Abstract
Background
Perianal Paget’s disease (PPD) is rare and mostly described in clinical literature as case reports or small series.
Methods
We investigated the clinicopathologic and immunohistochemical features of PPD in a total of 13 cases retrieved from multiple academic institutions.
Results
The median age at diagnosis was 75 (range 50–86) years. Males were predominant with a male to female ratio of 2.25:1. Four (30.8%) cases were classified as primary PPD due to lack of synchronous or metachronous underlying malignancies, while nine (69.2%) were classified as secondary PPD with concurrent invasive adenocarcinoma (n = 8) or tubular adenoma with high-grade dysplasia (n = 1). Immunohistochemically, there is no differential expression of CK7 or CK20 in Paget’s cells between primary and secondary PPD; however, GCDFP-15 was only positive in primary PPD (3/3 vs. 0/6, P = 0.012), while CDX2 was only positive in secondary PPD (0/3 vs. 7/7, P = 0.008), suggesting different cell origin. All patients received local surgical resection with or without adjuvant therapy. After a median follow-up of 47 months, one patient with secondary PPD (7.7%) died of disease progression from underlying adenocarcinoma.
Conclusions
PPD occurs in elderly patients with male predominance and is frequently associated with underlying malignancies. Differential expression of CDX2 and GCDFP-15 may help distinguishing primary vs. secondary PPD, which is important for management as the presence of an underlying malignancy impacts clinical course and prognosis. Surgical excision remains the major treatment strategy for PPD. Long-term follow-up is required to monitor the disease recurrence and metastasis.
Keywords: Paget’s disease, Perianal, Clinicopathologic features, Histology, Immunohistochemistry, Prognosis
Introduction
Extramammary Paget’s disease is a rare neoplastic condition of apocrine gland-bearing regions [1, 2]. The most frequently affected site is vulva, followed by perineal, perianal, scrotal and penile skin. Perianal Paget’s disease (PPD) involving perianal skin or anal mucosa accounts for less than 20% of extramammary Paget’s disease. Primary PPD is very rare. It is an indolent disease, but can recur with a recurrence rate of 44–60% [3, 4]. Up to 60% of PPD were associated with underlying malignancies [5, 6], in which the Paget’s cells represent intraepithelial spread of an existing dermal adnexal or visceral adenocarcinoma [7, 8]. Thus, the findings of PPD should prompt diligent search for an underlying malignancy [9–12]. The new WHO book classifies anal adenocarcinomas as primary if arising from mucosal glandular epithelium, which shares the same immunoprofile as colorectal adenocarcinoma (CK7+/−, CK20+/CDX2+), or from anal glands, which shares the same immunoprofile as skin adnexal carcinoma (CK7+/CK20−/CDX2-) [13]. Thus, proper diagnosis relies not only on immunoprofile, but also clinical information and macroscopic tumor location [14, 15]. PPD has been rarely described in literatures as single case report or small case series [9, 16–18]. Yet, much is still unknown due to its rarity. The main goal of this study is to conduct a multi-institutional study to investigate the clinical, histomorphological, immunohistochemical, and molecular genetic features of PPD.
Materials and methods
Patients
Thirteen patients with PPD were identified between 1999 and 2019 from three large medical centers (University of Rochester Medical Center, University of Florida College of Medicine, and Cedars-Sinai Medical Center) in the United States. Clinical data including patient demographics, medical history, presenting symptoms, physical examination, treatment, and outcome were collected through electronic medical record.
