Abstract
Background
Since the epidemiological profile of drug-induced liver injury (DILI) in China, especially the western of China, it has rarely been studied. The aim of this study was to analyze the characteristics of DILI patients in a large tertiary teaching hospital at Chongqing, a municipality in western China.
Material/Methods
The medical records of hospitalized patients which diagnosed with DILI between January 2011 and December 2016 were searched retrospectively, and demographic, clinical data, and laboratory data were retrieved for analysis.
Results
A total of 1811 patients had been diagnosed with DILI, accounting for 0.248% of the total admissions during the same period. Among the 1096 patients included in our analysis, DILI was caused by “medications” in 462 cases (42.15%), “herbs” in 391 cases (35.68%), and combined medications in 189 cases (17.24%). The profiles for each etiology were distinctive for age, sex, clinical features, laboratory features, and types and severity of DILI.
Conclusions
Our study provides a systematic etiological profile of DILI in Chinese patients, which can represent references for prevention, diagnosis and treatment, supporting and promoting efforts to ease the burden of this liver disease in China.
MeSH Keywords: Drug-Induced Liver Injury; Herbal Medicine; Medicine, Chinese Traditional
Background
Drug-induced liver injury (DILI), which can be caused by drugs, herbs, natural products, minerals, and other causes, and it leads to liver dysfunction and even to a life-threatening situation. Diagnosis is made by abnormal results on clinical tests of liver function, while reasonably excluding other reasons [1]. DILI progression can follow a predictable dose-dependent course (known as intrinsic DILI) or an unpredictable, non-dose-dependent course (also called idiosyncratic DILI) [2]. A large proportion of DILI cases occur as idiosyncratic events, and studies have shown the disease is the manifestation of the complex interplay between potentially immunogenic drugs or metabolites and the host’s immune response [3,4]. Most of DILI cases resolve upon withdrawal of the causative agent, but up to 20% of patients continue to progress and develop to chronic DILI [5]. Unfortunately, the global and country-specific incidence and prevalence of DILI is still only partially known, making it one of the most globally challenging disorders facing gastroenterologists [6].
A population-based case-control study from the UK determined the crude incidence rate of DILI was 2.4 cases per 100 000 person years [7]. In an area of France, the incidence was estimated at 14 cases per 100 000 persons, corresponding to a number of events 16 times higher than that collected by pharmacovigilance centers [8]. More recently, a study in Korea estimated the incidence of DILI-related hospitalization at a teaching hospital to be 12 cases per 100 000 [9], while a prospective in Iceland population-based study found that the crude incidence of approximately 19 cases per 100 000 a year [10]. To date, the rates and epidemiological profile of DILI in western of China have rarely been studied before.
Some studies in Chinese cohorts have attempted to shed light on the country-specific features of DILI. A population-based prospective study with tuberculosis patients showed a cumulative incidence of 2.55% for DILI caused by anti-tuberculosis drugs, indicating a substantial negative impact among this disease population [11]. In addition, a meta-analysis form Chinese literature found differences in the etiologies of DILI in China [12]. Thus, the aim of the present study was to find the detailed prevalence and incidence profile of DILI in China by assessing cases in a large tertiary teaching hospital at Chongqing, a municipality in western China. Herein, we present the incidence rate in the hospital, also the causes (the causative drug), clinical features and risk factors of DILI among a western of China population.
Material and Methods
This study used routinely collected clinical data in a de-identified format; as such, the Ethics Committee of Southwest Hospital of the Army Medical University waived the requirements for each patient consent or review by the Ethics Committee.
Patients and data collection
The medical records database of Southwest Hospital of the Army Medical University was searched retrospectively for patients who had been hospitalized with the diagnosis of DILI caused by medications (drugs), herbs (herbs remedies and traditional Chinese medicines), traditional remedies or other between January 2011 and December 2016. Patients were considered for study inclusion unless their diagnosis results of DILI met the following criteria [6,13]: i) documented exposure of drug intake resulting in hepatotoxicity, defined as recent onset abnormalities in liver tests (bilirubin >2 mg/dL and/or aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels more than 3 times the upper limit of normal (ULN) and/or alkaline phosphatase (ALP) levels greater than 2 times the ULN); and ii) exclusion of other possible causes, for instance viral hepatitis (hepatitis A, B, C, D, and E), alcoholic hepatitis, cirrhosis (diagnosed either radiologically or clinically), autoimmune hepatitis, or liver tumors. The results of liver tests given immediately upon admission were used for the analysis [14,15]. At the Southwest Hospital, the ULN for both ALT and AST levels are 42 U/L, and the ULN for ALP level is 114 U/L. Drug causality assessment was done by applying the Roussel Uclaf Causality Assessment Method (RUCAM) retrospectively [6,16], SZ and LS applied RUCAM to assess the causality respectively, and disagreements were resolved by involving YL and a chief physician of liver diseases in the hospital. Patients without demographic data, who lacked data for the precise chronology of medication records, who did not meet the onset time standard of the RUCAM, or which were categorized as “unlikely” and “excluded” according to the RUCAM score were excluded.
Clinical and laboratory data were retrieved from the medical records of all patients selected for study inclusion. Clinical data included age, sex, alcohol drinker status, symptoms and signs (i.e., presence of jaundice, skin rashes, encephalopathy, and ascites), causal agents, duration of drug exposure to recognition, duration of drug cessation to recognition, concomitant drugs, comorbid conditions, duration of treatment, and therapeutic outcome. Laboratory data included levels of AST, ALT, ALP, gamma-glutamyl transpeptidase (GGT), total bilirubin (TBIL), total proteins (TP), albumin (ALB) and serum creatinine (SCr), as well as prothrombin time (PT), prothrombin activity (PTA) and international normalized ratio (INR).
