Abstract
Background
Cocaine withdrawal activates stress systems. Females are more vulnerable to relapse to cocaine use and more sensitive to withdrawal-induced negative affect. Delta opioid receptors modulate anxiety-like behavior during cocaine withdrawal in rats. This study measured the time course of gene regulation of one of the main stress peptides, corticotropin-releasing factor (CRF), and its type 1 receptor in male and female rats as well as the ability of the delta opioid receptor agonist SNC80 to normalize cocaine withdrawal-induced changes in CRF mRNA.
Methods
Rats were injected with cocaine or saline 3 times daily for 14 days. Brains were collected 30 minutes, 24 hours, 48 hours, 7 days, and 14 days following the last injection. The paraventricular nucleus of the hypothalamus, central amygdala, and bed nucleus of the stria terminalis were processed for quantitative reverse transcriptase PCR measurement of CRF and CRFR1 mRNA. Additional rats received SNC80 during early cocaine withdrawal, and CRF mRNA was measured in the central amygdala.
Results
CRF mRNA was elevated in the central amygdala at 24 hours and the paraventricular nucleus at 48 hours of cocaine withdrawal in males and females. Significant sex differences in cocaine-induced CRF upregulation were found in the bed nucleus of the stria terminalis at 30 minutes and 24 hours. SNC80 administration attenuated the increase in CRF mRNA in the central amygdala of female rats only.
Conclusions
CRF mRNA regulation during cocaine withdrawal is sex, time, and brain region dependent. Administration of a delta opioid receptor agonist during early withdrawal may ameliorate stress-related negative affect in females by abrogating the induction of CRF mRNA.
Keywords: amygdala, bed nucleus of the stria terminalis, CRF, delta opioid receptor, sex differences
Significance Statement.
There currently is no medication for treatment of cocaine use disorder. Cocaine use and withdrawal activate stress systems. The withdrawal syndrome, characterized by depressed mood, anxiety, and drug craving, can trigger relapse and is worse in women than men. To better understand how to prevent relapse to cocaine, it is important to understand the biological components of withdrawal and how they differ between sexes. One way to mitigate the negative emotional states of withdrawal may be the use of therapeutics that target delta opioid receptors.
Introduction
Cocaine abuse and dependence are major public health problems with serious societal and economic consequences. Preventing cycles of relapse to drug use is the main goal of treatment for substance use disorders, yet there is currently no US Food and Drug Administration-approved pharmacological therapy for cocaine use disorder. Stress and withdrawal-induced negative affect greatly contribute to relapse vulnerability, particularly in females. Chronic cocaine use and withdrawal persistently activate the hypothalamic-pituitary-adrenal (HPA) axis (Kuhn and Francis, 1997; Richter and Weiss, 1999; Koob, 2008) and involve the extended amygdala, which consists of the interconnected central amygdala (CeA), bed nucleus of the stria terminalis (BNST), and nucleus accumbens shell. Interaction between the HPA axis and extended amygdala stress systems is important in both the development of cocaine addiction and the withdrawal syndrome following cocaine cessation (Sarnyai et al., 1992; Goeders, 1997).
A major component of both of these stress systems is the neuropeptide corticotropin-releasing factor (CRF). The paraventricular nucleus of the hypothalamus (PVN) of the HPA axis and the extended amygdala contain a large number of CRF cell bodies (Swanson et al., 1983). Alterations in CRF mRNA (Zhou et al., 1996, 2003a, 2003b; Maj et al., 2003; Erb et al., 2004; Mantsch et al., 2007; Rudoy et al., 2009) and peptide content (Sarnyai et al., 1995; Richter and Weiss, 1999; Zorrilla et al., 2001; Maj et al., 2003) have been reported in these brain regions at different time points following chronic cocaine administration. The majority of these studies have investigated male rats. As female humans and rats progress from cocaine abuse to dependence more quickly (Griffin et al., 1989; Becker and Hu, 2008), are more sensitive to the subjective effects of cocaine (Lukas et al., 1996; Sofuoglu et al., 1999; Back et al., 2005), and are more vulnerable to relapse (Erb et al., 1998; Hudson and Stamp, 2011), all stages of addiction involving stress systems, it is important to consider the underlying mechanisms responsible for these sex differences (Lynch et al., 2002; Lynch, 2018).
Delta opioid receptors (DOR) play an important role in not only analgesia but also regulation of mood. DOR signaling is disrupted by chronic cocaine use, which may contribute to the negative effect experienced during cocaine withdrawal (Perrine et al., 2008). Indeed, cocaine withdrawal-induced anxiety is reduced by the selective DOR agonist SNC80 in both male and female rats (Perrine et al., 2008; Ambrose-Lanci et al., 2010). The molecular or cellular mechanisms underlying the ability of DOR agonists to reduce cocaine withdrawal-induced anxiety remain unknown. DOR and CRF are colocalized in the central and basolateral amygdala; DOR are found on dendrites of CRF-containing neurons and in axon terminals targeting CRF neurons (Reyes et al., 2017). This anatomical arrangement suggests that DOR and CRF systems interact to regulate stress-related responses, and this interaction may be implicated in negative affective states during cocaine withdrawal.
