Abstract
Background
There is a need to identify effective and safe treatments for depression in children and adolescents. While tricyclic drugs are effective in treating depression in adults, individual studies involving children and adolescents have been equivocal. Prescribing of tricyclic drugs for depression in children and adolescents is now uncommon, but an accurate estimate of their efficacy is helpful as a comparator for other drug treatments for depression in this age group. This is an update of a Cochrane review first published in 2000 and updated in 2002, 2006 and 2010.
Objectives
To assess the effects of tricyclic drugs compared with placebo for depression in children and adolescents and to determine whether there are differential responses to tricyclic drugs between child and adolescent patient populations.
Search methods
We conducted a search of the Cochrane Depression, Anxiety and Neurosis Review Group's Specialised Register (CCDANCTR) (to 12 April 2013), which includes relevant randomised controlled trials from the following bibliographic databases: the Cochrane Central Register of Controlled Trials (CENTRAL) (all years), EMBASE (1974‐), MEDLINE (1950‐) and PsycINFO (1967‐). The bibliographies of previously published reviews and papers describing original research were cross‐checked. We contacted authors of relevant abstracts in conference proceedings of the American Academy of Child and Adolescent Psychiatry, and we handsearched the Journal of the American Academy of Child and Adolescent Psychiatry (1978 to 1999).
Selection criteria
Randomised controlled trials comparing the efficacy of orally administered tricyclic drugs with placebo in depressed people aged 6 to 18 years.
Data collection and analysis
One of two review authors selected the trials, assessed their quality, and extracted trial and outcome data. A second review author assessed quality and checked accuracy of extracted data. Most studies reported multiple outcome measures including depression scales and clinical global impression scales. For each study, we took the best available depression measure as the index measure of depression outcome. We established predetermined criteria to assist in the ranking of measures. Where study authors reported categorical outcomes, we calculated individual and pooled risk ratios for non‐improvement in treated compared with control subjects. For continuous outcomes, we calculated pooled effect sizes as the number of standard deviations by which the change in depression scores for the treatment group exceeded those for the control group.
Main results
Fourteen trials (590 participants) were included. No overall difference was found for the primary outcome of response to treatment compared with placebo (risk ratio (RR) 1.07, 95% confidence interval (CI) 0.91 to 1.26; 9 trials, N = 454). There was a small reduction in depression symptoms (standardised mean difference (SMD) ‐0.32, 95% CI ‐0.59 to ‐0.04; 13 trials, N = 533), but the evidence was of low quality. Subgroup analyses suggested a small reduction in depression symptoms among adolescents (SMD ‐0.45, 95% CI ‐0.83 to ‐0.007), and negligible change among children (SMD 0.15, 95% CI ‐0.34 to 0.64). Treatment with a tricyclic antidepressant caused more vertigo (RR 2.76, 95% CI 1.73 to 4.43; 5 trials, N = 324), orthostatic hypotension (RR 4.86, 95% CI 1.69 to 13.97; 5 trials, N = 324), tremor (RR 5.43, 95% CI 1.64 to 17.98; 4 trials, N = 308) and dry mouth (RR 3.35, 95% CI 1.98 to 5.64; 5 trials, N = 324) than did placebo, but no differences were found for other possible adverse effects. Wide CIs and the probability of selective reporting mean that there was very low‐quality evidence for adverse events.
There was heterogeneity across the studies in the age of participants, treatment setting, tricyclic drug administered and outcome measures. Statistical heterogeneity was identified for reduction in depressive symptoms, but not for rates of remission or response. As such, the findings from analyses of pooled data should be interpreted with caution.
We judged none of these trials to be at low risk of bias, with limited information about many aspects of risk of bias, high dropout rates, and issues regarding measurement instruments and the clinical usefulness of outcomes, which were often variously defined across trials.
Authors' conclusions
Data suggest tricyclic drugs are not useful in treating depression in children. There is marginal evidence to support the use of tricyclic drugs in the treatment of depression in adolescents.
Plain language summary
Tricyclic drugs for depressed children and adolescents
Depression affects about one in 20 young people, and can contribute to a variety of negative outcomes, such as poor academic functioning and difficulties in peer and family relationships. Depression also increases the risk for substance use, self harm and suicide. Beginning with imipramine, tricyclic drugs were developed from the late 1950s to alleviate the symptoms of depression. They were designed to enhance the availability of serotonin and noradrenaline to brain cells. Tricyclic drugs were first prescribed to children and adolescents for depression in the early 1960s, but were more commonly prescribed to children for the treatment of bedwetting. Since this Cochrane review was first published in 2000, tricyclic drugs have been replaced in most countries by newer‐generation antidepressants.
This review contained 14 trials (with 590 participants) and tested the effectiveness of tricyclic drugs against placebo. Trial data were available for amitriptyline, desipramine, imipramine and nortriptyline. Based on nine trials (454 participants), there was no evidence that tricyclic drugs lead to higher rates of remission or response than placebo. Based on 13 of the trials (533 participants), there was evidence that people treated with a tricyclic drug had lower depression severity scores than those on placebo, however, the size of this difference was small. Consistent with their known mechanism of action, tricyclic drugs were more likely than placebo to cause vertigo, symptoms of lowered blood pressure, tremor and dry mouth. Subgroup analyses of six trials involving only adolescents (239 participants) and two trials involving only children (77 participants) found no evidence of differential rates of remission or response between the age groups. In contrast, there was lowering of depression scores in eight trials involving only adolescents (414 participants) and no lowering of depression scores in three trials involving only children (64 participants).
Most of the included studies were conducted in the era before standard methods for conducting treatment trials for depression in children and adolescents came about. There were considerable differences between the studies with regards to the clinical tools and methods used in assessment of improvement. Most trials were small. Only two trials produced a definitive result for depressive symptoms, and no trial produced a definitive result for response or remission. There was typically insufficient information to judge the quality of the trials accurately. With these limitations, it is difficult to answer questions about the effectiveness and safety of tricyclic drugs for treating depression in children and adolescents. Current evidence suggests that the situation is much the same for newer‐generation antidepressants. Clinicians need to provide accurate information to children and adolescents, and their families, about the uncertainties regarding the benefits and risks of antidepressants as a treatment option for depression. Tricyclic drugs do not seem useful for treating children before puberty, and are, at most, of moderate benefit for adolescents.
Summary of findings
Summary of findings for the main comparison. Tricyclic antidepressants for depression in children and adolescents.
| Tricyclic drugs for depression in children and adolescents | ||||||
| Patient or population: children and adolescents with depression Settings: inpatients or outpatients Intervention: tricyclic drugs | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Tricyclic drugs | |||||
|
Achieved recovery according to predetermined criteria (no longer meeting criteria for depression on K‐SADS or meeting a priori criteria for response on a depression checklist, assessed between 4 and 10 weeks of onset of treatment) |
450 per 1000 | 482 per 1000 (410 to 567) | RR 1.07 (0.91 to 1.26) | 453 (9 studies) | ⊕⊕⊝⊝ low1,2 | |
|
Change in depression checklist scores (BID, CDI, CDRS, DACL or HAM‐D from baseline to between 2 and 10 weeks) |
The mean depression checklist score in the intervention groups was
0.32 standard deviations lower
(0.59 to 0.04 lower) This equates to a 3.3 (6.1 to 0.4) point reduction on the CDRS‐R (range 17 to 113) |
533 (13 studies) | ⊕⊕⊝⊝ low1,2,3 | SMD ‐0.32 (‐0.59 to ‐0.04) Subgroup analyses for children and adolescents found SMDs of 0.15 and ‐0.45, respectively, but the CIs overlapped |
||
|
Change in clinical global assessment scale scores (CGI scale or CGAS rated between 4 and 10 weeks of onset of treatment) |
The mean clinical global assessment scale score in the intervention groups was
0.1 standard deviations lower
(0.4 lower to 0.2 higher) This equates to 3 points lower to 1.5 points higher on the CGAS (range 0‐100) |
180 (5 studies) | ⊕⊕⊝⊝ low1,2,4 | SMD ‐0.1 (‐0.4 to 0.2) | ||
|
Number of withdrawals (occurring between 4 and 10 weeks of onset of treatment) |
200 per 1000 | 292 per 1000 (179 to 440) | OR 1.65 (0.87 to 3.14) | 462 (8 studies) | ⊕⊕⊝⊝ low1,5 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BID: Bellevue Index of Depression; CDI: Children's Depression Inventory; CDRS: Children's Depression Rating Scale; CGAS: Clinical Global Assessment Scale; CGI: Clinical Global Impression; CI: confidence interval; DACL: Depressive Adjective Checklist; HAM‐D: Hamilton Depression Rating Scale; OR: odds ratio; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Study methodology not robust in many studies, primarily due to age of study 2 Confidence intervals for analysis include both appreciable benefit and appreciable harm 3 Not all confidence intervals overlap. I2 is 50%. 4 Small sample size; less than 400 participants 5 Wide confidence intervals
Background
Description of the condition
Depression is a common, yet under‐recognised, problem in young people. Estimated prevalence ranges from 0.4% to 2.5% in primary school children, and from 0.4% to 8.3% in adolescents (Birmaher 1996). A study of registry data for a large number of American children over a 30‐year period indicated that the rate of depression has consistently been around 2.8% for under 13 and 5.6% for 13‐ to 18‐year olds (Costello 2006). Important consequences of depression in this age group include social dysfunction, academic underachievement and suicidal behaviour. Consequently, adequate detection and treatment of depressed adolescents is an important strategy for curbing the rising rate of youth suicide seen in many developed countries (Rosenberg 1989).
Description of the intervention
Tricyclic drugs were the first‐line pharmacological treatment for depression prior to the introduction of selective serotonin re‐uptake inhibitors (SSRIs) in the 1990s. A variety of tricyclic drugs with different dose ranges and half lives were introduced to the market over the years. The two most commonly studied tricyclic drugs in children and adolescents are imipramine and amitriptyline. Both are typically prescribed in doses of 1 to 5 mg/kg/day. Half lives of medications in children are inversely related to the age. Average half lives in children and adolescents of imipramine and amitriptyline are 16 and 24 hours, respectively (Werry 1999). Tricyclic drugs are metabolised in the liver by cytochrome P450 (CYP450) 2D6 and 1A2. Hence, CYP450 2D6 or 1A2 inhibitors, such as fluoxetine, paroxetine, cimetidine, haloperidol or phenothiazines, can increase plasma levels of tricyclic drugs. Methylphenidate, a drug used to treat attention deficit hyperactivity disorder (ADHD), may also inhibit metabolism of tricyclic drugs. Other reported interactions include; increased risk of seizure in combination with tramadol, paralytic ileus or hyperthermia if used with anticholinergic medications, increased sympathomimetic effects if given with sympathomimetic drugs and precipitating a manic state if switched over from or added to another antidepressant. Tricyclic drugs can also inhibit the antihypertensive effect of clonidine (Stahl 2006).
