Abstract
Background
Several studies have reported the use of acellular dermal matrix in breast reconstruction. However, the primary role of acellular dermal matrix in these studies was to support the implant; there are no reports on the use of acellular dermal matrix exclusively as volume replacement. Thus, we aimed to evaluate the safety and effectiveness of filling of the defect with acellular dermal matrix in oncoplastic breast-conserving surgery.
Methods
We prospectively recruited 120 adult breast cancer patients who were scheduled to undergo oncoplastic breast-conserving surgery with acellular dermal matrix filling from 2017 to 2018. Intraoperatively, diced human acellular dermal matrix measuring 3–5 mm was used on each side to fill in the excisional defect immediately. After 6 months, satisfaction of the patients and surgeons with overall and cosmetic outcomes was evaluated with a survey using a 10-point scale. Postoperative complications were assessed at 2 weeks and 6 months postoperatively.
Results
Of the 117 patients who were evaluated for their satisfaction, 94.0% were strongly satisfied with the cosmetic outcomes and 90.4% were strongly satisfied overall. Patient overall satisfaction scores were higher than surgeon satisfaction scores (p < 0.001). Of the 117 patients who underwent evaluation of complications 6 months postoperatively, six (5.1%) had hematoma and seven (6.0%) had seroma. The overall reoperation rate due to complications was 8.5%. Only two patients needed acellular dermal matrix removal due to hematoma and inflammation.
Conclusion
Oncoplastic breast-conserving surgery with acellular dermal matrix filling of defects can be performed safely with high cosmetic satisfaction.
Trial registration
ICTRP, KCT0003886; retrospectively registered May 3, 2019, http://apps.who.int/trialsearch/Trial2.aspx?TrialID=KCT0003886
Keywords: Acellular dermis, Breast-conserving surgery, Breast cancer, Breast reconstruction
Background
Oncoplastic breast-conserving surgery (BCS) has been developed to overcome the cosmetic disadvantages of conventional BCS and has become a commonly used procedure in patients with breast cancer [1]. Oncoplastic BCS can be divided into volume displacement and volume replacement techniques [2]. In Asian women, the size of the breast is relatively small, whereas the size of the tumor is large [3–5]. Therefore, volume displacement with the patient’s own breast tissue only is difficult to achieve cosmetically satisfying results. In small to medium-sized breasts, the replacement technique has shown better results [6].
Acellular dermal matrix (ADM) contains collagen, elastin, proteoglycans, laminin, and a basement membrane. These materials serve as pillars for reepithelialization, neovascularization, and fibroblast infiltration [7]. ADM does not evoke an immune response and is widely used in burn care and reconstructive surgery [7–9]. In breast surgery, ADM is used in more than 75% of immediate tissue expander reconstruction procedures to support the implant [10]. However, the role of ADM in these procedures is only supportive, and no method has been described as filling the defects during BCS exclusively with ADM. Before starting this study, we performed BCS using ADM in 15 cases. None of the patients had immediate postoperative surgical site infections or red breast syndrome (RBS). In these cases, we rolled the ADM plate into a cylindrical shape or cut it into a crepe cake. Since each surgeon designed the ADM for each operation, the results were different according to the operator’s scissoring skills. In addition, because it is difficult to reproduce the shape of the breast, which changes according to a woman’s posture, some patients complained that lumps were detectable through palpation in certain positions. Therefore, we found that filling the breast defect using small cubes was ideal. We, therefore, performed oncoplastic BCS using immediate diced ADM filling, and aimed to evaluate its safety and the levels of patient satisfaction.
