Key Points
A randomized placebo-controlled trial of the cysteinyl leukotriene inhibitor montelukast did not demonstrate an improvement in pain.
Abstract
Cysteinyl leukotrienes (CysLTs) are lipid mediators of inflammation. In patients with sickle cell disease (SCD), levels of CysLTs are increased compared with controls and associated with a higher rate of hospitalization for pain. We tested the hypothesis that administration of the CysLT receptor antagonist montelukast would improve SCD-related comorbidities, including pain, in adolescents and adults with SCD. In a phase 2 randomized trial, we administered montelukast or placebo for 8 weeks. The primary outcome measure was a >30% reduction in soluble vascular cell adhesion molecule 1 (sVCAM), a marker of vascular injury. Secondary outcome measures were reduction in daily pain, improvement in pulmonary function, and improvement in microvascular blood flow, as measured by laser Doppler velocimetry. Forty-two participants with SCD were randomized to receive montelukast or placebo for 8 weeks. We found no difference between the montelukast and placebo groups with regard to the levels of sVCAM, reported pain, pulmonary function, or microvascular blood flow. Although montelukast is an effective treatment for asthma, we did not find benefit for SCD-related outcomes. This clinical trial was registered at www.clinicaltrials.gov as #NCT01960413.
Visual Abstract
Introduction
Inflammation is critical to sickle cell disease (SCD) pathogenesis. Inflammatory mediators attract and activate white blood cells, promote vascular adhesion and coagulation, and sensitize neurons in the peripheral and central nervous system; all of these processes can worsen acute and chronic SCD pain.1 Reduction of inflammation, including with therapies traditionally used to treat allergic inflammation, is a proven strategy to treat or prevent SCD pain.2,3
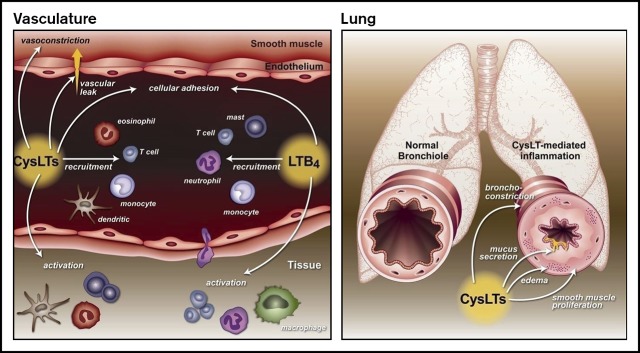
Leukotrienes (LTs), a unique family of lipids known for their role in promoting inflammation in asthma and other allergic disorders, are a potential therapeutic target for patients with SCD.4,5 The LT pathway begins with the enzymatic actions of 5-lipoxygenase on arachidonic acid, which leads to the generation of leukotriene A4 (LTA4), a common LT precursor. LTA4 is then converted to LTB4 or cysteinyl leukotrienes (CysLTs; LTC4, LTD4, and LTE4). LTB4 is primarily a leukocyte chemoattractor and activator.5 CysLTs act in the airway to promote bronchoconstriction, airway remodeling, mucus production, and smooth muscle proliferation,6-8 actions that are the basis for CysLT’s role in asthma. Clinically, levels of CysLTs correlate with asthma severity,9 asthma exacerbations,10 allergen challenge,11,12 and exercise-induced bronchoconstriction.13 In addition to the airways, CysLTs have actions elsewhere. In the vasculature, CysLTs mediate vasoconstriction14 and upregulation of cell adhesion molecules15 and increase vascular permeability.6 In the nervous system, CysLTs interact with the dorsal root ganglion to promote the development of neuropathic pain.16 Multiple cell types produce CysLTs. In the periphery, CysLTs are produced primarily by leukocytes, including mast cells, macrophages, basophils, and eosinophils. In the central nervous system, CysLTs can be produced by and bind to microglial cells, which are resident mononuclear phagocytes that are important to neuronal plasticity.17 Previously, our group examined levels of CysLTs in patients with SCD and found that they are increased compared with healthy controls, and higher levels correlate with increased rates of hospitalization for pain.18,19 Notably, these levels were not associated with asthma diagnosis. During an acute pain episode, levels of CysLTs increased further.20
Based on these data and the known actions of CysLTs, including suggestions of the involvement of CysLTs in neuropathic pain,17,21 we sought to test the hypothesis that blocking CysLTs would reduce SCD-related comorbidities, including pain. In a phase 2 study, we randomized adolescents and adults with SCD, but without asthma, to an 8-week course of montelukast or placebo. All participants were required to be on stable-dose hydroxyurea, because it is a well-established therapy for SCD pain. Our primary outcome measure was a marker of vascular injury, soluble vascular cell adhesion molecule 1 (sVCAM). Secondary measures included daily pain, pulmonary function, and microvascular blood flow, as measured by laser Doppler velocimetry (LDV).