How the intervention might work
Depression, at least for some patients, is associated with a lower level of noradrenaline and serotonin at certain brain receptor sites. Tricyclic drugs have been shown to inhibit the cellular uptake and inactivation of serotonin and noradrenaline leading to an increased noradrenergic and serotonergic neurotransmission in the brain. This is believed to be the mechanism of action of tricyclic drugs in the treatment of depression (Feighner 1999).
Why it is important to do this review
The current publication is an update of a Cochrane review first published in 2000 and previously updated in 2008. The earlier version indicated that tricyclic drugs are not useful in treatment of depression in children. The meta‐analysis needed to be updated given that there is an additional trial that has not been included in earlier the version. There has almost certainly been a shift away from the prescribing of tricyclic drugs to children and adolescents in favour of newer‐generation antidepressants. An updated review of newer‐generation antidepressants for depressive disorders in children and adolescents was published in 2012 (Hetrick 2012). Knowledge of the efficacy of tricyclic drugs remains relevant, however, for providing a baseline against which these newer agents may be compared.
Objectives
To assess the effects of tricyclic drugs compared with placebo for depression in children and adolescents.
To determine whether there are differential responses to tricyclic drugs between child and adolescent patient populations.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing the efficacy of tricyclic antidepressants versus placebo in depressed people aged 6 to 18 years.
Types of participants
Studies were included if they described people between 6 and 18 years who were identified as suffering from a unipolar depressive illness confirmed by Diagnostic and Statistical Manual of Mental Disorders (DSM) (APA 2000), or International Statistical Classification of Diseases (ICD) (WHO 1992), criteria.
Studies of mixed adolescents and adults were not included because it was not possible to separate out the data on the adolescents.
Participants could be treated as inpatients or outpatients.
Studies were excluded if participants had IQ levels less than 80.
While we applied no other exclusion criteria, individual studies commonly excluded people with significant comorbidities such as schizophrenia, and people with high risk of suicide, bipolar disorder, pervasive developmental disorder, substance abuse, and people with serious medical conditions. Studies variably excluded conditions such as obsessive compulsive disorder, conduct disorder and attention deficit hyperactivity disorder. It is likely, therefore, that patterns of comorbidity differed across studies.
Types of interventions
Experimental intervention
Any orally administered tricyclic drug (and no other pharmacological intervention). No restrictions on dose, frequency or duration of treatment were applied. There are at least 27 drugs classified as tricyclic drugs, but those that might be prescribed for depression in children and adolescents are limited to amitriptyline, chlorimipramine, desipramine, dothiepin, imipramine, nortriptyline, protriptyline and trimipramine. None is specifically approved for the treatment of depression in children and adolescents, and none is specifically contraindicated in this population.
Control intervention
Placebo.
Types of outcome measures
Primary outcomes
Achieved remission/response as per predetermined criteria (e.g. Hamilton Rating Scale for Depression score < 9 or > 49% reduction in Hamilton Rating Scale for Depression score from baseline (Keller 2001)).
Change in depressive checklist scores.
Side effects.
Secondary outcomes
Global improvement.
Discontinuation rates.
In the era in which most of these trials were conducted it was common to use multiple depression rating scales, with none identified as the primary outcome measure.
For the purposes of pooling results to obtain an aggregate outcome, a single 'best available' outcome measure was chosen for each study. The order of selection was predetermined by the rating of each instrument over the following five criteria: appropriateness to children and adolescents; reliability; construct validity; agreement with clinical interview; track record in psychopharmacological research. Most of the data for this rating were obtained from a review by Petti (Petti 1985).
The hierarchy of selection for analysis, and the number of criteria met by each rating scale (in parentheses), were as follows.
Schedule for Affective Disorders and Schizophrenia for School‐Age Children (Kiddie‐SADS), combined child and parent report (5).
Children's Depression Rating Scale (CDRS) (4).
Bellevue Index of Depression (BID) (3).
Children's Depression Inventory (CDI) (3).
Hamilton Depression Rating Scale (HAM‐D) (3).
Depressive Adjective Checklist (DACL) (2).
Side effects were examined where papers reported on any standardised side effect rating scale.
Search methods for identification of studies
The Cochrane Depression, Anxiety and Neurosis Review Group's Specialised Register (CCDANCTR)
The Cochrane Depression, Anxiety and Neurosis Group (CCDAN) maintain two clinical trials registers at their editorial base in Bristol, UK: a references register and a studies‐based register. The CCDANCTR‐References Register contains over 31,500 reports of RCTs in depression, anxiety and neurosis. Approximately 65% of these references have been tagged to individual, coded trials. The coded trials are held in the CCDANCTR‐Studies Register and records are linked between the two registers through the use of unique Study ID tags. Coding of trials is based on the EU‐Psi coding manual, using a controlled vocabulary (please contact the CCDAN Trials Search Coordinator for further details). Reports of trials for inclusion in the Group's registers are collated from routine (weekly), generic searches of MEDLINE (1950‐), EMBASE (1974‐) and PsycINFO (1967‐); quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) and review‐specific searches of additional databases. Reports of trials are also sourced from international trials registers via the World Health Organization's trials portal (the International Clinical Trials Registry Platform (ICTRP)); pharmaceutical companies; and handsearching of key journals, conference proceedings and other (non‐Cochrane) systematic reviews and meta‐analyses.
Details of CCDAN's generic search strategies (used to identify RCTs) can be found on the Group's website.
Electronic searches
The CCDANCTR‐Studies Register was searched (to 12 April 2013) using the following terms:
Diagnosis = Depress* or Dysthymi* or "Adjustment Disorder*" or "Mood Disorder*" or "Affective Disorder" or "Affective Symptoms" and Age‐Group = Child or Adolescent and Free‐text = Tricyclic* or TCA* or Amersergide or Amineptine or Amitriptylin* or Amitriptylinoxide or Amoxapine or Butriptyline or Cianopramine or (Clomipramin* or Clorimipramine or Chlorimipramin* or Chlomipramin*) or Demexiptiline or Desipramine or Dibenzipin or Dimetacrin* or (Dosulepin or Dothiepin) or Doxepin or Imipramin* or Iprindole or Lofepramin* or Melitracen or Metapramine or Nortriptyline or Noxiptilin* or Opipramol or Pipofezin* or Propizepine or Protriptylin* or Quinupramine or Tianeptin* or Trimipramin* and Intervention=placebo*
The CCDANCTR‐References Register was searched (to 12 April 2013) using a more sensitive set of terms to find additional untagged/uncoded references:
Title/Abstract/Keywords = Depress* or Dysthymi* or "Adjustment Disorder*" or "Mood Disorder*" or "Affective Disorder*" or "Affective Symptoms" and Title/Abstract/Keywords = (adolesc* or preadolesc* or pre‐adolesc* or boy* or girl* or child* or infant* or juvenil* or minors or school* or pediatri* or paediatri* or pubescen* or student* or teen* or young or youth* or school* or high‐school or “high school” or college or undergrad*) and Free‐text = (Tricyclic* or TCA* or Amersergide or Amineptine or Amitriptylin* or Amitriptylinoxide or Amoxapine or Butriptyline or Cianopramine or (Clomipramin* or Clorimipramine or Chlorimipramin* or Chlomipramin*) or Demexiptiline or Desipramine or Dibenzipin or Dimetacrin* or (Dosulepin or Dothiepin) or Doxepin or Imipramin* or Iprindole or Lofepramin* or Melitracen or Metapramine or Nortriptyline or Noxiptilin* or Opipramol or Pipofezin* or Propizepine or Protriptylin* or Quinupramine or Tianeptin* or Trimipramin*)
There was no restriction on date, language or publication status applied to the search.
(Please see Appendix 1 for search strategies used in earlier versions of this review.)
Searching other resources
Handsearches
Bibliographies of previously published reviews and papers describing original research were cross‐checked. Current Contents was screened for recent publications.
We handsearched the Journal of the American Academy of Child and Adolescent Psychiatry (1978‐1999) to identify RCTs.
Personal communication
We contacted authors of abstracts describing 'work in progress' identified in conference proceedings of the American Academy of Child and Adolescent Psychiatry to determine whether they held data that could be included in the meta‐analysis.
Data collection and analysis
Selection of studies
The selection of studies for inclusion in the review was performed independently by the two review authors (PH and MM). We obtained the full article when a trial seemed eligible for inclusion from the title or abstract. It was subsequently assessed to determine relevance to this review based on the inclusion criteria. We have reported the reasons for exclusion of trials in the Characteristics of excluded studies table.
Data extraction and management
MM extracted the data for the most recent update of the review. All other data were extracted by DH. All data were checked for accuracy by PH.
Statistical analysis was undertaken in accordance with the guidelines for statistical analysis in theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Summary statistics were pooled statistically using the meta‐analytic methods implemented in Review Manager (RevMan) software (RevMan 2011).
Assessment of risk of bias in included studies
Two review authors (DH and PH) independently assessed methodological quality of included studies using The Cochrane Collaboration's 'Risk of bias' tool as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The following items were assessed.
Sequence generation: was the allocation sequence adequately generated?
Allocation concealment: was allocation adequately concealed?
Blinding of participants, personnel and outcome assessors for each main outcome or class of outcomes: was knowledge of the allocated treatment adequately prevented during the study?
Incomplete outcome data for each main outcome or class of outcomes: were incomplete outcome data adequately addressed?
Selective outcome reporting: were reports of the study free of suggestion of selective outcome reporting?
Other sources of bias: was the study apparently free of other problems that could put it at a high risk of bias?
We included quotations from the text of included studies; comments on how we assessed the risk of bias; and judgements as follows: low risk of bias, unclear risk of bias, high risk of bias.
If disputes arose as to which judgement should be given, then resolution was achieved after consulting with a third party.
Measures of treatment effect
Odds ratio (OR) was used for comparing dichotomous data and standardised mean differences (SMD) for the analysis of continuous data.
Unit of analysis issues
Cluster‐randomised trials
Should any cluster randomised trials be identified in future updates of this review, they will be included as long as proper adjustment for the intra‐cluster correlation can be undertaken as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Cross‐over trials
Due to the risk of carry‐over effects in cross‐over trials, only data from the first phase of the study were used.
