Abstract
Introduction
Antidepressant drugs are effective therapies for major depressive disorder; however, they are frequently associated with side effects. Although there is some evidence for a relationship between genetic variation and side effects, little is known regarding the role of dynamic molecular factors as moderators of side effects. The aim of this study was to assess microRNA (miRNA) changes associated with side effects during escitalopram treatment and their downstream effects on target gene expression.
Methods
A total 160 patients with major depressive disorder from the CAN-BIND-1 cohort were included. Side effects were assessed with the Toronto Side Effect Scale after 2 weeks of treatment with escitalopram. We assessed the relationship between side effects and changes in peripheral expression of miRNAs between baseline and week 2. For miRNA whose expression changed, we used target prediction algorithms to identify putative messenger RNA (mRNA) targets and assessed their expression.
Results
Nausea was experienced by 42.5% of patients. We identified 45 miRNAs whose expression changed on initiation of escitalopram treatment, of which 10 displayed a negative association with intensity of nausea (miR15b-5p, miR17-5p, miR20a-5p, miR20b-5p, miR103a-3p, miR103b, miR106a-5p, miR182-5p, miR185-5p, and miR660-5p). Additionally, we found negative associations between 4 microRNAs (miR20a-5p, miR106a-5p, miR185-5p, miR660-5p) and mRNA targets. The expression of the miR185-5p target, CAMK2δ was significantly decreased [log 2 mean = −0.048 (0.233)] between weeks 0 and 2 (P = .01)].
Conclusions
We identified an overexpression of miR185-5p during escitalopram treatment of major depressive disorder, which was negatively associated with intensity of nausea, and identified a potential mRNA target that may mediate this effect.
Keywords: major depressive disorder, side effects, miRNA, antidepressant
Significance Statement.
While there are several studies focusing on genetic variation as predictors of side effects, there is little information on the possible role of dynamic molecular factors as side effect moderators. MicroRNAs (miRNA) are particularly good dynamic molecular factors to investigate for a relationship with antidepressant side effects. This study is the first, to our knowledge, to examine the role of miRNAs in the emergence of side effects during antidepressant treatment. We found negative associations between intensity of nausea and expression of miRNAs.
Introduction
Major depressive disorder (MDD) has a lifetime prevalence of approximatively 20% in the general population (Hasin et al., 2018) and may evolve into a recurrent or chronic course (Eaton et al., 2008), with a negative impact on general functioning and quality of life (IsHak et al., 2015).
Antidepressant drugs are effective therapies for MDD (Cipriani et al., 2018). However, the majority of patients treated with antidepressants experience 1 or more side effects. These side effects, which demonstrate high variability among individuals, often create barriers to achieving remission of depressive symptoms and contribute to relapse and recurrence. Indeed, poor remission rates and high relapse and recurrence rates are associated with drug discontinuation or poor compliance due to side effects (Hodgson et al., 2012; Crawford et al., 2014; De las Cuevas et al., 2014; Fabbri et al., 2018). Although selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors have better safety profiles than classical tricyclic antidepressants (Wang et al., 2018), side effects are still reported frequently.
The most common side effects associated with SSRIs and serotonin-norepinephrine reuptake inhibitors are gastrointestinal, sexual dysfunction, weight gain, dry mouth, sweating, and hyponatremia (Wang et al., 2018). Additionally, they may occur at various time points following intake of antidepressants (Kelly et al., 2008). For example, SSRI-associated side effects such as insomnia, sexual dysfunction, and drowsiness are often considered acute but may still persist at 3 months. Some side effects such as nausea start early in the course of treatment but dissipate within 1–2 weeks. Other side effects such as weight gain are not present initially but emerge over time (Hu et al., 2004; Kelly et al., 2008). The prevalence of side effects associated with SSRI treatment can be up to 64% (Bet et al., 2013). Patient- and treatment-related factors like age (Naranjo et al., 1995; Draper and Berman, 2008; Stone et al., 2009; Bet et al., 2013), depression and anxiety severity (Uher et al., 2009; Bet et al., 2013), as well as drug class and dose (Trindade et al., 1998; Stahl et al., 2005; Watanabe et al., 2010) can influence the occurrence and severity of side effects.
Individual factors, such as genetic makeup, explain a part of the variance in antidepressant-related side effects (Tansey et al., 2013). For instance, poor metabolizer status of CYPP450 (2C19) associates with a higher risk of side effects early in treatment (particularly during the first 2–4 weeks) (Fabbri et al., 2018). Genetic polymorphisms in the serotonin transporter, serotonin receptor genes (Garfield et al., 2014; Basu et al., 2015), and the ABCB1 gene (Schatzberg et al., 2015; Bet et al., 2016) also seem to be associated with side effects.
