Abstract
The relationship of plasma concentration of per- and polyfluoroalkyl substances (PFAS) with blood pressure (BP) is uncertain. This study examined cross-sectional and prospective associations of PFAS with BP and hypertension. We quantified plasma PFAS concentrations from 957 participants enrolled in the lifestyle and placebo arms of the Diabetes Prevention Program (DPP), a randomized controlled trial with approximately 15 years of follow-up. We used multivariable linear and logistic regressions to test cross-sectional associations of six PFAS, including perfluorooctanesulfonic acid (PFOS), perfluorooctanoic acid (PFOA), perfluorohexane sulfonic acid (PFHxS), N-ethyl-perfluorooctane sulfonamido acetic acid (EtFOSAA), N-methyl-perfluorooctane sulfonamido acetic acid (MeFOSAA), and perfluorononanoic acid (PFNA), with BP and hypertension prevalence, respectively, at baseline. We used generalized linear mixed models to estimate longitudinal associations between baseline PFAS and the rate of BP changes, and Cox-Proportional hazard models to estimate risk of developing hypertension relative to baseline PFAS. Models were adjusted for baseline age, sex, race/ethnicity, treatment arm, educational attainment, income, marital status, smoking habit, alcohol drinking, and diet. We tested for effect modification by the treatment arm and sex, and accounted for multiple comparisons using the False-Discovery Rate (FDR). PFAS concentrations and hypertension prevalence within the study population (65.3% female, 57.7% White, 65.3% aged 40–59 years) were comparable to the general U.S. population. Cross-sectionally, we found small but statistically significant associations of baseline plasma concentrations of PFOA with systolic BP (β per doubling: 1.49 mmHg, 95% CI: 0.29, 2.70); and MeFOSAA with hypertension (RR=1.09 per doubling, 95% CI: 1.01, 1.19). Estimates were not statistically significant after FDR adjustment. Longitudinally, we observed null associations in the placebo arm, but some inverse associations of baseline PFOS and MeFOSAA with systolic BP in the lifestyle arm, perhaps due to regression toward the mean. Baseline PFAS concentrations also were not prospectively associated with hypertension risk. Overall, there were modest and mostly null associations of plasma PFAS concentrations with BP and hypertension.
Keywords: per- and polyfluoroalkyl substances, blood pressure, hypertension, prediabetic adults, longitudinal study, Diabetes Prevention Program
1. Introduction
Increasing blood pressure (BP) has a strong, graded association with higher risk of cardiovascular disease (CVD) (Kjeldsen 2018). While the role of many modifiable risk factors for hypertension including body mass index (BMI), cigarette smoking, physical activity, and diet, have been widely studied, knowledge about the potential contribution from environmental contaminants is limited. Per- and polyfluoroalkyl substances (PFAS) are a family of synthetic chemicals with ubiquitous human exposure and previously shown to be associated with CVD risk (Huang et al. 2018; Shankar et al. 2012) as well as several CVD risk factors including thyroid disease (Kim et al. 2018; Melzer et al. 2010; Shrestha et al. 2015), elevated lipids (Costa et al. 2009; Eriksen et al. 2013; Fisher et al. 2013; Fu et al. 2014; Lin et al. 2019; Nelson et al. 2010; Sakr et al. 2007; Steenland et al. 2010; Winquist and Steenland 2014); and higher body mass index (Cardenas et al. 2017; Andres Cardenas et al. 2018; Liu et al. 2018).
In addition, PFAS have a hypothesized relationship with BP because of their potential to increase oxidative stress and impair vasodilation (Ceriello 2008; Huang et al. 2013; Panaretakis et al. 2001; Wielsoe et al. 2015). We identified six observational studies in adults on the association between PFAS and BP with conflicting findings. Cross-sectional analyses using data from the U.S. general population sampled in the National Health and Nutrition Examination Survey (NHANES) (2003–2006, N=2200) (Min et al. 2012) and from a highly exposed population in China (China C8 Health Project, N=1612) (Bao et al. 2017) found elevated BP and increased risk of hypertension with higher serum concentrations of PFOS, perfluorooctanoic acid (PFOA), and perfluorononanoic acid (PFNA). However, these findings were not reproduced among U.S. children (NHANES 1999–2000/2003–2008; N=1655) (Geiger et al. 2014) nor in prospective studies conducted among middle-aged adults in Sweden (N=187) (Donat-Vargas et al. 2019) or in occupational and community residents from polluted areas in the USA (US C8 Health Project, N=32,254) (Winquist and Steenland 2014). Two studies with smaller sample sizes even reported weak inverse, cross-sectional associations of PFOS and PFNA with hypertension (Christensen et al. 2016; Donat-Vargas et al. 2019).
To better evaluate the relationship of PFAS with BP and hypertension, we used data from the Diabetes Prevention Program (DPP), which is a multi-center randomized controlled trial in the U.S. that collected repeated blood samples and measured BP over 15 years of follow-up. This well-characterized cohort of pre-diabetic and overweight adults allowed us to examine both cross-sectional and prospective relationships within the same population while considering important baseline modifiable risk factors for CVD and potential effect modification by sex and lifestyle intervention. We hypothesized that associations of plasma PFAS concentrations with BP and hypertension would be positive but weak given previous reports and that the initial DPP lifestyle intervention would attenuate any adverse effect of PFAS on BP outcomes.
2. Methods
2.1. Study population
The Diabetes Prevention Program (DPP, ClinicalTrails.gov number, NCT00004992) was a randomized, controlled clinical trial conducted at 27 clinical centers around the United States from 1996 to 2001; the study goal was to assess the effect of lifestyle intervention or medication (metformin) on delaying or preventing type 2 diabetes. A detailed description on the recruitment is presented in the Supplementary Materials (Figure S1) and the study design is available elsewhere (Diabetes Prevention Program Research Group 1999). Participants from the lifestyle intervention arm (N=1,079) received intensive coaching in diet modification (reducing fat and calorie intakes), moderate physical activity (150 min per week) and behavioral changes (Diabetes Prevention Program Research Group 2002); while participants from the metformin (N=1,073) and medication placebo (N=1,082) arms took 850 mg of metformin or placebo-medication, respectively, twice a day and received standard advice about diet and physical activity. At the end of the study period of DPP (average 2.8 years of follow-up), participants in the lifestyle arm had 58% lower likelihood of developing type 2 diabetes compared to those in the placebo arm; while those in the metformin arm had 31% lower risk (Diabetes Prevention Program Research 2002). After the DPP ended, the DPP Outcomes Study (DPPOS, 2002–2014, ClinicalTrails.gov number, NCT00038727) enrolled 88% of the surviving DPP participants for ongoing follow-up.
In this analysis, we included 957 participants from the lifestyle (N=481) and medication placebo (N=476) arms who had blood samples available (≥400 μL) in the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) central repository (https://repository.niddk.nih.gov/home). We did not include participants from the metformin arm because of limited funding and unknown kinetics between PFAS and this medication. We requested blood samples from three time points: baseline (1996–1999), DPP second annual visit (1998–2001, referred to as year 2 in the rest of the paper), and DPPOS tenth annual visit (2010–2013, referred to as year 14 in the rest of the paper). The study flow chart (Supplementary Materials, Figure S1) depicts the numbers of participants at each time point of follow-up. All DPP/DPPOS participants provided written informed consent. This study used de-identified data from the February 2008 Full Scale Data Release available from NIDDK Central Repository (Diabetes Prevention Program 2008) and was approved by the Institutional Review Board at Harvard Pilgrim Health Care. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory did not constitute engagement in human subjects research.
