Abstract
Homotypic and heterotypic interactions between cells are of crucial importance in multicellular organisms for the maintenance of physiological functions. Accordingly, changes in cell-to-cell communication contribute significantly to tumor development. Cancer cells engage the different components of the tumor microenvironment (TME) to support malignant proliferation, escape immune control, and favor metastatic spreading. The interaction between cancerous and non-cancerous cell types within tumors occurs in many ways, including physical contact and paracrine signaling. Furthermore, local and long-range transfer of biologically active molecules (e.g., DNA, RNA, and proteins) can be mediated by small extracellular vesicles (EVs) and this has been shown to influence many aspects of tumor progression. As it stands, there is a critical need for suitable experimental systems that enable modeling the cell-to-cell communications occurring in cancer. Given their intrinsic complexity, animal models represent the ideal system to study cell-to-cell interaction between different cell types; however, they might make difficult to assess individual contribution to a given phenotype. On the other hand, simplest experimental models (i.e., in vitro culture systems) might be of great use when weighing individual contributions to a given phenomenon, yet it is imperative that they share a considerable number of features with human cancer. Of the many culture systems available to the scientific community, patient-derived organoids already proved to faithfully recapitulate many of the traits of patients’ disease, including genetic heterogeneity and response to therapy. The organoid technology offers several advantages over conventional monolayer cell cultures, including the preservation of the topology of cell-to-cell and cell-to-matrix interactions as observed in vivo. Several studies have shown that organoid cultures can be successfully used to study interaction between cancer cells and cellular components of the TME. Here, we discuss the potential of using organoids to model the interplay between cancer and non-cancer cells in order to unveil biological mechanisms involved in cancers initiation and progression, which might ultimately lead to the identification of novel intervention strategy for those diseases.
Keywords: tumor microenvironment, co-culture systems, extracellular vesicles, organoids, extracellular matrix
Introduction
Homotypic and heterotypic interactions between cells are of crucial importance in multicellular organisms for the maintenance of physiological functions, including embryonic development, neurotransmission, wound healing, and inflammation. An individual cell can interact with a heterogeneous group of cells in multiple ways, that is through physical contact, ligand–receptor interactions, cellular junctions or through secreted mediators. Non-cellular components of the extracellular microenvironment add another layer of complexity to this phenomenon. Indeed, the extracellular matrix (ECM) has many functions besides providing mechanical support for organs, tissues, and individual cells. ECM, which is rich in collagens, proteoglycans, hyaluronic acid, laminins and fibronectin, is constantly remodeled in physiological processes, especially during development and wound repair, as well as in many diseases. Its degradation and reassembly regulate cell proliferation, differentiation, survival, and migration (Daley et al., 2008). ECM proteins can bind either to adhesion receptors (e.g., integrins) to transduce intracellular signals or growth factors to regulate their presentation to cells (Hynes, 2009). The cell communication process and its regulation acquire major importance in the cancer contest, where cancer cells interact and communicate with a variety of non-cancerous cells, including normal epithelial cells, immune cells, endothelial cells and fibroblasts, which collectively constitute the tumor microenvironment (TME). In addition, recent evidences suggest a major role for a cell-to-cell communication system that involve extracellular vesicles (EVs) transferring biologically active molecules, which can substantially modify the cellular behavior of recipient cells (Ratajczak et al., 2006; Valadi et al., 2007). As it stands, complex interactions that occur in the TME need to be taken into account when modeling cancer. Driven by recent advances in cell culture technology and EVs isolation methods, we review how next generation culture systems, in particular organoids, represent a promising tool to help modeling cell-to-cell communication. Finally, we highlight some of the challenges that researchers are still facing with organoid cultures, including lack of standardization of ECM and media being used to support organoid formation and expansion.
Models to Study Cancer Cells Interactions Within the TME
Animal models represent the gold standard to study interactions between cancer cells and the microenvironment. Transplantation of patient-derived tumor’ tissue or cells in immunocompromised mice (Patient-derived xenografts, PDXs) is widely used in preclinical studies to model drug responses. Even if PDXs are considered a good avatar of primary human tumors, recent evidences suggest that generation of xenografts is associated to selection of genetic clones (Eirew et al., 2015; Ben-David et al., 2017). Furthermore, PDXs fail to properly recapitulate the interaction with the human immune system due to species-specific differences. Genetically engineered mouse models (GEMMs) spontaneously develop tumors in an immune-proficient microenvironment, yet their fast progression and homogeneous genetic background fail to replicate properly the heterogeneity of human diseases (Cheon and Orsulic, 2011). Humanized immune reconstituted (HIR) mice offer the possibility of transplanting patients’ tumors in a host that at least partially recapitulate the human immune system, thus providing a more realistic representation of the disease. Nonetheless, the use of HIR mice models is less straightforward, due to species-specific differences and incorrect representation of the patients’ immune system (Rangarajan and Weinberg, 2003).
Although animal models provide a more realistic platform to model the disease as a whole, their intrinsic complexity makes difficult assessing individual contributions to a given phenotype. On the other hand, in vitro two-dimensional (2D) and three-dimensional (3D) cell culture systems represent a facile platform to understand causative relationships in cancer through different type of perturbation analyses (i.e., genetic and non-genetic).
Conventional monolayer cell culture systems have been of tremendous importance for the current understanding of many diseases, including cancers; however, they suffer from several limitations making them inappropriate to correctly model patients’ tumors. A comprehensive evaluation of the advantages of 3D culture systems over 2D systems is beyond the scope of this review, and it has been extensively described elsewhere (Fong et al., 2016; Avnet et al., 2019; D’Agosto et al., 2019; Yang et al., 2019).
The most commonly used 3D culture models are spheroids and organoids. Spheroids are cell aggregates or spheres cultured primarily in suspension, which are likely enriched for stem-like cell population (Weiswald et al., 2015). This technology can be applied to both cancer cell lines and patient-derived tumor cells, but it is not applicable to normal cells from many tissues. The use of spheroids ranges from drug screening to modeling immune interactions (Katt et al., 2016). Spheroids partially compensate deficiencies of monolayer cultures; the formation of an aggregate creates a gradient of nutrients, oxygen and metabolites, and models cell-to-cell and cell-to-matrix interactions (Costa et al., 2016). Beyond a few exceptions (i.e., secretory acini spheroids) (Wu et al., 2011), the random aggregations of cells, and the consequent lack of organization in tissue-like structures, makes spheroids poor models of epithelial tissues (Torras et al., 2018). On the other hand, we defined organoids as 3D cultures derived directly from the dissociation of specialized epithelial tissues, from embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs), all showing self-renewal and self-organization capabilities (Lancaster and Knoblich, 2014). Furthermore, organoids are capable of preserving many relevant features of in vivo tissue physiology. Studies of tissue morphogenesis and early 3D cultures of mouse mammary primary cells (Moscona and Moscona, 1952; Bissell, 1981) set the ground for the subsequent development of the organoid technology. The laboratory of Mina Blissell was the first to show that primary epithelial cells from mouse mammary glands could self-organize in glandular structures and express milk proteins when cultured in a basement membrane matrix (BM). The BM used in that seminal work was isolated from mouse Engelbreth-Holm-Swarm (EHS) sarcoma (Barcellos-Hoff et al., 1989), currently branded as Matrigel® (Swarm, 1963; Orkin et al., 1977), and mainly composed by a mixture of collagen type IV, laminin, heparan sulfate proteoglycans, and entactin.
