Abstract
Native Americans face disproportionate exposures to environmental pollution including exposures through traditional subsistence practices such as fishing and shellfish harvesting. Prior studies in the Salish Sea region of the Pacific Northwest have found high levels of contaminants including polycyclic aromatic hydrocarbons (PAHs) in clams. Conventional biomonitoring methods require removal of clams from the environment which can affect clam abundance and deplete an important cultural and subsistence food. Traditional predictive methods for contaminant bioaccumulation are often inaccurate because of reliance on site specific sediment characteristics. The freely dissolved fraction (Cfree) of contaminants in sediment pore water is a better predictor of contaminant bioavailability than bulk sediment concentration. In this study, the collection of butter clams (Saxidomus giganteus) was spatially and temporally paired with deployment of sediment pore water passive samplers at 4 locations in the Puget Sound region of the Salish Sea in the Pacific Northwest, USA, within adjudicated usual and accustomed tribal fishing grounds and stations. Clams and passive samplers were analyzed for 62 individual PAHs. A linear regression model was constructed to predict PAH concentrations in the edible fraction of butter clams from Cfree in porewater. PAH concentrations can be predicted from the freely dissolved PAH concentration in porewater using the following equation:
The model predicted clam concentrations of PAHs based only on sediment pore water concentrations within a factor of 1.9 ± 0.2 on average. Carcinogenic risk assessment showed potentially unacceptable risk from consuming clams at one of the locations. This model offers a simplified, cost effective, and low impact approach to assess contaminant levels in butter clams which are an important traditional food.
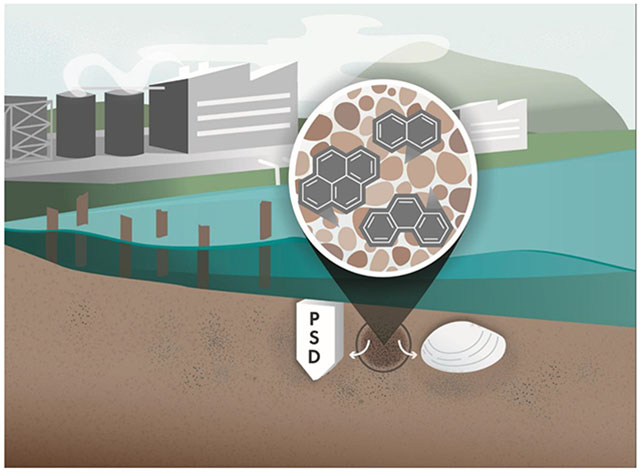
Graphical abstract.
Sediment pore water passive sampling devices (PSDs) were spatially and temporally paired with collection of 5 butter clams at each of 4 locations on adjudicated usual and accustomed tribal fishing grounds in the Salish Sea Region of the Pacific Northwest. Both sediment pore water and clams were analyzed for 62 individual PAHs.
Introduction
In order to calculate human health risk from seafood ingestion, it is necessary to assess contaminant accumulation in organisms. For Native American tribes of the Salish Sea region of the Pacific Northwest, shellfish have been an integral part of the way of life since time immemorial providing a source of both physical and cultural sustenance[1]. Concerns have arisen regarding the impact of anthropogenic contaminants on the safety of natural resources including clams [2, 3]. Previous work in the Salish Sea region has shown that carcinogenic risk from shellfish consumption may be driven by levels of polycyclic aromatic hydrocarbons (PAHs) in clams[1].
Given the importance of subsistence shellfish consumption, Native American tribes, government agencies, and researchers have been working together to better understand these potential human health risks from shellfish [4]. Furthermore, tribes of the Salish Sea region recognize the importance of protecting and sustaining the environment and the natural resources within the environment to protect the identity of their peoples. Subsequently, they have supported efforts to improve the monitoring of contaminants in shellfish.
Directly measuring contaminant levels in organisms is time and resource intensive because of the difficulty of locating and processing the organisms. Additionally, removing organisms from the environment for testing may deplete populations and could be harmful to ecosystems. These considerations have led to the development of predicative tools for determining contaminant levels in organisms [5, 6]. Conventional predictive methods for benthic organisms use total sediment concentrations and empirically derived biota–sediment accumulation factors (BSAF) [7]. However, these methods are often unreliable likely because of uncertainty in site-specific sediment parameters. For instance, variations in the fraction and type of organic carbon can influence the bioavailable fraction of chemical [8, 9]. Several studies have shown that the presence of black carbon, pitch, and other highly hydrophobic materials in the sediment may result in overestimation of the fraction of the chemical that is bioavailable to sediment-dwelling organisms and thus overestimation of chemical bioaccumulation in those organisms [10–12].
