Abstract
Introduction
Chronic trauma repair is an important issue affecting people’s healthy lives. Thermo-sensitive hydrogel is injectable in situ and can be used to treat large-area wounds. In addition, antioxidants play important roles in promoting wound repair.
Methods
The purpose of this research was to prepare a novel thermo-sensitive hydrogel-poly(N-isopropyl-acrylamide)/poly(γ-glutamic acid) (PP) loaded with superoxide dismutase (SOD) to improve the effect for trauma treatment. The micromorphology of the hydrogel was observed by scanning electron microscope and the physical properties were measured. The biocompatibility of hydrogel was evaluated by MTT experiment, and the effect of hydrogel on skin wound healing was evaluated by in vivo histological staining.
Results
Gelling behavior and differential scanning calorimeter outcomes showed that the PP hydrogels possessed thermo-sensitivity at physiological temperature and the phase transformation temperature was 28.2°C. The high swelling rate and good water retention were conducive to wound healing. The activity of SOD in vitro was up to 85% at 10 h, which was advantageous to eliminate the superoxide anion. MTT assay revealed that this hydrogel possessed good biocompatibility. Dressings of PP loaded with SOD (SOD-PP) had a higher wound closure rate than other treatments in vivo in diabetic rat model.
Discussion
The SOD-PP thermo-sensitive hydrogels can effectively promote wound healing and have good application prospects for wound repair.
Keywords: thermo-sensitive hydrogels, wound dressing, superoxide dismutase, poly(γ-glutamic acid)
Introduction
Chronic wound repair is a long-term process of tissue remodeling that severely affects the body’s health and living quality.1,2 Chronic trauma is often associated with vascular disease, venous insufficiency, and diabetes. In chronic inflammatory conditions, a large number of neutrophils might appear and inflammatory mediators are generated, including reactive oxygen species (ROS), reactive nitrogen radicals (RNS), and their derivatives.3,4 Oxidative stress is triggered when excess free radicals are produced and can lead to local and overall inflammatory pathophysiologic effects,5 eventually forming chronic wounds such as diabetic foot.6 Superoxide dismutase (SOD) as a kind of antioxidant can inhibit or reduce ROS production and is beneficial to the improvement of diseases caused by oxidative stress.7 So wound dressings with antioxidant properties should be a feasible therapeutic method.
Hydrogels are a class of materials with the properties of a 3-D network, swelling, and maintaining optimal moisture in the area.8 Because of the hydrophilicity of hydrogels, they may create a network with bound water and free water. So hydrogels lose liquidity and keep the shape stable.9 Hydrogel dressing is a new biological material and has the characteristics of good biocompatibility, strong moisture absorption, good moisture retention and antibacterial activity. It has been widely studied and applied in the biomedical field.10,11 Although the traditional dressing has some advantages for wound healing, it is not suitable for large areas and irregular wounds because of lack of environmental sensitivity, especially thermo-sensitivity. If the hydrogels are sensitive to temperature, they can have many advantages for clinical applications, such as flexibility and injectability12,13 and similarity to the extracellular matrix for supporting cell attachment, proliferation, migration as well as differentiation.14
Poly(N-isopropyl-acrylamide) (PNIPAM) has attracted wide attention because of its sensitivity to temperature,15 especially in the field of biomedicine. Thermo-sensitive hydrogel based on the properties of phase temperature sensitivity can be used for tissue wound repair.16,17 The lowest critical transition temperature of PNIPAM is 32°C,15 which is close to the human body temperature and suitable for biomedical materials and drug loading.18 A family of thermosensitive hydrogels based on N-isopropylacrylamide (NIPAM) macromer by Chen et al was synthesized for drug delivery.19 So a PNIPAM-based thermo-sensitive hydrogel could be loaded with SOD for clinical injection in situ. But PNIPAM as synthetic polymers could induce minimal inflammatory reaction and decrease biocompatibility.20 One way to improve biocompatibility is to combine PNIPAM with natural materials.
Poly(γ-glutamic acid) (γ-PGA), a natural biopolymer that is water-soluble, non-toxic, edible and biodegradable21,22 has shown potential in tissue engineering. The structure of the polymer containing a large number of carboxyl groups23 can load drugs21 and has strong hydrophilicity,24 so it can improve the hydrophobicity and SOD loading of the hydrogels. Zhuang et al25 prepared a poly(γ-glutamic acid)-based hydrogel loaded with superoxide dismutase (SOD) to accelerate wound healing. But this kind of hydrogel lack of injectability and is not conducive to irregular wounds.
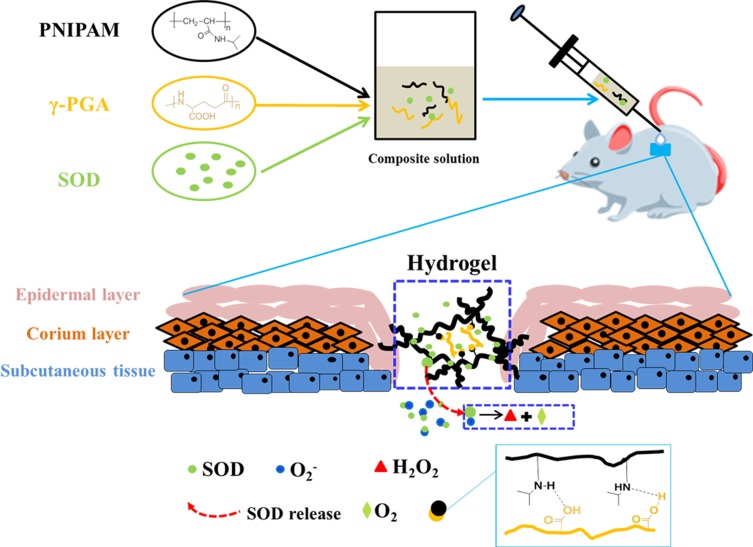
In this study, we prepared PNIPAM/γ-PGA loaded with SOD thermo-sensitive hydrogel (SOD-PP) to promote wound healing. The system might be used to encapsulate SOD at room temperature in solution and then solidify in situ after injection. The work integrates the advantages of SOD, PNIPAM and γ-PGA to reduce oxidative stress, to confer thermo-sensitivity and to create a moist microenvironment, which are beneficial for healing chronic wounds (Figure 1).
Figure 1.
Graphic. Schematic illustration of over research design.