Methods
Patients
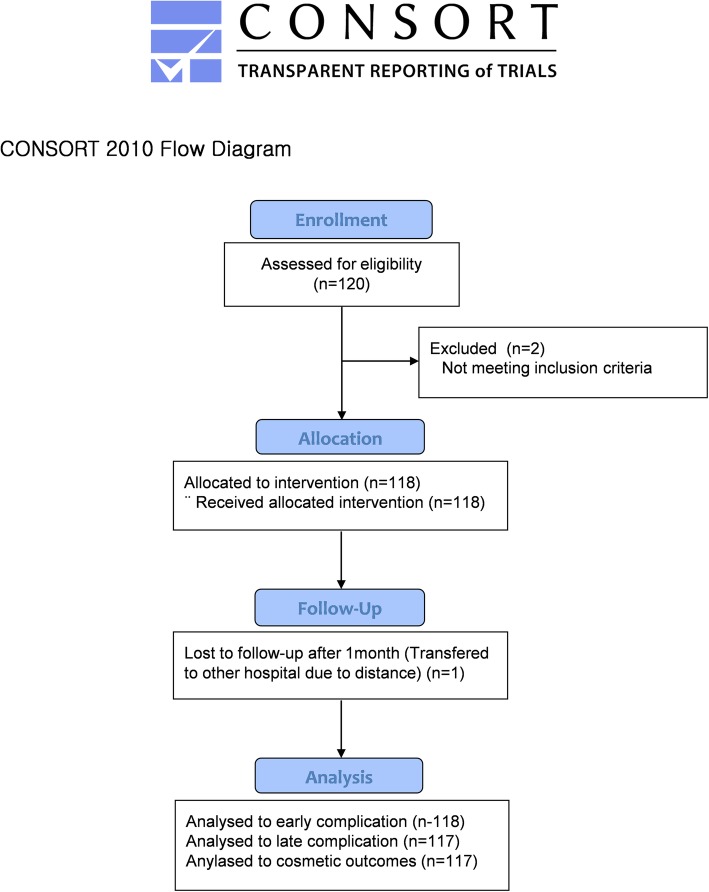
This prospective study consecutively enrolled 120 patients aged 20–80 years with breast cancer who desired BCS between December 2017 and August 2018. All patients agreed to the volume replacement procedure with ADM. We included patients regardless of the size and location of the mass in the breast. Patients with bilateral and multicentric breast cancer were excluded. Further exclusion criteria were infectious diseases, autoimmune diseases, blood clotting disorders, and inflammatory infections before the operation (Fig. 1).
Fig. 1.
Flow diagram of participants
We recorded demographic data, medical history, tumor stage, type and location, resection volume, and the administration of neoadjuvant chemotherapy for all patients.
All participants in this study provided written informed consent for the storage of their medical information in the database and the use of this information for research purposes. The study protocol was approved by the Institutional Review Board of the Catholic University of Korea (VC17OESI0168). All procedures were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments.
Procedure
In this study, the crosslinked human ADM (Megaderm®, L&C Bio, Seoul, Korea) derived from donated human skin in USA tissue banks following the guidelines of the American Association of Tissue Banks and the US Food and Drug Administration was used [6]. After removal of the epidermal and dermal cells from the fresh human cadaver skin, electron beam irradiation is applied to the remaining acellular dermal layer to remove viruses, bacteria, and spores. The product is a 5 × 6 cm square plate with a thickness of 3–5 mm. Each ADM square was diced and packed by the manufacturer and was immersed in normal saline for 30 min in the operating room (Fig. 2).
Fig. 2.
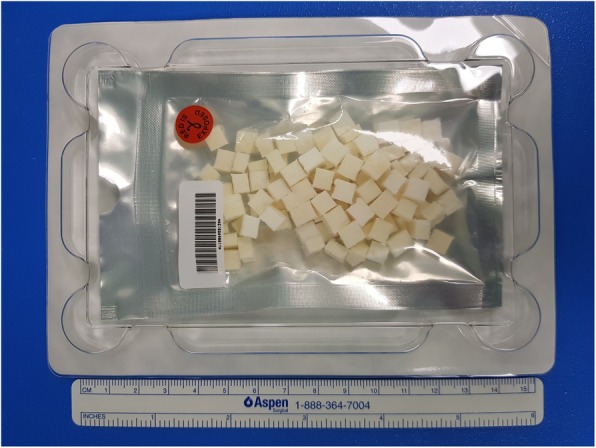
Diced acellular dermal matrix
An intravenous second-generation cephalosporin (cefotetan) was used as the preoperative antibiotic. In patients with a history of allergy to cephalosporin, we used ciprofloxacin instead. A skin incision of 3–12 cm was made above the tumor mass, depending on the size. A 5–7-mm-thick subcutaneous fat flap was made, and the mass was excised enough to obtain a negative tumor margin. The excision cavity was then filled with the diced ADM pieces. We then covered the ADM-filled cavity by approximating the breast tissue and subcutaneous fat above it to prevent the ADM from abutting the skin directly (Fig. 3). The amount of ADM was chosen in such a way that it sufficiently filled the cavity but did not cause bulging of the breast. Only one pack of the product was used per person. If the defect was too large to be filled with diced ADM pieces alone, additional minimal tissue displacement was performed. All procedures were performed by three surgeons with over 7 years of experience in oncologic breast surgery.
