Abstract
Context
Ultrasound imaging provides a cost-effective method of measuring quadriceps morphology, which may be related to self-reported function after anterior cruciate ligament reconstruction (ACLR).
Objective
To compare quadriceps morphology and strength between limbs in individuals with ACLR and matched control limbs and determine their associations with self-reported function.
Design
Cross-sectional study.
Setting
Research laboratory.
Patients or Other Participants
Forty-two individuals with ACLR (females = 66%; age = 21.8 ± 2.6 years; time since ACLR = 50.5 ± 29.4 months) and 37 controls (females = 73%; age = 21.7 ± 1.2 years).
Main Outcome Measure(s)
Quadriceps peak torque (PT) and rate of torque development were assessed bilaterally. Ultrasonography was used to measure the cross-sectional area (CSA) and echo intensity (EI) of the rectus femoris, vastus lateralis (VL), and vastus medialis. Self-reported function was assessed via the International Knee Documentation Committee (IKDC) score and Knee Injury and Osteoarthritis Outcome Score (KOOS) subscales. Paired-samples t tests were calculated to compare involved and uninvolved limbs. Independent t tests were conducted to compare groups (α = .05). Linear regression was performed to analyze associations between quadriceps function and self-reported function after accounting for time since ACLR, activity level, and sex, and models for EI added subcutaneous fat as a covariate.
Results
Isometric PT did not differ between limbs or groups. Involved limbs had a lower rate of torque development compared with the control (P = .01) but not the uninvolved limbs (P = .08). Vastus lateralis CSA was smaller in the involved than in the uninvolved (P < .01) but not the control limbs (P = .10). Larger VL CSA (ΔR2 = 0.103) and lower VL EI (ΔR2 = 0.076) were associated with a higher IKDC score (P < .05). Larger VL CSA was associated with greater KOOS Symptoms (ΔR2 = 0.09, P = .043) and Sport and Recreation (ΔR2 = 0.125, P = .014) scores. Lower VL EI was associated with higher KOOS Symptoms (ΔR2 = 0.104, P = .03) and Quality of Life (ΔR2 = 0.113, P = .01) scores. Quadriceps PT and rate of torque development were not associated with IKDC or KOOS subscale scores.
Conclusions
Quadriceps morphology was associated with self-reported function in individuals with ACLR and may provide unique assessments of quadriceps function.
Keywords: knee, muscle, torque, ultrasound
Key Points
Interlimb deficits in quadriceps muscle size and quality without deficits in quadriceps strength were evident in individuals who were, on average, 4 years out from anterior cruciate ligament reconstruction.
Larger vastus lateralis size and lower echo intensity were associated with greater International Knee Documentation Committee and Knee Injury and Osteoarthritis Outcome Score Symptoms, Sport and Recreation, and Quality of Life subscale scores, while quadriceps isometric peak torque and rate of torque development were not.
Using ultrasound to assess quadriceps size and echo intensity is a cost-effective and reliable method of imaging that may supplement knee-extensor strength assessments in individuals after anterior cruciate ligament injuries.
Anterior cruciate ligament (ACL) injury is one of the most common joint injuries in the United States, occurring in more than 250 000 individuals annually.1 Of these individuals, more than 100 000 undergo surgical reconstruction and structured rehabilitation to restore knee-joint stability and self-reported function and return to sports participation.2 However, despite structured rehabilitation programs, individuals with ACL reconstruction (ACLR) consistently report deficits in knee function, which can influence whether they return to sport participation.3 Moreover, these deficits exist despite the completion of a rehabilitation program.4 Therefore, to optimize rehabilitation after ACLR, identifying modifiable factors that contribute to a lower level of self-reported function is important.
Quadriceps weakness is among the notable changes after ACLR and persists after rehabilitation is completed.5 Quadriceps strength deficits contribute to the risk of posttraumatic knee osteoarthritis (KOA)6 and are associated with a lower level of self-reported function after reconstruction.7 Self-reported function is commonly assessed using the International Knee Documentation Committee (IKDC) and Knee Injury and Osteoarthritis Outcome Score (KOOS) survey instruments, which provide scores from 0 to 100 and have been validated for use in individuals with ACLR.8,9 Many indices of quadriceps function are measured using isokinetic dynamometry (eg, isometric and isokinetic peak torques [PTs] and rate of torque development [RTD]). However, previous studies have produced mixed results on the association between quadriceps function and self-reported function using the IKDC score,5,7,10 and significant correlations were weak to moderate, leaving a substantial proportion of unexplained variance in the IKDC score.11,12 As such, quadriceps strength assessments may not comprehensively evaluate the contribution of muscle function to self-reported function after ACLR.
Quadriceps atrophy is a common consequence after ACLR and is linked to both muscle weakness13 and poor self-reported functioning.13,14 However, it is unlikely that muscle atrophy fully characterizes reductions in muscle strength, as increases in intramuscular fat and noncontractile tissue content independently contribute to impairments in muscle strength and function.15–17 Limited evidence exists on alterations in quadriceps intramuscular fat content in the involved and uninvolved limbs of individuals with unilateral ACLR,18 and whether their intramuscular fat content would differ from that of healthy control participants remains unknown. However, greater quadriceps intramuscular fat has been shown in individuals with KOA compared with control participants.19 Specifically, greater quadriceps intramuscular fat content in individuals with KOA was associated with lesser self-reported function and strength and worse structural joint damage,19 yet it is unclear if quadriceps intramuscular fat content is associated with self-reported function in individuals with ACLR.