Etiology of DILI
Each patient’s history of medications, including prescription, non-prescription, herbal and dietary supplement products, was obtained from the medical records. DILI of the patients was classified by the causation of the following classifications: medication, herb, health food or dietary supplement, combination, and other [9]. The medication category was subdivided according to the organ system or the mechanism of action. The herb category was subdivided as Chinese patent medicine (herbal preparations approved by the National Health commission of China), Chinese herbal medicine (herbal medicines prescribed by a herb doctor or pharmacist) or medicinal herbs or plants that were recommended or instructed by an unauthorized people [9]. Vitamins, amino acids, and other materials used to provide nutrients were classified as healthy food or dietary supplement, the combination category represented cases with more than 2 causative agents that fell into the different categories and sub-categories aforementioned, and the other category represented cases with causative agent that could not treated as other categories defined.
Causality assessment
Causality assessment was based on RUCAM, which is used more widely by clinicians than other instruments. For the patients with no RUCAM scores in the medical records, 2 clinical pharmacists who were trained by doctors from our hospitals on the RUCAM scale assessed the scores according to the information in the patient’s medical records retrospectively, and resolved any disagreements by discussion or by involving doctors who trained them. The RUCAM score card system is differentiated according to hepatocellular injuries or cholestatic injuries or mixed injuries, and it semi-quantitatively estimated the causality according to the scores based on timing drug usage and liver biochemistry washout, also the risk factors for DILI, possible medications, other diagnoses may resulting in liver injury, then rechallenge information, and scores are grouped into likelihood levels of “excluded” (score ≤0), “unlikely” (1–2), “possible” (3–5), “probable” (6–8), and “highly probable” (>8).
Types of DILI
Types of liver injury were determined from the R values calculated with the data of initial liver tests given at hospital presentation. According to the 2014 guideline [6], the R value was defined as [(serum ALT/ULN)/(serum ALP/ULN)], it is the ratio of ALT activity expressed as the fold elevation over its ULN laboratory range to ALP. R ≥5 was defined as hepatocellular injury, R ≤2 as cholestatic injury, and 2< R <5 as mixed injury. Besides, patients with AST or ALT ≥3 UNL and TBIL ≥2 UNL without evidence of cholestasis (ALP ≤2 UNL) were considered to be consistent with the Hy’s law [17,18], and at the Southwest Hospital, the ULN for TBIL level is 21 μmol/L.
Severity of DILI
The severity of DILI was rate according to the livertox.nih.gov for DILIN severity score [19]. Briefly, mild DILI (grade 1) was defined by raised serum aminotransferase or ALP levels or both, but TBIL level <2.5 mg/dL and no coagulopathy (INR <1.5); moderate DILI (grade 2) was defined by raised serum aminotransferase or ALP levels or both and TBIL level >2.5 mg/dL or coagulopathy (INR >1.5) without hyperbilirubinemia; moderate to severe DILI (grade 3) was defined by raised serum aminotransferase or ALP levels and TBIL level >2.5 mg/dL and hospitalization (or prolonged preexisting hospitalization) because of the DILI; severe DILI (grade 4) was defined by raised serum aminotransferase or ALP levels and TBIL >2.5 mg/dL and at least 1 of the following: 1) prolonged jaundice and symptoms beyond 3 months, or 2) signs of hepatic decompensation (INR >1.5, ascites, encephalopathy), or 3) other organ failure believed to be related to DILI; death or liver transplantation for DILI was considered fatal DILI (grade 5).
Statistical analysis
The baseline clinical and laboratory data of the patients were described as median (range) for continuous variables and percentages for categorical variables. Non-normally distributed parameters were compared using Kruskal-Wallis, and for continuous variables, the Scheffé method was used (post-hoc test). Categorical variables were compared using the Pearson’s χ2 test. A P-value <0.05 was regarded as indicating statistical significance. All statistical analyses were performed on a personal computer with the statistical package SPSS for Windows (version 22.0).
Results
Patient characteristics
From January 2011 to December 2016, there were 729 229 admissions to the Southwest Hospital, of which 1811 patients (0.248%) were with a diagnosis of DILI. Screening of the demographic and clinical data led to 1096 patients (60.52%) being selected for study inclusion and data analysis, and the patients mostly represent Chinese Han population living in Southwest of China. DILI patients who were excluded from the analysis represented other competing cases of viral hepatitis (229 patients), alcoholic hepatitis (41 patients), cirrhosis (40 patients), autoimmune hepatitis (87 patients) and liver tumors (7 patients). An additional 311 patients were excluded due to lack of demographic or clinical data, not meeting the onset time standard of RUCAM, or being classified as “unlikely” or “excluded” by the RUCAM score.
The distribution of the included DILI cases over the study period is shown in Table 1; summaries of demographic, clinical, and laboratory variables are shown in Table 2. The age distribution represented adolescent to elderly, with 5.02% being <20 years old, 10.86% being 20 to 29 years old, 14.46% being 30 to 39 years old, 26.09% being 40 to 49 years old, 20.13% being 50 to 59 years old, and 23.54% being ≥60 years old. Since there were only 47 patients younger than 18 years old (4.29%), we did not separately analyze the data of children and adults. There were 296 patients who drank alcohol, but without alcoholic liver disease, so they were included in our analysis. The most frequent comorbidities among the total cases were kidney disease (112 patients; 10.22%), diabetes (113 patients; 10.31%), high blood pressure (121 patients; 11.04%) and infection (210 patients; 19.16%); other comorbid conditions included depression, gastritis, leukemia, gout, arthritis and hyperthyroidism. We did not have clear data on the incidence of chronic DILI, but 1 patient was definitely diagnosed as chronic DILI and 16 patients were hospitalized for more than 60 days. There were only 18 patients who underwent liver biopsy, and due to the small number, we did not make a separate analysis. Clinical outcomes ranged from cure (clinical symptoms disappeared and liver function biochemical laboratory parameters returned to normal in 148 patients [13.5%]) to death (1 patient; [0.09%]), with 881 patients (80.38%) showing improvement (clinical symptoms disappeared and liver function biochemical laboratory parameters got better), 6 patients (0.55%) were discharged because of treatment failure (liver function biochemical laboratory parameters unchanged or got worse), and 60 patients (5.47%) being discharged with insufficient treatment, which means the patients left the hospital before their treatment was complete. As in China, many patients with serious diseases and ineffective treatment will choose to give up treatment and be discharged, so, we only know 1 patient who died in hospital from the medical records database, and we do not know the final outcome of the 66 patients with treatment failure or without sufficient treatment.