Histomorphological evaluation and immunohistochemistry
Hematoxylin and eosin (H&E)-stained slides from all cases were reviewed by two experienced gastrointestinal pathologists to confirm diagnosis and for histopathologic analysis. Immunohistochemistry was performed on 4-μm-thick slides prepared from formalin fixed, paraffin embedded tissue blocks on an automated immunostainer (Ventana BenchMark Ultra) according to the manufacturer’s instructions. Heat induced epitope retrieval (HIER) was performed for antigen retrieval. Appropriate controls were used throughout. The following antibodies were used: cytokeratin 7 (CK7, Cat# GA619, Agilent, Santa Clara, CA), cytokeratin 20 (CK20, Cat# GA777, Agilent, Santa Clara, CA), CDX2 (Cat# DAK-CDX2, Agilent, Santa Clara, CA), carcinoembryonic antigen (CEA, Cat# GA622, Agilent, Santa Clara, CA), MUC1 (Cat# CM319B, Biocare Medical, Pacheco, CA), MUC2 (Cat# PA0155, Leica, Buffalo Grove, IL), GCDFP-15 (Cat# GA077, Agilent, Santa Clara, CA), GATA3 (Cat# CM405B, Biocare Medical, Pacheco, CA), and p40 (Cat# ACI3066, Biocare Medical, Pacheco, CA). Fisher’s exact test was used to compare frequencies between two groups (primary vs. secondary PPD). A P-value of < 0.05 was considered statistically significant.
Results
Clinical characteristics
The clinical characteristics of all 13 cases were summarized in Table 1. The median age at diagnosis was 75 (range 50–86) years. Males were predominant with a male to female ratio of 2.25:1. Clinical presentations included itching and irritation of the perianal area. Physical examination often showed erythema, plaques, ulceration, fissure, fistula, or mass lesions. Four (4/13, 30.8%) patients presented as primary PPD with no synchronous or metachronous underlying anorectal malignancies, while nine (9/13, 69.2%) were classified as secondary PPD due to concurrent invasive adenocarcinoma (n = 8) or tubular adenoma with high-grade dysplasia (n = 1). In addition, five patients had other malignancies, including basal cell carcinoma, breast carcinoma, urothelial carcinoma in-situ, chronic myeloid leukemia, and follicular lymphoma.
Table 1.
Demographics and clinical features of 13 patients with perianal Paget’s disease
| Case | Age (years) | Sex | Clinical presentation | Other malignancies | Underlying malignancy (Immunoprofile) | Metastasis | Outcome | Follow up (month) | Treatment other than surgery |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 75 | M | Perianal erosive plaque | Chronic myeloid leukemia | None | N/A | Dieda | 60 | Radiation |
| 2 | 79 | F | Discomfort | Follicular lymphoma; breast carcinoma; urothelial carcinoma in-situ | None | N/A | Alive | 83 | None |
| 3 | 61 | M | Itching and irritation of the perianal area; erythema with whitish plaques | None | None | N/A | Alive | 31 | None |
| 4 | 86 | M | Perianal lesion | Basal cell carcinoma | None | N/A | Alive | 4 | |
| 5 | 81 | M | Discomfort | None |
Mixed moderately differentiated adenocarcinoma with mucinous features and neuroendocrine (large cell) carcinoma (CK7+/CK20+/CDX2+) |