Building on previous work, this study aimed to provide a comprehensive time course analysis of gene regulation of CRF and its type 1 receptor in the CeA, PVN, and BNST of both male and female rats following chronic cocaine administration. Our preliminary studies did not find CRF or CRF-R1 mRNA regulation in the nucleus accumbens shell; therefore, this region was excluded from further study. An additional aim of this study was to investigate the ability of DOR agonist SNC80 to normalize cocaine withdrawal-induced increases in CRF gene expression in the CeA given its ability to reduce withdrawal-induced anxiety.
Methods
Animals
Male and female Sprague Dawley rats (7 weeks old on arrival, Charles River Laboratories) were housed in pairs of the same sex in a room on a 12-hour-light/-dark cycle (lights on at 7:00 am) for at least 1 week before experiments began. Rats had access to standard rat chow and water ad libitum. All procedures were in compliance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and approved by Temple University’s Institutional Animal Care and Use Committee.
Drugs
Cocaine HCl (generously supplied by the National Institute on Drug Abuse drug supply program) was dissolved in 0.9% normal saline and injected i.p. in a volume of 1 mL/kg body weight. SNC80 ((+)-4-[(αR)-α-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide) (Tocris Bioscience, Minneapolis, MN) was dissolved in 2 µL of 1 M HCl per milligram SNC80, and sterile water was slowly added with vortexing to achieve a concentration of 10 mg/mL. SNC80 was injected s.c. in a volume of 1 mL/kg body weight. Rats were injected with saline or 15 mg/kg cocaine 3 times per day, 1 hour apart (i.e., binge pattern) starting at 9:30 am for 14 days. An additional cohort of rats was injected with saline or cocaine for 14 days, followed by 10 mg/kg SNC80 or vehicle 8 hours and 20 hours (2 SNC80 injections total) following the last cocaine or saline injection. Brains were obtained 24 hours post last cocaine or saline injection.
Brain Tissue Collection
Rats were killed 30 minutes, 24 hours, 48 hours, 7 days, or 14 days following the last cocaine or saline injection by brief CO2 anesthesia followed by decapitation. Brains were removed, flash frozen in isopentane (−35°C to −40°C), and stored at −80°C. Frozen brains were sliced on a cryostat microtome, and 1-mm punches were used to dissect bilateral PVN, BNST, and CeA (supplementary Figure 1) (Paxinos and Watson, 2007) from 2 slices 300 µm thick. Tissue punches were placed in RNAlater-ICE (Invitrogen, Waltham, MA) overnight at −20°C before storage at −80°C.
Quantitative Reverse Transcriptase Polymerase Chain Reaction
Total RNA was isolated using a Quick-RNA Miniprep kit (Zymo Research, Irvine, CA), and RNA concentration was measured using a NanoDrop 2000 spectrophotometer. Samples were diluted to the same RNA concentration before cDNA was synthesized using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Waltham, MA). Quantitative RT-PCR was performed using TaqMan Fast Advanced Master Mix and TaqMan Gene Expression Assays for CRF (Rn01462137_m1), CRFR1 (Rn00578611_m1), and the control 18S rRNA (Hs99999901_s1). Relative fold change was calculated using the ΔΔCt method (Livak and Schmittgen, 2001). To facilitate analysis of sex differences, quantitative fluorescent data were collected simultaneously from samples from 1 brain region at a single time point from both sexes. For time course data within individual sexes, mRNA levels in cocaine-injected rats were normalized to saline controls of the same sex at the same time point. To identify sex differences, mRNA levels in cocaine-injected males, cocaine-injected females, and saline-injected females were all normalized to male saline controls, which did not differ across the time course.
Statistics
Statistical analyses were performed with GraphPad Prism software using 2-way ANOVA (cocaine × time, cocaine × sex, or cocaine × SNC80), followed by Sidak post tests for planned comparisons between saline- and cocaine-injected rats at individual time points. P < .05 was considered significant.
