Abstract
Background
Basolateral amygdalar projections to the prefrontal cortex play a key role in modulating behavioral responses to stress stimuli. Among the different neuromodulators known to impact basolateral amygdalar-prefrontal cortex transmission, the corticotrophin releasing factor (CRF) is of particular interest because of its role in modulating anxiety and stress-associated behaviors. While CRF type 1 receptor (CRFR1) has been involved in prefrontal cortex functioning, the participation of CRF type 2 receptor (CRFR2) in basolateral amygdalar-prefrontal cortex synaptic transmission remains unclear.
Methods
Immunofluorescence anatomical studies using rat prefrontal cortex synaptosomes devoid of postsynaptic elements were performed in rats with intra basolateral amygdalar injection of biotinylated dextran amine. In vivo microdialysis and local field potential recordings were used to measure glutamate extracellular levels and changes in long-term potentiation in prefrontal cortex induced by basolateral amygdalar stimulation in the absence or presence of CRF receptor antagonists.
Results
We found evidence for the presynaptic expression of CRFR2 protein and mRNA in prefrontal cortex synaptic terminals originated from basolateral amygdalar. By means of microdialysis and electrophysiological recordings in combination with an intra-prefrontal cortex infusion of the CRFR2 antagonist antisauvagine-30, we were able to determine that CRFR2 is functionally positioned to limit the strength of basolateral amygdalar transmission to the prefrontal cortex through presynaptic inhibition of glutamate release.
Conclusions
Our study shows for the first time to our knowledge that CRFR2 is expressed in basolateral amygdalar afferents projecting to the prefrontal cortex and exerts an inhibitory control of prefrontal cortex responses to basolateral amygdalar inputs. Thus, changes in CRFR2 signaling are likely to disrupt the functional connectivity of the basolateral amygdalar-prefrontal cortex pathway and associated behavioral responses.
Keywords: basolateral amygdala, CRFR2, glutamatergic transmission, prefrontal cortex
Significance Statement.
Corticotrophin-releasing factor (CRF), through its action on CRF type 1 and CRF type 2 receptors, is central for the regulation of adaptive responses to stressors. However, the mechanism by which CRF receptor signaling modulates synaptic transmission remains elusive, especially within the corticolimbic circuitry. Here, we found that CRF type 2 receptor is expressed in basolateral amygdalar terminals projecting to the prefrontal cortex and is functionally positioned to limit the strength of amygdalar transmission via inhibition of glutamate release.
Introduction
The basolateral amygdala (BLA) plays a critical role in modulating anxiety and stress-associated behaviors (Jaferi and Bhatnagar, 2007), in part through its regulation of prefrontal cortex (PFC) response to emotional stimuli (Morgan and LeDoux, 1995; Garcia et al., 1999; Davis and Whalen, 2001; Gilmartin and Helmstetter, 2010; Milad and Quirk, 2012). Among the different neuromodulators known to impact BLA-PFC transmission (Floresco and Tse, 2007; Rodrigues et al., 2009; Tejeda et al., 2015; Hervig et al., 2017), the corticotrophin releasing factor (CRF) is of special interest because of its role in regulating behavioral responses to stressors (Heinrichs et al., 1995; Koob and Heinrichs, 1999) by integrating the endocrine and neuronal systems (Vale et al., 1981). CRF receptor activation has been shown to modulate neuronal excitability in the BLA (Rainnie et al., 1992) and glutamatergic synaptic transmission in the PFC (Liu et al., 2015). Interestingly, the facilitatory effect of CRF onto PFC output neurons is mediated by activation of CRF type 1 receptor (CRFR1) and requires intact BLA inputs (Shekhar et al., 2005).
While CRFR1 is widely expressed throughout the brain (De Souza et al., 1985; Lovenberg et al., 1995; Van Pett et al., 2000) and its action has been well documented (Liu et al., 2004, 2005; Jaferi and Bhatnagar, 2007; Miguel et al., 2014, Hupalo et al., 2016; Uribe-Mariño et al., 2016), the distribution of CRF type 2 receptors (CRFR2) is more discrete and its functional impact remains unclear (Van Pett et al., 2000; Guan et al., 2014). For instance, CRFR2 modulation of synaptic transmission through diverse mechanisms has been described in the amygdala (Liu et al., 2004; Fu et al., 2008), hippocampus (Pollandt et al., 2006), and ventral tegmental area (Williams et al., 2014). However, the role of CRFR2 in the synaptic transmission in the PFC is not known. Thus, the goal of the present study is to determine the expression of CRFR2 in PFC synaptic terminals originated from the BLA and its role in modulating BLA transmission to the PFC. To address these questions, we utilized biochemical and histochemical approaches in combination with in vivo microdialysis and electrophysiological measures to determine whether the expression of CRFR2 is functionally positioned to limit the strength of BLA transmission via inhibition of glutamate release in the PFC.
Materials and Methods
Animals
Male Sprague-Dawley rats (270–300 g) were used. The experimental protocols were approved by the Bioethical Committee of the Faculty of Biological Sciences of Pontificia Universidad Católica de Chile. Electrophysiological experiments were performed following the USPHS Guide for Care and Use of Laboratory Animals and were approved by the Rosalind Franklin University Institutional Animal Care and Use Committee for the care and use of laboratory animals.