Magnetic resonance imaging (MRI) is commonly used to quantify quadriceps muscle size in individuals with ACLR13,20 and intramuscular fat content in individuals with KOA.19 However, MRI is not widely available and incurs a substantial financial cost. Conversely, ultrasonography provides a cost-effective and portable method that has high test-retest reliability for measuring skeletal muscle size and echo intensity (EI).21,22 Recent advances in ultrasound technology provide panoramic imaging capabilities, permitting visualization of the entire cross-sectional area (CSA) of larger muscles (eg, vastus lateralis [VL]). Additionally, intramuscular fat can be estimated using EI, which is the average grayscale of all pixels within the CSA of a muscle.16,23 Measurements of EI provide an indication of noncontractile tissues within a muscle, such as intramuscular fat infiltration and connective tissue16; a higher EI represents greater intramuscular fat content. Higher quadriceps EI is associated with lower muscle strength in adult males without ACLR21 and predicts mobility limitations in the elderly.17 However, quadriceps ultrasonography (ie, CSA and EI) has not been used to evaluate quadriceps function in individuals with ACLR.
Therefore, the purpose of our study was to (1) determine the associations of quadriceps CSA and EI and strength with self-reported function in the ACLR limb and (2) compare quadriceps morphology (CSA and EI) and strength between the limbs of individuals with unilateral ACLR and with the limbs from a control group. We hypothesized that (1) smaller quadriceps muscle size and less quality and strength would be associated with a lower level of self-reported function (IKDC score and KOOS subscale scores) and (2) individuals with ACLR would have smaller quadriceps CSA and higher EI (ie, greater intramuscular fat content) in the ACLR limb compared with the uninvolved and control limbs.
METHODS
Experimental Design
The data are from a larger cross-sectional investigation of biomechanical and neuromuscular alterations after ACLR.24 Outcomes were obtained from 2 testing sessions that included assessments of gait biomechanics, quadriceps strength, and self-reported function as well as quadriceps and femoral cartilage ultrasound imaging. The data reported here were obtained during a single session.
Participants
We recruited 42 individuals with primary unilateral ACLR for this study (Table 1). A control group of 37 individuals without ACLR (Table 1) was also recruited because recent evidence25 demonstrated bilateral impairments in quadriceps function in individuals with ACLR versus matched control participants. Therefore, comparisons with only the uninvolved limb in individuals with ACLR may underestimate the magnitude of quadriceps impairment. All participants were recreationally active, defined as exercising for a minimum of 30 minutes per day and at least 3 times per week,25 and between the ages of 18 and 30 years old. Individuals with ACLR were considered eligible if they (1) were a minimum of 6 months since surgical reconstruction and cleared by a physician to resume full physical activity, (2) had not experienced graft rupture or undergone revision surgery, and (3) had not sustained a lower extremity injury for at least 6 months before testing and had no history of lower extremity surgery other than ACLR. Participants with ACLR were cohort matched to control participants based on sex, age (±1 year), body mass index (±1 kg/m2), and Tegner score (±1 unit), and their demographics are reported in Table 1. Healthy control participants were also excluded if they had any lower extremity injury in the 6 months before testing or if they gave any history of lower extremity fracture or surgery. All methods were approved by the university's institutional review board, and all participants provided informed written consent before the study.
Table 1.
Participant Demographics
| Characteristic |
Group |
|
| Anterior Cruciate Ligament Reconstruction (n = 42) | Control (n = 37) | |
| Mean ± SD | ||
| Mass, kg | 69.7 ± 15.3 | 68.2 ± 12.2 |
| Height, m | 1.71 ± 0.10 | 1.70 ± 0.09 |
| Age, y | 21.8 ± 2.6 | 21.7 ± 1.2 |
| Tegner activity level (range = 0–10) | 7.0 ± 1.5 | 6.9 ± 1.1 |
| International Knee Documentation Committee score (range = 0–100) | 85.8 ± 11.3 | 99.7 ± 0.45 |
| Knee Injury and Osteoarthritis Outcome Score (subscale ranges = 0–100) | ||
| Pain | 93.2 ± 9.2 | 99.7 ± 1.1 |
| Symptoms | 87.1 ± 8.1 | 98.0 ± 4.4 |
| Activities of Daily Living | 97.9 ± 3.7 | 99.9 ± 0.5 |
| Sport and Recreation | 85.8 ± 12.1 | 98.0 ± 6.0 |
| Quality of Life | 75.6 ± 17.7 | 98.5 ± 6.0 |
| Time since reconstruction, mo | 50.5 ± 29.42 | NA |
| No. |
||
| Female | 20 | 20 |
| Concomitant meniscal injury | 21 | NA |
| Graft type | ||
| Patellar tendon | 20 | NA |
| Hamstrings | 11 | NA |
| Allograft | 11 | NA |
Abbreviation: NA, not applicable.
Ultrasound Imaging
Panoramic cross-sectional ultrasound images (LogiqE, GE Healthcare, Milwaukee, WI) of the vastus medialis (VM), VL, and rectus femoris (RF) were obtained, while all participants lay supine and fully relaxed on a padded treatment table with their knees fully extended (Figures 1 through 3). Vastus medialis images were obtained at 80% of the distance from the anterior-superior iliac spine to the medial border of the patella. Vastus lateralis and RF images were obtained at 50% of the distance from the anterior-superior iliac spine to the lateral border of the patella and the superior margin of the patella, respectively.26 The transducer was aligned in the transverse plane and moved along the entire width of the muscle to capture a full panoramic view of the RF, VL, and VM CSA (12 MHz, gain: 50, depth: 4.5 cm [RF, VL] and 6.0 cm [VM]).
Figure 1.
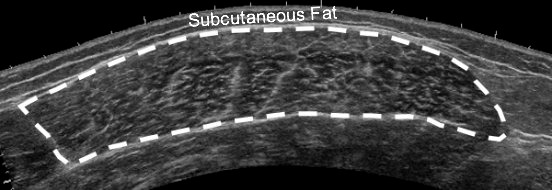
Example of a panoramic ultrasound image of the quadriceps muscle. The interface between the hyperechoic epimysium and hypoechoic muscle tissue was traced, and the resulting area was defined as the vastus lateralis cross-sectional area. Echo intensity was derived from the average grayscale of all the pixels within the region of interest.