Results
Time Course of CRF Gene Expression in the CeA, PVN, and BNST
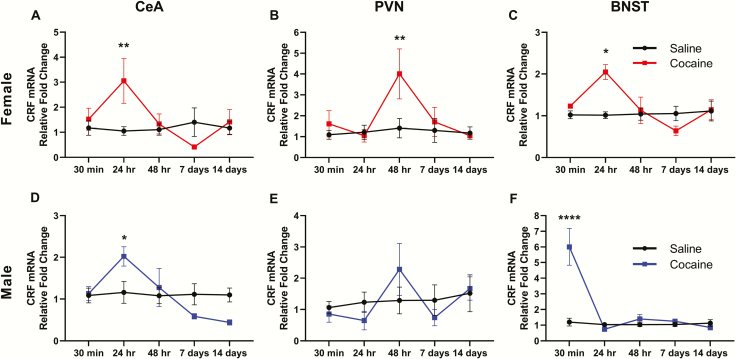
CRF mRNA was measured 30 minutes, 24 hours, 48 hours, 7 days, and 14 days following 14 days of binge-pattern cocaine or saline injections in male and female rats. In the CeA of female rats (Figure 1A), 2-way ANOVA indicated a significant interaction (F[4,38] = 3.240, P = .0221) between the main effects of cocaine (F[1,38] = 2.035, P = .1619) and time (F[4,38] = 2.020, P = .1112). Post hoc analysis revealed that CRF mRNA was elevated compared with saline-injected controls 24 hours following cocaine (3.05-fold, P = .0084) but not at other time points (supplementary Table 1). Similarly, 2-way ANOVA showed an increase in CRF mRNA in the CeA of cocaine-injected males vs saline-injected controls (Figure 1D) (interaction F[4,48] = 3.884, P = .0082; cocaine F[1,48] = 0.0107, P = .9181; time F[4,48] = 4.436, P = .0039), which was significant at 24 hours only (2.02-fold, post hoc P = .0255) (supplementary Table 1).
Figure 1.
Withdrawal from chronic cocaine administration increases corticotropin-releasing factor (CRF) mRNA in a time- and brain region-specific manner in female (top) and male (bottom) rats. Fold changes in CRF mRNA compared with saline-injected controls of the same sex are shown for 5 time points after the last injection of saline or cocaine in the central amygdala (CeA; A, D), paraventricular nucleus (PVN; B, E), and bed nucleus of the stria terminalis (BNST; C, F). Post hoc tests: *P < .05, **P < .01, ****P < .0001. n = 5–7 rats/time point/group.
No significant differences in CRF mRNA levels between cocaine- and saline-injected males were found in the PVN at any time point (Figure 1E; supplementary Table 1). In the PVN of female rats (Figure 1B), 2-way ANOVA found a significant effect of time (F[4,48] = 3.039, P = .0287; cocaine F[1,38] = 3.784, P = .0592; interaction F[4,48] = 2.220, P = .0850), and post hoc analysis indicated that CRF mRNA was significantly elevated in cocaine-injected females compared with saline-injected controls (4.01-fold, post hoc P = .0081) 48 hours following the last injection (supplementary Table 1).
In the BNST of female rats (Figure 1C), 2-way ANOVA revealed a significant interaction (F[4,39] = 3.027, P = .0288) between time (F[4.39] = 2.624, P = .0493) and cocaine (F[1,39] = 2.274, P = .1396). Compared with saline-injected controls, CRF mRNA was significantly elevated (2.05-fold, post hoc P = .0105) 24 hours following the last cocaine injection (supplementary Table 1). In the BNST of male rats (Figure 1F), 2-way ANOVA indicated a significant interaction (F[4,46] = 15.58, P < .0001) as well as significant main effects of time (F[4,46] = 17.29, P < .0001) and cocaine (F[1,46] = 16.32, P = .0002). Compared with saline controls, CRF mRNA was elevated in the BNST of male rats 30 minutes post cocaine (6.01-fold, post hoc P < .0001) but not at other time points (supplementary Table 1).
Time Course of CRFR1 Gene Expression in CeA, PVN, and BNST
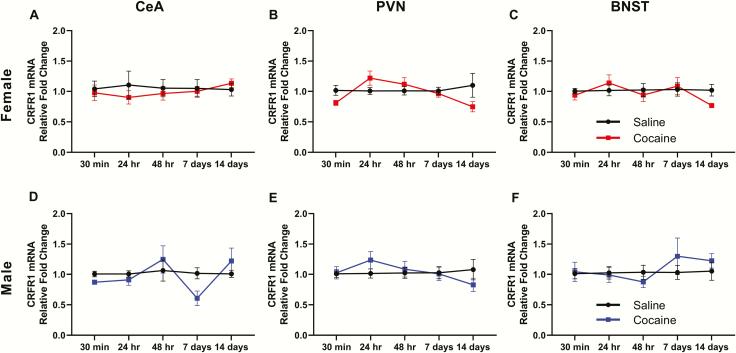
CRFR1 mRNA was measured 30 minutes, 24 hours, 48 hours, 7 days, and 14 days following 14 days of binge-pattern cocaine or saline injections in both males and females. No significant differences (supplementary Table 1) were found in CRFR1 expression between cocaine- and saline-injected male (Figure 2D–F) or female (Figure 2A–C) rats in the CeA, PVN, or BNST.
Figure 2.
