s). However, we stress that the subgroup analysis does not take into account the randomisation processes, so these results must be considered with caution.
Sensitivity analysis
We carried out sensitivity analyses to explore heterogeneity. We conducted subgroup analyses for subsets of participants. We had planned to analyse rimantadine and amantadine separately and together. However, when we identified the use of different antivirals being used as a control, we performed a subgroup analysis. We separated the trials in which the comparison was made using different antiviral medications from those in which the control was made with placebo or other drugs. We also carried out subgroup analyses for subsets of immunised and non‐immunised participants, as well as according to the dosages of antivirals tested in the trials.
Results
Description of studies
Results of the search
We retrieved a total of 33 records in this updated search. Out of a total of 205 abstracts, titles and studies that we retrieved through all the searches, 195 were written in English, three in Russian, two in Czech, three in German, one in French and one in Japanese. We discarded 129 studies. We assessed the remaining 78 articles in detail. It was necessary to contact 46 trial authors to verify that their studies met our selection criteria. We included 12 trials in this review. All of them are published trials and are described in the Characteristics of included studies table. We added another 38 trials in 2011 when we updated this review; we excluded all of them and our conclusions remain unchanged.
We did not identify any new trials for inclusion in this 2014 update. We excluded 20 new trials (Anton 2011; Atiee 2012; Bacosi 2002; Cayley 2012; Cheng 2012; De Vincenzo 2012; Escuret 2012; Gatwood 2012; Hayden 2012; Hsu 2012; Ison 2013; Jiang 2013; Lopez‐Medrano 2012; Louie 2012; Michiels 2013; Sampaio 2011; Santesso 2013; Shah 2012; Singer 2011; Yuen 2012).
Included studies
The 12 included studies were all randomised trials (Clover 1986; Clover 1991; Crawford 1988; Finklea 1967; Gravenstein 2005; Hall 1987; Kitamoto 1968; Kitamoto 1971; Monto 1995; Patriarca 1984; Payler 1984; Schilling 1998); 11 were blinded and one was unblinded (Schilling 1998). The methods of randomisation and the follow‐up period were poorly described in all studies, although we could estimate that follow‐up ranged from eight to 120 days. We classified the included trials into two major groups: those conducted in children and those in the elderly.
Trials in children
Eight selected studies looked at the following.
Treatment with amantadine (Kitamoto 1968; Kitamoto 1971) and rimantadine (Hall 1987).
Prophylaxis with amantadine (Finklea 1967; Payler 1984) and rimantadine (Clover 1986; Clover 1991; Crawford 1988).
Adverse effects due to amantadine (Kitamoto 1968; Kitamoto 1971) and rimantadine (Clover 1986; Crawford 1988; Hall 1987).
For treatment trials and the outcome fever on day three of treatment, the amantadine arm size was 51 and the control arm size was 53 children (Kitamoto 1968; Kitamoto 1971). The rimantadine arm size was 37 and the control arm size was 32 children (Hall 1987). For the other outcomes, cough on day seven, malaise on day six and eye symptoms on day five, we selected just one trial (Hall 1987). The rimantadine arm size was 37 and control arm size was 32 children for each of these outcomes.
In the five prophylaxis trials, we applied wider age ranges for children than the definition stated in the protocol (participants up to 16 years of age). These trials included older participants who were adolescents by the WHO definition (WHO 2007). Data regarding the proportion of the subgroup which strictly fulfilled the age criterion were not available in these studies or by contacting the trial authors. The respective age ranges were one to 17 years (Clover 1991), 13 to 19 years (Payler 1984), one to 18 years (Clover 1986; Crawford 1988), and eight to 19 years of age (Finklea 1967). The amantadine arm size was 368 (Finklea 1967 (104); Payler 1984 (264)) and the control arm size was 373 children (Finklea 1967 (133); Payler 1984 (240)). The rimantadine arm size was 84 (Clover 1986 (35); Clover 1991 (22); Crawford 1988 (27)) and the control arm size was 94 participants (Clover 1986 (41); Clover 1991 (24); Crawford 1988 (29)).
Reported adverse effects of amantadine included exanthema, malaise, muscular limb pain, headache, arrhythmia and stimulation/insomnia. The antiviral arm size was 264 children (Kitamoto 1968 (75); Kitamoto 1971 (189)) and the control arm size was 335 (Kitamoto 1968 (84); Kitamoto 1971 (251)).
A reported adverse effect of amantadine was dyspnoea. The antiviral arm size was 75 and the control arm size was 84 children (Kitamoto 1968). For the adverse effects of hyperreactivity and tinnitus the rimantadine arm size was 27 and the control arm size was 29 children (Crawford 1988).
Nausea/vomiting, diarrhoea and dizziness were described as possible adverse effects for both antivirals. For nausea/vomiting, the amantadine arm size was 264 children (Kitamoto 1968 (75); Kitamoto 1971 (189)) and the control arm size was 335 (Kitamoto 1971 (251); Kitamoto 1968 (84)). The rimantadine arm size was 38 (Crawford 1988 (1); Hall 1987 (37)) and the control arm size was 61 (Crawford 1988 (29); Hall 1987 (32)).
For diarrhoea and dizziness the amantadine arm size was 264 children (Kitamoto 1968 (75); Kitamoto 1971 (189)) and the control arm size was 335 (Kitamoto 1968 (84), Kitamoto 1971 (251)). The rimantadine arm size was 27 and the control arm size was 29 children for these adverse effects (Crawford 1988).
Trials in the elderly
We selected three trials in this age group that reported on prophylaxis with rimantadine; we did not select any treatment trials. We studied the following outcomes.
Prophylaxis of laboratory and clinical infection (Monto 1995; Patriarca 1984).
Adverse reactions (Monto 1995; Patriarca 1984).
Different doses of rimantadine as a prophylactic antiviral (Monto 1995).
Comparison to other antivirals in the prophylaxis of influenza (Gravenstein 2005; Schilling 1998).
For prophylaxis of laboratory and clinical infection, the rimantadine (200 mg/day) arm size was 44 (Monto 1995 (26); Patriarca 1984 (18)) and the placebo arm size was 31 participants (Monto 1995 (14); Patriarca 1984 (17)). The trial authors stated they limited this analysis to vaccinated participants in nursing homes with confirmed influenza, as it provided an estimate of the additional protective efficacy of rimantadine. The sample studied by Patriarca 1984 was made up of previously vaccinated participants, so all the participants were analysed (Monto 1995; Patriarca 1984).
In the adverse reaction studies focusing on stimulation/insomnia, confusion, fatigue, nausea, depression, loss of appetite and vomiting, the rimantadine (200 mg/day) arm size was 150 (Monto 1995 (132); Patriarca 1984 (18)) and the placebo arm size was 83 participants (Monto 1995 (66); Patriarca 1984 (17)). All randomly assigned participants were analysed.
In the adverse reaction study focusing on headache, impaired concentration, rash or allergic reaction, seizures or clonic twitching, the rimantadine (200 mg/day) arm size was 132 and the placebo arm size was 66 participants (Monto 1995).
In another adverse reaction study focusing on dizziness and anxiety, the rimantadine (200 mg/day) arm size was 18 and the placebo arm size was 17 participants (Patriarca 1984).
In the unique study evaluating different doses of rimantadine as a prophylactic drug for clinical and confirmed influenza A, the rimantadine (100 mg/day) arm size was 28 and the rimantadine (200 mg/day) arm size was 26 participants (Monto 1995).
Only one selected study focused on adverse effects related to different doses of rimantadine. The studied effects were confusion, depression, impaired concentration, insomnia or sleeplessness, loss of appetite, rash or allergic reaction, seizure or clonic twitching, dry mouth, fatigue or drowsiness, headache, body weakness and debility. The 100 mg/day arm size was 130 and the 200 mg/day arm size was 132 participants (Monto 1995).
We selected two trials for the comparison of rimantadine to another antiviral and the participants were also the elderly (Gravenstein 2005; Schilling 1998). The rimantadine arm size was 254 and the zanamivir arm size was 291 participants. No study used amantadine for this kind of comparison.
Excluded studies
We excluded 212 studies for the following reasons.
They were carried out in different age groups.
They were not controlled trials.
They assessed other drugs.
They were non‐human or laboratory studies.
We excluded 20 new trials in this 2014 update (Anton 2011; Atiee 2012; Bacosi 2002; Cayley 2012; Cheng 2012; De Vincenzo 2012; Escuret 2012; Gatwood 2012; Hayden 2012; Hsu 2012; Ison 2013; Jiang 2013; Lopez‐Medrano 2012; Louie 2012; Michiels 2013; Sampaio 2011; Santesso 2013; Shah 2012; Singer 2011; Yuen 2012).
Risk of bias in included studies
The overall risk of bias is presented graphically in Figure 1 and summarised in Figure 2.
1.
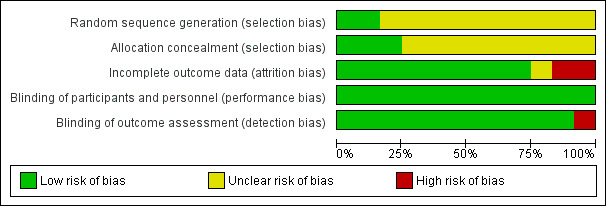
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
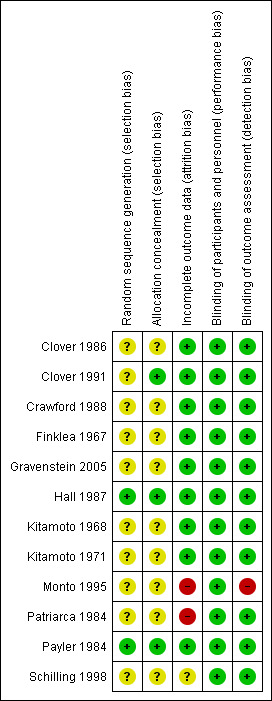
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The trial authors of the 12 included studies stated that participants had been randomly allocated into treatment or control groups. In two of the studies we obtained the following information by contacting the trial authors (Hall 1987; Payler 1984). Hall reported that a computer system was used to randomise participants. The university pharmacy was chosen to allocate and store the study drugs (Hall 1987). In Payler's study, randomisation had been carried out by the statistical department of a pharmaceutical company, which kept the key to the randomisation and only when the study was analysed was the code broken (Payler 1984). There was no mention of any particular randomisation method in the other studies.
Blinding
Ten studies were described as double‐blinded (Clover 1986; Clover 1991; Crawford 1988; Finklea 1967; Gravenstein 2005; Hall 1987; Kitamoto 1968; Kitamoto 1971; Monto 1995; Patriarca 1984). However, only in one trial were blinded people listed (Monto 1995). Although there was no blinding stated in Payler 1984, we judged that the outcome was not likely to be influenced by a lack of blinding. Schilling 1998 was described as an unblinded study; we also judged that the outcomes were unlikely to be influenced by a lack of blinding.
Incomplete outcome data
There were no missing participants in either Kitamoto 1971, Kitamoto 1968 or Payler 1984. The review authors considered that the reasons for missing outcome data were unlikely to be related to true outcome in the following studies: Clover 1986; Clover 1991; Crawford 1988; Finklea 1967; Gravenstein 2005. In the Hall 1987 trial, we considered that the proportion of missing outcomes compared with observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate. On the other hand, we considered the reasons for missing outcome data likely to be related to the true outcome data in two studies (Monto 1995; Patriarca 1984). In Schilling 1998, there was insufficient reporting of exclusion.
Selective reporting
The review authors did not identify any possible sources of reporting biases.
Other potential sources of bias
The review authors did not identify any other possible sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Primary outcomes in children
Amantadine and rimantadine compared to control (placebo and acetaminophen) in the treatment of influenza A in children
In the protocol, we originally planned to study the drug effect on reduction of fever and cough as they are considered the best predictors of influenza diagnosis. After collecting data, we verified that specific timelines for reduction of signs and symptoms were not reported in the included trials. We searched for another way to present an estimation of the response to amantadine and rimantadine in patients with influenza. For this unplanned analysis, we considered the available data and arbitrarily chose a day of antiviral use to evaluate the response to the treatment. This choice was based on the Eccle 2005 study in which clinical manifestations were classified into early and later symptoms. Typically fever may last four to eight days, so we chose day three of treatment as the cut‐off point to which it could be considered that the response to the drug would be useful. Cough is considered a later manifestation that develops slowly and can still be present a week later (Eccle 2005). In the same way, we chose day seven of treatment as the cut‐off point by when the response to the drug could be considered useful.
We also decided to include other treatment outcomes as they were available in Hall's electronic correspondence to us. In the same way, we arbitrarily chose a day of antiviral use to evaluate the response to the treatment to make this unplanned analysis: 'malaise on day six', as it begins early but could still be present for one or two weeks (Eccle 2005; Smith 2006), and 'eye manifestations on day five', as it can occur early on in the course of the illness (Treanor 2005; Wright 2004)
Amantadine was compared to placebo: 104 participants (Kitamoto 1968; Kitamoto 1971), and rimantadine to acetaminophen: 69 participants (Hall 1987).
There was a protective effect of amantadine and rimantadine in the occurrence of fever on day three of antiviral treatment, when trials using both antivirals were combined: 173 participants, risk ratio (RR) 0.39; 95% confidence interval (CI) 0.20 to 0.79 (Analysis 1.1) (Hall 1987; Kitamoto 1968; Kitamoto 1971).
1.1. Analysis.
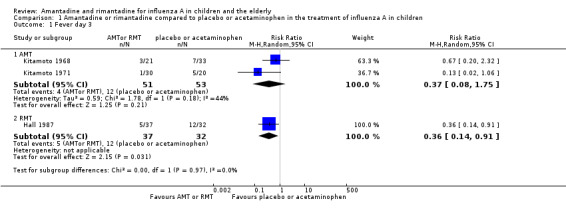
Comparison 1 Amantadine or rimantadine compared to placebo or acetaminophen in the treatment of influenza A in children, Outcome 1 Fever day 3.
The baseline risk of fever on day three of treatment was 0.28, calculated on the basis of the control group risk (CGR). The number of children needed to treat to benefit (NNTB) to prevent one case of fever on day three of treatment was six (95% CI 4 to 17) (Analysis 1.1).
We also verified a protective effect of rimantadine for this outcome: RR 0.36; 95% CI 0.14 to 0.91 (Analysis 1.1.2). The baseline risk of fever on day three of treatment was 0.38, calculated on the basis of the CGR. The NNTB was five (95% CI 3 to 25) (Analysis 1.1). Just one trial with 69 participants reported this outcome (Hall 1987).
We observed no protective effect of amantadine in the occurrence of fever on day three of treatment: 104 participants, RR 0.37; 95% CI 0.08 to 1.75 (Analysis 1.1.1) (Kitamoto 1968; Kitamoto 1971).
We saw no protective effect of rimantadine regarding the occurrence of any of the following outcomes assessed: malaise on day six (RR 1.04; 95% CI 0.63 to 1.70) (Analysis 1.2), cough on day seven (RR 0.83; 95% CI 0.63 to 1.10) (Analysis 1.3), conjunctivitis on day five (RR 0.17; 95% CI 0.01 to 3.49) (Analysis 1.4), and cases of pain on movement and visual distortion on day five (RR 0.58; 95% CI 0.10 to 3.24) (Analysis 1.5). Just one study with 69 participants reported these outcomes (Hall 1987).
1.2. Analysis.
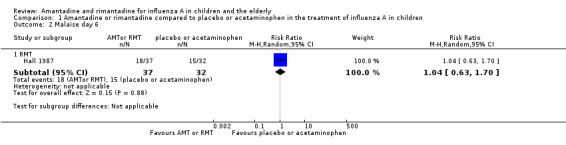
Comparison 1 Amantadine or rimantadine compared to placebo or acetaminophen in the treatment of influenza A in children, Outcome 2 Malaise day 6.
1.3. Analysis.
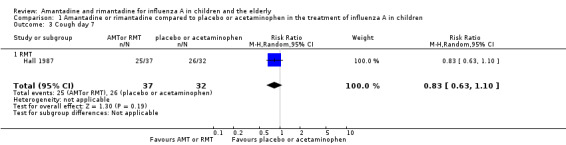
Comparison 1 Amantadine or rimantadine compared to placebo or acetaminophen in the treatment of influenza A in children, Outcome 3 Cough day 7.
1.4. Analysis.
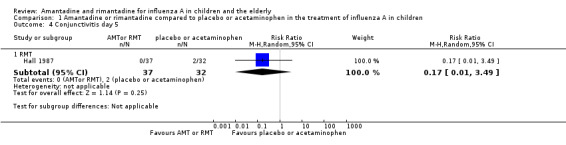
Comparison 1 Amantadine or rimantadine compared to placebo or acetaminophen in the treatment of influenza A in children, Outcome 4 Conjunctivitis day 5.
1.5. Analysis.
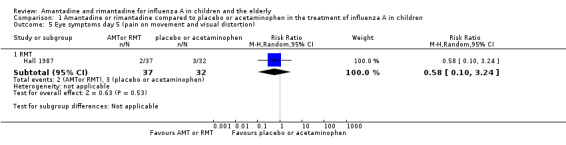
Comparison 1 Amantadine or rimantadine compared to placebo or acetaminophen in the treatment of influenza A in children, Outcome 5 Eye symptoms day 5 (pain on movement and visual distortion).
No selected studies reported the use of amantadine for these latter outcomes.
Amantadine and rimantadine compared to control in the treatment of influenza A in the elderly
There was no study selected for this comparison.
Amantadine and rimantadine compared to control (placebo and to specific treatment) in the prophylaxis of influenza A in children
Amantadine was compared to placebo and specific treatment (Finklea 1967; Payler 1984) and rimantadine to placebo (Clover 1986; Clover 1991; Crawford 1988).
The amantadine (Finklea 1967; Payler 1984) and rimantadine trials (Clover 1986; Clover 1991; Crawford 1988) were heterogeneous (Chi2 test 9.27, P value = 0.05, I2 statistic 56.8%) and could not be combined.
A protective effect of amantadine was observed with 773 participants, RR 0.11; 95% CI 0.04 to 0.30 (Analysis 2.1.1). The baseline risk of influenza was 0.10, calculated on the basis of the CGR. The NNTB was 12 (95% CI 9 to 17) for a period ranging from 14 (Payler 1984) to 18 weeks (Finklea 1967).
2.1. Analysis.
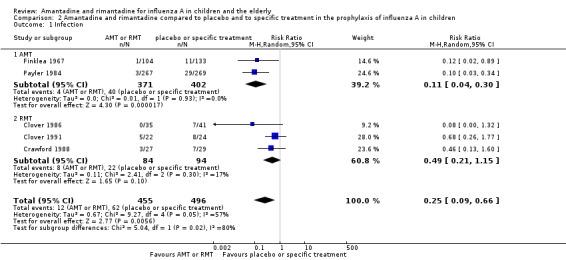
Comparison 2 Amantadine and rimantadine compared to placebo and to specific treatment in the prophylaxis of influenza A in children, Outcome 1 Infection.
On the other hand, no protective effect of rimantadine was seen in the prophylaxis of cases of influenza: 178 participants (RR 0.49; 95% CI 0.21 to 1.15) (Analysis 2.1.2) (Clover 1986; Clover 1991; Crawford 1988).
Use of different doses of amantadine and rimantadine for prophylaxis and treatment of influenza in children
There was no selected study conducted in children for this comparison.
Amantadine and rimantadine compared to other antivirals in children
There was no selected study conducted in children for this comparison
Amantadine and rimantadine compared to control (placebo and zanamivir) in the prophylaxis of influenza A in the elderly
Rimantadine was compared to placebo (Monto 1995; Patriarca 1984) and to zanamivir (Schilling 1998). No protective effect of rimantadine was seen regarding the prophylaxis of influenza in the elderly: 191 participants, RR 0.74; 95% CI 0.13 to 4.07 (Analysis 3.1).
3.1. Analysis.
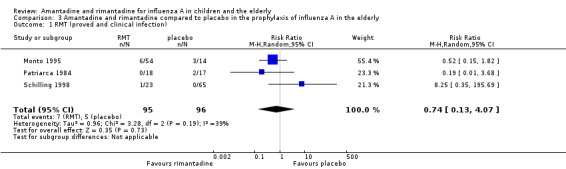
Comparison 3 Amantadine and rimantadine compared to placebo in the prophylaxis of influenza A in the elderly, Outcome 1 RMT (proved and clinical infection).
Although care must be taken in the interpretation of the Chi2 test due to its low power in detecting heterogeneity in meta‐analyses, we should emphasise the high P value observed in this comparison, considered alongside the I2 statistic value under 50%: Chi2 test 3.28; P value = 0.19, I2 statistic 39%. We decided to explore the reasons for these findings as if the studies were heterogeneous, even though it would result in smaller samples impairing the ability to reach any definitive conclusion (Monto 1995; Patriarca 1984; Schilling 1998).
Monto and Patriarca analysed previously vaccinated participants in blinded trials and used a placebo as control (Monto 1995; Patriarca 1984). Schilling did not state if the participants were vaccinated, although it was stated that the majority of the studied population had been previously immunised (Schilling 1998). This was an unblinded trial in which another antiviral (zanamivir) was used as a control drug.
When we excluded this study (Schilling 1998), the remaining trials, Monto 1995 and Patriarca 1984 were shown to be homogeneous but no protective effect of rimantadine prophylaxis in the occurrence of cases of influenza persisted (103 participants, RR 0.45; 95% CI 0.14 to 1.41) (Analysis 3.2).
3.2. Analysis.
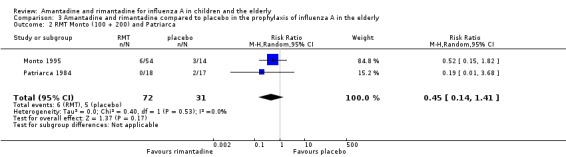
Comparison 3 Amantadine and rimantadine compared to placebo in the prophylaxis of influenza A in the elderly, Outcome 2 RMT Monto (100 + 200) and Patriarca.
Monto 1995 used two different doses of rimantadine in his trial (100 mg/day and 200 mg/day) and Patriarca 1984 used the conventional dose of 200 mg/day. Schilling 1998 used a single dose of 100 mg/day. We also combined Monto's 200 mg/day subgroup with Patriarca's study in which the same dose was administered, but again no protective effect of rimantadine was observed in the prophylaxis of influenza: eight participants, RR 0.44; 95% CI 0.12 to 1.63) (Analysis 3.3) (Monto 1995; Patriarca 1984; Schilling 1998).
3.3. Analysis.
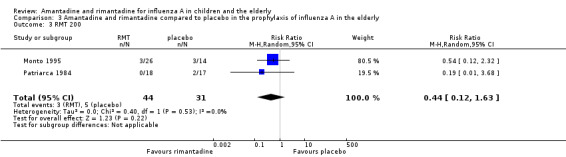
Comparison 3 Amantadine and rimantadine compared to placebo in the prophylaxis of influenza A in the elderly, Outcome 3 RMT 200.
Schilling's sample and Monto's 100 mg/day subgroup were heterogeneous and could not be combined (Chi2 test 2.55, P value = 0.11, I2 statistic 60.8%) (Monto 1995; Schilling 1998).
There was no amantadine study selected for comparison.
Use of different doses of amantadine and rimantadine for prophylaxis and treatment of influenza A in the elderly
A reduced rimantadine dose of 100 mg/day was comparable to the full dose of 200 mg daily for prophylaxis of influenza in the elderly, although a wide CI was verified (54 participants, RR 0.93; 95% CI 0.21 to 4.20) (Analysis 4.1). It should be emphasised that there were few data available for these comparisons (Monto 1995).