While there are several studies focusing on genetic variation as predictors of side effects (Adkins et al., 2012; Clark et al., 2012; Schatzberg et al., 2015; Amitai et al., 2016; Fabbri et al., 2018), there is little information on the possible role of dynamic molecular factors as side effect moderators. MicroRNAs (miRNA) are particularly good dynamic molecular factors to investigate for a relationship with antidepressant side effects. These small, single-stranded, non-coding RNAs are 17 to 22 nucleotides in length and modulate the expression of messenger RNA (mRNA) through mRNA degradation and inhibition of protein translation. As such, they play a crucial role in regulating the processes leading to metabolite and protein production (Fiori et al., 2017, 2018). The aim of this study was to assess changes in miRNA expression associated with side effects emerging within the first 2 weeks of escitalopram treatment and their downstream effects on target gene expression.
Materials and Methods
Population
The cohort used in this study was previously described in detail (Kennedy et al., 2019). A baseline clinical assessment was completed by 211 participants with MDD who began treatment with escitalopram immediately following baseline assessment. Of this group, sequencing was performed on 205 participants at baseline and 170 after 2 weeks of escitalopram treatment. Participants between 18 and 61 years of age who scored 21 or more on the Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979) were recruited from physician referrals or advertisements at 6 academic centers in Canada between August 2013 and December 2016. The Mini-International Neuropsychiatric Interview (Sheehan et al., 1998) Version 6.1 was administered to confirm or rule out MDD status and the presence or absence of other psychiatric comorbidities. Exclusion criteria included bipolar disorder, high suicidal risk, psychosis, drug dependency, pregnancy or breastfeeding, and failure to respond after 4 or more adequate pharmacologic interventions in the current episode or to a previous trial of escitalopram. Adequate dose and duration were used to calculate the resistance scores using the Antidepressant Treatment History Form (Sackeim, 2001). A score of 3 or higher constituted “resistance” for an individual. All participants provided written informed consent, and ethics approval was obtained at each center. The trial was registered at ClinicalTrials.gov (identifier: NCT01655706).
Clinical Assessment
In addition to the MADRS, the Toronto Side Effect Scale (TSES) (Vanderkooy et al., 2002), a 32-item instrument, was used to elicit adverse events after 2 weeks of treatment. The TSES is used to establish incidence, frequency, and severity of CNS, gastrointestinal, and sexual side effects. For each side effect, frequency (1 = never; 2 = sometimes; 3 = about half the time; 4 = often; 5 = everyday) and severity (1 = no trouble; 2; 3; 4; 5 = extreme trouble) were measured on a 5-point Likert scale. An “intensity” score was derived by multiplying frequency by severity for each side effect. At week 2, a total of 160 patients completed the TSES questionnaire, with 23 participants having missing subscores (Table 1). We did not include male-specific side effects in our analyses (we did not use the men’s specific subscale of the TSES).
Table 1.
Population characteristics
| n | Average (SD) | Minimum | Maximum | |||
|---|---|---|---|---|---|---|
| Age | 160 | 35.84 (12.64) | 18 | 61 | ||
| Gender | 160 | Female: 100 (62.5%) Male: 60 (37.5%) | ||||
| MADRS week 0 | 160 | 29.82 (5.53) | 21 | 47 | ||
| Intensity side effects week 2 | Abdominal pain | 159 | 11.89 (2.66) | 1 | 15 | |
| Agitation | 160 | 3.02 (4.41) | 1 | 25 | ||
| Anorgasmia | 156 | 2.09 (3.47) | 1 | 25 | ||
| Blurred vision | 160 | 1.45 (1.75) | 1 | 15 | ||
| Constipation | 159 | 1.74 (2.45) | 1 | 16 | ||
| Decreased appetite | 159 | 3.03 (5.21) | 1 | 25 | ||
| Decreased libido | 159 | 2.65 (4.59) | 1 | 25 | ||
| Decreased sleep | 159 | 3.2 (5.21) | 1 | 25 | ||
| Diarrhea | 159 | 2.09 (2.95) | 1 | 16 | ||
| Drowsiness/ somnolence | 159 | 4.59 (5.95) | 1 | 25 | ||
| Dry mouth | 160 | 2.50 (3.48) | 1 | 25 | ||
| Dyspepsia | 159 | 2.50 (3.58) | 1 | 25 | ||
| Dizziness | 160 | 2.09 (2.95) | 1 | 20 | ||
| Edema | 160 | 1.28 (2.24) | 1 | 25 | ||
| Flushing | 160 | 1.48 (1.78) | 1 | 15 | ||
| Headache | 159 | 2.79 (3.22) | 1 | 20 | ||
| Increased appetite | 160 | 1.93 (3.12) | 1 | 20 | ||
| Increased libido | 158 | 1.22 (.98) | 1 | 9 | ||
| Increased sleep | 160 | 2.97 (4.82) | 1 | 25 | ||
| Nausea | 159 | 3.40 (3.94) | 1 | 20 | ||
| Nervousness | 160 | 3.13 (4.65) | 1 | 20 | ||
| Other | 144 | 1.85 (2.92) | 1 | 20 | ||
| Postural hypotension | 160 | 1.53 (1.80) | 1 | 15 | ||
| Sweating | 159 | 2.33 (3.50) | 1 | 20 | ||
| Tremor | 160 | 1.62 (1.92) | 1 | 15 | ||
| Myoclonus | 160 | 1.36 (1.58) | 1 | 12 | ||
| Weight gain | 158 | 1.30 (1.36) | 1 | 16 | ||
| Weight loss | 160 | 1.21 (1) | 1 | 9 | ||
| Weakness/fatigue | 160 | 3.73 (5.26) | 1 | 25 |
Abbreviations: MADRS, Montgomery-Asberg Depression Rating Scale.