Preparation of PFC Synaptosomes
Purified synaptosomes of PFC, devoid of the postsynaptic density, were prepared on a discontinuous Percoll gradient as described (Rodrigues et al., 2005; Ciruela et al., 2006; Slater et al., 2016). After decapitation, the PFC, including both infra- and prelimbic regions of the PFC, according to the Atlas of Paxinos and Watson (1986) was dissected out of coronal slices of 4 animals per sample. The coordinates to dissect PFC were AP: 3.7–2.7 mm from bregma, ML: 1.0 mm, and DV: 3.0–6.0 mm from the skull. These animals were exclusively used for synaptosomal preparation. The extracted tissue was placed in a glass Potter homogenizer with 10 mM HEPES, 320 mM sucrose, and 3 mM EDTA, pH 7.4, and centrifuged at 1000 g for 10 minutes at 4°C. The supernatant was centrifuged at 17 000 g for 20 minutes at 4°C. The obtained pellet was resuspended and centrifuged in a Percoll gradient (PVP-silica colloid; Sigma Aldrich, St Louis, MO) at 15 000 g for 20 minutes at 4°C. The synaptosomal fraction was dissolved (in an equal volume to the fraction obtained) in 320 mM sucrose solution for immunofluorescence. The synaptosomal protein concentration was determined by Micro BCA Protein Assay Kit (Thermo Fisher).
Immunofluorescence in PFC Synaptosomes
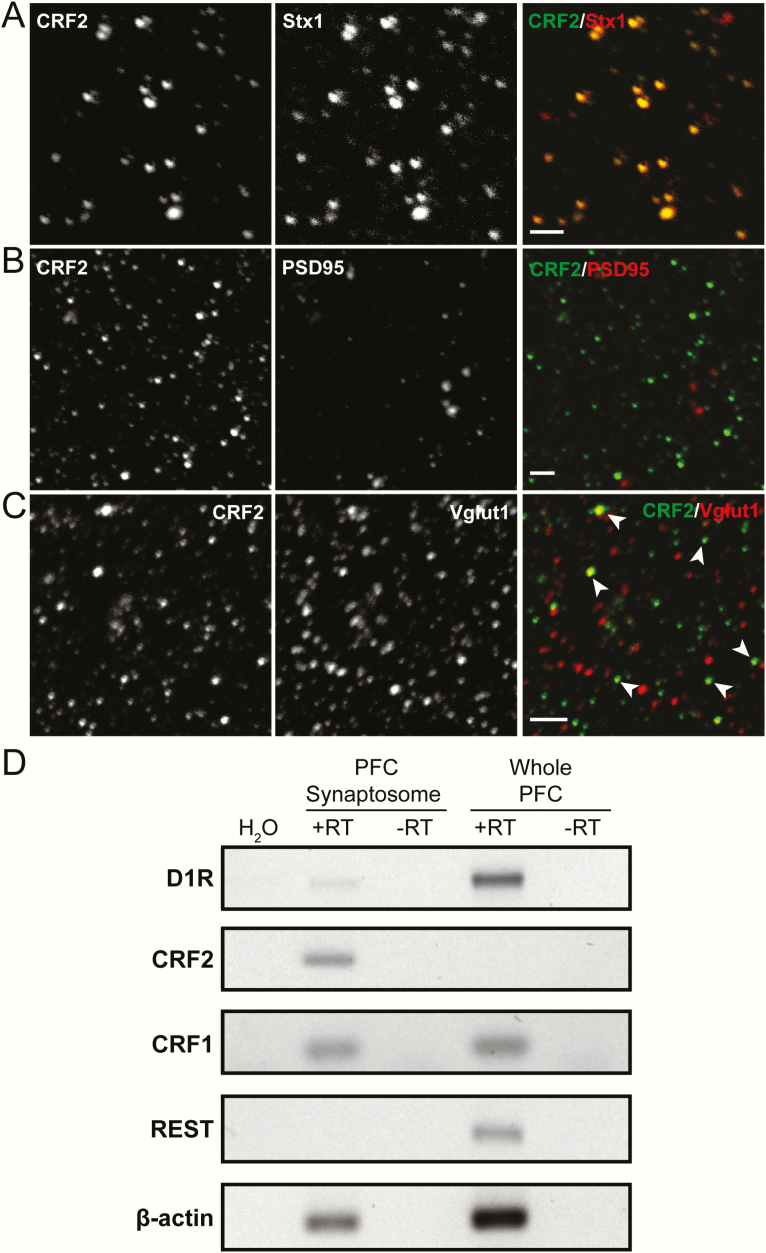
Immunofluorescence in synaptosomes was performed as previously described (Ciruela et al., 2006; Slater et al., 2016). Synaptosomes from PFC (15 μg of synaptosomal protein) were seeded on coverslips coated with poly-L-lysine (Sigma Aldrich) and fixed with 4% PFA/10% sucrose for 15 minutes, permeabilized with 0.2% Triton X-100, and incubated for 1 hour with blocking solution (4% bovine serum albumin in phosphate buffered saline). The synaptosomes were incubated 1 hour at room temperature with primary antibodies and thereafter for 1 hour with the secondary antibodies (1:200; Invitrogen). The primary antibodies used were mouse anti-syntaxin 1 (1:2000; MAB 336; Millipore), mouse anti-PSD95 (1:1000; 75-028; UC Davis/NIH NeuroMab Facility), goat anti-CRFR2 (1:200; SC-1826; Santa Cruz Biotechnology), and mouse anti-vesicular glutamate transporter 1 (1:500; 75-066; UC Davis/NIH NeuroMab Facility). The images were captured with a 100× objective in a confocal microscope (Olympus, Fluoview FLV1000) and analyzed with FLUOVIEW v6.0 software. Each synaptosomal preparation was obtained from 4 animals, and photographs for quantification were taken with 60× from 8 different subareas in each coverslip.