Compared to total sediment concentration, the freely dissolved fraction of chemical (Cfree) in the interstitial pore space of sediment (sediment pore water) is a better indicator of bioavailability, bioaccumulation, and toxicity for benthic organisms[13, 14]. Passive sampling devices (PSDs) directly measure Cfree in sediment pore water thereby inherently accounting for site specific sediment characteristics[15]. PSDs therefore offer an improvement in predictive capabilities for bioaccumulation compared to BSAFs[16–19]. A few studies have used passive sampling devices in water to predict contaminant levels in crayfish and mussels[20–22]. More studies have used passive sampling derived sediment pore water concentrations to predict accumulation in benthic organisms, primarily in worms[10, 12, 19, 23, 24]. Muijs and Jonker, 2012 reported that passive sampling methods improved prediction of polycyclic aromatic hydrocarbons (PAHs) in benthic worms compared to traditional methods using BSAF values and total sediment concentrations[24]. Fernandez and Gschwend, 2015 measured levels of 3 PAHs in sediment and the muscle portion of steamer clams (Mya arenaria) from locations in Massachusetts, USA, and compared the ability of passive samplers to predict levels of these 3 PAHs in the clams to the BSAF method. They found that the passive sampling approach was significantly more accurate than the BSAF method, and that the BSAF method consistently over predicted PAH levels in clams by average factors of 10-44 [10].
In the present study we expand on the work of Fernandez and Gschwend, 2015 by using sediment pore water passive samplers to predict accumulation of 62 PAHs in the edible fraction of butter clams (Saxidomus giganteus). Butter clams were selected by tribal governments and collected from 4 locations in the Puget Sound region of the Salish Sea in the Pacific Northwest, USA. Specific sampling locations were selected by the tribes and were within adjudicated usual and accustomed tribal fishing grounds and stations. In addition to building a predictive model, a quantitative human health risk assessment was also performed to determine the potential cancer risk due to PAHs associated with consuming butter clams from this area. Ultimately, the development of a more accurate and relatively simple predictive model could allow for an increased ability to monitor pollutant levels in shellfish without having to remove them from the ecosystem.
Materials and Methods
Study location
Sampling occurred on four separate beaches within the adjudicated usual and accustomed tribal fishing grounds and stations in the Puget Sound region of the Salish Sea in Northwestern Washington, USA. Specific beaches (locations and names withheld at request of tribal governments) were selected with the guidance of local tribal members based on their use as clamming grounds. Beach 1 (B1) and Beach 2 (B2) were located in close proximity to oil refineries while Beach 3 (B3) and Beach 4 (B4) were located within 5km of B1/B2.
Field Sampling
A scientific collection permit was obtained from Washington State (Permit# 14-274). After extensive discussion with tribal leaders, the butter clam was selected as the test species because of its importance to the tribes. Local tribal members worked with the research team to harvest and identify butter clams. At each of the 4 sites, 5 clams were collected for a total of 20 clams. Following collection, clams were rinsed with ambient water followed by deionized water, stored in either an amber glass jar or a sealed Teflon bag, and transported back to the lab on ice. Immediately following removal of the clams, sediment passive sampling devices (PSDs) containing low density polyethylene (LDPE) were placed in the clam holes and deployed for a total of 29 days. These sediment passive sampling devices have been previously described in detail and are shown in Figure S1[25]. The LDPE strips were transported back to the laboratory in glass jars in a cooler. Transport stability studies performed by Donald et al. confirm that transport times (<24h) and temperature (<20°C) should not result in the loss of PAHs from LDPE[26].
