Abstract
Context
Persistent neuromuscular deficits in the surgical limb after anterior cruciate ligament reconstruction (ACLR) have been repeatedly described in the literature, yet little is known regarding their association with physical performance and patient-reported function.
Objective
To describe (1) interlimb differences in neuromuscular and functional outcomes and (2) the associations of neuromuscular outcomes with measures of physical and knee-related patient-reported function.
Design
Cross-sectional study.
Setting
Laboratory.
Patients or Other Participants
Thirty individuals after primary, unilateral ACLR (19 males; age = 21.5 years [range, 14–41 years]; 8 months [range = 6–23 months] postsurgery).
Main Outcome Measure(s)
Knee-extensor isometric and isokinetic peak torque was measured with an isokinetic dynamometer. Cross-sectional area (CSA) was measured bilaterally for each of the quadriceps muscles via magnetic resonance imaging. We measured quadriceps central activation bilaterally via the superimposed-burst technique. Physical performance (single-legged hop tests, step length via spatiotemporal gait analysis) and patient-reported outcomes (International Knee Documentation Committee questionnaire and Knee Injury and Osteoarthritis Outcome Score Sport and Recreation subscale) were also recorded. We conducted Wilcoxon signed rank tests to identify interlimb differences. Spearman ρ correlation analyses revealed associations between limb symmetry and neuromuscular and functional outcomes, as well as with patient-reported function.
Results
Deficits in the surgical limb as compared with the nonsurgical limb were present for all outcomes (P values < .05). Greater single-legged hop-test symmetry (83%) was significantly correlated with greater symmetry in knee-extensor isometric (63%; rs = 0.567, P = .002) and isokinetic (68%; rs = 0.540, P = .003) strength, as well as greater cross-sectional area of the vastus medialis (78%; rs = 0.519, P = .006) and vastus lateralis (82%; rs = 0.752, P < .001). A higher International Knee Documentation Committee questionnaire score (82.2) was significantly correlated with greater symmetry in knee-extensor isokinetic strength (rs = 0.379, P = .039).
Conclusions
Although deficits were observed in the surgical limb for all neuromuscular measures, greater symmetry in the size and strength of the quadriceps, rather than activation, was more strongly associated with physical performance after ACLR. Greater symmetry in strength was also more strongly associated with patient-reported function.
Keywords: rehabilitation, knee injury, neuromuscular function, self-reported function, limb symmetry
Key Points
After anterior cruciate ligament reconstruction, the surgical limb exhibited deficits in quadriceps muscle size, strength, and activation.
Compared with greater activation, larger and stronger quadriceps muscles led to more successful physical function outcomes in the surgical limb.
Greater quadriceps isokinetic strength symmetry was the only outcome that was associated with improved patient-reported knee function.
Anterior cruciate ligament (ACL) sprains are one of the most debilitating knee-joint injuries. Nearly 300 000 injuries occur in the United States per year, and their economic effect exceeds $2 billion in US health care dollars.1 Anterior cruciate ligament reconstruction (ACLR) is a surgical procedure performed to stabilize the injured joint and facilitate functional recovery. Unfortunately, functional recovery is not always achieved, and 25% to 45% of individuals fail to return to their preinjury activity levels.2,3 These individuals face undesirable neuromusculoskeletal sequelae that likely result from peripheral and central (mal)adaptations (ie, muscle weakness, atrophy, and activation deficits) that contribute to an increased risk for reinjury,4,5 the development of early-onset osteoarthritis,6,7 and decreased quality of life.8 Given that the most often-stated goal for ACLR patients is to return to their preinjury activity levels, efforts to elucidate the functional consequences of neuromuscular dysfunction must be undertaken.
Strength deficits of the extensor musculature surrounding the reconstructed knee (eg, quadriceps femoris muscle group) are consistently observed, even years after surgery.9,10 This strength loss can be largely attributed to an inability to voluntarily activate the quadriceps muscle group—known as voluntary activation deficit—which compromises strength and limits the dose of rehabilitation that can be delivered. Additionally, magnetic resonance imaging (MRI) studies showed that the muscles surrounding the reconstructed knee experienced significant atrophy months to years after ACLR.11–14 Together, the reductions in muscle size and voluntary activation account for approximately 60% of the variance in quadriceps strength 6 months after ACLR.12 Addressing these deficits in quadriceps muscle strength, size, and activation is crucial in early rehabilitation before and during progression into functional tasks in later rehabilitation. Although others have described deficits in quadriceps muscle strength, size, and activation,13–16 the relationships of these factors to decreased function remain less clear,14 especially when considering values of the surgical limb only versus symmetry. Kuenze et al14 found that patient-reported function was associated with the volume of the vastus intermedius and not isometric strength, but the association was weak. Our study was intended to provide a more comprehensive approach to understanding these relationships by assessing additional clinically relevant functional outcomes.
Functional outcomes include hop-test results, spatiotemporal gait variables, and patient-reported outcomes. Specifically, hop tests are the most common tests of functional stability after ACLR,17 and performance is significantly predicted by quadriceps strength.18 Thus, hop testing may serve as an alternative for clinics that lack access to dynamometers. Additionally, greater symmetry on hop tests conducted 6 months post-ACLR predicted greater self-reported knee function at 1 year.19 As an alternative to motion capture, spatiotemporal gait analysis using a portable walking system (GAITRite; CIR Systems, Inc, Franklin, NJ) has shown early promise in detecting gait asymmetries. The GAITRite system is a clinical tool that only requires the patient to walk along a mat while the computer measures spatiotemporal variables. Step length of the surgical limb measured using GAITRite exhibited moderate to high positive correlations with patient-reported function at multiple time points after ACLR.20 Furthermore, reduced step length has been associated with reduced knee-joint loading and reduced quadriceps strength after ACLR.21 Lastly, patient-reported outcome measures are an emerging aspect of clinical research and are important to collect during rehabilitation, given that individual patients' perceptions of the recovery process may serve as key components of clinical testing when predicting or explaining the extent of recovery.