Corticotropin-releasing factor (CRF)R1 mRNA expression during withdrawal from chronic cocaine administration in female (top) and male (bottom) rats. Fold changes in CRFR1 mRNA compared with saline-injected controls of the same sex are shown for 5 time points after the last injection of saline or cocaine in the central amygdala (CeA; A, D), paraventricular nucleus (PVN; B, E), and bed nucleus of the stria terminalis (BNST; C, F). n = 5–7 rats/time point/group.
Sex Differences in CRF and CRFR1 Gene Regulation
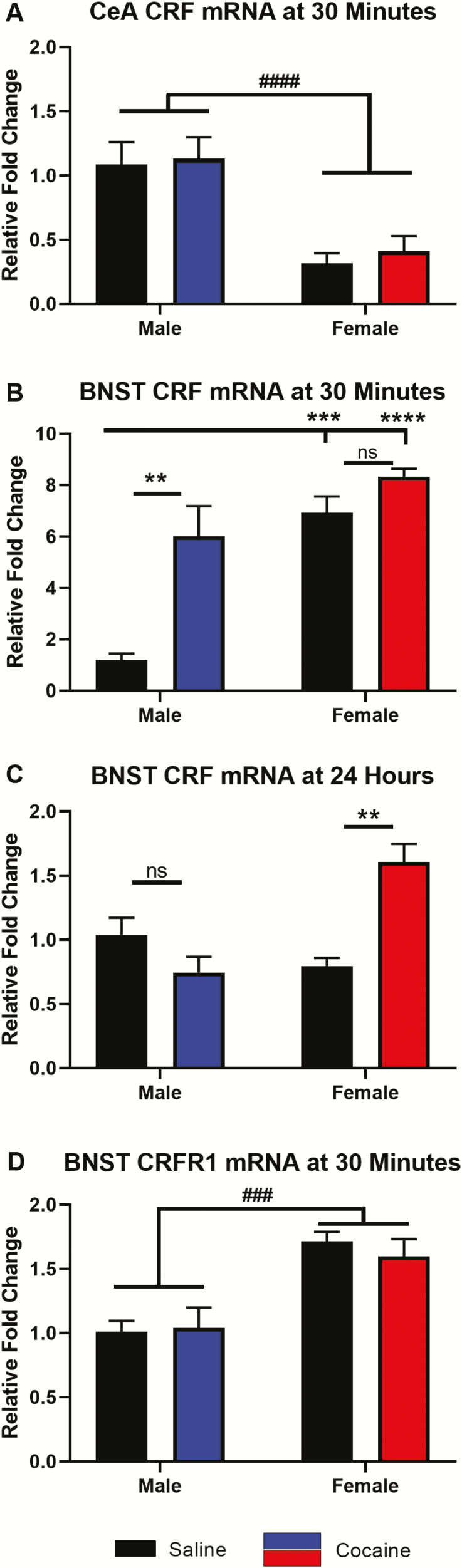
At the majority of time points and brain regions, males and females did not differ significantly when analyzed together (i.e., cocaine-injected males, cocaine-injected females, and saline-injected females were all normalized to male saline-injected controls); however, some sex differences did emerge. Thirty minutes following the last injection, 2-way ANOVA indicated a significant main effect of sex (F[1,17] = 25.73, P < .0001; cocaine F[1,17] = 0.2262, P = .6404; interaction F[1,17] = 0.0271, P = .8712), and post hoc tests showed that the CeA of both saline- and cocaine-injected females contained less CRF mRNA compared with male saline controls (0.317-fold, P = .0073 and 0.411-fold, P = .0190, respectively) (Figure 3A).
Figure 3.
Sex differences in regulation of corticotropin-releasing factor (CRF) and CRFR1 mRNAs during early withdrawal from chronic cocaine administration. Fold changes (compared with male saline controls) in CRF (A–C) and CRFR1 (D) mRNAs are shown in the central amygdala (CeA; A) and bed nucleus of the stria terminalis (BNST; B–D) of male and female rats either 30 minutes (A, B, D) or 24 hours (C) following the last injection. ANOVA main effect of sex: ###P < .001, #### P < .0001. Post hoc tests: **P < .01, ***P < .001, ****P < .0001, n = 5–7 rats/group.
In the BNST 30 minutes following the last injection, 2-way ANOVA revealed a significant interaction between the main effects of sex and cocaine (interaction F[1,16] = 5.319, P = .0348; sex F[1,16] = 29.87, P < .0001; cocaine F[1,16] = 17.83, P = .0006). Cocaine did not regulate CRF mRNA at 30 minutes in the BNST of females analyzed alone (Figure 1C), but compared with male saline controls, post hoc tests showed the BNST contained significantly higher amounts of CRF mRNA in both saline- (6.92-fold, P = .0001) and cocaine-injected (8.33-fold, P < .0001) females (Figure 3B). Additionally, the BNST of both saline- and cocaine-injected female rats contained significantly more CRFR1 mRNA than that of saline- and cocaine-injected males 30 minutes following the last injection (Figure 3D) (2-way ANOVA, sex F[1,16] = 24.88, P = .0001; interaction F[1,16] = 0.3297, P = .5738; cocaine F[1,16] = 0.1153, P = .7386).