Table 1.
Distribution of DILI cases during the study period.
| Year | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 |
|---|---|---|---|---|---|---|
| Medications | 35 | 81 | 92 | 91 | 103 | 59 |
| Herbs | 55 | 55 | 64 | 59 | 95 | 64 |
| Health foods or dietary supplements | 4 | 3 | 6 | 5 | 5 | 3 |
| Combined | 14 | 18 | 64 | 44 | 21 | 28 |
| Others | 2 | 7 | 4 | 4 | 4 | 7 |
| Total | 110 | 164 | 230 | 203 | 228 | 161 |
Table 2.
Demographics, clinical, and laboratory parameters of the 1096 cases of DILI.
| Variable | N |
|---|---|
| Total numbers of DILI | 1096 |
| Age* | 47 (2, 87) |
| Male** | 482 (43.98) |
| Alcohol drinker** | 296 (27.01) |
| Duration, days* | |
| Time from drug exposure to DILI recognition | 30 (1, 1825) |
| Time from drug cessation to DILI recognition | 10 (0, 365) |
| Duration of treatment | 14 (1, 97) |
| Types** | |
| Hepatocellular | 417 (38.05) |
| Mixed | 265 (24.18) |
| Cholestatic | 414 (37.77) |
| Clinical characteristics** | |
| Jaundice | 562 (51.28) |
| Skin rashes | 261 (23.81) |
| Encephalopathy | 48 (4.38) |
| Ascites | 53 (4.84) |
| Etiology** | |
| Medications | 462 (42.15) |
| Herbs | 391 (35.68) |
| Health food or dietary supplement | 26 (2.37) |
| Combined | 189 (17.24) |
| Others | 28 (2.55) |
| Laboratory findings* | |
| AST, U/L | 125 (9, 3967) |
| ALT, U/L | 175.5 (5, 9326) |
| ALP, U/L | 144 (34, 2200) |
| GGT, U/L | 125 (7, 3283) |
| TBIL, μmol/L | 24.6 (1.2, 728) |
| DBIL, μmol/L | 8.86 (0.3, 476) |
| TP, g/L | 68.4 (27, 366) |
| ALB, g/L | 38.3 (10.8, 60.5) |
| PT, s | 11.6 (7.6, 98.4) |
| INR | 1 (0.01, 133.1) |
| PTA, % | 96.1 (0.84, 248) |
| SCr. μmol/L | 61 (3.7, 533) |
| RUCAM** | |
| Highly probable | 207 (18.89) |
| Probable | 652 (59.49) |
| Possible | 237 (21.62) |
| Severity** | |
| Grade 5 | 12 (1.1) |
| Grade 4 | 37 (3.38) |
| Grade 3 | 358 (32.66) |
| Grade 2 | 49 (4.47) |
| Grade 1 | 640 (58.39) |
| Hy’s cases** | 272 (24.82) |
Continuous variables are expressed as median (range);
categorical variables are expressed as number (%).
Etiology of DILI
The distribution of DILI etiologies among our study population, categorized by the specific causative agents, is shown in Table 3. The most common agent causing drug-induced liver injury was medication (462 patients; 42.15%), followed closely by herbs (391 patients; 35.68%). Of note, 103 out of the 172 patients (59.88%) with DILI caused by antimicrobial agents were caused specifically by antitubercular agents, and for the analgesics-caused DILI, only 5 cases were caused specifically by acetaminophen.
Table 3.
Distribution of the main drugs suspected in 1096 cases of drug-induced liver disease.
| Etiology | N* | Causal agents (N) |
|---|---|---|
| Medications | 462 (42.15) | |
| Antimicrobial | 172 (15.69) | Moxifloxacin (7), levofloxacin (13), metronidazole (1), ornidazole (1), amoxicillin (1), sulbenicillin (2), flucloxacillin (2), piperacillin (3), aztreonam (2), cefotiam (2), cefoperazone (2), cefmenoxime (1), cefixime (1), azithromycin (2), clarithromycin (1), vancomycin (3), meropenem (2), teicoplanin (2), clindamycin (2), fluconazole (5), ketoconazole (2), voriconazole (12), zidovudine (1), isoniazid (30), rifampicin (23), pyrazinamide (22), ethambutol (22), albendazole (1), lamivudine (2), tenofovir (1), thalidomide (1) |
| Endocrine/immune system | 141 (12.86) | Hydrocortisone (13), methylprednisolone (10), dexamethasone (5), levothyroxine sodium (3), methimazole (4), propylthiouracil (8), insulin (2), metformin (12), progestin (5), gestrinone (1), methotrexate (23), leflunomide (14), tacrolimus (6), cyclosporine (7), colchicine (7), cyclophosphamide (9), azathioprine (4), allopurinol (5), cetirizine (1), ebastine (1), ketotifen (1) |
| Analgesic | 37 (3.38) | Acetaminophen (15), diclofenac (7), meloxicam (5), ibuprofen (3), celecoxib (4), ketoprofen (3) |
| Digestive system | 22 (2.01) | Omeprazole (6), lansoprazole (4), rabeprazole (3), sulfasalazine (4), mesalazine (1), diammonium glycyrrhizinate (3) |
| Cardiovascular/blood system | 21 (1.92) | Atorvastatin (9), simvastatin (5), edaravone (1), digoxin (1), amlodipine (2), hemocoagulase (2), granulocyte colony-stimulating factor (1) |