The extent to which neuromuscular function relates to these functional outcomes after ACLR, including whether the effect of neuromuscular function on functional performance is similar to its effect on patient self-reported function, remains under investigation. Therefore, the primary purpose of our study was to determine if associations existed between neuromuscular and clinically relevant functional outcomes in individuals after ACLR. As a first step, we determined the extent to which interlimb deficits were present by quantifying asymmetries in neuromuscular outcomes (quadriceps strength, size, and activation). Second, we determined associations of limb asymmetries with measures of physical and patient-reported function (hop test and step-length asymmetries and patient-reported knee function) in individuals after ACLR. We hypothesized that (1) significant interlimb differences in neuromuscular outcomes would be present and (2) greater symmetry in knee-extensor strength, quadriceps cross-sectional area (CSA), and central activation would be associated with greater symmetry in physical function, as well as improved patient-reported function.
METHODS
Study Design
This was a cross-sectional study in which individuals who were 6 months to 2 years post-ACLR underwent assessments in the following order: quadriceps isometric strength and central activation, isokinetic strength, functional tests, patient-reported outcomes, and magnetic resonance imaging (MRI) of the lower extremity. Before the MRI, each patient was given time to rest and complete the patient-reported outcome questionnaires. This study was approved by the review board of the investigator's institution.
Participants
Individuals (male and female) who had undergone primary ACLR were included based on the following inclusion and exclusion criteria. Inclusion criteria were (1) age 14 to 55 years, as individuals ≥14 years are considered skeletally mature and undergo standard adult reconstructive procedures for the ACL with little risk of growth plate disturbance22; (2) history of unilateral ACLR (with or without meniscal involvement) within the past 6 months to 2 years with ipsilateral quadriceps tendon or bone–patellar tendon–bone autografts; and (3) ACLR performed by one of the fellowship-trained sports medicine orthopaedic surgeons at our institution. Exclusion criteria were (1) a history of lower extremity injury or surgery other than ACLR to either the involved or uninvolved limb within the past 6 months; (2) inability to walk without assistance from an orthotic or knee brace or another person; (3) self-reported knee arthritis that would limit range of motion at the knee joint; (4) any contraindications to MRI, such as metal implants, pacemakers, claustrophobia, etc; or (5) pregnancy. Written informed consent was obtained from all participants (or the participant's legal guardian if the participant was <18 years old) before data collection.
Neuromuscular Outcome Measures
Strength
Bilateral quadriceps isometric and isokinetic strength was measured on an isokinetic dynamometer (System 4 Pro; Biodex Medical Systems, Inc, Shirley, NY). The nonsurgical limb was always tested first. Before testing, each participant underwent a period of familiarization and warm-up consisting of submaximal knee-extension contractions at 25%, 50%, and 75% of perceived maximal effort. After familiarization, 3 maximal contractions were performed with participants positioned in the dynamometer with the hips flexed to 85° and the knees flexed to 90°.6 The axis of the dynamometer was aligned with the knee-joint axis of rotation, and the lever arm was secured to the test leg proximal to the lateral malleolus. Stabilization in the dynamometer was maintained with straps at the chest, hips, and knee. Participants were encouraged with auditory feedback and motivation to develop torque as hard and fast as possible in order to produce maximal contractions, separated by 60 seconds of rest. For isometric testing, peak torque was identified during the maximum voluntary isometric contraction (MVIC), and then the mean of a 100-millisecond epoch recorded around the peak was used for analysis. The MVIC was normalized to body mass (newton meters per kilogram). Central activation was tested simultaneously during MVIC testing. (See “Central Activation” section below.) After activation testing, isokinetic testing of concentric knee-extensor and -flexor strength was performed bilaterally via 5 repetitions at angular velocities of 60°/s through a full range of motion and using gravity correction.10 Peak torque was normalized to body mass.
