Key Points
AH-t has higher degree of aneuploidy and KMT2D and KMT2B mutations and inferior survival compared with AH-DN.
Patients with blastoid/pleomorphic MCL with Ki-67 ≥50% identifies an ultra-high risk subset with a distinct mutation profile.
Abstract
Blastoid and pleomorphic mantle cell lymphomas (MCLs) are variants of aggressive histology MCL (AH-MCL). AH-MCL can arise de novo (AH-DN) or transform from prior classic variant MCL (AH-t). This study is the first integrated analysis of clinical and genomic characteristics of AH-MCL. Patient characteristics were collected from diagnosis (AH-DN) and at transformation (AH-t). Survival after initial diagnosis (AH-DN) and after transformation (AH-t) was calculated. Regression tree analysis was performed to evaluate prognostic variables and in univariate and multivariate analyses for survival. Whole-exome sequencing was performed in evaluable biopsy specimens. We identified 183 patients with AH-MCL (108 were AH-DN, and 75 were AH-t; 152 were blastoid, and 31 were pleomorphic). Median survival was 33 months (48 and 14 months for AH-DN and AH-t, respectively; P = .001). Factors associated with inferior survival were age (≥72 years), AH-t category, Ki-67 ≥50% and poor performance status. AH-t had a significantly higher degree of aneuploidy compared with AH-DN. Transformed MCL patients exhibited KMT2B mutations. AH-MCL patients with Ki-67 ≥50% had exclusive mutations in CCND1, NOTCH1, TP53, SPEN, SMARCA4, RANBP2, KMT2C, NOTCH2, NOTCH3, and NSD2 compared with low Ki-67 (<50%). AH-t patients have poor outcomes and distinct genomic profile. This is the first study to report that AH-MCL patients with high Ki-67 (≥50%) exhibit a distinct mutation profile and very poor survival.
Visual Abstract
Introduction
Although treatment options for patients with mantle cell lymphoma (MCL) have significantly improved, relapses are frequent.1 Detailed histopathologic evaluation of tissue biopsy specimens is pivotal for the diagnosis of MCL. Aggressive histology MCL2 (AH-MCL) is generally dichotomized according to the World Health Organization classification into blastoid (medium sized, fine chromatin, round nuclei, and resemble lymphoblasts) or pleomorphic (large size, irregular nuclei, anaplastic cells resembling diffuse large B-cell lymphoma) morphologic variants.3 Intermediate forms4 of MCL have also been described. Patients with AH-MCL can present at the time of initial diagnosis of MCL (ie, de novo [AH-DN]) or at the time of transformation from classical morphology (ie, AH-t). Transformation in MCL is clonally related to the original MCL clone.5
Previous studies have shown that patients with AH-MCL exhibit inferior survival6-9 and inferior response to intensive chemoimmunotherapy10-12 (despite the addition of cytosine arabinoside or autologous stem cell transplantation [SCT] as consolidation)13,14 or ibrutinib-based regimens15,16 compared with classic MCL. Frequency of central nervous system (CNS) relapses is higher in patients with AH-MCL.17,18 Few studies have reported that patients with AH-MCL exhibit the following features: loss or decreased expression of CD519; cyclin D1 alternative splicing lacking the 3′ untranslated region20,21 (truncated cyclin D1); TP53,22-24 NSD2, NOTCH1,25 NOTCH2,26 and CDKN2A27 mutations; upregulated miR-15b28; additional chromosomal aberrations29 (deletions of 1p, 13q and 17p, 3q gains and alterations in 10p)30; complex karyotype31; high Ki-678; reduced number of follicular dendritic cells in involved tissues32; c-MYC amplification33; or overexpression of MYC by immunohistochemistry.34 Recently, one study35 reported that the biochemical composition of AH-MCL cells is significantly different from that of classic MCL, with a higher degree of absorbance intensity of protein moiety in spectra using the Synchrotron Fourier transformed infrared micro-spectroscopy technique and principal component analysis of tissues. Another study36 showed that decreased expression of BACH2 (BTB and CNC homology-2; a B cell–specific transcription factor) is associated with drug resistance and blastoid MCL.
Because an integrated analysis of genomic, clinical characteristics, outcomes, and treatments of AH-MCL has not been reported, we envisaged the current study to evaluate the prognostic factors, survival outcomes, and genomic characteristics from a large cohort of patients with AH-DN and AH-t MCL.
Patients and methods
This study included 183 patients with a confirmed diagnosis via biopsy results with AH-MCL (blastoid or pleomorphic) and treated at The University of Texas MD Anderson Cancer Center between the years 1992 and 2018. A retrospective study protocol (allowing molecular studies and chart review) was approved by the Institutional Review Board in accordance with the Declaration of Helsinki, and a waiver of informed consent was obtained. Histopathology pattern (blastoid or pleomorphic or classic) was independently reviewed and confirmed by hematopathology collaborators. Only patients with available information regarding treatments, clinical characteristics, and response were included in the final survival analysis.
The primary objective of the study was to analyze the overall survival (OS), which was assessed from the date of diagnosis of AH-MCL until death or the date of last follow-up. For patients with AH-t, OS was calculated from the date of transformation to the date of death or the date of last follow-up. Failure-free survival (FFS) was assessed from the time of the initiation of first-line treatment of AH-MCL to the date of first disease recurrence, switch to second-line therapy, death, or last follow-up.
Statistical analysis
Univariate and multivariate Cox proportional hazards models were performed to identify specific characteristics of AH-MCL that are predictive of survival outcome. Variables with P ≤ .25 in the univariate analysis were entered into a multivariate model. The median survival and survival probabilities were analyzed by using the Kaplan-Meier method, and differences were calculated with the log-rank test. Classification and regression tree analysis were used to identify the optimal cutoff points for specific parameters associated with survival; we subsequently identified prognostic factors that could independently predict survival in patients with AH-MCL. P < .05 was considered to be statistically significant. Statistical analyses were performed by using STATA/SE statistical software version 14.1 (StataCorp LLC, College Station, TX).
Whole-exome sequencing and somatic mutation analysis
Whole-exome sequencing (WES) was performed on a total of 81 evaluable samples at the time of their histologic diagnosis (AH-MCL, n = 39; nonaggressive MCL, n = 42) and among these, germline matched controls were available for 32 patients. Details regarding the WES method and data analysis are given in the supplemental File and our previous study.37 Briefly, DNA was extracted from formalin-fixed paraffin-embedded tissues. Indexed libraries were prepared from sheared DNA using the Agilent SureSelect Reagent Kit (Agilent Technologies, Santa Clara, CA). Exome capture was then performed by using the Agilent SureSelect Human All Exon V3.0 kit. Library concentrations were normalized, and the libraries were multiplexed as 6 libraries per pool. Sequencing was performed on the HiSeq4000 Sequencer (Illumina, San Diego, CA), one capture (6 samples) per lane using the 76 bp paired-end configuration. Sequenced reads were aligned to the human genome reference hg19 by using Burrows-Wheeler Alignment. MuTect (v1.1.4)38 was applied to identify somatic point mutations, and Pindel (v0.2.4)39 was applied to identify small insertion and deletions. Platypus (v0.8.1) was used to call germline single-nucleotide polymorphisms. DNA copy number analysis was conducted by using ExomeLyzer followed by circular binary segmentation.