2.2. Exposures: Plasma concentrations of PFAS
Baseline, year 2 and year 14 blood samples from the DPP biorepository were retrieved for quantification at the CDC (National Center for Environmental Health, Division of Laboratory Sciences). Plasma PFAS concentrations were measured using a modified version of the online solid-phase extraction coupled to high-performance liquid chromatography-isotope dilution-tandem mass spectrometry method described before (Kato et al. 2011). We quantified five linear and branched isomers of PFOS and PFOA— n-perfluorooctane sulfonate (n-PFOS), perfluoromethylheptane sulfonates (Sm-PFOS), perfluorodimethylhexane sulfonates (Sm2-PFOS), n-perfluorooctanoate (n-PFOA), and branched perfluorooctanoates (Sb-PFOA). The limit of detection (LOD) was 0.1 ng/mL for all PFAS examined. We replaced concentrations below the LOD with LOD/√2 (Hornung and Reed 1990) for subsequent statistical analyses, and calculated total PFOS and total PFOA as the sum of their linear and branched isomers using the method used in NHANES (Centers for Disease Control and Prevention 2019). This current analysis included the six most detected PFAS with >90% detection frequency at baseline: PFOS, PFOA, perfluorohexane sulfonic acid (PFHxS), N-ethyl-perfluorooctane sulfonamido acetic acid (EtFOSAA), N-methyl-perfluorooctane sulfonamido acetic acid (MeFOSAA), and PFNA. We calculated a molar sum of the 6 PFAS to generate a sum PFAS variable for easier interpretation, however, we recognized this molar sum value is an imperfect way to obtain a total for the measured PFAS compounds with high detection rates as it does not address potency differences nor account unmeasured PFAS in blood, thus we only showed the result of sum PFAS in the supplementary material as a reference.
2.3. Outcomes: BP measurements
We extracted all BP measurements available from the NIDDK Repository for each participant. Study staff measured systolic and diastolic BP semiannually according to standard protocols (Diabetes Prevention Program Outcomes Study Research Group et al. 2013). Participants were seated in a chair for 5 minutes before having BP measurements taken twice using a standard mercury manometer separated by 30 seconds, the mean of which was used for this analysis. Pulse pressure was calculated as the difference between systolic and diastolic BP. We defined hypertension at each timepoint as self-reported hypertension diagnosis, use of anti-hypertensive medications, or systolic/diastolic BP ≥140/90 mmHg (or ≥130/80 mmHg for those with diabetes).
2.4. Covariates
We evaluated a priori potential confounders likely to be correlated with plasma PFAS concentrations and BP, including baseline age, sex, race/ethnicity, treatment assignment (for post-baseline analyses), education, income, marital status, alcohol drinking, smoking, and Dietary Approaches to Stop Hypertension (DASH) diet score. We drew directed acyclic graph to evaluate the underlying causal relationship. Research staff assessed participants’ characteristics using structured questionnaire. We used self-reported dietary intake during the previous 12 months captured using a validated semiquantitative food frequency questionnaire to calculate DASH diet score (range 0 to 9 points), which is composed of a linear index of nine nutrient components, including sodium, cholesterol, saturated fat, total fat, protein, calcium, magnesium, potassium, and fiber (Lin et al. 1999; Mellen et al. 2008). Additional baseline covariates considered included BMI, menopause status, physical activity, and estimated glomerular filtration rate (eGFR), calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (Levey et al. 2009).
2.5. Statistical analysis
We used descriptive analyses to examine participants’ characteristics at baseline (Table 1) and plotted mean levels of BP and hypertension rate over time for each of the two treatment groups. We used Chi-square or Kruskal-Wallis tests to compare BP, hypertension prevalence, and plasma PFAS concentrations between treatment arms at baseline, year 2, and year 14 (Table 2). We evaluated distributions of continuous variables using the Shapiro-Wilk test. Plasma PFAS concentrations were right-skewed, thus we log-2 transformed and grouped them into quartiles for some analyses. We assessed the trend across the three repeated measures using group-based latent class trajectory modeling (Jones et al. 2001), see Supplementary Material Appendix 1 for details.
Table 1.
Baseline characteristics of study participants (N=957)
| Characteristics | %, or mean ± STD |
|---|---|
| Group assignment | |
| .Lifestyle | 50.3 |
| .Placebo | 49.7 |
| Sex | |
| . Male | 34.7 |
| . Female | 65.3 |
| Race/ethnicity | |
| . Non-Hispanic white | 57.7 |
| . African American | 19.2 |
| . Hispanic of any race | 18.7 |
| . All others | 4.4 |
| Age (years) | |
| . <40 | 11.7 |
| . 40–44 | 11.2 |
| . 45–49 | 22.3 |
| . 50–54 | 17.5 |
| . 55–59 | 14.3 |
| . 60–64 | 11.2 |
| . >65 | 11.9 |
| Educational attainment | |
| . < High school | 4.7 |
| . High school/GED | 20.9 |
| . College | 49.0 |
| . Graduate school | 25.4 |
| Marital Status | |
| . Married/Cohabitating | 67.6 |
| . Single | 11.9 |
| . Divorced | 15.9 |
| . Widowed | 4.6 |
| Annual Income | |
| . $20,000 | 14.6 |
| . $20,000 - <$35,000 | 17.5 |
| . $35,000 - <$50,000 | 20.3 |
| . $50,000 - <$75,000 | 19.2 |
| . ≥ $75,000 | 22.4 |
| . Refused to answer | 8.1 |
| Alcohol consumption | |
| . Nondrinker | 54.1 |
| . <1 drink/week | 16.7 |
| . ≥1 drink/week | 29.2 |
| Current smoker | 5.4 |
| DASH Diet Score (0–9 range) | 2.5 ± 1.6 |
| Menopausal, % among female participants | 54.4 |
| Body mass index (kg/m2) | 33.5 ± 5.8 |
Table 2.