Figure 3.
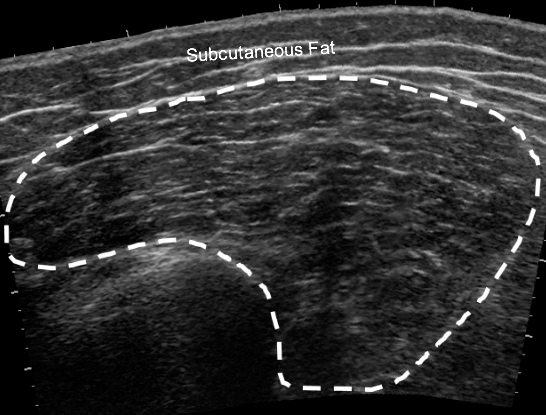
Example of a panoramic ultrasound image of the vastus medialis muscle.
Figure 2.
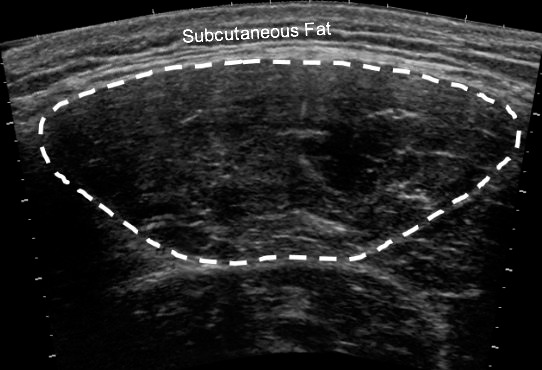
Example of a panoramic ultrasound image of the rectus femoris muscle.
Quadriceps Strength Testing
Quadriceps strength was assessed using a Humac NORM isokinetic dynamometer (Computer Sports Medicine Inc, Stoughton, MA) bilaterally for both groups. For strength testing, participants were secured at the torso, hip, thigh, and shank with the hip positioned at 85° of flexion and the knee at 45° of flexion.25,27 We chose the testing position based on a previous study25 that demonstrated bilateral impairments in quadriceps function (ie, RTD during the first 0–100 milliseconds after torque onset, isokinetic PT) in individuals with ACLR compared with control participants at 45° of knee flexion. Furthermore, earlier investigators27 showed stronger associations between strength and physical function at 45° than at 90° of knee flexion. All participants were given a standardized warmup protocol, which consisted of contractions at 25%, 50%, 75%, and 1 trial at 100% effort with a 1-minute rest between trials. After the warmup protocol, participants completed 3 maximal voluntary isometric contractions and were instructed to extend the knee as hard and fast as possible while receiving oral encouragement and visual feedback throughout the entire trial. The involved and uninvolved limbs of the ACLR group were compared with the dominant limb (ie, the leg used to kick a ball) of control participants.
Data Reduction
Ultrasound images were assessed using ImageJ Software (National Institutes of Health, Bethesda, MD). Regions of interest were selected by tracing the entire muscle CSA on the panoramic images while excluding the hyperechoic fascia (Figure 1). Echo intensity was calculated as the mean of the grayscale value (arbitrary unit [AU] range = 0 [black] to 255 [white]) of all the pixels within the region of interest and was averaged across 3 images for each muscle. Higher EI values indicate lower muscle quality and greater noncontractile tissue within the muscle.26 Subcutaneous fat thickness of each muscle was measured as the straight-line distance between the superficial aponeurosis and the skin-muscle interface at 3 sites (lateral, center, medial; Figure 1) as described by Stock et al.23 The distances in the 3 measures were averaged for analysis. To determine intrarater and interrater reliability, 2 raters blinded to limb injury status independently assessed each image 3 times, with the average value used in further analyses.
Torque data were sampled at 2000 Hz and normalized to body mass (Nm/kg). The torque signal was gravity corrected, and onset was defined as the point when torque exceeded 2.5 standard deviations of the resting signal using a custom written LabVIEW program (National Instruments, Austin, TX). Isometric PT was calculated as the maximum torque produced, and RTD was calculated as the slope of the line of the torque-time curve during the first 0–100 milliseconds after torque onset (RTD100). We selected this RTD time interval (0–100 milliseconds) because it is associated with neural contributors to force production such as motor-unit recruitment and firing rate.28
Self-Reported Function
Self-reported function was assessed using the IKDC and KOOS.8,9 These surveys are valid and reliable instruments for evaluating symptoms, function, and sports activities that are associated with knee-joint injuries. The KOOS survey consists of multiple subscales for evaluating pain, activities of daily living (ADL), function in sport and recreation (Sport/Rec) and knee-related quality of life (QOL).8,9 For the IKDC, participants were scored on a scale of 0 to 100, with a score of 100 indicating the highest level of subjective knee function.8 For each KOOS subscale, scoring is on a scale of 0 to 4, which is then transformed to a 0 to 100 scale, where 0 indicates extreme knee problems and 100 indicates no knee problems.9
Statistical Analyses
The data were inspected for normality using the Shapiro-Wilk test and for outliers using boxplots. To assess the analyses of the ultrasound images, we calculated the intrarater intraclass correlation coefficient (ICC [3,1]), interrater reliability (ICC [2,k]), and standard error of the measurement. Dependent variables (quadriceps strength, IKDC score, KOOS score, ultrasound measures) were compared between the involved and uninvolved limbs of the ACLR group using paired-samples t tests. The involved and uninvolved limbs were compared with the dominant limb in the control cohort using independent-samples t tests. We adjusted the α level to .017 to account for multiple comparisons. Cohen d effect sizes were calculated for limb comparisons and interpreted as small (0.2–0.5), medium (0.5–0.8), and large (>0.8). Stepwise linear regression was used to determine the unique association (ΔR2) of quadriceps strength (PT, RTD100) and morphology (CSA and EI) with self-reported physical function (IKDC and KOOS) after accounting for time since ACLR, Tegner score, and sex (α = .05). Preliminary analyses indicated that the time since ACLR and Tegner physical activity score were associated with the IKDC score and thus were considered as covariates. Sex was also a covariate because females had smaller CSAs and higher EIs in the preliminary analyses. Finally, models that assessed EI also included subcutaneous fat thickness as a covariate because of the known confounding effect of fat thickness on EI measurements.22,23
RESULTS
All data were normally distributed and treated as such, and no outliers were identified. Participant demographics are listed in Table 1 and were not different between groups. Intrarater reliability was good to excellent for muscle CSA (ICC [3,1] range = 0.85–0.94; SEM range = 98–135 mm2) and excellent for EI (ICC [3,1] range = 0.93–0.94; SEM range = 1.2–1.9 AU; Table 2).