Lymph nodes and liver | Dieda | 79 | None |
| 6 | 53 | F | Acute anal fissure, anal lesion | Basal cell carcinoma; breast carcinoma |
Adenocarcinoma, well differentiated (CK7−/CK20+/CDX2+) |
None | Alive | 61 | None |
| 7 | 86 | M | Discomfort | None |
Adenocarcinoma, poorly differentiated (CK7+/CK20-) |
None | Alive | 52 | None |
| 8 | 69 | F | None (detected during routine colon cancer screening biopsy) | None |
Adenocarcinoma, moderately differentiated (CK7+/CK20+/CDX2+) |
None | Alive | 16 | None |
| 9 | 83 | M | Perianal rash and lesion with ulceration | Basal cell carcinoma |
Adenocarcinoma, moderately differentiated (CK7−/CK20+/CDX2+) |
None | Alive | 7 | Chemoradiation |
| 10 | 69 | F | Anal/perianal rash and lesion | None |
Adenocarcinoma with neuroendocrine and signet ring features (CK7+/CK20+/CDX2+) |
None | Alive | 21 | None |
| 11 | 50 | M | Anal fistula | None | Adenocarcinoma with mucinous features | Lymph nodes | Alive | 227 | Chemotherapy |
| 12 | 70 | M | Perianal lesion | None |
Mucinous adenocarcinoma (CK7+/CK20+/CDX2+) |
Lymph nodes, liver and bone | Alive | 47 | Chemoradiation |
| 13 | 77 | M | None | None |
Tubular adenoma with high-grade dysplasia (CK7−/CK20+/CDX2+) |
None | Alive | 6 | None |
aCase #1 died of chronic myeloid leukemia. Case #5 died of disease progression. N/A, not applicable
Pathologic findings
Histologically, all PPD cases showed intraepithelial infiltration by sheets and clusters of large atypical neoplastic cells. The intraepidermal Paget’s cells were large, hyperchromatic and pleomorphic with clear or pale cytoplasm, and occasionally prominent nucleoli. For cases classified as primary, the Paget’s cells were mostly singly dispersed, with occasional visible intracytoplasmic mucin and rare glandular formation (Fig. 1a-c). In contrast, for cases classified as secondary, the Paget’s cells were more mucinous, frequently with eccentric nuclei and signet ring cell appearance, resembling the underlying carcinomatous cells (Figs. 2 and 3). Interestingly, mucinous differentiation was noted in 3 of 8 underlying adenocarcinomas. In one case where only tubular adenoma with high-grade dysplasia was identified, the Paget’s cells were also mucinous with signet ring like appearance, resembling some of the high-grade dysplastic cells (Fig. 2d, e). Of note, two underlying adenocarcinomas had neuroendocrine differentiation, among which one showed both mucinous and neuroendocrine components (Fig. 2a-c).
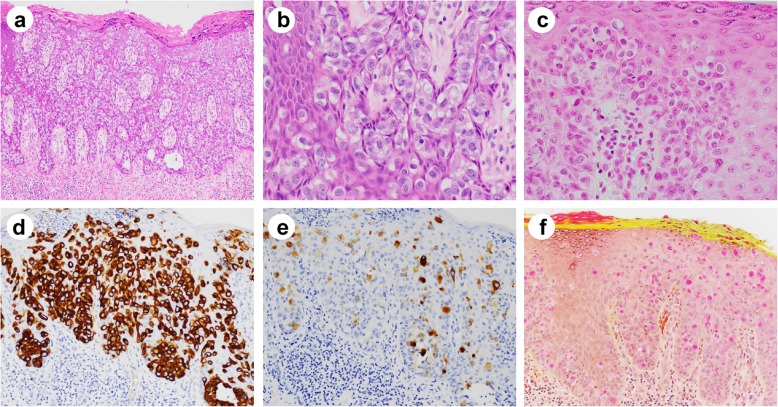
Fig. 1.
Histologic features and special stains of Paget’s cells in primary perianal Paget’s disease. a, b Paget’s cells in primary perianal Paget’s disease are large, hyperchromatic with pale cytoplasm and prominent nucleoli (hematoxylin and eosin; a, × 100; b, × 400). The Paget’s cells in primary perianal Paget’s disease (c, hematoxylin and eosin) are positive for CK7 (d) and GCDFP-15 (e) by immunohistochemistry. f Mucicarmine stain highlights intracellular mucin. Magnifications: c-f, × 200
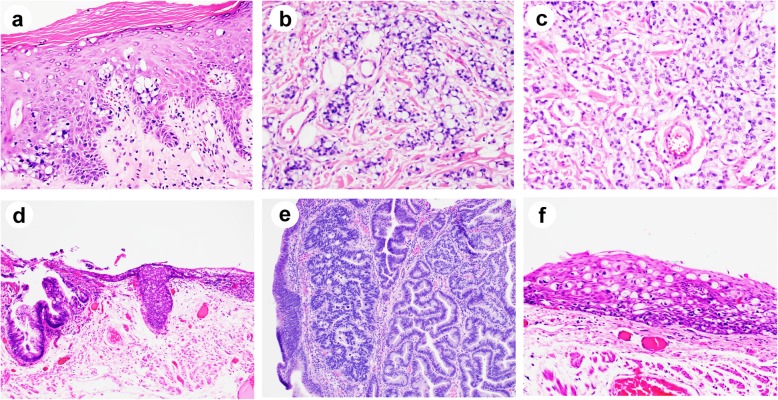
Fig. 2.