Few years later, the same method was applied to the propagation of human cells derived from both normal and tumoral tissues (Petersen et al., 1992). Notwithstanding the important earlier studies, the first organotypic models were reported in 2008 and in 2009 to enable growing cortical neurons (Eiraku et al., 2008) and intestinal epithelium (Sato et al., 2009), respectively. In 2009, the Clevers’ group (Sato et al., 2009) described the method for the generation of organoid cultures from individual Lgr5+ stem cells isolated from mouse intestinal tissue. The Lgr5+ cells are embedded in Matrigel® and overlaid with a defined culture medium supplemented with several growth factors and morphogens that substitute for stromal cues. The resulting organoids consisted of a monolayer of epithelial cells surrounding a central lumen as well as of protrusions containing stem cells and differentiated Paneth cells that form the stem cells niche. Hereafter, the procedure was adapted for the generation of organoids from human small intestine (Sato et al., 2011), which showed some differences compared to mouse organoids: human organoids exhibited a more cyst-like morphology with a concomitant increased need for Wnt signaling stimulation. The establishment of organoid cultures have been later reported from several human normal epithelial tissues (Barker et al., 2010; Sato et al., 2011; Huch et al., 2013a, b; Lancaster et al., 2013; Pringle et al., 2016). The possibility of growing normal epithelial cells without the need for genetic immortalization (e.g., through exogenous expression of viral genes) probably represents one of the major advantages of the organoid technology. Normal tissue-derived organoids might be used to study the mutual influence between healthy and cancer cells and to discover cancer-specific interactions within the TME. Nonetheless, they offer new opportunities for regenerative medicine applications (Grassi et al., 2019). Building on the seminal studies of the Clevers’ group, modifications of the original procedure were used for culturing human organoids derived from hPSCs (Spence et al., 2011) as well as from neoplastic tissues. To date, patient-derived organoids have been established from different cancer tissues, including colon (Sato et al., 2011), brain (Linkous et al., 2019), gastric (Wang K. et al., 2014), pancreatic (Boj et al., 2015), prostate (Drost et al., 2016), liver (Broutier et al., 2017), breast (Sachs et al., 2018), esophageal (Li et al., 2018), kidney (Schutgens et al., 2019), and lung (Sachs et al., 2019) cancers. Another organoid culture system has been reported by the Calvin Kuo’s lab, known as air liquid interface (ALI) system (Ootani et al., 2009). In this system, organoids are grown in a stromal matrix constituted by type I collagen that has an apical side, exposed to the air, and a basolateral side in contact with the media (Ootani et al., 2009). Although they first applied it to mouse-derived normal intestinal epithelia, the same method was then applied to normal and tumoral tissues derived from both mouse and human (Li et al., 2014; Usui et al., 2016, 2018; Yokobori et al., 2016; Giandomenico et al., 2019).
Tumor Organoid Co-Cultures Model the Interactions With TME
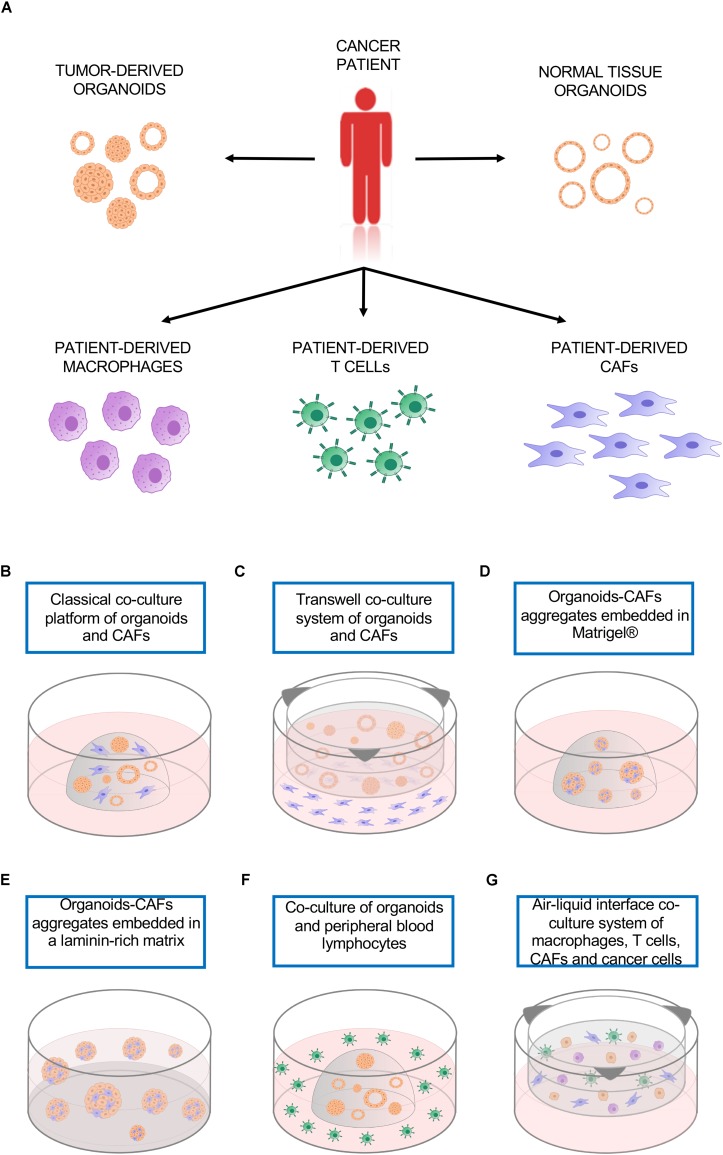
Organotypic cultures, like 2D cell lines, usually contain only one cell type representing the neoplastic epithelium, therefore lacking the multi-cellular representation of the TME. This limitation can be partly solved by the ability of patient-derived cancer organoids to be easily co-cultured with a variety of cell types, including patient-derived immune cells or cancer-associated fibroblast (CAFs) (Figure 1A; Lee et al., 2007; Marusyk et al., 2016; Ohlund et al., 2017; Dijkstra et al., 2018; Neal et al., 2018; Seino et al., 2018). 3D co-culture system offers a promising tool to model the interactions between tumor cells and the other cells that compose the TME.
FIGURE 1.
Co-culture platforms. (A) Tumor organoids as well as cultures of different non-neoplastic cell types can be established from the same patient and used to model cell-to-cell interactions. (B) Classical co-culture platform in which organoids and CAFs are embedded in the same drop of Matrigel®. (C) Transwell co-culture system in which organoids are embedded in a matrix on top of a transwell insert, CAFs are plated as monolayer culture. (D) Organoids-CAFs aggregates embedded in a drop of Matrigel®. (E) Organoids-CAFs aggregates embedded in a laminin-rich matrix on a Matrigel® precoated well. (F) Co-culture of organoids and peripheral blood lymphocytes, the organoids are embedded in a drop of Geltrex® while the lymphocytes are in suspension in the culture media. (G) Air-liquid interface co-culture system, patient-derived macrophages, T cells, CAFs, and cancer cells are embedded in collagen type I on top of a permeable insert precoated with collagen matrix. CAFs, cancer-associated fibroblasts.
Co-cultures With CAFs
Cancer-associated fibroblast constitute the major cellular component of the TME in many tumors (LeBleu and Kalluri, 2018) and can be recruited at the tumor site or differentiated from resident mesenchymal cells. Their increased expression/secretion of tumor-promoting factors, collagen, and other ECM proteins distinguish them from the normal fibroblast population. CAFs influence tumor growth and metastasis by several mechanisms. Via matrix synthesis and remodeling, they can modulate ECM stiffness and hypoxia facilitating tumor cells mobility and invasion. Through secreted molecules, such as growth factors, cytokines, chemokines and microRNAs (miRNAs), CAFs support tumor growth and induce resistance to therapy (Karagiannis et al., 2010; Zhang et al., 2018). Moreover, a direct cell-to-cell contact between CAFs and cancer cells, mediated by N-cadherin/E-cadherin adhesion complex, has been recently proved to promote tumor invasion (Labernadie et al., 2017). However, CAFs targeting experiments evidenced a more complex response than expected. For instance, the ablation of contractile isoform of alpha smooth muscle actin (α-SMA) cells in a mouse model of pancreatic ductal adenocarcinoma (PDAC) was found to reduce cancer desmoplasia, yet resulted in more aggressive and highly undifferentiated tumors, suggesting that the presence of this cell type influence the trajectory of tumor development and progression (Ozdemir et al., 2014). Similar results were obtained with the deletion of Sonic Hedgehog I, a ligand that enhances the formation of fibroblast-rich desmoplastic stroma in PDAC (Rhim et al., 2014). The contradictory results evidence the need to investigate more in deep the different subgroups of CAFs and their functions.