Blood pressure, hypertension status, and plasma PFAS concentrations at three time points during DPP/DPPOS follow-up
| Baseline (1996–1999) | DPP Year 2 (1998–2001) | DPPOS Year 10 (2010–2013) | |||
|---|---|---|---|---|---|
| All | Lifestyle arm | Placebo arm | |||
| Sample size, N | 957 | 956 | 480 | 476 | 677 |
| Years since enrollment, mean ± STD | 0 | 2.0 ± 0.0 | 14.0 ± 0.8 | ||
| Blood pressure, mean ± STD | |||||
| . Systolic, mmHg | 124.4 ± 14.7 | 122.5 ± 14.5c | 120.8 ± 14.5 | 124.2 ± 14.4 | 121.6 ± 15.0 |
| . Diastolic, mmHg | 78.5 ± 9.1 | 76.4 ± 9.0c | 75.5 ± 9.0 | 77.4 ± 9.0 | 70.1 ± 9.5 |
| . Pulse pressure, mmHg | 45.9 ± 11.7 | 46.0 ± 11.5c | 45.3 ± 11.4 | 46.8 ± 11.5 | 51.2 ± 13.2 |
| Change of blood pressure from baseline, mean ± STD | |||||
| . Systolic, mmHg | -- | −1.9 ± 13.5c | −2.4 ± 17.7 | ||
| . Diastolic, mmHg | -- | −2.1 ± 8.8c | −8.2 ± 11.0 | ||
| . Pulse pressure, mmHg | -- | 0.2 ± 11.3c | 5.8 ± 13.3 | ||
| Hypertension status, % | |||||
| . SBP ≥140mmHg or DBP ≥90mmHg | 21.3 | 15.5c | 12.3 | 18.7 | 11.1 |
| . Hypertension diagnosisa, % | 20.1 | 21.3 | 49.0 | ||
| . Hypertensionb, % | 31.0 | 30.0c | 26.5 | 33.4 | 51.7 |
| Plasma PFAS concentrations, Median (IQR) | |||||
| . PFOS, ng/mL | 26.7 (17.4-40.3) | 27.6 (19.6-38.9) | 9.8 (5.9–14.8) | ||
| . PFOA, ng/mL | 4.9 (3.5–6.7) | 5.7 (4.0–8.0)c | 5.4 (3.8, 7.8) | 5.9 (4.2, 8.2) | 2.8 (2.0–3.8) |
| . PFHxS, ng/mL | 2.3 (1.4–3.8) | 2.4 (1.5–3.9) | 1.7 (1.0–2.7) | ||
| . EtFOSAA, ng/mL | 1.1 (0.6–2.1) | 1.0 (0.5–1.8) | <LOD (<LOD)d | ||
| . MeFOSAA, ng/mL | 1.0 (0.6–1.7) | 1.1 (0.7–1.7) | <LOD (<LOD-0.3)d | ||
| . PFNA, ng/mL | 0.6 (0.4–0.8) | 0.6 (0.4–0.9) | 0.9 (0.6–1.5) | ||
Note:
Defined as having self-reported hypertension diagnosis or use of anti-hypertension medication
Defined as self-reported hypertension diagnosis, use of antihypertensive medication, systolic/diastolic blood pressure ≥140/90 mmHg or ≥130/80 mmHg for those with diabetes
Level differed across group assignment, p<0.05 using Chi-square test or Kruskal-Wallis Test.
The limit of detection (LOD) was 0.1 ng/mL.
We performed cross-sectional (Figure 1) and longitudinal analyses (Figure 2) of the associations between PFAS concentrations (six main PFAS and five isomers of PFOS and PFOA) and BP (systolic, diastolic and pulse pressure) and examined the prospective hazard of developing hypertension relative to plasma PFAS concentrations measured at baseline (Table 3). Each model included a total of 11 iterations (six PFAS and five isomers), and we accounted for multiple testing by adjusting the false-discovery rate (FDR) at 5% using the Benjamini and Hochberg step-up method (Benjamini and Hochberg 1995).
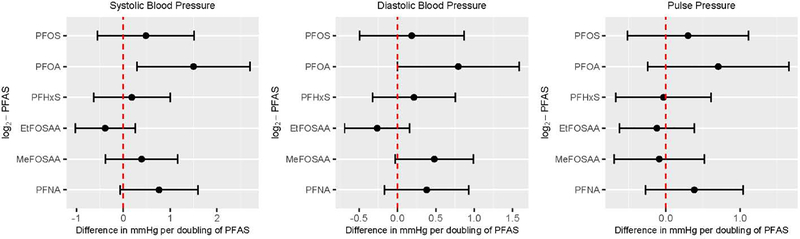
Figure 1.
Cross-sectional associations of plasma PFAS concentrations with blood pressure and hypertension prevalence among DPP/DPPOS participants (N=765)
Note: Estimated using multivariable linear regression among participants with no hypertension diagnosis and who were not taking anti-hypertensive medication at baseline. All models adjusted for age, sex, race, treatment assignment, education, income, marital status, alcohol drinking, smoking, and DASH diet score.
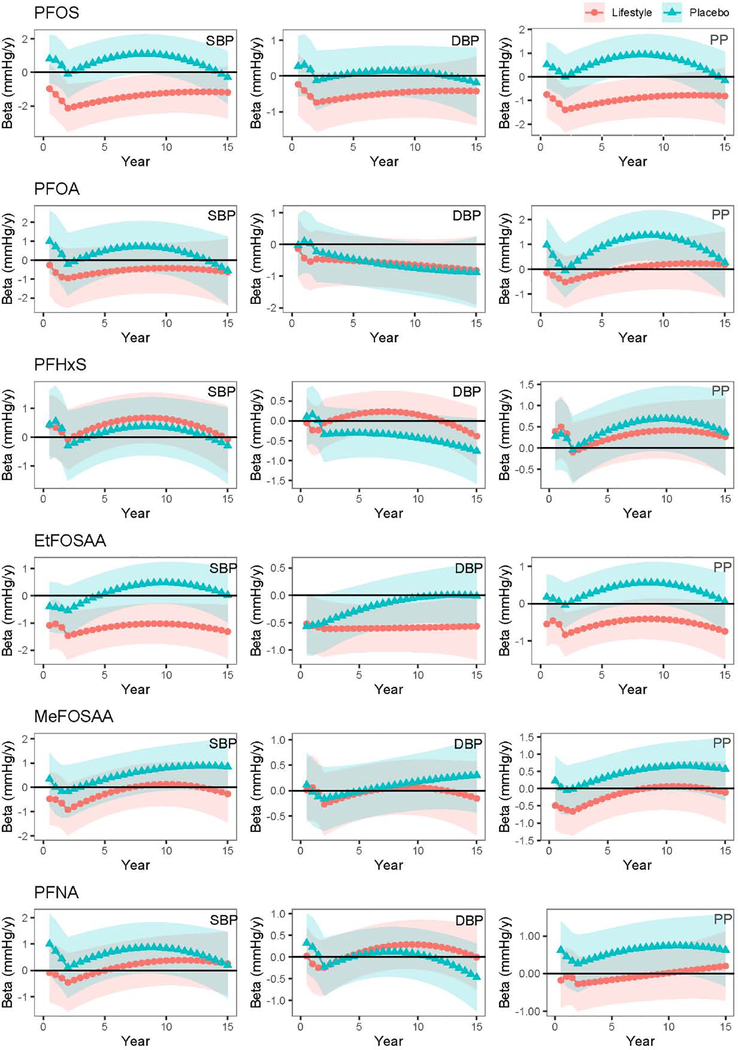
Figure 2.
Longitudinal associations between baseline plasma PFAS concentrations and BP trajectories over 15 years of follow-up in DPP/DPPOS (N=940)
Note: Effect estimates (markers) and 95% confidence interval (band) are interpreted as rate of blood pressure change (mmHg/y) per doubling of plasma PFAS concentrations measured at baseline. All models adjusted for age, sex, race, treatment assignment, education, income, marital status, alcohol drinking, smoking, and DASH diet score.
Table 3.
Prospective hazard ratio (HR) of hypertension relative to baseline plasma PFAS concentration (per doubling increase) among participants without hypertension at baseline
| Cross-sectional Risk (N=957) | Prospective Hazard (N=660) | ||
|---|---|---|---|
| Log-2 PFAS analytes | RR (95% CI) | HR (95% CI) | Effect modification by treatment arm (p-value)b |
| . PFOS | 1.06 (0.96, 1.17) | 0.95 (0.84, 1.06) | 0.25 |
| . PFOA | 1.08 (0.96, 1.21) | 0.95 (0.83, 1.08) | 0.33 |
| . PFHxS | 1.04 (0.96, 1.13) | 0.94 (0.86, 1.03) | 0.91 |
| . EtFOSAA | 0.98 (0.92, 1.04) | 0.98 (0.92, 1.06) | 0.12 |
| . MeFOSAA | 1.09 (1.01, 1.19) | 0.95 (0.87, 1.03) | 0.93 |
| . PFNA | 1.04 (0.95, 1.14) | 0.99 (0.91, 1.08) | 0.52 |
Note:
Hypertension defined as self-reported hypertension diagnosis, use of antihypertensive medication, systolic/diastolic blood pressure ≥140/90 mmHg or ≥130/80 mmHg for those with diabetes; All models adjusted for age, sex, race, treatment assignment, education, income, marital status, alcohol drinking, smoking, and DASH diet score.