Central Activation
As noted, central activation and isometric strength were tested simultaneously. After the skin was cleaned with alcohol pads, two 7- × 13-cm self-adhesive electrodes (Dura-Stick II; Chattanooga Group, Hixson, TN) were placed in bipolar fashion using the vastus medialis obliquus configuration.23 A 10-pulse train electrical stimulus was manually delivered using a square-wave stimulator (model S88; Grass Technologies Corp, West Warrick, RI) and a stimulation isolation unit (model SIU8T; Grass Technologies Corp) during MVICs once a maximal contraction was visually identified by the tester. (Participants did not receive visual feedback.) A standard intensity of 150 V was used for all participants. During the submaximal isometric trials, the intensity of electrical stimulation was superimposed at 25%, 50%, and 75% of 150 V to familiarize participants. Other stimulation settings were a 200-millisecond train of 10 stimuli, at 50 pulses per second, with a pulse duration of 0.6 milliseconds and 0.01-millisecond pulse delay. The stimulator and dynamometer were interfaced with a personal computer through a commercially available hardware system (model MP150; Biopac Systems, Inc, Goleta, CA). Data were sampled at 2000 Hz and analyzed using commercially available software (AcqKnowledge version 4.4; Biopac Systems, Inc). For analysis, peak torque was identified during the superimposed burst (SIB). We used the mean of a 100-millisecond epoch around the peak torque to calculate central activation via the following equation:
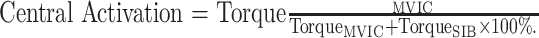 |
Cross-Sectional Area
Magnetic resonance imaging was conducted to compare the maximum CSA of each knee-extensor muscle between the surgical and nonsurgical limbs of each patient. All imaging was performed after the neuromuscular and functional testing using a 3.0-T MRI scanner (Prisma; Siemens AG, Munich, Germany) and a large body coil. The T1 axial fast-spin imaging without fat saturation was performed of the bilateral thighs from the knee-joint line to the proximal subtrochanteric femur (repetition time = 626 milliseconds, echo time = 10 milliseconds, echo train length = 3, 5-mm slice thickness, 0.5-mm interslice gap, 40-cm field of view, 384 × 384 matrix, and total scan length of 36 cm). Muscle and nonmuscle tissues were readily differentiated based on the conspicuity of fat planes on the T1 images, and the CSA of each of the quadriceps muscles was measured on all consecutive slices by drawing a freehand region of interest using publicly available software (Osirix; Pixmeo SARL, Geneva, Switzerland). Once the slice with the maximum CSA was identified, the respective CSA measurement was averaged with that of the 2 adjacent slices and used in data analysis. This was done for each quadriceps muscle. All images were analyzed by 2 evaluators who were blinded to the graft type and side of surgery. Using a 2-way mixed-effects model, we determined interrater reliability to be excellent (intraclass correlation coefficient = 0.914; 95% confidence interval = 0.823, 0.959).
Functional Outcome Measures
Hop Testing
Dynamic knee function was measured via the single-legged hop test for distance conducted on the nonsurgical limb first, followed by the surgical limb. This test is a safe, reliable, and valid measure in individuals after ACLR.24 The single-legged hop test was performed with the participant standing on 1 leg behind a marked starting line and hopping once as far as possible. For a trial to be considered successful, the participant must land on the tested limb in a controlled manner. The trial was repeated if the landing was not stable or the contralateral limb touched the ground. Hop distance was measured from the starting line to the participant's heel (to the nearest centimeter) using a standard tape measure. Three successful trials were averaged and used in the analyses.
Spatiotemporal Gait Analysis
Participants walked over a 14-ft (4.3-m) portable walking system (GAITRite) designed to measure spatiotemporal variables related to each individual's gait pattern. Once familiarized with the system, participants walked at their self-selected speed for 3 trials. They were not permitted to use any assistive devices during the trials. The average step length for each limb was calculated and used for the analyses.
Patient-Reported Outcome Measures
Participants completed the subjective portion of the International Knee Documentation Committee (IKDC) questionnaire, which is available online at the American Orthopedic Society for Sports Medicine Web site.25 The IKDC form is a reliable and valid tool for measuring self-reported knee function.26 It contains 18 items representing 3 domains: symptoms, sports, and daily activities. The total score is calculated by totaling the individual items and then converting the raw score to a scale of 0 to 100, in which 100 represents no limitation in knee functioning.
Additionally, participants completed the Knee Injury and Osteoarthritis Outcome Score (KOOS) questionnaire. The KOOS is a reliable and valid patient-centered measure for individuals after ACLR.27,28 The KOOS total score ranges from 0 to 100, with 0 representing extreme knee problems and 100 representing no knee problems. We used only the Sport and Recreation (Sport/Rec) subscale score for the correlation analysis because it is one of the only 2 subscales that demonstrated unidimensional measurement properties in individuals post-ACLR.29
Statistical Analyses
Because of their robustness to the possibility of nonnormal data and outliers, we determined a priori that only nonparametric statistical tests would be used for all analyses. Wilcoxon signed rank tests were performed to assess interlimb differences for all outcome measures. Spearman ρ correlation analyses were conducted to identify associations between symmetry in neuromuscular measures (knee-extensor strength, quadriceps CSA, central activation) and symmetry in functional measures (hop testing and step length). Additionally, the Spearman ρ was used to determine associations between the neuromuscular measures and patient-reported outcomes (IKDC and KOOS Sport/Rec). The strength of correlations was categorized as low (<0.25), fair (0.25–0.49), moderate to good (0.50–0.74), or good to excellent (>0.75).30 The α level was set a priori at .05 and used to describe significant correlations. All analyses were performed using SPSS (version 24; IBM Corp, Armonk, NY).
RESULTS
Thirty individuals with a history of ACLR participated in this study. On the Tegner Activity Scale, this sample reported high levels of intense activity at preinjury (median = 9 [range = 3–10]) and postsurgery (median = 7 [range = 3–9]). All demographic and other patient-reported outcome data are provided in Table 1.
Table 1.
Participant Characteristics
| Demographic Variable |
Value |
| No. (%) | |
| Sex | |
| Male | 19 (63) |
| Female | 11 (37) |
| Median (Range) |
|
| Age, y | 22 (14–41) |
| Height, cm | 174.0 (153.7–195.6) |
| Mass, kg | 76.3 (37.4–120.2) |
| Body mass index, kg/m2 | 23.7 (14.8–40.3) |
| Time since surgery, mo | 8.0 (6.0–23.0) |
| Gait speed, m/s | 1.24 (0.84–1.54) |
| International Knee Documentation Committee score | 82.2 (54.0–94.3) |
| KOOS Sport and Recreation score | 75.0 (45.0–100) |
| KOOS Quality of Life score | 68.8 (18.8–93.8) |
| No. (%) |
|
| Contact mechanisma | 12 (40) |
| Meniscal involvement | |
| Meniscectomy | 13 (43) |
| Meniscal repair | 4 (13) |
| Autograft type | |
| Quadriceps tendon | 15 (50) |
| Patellar tendon | 15 (50) |
Abbreviation: KOOS, Knee Injury and Osteoarthritis Outcome Score.