Fig. 3.
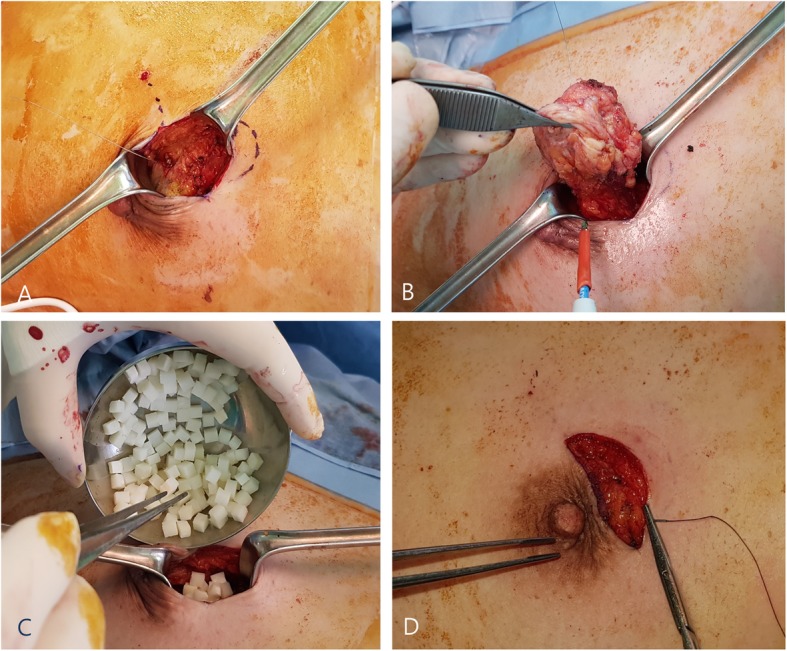
A subcutaneous fat flap was created after skin incision (a). Lumpectomy with wire localization was performed for the mass (b). The excision cavity was filled with soaked acellular dermal matrix pieces (c). The subcutaneous fat above the cavity was approximated to prevent abutting (d)
Survey of satisfaction and complications
The primary endpoint of the study was patient and surgeon satisfaction with the surgery outcomes 6 months postoperatively. Both cosmetic satisfaction and overall satisfaction were assessed on a 10-point scale using a questionnaire: scores of 1–4 were classified as dissatisfied; 5–6, neutral; 7–8, satisfied; and 9–10, strongly satisfied. The satisfaction of the patients and the surgeons was evaluated separately.
The secondary endpoint was postoperative complications. Complications were assessed 2 weeks and 6 months after surgery and were defined as the need for any kind of intervention.
Statistical analysis was performed using the paired samples t test to compare patient satisfaction with operator satisfaction. The chi-square test was used to compare the complication rate in subgroup analysis. A two-tailed p value less than 0.05 was considered statistically significant. Statistical analyses were performed using the R software (R Core Team [2013] R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/).
Results
A total of 118 patients were included in the analysis. Their mean age was 51.7 years. The clinical characteristics of the patients are shown in Table 1. One patient was lost to follow-up 1 month after the operation, resulting in 117 patients completing the satisfaction questionnaire and complication evaluation after 6 months.
Table 1.
Clinical characteristics of breast cancer patients (N=118) undergoing volume replacement with diced acellular dermal matrix
| Characteristics | N (%) |
|---|---|
| Age (years) | |
| 20–50 | 49 (44.9%) |
| > 50 | 69 (55.1%) |
| T stage | |
| Tis | 17 (14.4%) |
| T1 | 63 (53.4%) |
| T2 | 14 (11.9%) |
| ypTis | 1 (0.8%) |
| ypT0 | 4 (3.4%) |
| ypT1 | 7 (5.9%) |
| ypT2 | 9 (7.6%) |
| ypT3 | 3 (2.5%) |
| Pathology | |
| DCIS | 17 (14.4%) |
| IDC | 88 (74.6%) |
| ILC | 2 (1.7%) |
| Others | 11 (9.3%) |
| Location of cancer in breast | |
| Upper | 88 (74.6%) |
| Central | 10 (8.5%) |
| Lower | 20 (16.9%) |
| Resection volume (cm3) | |
| 0–50 | 62 (52.5%) |
| 50–100 | 32 (27.1%) |
| > 100 | 24 (20.3%) |
DCIS ductal carcinoma in situ, IDC invasive ductal carcinoma, ILC invasive lobular carcinoma
The mean satisfaction score for the cosmetic outcome on a 10-point scale was 9.7 (± 0.8) in the patient group and 9.7 (± 0.8) in the surgeon group (p = 0.250). The average score for overall satisfaction was 9.4 (± 1.0) in the patient group and 9.5 (± 1.1) in the surgeon group (p = 0.001). The responses in the cosmetic and overall satisfaction questionnaire revealed that more than 90% of the patients were strongly satisfied (Table 2).