Table 2.
Reliability of the Ultrasound Image Analyses
| Measure |
Intrarater |
Interrater |
||
| ICC (3,1) |
SEM |
ICC (2,k) |
SEM |
|
| Vastus lateralis | ||||
| CSAa | 0.845 | 134.57 | 0.811 | 146.97 |
| EIa | 0.935 | 1.20 | 0.904 | 1.61 |
| Rectus femoris | ||||
| CSA | 0.865 | 97.94 | 0.845 | 105.45 |
| EI | 0.944 | 1.84 | 0.945 | 1.95 |
| Vastus medialis | ||||
| CSA | 0.846 | 149.89 | 0.816 | 162.31 |
| EI | 0.928 | 1.91 | 0.924 | 2.01 |
Abbreviations: CSA, cross-sectional area; EI, echo intensity; ICC, intraclass correlation coefficient; SEM, standard error of the measurement.
All CSA values were reported in mm.2
All EI ranges = 0–255.
Limb Comparison
Comparisons of ultrasound measures and quadriceps strength between limbs are listed in Table 3. Vastus lateralis CSA was smaller in the involved compared with the uninvolved limb (t41 = 3.741, P < .01, d = 0.577) but not compared with control limbs (t77 = 1.67, P = .10). Vastus lateralis EI was higher (ie, worse quality) in the involved limb than in the uninvolved limb (t41 = 2.897, P = .012, d = 0.447) but not in the control limbs (t77 = 0.05, P = .96). Rectus femoris CSA was not different in the involved versus the uninvolved limb (t41 = 1.79, P = .08) or versus the control limbs (P = .50). Rectus femoris EI did not differ between limbs or compared with control limbs (P = .71 and P = .52, respectively). No differences were found in VM CSA between limbs (P = .10) or compared with control limbs (P = .80). Vastus medialis EI did not differ between limbs (P = .39) or compared with control limbs (P = .82).
Table 3.
Comparisons of the Anterior Cruciate Ligament Reconstruction Group's Involved and Uninvolved Limbs and the Healthy Control Group's Limbs, Mean (95% Confidence Interval)a
| Measure |
Group |
||
| Anterior Cruciate Ligament Reconstruction |
Healthy Control |
||
| Involved Limb |
Uninvolved Limb |
||
| Vastus lateralis | |||
| CSAb | 2161.76 (1993.35, 2330.17)d | 2383.23 (2196.16, 2570.30) | 2367.51 (2194.49, 2540.52) |
| EIc | 64.10 (60.94, 67.26)d | 62.11 (58.42, 65.80) | 63.93 (60.03, 67.83) |
| Rectus femoris | |||
| CSA | 766.05 (709.03, 823.07)d | 804.13 (753.20, 855.06) | 803.93 (727.57, 880.28) |
| EI | 66.17 (62.91, 69.42) | 65.92 (62.39, 69.44) | 66.10 (62.38, 69.82) |
| Vastus medialis | |||
| CSA | 2060.94 (1869.74, 2252.14) | 2147.92 (1969.51, 2326.34) | 2095.22 (1911.37, 2279.06) |
| EI | 63.06 (59.07, 67.05) | 63.48 (59.28, 67.67) | 62.83 (58.91, 66.75) |
| Peak torque, Nm/kg | 2.31 (2.11, 2.51) | 2.34 (2.13, 2.55) | 2.59 (2.36, 2.82) |
| RTD100, Nm/kg/s | 12.02 (10.15, 13.89)e | 11.03 (9.62, 12.44)e | 15.35 (13.09, 17.60) |
Abbreviations: CSA, cross-sectional area; EI, echo intensity; RTD100, rate of torque development during the first 0–100 milliseconds after torque onset.
Paired-samples t tests were used to compare the involved and uninvolved limbs of the reconstruction group, and independent-samples t tests were used to compare the involved and uninvolved limbs of the reconstruction group with the matched control limbs (significance = P < .017).
All CSA values were reported in mm.2
All EI values were reported in arbitrary units.
Difference compared with the uninvolved limb.
Difference compared with the control limb.
No differences in isometric PT were present between the ACLR group's limbs (t41 = 0.455, P = .69) or compared with control limbs (t77 = 1.775, P = .08). There were no differences in RTD100 between the involved and uninvolved limbs (t77 = 1.775, P = .08), but both limbs had less RTD100 than control limbs (t77 = 2.247, P = .01, d = 0.505 and t77 = 3.271, P < .01, d = 0.727, respectively).
Quadriceps Morphology and Strength and IKDC Score
Covariates were entered into the regression model first and explained 26.3% of the variance in IKDC score (F4,37 = 3.296, total R2 = 0.263, P = .021; Table 4). After accounting for covariates, a larger VL CSA was associated with a higher IKDC score (ΔR2 = 0.103, PΔ = .02, total R2 = 0.360), and a lower VL EI was associated with a higher IKDC score (ΔR2 = 0.081, PΔ = .042, total R2 = 0.344). Injured-limb PT and RTD100 were not associated with IKDC score after accounting for covariates (ΔR2 = 0.009, PΔ = .508 and ΔR2 = 0.033, PΔ = .201, respectively).