Results
Patients and disease characteristics
Patient and disease characteristics of 183 patients with AH-MCL at the time of initial diagnosis (AH-DN; n = 108) and at the time of diagnosis of transformation (AH-t; n = 75) were analyzed (Table 1). AH-MCL was either of blastoid (n = 152) or pleomorphic (n = 31) morphology (supplemental Figure 1). The median age of patients was 65 years (31-95 years), and 75% were male. Stage 4 disease was recorded in 72% of patients, and gastrointestinal involvement was noted in 17% of patients. The simplified Mantle Cell Lymphoma International Prognostic Index score was high risk in all patients. The median Ki-67 was 70% (range, 10%-100%), 60% had κ light chains, and 45% had lactate dehydrogenase (LDH) levels over the upper limit of normal. Thirty-eight patients had karyotype information available, and 74% had complex karyotype. SOX-11 expression was available in 37 patients, and 81% were positive. Fluorescence in situ hybridization (FISH) testing for TP53 was positive in 11 patients, and MYC translocation was detected in 8 patients (FISH information not available in 81% of patients). Patients with AH-DN were previously untreated at diagnosis of AH-MCL, whereas AH-t patients had received prior therapies for classic variant MCL before transforming to AH-MCL (median, 2; range, 1-8).
Table 1.
Clinical characteristics, treatments in patients at the time of diagnosis of AH-MCL (includes blastoid and pleomorphic histology): overall, AH-DN, and AH-t
| Characteristic | Overall (N = 183) | AH-DN (n = 108) | AH-t (n = 75) | P |
|---|---|---|---|---|
| Age, median (range), y | 65 (31-95) | 63 (31-83) | 67 (39-95) | .009 |
| Sex, male/female, n (%) | 137 (75)/46 (25) | 80 (74)/28 (26) | 57 (76)/18 (24) | .76 |
| Histology type, n (%) | .187 | |||
| Blastoid/pleomorphic | 152 (83)/31 (17) | 93 (86)/15 (14) | 59 (79)/16 (21) | |
| PS-ECOG, n (%) | <.001 | |||
| 0 | 40 (22) | 12 (11) | 28 (38) | |
| 1 | 103 (57) | 76 (71) | 27 (36) | |
| 2 | 27 (15) | 17 (16) | 10 (13) | |
| 3 | 5 (3) | 2 (2) | 3 (4) | |
| 4 | 6 (3) | 0 (0) | 6 (8) | |
| B symptoms, yes/no, n (%) | 35 (19)/147 (81) | 23 (21)/84 (78) | 12 (16)/63 (84) | .35 |
| Leukemic phase, yes/no, n (%) | 49 (27)/132 (73) | 44 (41)/64 (59) | 5 (7)/68 (93) | <.001 |
| Bone marrow involved by MCL, yes/no, n (%) | 106 (67)/53 (33) | 85 (79)/23 (21) | 21 (41)/30 (59) | <.001 |
| CNS involvement, yes/no, n (%) | 8 (5)/175 (95) | 4 (4)/104 (96) | 4 (5)/71 (95) | .71 |
| Light chain type, κ/λ, n (%) | 90 (60)/59 (40) | 59 (63)/34 (37) | 31 (55)/25 (45) | .32 |
| Ki-67, median (range), % | 70 (10-100) | 67 (10-100) | 70 (10-100) | .154 |
| LDH above the ULN, yes/no, median (range), IU/L | 79 (45)/96 (55) | 52 (49)/53 (51) | 27 (38)/43 (61) | .15 |
| SOX-11 expression (±), n (%) | 30 (81)/7 (19) | 11 (85)/2 (15) | 19 (80)/5 (20) | .99 |
| WBC, median (range), 103/µL | 6.2 (1-205) | 7 (1-205) | 5 (2-85) | .002 |
| Serum LDH, median (range), IU/L | 574 (202-42 000) | 609 (202-42 000) | 550 (214-30 000) | .60 |
| Serum β2-microglobulin, median (range), mg/dL | 3 (1-19) | 3 (2-19) | 3 (1-8) | .907 |
| Hemoglobin, median (range), g/dL | 12 (7-16) | 12 (7-16) | 13 (7-15) | .46 |
| Platelet count, median (range), 103/µL | 163 (8-615) | 189 (8-615) | 152 (17-427) | .05 |
| Absolute monocyte count, median (range), 103/µL | 0.5 (0.07-25) | 0.5 (0.07-25) | 0.5 (0.1-3) | .99 |
| Absolute lymphocyte count, median (range), 103/µL | 1.1 (0.1-78) | 1 (0.1-78) | 1 (0.1-11) | .03 |
| First-line treatments, n (%) | <.001 | |||
| R-HCVAD based | 59 (36) | 51 (50) | 8 (13) | |
| R-HCVAD based and SCT consolidation | 14 (8) | 13 (13) | 1 (2) | |
| R-chemotherapy | 37 (23) | 28 (28) | 9 (15) | |
| R-chemotherapy and SCT consolidation | 7 (4) | 6 (6) | 1 (2) | |
| R-lenalidomide with/without other agents | 8 (5) | 1 (1) | 7 (12) | |
| R-lenalidomide with/without other agents and SCT | 1 (1) | 0 (0) | 1 (2) | |
| Ibrutinib/other BTK inhibitor | 16 (10) | 0 (0) | 16 (26) | |
| Ibrutinib/other BTK inhibitor and SCT consolidation | 3 (2) | 0 (0) | 3 (5) | |
| Miscellaneous | 18 (12) | 3 (3) | 15 (25) | |
| Response to first-line treatment, CR/no CR, n (%) | 90 (56)/72 (44) | 75 (74)/27 (26) | 15 (25)/45 (75) | <.001 |
PS-ECOG, performance status, Eastern Cooperative Oncology Group; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; R-HCVAD, rituximab with hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and cytosine arabinoside; ULN, upper limit of normal; WBC, white blood cell count.
Patients with AH-DN exhibited significant differences compared with those with AH-t, including lower median age (63 vs 67 years) in AH-t (P = .009) and lower frequency of patients with a poor performance status, frequent leukemic phase, and bone marrow involvement (P < .001 in each group). Ki-67%, blastoid/pleomorphic histology, and other characteristics were not significantly different.
In subset analysis, patient characteristics between the blastoid and pleomorphic categories (Table 2) were similar. However, the frequency of AH-t category patients was higher with the pleomorphic variant than with the blastoid variant (52% vs 39%).
Table 2.