Effect modification of baseline PFAS concentration by treatment arm assessed by adding a multiplicative term between plasma PFAS concentration and treatment assignment in the Cox-Proportional Hazard model.
For cross-sectional analyses of associations between PFAS and BP, we used measurements taken at baseline and restricted the sample to participants without hypertension diagnosis nor on anti-hypertensive medications. We used linear regression models to estimate the difference in mean BP levels per doubling of PFAS concentration. Covariates and confounders considered in the model included age, sex, race/ethnicity, educational attainment, income, marital status, alcohol consumption, smoking status, DASH diet score. We tested the effect of physical activity, eGFR, menopause status, and BMI on the cross-sectional associations by adding them into the model, one at a time. We used complete case analysis for all models, as missingness was <10% (See Supplementary Materials Table S1 for number of missing for each covariates). To relax the linearity assumption, we replaced continuous plasma PFAS concentrations with a categorical variable (quartiles, see Supplementary Materials Table S2 for quartile ranges), and also ran generalized additive models (GAM) including smoothing splines with 4 degrees of freedom on plasma PFAS concentrations. We also evaluated the cross-sectional relationships between PFAS and BP measurements taken at year 2 and year 14 among participants who did not previously have a hypertension diagnosis and were not taking anti-hypertensive medications, adjusting for participants’ characteristics at the respective time point. In the sensitivity analysis, we did not exclude participants with hypertension diagnosis or on anti-hypertensive medications, instead, we added a constant of 10 mmHg and 5 mmHg to participants’ systolic and diastolic BP for those who received hypertensive treatment at baseline, following the method previous reported (Tobin et al. 2005). To test for reverse causation of the above cross-sectional relationships, we regressed baseline BP, as independent variable, on later PFAS concentrations as dependent variables. For the hypertension outcome, we used Poisson regression with robust variance (Zou 2004) to estimate the cross-sectional relative risk (RR) at baseline relative to baseline plasma PFAS concentrations.
For the longitudinal analysis, we used generalized linear mixed-effect models with random intercepts and slopes to model the relationship between baseline plasma PFAS concentrations and mean BP trajectories during the approximately 15 years of follow-up. We included all participants (N=957) and did not exclude participants with hypertension diagnosis or on anti-hypertensive medications;, we added a constant of 10 mmHg and 5 mmHg to participants’ systolic and diastolic BP at timepoints when they reported hypertensive treatment. We first used fractional polynomials (FP) to obtain the best fitted average trajectory for BP and then added baseline plasma PFAS concentration into the longitudinal model to evaluate the effect of baseline PFAS on BP trajectories. Visual inspection of the best-fitted FP curve suggested stratification by treatment arms and that two knots points would sufficiently describe the BP change over time. To provide parameter estimates more relevant to the timing of the intervention, we applied the piecewise linear function in longitudinal models, segregating data into two timeframes (before year 2 and after year 2). This method produced trajectories similar to that of the best-fitted FP curves and accounted for the intensive changes in BP after the initiation of DPP intervention (Figure S2). A detailed description of the longitudinal model is presented in Appendix 2 of the Supplementary Materials. For all models, we tested model assumptions and identified the unstructured covariance model as the most optimal fit based on Akaike information criteria. The final model included baseline covariates and confounders used in the cross-sectional model and study arm, as well as a square term for time for each of the two timeframe and cross-product terms between time and log-2 transformed baseline PFAS concentrations. The regression coefficient for the cross-product term estimates average slope of BP change in mmHg/year per doubling increase in baseline PFAS concentrations. For the hypertension outcome, we used Cox-proportional hazard models to estimate hazard ratio (HRs) of developing hypertension prospectively relative to baseline plasma PFAS concentrations. We evaluated the proportional hazard assumption using Martingale-based residuals (Lin et al. 1993). We performed a sensitivity analysis restricting the time of follow-up in the Cox-proportional hazard models to 0 to 10 years, when more than 80% of the participants remained in the follow-up.
For all models, we evaluated effect modification by sex and treatment assignment by adding a multiplicative term between the effect modifier and plasma PFAS concentration and considered an evidence for effect modification if p-values of the multiplicative term was <0.10. We presented stratified effect estimates if effect modification was suggested. We used SAS 9.4 for all analyses.
3. Results
3.1. Population demographics
Table 1 summarizes participants’ characteristics at baseline. Similar to the overall DPP study population the majority of the 957 prediabetic adults included in this analysis were female (65.3%), non-Hispanic white (57.7%), college educated or higher (74.4%), married (67.6%), and non-current smoker (94.6%). About equal proportions of participants were randomized to the lifestyle (50.3%) and the placebo (49.7%) arms, with comparable demographic characteristics across the two arms (data not shown). Participants had baseline plasma PFAS concentrations similar to median serum concentrations reported among the U.S. general population sampled in NHANES 1999–2000 (Centers for Disease Control and Prevention 2019). All six PFAS were frequently detected (93~100%) with PFOS (26.7 ng/mL) and PFOA (4.9 ng/mL) having the highest concentrations. As expected, plasma PFAS concentrations remained similar at year 2, considering the relatively long half-lives of elimination in humans of several PFAS, but declined by more than 50% at year 14 for most PFAS except for PFNA (Table 2, see Supplementary Materials Figure S3 for the correlation heat map between the repeated measurements of the 6 PFAS). There was no significant difference in the median concentrations and rate of change across treatment arms at the three time points of PFAS measurements. For PFOS, PFOA, PFHxS, and PFNA, group-based latent-class modeling identified distinct trajectories that differed only by the starting baseline concentration but not the slope of PFAS concentration change (see Supplementary Materials Appendix 1), which supported the use of baseline PFAS concentrations to account for variations in PFAS concentrations in subsequent analyses. Baseline concentrations of EtFOSAA and MeFOSAA were also different across the distinct trajectories. Consistent with our previous reports, (Cardenas et al. 2017; A. Cardenas et al. 2018; Cardenas et al. 2019; Lin et al. 2019) due to variations in the exposure patterns, we observed differences in PFAS concentrations by sex, age, and race/ethnicity (Table S2).
Participants’ systolic and diastolic BP did not differ significantly between the two treatment arms at baseline, indicating successful randomization, with overall mean ± SD systolic BP being 124.4 ± 14.7 mmHg and diastolic 78.5 ± 9.1 mmHg (Table 2). BP decreased after study initiation, with participants from the lifestyle arm having experienced more reduction during the DPP phase (baseline to year 4) and returning to levels comparable to the placebo arm in the subsequent years of follow-up during DPPOS (see Supplementary Materials Figure S2 for the trend over time). The mean ± SD pulse pressure increased over time, from 45.9 ± 11.7 mmHg at baseline to 51.2 ± 13.2 mmHg at year 14. The percentage of participants with hypertension was 31.0% at baseline, 30.0% at year 2, and 51.7%, at year 14, comparable to the age-specific prevalence trend in the US (Booth et al. 2017).