Studies with multiple treatment groups
Where studies had additional arms that were not tricyclic antidepressants, we only included the data relating to the tricyclic and placebo arms in the review. If a study had more than two arms that met the inclusion criteria, for example two tricyclic antidepressants and a placebo arm, then the data from the placebo arm would be split equally between to produce two (or more) pairwise comparisons.
Dealing with missing data
Mean change scores were typically not reported, and were therefore derived from the difference between mean baseline and follow‐up scores. The standard deviation for such derived change in scores was calculated using the test‐retest reliability correlations reported in the paper for the relevant instrument or a value of 0.9 as estimated from the placebo group in Kramer and Feguine (Kramer 1981), and from each group in Petti and Law (Petti 1982).
Assessment of heterogeneity
Heterogeneity was determined primarily on clinical grounds, based on the variability in depression measures used across the studies. We assessed statistical heterogeneity of intervention effects by visually inspecting the overlap of confidence intervals (CI) on the forest plots, tested for heterogeneity using the Chi2 test, and quantified heterogeneity using the I2 statistic (Higgins 2003). Categories suggested in Higgins 2011 (Chapter 9.5.2) are used to help interpret the degree of heterogeneity (0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; 75% to 100% considerable heterogeneity).
Assessment of reporting biases
Included studies were carried out in the era prior to the establishment of clinical trials registers. Therefore, the investigation of selective reporting was not possible.
Data synthesis
Because of the expected heterogeneity of outcome measures used across the studies, pooling of effect sizes and risk ratios (RR) was based on the random‐effects model. Where trials did not report data suitable for meta‐analysis, treatment estimates or raw data (as appropriate for each outcome) for each individual study are reported in additional tables.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were conducted for data specifically involving children aged 6 to 12 years and data involving adolescents aged 12 to 18 years. The reason for doing so is that there is anecdotal evidence for differential responses to tricyclic drugs in the two age bands.
Sensitivity analysis
Post‐hoc sensitivity analyses were undertaken to investigate the inclusion of trials that included participants who had already failed to respond to at least one antidepressant drug.
Results
Description of studies
Results of the search
The search on the CCDANCTR‐Studies Register produced 22 studies. The search on the CCDANCTR‐References Register produced 155 references. Of these 14 studies were selected for inclusion, four studies were excluded, and one study awaits classification.
The studies included, span the period 1981 to 2001.
Included studies
Fourteen relevant RCTs were included; for full details see Characteristics of included studies.
One new study was found since the publication of the previous version of the review. More details can be found below under: 'New studies found at this update'.
One study awaits classification (Berard 1998). To date, we have not been successful in contacting the author to obtain further information.
It was not necessary to contact trialists for missing data as the published reports of the included studies contributed the data required for the primary analyses.
Design
All included studies employed a randomised double‐blind design with a placebo control. One study included a third arm comprising alprazolam (Bernstein 1990), while another included a third arm comprising paroxetine (Keller 2001). One study included concomitant cognitive behaviour therapy in both the treatment and control arms of the trial (Bernstein 2000). One study employed a cross‐over design (Kashani 1984). All but one study were single‐centre trials (Keller 2001).
Sample sizes
Sample sizes ranged from seven (Petti 1982), to 182 (Keller 2001), participants.
Setting
One study was conducted in Canada, the remainder were conducted in the USA. Participants were outpatients in eight trials, inpatients of child or adolescent psychiatric units in five trials, and a mixture of inpatients and outpatients in one trial.
Participants
Eight trials were directed to adolescents (aged 12 years and over), four trials were directed to children (aged 11 years and under) and two trials involved participants spanning childhood and adolescence. Gender was reported in 11 of 14 studies, yielding 297 female and 235 male participants. Exclusion criteria were inconsistent across studies. Most studies sought to exclude people with comorbid schizophrenia, bipolar disorder, pervasive developmental disorder, substance abuse and serious medical conditions, but studies variably excluded conditions such as obsessive compulsive disorder, conduct disorder and attention deficit hyperactivity disorder. It is likely, therefore, that patterns of comorbidity differed across studies.
Interventions
Six trials involved imipramine, four trials involved amitriptyline, two trials involved desipramine and two trials involved nortriptyline. The control treatment in all cases was inactive placebo. Methods of determining doses, where reported, ranged in sophistication from fixed milligram per kilogram doses to weekly plasma level monitoring. The number of doses of drug per day ranged from one to three.
Outcomes
Heterogeneous and generally multiple methods were used for measuring response to treatment or changes in depressive symptoms. No single measure was used sufficiently often across studies to warrant consideration as a 'gold standard' measure. Our strategy for managing the heterogeneity of outcome measures is described under Types of outcome measures. Clinical global assessment scale changes were reported by a sufficient number of studies to enable these data to be pooled.
Follow‐up intervals ranged from 4 to 10 weeks. The shorter follow‐up intervals are arguably insufficient to determine treatment responsiveness adequately.
Excluded studies
Four studies were excluded (see Characteristics of excluded studies). Although improvement in depression was an outcome in the study reported by Berney et al., the trial was directed to young people referred for school refusal (Berney 1981). Less than half of the sample experienced clinically significant depressive symptoms and it was not feasible to obtain data for this subgroup of participants. The study reported by Preskorn did not employ a placebo control (Preskorn 1982). The focus of the study was to report the response to varying plasma levels of imipramine. In the study reported by Sallee et al. clomipramine was administered intravenously in a bolus, therefore, the study fell outside the inclusion criteria for the review (Sallee 1997). Lucas 1965 was excluded because the participants were not diagnosed with depression.
Ongoing studies
No ongoing studies were identified.
Studies awaiting classification
One study awaits classification (Berard 1998) (see Characteristics of studies awaiting classification). Further information is required, but we have, to date, been unsuccessful in contacting the author.
New studies found at this update
One new study has been added to the review (Bernstein 2000). The trial was directed to young people referred for school refusal, but presence of a depressive disorder ascertained by structured diagnostic interview was a requirement for inclusion in the study, and measures of depressive symptoms were included in the outcomes.
Risk of bias in included studies
See: Characteristics of included studies. For graphical representations of the overall risk of bias in included studies, see Figure 1 and Figure 2.
1.
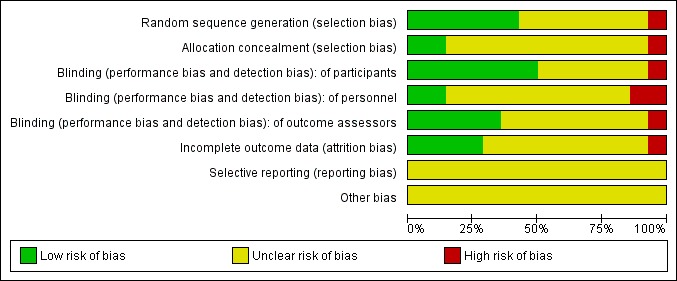
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
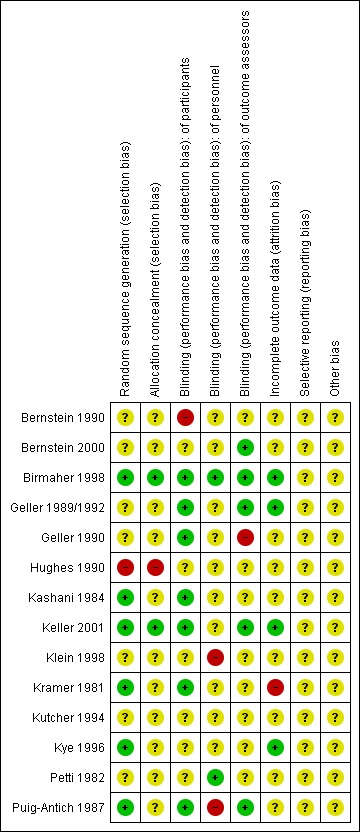
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All the included studies except for Birmaher 1998 and Keller 2001 were unclear about concealment of allocation. This was identified as a source of potential selection bias as detailed in the Characteristics of included studies table.
Blinding
Seven of the 14 studies were rated as at low risk of unblinding of participants, the remainder were rated as uncertain risk. Two studies were rated as at low risk for unblinding of study personnel, three were rated as high risk and nine were rated as uncertain risk. Five studies were rated as at low risk of unblinding of outcome assessors, one was rated high risk and eight were rated as uncertain risk.
Incomplete outcome data
Only one study did not report on attrition (Kramer 1981). Eight studies stated the reasons for withdrawal without modifying their analysis and five studies completed an intention‐to‐treat analysis.
Selective reporting
Included studies were carried out in the era prior to the establishment of clinical trials registers. Therefore, systematic investigation of selective reporting of data was not possible. All included studies reported multiple outcome measure, and, in the main, there were no statistical differences favouring tricyclic drugs over placebo. It is unlikely, therefore, that non‐significant outcomes were suppressed. Side effects were systematically reported in only five of 14 studies; therefore, it is possible that important side effect or adverse event data have not been reported.
Other potential sources of bias
None were identified.
Effects of interventions
See: Table 1
Comparison 1: tricyclic versus placebo
Primary outcomes
1. Achieved remission/response as per predetermined criteria
Compared with placebo, there was no difference in the percentage of participants who achieved remission when taking a tricyclic drug (9 trials; N = 454; RR 1.07, 95% CI 0.91 to 1.26) (Analysis 1.1). RR for individual studies ranged from 0.43 to 1.44, but no individual study result reached statistical significance. There was general consistency in study results (Chi2 = 5.41, degrees of freedom (df) = 8 (P value = 0.71); I2 = 0%).
1.1. Analysis.
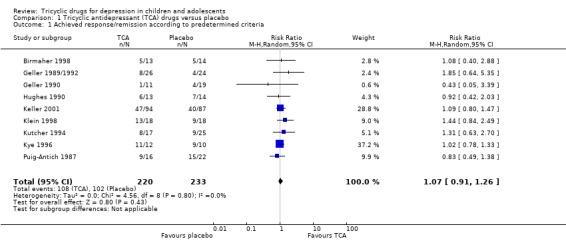
Comparison 1 Tricyclic antidepressant (TCA) drugs versus placebo, Outcome 1 Achieved response/remission according to predetermined criteria.
2. Change in depressive checklist scores
Compared with placebo, there was a small reduction in depression symptom scores for participants taking a tricyclic drug (13 trials; N = 533; SMD ‐0.32, 95% CI ‐0.59 to ‐0.04) (Analysis 1.2). The majority of estimates were in the same direction, favouring tricyclic drugs, but there was significant heterogeneity (Chi2 = 24.20, df = 12 (P value = 0.02); I2 = 50%). The treatment‐placebo difference was only statistically significantly different in two studies (Klein 1998; Kramer 1981).