Comparison of clinical characteristics of patients with AH-MCL (blastoid vs pleomorphic MCL), including all patients, AH-DN, and AH-t
| Characteristic | Blastoid (n = 152) | Pleomorphic (n = 31) | P |
|---|---|---|---|
| Age, median (range), y | 65 (31-95) | 65 (39-85) | .46 |
| Sex, male/female, n (%) | 113 (74)/39 (26) | 24 (77)/7 (23) | .71 |
| AH-MCL category, n (%) | |||
| AH-DN/AH-t | 93 (61)/59 (39) | 15 (48)/16 (52) | .18 |
| PS-ECOG, n (%) | .51 | ||
| 0 | 30 (20) | 10 (32) | |
| 1 | 87 (58) | 16 (52) | |
| 2 | 24 (16) | 3 (10) | |
| 3 | 4 (3) | 1 (3) | |
| 4 | 5 (3) | 1 (3) | |
| B symptoms, yes/no, n (%) | 30 (20)/121 (80) | 5 (17)/26 (83) | .63 |
| Leukemic phase, yes/no, n (%) | 43 (28)/108 (72) | 6 (20)/24 (80) | .34 |
| Bone marrow involved by MCL, yes/no, n (%) | 93 (69)/41 (31) | 13 (52)/12 (48) | .09 |
| CNS involvement, yes/no, n (%) | 5 (3)/147 (97) | 3 (10)/28 (90) | .136 |
| Light chain type, κ/λ, n (%) | 74 (59)/51 (41) | 16 (67)/8 (33) | .49 |
| Ki-67, median (range), % | 70 (10-100) | 70 (30-100) | .76 |
| LDH above the ULN, yes/no, median (range), IU/L | 68 (46)/80 (54) | 11 (41)/16 (59) | .61 |
| SOX-11 expression (±), n (%) | 23 (77)/7 (23) | 7 (100)/0 (0) | .30 |
| WBC, median (range), 103/µL | 6 (1-205) | 6 (2-98) | .71 |
| Serum LDH, median (range), IU/L | 581 (202-42 000) | 564 (314-5122) | .82 |
| Serum β2-microglobulin, median (range), mg/dL | 3 (1-19) | 3 (2-12) | .57 |
| Hemoglobin, median (range), g/dL | 12 (7-16) | 12 (7-15) | .94 |
| Platelet count, median (range), 103/µL | 162 (8-500) | 171 (12-615) | .56 |
| Absolute monocyte count, median (range), 103/µL | 0.56 (0.07-25) | 0.62 (0.17-5) | .08 |
| Absolute lymphocyte count, median (range), 103/µL | 1 (0.10-75) | 1 (0.16-78) | .74 |
| First-line treatments, n (%) | .138 | ||
| R-HCVAD based | 48 (36) | 11 (39) | |
| R-HCVAD based and SCT consolidation | 14 (10) | 0 (0) | |
| R-chemotherapy | 32 (24) | 5 (18) | |
| R-chemotherapy and SCT consolidation | 7 (5) | 0 (0) | |
| R-lenalidomide with/without other agents | 6 (4) | 2 (7) | |
| R-lenalidomide with/without other agents and SCT | 0 (0) | 1 (4) | |
| Ibrutinib/other BTK inhibitor | 11 (8) | 5 (18) | |
| Ibrutinib/other BTK inhibitor and SCT consolidation | 3 (2) | 0 (0) | |
| Miscellaneous | 14 (10) | 4 (14) | |
| Response to first-line treatment, CR/no CR, n (%) | 76 (57)/58 (43) | 14 (50)/14 (50) | .51 |
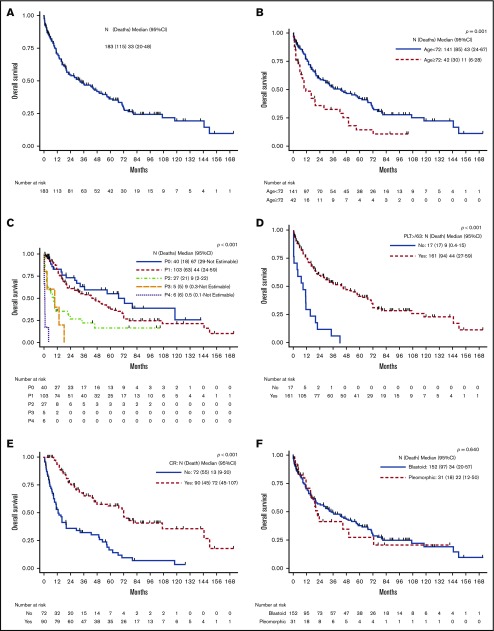
Survival and prognostic factors associated with survival
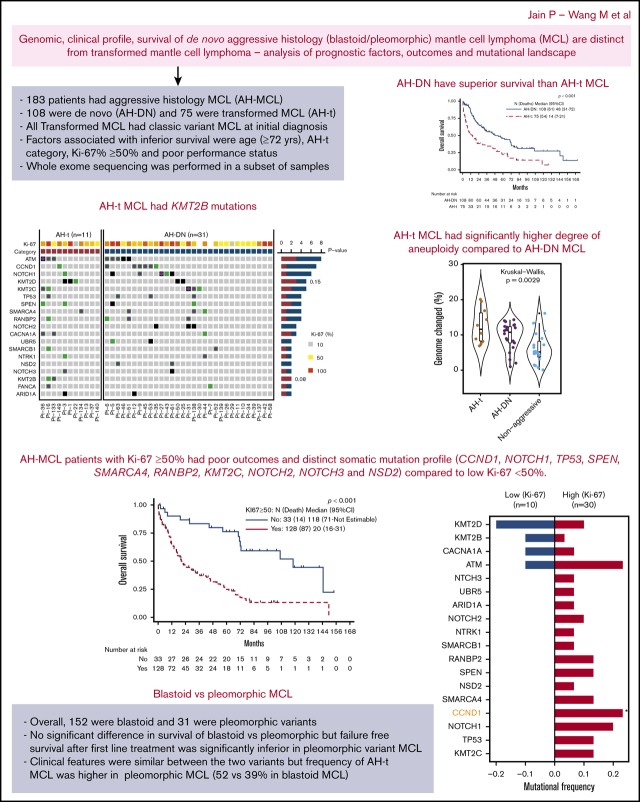
The median follow-up after the diagnosis of AH-MCL was 19.6 months (range, 1-168 months). Median survival after the diagnosis of AH-MCL was 33 months (95% CI, 20-48) (Figure 1A). At the time of last follow-up, 63% patients had died (70% due to progressive disease). Patients with the AH-t category had significantly inferior survival compared with those with AH-DN; median survival was 14 vs 48 months, respectively (P < .001) (Figure 2A). In univariate analysis, patients with advanced age (Figure 1B), poor performance status (Figure 1C), presence of B symptoms, CNS involvement, higher Ki-67%, lower hemoglobin levels, higher LDH levels, higher β2-microglobulin levels, and lower platelet count (<63 000 × 103/μL) (Figure 1D) had a significantly increased risk of death (Table 3). The statistically validated optimal cutoff values for various prognostic variables were incorporated into the logistic regression model. We identified that age ≥72 years, LDH level ≥1519 IU/L, hemoglobin level <14 gm/dL, β2-microglobulin level ≥4 mg/dL, platelet count <63 000 × 103/μL, and failure to achieve complete remission (CR) after first-line treatment (Figure 1E) were significantly associated with an increased risk of death.
Figure 1.