Biological Assessment
At both the baseline visit (week 0) and after 2 weeks of treatment, blood and urine samples were obtained for molecular analysis.
RNA Extraction
Whole blood for RNA analysis was collected at baseline and after 2 weeks in ethylenediaminetetraacetic acid tubes and filtered using LeukoLOCK filters (Life Technologies). Total RNA was extracted using a modified version of the LeukoLOCK Total RNA Isolation System protocol, which included DNase treatment to remove genomic DNA. The quality of RNA was assessed using the Agilent 2200 Tapestation, and only samples with an RNA integrity number ≥6.0 were used. The same RNA sample was used for both small RNA and mRNA sequencing.
Small RNA Sequencing
All libraries were prepared using the NEB small RNA protocol following the manufacturer’s instructions. Samples were sequenced at the McGill University and Genome Quebec Innovation Centre (Montreal, Canada) using the Illumina HiSeq4000 with 50nt single-end reads. All sequencing data were processed using CASAVA 1.8+ (Illumina) and extracted from FASTQ files. The Fastx toolkit was used to trim the Illumina adapter sequences. Additional filtering based on defined cutoffs was applied, including: (1) Phred quality (Q) mean scores higher than 30, (2) reads between 15 and 40 nt in length, (3) adapter detection based on perfect 10-nt match, and (4) removal of reads without detected adapter. Additionally, we used Bowtie (Song et al., 2014) to align reads to the human genome (GRCh37) (Kent et al., 2002) and ncPRO-seq (Chen et al., 2012) in combination with miRBase (V20) to match them to known miRNA sequences (Kozomara and Griffiths-Jones, 2011, 2014). Furthermore, all sequencing data were normalized with the Bioconductor- DESeq2 package (Love et al., 2014), using a detection threshold of 10 counts per miRNA.
mRNA Sequencing
All libraries were prepared using the Illumina TruSeq mRNA stranded protocol following the manufacturer’s instructions. Samples were sequenced at the McGill University and Genome Quebec Innovation Centre (Montreal, Canada) using the Illumina HiSeq4000 with 100-nt paired-end reads. FASTXToolkit and Trimmomatic were used for quality and adapter trimming, respectively. Tophat2 using bowtie2 was used to align the cleaned reads to reference genome. Reads that lost their mates through the cleaning process were aligned independently from the reads that still had pairs. Quantification on each gene’s expression was estimated using HTSeq-count and a reference transcript annotation from ENSEMBL. Counts for the paired and orphaned reads for each sample were added to each other. Normalization was conducted on the resulting gene matrix using DESeq2.
Target Prediction
We used 5 target prediction algorithms—miRWalk 2.0, miRanda, RNA22, RNAhybrid, and Targetscan (Rehmsmeier et al., 2004; Lewis et al., 2005; Miranda et al., 2006; Betel et al., 2010; Dweep and Gretz, 2015)—to identify putative mRNA targets of miRNAs and selected the top 100 targets for each miRNA. Although miRNAs can bind to other regions of mRNAs, we restricted our searches to the 3’ untranslated region of target mRNAs.
Statistical Analysis
Sociodemographic and clinical characteristics are presented using means and SDs for continuous variables and frequency distributions for categorical variables. We used log 2-transformed data for all miRNA and mRNA expression values. For each miRNA, we compared expression at each timepoint (week 0 and 2) with a repeated-measure ANOVA to identify those that changed significantly over time. For these miRNAs, we assessed the association between the change of expression of each miRNA (between week 0 and week 2) and course of intensity of each side effect between week 0 and week 2 (dependent variable) using linear regressions. To assess the role of miRNAs on predicted mRNA targets, we identified negative associations between week 0 and week 2 using linear regressions. For mRNA, we assessed the change of expression between week 0 and week 2 using a repeated-measure ANOVA. We used a false discovery rate threshold of 5% for each multiple comparison. Statistical analyses were performed with SPSS 25.0 (IBM Corp., released 2017. IBM SPSS Statistics for Mac, Version 25.0. Armonk, NY).
Results
Demographic Data
The sample included in this study comprised 160 patients who had TSES data available at week 2. Participants had a mean (SD) age of 35.84 (12.4) years, 62.5% were female, and their mean MADRS score prior to treatment was 29.82 (5.53) (Table 1). These patients were representative of the total sample (n = 211) included in the study at baseline (Kennedy et al., 2019) as they did not differ in age (35.84 [12.64] vs 35.34 [12.6], P = .74), sex ratio (female: 100 [62.5%] vs 133 [63%], P = .95), or mean baseline MADRS scores (29.82 [5.53] vs 29.98 [5.53], P = .85).