1.2. Analysis.
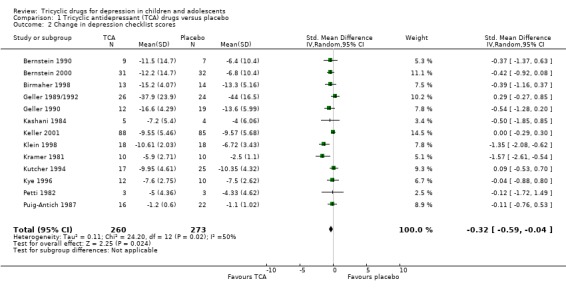
Comparison 1 Tricyclic antidepressant (TCA) drugs versus placebo, Outcome 2 Change in depression checklist scores.
3. Adverse events
Only five studies systematically reported side effects. There were no differences between treatment and control groups on the following side effects: tiredness, sleep problems, headache, palpitations, perspiration, constipation or problems with micturition (Analysis 1.3).
1.3. Analysis.
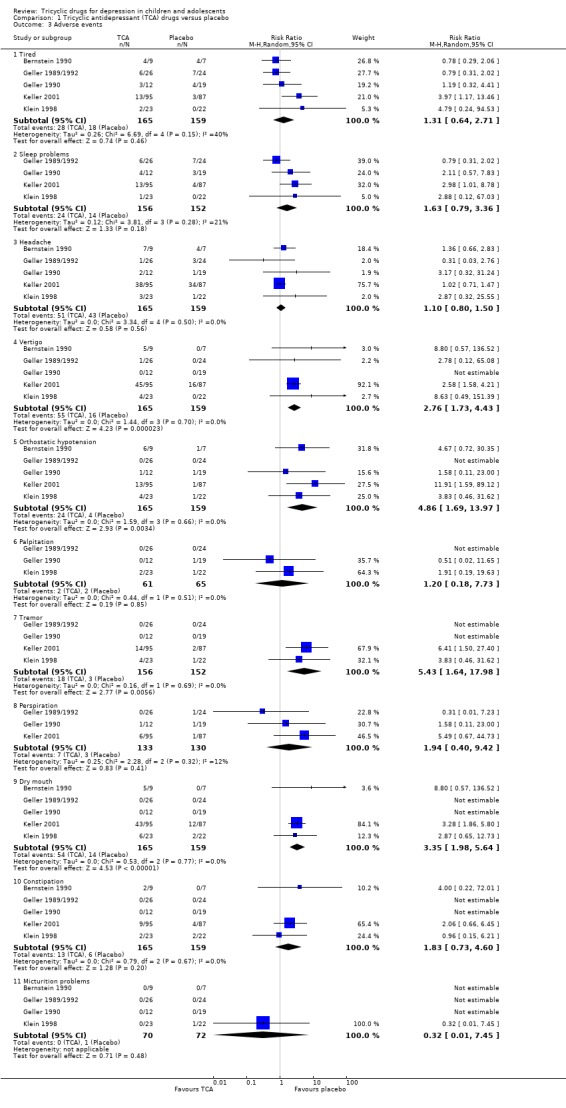
Comparison 1 Tricyclic antidepressant (TCA) drugs versus placebo, Outcome 3 Adverse events.
More people receiving tricyclic drugs experienced vertigo than those given placebo (RR 2.76, 95% CI 1.73 to 4.43; 5 trials, N = 324) (Analysis 1.3). More people in the tricyclic drugs group experienced orthostatic hypotension than in the placebo control group (RR 4.86, 95% CI 1.69 to 13.97; 5 trials, N = 324) (Analysis 1.3). More people in the tricyclic drugs group experienced tremor than in the placebo group (RR 5.43, 95% CI 1.64 to 17.98; 4 trials, N = 308) (Analysis 1.3); and more people in the tricyclic drugs group experienced dry mouth than in the placebo group (RR 3.35, 95% CI 1.98 to 5.64; 5 trials, N = 324) (Analysis 1.3).
Secondary outcomes
4. Global improvement
The effect size was calculated for change in clinical global assessment scale scores for five studies (180 participants). There was no difference between tricyclic drug treatment and placebo control (SMD ‐0.10, 95% CI ‐0.40 to 0.20) (Analysis 1.4).
1.4. Analysis.
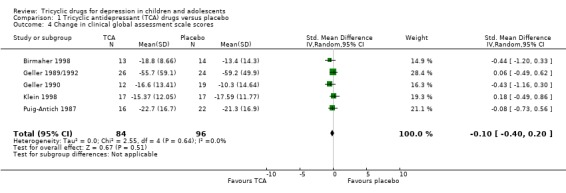
Comparison 1 Tricyclic antidepressant (TCA) drugs versus placebo, Outcome 4 Change in clinical global assessment scale scores.
5. Discontinuation rates
There was no difference in the percentages of participants who withdrew from placebo control treatment compared with tricyclic drug treatment (8 trials, N = 462; RR 1.48, 95% CI 0.94 to 2.31) (Analysis 1.5).
1.5. Analysis.
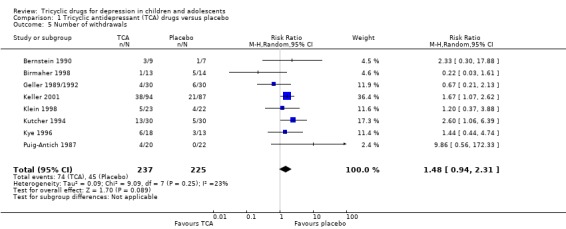
Comparison 1 Tricyclic antidepressant (TCA) drugs versus placebo, Outcome 5 Number of withdrawals.
Subgroup analyses
Subgroup analyses were conducted for studies reporting data exclusively on adolescent or children.
Children aged 6 to 12 years
Achieved remission/response as per predetermined criteria: compared with placebo, there was no difference in the percentage of children who achieved remission/response criteria when taking a tricyclic drug (2 trials; N = 77; RR 1.19, 95% CI 0.61 to 2.34) (Analysis 2.1).
2.1. Analysis.
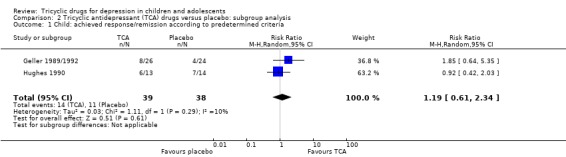
Comparison 2 Tricyclic antidepressant (TCA) drugs versus placebo: subgroup analysis, Outcome 1 Child: achieved response/remission according to predetermined criteria.
Change in depression checklist scores: compared with placebo, there was no difference in change in depression symptom scores for children taking a tricyclic drug (3 trials; N = 65; SMD 0.15, 95% CI ‐0.34 to 0.64) (Analysis 2.2).
2.2. Analysis.
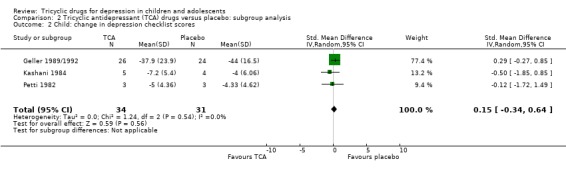
Comparison 2 Tricyclic antidepressant (TCA) drugs versus placebo: subgroup analysis, Outcome 2 Child: change in depression checklist scores.
Adolescents aged 12 to 18 years
Achieved remission/response as per predetermined criteria: compared with placebo, there was no difference in the percentage of adolescents who achieved remission/response criteria when taking a tricyclic drug (6 trials; N = 339; RR 1.01, 95% CI 0.85 to 1.19) (Analysis 2.3).
2.3. Analysis.
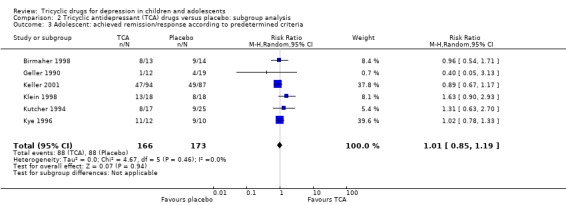
Comparison 2 Tricyclic antidepressant (TCA) drugs versus placebo: subgroup analysis, Outcome 3 Adolescent: achieved remission/response according to predetermined criteria.
Change in depression checklist scores: compared with placebo, there was a small reduction in depression symptom scores for adolescents taking a tricyclic drug (8 trials; N = 414; SMD ‐0.45, 95% CI ‐0.83 to ‐0.07) (Analysis 2.4).
2.4. Analysis.
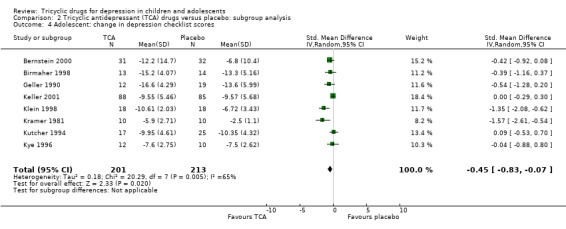
Comparison 2 Tricyclic antidepressant (TCA) drugs versus placebo: subgroup analysis, Outcome 4 Adolescent: change in depression checklist scores.
Sensitivity analyses
Sensitivity analyses were undertaken to investigate the effect of trials involving participants who had already failed to respond to at least one antidepressant drug.
Because the Birmaher study concerned people who had already failed to respond to at least one antidepressant treatment (Birmaher 1998), and included some people outside the study age range, values were recalculated with the Birmaher data removed. The RR remission/response and the SMD for reduction in depression checklist scores remained unchanged.
Discussion
Summary of main results
The review has yielded equivocal results. On primary outcomes, low‐quality evidence showed a small effect favouring tricyclic drug over placebo in the reduction of depressive symptoms while there was no difference in response/remission rates. On secondary outcomes, low‐quality evidence showed no difference in global assessment scores; while consistent with the known pharmacological action of tricyclic drugs, certain side effects, such as orthostatic hypotension and dry mouth, were more common with active treatment than placebo. Subgroup analyses showed the effect of tricyclic drugs on reducing depressive symptoms in adolescents was in the range negligible to moderate, while for children the effect was in the range small favouring tricyclic drug to moderate favouring placebo. To place the effect of tricyclic drug treatment in perspective, 482/1000 participants might be expected to respond or remit with tricyclic treatment compared with 450/1000 treated with placebo, yielding a net increase of 32/1000.