Biotinylated Dextran Amine (BDA) Injections and Immunohistochemistry
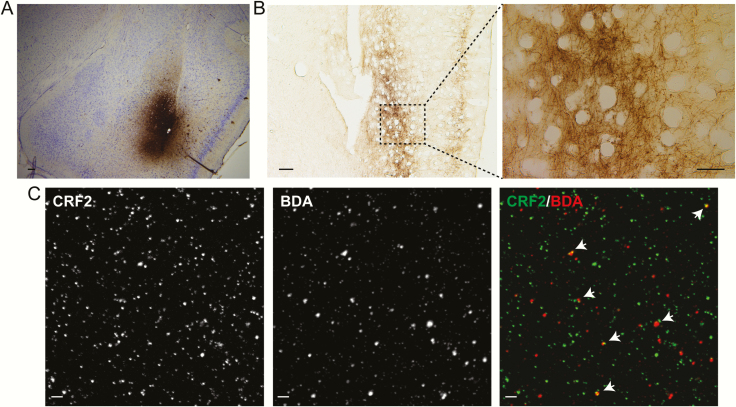
Rats (4 rats for each sample) were anesthetized with isofluorane (4% for induction and 1–1.5% for maintenance) and stereotaxically injected with 1 μL of 10% BDA 10 kDa (Thermo Fisher) at a rate of 0.1 μL/30 s with a 33-G Hamilton syringe in the BLA (AP = 2.8 mm, ML = 4.8 mm, and DV = 8.2 mm from Bregma). Seven days after the surgery, the animals were decapitated to prepare synaptosomes from the PFC or perfused for immunohistochemistry. Animals were perfused intracardially with 4% paraformaldehyde (PFA), and brains were postfixed in 4% PFA overnight and then kept in 20% sucrose for 48 hours. Brains were sliced in 30-μm coronal sections with a cryostat (Leica CM 1510, Wetzlar, Germany). BDA immunohistochemistry was performed as described (55). Coronal sections were mounted on gelatin-coated slides and coverslip using Entellan (Merck), and the images were captured in an epifluorescence microscope (Nikon, FLV1000). In the case of PFC synaptosomes prepared from BDA-injected animals, they were processed for immunofluorescence using the secondary antibody Streptavidin-AlexaFluor647 (1:200; Thermo Fisher).
RT-PCR
Total RNA from whole PFC (1 rat per sample) and synaptosomal preparation (4 rats per sample) from PFC were isolated using Trizol reagent (Invitrogen). The reverse transcriptase enzyme used was the RevertAid Reverse Transcriptase (Thermo Fisher). The primers used are listed in Table 1. Each PCR program was carried out with Platinum Taq DNA Polymerase (Invitrogen) for 30 to 35 cycles, and the PCR product sizes were evaluated by agarose gel staining with SYBR Safe DNA gel stain (ThermoFisher) and confirmed by using the Macrogene sequencing service (Macrogene, Seoul, Korea).
Table 1.
Primers used for RT-PCR
| Gene | Forward primer | Reverse primer |
|---|---|---|
| D1R | 5´-CATGCCAAGAATTGCCAGACC 3´ | 5´-CTCTTCCTTCTTCAGGTCCTC-3´ |
| CRFR2 | 5´-AAGAGCTGCTTTTGGACGGCT-3´ | 5´-GGCGATTCGGTAATGCAGGTC-3´ |
| CRFR1 | 5´-TCCACCTCCCTTCAGGATCA-3´ | 5´-TGCAGGCCAGAAACATTGC-3´ |
| REST | 5´-TACACGGCACACCTGAAGCACC-3´ | 5´-TTGCGTGTCGGGTCACTTCGTG-3´ |
| β-Actin | 5´-CACCCGCGAGTA CAACCTTC-3´ | 5´-CCCATACCCACCATCACACC-3´ |
RC-PCR, Reverse transcription polymerase chain reaction.
In Vivo Microdialysis
Animals (total of 17 rats exclusively used for in vivo microdialysis experiments) were anesthetized with 8% chloral hydrate (400 mg/kg, i.p.) and placed in a stereotaxic apparatus. The body temperature was maintained by an electrical blanket at 37°C, and the anesthesia was maintained at 0.8 µL/min by an electrical infusion pump (BASi). Microdialysis probes, 2 mm long (MAB 2.14.2, Microbiotech), were implanted in the PFC (AP = 3.2 mm, ML = 0.7 mm, and DV = 5.0 mm from Bregma), and CMA 11 (CMA Microdialysis AB) were implanted in the BLA (AP = 2.8 mm, ML = 4.8 mm, and DV = 8.2 mm from Bregma). The microdialysis protocol used was previously described (Vega-Quiroga et al., 2018). Artificial cerebrospinal fluid (aCSF) was perfused through the microdialysis probes at 2 µL/min. After the stabilization period (90 minutes), samples were collected every 10 minutes from the PFC. At the time indicated, 70 mM K+-aCSF was perfused through the microdialysis probe in the BLA for 10 minutes. Antisauvagine-30 (1 µM, Tocris), CP154,526 (1 µM, Tocris), or kynurenic acid (1 mM, Sigma) was perfused intra-BLA, as indicated in the figures. At the end of the microdialysis protocol, animals were decapitated and the brains were removed and stored in 4% PFA for verification of the microdialysis probe placements. The determination of glutamate was performed using HPLC-fluorometric determination, as previously described (Sotomayor-Zárate et al., 2010).