Pancreatic cancer organoids exemplify a culture system that can be used to model interaction between cancer cells and CAFs. Ohlund et al. (2017) developed a co-culture system that is able to recapitulate some of the features of the desmoplastic reaction as observed in patients. They demonstrated the existence of two CAFs subpopulations, which are differentially located in tumor tissues and display distinct phenotypes. Those populations were defined as myofibroblasts (myCAFs), proximal to the neoplastic cells and characterized by high levels α-SMA, and as pro-inflammatory CAFs (iCAFs), which are distally located from the cancer cells and have low expression of α-SMA and high expression of IL-6. Using two different types of co-culture, they demonstrated the possibility of modeling CAFs subtypes using an in vitro system. The co-culture was established using pancreatic stellate cells, which are resident mesenchymal cells expressing fibroblast-activation protein α (FAP), and pancreatic organoids established either from patients or mouse tumors. When both cancer cells and fibroblasts are embedded together in Matrigel® (Figure 1B), the close contact induced an activation of quiescent PSCs, with the acquisition of myCAFs phenotype. When the two cell types are physically separated (Figure 1C), yet sharing the growth medium, there was emergence of the iCAFs phenotype. The results obtained with the two co-culture methods sustained the concept of CAFs intratumoral heterogeneity, further supported by recent single cell RNA sequencing data on bulk PDAC tissue, that identified a third subtype, named antigen presenting CAFs (apCAFs), characterized by the expression of MHC class II related genes. Interestingly, all these subtypes are dynamic and reversible (Elyada et al., 2019).
An additional type of co-culture has been employed to investigate the role of CAFs in supporting the growth of pancreatic cancer organoids (Seino et al., 2018). In this study, pancreatic organoids and CAFs were dissociated into single cells and then aggregated before being transferred in a Matrigel®/collagen mixture (Figure 1D). This physical attachment allowed the CAFs to supply the organoids with the niche factor necessary for their growth. In agreement with these findings, a 3D co-culture model of prostate organoids and stroma enhanced the formation and induced a change in the morphology of organoids from spherical shape in mono-culture to branched acini structure in co-culture, more similar to what is observed in vivo (Richards et al., 2019).
Cancer-associated fibroblast represent an abundant non-neoplastic cell type also in breast cancers, in which they are implicated in tumor progression and resistance to both chemo- and targeted-therapies (Paraiso and Smalley, 2013). A 3D co-culture approach has been employed to investigate the modifications in gene expression and cell metabolism of breast cancer cells induced by the interaction with CAFs. After pre-coating the plate with a thin layer of Matrigel®, the two cell types were aggregated and cultured on top of the laminin-rich ECM (Lee et al., 2007; Figure 1E). This method uncovered the effects of CAFs in attenuating the accumulation in epithelial cells of Lapatinib, a tyrosine kinase inhibitor used in the treatment of breast cancer (Marusyk et al., 2016).
Co-cultures With Immune Cells
Immune cells have antithetic functions in the TME. Innate and adaptive immune systems both exert their influence on TME and in turn are affected by TME. Macrophages, one of the most abundant cell types in the tumor stroma, are innate immune system phagocytic cells that can have pro- or anti-tumor activity. M1 macrophages are pro-inflammatory and considered tumor suppressive, but when they interact with the TME they undergo a phenotypic switch toward the M2 phenotype, known to be anti-inflammatory and tumor promoting. The integration of peripheral blood-derived macrophages in a 3D co-culture system of squamous cell carcinoma allowed the modulation of the macrophages phenotype. The classical protocol for polarization of macrophages involve the use of recombinant INF-γ and LPS to induce the M1 phenotype, while IL-4 stimulation promotes the M2 phenotype. Interestingly, the prolonged co-culture with cancer cells was sufficient to cause the spontaneous switch of macrophages phenotype toward M2, even in absence of IL-4 (Linde et al., 2012). Adaptive immune cells, like CD8+ T cells, are subjected to TME influence as well. CD8+ T cells are major actors of anti-cancer immunity; their infiltration in TME has been reported to correlate with better prognosis in several malignancies such as melanoma, ovarian, pancreatic, breast and colorectal cancers (Fukunaga et al., 2004; Sato et al., 2005; Neagu, 2012; Ziai et al., 2018). CD8+ T cells act by recognizing tumor-associated antigens presented to their T cell receptor by major histocompatibility complex (MHC). However, tumors often activate immune evasion mechanisms that include secretion of immunosuppressive cytokines, MHC downregulation or resistance to T cell-mediated cell death (Khong and Restifo, 2002). The programmed death 1 (PD-1) immune checkpoint is a key player in the tumor-mediated immune evasion mechanisms. When PD-1 interacts with one of its ligands, PD-L1 or PD-L2, activates the recruitment of SHP-2, which functions suppressing T-cell receptor signaling, inducing tumor-specific T-cells exhaustion or apoptosis. In tumor, PD-L1 is expressed not only by cancer cells but also by cells of TME or antigen presenting cells. PD-1/PD-L1 complex is one of the targets of cancer immunotherapy based on immune checkpoint inhibitors (ICI), which aims at unleashing anti-tumor responses by activating immune cells. Unfortunately, only subsets of epithelial cancers patients have shown benefit from cancer immunotherapies based on ICI. While genome sequencing data have largely contributed to the identification of biomarkers predictor of response, the molecular mechanisms of sensitivity/resistance are still unclear. Recently, Dijkstra et al. (2018) described a patient personalized in vitro model that enable the induction and the analysis of tumor specific T cell responses. Organoids were established from mismatch repair deficient colorectal cancer and from non-small-cell lung cancer patients, while T lymphocytes were isolated from patient’ peripheral blood. The co-culture of tumor organoids and peripheral blood lymphocytes (Figure 1F) allowed the expansion of tumor-reactive T cells, which were able to kill tumor organoids but not the ones derived from healthy tissue. This platform permits the study of tumor and T cells interactions with the potential to uncover the determinants of sensitivity or resistance to immunotherapy in a personalized manner. Another platform has been described by Jenkins et al. (2018) and is based on a 3D microfluidic device, which enabled preservation of both tumor infiltrating lymphoid and myeloid cells along with tumor-spheroids. Using this system, patient-derived organotypic tumor spheroids proved to be a good model to study tumor-immune interactions and responses to PD-1 blockade. In addition, a peculiar co-culture method based on ALI system (Neal et al., 2018) permitted the propagation of patient-derived organoids and tumor-infiltrating lymphocytes. In this system, minced tumor tissues are resuspended in type I collagen and plated on a permeable insert precoated with collagen matrix (Figure 1G). The ALI culture system was found to preserve the native stromal populations, including fibroblasts, macrophages and lymphocytes, for up to 2 months. Importantly, tumor-infiltrating lymphocytes maintained the T cell receptor repertoire observed in patients and the patient-derived organoids response to anti-PD-1 Nivolumab in vitro was comparable to what has been observed in clinical trials.
New Horizons of Organoids Application: Modeling EVs Release
Communication between cancerous cells and TME is also achieved through release of extracellular vescicles (EVs), a heterogeneous population of double-layer phospholipid membrane vesicles released by most cell types. The different populations of EVs can be discriminated by vesicle size, biogenesis, and release pathway. Microvesicles are vesicles with a diameter ranging from 200 to 2000 nm that originate directly from the budding of the cellular membrane (Li et al., 2012). Exosomes are smaller vesicles, with a diameter less than 200 nm, derived from intracellular endosomal compartment, called multivesicular body, and released by the fusion of the multicellular body with the plasma membrane (Kowal et al., 2014). Another type of EVs is represented by apoptotic bodies that originate during the late stages of programmed cell death and containing part of cytoplasm (Turiak et al., 2011). Several findings support the hypothesis that the bioactive molecules encapsulated in the EVs are capable of modifying the functions of the recipient cells. Tumor-derived EVs, also referred as oncosomes due to the fact that their cargo is composed by oncogenic macromolecules (Meehan et al., 2016), have been proven to induce changes in the phenotype of neighboring as well as distant cells, promoting tumor growth and local invasion in different cancer types (Webber et al., 2015; Fang et al., 2018; Naito et al., 2019). In addition, atypically large (1–10 μm in diameter) EVs derived from amoeboid tumor cells have been recently identified in the circulation of different mouse models of prostate cancer as well as in the biological fluids of metastatic prostate cancer patients (Di Vizio et al., 2012). This so-called large oncosomes (LOs) are not detected in samples from healthy donors and appear to originate from the shedding of non-apoptotic plasma membrane blebs (Minciacchi et al., 2015, 2017).