Methods
Study conduct
This was a double-blind placebo-controlled phase 2 trial conducted at Vanderbilt University and the Medical College of Wisconsin/Froedtert Hospital. This clinical trial was registered at www.clinicaltrials.gov as #NCT01960413 (IND number 76997). The study was approved by the Institutional Review Boards at Vanderbilt University and the Medical College of Wisconsin. Study participants were block randomized (≥25 or <25 years) to receive montelukast or placebo for 8 weeks. Key eligibility criteria were HbSS/HbSβ0 thalassemia, age 16 to 60 years, on a stable dose of hydroxyurea for ≥2 months, ≥2 episodes of pain in the last 12 months, and no diagnosis of asthma.
Drug formulation
Montelukast, 10 mg, was ground and combined with microcrystalline cellulose to pack a size #1 pink opaque capsule, so that each capsule contained 10 mg of montelukast. Placebo capsules were also size #1 opaque pink and contained only microcrystalline cellulose.
Biomarkers
Blood samples for inflammatory markers were collected prerandomization, at randomization, and at weeks 4 and 8. Whole-blood samples were collected in a 5-mL light blue top citrate tube and centrifuged at 1500g for 15 minutes. Plasma was aliquoted into 4 Eppendorf tubes and immediately frozen at −80°C. Clean-catch urine samples were collected by standard methods. Urine was processed within 2 hours of collection by centrifugation (1500g, 15 minutes) to remove particulates. Urine specimens were aliquoted and frozen at −80°C. Samples were stored frozen at −80°C until shipped or analyzed. Samples from Nashville were batched and shipped on dry ice overnight by FedEx to the laboratory of J.J.F. in Milwaukee, Wisconsin. Cytokines interleukin-1β, interleukin-6, tumor necrosis factor-α, and interferon-γ were measured with a high-sensitivity human-specific multiplex cytokine assay kit (Life Technologies). To determine plasma levels of human-specific sVCAM, soluble E-selectin (sE-sel), and soluble P-selectin (sP-sel), enzyme-linked immunosorbent assay kits DVC00, DSLE00, and BBE6 were used, respectively (R&D Systems/Bio-Techne). Urine levels of LTE4 were measured by enzyme-linked immunosorbent assay and normalized to urine creatinine levels determined by colorimetric assay (both from Cayman Chemical).
Diary
Study participants completed a daily diary in which they were asked to rate their maximum daily pain score on a scale from 0 to 9 (0 is no pain and 9 is greatest pain) and whether they were having a “crisis.” The diary was initiated in a 14-day lead-in period to establish a baseline, and it was continued through the study.