Materials and Methods
Material
γ-PGA (Mw=460,000) was provided by the laboratory of microbial catalytic synthesis, College of Life Science, Nankai University. N-isopropyl acrylamide (NIPAM) was from J&K Scientific Ltd. (Beijing). Azobisisobutyronitrile (AIBN) was from Beijing Hua Wei Rui Branch Chemical Co. (China). Cu-Zn superoxide dismutase (SOD) was from Zhejiang Conkey Biological Technology Co. (Hangzhou, China) and streptozotocin (STZ) was from Sigma-Aldrich (St. Louis, MO, USA). Tegaderm, a commercial product for wound healing, was from 3M (3M Health Care) for comparative purposes.
Preparation of PP Composite Solutions
Firstly, the PNIPAM was synthesized by free radical polymerization with NIPAM as a monomer and AIBN as an initiator according to the method in the supporting information section S1 and the PNIPAM polymer was confirmed byH1NMR spectra, FTIR spectrum and gel permeation chromatography in the supporting information section S2. The results in Fig S1 have shown that PNIPAM can be successfully synthesized by this method. Then, the PNIPAM and γ-PGA with different weight ratios (50/50, 75/25, 100/0) were dissolved in 1 mL deionized water under ultrasonication. The total concentration of the polymer solution was 20 mg/mL. The prepared mixture was then kept at 4°C for 12 hrs.
Observation of Micromorphology
To observe the micromorphology of the composite hydrogels, the cross sections of hydrogel were observed using scanning electron microscopy (SEM) at an accelerating voltage of 10 kV.
Preparation of SOD-PP Composite Solutions
PNIPAM and γ-PGA with different weight ratios (50/50, 75/25, 100/0) were dissolved in 1 mL deionized water by ultrasonication. An amount of 2 mg SOD was added, and the prepared mixture was then kept at 4°C for 12 hrs.
Gelation Properties of Composite Solutions
The tube inversion method was used to test the gel formation of composite solutions. Briefly, the composite solutions of PP were placed in a water bath at 37°C for 10 mins, and the flow of the solution was observed by reversing the test tube. To further determine the gelling temperature, differential scanning calorimetry (DSC) was used (DSC 204, NETZSCH, Germany). The scanning temperature range was 0–50°C, and the scanning speed was 1°C/min. And storage (G′) and loss (G″) modulus was obtained by a rheometer at 10–40°C with a constant frequency of 1 Hz. The samples were tested under angular frequency sweep mode with a fixed shear strain of 1%.16,26,27
Determination of Swelling Ratio, Water Retention
The formed hydrogels were placed in phosphate-buffered saline (PBS; pH=7.4) at 37°C. The mass of swelled hydrogel was obtained after 24 hrs. Then, the swelling ratio (%) was calculated according to the formula after recording the weight of dry hydrogel (W0) and the swelled hydrogel (W).28
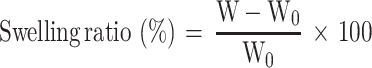 |
To determine the water retention, the hydrogels were weighted per hour. The water retention (%) was calculated according to the formula as follows after the weight of hydrogel for x hour (Wx), the weight of the dry hydrogel (W’) and the weight of the swelled hydrogel (W) were obtained.
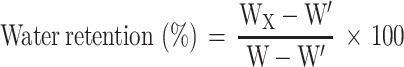 |
Determination of SOD Release, Scavenging Effect on SOD Radicals and SOD Activity in vitro
Different ratios of hydrogel SOD-PP with 2 cm3 volume were fabricated and soaked into release medium (10 mL PBS and pH=7.4) at 37°C. An amount of 10 mL buffer was refreshed and equal volumes of release medium were added at certain time intervals. To determine the concentration of the released SOD, the collected solution was spectrophotometrically analyzed at 595 nm according to the Bradford method.29,30
For the scavenging effect of superoxidant radicals and SOD activity in vitro, the mixture was prepared as described.31 This method is based on the inhibition of pyrogallol autoxidation. The inhibition of the pyrogallol autoxidation is proportional to the activity of the SOD present in the sample (n = 3). The SOD sample and distilled water were added to the 50 mmol Tris–HCl buffer solution (pH 8.20, including 0.1 mmol EDTA) and stirred to prepare the sample group and control group, respectively. Then, 6 mmol pyrogallol was added to two solutions and immediately stirred. The absorption of the control group (Acontrol) and sample group (Asample) was recorded at 299 nm, and the scavenging effect to superoxide radicals was defined with the following formula. After obtaining the scavenging effect (α), the volume of the sample solution (v) and the volume of the whole solution (V), the SOD activity could be calculated as follows:
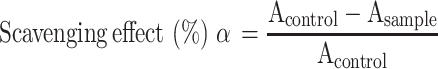 |
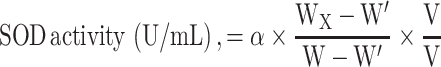 |
Evaluation of Cytocompatibility
To investigate the cytocompatibility of the hydrogels, the extraction method was used according to the national standard GB/T 16886.5–2003.32 Briefly, because the previous studies and related literature showed that the SOD concentration of 2 mg/mL had good cellular compatibility,33,34 the hydrogel without SOD was prepared by placing the gel solution in a water bath at 37°C. The hydrogel was immersed in a full medium (the ratio of film area to the medium solution was 1.5 cm2/mL) in 24 hrs at 37°C. Then, the extract solution was obtained. Afterwards, 3T3 fibroblasts were seeded on 48-well plates at 1×104 cells/well. After culturing for 12 hrs with DMEM (10% fetal bovine serum, 1% antibiotic), the medium was replaced with 200 μL hydrogel extract solution prepared by immersing 100 mg hydrogel (under UV for 24 hrs) in 10 mL DMEM at 37°C for 24 hrs. Cells were incubated for 1, 3, and 5 d at 37°C. Medium was refreshed every 2 days. Methylthiazol tetrazolium (MTT) assay was used to detect cell viability. An amount of 50 μL of 5 mg/mL MTT was added to each well. After incubating at 37°C for 4 hrs, the medium containing the MTT solution was removed and 200 μL dimethylsulfoxide (DMSO) was added. The solution was cultured under shaking conditions at 37°C for 20 mins and measured at 490 nm.