3.2. Cross-sectional associations between PFAS and BP
Among 765 participants without hypertension at baseline, univariate analysis showed small but statistically significant associations between plasma concentrations of PFOA and PFNA with systolic BP [βper doubling of PFOA: 1.72 mmHg (95% CI: 0.55, 2.90); βper doubling of PFNA: 1.12 mmHg (95% CI: 0.34, 1.89)]; and MeFOSAA with diastolic BP (βper doubling of MeFOSAA: 0.63 mmHg (95% CI: 0.15, 1.11) (Supplementary Materials, Table S3). After accounting for covariates and confounders, all effect estimates attenuated and only the association between PFOA and systolic BP [βper doubling of PFOA: 1.49 mmHg (95% CI: 0.29, 2.70)] remained statistically significant at a 5% type I error rate before accounting for multiple comparisons. Figure 1 summarizes the covariate-adjusted associations between of PFAS on BP. We examined linear and branched isomers of PFOA separately and found the association to be mainly driven by the linear isomer, which was detected at higher concentration compared to the branched isomers (Supplementary Materials, Table S3). Among all covariates considered, age had the strongest positive association with BP (data not shown). Additional adjustment for physical activity, BMI, eGFR, and menopause status (among females only) in the model did not change the effect estimate substantially. A GAM with a non-linear spline term did not significantly improve the model fit. Using quartiles, individuals in the 3rd and 4th quartiles of plasma PFOA concentrations (3.0–4.4 ng/mL and 4.5–6.6 ng/mL, respectively) had higher systolic BP compared to the 1st quartile (0.6 to 2.9 ng/mL), i.e. βQ4 vs Q1: 2.38 mmHg (95% CI: −0.30, 5.07), βQ3 vs Q1: 2.49 mmHg (95% CI: −0.11, 5.09); although effect estimates were moderate in magnitude, the confidence interval included the null (Supplementary Materials, Table S4). We did not observe evidence of effect modification by sex for any associations at baseline; the p-values of the multiplicative term between PFAS and sex were all >0.10, but we did observe stronger associations between PFOA and systolic BP among males compared to females (Supplementary Materials, Table S5). Sensitivity analysis using modified BP (adding 10 mmHg to systolic and 5 mmHg and diastolic BP) to include participants on anti-hypertensive medications at baseline yielded similar effect estimates but wider confidence intervals and none of the associations were statistically significant (data not shown). After we accounted for multiple testing using FDR, none of the covariate adjusted parameter estimates was statistically significant. When we tested for reverse causation by replacing PFAS as the dependent and BP as the independent variable in the multivariable linear regression models, baseline BP was not associated with change of PFAS concentrations from baseline to year 2 nor from baseline to year 14 (data not shown).
In separate multivariate linear regression models, we repeated the cross-sectional analyses at year 2 (N=752) and year 14 (N=346) and did not find any statistically significant associations (Table S3).
3.3. Longitudinal associations between PFAS and BP
We modelled the BP trajectories during the approximately 15 years of follow up and examined the extent to which the trajectories differed by baseline plasma PFAS concentrations among all 957 participants. Longitudinal models with interaction terms between PFAS concentrations and treatment assignment had better model fits (smaller AIC and BIC) compared to models without the interaction term. Thus, we stratified all longitudinal analysis by treatment arms. The result showed mostly null longitudinal association between baseline PFAS concentration with trajectories of BP. Figure 2 showed the rate of BP change over time relative to each doubling of baseline PFAS concentrations. We did observe higher baseline PFOS and EtFOSAA concentrations to be associated with more negative rate of BP change in the lifestyle arm [at year 2, βPFOS: −2.13 mmHg/year (95% CI: −3.54, −0.71); βEtFOSAA: −1.47 mmHg/year (95% CI: −2.41, −0.59) per doubling of baseline PFAS concentrations], but not in the placebo arm [at year 2, βPFOS: −0.01 mmHg/year (95% CI: −1.51, 1.33); βEtFOSAA: −0.54 mmHg/year (95% CI: −1.45, 0.37)]. However, we did not find evidence for effect modification by study arm or sex.
3.4. Baseline plasma PFAS concentrations and hypertension
We tested whether baseline plasma PFAS concentrations was associated with prevalence and incidence of hypertension, which was self-reported hypertension diagnosis, use of anti-hypertensive medications, or systolic/diastolic BP ≥140/90 mmHg (or ≥130/80 mmHg for those with diabetes). At baseline , we found 9% higher prevalence of hypertension per doubling of MeFOSAA [RR per doubling=1.09 (95% CI: 1.01, 1.19), Table 3]. Using concentration quartiles, the RR was 59% higher comparing the 4th to the 1st quartile [RRQ4 vs Q1=1.59 (95% CI: 1.05, 2.41), Supplementary Materials, Table S6]. The cross-sectional association of PFOA and hypertension was not statistically significant [RRQ4 vs Q1=1.24 (95% CI:0.91, 1.68), Supplementary Materials, Table S6], but was modified by sex (Supplementary Materials, Table S7); among male participants, each doubling of plasma PFOA concentration was associated with 27% higher hypertension prevalence [RR=1.27 (95% CI: 1.06, 1.54)] while the association was null among females [RR=0.99 (95% CI: 0.85, 1.15)]. Among participants who did not have hypertension at baseline (N=660), there was no significant association between hypertension incidence at the approximately 15 years of follow-up with respect to baseline plasma concentrations for any PFAS, and there was no effect modification by treatment assignment (all interaction p-value > 0.10) (Table 3). Findings remained consistent in sensitivity analyses when restricting the study follow-up time to 10 years (data not shown). We additionally examined the incidence of hypertension up to year 2 of DPP and found consistent null associationa (Table S6).
4. Discussion
In this population of overweight and prediabetic adults in the USA, whose hypertension prevalence and plasma PFAS concentrations were comparable with the general U.S. population of the same age range, we detected some cross-sectional but mostly null longitudinal associations between plasma PFAS concentrations with BP and hypertension. At baseline, we found elevated systolic BP with higher plasma PFOA concentration and increased baseline prevalence of hypertension with MeFOSAA and PFOA (among men only). However, associations were not statistically significant after accounting for multiple testing.