In Vivo Recordings of BLA-Evoked Local Field Potential (LFP) Responses in Medial PFC
All LFP recordings in the medial PFC were obtained using a concentric bipolar electrode (SNE-100 × 50 mm; Rhodes Medical Instruments Inc., Summerland, CA) attached to a 28-gauge cannula to enable local administration of CRF receptor antagonists (aCSF as control) while stimulating the BLA, as previously described (Caballero et al., 2014; Thomases et al., 2014). Briefly, rats were anesthetized with 8% choral hydrate (400 mg/kg, i.p.), fixed in a stereotaxic apparatus (ASI Instruments, Warren, MI), and maintained at 37–38°C (Physitemp Instruments, Clifton, NJ) while a steady supplementary level of anesthesia (400 μL/h, i.p.) was delivered throughout the recording session. The intensity of BLA stimulation was chosen from the minimal current (0.5–0.7 mA range) needed to elicit a reliable LFP response with <15% variability in slope and amplitude. Typically, single evoked pulses (300-μs square pulses) were delivered every 15 seconds through a computer-controlled pulse generator (Master-8 AMPI, Jerusalem, Israel) during baseline recording, and changes in the slope of LFP potentiation were assessed following a protocol of high-frequency stimulation (HFS; 50 pulses at 100 Hz/15 s × 4) delivered into the BLA. Single PFC infusions of 1.0 μL aCSF alone or in combination with Antisauvagine-30 (300 nM) or CP154,526 (1 μM) were delivered at a rate of 0.1 μL/min prior to BLA HFS. The chemical composition of the aCSF solution was (in mM): 122.5 NaCl, 3.5 KCl, 25 NaHCO3, 1 NaH2PO4, 2.5 CaCl2, 1 MgCl2, 20 glucose, 1 ascorbic acid (pH: 7.40, 295–305 mOsm). All time-course plots summarizing the effects of the HFS shown in the figures were created using a bin size window of 2 minutes (i.e., mean slope value from 8 field responses per data point). At the end of the recording sessions, animals were killed and the brains removed for histological assessment of the recording and stimulating sites, as previously described (Thomases et al., 2014).
Statistical Analyses
Statistical analyses were performed with the statistical software GraphPad Prism 6 (GraphPad Software). The data are expressed as the mean ± SEM. All plots in the LFP experiments are the normalization of the HFS drive LFP in the PFC every 2 minutes. The microdialysis and HFS-driving LFP experiments were analyzed with 1-way ANOVA or 2-way ANOVA, followed by Tukey post-hoc test.
Results
CRFR2 and Its mRNA Are Present in PFC Glutamatergic Terminals From BLA
We first determined whether CRFR2 distribution in the PFC is presynaptic using a synaptosomal preparation devoid of postsynaptic elements (Rodrigues et al., 2005). Immunofluorescence conducted in PFC synaptosomes enriched in presynaptic elements revealed the presence of CRFR2 (Fig. 1A–C), as shown by co-staining with syntaxin 1 (presynaptic marker) (Fig. 1A), and the staining for CRFR2 did not colocalize with PSD95 (postsynaptic marker) (Fig. 1B). Overall, 85.7% ± 7.3% of PFC synaptosomes bearing CRFR2 were positive for syntaxin 1. In addition, the 62% ± 7.8% of PFC synaptosomes positive for CRFR2 stain were positive for the vesicular glutamate transporter 1 (glutamatergic neuronal marker) (Fig. 1C). These results indicate that CRFR2 is present in glutamatergic terminals in the PFC.
Figure 1.
CRF type 2 receptor (CRFR2) are expressed in PFC presynaptic terminals. (A–C) Confocal images showing immune detection of CRFR2 in PFC synaptosomes, devoid of postsynaptic elements. (A) Immunofluorescence detection of CRFR2 (green) in PFC presynaptic terminals (identified as syntaxin 1 positive; red) (scale bar = 2 µm). (B) Immunofluorescence detection of CRFR2 (green) in PFC synaptosomes with a postsynaptic terminal marker (identified as PSD95 positive; red) (scale bar = 2 µm). (C) Immunofluorescence detection of CRFR2 (green) in PFC glutamate presynaptic terminals (identified as vesicular glutamate transporter 1 positive; red) (scale bar = 5 µm). The arrows indicate some of the colocalized synaptosomes in the image. (D) RT-PCR analysis for D1R, CRFR2, CRFR1, REST, and β-actin in RNA extracted from PFC synaptosomes and whole PFC tissue.
Next, we asked whether mRNA for CRFR2 could be found in the PFC synaptic terminals. Data from mRNA extracted from PFC synaptosomes (devoid of postsynaptic elements) and from whole PFC tissue indicate that CRFR2 mRNA is detectable only in the PFC synaptosomal preparation (Fig. 1D). It is worth mentioning that the synaptosomal fraction is a small percentage of the whole PFC preparation, explaining why CRFR2 mRNA was observed only in the synaptosomal preparation and not in the whole PFC preparation. Instead, CRFR1 mRNA was found in both PFC synaptosomes and whole PFC tissue. To further evaluate the quality of the mRNA samples, we analyzed the presence of mRNA for dopamine D1 receptor and RE1-silencing transcription factor (Chong et al., 1995; Palm et al., 1998). The mRNA for dopamine D1 receptor was present in both preparations but in higher amounts in whole PFC tissue samples. RE1-silencing transcription factor mRNA was present only in whole PFC tissue, indicating that the synaptosomal preparation is clean of nonsynaptosomal elements. These results indicate that CRFR2 is expressed exclusively in PFC synaptic terminals. Finally, we injected the anterograde tracer BDA into the BLA to determine whether PFC glutamatergic terminals bearing CRFR2 originate from the BLA (Fig. 2A–B). Overall, 11.6% ± 0.6% of PFC synaptosomes prepared from BDA-injected animals (n = 3) showed immunoreactivity for CRF2 (Fig. 2C). Together, these results indicate that some CRFR2 are expressed in PFC glutamatergic terminals originated in the BLA.
Figure 2.CRF type 2 receptor (CRFR2).
are expressed in PFC synaptic terminals originated in BLA. (A) Coronal brain sections showing the injection site of BDA in BLA (scale bar = 100 µm). (B) Coronal brain sections showing labeled BDA fibers in PFC (scale bar = 200 µm). (C) Confocal images of PFC synaptosomes obtained from a BDA-injected animal and subjected to immunofluorescence for CRFR2 (green) and BDA (red) (scale bar = 2 µm). Arrows depict double-labeled synaptosomes.