Extracellular vescicles are also released by stromal cells in the TME to exert their influence on cancer cells. ANXA6+ (Annexin 6/LDL receptor-related protein-1/thrombospondin-1 complex) CAFs-secreted EVs enhanced tumor cell aggressiveness in pancreatic cancer (Leca et al., 2016). Fibroblast-derived exosomes induced mobilization, invasion (Luga et al., 2012), resistance to therapy and tumor recurrence in breast cancer (Boelens et al., 2014), while CAFs EVs-mediated transfer of proteins and lipids enhanced cancer proliferation rate of human prostate cancer and melanoma cell lines (Santi et al., 2015). The EVs transfer of surface receptors or intracellular material has been also associated with immunosuppressive and tumor-promoting functions. The expression of immunomodulatory molecules on exosomes surface contributes to tumor immunosuppression. Fas ligand on exosomes induces T-cell apoptosis in a prostate cancer cell model (Abusamra et al., 2005), while expression of PD-L1 suppresses the function of CD8+ T cells thereby enhancing tumor growth in a xenograft mouse model of melanoma (Chen et al., 2018). Moreover, melanoma EVs interact with B lymphocytes in lymph nodes and mediate alteration of antitumor immunity promoting cancer progression (Pucci et al., 2016). Additional evidences proved that lung cancer-derived exosomes engineered to overexpressed CD40 ligand, part of a critical signaling for the activation of dendritic cells, induced dendritic cells maturation with a consequent increase in T cells proliferation and antitumor activity (Wang J. et al., 2014).
Based on the “Seed and Soil” hypothesis proposed by Paget in 1889, several works have been published demonstrating that tissues distant from the site of the primary tumor, undergo processes of adaptation to become permissive for the growth of cancer cells. The Lyden’ s group unveiled the role of exosomes in this process (Peinado et al., 2012; Costa-Silva et al., 2015; Hoshino et al., 2015). They proved that the transfer of MET oncoprotein by melanoma-derived exosomes is able to educate bone marrow progenitor cells to become pro-metastatic, leading to the discovery of an exosome signature with prognostic and therapeutic potential (Peinado et al., 2012). More recently, they described the sequential steps necessary for the formation of the pre-metastatic niche in pancreatic cancer, uncovering MIF as potential prognostic marker for liver metastasis (Costa-Silva et al., 2015). Moreover, proteomic analysis of tumor-derived exosomes revealed how the exosomal integrin signature is determinant for the formation of the pre-metastatic niche in specific tissues, explaining why tumors may have distinct metastatic tropisms (Hoshino et al., 2015). Tumor-derived EVs are also implicated in the remodeling of the ECM. EVs isolated from ovarian cancer cell lines were able to compromise the mesothelium integrity and to promote peritoneal dissemination of tumor cells in mice (Yokoi et al., 2017).
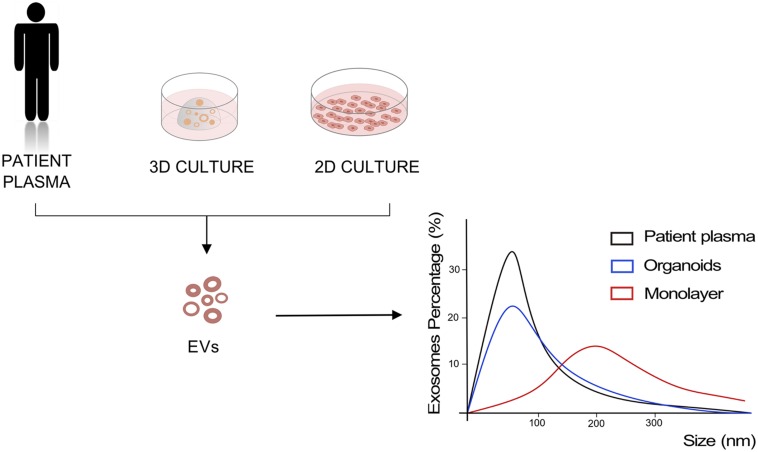
Given increasing evidences of a critical role of EVs in driving cancer progression, implementation and refinement of in vitro system to model EV-mediated cell communication is urgently needed. Monolayer cell cultures have been historically used to investigate the content and role of EVs in cancer, and most of data reported above have been generated using 2D cell culture systems. Rocha et al. (2019) have recently compared biochemical features, small RNA transcriptome, and proteome of EVs released by two gastric cell lines when cultured in 2D and 3D conditions to uncover the effects of the culture system on EVs. They observed that 3D culture conditions affect the size of the released EVs, inducing the production of smaller vesicles, with size and concentrations comparable to the exosomes isolated from patient plasma. In addition, Ewing’s sarcoma type 1 EVs isolated from 3D cultures proved to have higher similarity to the EVs isolated from patient plasma compared to the same cancer cells cultured as monolayer (Villasante et al., 2016; Figure 2). A recent study demonstrated the ability of organoids to intake and respond to EVs (Ke et al., 2017). They showed how human normal gastric organoids responded to tumor-derived EVs content by acquiring a neoplastic phenotype. EVs-treated organoids showed increased proliferation and smaller lumens with multilayered morphology that is typical of neoplastic organoids, along with decreased expression of the tumor suppressors PTEN and AIFM3. In a colorectal cancer organotypic model, EVs purified from human colon fibroblasts grown in hypoxic conditions were able to induce increase in number of neoplastic organoids, suggesting a role of fibroblast-derived EVs in tumorigenesis (Szvicsek et al., 2019). In the same work, the introduction of Apc mutation into mouse small intestinal organoids by CRISPR-Cas9, and the consequent activation of Wnt pathway, enhanced EVs secretion already at the adenoma stage of the intestinal tumorigenesis (Szvicsek et al., 2019).
FIGURE 2.
Models to study EVs. Representation of EVs size analysis for 2D and 3D culture of cancer cells compared to EVs derived from patient plasma.
Another crucial advantage of 3D culture system is the possibility of modeling cell-to-matrix interactions, which appear to influence release of EVs. Indeed, it has been shown that matrix supporting organoids growth influences the production of EVs, specifically the enrichment of collagen type I increased the release of EVs in the culture media by colorectal cancer-derived organoids (Szvicsek et al., 2019).
Additionally, tumor-derived EVs presence in body fluids makes them a promising source of cancer biomarkers. EVs miRNA signatures already proved to have prognostic value in several cancer types, including colorectal cancer (Fu et al., 2018), pancreatic cancer (Goto et al., 2018), prostate cancer (Valentino et al., 2017), non-small cell lung cancers (Liu et al., 2017), and glioblastoma (Skog et al., 2008). A novel approach using organoid media supernatant has been proposed for the identification of EVs proteins present exclusively in the blood of PDAC patients, and not in patients disease-free or with benign conditions (Huang et al., 2019). The subsequent validation of EV proteins obtained from media supernatant in clinical samples demonstrated the ability of the organoid platform to enable the identification of cancer biomarkers for PDAC patients. Moreover, recent evidences suggest EVs potential role in early disease detection (Melo et al., 2015) and prediction of response to treatment (Ciravolo et al., 2012; Wei et al., 2014; Rodrigues-Junior et al., 2019). The ability to enter other cells and deliver a functional cargo, even at distant sites, without inducing toxicity makes them good therapeutic agents vehicles (Ferguson and Nguyen, 2016). EVs can be engineered to target specific cell types, deliver chemotherapeutic agents (Kim et al., 2016) and enhance antitumor immune response (Besse et al., 2016). In conclusion, although patient-derived organoids might be a promising system to study cell-to-cell interactions, additional efforts from the scientific community are expected to finally assess their potential to model EV-based communication in cancers. Given the heterogeneity of matrices used in 3D culture systems, greater efforts are also expected to analyze the different effects of matrices on the migration and biological effect of EVs.
Organotypic System Challenges
Organoids are the in vitro system with a higher degree of similarity with the tissue of origin as compared to conventional monolayer cultures. Integration of different cell types through co-culture systems may help modeling the cell-to-cell interactions observed in vivo. However, beyond the evident advantages offered by the system, some challenges still need to be solved.