OS in patients with AH-MCL, including AH-DN and AH-t. (A) Median survival after diagnosis was 33 months. This included all patients with AH-MCL. (B) Median survival was significantly longer in patients aged <72 years (43 months) compared with those aged ≥72 years (11 months) (P < .001). The cutoff point of 72 years was based on classification and regression tree analysis. (C) Median survival was significantly inferior in patients with poor Eastern Cooperative Oncology Group performance status compared with patients with good performance status at the time of diagnosis of AH-MCL (P < .001). (D) Median survival was significantly shorter in patients with a platelet count <63 000 × 103/μL (9 months) vs ≥63 000 × 103/μL (44 months) (P < .001). The cutoff point of 63 000 × 103/μL was based on classification and regression tree analysis. (E) Median survival was significantly longer in patients who achieved CR after first-line treatment following the diagnosis of AH-MCL (72 months) compared with those patients who did not achieve CR (13 months) after first-line therapy (P < .001). (F) Median survival was not statistically different in patients with blastoid vs pleomorphic variants; however, there was a clear trend of better survival in the blastoid category (median survival, 34 vs 22 months in blastoid vs pleomorphic, respectively; P = .640).
Figure 2.
Comparison of OS and genomic profile (oncoprint, copy number analysis, and mutation signature) of AH-MCL subgroups: AH-DN vs AH-t. (A) Median survival was significantly longer in AH-DN than in AH-t (48 vs 14 months; P < .001). (B) Oncoprint showing pattern of somatic mutations in AH-DN (n = 31) and AH-t (n = 11) categories. Differences in the 2 groups were not statistically significant. CCND1, NOTCH1, NOTCH2, TP53, NSD2 UBR5, SMARCA4, and RANBP2 mutations were predominant in the AH-DN group, whereas KMT2D, KMT2B, and CACNA1A were predominant in the AH-t group. (C) Copy number gain (blue) and losses (red) are shown between AH-DN and AH-t categories. More frequent copy number losses were noted at chromosome 17p in the AH-DN category. (D) Mutation signature and total mutation burden is shown between the AH-DN and AH-t groups. Mutation signature 6 was predominant in the AH-t group, whereas mutational burden was higher in the AH-DN group compared with the AH-t group (P = not significant).
Table 3.
Univariate and multivariate analyses of factors associated with OS after the diagnosis of AH-MCL
| Variable | n | Events | Univariate* | Multivariate† | ||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |||
| Age, y | 183 | 115 | 1.02 | 1-1.05 | .02 | |||
| WBC, ×109/L | 177 | 111 | 1.01 | 1-1.01 | <.001 | 1.00 | 1.00-1.01 | .40 |
| Hemoglobin, g/dL | 178 | 111 | 0.85 | 0.78-0.92 | <.001 | |||
| Platelet count, ×109/L | 178 | 111 | 1.00 | 0.99-1.00 | <.001 | |||
| LDH, IU/L | 176 | 110 | 1.00 | 1.00-1.00 | .003 | |||
| β2-microglobulin, mg/L | 112 | 70 | 1.13 | 1.06-1.20 | <.001 | |||
| Ki-67, % | 161 | 101 | 1.02 | 1.01-1.02 | <.001 | |||
| AH-MCL category | <.001 | |||||||
| AH-DN‡ | 108 | 61 | 1.00 | 1.00-1.00 | ||||
| AH-t | 75 | 54 | 2.15 | 1.49-3.13 | 3.62 | 2.03-6.46 | <.001 | |
| AH-MCL subcategory | <.001 | |||||||
| Blastoid-DN | 93 | 53 | 1.00 | 1.00-1.00 | ||||
| Pleomorphic-DN | 15 | 8 | 1.17 | 0.56-2.47 | ||||
| AH-t | 75 | 54 | 2.20 | 1.49-3.24 | ||||
| PS-ECOG | <.001 | |||||||
| 0‡ | 40 | 18 | 1.00 | 1.00-1.00 | ||||
| 1 | 103 | 63 | 1.40 | 0.83-2.37 | 2.80 | 1.40-5.60 | .004 | |
| 2 | 27 | 21 | 3.34 | 1.77-6.30 | 5.83 | 2.46-13.81 | <.001 | |
| 3 | 5 | 5 | 7.00 | 2.54-19.31 | 4.82 | 1.15-20.29 | .032 | |
| 4 | 6 | 6 | 40.6 | 14.30-115.28 | 15.7 | 4.16-59.51 | <.001 | |
| B symptoms | .007 | |||||||
| No‡ | 147 | 90 | 1.00 | 1.00-1.00 | ||||
| Yes | 35 | 24 | 1.86 | 1.18-2.93 | 1.11 | 0.55-2.23 | .77 | |
| CNS involvement | .02 | |||||||
| No‡ | 175 | 109 | 1.00 | 1.00-1.00 | ||||
| Yes | 8 | 6 | 2.59 | 1.12-5.98 | 2.36 | 0.64-8.75 | .19 | |
| LDH (above the ULN), IU/L | <.001 | |||||||
| No | 96 | 50 | 1.00 | 1.00-1.00 | ||||
| Yes | 79 | 60 | 2.35 | 1.59-3.48 | ||||
| Age ≥72 y | <.001 | |||||||
| No‡ | 141 | 85 | 1.00 | 1.00-1.00 | ||||
| Yes | 42 | 30 | 2.00 | 1.31-3.06 | 1.97 | 1.18-3.27 | .009 | |
| Ki-67 ≥50% | <.001 | |||||||
| No‡ | 33 | 14 | 1.00 | 1.00-1.00 | ||||
| Yes | 128 | 87 | 3.77 | 2.09-6.81 | 2.49 | 1.32-4.69 | .005 | |
| LDH ≥1519 IU/L | <.001 | |||||||
| No‡ | 159 | 93 | 1.00 | 1.00-1.00 | ||||
| Yes | 17 | 17 | 4.50 | 2.61-7.77 | 1.52 | 0.59-3.93 | .39 | |
| β2-microglobulin ≥4 mg/L | .006 | |||||||
| No | 73 | 41 | 1.00 | 1.00-1.00 | ||||
| Yes | 39 | 29 | 1.96 | 1.20-3.20 | ||||
| Hemoglobin ≥14 g/dL | .004 | |||||||
| No‡ | 149 | 98 | 1.00 | 1.00-1.00 | ||||
| Yes | 29 | 13 | 0.44 | 0.24-0.78 | 0.89 | 0.44-1.82 | .75 | |
| Platelet count ≥63 × 109/L | <.001 | |||||||
| No‡ | 17 | 17 | 1.00 | 1.00-1.00 | ||||
| Yes | 161 | 94 | 0.24 | 0.14-0.42 | 0.39 | 0.19-0.79 | .009 | |
| Response to first-line treatment | <.001 | |||||||
| No CR | 72 | 55 | 1.00 | 1.00-1.00 | ||||
| CR | 90 | 45 | 0.29 | 0.19-0.43 | ||||
Bold values are statistically significant.
Factors not significant in univariate analysis are not shown: absolute monocyte count, absolute lymphocyte count, sex, histology type blastoid or pleomorphic, leukemic phase, bone marrow involvement, SOX-11, light chain type, and type of first-line treatment given.
Variables with >25% of missing values were excluded from this analysis.
Reference in multivariate analysis.
We also compared survival between the blastoid and the pleomorphic variants. There was no significant difference when blastoid MCL was compared with pleomorphic MCL (median, 34 vs 22 months, respectively; P = .640) (Figure 1F), and the difference was not significant when patients with blastoid morphology were compared with pleomorphic within the AH-DN and AH-t categories (supplemental Figure 2A-B).