Literature on the relationship between PFAS and BP is relatively limited. Histological observations from animal studies showed perfluorooctane sulfonic acid (PFOS) exposure induced the production of reactive oxygen species which may lead to actin filament remodeling and endothelial permeability change in human microvascular endothelial cells (Qian et al. 2010). In vivo evidence suggests PFAS exposure causes oxidative stress in the liver and endothelial cells which are the major underlying mechanism responsible for most PFAS-induced health effects (Huang et al. 2013; Panaretakis et al. 2001; Yao and Zhong 2005). Oxidative stress has been proposed to be a key player in the pathogenesis of hypertension (Montezano and Touyz 2012; Rodrigo et al. 2011). While endothelial cells regulate arterial relaxation, and PFAS-induced oxidative stress might lead to impaired vasodilation (Ceriello 2008; Huang et al. 2013; Panaretakis et al. 2001). Previous cross-sectional findings from epidemiologic studies on the associations of PFAS with BP were inconsistent, with two reported positive associations (Bao et al. 2017; Min et al. 2012) and other studies showed null findings (Christensen et al. 2016; Donat-Vargas et al. 2019; Geiger et al. 2014; Winquist and Steenland 2014). Among the two studies that reported positive associations, the magnitude of associations was in ranges comparable to what we detected in our study. The cross-sectional analysis of 2934 adults age >20 years sampled in NHANES 2003–2004 and 2005–2006 (median PFOA=4.9 ng/mL at baseline) showed each natural log unit (approximately 2.71 times) increase in serum PFOA was associated with 2.49 mmHg (SE=0.63) higher systolic BP and 71% higher (OR=1.71, 95% CI: 1.23 to 2.36) odds of hypertension comparing the fourth to the first quartile of PFOA (Min et al. 2012). The other study among 1,612 adults in China showed each natural log unit increase in the serum concentrations of various PFAS isomers was associated with 1 to 5 mmHg increase in systolic BP [e.g. βPFOS=4.48 (95% CI: 3.55, 6.12); βPFOA=1.16 (95% CI: 0.25, 3.13); βPFNA=3.01 (95% CI: 1.79, 4.23)] and 19% to 24 % increase in the odds of hypertension [e.g. ORPFOS=1.24 (95% CI: 1.08, 1.44); βPFNA=1.19 (95% CI: 1.04, 1.36)] (Bao et al. 2017). We should note that our study samples were pre-diabetic adults with elevated BMI who had higher risk to develop hypertension and CVD, thus, we should be careful when directly comparing our findings with those from adults sampled from the general population. Since participants in our study may experience more competing risks for adverse BP outcomes compared to healthy adults with normal BMI, any associations between PFAS and BP might be attenuated. This was a possible explanation for the weaker or null associations we observed in our study compared to the previous studies.
Our prospective analysis was the first, as far as we are aware, to examine the longitudinal change in BP relative to plasma PFAS concentration. We found that plasma PFOS and EtFOSAA concentrations at baseline were associated with more negative rate of systolic BP changes in the lifestyle but not the placebo group. We observed null effect estimates for all other longitudinal associations and did not detect effect modification by treatment arm nor sex. Previously, there were two longitudinal studies that examined the associations between PFAS and BP using population-level, rather than individual level, dataand both yielded mostly null results (Donat-Vargas et al. 2019; Winquist and Steenland 2014). The retrospective analysis on the U.S. C8 Health Project participants (N=32,254) did find some evidence of elevated hazard of hypertension with higher cumulative PFOA exposure among some subgroups (20–39 years old female and 40–59 years old male age groups), however, the trend of hazard was not monotonic across quartiles and the study used environmental monitoring data rather than individual biomarker measurements (Winquist and Steenland 2014)The inverse longitudinal associations we observed of PFOS and EtFOSAA with BP trajectories in the lifestyle group could be a result of regression toward the mean, a statistical phenomenon where a more extreme value from the population mean can experience a greater change toward the mean (Bland and Altman 1994). This finding could also suggest a more beneficial effect from lifestyle intervention for participants with higher plasma PFAS concentrations prior to study initiation.
Overall, our study showed no apparent association between plasma PFAS concentrations with BP and hypertension among prediabetic adults with elevated BMI. The fact that we observed only cross-sectional but not longitudinal association (in the placebo group) led us to use caution when interpreting the cross-sectional findings. Test of reverse causation did not suggest variations in BP could lead to variations in plasma PFAS concentration. Other potential drivers of the cross-sectional associations included unmeasured confounder such as consumption of packaged and processed food. We did adjust for DASH diet score in our analysis, which accounted for salt intake but not exposures to other chemicals or additives in processed and packaged food. It was also possible the underlying driver of the cross-sectional association was modified after the initiation of DPP intervention, diminishing the effect of PFAS on BP in the longitudinally follow-up. All participants received healthy lifestyle recommendations, which might have led the study participants to alter their diet, physical activity, and health awareness levels that could influence both PFAS exposures and BP control. Since we did not have a clear understanding of the temporal relationship of each variable nor did we have adequate measures of the time-varying confounders, we adjusted only for confounders measured at baseline in the longitudinal models. Our previous analyses of the same population showed that the DPP lifestyle intervention modified several adverse health outcomes associated with PFAS exposure, including increases in weight, body size, glycemic indicators, diabetes and total cholesterol (Cardenas et al. 2017; Andres Cardenas et al. 2018; Cardenas et al. 2019; Lin et al. 2019). For the mostly null associations we observed in this analysis between plasma PFAS concentrations and BP and hypertension, we did not expect and did not detect effect modification by lifestyle intervention, which further suggested the lack of a causal relationship of PFAS with BP and hypertension. Our study had several strengths. First, we evaluated sex-specific and isomer-specific effects systematically. Sex- and isomer-specific elimination and toxicity of certain PFAS had been reported in animal studies (Harada et al. 2005; Kudo et al. 2002; Loveless et al. 2006). We detected a sex-specific association between PFOA and hypertension prevalence at baseline, warranting further evaluation in other study populations. Potential mechanisms for having lower plasma PFAS concentration among females include transferring of PFAS to fetus during pregnancy (Berg et al. 2014), excretion via breast milk (Thomsen et al. 2010), or blood loss from menstruation (Wong et al. 2014). Another strength was the well-characterized data with repeated measures of plasma PFAS concentrations and BP of the same cohort, which was not available in previous studies. We performed careful assessment of participants’ prescription records to ensure the anti-hypertensive medication use was intended for BP control. The unique study design allowed us to test effect modification by lifestyle intervention, while the longitudinal follow-up data enabled us to examine both cross-sectional and prospective associations at the same time, reducing the bias from reverse causation.
Some limitations of the study include the potential for residual confounding and limited external validity based on participants’ characteristics. One of the residual confounders we did not account for was the inter-individual variation in PFAS absorption and elimination; though, we did control for eGFR to account for kidney function, which determines PFAS elimination efficiency. Our results reflected the associations observed among pre-diabetic adults with elevated BMI, who have higher risk for hypertension, thus, the results of our study may not be generalizable to a healthy cohort with normal weight as their lifestyle and exposure pattern to PFAS might be different. It was also possible that any true associations between PFAS and BP was masked by the other competing risks factor prevalent in study population, leading to an observed null association in our study population. However, given the current global obesity epidemic, our study provides relevant and valuable evidences on the risk of PFAS exposure on BP among an overweight/obese population with high-risk for cardiometabolic disease. Another limitation on external validity was that our study population was recruited between 1996 to 1999, and since then serum concentrations of the legacy PFAS, notably PFOS and PFOA, have declined over time among the U.S. general population (Centers for Disease Control and Prevention 2019) due to manufacture’s phasing out. Though our analysis could not reflect the current PFAS exposure level among the US population, its findings offered an opportunity to assess the potential risk at a higher exposure level.
5. Conclusions
Among an ethnically diverse population of prediabetic adults in the USA, we found mostly null associations between the PFAS examined with BP and hypertension. We detected weak positive cross-sectional associations between plasma PFOA concentration with elevated systolic BP and MeFOSAA with hypertension, with magnitudes of effects similar to those previously reported. However, the lack of longitudinal associations suggested the detected cross-sectional associations could be due to residual confounding.
Supplementary Material
Highlights.