4.1. Analysis.
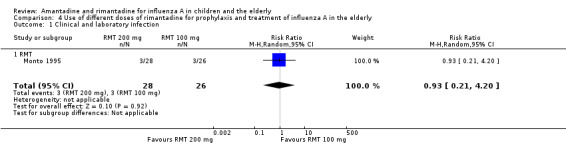
Comparison 4 Use of different doses of rimantadine for prophylaxis and treatment of influenza A in the elderly, Outcome 1 Clinical and laboratory infection.
There was no selected study using different doses of rimantadine in the elderly, nor any selected trial comparing different doses of amantadine for prophylaxis and treatment of influenza in the elderly.
Amantadine and rimantadine compared to other antivirals in the elderly
In Gravenstein's but not in Schilling's study an identical placebo was used (Gravenstein 2005; Schilling 1998). When rimantadine was compared to zanamivir it was shown that zanamivir prevented influenza A more effectively than rimantadine in the elderly (Analysis 5.1).
5.1. Analysis.
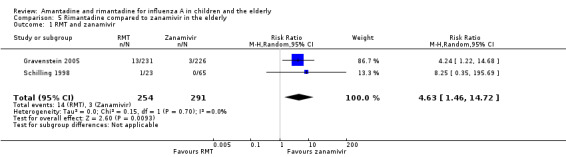
Comparison 5 Rimantadine compared to zanamivir in the elderly, Outcome 1 RMT and zanamivir.
There was no amantadine trial selected for this comparison in the elderly.
Adverse effects of amantadine and rimantadine compared to control (placebo and acetaminophen) in children
Amantadine was compared to placebo (Kitamoto 1968; Kitamoto 1971). Rimantadine was compared to placebo (Clover 1986; Crawford 1988) and to acetaminophen (Hall 1987).
Amantadine was not related to a higher risk of the following adverse effects in two trials with 599 participants: diarrhoea (RR 0.79; 95% CI 0.42 to 1.47) (Analysis 6.1), exanthema (RR 0.69; 95% CI 0.21 to 2.34) (Analysis 6.2), muscular limb pain (RR 0.85; 95% CI 0.46 to 1.59) (Analysis 6.3), headache (RR 0.73; 95% CI 0.52 to 1.03) (Analysis 6.4) and stimulation and insomnia (RR 0.46; 95% CI 0.12 to 1.74) (Analysis 6.5) (Kitamoto 1968; Kitamoto 1971).
6.1. Analysis.
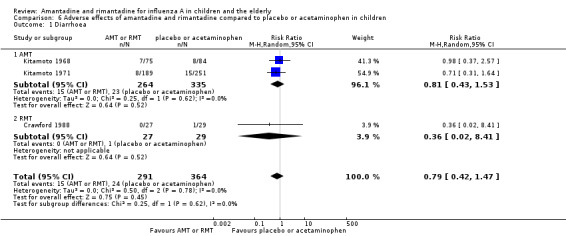
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 1 Diarrhoea.
6.2. Analysis.
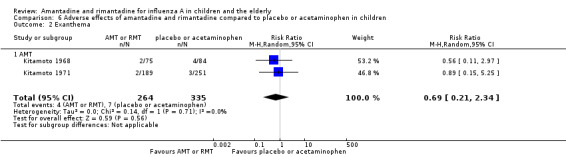
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 2 Exanthema.
6.3. Analysis.
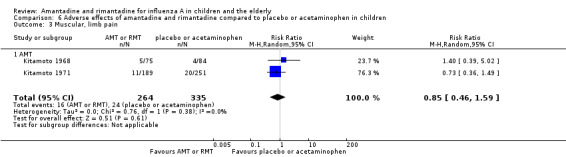
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 3 Muscular, limb pain.
6.4. Analysis.
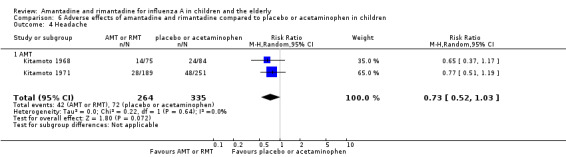
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 4 Headache.
6.5. Analysis.
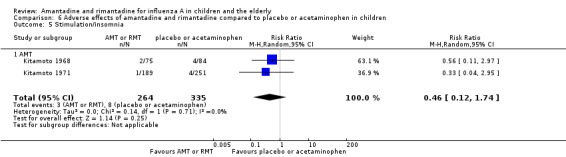
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 5 Stimulation/insomnia.
In the same way, amantadine was not related to the outcomes dizziness and dyspnoea. For dizziness there were 655 participants in two studies (Kitamoto 1968; Kitamoto 1971). The RR was 6.63 (95% CI 0.32 to 137.33) (Analysis 6.6.1) and for dyspnoea there were 159 participants in just one trial (Kitamoto 1968). The RR was 0.37 (95% CI 0.02 to 9.02) (Analysis 6.7).
6.6. Analysis.
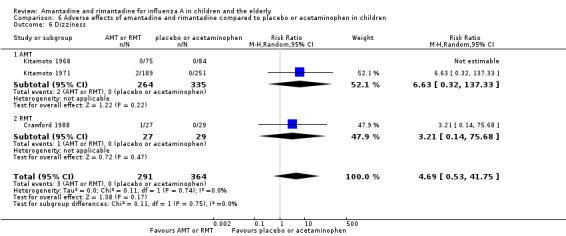
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 6 Dizziness.
6.7. Analysis.
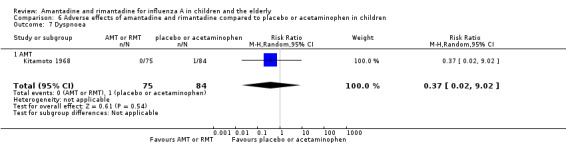
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 7 Dyspnoea.
The studies were heterogeneous for the outcomes malaise (Chi2 test 3.75, P value = 0.05, I2 statistic 73.3%) and nausea/vomiting (Chi2 test 4.26, P value = 0.04, I2 statistic 76.5%), although it seems that the author had used the same protocol. Nevertheless, the heterogeneity for the outcome nausea/vomiting does not seem to be relevant, as amantadine could be related either to an increase or to a reduction in the occurrence of this adverse effect (Kitamoto 1968; Kitamoto 1971).
No cases of arrhythmia were reported in those two trials.
Rimantadine was not related to a higher risk of any of the following adverse effects assessed: central nervous system (CNS) symptoms: one study, 76 participants (RR 0.23; 95% CI 0.01 to 4.70) (Analysis 6.8); change in behaviour: one study, 76 participants (RR 0.23; 95% CI 0.01 to 4.70) (Analysis 6.9); diarrhoea: one study, 56 participants (RR 0.36; 95% CI 0.02 to 8.41) (Analysis 6.1.2); dizziness: one study, 56 participants (RR 3.21; 95% CI 0.14 to 75.68) (Analysis 6.6.2); gastro‐intestinal (GI) manifestations: one study, 76 participants (RR 1.17; 95% CI 0.08 to 18.05) (Analysis 6.10); hyperactivity: one study, 56 participants (RR 0.36; 95% CI 0.02 to 8.41) (Analysis 6.11); tinnitus: one study, 56 participants (RR 3.21; 95% CI 0.14 to 75.68) (Analysis 6.12); cerebellar ataxia: one study, 69 participants (RR 2.61; 95% CI 0.11 to 61.80) (Analysis 6.13) (Clover 1986; Crawford 1988; Hall 1987).
6.8. Analysis.
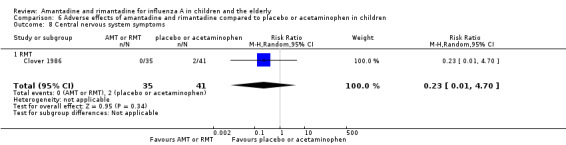
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 8 Central nervous system symptoms.
6.9. Analysis.
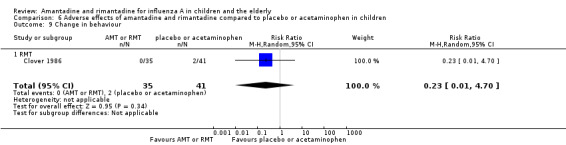
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 9 Change in behaviour.
6.10. Analysis.
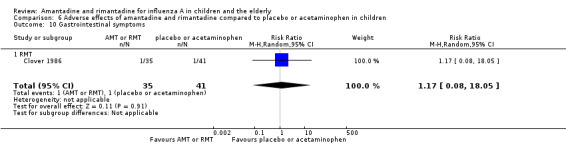
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 10 Gastrointestinal symptoms.
6.11. Analysis.
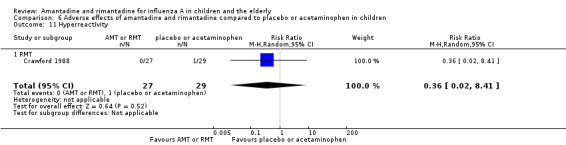
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 11 Hyperreactivity.
6.12. Analysis.
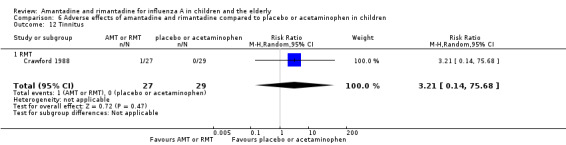
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 12 Tinnitus.
6.13. Analysis.
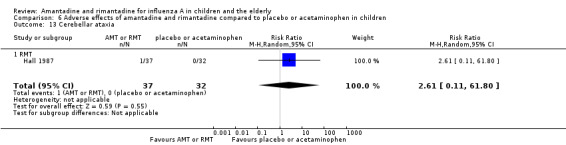
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 13 Cerebellar ataxia.
As it was stated, each one of the adverse effects described above was studied in just one included trial, except for nausea and vomiting (Crawford 1988; Hall 1987). In the same way, rimantadine was not related to a higher risk of nausea and vomiting: two studies, 125 participants (RR 0.96; 95% CI 0.10 to 9.01) (Analysis 6.15.2).
6.15. Analysis.
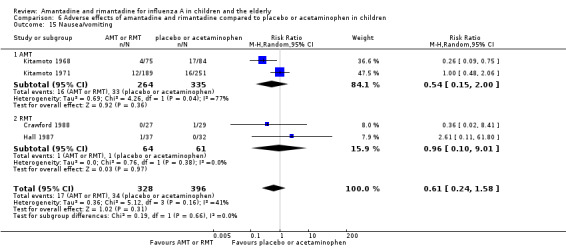
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 15 Nausea/vomiting.
Adverse effects related to different doses of amantadine and rimantadine in children
There were no selected studies conducted in children for this comparison.
Adverse effects of amantadine and rimantadine compared to control (placebo) in the elderly
There were two selected studies for these outcomes, both using rimantadine and placebo (Monto 1995; Patriarca 1984).
No effect of rimantadine was seen regarding any of the adverse outcomes assessed in the combined studies: stimulation and insomnia (233 participants, RR 1.61; 95% CI 0.43 to 6.02) (Analysis 7.1), confusion (233 participants, RR 0.79; 95% CI 95% 0.40 to 1.56) (Analysis 7.2), fatigue (233 participants, RR 0.81; 95% CI 0.41 to 1.60) (Analysis 7.3) and vomiting (233 participants, RR 0.99; 95% CI 0.38 to 2.60) (Analysis 7.4) (Monto 1995; Patriarca 1984).
7.1. Analysis.
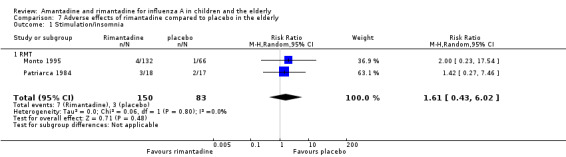
Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 1 Stimulation/insomnia.
7.2. Analysis.
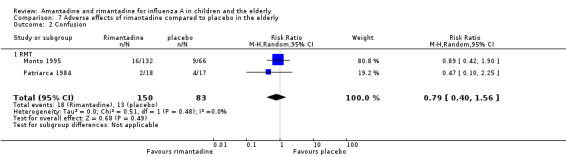
Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 2 Confusion.
7.3. Analysis.
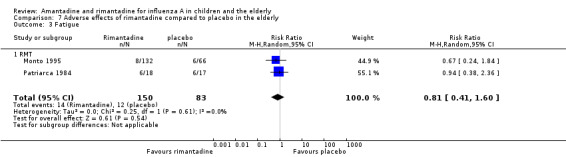
Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 3 Fatigue.
7.4. Analysis.
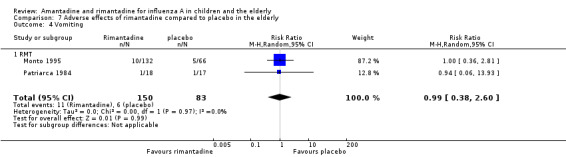
Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 4 Vomiting.
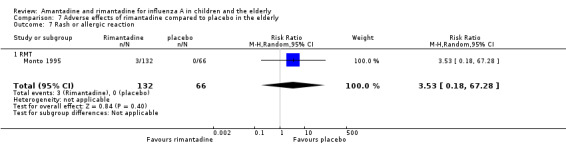
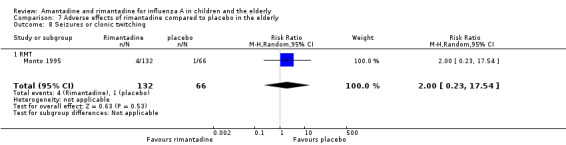
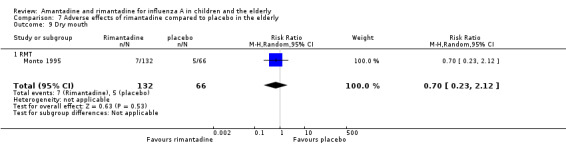
In the same way, rimantadine was not related to the outcomes studied by Monto: headache (198 participants, RR 0.83; 95% CI 0.21 to 3.38) (Analysis 7.5); impaired concentration (198 participants, RR 0.50; 95% CI 0.10 to 2.41) (Analysis 7.6); rash or allergic reaction (198 participants, RR 3.53; 95% CI 0.18 to 67.28) (Analysis 7.7); seizures or clonic twitching (198 participants, RR 2.00; 95% CI 0.23 to 17.54) (Analysis 7.8) and dry mouth (198 participants, RR 0.70; 95% CI 0.23 to 2.12) (Analysis 7.9), as well as in those studied by Patriarca: dizziness (35 participants, RR 0.94; 95% CI 0.15 to 5.97) (Analysis 7.10) and anxiety (35 participants, RR 2.83; 95% CI 0.92 to 8.74) (Analysis 7.11) (Monto 1995; Patriarca 1984).
7.5. Analysis.
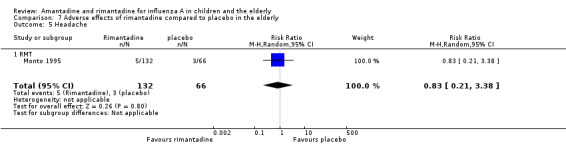
Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 5 Headache.
7.6. Analysis.
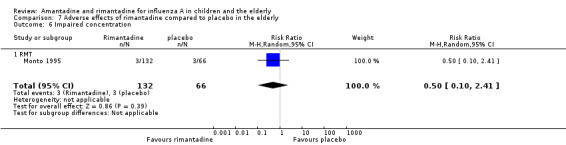
Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 6 Impaired concentration.
7.7. Analysis.
Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 7 Rash or allergic reaction.
7.8. Analysis.
Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 8 Seizures or clonic twitching.
7.9. Analysis.
Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 9 Dry mouth.
7.10. Analysis.
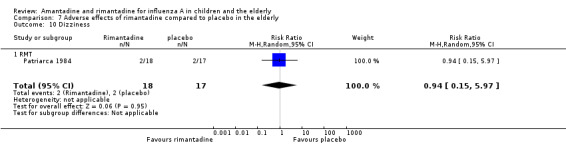
Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 10 Dizziness.
7.11. Analysis.
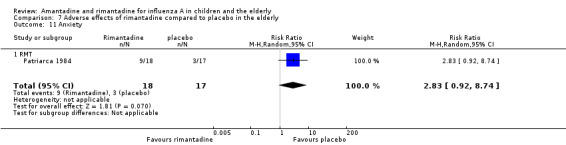
Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 11 Anxiety.
The articles were heterogeneous just for the occurrence of nausea (test for heterogeneity: Chi2 test 2.02; P value = 0.16; I2 statistic 50.5%). Nevertheless, this heterogeneity does not seem to be relevant as rimantadine could be related either to an increase or to a reduction in the occurrence of nausea in each one of the studies (Patriarca 1984: 35 participants, RR 5.67; 95% CI 0.76 to 42.32 and Monto 1995: 198 participants, RR 1.17; 95% CI 0.47 to 2.90) (Analysis 7.12).
7.12. Analysis.
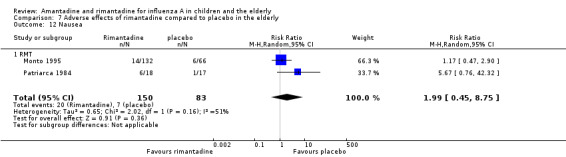
Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 12 Nausea.
It is important to stress the small samples studied in both trials. There was no amantadine trial selected for comparison.
Adverse effects related to different doses of amantadine and rimantadine in the elderly
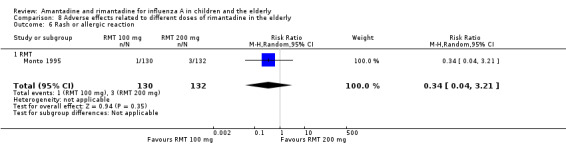
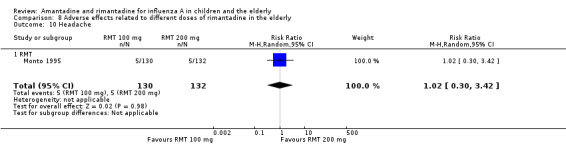
There was no protective effect of a reduced dose of rimantadine in the occurrence of the following adverse reactions in the elderly: one study with 262 participants: confusion (RR 0.82; 95% CI 0.41 to 1.65) (Analysis 8.1), depression (RR 0.44; 95% CI 0.12 to 1.65) (Analysis 8.2), impaired concentration (RR 0.68; 95% CI 0.11 to 3.98) (Analysis 8.3), insomnia or sleeplessness (RR 1.02; 95% CI 0.26 to 3.97) (Analysis 8.4), loss of appetite (RR 0.62; 95% CI 0.27 to 1.46) (Analysis 8.5), rash or allergic reaction (RR 0.34; 95% CI 0.04 to 3.21) (Analysis 8.6), seizures or clonic twitching (RR 0.11; 95% CI 0.01 to 2.07) (Analysis 8.7), dry mouth (RR 1.16; 95% CI 0.43 to 3.11) (Analysis 8.8), fatigue or drowsiness (RR 1.14; 95% CI 0.45 to 2.87) (Analysis 8.9), headache (RR 1.02; 95% CI 0.30 to 3.42) (Analysis 8.10) and body weakness or debility (RR 0.91; 95% CI 0.38 to 2.18) (Analysis 8.11) (Monto 1995).
8.1. Analysis.
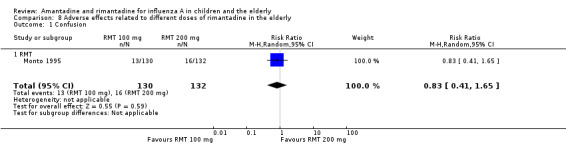
Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 1 Confusion.
8.2. Analysis.
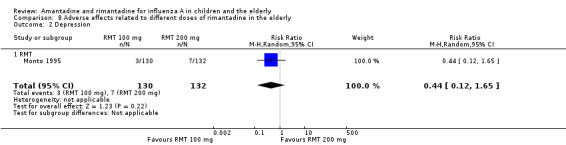
Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 2 Depression.
8.3. Analysis.
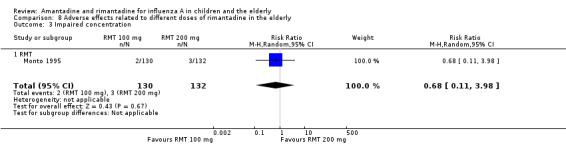
Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 3 Impaired concentration.
8.4. Analysis.
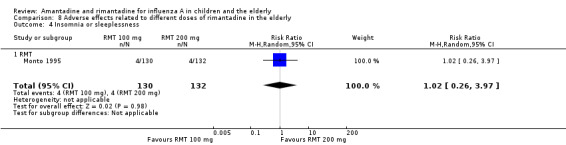
Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 4 Insomnia or sleeplessness.
8.5. Analysis.
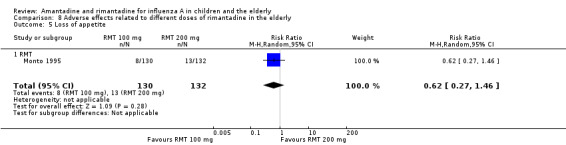
Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 5 Loss of appetite.
8.6. Analysis.
Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 6 Rash or allergic reaction.
8.7. Analysis.
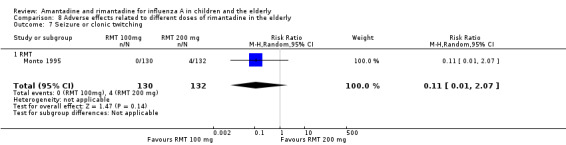
Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 7 Seizure or clonic twitching.
8.8. Analysis.
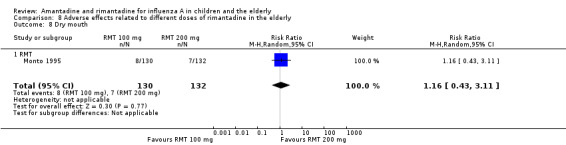
Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 8 Dry mouth.
8.9. Analysis.
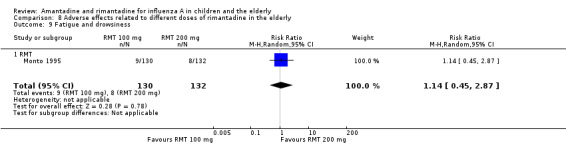
Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 9 Fatigue and drowsiness.
8.10. Analysis.
Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 10 Headache.
8.11. Analysis.
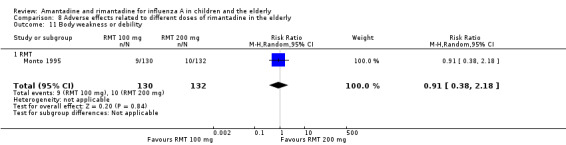
Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 11 Body weakness or debility.
There was no amantadine trial selected for this comparison in the elderly.
Additional outcome (children plus the elderly)
Rimantadine compared to control (placebo) in the prophylaxis of influenza A in children and the elderly
Originally in the protocol we planned only to make the above 12 comparisons. However, whilst analysing the data we considered doing an additional comparison and put the two age groups together. As the small samples studied in rimantadine trials for prophylaxis might have influenced the observed results, we tried to overcome this limitation by combining the trials with rimantadine in children and in the elderly. Rimantadine had no proven effect in preventing influenza in either age group but could be effective when we combined the results from both groups. However, it must be stressed that extraneous characteristics between those groups, other than age or previous immunisations, may have occurred, impairing generalisation of these results. There were five studies selected for this comparison with 156 patients in the treatment group and 125 in the placebo control group (Clover 1986; Clover 1991; Crawford 1988; Monto 1995; Patriarca 1984). The combination of the trials showed a protective effect of rimantadine in preventing influenza A (281 participants, RR 0.49; 95% CI 0.27 to 0.92) (Analysis 9.1).