Table 2.
Satisfaction with cosmetic and overall surgical outcomes in patients (N = 117) using acellular dermal matrix
| Cosmetic outcome | Overall outcome | ||||
|---|---|---|---|---|---|
| Score |
Surgeon N(%) |
Patient N(%) |
Surgeon N(%) |
Patient N(%) |
|
| Strongly satisfied | 10 | 91 (77.8) | 92 (78.6) | 78 (66.7) | 83 (70.9) |
| 9 | 16 (13.7) | 18 (15.4) | 23 (19.7) | 23 (19.7) | |
| Satisfied | 8 | 8 (6.8) | 5 (4.3) | 9 (7.7) | 6 (5.1) |
| 7 | 1 (0.9) | 0 | 3 (2.6) | 0 | |
| Neutral | 6 | 0 | 1 (0.9) | 2 (1.7) | 4 (3.4) |
| 5 | 0 | 1 (0.9) | 1 (0.9) | 1 (0.9) | |
| Dissatisfied | 4 | 1 (0.9) | 0 | 1 (0.9) | 0 |
| 3 | 0 | 0 | 0 | 0 | |
| 2 | 0 | 0 | 0 | 0 | |
| 1 | 0 | 0 | 0 | 0 | |
| Average score | 9.7 | 9.7 | 9.4 | 9.5 | |
A total of 24 patients (20.5%) developed a complication until 6 months follow-up. Among the patients with a complication, 10 patients (8.5%) underwent reoperation. Two patients required removal of the ADM filling due to RBS and hematoma, respectively. The different complications with their rates after 2 weeks and 6 months are summarized in Tables 3 and 4, respectively.
Table 3.
Postoperative complication rates in 118 breast cancer patients after volume replacement on postoperative day 14
| Incidence | Reoperation | ADM removal | |
|---|---|---|---|
| Seroma | 4 (3.4%) | 0 | 0 |
| Red breast syndrome | 1 (0.8%) | 0 | 0 |
| Infection | 0 | 0 | 0 |
| Hematoma | 6 (5.1%) | 4 (3.4%) | 0 |
| Wound edge necrosis | 1 (0.8%) | 1 (0.8%) | 0 |
| Fat necrosis | 1 (0.8%) | 1 (0.8%) | 0 |
| Total | 13 (11.0%) | 6 (5.1%) | 0 |
Table 4.
Postoperative complication rates in 117 breast cancer patients after volume replacement 6 months postoperatively
| Incidence | Reoperation | ADM removal | |
|---|---|---|---|
| Seroma | 7 (6.0%) | 0 | 0 |
| Red breast syndrome | 3 (2.5%) | 1 (0.9%) | 1 (0.9%) |
| Infection | 3 (2.5%) | 0 | 0 |
| Hematoma | 6 (5.1%) | 4 (3.4%)* | 1 (0.9%) |
| Wound edge necrosis | 1 (0.9%) | 1 (0.9%) | 0 |
| Fat necrosis | 4 (3.4%) | 4 (3.4%) | 0 |
| Total | 24 (20.5%) | 10 (8.5%) | 2 (1.7%) |
The most common postoperative complication was a seroma. Four patients developed a seroma within 2 weeks of surgery, and three patients developed a seroma after chemotherapy. The condition improved in all patients after aspiration. RBS occurred in three patients (2.5%): one case occurred within 2 weeks after surgery and two cases developed after radiotherapy (RT). The condition in two patients improved after receiving antihistamine treatment, but one patient who developed RBS after RT had to undergo ADM removal.