Histologic features of secondary perianal Paget’s disease cases associated with underlying malignancy (hematoxylin and eosin). a-c High magnification (× 200) showed intraepithelial Paget’s cells (a) and underlying invasive adenocarcinoma with signet ring cell feature (b) and neuroendocrine feature (c). d Low magnification (× 100) showed intraepithelial Paget’s cells associated with a tubular adenoma with high-grade dysplasia. Higher magnification views (× 200) of (d) showed tubular adenoma with high-grade dysplasia (e) and Paget’s cells (f)
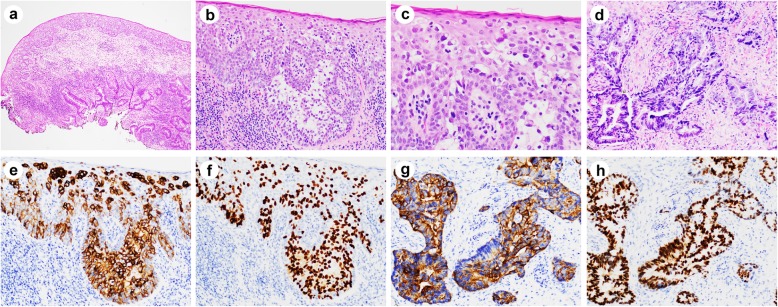
Fig. 3.
Histologic and immunohistochemical features of secondary perianal Paget’s disease with associated invasive adenocarcinoma. a Low magnification showed intraepithelial Paget’s cells and underlying conventional invasive adenocarcinoma. b-d High magnification view of (a) showed Paget’s cells (b, c) and invasive adenocarcinoma (d). Paget’s cells are positive for CK20 (e) and CDX2 (f) by immunohistochemistry. Underlying adenocarcinoma cells are also positive for CK20 (g) and CDX2 (h) by immunohistochemistry. Magnifications: a, × 40; b, × 200; c, × 400; d-h, × 200
Immunohistochemical profile
Immunohistochemical studies were performed in all cases except one (Table 2). The Paget’s cells were frequently positive for CK7 (9/12; 75%), CK20 (9/12; 75%), CDX2 (7/10; 70%), CEA (6/7; 85.7%), MUC1 (5/8; 62.5%), MUC2 (8/8; 100%), GATA3 (4/7, 57.1%), and GCDFP-15 (3/9; 33.3%) (Fig. 1d, e). Mucicarmine stain highlighted intracellular mucin in all of the cases tested (Fig. 1f). Secondary Paget’s cells shared the same immunoprofile as the invasive adenocarcinoma component, suggesting the same tumor origin (Fig. 3e-h). Interestingly, while there was no differential expression of CK7, CK20, CEA, MUC1, MUC2 between primary and secondary PPD, GCDFP-15 was only positive in primary PPD (3/3 vs. 0/6, P = 0.012), while CDX2 was only positive in secondary PPD (0/3 vs. 7/7, P = 0.008). GATA3 was positive in all primary PPD (3/3, 100%), and 1 of 4 secondary PPD (1/4, 25%) tested. All cases were negative for squamous cell markers such as p40 or p63. SOX10, MelanA, p53, p16, HER2, PAX8 were also tested in some of the cases and were all negative (data not shown). Immunoprofiling of 8 cases with underlying malignancies (Table 1) indicated that only one case (case #7) is likely anal gland origin (CK7+/CK20-), while the others are likely colorectal primary (CK20+/CDX2+).
Table 2.