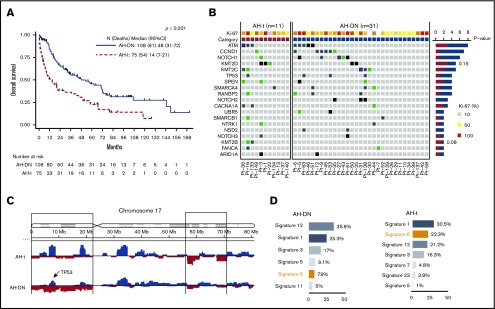
Furthermore, patients with Ki-67 ≥50% (n = 128) (Figure 3A) exhibited a significantly shorter survival (median, 20 vs 118 months, respectively; P < .001) compared with those with low Ki-67 (n = 33; Ki-67 <50%). Using multivariate analysis, we then showed that patients with Ki-67 ≥50% (hazard ratio [HR], 2.49; 95% CI, 1.32-4.69; P = .005), AH-t category (HR, 3.62; 95% CI, 2.03-6.46; P < .001), poor performance status (HR, 15.7; 95% CI, 4.16-59.51; P < .001), and age ≥72 years (HR, 1.97; 95% CI, 1.18-3.27; P = .009) were independently associated with an increased risk of death (Table 3); platelet count ≥63 000 × 103/μL (HR, 0.39; 95% CI, 0.19-0.79; P = .009) had decreased risk of death.
Figure 3.
Comparative analysis of survival and mutation spectrum according to Ki-67 (low [< 50%] vs high [≥50%]) in AH-MCL and genomic profile in patients with AH-MCL (AH-DN and AH-t) compared with nonaggressive MCL (classic variant). (A) Median survival was significantly longer in patients with low Ki-67 (118 months) vs high Ki-67 in AH-t (20 months) (P < .001). The cutoff point of 50% was based on classification and regression tree analysis. (B) Median FFS was significantly longer in patients with low Ki-67 (27 months) vs high Ki-67 (10 months; P < .001). The cutoff point of 50% was based on classification and regression tree analysis. (C) Pattern of somatic mutation distribution in AH-MCL (n = 42) vs nonaggressive MCL (n = 39). All alterations were identified by using WES. Mutation frequencies are shown as nonaggressive (light blue) and aggressive (dark blue). Genes with nonsynonymous mutations or copy number alterations in ≥2 patients are listed. CCND1 gene was mutated more frequently in the aggressive group. Mutations of UBR5, NOTCH2, and NOTCH3 were exclusively observed in the aggressive group. (D) Violin plots depicting remarkable variation in the degree of aneuploidy in AH-t vs AH-DN vs nonaggressive MCL groups. Significantly higher degree of aneuploidy was observed in AH-t and AH-DN MCL, compared with the nonaggressive category (P < .0021). Kruskal-Wallis test. Boxes in the box plot indicate interquartile range and the center line the median. (E) Pattern of somatic mutations in high (n = 30) and low (n = 10) Ki-67 categories. Differences in the 2 groups were statistically significant with a distinct somatic mutation profile in patients with high Ki-67. The bar graphs on either side show the accumulated counts of somatic alterations for each specific gene in their group. Almost all of the somatic mutations were exclusive in patients in the high Ki-67 group. CCND1 mutations were significantly higher in the high Ki-67 group. (F) The schematic diagram shows the CCND1 and NSD2 protein domains and the positions of specific mutations. The length of the line that connects the mutation annotation to the protein is directly proportional to the number of samples with the mutation. The most recurrent mutations are shown in the diagram.
Patient treatments, response, and FFS
Patients received various treatments as a first-line therapy for AH-MCL, described in Table 1. Twenty patients did not have treatment information available and were excluded from FFS analysis. Among the 25 patients who received SCT in the first-line treatment, 16 were autologous and 9 were allogeneic. Overall response rate was 78%, and CR was 56%. Response rates according to the treatment type (excluding SCT) were 86%, 75%, 69%, 50%, and 50% in rituximab with hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and cytosine arabinoside (R-HCVAD)–based regimens, R-chemotherapy, ibrutinib or other Bruton's tyrosine kinase (BTK) inhibitors, R-lenalidomide–based regimens, and miscellaneous regimens, respectively. Survival rates according to the treatment type are shown in supplemental Figure 3A (P = .179).
When patients with AH-DN and AH-t were compared, there was a significant difference in the distribution of treatment types (P < .001) (Table 1). A higher proportion of patients with AH-DN received intensive chemoimmunotherapy or R-chemotherapy with SCT compared with patients with AH-t who received ibrutinib/BTK inhibitors. The CR rate was significantly higher in AH-DN (74% vs 25%) than in AH-t (P < .001).
We then analyzed the FFS with respect to various prognostic factors and response and type of first-line treatments in AH-MCL patients, summarized in supplemental Figures 3B and 4 and supplemental Table 1. Median FFS was 13 months, and patients with AH-DN had significantly longer FFS compared with those in the AH-t category (supplemental Figure 4B). Patients with Ki-67 ≥50% had significantly shorter FFS (P < .004) (Figure 3B).
When patients with blastoid variants were compared vs those with pleomorphic variants, there was no significant difference in terms of distribution of treatments and rate of CR (57% vs 50%, respectively), but FFS was significantly longer in the blastoid category compared with the pleomorphic category (supplemental Figure 4F). Within the AH-DN category, the blastoid variant had longer FFS compared with the pleomorphic variant but not among the AH-t category (supplemental Figure 2C-D).
In univariate analysis, factors associated with increased risk of failing first-line treatments were low hemoglobin, higher Ki-67, AH-t category, LDH level ≥1519 IU/L, CNS involvement, pleomorphic variant, age ≥72 years, platelet count <63 000 × 103/μL, and lack of CR after first-line treatment (supplemental Figure 4C-E). In multivariate analysis, patients with Ki-67 ≥50%, AH-t category, and CNS involvement were associated with shorter FFS (supplemental Table 1).
Genomic features of AH-MCL and its subcategories
To demonstrate the genomic features of AH-MCL, we performed WES in evaluable samples (n = 42 [AH-DN, n = 31; AH-t, n = 11]) and compared these with a separate set of 39 patients with classic variant MCL which never transformed to AH-MCL (supplemental Table 2). Exclusively mutated genes in AH-MCL compared with the classic variant were NOTCH2, NOTCH3, and UBR5, whereas the frequency of other gene mutations was not significantly different (Figure 3C; supplemental Figure 5A). CCND1 mutations were enriched in AH-MCL (10 times more frequent in AH-MCL; P = .07). Copy number analysis revealed a decreasing degree of aneuploidy in AH-t followed by AH-DN and classic variant (P = .002) (Figure 3D). Mutation signature 6, associated with defective DNA mismatch repair, was preferentially observed in AH-MCL (21.4%) compared with classic variant (12%) (supplemental Figure 5B).
On comparing AH-DN vs AH-t (Figure 2B-D), there was no statistically significant difference in the somatic mutation profile of AH-DN vs AH-t; however, CCND1, NOTCH1, TP53, NOTCH2, SMARCA4, RANBP2, UBR5, and NSD2 mutations were predominantly observed in the AH-DN group and KMT2D, KMT2B, and CACNA1A in the AH-t group. More frequent copy number losses were noted at chromosome 17p in the AH-DN category, and mutation signature 6 (associated with defective DNA mismatch repair) was predominant in the AH-t category, indicating higher degree of genomic instability.