The relationship between PFAS and blood pressure is unclear
The study examined this relationship among prediabetic adults in US over 15 years
There were small associations between PFAS and BP at baseline
Baseline PFAS were not associated with longitudinal changes in BP over time
Baseline PFAS were not associated with incidence of hypertension over time
Acknowledgements
The authors would like to express their gratitude to K. Kato, T. Jia, and the late X. Ye for performing the quantification of PFAS biomarkers at the Centers for Disease Control and Prevention (CDC), S. Edelstein at the Diabetes Prevention Program and Outcome Study (DPPOS) Coordinating Center for data clarification, and J. Thompson in the Department of Population Medicine at Harvard Pilgrim Health Care Institute for providing administrative support for this project. The Diabetes Prevention Program (DPP) was conducted by the DPP Research Group and supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the General Clinical Research Center Program, the National Institute of Child Health and Human Development (NICHD), the National Institute on Aging (NIA), the Office of Research on Women’s Health, the Office of Research on Minority Health, the Centers for Disease Control and Prevention (CDC), and the American Diabetes Association. The data [and samples] from the DPP were supplied by the NIDDK Central Repositories (project number 1X01DK104234). The authors acknowledge all participants in DPP and DPPOS who made this study possible. All persons named in the Acknowledgments section have provided the corresponding author with written permission to be named in the manuscript.
Funding source
Support for this research was provided by grants from the US National Institute of Environmental Health Sciences, National Institutes of Health (R01ES024765).
Abbreviations
- BMI
Body mass index
- BP
Blood pressure
- CDC
Centers for Disease Control and Prevention
- CVD
Cardiovascular disease
- DASH
Dietary Approaches to Stop Hypertension
- DPP
Diabetes Prevention Program
- DPPOS
Diabetes Prevention Program Outcomes Study
- eGFR
Estimated glomerular filtration rate
- EtFOSAA
N-ethyl-perfluorooctane sulfonamido acetic acid
- FDR
False-discovery rate
- FP
Fractional polynomials
- GAM
Generalized additive model
- HR
Hazard ratio
- LOD
limit of detection
- MeFOSAA
N-methyl-perfluorooctane sulfonamido acetic acid
- NHANES
National Health and Nutrition Examination Survey
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- n-PFOA
n-perfluorooctanoate
- n-PFOS
n-perfluorooctane sulfonate
- PFAS
Per- and polyfluoroalkyl substances
- PFHxS
perfluorohexane sulfonic acid
- PFNA
Perfluorononanoic acid
- PFOA
Perfluorooctanoic acid
- PFOS
Perfluorooctane sulfonic acid
- RR
Relative risk
- Sb-PFOA
branched perfluorooctanoates
- Sm2-PFOS
Perfluorodimethylhexane sulfonates
- Sm-PFOS
Perfluoromethylheptane sulfonates
Footnotes
Declaration of interests
☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare they have no actual or potential competing financial interests. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Institutes of Health or the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services. This manuscript was not prepared under the auspices of the DPP and does not represent analyses or conclusions of the DPP Research Group, the NIDDK Central Repositories, or the NIH. The authors declare they have no actual or potential competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bao WW, Qian ZM, Geiger SD, Liu E, Liu Y, Wang SQ, et al. 2017. Gender-specific associations between serum isomers of perfluoroalkyl substances and blood pressure among chinese: Isomers of c8 health project in china. Sci Total Environ 607–608:1304–1312. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, B 57:289–300. [Google Scholar]
- Berg V, Nost TH, Huber S, Rylander C, Hansen S, Veyhe AS, et al. 2014. Maternal serum concentrations of per- and polyfluoroalkyl substances and their predictors in years with reduced production and use. Environ Int 69:58–66. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. 1994. Regression towards the mean. BMJ 308:1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JN 3rd, Li J, Zhang L, Chen L, Muntner P, Egan B. 2017. Trends in prehypertension and hypertension risk factors in us adults: 1999–2012. Hypertension 70:275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas A, Gold DR, Hauser R, Kleinman KP, Hivert MF, Calafat AM, et al. 2017. Plasma concentrations of per- and polyfluoroalkyl substances at baseline and associations with glycemic indicators and diabetes incidence among high-risk adults in the diabetes prevention program trial. Environ Health Perspect 125:107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas A, Hauser R, Gold DR, Kleinman KP, Hivert MF, Fleisch AF, et al. 2018. Association of perfluoroalkyl and polyfluoroalkyl substances with adiposity. JAMA Netw Open 1:e181493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas A, Hauswer R, Gold DR, Kleinman KP, Hivert M-F, Fleisch AF, et al. 2018. Association of perfluoroalkyl and polyfluoroalkyl substances with adiposity. JAMA Network Open 1:e181493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas A, Hivert MF, Gold DR, Hauser R, Kleinman KP, Lin PD, et al. 2019. Associations of perfluoroalkyl and polyfluoroalkyl substances with incident diabetes and microvascular disease. Diabetes Care 42:1824–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2019. Fourth report on human exposure to environmental chemicals, updated tables. Atlanta, GA:U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. [Google Scholar]
- Ceriello A 2008. Possible role of oxidative stress in the pathogenesis of hypertension. Diabetes Care 31 Suppl 2:S181–184. [DOI] [PubMed] [Google Scholar]
- Christensen KY, Raymond M, Thompson BA, Anderson HA. 2016. Perfluoroalkyl substances in older male anglers in wisconsin. Environ Int 91:312–318. [DOI] [PubMed] [Google Scholar]
- Costa G, Sartori S, Consonni D. 2009. Thirty years of medical surveillance in perfluooctanoic acid production workers. J Occup Environ Med 51:364–372. [DOI] [PubMed] [Google Scholar]
- Diabetes Prevention Program Outcomes Study Research Group, Orchard TJ, Temprosa M, Barrett-Connor E, Fowler SE, Goldberg RB, et al. 2013. Long-term effects of the diabetes prevention program interventions on cardiovascular risk factors: A report from the dpp outcomes study. Diabet Med 30:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research G. 2002. The diabetes prevention program (dpp): Description of lifestyle intervention. Diabetes Care 25:2165–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group. 1999. The diabetes prevention program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 22:623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group. 2002. The diabetes prevention program (dpp): Description of lifestyle intervention. Diabetes Care 25:2165–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donat-Vargas C, Bergdahl IA, Tornevi A, Wennberg M, Sommar J, Koponen J, et al. 2019. Associations between repeated measure of plasma perfluoroalkyl substances and cardiometabolic risk factors. Environ Int 124:58–65. [DOI] [PubMed] [Google Scholar]
- Eriksen KT, Raaschou-Nielsen O, McLaughlin JK, Lipworth L, Tjonneland A, Overvad K, et al. 2013. Association between plasma pfoa and pfos levels and total cholesterol in a middle-aged danish population. PLoS One 8:e56969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Arbuckle TE, Wade M, Haines DA. 2013. Do perfluoroalkyl substances affect metabolic function and plasma lipids?--analysis of the 2007–2009, canadian health measures survey (chms) cycle 1. Environ Res 121:95–103. [DOI] [PubMed] [Google Scholar]