PSD preparation and extraction
Additive free LDPE strips were constructed as described in Anderson et al. [27]. Fluorene-d10 was added to the passive samplers prior to deployment for determination of in-situ sampling rates[15]. PSDs were stored at −20°C prior to sample processing which is described in detail by Allan et al. [28]. In brief, PSDs were spiked with 7 deuterated surrogate extraction standards followed by dialysis in n-hexane and quantitative concentration. Surrogate extraction standards included: naphthalene-d8, acenaphthylene-d8, phenanthrene-d10, fluoranthene-d10, chrysene-d12, benzo[a]pyrene-d12 and benzo[ghi]perylene-d12. Surrogate recoveries ranged from 45% for naphthalene-d8 (RSD 11%) to 110% for benzo[ghi]perylene-D12 (RSD 15%). All solvents used were Optima® grade or better (Fisher Scientific, Pittsburgh, PA), and standards were purchased at purities ≥ 97%. Standards were prepared as singles or simple mixes in ethyl acetate, n-hexane, or isooctane (Fisher Scientific, optima grade) at concentrations typically between 1mg/L and 10mg/L.
Clam processing and extraction
Clam tissue was homogenized via freeze fracture using a Robot Coupe Blixer-2 food processor and liquid N2. After homogenization, tissue was transferred into an amber glass screw-top jar and stored in the dark at −20°C. Prior to use, and between samples, all equipment was washed with soap and water and then rinsed with high purity water, acetone, and hexane. Extraction of PAHs from clam followed a modified QuECHERS method as described previously [21]. Seven individual extraction surrogates (same as LDPE extraction) were added to 1.0 g (± 2%) wet weight of homogenized clam tissue. Surrogate recoveries were 39%, 49%, 44%, 46%, 61%, 73%, and 68% for naphthalene-d8, acenaphthylene-d8, phenanthrene-d10, fluoranthene-d10, chrysene-d12, benzo(a)pyrene-d12, and benzo(ghi)perylene-d12, respectively.
Chemical Analysis
Samples were analyzed for 62 PAHs (Table S1) using an Agilent 7890A gas chromatograph (GC) with an Agilent 7000C MS/MS as described in Anderson et al. [29]. Use of an Agilent Select PAH column (30m x 250μm x 0.15μm) allowed for enhanced separation of compounds. For all PAHs, at least a 5-point calibration was employed spanning 4 orders of magnitude (1μg/L – 10mg/L) with correlations ≥0.98. GC oven and MS parameters along with a comprehensive list of method detection limits (MDLs) can be found in the Supplemental information (Table S1).
Porewater concentration calculation
The freely dissolved concentration of PAHs in the sediment pore water was calculated from extract concentration using methods described by Huckins et al[15]. Briefly, performance reference compounds were utilized to determine chemical specific sampling rates with additional corrections for the effect of temperature and salinity on the partition coefficients between the sampler and water. These calculations are described in detail in the supplemental information equation S1–S7.
Quality control
Quality control samples including field, trip, instrument, cleaning, reagent, and laboratory blanks as well as laboratory duplicates and over-spikes accounted for over 30% of the total samples analyzed. Clam extraction blanks contained measurable amounts of several low molecular weight PAHs making up on average 15% of Σ62PAHs in the clams and are shown in Table S4. Average extraction blank concentrations were subtracted from PAH concentrations measured in clam samples. Field blanks for the passive samplers contained quantifiable levels of several low molecular weight PAHs making up on average 13% of Σ62 PAHs in sediment pore water and are shown in Table S4. Average field blank levels for the passive samplers were subtracted from PAH concentrations measured in sediment pore water samples. Instrument blanks and reagent blanks were below levels of detection for all analytes. Perylene-d12 was used as the instrumental internal standard. Continuing calibration verifications samples consisting of 51 individual PAHs at 500 μg/L were run every 12 samples or less and all met our data quality objectives where >80% of target analytes must be within 20% of the known value.
Human health risk assessment
The relative potency factor approach, described by the US EPA, was utilized to determine the carcinogenic risk associated with consuming the clams analyzed in this study. Risk assessment was performed for both subsistence consumers and consumers in the general US population[30]. Subsistence consumer ingestion rates (100 g/d) were obtained from a previously reported study based on a tribal community located in the Puget Sound region of the Salish Sea in Washington, USA[1]. Average shellfish ingestion rates for the general adult population in the Pacific region (4.6 g/d) was obtained from the US EPA[31]. Excess lifetime cancer risk was assessed based on the following EPA standardized assumptions for an adult (70 kg) using an exposure frequency of 365 days/year for an exposure duration of 70 years. The average benzo[a]pyrene equivalence (BaPeq) factor for each beach was multiplied by the slope factor of 7.3 mg/kg×d based on the latest US EPA guidance[30]. Detailed equations for these calculations can be found in the Supplemental information equation S8–S10.