Pulmonary function test
Spirometry, diffusion capacity of carbon monoxide, and lung volumes were tested according to American Thoracic Society criteria.22,23 We obtained pre- and postbronchodilator measurements (albuterol, 4 puffs from a metered dose inhaler administered by an aerochamber), using a calibrated spirometer. While seated, the participants were encouraged to perform between 5 and 8 maneuvers to obtain 3 acceptable tracings. Up to 5 maneuvers were repeated after inhalation of an albuterol inhaler. The adequacy of the blows was judged using Epidemiologic Standardization Criteria: a minimum duration of 6 seconds, back extrapolated volume <5%, forced vital capacity (FVC) within 5% or 200 cc, and assessment by the technician of the adequacy of the effort. For lung volumes, patients were tested with body plethysmography. To ensure American Thoracic Society criteria were met across the 2 participating sites for spirometry, test results were overseen a single pulmonologist.
LDV
We measured changes in blood flow with LDV (PeriFlux System 5000) assessment of the microcirculation in the patient’s forearm using standard methodology prerandomization and again at week 8.24 LDV is a noninvasive methodology that can be performed at the bedside. Two probes were adhered to the participant’s forearm, each at a position not overlying a visible large blue vein or a palpable vein and where the baseline flow is <20 PU, and a blood pressure cuff is placed on the upper arm. To characterize the hyperemic blood flow after temporary occlusion of flow (postocclusion reactive hyperemia), once a stable LDV tracing was obtained for 30 seconds, the cuff was inflated to 200 mm Hg for 3 minutes and then the pressure was released. Readings were recorded after release of occlusion to capture a postocclusive peak in the LDV tracing and after the flow becomes stable (∼2-4 minutes). The LDV tests were performed at the Medical College of Wisconsin.
Statistical analysis
Data analysis.
Continuous variables are reported as median, range, or interquartile range (IQR), and categorical variables are reported as frequencies (%). To examine the differences between the control and montelukast groups, the Fisher’s exact test was used for categorical variables, and the Mann-Whitney U test was used for continuous variables. For the primary outcome (plasma levels of sVCAM), a mixed model intent-to-treat analysis was performed to examine for trends or group differences over time. Logarithm base 10 was applied to the primary outcome to satisfy parametric assumptions about the link function. Intercept and slope (measured in weeks) were included as the random terms to estimate the deviation of each participant from the overall group, the covariance structure used was unstructured, and parameters were estimated using maximum likelihood method.
Power analysis.
We planned to accrue 25 participants in each arm. From preliminary data, the mean sVCAM level is 353 ng/mL, with a standard deviation of 104 ng/mL. If we had similar variability in the proposed study, we would have been able to detect a change ≥62 ng/mL (or 18%) with 80% power. A 2-sided P < .05 was considered significant. All analyses were conducted using SAS 9.4 or SPSS Version 24.
Results
A total of 78 patients signed consent and was screened (supplemental Figure). Of these, 19 participants failed screening and 13 withdrew before randomization, resulting in a total of 46 eligible participants who were randomized. After the randomization, 5 participants did not continue: 2 subjects were lost to follow-up, 2 participants withdrew themselves from the study, and 1 subject was withdrawn by the principal investigator. Only 1 of these subjects had a postrandomization measure of the primary outcome, sVCAM. A total of 41 participants completed the study (Table 1). Of those, 20 received montelukast, and 21 received placebo. Seventeen participants were male, and median age was 26.5 years. There were no differences in age, sex, laboratory values, or number of painful episodes requiring hospital admission in the 3 years prior to study between the montelukast and control groups.
Table 1.
Patient demographics
| Placebo (n = 21) | Montelukast (n = 20) | P | |
|---|---|---|---|
| Male, n (%) | 8 (38) | 9 (45) | .76 |
| Age, median (range), y | 27 (19-52) | 26.5 (16-64) | .96 |
| Hospitalizations in last 3 y, n | 2 (0-3) | 3 (1.5-7) | .13 |
| ED visits in last 3 y, n | 2 (1-4) | 5 (1.5-7) | .16 |
| Admissions for pain crises in last 3 y, n | 7.5 (4-15) | 10 (6.5-20) | .46 |
| WBC, ×109/L | 7.1 (5.5-8.6) | 7.6 (5.5-9.4) | .50 |
| Hb, g/dL | 8.7 (8.2-9.7) | 9.1 (8.3-9.9) | .34 |
| Reticulocyte count (%), % | 8.85 (5.50-13.1) | 9.15 (8.35-9.9) | .53 |
Hb, hemoglobin; WBC, white blood cell count.