For side effects, all but tiredness, perspiration and micturition problems occurred in the tricyclic group more frequently than the placebo group, but only the difference in rates of vertigo, orthostatic hypotension, tremor and dry mouth reached statistical significance. Based on these data, side effects are not likely to be the main barrier to the clinical usefulness of tricyclic antidepressants in juvenile depression. However, only a minority of studies reported side effects in a systematic fashion. We consider this an important omission. No serious adverse events were reported in the trials. That said, a spate of sudden deaths in children associated with apparently therapeutic doses of tricyclic drugs was reported through the 1990s (Varley 1997). In accidental or deliberate overdose, tricyclic drugs may cause seizures, coma and cardiac arrhythmias (Olgun 2009). Such problems are unlikely to occur, however, if the amount ingested of, for example, amitriptyline is less than 5 mg/kg (McFee 2001). The equivocal benefits of tricyclic drugs for depression in children and adolescents is outweighed by the small but important risk of toxicity at therapeutic doses, and the greater risk of toxicity in overdose.
Overall completeness and applicability of evidence
The results of this meta‐analysis suggest that tricyclic drugs do not increase the likelihood that children and adolescents who are depressed will remit or respond. Tricyclic drugs may lead to a small reduction in depression symptoms in adolescents, but not children. The latter result makes intuitive sense, since it should be expected that samples of depressed adolescents would show a pattern of response to tricyclic drugs that moves towards the response seen in adult populations. The systematic review has included studies covering a variety of study populations in different sites treated with a range of tricyclic drugs in various doses. Nevertheless, it is by no means a comprehensive study of all possible participants, investigations and outcomes. Use of tricyclic drugs in children and adolescents has significantly diminished since the first version of this study was published, to be replaced by the prescription of SSRI drugs and other newer‐generation antidepressants. A companion Cochrane review of 16 eligible RCTs of newer‐generation antidepressants for depression in children and adolescents found an RR for response or remission of 1.18 (95% CI 1.08 to 1.28) (Hetrick 2012). The magnitude of reduction in depression symptoms cannot be directly compared between the tricyclic and newer‐generation antidepressant reviews, because different methods were used to analyse the data. While superiority of SSRI drugs as a class over placebo was statistically significant, the authors of the review correctly identified that the effect was small and of uncertain clinical significance. While SSRIs have been shown to increase the risk of suicide‐related behaviours in the short term compared with placebo (Hetrick 2012), there is a body of evidence demonstrating that unlike the situation with tricyclic drugs, fatal outcomes with SSRIs overdose are extremely rare (Barbey 1998; Hayes 2010; Klein‐Schwartz 1996; Phillips 1997). SSRIs therefore, offer a safer alternative than tricyclic drugs and are possibly more effective in children and adolescents, although the benefits of treatment are modest.
Quality of the evidence
Fourteen studies examining the efficacy of tricyclic drugs in the treatment of child and adolescent depression were amenable to analysis using a meta‐analytic approach. Nine studies (454 participants) reported response or remission data. The sample size just has sufficient power at the 80% level to detect a significant RR for failure to recover or respond of 0.50 or less. Most of the included studies were conducted in the era before methodology of treatment trials for depression in children and adolescents was standardised. As such, there was considerable heterogeneity in the clinical tools and methods used in the assessment of improvement. It was unusual in this era for investigators to specify a primary outcome measure. We have attempted to overcome this by using a systematic approach to selecting the highest quality outcome measure. Variable and usually short period of follow‐up (2 to 10 weeks) should also be considered a limitation. Nevertheless, the results of the studies were consistent. Only two of 14 studies show a positive treatment effect on any measure. The studies were also conducted in the era before standardised reporting of trial methodology (e.g. Consolidated Standards of Reporting Trials (CONSORT)). Risk of bias was, therefore, difficult to determine across most domains because of inadequate reporting. That said, blinding of participants was reported most frequently, while concealment of allocation was reported least frequently.
Potential biases in the review process
Studies published in languages other than English may have been missed in the process of the literature search. We included only orally administered tricyclic drugs as this reflects the manner in which these drugs are typically delivered. However, this decision precluded the possibility of identifying that tricyclic drugs are more effective if administered by other routes. The original published version of this review (Hazell 1995), which was based on a smaller number of trials, concluded that tricyclic drugs were unlikely to be beneficial for depression in children and adolescents. It is possible that we have allowed that conclusion to influence the interpretation of more recent trial data.
Agreements and disagreements with other studies or reviews
The results are consistent with findings of the earlier review by Hazell et al (Hazell 1995). However, unlike Hazell 1995, the present review has sufficient power at the 80% level to detect a significant OR for failure to improve of 0.50 or less. The 95% CI indicated that any improvement in depression rating scores in the treated group compared with the control group is likely to be less than 0.59 standard deviations, which is lower than the estimate of 0.86 reported by Hazell et al. (Hazell 1995). The pooled estimate effect size of 0.32 was statistically significant, but is well below the two standard deviation difference required to produce a 50% reduction in depression rating scores from baseline to follow‐up (Byrne 1989). Again, we conclude that the data indicate no clinically significant treatment effect.
A study by Sallee et al. involving intravenous administration of clomipramine to depressed adolescents aged between 14 and 18 years was excluded from the present analyses owing to its novel and experimental nature (Sallee 1997). However, the authors reported a statistically non‐significant trend favouring treatment, with only one of eight actively treated adolescents failing to achieve 50% reduction in HAM‐D scale scores after six days, compared with five of eight adolescents treated with saline (Chi2 = 2.4, df = 1, P value = 0.12). This method of administration warrants further investigation in a larger sample, and may have special applicability to severely affected adolescents for whom rapid reduction in depressive symptoms is sought.
There are several plausible neuropharmacological explanations why tricyclic drugs are not efficacious for children or adolescent depression (Ryan 1992). In children, there is incomplete maturation of the neurotransmitter systems involved in the control of affect. The noradrenergic system does not fully develop until early adulthood, while the more rapid hepatic metabolism of tricyclic compounds in children shifts the ratio of noradrenergic to serotonergic activity in the direction of noradrenergic activity. In addition, adolescents have high ketosteroid levels, which also affect noradrenergic transmitter systems. In theory at least, selective serotonergic compounds may be expected to have greater efficacy than noradrenergic compounds in juveniles. This is supported by the preliminary evidence for efficacy of intravenously administered clomipramine (Sallee 1997), which is highly serotonergic compared with other tricyclic compounds, and the positive treatment effect reported in one of two published trials of the SSRI, fluoxetine (Emslie 1997). The hormonal milieu of adolescent brains may also influence neurotransmitter activity, but the mechanism is unknown. There is also a possibility that childhood‐onset depressive illness is aetiologically distinct from adult‐onset depressive illness, and that adults who are depressed with a childhood onset of their disorder may also be relatively non‐responsive to tricyclic drugs (Jensen 1992). The likelihood of a substantial level of psychiatric comorbidity among depressed children and adolescents may also confound their response to treatment.
The data from the present review do suggest an increasing responsiveness to tricyclic drugs with increasing age. The role of the onset of puberty in mediating the response to tricyclic drugs warrants further investigation.
Authors' conclusions
Implications for practice.
These data suggest that tricyclic drugs are not useful for treating depression in prepubertal children. There may be a place for the use of tricyclic drugs in adolescent depression, although the treatment effects are likely to be modest. While it is tempting to argue that tricyclic drugs should be considered second‐line treatment for depression in adolescents after other pharmacotherapy or psychotherapy has failed, the one trial that used tricyclic drugs in this context demonstrated no evidence of treatment benefit (Birmaher 1998).
Implications for research.
We consider further replication studies using 'traditional' tricyclic drugs with mixed noradrenergic and serotonergic activity to be unwarranted. Pharmacological research with newer‐generation antidepressants has yielded more favourable results, but the effects are modest and of uncertain clinical significance (Hetrick 2012). Treatment research should examine other widely adopted strategies such as family therapy, supportive psychotherapy and specific psychotherapies. The benefits of hospitalisation over day hospitalisation or outpatient treatment should also be considered.
What's new
| Date | Event | Description |
|---|---|---|
| 13 June 2013 | New search has been performed | Methodology updated |
| 13 June 2013 | New citation required but conclusions have not changed | Searches updated and one new study incorporated |
History
Protocol first published: Issue 1, 1996 Review first published: Issue 3, 2000
| Date | Event | Description |
|---|---|---|
| 12 March 2010 | Amended | Contact author's details updated. |
| 6 November 2008 | Amended | Converted to new review format. |
| 12 February 2008 | Amended | An updated search was run on 12/2/2008 but no new studies were identified. |
| 26 February 2002 | New citation required and conclusions have changed | Substantive amendment |
Notes
We do not anticipate further trials will be undertaken in this area and so we will not seek to update this review routinely in future. If we become aware of any new trials, we will revisit this decision.
Acknowledgements
The work of conducting this review was supported in part by a Research Infrastructure Grant from the University of Newcastle, awarded to the Newcastle Systematic Review Group. We also acknowledge the assistance of Jane Robertson, Diane Heathcote, David Henry and Dianne O'Connell in preparing earlier versions of this review.
CRG Funding Acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Depression, Anxiety and Neurosis Group.
Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service or the Department of Health.
Appendices
Appendix 1. Previous search strategies
We searched the literature using CD ROM Silver Platter and on‐Line MEDLINE (1966‐1997), EMBASE and Excerpta Medica (June 1974‐1997) databases. Terms used for the search were: exploded terms for child and depression; subject headings of antidepressant drugs, tricyclic and affective disorders; individual tricyclic drugs by name; names of well‐known researchers in the field; and school phobia. We searched the trials database of the Cochrane Collaboration Depression, Anxiety and Neurosis Group (CCDANCTR). Abstracts in English (of English and non‐English papers) were reviewed.