Statistical Methods
Statistical analyses of PAH data were performed using Microsoft Excel, 2016 and JMP Pro 13. If individual PAH concentrations were below the limit of detections in either the clam or sediment pore water, the corresponding limits of detection were substituted for modeling purposes. This occurred in 163 of 436 (37%) individual paired (sediment pore water and clam) PAH measurements. The vast majority (93%) of these were detections of PAHs in the sediment pore water but not in the clams resulting in substitutions of clam detection limits. Of these, 89% were 2- and 3- ring PAHs. Statistical analysis of correlations between PAH concentrations in clams and sediment pore water showed that a regression model with a square root function appeared to be the best fit for the data. Specifically, this regression captured the non-linear form of the line at elevated sediment pore water concentrations. Additional factors were explored including individual site locations, clam size/age, and Kow values.
Results & Discussion
PAH concentrations in sediment and clams
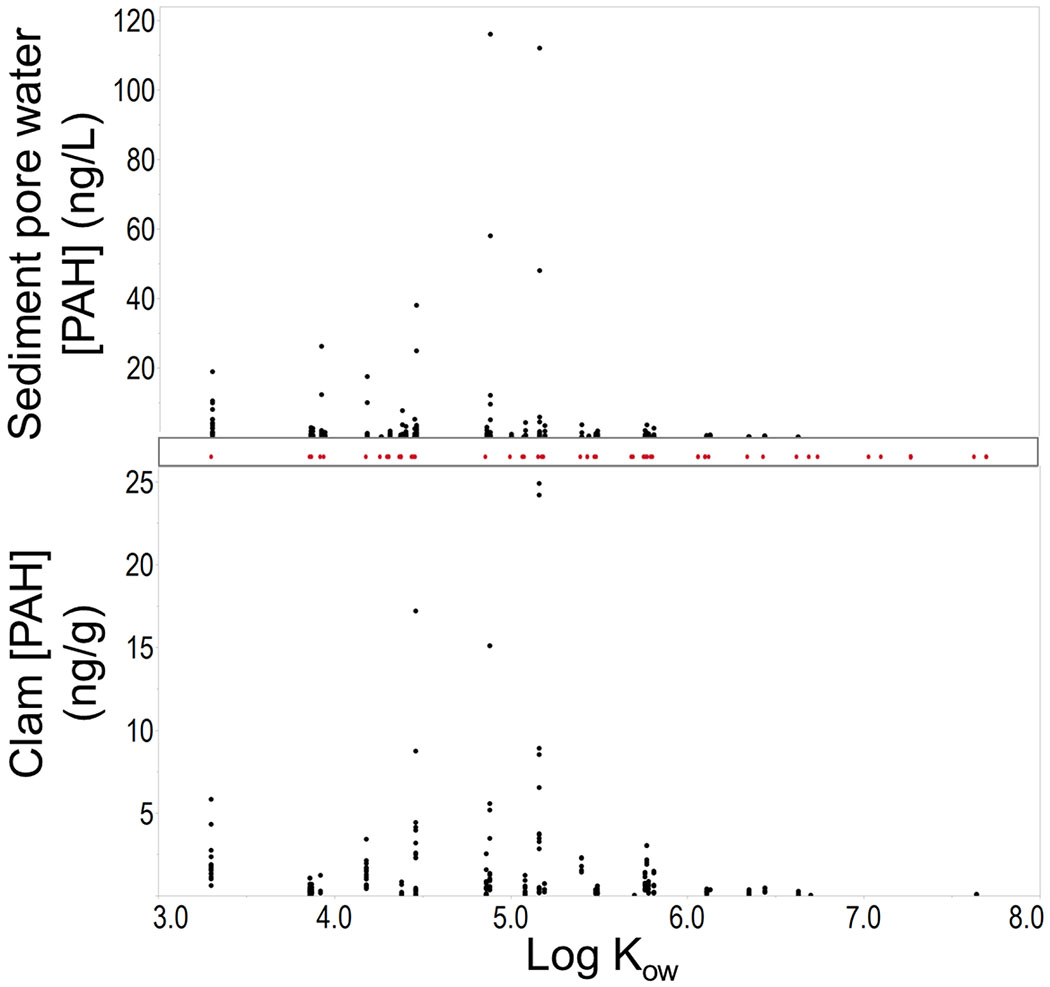
Of the 62 PAHs analyzed, 42 different PAHs were detected in at least one sediment pore water or clam sample. Concentrations of PAHs in sediment pore water and clams were correlated (Table 1). PAHs with log Kow values between 4.0 and 5.5 were elevated in both clams and sediment pore water. This indicated that freely dissolved PAHs in the sediment pore water were more likely to bioaccumulate in clams (Figure 1). As expected, PAHs with log Kow values greater than 6.0 were rarely detected or detected at low levels in both clam and sediment pore water, illustrating that these high molecular PAHs were likely bound to sediment and not readily available for bioaccumulation.