Unless otherwise noted, data are median (IQR).
Plasma markers of vaso-occlusive crisis–associated tissue injury
The mixed-model analysis, used for an intent-to-treat analysis (46 total patients, 42 of whom had an end-of-study sVCAM measurement), found that there was no significant group difference or trend over time. The change in plasma levels in sVCAM before and after montelukast vs placebo was not significantly different between the groups (P = .16; Table 2). There also was no difference in plasma sP-sel levels (P = .98) or sE-sel levels (P = .88) between the montelukast and placebo groups before and after 8 weeks of treatment (Table 2).
Table 2.
Outcome measures in patients treated with placebo and montelukast
| Placebo (n = 20) | Montelukast (n = 20) | P | |
|---|---|---|---|
| sVCAM, ng/mL | |||
| Prerandomization | 945.0 (814.0-1254.5) | 956.0 (694.0-1080.0) | .44 |
| Week 8 | 900.5 (865.5-1403.5) | 908.5 (668.0-1158.5) | .37 |
| % Change | 4.17 (−3.3 to +12.1) | −3.2 (−13.6 to +11.6) | .16 |
| sP-sel, ng/mL | |||
| Prerandomization | 44.5 (35.0-63.5) | 49.5 (43.0-62.5) | .24 |
| Week 8 | 46.5 (31.0-52.5) | 49.5 (35.5-59.5) | .18 |
| % Change | −6.2 (−21.1 to +14.7) | −7.7 (−21.4 to +11.2) | .98 |
| sE-sel, ng/mL | |||
| Prerandomization | 45.0 (31.5-61.0) | 52.5 (33.0-65.5) | .47 |
| Week 8 | 44.0 (30.5-55.0) | 49.5 (34.0-55.0) | .53 |
| % Change | −7.4 (−14.8 to 0) | −2.5 (−18.5 to +8.9) | .88 |
| LTE4, pg/mg | |||
| Prerandomization | 2145.5 (1607.0-2643.0) | 2055.5 (1579.0-2493.5) | .66 |
| Week 8 | 1920.5 (1666.0-2339.0) | 1787.5 (1480.5-2196.5) | .39 |
| % Change | −3.1 (−21.3 to +33.0) | −10.0 (−29.7 to +28.6) | .74 |
| Daily mean pain score | |||
| Prerandomization | 5.0 (3.8-5.9) | 4.3 (4.0-5.0) | .20 |
| Postrandomization | 5.2 (3.9-5.8) | 4.6 (3.2-6.3) | .29 |
| % Change | −3.8 (−12.9 to +15.4) | 5.7 (−7.7 to +31.8) | .42 |
| Daily median pain score | |||
| Prerandomization | 5.0 (4.0-6.0) | 4.0 (4.0-5.0) | .29 |
| Postrandomization | 5.0 (4.0-6.0) | 4.0 (3.0-5.5) | .20 |
| % Change | 0 (−16.7 to +15.5) | 0 (0-33.3) | .53 |
| Pain days with pain score ≥5, % | |||
| Prerandomization | 15.5 (6.7-42.9) | 13.4 (0-23.8) | .31 |
| Postrandomization | 14.9 (3.5-31.5) | 4 (1.8-13.2) | .09 |
| % Change | −1.5 (−4.7 to +1.4) | −2.5 (−12.5 to +2.6) | .78 |
| Days with pain crisis episode, % | |||
| Prerandomization | 4.6 (0-16.7) | 0 (0-0) | .024 |
| Postrandomization | 3.5 (0-15.8) | 0 (0-2.5) | .030 |
| % Change | 0 (−4.6 to +3.5) | 0 (0-1.5) | .58 |
| FEV1 predicted, % | |||
| Prerandomization | 83.0 (74.0-91.0) | 92.0 (81.0-100.5) | .06 |
| Week 8 | 82.0 (70.0-94.0) | 86.5 (77.0-100.0) | .22 |
| % Change | −1.2 (−4.1 to +4.1) | −2.2 (−8.6 to +1.64) | .84 |
| FVC predicted, % | |||
| Prerandomization | 82.0 (80.0-95.0) | 96 (87.5-106.0) | .024 |
| Week 8 | 84 (75-99) | 92.5 (85.5-103.5) | .09 |
| % Change | 1.0 (−1.9 to +4.0) | −1.5 (−4.3 to +3.8) | .35 |
| FEV1/FVC predicted, % | |||
| Prerandomization | 83.0 (78.0-98.0) | 86.0 (80.0-93.5) | .73 |
| Week 8 | 84.0 (75.0-98.0) | 85.0 (78.0-96.5) | .0 |