Epithelial organoid cultures usually need to be grown in a matrix that allows the formation of 3D structures and sustains their proliferation. The most commonly used matrices are biologically derived materials such as rat tail collagen I and Matrigel® (Table 1). Animal-derived matrices suffer from lot-to-lot variability, both in terms of composition and structure, and are unsuitable for clinical applications (Vukicevic et al., 1992; Hughes et al., 2010). Recently, efforts have been made toward establishing synthetic ECM analogs able to replace animal-derived matrices, with the aim to obtain more characterized and less variable matrix composition, with translational potential. Synthetically produced matrices can be composed by natural or non-natural polymers. An example of natural polymers matrix is the hyaluronic acid (HA) hydrogel, a highly biocompatible material that guarantee batch-to-batch consistency and reproducible results (Burdick and Prestwich, 2011). On the other hand, non-natural polymers like polyethylene glycol (PEG) are less expensive, highly reproducible, but lack biological features necessary to reproduce natural ECM, to overcome this issue and to improve functionality, natural peptides need to be crosslinked to the scaffold (Worthington et al., 2015). Gjorevski et al. (2016) developed a synthetic hydrogel for intestinal stem cells organoids generation and growth. PEG hydrogel supplemented with an RGD (Arg-Gly-Asp) peptide supported the expansion of intestinal stem cells, preserving the stemness partly lost during Matrigel® culture. Moreover, they proved that matrix stiffness and composition influence the formation and the differentiation of organoids. For this reason, they proposed the use of a mechanically static PEG to grow intestinal stem cells as spheroids, that successively need to be partially replaced by more degradable PEG to obtain the softness required for the cell’s differentiation and the formation of 3D structures. A different synthetic matrix, composed by PEG with maleimide groups at each terminus (PEG-4MAL), has been also applied for the growth, expansion and differentiation of human intestinal organoids (Cruz-Acuna et al., 2017). An initial 2D culture on Matrigel®-coated substrate was necessary before the encapsulation of cells in PEG-4MAL. The synthetic matrix has been engineered to obtain the optimal polymer density to support the generation of organoids derived from both ESCs and iPSCs. Furthermore, the delivery of human intestinal organoids resuspended in PEG-4MAL hydrogel into injured intestinal mucosa, improved wound repair in a mouse model, suggesting hydrogel as potential vehicle for clinical applications. To overcome the limitations of PEG hydrogel, a fibrin/laminin hydrogel has been developed for the generation and the expansion of epithelial organoids (Broguiere et al., 2018). Fibrin/laminin hydrogel proved to support long-term expansion of organoids established from different tissues, maintaining the same architecture observed in Matrigel®, giving proof to be a valid candidate for Matrigel® replacement.
TABLE 1.
Most commonly used matrices for the growth of 3D cultures.
| Matrix | Composition | Derivation | Applications |
| Matrigel® | collagen type IV, laminin, heparan sulfate proteoglycans, entactin/nidogen, and a number of growth factors | murine Englebreth-Holm-Swarm sarcoma tumors | 3D cell culture |
| Collagen type I | collagen type I | rat tail | 3D cell culture |
| HA hydrogel | hyaluronic acid | synthetic | 3D cell culture; clinical applications |
| PEG hydrogel | polyethylene glycol (PEG) hydrogel supplemented with an RGD (Arg-Gly-Asp) peptide | synthetic | 3D cell culture; clinical applications |
| PEG-4MAL hydrogel | PEG with maleimide groups at each terminus | synthetic | 3D cell culture; clinical applications |
| Fibrin/laminin hydrogel | fibrin, laminin | synthetic | 3D cell culture; clinical applications |
The other critical aspect of the organoids system is the formulation of an optimal culture media that contains the niche factors necessary for the establishment and the long-term culture of organoids. Wnt3a and R-spondin1 have been proven to be essential niche components for the establishment of patient-derived organoids from different tissues, like pancreas, liver, stomach and intestine (Clevers and Nusse, 2012). Although in the first organoid protocol it was reported the use of purified proteins (Sato et al., 2009), currently, the production of these two factors in many laboratories relies on eukaryotic cell culture engineered to secrete Wnt3a and R-spondin into their culture media due to economical constraints. The obtained conditioned media are then used for the production of the organoid culture media. Clearly, the use of conditioned media introduces elements of variability in the culture system and makes difficult comparing data generated from different laboratories. Moreover, the presence of other macromolecules, apart from the protein of interest, and batch-to-batch variability of protein activity may affect the organoid growth. Replacing conditioned media with recombinant proteins displaying similar activity may lead to the definition of standardized media conditions for the establishment of patient-derived organoids. Efforts have been recently made in this direction with the production of recombinant R-spondin (Urbischek et al., 2019) and Wnt surrogate (Janda et al., 2017).
Concluding Remarks
The advent of 3D culture systems is enabling the systematic generation of in vitro models from a variety of cancers and normal tissues. Increasing evidences suggest that those cultures are genetically stable and faithfully recapitulate major molecular features of parental tissues. The integration of these new systems with improved methods for isolation and characterization of EVs offers now the opportunity of exploring the role of unconventional ways of communications between cells, which has been shown to influence many aspects of cancer biology, including metastasis. Ultimately, the possibility to isolate and culture both tumoral and non-tumoral cells from the same patient permits the assessment of malignant specific features that has never been possible before using in vitro system for many cancer types.
Author Contributions
EF wrote the manuscript and performed literature search. LV prepared the figures. EF and LV drafted the manuscript. VC devised the idea and finalized the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all the members of the Corbo’s lab for support and critical review of the manuscript.
Footnotes
Funding. VC was supported by the Associazione Italiana Ricerca sul Cancro (AIRC; Grant No. 18178), while both EF and LV were supported by the Fondazione Cariverona.
References
- Abusamra A. J., Zhong Z., Zheng X., Li M., Ichim T. E., Chin J. L., et al. (2005). Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol. Dis. 35 169–173. 10.1016/j.bcmd.2005.07.001 [DOI] [PubMed] [Google Scholar]
- Avnet S., Lemma S., Cortini M., Di Pompo G., Perut F., Baldini N. (2019). Pre-clinical models for studying the interaction between mesenchymal stromal cells and cancer cells and the induction of stemness. Front. Oncol. 9:305. 10.3389/fonc.2019.00305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff M. H., Aggeler J., Ram T. G., Bissell M. J. (1989). Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development 105 223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N., Huch M., Kujala P., van de Wetering M., Snippert H. J., van J. H. (2010). Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6 25–36. 10.1016/j.stem.2009.11.013 [DOI] [PubMed] [Google Scholar]
- Ben-David U., Ha G., Tseng Y. Y., Greenwald N. F., Oh C., Shih J. (2017). Patient-derived xenografts undergo mouse-specific tumor evolution. Nat. Genet. 49 1567–1575. 10.1038/ng.3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besse B., Charrier M., Lapierre V., Dansin E., Lantz O., Planchard D. (2016). Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology 5:e1071008. 10.1080/2162402x.2015.1071008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell M. J. (1981). The differentiated state of normal and malignant cells or how to define a “normal” cell in culture. Int. Rev. Cytol. 70 27–100. 10.1016/s0074-7696(08)61130-4 [DOI] [PubMed] [Google Scholar]