Side effects reported in this study primarily involved CNS, gastrointestinal, and sexual complaints (Table 1). The most common side effects experienced, at least occasionally, were drowsiness (45.9%), nausea (42.5%), and headaches (35.6%). Those with greatest severity were drowsiness (1.75; SD 1.11), nausea (1.60; SD 0.86), and weakness (1.57; SD 0.975).
Change in miRNA expression between week 0 and week 2 following intake of SSRI (escitalopram) treatment
We detected 356 miRNAs that passed our filtering criteria at weeks 0 and 2. Of these, 45 displayed differential expression between weeks 0 and 2 (false discovery rate < 0.05) (supplementary Table 1).
Association Between Side Effects and miRNA Expression Change Between Week 0 and Week 2
Among all side effects, only the intensity of nausea was associated with changes of miRNA expression between week 0 and week 2. Of the 45 miRNAs that showed significant changes between week 0 and week 2, we identified 12 where difference in expression between the 2 timepoints was significantly associated with reports of nausea (Table 2). Of these, 10 remained significant once covariates (age and gender) were accounted for. All were negatively associated with intensity of nausea (Table 2).
Table 2.
Association between nausea and change in miRNA expression (log 2) between week 0 and week 2
| Unadjusted | Adjusteda | |||||||
|---|---|---|---|---|---|---|---|---|
| miRNA (n = 160) | Week 0 (SD) | Week 2 (SD) | Delta week 0 week 2 (SD) | Nausea week 2 (n = 159) | β | P valueb | β | P valueb |
| miR20b-5p | 9.09 (0.64) | 9.23 (0.6) | 0.14 (0.59) | 3.40 (3.94) | −0.278 | .009 | −0.262 | .011 |
| miR106a-5p | 7.98 (0.71) | 8.14 (0.67) | 0.17 (0.68) | −0.276 | .009 | −0.248 | .011 | |
| miR20a-5p | 11.49 (0.57) | 11.65 (0.57) | 0.16 (0.57) | −0.264 | .009 | −0.266 | .011 | |
| miR17-5p | 11.52 (0.49) | 11.65 (0.47) | 0.13 (0.48) | −0.268 | .009 | −0.248 | .011 | |
| miR185-5p | 12.85 (0.46) | 12.96 (0.45) | 0.11 (0.42) | −0.253 | .009 | −0.238 | .027 | |
| miR103a-3p | 13.87 (0.37) | 13.96 (0.35) | 0.1 (0.32) | −0.231 | .019 | −0.209 | .039 | |
| miR103b | 13.87 (0.37) | 13.96 (0.35) | 0.1 (0.32) | −0.231 | .019 | −0.209 | .039 | |
| miR1301-3p | 7.26 (0.53) | 7.13 (0.49) | −0.13 (0.56) | 0.217 | .03 | 0.201 | .05 | |
| miR182-5p | 11.7 (0.69) | 11.83 (0.69) | 0.13 (0.55) | −0.217 | .03 | −0.181 | .028 | |
| miR15b-5p | 11.53 (0.62) | 11.71 (0.58) | 0.16 (0.65) | −0.211 | .008 | −0.17 | .034 | |
| miR93-5p | 12.02 (0.45) | 12.15 (0.43) | 0.13 (0.41) | −0.203 | .041 | −0.186 | .077 | |
| miR660-5p | 6.57 (0.62) | 6.74 (0.58) | 0.17 (0.64) | −0.200 | .041 | −0.21 | .013 | |
Abbreviation: miRNA, microRNA.
False discovery rate (0.05) correction.
aAge and gender.
bFalse Discovery Rate (0.05) correction for multiple comparisons.
Targets of Nausea-Associated miRNAs
We next tested for significant associations between these 10 miRNAs and their predicted mRNA targets. To generate a list of predicted mRNA targets, we used miRWalk, RNA22, miRanda, RNAhybrid, and Targetscan and identified a total of 19 targets that were predicted by all 5 algorithms (miR17-5p [3], miR20a-5p [1], miR20b-5p [2], miR106a-5p [3], miR185-5p [4], and miR660-5p [6]) (supplementary Table 2).
As miRNA primarily negatively regulates mRNA levels, we focused our analysis on miRNA-mRNA pairs that displayed negative correlations between week 0 and week 2. We found negative associations for (1) miR20a-5p with 1 mRNA (LDLRAD4), (2) miR106a-5p with 1 mRNA (CSNK1A1), (3) miR185-5p with 1 mRNA (CAMK2δ), and (4) miR660-5p with 2 mRNAs (HNRNPU, ASXL2) (Table 3).
Table 3.
Association between change in miRNA and target mRNAs between week 0 and week 2 (negative correlations only)
| Linear regression (adjusted)a | |||
|---|---|---|---|
| miRNA | Target (mRNA) | β | P valueb |
| miR20a-5p | LDLRAD4 | −0.26 | .001 |
| miR106a-5p | CSNK1A1 | −0.29 | .003 |
| miR185-5p | CAMK2δ | −0.24 | .005 |
| miR660-5p | HNRNPU | −0.24 | .006 |
| ASXL2 | −0.22 | .006 | |
Abbreviations: mRNA, messenger RNA; miRNA, microRNA.