| % Change | 0 (−3.9 to +1.4) | 1.8 (−1.7 to +2.9) | .27 |
| TLC predicted, % | |||
| Prerandomization | 88.0 (79.0-92.0) | 99.0 (88.0-114.0) | .044 |
| Week 8 | 88.0 (79.0-97.0) | 94.0 (87.0-109.0) | .17 |
| % Change | 2.9 (0-5.1) | 0 (−9.1 to +6.8) | .65 |
| TH1, s | |||
| Prerandomization | 3.2 (2.5-4.1) | 4.3 (2.6-7.4) | .9755 |
| Week 8 | 2.5 (2.3-2.9) | 3.1 (2.5-7.6) | .10 |
| % Change | −34.1 (−49.2 to −18.0) | 2.9 (−8.8 to +74.8) | .07 |
Twenty-one patients underwent LDV (10 placebo and 11 montelukast). TLC predicted values were available for 20 patients (9 placebo and 11 montelukast). Data are median (IQR).
FEV1, forced expiratory volume in 1 second; TH1, time to half-peak of hyperemia.
Urine measurements
Levels of urine LTE4 were not significantly different between the montelukast and placebo groups before and after 8 weeks of treatment (P = .74; Table 2).
Pain outcomes
Prior to randomization, when participants maintained a 14-day pain diary, there were fewer reported pain crisis episodes in the montelukast group compared with the placebo group. There was no change in any of the pain outcomes after 8 weeks of treatment compared with before treatment in the montelukast and placebo groups (Table 2). We also examined the montelukast-treated group by itself to determine whether any of the biomarkers pretreatment would predict pain response to montelukast but did not find any association between pre-LTE4, sVCAM, p-selectin, substance P levels, or white blood cell count and improvement in any pain parameter (percentage change in mean or median pain score or percentage of days with pain score > 5; data not shown).
Pulmonary function
There was a difference in FVC between the montelukast and placebo groups prerandomization (P = .024). There was no significant group difference in the percentage of change in forced expiratory volume in 1 second, FVC, or forced expiratory volume in 1 second/FVC (Table 2).
LDV
Twenty-one participants in Milwaukee completed the LDV test: 10 in the placebo group and 11 in the montelukast group. There was no significant increase in microvascular blood flow in any measured parameter using LDV in the group receiving montelukast vs placebo (P = .07). The group comparisons of time to half-peak of hyperemia are shown in Table 2.
Discussion
Montelukast is a CysLT receptor 1 inhibitor that is widely used for the treatment of asthma. Members of our investigative group have demonstrated that a diagnosis of asthma is associated with an increased rate of pain, acute chest syndrome, and mortality in children with SCD.25,26 It would not be surprising if montelukast improved asthma symptoms in patients with SCD. However, in this study, we sought to determine whether montelukast would improve SCD comorbidities, such as inflammation and pain, independent of its effects in the lung. We found that, in subjects treated with montelukast vs placebo, there was no difference in markers of vascular inflammation, pain, pulmonary function, or microvascular blood flow.