- Boelens M. C., Wu T. J., Nabet B. Y., Xu B., Qiu Y., Yoon T. (2014). Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell 159 499–513. 10.1016/j.cell.2014.09.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boj S. F., Hwang C. I., Baker L. A., Chio I. I., Engle D. D., Corbo V. (2015). Organoid models of human and mouse ductal pancreatic cancer. Cell 160 324–338. 10.1016/j.cell.2014.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broguiere N., Isenmann L., Hirt C., Ringel T., Placzek S., Cavalli E. (2018). Growth of epithelial organoids in a defined hydrogel. Adv. Mater. 30:e1801621. 10.1002/adma.201801621 [DOI] [PubMed] [Google Scholar]
- Broutier L., Mastrogiovanni G., Verstegen M. M., Francies H. E., Gavarro L. M., Bradshaw C. R. (2017). Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med. 23 1424–1435. 10.1038/nm.4438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick J. A., Prestwich G. D. (2011). Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 23 H41–H56. 10.1002/adma.201003963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Huang A. C., Zhang W., Zhang G., Wu M., Xu W. (2018). Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 560 382–386. 10.1038/s41586-018-0392-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon D. J., Orsulic S. (2011). Mouse models of cancer. Annu. Rev. Pathol. 6 95–119. 10.1146/annurev.pathol.3.121806.154244 [DOI] [PubMed] [Google Scholar]
- Ciravolo V., Huber V., Ghedini G. C., Venturelli E., Bianchi F., Campiglio M. (2012). Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J. Cell. Physiol. 227 658–667. 10.1002/jcp.22773 [DOI] [PubMed] [Google Scholar]
- Clevers H., Nusse R. (2012). Wnt/beta-catenin signaling and disease. Cell 149 1192–1205. [DOI] [PubMed] [Google Scholar]
- Costa E. C., Moreira A. F., de Melo-Diogo D., Gaspar V. M., Carvalho M. P., Correia I. J. (2016). 3D tumor spheroids: an overview on the tools and techniques used for their analysis. Biotechnol. Adv. 34 1427–1441. 10.1016/j.biotechadv.2016.11.002 [DOI] [PubMed] [Google Scholar]
- Costa-Silva B., Aiello N. M., Ocean A. J., Singh S., Zhang H., Thakur B. K. (2015). Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 17 816–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Acuna R., Quiros M., Farkas A. E., Dedhia P. H., Huang S., Siuda D. (2017). Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat. Cell Biol. 19 1326–1335. 10.1038/ncb3632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agosto S., Andreani S., Scarpa A., Corbo V. (2019). Preclinical Modelling of PDA: Is Organoid the New Black? Int. J. Mol. Sci 20:2766. 10.3390/ijms20112766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley W. P., Peters S. B., Larsen M. (2008). Extracellular matrix dynamics in development and regenerative medicine. J. Cell Sci. 121 255–264. 10.1242/jcs.006064 [DOI] [PubMed] [Google Scholar]
- Di Vizio D., Morello M., Dudley A. C., Schow P. W., Adam R. M., Morley S. (2012). Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am. J. Pathol. 181 1573–1584. 10.1016/j.ajpath.2012.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra K. K., Cattaneo C. M., Weeber F., Chalabi M., van de Haar J., Fanchi L. F. (2018). Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell 174 1586–1598.e12. 10.1016/j.cell.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drost J., Karthaus W. R., Gao D., Driehuis E., Sawyers C. L., Chen Y., et al. (2016). Organoid culture systems for prostate epithelial and cancer tissue. Nat. Protoc. 11 347–358. 10.1038/nprot.2016.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiraku M., Watanabe K., Matsuo-Takasaki M., Kawada M., Yonemura S., Matsumura M. (2008). Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3 519–532. 10.1016/j.stem.2008.09.002 [DOI] [PubMed] [Google Scholar]
- Eirew P., Steif A., Khattra J., Ha G., Yap D., Farahani H. (2015). Dynamics of genomic clones in breast cancer patient xenografts at single-cell resolution. Nature 518 422–426. 10.1038/nature13952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elyada E., Bolisetty M., Laise P., Flynn W. F., Courtois E. T., Burkhart R. A. (2019). Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 9 1102–1123. 10.1158/2159-8290.CD-19-0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang T., Lv H., Lv G., Li T., Wang C., Han Q. (2018). Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat. Commun. 9:191. 10.1038/s41467-017-02583-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S. W., Nguyen J. (2016). Exosomes as therapeutics: the implications of molecular composition and exosomal heterogeneity. J. Control. Release 228 179–190. 10.1016/j.jconrel.2016.02.037 [DOI] [PubMed] [Google Scholar]
- Fong E. L., Harrington D. A., Carson F.-C., Yu H. (2016). Heralding a new paradigm in 3D tumor modeling. Biomaterials 108 197–213. 10.1016/j.biomaterials.2016.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu F., Jiang W., Zhou L., Chen Z. (2018). Circulating exosomal miR-17-5p and miR-92a-3p predict pathologic stage and grade of colorectal cancer. Transl. Oncol. 11 221–232. 10.1016/j.tranon.2017.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga A., Miyamoto M., Cho Y., Murakami S., Kawarada Y., Oshikiri T. (2004). CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas 28 e26–e31. 10.1097/00006676-200401000-00023 [DOI] [PubMed] [Google Scholar]
- Giandomenico S. L., Mierau S. B., Gibbons G. M., Wenger L. M. D., Masullo L., Sit T. (2019). Cerebral organoids at the air-liquid interface generate diverse nerve tracts with functional output. Nat. Neurosci. 22 669–679. 10.1038/s41593-019-0350-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjorevski N., Sachs N., Manfrin A., Giger S., Bragina M. E., Ordonez-Moran P. (2016). Designer matrices for intestinal stem cell and organoid culture. Nature 539 560–564. 10.1038/nature20168 [DOI] [PubMed] [Google Scholar]
- Goto T., Fujiya M., Konishi H., Sasajima J., Fujibayashi S., Hayashi A. (2018). An elevated expression of serum exosomal microRNA-191, - 21, -451a of pancreatic neoplasm is considered to be efficient diagnostic marker. BMC Cancer 18:116. 10.1186/s12885-018-4006-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi L., Alfonsi R., Francescangeli F., Signore M., De Angelis M. L., Addario A. (2019). Organoids as a new model for improving regenerative medicine and cancer personalized therapy in renal diseases. Cell Death Dis. 10:201. 10.1038/s41419-019-1453-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A., Costa-Silva B., Shen T. L., Rodrigues G., Hashimoto A., Tesic Mark M. (2015). Tumour exosome integrins determine organotropic metastasis. Nature 527 329–335. 10.1038/nature15756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Bockorny B., Paul I., Akshinthala D., Gandarilla O., Bose A. (2019). Pancreatic tumor organoids for modeling in vivo drug response and discovering clinically-actionable biomarkers. bioRxiv [Preprint]. 10.1101/513267 [DOI] [Google Scholar]
- Huch M., Bonfanti P., Boj S. F., Sato T., Loomans C. J., van de Wetering M. (2013a). Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 32 2708–2721. 10.1038/emboj.2013.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch M., Dorrell C., Boj S. F., van Es J. H., Li V. S., van de Wetering M. (2013b). In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494 247–250. 10.1038/nature11826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C. S., Postovit L. M., Lajoie G. A. (2010). Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10 1886–1890. 10.1002/pmic.200900758 [DOI] [PubMed] [Google Scholar]
- Hynes R. O. (2009). The extracellular matrix: not just pretty fibrils. Science 326 1216–1219. 10.1126/science.1176009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda C. Y., Dang L. T., You C., Chang J., de Lau W., Zhong Z. A. (2017). Surrogate Wnt agonists that phenocopy canonical Wnt and beta-catenin signalling. Nature 545 234–237. 10.1038/nature22306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins R. W., Aref A. R., Lizotte P. H., Ivanova E., Stinson S., Zhou C. W. (2018). Ex vivo profiling of PD-1 blockade using organotypic tumor spheroids. Cancer Discov. 8 196–215. 10.1158/2159-8290.CD-17-0833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis G. S., Pavlou M. P., Diamandis E. P. (2010). Cancer secretomics reveal pathophysiological pathways in cancer molecular oncology. Mol. Oncol. 4 496–510. 10.1016/j.molonc.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katt M. E., Placone A. L., Wong A. D., Xu Z. S., Searson P. C. (2016). In vitro tumor models: advantages, disadvantages, variables, and selecting the right platform. Front. Bioeng. Biotechnol. 4:12. 10.3389/fbioe.2016.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke X., Yan R., Sun Z., Cheng Y., Meltzer A., Lu N. (2017). Esophageal adenocarcinoma-derived extracellular vesicle micrornas induce a neoplastic phenotype in gastric organoids. Neoplasia 19 941–949. 10.1016/j.neo.2017.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khong H. T., Restifo N. P. (2002). Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat. Immunol. 3 999–1005. 10.1038/ni1102-999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. S., Haney M. J., Zhao Y., Mahajan V., Deygen I., Klyachko N. L. (2016). Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine 12 655–664. 10.1016/j.nano.2015.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal J., Tkach M., Thery C. (2014). Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 29 116–125. 10.1016/j.ceb.2014.05.004 [DOI] [PubMed] [Google Scholar]