Creation of Skin Wounds on Diabetic Rats
STZ can destroy pancreatic beta cells and block the synthesis and release of insulin. It can lead to type I diabetes with a one-time injection after 2–3 days.35 Sprague–Dawley rats were selected (male, weight 300–350 g; Laboratory Animal Center of Academy of Military Medical Sciences, Beijing, China) and injected with STZ to induce diabetes. The type I diabetic rat model was established as previously described.25 Then, square wounds (side length 1 cm) were created on the left and right sides of the back spine of every rat. The control group was covered with 3M wound dressing. The experimental groups were treated with thermo-sensitive hydrogel (PP and SOD-PP) via injection, and both groups were similarly covered with 3M wound dressing. The 3M wound dressing and hydrogel in wounds were changed every 3 days.
Evaluation of Wound Healing in vivo
The experimental procedures involving laboratory animals were approved by the Animal Care Committee and followed the regulations of the Administration of Affairs Concerning Experimental Animals at Nankai University (Tianjin, China). Sprague–Dawley rats used in the experiments were obtained from the Laboratory Animal Research Center of Nankai University and housed in a temperature-controlled environment under a light/dark cycle. Wound closure was measured at days 7, 14, and 21. The wound was disinfected and the digital camera was used to record wound healing. After removing the wound tissue, it was washed 3 times with normal saline and fixed for 6 hrs in 4% paraformaldehyde and dehydrated overnight with 30% sucrose solution. Then, 6-μm cryosections of the trauma samples were prepared. Sections were stained with hematoxylin and eosin (H&E) for observing inflammatory reaction and Masson’s trichrome for collagen secretion and compared with native tissues.
Statistical Analysis
Data are expressed as mean±SD. Significance differences between two groups were analyzed by Student’s t test. P < 0.05 was considered statistically significant.
Results
Micromorphology of PP Hydrogels with Different Ratios
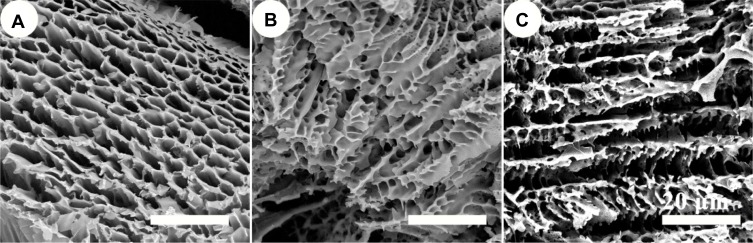
In clinical treatment, a wound dressing must have a porous structure to facilitate the transfer of nutrients, oxygen and water as well as the removal of cellular metabolites. So the three-dimensional porous structure of the hydrogels is advantageous in wound healing. To observe and confirm the microstructure, the cross-section of the various ratios of PP hydrogels was investigated using SEM (Figure 2). It indicated that there were well-interconnected porous structures in all hydrogels, and the mean pore size was about 10–15 μm. Therefore, PP hydrogel possessed porosity. They can meet the need of nutrient supply to cells, metabolite dispersal.
Figure 2.
SEM micrographs of PP hydrogels with different ratios (A) PP 100/0; (B) PP 75/25; (C) PP 50/50.
Gelation Property of Polymers with Different Formulation
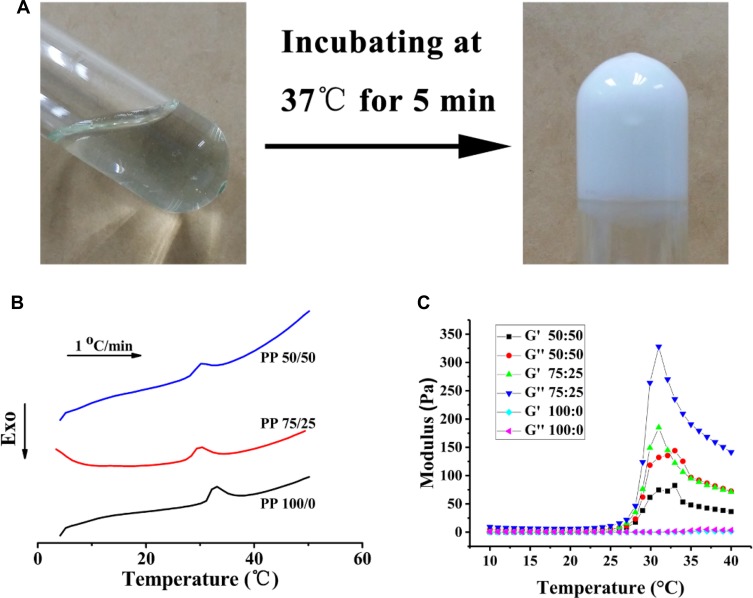
The matching of gelation temperature and the physiological temperature is beneficial to the healing of wound after hydrogel injection. The thermo-sensitive hydrogels are accompanied by a certain phase transition with the temperature change. The DSC method is used to determine the phase transition temperature. Figure 3A shows that the hydrogel formed at 37°C. With an increase of temperature, the three gel systems had an endothermic peak, and γ-PGA reduced the shift of endothermic peak from 32°C to 28.2°C (Figure 3B). A large number of hydrogen bonds may be formed between γ-PGA and H2O, which affects the temperature change of gel formation.36,37 To quantitatively characterize the thermosensitivity of the prepared hydrogel, we conducted rheological tests with different ratios sample to measure the changes of its storage modulus (G′) and loss modulus (G″) with temperature. As shown in Figure 3C and Fig S2, at lower temperatures, G′ was larger than G″, indicating a liquid-like sol state, whereas with the heating process, G″ increased significantly faster than G′, indicating a solid-like gel state. The crossover point at 28°C was defined as the gelation temperature.
Figure 3.
The gel-forming properties of poly(N-isopropyl-acrylamide)/poly(γ-glutamic acid) (PP); (A) phase transformation at 37°C (PP 75:25); (B) Differential scanning calorimetry thermodynamic diagram of hydrogels with different ratios (PP 50:50, 75:25, 100:0); (C) Storage (G′) and loss (G″) modulus changes of hydrogels with various ratios (PP 50:50, 75:25, 100:0) with temperature variation.