Table 4.
Summary of Covariates
| Covariate |
International Knee Documentation Committee Score |
Knee Injury and Osteoarthritis Outcome Subscale Scores |
||||||||||
| Pain |
Symptoms |
Activities of Daily Living |
Sport and Recreation |
Quality of Life |
||||||||
| β Value |
t Value |
β Value |
t Value |
β Value |
t Value |
β Value |
t Value |
β Value |
t Value |
β Value |
t Value |
|
| Models for cross-sectional area | ||||||||||||
| Time since ACLR, mo | 0.091 | 1.673 | 0.033 | 0.633 | 0.035 | 0.433 | 0.002 | 0.064 | 0.134 | 2.174a | 0.258 | 3.368a |
| Tegner score (range = 0–10) | 2.915 | 2.944a | 0.528 | 0.559 | 1.191 | 0.152 | 0.648 | 1.220 | 1.260 | 1.120 | 3.227 | 2.304a |
| Sex (female = 0, male = 1) | 1.987 | 0.598 | 1.762 | 0.556 | 4.438 | 0.113 | 2.014 | 1.131 | 4.703 | 1.247 | 0.869 | 0.185 |
| Models for vastus lateralis echo intensity | ||||||||||||
| Time since ACLR, mo | 0.091 | 1.656 | 0.033 | 0.629 | 0.035 | 0.436 | 0.002 | 0.074 | 0.134 | 2.164a | 0.258 | 3.327a |
| Tegner score (range = 0–10) | 3.063 | 2.955a | 0.674 | 0.682 | 1.306 | 1.531 | 0.966 | 0.072 | 1.493 | 1.273 | 3.097 | 2.107a |
| Sex (female = 0, male = 1) | 2.077 | 0.089 | 1.851 | 0.095 | 4.508 | 1.632 | 2.209 | 1.307 | 4.846 | 1.276 | 0.790 | 0.166 |
| Fat thickness, mm | −2.483 | 4.614 | −2.452 | −0.557 | −1.940 | −0.511 | −5.345 | −2.300a | −3.930 | −0.753 | 2.183 | 0.334 |
Abbreviation: ACLR, anterior cruciate ligament reconstruction.
Significance = P < .05.
Quadriceps Morphology and Strength and KOOS Subscale Scores
Covariates were entered into the regression model first and explained 15.1% of the variance in KOOS Symptoms score (F4,37 = 1.65, total R2 = 0.151, P = .182), 19.3% of the variance in Sport/Rec score (F4,37 = 2.819, total R2 = 0.193, P = .05), and 32% of the variance in KOOS QOL score (F4,37 = 4.353, total R2 = 0.320, P = .006; Tables 3 and 4). After we accounted for covariates, a larger VL CSA was associated with a higher KOOS Symptoms score (ΔR2 = 0.09, PΔ = .043, total R2 = 0.236) and Sport/Rec score (ΔR2 = 0.125, PΔ = .014, total R2 = 0.299). Lower VL EI was associated with a higher KOOS Symptoms score (ΔR2 = 0.110, PΔ = .026, total R2 = 0.262) and KOOS QOL subscale score (ΔR2 = 0.111, PΔ = .012, total R2 = 0.431). Injured-limb PT and RTD100 did not predict any KOOS subscale scores after covariates were accounted for (Table 5).
Table 5.
Associations Between Quadriceps Strength, Ultrasound Measures, and Self-Reported Outcome Scores
| Variable |
International Knee Documentation Committee Score |
Knee Injury and Osteoarthritis Outcome Subscale Scores |
||||||||||
| Pain |
Symptoms |
Activities of Daily Living |
Sport and Recreation |
Quality of Life |
||||||||
| β Value |
ΔR2 |
β Value |
ΔR2 |
β Value |
ΔR2 |
β Value |
ΔR2 |
β Value |
ΔR2 |
β Value |
ΔR2 |
|
| Peak torque, Nm/kg | −1.944 | 0.009 | 0.670 | 0.002 | 1.989 | 0.016 | 0.882 | 0.008 | 1.336 | 0.004 | −0.459 | <0.010 |
| Rate of torque development, Nm/kg/s | 0.381 | 0.033 | 0.192 | 0.012 | 0.071 | 0.002 | 0.145 | 0.02 | 0.084 | 0.001 | −0.04 | 0.001 |
| Vastus lateralis | ||||||||||||
| Cross-sectional area, mm2 | 0.007 | 0.103a | 0.004 | 0.046 | 0.005 | 0.09 | 0.009 | 0.024 | 0.009 | 0.125a | 0.005 | 0.021 |
| Echo intensity, arbitrary units | −0.298 | 0.081a | −0.174 | 0.037 | −0.268 | 0.110a | 0.002 | <0.010 | −0.224 | 0.037 | −0.538 | 0.111a |
Abbreviation: β, weight, unstandardized.
P < .05. Covariates = time since anterior cruciate ligament reconstruction, sex, and Tegner physical activity level. Subcutaneous fat thickness was added as a covariate for the echo intensity regression models.