- Labernadie A., Kato T., Brugues A., Serra-Picamal X., Derzsi S., Arwert E. (2017). A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat. Cell Biol. 19 224–237. 10.1038/ncb3478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster M. A., Knoblich J. A. (2014). Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345:1247125. 10.1126/science.1247125 [DOI] [PubMed] [Google Scholar]
- Lancaster M. A., Renner M., Martin C. A., Wenzel D., Bicknell L. S., Hurles M. E. (2013). Cerebral organoids model human brain development and microcephaly. Nature 501 373–379. 10.1038/nature12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBleu V. S., Kalluri R. (2018). A peek into cancer-associated fibroblasts: origins, functions and translational impact. Dis. Model. Mech. 11:dmm029447. 10.1242/dmm.029447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leca J., Martinez S., Lac S., Nigri J., Secq V., Rubis M. (2016). Cancer-associated fibroblast-derived annexin A6+ extracellular vesicles support pancreatic cancer aggressiveness. J. Clin. Invest. 126 4140–4156. 10.1172/JCI87734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G. Y., Kenny P. A., Lee E. H., Bissell M. J. (2007). Three-dimensional culture models of normal and malignant breast epithelial cells. Nat. Methods 4 359–365. 10.1038/nmeth1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Antonyak M. A., Zhang J., Cerione R. A. (2012). RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells. Oncogene 31 4740–4749. 10.1038/onc.2011.636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Francies H. E., Secrier M., Perner J., Miremadi A., Galeano-Dalmau N. (2018). Organoid cultures recapitulate esophageal adenocarcinoma heterogeneity providing a model for clonality studies and precision therapeutics. Nat. Commun. 9:2983. 10.1038/s41467-018-05190-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Nadauld L., Ootani A., Corney D. C., Pai R. K., Gevaert O. (2014). Oncogenic transformation of diverse gastrointestinal tissues in primary organoid culture. Nat. Med. 20 769–777. 10.1038/nm.3585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde N., Gutschalk C. M., Hoffmann C., Yilmaz D., Mueller M. M. (2012). Integrating macrophages into organotypic co-cultures: a 3D in vitro model to study tumor-associated macrophages. PLoS One 7:e40058. 10.1371/journal.pone.0040058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkous A., Balamatsias D., Snuderl M., Edwards L., Miyaguchi K., Milner T. (2019). Modeling patient-derived glioblastoma with cerebral organoids. Cell Rep. 26 3203–3211.e5. 10.1016/j.celrep.2019.02.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Yu Z., Yuan S., Xie W., Li C., Hu Z. (2017). Circulating exosomal microRNAs as prognostic biomarkers for non-small-cell lung cancer. Oncotarget 8 13048–13058. 10.18632/oncotarget.14369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luga V., Zhang L., Viloria-Petit A. M., Ogunjimi A. A., Inanlou M. R., Chiu E. (2012). Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 151 1542–1556. 10.1016/j.cell.2012.11.024 [DOI] [PubMed] [Google Scholar]
- Marusyk A., Tabassum D. P., Janiszewska M., Place A. E., Trinh A., Rozhok A. I. (2016). Spatial proximity to fibroblasts impacts molecular features and therapeutic sensitivity of breast cancer cells influencing clinical outcomes. Cancer Res. 76 6495–6506. 10.1158/0008-5472.CAN-16-1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan B., Rak J., Di Vizio D. (2016). Oncosomes - large and small: what are they, where they came from? J. Extracell. Vesicles 5:33109. 10.3402/jev.v5.33109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo S. A., Luecke L. B., Kahlert C., Fernandez A. F., Gammon S. T., Kaye J. (2015). Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 523 177–182. 10.1038/nature14581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minciacchi V. R., Spinelli C., Reis-Sobreiro M., Cavallini L., You S., Zandian M. (2017). MYC mediates large oncosome-induced fibroblast reprogramming in prostate cancer. Cancer Res. 77 2306–2317. 10.1158/0008-5472.can-16-2942 [DOI] [PubMed] [Google Scholar]
- Minciacchi V. R., You S., Spinelli C., Morley S., Zandian M., Aspuria P. J. (2015). Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget 6 11327–11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona A., Moscona H. (1952). The dissociation and aggregation of cells from organ rudiments of the early chick embryo. J. Anat. 86 287–301. [PMC free article] [PubMed] [Google Scholar]
- Naito Y., Yamamoto Y., Sakamoto N., Shimomura I., Kogure A., Kumazaki M. (2019). Cancer extracellular vesicles contribute to stromal heterogeneity by inducing chemokines in cancer-associated fibroblasts. Oncogene 38 5566–5579. 10.1038/s41388-019-0832-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neagu M. (2012). The immune system–a hidden treasure for biomarker discovery in cutaneous melanoma. Adv. Clin. Chem. 58 89–140. 10.1016/b978-0-12-394383-5.00011-4 [DOI] [PubMed] [Google Scholar]
- Neal J. T., Li X., Zhu J., Giangarra V., Grzeskowiak C. L., Ju J. (2018). Organoid modeling of the tumor immune microenvironment. Cell 175 1972–1988.e16. 10.1016/j.cell.2018.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlund D., Handly-Santana A., Biffi G., Elyada E., Almeida A. S., Ponz-Sarvise M. (2017). Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 214 579–596. 10.1084/jem.20162024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ootani A., Li X., Sangiorgi E., Ho Q. T., Ueno H., Toda S. (2009). Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. 15 701–706. 10.1038/nm.1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin R. W., Gehron P., McGoodwin E. B., Martin G. R., Valentine T., Swarm R. (1977). A murine tumor producing a matrix of basement membrane. J. Exp. Med. 145 204–220. 10.1084/jem.145.1.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdemir B. C., Pentcheva-Hoang T., Carstens J. L., Zheng X., Wu C. C., Simpson T. R. (2014). Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25 719–734. 10.1016/j.ccr.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraiso K. H., Smalley K. S. (2013). Fibroblast-mediated drug resistance in cancer. Biochem. Pharmacol. 85 1033–1041. 10.1016/j.bcp.2013.01.018 [DOI] [PubMed] [Google Scholar]
- Peinado H., Aleckovic M., Lavotshkin S., Matei I., Costa-Silva B., Moreno-Bueno G. (2012). Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 18 883–891. 10.1038/nm.2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. W., Ronnov-Jessen L., Howlett A. R., Bissell M. J. (1992). Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 89 9064–9068. 10.1073/pnas.89.19.9064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle S., Maimets M., van der Zwaag M., Stokman M. A., van Gosliga D., Zwart E. (2016). Human salivary gland stem cells functionally restore radiation damaged salivary glands. Stem Cells 34 640–652. 10.1002/stem.2278 [DOI] [PubMed] [Google Scholar]
- Pucci F., Garris C., Lai C. P., Newton A., Pfirschke C., Engblom C. (2016). SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science 352 242–246. 10.1126/science.aaf1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan A., Weinberg R. A. (2003). Opinion: comparative biology of mouse versus human cells: modelling human cancer in mice. Nat. Rev. Cancer 3 952–959. 10.1038/nrc1235 [DOI] [PubMed] [Google Scholar]
- Ratajczak J., Miekus K., Kucia M., Zhang J., Reca R., Dvorak P., et al. (2006). Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 20 847–856. 10.1038/sj.leu.2404132 [DOI] [PubMed] [Google Scholar]