Because Ki-67 is an important prognostic marker in MCL, we wanted to evaluate whether the genomic profile of AH-MCL differed based on this index. Patients with high Ki-67 (≥50%) showed high tumor mutation burden and exhibited exclusive mutations in CCND1, NOTCH1, TP53, SPEN, SMARCA4, RANBP2, KMT2C, NOTCH2, NOTCH3, and NSD2 compared with low Ki-67 (<50%) (Figure 3E; supplemental Figure 5C). Because CCND1 and NSD2 mutations were enriched in high Ki-67 AH-MCL, we present the CCND1 protein and locations of point mutations in the CCND1 and NSD2 genes in Figure 3F. Interestingly, 8 of 9 CCND1 mutations were located in the N-terminal cyclin domain, and the NSD2 mutations were clustered at the c-terminal domain.
We also analyzed 1 patient with a paired sample (classic to AH-MCL) in supplemental Figure 5D. CDH19 and CHD1L truncating mutations emerged at transformation, whereas KMT2D and TRAF2 mutations were predominant at the transformation phase. Of note, we could not compare the genomic features of the blastoid vs pleomorphic variants due to lack of evaluable samples in the 2 groups.
Discussion
To the best of our knowledge, this analysis is the first and largest integrated study focusing on the clinical and genomic characteristics of blastoid/pleomorphic or AH-MCL. In general, patients with AH-MCL have inferior outcomes compared with those having the classic variant of MCL.6,9,40 Previous studies with a limited number of patients have identified some prognostic features associated with poor outcome (complex karyotype,31 TP53 mutations23) in AH-MCL. Patients with classic MCL who transform to AH-MCL after ibrutinib treatment exhibit very poor outcomes.41 In addition, patients with AH-MCL or those with high Ki-67 continue to relapse after ibrutinib/rituximab therapy.16
We have shown that the degree of aneuploidy and mutation burden were significantly higher in AH-MCL compared with classic MCL. Patients with AH-MCL frequently exhibited CCND1,21 NOTCH1, and SMARCA4 gene mutations, which are associated with lack of response to ibrutinib/venetoclax.42 It is interesting to note that CCND1 cytoplasmic localization is associated with increased adhesion and invasiveness of MCL cells,43 and truncated cyclin D1 was associated with AH-MCL.21 We showed that CCND1 gene mutations were enriched in AH-MCL. Furthermore, the presence of NOTCH2, UBR5, and NOTCH3 was exclusive to AH-MCL.
We also found a preponderance of CCND1, NOTCH2, and NSD2 mutations in AH-DN, whereas KMT2B mutations were predominant in AH-t MCL, suggesting that the epigenetic perturbations are predominant pathogenic mechanisms in transformed MCL. Patients with AH-t exhibit the highest degree of aneuploidy compared with AH-DN and the classic variant, and this difference could be related to previous treatments the patients received before transformation. Furthermore, it is highly interesting that mutation signature 6 associated with DNA mismatch repair deficiency was predominantly observed in AH-MCL and particularly in AH-t; this finding suggests that microsatellite instability status and checkpoint inhibition can be explored further in transformed MCL for potential therapeutic relevance.44
In addition, our data indicate an “ultra-high-risk” subset of patients with AH-MCL, Ki-67 proliferation index ≥50% with poor survival carrying a distinct mutation profile (CCND1, NOTCH1, TP53, SPEN, SMARCA4, RANBP2, KMT2C, NOTCH2, and NSD2 mutations), compared with patients with low Ki-67 (<50%). We chose the cutoff of 50% Ki-67 after statistical validation with classification and regression tree analysis; however, almost 80% of patients with AH-MCL exhibited Ki-67 of 50%, and only 24% had mutation analysis data available. Moreover, we hypothesize that only those patients with Ki-67 ≥50% and more than one pathogenic mutation belong to ultra-high-risk MCL, and therefore these findings should be validated in prospective studies with a larger sample size. Unfortunately, none of these mutations was potentially targetable with the currently available agents, and we could not change treatment management in any of the patients with these data; however, our data provide further directions to study epigenetic perturbations and cell cycle dysregulatory pathways in AH-MCL. We are conducting dedicated studies for detailed dissection of epigenetic pathways in AH-MCL.
Furthermore, we have systematically explored survival and prognostic factors in AH-MCL. Patients with AH-DN exhibited superior survival compared with those in the AH-t category. In addition, FFS and response rates after first-line treatments were inferior in AH-t. Some possible explanations for the superior survival of patients with AH-DN compared with AH-t MCL are because patients with AH-t MCL were previously treated with various lines of systemic therapies and therefore are expected to have poorer outcome; furthermore, AH-DN patients exhibited a preponderance of leukemic phase, higher frequency of patients undergoing SCT, higher CR rates after first-line therapy, and were previously untreated. Although we do not have IGHV somatic hypermutation status results on patients in this study, it is generally believed that patients with leukemic phase MCL45 have mutated IGHV46 and exhibit an indolent disease course. Previous studies have shown that cytarabine-based regimens and consolidation with an autologous SCT in AH-MCL can improve outcomes.13 Similar to OS, FFS was significantly longer in the AH-DN group, and this finding could be related to the higher CR rates attained after first-line treatment and frequent use of SCT in AH-DN group.
Among the first-line treatments received by these patients, it seems that the R-HCVAD–based regimen has a superior overall response rate of 86% compared with other treatments. The overall response rate of 86% is similar to results observed in a previous study in which cytarabine was added, alternating with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone before autologous SCT.6 Patients with AH-MCL have inferior responses to ibrutinib-based treatments and frequently progress. Nevertheless, our data show that intensive chemoimmunotherapy followed by SCT as consolidation provides superior survival compared with other treatments; in the absence of SCT, however, the median FFS was similar in the ibrutinib-based regimen compared with R-HCVAD alone. One of the inherent limitations of analyzing response data from this study is that the treatments were heterogeneous, conducted at various time points, and response assessment was not uniform. For the ultra-high-risk category of MCL patients, early SCT or early administration of anti-CD19 chimeric antigen receptor T-cell therapy might prove beneficial, but this hypothesis requires further validation from ongoing clinical trials. Future clinical trials will address these questions; until then, intensive chemoimmunotherapy followed by autologous SCT consolidation remains an optimal choice to treat these patients.
Finally, this study provides the first direct comparison of clinical characteristics of blastoid vs pleomorphic MCL from a large number of patients. Although the number of patients with pleomorphic histology (n = 31) is much less than those in the blastoid category (n = 152), we did not detect any significant differences in their clinical features. In one previous study8 with 62 patients with blastoid cytology (43 were pleomorphic and 19 were blastoid), study clarification among AH-t vs AH-DN was not described. It is generally hypothesized that patients with the blastoid variant have a poorer prognosis than those with the pleomorphic variant; however, we identified that patients with pleomorphic MCL have a significantly inferior FFS and a trend for worse OS compared with those with blastoid MCL. CNS involvement was more frequent (10% in pleomorphic vs 3% in blastoid) and proportion of transformed patients was higher (52%) in the pleomorphic group. This suggests that patients with pleomorphic variant MCL have a much poorer prognosis than those with blastoid MCL. Limited number and poor quality of samples precluded us from performing genomic profiling in pleomorphic MCL; hence, a dedicated comparison of genomic features of pleomorphic vs blastoid was not possible.