| Mental/nervous system | 13 (1.19) | Paroxetine (2), olanzapine (1), flupenthixol (1), melitracen (1), carbamazepine (2), gabapentin (1), escitalopram (1), alprazolam (1), clonazepam (1), diazepam (2) |
| Antitumor | 33 (3.01) | Crizotinib (4), pemetrexed (4), gefitinib (4), dasatinib (2), irinotecan (2), cytarabine (4), daunorubicin (6), mitoxantrone (2), pegaspargase (5) |
| Others | 22 (2.01) | Cold medications (19), urografin (1), fat emulsion (2) |
| Herbs | 391 (35.68) | |
| Chinese patent medicine | 94 (8.58) | Sheng Fa granule (7), Bu Shen Yi Shou capsule (13), Compound Chen Xiang Wei tablet (2), Yang Fa Shen Xue capsule (4), San Huang tablet (1), Aplotaxis carminative pill (1), Xian Ling Gu Bao tablets (3), Bai Ling tablet (1), Gu Kang capsule (1), Teng Huang Jian Gu tablet (1), Pai Shi Li Dan tablet (2), Dan Lu Tong Du tablet (4), Xiao Ying granule (2), Ru Ning tablet (3), Xiao He tablet (13), Jie Gu Qi Li tablet (1), Xian Ling Gu Bao capsule (1), Bu Shen capsule (3), Hong Jin Xiao Jie capsule (1), Yi Qi Yang Xue oral solution (2), Lumbar pain capsule (1), Zheng Qing Feng Tong Ning tablet (1), Nei XiaoLuo Li pill (1), Xiao Yao granule (1), Liu Wei Di Huang pill (1), Bu Zhong Yi Qi pill (1), Gui Fu Di Huang pill (1), Jiu Wei Qiang Huo pill (1), Huo Ba Hua Gen tablet (1), Yin Shen Tong Luo capsule (1), She Xiang Bao Xin pill (3), Tong Zi Su Run Hong capsule (2), Ke Zhi capsule (1), Zu Shi Ma tablet (4), Bulleyaconitine A injection (3), Xue Shuan Xin capsule (1), Tripterygium glycosides tablet (1), Ge Tong Tong Luo capsule (1), He Wei Zhi Tong capsule (1), Zhi Bai Di Huang pill (1) |
| Chinese herbal medicine | 198 (18.07) | Prescriptions of traditional Chinese medicine that consisted of two or more of the following herbs: polygonum multiflorum, atractylodes, gardenia, glycyrrhiza, rhubarb, virgate wormwood, poria, radix clematidis, leech, hypericum japonicum, semen plantaginis, lysimachia, angelica sinensis, ligusticum chuanxiong hort, saffron crocus, radix paeoniae alba, angelica root, salvia miltiorrhiza, lumbricus, radix sileris, phellodendron amurense, gallinaceous bone grass, chrysanthemum, schisandra chinensis, rhizoma cyperi, radix curcumae, bupleurum marginatum |
| Medicinal herbs or plants | 99 (9.03) | Powder, pill, water solution, medicinal liquor of the following herbs or plants: artemisia apiacea (4), folium ginkgo (2), polygonum multiflorum (49), lanatechead saussurea herb with flower (1), panax notoginseng (6), asiaticoside (2), purslane (1), honeysuckle (1), dandelion (1), dioscorea bulbifera (1), scutellariae barbatae (2), oldenlandia diffusa (2), paris polyphylla (2), donkey-hide gelatin (1), eucommia (2), gynostemma pentaphylla (1), cannabis (5), semen raphani (2), asparagus (1), scorpion (5), american ginseng (3), red ginseng (2), tripterygium hypoglaucum (3) |
| Health food or dietary supplement | 26 (2.37) | Weight-loss pills (2), health or sexual enhancement products (21), albumen powder (1), viaminate (1), calcium (1) |
| Combined | 189 (17.24) | Combinations of medications that work on different systems (79), or of medications and herbs (110) |
| Others | 28 (2.55) | Hair dye (3), toxic food (5), pesticide (12), etc. (8) |
Number (%).
The proportion of etiologies was significantly different between age groups. The median age was significantly lower in the medications group (P<0.001; Figure 1), and the incidence of liver injury caused by herbs was significantly higher among female in the other group compared with the medication group (P<0.05). The frequency of jaundice was significantly higher in the herb group, the health food or dietary supplement group and the combination group (P<0.001). Occurrences of skin rashes, encephalopathy and ascites were significantly higher in the medication group and the combination group (P<0.05). The median levels of ALT and AST of the herb group were higher than the medication group (P<0.001), and TBIL of the herb group and the health food or dietary supplement group was higher than the other groups (P<0.001), and DBIL of the herb group was higher than the other groups (P<0.001). In addition, the frequency of Hy’s cases was higher in the herb group and the health foods or dietary supplement group (P<0.001), see Table 4.
Figure 1.
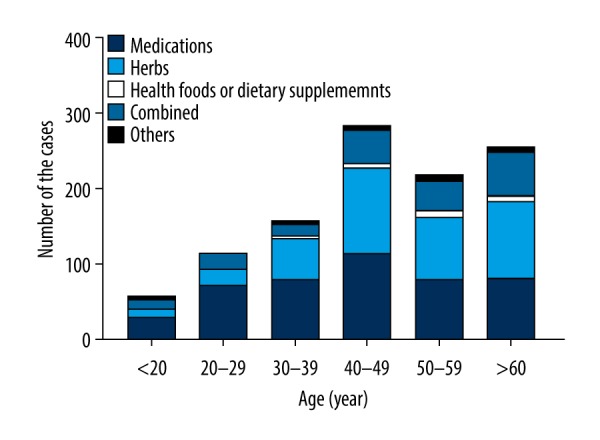
Age distribution of 1096 cases of drug-induced liver injury.