Contact mechanism was determined via participant's self-report of mechanism of injury.
The surgical limb was weaker and smaller and had less activation than the nonsurgical limb. Additionally, scores on the single-legged hop test were lower and step lengths were shorter for the surgical limb (Table 2).
Table 2.
Neuromuscular and Functional Outcome Variables Between Limbs
| Variable |
Limb, Median (Range) |
P Value |
|
| Surgical |
Nonsurgical |
||
| Strength and activation | |||
| Knee-extensor, Nm/kg | |||
| Maximum voluntary isometric contraction | 1.22 (0.62–2.43) | 2.02 (1.03–3.71) | <.001 |
| Torque, 60°/s | 1.39 (0.51–2.80) | 2.01 (1.06–3.56) | <.001 |
| Central activation, % | 86.8 (72.7–98.2) | 95.1 (80.9–101.9) | <.001 |
| Cross-sectional area, cm2 | |||
| Vastus medialis | 19.6 (9.9–32.9) | 26.0 (15.6–39.0) | <.001 |
| Vastus lateralis | 26.0 (13.7–40.6) | 33.3 (21.9–47.1) | <.001 |
| Vastus intermedius | 21.5 (10.4–35.4) | 27.8 (16.2–39.6) | <.001 |
| Rectus femoris | 13.4 (5.6–18.2) | 12.8 (6.6–19.8) | .001 |
| Functional tests, cm2 | |||
| Single-legged hop | 87.0 (15.0-182.7) | 112.3 (43.3–201.3) | <.001 |
| Step length | 69.6 (54.1–91.4) | 70.9 (53.3–95.3) | .047 |
Consistent with previous work14 demonstrating a strong relationship between greater quadriceps size and strength, we observed significant associations between increased isometric strength and larger quadriceps muscle size for the vastus medialis (rs = 0.603, P = .001), vastus lateralis (rs = 0.479, P = .010), vastus intermedius (rs = 0.488, P = .007), and rectus femoris (rs = 0.632, P < .001).
Greater symmetry in hop-test scores was significantly associated with greater symmetry in knee-extensor strength and with the CSAs of the vastus medialis and the vastus lateralis (Table 3). Additionally, greater IKDC scores were significantly associated with greater symmetry in isokinetic knee-extensor strength. Central-activation symmetry did not correlate with any functional outcomes.
Table 3.
Correlation Coefficients Between Measures of Symmetry in Neuromuscular and Functional Outcomes
| Variable |
Symmetry |
International Knee Documentation Committee Score |
Knee Injury and Osteoarthritis Outcome Sport and Recreation Score |
|
| Single-Legged Hop |
Step Length |
|||
| Maximal voluntary isometric contraction | 0.567a | −0.065 | −0.038 | 0.111 |
| Isokinetic testing at 60°/s | 0.540a | 0.185 | 0.379a | 0.337 |
| Central activation | 0.199 | 0.208 | −0.221 | 0.063 |
| Cross-sectional area | ||||
| Vastus medialis | 0.519a | −0.063 | 0.285 | 0.265 |
| Vastus lateralis | 0.752a | −0.221 | 0.222 | 0.264 |
| Vastus intermedius | 0.181 | −0.244 | −0.157 | −0.053 |
| Rectus femoris | 0.212 | −0.197 | 0.194 | 0.065 |
Significant correlation. Spearman ρ correlation coefficients are presented.
As a subanalysis, we investigated the correlations between neuromuscular and functional outcomes in the surgical limb only (Table 4). Hop-test performance was significantly and positively correlated with knee-extensor strength and with the CSAs of all quadriceps muscles. Also, greater step length was significantly associated with greater central activation and CSAs of the vastus intermedius and rectus femoris in the surgical limb.
Table 4.
Correlation Coefficients Between Neuromuscular Outcomes and Function in the Surgical Limb
| Variable |
Single-Legged Hop Test |
Step Length |
International Knee Documentation Committee Score |
Knee Injury and Osteoarthritis Outcome Sport and Recreation Score |
| Maximal voluntary isometric contraction | 0.800a | 0.315 | −0.025 | 0.019 |
| Isokinetic testing at 60°/s | 0.758a | 0.124 | 0.256 | 0.214 |
| Central activation | 0.206 | 0.383a | −0.204 | −0.052 |
| Cross-sectional area | ||||
| Vastus medialis | 0.562a | 0.308 | 0.171 | 0.161 |
| Vastus lateralis | 0.456a | 0.320 | 0.175 | 0.231 |
| Vastus intermedius | 0.417a | 0.487a | −0.013 | −0.023 |
| Rectus femoris | 0.554a | 0.525a | 0.012 | 0.049 |
Significant correlation. Spearman ρ correlation coefficients are presented.