CCDANCTR‐Studies (searched up to 12/2/2008) Diagnosis = Depress* or Dysthymi* or "Adjustment Disorder*" or "Mood Disorder*" or "Affective Disorder" or "Affective Symptoms" and Age‐Group = Child or Adolescent and Free‐text = "Tricyclic Drugs" or Amersergide or Amineptine or Amitriptyline or Amoxapine or Butriptyline or Clomipramine or Clorimipramine or Demexiptiline or Desipramine or Dibenzipin or Dothiepin or Doxepin or Imipramine or Lofepramine or Melitracen or Metapramine or Nortriptyline or Noxiptiline or Opipramol or Protriptyline or Quinupramine or Tianeptine or Trimipramine and Intervention = Placebos
CCDANCTR‐References (searched up to 12/2/2008) Keyword = Depress* or Dysthymi* or "Adjustment Disorder*" or "Mood Disorder*" or "Affective Disorder" or "Affective Symptoms" and Free‐text = child* or adolesc* or pediatr* or paediatr* or juvenil* or school* or pup* and Free‐text = "Tricyclic Drugs" or Amersergide or Amineptine or Amitriptyline or Amoxapine or Butriptyline or Clomipramine or Clorimipramine or Demexiptiline or Desipramine or Dibenzipin or Dothiepin or Doxepin or Imipramine or Lofepramine or Melitracen or Metapramine or Nortriptyline or Noxiptiline or Opipramol or Protriptyline or Quinupramine or Tianeptine or Trimipramine
Data and analyses
Comparison 1. Tricyclic antidepressant (TCA) drugs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Achieved response/remission according to predetermined criteria | 9 | 453 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.91, 1.26] |
| 2 Change in depression checklist scores | 13 | 533 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.59, ‐0.04] |
| 3 Adverse events | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Tired | 5 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.64, 2.71] |
| 3.2 Sleep problems | 4 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.79, 3.36] |
| 3.3 Headache | 5 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.80, 1.50] |
| 3.4 Vertigo | 5 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 2.76 [1.73, 4.43] |
| 3.5 Orthostatic hypotension | 5 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 4.86 [1.69, 13.97] |
| 3.6 Palpitation | 3 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.18, 7.73] |
| 3.7 Tremor | 4 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 5.43 [1.64, 17.98] |
| 3.8 Perspiration | 3 | 263 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [0.40, 9.42] |
| 3.9 Dry mouth | 5 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 3.35 [1.98, 5.64] |
| 3.10 Constipation | 5 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [0.73, 4.60] |
| 3.11 Micturition problems | 4 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.45] |
| 4 Change in clinical global assessment scale scores | 5 | 180 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.40, 0.20] |
| 5 Number of withdrawals | 8 | 462 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.94, 2.31] |
Comparison 2. Tricyclic antidepressant (TCA) drugs versus placebo: subgroup analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Child: achieved response/remission according to predetermined criteria | 2 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.61, 2.34] |
| 2 Child: change in depression checklist scores | 3 | 65 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.34, 0.64] |
| 3 Adolescent: achieved remission/response according to predetermined criteria | 6 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.85, 1.19] |
| 4 Adolescent: change in depression checklist scores | 8 | 414 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.83, ‐0.07] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bernstein 1990.
| Methods | Randomised double‐blind control trial of imipramine, alprazolam and placebo | |
| Participants | Outpatients aged 7‐17 years referred with school refusal Gender unknown 9 active treatment, 7 placebo 3 withdrawals |
|
| Interventions | Imipramine up to 200 mg/day | |
| Outcomes | Children's Depression Rating Scale Number of withdrawals Side effect rating scale Follow‐up interval 8 weeks | |
| Notes | Study quality score = 22 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as randomised but no further description was provided |
| Allocation concealment (selection bias) | Unclear risk | No description was provided |
| Blinding (performance bias and detection bias) of participants | High risk | Remarkable variation in frequency and dose of medications between study arms |
| Blinding (performance bias and detection bias) of personnel | Unclear risk | No description was provided |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | No description was provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number and reason for withdrawal was provided but analysis was not modified |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Other bias | Unclear risk | Baseline observation and previous treatment was not stated |
Bernstein 2000.
| Methods | Randomised double‐blind control trial of imipramine versus placebo each in combination with cognitive behavioural therapy | |
| Participants | Outpatients aged 12‐18 years referred with school refusal 38 females, 25 males 31 active treatment, 32 placebo 16 withdrawals |
|
| Interventions | Imipramine up to 3 mg/kg in 2 divided dose | |
| Outcomes | Anxiety Rating for Children‐Revised Children's Depression Rating Scale‐Revised Revised Children's Manifest Anxiety Scale Beck Depression Inventory School attendance Side Effects Rating Clinical Global Impressions Follow‐up interval 2 weeks Family Adaptability and Cohesion Evaluation Scale II |
|
| Notes | Study quality score = 28 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as random but no further description was provided |
| Allocation concealment (selection bias) | Unclear risk | No description was provided |
| Blinding (performance bias and detection bias) of participants | Unclear risk | No description was provided |
| Blinding (performance bias and detection bias) of personnel | Unclear risk | No description was provided |
| Blinding (performance bias and detection bias) of outcome assessors | Low risk | Assessors were an independent child psychiatrist who was not involved with the earlier stages of the study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number and reason for withdrawal was provided but analysis was not modified |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Other bias | Unclear risk | Previous treatment was not stated |
Birmaher 1998.
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | Inpatients aged 12‐18 years who had not responded to trial of at least 1 other antidepressant with or without lithium augmentation 19 female, 8 male 13 active treatment, 14 placebo 6 withdrawals | |
| Interventions | Amitriptyline titrated up to a maximum of 5 mg/kg/day in 3 divided doses | |
| Outcomes | Depression items on K‐SADS‐P for dichotomous data Hamilton Depression Rating Scale for continuous data Clinical Global Assessment Scale Number of withdrawals Follow‐up interval 10 weeks | |
| Notes | Study quality score = 28 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Efron's biased coin design was used in order to match for sex and age |
| Allocation concealment (selection bias) | Low risk | ‐ |
| Blinding (performance bias and detection bias) of participants | Low risk | Visually identical tablets were used |
| Blinding (performance bias and detection bias) of personnel | Low risk | Side effects were monitored by an independent paediatric team. The only non‐blind investigator who monitored response to treatment, dose and side effects was not involved in outcome investigation |
| Blinding (performance bias and detection bias) of outcome assessors | Low risk | Side effects were monitored by an independent paediatric team. The only non‐blind investigator who monitored response to treatment, dose and side effects was not involved in outcome investigation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis was performed |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Other bias | Unclear risk | ‐ |
Geller 1989/1992.
| Methods | Randomised double‐blind placebo‐controlled trial using fixed plasma level | |
| Participants | Outpatients aged 6‐12 years 15 female, 35 male 26 active treatment, 24 control 10 withdrawals | |
| Interventions | Nortriptyline. Dose calculated to obtain steady state plasma levels of 60‐100 ng/mL | |
| Outcomes | Depression items from the K‐SADS Clinical Global Assessment Scale Number of withdrawals Side effect rating scale Follow‐up interval 8 weeks | |
| Notes | Study quality score = 28 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as random but no further description was provided |
| Allocation concealment (selection bias) | Unclear risk | No description was provided |
| Blinding (performance bias and detection bias) of participants | Low risk | Visually identical capsules given at the same frequency was used |
| Blinding (performance bias and detection bias) of personnel | Unclear risk | No description was provided |
| Blinding (performance bias and detection bias) of outcome assessors | Low risk | Different raters who had established inter‐rater reliability were used throughout the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary analysis was based on all cases randomised |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Other bias | Unclear risk | Current and previous treatments were not stated |
Geller 1990.
| Methods | Randomised double‐blind placebo‐controlled trial using fixed plasma level | |
| Participants | Depressed outpatients aged 12‐17 years Gender unknown 12 active treatment, 19 placebo 4 withdrawals | |
| Interventions | Nortriptyline in a dose sufficient to maintain steady state plasma levels at 60‐100 ng/mL | |
| Outcomes | Children's Depression Rating Scale Number of withdrawals Side effect rating scale Follow‐up interval 8 weeks | |
| Notes | Study abandoned at midpoint owing to very low likelihood of demonstrating statistically significant treatment effect Study quality score = 25 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as random but no further description was provided |
| Allocation concealment (selection bias) | Unclear risk | No description was provided |
| Blinding (performance bias and detection bias) of participants | Low risk | Visually identical capsules given at the same frequency were used |
| Blinding (performance bias and detection bias) of personnel | Unclear risk | No description was provided |
| Blinding (performance bias and detection bias) of outcome assessors | High risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers and reasons for withdrawals were provided but analysis was not modified |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Other bias | Unclear risk | Concurrent and previous treatments were not stated |
Hughes 1990.
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | Depressed inpatients aged 6‐12 years Gender unknown 13 active treatment, 14 placebo 4 withdrawals | |
| Interventions | Imipramine. Dose not stated | |
| Outcomes | Children's Depression Rating Scale Follow‐up interval 6 weeks | |
| Notes | Study quality score = 18 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not stated |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) of participants | Unclear risk | No description was provided |
| Blinding (performance bias and detection bias) of personnel | Unclear risk | No description was provided |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | No description was provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers and reasons for withdrawals were provided but analysis was not modified |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Other bias | Unclear risk | Previous or concurrent treatments were not stated |
Kashani 1984.
| Methods | Randomised placebo‐controlled cross‐over trial | |
| Participants | 9 prepubertal children admitted to an inpatient unit, mean age 10.8 years (range 9‐12 years) 1 female, 8 male No withdrawals | |
| Interventions | Amitriptyline fixed dose 1.5 mg/kg | |
| Outcomes | Bellevue Index of Depression Follow‐up interval 4 weeks | |
| Notes | Study quality score = 22 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study was described as random‐order cross‐over design. A random assignment schedule was provided by the drug company |
| Allocation concealment (selection bias) | Unclear risk | No description was provided |
| Blinding (performance bias and detection bias) of participants | Low risk | Visually identical tablets for both arms of the study were provided by the drug company |
| Blinding (performance bias and detection bias) of personnel | Unclear risk | No description was provided |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Primary analysis was based on all cases as randomised |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Other bias | Unclear risk | Current and past treatment, comorbidity and level of compliance was not stated |
Keller 2001.
| Methods | Randomised double‐blind 3‐arm placebo‐controlled trial that also included the serotonin reuptake inhibitor drug, paroxetine | |
| Participants | Psychiatric outpatients aged 12‐18 years 69 males and 113 females randomised to receive imipramine or placebo. A further 35 males and 58 males received paroxetine | |
| Interventions | Imipramine 200‐300 mg/day in divided doses | |
| Outcomes | Hamilton Rating Scale for Depression Schedule for Affective Disorders and Schizophrenia for Adolescents Lifetime version Clinical Global Impression scale Follow‐up interval 8 weeks | |
| Notes | Study quality score = 28 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out using a computer‐generated table |
| Allocation concealment (selection bias) | Low risk | ‐ |
| Blinding (performance bias and detection bias) of participants | Low risk | Visually identical capsules were used for all 3 arms of the study |
| Blinding (performance bias and detection bias) of personnel | Unclear risk | No description was provided |
| Blinding (performance bias and detection bias) of outcome assessors | Low risk | Assessors were blind to the subject allocation and used a standard assessment toll |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A last observation carried forward dataset was analysed along with a completer dataset |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Other bias | Unclear risk | Level of compliance was not stated |
Klein 1998.
| Methods | Randomised placebo‐controlled trial | |
| Participants | Psychiatric outpatients aged 13‐17 years 30 female, 15 male 18 active treatment, 18 placebo 9 withdrawals | |
| Interventions | Desipramine titrated to a maximum of 300 mg/day | |
| Outcomes | Clinician interview for dichotomous data Hamilton Depression Rating Scale for continuous data Clinical Global Assessment Scale Number of withdrawals Side effect rating scale Follow‐up interval 6 weeks | |
| Notes | Study quality score = 24 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as random but no further description was provided |
| Allocation concealment (selection bias) | Unclear risk | No description was provided |
| Blinding (performance bias and detection bias) of participants | Unclear risk | No description was provided |
| Blinding (performance bias and detection bias) of personnel | High risk | Medication was prescribed by a psychiatrist who also monitored the side effects and provided supportive therapy |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | No description was provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number and reason for withdrawal was given but analysis was not modified |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Other bias | Unclear risk | Level of compliance was not assessed |
Kramer 1981.