- Fu Y, Wang T, Fu Q, Wang P, Lu Y. 2014. Associations between serum concentrations of perfluoroalkyl acids and serum lipid levels in a chinese population. Ecotoxicol Environ Saf 106:246–252. [DOI] [PubMed] [Google Scholar]
- Geiger SD, Xiao J, Shankar A. 2014. No association between perfluoroalkyl chemicals and hypertension in children. Integr Blood Press Control 7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada K, Inoue K, Morikawa A, Yoshinaga T, Saito N, Koizumi A. 2005. Renal clearance of perfluorooctane sulfonate and perfluorooctanoate in humans and their species-specific excretion. Environ Res 99:253–261. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. 1990. Estimation of average concentration in the presence of nondetectable values. Applied Occupational and Environmental Hygiene 5:46–51. [Google Scholar]
- Huang M, Jiao J, Zhuang P, Chen X, Wang J, Zhang Y. 2018. Serum polyfluoroalkyl chemicals are associated with risk of cardiovascular diseases in national us population. Environ Int 119:37–46. [DOI] [PubMed] [Google Scholar]
- Huang Q, Zhang J, Martin FL, Peng S, Tian M, Mu X, et al. 2013. Perfluorooctanoic acid induces apoptosis through the p53-dependent mitochondrial pathway in human hepatic cells: A proteomic study. Toxicol Lett 223:211–220. [DOI] [PubMed] [Google Scholar]
- Jones BL, Nagin DS, Roeder K. 2001. A sas procedure based on mixture models for estimating developmental trajectories. Sociological Methods & Research 29:374–393. [Google Scholar]
- Kato K, Basden BJ, Needham LL, Calafat AM. 2011. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J Chromatogr A 1218:2133–2137. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Moon S, Oh BC, Jung D, Ji K, Choi K, et al. 2018. Association between perfluoroalkyl substances exposure and thyroid function in adults: A meta-analysis. PLoS One 13:e0197244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjeldsen SE. 2018. Hypertension and cardiovascular risk: General aspects. Pharmacol Res 129:95–99. [DOI] [PubMed] [Google Scholar]
- Kudo N, Katakura M, Sato Y, Kawashima Y. 2002. Sex hormone-regulated renal transport of perfluorooctanoic acid. Chem Biol Interact 139:301–316. [DOI] [PubMed] [Google Scholar]
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. 2009. A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DY, Wei LJ, Ying Z. 1993. Checking the cox model with cumulative sums of martingale-based residuals. Biometrika 80:557–572. [Google Scholar]
- Lin PD, Cardenas A, Hauser R, Gold DR, Kleinman KP, Hivert MF, et al. 2019. Per- and polyfluoroalkyl substances and blood lipid levels in pre-diabetic adults-longitudinal analysis of the diabetes prevention program outcomes study. Environ Int 129:343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PH, Windhauser MM, Plaisted CS, Hoben KP, McCullough ML, Obarzanek E. 1999. The linear index model for establishing nutrient goals in the dietary approaches to stop hypertension trial. Dash collaborative research group. J Am Diet Assoc 99:S40–44. [DOI] [PubMed] [Google Scholar]
- Liu G, Dhana K, Furtado JD, Rood J, Zong G, Liang L, et al. 2018. Perfluoroalkyl substances and changes in body weight and resting metabolic rate in response to weight-loss diets: A prospective study. PLoS Med 15:e1002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveless SE, Finlay C, Everds NE, Frame SR, Gillies PJ, O’Connor JC, et al. 2006. Comparative responses of rats and mice exposed to linear/branched, linear, or branched ammonium perfluorooctanoate (apfo). Toxicology 220:203–217. [DOI] [PubMed] [Google Scholar]
- Mellen PB, Gao SK, Vitolins MZ, Goff DC Jr. 2008. Deteriorating dietary habits among adults with hypertension: Dash dietary accordance, nhanes 1988–1994 and 1999–2004. Arch Intern Med 168:308–314. [DOI] [PubMed] [Google Scholar]
- Melzer D, Rice N, Depledge MH, Henley WE, Galloway TS. 2010. Association between serum perfluorooctanoic acid (pfoa) and thyroid disease in the u.S. National health and nutrition examination survey. Environ Health Perspect 118:686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min JY, Lee KJ, Park JB, Min KB. 2012. Perfluorooctanoic acid exposure is associated with elevated homocysteine and hypertension in us adults. Occup Environ Med 69:658–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montezano AC, Touyz RM. 2012. Oxidative stress, noxs, and hypertension: Experimental evidence and clinical controversies. Ann Med 44 Suppl 1:S2–16. [DOI] [PubMed] [Google Scholar]
- Nelson JW, Hatch EE, Webster TF. 2010. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general u.S. Population. Environ Health Perspect 118:197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaretakis T, Shabalina IG, Grander D, Shoshan MC, DePierre JW. 2001. Reactive oxygen species and mitochondria mediate the induction of apoptosis in human hepatoma hepg2 cells by the rodent peroxisome proliferator and hepatocarcinogen, perfluorooctanoic acid. Toxicol Appl Pharmacol 173:56–64. [DOI] [PubMed] [Google Scholar]
- Qian Y, Ducatman A, Ward R, Leonard S, Bukowski V, Lan Guo N, et al. 2010. Perfluorooctane sulfonate (pfos) induces reactive oxygen species (ros) production in human microvascular endothelial cells: Role in endothelial permeability. J Toxicol Environ Health A 73:819–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo R, Gonzalez J, Paoletto F. 2011. The role of oxidative stress in the pathophysiology of hypertension. Hypertens Res 34:431–440. [DOI] [PubMed] [Google Scholar]
- Sakr CJ, Leonard RC, Kreckmann KH, Slade MD, Cullen MR. 2007. Longitudinal study of serum lipids and liver enzymes in workers with occupational exposure to ammonium perfluorooctanoate. J Occup Environ Med 49:872–879. [DOI] [PubMed] [Google Scholar]
- Shankar A, Xiao J, Ducatman A. 2012. Perfluorooctanoic acid and cardiovascular disease in us adults. Arch Intern Med 172:1397–1403. [DOI] [PubMed] [Google Scholar]
- Shrestha S, Bloom MS, Yucel R, Seegal RF, Wu Q, Kannan K, et al. 2015. Perfluoroalkyl substances and thyroid function in older adults. Environ Int 75:206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Fletcher T, Savitz DA. 2010. Epidemiologic evidence on the health effects of perfluorooctanoic acid (pfoa). Environ Health Perspect 118:1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen C, Haug LS, Stigum H, Froshaug M, Broadwell SL, Becher G. 2010. Changes in concentrations of perfluorinated compounds, polybrominated diphenyl ethers, and polychlorinated biphenyls in norwegian breast-milk during twelve months of lactation. Environ Sci Technol 44:9550–9556. [DOI] [PubMed] [Google Scholar]
- Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. 2005. Adjusting for treatment effects in studies of quantitative traits: Antihypertensive therapy and systolic blood pressure. Stat Med 24:2911–2935. [DOI] [PubMed] [Google Scholar]
- Wielsoe M, Long M, Ghisari M, Bonefeld-Jorgensen EC. 2015. Perfluoroalkylated substances (pfas) affect oxidative stress biomarkers in vitro. Chemosphere 129:239–245. [DOI] [PubMed] [Google Scholar]
- Winquist A, Steenland K. 2014. Modeled pfoa exposure and coronary artery disease, hypertension, and high cholesterol in community and worker cohorts. Environ Health Perspect 122:1299–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong F, MacLeod M, Mueller JF, Cousins IT. 2014. Enhanced elimination of perfluorooctane sulfonic acid by menstruating women: Evidence from population-based pharmacokinetic modeling. Environ Sci Technol 48:8807–8814. [DOI] [PubMed] [Google Scholar]
- Yao X, Zhong L. 2005. Genotoxic risk and oxidative DNA damage in hepg2 cells exposed to perfluorooctanoic acid. Mutat Res 587:38–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.