DISCUSSION
The purpose of our study was to describe interlimb differences and associations between quadriceps neuromuscular outcomes and clinically relevant functional outcomes in individuals after ACLR. Significant deficits were present in all measures of neuromuscular and physical function in the surgical limb compared with the nonsurgical limb. Single-legged hop symmetry was the only variable that was significantly and positively associated with several measures of neuromuscular symmetry (quadriceps strength and CSA). The IKDC score demonstrated a significant and positive association only with isokinetic strength symmetry. Whether investigating interlimb symmetry or surgical-limb values in isolation, we suggest that larger and stronger quadriceps muscles are more strongly associated with physical function; thus, restoring quadriceps muscle function during rehabilitation may serve as a foundational target before the focus is placed on functional performance.
Neuromuscular Deficits
Neuromuscular dysfunction after ACLR has been well documented, particularly deficits in knee-extensor strength,9,10 quadriceps muscle size,11–14 and quadriceps central activation.31 To replicate these findings, we first described deficits in the surgical limb compared with the nonsurgical limb. Similar to previous researchers, we observed significant deficits in neuromuscular variables of the surgical limb. Although Wellsandt et al27 noted that both limbs were affected by ACLR, much debate surrounds the reporting of limb-symmetry indices versus surgical-limb raw values.27,28 Therefore, we conducted subanalyses using surgical-limb values to complement our analyses using symmetry measures. Consistent with the findings of Norte at al,32 we identified stronger associations between strength and functional outcomes than limb-symmetry indices in the surgical limb. Particularly for the association between strength and hop-test results, the results justified the use of surgical-limb values. Interestingly, although we did show a significant association between isometric strength and IKDC values, no participants in this study demonstrated the clinically meaningful normalized strength threshold value (>3.0 Nm/kg) in the surgical limb that has been associated with better patient-reported function.33 The IKDC scores for males (80.4) and females (79.9) in this sample were below normative values.34
Participants demonstrated reduced central activation after ACLR.31 In the surgical limb (86.7%), central activation was similar to what has been extensively reported in the literature (87.3%, according to a systematic review of 15 studies31). Additionally, our investigation showed a deficit in the nonsurgical limb (93.1%), which was similar to but not quite as low as that reported in the systematic review (89.1%). At the time point evaluated in our study (median of 8 months post-ACLR), central activation was not associated with measures of strength symmetry. This is contrary to other findings.18 It is possible that quadriceps-activation deficits may be more strongly related to strength in the earlier stages after ACLR and less so in the later stages.35 Thus, muscle size may be a more appropriate target during rehabilitation in the later stages of recovery after ACLR. Muscle atrophy can be addressed using modalities, such as neuromuscular electrical stimulation, and specific modes of exercise, including eccentric strength training.
Hop Testing
Clinically feasible and relatively easy to conduct, the hop test is the most common method of assessing functional stability after ACL injury and reconstruction.17 Hop tests are safe, reliable, and valid measures of knee stability.24 Improved performance is believed to indicate a reduced risk of reinjury, although few authors have investigated this relationship.36 Like muscle-strength tests, hop tests are often used to determine readiness to return to activity. Ideally, hop tests should be conducted on both limbs to evaluate symmetry; readiness to return to activity is evident with a limb-symmetry index ≥90%. Hop-test scores in our sample were below the normative values established by Gokeler et al28 for both males and females. Because greater hop-test distance was significantly associated with greater knee-extensor strength and quadriceps size in this study and predicted quadriceps strength in other studies,18 we are confident that these tests are useful for not only dynamic knee function but also quadriceps strength. They may be especially useful when access to strength-testing equipment is limited.
Gait Analysis
We evaluated gait because of the emerging evidence that walking mechanics contribute to the development of posttraumatic osteoarthritis. More specifically, unloading (reduced vertical ground reaction forces) of the surgical limb is common after ACLR and not ideal for long-term cartilage health.37,38 Cross-sectional studies39,40 revealed that insufficient quadriceps strength and rate of torque development were associated with unloading. Unfortunately, these small, but significant, degrees of weight shifting during gait are not always clinically observable. The GAITRite is a relatively inexpensive and much more efficient tool than high-tech motion capture systems and may, therefore, be a useful indicator of gait dysfunction after ACLR. In the present study, reduced step length was used as a surrogate measure of unloading of the surgical limb and favoring the nonsurgical limb. Reduced step length has been associated with decreased knee-joint loading during walking in uninjured individuals.41 In patients with ACL-reconstructed knees, reduced step length during running was also associated with decreased knee-joint loading and quadriceps force.21 Similar associations of reduced step length and ground reaction forces have been observed in other clinical cohorts, such as individuals with obesity41 or stroke.42 Step-length asymmetry was small, but present, as indicated by the statistically significant difference between limbs. Future researchers (with currently unpublished data) will determine the strength of correlations between step length and other spatiotemporal variables with ground reaction forces. We hypothesized that reduced neuromuscular symmetry would be related to reduced step-length symmetry; however, this association was not found. On the contrary, when evaluating the surgical limb, greater activation and muscle size in the surgical limb were significantly correlated with greater step length. More specifically, greater CSA of the rectus femoris was associated with greater step length, which may reflect the importance of hip flexion during gait. The hip flexors have a vital role in the late swing phase of gait, supporting the limb just before heel strike. A reduction in hip-flexion angle has been observed in individuals with ACLR compared with control participants and theorized to be related to greater reliance on the hip-extensor muscles.43 Perhaps the focus should be on hip-flexor activation and range of motion to generate a greater step length by the surgical limb, but this requires further study.