Table 4.
Comparison of characteristics according to the etiology.
| Variable | Medications | Herbs | Health foods or dietary supplements | Combined | Others | p |
|---|---|---|---|---|---|---|
| Total numbers of DILI | 462 | 391 | 26 | 189 | 28 | |
| Age* | 44 (11, 87) | 49 (6, 79) | 53 (29, 79) | 50 (2, 86) | 51 (15, 80) | <0.001 |
| Male** | 238 (51.52) | 147 (37.6) | 69 (34.62) | 77 (40.74) | 12 (42.86) | <0.05 |
| Alcohol drinker** | 130 (28.14) | 113 (28.90) | 9 (34.62) | 40 (21.16) | 4 (14.29) | NS |
| Duration, daysI | ||||||
| Time from drug exposure to DILI recognition | 32 (1, 1825) | 30 (1, 1642) | 28 (3, 547) | 30 (1, 1460) | 24 (2, 547) | NS |
| Time from drug cessation to DILI recognition | 11 (0, 365) | 10 (0, 365) | 10 (1, 330) | 9 (0, 365) | 12 (1, 365) | NS |
| Duration of treatment | 13 (1, 97) | 14 (1, 81) | 16 (2, 41) | 13 (1, 84) | 10 (1, 42) | NS |
| Clinical characteristics** | ||||||
| Jaundice | 110 (23.81) | 306 (78.26) | 21 (80.77) | 120 (63.49) | 5 (17.86) | <0.001 |
| Skin rashes | 133 (28.79) | 53 (13.55) | 1 (3.85) | 72 (38.1) | 2 (7.14) | <0.001 |
| Encephalopathy | 32 (6.93) | 2 (0.51) | 0 | 14 (7.41) | 0 | <0.001 |
| Ascites | 26 (5.63) | 13 (3.32) | 0 | 13 (6.88) | 1 (3.57) | <0.05 |
| Laboratory findings* | ||||||
| AST, U/L | 84 (9, 3967) | 237 (16, 2363) | 337 (16, 1965) | 96 (12, 3790) | 275 (24, 1638) | <0.001 |
| ALT, U/L | 109 (5, 3298) | 325 (10, 2744) | 301 (12, 1688) | 118 (5, 9326) | 276 (12, 1820) | <0.001 |
| ALP, U/L | 131 (34, 2200) | 156 (41, 1165) | 129 (50, 1109) | 142 (37, 1314) | 160 (55, 760) | NS |
| GGT, U/L | 98 (7, 3583) | 147 (9, 2493) | 178 (12, 856) | 115 (10, 1262) | 180 (28, 635) | NS |
| TBIL, μmol/L | 14.5 (1.2, 585) | 87 (4, 728) | 96.7 (10.6, 401) | 16.8 (2.8, 633) | 26.8 (7.2, 535) | <0.001 |
| DBIL, μmol/L | 4.2 (0.4, 428) | 51.6 (0.3, 476) | 48.1 (2, 249) | 5.1 (0.3, 476) | 10.9 (1.2, 193) | <0.001 |
| TP, g/L | 68 (27, 366) | 69 (38.5, 169) | 70.4 (54.6, 94.5) | 66.2 (29, 93.9) | 70.4 (48.6, 96) | NS |
| ALB, g/L | 37.9 (10.8, 60.5) | 39 (22.8, 52.8) | 38.8 (19.9, 48.8) | 37.8 (11, 54.3) | 38 (18.8, 46.8) | NS |
| PT, s | 11.7 (9, 86.5) | 11.6 (7.6, 98.4) | 11.4 (10.1, 21.7) | 11.5 (8.3 47.9) | 11.3 (9.5, 35) | NS |
| INR | 1.01 (0.01, 26.7) | 0.99 (0.6, 133) | 0.99 (0.84, 1.82) | 0.99 (0.7, 4.17) | 0.97 (0.8, 1.42) | NS |
| PTA, % | 92 (5.6, 213) | 99 (0.84, 248) | 108 (34, 136) | 98 (16.5, 176) | 109 (54.5, 141) | NS |
| SCr, μmol/L | 61.8 (3.7, 533) | 59.2 (21, 327) | 65.4 (36.3, 167) | 63 (23.2, 368) | 68.5 (32, 411) | NS |
| Hy’s cases** | 58 (12.55) | 156 (39.9) | 9 (34.62) | 45 (23.81) | 4 (14.29) | <0.001 |
| R values | 2.28 (0.07, 117) | 5.25 (0.12, 80.4) | 5.7 (0.14, 34.5) | 2.42 (0.05, 224) | 4.19 (0.2, 1.27) | 0.001 |
Continuous variables are expressed as median values (range);
categorical variables are expressed as number (%).
Causality assessment
Statistics showed RUCAM scores of the patients ranged from 3 to 14, with a median of 7. Causality assessment showed the likelihood levels of highly probable in 207 patients (18.89%), probable in 652 patients (59.49%) and possible in 237 patients (21.62%) (Table 2). The distribution of cases with different etiologies according to causality is shown in Figure 2. Comparison of etiology with causality revealed that the frequency of highly probable cases was lower in the herb group (P<0.001), and the frequency of probable was higher in the herb group and the combination group (P<0.001). The other group did not show any differences (P>0.05).
Figure 2.
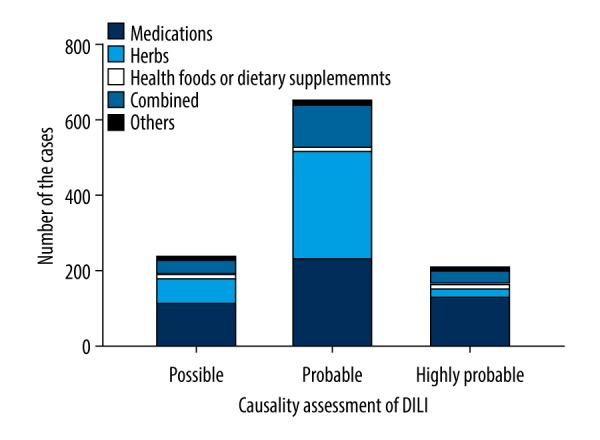
Causative drugs in 1096 cases of drug-induced liver injury (DILI) according to drug class and possibility of injury.