Determination of Swelling Ratio and Water Retention of PP Hydrogel
Hydrogel dressing can effectively absorb wound exudates and keep the wound clean.38 Good swelling ability could promote wound healing.39 The swelling rates were 1470±110%, 980±154%, 384±41% for PP 50/50, 75/25, 100/0, respectively (Figure 4A). Wound dressings should keep the wound surface moist, which is helpful for wound repair. The water content was 32±0.9%, 68±1.6% and 61±1.2% for the PP 100/0, 75/25, 50/50 gel system, respectively, after 40 hrs (Figure 4B). Compared with the pure PNIPAM hydrogel, adding γ-PGA improved the swelling ratio and the water retention performance of the system.
Figure 4.
Characteristics of PP hydrogels with different ratios. (A) Swelling behavior of hydrogels in phosphate-buffered saline (pH=7.4) (n=3); (B) Determination of water retention of hydrogels (n=3). Data are mean±SD. *P < 0.05, **P< 0.01.
With the increase of γ-PGA, the number of hydrophilic carboxyls was enhanced, leading to an increase in swelling rate and water retention of the materials.40 However, when the content of γ-PGA was too high, the free γ-PGA increased. They can not participate in the formation of hydrogel network structure, which resulted in decreasing water retention. Therefore, from the results of swelling behavior and water retention, PP 75/25 was better than other different ratios. Wang prepared poly(N-vinyl pyrrolidone)/carboxymethyl cellulose (PVP/CMC) hydrogel by radiation, and the water retention rate was 54.5% after 6 hrs for PVP/CMC = 6:4.41 Nevertheless, the water content of PP 75/25 was still 51±0.9% after 70 hrs. Therefore, the PP gel system has better performance to keep a moist environment.
Determination of SOD Release, Scavenging Effect on Superoxidant Radicals and SOD Activity in vitro
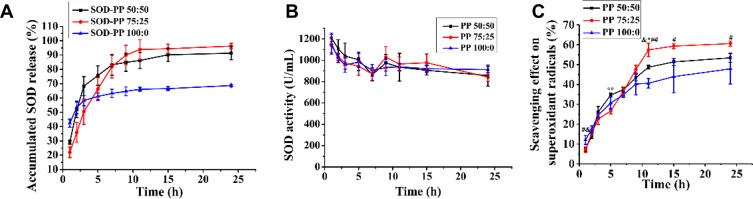
Hydrogels are often used as a drug carrier for sustained and controlled release.42 Figure 5A shows SOD release in three different ratios of PP hydrogels. The accumulated SOD release of PP groups were both up to 85% and the pure PNIPAM group was only 65% after 24 hrs. The interaction between the carboxyl in γ-PGA and the amino in SOD was enhanced, which increased the amount of SOD loaded into the composite hydrogel. The effect of the SOD release was better with PP 75/25. The SOD activities of the three components did not significantly differ (Figure 5B). After 24 h, the activity SOD was about 85%, so the PP hydrogel system could maintain the SOD activity at a higher level. After 24 h, the scavenging effect of the superoxide radical was 47.8±7.6%, 53.6±2.8%, 57.3±1.9% for the PP 100/0, 75/25, 50/50 gel system, respectively (Figure 5C). The superoxide radical scavenging effect was better with PP 75/25. The increase in scavenging ability was related to the accumulated release rate of SOD. Therefore, the addition of γ-PGA could improve the anti-oxidative property of the hydrogel system by increasing the amount of SOD.
Figure 5.
(A) Accumulated superoxide dismutase (SOD) release from hydrogels in phosphate-buffered saline (n=3); (B) Activity of released SOD (n=3); (C) Accumulated scavenging effect on superoxide radicals of hydrogels (n=3). &, # and * represent the comparison between the group PP 50:50 and PP 100:0, the group PP 75:25 and PP 100:0, the group PP 50:50 and PP 75:25, respectively. &, # or *P < 0.05, ## or **P< 0.01.
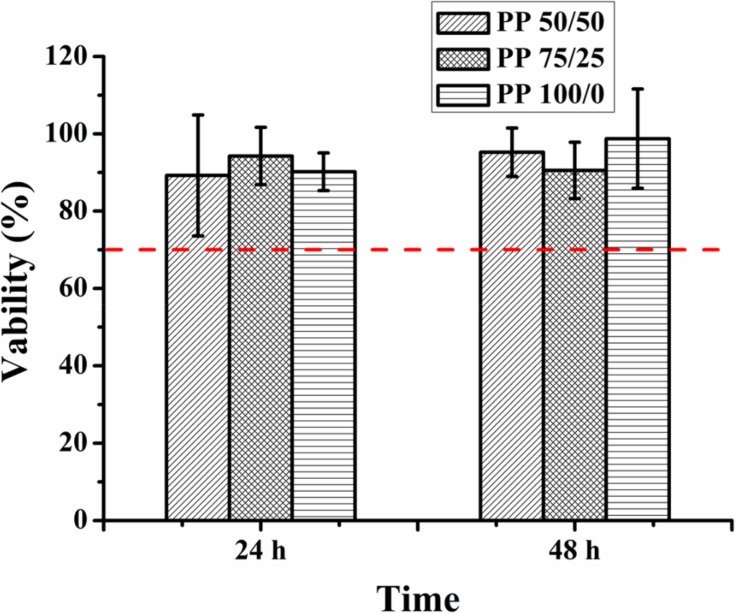
Evaluation of Cytocompatibility of PP Hydrogels
Excellent biocompatibility is one of the most important characteristics of materials in biomedical application. The cell relative survival rate of three components was greater than 70% after 24 and 48 hrs, respectively (Figure 6) (relative survival rate greater than 70% could be considered non-toxic43).
Figure 6.
Cytotoxicity of the extracting solution of the PP hydrogels with different component contents (n=6).
γ-PGA is a product of microbial fermentation that has good biological biocompatibility44,45 and the unreacted NIPAM monomer and initiator was removed by dialysis, so the synthetic hydrogels had fewer side effects on cytocompatibility. Thus, PP hydrogels in our experiment had good biocompatibility and the synthesis method we adopted in this experiment was feasible.
Evaluation of Wound Healing in vivo
In the process of wound healing, excessive ROS would cause oxidative stress and cause chronic wounds, such as diabetic foot. The results of the previous experiment showed that when the ratio of PNIPAM to γ-PGA was 75:25, the hydrogel had good swelling rate, water retention and SOD release capacity. This experiment selected the PP hydrogel (75:25) loaded with SOD to study the effects of chronic wound healing in a diabetic rat model.