CRF2 Negatively Controls Glutamate Release in the BLA-PFC Circuit
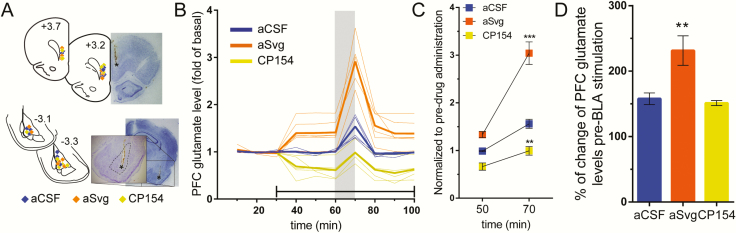
To determine the role of CRFR2 in PFC glutamatergic terminals originated in the BLA, we conducted in vivo microdialysis measure in the PFC and assessed how the local infusion of the CRFR2 antagonist antisauvagine-30 (aSvg, 1 µM) alters glutamate levels elicited following BLA stimulation (Fig. 3). We found that PFC infusion of aSvg significantly increased basal glutamate levels (Fig. 3B–C), an effect that was further potentiated following BLA stimulation (Fig. 3B, D). In contrast, PFC infusion of the CRFR1 antagonist CP154,526 (CP154, 1 µM) significantly reduced basal glutamate levels (Fig. 3B–C), yet the extent of glutamate increase following BLA stimulation resembles that of aCSF controls (Fig. 3B, D). Collectively, these results indicate that local prefrontal CRFR1 and CRFR2 contribute to regulate basal glutamate levels, but only the CRFR2 apparently exerts an inhibitory control on BLA-evoked glutamate release in the PFC.
Figure 3.
PFC infusion of the CRF type 2 receptor (CRFR2) antagonist aSvg-30 (aSvg) enhanced BLA-induced glutamate release in the PFC. (A) Brain coronal sections showing the placement of the microdialysis probes in BLA (−3.1 mm to −3.3 mm from bregma) (bottom) and PFC (3.7 mm to 3.2 mm from bregma) (top). (B) Measurement of PFC extracellular glutamate levels using in vivo microdialysis. The vertical gray bar indicates the time of BLA stimulation with local perfusion of 70 mM K+-aCSF, and the horizontal black line indicates the time of intra-PFC infusion of the antagonists. PFC glutamate levels in the presence of aCSF (n = 6), 1 µM of the CRFR2 antagonist aSvg (n = 6), and 1 µM of the CRFR1 antagonist CP154,526 (CP154, n = 5). Changes in PFC glutamate levels were normalized to the first 3 values for each condition. (C) Normalized glutamate levels to the pre-drug infusion period (20 minutes) summarizing the mean values obtained in the PFC before BLA stimulation (50 minutes) and 10 minutes post-BLA stimulation (70 minutes; gray area in B). So, 2-way ANOVA revealed a main effect of treatment (F2,14 = 60.6, P < .0001), a main effect of time (F1,14 = 80.8, P < .0001), and treatment × time interaction (F2,14 = 19.6, P < .0001; ***P < .0005, **P < .005 vs aCSF, Tukey post-hoc test). (D) Effects of aSvg and CP154 infusion on BLA-induced PFC glutamate increase. One-way ANOVA revealed a main effect of BLA stimulation (F2,14 = 8.9, P = .003; **P < .005 vs aCSF, Tukey post-hoc test).
Prefrontal CRFR2 Signaling Limits Afferent Transmission Originated From BLA in Vivo
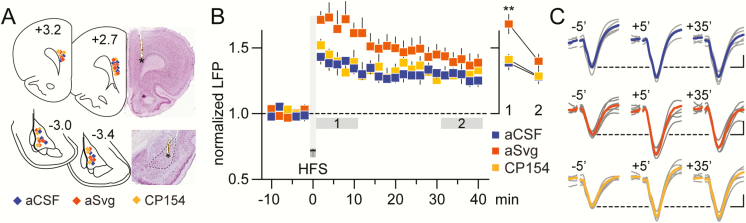
We next examined the effects of PFC infusion of the CRFR2 antagonist aSvg (300 nM) on prefrontal LFP responses elicited from the BLA following a protocol of HFS (Fig. 4) (Caballero et al., 2014). As shown previously, HFS of the BLA induces a sustained potentiation of LFP responses in the PFC (Caballero et al., 2014; Thomases et al., 2014). Relative to aCSF controls, PFC infusion of aSvg markedly facilitated the amplitude of LFP potentiation (Fig. 4B–C). However, PFC infusion of the CRFR1 antagonist CP154 failed to disrupt the pattern of LFP potentiation (Fig. 4B–C). Collectively, these results reveal that CRFR2 signaling is functionally positioned in the PFC to limit the gain of BLA transmission, whereas CRFR1 does not play a major role in this process.
Figure 4.
PFC blockade of CRF type 2 receptor (CRFR2) enhanced afferent transmission originated from the BLA. (A) Brain coronal sections showing the location of BLA-stimulating electrode placement (−3.0 mm to −3.0 mm from bregma) (bottom) and location of PFC recording sites (3.2 mm to 2.7 mm from bregma) (top) for this cohort of experiments. Inset images are coronal sections showing examples of the anatomical location (asterisks) of the recording (top: PFC) and stimulating (bottom: BLA) electrodes. (B) Typically, high-frequency stimulation (HFS; 4 trains of 50 pulses at 100 Hz/15 s) of the BLA elicits robust facilitation of BLA-evoked local field potential (LFP) responses in the PFC. Relative to aCSF controls (n = 6), PFC infusion of the CRFR2 antagonist aSvg (300 nM, n = 8) further enhanced the response of BLA-evoked LFP in the PFC. Yet, PFC infusion of the CRFR1 antagonist CP154 (1 μM, n = 7) did not elicit any apparent changes in the pattern of BLA-evoked LFP facilitation. So, 2-way ANOVA revealed main effects of treatment (F2,36 = 9.51, P < .001) and time (F1,36 = 14.53, P < .001; first (1) vs last (2) 10 minutes post-HFS; **P < .005 vs aCSF or CP154, Tukey post-hoc test). (C) Example traces of BLA-evoked LFP recorded in the PFC at baseline (−5’) and after HFS at 2 time points (+5’ and +35’) to illustrate the enhanced facilitation of the response following PFC infusion of the CRFR2 antagonist aSvg (calibration bars = 5 mV/25 ms).