Types of DILI
Hepatocellular, mixed and cholestatic DILI were present in 417 (38.05%), 265 (24.18%) and 414 (37.77%) patients, respectively (Table 2). According to the medical records database of the hospital, the patient who died was cholestatic DILI, and 7 of the 11 patients underwent liver transplantation were hepatocellular DILI, 3 were cholestatic DILI and 1 mixed DILI. The distribution of cases with different etiologies according to types of DILI is shown in Figure 3. Comparison of etiology with DILI types revealed that frequency of hepatocellular DILI was higher in the herb group and the health food or dietary supplement group (P<0.001), and frequency of cholestatic DILI was higher in the medication group and the combination group (P<0.001).
Figure 3.
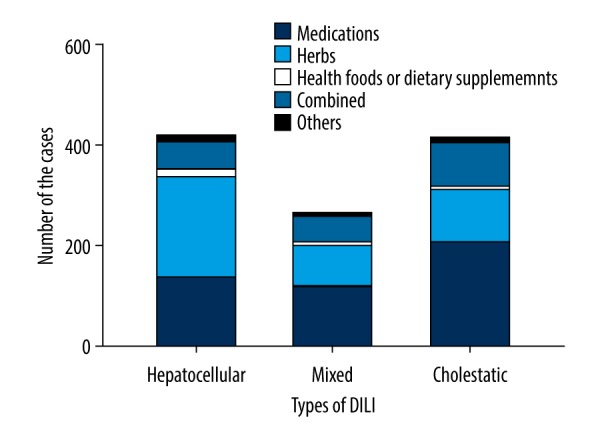
Distribution of drug-induced liver injury (DILI) cases with different etiologies according to types of DILI.
Severity of DILI
The distribution of DILI grades was 640 patients (58.39%) with grade 1, 49 patients (4.47%) with grade 2, 358 patients (32.66%) with grade 3, 37 patients (3.38%) with grade 4, and 12 patients (1.1%) with grade 5 (Table 2). The distribution of cases with different etiologies according to severity of DILI is shown in Figure 4. Comparison of etiology with severity of DILI revealed that frequency of grade 3 DILI was lower in the medication group and higher in the herbs group (P<0.001), frequency of grade 4 DILI was lower in the combined group and higher in the herbs group (P<0.001), and frequency of grade 5 DILI was higher in the combined group (P<0.001).
Figure 4.
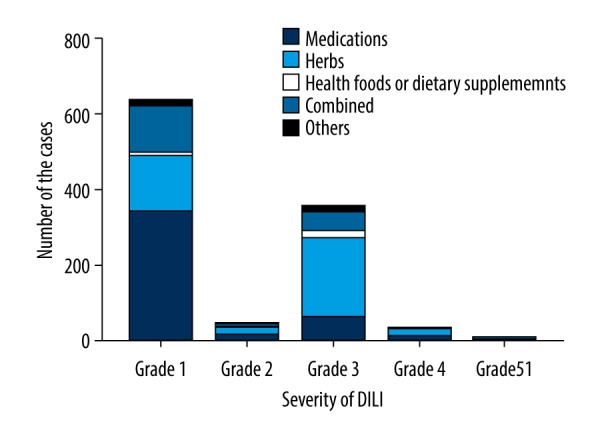
Distribution of drug-induced liver injury (DILI) cases with different etiologies according to severity of DILI.
Discussion
The majority of patients (93.9%) in this study were either cured or showed improvement following clinical intervention; as such, assessing the influence of etiology on outcomes was untenable. Besides, we acknowledge that RUCAM should be used prospectively for timely collection of the relevant data, but unfortunately, it is often used late, after the onset of the liver injury, reducing the chance to detect new hepatotoxins and increasing inter-rater variability. Nevertheless, retrospective but careful RUCAM-based analyses of well documented DILI and HILI cases can provide high causality degrees. In our study, although we use RUCAM retrospectively, we tried our best to reduce relevant judgment bias [20]. The most common causative agents identified were medications and herbs, and the largest category among the medications was antimicrobial agents, which also represent the most common type of drugs that have been reported for DILI [21,22]. Previous studies by others have described the clinical phenotype of DILI caused by different types of antibiotics, revealing a high variability [23]; for example, amoxicillin-clavulanate produce a delayed onset, while cefazolin has a latency period of 1 to 3 weeks after exposure (with a self-limited, moderate to severe clinical course) [24–26] and DILI caused by nitrofurantoin can manifest a few years after treatment [27].
Clinicians presented with patients having symptoms and signs of DILI should initiate prompt cessation of any suspected drug, given the severe consequences of adverse liver reactions if left unchecked [23]. For pediatric patients, the clinical staff needs to maintain particular awareness of the potential risk of liver injury associated with ceftriaxone, cotrimoxazole and clarithromycin, even when these drugs are administered for short periods [28]. The largest category of DILI-related antimicrobial agents identified in our Chinese patient population was antitubercular agents, which are also the most prevalent hepatotoxic agents in many countries. Studies by others have indicated that all patients on antimicrobial agents should be considered for universal liver monitoring, particularly during the first 8 weeks of treatment [29], and genetic factors associated with antitubercular DILI have been reported which may help in these efforts [30,31].