9.1. Analysis.
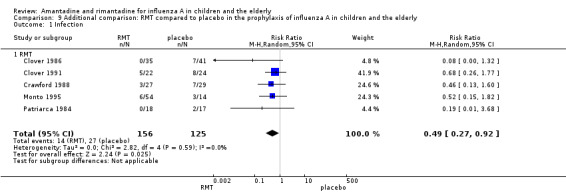
Comparison 9 Additional comparison: RMT compared to placebo in the prophylaxis of influenza A in children and the elderly, Outcome 1 Infection.
The baseline risk of influenza A was 0.22, calculated on the basis of the CGR. The NNTB was 9.09 (95% CI 6.25 to 50). We should emphasise that the follow‐up period ranged from 3 to 11 weeks.
The following secondary outcomes appeared in the protocol but in the end we did not consider them in the analysis, as they were not reported in the included trials: patients' well‐being, admission to hospital, general practitioner (GP) visits and other drugs used. We could not analyse deaths. Although cited by Monto 1995, they were included among other causes of withdrawal.
Discussion
Summary of main results
We used a comprehensive search strategy and made every effort to identify relevant studies. In the majority of our comparisons, drawing definitive conclusions was impaired by the small number of studies and participants. The studies demonstrated a decreased incidence of influenza A in children using amantadine during a period ranging from 14 to 18 weeks. The number needed to treat to benefit (NNTB) indicates that for every nine to 17 children receiving amantadine, one case of influenza A can be prevented.
Rimantadine had no proven effect in preventing influenza in either age group but could be effective when we combined the results of both groups. Nevertheless, any inferences from combining these groups must be treated with considerable caution, as they are different clinical groups combined with a small number of studies. Extraneous characteristics between those groups, other than age or previous immunisations, may also have occurred impairing generalisation of these results. Multiple comparisons should also be taken into account in the interpretation of these results.
When amantadine and rimantadine were combined, they appeared to prevent the occurrence of fever on day three in children. However, when analysed separately, this effect was confirmed only for rimantadine. It must be emphasised that there was just one rimantadine trial selected for this outcome (Hall 1987), in which the baseline risk for the occurrence of fever on day three was 38%. For every five children (ranging from three to 25) treated with rimantadine in this unique small sample, it would be possible to prevent one case of fever on day three of treatment.
Overall completeness and applicability of evidence
It could be suggested that amantadine is well tolerated by children, as its use was not related to an increase in the occurrence of the analysed adverse effects. Nevertheless, it may be difficult to distinguish between an adverse effect of the drug and a clinical manifestation of influenza itself. The outcomes muscular pain, headache, malaise, diarrhoea and nausea/vomiting may be adverse effects of amantadine as well as clinical manifestations of influenza in children (MS 2006). In the same way, the outcome dyspnoea (as in Kitamoto 1968) may also occur due to other respiratory diseases, such as asthma, since an asthmatic episode may be triggered by respiratory viruses. So we must emphasise that adverse effects of the drug and clinical manifestations of influenza may had been confounded, since the selected trials were carried out in ill children.
Rimantadine, administered exclusively on a prophylactic basis, was not related to an increase in the occurrence of the analysed adverse effects. In contrast to amantadine studies, just nausea/vomiting could be confounded with influenza manifestations. The other adverse effects could not be confounded, as two of the three selected studies were about prophylaxis and were conducted in children without influenza (Clover 1986; Crawford 1988). The third study was the only one carried out in children with influenza (Hall 1987). Cerebellar ataxia and nausea/vomiting were the studied adverse effects in this trial. Cerebellar ataxia could not be confounded as it had not been described as an influenza manifestation. Cases of nausea/vomiting, which were also cited by Crawford, could have been confounded with influenza manifestations in Hall's article. The side effects nausea/vomiting were described in two studies (Crawford 1988; Hall 1987), while all the other adverse effects were mentioned in just one: diarrhoea, dizziness, hyperreactivity, tinnitus (Crawford 1988), gastrointestinal (GI) symptoms, central nervous system (CNS) symptoms, changes in behaviour (Clover 1986), and cerebellar ataxia (Hall 1987). Rimantadine also was considered to be well tolerated by the elderly, since it was not related to an increase in the incidence of adverse effects in this age group. However, the studied samples were even smaller in the elderly than in the children's age group and this fact may have influenced our results (Monto 1995; Patriarca 1984).
When analysing the adverse reactions to the antivirals, we could not even try to overcome the limitation of the small number of articles and the small samples studied by combining the results of both age groups, as the trial authors had described different outcomes (Clover 1986; Crawford 1988; Hall 1987; Kitamoto 1968; Kitamoto 1971; Monto 1995; Patriarca 1984).
Comparison of different doses of antiviral drugs was available only for rimantadine and was tested in only one study related to the elderly group. There was no selected trial regarding the treatment either in children or in participants using amantadine in both age groups. Both doses were shown to be comparable in the prophylaxis of influenza as well as in the occurrence of adverse effects, with no proven efficacy (Monto 1995).
Data for comparison to other antivirals were available just for rimantadine and zanamivir for prophylaxis of influenza A in the elderly group. This fact allowed a comparison of drugs of the two different classes of antivirals: M2 ion channel inhibitors and neuraminidase inhibitors. Zanamivir more effectively prevented influenza A in the elderly group (Gravenstein 2005; Schilling 1998).
These antivirals proved to be effective prophylactics against influenza in the 1968 Hong Kong pandemic and in the 1977 pandemic‐like event 'Russian influenza'. Although the same resistance marker (Ser31Asn) was present in two isolates of influenza A (H5N1) obtained from patients in China in 2003 and in one lineage of avian and human H5N1 viruses in Thailand, Vietnam and Cambodia, most tested isolates from a second lineage that had been circulating in Indonesia, China, Mongolia, Russia and Turkey appear to be sensitive to amantadine (Hayden 2005; Li 2014). Furthermore, the next pandemic virus may be one that, like H2N2, is susceptible to this class of drug. If the circulating strain were known to be susceptible to M2 inhibitors, these drugs would offer a less costly alternative to other antivirals (neuraminidase inhibitors) for prophylaxis against influenza.
Quality of the evidence
We selected a total of 12 randomised controlled trials (RCTs) (2494 participants: 1586 children and adolescents and 908 elderly participants).
The main factors that affect the strength of evidence are the sparsity of data and the unclear risk of selection bias (Clover 1986; Clover 1991; Crawford 1988; Finklea 1967; Gravenstein 2005; Kitamoto 1968; Kitamoto 1971; Monto 1995; Patriarca 1984; Schilling 1998). We classified two of these studies, both in the elderly, as high risk of bias because of incomplete outcome data (Monto 1995; Patriarca 1984) and a high probability of detection bias (Monto 1995). We considered two trials, both in children and adolescents, to have a low risk of bias (Hall 1987; Payler 1984).
Potential biases in the review process
The use of unpublished data, obtained in electronic correspondence with two of the 12 contact trial authors (Hall 1987; Payler 1984), was the only identified potential bias in this review process.
Agreements and disagreements with other studies or reviews
Although another Cochrane review carried out in adults showed that both amantadine and rimantadine are efficacious and safe in the prophylaxis and treatment of influenza A symptoms (Jefferson 2006b), we could not reach the same conclusion in children and the elderly, except for prophylaxis with amantadine in children. This antiviral was effective in preventing influenza A in children. As in the adults review, rimantadine shortens the duration of fever in children. In 2012, the editorial team considered that the question addressed by this Cochrane review dealing with adults was no longer relevant to decision‐making, as amantadine and rimantadine for influenza A in adults had been replaced by neuraminidase inhibitors and were no longer used.
The M2 ion channel inhibitors are increasingly subject to viral resistance (Goodman 2006; Sleeman 2013). Nevertheless, we consider that in these two especially vulnerable age groups, we should continue to assess the susceptibility of any influenza A outbreak virus to all antiviral drugs, as they may be the first line of defence before an effective vaccine becomes available (Sleeman 2013).
Authors' conclusions
Implications for practice.
The quality of evidence currently available does not provide strong support for amantadine and rimantadine use to treat and prevent influenza in children and the elderly.
Amantadine was effective in preventing influenza A in children but the safety of the drug was not well established. Currently, rimantadine cannot be recommended as a prophylactic drug for either age group. Nevertheless, if we consider: 1) that it is a safe drug, 2) the results of the combined age groups and 3) the possibility that the next pandemic virus is susceptible to this class of drug, as indicated in former pandemics, we can still consider this 'old' drug as a less costly alternative to neuraminidase inhibitors.
Our conclusions regarding the effectiveness of both antivirals for the treatment of influenza A in children were limited to a proven benefit of rimantadine in the abatement of fever by day three of treatment. This benefit does not seem to justify a recommendation for using rimantadine to treat all children with influenza A. We could not reach a conclusion regarding amantadine in the elderly, or antiviral treatment in this age group, as no trials fulfilled our selection criteria.
Implications for research.
Definitive conclusions may have been impaired by the small number of selected studies and the small sample numbers used. Further research is necessary for the following.
Treatment
Amantadine for the treatment of influenza A in children to increase the sample numbers and the power of the studies.
Rimantadine for the treatment of influenza A in children in order to confirm the observed result from the only selected study and to see if the drug could be useful in treating other clinical manifestations of influenza.
Amantadine and rimantadine for the treatment of influenza A in the elderly, as no identified studies fulfilled our inclusion criteria.
Prophylaxis
Rimantadine in children to increase the sample numbers and the power of the studies, in order to achieve more definitive conclusions.
Amantadine in the elderly, as there were no identified studies fulfilling our inclusion criteria for this age group.
Rimantadine in the elderly to increase the sample numbers and the power of the studies, in order to achieve more definitive conclusions.
Adverse effects
Amantadine in children without influenza to avoid confounding adverse reactions of the antiviral with clinical manifestations of influenza.
Rimantadine in the elderly to increase the sample numbers and the power of the studies.
Different doses of amantadine and rimantadine
Further information is necessary on both drugs in both age groups.
Feedback
Amantadine and rimantadine for influenza A in children and the elderly, 24 January 2008
Summary
A year ago CDC provided a recommendation not to use these drugs for 'flu supporting this recommendation by newly acquired resistance of the virus. I believe that this recommendation ought to be at least discussed in the review and better, addressed e.g. by analysis of RCTs data for time periods e.g. before 2000 and after etc. Also it would be nice to have the abstract rich with data, not just a statement. Submitter agrees with default conflict of interest statement: I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
We do agree that the issue of viral resistance is of utmost importance. We have stressed this concern in the Background and in the Discussion sections. We expect, from what is written in the text, that readers would be aware of the problem.
Background: ...Both drug classes have shown partial effectiveness for prevention and treatment of influenza A viruses, although neuraminidase inhibitors are less likely to promote the development of drug‐resistant influenza (Moscona 2005).
Discussion: Data on comparison to other antivirals was available just for rimantadineand zanamivir for prophylaxis of influenza A in the elderly group. This fact allowed a comparison of drugs of the two different classes of antivirals: M2 ion channel inhibitors and neuraminidase inhibitors. Zanamivir more effectively prevented influenza A in the elderly group (Gravenstein 2005; Schilling 1998). Although the M2 ion channel inhibitors are increasingly subject to viral resistance (Goodman 2006) it does not mean that we should abandon amantadineand rimantadine. These antivirals proved effective for prophylaxis against influenza illness in the 1968 pandemic of “Hong Kong Influenza” and in 1977 pandemic‐like event involving “Russian influenza”. Although the same resistance marker (Ser31Asn) was present in two isolates of influenza A (H5N1) obtained from patients in China in 2003 and in one lineage of avian and human H5N1 viruses in Thailand, Vietnam andCambodia, most tested isolates from a second lineage that has been circulating in Indonesia, China, Mongolia, Russia andTurkey appear to be sensitive to amantadine (Hayden 2005). Furthermore, the next pandemic virus may be one that, like H2N2, is susceptible to this class of drugs. If the circulating strain were known to be susceptible to M2 inhibitors, these drugs would offer a less costly alternative to other antivirals (neuraminidase inhibitors) for prophylaxis against illness.
Contributors
Vasiliy Vlassov Feedback comment added 12 June 2008
What's new
| Date | Event | Description |
|---|---|---|
| 7 October 2014 | New search has been performed | Searches conducted. We did not identify any new trials for inclusion. We excluded 20 new trials (Anton 2011; Atiee 2012; Bacosi 2002; Cayley 2012; Cheng 2012; De Vincenzo 2012; Escuret 2012; Gatwood 2012; Hayden 2012; Hsu 2012; Ison 2013; Jiang 2013; Lopez‐Medrano 2012; Louie 2012; Michiels 2013; Sampaio 2011; Santesso 2013; Shah 2012; Singer 2011; Yuen 2012). |
| 7 October 2014 | New citation required but conclusions have not changed | Our conclusions remain unchanged. |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 1, 2008
| Date | Event | Description |
|---|---|---|
| 27 June 2011 | New search has been performed | Searches updated. No new trials fulfilled our inclusion criteria. We excluded 38 new trials (Bantia 2010; Boltz 2010; Brammer 2009; Burch 2009; Cady 2011; Carter 2008; Cayley 2010; Chawla 2009; Chen 2007; Cheng 2009; Choi 2009; Chou 2008; Cowling 2008; Curran 2010; De la Camara 2007; DeLaney 2010; Falagas 2010; Farlow 2008; Fiore 2008; Guo 2007; Hota 2007; Kalia 2008; Kim 2011; Kirkby 2010; Korenke 2008; Langlet 2009; Matheson 2007; Miyachi 2011; Moffat 2008; Morrison 2007; Nuesch 2007; Sato 2008; Simeonova 2009; Tappenden 2009; Thomas 2008; Wailoo 2008; Welton 2008; Whitley 2007). |
| 29 April 2011 | New citation required but conclusions have not changed | Our conclusions remain unchanged. |
| 13 May 2009 | Amended | No changes ‐ republished to fix technical problem. |
| 12 June 2008 | Feedback has been incorporated | Feedback comment added. |
| 25 May 2008 | Amended | Converted to new review format. |
| 26 July 2007 | New search has been performed | Searches conducted. |
Acknowledgements
The authors would like to thank Amanda Burls, Rebecca Mears, David Moore, Lisa Gold and Karen Elley for the use of their protocol. We also would like to thank Tom Jefferson and Richard Stubbs for comments provided on the draft protocol. We acknowledge Elizabeth Dooley from the Cochrane Acute Respiratory Infections Group for helping us in all phases of the review process; Ruth Foxlee and Sarah Thorning, for their essential help with the search strategy, the Iberoamerican Cochrane Centre and especially the kindness of Marta Roque, who helped us in the statistical and methodological aspects of the review. We also acknowledge Raimundo Santos, Vladmír Plesnik, Oleg Borisenko and Stuko Nakano for the assessment and translation of the essential topics for this review from the clinical trials published in German, French, Czech, Russian and Japanese. We also thank Jonathan Haliburton for reviewing the English version of this manuscript. The review authors wish to thank Caroline Hall, David Payler and Vladmír Plesnik, who generously provided us with unpublished trial data. Finally, we wish to thank the following referees who gave their permission to be acknowledged for commenting on this review: Maryann Napoli, Nelcy Rodriguez and Tom Jefferson.
Appendices
Appendix 1. MEDLINE search strategy
We combined the MEDLINE search strategy with the Cochrane highly sensitive search strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011).
MEDLINE (OVID) 1 exp Influenza, Human/ 2 influenza*.tw. 3 flu.tw. 4 exp Influenzavirus A/ 5 or/1‐4 6 exp Amantadine/ 7 amantadine.tw,nm. 8 symmetrel.tw,nm. 9 Rimantadine/ 10 rimantadine.tw,nm. 11 flumadine.tw,nm. 12 or/6‐11 13 5 and 12
Appendix 2. EMBASE.com search strategy
#13. #9 AND #12 #12. #10 OR #11 #11. random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR 'cross‐over':ab,ti OR volunteer*:ab,ti OR allocat*:ab,ti OR assign*:ab,ti OR ((singl* OR doubl*) NEAR/2 (blind* OR mask*)):ab,ti #10. 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp #9. #3 AND #8 #8. #4 OR #5 OR #6 OR #7 #7. rimantadine:ab,ti OR flumadine:ab,ti #6. 'rimantadine'/de #5. amantadine:ab,ti OR symmetrel:ab,ti #4. 'amantadine'/de #3. #1 OR #2 #2. influenza*:ab,ti OR flu:ab,ti #1. 'influenza'/de OR 'influenza virus a'/exp
Appendix 3. Previous searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2007, Issue 3); MEDLINE (1966 to July 2007); and EMBASE (1980 to July 2007).
The MEDLINE and CENTRAL search strategies are shown below. We combined the MEDLINE search string with the Cochrane highly sensitive search strategy phases one and two as published in Appendix 5b of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2005). We adapted the search strategy to search EMBASE.
MEDLINE (OVID) 1 exp INFLUENZA/ 2 influenza.mp. 3 or/1‐2 4 exp AMANTADINE/ 5 amantadine.mp. 6 exp RIMANTADINE/ 7 rimantadine.mp. 8 or/4‐7 9 3 and 8
EMBASE (Embase.com) 1 exp INFLUENZA/ 2 influenza.ti. or influenza.ab. 3 or/1‐2 4 exp AMANTADINE/ 5 amantadine.ti. or amantadine.ab. 6 exp RIMANTADINE/ 7 rimantadine.ti. or rimantadine.ab. 8 or/4‐7 9 3 and 8 10 Randomized Controlled Trial/ 11 Controlled Study/ 12 exp RANDOMIZATION/ 13 Single Blind Procedure/ 14 Double Blind Procedure/ 15 Crossover Procedure/ 16 Phase 3 Clinical Trial/ 17 Phase 4 Clinical Trial/ 18 or/10‐17 19 9 and 18
Data and analyses
Comparison 1. Amantadine or rimantadine compared to placebo or acetaminophen in the treatment of influenza A in children.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fever day 3 | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 AMT | 2 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.08, 1.75] |
| 1.2 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.14, 0.91] |
| 2 Malaise day 6 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.63, 1.70] |
| 3 Cough day 7 | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.63, 1.10] |
| 3.1 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.63, 1.10] |
| 4 Conjunctivitis day 5 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.01, 3.49] |
| 5 Eye symptoms day 5 (pain on movement and visual distortion) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.10, 3.24] |
Comparison 2. Amantadine and rimantadine compared to placebo and to specific treatment in the prophylaxis of influenza A in children.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Infection | 5 | 951 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.09, 0.66] |
| 1.1 AMT | 2 | 773 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.04, 0.30] |
| 1.2 RMT | 3 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.21, 1.15] |
Comparison 3. Amantadine and rimantadine compared to placebo in the prophylaxis of influenza A in the elderly.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 RMT (proved and clinical infection) | 3 | 191 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.13, 4.07] |
| 2 RMT Monto (100 + 200) and Patriarca | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.14, 1.41] |
| 3 RMT 200 | 2 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.12, 1.63] |
| 4 RMT 100 | 2 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.10, 21.10] |
3.4. Analysis.
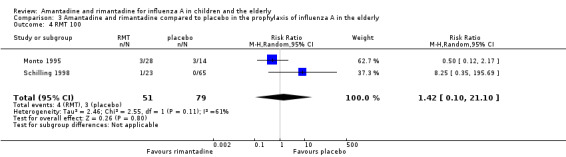
Comparison 3 Amantadine and rimantadine compared to placebo in the prophylaxis of influenza A in the elderly, Outcome 4 RMT 100.
Comparison 4. Use of different doses of rimantadine for prophylaxis and treatment of influenza A in the elderly.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical and laboratory infection | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.21, 4.20] |
| 1.1 RMT | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.21, 4.20] |
Comparison 5. Rimantadine compared to zanamivir in the elderly.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 RMT and zanamivir | 2 | 545 | Risk Ratio (M‐H, Random, 95% CI) | 4.63 [1.46, 14.72] |
Comparison 6. Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diarrhoea | 3 | 655 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.42, 1.47] |
| 1.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.43, 1.53] |
| 1.2 RMT | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.02, 8.41] |
| 2 Exanthema | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.21, 2.34] |
| 2.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.21, 2.34] |
| 3 Muscular, limb pain | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.46, 1.59] |
| 3.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.46, 1.59] |
| 4 Headache | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.52, 1.03] |
| 4.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.52, 1.03] |
| 5 Stimulation/insomnia | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.12, 1.74] |
| 5.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.12, 1.74] |
| 6 Dizziness | 3 | 655 | Risk Ratio (M‐H, Random, 95% CI) | 4.69 [0.53, 41.75] |
| 6.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 6.63 [0.32, 137.33] |
| 6.2 RMT | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 3.21 [0.14, 75.68] |
| 7 Dyspnoea | 1 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 9.02] |
| 7.1 AMT | 1 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 9.02] |
| 8 Central nervous system symptoms | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.01, 4.70] |
| 8.1 RMT | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.01, 4.70] |
| 9 Change in behaviour | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.01, 4.70] |
| 9.1 RMT | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.01, 4.70] |
| 10 Gastrointestinal symptoms | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.08, 18.05] |
| 10.1 RMT | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.08, 18.05] |
| 11 Hyperreactivity | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.02, 8.41] |
| 11.1 RMT | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.02, 8.41] |
| 12 Tinnitus | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 3.21 [0.14, 75.68] |
| 12.1 RMT | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 3.21 [0.14, 75.68] |
| 13 Cerebellar ataxia | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 2.61 [0.11, 61.80] |
| 13.1 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 2.61 [0.11, 61.80] |
| 14 Malaise | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.41, 1.96] |
| 14.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.41, 1.96] |
| 15 Nausea/vomiting | 4 | 724 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.24, 1.58] |
| 15.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.15, 2.00] |
| 15.2 RMT | 2 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.10, 9.01] |
| 16 Arrhythmia | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
6.14. Analysis.
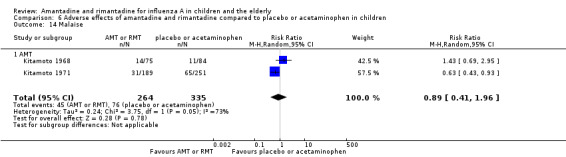
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 14 Malaise.
6.16. Analysis.
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 16 Arrhythmia.
Comparison 7. Adverse effects of rimantadine compared to placebo in the elderly.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stimulation/insomnia | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.43, 6.02] |
| 1.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.43, 6.02] |
| 2 Confusion | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.40, 1.56] |
| 2.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.40, 1.56] |
| 3 Fatigue | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.41, 1.60] |
| 3.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.41, 1.60] |
| 4 Vomiting | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.38, 2.60] |
| 4.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.38, 2.60] |
| 5 Headache | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.21, 3.38] |
| 5.1 RMT | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.21, 3.38] |
| 6 Impaired concentration | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.10, 2.41] |
| 6.1 RMT | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.10, 2.41] |
| 7 Rash or allergic reaction | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 3.53 [0.18, 67.28] |
| 7.1 RMT | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 3.53 [0.18, 67.28] |
| 8 Seizures or clonic twitching | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.23, 17.54] |
| 8.1 RMT | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.23, 17.54] |
| 9 Dry mouth | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.7 [0.23, 2.12] |
| 9.1 RMT | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.7 [0.23, 2.12] |
| 10 Dizziness | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.15, 5.97] |
| 10.1 RMT | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.15, 5.97] |
| 11 Anxiety | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 2.83 [0.92, 8.74] |
| 11.1 RMT | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 2.83 [0.92, 8.74] |
| 12 Nausea | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.45, 8.75] |
| 12.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.45, 8.75] |
| 13 Depression | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.53, 4.98] |
| 13.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.53, 4.98] |
| 14 Loss of appetite | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.56, 2.17] |
| 14.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.56, 2.17] |
7.13. Analysis.
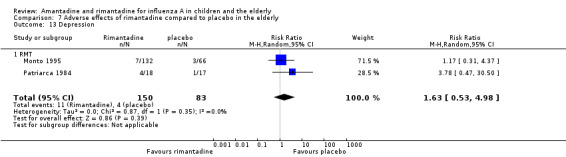
Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 13 Depression.