False discovery rate (0.05) correction.
aAge and gender.
bFalse Discovery Rate (0.05) correction for multiple comparisons.
Change of Target mRNA Expression Between Week 0 and Week 2
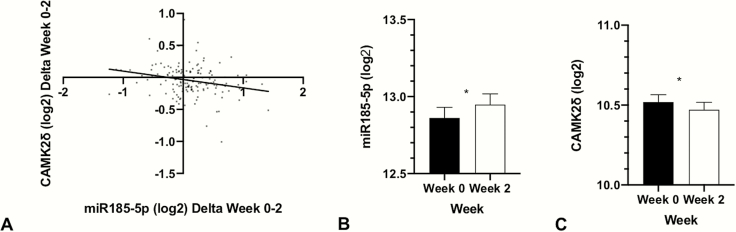
Expression of the miR-185-5p target CAMK2δ (log 2 mean = −0.048 [0.233]) decreased between weeks 0 and 2 (adjusted P = .01) (Figure 1). There were no changes in expression of the other target mRNAs over this period of time (Table 4).
Figure 1.
Changes in miR185-5p and CAMK2δ over time. (A) Association between miR185-5p and CAMK2δ. (B) Changes of miR185-5p expression (log 2) between week 0 and week 2. (C) Changes of CAMK2δ expression (log 2) between week 0 and week 2. *P < .05; CAMK2δ, calcium/calmodulin (Ca2+ /CaM)-dependent serine/threonine kinase 2δ; miRNA, micro RNA.
Table 4.
Change in mRNA expression (each miRNA selected) between week 0 and week 2.
| mRNA | miRNA | Week 0 (SD) | Week 2 (SD) | P valuea |
|---|---|---|---|---|
| LDLRAD4 | miR20a-5p | 9.999 (0.288) | 9.988 (0.258) | .57 |
| CAMK2δ | miR185-5p | 10.519 (0.287) | 10.471 (0.290) | .01 |
| CSSNK1A1 | miR106-5p | 11.904 (0.185) | 11.890 (0.179) | .39 |
| HNRNPU | miR660-5p | 13.917 (0.177) | 13.904 (0.157) | .56 |
| ASXL2 | 11.431 (0.410) | 11.416 (0.372) | .56 |
Abbreviations: mRNA, messenger RNA; miRNA, microRNA.
Log 2-adjusted counts are shown for expression.
aFalse discovery rate (0.05) correction for multiple comparisons.
Discussion
To our knowledge, this study is the first to examine the role of miRNAs in the emergence of side effects during antidepressant treatment. We identified a negative association between the intensity of nausea and differential expression of 10 miRNAs between week 0 and week 2 of SSRI treatment. Additionally, we showed that the increased expression of miR185-5p was negatively associated with intensity of nausea and that its levels were negatively associated with the expression of a predicted target, calcium/calmodulin (Ca2+ /CaM)-dependent serine/threonine kinase 2δ (CAMK2δ), whose expression decreased between week 0 and week 2.
These findings are interesting when considering nausea/vomiting mechanisms. Nausea is one of the most prevalent side effects, occurring in up to 26% of patients treated with SSRIs (Trindade et al., 1998). Nausea and vomiting result in part from the stimulation of the chemoreceptor zone located in the fourth ventricle (area postrema) (David and Gourion, 2016). The tip of the fourth ventricle in the area postrema is free of blood-brain barrier, allowing access to toxins, chemicals, and also neurotransmitters (Miller and Leslie, 1994). This zone contains a number of different neurotransmitter receptors, but it is rich in serotoninergic 5-HT3 receptors. Drugs that produce nausea/vomiting activate the serotonin 5-HT3 receptors, among others, and stimulation of 5-HT3 receptors in the intrinsic plexus of the intestine or in the vagus induces vomiting (Nutt, 1997). It has also been suggested that the neurokinin receptor NK1 interacts with the 5-HT3 receptor to potentiate the signal (Navari and Aapro, 2016).
CAMKs constitute a family of 81 proteins in the human proteome that play a central role in cellular signaling by transmitting Ca2+ signals (Manning et al., 2002). Kinases in this protein family are activated through binding of Ca2+/CaM to regulatory regions that either flank the catalytic domain or are located in regulatory molecules (Swulius and Waxham, 2008). Four CAMK2 isozymes (α, β, γ, and δ) are expressed in humans. The α and β isoforms are brain specific (Erondu and Kennedy, 1985). The γ and δ isoforms are expressed in most tissues (Hudmon and Schulman, 2002; Rellos et al., 2010). There is some evidence to support a relationship between CAMK2 and vomiting through activation of the Ca2+-CAMK2-ERK1/2 pathway (Zhong et al., 2014,2016). In our study, we found that onset of nausea was negatively associated with an increased expression of miR185-5p and that its levels were negatively associated with the expression of a predicted target, CAMK2δ, whose expression decreased between week 0 and week 2. Our results suggest that the negative association between increased expression of miR185-5p and intensity of nausea could be linked with the decreased expression of CAMK2δ.