As the use of Chinese herbal medicine continues to spread worldwide, the associated liver injuries, or potential for such, have attracted attention of clinicians and researchers alike. It is often difficult to characterize liver injury attributable to herbal medicine because of the complexity of marketed products and under-reporting by the patients who use them [32,33]. Analysis of reported cases highlighted numerous specific herbal products with hepatotoxic potential, including Radix Scutellariae and Glycyrrhiza Uralensis, and firmly established the causality in such cases for some [34]. In our study, 60 of the DILI cases were associated with patent medications, herbal medicine, folk remedies, or health foods that contained polygonum multiflorum, which is gaining popularity for treatment of many conditions, particularly those associated with aging [35]. Polygonum multiflorum can cause liver damage to various extents, even up to death, and long-term use and overdose increases the risk of both; however, active treatment can lead to cure [36]. Thus, consumers should be alerted to the potential of liver damage when taking preparations containing polygonum multiflorum.
As has been found with some of the previously studies of DILI [9,37], our study population showed a different distribution of sex among the DILI cases with different etiologies. For the factors of age and sex, the differences observed in our study population might have been related to the patients’ medication habits. Clinical features were different among the DILI cases with different etiologies, but across all etiologies jaundice was the most common symptom. A previous study showed that the frequencies of jaundice, skin rashes, encephalopathy and ascites were different between patients using anti-tuberculous drugs and patients not using them [10]. In our study, jaundice was higher in patients using herbs, health foods, and combinations, while skin rashes, encephalopathy and ascites were higher in patients using western medications and combinations. For laboratory features, the reported DILI-related changes have mostly varied among the different studies [8–10,38]. However, the liver biochemistry parameters have shown consistent differences the correspond to the different types of DILI classified by etiology, severity or other classification methods; the detailed mechanisms remain unknown. In general, the patients in our study with herb-induced liver injuries showed higher values of ALT, AST, TBIL, and DBIL. In addition, DILI with etiologies of herbs and health foods or dietary supplements presented higher frequencies of hepatocellular injury DILI, as compared to the other etiologies. Besides, in our study, there were 2.1% patients with severity of grade 4~5 DILI.
Conclusions
Our hospital treated 1811 patients diagnosed with DILI between 2011 and 2016, accounting for 0.248% of the total hospital admissions. Among the 1096 cases included in our analysis, the etiologies of DILI were most frequently medications (462 patients; 42.15%) and herbs (391 patients; 35.68%), followed by health foods or dietary supplements (26 patients; 2.37%), combinations (189 patients; 17.24%) and others (28 patients; 2.55%). Differences in distributions of age, sex, clinical features, laboratory features, and types and severity of DILI in patients corresponded with different etiologies of DILI in our Chinese patient cohort. These data not only reveal novel characteristics of DILI in Chinese patients but may serve as references for prevention, diagnosis and treatment of this disease in China.
Abbreviations
- DILI
drug-induced liver injury
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- ULN
upper limit of normal
- ALP
alkaline phosphatase
- RUCAM
Roussel Uclaf Causality Assessment Method
- GGT
gamma-glutamyl transpeptidase
- TBIL
total bilirubin
- DBIL
direct bilirubin
- TP
total proteins
- ALB
albumin
- SCr
serum creatinine
- PT
prothrombin time
- INR
international normalized ratio
- PTA
prothrombin activity
Footnotes
Conflicts of interest
None.
Source of support: This work was supported by grants from Army Medical University 2017MPRC-09
References
- 1.Vuppalanchi R, Liangpunsakul S, Chalasani N. Etiology of new-onset jaundice: How often is it caused by idiosyncratic drug-induced liver injury in the United States? Am J Gastroenterol. 2007;102(3):558–62. doi: 10.1111/j.1572-0241.2006.01019.x. [DOI] [PubMed] [Google Scholar]
- 2.Fisher K, Vuppalanchi R, Saxena R. Drug-induced liver injury. Arch Pathol Lab Med. 2015;139(7):876–8. doi: 10.5858/arpa.2014-0214-RA. [DOI] [PubMed] [Google Scholar]
- 3.Fontana RJ. Pathogenesis of idiosyncratic drug-induced liver injury and clinical perspectives. Gastroenterol. 2014;146(4):914–28. doi: 10.1053/j.gastro.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dara L, Liu ZX, Kaplowitz N. Mechanisms of adaptation and progression in idiosyncratic drug induced liver injury, clinical implications. Liver Int. 2016;36(2):158–65. doi: 10.1111/liv.12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayashi PH, Fontana RJ. Clinical features, diagnosis, and natural history of drug-induced liver injury. Semin Liver Dis. 2014;34(02):134–44. doi: 10.1055/s-0034-1375955. [DOI] [PubMed] [Google Scholar]
- 6.Chalasani NP, Hayashi PH, Bonkovsky HL, et al. ACG Clinical Guideline: The diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109(7):950–66. doi: 10.1038/ajg.2014.131. [DOI] [PubMed] [Google Scholar]
- 7.De Abajo FJ, Montero D, Madurga M, et al. Acute and clinically relevant drug-induced liver injury: A population-based case-control study. Brit J Clin Pharmacol. 2004;58(1):71–80. doi: 10.1111/j.1365-2125.2004.02133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sgro C, Clinard F, Ouazir K, et al. Incidence of drug-induced hepatic injuries: A French population – based study. Hepatol. 2002;36(2):451–55. doi: 10.1053/jhep.2002.34857. [DOI] [PubMed] [Google Scholar]
- 9.Suk KT, Kim DJ, Kim CH, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012;107(9):1380–87. doi: 10.1038/ajg.2012.138. [DOI] [PubMed] [Google Scholar]