Immunohistochemical profile of perianal Paget’s disease
| Primary | Secondary | P value | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | ||
| Cytokeratin 7 | + | + | + | + | + | – | + | + (F) | – | + | ND | + | – | NS |
| Cytokeratin 20 | – | – | + | + (F) | + | + | – | + | + | + | ND | + | + | NS |
| CDX2 | – | – | ND | – | + | + | ND | + | + | + | ND | + | + | 0.008 |
| MUC1 | + | + | ND | + | + | – | ND | – | ND | ND | ND | + | – | NS |
| MUC2 | + | + | ND | + (F) | + | + | ND | + | ND | ND | ND | + | + | NS |
| CEA | + | – | ND | + (F) | ND | + | + | + | ND | + | ND | ND | + | NS |
| GCDFP-15 | + (F) | + | ND | + (F) | – | – | ND | – | ND | – | ND | – | – | 0.012 |
| P40 | – | – | ND | – | – | – | ND | – | ND | ND | ND | ND | – | NS |
| Mucicarmine | + | + | + | + | ND | + | + | + | ND | ND | ND | ND | + | NS |
| GATA3 | + | + | ND | + | ND | – | ND | – | ND | ND | ND | – | + | NS |
(F) Focally positive, ND Not done, NS Not significant
Mismatch repair proteins (MMR) immunohistochemistry and/or microsatellite instability PCR were performed in three invasive adenocarcinomas and all showed to be MMR proficient. Next-generation sequencing using a 74 cancer-related gene panel was performed in one case of invasive adenocarcinoma with neuroendocrine and signet ring cell features (case #10) but did not reveal any known cancer-related genetic mutations in the panel.
Treatment and follow-up
All patients received local/extensive surgical excision of the perianal lesions as well as underlying adenocarcinomas. Four patients, including 1 primary PPD and 3 secondary PPD, received radiation and/or chemotherapy after the surgery (Table 1). After a median follow-up of 47 (range 4–227) months, all patients with primary PPD survived except one died of other malignancy (chronic myeloid leukemia). Majority (8/9, 88.9%) of patients with secondary PPD survived, yet 2 (22.2%) patients had recurrence of underlying adenocarcinoma, 3 (33.3%) developed lymph node, liver or bone metastasis, and 1 (case #5, 11.1%) died of underlying adenocarcinoma with nodal and liver metastasis. Overall, the total mortality of PPD was 15.4% and disease-specific mortality was 7.7%.
Discussion
In this multi-institutional study, we analyzed a series of PPD to characterize its clinicopathologic and immunophenotypic features. We found that PPD occurs in elderly patient with male predominance and is frequently associated with underlying adenocarcinoma. A panel of immunomarkers (CK7, CK20, CDX2, GCDFP-15) plus mucin stain not only can help with diagnoses but also predict the presence of underlying malignancies. Long-term follow-up after local excision is required to monitor the disease recurrence and metastasis.
PPD must be differentiated from other squamous intraepithelial lesions, including squamous cell carcinoma and melanoma. Squamous lesions are positive for p63, p40 and high molecular weight keratin CK5/6. They are usually associated with Human papillomavirus (HPV) infection and p16 overexpression [19, 20]. Melanocytic lesions are usually positive for S100, SOX10, HMB45 and MelanA, but negative for cytokeratins [21], although rare cases can lose melanocytic markers and gain aberrant expression of cytokeratins [22]. Atypical regenerative basal keratinocytes sometimes can be mistaken as Paget’s cells; however, basal keratinocytes have intercellular bridges and are positive for squamous markers.