7.14. Analysis.
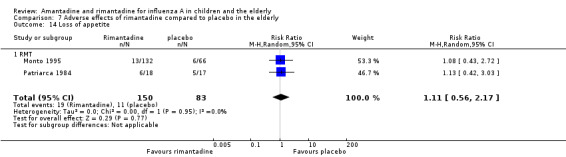
Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 14 Loss of appetite.
Comparison 8. Adverse effects related to different doses of rimantadine in the elderly.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Confusion | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.41, 1.65] |
| 1.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.41, 1.65] |
| 2 Depression | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.12, 1.65] |
| 2.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.12, 1.65] |
| 3 Impaired concentration | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.11, 3.98] |
| 3.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.11, 3.98] |
| 4 Insomnia or sleeplessness | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.26, 3.97] |
| 4.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.26, 3.97] |
| 5 Loss of appetite | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.27, 1.46] |
| 5.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.27, 1.46] |
| 6 Rash or allergic reaction | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.04, 3.21] |
| 6.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.04, 3.21] |
| 7 Seizure or clonic twitching | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 2.07] |
| 7.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 2.07] |
| 8 Dry mouth | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.43, 3.11] |
| 8.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.43, 3.11] |
| 9 Fatigue and drowsiness | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.45, 2.87] |
| 9.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.45, 2.87] |
| 10 Headache | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.30, 3.42] |
| 10.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.30, 3.42] |
| 11 Body weakness or debility | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.38, 2.18] |
| 11.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.38, 2.18] |
Comparison 9. Additional comparison: RMT compared to placebo in the prophylaxis of influenza A in children and the elderly.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Infection | 5 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.27, 0.92] |
| 1.1 RMT | 5 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.27, 0.92] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Clover 1986.
| Methods | Randomised, parallel, double‐blind comparison of rimantadine with PB. The trial took place during an outbreak of influenza A/H1N1 in Oklahoma Study duration: 5 weeks Patients and providers were blinded. Outcome assessor method of blinding was unclear Dropouts: 3 families who moved outside the study area, 1 in the placebo group whose parents attributed the 'medication' to the reducing of the child's performance at school and 1 in the rimantadine group due to a non‐influenza illness in a 4‐year‐old child Co‐interventions and other potential confounders were not observed | |
| Participants | There was a total of 146 participants, including 76 children, which was our subgroup of interest Inclusion criteria: children within 35 families during a naturally occurring outbreak of influenza A Exclusion criteria: if any family member was known to have cardiac, pulmonary, or neurologic disease; if a female family member was pregnant or actively trying to become pregnant; if any family member had received the influenza vaccine during the past year; if any member was taking medications that might interfere with the study Gender: both females and males were included (proportion not specified) Disease stage: rimantadine was administered as a prophylactic when influenza A was identified within community | |
| Interventions | Rimantadine: 5 mg/kg/d, max: 100 mg/ d (< 10 years) or 200 mg/ d (> 10 years). Oral route. Duration: 5 weeks | |
| Outcomes | Laboratory‐proven infection cases and reported adverse effects | |
| Notes | 1 to 18 years old | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It is reported that "children ... received either rimantadine or PB in a double‐blind, random assignment". Nevertheless, the randomisation method is not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment is not clearly described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for missing outcome data are unlikely to be related to true outcome |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is stated that "children ... received either rimantadine or PB in a double‐blind, random assignment" but the specific people who were blinded are not listed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | It is stated that "children ... received either rimantadine or PB in a double‐blind, random assignment" but the specific people who were blinded are not listed |
Clover 1991.
| Methods | Randomised, parallel, comparison of rimantadine with PB. Multicentre trial that took place during an influenza season for 3 to 4 weeks after the start of treatment Patients were blinded. Outcome assessor blinding was unclear Dropouts: none (in the subgroup of interest) Co‐interventions and other potential confounders were not observed | |
| Participants | There was a total of 84 participants, including 46 children, which was our subgroup of interest Inclusion criteria: children within families consisting of 2 to 5 members with at least 1 adult (ranging in age from 18 to 75 years and 1 child aged between 1 to 17 years during a naturally occurring outbreak of influenza A Exclusion criteria: participants who had a history of amantadine hypersensitivity, chronic respiratory disease, severe medical illness, neuropsychiatric disorder; were pregnant or lactating; had a recently documented influenza A virus infection; required long‐term drug therapy with amantadine or drugs that could interfere with rimantadine or with clinical assessments (e.g. aspirin, tranquillisers, antihistamines and decongestants Gender: unclear Disease stage: all the eligible participants were given the assigned drug as soon as influenza was first recognised in family members (the index patient) and after the member had been evaluated by a study nurse | |
| Interventions | Rimantadine: 5 mg/kg/d, max: 150 mg/d (= or < 10 years or weighing less than 30 kg) or 200 mg/d (> 9 years who weighed more than 30 kg). Oral route. Duration: 10 days | |
| Outcomes | The outcome of interest was laboratory‐proven infection cases | |
| Notes | 1 to 17 years old | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors stated it was a randomised study and that randomisation is described in another article (Hayden 1989): "all eligible family members ... randomly assigned as a block to receive either rimantadine or PB". The method used is not described |
| Allocation concealment (selection bias) | Low risk | Randomisation was carried out in one of the centres where this multicentric trial was conducted |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for missing outcome data are unlikely to be related to true outcome |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors stated it was a double‐blinded trial as described in the other article (Hayden 1989): "the study was double‐blind ... trial". Nevertheless, the specific people who were blinded are not listed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Authors stated it was a double‐blinded trial as described in the other article (Hayden 1989): "the study was double‐blind ... trial". Nevertheless, the specific people who were blinded are not listed |
Crawford 1988.
| Methods | Randomised, parallel, double‐blind trial in which prophylactic efficacy of rimantadine against influenza A infection in children was evaluated. Rimantadine was compared to PB. The trial took place during a naturally occurring outbreak of influenza A (H3N2) in Oklahoma City, USA, from November, 1984 to March, 1985 Study duration: 5 weeks Withdrawal: 3 children in the rimantadine group were found post‐study to have had documented influenza A infection before or on the day of institution of prophylaxis and were excluded from the analysis. 17 people from 5 families withdrew because of relocation or refusal to have a second blood specimen drawn. Their age group was not stated | |
| Participants | There was a total of 110 participants from 29 families, including 56 children, which was our subgroup of interest Inclusion criteria: children within 29 families during a naturally occurring outbreak of influenza A infection Exclusion criteria: if any family member was known to have cardiac, pulmonary or neurologic disease; if a female family member was pregnant or actively trying to become pregnant; if any family member had received the influenza vaccine during the past year; if any member was taking medications that might interfere with the study Gender: both females and males were included (proportion not specified) Disease stage: rimantadine was administered as a prophylactic when influenza A was identified within community | |
| Interventions | Rimantadine: 5 mg/kg/d, max: 100 mg/d (< 10 years) or 200 mg/d (> 10 years). Oral route | |
| Outcomes | Laboratory‐proven infection cases. Adverse effects | |
| Notes | 1 to 18 years old | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors stated it was a "a randomised ... clinical trial" although randomisation methods are not described |
| Allocation concealment (selection bias) | Unclear risk | The authors state that their "study design has been previously reported" (Clover 1986) but even in that trial, the method of concealment is not clearly described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for missing outcome data unlikely to be related to outcome |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors stated it was "a double‐blind PB controlled clinical trial". Nevertheless, the specific people who are blinded are not listed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Authors stated it was "a double‐blind PB controlled clinical trial". Nevertheless, the specific people who are blinded are not listed |
Finklea 1967.
| Methods | Randomised, parallel, double‐blind, trial in which amantadine was used as prophylaxis in naturally occurring acute respiratory illness. Amantadine was compared to PB. The trial took place between February 1965 to June 1965 The method of blinding is unclear Study duration: 18 weeks Withdrawal was the same for the 2 groups ‐ discharge from school (19%). The proportion was not stated | |
| Participants | There were 293 participants from both sexes (proportion not stated), from 8 to 19 years of age. The participants were volunteers at a school for intellectually handicapped but educable children. Sera pairs tests were obtained in 237 children. Exclusion criteria: children receiving tranquillisers, sympathomimetic amines or anticonvulsives Co‐morbid conditions: intellectually handicapped children | |
| Interventions | Amantadine: 1 to 2.5 mg/kg (pre‐puberal: 60 mg/dose, 2 x/d, during the first week and 1 x/d during the rest of the period of the study. Older children: 100 mg/dose, 2 x/d, during the first week and 1 x/d during the rest of the period of the study | |
| Outcomes | 4‐fold rises in CF and/or HI tilter against A2/AA/1/65 | |
| Notes | 8 to 19 years old | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors stated "volunteers were assigned to amantadine or the PB group by randomisation", although randomisation method is not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment is not clearly described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It is stated that "The rate of withdrawal ... (the same for the two groups) was small. The reason for withdrawal was discharge from school" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Although it was "a double‐blind study", the specific people who are blinded are not listed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Although the trial is described as "a double‐blind study", the specific people who are blinded are not listed |
Gravenstein 2005.
| Methods | Randomised, parallel, double‐blind comparison of rimantadine with zanamivir. Identical PB (inhaled or tablets) were used. The trial took place in nine long‐term care facilities in the United States over 3 winter seasons. The study was conducted over multiple influenza seasons, therefore some participants were randomised more than once Study duration: 3 winter seasons Co‐interventions and other potential confounders were not observed | |
| Participants | There were 231 participants in the rimantadine group and 226 in the zanamivir group (intention‐to‐treat population) of both sexes (29% female in rimantadine group and 30% female in zanamivir group). More than 75% of the participants were 65 years of age or older (90% in rimantadine group and 89% in zanamivir group) | |
| Interventions | Upon an influenza outbreak participants were randomised (1:1) to inhaled zanamivir plus PB or inhaled PB plus zanamivir 100 mg tablets for 14 days | |
| Outcomes | The outcome of interest was laboratory‐proven infection cases | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors describe the trial as "a randomised, parallel comparison of rimantadine with zanamivir" but randomisation methods are not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment is not clearly described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for missing outcome data are unlikely to be related to the true outcomes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial is described as a "double‐blind comparison of rimantadine with zanamivir. Identical PB (inhaled or tablets) were used". Nevertheless, the specific people who are blinded are not listed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial is described as a "double‐blind comparison of rimantadine with zanamivir. Identical PB (inhaled or tablets) were used". Nevertheless, the specific people who are blinded are not listed |
Hall 1987.
| Methods | Randomised, parallel, double‐blind comparison of rimantadine with acetaminophen Study duration: 7 days 1 patient dropped out, due to AE Co‐interventions and other potential confounders were not observed | |
| Participants | 69 children were included, 40 females and 29 males Inclusion criteria: clinical illness and viral isolation Exclusion criteria: previously unhealthy aged 1 to 15 years Disease stage: clinical illness and laboratory‐confirmed infection | |
| Interventions | Rimantadine: 6.6 mg/kg/d, max: 150 mg/d (< 9 years) and 200 mg/d (>= 9 years), 2 x/d; by oral route, for 5 days | |
| Outcomes | Mean symptom score of: fever, conjunctivitis, eye symptoms (pain on movement, fever up to 3rd day, conjunctivitis up to 3rd day, eyes symptoms (pain on movement and visual distortion); cough up to 7th day; malaise up to 6th day; CNS symptoms | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | It is stated in the published study that "Patients were assigned to the rimantadine or acetaminophen treatment group under a double‐blind, randomised allocation". The investigators also reported in their correspondence to the review authors that a computer random system was used to randomise participants |
| Allocation concealment (selection bias) | Low risk | Participants and investigators enrolling participants could not foresee assignment because a pharmaceutical‐controlled randomisation was used to conceal allocation, as stated in the authors' correspondence to the review authors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 "child receiving rimantadine complained of nausea and vomiting and withdrew from the study on the second day". The proportion of missing outcomes compared with observed event risk is not enough to have a clinically relevant impact on the intervention effect estimate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Although "patients were assigned to the rimantadine or acetaminophen treatment group under a double‐blind, randomised allocation", the specific people who are blinded are not listed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Although "patients were assigned to the rimantadine or acetaminophen treatment group under a double‐blind, randomised allocation", the specific people who are blinded are not listed |
Kitamoto 1968.
| Methods | Randomised, parallel, double‐blind comparison of amantadine with PB. This trial took place during an outbreak of influenza in Japan Study duration: 7 days Patient, provider and outcome assessor method of blinding is unclear Dropouts: none Co‐interventions and other potential confounders were not observed | |
| Participants | There were 355 participants. Although the proportions are not cited, it is stated that the groups are comparable in the following criteria: sex, age, influenza vaccination history, distribution and geometric mean of HI and CF titre in acute sera, interval between onset of symptoms and start of treatment and maximum body temperature before the treatment 158 participants of both genders met the age criteria. 91 children were cases of clinical influenza with serological confirmation. The proportion of males and females was not stated Inclusion criteria: respiratory symptoms evident within the 2nd day of illness Disease stage: clinical symptoms within 2nd day of illness | |
| Interventions | Amantadine: 50 mg/d (1 to 2 years old); 100 mg/d (3 to 5 years old); 150 mg/d (6 to 10 years old), by oral route, for 7 days | |
| Outcomes | Fever up to 4th day. AE: nausea/vomiting; diarrhoea; exanthema; malaise; muscular, limb pain; headache; dyspnoea; cyanosis; stimulation/insomnia; dizziness; arrhythmia | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that "amantadine or PB was given to the patient at random", although randomisation method is not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment is not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing patients, although "four cases were shown to be influenza B and were excluded from statistical analysis" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Although "amantadine or PB was given to the patient at random by double‐blind method" the specific people who are blinded are not listed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Although "amantadine or PB was given to the patient at random by double‐blind method" the specific people who are blinded are not listed |
Kitamoto 1971.
| Methods | Randomised, parallel, double‐blind comparison of amantadine with PB. The trial took place during an outbreak of influenza in the winter of 1968 to 1969 in Japan Study duration: at least 7 days Patient, provider and outcome assessor method of blinding was unclear Dropouts were not stated Co‐interventions and other potential confounders: concomitant administration of antipyretics. An analyses with patients who received concomitant antipyretics was also performed | |
| Participants | Of the 737 participants, 155 participants of both genders met the inclusion criteria. Although the proportions are not cited, it is stated that the groups are comparable in the following criteria: sex, age, influenza vaccination history, distribution and geometric mean of HI and CF titre in acute sera, interval between onset of symptoms and start of treatment and maximum body temperature before the treatment Inclusion criteria: respiratory symptoms evident within the 2nd day of illness Disease stage: clinical symptoms within 2nd day of illness | |
| Interventions | Amantadine: 50 mg/d (1 to 2 years old); 100 mg/d (3 to 5 years old); 150 mg/d (6 to 10 years old), by oral route, for 7 days | |
| Outcomes | Fever up to 4th day. AE: nausea/vomiting; diarrhoea; exanthema; malaise; muscular, limb pain; headache; stimulation/insomnia; dizziness; arrhythmia | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The author states "patients were given amantadine or PB according to randomly distributed individual code of the double‐blind method", although the randomisation method is not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment is not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although there were no missing outcome data, the author states that "only patients with Hong Kong influenza in whom medication was started within 2 days were included in statistical analysis". "In order to exclude the possible influence of concomitantly administered antipyretics on the defervescent effect of amantadine the same analysis was performed with 134 Hong Kong influenza patients who had received no concomitant antipyretics" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The author states "patients were given amantadine or PB according to randomly distributed individual code of the double‐blind method". Nevertheless, the specific people who are blinded are not listed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The author states "patients were given amantadine or PB according to randomly distributed individual code of the double‐blind method". Nevertheless, the specific people who are blinded are not listed |
Monto 1995.
| Methods | Randomised, parallel, double‐blind comparison of 2 different doses of rimantadine with PB. The trial took place during an outbreak of influenza A/H3N2 during 1993 Study duration: 8 weeks Dropouts: 62% withdrew because of side effects, death, discharge, hospitalisation, physician's request and refusal to continue participation Co‐interventions and other potential confounders were not observed | |
| Participants | A total of 328 participants, 275 females and 53 males were included Inclusion criteria: residents of 10 nursing homes who agreed to participate in the study Exclusion criteria: patients with significant renal or hepatic disease Disease stage: rimantadine was administered as prophylaxis | |
| Interventions | Rimantadine: 100 mg/d; rimantadine: 200 mg/d; PB. Ratio: 2:2:1. Duration: up to 8 weeks | |
| Outcomes | Death. AEs: dry mouth, drowsiness/fatigue, headache, irritability, dizziness/light headedness, nausea/vomiting, abdominal pain, body weakness or disability, confusion, depression, impaired concentration, insomnia or sleeplessness, loss of appetite, rash or allergic reaction, seizure or clonic twitching | |
| Notes | 3 groups: rimantadine 100 amantadine 200 and PB | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although the authors state that the participants were randomly assigned to receive active medication (100 or 200 mg of rimantadine per day) or placebo, the randomisation method is not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment is not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Authors stated that an "increased risk of withdrawal from the study only on the basis of perceived side effects was demonstrated among participants in both groups receiving active medication, especially the 200 mg/day group, compared with the placebo group; however, these associations were not statistically significant". The reasons for missing outcome data are likely to be related to true outcome |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is stated that "staff and residents were blinded to group assignment" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding is stated. The outcome is likely to be influenced by lack of blinding |
Patriarca 1984.
| Methods | Randomised, parallel, double‐blind comparison of rimantadine with PB. The trial took place during an outbreak of influenza A (H3N2). Viruses were isolated from patients in the community. The study was conducted from early January to 6 April 1983 Patient, provider and outcome assessor method of blinding is unclear | |
| Participants | 35 participants, 68 to 102 years old, of non‐specified gender, all of whom had been vaccinated the previous autumn Inclusion criteria: residents of 3 nursing homes who agreed to participate in the study Exclusion criteria: patients with medical conditions that might increase the severity of side effects or require careful adjustments in the dosage of rimantadine, which include: significant renal impairment (SCr > 2 mg/d) or liver disease, acute congestive heart failure, seizure disorders, psychosis, severe pitting oedema, orthostatic hypotension and conditions requiring central nervous system stimulants Disease stage: rimantadine was administered as prophylaxis | |
| Interventions | Rimantadine: 100 mg twice a day; PB. Duration: 80 (+/‐ 4.9) days prophylaxis | |
| Outcomes | Adverse reactions: anxiety, confusion, insomnia, anorexia, fatigue, dizziness, nausea and vomiting | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors stated that "participants ... were randomly assigned to receive either rimantadine or PB". Nevertheless, randomisation method is not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment is not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | It was cited that 2 participants from the intervention group withdrew because of side effects. 1 suffered a generalised convulsion of undetermined aetiology (a participant with an underlying idiopathic seizure disorder). 3 later withdrew for no described reasons. 2 participants from the PB group also withdrew. Reasons for missing outcome data are likely to be related to the true outcome, with imbalance in reasons for missing data across intervention and control groups |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is stated that "a double‐blind, placebo‐control trial" was conducted. Nevertheless, the specific people who were blinded are not listed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | It is stated that "a double‐blind, placebo‐control trial" was conducted. Nevertheless, the specific people who were blinded are not listed |
Payler 1984.
| Methods | Randomised, parallel trial; blinding is not stated. Amantadine used as prophylaxis in naturally occurring acute respiratory illness. Amantadine was compared to no specific treatment. The trial took place in the autumn of 1982 Study duration: 14 days Patients excluded from analysis were similar in the 2 groups and the reasons were: students were day boys from whom samples were not available; students infected before the start of amantadine; compliance failures | |
| Participants | There were 604 randomised students and 536 were analysed. All of them were male, from 13 to 19 years of age. The participants were students of a boarding school. Once the influenza A outbreak had been detected, samples were taken from all boys who were sufficiently unwell to be absent from lessons even if they did not have a fever. Nasopharyngeal aspirates were examined for viruses by rapid immunofluorescent microscopy and tissue culture. Once outbreaks had been identified, only culture methods were used | |
| Interventions | Amantadine: 100 mg/ dose, 1 x/d, during the 14 days | |
| Outcomes | Clinical and laboratory‐proven influenza A | |
| Notes | 13 to 19 years old | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | In correspondence with the review authors, the study authors reported that randomisation had been carried out by the statistical department of a pharmaceutical company |
| Allocation concealment (selection bias) | Low risk | Participants and investigators enrolling participants could not foresee assignment because a pharmaceutical company‐controlled randomisation was used to conceal allocation. They kept the key to the randomisation and only when the study was analysed was the code broken, as stated in the study authors' correspondence with the review authors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Although there was no blinding stated, the review authors judge that the outcome is not likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Although there was no blinding stated, the review authors judge that the outcome is not likely to be influenced by the lack of blinding |
Schilling 1998.