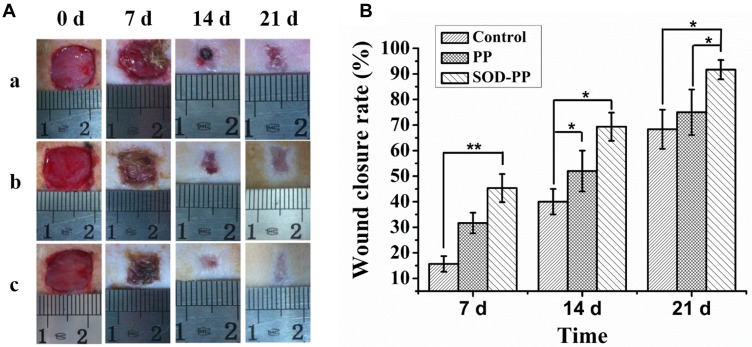
To evaluate the efficacy of SOD-PP hydrogel dressing to promote wound healing, wound repair was evaluated on days 0, 7, 14 and 21 after the hydrogel was injected into wound site (Fig S3) and the wound healing rate was calculated. The wound area of experimental groups (group b and c) was less than the control group after 7, 14 and 21 days (Figure 7A), so the thermo-sensitive hydrogel dressing could promote wound healing and the wound area of all groups was decreased with time. The effects of SOD-PP were the best among all groups, followed by PP. On day 7, the closure rate of wounds was higher with SOD-PP (45±5.5%) than PP (31±4.0%) and control (16±3.1%) dressings (Figure 7B). Furthermore, the resulting showed a treatment effect in wounds treated with SOD-PP (52±8.0%) and PP (69±5.5%) as compared with control wounds (40±5.0%) on day 14. After 21 days, the wound healing rate of the control, PP and SOD-PP groups was 68±7.6%, 75±8.9% and 92±3.8%, respectively. In brief, SOD-PP had a better treatment effect on wounds than the other two groups.
Figure 7.
(A) Representative photographs of wounds in diabetic rats treated with control (a), PP (b), and SOD-PP (c) on days 0, 7, 14, and 21 after surgical excision of the skin; (B) Percentage wound closure at various times (n=3). Data are mean±SD. *P < 0.05, **P< 0.01.
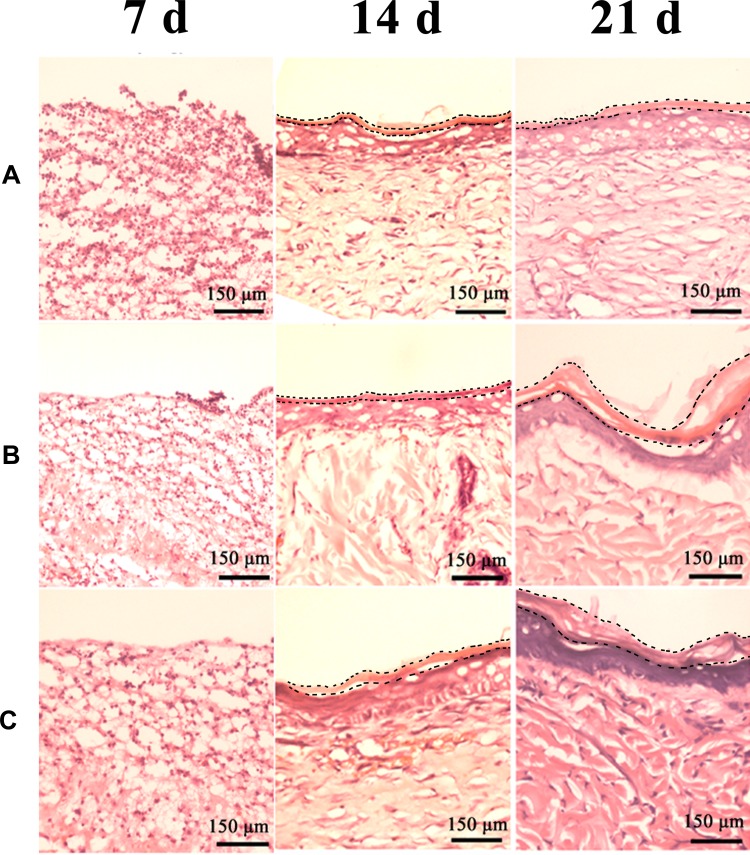
H&E staining was used to evaluate the effect of wound healing, including inflammation and epithelialization. Inflammatory cells were fewer in PP and SOD-PP groups than the control group on day 7 (Figure 8). As expected, the numbers of inflammation cells with PP and SOD-PP continued to decrease with time, whereas the inflammatory conditions remained severe in the control group. After 21 days, re-epithelialization in the wounds was almost complete in all groups but more obvious in the SOD-PP group. The results of H&E staining were consistent with the evaluation of wound healing in vivo, and SOD-PP could accelerate the process of epithelialization that is closest to natural epithelialization (Fig S4A). Epithelialization benefited from the SOD-sustained release from thermo-sensitive hydrogels and decreased concentration of superoxide anion (O2−) via catalyzing O2− into oxygen and H2O2.
Figure 8.
H&E-stained slices of wound sites on day 7, 14, 21 (A) control, (B) PP, (C) SOD-PP.
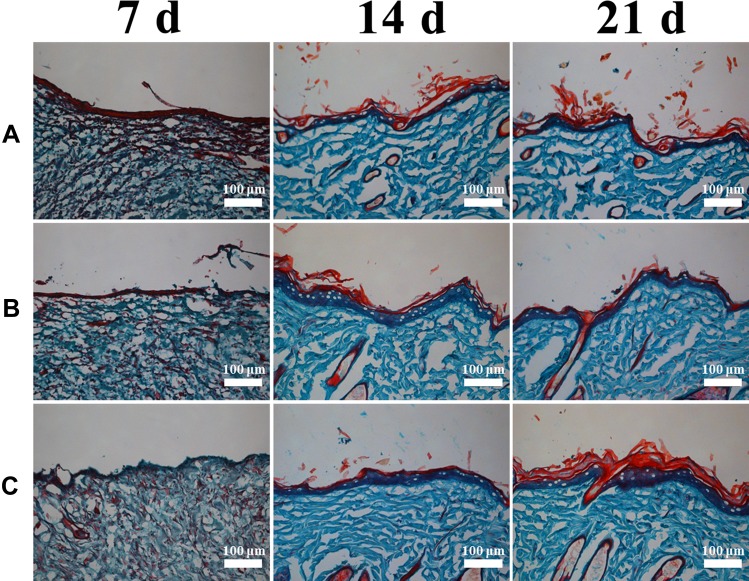
Type I collagen is the main form of collagen protein in the native skin (Fig S4B), mainly produced by fibroblasts.32,46 Collagen deposition and the formation of granulation tissue play a crucial role for tissue reconstruction in wound healing. Granulation tissue and collagen were formed during the progression of wound healing (Figure 9). On day 7, collagen deposition did not differ in all groups. On day 14, the SOD-PP group showed denser collagen, and the epidermal layer in all groups was the thickest. After 21 days, collagen and epidermis thickness increased markedly in all groups, but the SOD-PP group had the largest collagen formation and the thickest epidermis layer. Therefore, thermo-hydrogels loaded with SOD could enhance the capability of wound healing by promoting collagen formation and epidermal formation.