Another difference between sexes became apparent in the BNST 24 hours following the last injection (2-way ANOVA, interaction F[1,16] = 19.27, P = .0005; sex F[1,16] = 6.059, P = .0256; cocaine F[1,16] = 4.235, P = .0563). While CRF mRNA levels in saline- and cocaine-injected males did not differ (Figure 1F), CRF mRNA was higher in cocaine-injected females (1.61-fold) compared with saline-injected females (0.797-fold, P = .0041) (Figure 3C) when normalized to saline control males.
Effect of SNC80 Administration on CRF mRNA Levels in the Central Amygdala and Bed Nucleus of the Stria Terminalis
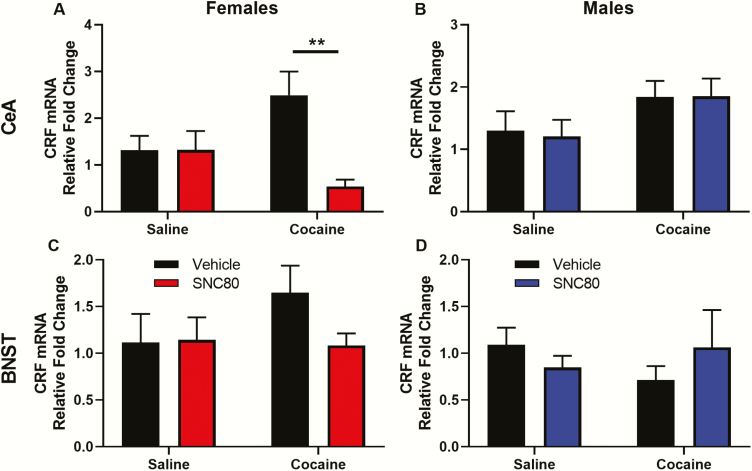
The effect of SNC80 on CRF mRNA levels in the CeA and BNST was assessed in male and female rats 24 hours following the last injection. In the CeA of females, 2-way ANOVA found a significant interaction (F[1,23] = 7.189, P = .0133) between the main effects of SNC80 (F[1,23] = 7.042, P = .0142) and cocaine (F[1,23] = 0.2878 P = .5968). Post hoc tests showed CRF mRNA levels in the CeA of cocaine-injected rats treated with SNC80 were significantly lower compared with cocaine-injected rats given vehicle (P = .0058; Figure 4A). In contrast to the findings in females, SNC80 administration did not regulate CRF mRNA levels in the CeA of male rats 24 hours following the last injection (Figure 4B; supplementary Table 1).
Figure 4.
SNC80 attenuates cocaine withdrawal-induced increases in corticotropin-releasing factor (CRF) mRNA in the central amygdala of female rats. Fold changes in CRF mRNA (compared with saline + vehicle controls) in the central amygdala (A, B) and bed nucleus of the stria terminalis (C, D) are shown for female (A, C) and male (B, D) rats injected with SNC80 or vehicle following chronic cocaine or saline administration. Brain tissue was obtained 24 hours following the last cocaine or saline injection. Post hoc tests: **P < .01. n = 6–8 rats/group.
CRF mRNA in the BNST of female rats was higher 24 hours following chronic cocaine and normalized by SNC80 administration (Figure 4C); however, this did not reach statistical significance due to variability between animals. CRF mRNA levels in the BNST of males were not regulated by SNC80 administration (Figure 4D; supplementary Table 1).
Discussion
This study investigated the regulation of CRF and CRFR1 gene expression during early and protracted withdrawal from chronic cocaine administration. CRF mRNA was significantly higher in the CeA of both male and female rats injected with cocaine compared with those injected with saline 24 hours after the last injection. Regardless of treatment group, the CeA of female rats contained less CRF mRNA than males, and the BNST of females contained more CRFR1 mRNA than males when measured 30 minutes after the last injection. Additional sex differences in CRF regulation emerged in the BNST. Thirty minutes following the last injection, CRF mRNA levels in cocaine-injected males and both saline- and cocaine-injected females were higher than that of saline-injected male controls. Twenty-four hours later, CRF mRNA content returned to control levels in males and saline-injected females, while CRF mRNA remained elevated in cocaine-injected females.