DISCUSSION
The purpose of our study was to determine the associations of quadriceps CSA, EI, and strength of the involved limb with self-reported function in individuals with ACLR. Further, we sought to compare quadriceps morphology (CSA and EI) and strength between the involved and uninvolved limbs in individuals with ACLR and with a control limb. We hypothesized that less quadriceps strength (PT, RTD100), smaller size (CSA), and lower EI would be associated with lower levels of self-reported function as assessed by the IKDC and KOOS subscales. We also hypothesized that individuals with ACLR would have smaller quadriceps CSA, higher EI (ie, greater intramuscular fat), and less strength in both limbs compared with a matched control limb. After accounting for time since ACLR, sex, and Tegner physical activity level, we found that a larger VL CSA and lower VL EI were associated with higher self-reported function. Strength characteristics of the ACLR limb were not associated with IKDC or KOOS subscale scores after accounting for covariates. Vastus lateralis CSA was smaller in the ACLR limb than in the uninvolved limb but not smaller than the control limb. No differences were present between limbs for any strength variables.
Contrary to our hypothesis, no differences in isometric quadriceps strength were demonstrated between the involved and uninvolved limbs. However, both limbs had lower quadriceps RTD100 compared with control limbs, which partially agreed with our hypothesis and is consistent with previous findings.10 Earlier researchers29 indicated that activation deficits occurred bilaterally and persisted for several years after ACLR. Thus, the lack of interlimb deficits in RTD100 in our sample highlights the importance of comparisons with a matched control group when evaluating quadriceps function. Further, RTD100 indicates neural contributions (ie, motor-unit recruitment, rate coding) to rapid force production.28 Therefore, less RTD100 bilaterally in individuals with ACLR versus healthy control participants suggests that neural deficits remain unresolved for several years after ACLR. Interestingly, individuals with ACLR had smaller VL CSA in the involved limb than in the uninvolved limb without interlimb differences in strength. Similar findings were reported by Lepley et al,30 who identified interlimb deficits in quadriceps muscle volume in the absence of interlimb isometric torque deficits among a cohort an average of 6 years after ACLR. Thus, our results and recent findings30 indicate that maximal isometric strength may not comprehensively evaluate quadriceps impairment, particularly in individuals several years after ACLR. Measures of quadriceps muscle morphology may offer an additional or alternative method of detecting interlimb deficits in quadriceps function compared with maximal strength characteristics (ie, PT, RTD) in individuals with ACLR.
Moreover, assessing maximal strength allows for only gross estimates of the overall force-generating capacity of the knee-extensor muscle group and may not comprehensively evaluate quadriceps dysfunction. Maximal strength assessments also assume a maximal volitional effort and thus may underestimate the magnitude of quadriceps dysfunction in poorly motivated participants.5 Also, maximal strength assessments are contraindicated in the early postoperative phases (ie, 1–2 months) after ACLR, limiting the clinician's ability to monitor quadriceps function immediately after surgery.31 Alternatively, ultrasound measures of muscle morphology are not contraindicated during an early rehabilitation stage, which may be advantageous when maximal strength assessments are not feasible while the graft is still healing.31 Further, ultrasonography is not confounded by a participant's volitional effort and is sensitive to changes in muscle morphology over time.32 Therefore, ultrasound imaging of the quadriceps may provide clinicians with unique measurements of muscle function that can be tracked consistently throughout and after rehabilitation.
In this sample, quadriceps strength characteristics (PT, RTD100) were not associated with self-reported function, which was contrary to our hypothesis and inconsistent with the previous literature. Multiple authors4,7,11,12 have found significant associations between quadriceps strength and self-reported function. However, the correlation coefficients reported in these studies ranged from weak to moderate, leaving a substantial amount of variance unexplained. Discrepancies in these results may be explained by differences in sample characteristics, with investigators often reporting substantial strength asymmetry, and our sample did not exhibit interlimb differences in strength. In addition, Bodkin et al11 showed that the strength of association between strength and self-reported measures of function (IKDC and KOOS subscale scores) depended on the time since ACLR, and stronger associations were reported in earlier time periods after ACLR. Our cohort of individuals with ACLR was, on average, 4 years postreconstruction, which may explain the nonsignificant associations between quadriceps strength and self-reported function. Thus, our findings may suggest that additional metrics of quadriceps function may contribute to self-reported function in the absence of measurable interlimb strength deficits in a cohort that is several years after surgery.
Larger VL CSA and lower EI were associated with greater self-reported function as measured by IKDC score after accounting for time since reconstruction, activity level, subcutaneous fat, and sex, yet this relationship was not observed for the other quadriceps muscles (ie, RF or VM). Previous researchers13 quantified quadriceps CSA in individuals with ACLR using MRI and found significant associations between vastus intermedius (VI) CSA and self-reported function. However, they did not assess interlimb differences in CSA, and associations were noted in a different portion of the quadriceps femoris (VI versus VL), which limits our ability to directly compare findings. It is possible that each head of the quadriceps femoris may have unique contributions to quadriceps function. Regardless, we observed similar associations between quadriceps CSA and IKDC score (ΔR2 = 0.103 versus 0.102, respectively). Future authors should image the VI when evaluating quadriceps morphology in individuals with ACLR.
We found associations between VL EI (ie, greater intramuscular fat) derived using ultrasound imaging and self-reported function in individuals with ACLR. However, few other studies of individuals with ACLR have been conducted that would allow us to directly contextualize our results. Conversely, quadriceps CSA and EI contributed to functional ability in young adults with obesity, older adults, and individuals with KOA.17,19,33 In individuals with KOA, greater quadriceps intramuscular fat was associated with greater disease severity and less strength.19 Similarly, greater thigh muscle fat infiltration was associated with higher odds of mobility limitations independent of muscle size and strength in older adults.31 Although ultrasound imaging does not provide a direct measurement of intramuscular fat, EI is a surrogate measurement and represents noncontractile components and fatty infiltration within the muscle.16,23 Therefore, assessments of CSA and EI may be important markers of quadriceps function in individuals with ACLR.