LTs are a unique family of lipids known for their roles in promoting inflammation.5 The production of LTs begins when 5-lipoxygenase converts arachidonic acid to LTA4, which is subsequently converted to LTB4 or CysLTs (LTC4, LTD4, and LTE4). LTB4 and CysLTs have been reported to be elevated in SCD patients compared with healthy controls, and levels of CysLTs increase further during acute pain episodes.18-20,27 One mechanism for increased LT production in SCD involves increased secretion of placenta growth factor by erythroid cells.28 Placenta growth factor released into the circulation targets mononuclear cells, including mast cells, causing hypoxia-independent activation of HIF-1α and stimulating LT production and cytochemokine expression.28
There is evidence that LTs can promote pain. In animal models, CysLTs and CysLT receptors are expressed by microglial cells in the spinal cord; their activation could, in turn, activate dorsal root ganglions.29 Studies in rat models of spinal cord injury and chronic constriction injury demonstrated that blocking CysLT receptor 1 with montelukast or pranlukast decreased the threshold at which mechanical and thermal withdrawal occurred during quantitative sensory testing.30 Another study confirmed these results, demonstrating that montelukast could prevent the development of neuropathic pain after ischemia-reperfusion injury in rats.31 In 1 of the few clinical studies to date, children treated with montelukast for the management of postoperative tonsillectomy pain required a smaller amount of rescue analgesics.32 In another study, montelukast had an antinociceptive effect in children and adolescents with dyspepsia and duodenal eosinophilia.33
The reason why montelukast did not affect pain in our study is not clear. Potentially, the increased levels of CysLTs that we demonstrated previously are a marker of acute pain events but are not involved in their pathogenesis. Acute and chronic pain in SCD has a unique and complex pathogenesis; the role of CysLTs that has been established in animal models and patients without SCD may not be the same in SCD. Other possibilities are (1) the confounding effects of nonsteroidal anti-inflammatory drugs or aspirin on pain or biomarkers, (2) montelukast does not result in therapeutic levels in the central nervous system, and (3) our sample size was not large enough to detect differences in pain, especially because our study was not powered to detect differences in pain. The lower percentage of days with pain crises in the montelukast group also could have affected our ability to find an effect of the drug. Finally, it is also possible that the biology accounting for the chronic nature of SCD pain may not be reversible with montelukast.
Although this study did not have positive findings, it was an important one to conduct given the preliminary evidence that demonstrated an association between elevated levels of CysLTs and acute SCD pain. Montelukast might still have an important role in the management of asthma in patients with SCD, and the findings of this study should not dissuade providers from using it in that setting.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Debora Nischik and Zora Jovanovich for contributions to the study.
This work was supported by US Food and Drug Administration grant R01 FD004117 (J.J.F. and M.R.D.).
Footnotes
Data sharing requests should be sent to Joshua J. Field (e-mail: jfield@versiti.org).
Authorship
Contribution: J.J.F. and M.R.D. conceived and designed the studies and interpreted the data; A.K., A.B., S.H.E., N.M., K.W., V.B., L.Z., and P.S. acquired and analyzed data; J.J.F. wrote the manuscript; and M.R.D., A.K., A.B., S.H.E., and P.S. edited the manuscript.
Conflict-of-interest disclosure: J.J.F. conducts research studies with Cyclerion Therapeutics and Rigel Pharmaceuticals and has been a paid consultant for Ironwood Pharmaceuticals. S.H.E. and N.M. are employees of and stockholders in Vanguard Therapeutics, Inc. The remaining authors declare no competing financial interests.
Correspondence: Joshua J. Field, Versiti Wisconsin, 8733 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: jfield@versiti.org.