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | Depressed adolescent inpatients aged 13‐17 years 13 female, 7 male 10 active treatment, 10 placebo No withdrawals | |
| Interventions | Amitriptyline to a maximum of 200 mg/day | |
| Outcomes | Depression Adjective Checklist Follow‐up interval 6 weeks | |
| Notes | Study quality score = 18 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study was described as double‐blind experimental design using a random distribution table provided by Merck, Sharp, and Dohme |
| Allocation concealment (selection bias) | Unclear risk | No description was provided |
| Blinding (performance bias and detection bias) of participants | Low risk | Matching medication schedule was followed |
| Blinding (performance bias and detection bias) of personnel | Unclear risk | No description was provided |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | No description was provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only number of withdrawal was provided |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Other bias | Unclear risk | Level of compliance, baseline observation and previous treatment was not provided |
Kutcher 1994.
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | Depressed outpatients aged 15‐20 years 42 female, 18 male 17 active treatment, 25 placebo 18 withdrawals | |
| Interventions | Desipramine 200 mg/day | |
| Outcomes | Hamilton Depression Rating Scale Number of withdrawals Follow‐up interval 6 weeks | |
| Notes | Study quality score = 24 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as random but no further description was provided |
| Allocation concealment (selection bias) | Unclear risk | No description was provided |
| Blinding (performance bias and detection bias) of participants | Unclear risk | Visually identical placebo was used |
| Blinding (performance bias and detection bias) of personnel | Unclear risk | No description was provided |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | No description was provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number and reason for withdrawal was given but analysis was not modified |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Other bias | Unclear risk | Concurrent treatment was not identified |
Kye 1996.
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | Depressed outpatients aged 12‐17 years 9 female, 22 male 12 active treatment, 10 placebo 9 withdrawals | |
| Interventions | Amitriptyline 5 mg/kg/day up to maximum 300 mg/day | |
| Outcomes | Hamilton Depression Rating Scale for dichotomous data Depression items from K‐SADS for continuous data Number of withdrawals Follow‐up interval 8 weeks | |
| Notes | Study quality score = 24 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Subjects were randomised using Efron's biased coin design to approximately match for age, sex and the presence of melancholia |
| Allocation concealment (selection bias) | Unclear risk | No description was provided |
| Blinding (performance bias and detection bias) of participants | Unclear risk | No description was provided |
| Blinding (performance bias and detection bias) of personnel | Unclear risk | No description was provided |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | No description was provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis was carried out |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Other bias | Unclear risk | Concurrent or previous treatment were not identified |
Petti 1982.
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | Depressed inpatients aged 6‐12 years 1 female, 5 male 3 active treatment, 3 placebo 1 withdrawal | |
| Interventions | Imipramine 5 mg/kg/day | |
| Outcomes | Children's Depression Inventory Follow‐up interval 6 weeks | |
| Notes | Study quality score = 22 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as random but no further description was provided |
| Allocation concealment (selection bias) | Unclear risk | No description was provided |
| Blinding (performance bias and detection bias) of participants | Unclear risk | Described as blind but no description was provided |
| Blinding (performance bias and detection bias) of personnel | Low risk | Visually identical tablets and uniform frequency was used |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | Described as blind but no description was provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number and reason for withdrawal was provided but analysis was not modified |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Other bias | Unclear risk | Previous treatment and comorbidity was not stated |
Puig‐Antich 1987.
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | Mixed inpatients and outpatients with mean age 9 years 16 female, 22 male 16 active treatment, 22 placebo 5 withdrawals | |
| Interventions | Imipramine to a maximum of 5 mg/kg/day | |
| Outcomes | Depression items from the K‐SADS Number of withdrawals Follow‐up interval 5 weeks | |
| Notes | Study quality score = 27 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a table of random permutations matching the 2 groups for age, sex and outpatient versus inpatient status |
| Allocation concealment (selection bias) | Unclear risk | The double‐dummy technique was used |
| Blinding (performance bias and detection bias) of participants | Low risk | Identical shape and frequency of tablets were used for different arms of the study |
| Blinding (performance bias and detection bias) of personnel | High risk | The clinical monitor and the research nurse could highly accurately guess the allocation of the participants |
| Blinding (performance bias and detection bias) of outcome assessors | Low risk | Outcome assessors were blind to side effects and other clinical observation which could potentially breach the blindness |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number and reason for withdrawal was provided but analysis was not modified |
| Selective reporting (reporting bias) | Unclear risk | ‐ |
| Other bias | Unclear risk | Previous treatment was not stated |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Berney 1981 | Study involved clomipramine directed to school refusal. Depression was not an inclusion criterion for the trial. More than half the sample had absent or dubious depressive symptoms |
| Lucas 1965 | None of the participants had a diagnosis of depression |
| Preskorn 1982 | Study was not a randomised control trial. Responses to varying doses of imipramine were compared in an open‐label study |
| Sallee 1997 | Tricyclic drug administered intravenously in a bolus, which is outside inclusion criterion for the review |
Characteristics of studies awaiting assessment [ordered by study ID]
Berard 1998.
| Methods | Randomised, placebo‐controlled trial |
| Participants | 136 participants, aged 12‐18 years |
| Interventions | Imipramine (dose not stated) Moclobemide (dose not stated) Placebo |
| Outcomes | Major depressive episode, progress monitored on days 7, 14, 28, 42 and 56 in the acute phase (Hospital Anxiety and Depression Scale, Montgomery‐Åsberg Depression Rating Scale) |
| Notes | Conference abstract, could not locate author to find further details |
Differences between protocol and review
The first version of this review was released in the era before review protocols were routinely published. The present version differs in approach from the earliest version in the following ways:
includes a statement of primary and secondary outcome measures;
includes a 'Risk of bias' table
Contributions of authors
Philip Hazell
Conceiving the review Designing the review Co‐ordinating the review Screening retrieved papers against inclusion criteria Appraising quality of papers Obtaining and screening data on unpublished studies Data management for the review Entering data into RevMan Analysis of data Interpretation of data Providing a clinical perspective Writing the review Securing funding for the review Performing previous work that was the foundation of current study
Mohsen Mirzaie
Screening retrieved papers against inclusion criteria Appraising quality of papers Abstracting data from papers Entering data into RevMan Analysis of data Interpretation of data Writing the review
Sources of support
Internal sources
Research Infrastructure Grant, University of Newcastle, Australia.
External sources
No sources of support supplied
Declarations of interest
There were no conflicts of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bernstein 1990 {published data only}
- Bernstein GA, Garfinkel BD, Borchardt CM. Comparative studies of pharmacotherapy for school refusal. Journal of the American Academy of Child and Adolescent Psychiatry 1990;29:773‐81. [DOI] [PubMed] [Google Scholar]
Bernstein 2000 {published data only}
- Bernstein GA, Anderson LK, Hektner JM, Realmuto GM. Imipramine compliance in adolescents. Journal of the American Academy of Child and Adolescent Psychiatry 2000;39(3):284‐91. [DOI] [PubMed] [Google Scholar]
- Bernstein GA, Borchardt CM, Perwien AR, Crosby RD, Kushner MG, Thuras PD, et al. Imipramine plus cognitive‐behavioural therapy in the treatment of school refusal. Journal of the American Academy of Child and Adolescent Psychiatry 2000;39(3):276‐83. [DOI] [PubMed] [Google Scholar]
- Bernstein GA, Hektner JM, Borchardt CM, McMillan MH. Treatment of school refusal: one‐year follow‐up. Journal of the American Academy of Child & Adolescent Psychiatry 2001;40(2):206‐13. [DOI] [PubMed] [Google Scholar]
- Bernstein GA, Warren SL, Massie ED, Thuras PD. Family dimensions in anxious‐depressed school refusers. Journal of Anxiety Disorders 1999;13(5):513‐28. [DOI] [PubMed] [Google Scholar]
- Layne AE, Bernstein GA, Egan EA, Kushner MG. Predictors of treatment response in anxious‐depressed adolescents with school refusal. Journal of the American Academy of Child & Adolescent Psychiatry 2003;42(3):319‐26. [DOI] [PubMed] [Google Scholar]
Birmaher 1998 {published data only}
- Birmaher B, Waterman GS, Ryan ND, Perel J, McNabb J, Balach L, et al. Randomized, controlled trial of amitriptyline versus placebo for adolescents with "treatment‐resistant" major depression. Journal of the American Academy of Child and Adolescent Psychiatry 1998;37:527‐35. [DOI] [PubMed] [Google Scholar]
Geller 1989/1992 {published data only}
- Geller B, Cooper TB, Graham DL, Fetner HH, Marsteller FA, Wells JM. Pharmacokinetically designed double‐blind placebo‐controlled study of nortriptyline in 6‐12 year‐olds with major depressive disorder. Journal of the American Academy of Child and Adolescent Psychiatry 1992;31:34‐44. [DOI] [PubMed] [Google Scholar]
- Geller B, Cooper TB, McCombs HG, Graham D, Wells J. Double‐blind, placebo‐controlled study of nortriptyline in depressed children using a "fixed plasma level" design. Psychopharmacology Bulletin 1989;25:101‐8. [PubMed] [Google Scholar]
Geller 1990 {published data only}
- Geller B, Cooper TB, Graham DL, Marsteller FA, Bryant DM. Double‐blind placebo‐controlled study of nortriptyline in depressed adolescents using a "fixed plasma level" design. Psychopharmacology Bulletin 1990;26:85‐90. [PubMed] [Google Scholar]
Hughes 1990 {published data only}
- Hughes CW, Preskorn SH, Weller E, Weller R, Hassanein R. Imipramine vs. placebo studies of childhood depression: baseline predictors of response to treatment and factor analysis of presenting symptoms. Psychopharmacology Bulletin 1998;24(2):275‐9. [PubMed] [Google Scholar]
- Hughes CW, Preskorn SH, Weller E, Weller RA, Hassanein R, Tucker S. The effect of concomitant disorders in childhood depression on predicting treatment response. Psychopharmacology Bulletin 1990;26:235‐8. [PubMed] [Google Scholar]
Kashani 1984 {published data only}
- Kashani JH, Shekim WO, Reid JC. Amitriptyline in children with major depressive disorder: a double‐blind crossover pilot study. Journal of the American Academy of Child and Adolescent Psychiatry 1984;23:348‐51. [DOI] [PubMed] [Google Scholar]
Keller 2001 {published data only}
- Keller MB, Ryan ND, Birmaher B, Klein RG, Strober M, Wagner KD, et al. Paroxetine and imipramine in the treatment of adolescent depression. Proceedings of the 151st Annual Meeting of the American Psychiatric Association (APA); 1998 May 30‐Jun 4; Toronto, ON, Canada. 1998:123.