The KOOS instrument is used to monitor disability in populations with or at risk for KOA and has been previously linked with quadriceps function after ACLR.9 The KOOS Symptoms, Sport/Rec, and QOL subscales assess subjective function during tasks ranging from higher-level functioning activities, such as running or jumping, to overall awareness of current knee problems and its effect on function. Contrary to our hypothesis, neither quadriceps PT nor RTD100 was associated with any KOOS subscale score. As mentioned previously, the strength of relationships between these 2 measures is influenced by the time since ACLR,11 and our sample was, on average, 4 years postreconstruction. Conversely, our results indicate that VL CSA was associated with the Symptoms and Sport/Rec subscale scores, and VL EI was associated with the Symptoms and QOL subscale scores. No associations were found between VL CSA and EI and pain or ADL subscale scores. However, most participants had higher scores for these domains, indicating fewer pain symptoms and ADL limitations, which were likely due to their age and the time since ACLR. Regardless, quadriceps CSA and EI were associated with IKDC and KOOS subscale scores while strength measurements were not. We reason that the greater associations between quadriceps morphology (CSA and EI) and self-reported function may be attributed to the types of activities and tasks assessed by the IKDC and KOOS, which are mainly submaximal (eg, climbing stairs). Thus, function during these tasks may not be fully explained by maximal strength. Evaluating quadriceps morphology and strength in combination may better characterize overall quadriceps function in individuals with ACLR.
This study should be interpreted in the context of its limitations. First, quadriceps muscle EI is a surrogate for intramuscular fat, which is commonly assessed using MRI. Previous authors22 have applied regression equations derived from MRI to predict the fat percentage. However, this was only evaluated in the RF, and the researchers cautioned about its use for other portions of the quadriceps femoris. Future studies would benefit from the development and validation of regression equations to predict the intramuscular fat percentage from VL EI. Second, CSA and EI were assessed at only a single site and may not fully represent the entire muscle. Evaluating CSA and EI at multiple sites to estimate muscle volume and quality may be beneficial when assessing quadriceps morphology. Additionally, we did not assess the VI. Future investigators should include this muscle in order to comprehensively evaluate quadriceps function. Furthermore, the graft types of our ACLR cohort were heterogeneous (20 patellar tendon, 11 hamstrings, 11 allograft), and 50% reported concomitant meniscal injury, which may have affected the magnitude and location of muscle impairment. However, whether graft type influences muscle morphology (ie, CSA and EI) after ACLR is unknown. Last, the cross-sectional design of this study limited our ability to ascertain if differences in muscle size existed before data collection. Future studies are needed to prospectively examine quadriceps CSA and EI before and after ACLR using ultrasonography.
CONCLUSIONS
Our findings suggest that larger VL CSA and lower EI are associated with higher self-reported function (ie, IKDC, KOOS Symptoms, Sport/Rec, and QOL scores) in individuals with ACLR, without interlimb deficits in strength. Moreover, our results demonstrate that quadriceps ultrasonography may have clinical utility as an objective assessment of quadriceps muscle dysfunction after ACLR. Developing rehabilitation strategies that target quadriceps CSA and EI may be beneficial for improving self-reported function after ACLR. Prospective research using ultrasound to measure and track quadriceps muscle CSA and EI throughout rehabilitation is needed.
ACKNOWLEDGMENTS
This study was supported by a research grant from the California State University Program for Research and Education in Biotechnology.
REFERENCES
- 1.Mather RC, III, Koenig L, Kocher MS, et al. Societal and economic impact of anterior cruciate ligament tears. J Bone Joint Surg Am. 2013;95(19):1751–1759. doi: 10.2106/JBJS.L.01705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filbay SR, Ackerman IN, Russell TG, Macri EM, Crossley KM. Health-related quality of life after anterior cruciate ligament reconstruction: a systematic review. Am J Sports Med. 2014;42(5):1247–1255. doi: 10.1177/0363546513512774. [DOI] [PubMed] [Google Scholar]
- 3.Logerstedt D, Di Stasi S, Grindem H, et al. Self-reported knee function can identify athletes who fail return-to-activity criteria up to 1 year after anterior cruciate ligament reconstruction: a Delaware-Oslo ACL cohort study. J Orthop Sports Phys Ther. 2014;44(12):914–923. doi: 10.2519/jospt.2014.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lepley LK. Deficits in quadriceps strength and patient-oriented outcomes at return to activity after ACL reconstruction: a review of the current literature. Sports Health. 2015;7(3):231–238. doi: 10.1177/1941738115578112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hart JM, Pietrosimone B, Hertel J, Ingersoll CD. Quadriceps activation following knee injuries: a systematic review. J Athl Train. 2010;45(1):87–97. doi: 10.4085/1062-6050-45.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tourville TW, Jarrell KM, Naud S, Slauterbeck JR, Johnson RJ, Beynnon BD. Relationship between isokinetic strength and tibiofemoral joint space width changes after anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42(2):302–311. doi: 10.1177/0363546513510672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pietrosimone B, Lepley AS, Harkey MS, et al. Quadriceps strength predicts self-reported function post-ACL reconstruction. Med Sci Sports Exerc. 2016;48(9):1671–1677. doi: 10.1249/MSS.0000000000000946. [DOI] [PubMed] [Google Scholar]
- 8.Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the international Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2001;29(5):600–613. doi: 10.1177/03635465010290051301. [DOI] [PubMed] [Google Scholar]