Patient-Reported Knee Function and Quality of Life
A recent surge in patient-reported outcomes research has revealed the strong influence of patient self-efficacy in ACL recovery. In many cases, knee function has been restored to normal or near normal, and yet, the individual is still not comfortable about returning to activity.44 In our study, the only neuromuscular measure significantly associated with IKDC scores was isokinetic strength symmetry, though this association was weak. Bodkin et al45 subdivided patients by time since surgery and failed to show relationships between IKDC scores and quadriceps strength in patients <2 years postsurgery. It is possible that patients cannot perceive the extent of their neuromuscular dysfunction, and the construct of the IKDC is directed more toward physical function (ie, performance of activities of daily living). We thought that perhaps the KOOS Sport/Rec subscale score would be significantly associated with neuromuscular dysfunction, but this was not confirmed. Nonetheless, further work is needed to develop and use patient-reported outcomes that can capture neuromuscular dysfunction after surgery, especially considering the relationship of neuromuscular dysfunction and longer-term complications.
Limitations
Limitations of this study were as follows: (1) Based on the cross-sectional study design, we were only able to draw conclusions at a single time point after surgery. It is likely that the strength of associations between neuromuscular and physical function changed later after ACLR. Thus, longitudinal investigations would provide more solid conclusions regarding the relationships between neuromuscular and physical functioning. (2) In the MVIC and central-activation testing, visual feedback to encourage maximal contraction was not provided to participants. We recognize that this may have lowered our estimates of strength and activation,46 but these underestimations would have been systematic across all participants and would, therefore, have had a limited effect on the associations presented. (3) In the imaging analyses, we did not estimate muscle volume, pennation angle, or physiological CSA. Although this is certainly a potential limitation, Marcon et al47 showed that quadriceps CSA was strongly correlated with quadriceps volume. (4) The CSA of the rectus femoris may have been underestimated in this study. Using our MRI protocol, the maximum CSA for the rectus femoris was often found on the most superior slice in the series. However, when compared with the nonsurgical limb, the rectus femoris CSA results were reasonable. (5) The SIB technique had limitations as a proxy for measuring central activation because the mechanisms underlying this measure are not fully understood. Emerging evidence6 evaluating central drive to the quadriceps through other mechanisms (eg, brain stimulation, spinal reflexes) offers promising evaluation techniques for further understanding the physiology of quadriceps inhibition.
CONCLUSIONS
This sample of individuals after ACLR showed deficits in the surgical limb for all measures of neuromuscular and physical function. Greater hop-distance symmetry was significantly associated with greater symmetry in knee-extensor strength and muscle size, and greater IKDC scores were significantly associated with greater symmetry in isokinetic strength. Interestingly, larger and stronger quadriceps muscles, rather than greater activation, led to more successful functional and self-reported outcomes. Further work is needed to understand if and the extent to which strength mediates the relationship between functional performance and self-reported outcomes after ACLR. In addition, it will be valuable to understand how certain aspects of gait and patient-reported outcomes are associated with quadriceps dysfunction after surgery.
ACKNOWLEDGMENTS
This research was supported by the National Athletic Trainers' Association Research & Education Foundation Award #1617DGP005, as well as the South Carolina Clinical & Translational Research Institute, with an academic home at the Medical University of South Carolina, through NIH grant numbers TL1 TR001451 and UL1 TR001450.
REFERENCES
- 1.Hootman JM, Albohm MJ. Anterior cruciate ligament injury prevention and primary prevention of knee osteoarthritis. J Athl Train. 2012;47(5):589–590. doi: 10.4085/1062-6050-47.5.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ardern CL, Taylor NF, Feller JA, Webster KE. Fifty-five per cent return to competitive sport following anterior cruciate ligament reconstruction surgery: an updated systematic review and meta-analysis including aspects of physical functioning and contextual factors. Br J Sports Med. 2014;48(21):1543–1552. doi: 10.1136/bjsports-2013-093398. [DOI] [PubMed] [Google Scholar]
- 3.Brophy RH, Schmitz L, Wright RW, et al. Return to play and future ACL injury risk after ACL reconstruction in soccer athletes from the Multicenter Orthopaedic Outcomes Network (MOON) group. Am J Sports Med. 2012;40(11):2517–2522. doi: 10.1177/0363546512459476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hui C, Salmon LJ, Kok A, Maeno S, Linklater J, Pinczewski LA. Fifteen-year outcome of endoscopic anterior cruciate ligament reconstruction with patellar tendon autograft for “isolated” anterior cruciate ligament tear. Am J Sports Med. 2011;39(1):89–98. doi: 10.1177/0363546510379975. [DOI] [PubMed] [Google Scholar]
- 5.Shelbourne KD, Gray T, Haro M. Incidence of subsequent injury to either knee within 5 years after anterior cruciate ligament reconstruction with patellar tendon autograft. Am J Sports Med. 2009;37(2):246–251. doi: 10.1177/0363546508325665. [DOI] [PubMed] [Google Scholar]