Figure 9.
Masson’s trichrome staining for collagen formation on day 7, 14, and 21 (A) control, (B) PP, (C) SOD-PP.
Discussion
Because of the existing deficiencies in current treatments for wound, the development of smart dressing is urgent. In the present study, composite hydrogels with thermo-sensitivity, capability for SOD and physical properties were successfully prepared to apply for skin wound repair. PNIPAM as a typical thermo-responsive polymer exhibits injection in situ at the physiological temperature. After PNIPAM was synthesized (Supplementary sections S1 and S2), the addition of γ-PGA gives PNIPAM physical properties. When the ratio of PNIPAM to γ-PGA in hydrogel is 75 to 25, hydrogels possess good water retention and good swelling ability (Figure 4). These results are related to forming network structure because of hydrogen bonds between amino-group in PNIPAM and carboxyl group in γ-PGA. When the proportion of amino-group in PNIPAM and carboxyl group in γ-PGA is appropriate, hydrogels have good physical properties. SOD could reduce the concentration of superoxide anion. Therefore, composite hydrogels integrating with SOD possess antioxidant properties (Figure 5).
γ-PGA has good biological biocompatibility, and the unreacted NIPAM monomer and initiator were removed after PNIPAM was synthesized, so the hydrogels had good cell compatibility (Figure 6). Re-epithelialization is a key parameter for wound closure. A moist environment for wounds is conductive to keratinocyte migration and epithelialization.47 In addition, thermo-sensitive hydrogel has strong shape adaptation after gelation and is similar to extracellular matrix,48 which is beneficial to hydrogel-tissue adhesiveness, cell adhesion, proliferation, migration and differentiation. Moreover, the hydrogels could be directly applied at the wound site and had no side effects on organisms.49 As compared with PP, SOD-PP could release SOD controllably after gelation, which could reduce free radicals of super oxygen and promote wound healing at the trauma site. After 21 days operation, the wound almost healed and the healing rate was up to 92±3.8% in the SOD-PP group. H&E and Masson’s trichrome staining results further indicate that SOD-PP could accelerate the process of epithelialization (Figure 8) and the SOD-PP group had the largest collagen formation and the thickest epidermis layer (Figure 9). In the SOD-PP group, the regenerated skin was closer to the natural tissue (Fig S4). Therefore, the thermo-sensitive hydrogel dressing loaded with SOD could promote wound healing effectively.
Conclusion
In this study, we prepared a thermo-sensitive hydrogel loaded with SOD for wound healing. The thermo-sensitive hydrogels exhibited greater water absorption, better moisture retention, sustained release of SOD and good biocompatibility, which was beneficial to wound repair. In vivo results showed that SOD-PP hydrogel was conducive to wound healing, wound epithelialization and tissue remodeling in diabetic rats. The preparation of thermo-sensitive hydrogel provides new ideas for designing intelligent hydrogels with environmental sensitivity, and the thermo-sensitive hydrogel loaded with SOD has good prospects for wound repair.
Acknowledgments
This work was supported by National Natural Science Foundation of China (Grant 81671842, 31870966, 81871782), National Key Research Development Program of China (2017YFC1103504), the Fundamental Research Funds for Central Universities, Nankai University (63191339), China Postdoctoral Science Foundation (2019M660989) and the Ph.D. Candidate Research Innovation Fund of Nankai University.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zeng Q, Han Y, Li H, Chang J. Design of a thermosensitive bioglass/agarose–alginate composite hydrogel for chronic wound healing. J Mater Chem B. 2015;3:8856–8864. doi: 10.1039/C5TB01758K [DOI] [PubMed] [Google Scholar]
- 2.Zhu C, Lei H, Fan D, et al. Novel enzymatic crosslinked hydrogels that mimic extracellular matrix for skin wound healing. J Mater Sci. 2018;53:5909–5928. doi: 10.1007/s10853-017-1956-y [DOI] [Google Scholar]
- 3.Komeri R, Thankam FG, Muthu J. Free radical scavenging injectable hydrogels for regenerative therapy. Mater Sci Eng C Mater Biol Appl. 2017;71:100–110. doi: 10.1016/j.msec.2016.09.087 [DOI] [PubMed] [Google Scholar]
- 4.Cheng YH, Chavez E, Tsai KL, et al. Effects of thermosensitive chitosan-gelatin based hydrogel containing glutathione on Cisd2-deficient chondrocytes under oxidative stress. Carbohydr Polym. 2017;173:17–27. doi: 10.1016/j.carbpol.2017.05.069 [DOI] [PubMed] [Google Scholar]
- 5.Mohanty C, Das M, Sahoo SK. Sustained wound healing activity of curcumin loaded oleic acid based polymeric bandage in a rat model. Mol Pharm. 2012;9:2801–2811. doi: 10.1021/mp300075u [DOI] [PubMed] [Google Scholar]
- 6.Brem H, Tomic-Canic M, Brem H, Tomic-canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117:1219–1222. doi: 10.1172/JCI32169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujiwara T, Dohi T, Maan ZN, et al. Age-associated intracellular superoxide dismutase deficiency potentiates dermal fibroblast dysfunction during wound healing. Exp Dermatol. 2019;28(4):485–492. doi: 10.1111/exd.2019.28.issue-4 [DOI] [PubMed] [Google Scholar]