- Keller MB, Ryan ND, Strober M, Klein RG, Kutcher SP, Birmaher B, et al. Efficacy of paroxetine treatment in the treatment of adolescent depression: a randomized, controlled trial. Journal of the American Academy of Child and Adolescent Psychiatry 2001;40(7):762‐72. [DOI] [PubMed] [Google Scholar]
- Wagner KD, Birmaher B, Carlson G, Clarke G, Emslie G, Geller B, et al. Safety of paroxetine and imipramine in the treatment of adolescent depression. Annual Meeting of New Clinical Drug Evaluation Program (NCDEU); 1998 Jun 11; Boca Raton (FL). 1998:Abstract No. 69.
Klein 1998 {published data only}
- Klein RG, Mannuzza S, Koplewicz HS, Tancer NK, Shah M, Liang V, et al. Adolescent depression: controlled desipramine treatment and atypical features. Depression & Anxiety 1998;7(1):15‐31. [DOI] [PubMed] [Google Scholar]
Kramer 1981 {published data only}
- Kramer AD, Feguine RJ. Clinical effects of amitriptyline in adolescent depression. Journal of the American Academy of Child and Adolescent Psychiatry 1981;20:636‐44. [DOI] [PubMed] [Google Scholar]
Kutcher 1994 {published data only}
- Boulos C, Kutcher S, Marton P, Simeon J, Ferguson B, Roberts N. Response to desipramine treatment in adolescent major depression. Psychopharmacology Bulletin 1991;27:59‐65. [PubMed] [Google Scholar]
- Kutcher S, Boulos C, Ward B, Marton P, Simeon J, Ferguson HB, et al. Response to desipramine treatment in adolescent depression: a fixed‐dose, placebo‐controlled trial. Journal of the American Academy of Child & Adolescent Psychiatry 1994;33(5):686‐94. [DOI] [PubMed] [Google Scholar]
Kye 1996 {published data only}
- Klein RG. Pharmacotherapy of adolescent depression. Pediatric Psychopharmacology 1992;15 Suppl 1, Pt A:227A. [DOI] [PubMed] [Google Scholar]
- Kye CH, Waterman GS, Ryan ND, Birmaher B, Williamson DE, Iyengar S, et al. A randomized, controlled trial of amitriptyline in the acute treatment of adolescent major depression. Journal of the American Academy of Child and Adolescent Psychiatry 1996;35:1139‐44. [DOI] [PubMed] [Google Scholar]
- Ryan ND, Puig‐Antich J. Affective illness in adolescence. In: Frances AJ, Hales RE editor(s). American Psychiatric Association Annual Review. Vol. 5, Washington, DC: American Psychiatric Press, 1986:420‐50. [Google Scholar]
Petti 1982 {published data only}
- Petti TA, Law W. Imipramine treatment of depressed children: a double‐blind pilot study. Journal of Clinical Psychopharmacology 1982;2:107‐10. [PubMed] [Google Scholar]
Puig‐Antich 1987 {published data only}
- Puig‐Antich J, Perel JM, Lupatkin W, Chambers WJ, Tabrizi MA, King J, et al. Imipramine in prepubertal major depressive disorders. Archives of General Psychiatry 1987;44:81‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Berney 1981 {published data only}
- Berney T, Kolvin I, Bhate SR, Garside RF, Jeans J, Kay B, et al. School phobia: a therapeutic trial with clomipramine and short‐term outcome. British Journal of Psychiatry 1981;138:110‐8. [DOI] [PubMed] [Google Scholar]
Lucas 1965 {published data only}
- Lucas AR, Lockett HJ, Grimm F. Amitriptyline in childhood depressions. Diseases of the Nervous System 1965;26(2):105‐10. [PubMed] [Google Scholar]
Preskorn 1982 {published data only}
- Preskorn SH, Weller EB, Weller RA. Depression in children: relationship between plasma imipramine levels and response. Journal of Clinical Psychiatry 1982;43:450‐3. [PubMed] [Google Scholar]
Sallee 1997 {published data only}
- Sallee FR, Vrindavanam NS, Deas‐Smith D, Carson, SW, Sethuraman G. Pulse intravenous clomipramine for depressed adolescents: double‐blind, controlled trial. American Journal of Psychiatry 1997;154:668‐73. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Berard 1998 {published data only}
- Berard 1998. Clinical trials and tribulations in the pharmacological treatment of adolescent depression. Proceedings of the XXIst Collegium Internationale Neuro Psychopharmacologicum; 1998 Jul 12‐16; Glasgow, Scotland. 1998.
Additional references
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM‐IV‐TR). Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
Barbey 1998
- Barbey JT, Roose SP. SSRI safety in overdose. Journal of Clinical Psychiatry 1998;59 Suppl:42‐8. [PubMed] [Google Scholar]
Birmaher 1996
- Birmaher B, Ryan ND, Williamson DE, Brent DA, Kaufman J, Dahl RE, et al. Childhood and adolescent depression: a review of the past 10 years. Part I. Journal of the American Academy of Child and Adolescent Psychiatry 1996;35:1427‐39. [DOI] [PubMed] [Google Scholar]
Byrne 1989
- Byrne MM. Meta‐analysis of early phase II studies with paroxetine in hospitalised depressed patients. Acta Psychiatrica Scandinavica, Supplementum 1989;350:138‐9. [DOI] [PubMed] [Google Scholar]
Costello 2006
- Costello EJ, Foley DL, Angold A. 10‐year research update review; the epidemiology of child and adolescent psychiatric disorders: ll. Developmental epidemiology. Journal of American Academy of Child and Adolescent Psychiatry 2006;45(1):8‐25. [DOI] [PubMed] [Google Scholar]
Emslie 1997
- Emslie GJ, Rush R, Weinberg WA, Kowatch WA, Hughes CW, Carmody T, et al. A double‐blind, randomized, placebo‐controlled trial of fluoxetine in children and adolescents with depression. Archives of General Psychiatry 1997;54:1031‐7. [DOI] [PubMed] [Google Scholar]
Feighner 1999
- Feighner JP. Mechanism of action of antidepressant medications. Journal of Clinical Psychiatry 1999;60(4):4‐11. [PubMed] [Google Scholar]
Hayes 2010
- Hayes BD, Klein‐Schwartz W, Clark RF, Muller AA, Miloradovich JE. Comparison of toxicity of acute overdoses with citalopram and escitalopram. Journal of Emergency Medicine 2010;39(1):44‐8. [DOI] [PubMed] [Google Scholar]
Hetrick 2012
- Hetrick S, McKenzie J, Cox R, Simmons M, Merry S. Newer generation antidepressants for depressive disorders in children and adolescents. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD004851.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jensen 1992
- Jensen PS, Ryan ND, Prien R. Psychopharmacology of child and adolescent major depression: present status and future directions. Journal of Child and Adolescent Psychopharmacology 1992;2:31‐45. [DOI] [PubMed] [Google Scholar]
Klein‐Schwartz 1996
- Klein‐Schwartz W, Anderson B. Analysis of sertraline‐only overdoses. American Journal of Emergency Medicine 1996;14(5):456‐8. [DOI] [PubMed] [Google Scholar]
McFee 2001
- McFee RB, Caraccio TR, Mofenson HC. Selected tricyclic antidepressant ingestions involving children 6 years old or less. Academic Emergency Medicine 2001;8(2):139‐44. [DOI] [PubMed] [Google Scholar]
Olgun 2009
- Olgun H, Yildirim ZK, Karacan M, Ceviz N. Clinical, electrocardiographic, and laboratory findings in children with amitriptyline intoxication. Pediatric Emergency Care 2009;25(3):170‐3. [DOI] [PubMed] [Google Scholar]
Petti 1985
- Petti T. Scales of potential use in the psychopharmacological treatment of depressed children and adolescents. Psychopharmacology Bulletin 1985;21:951‐77. [PubMed] [Google Scholar]
Phillips 1997
- Phillips S, Brent J, Kulig K, Heiligenstein J, Birkett M. Fluoxetine versus tricyclic antidepressants: a prospective multicentre study of antidepressant drug overdoses. The Antidepressant Study Group. Journal of Emergency Medicine 1997;15(4):439‐45. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rosenberg 1989
- Rosenberg ML, Eddy DM, Wolpert RC, Broumas EP. Developing strategies to prevent youth suicide. In: Pfeffer CR editor(s). Suicide Among Youth: Perspectives on Risk and Prevention. Washington, DC: American Psychiatric Press, 1989:203‐25. [Google Scholar]
Ryan 1992
- Ryan ND. The pharmacologic treatment of child and adolescent depression. Psychiatric Clinics of North America 1992;15:29‐39. [PubMed] [Google Scholar]
Stahl 2006
- Stahl SM. Essential Psychopharmacology. New York: Cambridge University Press, 2006. [Google Scholar]
Varley 1997
- Varley CK, McClellan J. Case study: two additional sudden deaths with tricyclic antidepressants. Journal of the American Academy of Child & Adolescent Psychiatry 1997;36(3):390‐4. [DOI] [PubMed] [Google Scholar]
Werry 1999
- Werry JS, Aman MG. Practitioner's Guide to Psychoactive Drugs for Children and Adolescents. New York: Springer, 1999. [Google Scholar]
WHO 1992
- World Health Organization (WHO). The ICD‐10 Classification of Mental and Behavioural Disorders. Geneva: WHO, 1992. [Google Scholar]
References to other published versions of this review
Hazell 1995
- Hazell PL, O'Connell D, Heathcote D, Robertson J, Henry D. Efficacy of tricyclic drugs in treating child and adolescent depression: a meta‐analysis. BMJ 1995;310:897‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]