- 6.Pietrosimone BG, Lepley AS, Ericksen HM, Gribble PA, Levine J. Quadriceps strength and corticospinal excitability as predictors of disability after anterior cruciate ligament reconstruction. J Sport Rehabil. 2013;22(1):1–6. doi: 10.1123/jsr.22.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Tourville TW, Jarrell KM, Naud S, Slauterbeck JR, Johnson RJ, Beynnon BD. Relationship between isokinetic strength and tibiofemoral joint space width changes after anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42(2):302–311. doi: 10.1177/0363546513510672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filbay SR, Ackerman IN, Russell TG, Macri EM, Crossley KM. Health-related quality of life after anterior cruciate ligament reconstruction: a systematic review. Am J Sports Med. 2014;42(5):1247–1255. doi: 10.1177/0363546513512774. [DOI] [PubMed] [Google Scholar]
- 9.Petersen W, Taheri P, Forkel P, Zantop T. Return to play following ACL reconstruction: a systematic review about strength deficits. Arch Orthop Trauma Surg. 2014;134(10):1417–1428. doi: 10.1007/s00402-014-1992-x. [DOI] [PubMed] [Google Scholar]
- 10.Undheim MB, Cosgrave C, King E, et al. Isokinetic muscle strength and readiness to return to sport following anterior cruciate ligament reconstruction: is there an association? A systematic review and a protocol recommendation. Br J Sports Med. 2015;49(20):1305–1310. doi: 10.1136/bjsports-2014-093962. [DOI] [PubMed] [Google Scholar]
- 11.Thomas AC, Wojtys EM, Brandon C, Palmieri-Smith RM. Muscle atrophy contributes to quadriceps weakness after ACL reconstruction. J Sci Med Sport. 2016;19(1):7–11. doi: 10.1016/j.jsams.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams GN, Buchanan TS, Barrance PJ, Axe MJ, Snyder-Mackler L. Quadriceps weakness, atrophy, and activation failure in predicted noncopers after anterior cruciate ligament injury. Am J Sports Med. 2005;33(3):402–407. doi: 10.1177/0363546504268042. [DOI] [PubMed] [Google Scholar]
- 13.Norte GE, Knaus KR, Kuenze C, et al. MRI-based assessment of lower-extremity muscle volumes in patients before and after ACL reconstruction. J Sport Rehabil. 2018;27(3):201–212. doi: 10.1123/jsr.2016-0141. [DOI] [PubMed] [Google Scholar]
- 14.Kuenze CM, Blemker SS, Hart JM. Quadriceps function relates to muscle size following ACL reconstruction. J Orthop Res. 2016;34(9):1656–1662. doi: 10.1002/jor.23166. [DOI] [PubMed] [Google Scholar]
- 15.Thomas AC, Wojtys EM, Brandon C, Palmieri-Smith RM. Muscle atrophy contributes to quadriceps weakness after anterior cruciate ligament reconstruction. J Sci Med Sport. 2016;19(1):7–11. doi: 10.1016/j.jsams.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams GN, Snyder-Mackler L, Barrance PJ, Buchanan TS. Quadriceps femoris muscle morphology and function after ACL injury: a differential response in copers versus non-copers. J Biomech. 2005;38(4):685–693. doi: 10.1016/j.jbiomech.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Abrams GD, Harris JD, Gupta AK, et al. Functional performance testing after anterior cruciate ligament reconstruction: a systematic review. Orthop J Sports Med. 2014;2(1):2325967113518305. doi: 10.1177/2325967113518305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmieri-Smith RM, Lepley LK. Quadriceps strength asymmetry after anterior cruciate ligament reconstruction alters knee joint biomechanics and functional performance at time of return to activity. Am J Sports Med. 2015;43(7):1662–1669. doi: 10.1177/0363546515578252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Logerstedt D, Grindem H, Lynch A, et al. Single-legged hop tests as predictors of self-reported knee function after anterior cruciate ligament reconstruction: the Delaware-Oslo ACL cohort study. Am J Sports Med. 2012;40(10):2348–2356. doi: 10.1177/0363546512457551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papuga MO, Beck CA, Kates SL, Schwarz EM, Maloney MD. Validation of GAITRite and PROMIS as high-throughput physical function outcome measures following ACL reconstruction. J Orthop Res. 2014;32(6):793–801. doi: 10.1002/jor.22591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowersock CD, Willy RW, DeVita P, Willson JD. Reduced step length reduces knee joint contact forces during running following anterior cruciate ligament reconstruction but does not alter inter-limb asymmetry. Clin Biomech (Bristol, Avon) 2017;43:79–85. doi: 10.1016/j.clinbiomech.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Fabricant PD, Kocher MS. Anterior cruciate ligament injuries in children and adolescents. Orthop Clin N Am. 2016;47(4):777–788. doi: 10.1016/j.ocl.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Pietrosimone BG, Selkow NM, Ingersoll CD, Hart JM, Saliba SA. Electrode type and placement configuration for quadriceps activation evaluation. J Athl Train. 2011;46(6):621–628. doi: 10.4085/1062-6050-46.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reid A, Birmingham TB, Stratford PW, Alcock GK, Giffin JR. Hop testing provides a reliable and valid outcome measure during rehabilitation after anterior cruciate ligament reconstruction. Phys Ther. 2007;87(3):337–349. doi: 10.2522/ptj.20060143. [DOI] [PubMed] [Google Scholar]
- 25.Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2001;29(5):600–613. doi: 10.1177/03635465010290051301. [DOI] [PubMed] [Google Scholar]