Discussion
We found compelling evidence revealing for the first time, to our knowledge, that CRFR2 is expressed in BLA afferents projecting to the PFC. Our results also show that CRFR2 is functionally positioned to limit the strength of BLA glutamatergic transmission to the PFC, possibly through a presynaptic inhibition of glutamate release.
CRF receptors are differentially distributed in the brain (Henckens et al., 2016), with CRFR2 displaying a more discrete expression than that of CRFR1. In the PFC, we observed that CRFR2 is expressed in glutamatergic terminals originated from the BLA. Similar presynaptic expression of CRFR2 has been found in distinct brain areas (Lawrence et al., 2002; Fu and Neugebauer, 2008), suggesting that changes in CRFR2 signaling may impact the strength of synaptic transmission in a region-specific manner. Our results also indicate the presence of CRFR2 mRNA in PFC synaptosomes, suggesting that CRFR2 expression could be locally regulated, as it has been shown for tyrosine-hydroxylase (Jiménez et al., 2002; Gervasi et al., 2016).
Prefrontal blockade of CRFR2, but not CRFR1, enhanced BLA-evoked glutamate release and LFP potentiation in the PFC. These results indicate CRFR2 signaling in the PFC plays a critical role in limiting the strength of glutamatergic transmission originated from the BLA. Although this inhibitory effect of CRFR2 in the PFC has not been previously reported, our results are consistent with published data showing an inhibitory role of CRFR2 signaling in the regulation of synaptic transmission. For example, CRFR2 activation decreases the amplitude of glutamatergic transmission in the lateral septum (Liu et al., 2004), and its antagonism increases the frequency of excitatory postsynaptic currents in the centro-lateral amygdala (Fu and Neugebauer, 2008). CRFR2 activation also diminishes the gain of glutamatergic transmission into ventral tegmental area dopamine neurons (Williams et al., 2014). However, CRFR2 can also facilitate glutamatergic transmission as revealed by its effects on the amplitude of excitatory postsynaptic currents in the central amygdala (Liu et al., 2004; Pollandt et al., 2006) and NMDA-mediated postsynaptic currents in dopamine neurons (Hahn et al., 2009). Collectively, CRFR2 signaling can exert both inhibitory and excitatory effects on glutamatergic transmission in a synapse-specific manner, yet the distinct mechanisms mediating such opposing actions remain unclear.
CRF receptors are known to be activated by CRF and urocortins (Bale and Vale, 2004). While CRFR1 binds to both CRF and urocortin I with high affinity (Chen et al., 1993, Perrin et al., 1993; Vaughan et al., 1995), CRFR2 binds with high affinity to urocortins I-III (Perrin et al., 1995). Both CRF and urocortins have been shown to modulate synaptic transmission in different brain areas (Gallagher et al., 2008), including the PFC, BLA, and lateral septum (Liu et al., 2004, 2005; Orozco-Cabal et al., 2008). Thus, changes in urocortin levels are likely to impact CRFR2 signaling and contribute to fine-tuning PFC output and behavior by modulating the activity of its major afferents, including inputs from the BLA. It has been shown that CRF is present in PFC neurons (Swanson et al., 1983; reviewed in Deussing and Chen, 2018), mainly in GABAergic interneurons (Yan et al. 1998). Urocortin I has also been found in the PFC (Bittencourt et al., 1999). In addition, there are other possible sources of CRF in PFC such as the input from central amygdala neurons that express CRF (Merali et al, 2008). Further studies are warranted to determine which endogenous ligand is responsible for driving CRFR2 signaling and limiting prefrontal response to BLA inputs.
In summary, CRFR2 in the PFC is functionally positioned to limit the gain of glutamatergic transmission originated from the BLA, which in turn suggests a more complex role of BLA-PFC pathway in emotion and other CRF-related behaviors. This inhibitory action of CRFR2 may provide a mechanism by which BLA transmission is regulated in the PFC during emotional activation. Further studies should address how modulation of BLA-PFC synapses by CRFR2 shapes decision-making processing in response to changes in emotional states (Uribe-Mariño et al., 2016; Moghaddam, 2016; Sun et al., 2019).
Acknowledgments
We thank Dr Alon Chen for providing insights on the synaptosomal mRNA detection method and Dr Annabell Segarra for helpful comments. The authors acknowledge the services provided by UC CINBIOT Animal Facility funded by PIA CONICYT* ECM-07.
This work was supported by Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT grants 1150244 and 1191274) to K.G. and National Institutes of Health grant R01-MH086507 to K.Y.T. H.E.Y. was supported by a doctoral fellowship from Comisión Nacional de Investigación Científica y Tecnológica de Chile (CONICYT).
Statement of InterestNone.