| Methods | Randomised, parallel, unblinded trial. Rimantadine and zanamivir were compared for prophylaxis of influenza A. The trial began in November 1996. The participants were volunteer residents of a nursing home for veterans and their spouses Drug administration: 14 days The number of respiratory illness was monitored until January 1997 | |
| Participants | 65 volunteers of both sexes received zanamivir and 23 rimantadine Age range: 50 to 95 years old and 75% older than 65 years of age The participants were volunteers residents of a nursing home for veterans and their spouses Inclusion criteria: volunteers living in a unit of the nursing home where outbreak of influenza was declared Exclusion criteria: symptoms of new respiratory illness within the previous 7 days of the declared outbreak | |
| Interventions | Rimantadine: 100 mg/dose, 1 x/day, during 14 days. Zanamivir: 10 mg inhaled bid and 4.4 mg intranasally bid | |
| Outcomes | Clinical and laboratory‐proven influenza A | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors stated that it was a "randomised unblinded study" but the randomisation method is not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment is not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There is insufficient reporting of exclusions. It is stated that "six volunteers receiving zanamivir withdrew. One withdrew due to mild adverse effects". The other reasons for withdrawal are not clear. It is also unclear if there were withdrawals among the rimantadine group |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Although it was a "randomised unblinded study", the review authors judge that the outcome is not likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Although it was a "randomised unblinded study", the review authors judge that the outcome is not likely to be influenced by the lack of blinding |
ACM: acetaminophen AE: adverse effects bid: twice a day CF: complement fixation CNS: central nervous system d: day GI: gastrointestinal HI: haemagglutination inhibition NC: not clear PB: placebo SCr: serum creatinine STGO: aspartate aminotransferase
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| AAPCID 2007 | Not a RCT |
| Allen 2006 | Not a RCT |
| Anonymous 2006 | Not a RCT |
| Anonymous 2007 | Article about oseltamivir and vaccination |
| Anton 2011 | Review article |
| Aoky 1985a | Pharmacokinetics study of amantadine and rimantadine |
| Aoky 1985b | Ages of participants were outside protocol age range |
| Aoky 1986 | Ages of participants were outside protocol age range |
| Atiee 2012 | Open‐label study of the pharmacokinetic interactions of peramivir with oseltamivir or rimantadine |
| Atmar 1990 | Ages of participants were outside protocol age range |
| Bacosi 2002 | Article about the treatment of hepatitis C |
| Baker 1969 | Ages of participants were outside protocol age range (participants were aged between 17 to 57 years old) |
| Bantia 2010 | Non‐human trial |
| Barr 2007a | Not a RCT |
| Barr 2007b | Not a RCT |
| Bauer 2007 | Non‐human trial |
| Belenky 1998 | Ages of participants were outside protocol age range (participants were aged between 17 to 57 years old) |
| Bloomfield 1970 | Ages of participants were outside protocol age range |
| Boltz 2010 | Review article about other antiviral drugs |
| Brady 1990 | Ages of participants were outside protocol age range |
| Brammer 2009 | Article focusing influenza surveillance |
| Bricaire 1990 | Analyses by age subgroups of interest were not available |
| Bryson 1990 | Insufficient data available |
| Burch 2009 | Systematic review about the use of other antivirals |
| Cady 2011 | Not a RCT |
| Callmander 1968 | Ages of participants were outside protocol age range (participants were 20 to 60 years old) |
| Carter 2008 | Review of the use of the influenza vaccine |
| Cayley 2010 | Article about neuraminidase inhibitors in healthy adults |
| Cayley 2012 | Review about neuraminidase inhibitors |
| Chawla 2009 | Article about strategies for pandemic preparedness |
| Chemaly 2006 | Not a RCT |
| Chen 2007 | Article about Chinese medical herbs |
| Cheng 2004 | The authors studied other antivirals, included other viral infections and the ages of participants were outside protocol age range |
| Cheng 2009 | Review study with different objectives |
| Cheng 2012 | Review article |
| Choi 2009 | Trial conducted in influenza isolates |
| Chou 2008 | Article about chronic hepatitis C |
| Cohen 1976 | Ages of participants were outside protocol age range (participants were aged between 20 to 39 years old) |
| Cohen 2006 | Study that compared patient access to pharmaceuticals in the UK and US |
| Cowling 2008 | Preliminary findings of non‐pharmaceutical intervention trial |
| Curran 2010 | Article about an influenza vaccine |
| Dawkins 1968 | Study assessing the prophylactic efficacy of an analogue of amantadine |
| De la Camara 2007 | Review study |
| De Vincenzo 2012 | Review article |
| DeLaney 2010 | Review study |
| Denys 1963 | Ages of human participants were outside protocol age range (participants were aged between 19 to 21 years old). Animals were also studied |
| Dolamore 2003 | Case‐control study |
| Dolin 1982 | Ages of participants were outside protocol age range (participants were aged between 18 to 45 years old) |
| Doyle 1998 | Ages of participants were outside protocol age range (participants were aged between 18 to 50 years old) |
| Drinevskii 1998 | Randomisation was not stated |
| Drinka 1998 | Groups characteristics not stated. Analyses by age subgroup of interest not available |
| Enger 2004 | Article about oseltamivir |
| Escuret 2012 | Ages of participants were outside protocol age range |
| Falagas 2010 | Review study |
| Farlow 2008 | Article about Alzheimer's |
| Fiore 2008 | Article about Glycyrrhiza species |
| Furuta 2005 | Study of the mechanism of action of T‐705 against influenza virus |
| Galabov 2006 | Non‐human trial |
| Galbraith 1969a | Analyses by age subgroups of interest were not available |
| Galbraith 1969b | Outcomes of interest were not studied |
| Galbraith 1971 | Analyses by age subgroups of interest were not available |
| Galbraith 1973 | Insufficient data available |
| Garman 2004 | Trial about drugs that inhibit the virus's neuramidase |
| Gatwood 2012 | Review study |
| Gerth 1966 | Not a RCT |
| Griffin 2004 | Pharmacological study |
| Guo 2007 | Review article |
| Hay 1986 | Study about the molecular basis for resistance of influenza A to amantadine |
| Hayden 1979 | Ages of participants were outside protocol age range |
| Hayden 1980 | Ages of participants were outside protocol age range |
| Hayden 1981 | Ages of participants were outside protocol age range |
| Hayden 1982 | Ages of participants were outside protocol age range |
| Hayden 1985 | Pharmacokinetics study in which ages of participants were outside protocol age range |
| Hayden 1986 | Ages of participants were outside protocol age range |
| Hayden 1989 | Analysis by age subgroups of interest was not available |
| Hayden 1991 | Analysis by age subgroups of interest was not available |
| Hayden 2000 | The drug studied was zanamivir |
| Hayden 2006 | Not a RCT |
| Hayden 2012 | Review study |
| Hornick 1969 | Ages of participants were outside protocol age range |
| Hota 2007 | Not a RCT |
| Hout 2006a | Study about the human immunodeficiency virus |
| Hout 2006b | Study about the human immunodeficiency virus |
| Hsu 2012 | Systematic review |
| Hurt 2007 | Not a RCT |
| Ilyushina 2005 | Not a RCT |
| Ilyushina 2006 | Study of whether combined therapy with 2 classes of anti‐influenza drugs could affect the emergence of resistant virus variants in vitro |
| Ilyushina 2007a | Non‐human trial |
| Ilyushina 2007b | Non‐human trial |
| Ison 2006 | Case series |
| Ison 2013 | Review about pharmacokinetics |
| Ito 2000 | Ages of participants were outside protocol age range |
| Ito 2006 | Study about influenza vaccination |
| Jefferson 2006a | Systematic review about antivirals for influenza in healthy adults |
| Jiang 2013 | Article about Chinese medicinal herbs |
| Jones 2006 | Trial in which a 20‐amino‐acid peptide was used |
| Kalia 2008 | Article about neurological diseases |
| Kantor 1980 | Ages of participants were outside protocol age range (participants were aged between 17 to 53 years old) |
| Kawai 2005 | Not a RCT |
| Khakoo 1981 | Amantadine and/or rimantadine were not tested in this trial |
| Kim 2011 | Article about the effect of corticosteroids treatment |
| Kirkby 2010 | Article about complementary and alternative medicine. Not a RCT |
| Kiso 2004 | Descriptive study to investigate oseltamivir resistance in children treated for influenza |
| Kitamoto 1969 | Duplicated results |
| Knight 1969 | Ages of participants were outside protocol age range |
| Knight 1970a | Ages of participants were outside protocol age range |
| Knight 1970b | Ages of participants were outside protocol age range |
| Knight 1981 | Ribavirin study in which ages of participants were outside protocol age range (participants were aged between 22 to 42 years old) |
| Korenke 2008 | Article about multiple sclerosis treatment |
| Krylov 1978 | Analysis by age subgroups of interest was not available |
| Kulichenko 2003 | Ages of participants were outside protocol age range |
| Langlet 2009 | Article about the use of antivirals for chronic hepatitis C |
| Le Tissier 2005 | Non‐human trial |
| Leeming 1969 | Insufficient data available |
| Leone 2005 | Article about the use of amantadine for traumatic brain injury |
| Leung 1979 | Outcomes of interest were not studied |
| Lim 2007 | Study about an influenza‐like illness |
| Lin 2006 | Study about neurologic manifestations in children with influenza B |
| Linder 2005 | The authors measured the rates of antiviral and antibiotic prescribing for patients with influenza |
| Lipatov 2007 | The study was conducted in influenza viruses isolated from poultry |
| Little 1976 | Analyses by age subgroups of interest were not available |
| Little 1978 | Article is about hyperreactivity and airway dysfunction in influenza infection and not about treatment or prevention of influenza |
| Lopez‐Medrano 2012 | Not a RCT |
| Louie 2012 | Article about an intravenous neuraminidase inhibitor drug for influenza A |
| Lutz 2005 | Study of a method for detecting and quantifying influenza A virus replication |
| Lynd 2005 | Not a RCT |
| Machado 2004 | Article was about the use of oseltamivir to control influenza complications after bone marrow transplantation |
| Mallia 2007 | Not a RCT |
| Maricich 2004 | Not a RCT |
| Mase 2007 | The study was conducted in influenza viruses isolated from poultry |
| Mate 1970 | Ages of participants were outside protocol age range |
| Mate 1971 | Ages of participants were outside protocol age range |
| Matheson 2007 | Systematic review of the use of neuraminidase inhibitors |
| Matsuya 2007 | Study of the synthesis and evaluation of dihydrofuran‐fused perhydrophenanthrenes as a new anti‐influenza agent |
| Matthews 2004 | Review article about treatment of viral hepatitis and oncological conditions |
| McCullers 2004 | Non‐human trial |
| McKay 2006 | Non‐human trial |
| Michiels 2013 | Article about oseltamivir and zanamivir |
| Mishin 2005 | Not a clinical trial |
| Miyachi 2011 | Insufficient data available |
| Moffat 2008 | Article about biophysical aspects of the influenza virus |
| Monto 1979 | Ages of participants were outside were outside protocol age (participants were aged between 18 to 24 years old) |
| Morrison 2007 | Ages of participants were outside protocol age range |
| Muldoon 1976 | Ages of participants were outside protocol age range |
| Nafta 1970 | A wider age range was considered. Analysis by age subgroups of interest was not available |
| Natsina 1994 | Randomisation was not stated. Additional information not available |
| Nuesch 2007 | Review study |
| O'Donoghute 1973 | Analysis by age subgroups of interest was not available |
| Obrosova‐Serova 1972 | Study about effectiveness of midantan and interferon inducers as means of non‐specific prevention of influenza |
| Oker‐Blom 1970 | Ages of participants were outside protocol age range (participants were aged between 20 to 28 years old) |
| Ong 2007 | Not a RCT |
| Pachucki 2004 | Article about a diagnostic test |
| Peiris 2004 | The aim of the authors was not to study amantadine and rimantadine to prevent or treat influenza |
| Pemberton 1986 | Article about amantadine resistance in clinical influenza A and virus isolates |
| Petterson 1980 | Insufficient data available |
| Pritchard 1989 | Article about the treatment of juvenile chronic arthritis with antivirals |
| Quarles 1981 | Ages of participants were outside protocol age range |
| Quilligan 1966 | Not a RCT |
| Rabinovich 1969 | Ages of participants were outside protocol age range |
| Reis 2006 | Article about neurologic effects of amantadine |
| Reuman 1989a | Ages of participants were outside protocol age range (participants were aged between 18 to 40 years old) |
| Reuman 1989b | Ages of participants were outside protocol age range (participants were aged between 18 to 55 years old) |
| Risenbrough 2005 | Not a RCT |
| Rose 1980 | Not a RCT |
| Rothberg 2005 | Not a RCT |
| Saito 2006 | Not a RCT |
| Sampaio 2011 | Article about the efficacy and safety of pardoprunox in patients with early Parkinson's disease |
| Santesso 2013 | Systematic review |
| Sato 2008 | Article about oseltamivir treatment |
| Sauerbrei 2006 | Not a RCT |
| Schapira 1971 | Analysis by age subgroups of interest was not available |
| Schmidt 2004 | Review article |
| Sears 1987 | Ages of participants were outside protocol age range (participants were aged between 18 to 40 years old) |
| Semlitsch 1992 | The purpose of this article was to study the acute effects of amantadine infusions on event‐related potentials |
| Serkedjieva 2007 | Non‐human trial |
| Shah 2012 | Review article |
| Shuler 2007 | Case‐control study |
| Shvetsova 1974 | The trial authors studied different populations. No information was available about clinical outcomes and confirmation of influenza diagnosis |
| Simeonova 2009 | Non‐human article |
| Singer 2011 | Review article |
| Skoner 1999 | Ages of participants were outside protocol age range (participants were aged between 18 to 50 years old) |
| Smorodintsev 1970a | Ages of participants were outside protocol age range |
| Smorodintsev 1970b | Ages of participants were outside protocol age range |
| Smorodintsev 1970c | Ages of participants were outside protocol age range (participants were aged between 18 to 30 years old) |
| Somani 1991 | Randomisation was not stated. The groups were not similar at baseline |
| Tajima 2006 | Study of aetiology and treatment in hospitalised children with pneumonia |
| Takemura 2005 | Not a study about influenza A |
| Tappenden 2009 | Systematic review |
| Terabayashi 2006 | Article about the inhibition of influenza‐virus‐induced cytopathy by sialyglycoconjugates |
| Thomas 2008 | Article about multiple sclerosis |
| Thompson 1987 | Insufficient data presented |
| Togo 1968 | Ages of participants were outside protocol age range |
| Togo 1970 | Ages of participants were outside protocol age range |
| Togo 1972 | The drug studied was cyclooctylamine |
| Townsend 2006 | Not a RCT |
| Van der Wouden 2005 | Not a RCT |
| Van Voris 1981 | Ages of participants were outside protocol age range |
| Van Voris 1985 | Study about 4 antibody techniques to assess influenza infection |
| Wailoo 2008 | Article about the use of neuraminidase inhibitors in adults |
| Webster 1986 | Non‐human trial |
| Welton 2008 | Not a RCT |
| Wendel 1966 | Ages of participants were outside protocol age range (participants were aged between 17 to 54 years old) |
| Whitley 2007 | Not a RCT |
| Wingfield 1969 | Ages of participants were outside protocol age range |
| Wong 2006 | Not a RCT |
| Wright 1976 | Analysis by age subgroups of interest was not available |
| Wultzler 2004 | Not a clinical trial |
| Yamaura 2003 | The antiviral studied was oseltamivir |
| Younkin 1983 | Ages of participants were outside protocol age range (participants were aged between 17 to 20 years old) |
| Yuen 2005 | Not a RCT |
| Yuen 2012 | Review article |
| Zeuzem 1999 | The purpose of the authors was to study treatment for chronic hepatitis C |
PB: placebo RCT: randomised controlled trial
Differences between protocol and review
Originally in the protocol we planned to study the drug effect on reduction of fever and cough, as they are considered the best predictors of influenza diagnosis. After collecting data, we verified that specific timelines for reduction of signs and symptoms were not reported in the included trials. So, we considered the available data and arbitrarily chose a day of antiviral use to evaluate the response to the treatment. This choice was based on Eccle's study in which clinical manifestations were classified into early and later symptoms (Eccle 2005).
We applied wider age ranges for children than the definition stated in the protocol (participants up to 16 years of age). Trials in older participants who were adolescents by the World Health Organization (WHO) definition were also included (WHO 2007). Data regarding the proportion of the subgroup which strictly fulfilled the age criterion in the protocol were not available in five studies or by contacting the trial authors. The respective age ranges were one to 17 years (Clover 1991), 13 to 19 years (Payler 1984), one to 18 years (Clover 1986; Crawford 1988), and eight to 19 years of age (Finklea 1967).
We planned only to make 12 comparisons. However, whilst analysing data we considered doing an additional comparison and put the two age groups together. As the small samples studied in rimantadine trials for prophylaxis might have influenced the observed results, we tried to overcome this limitation by combining the trials with rimantadine in children and in the elderly. It must be stressed that extraneous characteristics between those groups, other than age or previous immunisations, may have occurred, impairing generalisation of these results.
Contributions of authors
Márcia G Alves Galvão (MG) selected the trials, extracted data and was responsible of the methodological aspects of the review. Marilene Augusta Rocha Crispino Santos (MS) selected the trials, extracted data, was responsible of the methodological aspects of the review and supervised the day‐to‐day work of the review. Antonio Ledo Alves da Cunha (AC) was appointed as an arbitrator to solve disagreements between MG and MS on the selection of the trials. He supervised the work in all phases and provided his experience on the development of the review.
Declarations of interest
Márcia G Alves Galvão: none known. Marilene Augusta Rocha Crispino Santos: none known. Antonio Ledo Alves da Cunha: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Clover 1986 {published data only}
- Clover RD, Crawford SA, Abell TD, Ramsey CN Jr, Glezen WP, Couch RB. Effectiveness of rimantadine prophylaxis of children within families. American Journal of Diseases of Children 1986;140(7):706‐9. [CN‐00043175] [DOI] [PubMed] [Google Scholar]
Clover 1991 {published data only}
- Clover RD, Warner JL, Becker L, Davis A. Effect of rimantadine on the immune response to influenza A infections. Journal of Medical Virology 1991;34(1):68‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Crawford 1988 {published data only}
- Crawford SA, Clover RD, Abell TD, Ramsey CN, Glezen WP, Couch RB. Rimantadine prophylaxis in children: a follow up study. Pediatric Infectious Disease Journal 1988;7(6):379‐83. [3292997] [DOI] [PubMed] [Google Scholar]
Finklea 1967 {published data only}
- Finklea JF, Hennessy AV, Davenport FM. A field trial of amantadine prophylaxis in naturally‐occurring acute respiratory illness. American Journal of Epidemiology 1967;85:403‐12. [5337715] [DOI] [PubMed] [Google Scholar]
Gravenstein 2005 {published data only}
- Gravenstein S, Drinka P, Osterweil D, Schilling M, Krause P, Elliot M, et al. Inhaled zanamivir versus rimantadine for the control of influenza in a highly vaccinated long‐term care population. Journal of the American Medical Directors Association 2005;6:359‐66. [DOI] [PubMed] [Google Scholar]
Hall 1987 {published data only}
- Hall CB, Dolin R, Gala CL, Markovitz DM, Zhang YQ, Madore PH, et al. Children with influenza A infection: treatment with rimantadine. Pediatrics 1987;80:275‐82. [CN‐00049308] [PubMed] [Google Scholar]
Kitamoto 1968 {published data only}
- Kitamoto O. Therapeutic effectiveness of amantadine hydrochloride in influenza A2‐double‐blind studies. Japanese Journal of Tuberculosis and Chest Diseases 1968;15(1):17‐26. [CN‐00004214] [PubMed] [Google Scholar]
Kitamoto 1971 {published data only}
- Kitamoto O. Therapeutic effectiveness of amantadine hydrochloride in naturally occurring Hong Kong influenza ‐ double‐blind studies. Japanese Journal of Tuberculosis and Chest Diseases 1971;17(1):1‐7. [CN‐0000725] [PubMed] [Google Scholar]
Monto 1995 {published data only}
- Monto AS, Ohmit SE, Hornbuckle K, Pearce CL. Safety and efficacy of long‐term use of rimantadine for prophylaxis of type A influenza in nursing homes. Antimicrobial Agents and Chemotherapy 1995;39:2224‐8. [CN‐00121619] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patriarca 1984 {published data only}
- Patriarca PA, Kater NA, Kendal AP, Bregman DJ, Smith JD, Sikes RK. Safety of prolonged administration of rimantadine hydrochloride in prophylaxis of influenza A virus infections in nursing homes. Antimicrobial Agents and Chemotherapy 1984;26(1):101‐3. [CN‐00035517] [DOI] [PMC free article] [PubMed] [Google Scholar]
Payler 1984 {published data only}
- Payler DK, Purdham PA. Influenza A prophylaxis with amantadine in a boarding school. Lancet 1984;1(8375):502‐4. [DOI] [PubMed] [Google Scholar]
Schilling 1998 {published data only}
- Schilling M, Polvinelli L, Krause P, Gravenstein M, Ambrozaitis A, Jones HH, et al. Efficacy of zanamivir for chemoprophylaxis of nursing home influenza outbreaks. Vaccine 1998;16:1771‐4. [9778755] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
AAPCID 2007 {published data only}
- American Academy of Pediatrics Committee on Infectious Diseases. Antiviral therapy and prophylaxis for influenza in children. Pediatrics 2007;119(4):852‐60. [DOI] [PubMed] [Google Scholar]
Allen 2006 {published data only}
- Allen UD, Aoki FY, Stiver HG. The use of antiviral drugs for influenza: recommended guidelines for practitioners. Canadian Journal of infectious Diseases and Medical Microbiology 2006;17(5):273‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anonymous 2006 {published data only}