- 26.Higgins LD, Taylor MK, Park D, et al. Reliability and validity of the International Knee Documentation Committee (IKDC) Subjective Knee Form. Joint Bone Spine. 2007;74(6):594–599. doi: 10.1016/j.jbspin.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 27.Wellsandt E, Failla MJ, Snyder-Mackler L. Limb symmetry indexes can overestimate knee function after anterior cruciate ligament injury. J Orthop Sports Phys Ther. 2017;47(5):334–338. doi: 10.2519/jospt.2017.7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gokeler A, Welling W, Benjaminse A, Lemmink K, Seil R, Zaffagnini S. A critical analysis of limb symmetry indices of hop tests in athletes after anterior cruciate ligament reconstruction: a case control study. Orthop Traumatol Surg Res. 2017;103(6):947–951. doi: 10.1016/j.otsr.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Comins J, Brodersen J, Krogsgaard M, Beyer N. Rasch analysis of the Knee Injury and Osteoarthritis Outcome Score (KOOS): a statistical re-evaluation. Scand J Med Sci Sports. 2008;18(3):336–345. doi: 10.1111/j.1600-0838.2007.00724.x. [DOI] [PubMed] [Google Scholar]
- 30.Portney LG, Watkins MP. Foundations of Clinical Research: Application to Practice 3rd ed. Upper Saddle River, NJ: Pearson Prentice Hall; 2009. Experimental design; pp. 200–203. [Google Scholar]
- 31.Hart JM, Pietrosimone B, Hertel J, Ingersoll CD. Quadriceps activation following knee injuries: a systematic review. J Athl Train. 2010;45(1):87–97. doi: 10.4085/1062-6050-45.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norte GE, Hertel JN, Saliba SA, Diduch DR, Hart JM. Quadriceps and patient reported function in ACL-reconstructed patients: a principal component analysis. 2018] doi: 10.1123/jsr.2017-0080. [published online ahead of print June 25. J Sport Rehabil. doi: [DOI] [PubMed]
- 33.Kuenze C, Hertel J, Saliba S, Diduch DR, Weltman A, Hart JM. Clinical thresholds for quadriceps assessment after anterior cruciate ligament reconstruction. J Sport Rehabil. 2015;24(1):36–46. doi: 10.1123/jsr.2013-0110. [DOI] [PubMed] [Google Scholar]
- 34.Anderson AF, Irrgang JJ, Kocher MS, Mann BJ, Harrast JJ. The International Knee Documentation Committee Subjective Knee Evaluation Form: normative data. Am J Sports Med. 2006;34(1):128–135. doi: 10.1177/0363546505280214. [DOI] [PubMed] [Google Scholar]
- 35.Krishnan C, Williams GN. Factors explaining chronic knee extensor strength deficits after ACL reconstruction. J Orthop Res. 2011;29(5):633–640. doi: 10.1002/jor.21316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Losciale JM, Bullock G, Cromwell C, Ledbetter L, Pietrosimone L, Sell TC. Hop testing lacks strong association with key outcome variables after primary anterior cruciate ligament reconstruction: a systematic review. Am J Sports Med. 2019];2019:363546519838794. doi: 10.1177/0363546519838794. [published online ahead of print May 7. [DOI] [PubMed] [Google Scholar]
- 37.Wellsandt E, Gardinier ES, Manal K, Axe MJ, Buchanan TS, Snyder-Mackler L. Decreased knee joint loading associated with early knee osteoarthritis after anterior cruciate ligament injury. Am J Sports Med. 2016;44(1):143–151. doi: 10.1177/0363546515608475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pietrosimone B, Loeser RF, Blackburn JT, et al. Biochemical markers of cartilage metabolism are associated with walking biomechanics 6-months following anterior cruciate ligament reconstruction. J Orthop Res. 2017;35(10):2288–2297. doi: 10.1002/jor.23534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewek M, Rudolph K, Axe M, Snyder-Mackler L. The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon) 2002;17(1):56–63. doi: 10.1016/s0268-0033(01)00097-3. [DOI] [PubMed] [Google Scholar]
- 40.Blackburn JT, Pietrosimone B, Harkey MS, Luc BA, Pamukoff DN. Quadriceps function and gait kinetics after anterior cruciate ligament reconstruction. Med Sci Sports Exerc. 2016;48(9):1664–1670. doi: 10.1249/MSS.0000000000000963. [DOI] [PubMed] [Google Scholar]
- 41.Milner CE, Meardon SA, Hawkins JL, Willson JD. Walking velocity and step length adjustments affect knee joint contact forces in healthy weight and obese adults. J Orthop Res. 2018;36(10):2679–2686. doi: 10.1002/jor.24031. [DOI] [PubMed] [Google Scholar]
- 42.Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Arch Phys Med Rehabil. 2007;88(1):43–49. doi: 10.1016/j.apmr.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Noehren B, Wilson H, Miller C, Lattermann C. Long-term gait deviations in anterior cruciate ligament-reconstructed females. Med Sci Sports Exerc. 2013;45(7):1340–1347. doi: 10.1249/MSS.0b013e318285c6b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ardern CL, Osterberg A, Tagesson S, Gauffin H, Webster KE, Kvist J. The impact of psychological readiness to return to sport and recreational activities after anterior cruciate ligament reconstruction. Br J Sports Med. 2014;48(22):1613–1619. doi: 10.1136/bjsports-2014-093842. [DOI] [PubMed] [Google Scholar]
- 45.Bodkin S, Goetschius J, Hertel J, Hart J. Relationships of muscle function and subjective knee function in patients after ACL reconstruction. Orthop J Sports Med. 2017;5(7):2325967117719041. doi: 10.1177/2325967117719041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luc BA, Harkey MH, Arguelles GD, Blackburn JT, Ryan ED, Pietrosimone B. Measuring voluntary quadriceps activation: effect of visual feedback and stimulus delivery. J Electromyogr Kinesiol. 2016;26:73–81. doi: 10.1016/j.jelekin.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 47.Marcon M, Ciritsis B, Laux C, et al. Cross-sectional area measurements versus volumetric assessment of the quadriceps femoris muscle in patients with anterior cruciate ligament reconstructions. Eur Radiol. 2015;25(2):290–298. doi: 10.1007/s00330-014-3424-2. [DOI] [PubMed] [Google Scholar]