Another limitation of this study is the lack of FISH cytogenetics, immunoglobulin somatic hypermutation data, SOX-11 expression data, and an inadequate number of evaluable biopsy specimens for detailed genomic characterization in subsets of AH-MCL. Our molecular data are therefore largely hypothesis generating and need to be validated in larger patient cohorts. Nevertheless, to the best of our knowledge, this is the largest integrated study on this challenging aspect of MCL.
In conclusion, our analysis indicates that although outcomes are poor in blastoid/pleomorphic MCL, significant clinical and genomic heterogeneity exists within these patients. Our finding of CCND1 mutations in transformed MCL and ultra-high-risk MCL along with other epigenetic mutational perturbations requires further validation from larger patient cohorts. Blastoid/pleomorphic MCL patients remain a therapeutic challenge and therefore require dedicated prospective studies focusing on their disease biology and clinical trials with newer therapeutic modalities.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
Funding for these studies was provided in part by generous philanthropy donated to the MD Anderson Cancer Center B-Cell Lymphoma Moon Shot Program; the National Institutes of Health, National Cancer Institute (R21 CA202104) (M.L.W.); philanthropic funds from The Gary Rogers Foundation and the Fox Family Foundation; and the Institutional Research Grant from the University of Texas MD Anderson Cancer Center (L.W.).
Footnotes
Original data can be obtained by contacting the corresponding author (Michael L. Wang; e-mail: miwang@mdanderson.org).
Authorship
Contribution: M.L.W. (Principal Investigator) had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; P.J., R.K.-S., and M.L.W. conceived and designed the research; L.W. supervised the bioinformatics data analysis and data integration with clinical information; S.Z., L.W., R.W., G.H., J.Z., and X.S. performed analysis of the sequencing data; G.N.G. performed statistical data analysis; R.K.-S. and C.Y.O. reviewed pathology slides; P.J. and O.G.-P. reviewed patient charts and pathology results; P.J., S.Z., K.N., R.K.-S., L.W., and M.L.W. wrote and revised the manuscript and performed statistical analysis; all other authors participated in patient accrual, pathology sample collection, pathology reports, cytogenetic information, patient management, manuscript review, and data collection; and all authors critically reviewed and edited the manuscript for important intellectual content.
Conflict-of-interest disclosure: M.L.W. has served as a consultant and received research support from Pharmacyclics and Janssen. The remaining authors declare no competing financial interests.
Correspondence: Michael L. Wang, Department of Lymphoma and Myeloma, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: miwang@mdanderson.org.
References
- 1.Jain P, Wang M. Mantle cell lymphoma: 2019 update on the diagnosis, pathogenesis, prognostication, and management. Am J Hematol. 2019;94(6):710-725. [DOI] [PubMed] [Google Scholar]
- 2.Dreyling M, Klapper W, Rule S. Blastoid and pleomorphic mantle cell lymphoma: still a diagnostic and therapeutic challenge!. Blood. 2018;132(26):2722-2729. [DOI] [PubMed] [Google Scholar]
- 3.Chuang WY, Chang H, Chang GJ, et al. . Pleomorphic mantle cell lymphoma morphologically mimicking diffuse large B cell lymphoma: common cyclin D1 negativity and a simple immunohistochemical algorithm to avoid the diagnostic pitfall. Histopathology. 2017;70(6):986-999. [DOI] [PubMed] [Google Scholar]
- 4.Kimura Y, Sato K, Arakawa F, et al. . Mantle cell lymphoma shows three morphological evolutions of classical, intermediate, and aggressive forms, which occur in parallel with increased labeling index of cyclin D1 and Ki-67. Cancer Sci. 2010;101(3):806-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin CC, Medeiros LJ, Cromwell CC, et al. . Sequence analysis proves clonal identity in five patients with typical and blastoid mantle cell lymphoma. Mod Pathol. 2007;20(1):1-7. [DOI] [PubMed] [Google Scholar]
- 6.Hermine O, Hoster E, Walewski J, et al. ; European Mantle Cell Lymphoma Network . Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): a randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet. 2016;388(10044):565-575. [DOI] [PubMed] [Google Scholar]
- 7.Bernard M, Gressin R, Lefrère F, et al. . Blastic variant of mantle cell lymphoma: a rare but highly aggressive subtype. Leukemia. 2001;15(11):1785-1791. [DOI] [PubMed] [Google Scholar]
- 8.Hoster E, Rosenwald A, Berger F, et al. . Prognostic value of Ki-67 index, cytology, and growth pattern in mantle-cell lymphoma: results from randomized trials of the European Mantle Cell Lymphoma Network. J Clin Oncol. 2016;34(12):1386-1394. [DOI] [PubMed] [Google Scholar]
- 9.Kluin-Nelemans HC, Hoster E, Hermine O, et al. . Treatment of older patients with mantle-cell lymphoma. N Engl J Med. 2012;367(6):520-531. [DOI] [PubMed] [Google Scholar]
- 10.Damon LE, Johnson JL, Niedzwiecki D, et al. . Immunochemotherapy and autologous stem-cell transplantation for untreated patients with mantle-cell lymphoma: CALGB 59909. J Clin Oncol. 2009;27(36):6101-6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robak T, Huang H, Jin J, et al. ; LYM-3002 Investigators . Bortezomib-based therapy for newly diagnosed mantle-cell lymphoma. N Engl J Med. 2015;372(10):944-953. [DOI] [PubMed] [Google Scholar]
- 12.Chihara D, Cheah CY, Westin JR, et al. . Rituximab plus hyper-CVAD alternating with MTX/Ara-C in patients with newly diagnosed mantle cell lymphoma: 15-year follow-up of a phase II study from the MD Anderson Cancer Center. Br J Haematol. 2016;172(1):80-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geisler CH, Kolstad A, Laurell A, et al. ; Nordic Lymphoma Group . Nordic MCL2 trial update: six-year follow-up after intensive immunochemotherapy for untreated mantle cell lymphoma followed by BEAM or BEAC + autologous stem-cell support: still very long survival but late relapses do occur. Br J Haematol. 2012;158(3):355-362. [DOI] [PubMed] [Google Scholar]
- 14.Eskelund CW, Kolstad A, Jerkeman M, et al. . 15-Year follow-up of the Second Nordic Mantle Cell Lymphoma trial (MCL2): prolonged remissions without survival plateau. Br J Haematol. 2016;175(3):410-418. [DOI] [PubMed] [Google Scholar]
- 15.Rule S, Dreyling M, Goy A, et al. . Ibrutinib for the treatment of relapsed/refractory mantle cell lymphoma: extended 3.5-year follow-up from a pooled analysis. Haematologica. 2019;104(5):e211-e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain P, Romaguera J, Srour SA, et al. . Four-year follow-up of a single arm, phase II clinical trial of ibrutinib with rituximab (IR) in patients with relapsed/refractory mantle cell lymphoma (MCL). Br J Haematol. 2018;182(3):404-411. [DOI] [PubMed] [Google Scholar]