- Anonymous. Antiviral drugs in influenza: an adjunct to vaccination in some situations. Prescrire International 2006;15(81):21‐30. [PubMed] [Google Scholar]
Anonymous 2007 {published data only}
- Anonymous. Oseltamivir: new indication. Prevention of influenza in at‐risk children: vaccination is best. Prescrire International 2007;16(87):9‐11. [PubMed] [Google Scholar]
Anton 2011 {published data only}
- Anton A, Pumarola T. Influenza in immunocompromised patients: Considerations for therapy. Future Virology 2011;6:855‐68. [Google Scholar]
Aoky 1985a {published data only}
- Aoky FY, Sitar DS. Amantadine kinetics in healthy elderly men: implications for influenza prevention. Clinical Pharmacology and Therapeutics 1985;37(2):137‐4. [CN‐00186271] [DOI] [PubMed] [Google Scholar]
Aoky 1985b {published data only}
- Aoky FY, Silver HG, Sitar DS, Boudreault A, Ogilvie RI. Prophylactic amantadine dose and plasma concentration‐effect relationships in healthy adults. Clinical Pharmacology and Therapeutics 1985;37(2):128‐36. [CN‐00036657] [DOI] [PubMed] [Google Scholar]
Aoky 1986 {published data only}
- Aoky FY, Sitar DS, Milley EV. Potential of influenza vaccine and amantadine to prevent influenza A illness in Canadian Forces personnel 1980‐1983. Military Medicine 1986;151(9):459‐65. [CN‐00174577] [DOI] [PMC free article] [PubMed] [Google Scholar]
Atiee 2012 {published data only}
- Atiee G, Lasseter K, Baughman S, McCullough A, Collis P, Hollister A, et al. Absence of pharmacokinetic interaction between intravenous peramivir and oral oseltamivir or rimantadine in humans. Journal of Clinical Pharmacology 2012;52:1410‐9. [DOI] [PubMed] [Google Scholar]
Atmar 1990 {published data only}
- Atmar RL, Greenberg SB, Quarles JM, Wilson SZ, Tyler B, Feldman S, et al. Safety and pharmacokinetics of rimantadine small‐particle aerosol. Antimicrobial Agents and Chemotherapy 1990;34(11):2228‐33. [CN‐00073727] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bacosi 2002 {published data only}
- Bacosi M, Russo F, D'Innocenzo S, Santolamazza M, Miglioresi L, Ursitti A, et al. Amantadine and interferon in the combined treatment of hepatitis C virus in elderly patients. Hepatology Research 2002;22:231‐9. [DOI] [PubMed] [Google Scholar]
Baker 1969 {published data only}
- Baker LM, Shock MP, Iezzoni DG. The therapeutic efficacy of Symmetrel (amantadine hydrochloride) in naturally occurring influenza A2 respiratory illness. Journal of the American Osteopathic Association 1969;68(12):1244‐50. [CN‐00003808] [PubMed] [Google Scholar]
Bantia 2010 {published data only}
- Bantia S, Kellogg D, Parker CD, Babu YS. Combination of peramivir and rimantadine demonstrate synergistic antiviral effects in sub‐lethal influenza A (H3N2) virus mouse model. Antiviral Research 2010;88(3):276‐80. [DOI] [PubMed] [Google Scholar]
Barr 2007a {published data only}
- Barr IG, Hurt AC, Deed N, Iannello P, Tomasov C, Komadina N. The emergence of adamantane resistance in influenza A(H1) viruses in Australia and regionally in 2006. Antiviral Research 2007;75(2):173‐6. [DOI] [PubMed] [Google Scholar]
Barr 2007b {published data only}
- Barr IG, Hurt AC, Iannello P, Tomasov C, Deed N, Komadina N. Increased adamantane resistance in influenza A(H3) viruses in Australia and neighbouring countries in 2005. Antiviral Research 2007;73(2):112‐7. [DOI] [PubMed] [Google Scholar]
Bauer 2007 {published data only}
- Bauer K, Schrader C, Suess J, Wutzler P, Schmidtke M. Neuraminidase inhibitor susceptibility of porcine H3N2 influenza A viruses isolated in Germany between 1982 and 1999. Antiviral Research 2007;75(3):219‐26. [DOI] [PubMed] [Google Scholar]
Belenky 1998 {published data only}
- Belenky S, Gentile D, Doyle W, Patel A, Hayden F, Skoner D. Rimantadine effect on specific serum hemagglutination inhibition and nasal antibodies in experimental influenza virus exposure of adults. American Journal of Respiratory and Critical Care Medicine 1998;157(3):A173. [CN‐00428220] [Google Scholar]
Bloomfield 1970 {published data only}
- Bloomfield SS, Gaffney TE, Schiff GM. A design for the evaluation of antiviral drugs in human influenza. American Journal of Epidemiology 1970;91(6):568‐74. [CN‐00004457] [DOI] [PubMed] [Google Scholar]
Boltz 2010 {published data only}
- Boltz DA, Aldridge JR Jr, Webster RG, Govorkova EA. Drugs in development for influenza. Drugs 2010;70(11):1349‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brady 1990 {published data only}
- Brady MT, Sears SD, Pacini DL, Samorodin R, DePamphilis J, Oakes M, et al. Safety and prophylactic efficacy of low‐dose rimantadine in adults during an influenza A epidemic. Antimicrobial Agents and Chemotherapy 1990;34(9):1633‐6. [CN‐00073150] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brammer 2009 {published data only}
- Brammer L, Budd A, Cox N. Seasonal and pandemic influenza surveillance considerations for constructing multi component systems. Influenza and Other Respiratory Viruses 2009;3(2):51‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bricaire 1990 {published data only}
- Bricaire F, Hannoun C, Boissel JP. Prevention of influenza A. Effectiveness and tolerance of rimantadine hydrochloride [Prevention de la grippe A. Efficacite et tolerance du chlor hydrate de rimantadine]. Nouvelle Presse Médicale 1990;19(2):69‐72. [CN‐00065369] [PubMed] [Google Scholar]
Bryson 1990 {published data only}
- Bryson YJ, Monahan C, Pollack M, Shields WD. A prospective double‐blind study of side effects associated with the administration of amantadine for influenza A virus prophylaxis. Journal of Infectious Diseases 1980;141:543‐7. [CN‐00022658] [DOI] [PubMed] [Google Scholar]
Burch 2009 {published data only}
- Burch J, Paulden M, Conti S, Stock C, Corbett M, Welton NJ, et al. Antiviral drugs for the treatment of influenza: a systematic review and economic evaluation. Health Technology Assessment 2009;13(58):1‐265. [DOI] [PubMed] [Google Scholar]
Cady 2011 {published data only}
- Cady SD, Wang J, Wu Y, Degrado WF, Hong M. Specific binding of adamantane drugs and direction of their polar amines in the pore of the influenza M2 transmembrane domain in lipid bilayers and dodecylphosphocholine micelles determined by NMR spectroscopy. Journal of the American Chemical Society 2011;133(12):4274‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Callmander 1968 {published data only}
- Callmander E, Hellgren L. Amantadine hydrochloride as a prophylactic in respiratory infections. A double‐blind investigation of its clinical use and serology. Journal of Clinical Pharmacology and the Journal of New Drugs 1968;8(3):186‐9. [DOI] [PubMed] [Google Scholar]
Carter 2008 {published data only}
- Carter NJ, Plosker GL. Prepandemic influenza vaccine H5N1 (split virion, inactivated, adjuvanted) [Prepandrix]: a review of its use as an active immunization against influenza A subtype H5N1 virus. BioDrugs 2008;22(5):279‐92. [DOI] [PubMed] [Google Scholar]
Cayley 2010 {published data only}
- Cayley WE Jr. Neuraminidase inhibitors for influenza treatment and prevention in healthy adults. American Family Physician 2010;82(3):242‐4. [PubMed] [Google Scholar]
Cayley 2012 {published data only}
- Cayley Jr WE. Are neuraminidase inhibitors effective for preventing and treating influenza in healthy adults and children?. American Family Physician 2012;86:624‐6. [PubMed] [Google Scholar]
Chawla 2009 {published data only}
- Chawla R, Sharma RK, Bhardwaj JR. Influenza A (H1N1) outbreak and challenges for pharmacotherapy. Indian Journal of Physiology and Pharmacology 2009;53(2):113‐26. [PubMed] [Google Scholar]
Chemaly 2006 {published data only}
- Chemaly RF, Ghosh S, Bodey GP, Rohatgi N, Safdar A, Keating MJ el al. Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: a retrospective study at a major cancer center. Medicine (Baltimore) 2006;85(5):278‐87. [DOI] [PubMed] [Google Scholar]
Chen 2007 {published data only}
- Chen XY, Wu TX, Liu GJ, Wang Q, Zheng J, Wei J, et al. Chinese medicinal herbs for influenza. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD004559.pub3] [DOI] [PubMed] [Google Scholar]
Cheng 2004 {published data only}
- Cheng VCC, Tang BSF, Wu AKL, Chu CM, Yuen KY. Medical treatment of viral pneumonia including SARS in immunocompetent adult. Journal of Infection 2004;49(4):262‐73. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheng 2009 {published data only}
- Cheng HY. Assessing the quality of evidence from randomized, controlled dug and nutritional supplement trials conducted among nursing home residents between 1968 and 2004: what can we learn?. Journal of the American Medical Directors Association 2009;10(1):28‐35. [DOI] [PubMed] [Google Scholar]
Cheng 2012 {published data only}
- Cheng VCC, To KKW, Tse H, Hung IFN, Yuen KY. Two years after pandemic influenza A/2009/H1N1: What have we learned?. Clinical Microbiology Reviews 2012;25:223‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Choi 2009 {published data only}
- Choi WY, Kim SJ, Lee NJ, Kwon M, Yang IS, Kim Min‐Ji, et al. Amantadine‐resistant influenza A viruses isolated in South Korea from 2003 to 2009. Antiviral Research 2009;84(2):199‐202. [DOI] [PubMed] [Google Scholar]
Chou 2008 {published data only}
- Chou R, Carson S, Chan BKS. Pegylated interferons for chronic hepatitis C virus infection: an indirect analysis of randomized trials. Journal of Viral Hepatitis 2008;15(8):551‐70. [DOI] [PubMed] [Google Scholar]
Cohen 1976 {published data only}
- Cohen A, Togo Y, Khakoo R, Waldman R, Sigel M. Comparative clinical and laboratory evaluation of the prophylactic capacity of ribavirin, amantadine hydrochloride, and placebo in induced human influenza type A. Journal of Infectious Diseases 1976;133(Suppl):A114‐20. [CN‐0014092] [DOI] [PubMed] [Google Scholar]
Cohen 2006 {published data only}
- Cohen J, Cairns C, Paquette C, Faden L. Comparing patient access to pharmaceuticals in the UK and US. Applied Health Economics and Health Policy 2006;5(3):177‐87. [DOI] [PubMed] [Google Scholar]
Cowling 2008 {published data only}
- Cowling BJ, Fung ROP, Cheng CKY, Fang VJ, Chan KH, Seto WH, et al. Preliminary findings of a randomized trial of non‐pharmaceutical interventions to prevent influenza transmission in households. PloS One 2008;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Curran 2010 {published data only}
- Curran MP, Leroux‐Roels I. Inactivated split‐virion seasonal influenza vaccine (Fluarix®): a review of its use in the prevention of seasonal influenza in adults and the elderly. Drugs 2010;70(12):1519‐43. [DOI] [PubMed] [Google Scholar]
Dawkins 1968 {published data only}
- Dawkins AT Jr, Gallager LR, Togo Y, Hornick RB, Harris BA. Studies on induced influenza in man. II. Double‐blind study designed to assess the prophylactic efficacy of an analogue of amantadine hydrochloride. JAMA 1968;203:1095‐9. [CN‐00001969] [DOI] [PubMed] [Google Scholar]
De la Camara 2007 {published data only}
- Camara R, Lopez‐Jimenez J, Vallejo C, Vazquez L, Luis Perez J, Varo E, et al. Update on viral infections in immunocompromised patients. Enfermedades Infecciosas y Microbiologia Clinica 2007;25(Suppl 1):2‐11. [Google Scholar]
DeLaney 2010 {published data only}
- DeLaney E, Smith MJ, Harvey BT, Pelletier KJ, Aquino MP, Stone JM, et al. Extracorporeal life support for pandemic influenza: the role of extracorporeal membrane oxygenation in pandemic management. Journal of Extra‐Corporeal Technology 2010;42(4):268‐80. [PMC free article] [PubMed] [Google Scholar]
Denys 1963 {published data only}
- Denys A, Szram S, Tkaczewski W, Niedzielska H, Bochenska J, Kulawczyk M, et al. Antiviral activity of rimantadine, virological, pathomorphological, and clinical studies. Acta Microbiologica Polonica. Series A: Microbiologia Generalis 1973;5:217‐20. [CN‐00009559] [PubMed] [Google Scholar]
De Vincenzo 2012 {published data only}
- Vincenzo JP. The promise, pitfalls and progress of RNA‐interference‐based antiviral therapy for respiratory viruses. Antiviral Therapy 2012;17:213‐25. [DOI] [PubMed] [Google Scholar]
Dolamore 2003 {published data only}
- Dolamore MJ. Influenza prophylaxis in long‐tern care facility: a case‐control study of the risk factors for adverse drug reactions to amantadine. Current Therapeutic Research, Clinical and Experimental 2003;64(9):753. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dolin 1982 {published data only}
- Dolin R, Reichman RC, Madore HP, Maynard R, Linton PN, Webber‐Jones J. A controlled trial of amantadine and rimantadine in the prophylaxis of influenza A infection. New England Journal of Medicine 1982;307(10):580‐4. [CN‐00201667] [DOI] [PubMed] [Google Scholar]
Doyle 1998 {published data only}
- Doyle WJ, Skoner DP, Patel A, Hayden FG. Effect of rimantadine on induced specific serum haemagglutination‐inhibiting antibody and nasal IgA titres after experimental exposure of adults to influenza A virus. Antiviral Therapy 1998;3(1):19‐23. [CN‐00201667] [Google Scholar]
Drinevskii 1998 {published data only}
- Drinevskii VP, Osidak LV, Natsina VK, Afanas'eva OI, Mil'kint KK, Danini GV, et al. Chemotherapeutics for treatment of influenza and other viral respiratory tract infections in children [Khimiopreparaty v terapii grippa i drugikh respiratomykh infektsii u detei]. Antibiotiki i Khimioterapiia 1998;43(9):29‐34. [CN‐00332458] [PubMed] [Google Scholar]
Drinka 1998 {published data only}
- Drinka PJ, Gravenstein S, Schilling M, Krause P, Miller BA, Shult P. Duration of antiviral prophylaxis during nursing home outbreaks of influenza. A comparison of 2 protocols. Archives of Internal Medicine 1998;158(19):2155‐9. [CN‐00156407] [DOI] [PubMed] [Google Scholar]
Enger 2004 {published data only}
- Enger C, Nordstrom BL, Thakrar B, Sacks S, Rothman KJ. Health outcomes among patients receiving oseltamivir. Pharmacoepidemiology and Drug Safety 2004;13(4):227‐37. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Escuret 2012 {published data only}
- Escuret V, Cornu C, Boutitie F, Enouf V, Mosnier A, Bouscambert‐Duchamp M, et al. Oseltamivir‐zanamivir bitherapy compared to oseltamivir monotherapy in the treatment of pandemic 2009 influenza A (H1N1) virus infections. Antiviral Research 2012;96:130‐7. [DOI] [PubMed] [Google Scholar]
Falagas 2010 {published data only}
- Falagas ME, Vouloumanou EK, Baskouta E, Rafailidis PI, Polyzos K, Rello J. Treatment options for 2009 H1N1 influenza: evaluation of the published evidence. International Journal of Antimicrobial Agents 2010;35(5):421‐30. [DOI] [PubMed] [Google Scholar]
Farlow 2008 {published data only}
- Farlow MR, Graham SM, Alva G. Memantine for the treatment of Alzheimer's disease: tolerability and safety data from clinical trials. Drug Safety 2008;31(7):577‐85. [DOI] [PubMed] [Google Scholar]
Fiore 2008 {published data only}
- Fiore C, Eisenhut M, Krausse R, Ragazzi E, Pellati D, Armanini D, et al. Antiviral effects of Glycyrrhiza species. Phytotherapy Research 2008;22(2):141‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Furuta 2005 {published data only}
- Furuta Y, Takahashi K, Kuno‐Markawa M, Sangawa H, Uehara S, Kozaki K el al. Mechanism of action of T‐705 against influenza virus. Antimicrobial Agents and Chemotherapy 2005;49(3):981‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Galabov 2006 {published data only}
- Galabov AS, Simeonova L, Gegova G. Rimantadine and oseltamivir demonstrate synergistic combination effect in an experimental infection with type A (H3N2) influenza virus in mice. Antiviral Chemistry & Chemotherapy 2006;17(5):251‐8. [DOI] [PubMed] [Google Scholar]
Galbraith 1969a {published data only}
- Galbraith AW, Oxford JS, Schild GC, Watson GI. Protective effect of 1‐adamantanamine hydrochloride on influenza A2 infections in the family environment: a controlled double‐blind study. Lancet 1969;2(7629):1026‐8. [CN‐00003903] [DOI] [PubMed] [Google Scholar]
Galbraith 1969b {published data only}
- Galbraith AW, Oxford JS, Schild GC, Watson GI. Study of 1‐adamantanamine hydrochloride used prophylactically during the Hong‐Kong influenza epidemic in the family environment. Bulletin of the World Health Organization 1969;41(3):677‐82. [00004308] [PMC free article] [PubMed] [Google Scholar]
Galbraith 1971 {published data only}
- Galbraith AW, Oxford JS, Schild GC, Potter CW, Watson GI. Therapeutic effect of 1‐adamantanamine hydrochloride in naturally occurring influenza A 2‐Hong Kong infection. A controlled double‐blind study. Lancet 1971;2(7716):113‐5. [00006011] [DOI] [PubMed] [Google Scholar]
Galbraith 1973 {published data only}
- Galbraith AW, Schild GC, Potter CW, Watson GI. The therapeutic effect of amantadine in influenza occurring during the winter of 1971‐2 assessed by double‐blind study. Journal of the Royal College of General Practitioners 1973;23(126):34‐7. [CN‐00008567] [PMC free article] [PubMed] [Google Scholar]
Garman 2004 {published data only}
- Garman E, Laver G. Controlling influenza by inhibiting the virus's neuramidase. Lancet 2004;363(9409):617‐9. [MEDLINE: ] [Google Scholar]
Gatwood 2012 {published data only}
- Gatwood J, Meltzer MI, Messonnier M, Ortega‐Sanchez IR, Balkrishnan R, Prosser LA. Seasonal influenza vaccination of healthy working‐age adults: a review of economic evaluations. Drugs 2012;72:35‐48. [DOI] [PubMed] [Google Scholar]
Gerth 1966 {published data only}
- Gerth HJ. Influenza prevention with 1‐amino‐adamantan‐hydrochloride.II [Grippeprophylaxe mit 1‐Amino‐adamantan‐hydrochlorid]. Die Medizinische Welt 1966;2:96‐100. [CN‐00000687] [PubMed] [Google Scholar]
Griffin 2004 {published data only}
- Griffin SDC, Harvey R, Clarke DS, Barclay WS, Harris M, Rowlands DJ. A conserved basic loop in hepatitis C virus p7 protein is required for amantadine‐sensitive ion channel activity in mammalian cells but is dispensable for localization to mitochondria. Journal of General Virology 2004;85(2):451‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guo 2007 {published data only}
- Guo R, Pittler MH, Ernst E. Complementary medicine for treating or preventing influenza or influenza‐like illness. American Journal of Medicine 2007;120(11):923‐9. [DOI] [PubMed] [Google Scholar]
Hay 1986 {published data only}
- Hay AJ, Zambon MC, Wolstenholme AJ, Skehel JJ, Smith MH. Molecular basis of resistance of influenza A viruses to amantadine. Journal of Antimicrobial Chemotherapy 1986;18:19‐29. [00341317] [DOI] [PubMed] [Google Scholar]
Hayden 1979 {published data only}
- Hayden FG, Hall WJ, Douglas RGJ, Speers DM. Amantadine aerosols in normal volunteers; pharmacology and safety testing. Antimicrobial Agents and Chemotherapy 1979;16(5):644‐50. [CN‐00341319] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hayden 1980 {published data only}
- Hayden FG, Hall WJ, Douglas FG Jr. Therapeutic effects of aerolized amantadine in naturally acquired infection due to influenza A virus. Journal of Infectious Diseases 1980;141(5):535‐42. [CN‐00022657] [DOI] [PubMed] [Google Scholar]
Hayden 1981 {published data only}
- Hayden FG, Gwaltney JM Jr, Castle RL, Adams KF, Giordani F. Comparative toxicity of amantadine hydrochloride and rimantadine hydrochloride in healthy adults. Antimicrobial Agents and Chemotherapy 1981;19(2):226‐33. [CN‐00029040] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hayden 1982 {published data only}
- Hayden FG, Zylidnikov DM, Iljenko VI, Padolka YV. Comparative therapeutic effect of aerolized and oral rimantadine HCl in experimental human influenza A virus infection. Antiviral Research 1982;2(3):147‐53. [CN‐00029306] [DOI] [PubMed] [Google Scholar]
Hayden 1985 {published data only}
- Hayden FG, Minocha A, Spyker DA, Hoffman HE. Comparative single‐dose pharmacokinetics of amantadine hydrochloride and rimantadine hydrochloride in young and elderly adults. Antimicrobial Agents and Chemotherapy 1985;28(2):216‐21. [CN‐00341320] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hayden 1986 {published data only}
- Hayden FG, Monto AS. Oral rimantadine hydrochloride therapy of influenza A virus H3N2 subtype infection in adults. Antimicrobial Agents and Chemotherapy 1986;29(2):339‐41. [CN‐0017 4577] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hayden 1989 {published data only}
- Hayden FG, Belshe RB, Clover RB, Hay AJ, Oakes MG, Soo W. Emergence and apparent transmission of rimantadine‐resistant influenza A virus in families. New England Journal of Medicine 1989;321(25):1696‐702. [CN‐00064208] [DOI] [PubMed] [Google Scholar]
Hayden 1991 {published data only}
- Hayden FG, Sperber SJ, Belshe RB, Clover RD, Hay AJ, Pyke S. Recovery of drug‐resistant influenza A virus during therapeutic use of rimantadine. Antimicrobial Agents and Chemotherapy 1991;35:1741‐7. [CN‐00079594] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hayden 2000 {published data only}
- Hayden FG, Gubareva LV, Monto AS, Klein TC, Elliott MJ, Hammond JM, et al. Inhaled zanamivir for the prevention of influenza in families. New England Journal of Medicine 2000;343(18):1282‐9. [CN‐00399563] [DOI] [PubMed] [Google Scholar]
Hayden 2006 {published data only}
- Hayden FG, Pavia AT. Antiviral management of seasonal and pandemic influenza. Journal of Infectious Diseases 2006;194(Suppl 2):155‐61. [DOI] [PubMed] [Google Scholar]
Hayden 2012 {published data only}
- Hayden FG. Experimental human influenza: Observations from studies of influenza antivirals. Antiviral Therapy 2012;17:133‐41. [DOI] [PubMed] [Google Scholar]
Hornick 1969 {published data only}
- Hornick RB, Togo Y, Mahler S, Iezzoni D. Evaluation of amantadine hydrochloride in the treatment of A2 influenzal disease. Bulletin of the World Health Organization 1969;41(3):671‐6. [CN‐00004307] [PMC free article] [PubMed] [Google Scholar]
Hota 2007 {published data only}
- Hota S, McGeer A. Antivirals and the control of influenza outbreaks. Clinical Infectious Diseases 2007;45(10):1362‐8. [DOI] [PubMed] [Google Scholar]
Hout 2006a {published data only}
- Hout DR, Gomez LM, Pacyniak E, Miller JM, Hill MS, Stephens EB. A single amino acid substitution within the transmembrane domain of the human immunodeficiency virus type 1 Vpu protein renders simian‐human immunodeficiency virus (SHIV(KU‐1bMC33)) susceptible to rimantadine. Virology 2006;348(2):449‐61. [DOI] [PubMed] [Google Scholar]
Hout 2006b {published data only}
- Hout DR, Gomez ML, Pacyniak E, Gomez LM, Fegley B, Mulcahy ER el al. Substitution of the transmembrane domain of Vpu in simian‐human immunodeficiency virus (SHIVKU1bMC33) with that of M2 of influenza A results in a virus that is sensitive to inhibitors of the M2 ion channel and is pathogenic for pig‐tailed macaques. Virology 2006;344(2):541‐59. [DOI] [PubMed] [Google Scholar]
Hsu 2012 {published data only}
- Hsu J, Santesso N, Mustafa R, Brozek J, Chen YL, Hopkins JP, et al. Antivirals for treatment of influenza: a systematic review and meta‐analysis of observational studies. Annals of Internal Medicine 2012;156:512‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hurt 2007 {published data only}