Table 1.
Range of concentrations for ∑62 PAH in sediment pore water (ng/L) and the edible fraction of clam (ng/g) at beaches 1-4. Using the EPA RPF approach, the benzo[a]pyrene equivalence (ng/g) and corresponding excess lifetime cancer risk (ELCR) was calculated for both subsistence consumers (100g/d) and consumers in the general population (4.6g/d).
| Beach 1 | Beach 2 | Beach 3 | Beach 4 | |
|---|---|---|---|---|
| ∑62[PAH] Sediment pore water (ng/L) | 5.0-19 | 12-390 | 1.2-5.5 | 5.5-15 |
| ∑62[PAH] Clam (ng/g) | 12-18 | 25-81 | 4.1-4.7 | 2.7-4.4 |
| BaPeq Clam (ng/g) | 0.45-0.59 | 5.7-18 | 0.032-0.041 | 0.014-0.021 |
| ELCR Clam (subsistence) | 4.7-6.1 | 59-190 | 0.33-0.43 | 0.15-0.22 |
| ELCR Clam (general) | 0.22-0.24 | 2.7-8.6 | 0.015-0.020 | 0.0068-0.056 |
Figure 1.
Individual PAH concentrations in clams (ng/g) and in sediment pore water (ng/L) versus log Kow. Red points represent the 62 individual PAHs in the analytical method for both clams and sediment pore water.
Concentrations of PAHs in the sediment pore water were highest at B2 (12- 390ng/L). For context, PAH concentrations at B2 were comparable to, and in some cases higher than ∑62 PAH concentrations reported in sediment pore water at a PAH-contaminated Superfund site in Portland, Oregon (24-180ng/L)[25]. Concentrations of PAHs in clams were also highest at B2 (25-81ng/g), driven largely by 2 individual clams with concentrations of 68 ng/g and 81 ng/g. Of note, these 2 clams were located closest to a creosote covered wooden pier. It is possible that creosote, which contains high concentrations of PAHs, is partially responsible for these elevated levels[32].
Spatial differences in PAH concentrations were noted between the 4 sites, and these differences have important implications for shellfish harvesters. The average ∑62 PAH clam concentration at B2 (48 ng/g) was approximately 3 times higher than the average clam concentration at B1 (15 ng/g) and roughly 10 times higher than the average clam concentration at B3 (4.3 ng/g) and B4 (3.3 ng/g). Similar trends were seen for sediment pore water concentrations. PAH data for all clam and sediment pore water samples can be found in Tables S7 and S8, respectively. B2 is adjacent to petroleum refineries and B1 is approximately 1km away from the refineries. B3 and B4 are approximately 5km from the refineries and in a separate bay. Together, these results suggest a potential impact from the refineries on PAH concentrations in clams collected nearby. However, additional historical sources of PAHs in the area exist, including a non-contained waste facility, petroleum storage, and pulp mill.