- 17.Cheah CY, George A, Giné E, et al. ; European Mantle Cell Lymphoma Network . Central nervous system involvement in mantle cell lymphoma: clinical features, prognostic factors and outcomes from the European Mantle Cell Lymphoma Network. Ann Oncol. 2013;24(8):2119-2123. [DOI] [PubMed] [Google Scholar]
- 18.Gill S, Herbert KE, Prince HM, et al. . Mantle cell lymphoma with central nervous system involvement: frequency and clinical features. Br J Haematol. 2009;147(1):83-88. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z, Dong HY, Gorczyca W, et al. . CD5- mantle cell lymphoma. Am J Clin Pathol. 2002;118(2):216-224. [DOI] [PubMed] [Google Scholar]
- 20.Slotta-Huspenina J, Koch I, de Leval L, et al. . The impact of cyclin D1 mRNA isoforms, morphology and p53 in mantle cell lymphoma: p53 alterations and blastoid morphology are strong predictors of a high proliferation index. Haematologica. 2012;97(9):1422-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiestner A, Tehrani M, Chiorazzi M, et al. . Point mutations and genomic deletions in CCND1 create stable truncated cyclin D1 mRNAs that are associated with increased proliferation rate and shorter survival. Blood. 2007;109(11):4599-4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eskelund CW, Dahl C, Hansen JW, et al. . TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood. 2017;130(17):1903-1910. [DOI] [PubMed] [Google Scholar]
- 23.Greiner TC, Moynihan MJ, Chan WC, et al. . p53 mutations in mantle cell lymphoma are associated with variant cytology and predict a poor prognosis. Blood. 1996;87(10):4302-4310. [PubMed] [Google Scholar]
- 24.Halldórsdóttir AM, Lundin A, Murray F, et al. . Impact of TP53 mutation and 17p deletion in mantle cell lymphoma. Leukemia. 2011;25(12):1904-1908. [DOI] [PubMed] [Google Scholar]
- 25.Kridel R, Meissner B, Rogic S, et al. . Whole transcriptome sequencing reveals recurrent NOTCH1 mutations in mantle cell lymphoma. Blood. 2012;119(9):1963-1971. [DOI] [PubMed] [Google Scholar]
- 26.Beà S, Valdés-Mas R, Navarro A, et al. . Landscape of somatic mutations and clonal evolution in mantle cell lymphoma. Proc Natl Acad Sci U S A. 2013;110(45):18250-18255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinyol M, Hernandez L, Cazorla M, et al. . Deletions and loss of expression of p16INK4a and p21Waf1 genes are associated with aggressive variants of mantle cell lymphomas. Blood. 1997;89(1):272-280. [PubMed] [Google Scholar]
- 28.Arakawa F, Kimura Y, Yoshida N, et al. . Identification of miR-15b as a transformation-related factor in mantle cell lymphoma. Int J Oncol. 2016;48(2):485-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parrens M, Belaud-Rotureau MA, Fitoussi O, et al. . Blastoid and common variants of mantle cell lymphoma exhibit distinct immunophenotypic and interphase FISH features. Histopathology. 2006;48(4):353-362. [DOI] [PubMed] [Google Scholar]
- 30.Espinet B, Salaverria I, Beà S, et al. . Incidence and prognostic impact of secondary cytogenetic aberrations in a series of 145 patients with mantle cell lymphoma. Genes Chromosomes Cancer. 2010;49(5):439-451. [DOI] [PubMed] [Google Scholar]
- 31.Sarkozy C, Terré C, Jardin F, et al. . Complex karyotype in mantle cell lymphoma is a strong prognostic factor for the time to treatment and overall survival, independent of the MCL international prognostic index. Genes Chromosomes Cancer. 2014;53(1):106-116. [DOI] [PubMed] [Google Scholar]
- 32.Schrader C, Meusers P, Brittinger G, et al. . Growth pattern and distribution of follicular dendritic cells in mantle cell lymphoma: a clinicopathological study of 96 patients. Virchows Arch. 2006;448(2):151-159. [DOI] [PubMed] [Google Scholar]
- 33.Hu Z, Medeiros LJ, Chen Z, et al. . Mantle cell lymphoma with MYC rearrangement: a report of 17 patients. Am J Surg Pathol. 2017;41(2):216-224. [DOI] [PubMed] [Google Scholar]
- 34.Choe JY, Yun JY, Na HY, et al. . MYC overexpression correlates with MYC amplification or translocation, and is associated with poor prognosis in mantle cell lymphoma. Histopathology. 2016;68(3):442-449. [DOI] [PubMed] [Google Scholar]
- 35.Kolodziej M, Jesionek-Kupnicka D, Braun M, et al. . Classification of aggressive and classic mantle cell lymphomas using synchrotron Fourier Transform Infrared microspectroscopy. Sci Rep. 2019;9(1):12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H, Chen Z, Miranda RN, Medeiros LJ, McCarty N. Bifurcated BACH2 control coordinates mantle cell lymphoma survival and dispersal during hypoxia. Blood. 2017;130(6):763-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L, Yao Y, Zhang S, et al. . Metabolic reprogramming toward oxidative phosphorylation identifies a therapeutic target for mantle cell lymphoma. Sci Transl Med. 2019;11(491):eaau1167. [DOI] [PubMed] [Google Scholar]
- 38.Cibulskis K, Lawrence MS, Carter SL, et al. . Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013;31(3):213-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye K, Schulz MH, Long Q, Apweiler R, Ning Z. Pindel: a pattern growth approach to detect break points of large deletions and medium sized insertions from paired-end short reads. Bioinformatics. 2009;25(21):2865-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Räty R, Franssila K, Jansson SE, Joensuu H, Wartiovaara-Kautto U, Elonen E. Predictive factors for blastoid transformation in the common variant of mantle cell lymphoma. Eur J Cancer. 2003;39(3):321-329. [DOI] [PubMed] [Google Scholar]
- 41.Jain P, Kanagal-Shamanna R, Zhang S, et al. . Long-term outcomes and mutation profiling of patients with mantle cell lymphoma (MCL) who discontinued ibrutinib. Br J Haematol. 2018;183(4):578-587. [DOI] [PubMed] [Google Scholar]
- 42.Agarwal R, Chan YC, Tam CS, et al. . Dynamic molecular monitoring reveals that SWI-SNF mutations mediate resistance to ibrutinib plus venetoclax in mantle cell lymphoma. Nat Med. 2019;25(1):119-129. [DOI] [PubMed] [Google Scholar]
- 43.Body S, Esteve-Arenys A, Miloudi H, et al. . Cytoplasmic cyclin D1 controls the migration and invasiveness of mantle lymphoma cells. Sci Rep. 2017;7(1):13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma J, Setton J, Lee NY, Riaz N, Powell SN. The therapeutic significance of mutational signatures from DNA repair deficiency in cancer. Nat Commun. 2018;9(1):3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ondrejka SL, Lai R, Smith SD, Hsi ED. Indolent mantle cell leukemia: a clinicopathological variant characterized by isolated lymphocytosis, interstitial bone marrow involvement, kappa light chain restriction, and good prognosis. Haematologica. 2011;96(8):1121-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Navarro A, Clot G, Royo C, et al. . Molecular subsets of mantle cell lymphoma defined by the IGHV mutational status and SOX11 expression have distinct biologic and clinical features. Cancer Res. 2012;72(20):5307-5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.