- Hurt AC, Selleck P, Komadina S, Shaw R, Brown L, Barr IG. Susceptibility of highly pathogenic A(H5N1) avian influenza viruses to the neuraminidase inhibitors and adamantanes. Antiviral Research 2007;73(3):228‐31. [DOI] [PubMed] [Google Scholar]
Ilyushina 2005 {published data only}
- Ilyushina NA, Govorkova EA, Webster RG. Detection of amantadine‐resistant variants among avian influenza viruses isolated in North America and Asia. Virology 2005;341(1):102‐6. [DOI] [PubMed] [Google Scholar]
Ilyushina 2006 {published data only}
- IIyushina NA, Bovin NV, Webster RG, Govorkova EA. Combination chemotherapy, a potential strategy for reducing the emergence of drug‐resistant influenza A variants. Antiviral Research 2006;70(3):121‐31. [DOI] [PubMed] [Google Scholar]
Ilyushina 2007a {published data only}
- Ilyushina NA, Gorkova EA, Russell CJ, Hoffmann E, Webster RG. Contribution of H7 haemagglutinin to amantadine resistance and infectivity of influenza virus. Journal of General Virology 2007;88(4):1266‐74. [DOI] [PubMed] [Google Scholar]
Ilyushina 2007b {published data only}
- Ilyushina NA, Hoffmann E, Solomon R, Webster RG, Govorkova EA. Amantadine‐oseltamivir combination therapy for H5N1 influenza virus infection in mice. Antiviral Therapy 2007;12(3):363‐70. [PubMed] [Google Scholar]
Ison 2006 {published data only}
- Ison MG, Gubareva LV, Atmar RL, Treanor J, Hayden FG. Recovery of drug‐resistant influenza virus from immunocompromised patients: a case series. Journal of Infectious Diseases 2006;193(6):760‐4. [DOI] [PubMed] [Google Scholar]
Ison 2013 {published data only}
- Ison MG. Clinical use of approved influenza antivirals: therapy and prophylaxis. Influenza & Other Respiratory Viruses 2013;7(Suppl 1):7‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ito 2000 {published data only}
- Ito S, Iijima N, Kanemaki K, Hayashi S, Hujii S, Watanabe T, et al. Therapeutic efficacy of amantadine hydrochloride in patients with epidemic influenza A virus infection. Nihon Kokyuki Gakkai Zasshi [Journal of the Japanese Respiratory Society] 2000;38(12):897‐902. [CN: 00327106] [PubMed] [Google Scholar]
Ito 2006 {published data only}
- Ito Y, Sumi H, Kato T. Evaluation of influenza vaccination in health care workers, using rapid antigen detection test. Journal of Infection and Chemotherapy: Official Journal of the Japan Society of Chemotherapy 2006;12(2):70‐2. [DOI] [PubMed] [Google Scholar]
Jefferson 2006a {published data only}
- Jefferson T, Demicheli V, Rivetti D, Jones M, Pietrantonj C, Rivetti A. Antivirals for influenza in healthy adults: systematic review. Lancet 2006;367(9507):303‐13. [DOI] [PubMed] [Google Scholar]
Jiang 2013 {published data only}
- Jiang L, Deng L, Wu T. Chinese medicinal herbs for influenza. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD004559.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2006 {published data only}
- Jones JC, Turpin EA, Bultmann H, Brandt CR, Schultz‐Cherry S. Inhibition of influenza virus infection by a novel antiviral peptide that targets viral attachment to cells. Journal of Virology 2006;80(24):11960‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalia 2008 {published data only}
- Kalia LV, Kalia SK, Salter MW. NMDA receptors in clinical neurology: excitatory times ahead. Lancet Neurology 2008;7(8):742‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kantor 1980 {published data only}
- Kantor RJ, Stevens D, Potts DW, Noble GR. Prevention of influenza A/USSR/77 (H1N1): an evaluation of the side effects and efficacy of amantadine in recruits at Fort Sam Houston. Military Medicine 1980;145(5):312‐5. [CN‐00176323] [PubMed] [Google Scholar]
Kawai 2005 {published data only}
- Kawai N, Ikematsu H, Iwaki N, Satoh I, Kawashima T, Maeda T, et al. Factors influencing the effects of oseltamivir and amantadine for the treatment of influenza: a multicenter study from Japan of the 2002‐2003 influenza season. Clinical Infectious Diseases 2005;40:1309‐16. [DOI] [PubMed] [Google Scholar]
Khakoo 1981 {published data only}
- Khakoo RA, Watson GW, Waldman RH, Ganguly R. Effect of inosiplex (Isoprinosine (Reg. trademark)) on induced human influenza A infection. Journal of Antimicrobial Chemotherapy 1981;7(4):389‐97. [CN‐00192285] [DOI] [PubMed] [Google Scholar]
Kim 2011 {published data only}
- Kim SH, Hong SB, Yun SC, Choi WI, Ahn JJ, Lee YJ, et al. Corticosteroid treatment in critically ill patients with pandemic influenza A/H1N1 2009 infection: analytic strategy using propensity scores. American Journal of Respiratory and Critical Care Medicine 2011;183(9):1207‐14. [DOI] [PubMed] [Google Scholar]
Kirkby 2010 {published data only}
- Kirkby R, Calabrese C, Kaltman L, Monnier J, Herscu P. Methodological considerations for future controlled influenza treatment trials in complementary and alternative medicine. Journal of Alternative and Complementary Medicine 2010;16(3):275‐83. [DOI] [PubMed] [Google Scholar]
Kiso 2004 {published data only}
- Kiso M, Mitamura K, Sakai‐Tagawa Y, Shiraishi K, Kawakami C, Kimura K, et al. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet 2004;364(9436):759‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kitamoto 1969 {published data only}
- Kitamoto O. Early diagnosis and treatment of influenza. Naika. Internal Medicine 1969;23(6):1271‐6. [CN‐00003590] [PubMed] [Google Scholar]
Knight 1969 {published data only}
- Knight V, Fedson D, Baldini J, Douglas RG, Couch RB. Amantadine therapy of epidemic influenza A2‐Hong Kong. Antimicrobial Agents and Chemotherapy 1969;9:370‐1. [CN‐00320019] [PubMed] [Google Scholar]
Knight 1970a {published data only}
- Knight V, Fedson D, Baldini J, Douglas R, Couch R. Amantadine therapy of epidemic influenza A (Hong Kong). Infection and Immunity 1970;1:200‐4. [00203595] [DOI] [PMC free article] [PubMed] [Google Scholar]
Knight 1970b {published data only}
- Knight V, Fedson D, Baldini J, Douglas R, Couch R. Amantadine therapy of epidemic influenza A (Hong Kong). Infection and Immunity 1970;1:200‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knight 1981 {published data only}
- Knight V, McClung HW, Wilson SZ, Waters BK, Quarles JM, Cameron RW, et al. Ribavirin small‐particle aerosol treatment of influenza. Lancet 1981;2(8253):945‐9. [CN‐00026398] [DOI] [PubMed] [Google Scholar]
Korenke 2008 {published data only}
- Korenke AR, Rivey MP, Allington DR. Sustained‐release fampridine for symptomatic treatment of multiple sclerosis. Annals of Pharmacotherapy 2008;42(10):1458‐65. [DOI] [PubMed] [Google Scholar]
Krylov 1978 {published data only}
- Krylov VF, Ketilladze ES, Smagulova EG, Alekseeva AA, Nefelova MM. Use of rimantadine in familial foci during an epidemic of influenza caused by A1 virus [Primenenie rimantadina v semeinykh ochagakh v period epidemii grippa, vyzvannogo virusom A1]. Voprosy Virusologii 1978;3:277‐82. [CN‐00018933] [PubMed] [Google Scholar]
Kulichenko 2003 {published data only}
- Kulichenko LL, Kireyeva LV, Malyshikina EN, Wikman G. A randomized controlled study of Kan Jang versus amantadine in the treatment of influenza in Volgograd. Journal of Herbal Pharmacotherapy 2003;3(1):77‐93. [CN‐00473824] [PubMed] [Google Scholar]
Langlet 2009 {published data only}
- Langlet P, D'Heygere F, Henrion J, Adler M, Delwaide J, Van Vlierberghe H et al. Clinical trial: a randomized trial of pegylated‐interferon‐alpha‐2a plus ribavirin with or without amantadine in treatment‐naive or relapsing chronic hepatitis C patients. Alimentary Pharmacology & Therapeutics 2009;30(4):352‐63. [DOI] [PubMed] [Google Scholar]
Leeming 1969 {published data only}
- Leeming JT. Amantadine hydrochloride and the elderly. British Medical Journal 1969;1(639):313‐4. [CN‐00002734] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leone 2005 {published data only}
- Leone H, Polsonetti BW. Amantadine for traumatic brain injury: does it improve cognition and reduce agitation?. Journal of Clinical Pharmacy and Therapeutics 2005;30(2):101‐4. [DOI] [PubMed] [Google Scholar]
Le Tissier 2005 {published data only}
- Tissier PR, Carmignac DF, Lilley S, Sesay AK, Phelps CJ, Houston P, et al. Hypothalamic growth hormone‐releasing hormone (GHRH) deficiency: target ablation pf GHRH neurons in mice using a viral ion channel transgene. Molecular Endocrinology 2005;19(5):1251‐62. [DOI] [PubMed] [Google Scholar]
Leung 1979 {published data only}
- Leung P, McIntosh K, Chai H. Amantadine prophylaxis against influenza A/USSR in children with chronic asthma. Journal of Allergy and Clinical Immunology 1979;63(3):140. [CN‐00353210] [Google Scholar]
Lim 2007 {published data only}
- Lim WS. Pandemic flu: clinical management of patients with an influenza‐like illness during an influenza pandemic. Thorax 2007;62(Suppl 1):1‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2006 {published data only}
- Lin CH, Huang YC, Chi CH, Huang CG, Tsao KC, Lin TY. Neurologic manifestations in children with influenza B virus infection. Pediatric Infectious Disease Journal 2006;25(11):1081‐3. [DOI] [PubMed] [Google Scholar]
Linder 2005 {published data only}
- Linder JA, Bates DW, Platt R. Antivirals and antibiotics for influenza in the United States, 1995‐2002. Pharmacoepidemiology and Drug Safety 2005;14(8):531‐6. [DOI] [PubMed] [Google Scholar]
Lipatov 2007 {published data only}
- Lipatov AS, Evseenko VA, Yen HL, Zaykovskaya AV, Durimanov AG, Zolotykh SI, et al. Influenza (H5N1) viruses in poultry, Russian Federation, 2005‐2006. Emerging Infectious Diseases 2007;13(4):539‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Little 1976 {published data only}
- Little JW, Hall WJ, Douglas RG Jr, Hyde RW, Speers DM. Amantadine effect on peripheral airways abnormalities in influenza A study in 15 students with natural influenza A infection. Annals of Internal Medicine 1976;85(2):177‐82. [CN‐00014385] [DOI] [PubMed] [Google Scholar]
Little 1978 {published data only}
- Little JW, Hall WJ, Douglas RG Jr, Mudholkar GS, Speer DM, Patel K. Airway hyperreactivity and peripheral airway dysfunction in influenza A infection. American Review of Respiratory Disease 1978;118(2):295‐303. [CN‐00018956] [DOI] [PubMed] [Google Scholar]
Lopez‐Medrano 2012 {published data only}
- Lopez‐Medrano F, Carmen Farinas M, Payeras A, Pachon J. Antiviral treatment and vaccination for influenza A (H1N1) pdm09 virus: lessons learned from the pandemic. Enfermedades Infecciosas y Microbiologia Clinica 2012;30:49‐53. [DOI] [PubMed] [Google Scholar]
Louie 2012 {published data only}
- Louie JK, Yang S, Yen C, Acosta M, Schechter R, Uyeki TM. Use of intravenous peramivir for treatment of severe influenza A (H1N1) pdm09. PloS One 2012;7(6):e40261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lutz 2005 {published data only}
- Lutz A, Dyall J, Olivo PD, Pekosz A. Virus‐inducible reporter genes as a tool for detecting and quantifying influenza A virus replication. Journal of Virological Methods 2005;126(1‐2):13‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lynd 2005 {published data only}
- Lynd LD, Goeree R, O'Brien BJ. Antiviral agents for influenza: a comparison of cost‐effectiveness data. PharmacoEconomics 2005;23(11):1083‐106. [DOI] [PubMed] [Google Scholar]
Machado 2004 {published data only}
- Machado CM, Boas LSV, Mendes AVA, Rocha IF, Sturaro D, Dulley FL, et al. Use of oseltamivir to control influenza complications after bone marrow transplantation. Bone Marrow Transplantation 2004;34(2):111‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mallia 2007 {published data only}
- Mallia P, Johnston SL. Influenza infection and COPD. International Journal of Chronic Obstructive Pulmonary Disease 2007;2(1):55‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maricich 2004 {published data only}
- Maricich SM, Neul JL, Lotze TE, Cazacu AC, Uyeki TM, Demmler GJ, et al. Neurologic complications associated with influenza A in children during the 2003‐2004 influenza season in Houston, Texas. Pediatrics 2004;114(5):626‐33. [DOI] [PubMed] [Google Scholar]
Mase 2007 {published data only}
- Mase M, Eto M, Imai K, Tsukamoto K, Yamaguchi S. Characterization of H9N2 influenza A viruses isolated from chicken products imported into Japan from China. Epidemiology and Infection 2007;135(3):386‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mate 1970 {published data only}
- Mate J, Simon M, Juvancz I, Takatsy G, Hollos I, Farkas E. Prophylactic use of amantadine during Hong Kong influenza epidemic. Acta Microbiologica Academiae Scientiarum Hungaricae 1970;17(3):285‐96. [PubMed] [Google Scholar]
Mate 1971 {published data only}
- Mate J, Simon M, Juvancz I. Use of Viregyt (amantadine hydrochloride) in the treatment of epidemic influenza. Therapia Hungarica 1971;19(3):117‐21. [CN‐00006813] [PubMed] [Google Scholar]
Matheson 2007 {published data only}
- Matheson NJ, Harnden AR, Perera R, Sheikh A, Symmonds‐Abrahams M. Neuraminidase inhibitors for preventing and treating influenza in children. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD002744.pub2] [DOI] [PubMed] [Google Scholar]
Matsuya 2007 {published data only}
- Matsuya Y, Sasaki K, Ochiai H, Nemoto H. Synthesis and biological evaluation of dihydrofuran‐fused perhydrophenanthrenes as a new anti‐influenza agent having novel structural characteristic. Bioorganic and Medicinal Chemistry 2007;15(1):424‐32. [DOI] [PubMed] [Google Scholar]
Matthews 2004 {published data only}
- Matthews SJ, McCoy C. Peginterferon alfa‐2a: a review of approved and investigational uses. Clinical Therapeutics 2004;26(7):991‐1025. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McCullers 2004 {published data only}
- McCullers JA. Effect of antiviral treatment on the outcome of secondary bacterial pneumonia after influenza. Journal of Infectious Diseases 2004;190(3):519‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McKay 2006 {published data only}
- McKay T, Patel M, Pickles RJ, Johnson LG, Olsen JC. Influenza M2 envelope protein augments avian influenza hemagglutinin pseudotyping of lentiviral vectors. Gene Therapy 2006;13(8):715‐24. [DOI] [PubMed] [Google Scholar]
Michiels 2013 {published data only}
- Michiels B, Puyenbroeck K, Verhoeven V, Vermeire E, Coenen S. The value of neuraminidase inhibitors for the prevention and treatment of seasonal influenza: a systematic review of systematic reviews. PloS One 2013;8(4):e60348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mishin 2005 {published data only}
- Mishin VP, Hayden FG, Gubareva LV. Susceptibilities of antiviral‐resistant influenza viruses to novel neuraminidase inhibitors. Antimicrobial Agents and Chemotherapy 2005;49(11):4515‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miyachi 2011 {published data only}
- Miyachi K, Watanabe A, Iida H, Hattori H, Ukai H, Takano T, et al. Comparison of the efficacies of amantadine treatment of swine‐origin influenza virus A H1N1 and seasonal influenza H1N1 and H3N2 in Japan (2008‐2009). Journal of Infection and Chemotherapy: official journal of the Japan Society of Chemotherapy 2011;17(4):524‐9. [DOI] [PubMed] [Google Scholar]
Moffat 2008 {published data only}
- Moffat JC, Vijayvergiya V, Gao PF, Cross TA, Woodbury DJ, Busath DD. Proton transport through influenza A virus M2 protein reconstituted in vesicles. Biophysical Journal 2008;94(2):434‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Monto 1979 {published data only}
- Monto AS, Gunn RA, Bandyk MG, King CL. Prevention of Russian influenza by amantadine. JAMA 1979;241(10):1003‐7. [PubMed] [Google Scholar]
Morrison 2007 {published data only}
- Morrison D, Roy S, Rayner C, Amer A, Howard D, Smith JR, et al. A randomized, crossover study to evaluate the pharmacokinetics of amantadine and oseltamivir administered alone and in combination. PLoS One 2007;2(12):e1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muldoon 1976 {published data only}
- Muldoon RL, Stanley ED, Jackson GG. Use and withdrawal of amantadine chemoprophylaxis during epidemic influenza A. American Review of Respiratory Disease 1976;113:487‐91. [CN‐00203757] [DOI] [PubMed] [Google Scholar]
Nafta 1970 {published data only}
- Nafta I, Turcanu AG, Braun I, Companetz W, Simionescu A. Administration of amantadine for the prevention of Hong‐Kong influenza. Bulletin of the World Health Organization 1970;42(3):200‐4. [CN‐00004499] [PMC free article] [PubMed] [Google Scholar]
Natsina 1994 {published data only}
- Natsina VK, Drinevskii VP, Milkint KK. Remantadine in the treatment of influenza in children [Primenenie remantadina dlia lecheniia grippa u detei]. Vestnik Rossiiskoi Akademii Meditsinskikh Nauk Vestnik 1994;9:51‐5. [CN‐00110913] [PubMed] [Google Scholar]
Nuesch 2007 {published data only}
- Nuesch R. Antiviral treatment of influenza in humans. Therapeutische Umschau 2007;64(11):635‐41. [DOI] [PubMed] [Google Scholar]
O'Donoghute 1973 {published data only}
- O'Donoghute JM, Ray CG, Terry DW Jr, Beaty HN. Prevention of nosocomial influenza infection with amantadine. American Journal of Epidemiology. 1973;97(4):276‐82. [CN‐00008489] [DOI] [PubMed] [Google Scholar]
Obrosova‐Serova 1972 {published data only}
- Obrosova‐Serova NP, Fedorova GI, Glukhov PI, Shal'nov MI, Litvinov VG. Effectiveness of midantan and interferon inducers as means of non‐specific prevention of influenza [Izuchenie effektivnosti midantana i stimuliatorav interferona kak sredstv nespetsificheskoi profilaktiki grippa]. Antibiotiki 1972;17:734‐8. [CN‐00007887] [PubMed] [Google Scholar]
Oker‐Blom 1970 {published data only}
- Oker‐Blom N, Hovi T, Leinikki P, Palosuo T, Petterson R, Suni J. Protection of man from natural infection with influenza A2 Hong Kong virus by amantadine: a controlled field trial. British Medical Journal 1970;3(724):676‐8. [CN‐00004991] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ong 2007 {published data only}
- Ong AK, Hayden FG. John F. Enders lecture 2006: antivirals for influenza. Journal of Infectious Diseases 2007;196(2):181‐90. [DOI] [PubMed] [Google Scholar]
Pachucki 2004 {published data only}
- Pachucki CT, Kurshid MA, Nawrocki J. Utility of reverse transcriptase PCR for rapid diagnosis of influenza A virus infection and detection of amantadine‐resistant influenza A virus isolates. Journal of Clinical Microbiology 2004;42(6):2796‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peiris 2004 {published data only}
- Peiris JS, Yu WC, Leung CW, Cheung CY, Ng WF, Nicholls JM, et al. Re‐emergence of fatal human influenza A subtype H5N1 disease. Lancet 2004;21(363):617‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pemberton 1986 {published data only}
- Pemberton RM, Jennings R, Potter CW, Oxford JS. Amantadine resistance in clinical influenza A (H3N2) and (H1N1) virus isolates. Journal of Antimicrobial Chemotherapy 1986;18:135‐40. [CN‐00341484] [DOI] [PubMed] [Google Scholar]
Petterson 1980 {published data only}
- Petterson RF, Hellstrom PE, Penttinen K, Pyhala R, Tokola O, Vartio T, et al. Evaluation of amantadine in the prophylaxis of influenza A (H1N1) virus infection: a controlled field trial among young adults and high‐risk patients. Journal of Infectious Diseases 1980;142(3):377‐83. [CN‐00023981] [DOI] [PubMed] [Google Scholar]
Pritchard 1989 {published data only}
- Pritchard MH, Munro J. Successful treatment of juvenile chronic arthritis with a specific antiviral agent. British Journal of Rheumatology 1989;28(6):521‐4. [CN‐00064111] [DOI] [PubMed] [Google Scholar]
Quarles 1981 {published data only}
- Quarles JM, Couch RB, Cate TR, Goswick CB. Comparison of amantadine and rimantadine for prevention of type A (Russian) influenza. Antiviral Research 1981;1(3):149‐55. [CN‐00193509] [DOI] [PubMed] [Google Scholar]
Quilligan 1966 {published data only}
- Quilligan JJ Jr, Hirayama H, Baernstein HD Jr. The suppression of A2 influenza in children by the chemoprophylactic use of amantadine. Journal of Pediatrics 1966;69(4):572‐5. [CN‐00000801] [DOI] [PubMed] [Google Scholar]
Rabinovich 1969 {published data only}
- Rabinovich S, Baldini JT, Bannister R. Treatment of influenza. The therapeutic efficacy of rimantadine HCl in a naturally occurring influenza A2 outbreak. American Journal of the Medical Sciences 1969;257(5):328‐35. [CN‐00003292] [DOI] [PubMed] [Google Scholar]
Reis 2006 {published data only}
- Reis J, John D, Heimeroth A, Mueller HH, Oertel WH, Arndt T, et al. Modulation of human motor cortex excitability by single doses of amantadine. Neuropsychopharmacology 2006;31(12):2758‐66. [DOI] [PubMed] [Google Scholar]
Reuman 1989a {published data only}
- Reuman PD, Bernstein DI, Keely SP, Young EC, Sherwood, JR, Schiff GM. Differential effect of amantadine hydrochloride on systemic and local immune response to influenza A. Journal of Medical Virology 1989;27(2):137‐41. [CN‐00058423] [DOI] [PubMed] [Google Scholar]
Reuman 1989b {published data only}
- Reuman PD, Bernstein DI, Keefer MC, Young EC, Sherwood JR, Schiff GM. Efficacy and safety of low dosage amantadine hydrochloride as prophylaxis for influenza A. Antiviral Research 1989;11(1):27‐40. [CN‐00059636] [DOI] [PubMed] [Google Scholar]
Risenbrough 2005 {published data only}
- Risenbrough NA, Bowles SK, Simor AE, McGeer A, Oh PI. Economic evaluation of oseltamivir phosphate for postexposure prophylaxis of influenza in long‐term care facilities. Journal of the American Geriatrics Society 2005;53(3):444‐51. [DOI] [PubMed] [Google Scholar]
Rose 1980 {published data only}
- Rose HJ. Therapeutic effects of aerosolized amantadine in naturally acquired infection due to influenza A virus. Journal of Infectious Diseases 1980;141(5):535‐42. [CN‐00022657] [DOI] [PubMed] [Google Scholar]
Rothberg 2005 {published data only}
- Rothberg MB, Fisher D, Kelly B, Rose DN. Management of influenza symptoms in healthy children. Archives of Pediatrics & Adolescent Medicine 2005;159:1055‐62. [DOI] [PubMed] [Google Scholar]
Saito 2006 {published data only}
- Saito R, Li D, Shimomura C, Masaki H, Le MR, Nquyen HL, et al. An off‐seasonal amantadine‐resistant H3N2 influenza outbreak in Japan. Tohoku Journal of Experimental Medicine 2006;210(1):21‐7. [DOI] [PubMed] [Google Scholar]
Sampaio 2011 {published data only}
- Sampaio C, Bronzova J, Hauser RA, Lang AE, Rascol O, Witte SV, et al. Pardoprunox in early Parkinson's disease: Results from 2 large, randomized double‐blind trials. Movement Disorders 2011;26:1464‐76. [DOI] [PubMed] [Google Scholar]
Santesso 2013 {published data only}
- Santesso N, Hsu J, Mustafa R, Brozek J, Chen YL, Hopkins JP, et al. Antivirals for influenza: A summary of a systematic review and meta‐analysis of observational studies. Influenza and other Respiratory Viruses 2013;7:76‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sato 2008 {published data only}