Subgroup analysis of complications was performed. The incidence of complications was higher when the primary tumor was located in the central part of the breast. The complication rate for tumors located in the upper part of the breast was 17/88 (19.3%), 5/10 (50.0%) for tumors located in the central part, and 2/20 (10.0%) for tumors located in the lower part of the breast (p = 0.033). The complication rate increased as the resection volume increased. The complication rate was 4/62 (6.5%) for a resection volume less than 50 cm3, 7/32 (21.9%) for resection volumes between 50 and 100 cm3, and 13/24 (54.2%) for resection volumes greater than 100 cm3 (p < 0.001). The rate of complications in the neoadjuvant chemotherapy group was significantly higher at 45.8% (11/24) than that in the non-neoadjuvant group (13.8% [13/94]) (p = 0.002). Among the 12 patients with a history of smoking and the 8 patients with diabetes, none had complications.
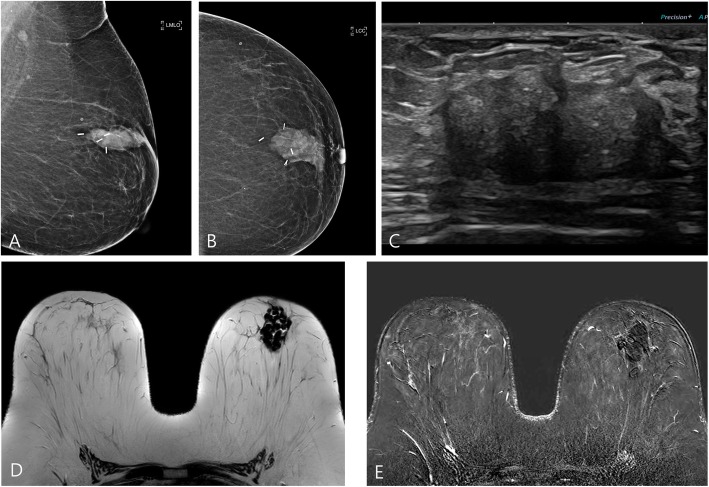
One hundred seventeen patients underwent mammography, breast ultrasonography (USG), and magnetic resonance imaging (MRI) for follow-up at 6 months after surgery (Fig. 4). ADM was most clearly observed by USG. Postoperatively, on the follow-up sonogram, the gap with the matrix disappeared naturally as the seroma was absorbed. In all the patients, except for two patients in whom ADM was surgically removed, no morphological changes were observed in the ADMs using USG at the 6-month and 1-year follow-up. In one patient, a rim-enhancing mass was found around the ADM on contrast-enhanced MRI. The core needle biopsy results were lymphohistiocytic cell infiltration with eosinophils and fibrosis. This mass was not seen on the 1-year follow-up MRI.
Fig. 4.
Six-month follow-up studies of a 42-year-old woman who underwent breast-conserving surgery with ADM. Mammography showed ADM as a mass with a well circumscribed margin on the left mediolateral oblique view (a) and left cranio-caudal view (b). The diced ADM was observed to the same echogenicity as fibroglandular parenchyma on B-mode ultrasonography (c). The ADM showed low signal intensity on T2-weighted imaging (d) and no enhancement on contrast-enhanced magnetic resonance imaging (e). ADM = acellular dermal matrix
Discussion
ADM can be classified as human, porcine, or bovine ADM, depending on the source. The lowest complication incidence in breast cancer patients is associated with human ADM [11].
When using ADM, inflammation without infection is called RBS and has been reported in 1.7–6% of cases [11–13]. RBS is a delayed-type hypersensitivity reaction [14, 15]. In this study, its incidence was 2.5%, which was comparable to that in other reports.
We used a 10-point scale to evaluate the satisfaction level, including overall satisfaction and satisfaction with the shape. Unlike in other studies, the surgeons also participated in the survey, and their responses were compared with those of the patients. The average patient satisfaction score was 9.8 (± 0.7), and the overall satisfaction score was 9.6 (± 1.0). The surgeons’ cosmetic evaluation was not different from that of their patients. Overall satisfaction was slightly worse in the surgeon group. Complications, such as RBS, which are not seen in other surgeries without ADM, may have affected this result.