The experiments described in this manuscript are based on an important foundation of previous work. Expanded by a thorough time-course analysis during withdrawal and by the addition of females treated in parallel to males, the present results support previous findings and extend those results in several ways. For example, 2 studies found an increase in CRF mRNA in the CeA of male rats 24 hours post cocaine (Maj et al., 2003; Erb et al., 2004), which is replicated here. In contrast, other studies have found an increase in CRF mRNA in the amygdala at 48 hours, not 24 hours, post cocaine (Zhou et al., 2003a; Rudoy et al., 2009). While the current study and others (Mantsch et al., 2007) show no significant differences in CRF mRNA in the PVN, another found a decrease in CRF mRNA in the hypothalamus 30 minutes following the last cocaine injection (Zhou et al., 1996). Congruent with the present study, previous work reported no differences in CRF mRNA in the BNST after 1, 3, or 10 days withdrawal (Erb et al., 2004). The reason(s) for discrepancies in CRF mRNA levels in the amygdala and hypothalamus are uncertain; however, differences may be due to the tissue included in the analysis. Studies that analyzed the whole amygdala found increases in CRF mRNA at 48 hours following chronic cocaine administration (Zhou et al., 2003a; Rudoy et al., 2009), while studies that selectively analyzed only the CeA agreed on an increase in CRF mRNA at 24 hours (Maj et al., 2003; Erb et al., 2004). It should be noted that other nuclei of the amygdala, such as the basolateral amygdala, also contain CRF and play a role in cocaine withdrawal-induced negative affect, which may have affected the study outcome. In similar fashion, results from studies investigating the whole hypothalamus (Zhou et al., 1996, 2003a, 2003b) differ from those that processed the PVN specifically (Mantsch et al., 2007; this study). Inter-animal variability in CRF mRNA expression levels in the PVN in the current study may also be a factor in the different outcomes. In terms of lack of changes in gene expression during long-term withdrawal, the present findings support several studies that showed no differences in CRF mRNA levels between rats given chronic saline or cocaine at longer withdrawal time points (i.e., 7, 10, or 14 days post cocaine) (Zhou et al., 1996, 2003a, 2003b; Erb et al., 2004). It should be noted that all of the above cited studies investigated male animals. To the authors’ knowledge, regulation of CRF mRNA during withdrawal from chronic cocaine has not been reported in females.
This study investigated the effect of the DOR agonist SNC80 on elevated CRF mRNA levels in the CeA during cocaine withdrawal. SNC80 administration significantly reduced elevated CRF mRNA levels in females but had no effect on CRF mRNA in males. This was unexpected given that DOR agonists can modulate anxiety-like behavior in both sexes (Perrine et al., 2006, 2008; Ambrose-Lanci et al., 2010; Randall-Thompson et al., 2010; van Rijn et al., 2010). Why SNC80 reduced CRF mRNA in females but not males is unclear. One possible explanation could be the timing of SNC80 administration. In previous behavioral studies, SNC80 was injected 30 to 60 minutes before anxiety testing. In the current study, SNC80 was injected 8 hours and 20 hours following the last cocaine or saline injection (i.e., 4 hours before brain collection). It is possible that the timing of CRF regulation by SNC80 differs between males and females. Sex differences in subcellular distribution of DORs have been documented in the hippocampus following stress (Mazid et al., 2016) and in the nucleus accumbens following chronic cocaine (Ambrose-Lanci et al., 2008). This may contribute to the difference in regulation of CRF by DOR agonists. It is likely that the effect of DOR agonists on CRF expression and, subsequently, downstream effects of CRF are modulated by a network of brain areas. Recent studies provide evidence of sex differences in stress-related brain circuits. Using cFos as a marker of neuronal activation, Bangasser and colleagues measured functional connectivity between stress-related brain regions and found sex differences in activated networks both in unstressed rats as well as in rats that received CRF by icv injection (Wiersielis et al., 2016; Salvatore et al., 2018). They further showed that behavioral responses to CRF, such as anxiety, are regulated by different brain area connections in males and females. Further studies investigating different SNC80 administration protocols or measuring CRF protein levels are needed to address the noted sex difference in CRF regulation by DOR agonists.
A growing body of literature highlights sex differences in the escalation of cocaine use, the subjective effects of cocaine, and susceptibility to relapse. One of the main triggers to relapse is stress, and sex differences in stress systems have emerged as a critical factor driving substance abuse and relapse in women. Cocaine-using women are more sensitive to CRF (Brady et al., 2009), and female rats show greater HPA axis activation in response to cocaine (Kuhn and Francis, 1997; Fox et al., 2006). Female rats are also more sensitive to stress-induced reinstatement of cocaine seeking (Erb et al., 1998; Anker and Carroll, 2010; Buffalari et al., 2012). One possible explanation for this is the difference in CRF receptor trafficking and signaling. In males, in response to CRF release, CRF receptors are internalized, while in females CRF receptors are internalized less, leaving more receptors on the cell membrane to continue signaling (Reyes et al., 2008; Bangasser et al., 2010; Valentino et al., 2013). Although these studies were performed in the locus coeruleus, it is probable that this phenomenon occurs elsewhere in the brain. Indeed, the locus coeruleus, CeA, PVN, and BNST are highly interconnected by CRF fibers (reviewed in Koob and Volkow, 2010). The current study identified the BNST as a region of significant sex-specific gene regulation induced by cocaine exposure and withdrawal. The BNST of female rats contains more CRF neurons than the BNST of male rats (Funabashi et al., 2004), and more BNST neurons are activated after an acute stressor in female rats than in males (Babb et al., 2013). The current study shows differential regulation of CRF gene expression between males and females following chronic cocaine and highlights that the BNST of both cocaine- and saline-injected female rats contained more CRFR1 mRNA than that of males.