This study investigated the expression of miRNAs and mRNAs in blood. While changes measured peripherally may not reflect the expression of these molecules in the brain, MDD is a systemic illness and side effects may arise as a result of treatment-induced peripheral molecular changes. Another limitation of this study is that we focused on side effects occurring early after the initiation of SSRI treatment. Other side effects, such as weight gain, are known to occur later in the course of treatment. Thus, future research on this topic should include points of assessment later in the treatment course.
Conclusions
We identified an overexpression of miR-185-5p during escitalopram treatment of MDD, which was negatively associated with intensity of nausea, and identified a potential mRNA target that may mediate this effect. Future studies are warranted to investigate the role of epigenetic factors in the mechanisms underlying side effects during antidepressant treatment in order to improve patient care and reduce discontinuation or poor compliance.
Supplementary Material
Acknowledgments
G.T. holds a Canada Research Chair (Tier 1) and is supported by grants from the Canadian Institute of Health Research (CIHR) (FDN148374, EGM141899, and ENP161427) and by the Fonds de recherche du Québec—Santé (FRQS) through the Quebec Network on Suicide, Mood Disorders and Related Disorders.
CAN-BIND is an Integrated Discovery Program carried out in partnership and with financial support from the Ontario Brain Institute, an independent nonprofit corporation funded partially by the Ontario government. The opinions, results, and conclusions are those of the authors and no endorsement by the Ontario Brain Institute is intended or should be inferred. Additional funding is provided by the Canadian Institutes of Health Research (CIHR), Lundbeck, Bristol-Myers Squibb, Pfizer, and Servier. Funding and/or in kind support is also provided by the investigators’ universities and academic institutions. All study medications are independently purchased at wholesale market values.
Statement of Interest
A.Y. has received speaker honoraria from AstraZeneca, Janssen, Lundbeck, Otsuka, and Servier unrelated to this work. B.F. has received a research grant from Pfizer. G.M. has received consulting fees from Pfizer, Lundbeck, Janssen, and Johnson & Johnson; disclosed honoraria for lectures for Lundbeck and Allergen; and has received research funding from the Ontario Brain Institute, Brain Canada, the Hotchkiss Brain Institute, and Canadian Institutes of Health Research. R.M. has received consulting and speaking honoraria from Allergan, Janssen, KYE, Lundbeck, Otsuka, Pfizer, and Sunovion, and research grants from CAN-BIND, CIHR, Janssen, Lallemand, Lundbeck, Nubiyota, OBI, OMHF, and Pfizer. S.K. has received research funding or honoraria from the following sources: Abbott, Alkermes, Allergan, BMS, Brain Canada, Canadian Institutes for Health Research (CIHR), Janssen, Lundbeck, Lundbeck Institute, Ontario Brain Institute, Ontario Research Fund (ORF), Otsuka, Pfizer, Servier, Sunovion, and Xian-Janssen.
L.F., R.L., D.M., J.F., and G.T. declare no competing interests.
References
- Adkins DE, Clark SL, Åberg K, Hettema JM, Bukszár J, McClay JL, Souza RP, van den Oord EJ (2012) Genome-wide pharmacogenomic study of citalopram-induced side effects in STAR*D. Transl Psychiatry 2:e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai M, Kronenberg S, Carmel M, Michaelovsky E, Frisch A, Brent D, Apter A, Chen A, Weizman A, Fennig S (2016) Pharmacogenetics of citalopram-related side effects in children with depression and/or anxiety disorders. J Neural Transm (Vienna) 123:1347–1354. [DOI] [PubMed] [Google Scholar]
- Basu A, Chadda RK, Sood M, Kaur H, Kukreti R (2015) Association of serotonin transporter (SLC6A4) and receptor (5HTR1A, 5HTR2A) polymorphisms with response to treatment with escitalopram in patients with major depressive disorder: a preliminary study. Indian J Med Res 142:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bet PM, Hugtenburg JG, Penninx BW, Hoogendijk WJ (2013) Side effects of antidepressants during long-term use in a naturalistic setting. Eur Neuropsychopharmacol 23:1443–1451. [DOI] [PubMed] [Google Scholar]
- Bet PM, Verbeek EC, Milaneschi Y, Straver DB, Uithuisje T, Bevova MR, Hugtenburg JG, Heutink P, Penninx BW, Hoogendijk WJ (2016) A common polymorphism in the ABCB1 gene is associated with side effects of PGP-dependent antidepressants in a large naturalistic Dutch cohort. Pharmacogenomics J 16:202–208. [DOI] [PubMed] [Google Scholar]