- 9.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS): development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88–96. doi: 10.2519/jospt.1998.28.2.88. [DOI] [PubMed] [Google Scholar]
- 10.Davis HC, Blackburn JT, Ryan ED, et al. Quadriceps rate of torque development and disability in individuals with anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon) 2017;46:52–56. doi: 10.1016/j.clinbiomech.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Bodkin S, Goetschius J, Hertel J, Hart J. Relationships of muscle function and subjective knee function in patients after ACL reconstruction. Orthop J Sports Med. 2017;5(7):2325967117719041. doi: 10.1177/2325967117719041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zwolski C, Schmitt LC, Quatman-Yates C, Thomas S, Hewett TE, Paterno MV. The influence of quadriceps strength asymmetry on patient-reported function at time of return to sport after anterior cruciate ligament reconstruction. Am J Sports Med. 2015;43(9):2242–2249. doi: 10.1177/0363546515591258. [DOI] [PubMed] [Google Scholar]
- 13.Kuenze CM, Blemker SS, Hart JM. Quadriceps function relates to muscle size following ACL reconstruction. J Orthop Res. 2016;34(9):1656–1662. doi: 10.1002/jor.23166. [DOI] [PubMed] [Google Scholar]
- 14.Krishnan C, Williams GN. Factors explaining chronic knee extensor strength deficits after ACL reconstruction. J Orthop Res. 2011;29(5):633–640. doi: 10.1002/jor.21316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukumoto Y, Ikezoe T, Yamada Y, et al. Skeletal muscle quality assessed from echo intensity is associated with muscle strength of middle-aged and elderly persons. Eur J Appl Physiol. 2012;112(4):1519–1525. doi: 10.1007/s00421-011-2099-5. [DOI] [PubMed] [Google Scholar]
- 16.Pillen S, Tak RO, Zwarts MJ, et al. Skeletal muscle ultrasound: correlation between fibrous tissue and echo intensity. Ultrasound Med Biol. 2009;35(3):443–446. doi: 10.1016/j.ultrasmedbio.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60(3):324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 18.Lindström M, Strandberg S, Wredmark T, Felländer-Tsai L, Henriksson M. Functional and muscle morphometric effects of ACL reconstruction: a prospective CT study with 1 year follow-up. Scand J Med Sci Sports. 2013;23(4):431–442. doi: 10.1111/j.1600-0838.2011.01417.x. [DOI] [PubMed] [Google Scholar]
- 19.Kumar D, Karampinos DC, MacLeod TD, et al. Quadriceps intramuscular fat fraction rather than muscle size is associated with knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(2):226–234. doi: 10.1016/j.joca.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norte GE, Knaus KR, Kuenze C, et al. MRI-based assessment of lower-extremity muscle volumes in patients before and after ACL reconstruction. J Sport Rehabil. 2018;27(3):201–212. doi: 10.1123/jsr.2016-0141. [DOI] [PubMed] [Google Scholar]
- 21.Mota JA, Stock MS. Rectus femoris echo intensity correlates with muscle strength, but not endurance, in younger and older men. Ultrasound Med Biol. 2017;43(8):1651–1657. doi: 10.1016/j.ultrasmedbio.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Young HJ, Jenkins NT, Zhao Q, Mccully KK. Measurement of intramuscular fat by muscle echo intensity. Muscle Nerve. 2015;52(6):963–971. doi: 10.1002/mus.24656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stock MS, Whitson M, Burton AM, Dawson NT, Sobolewski EJ, Thompson BJ. Echo intensity versus muscle function correlations in older adults are influenced by subcutaneous fat thickness. Ultrasound Med Biol. 2018;44(8):1597–1605. doi: 10.1016/j.ultrasmedbio.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Pamukoff DN, Montgomery MM, Holmes SC, Moffit TJ, Garcia SA, Vakula MN. Association between gait mechanics and ultrasonographic measures of femoral cartilage thickness in individuals with ACL reconstruction. Gait Posture. 2018;65:221–227. doi: 10.1016/j.gaitpost.2018.07.174. [DOI] [PubMed] [Google Scholar]
- 25.Pamukoff DN, Montgomery MM, Choe KH, Moffit TJ, Garcia SA, Vakula MN. Bilateral alterations in running mechanics and quadriceps function following unilateral anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2018;48(12):960–967. doi: 10.2519/jospt.2018.8170. [DOI] [PubMed] [Google Scholar]
- 26.Taniguchi M, Fukumoto Y, Kobayashi M, et al. Quantity and quality of the lower extremity muscles in women with knee osteoarthritis. Ultrasound Med Biol. 2015;41(10):2567–2574. doi: 10.1016/j.ultrasmedbio.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Krishnan C, Theuerkauf P. Effect of knee angle on quadriceps strength and activation after anterior cruciate ligament reconstruction. J Appl Physiol (1985) 2015;119(3):223–231. doi: 10.1152/japplphysiol.01044.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersen LL, Andersen JL, Zebis MK, Aagaard P. Early and late rate of force development: differential adaptive responses to resistance training? Scand J Med Sci Sports. 2010;20(1):e162–e169. doi: 10.1111/j.1600-0838.2009.00933.x. [DOI] [PubMed] [Google Scholar]
- 29.Urbach D, Nebelung W, Becker R, Awiszus F. Effects of reconstruction of the anterior cruciate ligament on voluntary activation of quadriceps femoris: a prospective twitch interpolation study. J Bone Joint Surg Br. 2001;83(8):1104–1110. doi: 10.1302/0301-620x.83b8.11618. [DOI] [PubMed] [Google Scholar]
- 30.Lepley AS, Grooms DR, Burland JP, Davi SM, Kinsella-Shaw JM, Lepley LK. Quadriceps muscle function following anterior cruciate ligament reconstruction: systemic differences in neural and morphological characteristics. Exp Brain Res. 2019;237(5):1267–1278. doi: 10.1007/s00221-019-05499-x. [DOI] [PubMed] [Google Scholar]
- 31.Fleming BC, Oksendahl H, Beynnon BD. Open- or closed-kinetic chain exercises after anterior cruciate ligament reconstruction? Exerc Sport Sci Rev. 2005;33(3):134–140. doi: 10.1097/00003677-200507000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 33.Vakula MN, Fisher KL, Garcia SA, et al. Quadriceps impairment is associated with gait mechanics in young adults with obesity. Med Sci Sports Exerc. 2019;51(5):951–961. doi: 10.1249/MSS.0000000000001891. [DOI] [PubMed] [Google Scholar]