References
- 1.Zhang D, Xu C, Manwani D, Frenette PS. Neutrophils, platelets, and inflammatory pathways at the nexus of sickle cell disease pathophysiology. Blood. 2016;127(7):801-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballas SK, Gupta K, Adams-Graves P. Sickle cell pain: a critical reappraisal. Blood. 2012;120(18):3647-3656. [DOI] [PubMed] [Google Scholar]
- 3.Glassberg J, Minnitti C, Cromwell C, et al. Inhaled steroids reduce pain and sVCAM levels in individuals with sickle cell disease: a triple-blind, randomized trial. Am J Hematol. 2017;92(7):622-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Field JJ, DeBaun MR. Asthma and sickle cell disease: two distinct diseases or part of the same process? Hematology Am Soc Hematol Educ Program. 2009;2009;45-53. [DOI] [PubMed] [Google Scholar]
- 5.Knight-Perry J, DeBaun MR, Strunk RC, Field JJ. Leukotriene pathway in sickle cell disease: a potential target for directed therapy. Expert Rev Hematol. 2009;2(1):57-68. [DOI] [PubMed] [Google Scholar]
- 6.Joris I, Majno G, Corey EJ, Lewis RA. The mechanism of vascular leakage induced by leukotriene E4. Endothelial contraction. Am J Pathol. 1987;126(1):19-24. [PMC free article] [PubMed] [Google Scholar]
- 7.Lynch KR, O’Neill GP, Liu Q, et al. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 1999;399(6738):789-793. [DOI] [PubMed] [Google Scholar]
- 8.Rabinovitch N, Zhang L, Gelfand EW. Urine leukotriene E4 levels are associated with decreased pulmonary function in children with persistent airway obstruction. J Allergy Clin Immunol. 2006;118(3):635-640. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC) Asthma prevalence and control characteristics by race/ethnicity--United States, 2002. MMWR Morb Mortal Wkly Rep. 2004;53(7):145-148. [PubMed] [Google Scholar]
- 10.Chavis C, van Vyve T, Chanez P, et al. Leukotriene E4 plasma levels in adult asthmatic patients with variable disease severity. Allergy. 1997;52(5):589-592. [DOI] [PubMed] [Google Scholar]
- 11.Kumlin M, Dahlén B, Björck T, Zetterström O, Granström E, Dahlén SE. Urinary excretion of leukotriene E4 and 11-dehydro-thromboxane B2 in response to bronchial provocations with allergen, aspirin, leukotriene D4, and histamine in asthmatics. Am Rev Respir Dis. 1992;146(1):96-103. [DOI] [PubMed] [Google Scholar]
- 12.Manning PJ, Rokach J, Malo JL, et al. Urinary leukotriene E4 levels during early and late asthmatic responses. J Allergy Clin Immunol. 1990;86(2):211-220. [DOI] [PubMed] [Google Scholar]
- 13.Kikawa Y, Miyanomae T, Inoue Y, et al. Urinary leukotriene E4 after exercise challenge in children with asthma. J Allergy Clin Immunol. 1992;89(6):1111-1119. [DOI] [PubMed] [Google Scholar]
- 14.Drazen JM, Austen KF, Lewis RA, et al. Comparative airway and vascular activities of leukotrienes C-1 and D in vivo and in vitro. Proc Natl Acad Sci USA. 1980;77(7):4354-4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Datta YH, Romano M, Jacobson BC, Golan DE, Serhan CN, Ewenstein BM. Peptido-leukotrienes are potent agonists of von Willebrand factor secretion and P-selectin surface expression in human umbilical vein endothelial cells. Circulation. 1995;92(11):3304-3311. [DOI] [PubMed] [Google Scholar]
- 16.Zhao B, Wang H, Li CX, et al. GPR17 mediates ischemia-like neuronal injury via microglial activation. Int J Mol Med. 2018;42(5):2750-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noguchi K, Okubo M. Leukotrienes in nociceptive pathway and neuropathic/inflammatory pain. Biol Pharm Bull. 2011;34(8):1163-1169. [DOI] [PubMed] [Google Scholar]