- Betel D, Koppal A, Agius P, Sander C, Leslie C (2010) Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol 11:R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Servant N, Toedling J, Sarazin A, Marchais A, Duvernois-Berthet E, Cognat V, Colot V, Voinnet O, Heard E, Ciaudo C, Barillot E (2012) ncPRO-seq: a tool for annotation and profiling of ncRNAs in sRNA-seq data. Bioinformatics 28:3147–3149. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, Leucht S, Ruhe HG, Turner EH, Higgins JPT, Egger M, Takeshima N, Hayasaka Y, Imai H, Shinohara K, Tajika A, Ioannidis JPA, Geddes JR (2018) Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 391:1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SL, Adkins DE, Aberg K, Hettema JM, McClay JL, Souza RP, van den Oord EJ (2012) Pharmacogenomic study of side-effects for antidepressant treatment options in STAR*D. Psychol Med 42:1151–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AA, Lewis S, Nutt D, Peters TJ, Cowen P, O’Donovan MC, Wiles N, Lewis G (2014) Adverse effects from antidepressant treatment: randomised controlled trial of 601 depressed individuals. Psychopharmacology (Berl) 231:2921–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DJ, Gourion D (2016) [Antidepressant and tolerance: determinants and management of major side effects]. Encephale 42:553–561. [DOI] [PubMed] [Google Scholar]
- De las Cuevas C, Peñate W, Sanz EJ (2014) Risk factors for non-adherence to antidepressant treatment in patients with mood disorders. Eur J Clin Pharmacol 70:89–98. [DOI] [PubMed] [Google Scholar]
- Draper B, Berman K (2008) Tolerability of selective serotonin reuptake inhibitors: issues relevant to the elderly. Drugs Aging 25:501–519. [DOI] [PubMed] [Google Scholar]
- Dweep H, Gretz N (2015) miRWalk2.0: a comprehensive atlas of microRNA-target interactions. Nat Methods 12:697. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Shao H, Nestadt G, Lee HB, Lee BH, Bienvenu OJ, Zandi P (2008) Population-based study of first onset and chronicity in major depressive disorder. Arch Gen Psychiatry 65:513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erondu NE, Kennedy MB (1985) Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J Neurosci 5:3270–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri C, et al. (2018) Effect of cytochrome CYP2C19 metabolizing activity on antidepressant response and side effects: meta-analysis of data from genome-wide association studies. Eur Neuropsychopharmacol 28:945–954. [DOI] [PubMed] [Google Scholar]
- Fiori LM, Lopez JP, Richard-Devantoy S, Berlim M, Chachamovich E, Jollant F, Foster J, Rotzinger S, Kennedy SH, Turecki G (2017) Investigation of miR-1202, miR-135a, and miR-16 in major depressive disorder and antidepressant response. Int J Neuropsychopharmacol 20:619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiori LM, Lin R, Ju C, Belzeaux R, Turecki G (2018) Using epigenetic tools to investigate antidepressant response. Prog Mol Biol Transl Sci 158:255–272. [DOI] [PubMed] [Google Scholar]
- Garfield LD, Dixon D, Nowotny P, Lotrich FE, Pollock BG, Kristjansson SD, Doré PM, Lenze EJ (2014) Common selective serotonin reuptake inhibitor side effects in older adults associated with genetic polymorphisms in the serotonin transporter and receptors: data from a randomized controlled trial. Am J Geriatr Psychiatry 22:971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, Grant BF (2018) Epidemiology of Adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry 75:336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson K, Mufti SJ, Uher R, McGuffin P (2012) Genome-wide approaches to antidepressant treatment: working towards understanding and predicting response. Genome Med 4:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XH, Bull SA, Hunkeler EM, Ming E, Lee JY, Fireman B, Markson LE (2004) Incidence and duration of side effects and those rated as bothersome with selective serotonin reuptake inhibitor treatment for depression: patient report versus physician estimate. J Clin Psychiatry 65:959–965. [DOI] [PubMed] [Google Scholar]
- Hudmon A, Schulman H (2002) Neuronal CA2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem 71:473–510. [DOI] [PubMed] [Google Scholar]
- IsHak WW, Mirocha J, James D, Tobia G, Vilhauer J, Fakhry H, Pi S, Hanson E, Nashawati R, Peselow ED, Cohen RM (2015) Quality of life in major depressive disorder before/after multiple steps of treatment and one-year follow-up. Acta Psychiatr Scand 131:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K, Posternak M, Alpert JE (2008) Toward achieving optimal response: understanding and managing antidepressant side effects. Dialogues Clin Neurosci 10:409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SH, et al. ; CAN-BIND Investigator Team , (2019) Symptomatic and functional outcomes and early prediction of response to escitalopram monotherapy and sequential adjunctive aripiprazole therapy in patients with major depressive disorder: a CAN-BIND-1 Report. J Clin Psychiatry 80. doi. 10.4088/JCP.18m12202 [DOI] [PubMed] [Google Scholar]
- Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D (2002) The human genome browser at UCSC. Genome Res 12:996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozomara A, Griffiths-Jones S (2011) miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res 39:D152–D157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozomara A, Griffiths-Jones S (2014) miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res 42:D68–D73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120:15–20. [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S (2002) The protein kinase complement of the human genome. Science 298:1912–1934. [DOI] [PubMed] [Google Scholar]
- Miller AD, Leslie RA (1994) The area postrema and vomiting. Front Neuroendocrinol 15:301–320. [DOI] [PubMed] [Google Scholar]
- Miranda KC, Huynh T, Tay Y, Ang YS, Tam WL, Thomson AM, Lim B, Rigoutsos I (2006) A pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell 126:1203–1217. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389. [DOI] [PubMed] [Google Scholar]
- Naranjo CA, Herrmann N, Mittmann N, Bremner KE (1995) Recent advances in geriatric psychopharmacology. Drugs Aging 7:184–202. [DOI] [PubMed] [Google Scholar]
- Navari RM, Aapro M (2016) Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med 374:1356–1367. [DOI] [PubMed] [Google Scholar]
- Nutt D. (1997) Mirtazapine: pharmacology in relation to adverse effects. Acta Psychiatr Scand Suppl 391:31–37. [DOI] [PubMed] [Google Scholar]
- Rehmsmeier M, Steffen P, Hochsmann M, Giegerich R (2004) Fast and effective prediction of microRNA/target duplexes. Rna 10:1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rellos P, Pike AC, Niesen FH, Salah E, Lee WH, von Delft F, Knapp S (2010) Structure of the CaMKIIdelta/calmodulin complex reveals the molecular mechanism of CaMKII kinase activation. Plos Biol 8:e1000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackeim HA. (2001) The definition and meaning of treatment-resistant depression. J Clin Psychiatry 62(Suppl 16):10–17. [PubMed] [Google Scholar]
- Schatzberg AF, DeBattista C, Lazzeroni LC, Etkin A, Murphy GM Jr, Williams LM (2015) ABCB1 genetic effects on antidepressant outcomes: a report from the iSPOT-D trial. Am J Psychiatry 172:751–759. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59(Suppl 20):22–33;quiz 34–57. [PubMed] [Google Scholar]
- Song L, Florea L, Langmead B (2014) Lighter: fast and memory-efficient sequencing error correction without counting. Genome Biol 15:509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl SM, Grady MM, Moret C, Briley M (2005) SNRIs: their pharmacology, clinical efficacy, and tolerability in comparison with other classes of antidepressants. CNS Spectr 10:732–747. [DOI] [PubMed] [Google Scholar]
- Stone M, Laughren T, Jones ML, Levenson M, Holland PC, Hughes A, Hammad TA, Temple R, Rochester G (2009) Risk of suicidality in clinical trials of antidepressants in adults: analysis of proprietary data submitted to US Food and Drug Administration. Bmj 339:b2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swulius MT, Waxham MN (2008) Ca(2+)/calmodulin-dependent protein kinases. Cell Mol Life Sci 65:2637–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey KE, Guipponi M, Hu X, Domenici E, Lewis G, Malafosse A, Wendland JR, Lewis CM, McGuffin P, Uher R (2013) Contribution of common genetic variants to antidepressant response. Biol Psychiatry 73:679–682. [DOI] [PubMed] [Google Scholar]
- Trindade E, Menon D, Topfer LA, Coloma C (1998) Adverse effects associated with selective serotonin reuptake inhibitors and tricyclic antidepressants: a meta-analysis. Cmaj 159:1245–1252. [PMC free article] [PubMed] [Google Scholar]
- Uher R, et al. (2009) Adverse reactions to antidepressants. Br J Psychiatry 195:202–210. [DOI] [PubMed] [Google Scholar]
- Vanderkooy JD, Kennedy SH, Bagby RM (2002) Antidepressant side effects in depression patients treated in a naturalistic setting: a study of bupropion, moclobemide, paroxetine, sertraline, and venlafaxine. Can J Psychiatry 47:174–180. [DOI] [PubMed] [Google Scholar]
- Wang SM, Han C, Bahk WM, Lee SJ, Patkar AA, Masand PS, Pae CU (2018) Addressing the side effects of contemporary antidepressant drugs: a comprehensive review. Chonnam Med J 54:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Omori IM, Nakagawa A, Cipriani A, Barbui C, McGuire H, Churchill R, Furukawa TA; MANGA (Meta-Analysis of New Generation Antidepressants) Study Group (2010) Safety reporting and adverse-event profile of mirtazapine described in randomized controlled trials in comparison with other classes of antidepressants in the acute-phase treatment of adults with depression: systematic review and meta-analysis. CNS Drugs 24:35–53. [DOI] [PubMed] [Google Scholar]
- Zhong W, Hutchinson TE, Chebolu S, Darmani NA (2014) Serotonin 5-HT3 receptor-mediated vomiting occurs via the activation of Ca2+/CaMKII-dependent ERK1/2 signaling in the least shrew (Cryptotis parva). Plos One 9:e104718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W, Chebolu S, Darmani NA (2016) Thapsigargin-induced activation of Ca(2+)-CaMKII-ERK in brainstem contributes to substance P release and induction of emesis in the least shrew. Neuropharmacology 103:195–210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.