- Rhim A. D., Oberstein P. E., Thomas D. H., Mirek E. T., Palermo C. F., Sastra S. A. (2014). Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25 735–747. 10.1016/j.ccr.2014.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards Z., McCray T., Marsili J., Zenner M. L., Manlucu J. T., Garcia J. (2019). Prostate stroma increases the viability and maintains the branching phenotype of human prostate organoids. iScience 12 304–317. 10.1016/j.isci.2019.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha S., Carvalho J., Oliveira P., Voglstaetter M., Schvartz D., Thomsen A. R. (2019). 3D cellular architecture affects microRNA and protein cargo of extracellular vesicles. Adv. Sci. 6:1800948. 10.1002/advs.201800948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Junior D. M., Tan S. S., de Souza Viana L., Carvalho A. L., Lim S. K., Iyer N. G., et al. (2019). A preliminary investigation of circulating extracellular vesicles and biomarker discovery associated with treatment response in head and neck squamous cell carcinoma. BMC Cancer 19:373. 10.1186/s12885-019-5565-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs N., de Ligt J., Kopper O., Gogola E., Bounova G., Weeber F. (2018). A living Biobank of breast cancer organoids captures disease heterogeneity. Cell 172 373–386.e10. 10.1016/j.cell.2017.11.010 [DOI] [PubMed] [Google Scholar]
- Sachs N., Papaspyropoulos A., Zomer-van Ommen D. D., Heo I., Bottinger L., Klay D. (2019). Long-term expanding human airway organoids for disease modeling. EMBO J. 38:e100300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi A., Caselli A., Ranaldi F., Paoli P., Mugnaioni C., Michelucci E., et al. (2015). Cancer associated fibroblasts transfer lipids and proteins to cancer cells through cargo vesicles supporting tumor growth. Biochim. Biophys. Acta 1853 3211–3223. 10.1016/j.bbamcr.2015.09.013 [DOI] [PubMed] [Google Scholar]
- Sato E., Olson S. H., Ahn J., Bundy B., Nishikawa H., Qian F. (2005). Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. U.S.A. 102 18538–18543. 10.1073/pnas.0509182102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Stange D. E., Ferrante M., Vries R. G., Van Es J. H., Van den Brink S. (2011). Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141 1762–1772. 10.1053/j.gastro.2011.07.050 [DOI] [PubMed] [Google Scholar]
- Sato T., Vries R. G., Snippert H. J., van de Wetering M., Barker N., Stange D. E. (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459 262–265. 10.1038/nature07935 [DOI] [PubMed] [Google Scholar]
- Schutgens F., Rookmaaker M. B., Margaritis T., Rios A., Ammerlaan C., Jansen J. (2019). Tubuloids derived from human adult kidney and urine for personalized disease modeling. Nat. Biotechnol. 37 303–313. 10.1038/s41587-019-0048-8 [DOI] [PubMed] [Google Scholar]
- Seino T., Kawasaki S., Shimokawa M., Tamagawa H., Toshimitsu K., Fujii M. (2018). Human pancreatic tumor organoids reveal loss of stem cell niche factor dependence during disease progression. Cell Stem Cell 22 454–467.e6. 10.1016/j.stem.2017.12.009 [DOI] [PubMed] [Google Scholar]
- Skog J., Wurdinger T., van Rijn S., Meijer D. H., Gainche L., Sena-Esteves M. (2008). Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10 1470–1476. 10.1038/ncb1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence J. R., Mayhew C. N., Rankin S. A., Kuhar M. F., Vallance J. E., Tolle K. (2011). Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470 105–109. 10.1038/nature09691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarm R. L. (1963). Transplantation of a murine chondrosarcoma in mice of different inbred strains. J. Natl. Cancer Inst. 31 953–975. [PubMed] [Google Scholar]
- Szvicsek Z., Oszvald A., Szabo L., Sandor G. O., Kelemen A., Soos A. A. (2019). Extracellular vesicle release from intestinal organoids is modulated by Apc mutation and other colorectal cancer progression factors. Cell. Mol. Life Sci. 76 2463–2476. 10.1007/s00018-019-03052-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torras N., Garcia-Diaz M., Fernandez-Majada V., Martinez E. (2018). Mimicking epithelial tissues in three-dimensional cell culture models. Front. Bioeng. Biotechnol. 6:197. 10.3389/fbioe.2018.00197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turiak L., Misjak P., Szabo T. G., Aradi B., Paloczi K., Ozohanics O. (2011). Proteomic characterization of thymocyte-derived microvesicles and apoptotic bodies in BALB/c mice. J. Proteomics 74 2025–2033. 10.1016/j.jprot.2011.05.023 [DOI] [PubMed] [Google Scholar]
- Urbischek M., Rannikmae H., Foets T., Ravn K., Hyvonen M., de la Roche M. (2019). Organoid culture media formulated with growth factors of defined cellular activity. Sci. Rep. 9:6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui T., Sakurai M., Enjoji S., Kawasaki H., Umata K., Ohama T. (2016). Establishment of a novel model for anticancer drug resistance in three-dimensional primary culture of tumor microenvironment. Stem Cells Int. 2016:7053872. 10.1155/2016/7053872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui T., Sakurai M., Umata K., Yamawaki H., Ohama T., Sato K. (2018). Preparation of human primary colon tissue-derived organoid using air liquid interface culture. Curr. Protoc. Toxicol. 75 22.6.1–22.6.7. 10.1002/cptx.40 [DOI] [PubMed] [Google Scholar]
- Valadi H., Ekstrom K., Bossios A., Sjostrand M., Lee J. J., Lotvall J. O. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9 654–659. 10.1038/ncb1596 [DOI] [PubMed] [Google Scholar]
- Valentino A., Reclusa P., Sirera R., Giallombardo M., Camps C., Pauwels P. (2017). Exosomal microRNAs in liquid biopsies: future biomarkers for prostate cancer. Clin. Transl. Oncol. 19 651–657. 10.1007/s12094-016-1599-5 [DOI] [PubMed] [Google Scholar]
- Villasante A., Marturano-Kruik A., Ambati S. R., Liu Z., Godier-Furnemont A., Parsa H. (2016). Recapitulating the size and cargo of tumor exosomes in a tissue-engineered model. Theranostics 6 1119–1130. 10.7150/thno.13944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukicevic S., Kleinman H. K., Luyten F. P., Roberts A. B., Roche N. S., Reddi A. H. (1992). Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp. Cell Res. 202 1–8. 10.1016/0014-4827(92)90397-q [DOI] [PubMed] [Google Scholar]
- Wang J., Wang L., Lin Z., Tao L., Chen M. (2014). More efficient induction of antitumor T cell immunity by exosomes from CD40L gene-modified lung tumor cells. Mol. Med. Rep. 9 125–131. 10.3892/mmr.2013.1759 [DOI] [PubMed] [Google Scholar]
- Wang K., Yuen S. T., Xu J., Lee S. P., Yan H. H., Shi S. T. (2014). Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat. Genet. 46 573–582. 10.1038/ng.2983 [DOI] [PubMed] [Google Scholar]
- Webber J. P., Spary L. K., Sanders A. J., Chowdhury R., Jiang W. G., Steadman R. (2015). Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene 34 290–302. 10.1038/onc.2013.560 [DOI] [PubMed] [Google Scholar]
- Wei Y., Lai X., Yu S., Chen S., Ma Y., Zhang Y. (2014). Exosomal miR-221/222 enhances tamoxifen resistance in recipient ER-positive breast cancer cells. Breast Cancer Res. Treat. 147 423–431. 10.1007/s10549-014-3037-0 [DOI] [PubMed] [Google Scholar]
- Weiswald L. B., Bellet D., Dangles-Marie V. (2015). Spherical cancer models in tumor biology. Neoplasia 17 1–15. 10.1016/j.neo.2014.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington P., Pochan D. J., Langhans S. A. (2015). Peptide hydrogels - versatile matrices for 3D cell culture in cancer medicine. Front. Oncol. 5:92. 10.3389/fonc.2015.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Peters-Hall J. R., Bose S., Pena M. T., Rose M. C. (2011). Human bronchial epithelial cells differentiate to 3D glandular acini on basement membrane matrix. Am. J. Respir. Cell Mol. Biol. 44 914–921. 10.1165/rcmb.2009-0329OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Yang S., Li X., Li B., Li Y., Zhang X. (2019). Tumor organoids: From inception to future in cancer research. Cancer Lett. 454 120–133. 10.1016/j.canlet.2019.04.005 [DOI] [PubMed] [Google Scholar]
- Yokobori T., Suzuki S., Miyazaki T., Sohda M., Sakai M., Tanaka N. (2016). Intestinal epithelial culture under an air-liquid interface: a tool for studying human and mouse esophagi. Dis. Esophagus 29 843–847. 10.1111/dote.12346 [DOI] [PubMed] [Google Scholar]
- Yokoi A., Yoshioka Y., Yamamoto Y., Ishikawa M., Ikeda S. I., Kato T. (2017). Malignant extracellular vesicles carrying MMP1 mRNA facilitate peritoneal dissemination in ovarian cancer. Nat. Commun. 8:14470. 10.1038/ncomms14470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Li L., Jiang H., Li Q., Wang-Gillam A., Yu J. (2018). Tumor-stroma IL1beta-IRAK4 feedforward circuitry drives tumor fibrosis, chemoresistance, and poor prognosis in pancreatic cancer. Cancer Res. 78 1700–1712. 10.1158/0008-5472.CAN-17-1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziai J., Gilbert H. N., Foreman O., Eastham-Anderson J., Chu F., Huseni M., et al. (2018). CD8+ T cell infiltration in breast and colon cancer: a histologic and statistical analysis. PLoS One 13:e0190158. 10.1371/journal.pone.0190158 [DOI] [PMC free article] [PubMed] [Google Scholar]