References
- Bale TL, Vale WW (2004) CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 44:525–557. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Vaughan J, Arias C, Rissman RA, Vale WW, Sawchenko PE (1999) Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J Comp Neurol 415:285–312. [PubMed] [Google Scholar]
- Caballero A, Thomases DR, Flores-Barrera E, Cass DK, Tseng KY (2014) Emergence of GABAergic-dependent regulation of input-specific plasticity in the adult rat prefrontal cortex during adolescence. Psychopharmacology (Berl) 231:1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Lewis KA, Perrin MH, Vale WW (1993) Expression cloning of a human corticotropin-releasing-factor receptor. Proc Natl Acad Sci U S A 90:8967–8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong J, Tapia-Ramírez J, Kim S, Toledo-Aral JJ, Zheng Y, Boutros MC, Altshuller YM, Frohman MA, Kraner SD, Mandel G (1995) REST: a mammalian silencer protein that restricts sodium channel gene expression to neurons. Cell 80:949–957. [DOI] [PubMed] [Google Scholar]
- Ciruela F, Casadó V, Rodrigues RJ, Luján R, Burgueño J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortés A, Canela EI, López-Giménez JF, Milligan G, Lluis C, Cunha RA, Ferré S, Franco R (2006) Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J Neurosci 26:2080–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ (2001) The amygdala: vigilance and emotion. Mol Psychiatry 6:13–34. [DOI] [PubMed] [Google Scholar]
- De Souza EB, Insel TR, Perrin MH, Rivier J, Vale WW, Kuhar MJ (1985) Corticotropin-releasing factor receptors are widely distributed within the rat central nervous system: an autoradiographic study. J Neurosci 5:3189–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deussing JM, Chen A (2018) The corticotropin-releasing factor family: physiology of the stress response. Physiol Rev 98:2225–2286. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Tse MT (2007) Dopaminergic regulation of inhibitory and excitatory transmission in the basolateral amygdala-prefrontal cortical pathway. J Neurosci 27:2045–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Neugebauer V (2008) Differential mechanisms of CRF1 and CRF2 receptor functions in the amygdala in pain-related synaptic facilitation and behavior. J Neurosci 28:3861–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher JP, Orozco-Cabal LF, Liu J, Shinnick-Gallagher P (2008) Synaptic physiology of central CRH system. Eur J Pharmacol 583: 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R, Vouimba RM, Baudry M, Thompson RF (1999) The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature 402:294–296. [DOI] [PubMed] [Google Scholar]
- Gervasi NM, Scott SS, Aschrafi A, Gale J, Vohra SN, MacGibeny MA, Kar AN, Gioio AE, Kaplan BB (2016) The local expression and trafficking of tyrosine hydroxylase mRNA in the axons of sympathetic neurons. RNA 22:883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Helmstetter FJ (2010) Trace and contextual fear conditioning require neural activity and NMDA receptor-dependent transmission in the medial prefrontal cortex. Learn Mem 17:289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X, Wan R, Zhu C, Li S (2014) Corticotropin-releasing factor receptor type-2 is involved in the cocaine-primed reinstatement of cocaine conditioned place preference in rats. Behav Brain Res 258:90–96. [DOI] [PubMed] [Google Scholar]
- Hahn J, Hopf FW, Bonci A (2009) Chronic cocaine enhances corticotropin-releasing factor-dependent potentiation of excitatory transmission in ventral tegmental area dopamine neurons. J Neurosci 29:6535–6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Menzaghi F, Merlo Pich E, Britton KT, Koob GF (1995) The role of CRF in behavioral aspects of stress. Ann N Y Acad Sci 771:92–104. [DOI] [PubMed] [Google Scholar]
- Henckens MJ, Deussing JM, Chen A (2016) Region-specific roles of the corticotropin-releasing factor-urocortin system in stress. Nat Rev Neurosci 17:636–651. [DOI] [PubMed] [Google Scholar]
- Hervig ME, Jensen NCH, Rasmussen NB, Rydbirk R, Olesen MV, Hay-Schmidt A, Pakkenberg B, Aznar S (2017) Involvement of serotonin 2A receptor activation in modulating medial prefrontal cortex and amygdala neuronal activation during novelty-exposure. Behav Brain Res 326:1–12. [DOI] [PubMed] [Google Scholar]
- Hupalo S, Berridge CW (2016) Working memory impairing actions of Corticotropin-Releasing Factor (CRF) neurotransmission in the prefrontal cortex. Neuropsychopharmacology 41:2733–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaferi A, Bhatnagar S (2007) Corticotropin-releasing hormone receptors in the medial prefrontal cortex regulate hypothalamic-pituitary-adrenal activity and anxiety-related behavior regardless of prior stress experience. Brain Res 1186:212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez CR, Eyman M, Lavina ZS, Gioio A, Li KW, van der Schors RC, Geraerts WP, Giuditta A, Kaplan BB, van Minnen J (2002) Protein synthesis in synaptosomes: a proteomics analysis. J Neurochem 81:735–744. [DOI] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC (1999) A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res 848:141–152. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Krstew EV, Dautzenberg FM, Rühmann A (2002) The highly selective CRF(2) receptor antagonist K41498 binds to presynaptic CRF(2) receptors in rat brain. Br J Pharmacol 136:896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yu B, Neugebauer V, Grigoriadis DE, Rivier J, Vale WW, Shinnick-Gallagher P, Gallagher JP (2004) Corticotropin-releasing factor and Urocortin I modulate excitatory glutamatergic synaptic transmission. J Neurosci 24:4020–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yu B, Orozco-Cabal L, Grigoriadis DE, Rivier J, Vale WW, Shinnick-Gallagher P, Gallagher JP (2005) Chronic cocaine administration switches corticotropin-releasing factor2 receptor-mediated depression to facilitation of glutamatergic transmission in the lateral septum. J Neurosci 25:577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Ota KT, Dutheil S, Duman RS, Aghajanian GK (2015) Ketamine Strengthens CRF-activated amygdala inputs to basal dendrites in mPFC layer V pyramidal cells in the prelimbic but not infralimbic subregion, a key suppressor of stress responses. Neuropsychopharmacology 40:2066–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, Oltersdorf T (1995) Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci U S A 92:836–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z, Anisman H, James JS, Kent P, Schulkin J (2008) Effects of corticosterone on corticotrophin-releasing hormone and gastrin-releasing peptide release in response to an aversive stimulus in two regions of the forebrain (central nucleus of the amygdala and prefrontal cortex). Eur J Neurosci 28:165–172. [DOI] [PubMed] [Google Scholar]