- 18.Field JJ, Krings J, White NL, et al. Urinary cysteinyl leukotriene E(4) is associated with increased risk for pain and acute chest syndrome in adults with sickle cell disease. Am J Hematol. 2009;84(3):158-160. [DOI] [PubMed] [Google Scholar]
- 19.Jennings JE, Ramkumar T, Mao J, et al. Elevated urinary leukotriene E4 levels are associated with hospitalization for pain in children with sickle cell disease. Am J Hematol. 2008;83(8):640-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Field JJ, Strunk RC, Knight-Perry JE, Blinder MA, Townsend RR, DeBaun MR. Urinary cysteinyl leukotriene E4 significantly increases during pain in children and adults with sickle cell disease. Am J Hematol. 2009;84(4):231-233. [DOI] [PubMed] [Google Scholar]
- 21.Okubo M, Yamanaka H, Kobayashi K, Fukuoka T, Dai Y, Noguchi K. Expression of leukotriene receptors in the rat dorsal root ganglion and the effects on pain behaviors. Mol Pain. 2010;6:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Thoracic Society American Thoracic Society. Single-breath carbon monoxide diffusing capacity (transfer factor). Recommendations for a standard technique--1995 update. Am J Respir Crit Care Med. 1995;152(6 Pt 1):2185-2198. [DOI] [PubMed] [Google Scholar]
- 23.Standardization of spirometry, 1994 update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107-1136. [DOI] [PubMed] [Google Scholar]
- 24.Bachir D, Maurel A, Beuzard Y, et al. Improvement of microcirculation abnormalities in sickle cell patients upon buflomedil treatment. Microvasc Res. 1993;46(3):359-373. [DOI] [PubMed] [Google Scholar]
- 25.Boyd JH, Macklin EA, Strunk RC, DeBaun MR. Asthma is associated with acute chest syndrome and pain in children with sickle cell anemia. Blood. 2006;108(9):2923-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyd JH, Macklin EA, Strunk RC, DeBaun MR. Asthma is associated with increased mortality in individuals with sickle cell anemia. Haematologica. 2007;92(8):1115-1118. [DOI] [PubMed] [Google Scholar]
- 27.Setty BN, Stuart MJ. Eicosanoids in sickle cell disease: potential relevance of neutrophil leukotriene B4 to disease pathophysiology. J Lab Clin Med. 2002;139(2):80-89. [DOI] [PubMed] [Google Scholar]
- 28.Patel N, Gonsalves CS, Yang M, Malik P, Kalra VK. Placenta growth factor induces 5-lipoxygenase-activating protein to increase leukotriene formation in sickle cell disease. Blood. 2009;113(5):1129-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okubo M, Yamanaka H, Kobayashi K, Noguchi K. Leukotriene synthases and the receptors induced by peripheral nerve injury in the spinal cord contribute to the generation of neuropathic pain. Glia. 2010;58(5):599-610. [DOI] [PubMed] [Google Scholar]
- 30.Zhou C, Shi X, Huang H, Zhu Y, Wu Y. Montelukast attenuates neuropathic pain through inhibiting p38 mitogen-activated protein kinase and nuclear factor-kappa B in a rat model of chronic constriction injury. Anesth Analg. 2014;118(5):1090-1096. [DOI] [PubMed] [Google Scholar]
- 31.Muthuraman A, Ramesh M, Sood S. Ameliorative potential of montelukast on ischemia-reperfusion injury induced vasculitic neuropathic pain in rat. Life Sci. 2012;90(19-20):755-762. [DOI] [PubMed] [Google Scholar]
- 32.Ince I, Yoruk O, Ahiskalioglu A, Aksoy M, Dostbil A, Celik M. Does montelukast have an effect on post-tonsillectomy pain control in children? A randomized trial study. Otolaryngol Head Neck Surg. 2015;153(2):269-274. [DOI] [PubMed] [Google Scholar]
- 33.Friesen CA, Kearns GL, Andre L, Neustrom M, Roberts CC, Abdel-Rahman SM. Clinical efficacy and pharmacokinetics of montelukast in dyspeptic children with duodenal eosinophilia. J Pediatr Gastroenterol Nutr. 2004;38(3):343-351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.