In 120 consecutive traditional BCSs that were performed before the new technique, complications such as seroma (10.8%), hematoma (2.5%), infection (5.8%), and fat necrosis (7.5%) occurred at 6 months of follow-up. The overall complication rate was 26.7% and reoperation was required in 7.5% of cases. Fat necrosis is a complication causing symptoms in the patient and sometimes requiring biopsy to differentiate it from malignancy. Fat necrosis occurs from 4.6 to 100%, depending on the surgical procedure [16, 17]. Our surgical method reported a lower fat necrosis rate (3.4%) than that of traditional BCS because it either did not make or minimized pedicled fat flap. Another advantage of ADM is that it is composed of cells that are resistant to radiation and have already been applied irradiation. Radiation therapy is also a risk factor of fat necrosis [18].
In this study, neoadjuvant chemotherapy, the central location of breast cancer, and high resection volume over 100 cm3 were risk factors for postoperative complications. However, we do not suggest selection criteria for this surgery. Seroma and hematoma accounted for 54.2% of all complications. Seroma with wound bulging or discharge improved after aspiration. Hematoma occurred in 6 patients: 5 cases occurred in the first 38 cases, and only 1 case occurred in the 80 subsequent cases. Four of these patients required reoperation. Therefore, it is necessary to consider the delicate hemostasis and prophylactic needle aspiration in patients with have associated risk factors.
One limitation of this study was that the neovascularization of ADM had not been confirmed histologically in our patients. After AMD implantation, neovascularization occurs between 1 and 6 months postoperatively [7]. During this period, most patients undergo chemo- and/or RT, which has already been shown to limit ADM remodeling [19]. In our study, 45.8% of all complications occurred between 2 weeks and 6 months.
Conclusions
Immediate filling of defects with ADM was a safe and satisfactory BCS procedure in breast cancer patients. However, patients who underwent volume resections of greater than 100 cm3 and neoadjuvant chemotherapy had relatively higher complication rates. Evaluation of long-term cosmetic satisfaction as well as new approaches to reduce complications in high-risk groups is warranted.
Abbreviations
- ADM
Acellular dermal matrix
- BCS
Breast-conserving surgery
- MRI
Magnetic resonance imaging
- RBS
Red breast syndrome
- RT
Radiotherapy
- USG
Ultrasonography
Authors’ contributions
YJS contributed to the study conception and design. HG contributed to the data acquisition and quality control. All authors contributed to the data analysis and interpretation. HG and YJS contributed to the statistical analysis. All authors contributed to the manuscript preparation. HG and YJS contributed to the manuscript editing. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of St. Vincent’s Hospital. Informed consent was obtained from all individual participants included in the study.
Consent for publication
Informed consent for publication was obtained.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weber WP, Soysal SD, Fulco I, Barandun M, Babst D, Kalbermatten D, Schaefer DJ, Oertli D, Kappos EA, Haug M. Standardization of oncoplastic breast conserving surgery. Eur J Surg Oncol. 2017;43:1236–1243. doi: 10.1016/j.ejso.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Chatterjee A, Gass J, Patel K, Holmes D, Kopkash K, Peiris L, Peled A, Ryan J, El-Tamer M, Reiland J. A consensus definition and classification system of oncoplastic surgery developed by the American Society of Breast Surgeons. Ann Surg Oncol. 2019;26:3436–3444. doi: 10.1245/s10434-019-07345-4. [DOI] [PubMed] [Google Scholar]
- 3.Yang JD, Lee JW, Kim WW, Jung JH, Park HY. Oncoplastic surgical techniques for personalized breast conserving surgery in breast cancer patient with small to moderate sized breast. J Breast Cancer. 2011;14:253–261. doi: 10.4048/jbc.2011.14.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogawa T. Usefulness of breast-conserving surgery using the round block technique or modified round block technique in Japanese females. Asian J Surg. 2014;37:8–14. doi: 10.1016/j.asjsur.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Yang JD, Bae SG, Chung HY, Cho BC, Park HY, Jung JH. The usefulness of oncoplastic volume displacement techniques in the superiorly located breast cancers for Korean patients with small to moderate-sized breasts. Ann Plast Surg. 2011;67:474–480. doi: 10.1097/SAP.0b013e318201fdf4. [DOI] [PubMed] [Google Scholar]