- 8.Yasasvini S, Anusa RS, Vedhahari BN, Prabhu PC, Ramyadevi D. Topical hydrogel matrix loaded with simvastatin microparticles for enhanced wound healing activity. Mater Sci Eng C. 2017;72:160–167. [DOI] [PubMed] [Google Scholar]
- 9.Ratner BD, Hoffman AS. Synthetic hydrogels for biomedical applications. Adv Drug Deliv Rev. 1976;54:3–12. [DOI] [PubMed] [Google Scholar]
- 10.Xiao M, Gao L, Chandrasekaran AR, et al. Bio-functional G-molecular hydrogels for accelerated wound healing. Materials Science and Engineering C. 2019;105:110067. doi: 10.1016/j.msec.2019.110067 [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Tu J, Wang D, et al. Programmable and multifunctional DNA-based materials for biomedical applications. Advan Mater. 2018;30(24):1703658. [DOI] [PubMed] [Google Scholar]
- 12.Wang W, Liu J, Li C, et al. Real-time and non-invasive fluorescence tracking of in vivo degradation of the thermosensitive PEGlyated polyester hydrogel. J Mater Chem B. 2014;2:4185–4192. doi: 10.1039/c4tb00275j [DOI] [PubMed] [Google Scholar]
- 13.Li L, Yan B, Yang J, Huang W, Chen L, Zeng H. Injectable self-healing hydrogel with antimicrobial and antifouling properties. ACS Appl Mater Interfaces. 2017;9:9221–9225. doi: 10.1021/acsami.6b16192 [DOI] [PubMed] [Google Scholar]
- 14.Dang Q, Liu K, Zhang Z, et al. Fabrication and evaluation of thermosensitive chitosan/collagen/α, β-glycerophosphate hydrogels for tissue regeneration. Carbohydr Polym. 2017;167:145–157. doi: 10.1016/j.carbpol.2017.03.053 [DOI] [PubMed] [Google Scholar]
- 15.Jeong B, Kim SW, Bae YH. Thermosensitive sol-gel reversible hydrogels. Adv Drug Deliv Rev. 2012;64:154–162. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Luan J, Shen W, Lei K, Yu L, Ding J. Injectable and thermosensitive hydrogel containing liraglutide as a long-acting anti-diabetic system. ACS Appl Mater Interfaces. 2016;8(45):30703–30713. doi: 10.1021/acsami.6b09415 [DOI] [PubMed] [Google Scholar]
- 17.Kim EJ, Choi JS, Kim JS, Choi YC, Cho YW. Injectable and thermosensitive soluble extracellular matrix and methylcellulose hydrogels for stem cell delivery in skin wounds. Biomacromolecules. 2016;17(1):4–11. doi: 10.1021/acs.biomac.5b01566 [DOI] [PubMed] [Google Scholar]
- 18.Baghaei B, Jafari SH, Khonakdar HA, Wagenknecht U, Heinrich G. Novel thermosensitive hydrogel composites based on poly(d,l-lactide-co-glycolide) nanoparticles embedded in poly(n-isopropyl acrylamide) with sustained drug-release behavior. J Appl Polym Sci. 2014;131:590–600. [Google Scholar]
- 19.Chen CH, Chang-yi K, Shih-hsien C, Shih-hsuan M, Chih-yen C,K,S, Jyh-ping C. Thermosensitive injectable hydrogel for simultaneous intraperitoneal delivery of doxorubicin and prevention of peritoneal adhesion. Int J Mol Sci. 2018;19(5):1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayakumar R, Prabaharan M, Sudheesh Kumar PT, Nair SV, Tamura H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv. 2011;29:322–337. doi: 10.1016/j.biotechadv.2011.01.005 [DOI] [PubMed] [Google Scholar]
- 21.Lee YH, Chang JJ, Yang MC, Chien CT, Lai WF. Acceleration of wound healing in diabetic rats by layered hydrogel dressing. Carbohydr Polym. 2012;88:809–819. doi: 10.1016/j.carbpol.2011.12.045 [DOI] [Google Scholar]
- 22.Kang HS, Park SH, Lee YG, Son TI. Polyelectrolyte complex hydrogel composed of chitosan and poly(γ-glutamic acid) for biological application: preparation, physical properties, and cytocompatibility. J Appl Polym Sci. 2006;103(1):386–394. doi: 10.1002/app.24623 [DOI] [Google Scholar]
- 23.Lim S-M, Kim J, Shim J-Y, Imm B-Y, Sung M-H, Imm J-Y. Effect of poly-γ-glutamic acids (PGA) on oil uptake and sensory quality in doughnuts. Food Sci Biotechnol. 2012;21:247–252. [Google Scholar]
- 24.Jaimes-aguirre J, Morales-avila E, Ocampo-garcía B, et al. Biodegradable poly(D, L-lactide-co-glycolide)/poly(L-γ-glutamic acid) nanoparticles conjugated to folic acid for targeted delivery of doxorubicin. Mater Sci Eng C Mater Biol Appl. 2017;76:743–751. doi: 10.1016/j.msec.2017.03.145 [DOI] [PubMed] [Google Scholar]
- 25.Zhuang H, Hong Y, Gao J, Chen S, Ma Y, Wang S. A poly(γ-glutamic acid) based hydrogel loaded with superoxide dismutase for wound healing. J Appl Polym Sci. 2015;132:42033. doi: 10.1002/app.v132.23 [DOI] [Google Scholar]
- 26.Zhang E, Guo Q, Ji F, Tian X, Cui J. Thermoresponsive polysaccharide-based composite hydrogel with antibacterial and healing-promoting activities for preventing recurrent adhesion after adhesiolysis. Acta Biomaterialia. 2018;74:439–453. doi: 10.1016/j.actbio.2018.05.037 [DOI] [PubMed] [Google Scholar]
- 27.Kim JK, Yoo C, Cha Y-H, Kim Y-H. Thermo-reversible injectable gel based on enzymatically-chopped low molecular weight methylcellulose for exenatide and FGF 21 delivery to treat types 1 and 2 diabetes. J Controlled Release. 2014;194:316–322. doi: 10.1016/j.jconrel.2014.09.014 [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Sui J, Wang Q, Yin Y, Zhang X. Injectable self-crosslinking HA-SH/Col I blend hydrogels for in vitro construction of engineered cartilage. Carbohydr Polym. 2018;190(57–66). doi: 10.1016/j.carbpol.2018.02.057 [DOI] [PubMed] [Google Scholar]