- Miguel TT, Gomes KS, Nunes-de-Souza RL (2014) Tonic modulation of anxiety-like behavior by corticotropin-releasing factor (CRF) type 1 receptor (CRF1) within the medial prefrontal cortex (mPFC) in male mice: role of protein kinase A (PKA). Horm Behav 66:247–256. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ (2012) Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol 63:129–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B. (2016) The complicated relationship of stress and prefrontal cortex. Biol Psychiatry 80:728–729. [DOI] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE (1995) Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci 109:681–688. [DOI] [PubMed] [Google Scholar]
- Orozco-Cabal L, Liu J, Pollandt S, Schmidt K, Shinnick-Gallagher P, Gallagher JP (2008) Dopamine and corticotropin-releasing factor synergistically alter basolateral amygdala-to-medial prefrontal cortex synaptic transmission: functional switch after chronic cocaine administration. J Neurosci 28:529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm K, Belluardo N, Metsis M, Timmusk T (1998) Neuronal expression of zinc finger transcription factor REST/NRSF/XBR gene. J Neurosci 18:1280–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. 2nd ed.Sydney, Australia:Academic Press. [Google Scholar]
- Perrin M, Donaldson C, Chen R, Blount A, Berggren T, Bilezikjian L, Sawchenko P, Vale W (1995) Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc Natl Acad Sci U S A 92:2969–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin MH, Donaldson CJ, Chen R, Lewis KA, Vale WW (1993) Cloning and functional expression of a rat brain corticotropin releasing factor (CRF) receptor. Endocrinology 133:3058–3061. [DOI] [PubMed] [Google Scholar]
- Pollandt S, Liu J, Orozco-Cabal L, Grigoriadis DE, Vale WW, Gallagher JP, Shinnick-Gallagher P (2006) Cocaine withdrawal enhances long-term potentiation induced by corticotropin-releasing factor at central amygdala glutamatergic synapses via CRF, NMDA receptors and PKA. Eur J Neurosci 24:1733–1743. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Fernhout BJ, Shinnick-Gallagher P (1992) Differential actions of corticotropin releasing factor on basolateral and central amygdaloid neurones, in vitro. J Pharmacol Exp Ther 263:846–858. [PubMed] [Google Scholar]
- Rodrigues RJ, Alfaro TM, Rebola N, Oliveira CR, Cunha RA (2005) Co-localization and functional interaction between adenosine A(2A) and metabotropic group 5 receptors in glutamatergic nerve terminals of the rat striatum. J Neurochem 92:433–441. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, LeDoux JE, Sapolsky RM (2009) The influence of stress hormones on fear circuitry. Annu Rev Neurosci 32:289–313. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Truitt W, Rainnie D, Sajdyk T (2005) Role of stress, corticotrophin releasing factor (CRF) and amygdala plasticity in chronic anxiety. Stress 8:209–219. [DOI] [PubMed] [Google Scholar]
- Slater PG, Noches V, Gysling K (2016) Corticotropin-releasing factor type-2 receptor and corticotropin-releasing factor-binding protein coexist in rat ventral tegmental area nerve terminals originated in the lateral hypothalamic area. Eur J Neurosci 43:220–229. [DOI] [PubMed] [Google Scholar]
- Sotomayor-Zárate R, Araya KA, Pereira P, Blanco E, Quiroz G, Pozo S, Carreño P, Andrés ME, Forray MI, Gysling K (2010) Activation of GABA-B receptors induced by systemic amphetamine abolishes dopamine release in the rat lateral septum. J Neurochem 114:1678–1686. [DOI] [PubMed] [Google Scholar]
- Sun T, et al. (2019) Basolateral amygdala input to the medial prefrontal cortex controls obsessive-compulsive disorder-like checking behavior. Proc Natl Acad Sci U S A 116:3799–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW (1983) Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 36:165–186. [DOI] [PubMed] [Google Scholar]
- Tejeda HA, Hanks AN, Scott L, Mejias-Aponte C, Hughes ZA, O’Donnell P (2015) Prefrontal cortical kappa opioid receptors attenuate responses to amygdala inputs. Neuropsychopharmacology 40:2856–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomases DR, Cass DK, Meyer JD, Caballero A, Tseng KY (2014) Early adolescent MK-801 exposure impairs the maturation of ventral hippocampal control of basolateral amygdala drive in the adult prefrontal cortex. J Neurosci 34:9059–9066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribe-Mariño A, Gassen NC, Wiesbeck MF, Balsevich G, Santarelli S, Solfrank B, Dournes C, Fries GR, Masana M, Labermeier C, Wang XD, Hafner K, Schmid B, Rein T, Chen A, Deussing JM, Schmidt MV (2016) Prefrontal cortex corticotropin-releasing factor receptor 1 conveys acute stress-induced executive dysfunction. Biol Psychiatry 80:743–753. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J (1981) Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 213:1394–1397. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE (2000) Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 428:191–212. [DOI] [PubMed] [Google Scholar]
- Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C (1995) Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature 378:287–292. [DOI] [PubMed] [Google Scholar]
- Vega-Quiroga I, Yarur HE, Gysling K (2018) Lateral septum stimulation disinhibits dopaminergic neurons in the antero-ventral region of the ventral tegmental area: Role of GABA-A alpha 1 receptors. Neuropharmacology 128:76–85. [DOI] [PubMed] [Google Scholar]
- Williams CL, Buchta WC, Riegel AC (2014) CRF-R2 and the heterosynaptic regulation of VTA glutamate during reinstatement of cocaine seeking. J Neurosci 34:10402–10414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XX, Baram TZ, Gerth A, Schultz L, Ribak CE (1998) Co-localization of corticotropin-releasing hormone with glutamate decarboxylase and calcium-binding proteins in infant rat neocortical interneurons. Exp Brain Res 123:334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]