In contrast to what have been reported before [23–25], we found that CK7/CK20 were variably expressed in both primary and secondary PPD, yet GCDFP-15 was only expressed in primary PPD while CDX2 was only positive in secondary PPD, indicating CDX2 and GCDFP-15 are most reliable markers to distinguish these two subtypes of PPD. Our results suggest that the presence of CDX2+/GCDFP-15- PPD should prompt a careful search for primary adenocarcinoma in the lower gastrointestinal tract as the underlying adenocarcinoma determines the outcome of the patient. GATA3 is a very sensitive marker for primary genital and vulvar extramammary Paget’s disease [26, 27]. It is also positive in primary PPD, although may not be used to differentiate from secondary PPD. In our study cohort, 8 cases had a concurrent invasion adenocarcinoma, while 1 case had only tubular adenoma with high-grade dysplasia. Immunohistochemical stains confirmed that the Paget’s cells shared the same immunoprofile as the adenocarcinoma component, suggesting pagetoid spread from the underlying carcinoma cells. Only one case of PPD with concurrent tubular adenoma was reported before [11]; however, in such a case close follow-up is recommended to exclude occult invasive carcinoma.
In our case series, more than two thirds of PPD are classified as secondary, slightly higher than previously reported [5, 6]. It seems that the underlying malignancy dominates the clinical course and prognosis. Treatment and management should be primarily directed towards the underlying invasive carcinoma in addition to addressing the anal skin lesion by a variety of modalities. Primary PPD appears to be more indolent and patients usually die of other unrelated conditions. Wide local excision of skin and subcutaneous tissue in the perianal region is generally recommended for the treatment of the non-invasive form of PPD [28–30]. Radiation therapy is considered a treatment strategy in patients who were poor surgical candidates [31]. Other non-surgical treatments such as 5-fluorouracil or topical imiquimod have been used either in non-invasive or recurrent PPD [18, 32, 33]. photodynamic therapy with topically applied 5-aminolevulinic acid has been reported to treat non-invasive PPD and achieved complete cure without recurrence [34]. Although only a few cases were tested in our cohort, targeted therapy may be offered in situations if tumor cells are MMR deficient or have targetable genetic mutations.
In summary, we presented a multicentric study on PPD to characterize its clinicopathologic and immunophenotypic features. PPD is frequently associated with underlying adenocarcinoma, sometimes even a precursor lesion such as tubular adenoma. Mucinous and neuroendocrine features are not uncommon in the underlying malignancies and the Paget’s cells frequently demonstrate signet ring cell or mucinous features. CDX2 and GCDFP-15 proved to be the best distinguishing markers for primary vs. secondary PPD. Such a distinction is prognostically significant as the outcome in patients with secondary PPD is primarily dependent upon the invasive adenocarcinoma. Future studies may be warranted to explore the molecular signatures of Paget’s cells, as well as the mechanisms of pathogenesis, either de novo, or secondary.
Acknowledgements
The authors would like to thank the histology laboratory of the Department of Pathology and Laboratory Medicine at University of Rochester Medical Center for technical expertise in performing the immunohistochemical stains.
Abbreviations
- H&E
Hematoxylin and eosin
- HPV
Human papillomavirus
- MMR
Mismatch repair proteins
- PPD
Perianal Paget’s disease
Author’s contributions
XL (Xiaoyan Liao), XL (Xiuli Liu), JL, XF and DZ contributed to project concept, data collection and manuscript preparation. All authors reviewed and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets generated and/or analyzed in this study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of each participating institution.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jinping Lai and Dongwei Zhang contributed equally to this work.