- 10.Björnsson ES, Bergmann OM, Björnsson HK, et al. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterol. 2013;144(7):1419–25. doi: 10.1053/j.gastro.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Shang P, Xia Y, Liu F, et al. Incidence, clinical features and impact on anti-tuberculosis treatment of anti-tuberculosis drug induced liver injury (ATLI) in China. PLoS One. 2011;6(7):e21836. doi: 10.1371/journal.pone.0021836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y, Yang L, Liao Z, et al. Epidemiology of drug-induced liver injury in China: A systematic analysis of the Chinese literature including 21 789 patients. Eur J Gastroen Hepatol. 2013;25(7):825–29. doi: 10.1097/MEG.0b013e32835f6889. [DOI] [PubMed] [Google Scholar]
- 13.Benichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol. 1990;11(2):272–76. doi: 10.1016/0168-8278(90)90124-a. [DOI] [PubMed] [Google Scholar]
- 14.Antoine DJ, Dear JW, Lewis PS, et al. Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatol. 2013;58(2):777–87. doi: 10.1002/hep.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woo HJ, Kim HY, Choi ES, et al. Drug-induced liver injury: A 2-year retrospective study of 1169 hospitalized patients in a single medical center. Phytomedicine. 2015;22(13):1201–5. doi: 10.1016/j.phymed.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Danan G, Benichou C. A novel method based on the conclusions of international consensus meetings: Application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–30. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 17.Temple R. Hy’s law: Predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf. 2006;15(4):241–43. doi: 10.1002/pds.1211. [DOI] [PubMed] [Google Scholar]
- 18.Robles-Diaz M, Lucena MI, Kaplowitz N, et al. Use of Hy’s law and a new composite algorithm to predict acute liver failure in patients with drug-induced liver injury. Gastroenterol. 2014;147(1):109–18. doi: 10.1053/j.gastro.2014.03.050. [DOI] [PubMed] [Google Scholar]
- 19.Drug-induced Liver Disease Study Group, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the management of drug-induced liver injury. J Clin Hepatol. 2015;31(11):1752–69. [Google Scholar]
- 20.Danan G, Teschke R. Drug-induced liver injury: Why is the Roussel Uclaf causality assessment method (RUCAM) still used 25 years after its launch? Drug Saf. 2018;41(8):735–43. doi: 10.1007/s40264-018-0654-2. [DOI] [PubMed] [Google Scholar]
- 21.Chalasani N, Bonkovsky HL, Fontana R, et al. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN prospective study. Gastroenterol. 2015;148(7):1340–52. doi: 10.1053/j.gastro.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leise MD, Poterucha JJ, Talwalkar JA. Drug-induced liver injury. Mayo Clin Proc. 2014;89(1):95–106. doi: 10.1016/j.mayocp.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 23.Björnsson ES. Drug-induced liver injury due to antibiotics. Scand J Gastroenterol. 2017;52(6–7):617–23. doi: 10.1080/00365521.2017.1291719. [DOI] [PubMed] [Google Scholar]
- 24.Alqahtani SA, Kleiner DE, Ghabril M, et al. Identification and characterization of cefazolin-induced liver injury. Clin Gastroenterol Hepatol. 2015;13(7):1328–36.e2. doi: 10.1016/j.cgh.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghabril M, Rockey DC, Gu J, et al. Amoxicillin-clavulanate-induced liver injury. Dig Dis Sci. 2016;61(8):2406–16. doi: 10.1007/s10620-016-4121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SH, Saide K, Farrell J, et al. Characterization of amoxicillin- and clavulanic acid-specific T cells in patients with amoxicillin-clavulanate-induced liver injury. Hepatol. 2015;62(3):887–99. doi: 10.1002/hep.27912. [DOI] [PubMed] [Google Scholar]
- 27.Kleiner DE, Chalasani NP, Lee WM, et al. Hepatic histological findings in suspected drug-induced liver injury: Systematic evaluation and clinical associations. Hepatol. 2014;59(2):661–70. doi: 10.1002/hep.26709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrajolo C, Verhamme KMC, Trifirò G, et al. Antibiotic-induced liver injury in paediatric outpatients: A case-control study in primary care databases. Drug Saf. 2017;40(4):305–15. doi: 10.1007/s40264-016-0493-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abbara A, Chitty S, Roe JK, et al. Drug-induced liver injury from anti-tuberculous treatment: A retrospective study from a large TB centre in the UK. BMC Infect Dis. 2017;17(1):231. doi: 10.1186/s12879-017-2330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang YS. Recent progress in genetic variation and risk of antituberculosis drug-induced liver injury. J Chin Med Assoc. 2014;77(4):169–73. doi: 10.1016/j.jcma.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Chen R, Zhang Y, Tang S, et al. The association between HLA-DQB1 polymorphism and antituberculosis drug-induced liver injury: A case-control study. J Clin Pharm Ther. 2015;40(1):110–15. doi: 10.1111/jcpt.12211. [DOI] [PubMed] [Google Scholar]
- 32.Rossi S, Navarro VJ. Herbs and liver injury: A clinical perspective. Clin Gastroenterol Hepatol. 2014;12(7):1069–76. doi: 10.1016/j.cgh.2013.07.030. [DOI] [PubMed] [Google Scholar]
- 33.Zheng EX, Navarro VJ. Liver injury from herbal, dietary, and weight loss supplements: A review. J Clin Transl Hepatol. 2015;3(2):93–98. doi: 10.14218/JCTH.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teschke R. Traditional Chinese Medicine induced liver injury. J Clin Transl Hepatol. 2014;2(2):80–94. doi: 10.14218/JCTH.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong H, Slain D, Cheng J, et al. Eighteen cases of liver injury following ingestion of polygonum multiflorum. Complement Ther Med. 2014;22(1):70–74. doi: 10.1016/j.ctim.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Lei X, Chen J, Ren J, et al. Liver damage associated with Polygonum multiflorum thunb: A systematic review of case reports and case series. Evid Based Complement Alternat Med. 2015;2015 doi: 10.1155/2015/459749. 459749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterol. 2008;135(6):1924–34. doi: 10.1053/j.gastro.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Devarbhavi H, Dierkhising R, Kremers WK, et al. Single-center experience with drug-induced liver injury from India: Causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105(11):2396–404. doi: 10.1038/ajg.2010.287. [DOI] [PubMed] [Google Scholar]