While changes in CRF mRNA during withdrawal from chronic cocaine have been described in males, this study identifies important sex differences in CRF and CRFR1 regulation in the CeA and particularly in the BNST. Perhaps these differences play a role in females’ increased vulnerability to cocaine withdrawal-induced negative affect and relapse. Since SNC80 mitigated cocaine withdrawal-induced increases in CRF mRNA in the CeA of females and mitigated withdrawal-induced anxiety (Perrine et al., 2008; Ambrose-Lanci et al., 2010), DOR agonists may be a valuable tool for managing withdrawal symptoms and preventing relapse, especially in females.
Supplementary Material
Acknowledgments
The authors thank Dr Thomas Rogers and Dr William Cornwell for their technical knowledge and Eva von Weltin and Rachel Denny for their experimental assistance.
This work was supported by the National Institutes of Health (grant nos. T32 DA007237, R01 DA018326, and P30 DA013429).
Statement of Interest
None.
References
- Ambrose-Lanci LM, Peiris NB, Unterwald EM, Van Bockstaele EJ (2008) Cocaine withdrawal-induced trafficking of delta-opioid receptors in rat nucleus accumbens. Brain Res 1210:92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose-Lanci LM, Sterling RC, Van Bockstaele EJ (2010) Cocaine withdrawal-induced anxiety in females: impact of circulating estrogen and potential use of delta-opioid receptor agonists for treatment. J Neurosci Res 88:816–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME (2010) Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug Alcohol Depend 107:264–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb JA, Masini CV, Day HE, Campeau S (2013) Sex differences in activated corticotropin-releasing factor neurons within stress-related neurocircuitry and hypothalamic-pituitary-adrenocortical axis hormones following restraint in rats. Neuroscience 234:40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Brady KT, Jackson JL, Salstrom S, Zinzow H (2005) Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology 180:169–176. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ (2010) Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry 15:877, 896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Hu M (2008) Sex differences in drug abuse. Front Neuroendocrinol 29:36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, McRae AL, Moran-Santa Maria MM, DeSantis SM, Simpson AN, Waldrop AE, Back SE, Kreek MJ (2009) Response to corticotropin-releasing hormone infusion in cocaine-dependent individuals. Arch Gen Psychiatry 66:422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, Baldwin CK, Feltenstein MW, See RE (2012) Corticotrophin releasing factor (CRF) induced reinstatement of cocaine seeking in male and female rats. Physiol Behav 105:209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J (1998) The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci 18:5529–5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Funk D, Borkowski S, Watson SJ, Akil H (2004) Effects of chronic cocaine exposure on corticotropin-releasing hormone binding protein in the central nucleus of the amygdala and bed nucleus of the stria terminalis. Neuroscience 123:1003–1009. [DOI] [PubMed] [Google Scholar]
- Fox HC, Garcia M Jr, Kemp K, Milivojevic V, Kreek MJ, Sinha R (2006) Gender differences in cardiovascular and corticoadrenal response to stress and drug cues in cocaine dependent individuals. Psychopharmacology 185:348–357. [DOI] [PubMed] [Google Scholar]
- Funabashi T, Kawaguchi M, Furuta M, Fukushima A, Kimura F (2004) Exposure to bisphenol A during gestation and lactation causes loss of sex difference in corticotropin-releasing hormone-immunoreactive neurons in the bed nucleus of the stria terminalis of rats. Psychoneuroendocrinology 29:475–485. [DOI] [PubMed] [Google Scholar]
- Goeders NE. (1997) A neuroendocrine role in cocaine reinforcement. Psychoneuroendocrinology 22:237–259. [DOI] [PubMed] [Google Scholar]
- Griffin ML, Weiss RD, Mirin SM, Lange U (1989) A comparison of male and female cocaine abusers. Arch Gen Psychiatry 46:122–126. [DOI] [PubMed] [Google Scholar]
- Hudson A, Stamp JA (2011) Ovarian hormones and propensity to drug relapse: a review. Neurosci Biobehav Rev 35:427–436. [DOI] [PubMed] [Google Scholar]
- Koob GF. (2008) A role for brain stress systems in addiction. Neuron 59:11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn C, Francis R (1997) Gender difference in cocaine-induced HPA axis activation. Neuropsychopharmacology 16:399–407. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Sholar M, Lundahl LH, Lamas X, Kouri E, Wines JD, Kragie L, Mendelson JH (1996) Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharmacology 125:346–354. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. (2018) Modeling the development of drug addiction in male and female animals. Pharmacol Biochem Behav 164:50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME (2002) Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology 164:121–137. [DOI] [PubMed] [Google Scholar]