References
- 1.Shepherd V, Davidson EJ, Davies-Humphreys J. Extramammary Paget’s disease. BJOG. 2005;112:273–279. doi: 10.1111/j.1471-0528.2004.00438.x. [DOI] [PubMed] [Google Scholar]
- 2.Heymann WR. Extramammary Paget’s disease. Clin Dermatol. 1993;11:83–87. doi: 10.1016/0738-081X(93)90101-H. [DOI] [PubMed] [Google Scholar]
- 3.Zollo JD, Zeitouni NC. The Roswell Park Cancer Institute experience with extramammary Paget’s disease. Br J Dermatol. 2000;142:59–65. doi: 10.1046/j.1365-2133.2000.03242.x. [DOI] [PubMed] [Google Scholar]
- 4.Sarmiento JM, Wolff BG, Burgart LJ, et al. Paget’s disease of the perianal region--an aggressive disease? Dis Colon Rectum. 1997;40:1187–1194. doi: 10.1007/BF02055165. [DOI] [PubMed] [Google Scholar]
- 5.Jensen SL, Sjolin KE, Shokouh-Amiri MH, et al. Paget’s disease of the anal margin. Br J Surg. 1988;75:1089–1092. doi: 10.1002/bjs.1800751113. [DOI] [PubMed] [Google Scholar]
- 6.Beck DE, Fazio VW. Perianal Paget’s disease. Dis Colon Rectum. 1987;30:263–266. doi: 10.1007/BF02556169. [DOI] [PubMed] [Google Scholar]
- 7.Lam C, Funaro D. Extramammary Paget’s disease: summary of current knowledge. Dermatol Clin. 2010;28:807–826. doi: 10.1016/j.det.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 8.McDaniel B, Brown F, Crane JS. Extramammary Paget Disease. StatPearls. Treasure Island: StatPearls Publishing; 2019. [PubMed] [Google Scholar]
- 9.Liao X, Mao W, Lin A. Perianal Paget’s disease co-associated with Anorectal adenocarcinoma: primary or secondary disease? Case Rep Gastroenterol. 2014;8:186–192. doi: 10.1159/000363177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al Hallak MN, Zouain N. Extramammary perianal Paget’s disease. Case Rep Gastroenterol. 2019;3:332–337. doi: 10.1159/000256382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chumbalkar V, Jennings TA, Ainechi S, et al. Extramammary Paget's disease of Anal Canal associated with rectal adenoma without invasive carcinoma. Gastroenterology Res. 2016;9:99–102. doi: 10.14740/gr727e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Fur R, Mears L, Dannawi Z. A peri-anal extramammary Paget's disease associated with two well-differentiated invasive intramucosal sigmoid carcinomas, a very rare case: an immunohistochemical and clinical review of extramammary Paget’s disease. Ann R Coll Surg Engl. 2004;86:W26–W31. doi: 10.1308/147363504772441976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO Classification of Tumours Editorial Board . Digestive system tumours. Lyon: International Agency for Research on Cancer; 2019. Anal adenocarcinoma; pp. 208–211. [Google Scholar]
- 14.Isik O, Aytac E, Brainard J, et al. Perianal Paget’s disease: three decades experience of a single institution. Int J Color Dis. 2016;31:29–34. doi: 10.1007/s00384-015-2342-3. [DOI] [PubMed] [Google Scholar]
- 15.Lee GC, Kunitake H, Stafford C, et al. High risk of proximal and local neoplasms in 2206 patients with Anogenital Extramammary Paget’s disease. Dis Colon Rectum. 2019;62:1283–1293. doi: 10.1097/DCR.0000000000001487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carbotta G, Sallustio P, Prestera A, et al. Perineal Paget’s disease: A rare disorder and review of literature. Ann Med Surg (Lond) 2016;9:50–52. doi: 10.1016/j.amsu.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stavrou M, Martin L, El-Madani F, et al. Perianal Paget’s disease-report of a rare case. Int J Surg Case Rep. 2012;3:483–485. doi: 10.1016/j.ijscr.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dos Santos JS, Bonafe GA, Pereira JA, et al. Rare perianal extramammary Paget disease successfully treated using topical Imiquimod therapy. BMC Cancer. 2018;18:921. doi: 10.1186/s12885-018-4815-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah KV. Human papillomaviruses and anogenital cancers. N Engl J Med. 1997;337:1386–1388. doi: 10.1056/NEJM199711063371911. [DOI] [PubMed] [Google Scholar]