- 6.Noguchi M, Yokoi-Noguchi M, Ohno Y, Morioka E, Nakano Y, Kosaka T, Kurita T. Oncoplastic breast conserving surgery: volume replacement vs. volume displacement. Eur J Surg Oncol. 2016;42:926–934. doi: 10.1016/j.ejso.2016.02.248. [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, Kim HG, Lee WJ. Characterization and tissue incorporation of cross-linked human acellular dermal matrix. Biomaterials. 2015;44:195–205. doi: 10.1016/j.biomaterials.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Bloemen MC, van Leeuwen MC, van Vucht NE, van Zuijlen PP, Middelkoop E. Dermal substitution in acute burns and reconstructive surgery: a 12-year follow-up. Plast Reconstr Surg. 2010;125:1450–1459. doi: 10.1097/PRS.0b013e3181d62b08. [DOI] [PubMed] [Google Scholar]
- 9.Baek WY, Byun IH, Kim YS, Lew DH, Jeong J, Roh TS. Patient satisfaction with implant based breast reconstruction associated with implant volume and mastectomy specimen weight ratio. J Breast Cancer. 2017;20:98–103. doi: 10.4048/jbc.2017.20.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorkin M, Qi J, Kim HM, Hamill JB, Kozlow JH, Pusic AL, Wilkins EG. Acellular dermal matrix in immediate expander/implant breast reconstruction: a multicenter assessment of risks and benefits. Plast Reconstr Surg. 2017;140:1091–1100. doi: 10.1097/PRS.0000000000003842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paprottka FJ, Krezdorn N, Sorg H, Konneker S, Bontikous S, Robertson I, Schlett CL, Dohse NK, Hebebrand D. Evaluation of complication rates after breast surgery using acellular dermal matrix: median follow-up of three years. Plast Surg Int. 2017;2017:1283735. doi: 10.1155/2017/1283735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ortiz JA. Clinical outcomes in breast reconstruction patients using a sterile acellular dermal matrix allograft. Aesthetic Plast Surg. 2017;41:542–550. doi: 10.1007/s00266-017-0817-z. [DOI] [PubMed] [Google Scholar]
- 13.Lewis P, Jewell J, Mattison G, Gupta S, Kim H. Reducing postoperative infections and red breast syndrome in patients with acellular dermal matrix-based breast reconstruction: the relative roles of product sterility and lower body mass index. Ann Plast Surg. 2015;74(Suppl 1):S30–S32. doi: 10.1097/SAP.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 14.Ganske I, Hoyler M, Fox SE, Morris DJ, Lin SJ, Slavin SA. Delayed hypersensitivity reaction to acellular dermal matrix in breast reconstruction: the red breast syndrome? Ann Plast Surg. 2014;73(Suppl 2):S139–S143. doi: 10.1097/SAP.0000000000000130. [DOI] [PubMed] [Google Scholar]
- 15.Wu PS, Winocour S, Jacobson SR. Red breast syndrome: a review of available literature. Aesthetic Plast Surg. 2015;39:227–230. doi: 10.1007/s00266-014-0444-x. [DOI] [PubMed] [Google Scholar]
- 16.Vasei N, Shishegar A, Ghalkhani F, Darvishi M. Fat necrosis in the breast: a systematic review of clinical. Lipids Health Dis. 2019;18:139. doi: 10.1186/s12944-019-1078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakada H, Inoue M, Furuya K, Watanabe H, Ikegame K, Nakayama Y, Ohmori M, Nakagomi H. Fat necrosis after breast-conserving oncoplastic surgery. Breast Cancer. 2019;26:125–130. doi: 10.1007/s12282-018-0901-5. [DOI] [PubMed] [Google Scholar]
- 18.Russo AL, Taghian AG. Fat necrosis of the breast in the accelerated partial breast irradiation era: the need for a universal grading system. Breast Cancer Res Treat. 2013;140:1–11. doi: 10.1007/s10549-013-2611-1. [DOI] [PubMed] [Google Scholar]
- 19.Myckatyn TM, Cavallo JA, Sharma K, Gangopadhyay N, Dudas JR, Roma AA, Baalman S, Tenenbaum MM, Matthews BD, Deeken CR. The impact of chemotherapy and radiation therapy on the remodeling of acellular dermal matrices in staged, prosthetic breast reconstruction. Plast Reconstr Surg. 2015;135:43e–57e. doi: 10.1097/PRS.0000000000000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.