- 29.Bradford MM. A rapid and sensitive and methods for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- 30.Xu X, Shen Y, Wang W, et al. Preparation and in vitro characterization of thermosensitive and mucoadhesive hydrogels for nasal delivery of phenylephrine hydrochloride. Eur J Pharm Biopharm. 2014;88:998–1004. doi: 10.1016/j.ejpb.2014.08.015 [DOI] [PubMed] [Google Scholar]
- 31.Chen P, Sun Y-J, Zhu Z-C, et al. A controlled release system of superoxide dismutase by electrospun fiber and its antioxidant activity in vitro. J Mater Sci. 2010;21:609–614. doi: 10.1007/s10856-009-3927-6 [DOI] [PubMed] [Google Scholar]
- 32.Cooperstein MA, Canavan HE. Assessment of cytotoxicity of (N-isopropyl acrylamide) and poly(N-isopropyl acrylamide)-coated surfaces. Biointerphases. 2013;8:1–12. doi: 10.1186/1559-4106-8-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiumiento A, Dominguez A, Lamponi S, Villalonga R, Barbucci R. Anti-inflammatory properties of superoxide dismutase modified with carboxymetil-cellulose polymer and hydrogel. J Mater Sci. 2006;17(5):427–435. doi: 10.1007/s10856-006-8470-0 [DOI] [PubMed] [Google Scholar]
- 34.Li Z, Wang F, Roy S, Sen CK, Guan J. Injectable, highly flexible, and thermosensitive hydrogels capable of delivering superoxide dismutase. Biomacromolecules. 2009;10(12):3306–3316. doi: 10.1021/bm900900e [DOI] [PubMed] [Google Scholar]
- 35.Li J, Shu Y, Hao T, et al. A chitosan–glutathione based injectable hydrogel for suppression of oxidative stress damage in cardiomyocytes. Biomaterials. 2013;34:9071–9081. doi: 10.1016/j.biomaterials.2013.08.031 [DOI] [PubMed] [Google Scholar]
- 36.Ren Y, Zhang Y, Sun W, et al. Methyl matters: an autonomic rapid self-healing supramolecular poly(N-methacryloyl glycinamide) hydrogel. Polymer. 2017;126:1–8. doi: 10.1016/j.polymer.2017.08.016 [DOI] [Google Scholar]
- 37.Ashraf S, Park HK, Park H, Lee SH. Snapshot of phase transition in thermoresponsive hydrogel PNIPAM: role in drug delivery and tissue engineering. Macromol Res. 2016;24:297–304. doi: 10.1007/s13233-016-4052-2 [DOI] [Google Scholar]
- 38.Ngadaonye JI, Geever LM, Killion J, Higginbotham CL. Development of novel chitosan-poly (N, N-diethylacrylamide) IPN films for potential wound dressing and biomedical applications. J Polymer Res. 2013;20:161. doi: 10.1007/s10965-013-0161-1 [DOI] [Google Scholar]
- 39.Ponrasu T, Veerasubramanian PK, Kannan R, Gopika S, Suguna L, Muthuvijayan V. Morin incorporated polysaccharide–protein (psyllium–keratin) hydrogel scaffolds accelerate diabetic wound healing in Wistar rats. RSC Adv. 2018;8:2305–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsao CT, Chang CH, Lin YY, et al. Evaluation of chitosan/poly(γ-glutamic acid) polyelectrolyte complex for wound dressing materials. Carbohydr Polym. 2011;84:812–819. doi: 10.1016/j.carbpol.2010.04.034 [DOI] [Google Scholar]
- 41.Wang M, Xu L, Hu H, et al. Radiation synthesis of PVP/CMC hydrogels as wound dressing. Nucl Instruments Meth Phys Res. 2007;265:385–389. doi: 10.1016/j.nimb.2007.09.009 [DOI] [Google Scholar]
- 42.Pacelli S, Acosta F, Chakravarti AR, et al. Nanodiamond-based injectable hydrogel for sustained growth factor release: preparation, characterization and in vitro analysis. Acta Biomater. 2017;58:479–491. doi: 10.1016/j.actbio.2017.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi L, Yang N, Zhang H, et al. A novel poly(γ-glutamic acid)/silk-sericin hydrogel for wound dressing: synthesis, characterization and biological evaluation. Mater Sci Eng C Mater Biol Appl. 2015;48:533–540. doi: 10.1016/j.msec.2014.12.047 [DOI] [PubMed] [Google Scholar]
- 44.Zhang L, Ma Y, Pan X, Chen S, Zhuang H, Wang S. A composite hydrogel of chitosan/heparin/poly (γ-glutamic acid) loaded with superoxide dismutase for wound healing. Carbohydr Polym. 2018;180:168–174. doi: 10.1016/j.carbpol.2017.10.036 [DOI] [PubMed] [Google Scholar]
- 45.Gentilini C, Dong Y, May JR, et al. Functionalized poly(γ-Glutamic acid) fibrous scaffolds for tissue engineering. Adv Healthc Mater. 2012;1:308–315. doi: 10.1002/adhm.201200036 [DOI] [PubMed] [Google Scholar]
- 46.Gould L, Abadir P, Brem H, et al. Chronic wound repair and healing in older adults: current status and future research. J Am Geriatr Soc. 2015;63(3):427–438. doi: 10.1111/jgs.13332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vogt PM, Hauser J, Rossbach O, et al. Polyvinyl pyrrolidone-iodine liposome hydrogel improves epithelialization by combining moisture and antisepis. A new concept in wound therapy. Wound Repair Regenerat. 2010;9:116–122. doi: 10.1046/j.1524-475x.2001.00116.x [DOI] [PubMed] [Google Scholar]
- 48.Gao Y, Kong W, Li B, et al. Fabrication and characterization of collagen-based injectable and self-crosslinkable hydrogels for cell encapsulation. Coll Surf B Biointerfaces. 2018;167:448–456. doi: 10.1016/j.colsurfb.2018.04.009 [DOI] [PubMed] [Google Scholar]
- 49.Miguel SP, Ribeiro MP, Brancal H, Coutinho P, Correia IJ. Thermoresponsive chitosan–agarose hydrogel for skin regeneration. Carbohydr Polym. 2014;111:366–373. doi: 10.1016/j.carbpol.2014.04.093 [DOI] [PubMed] [Google Scholar]