- Maj M, Turchan J, Smiałowska M, Przewłocka B (2003) Morphine and cocaine influence on CRF biosynthesis in the rat central nucleus of amygdala. Neuropeptides 37:105–110. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Taves S, Khan T, Katz ES, Sajan T, Tang LC, Cullinan WE, Ziegler DR (2007) Restraint-induced corticosterone secretion and hypothalamic CRH mRNA expression are augmented during acute withdrawal from chronic cocaine administration. Neurosci Lett 415:269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazid S, Hall BS, Odell SC, Stafford K, Dyer AD, Van Kempen TA, Selegean J, McEwen BS, Waters EM, Milner TA (2016) Sex differences in subcellular distribution of delta opioid receptors in the rat hippocampus in response to acute and chronic stress. Neurobiol Stress 5:37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2007) The Rat Brain in Stereotaxic Coordinates Sixth Edition. San Diego: Elsevier Acad Press. [Google Scholar]
- Perrine SA, Hoshaw BA, Unterwald EM (2006) Delta opioid receptor ligands modulate anxiety-like behaviors in the rat. Br J Pharmacol 147:864–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine SA, Sheikh IS, Nwaneshiudu CA, Schroeder JA, Unterwald EM (2008) Withdrawal from chronic administration of cocaine decreases delta opioid receptor signaling and increases anxiety- and depression-like behaviors in the rat. Neuropharmacology 54:355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall-Thompson JF, Pescatore KA, Unterwald EM (2010) A role for delta opioid receptors in the central nucleus of the amygdala in anxiety-like behaviors. Psychopharmacology 212:585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BA, Valentino RJ, Van Bockstaele EJ (2008) Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology 149:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BA, Kravets JL, Connelly KL, Unterwald EM, Van Bockstaele EJ (2017) Localization of the delta opioid receptor and corticotropin-releasing factor in the amygdalar complex: role in anxiety. Brain Struct Funct 222:1007–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter RM, Weiss F (1999) In vivo CRF release in rat amygdala is increased during cocaine withdrawal in self-administering rats. Synapse 32:254–261. [DOI] [PubMed] [Google Scholar]
- Rudoy CA, Reyes AR, Van Bockstaele EJ (2009) Evidence for beta1-adrenergic receptor involvement in amygdalar corticotropin-releasing factor gene expression: implications for cocaine withdrawal. Neuropsychopharmacology 34:1135–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore M, Wiersielis KR, Luz S, Waxler DE, Bhatnagar S, Bangasser DA (2018) Sex differences in circuits activated by corticotropin releasing factor in rats. Horm Behav 97:145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnyai Z, Bíró E, Penke B, Telegdy G (1992) The cocaine-induced elevation of plasma corticosterone is mediated by endogenous corticotropin-releasing factor (CRF) in rats. Brain Res 589:154–156. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Bíró E, Gardi J, Vecsernyés M, Julesz J, Telegdy G (1995) Brain corticotropin-releasing factor mediates ‘anxiety-like’ behavior induced by cocaine withdrawal in rats. Brain Res 675:89–97. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK (1999) Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol 7:274–283. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW (1983) Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 36:165–186. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E, Bangasser D (2013) Sex-specific cell signaling: the corticotropin-releasing factor receptor model. Trends Pharmacol Sci 34:437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn RM, Brissett DI, Whistler JL (2010) Dual efficacy of delta opioid receptor-selective ligands for ethanol drinking and anxiety. J Pharmacol Exp Ther 335:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersielis KR, Wicks B, Simko H, Cohen SR, Khantsis S, Baksh N, Waxler DE, Bangasser DA (2016) Sex differences in corticotropin releasing factor-evoked behavior and activated networks. Psychoneuroendocrinology 73:204–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, LaForge KS, Maggos CE, Ho A, Kreek MJ (1996) Corticotropin-releasing factor and type 1 corticotropin-releasing factor receptor messenger RNAs in rat brain and pituitary during “binge”-pattern cocaine administration and chronic withdrawal. J Pharmacol Exp Ther 279:351–358. [PubMed] [Google Scholar]
- Zhou Y, Spangler R, Ho A, Kreek MJ (2003a) Increased CRH mRNA levels in the rat amygdala during short-term withdrawal from chronic ‘binge’ cocaine. Brain Res Mol Brain Res 114:73–79. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, Schlussman SD, Ho A, Kreek MJ (2003b) Alterations in hypothalamic-pituitary-adrenal axis activity and in levels of proopiomelanocortin and corticotropin-releasing hormone-receptor 1 mRNAs in the pituitary and hypothalamus of the rat during chronic ‘binge’ cocaine and withdrawal. Brain Res 964:187–199. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F (2001) Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology 158:374–381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.