- Sato M, Saito R, Sato I, Tanabe N, Shobugawa Y, Sasaki A, et al. Effectiveness of oseltamivir treatment among children with influenza A or B virus infections during four successive winters in Niigata City, Japan. Tohoku Journal of Experimental Medicine 2008;214(2):113‐20. [DOI] [PubMed] [Google Scholar]
Sauerbrei 2006 {published data only}
- Sauerbrei A, Haertl A, Brandstaedt A, Schmidtke M, Wutzler P. Utilization of the embryonated egg for in vivo evaluation of the anti‐influenza virus activity of neuraminidase inhibitors. Medical Microbiology and Immunology 2006;195(2):65‐71. [DOI] [PubMed] [Google Scholar]
Schapira 1971 {published data only}
- Schapira M, Oxford JS, Galbraith AW. Therapeutic effect of 1‐adamantanamine hydrochloride in naturally. Journal of the Royal College of General Practitioners 1971;21(113):695‐7. [CN‐00006890] [PMC free article] [PubMed] [Google Scholar]
Schmidt 2004 {published data only}
- Schmidt AC. Antiviral therapy for influenza: a clinical and economic comparative review. Drugs 2004;64(18):2031‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sears 1987 {published data only}
- Sears SD, Clements ML. Protective efficacy of low dose amantadine in adults challenge with wild‐type influenza A virus. Antimicrobial Agents and Chemotherapy 1987;31(10):1470‐3. [CN‐00052243] [DOI] [PMC free article] [PubMed] [Google Scholar]
Semlitsch 1992 {published data only}
- Semlitsch HV, Anderer P, Saletu B. Topographic mapping of long latency "cognitive" event‐related potentials (P 300): a double‐blind, placebo‐controlled study with amantadine in mild dementia. Journal of Neural Transmission. Parkinson's Disease and Dementia Section 1992;4:319‐36. [CN‐00087210] [DOI] [PubMed] [Google Scholar]
Serkedjieva 2007 {published data only}
- Serkedjieva J, Toshkova R, Antonova‐Nikolova S, Stefanova T, Teodosieva A, Ivanova I. Effect of a plant polyphenol‐rich extract on the lung protease activities of influenza‐virus‐infected mice. Antiviral Chemistry & Chemotherapy 2007;18(2):75‐82. [DOI] [PubMed] [Google Scholar]
Shah 2012 {published data only}
- Shah DP, Ghantoji SS, Mulanovich VE, Ariza‐Heredia EJ, Chemaly RF. Management of respiratory viral infections in hematopoietic cell transplant recipients. American Journal of Blood Research 2012;2:203‐18. [PMC free article] [PubMed] [Google Scholar]
Shuler 2007 {published data only}
- Schuler CM, Iwamoto M, Bridges CB, Marin M, Neeman R, Gargiullo P, et al. Vaccine effectiveness against medically attended, laboratory‐confirmed influenza among children aged 6 to 59 months, 2003‐2004. Pediatrics 2007;119(3):587‐95. [DOI] [PubMed] [Google Scholar]
Shvetsova 1974 {published data only}
- Shvetsova EG, Malysheva AM, Karapats NM, Oleinikova EV, Vasil'eva RI. Comparative study of the epidemiological efficacy of specific and nonspecific influenza preventive agents [Sravnitel'noe izuchenie epidemiologicheskoi effektivnosti speisificheskikh i nespetsificheskikh sredstv profilaktiki grippa]. Zhurnal Mikrobiologii Epidemiologii i Immunobiologii [Journal of Microbiology, Epidemiology and Immunobiology] 1974;51(4):47‐51. [CN‐00010299] [PubMed] [Google Scholar]
Simeonova 2009 {published data only}
- Simeonova L, Gegova G, Galabov AS. Rimantadine and oseltamivir combination effects in a therapeutic course of application against influenza a (H3N2) in mice. Antiviral Research 2009;82(2):A37. [DOI] [PubMed] [Google Scholar]
Singer 2011 {published data only}
- Singer B, Ross AP, Tobias K. Oral fingolimod for the treatment of patients with relapsing forms of multiple sclerosis. International Journal of Clinical Practice 2011;65:887‐95. [DOI] [PubMed] [Google Scholar]
Skoner 1999 {published data only}
- Skoner DP, Gentile DA, Patel A, Doyle WJ. Evidence for cytokine mediation of disease expression in adults experimentally infected with influenza A virus. Journal of Infectious Diseases 1999;180(1):10‐4. [CN‐00163791] [DOI] [PubMed] [Google Scholar]
Smorodintsev 1970a {published data only}
- Smorodintsev AA, Zlydnikov DM, Kiselva AM, Romanov JA, Kanantsev AP, Rumovsky VI. Evaluation of amantadine in artificially induced A2 and B influenza. JAMA 1970;213(9):1448‐54. [CN‐00004739] [PubMed] [Google Scholar]
Smorodintsev 1970b {published data only}
- Smorodintsev AA, Karpuhin GI, Zlydnikov DM, Malyseva AM, Svecova EG, Burov SA, et al. The prophylactic effectiveness of amantadine hydrochloride in an epidemic of Hong Kong influenza in Leningrad in 1969. Bulletin of the World Health Organization 1970;42(6):865‐72. [CN‐00005082] [PMC free article] [PubMed] [Google Scholar]
Smorodintsev 1970c {published data only}
- Smorodintsev AA, Karpuhin GI, Zlydnikov DM. The prospect of amantadine for prevention of influenza A in humans (effectiveness of amantadine during influenza A2/Hong Kong epidemics in January‐February 1969 in Leningrad). Annals of the New York Academy of Sciences 1970;173:44‐73. [Google Scholar]
Somani 1991 {published data only}
- Somani SK, Degelau J, Cooper SL, Guay DRP, Ehresman D, Zaske D. Comparison of pharmacokinetic and safety profiles of amantadine 50 and 100 mg daily doses in elderly nursing home residents. Pharmacotherapy 1991;11(6):440‐66. [CN‐00441230] [PubMed] [Google Scholar]
Tajima 2006 {published data only}
- Tajima T, Kakayama E, Kondo Y, Hirai F, Ito H, Iitsuka T, et al. Etiology and clinical study of community‐acquired pneumonia in 157 hospitalized children. Journal of Infection and Chemotherapy: official journal of the Japan Society of Chemotherapy 2006;12(6):372‐9. [DOI] [PubMed] [Google Scholar]
Takemura 2005 {published data only}
- Takemura Y, Ishida H, Saitoh H, Kure H, Kakoi H, Ebisawa K, et al. Economic consequence of immediate testing for C‐reactive protein and leukocyte count in new outpatients with acute infection. Clinica Chimica Acta: International Journal of Clinical Chemistry 2005;360(1‐2):114‐21. [DOI] [PubMed] [Google Scholar]
Tappenden 2009 {published data only}
- Tappenden P, Jackson R, Cooper K, Rees A, Simpson E, Read R, et al. Amantadine, oseltamivir and zanamivir for the prophylaxis of influenza (including a review of existing guidance no. 67): A systematic review and economic evaluation. Health Technology Assessment 2009;13(11):iii‐148. [DOI] [PubMed] [Google Scholar]
Terabayashi 2006 {published data only}
- Terabayashi T, Morita M, Ueno M, Nakamura T, Urashima T. Inhibition of influenza‐virus‐induced cytopathy by sialylglycoconjugates. Carbohydrate Research 341;13:2246‐53. [DOI] [PubMed] [Google Scholar]
Thomas 2008 {published data only}
- Thomas T, Banwell B. Multiple sclerosis in children. Seminars in Neurology 2008;28(1):69‐83. [DOI] [PubMed] [Google Scholar]
Thompson 1987 {published data only}
- Thompson J, Fleet W, Lawrence E, Pierce E, Morris L, Wright P. A comparison of acetaminophen and rimantadine in the treatment of influenza A infection in children. Journal of Medical Virology 1987;21(3):249‐55. [CN‐00047234] [DOI] [PubMed] [Google Scholar]
Togo 1968 {published data only}
- Togo Y, Hornick RB, Dawkins AT Jr. Studies on induced influenza in man. I. Double‐blind studies designed to assess prophylactic efficacy of amantadine hydrochloride against a2/Rockville/1/65 strain. JAMA 1968;203(13):1089‐94. [CN‐00001827] [DOI] [PubMed] [Google Scholar]
Togo 1970 {published data only}
- Togo Y, Hornick RB, Felitti VJ, Kaufman ML, Dawkins AT Jr, Kilpe VE, et al. Evaluation of therapeutic efficacy of amantadine in patients with naturally occurring A2 influenza. JAMA 1970;211(7):1149‐56. [CN‐00004092] [PubMed] [Google Scholar]
Togo 1972 {published data only}
- Togo Y, Schwatrz AR, Tominaga S, Hornick RB. Cyclooctylamine in the prevention of experimental human influenza. JAMA 1972;220(6):837‐41. [CN‐00007054] [PubMed] [Google Scholar]
Townsend 2006 {published data only}
- Townsend KA, Eiland LS. Combating influenza with antiviral therapy in the pediatric population. Pharmacotherapy 2006;26(1):95‐103. [DOI] [PubMed] [Google Scholar]
Van der Wouden 2005 {published data only}
- Wouden JC, Bueving HJ, Poole P. Preventing influenza: an overview of systematic reviews. Respiratory Medicine 2005;99(11):1341‐9. [DOI] [PubMed] [Google Scholar]
Van Voris 1981 {published data only}
- Voris LP, Betts RF, Hayden FG, Christmas WA, Douglas RG Jr. Successful treatment of naturally occurring influenza A/USSR/77 H1N1. JAMA 1981;245(11):1128‐31. [CN‐00024393] [DOI] [PubMed] [Google Scholar]
Van Voris 1985 {published data only}
- Voris LP, Betts RF, Menegus MA, Murphy BR, Roth FK, Douglas RG Jr. Serological diagnosis of influenza A/USSR/77 H1N1 infection: value of ELISA compared to other antibody techniques. Journal of Medical Virology 1985;16(4):315‐20. [CN‐00039377] [DOI] [PubMed] [Google Scholar]
Wailoo 2008 {published data only}
- Wailoo AJ, Sutton AJ, Cooper NJ, Turner DA, Abrams KR, Brennan A, et al. Cost‐effectiveness and value of information analyses of neuraminidase inhibitors for the treatment of influenza. Value in Health 2008;11(2):160‐71. [DOI] [PubMed] [Google Scholar]
Webster 1986 {published data only}
- Webster R, Kawaoka Y, Bean W. Vaccination as strategy to reduce the emergence of amantadine and rimantadine‐resistant strains of A/Chick/Pennsylvania/83 (H5N2) influenza virus. Journal of Antimicrobial Chemotherapy 1986;18:157‐64. [CN‐00341633] [DOI] [PubMed] [Google Scholar]
Welton 2008 {published data only}
- Welton NJ, Cooper NJ, Ades AE, Lu G, Sutton AJ. Mixed treatment comparison with multiple outcomes reported inconsistently across trials: evaluation of antivirals for treatment of influenza A and B. Statistics in Medicine 2008;27(27):5620‐39. [DOI] [PubMed] [Google Scholar]
Wendel 1966 {published data only}
- Wendel HA, Snyder MT, Pell P. Trial of amantadine in epidemic in influenza. Clinical Pharmacology and Therapeutics 1966;7(1):38‐43. [CN‐00000530] [DOI] [PubMed] [Google Scholar]
Whitley 2007 {published data only}
- Whitley RJ. The role of oseltamivir in the treatment and prevention of influenza in children. Expert Opinion on Drug Metabolism and Toxicology 2007;3(5):755‐67. [DOI] [PubMed] [Google Scholar]
Wingfield 1969 {published data only}
- Wingfield WL, Pollack D, Grunert RR. Therapeutic efficacy of amantadine HCl and rimantadine HCl in naturally occurring influenza A2 respiratory illness in man. New England Journal of Medicine 1969;281(11):579‐84. [DOI] [PubMed] [Google Scholar]
Wong 2006 {published data only}
- Wong SS, Yuen KY. Avian influenza virus infections in humans. Chest 2006;129(1):156‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 1976 {published data only}
- Wright PF, Khaw KT, Oxman MN, Shwachman H. Evaluation of the safety of amantadine‐HCL and the role of respiratory viral infections in children with cystic fibrosis. Journal of Infectious Diseases 1976;134(2):144‐9. [CN‐00208364] [DOI] [PubMed] [Google Scholar]
Wultzler 2004 {published data only}
- Wultzler P, Kossow KD, Lode H, Ruf BR, Scholz H, Vogel GE, et al. Antiviral treatment and prophylaxis of influenza in primary care: German recommendations. Journal of Clinical Virology 2004;31(2):84‐91. [Unique identifier: 15364262] [DOI] [PubMed] [Google Scholar]
Yamaura 2003 {published data only}
- Yamaura K, Yoshihara M. Investigation of the reconsultation rate and pharmacoeconomic evaluation of period of influenza treatment by oseltamivir. Yakugaku Zasshi: Journal of the Pharmaceutical Society of Japan 2003;123(10):887‐91. [DOI] [PubMed] [Google Scholar]
Younkin 1983 {published data only}
- Younkin SW, Betts RF, Roth FK, Douglas RG Jr. Reduction in fever and symptoms in young adults with influenza A/Brazil/78 H1N1 infection after treatment with aspirin or amantadine. Antimicrobial Agents and Chemotherapy 1983;23(4):577‐82. [CN‐00031339] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yuen 2005 {published data only}
- Yuen KY, Wong SS. Human infection by avian influenza A H5N1. Hong Kong Medical Journal 2005;11(3):189‐99. [PubMed] [Google Scholar]
Yuen 2012 {published data only}
- Yuen C, Chan Y, Tambyah PA. Preflucel (registered trademark): a Vero‐cell culture‐derived trivalent influenza vaccine. Expert Review of Vaccines 2012;11:759‐73. [DOI] [PubMed] [Google Scholar]
Zeuzem 1999 {published data only}
- Zeuzem S, Teuber G, Naumann U, Berg T, Raedle J, Hartmann S, et al. Randomised, double‐blind, placebo‐controlled trial of interferon‐alfa with and without amantadine as initial treatment for chronic hepatitis C. Hepatology 1999;30(4):200A. [CN‐00271208] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Ahovuo‐Saloranta 2014
- Ahovuo‐Saloranta A, Rautakorpi U‐M, Borisenko OV, Liira H, Williams Jr JW, Mäkelä M. Antibiotics for acute maxillary sinusitis in adults. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD000243.pub3] [DOI] [PubMed] [Google Scholar]
Antanova 2012
- Antonova EN, Rycroft CE, Ambrose CS, Heikkinen T, Principi N. Burden of paediatric influenza in Western Europe: a systematic review. BMC Public Health 2012;12:968‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barik 2012
- Barik S. New treatments for influenza. BMC Medicine 2012;13(10):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bitko 2007
- Bitko V, Musiyenko A, Barik S. Viral infection of the lungs through the eye. Journal of Virology 2007;81:783‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonn 1997
- Bonn D. Spared an influenza pandemic for another year?. Lancet 1997;349(9044):36. [DOI] [PubMed] [Google Scholar]
Bright 2005
- Bright RA, Medina MJ, Xu X, Perez‐Oronoz G, Wallis TR, Davis XM, et al. Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: a cause for concern. Lancet 2005;366:1175‐81. [DOI] [PubMed] [Google Scholar]
Bright 2006
- Bright RA, Shay DK, Shu B, Cox NJ, Klimov AI. Adamantane resistance among influenza A viruses isolated early during the2005‐2006 influenza season in the United States. JAMA 2006;295:891‐4. [DOI] [PubMed] [Google Scholar]
CCI 2006
- Centro Cochrane Iberoamericano. Introduction to systematic reviews [Curso de introducción a las revisiones sistemáticas]. http://www.cochrane.es/ 2006 (accessed 12 January 2007).
Cheung 2002
- Cheung CY, Poon LLM, Lau AS, Luk W, Lau YL, Shortridge KF, et al. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease?. Lancet 2002;360:1831‐7. [DOI] [PubMed] [Google Scholar]
Demicheli 2000
- Demicheli V, Jefferson T, Rivetti D, Deeks J. Prevention and early treatment of influenza in health adults. Vaccine 2000;18:957‐1030. [DOI] [PubMed] [Google Scholar]
Demicheli 2012
- Demicheli V, Jefferson T, Al‐Ansary LA, Ferroni E, Rivetti A, Pietrantonj C. Vaccines for preventing influenza in healthy children. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD004879.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deyde 2007
- Deyde VM, Xu X, Bright RA, Shaw M, Smith CB, Zhang Y, et al. Surveillance of resistance to adamantanes among influenza A(H3N2) and A(H1N1) viruses isolated worldwide. Journal of Infectious Diseases 2007;196(2):249‐57. [DOI] [PubMed] [Google Scholar]
Dolin 2005
- Dolin R. Influenza‐interpandemic as well as pandemic disease. New England Journal of Medicine 2005;353(24):2535‐7. [DOI] [PubMed] [Google Scholar]
Eccle 2005
- Eccle R. Understanding the symptoms of the common cold and influenza. Lancet Infectious Diseases 2005;5:718‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fletcher 2006
- Fletcher RH, Fletcher SW. Epidemiologia Clínica: Elementos Essenciais. 4th Edition. Porto Alegre, Brazil: Artmed, 2006. [Google Scholar]
Goodman 2006
- Goodman C, Mukherjee D, Faulkner E. How effective would antiviral vaccination and antiviral drug prevention and treatment strategies be for reducing the impact of the next influenza pandemic. Copenhagen, WHO Regional Office for Europe (Health Evidence Network report; http://www.euro.who.int/en/data‐and‐evidence/evidence‐informed‐policy‐making/publications/pre2009/how‐effective‐would‐antiviral‐vaccination‐and‐antiviral‐drug‐prevention‐and‐treatment‐strategies‐be‐for‐reducing‐the‐impact‐of‐the‐next‐influenza‐pandemic 2006 (accessed 20 October 2007).
Hayden 2004
- Hayden FG. Pandemic influenza: is an antiviral response realistic?. Pediatric Infectious Disease Journal 2004;23(Suppl):262‐9. [DOI] [PubMed] [Google Scholar]
Hayden 2005
- Hayden FG, Klimov A, Tashiro M, Hay A, Monto A, McKimm‐Breschkin J, et al. Neuraminidase inhibitor susceptibility network position statement: antiviral resistance in influenza A/H5N1 viruses. Antiviral Therapy 2005;10(8):873‐7. [PubMed] [Google Scholar]
Hayden 2006b
- Hayden FG. Antiviral resistance in influenza viruses ‐ implications for management and pandemic response. New England Journal of Medicine 2006;354(8):785‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jefferson 2006b
- Jefferson T, Demicheli V, Pietrantonj C, Rivetti D. Amantadine and rimantadine for influenza A in adults. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD001169.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jefferson 2010
- Jefferson T, Pietrantonj C, Al‐Ansary LA, Ferroni E, Thorning S, Thomas RE. Vaccines for preventing influenza in the elderly. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD004876.pub3] [DOI] [PubMed] [Google Scholar]
Jefferson 2014
- Jefferson T, Jones MA, Doshi P, Mar CB, Hama R, Thompson MJ, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD008965.pub4] [DOI] [PubMed] [Google Scholar]
Kelso 2013
- Kelso JK, Halder N, Milne GJ. Vaccination strategies for future influenza pandemics: a severity‐based cost effectiveness analysis. BMC Infectious Diseases 2013;11:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Li 2014
- Li Q, Zhou L, Zhou M, Chen Z, Li F, Wu H, et al. Epidemiology of human infections with avian influenza A(H7N9) virus in China. New England Journal of Medicine 2014;370:520‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Monto 2008
- Monto AS. Epidemiology of influenza. Vaccine 2008;Suppl(4):D45–8. [DOI] [PubMed] [Google Scholar]
Moscona 2005
- Moscona A. Neuraminidase inhibitors for Influenza. New England Journal of Medicine 2005;353:1363‐73. [DOI] [PubMed] [Google Scholar]
MS 2006
- Ministério da Saúde 2006 (Brazilian Ministry of Health 2006). Human influenza and avian influenza [Influenza humana e influenza aviária]. Série F. Comunicação e Educação em Saúde 2006.
Nicholson 1992
- Nicholson K. Clinical features of influenza. Seminars in Respiratory Infections 1992;7(1):26‐37. [PubMed] [Google Scholar]
Noah 2013
- Noah DL, Noah JH. Adapting global influenza management strategies to address emerging viruses. American Journal of Physiology. Lung Cellular and Molecular Physiology 2013;305:108‐17. [DOI] [PubMed] [Google Scholar]
Pineda Solas 2006
- Pineda Solas A, Bernaola Iturbe E, Martinon‐Torres F, Baca Cots M, Juan Martin F, Gomez Campdera JA, et al. Recommendations of the Vaccine Advisory Committee of the Spanish Association of Pediatrics: influenza vaccination campaign 2006‐2007. Anales de Pediatría 2006;65(3):252‐5. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Sasaki 2011
- Sasaki S, Sullivan M, Narvaez C, Holmes T, Furman D, Zheng NY, et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine‐specific antibodies. Journal of Clinical Investigation 2011;121(8):3109‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sleeman 2013
- Sleeman K, Guo Z, Barnes J, Shaw M, Stevens J, Gubareva L. R292K Substitution and drug susceptibility of Influenza A (H7N9) viruses. Emerging Infectious Diseases 2013;9(9):1521‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2006
- Smith NM, Bresee JJ, Shay DK, Uyeki TM, Cox NJ, Strikas RA. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practice (ACIP). Morbidity and Mortality Weekly Report: Recommendations and Reports/Centers for Disease Control 2006;55(RR10):1‐42. [ISSN 1545‐8601 (Electronic)] [PubMed] [Google Scholar]
Thomas 2011
- Thomas RE, Russell M, Lorenzetti D. Interventions to increase influenza vaccination rates of those 60 years and older in the community. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD005188.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Treanor 2005
- Treanor JJ. Influenza viruses. In: Mandell, Bennett, & Dolin editor(s). Principles and Practice of Infectious Diseases. 6th Edition. Vol. 2, W.B. Saunders, an imprint of Elsevier, 2005:2060‐85. [Google Scholar]
WHO 2003
- World Health Organization. Influenza. Geneva, World Health Organization, 2003 (Fact Sheet No. 211). http://www.who.int/mediacentre/factsheets/fs211/en/print.html 2003 (accessed 20 October 2007).
WHO 2007
- World Health Organization. Child and adolescent health and development. http://www.who.int/child‐adolescent‐health/over.htm 2007 (20 accessed January 2007).
WHO 2010a
- World Health Organization. Pandemic (H1N1) 2009 ‐ update 112. http://www.who.int/csr/don/2010_08_06/en/ 2010 (accessed 14 August 2011).
WHO 2010b
- World Health Organization. Essential medicines selection. Unedited report of the supplementary meeting of the expert committee on the selection and use of essential medicines. http://www.who.int/selection_medicines/committees/expert/emergency_session/unedited_Emergency_report.pdf 2010 (accessed 14 August 2011).
WHO 2011
- World Health Organization. Global Influenza Surveillance and Response System (GISRS). http://www.who.int/influenza/gisrs_laboratory/en 2011 (accessed in 3 December 2011).
WHO 2012
- World Health Organization. Laboratory methodologies for testing the antiviral susceptibility of influenza viruses. http://www.who.int/influenza/gisrs_laboratory/antiviral_susceptibility/en/ 2012 (accessed 20 August 2012).
Wiselka 1994
- Wiselka M. Influenza: diagnosis, management and prophylaxis. BMJ 1994;308:1341‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 2004
- Wright P. Influenza viruses. In: Behrman RE, Kliegman RM, Hall BJ editor(s). Nelson Textbook of Pediatrics of Pediatrics. 17th Edition. Saunders (an imprint of Elsevier), 2004:1072‐5. [Google Scholar]
Yang 2010
- Yang M, Dong BR, Wu HM, Li T, Liu GJ. Interventions for treating influenza: an overview of Cochrane systematic reviews. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD008799] [DOI] [Google Scholar]
Ziegler 1999
- Ziegler T, Hemphill ML, Ziegler ML, Perez‐Oronoz G, Klimov AI, Hampson AW, et al. Low incidence of rimantadine resistance infield isolates of influenza A viruses. Journal of Infectious Diseases 1999;4:935–9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Alves Galvão 2008
- Alves Galvão MG, Rocha Crispino Santos MA, Alves da Cunha AJL. Amantadine and rimantadine for influenza A in children and the elderly. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD002745.pub2] [DOI] [PubMed] [Google Scholar]
Alves Galvão 2012
- Alves Galvão MG, Rocha Crispino Santos MA, Alves da Cunha AJL. Amantadine and rimantadine for influenza A in children and the elderly. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD002745.pub3] [DOI] [PubMed] [Google Scholar]