- 20.Abramowitz L, Jacquard AC, Jaroud F, et al. Human papillomavirus genotype distribution in anal cancer in France: the EDiTH V study. Int J Cancer. 2011;129:433–439. doi: 10.1002/ijc.25671. [DOI] [PubMed] [Google Scholar]
- 21.Chute DJ, Cousar JB, Mills SE. Anorectal malignant melanoma: morphologic and immunohistochemical features. Am J Clin Pathol. 2006;126:93–100. doi: 10.1309/DVWLTV8FFKC3L80H. [DOI] [PubMed] [Google Scholar]
- 22.Bell PD, Israel AK, Dunn AL, et al. Primary dedifferentiated Amelanotic Anorectal melanoma: report of a rare case. Int J Surg Pathol. 2019;27:923–928. doi: 10.1177/1066896919857148. [DOI] [PubMed] [Google Scholar]
- 23.Wang YC, Li AF, Yang SH, et al. Perianal Paget’s disease: the 17-year-experience of a single institution in Taiwan. Gastroenterol Res Pract. 2019;2603279. [DOI] [PMC free article] [PubMed]
- 24.Goldblum JR, Hart WR. Perianal Paget’s disease: a histologic and immunohistochemical study of 11 cases with and without associated rectal adenocarcinoma. Am J Surg Pathol. 1998;22:170–179. doi: 10.1097/00000478-199802000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Nowak MA, Guerriere-Kovach P, Pathan A, et al. Perianal Paget’s disease: distinguishing primary and secondary lesions using immunohistochemical studies including gross cystic disease fluid protein-15 and cytokeratin 20 expression. Arch Pathol Lab Med. 1998;122:1077–1081. [PubMed] [Google Scholar]
- 26.Zhao M, Zhou L, Sun L. GATA3 is a sensitive marker for primary genital extramammary Paget disease: an immunohistochemical study of 72 cases with comparison to gross cystic disease fluid protein 15. Diagn Pathol. 2017;12:51. doi: 10.1186/s13000-017-0638-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morbeck D, Tregnago AC, Baiocchi G, et al. GATA3 expression in primary vulvar Paget disease: a potential pitfall leading to misdiagnosis of pagetoid urothelial intraepithelial neoplasia. Histopathology. 2017;70:435–441. doi: 10.1111/his.13086. [DOI] [PubMed] [Google Scholar]
- 28.Kyriazanos ID, Stamos NP, Miliadis L, et al. Extra-mammary Paget's disease of the perianal region: a review of the literature emphasizing the operative management technique. Surg Oncol. 2011;20:e61–e71. doi: 10.1016/j.suronc.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Coldiron BM, Goldsmith BA, Robinson JK. Surgical treatment of extramammary Paget's disease. A report of six cases and a reexamination of Mohs micrographic surgery compared with conventional surgical excision. Cancer. 1991;67:933–938. doi: 10.1002/1097-0142(19910215)67:4<933::AID-CNCR2820670413>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 30.Hendi A, Brodland DG, Zitelli JA. Extramammary Paget's disease: surgical treatment with Mohs micrographic surgery. J Am Acad Dermatol. 2004;51:767–773. doi: 10.1016/j.jaad.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Mann J, Lavaf A, Tejwani A, et al. Perianal Paget disease treated definitively with radiotherapy. Curr Oncol. 2012;19:e496–e500. doi: 10.3747/co.19.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato H, Watanabe S, Kariya K, et al. Efficacy of low-dose 5-fluorouracil/cisplatin therapy for invasive extramammary Paget’s disease. J Dermatol. 2018;45:560–563. doi: 10.1111/1346-8138.14247. [DOI] [PubMed] [Google Scholar]
- 33.Molina GE, Khalifian S, Mull JL, et al. Topical combination of fluorouracil and Calcipotriene as a palliative therapy for refractory Extramammary Paget disease. JAMA Dermatol. 2019;155:599–603. doi: 10.1001/jamadermatol.2018.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li L, Deng Y, Zhang L, et al. Treatment of perianal Paget's disease using photodynamic therapy with assistance of fluorescence examination: case report. Lasers Med Sci. 2009;24:981–984. doi: 10.1007/s10103-009-0653-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed in this study are available from the corresponding author upon reasonable request.