PAH profiles
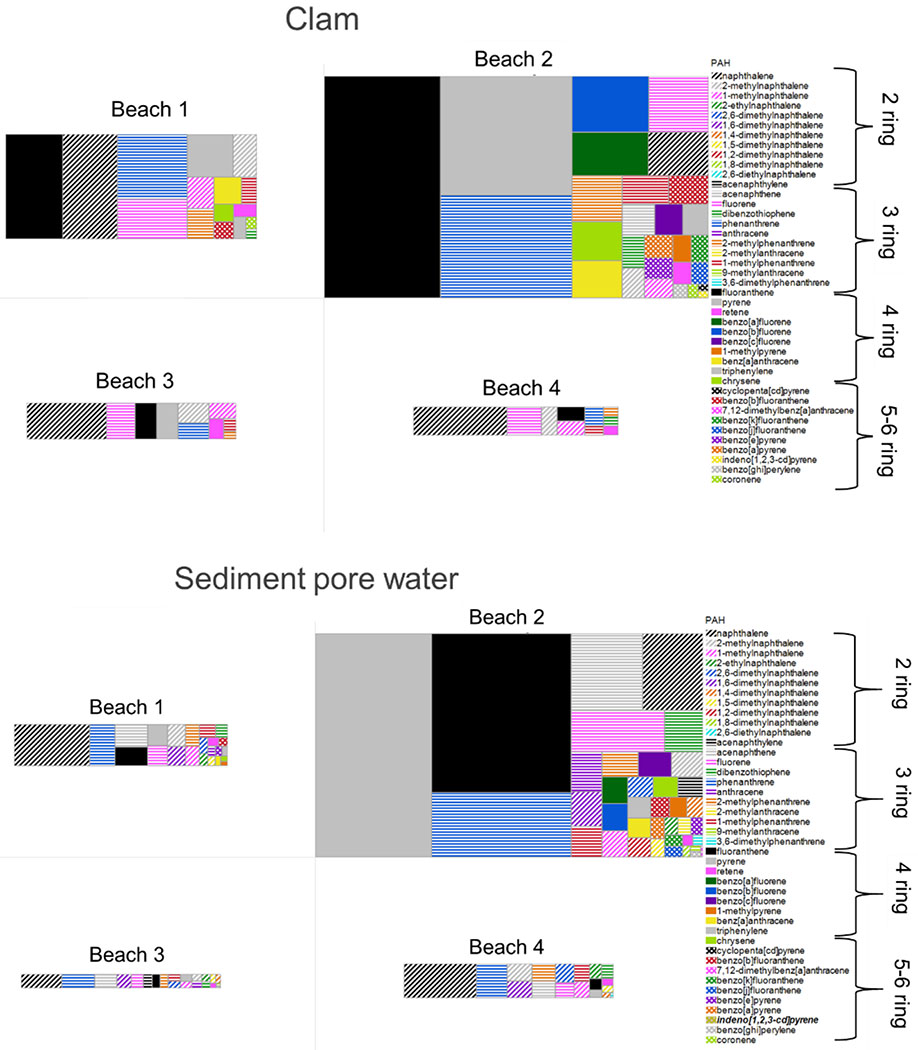
Profiles of PAHs in clams from the 4 different beaches shows high variability (Figure 2a). At B2, fluoranthene, pyrene, and phenanthrene accounted for approximately half of ∑PAH. Additionally, several 4-ring PAHs were detected in clams from B2, the site nearest to the oil refinery, that were not detected at any of the other beaches. Two of these 4-ring PAHs were benzo[a]pyrene and benzo[c]fluorene. This is noteworthy because larger PAHs are generally more carcinogenic than 2- and 3-ring PAHs. In the case of benzo[c]fluorene, the relative potency factor for cancer risk assessment is 20 times higher than that of benzo[a]pyrene[30]. Detections of 4-6 ring PAHs were lower at B1 compared to B2 and ∑PAH was driven by fluoranthene, naphthalene, and phenanthrene. At B3 and B4, individual PAH profiles were very similar showing mostly 2- and 3-ring PAHs with naphthalene and fluorene accounting for approximately half of ∑PAH. PAH profiles in sediment pore water were similar to PAH profiles in clams, but were comparatively elevated in 2 and 3-ring PAHs.
Figure 2.
PAH profile in clams and sediment pore water. The size of the entire rectangle at each location indicates the relative ∑62 PAH concentration for the clam and sediment pore water separately. The size of the individual colored rectangles indicates the relative individual PAH concentration as a fraction of the ∑62 PAH concentration for each beach location. As such the size of the rectangles are directly comparable between beaches when comparing clams or sediment pore water separately. The striped pattern indicates 2- and 3-ring PAHs, the solid pattern 4-ring PAHs, and the checker pattern 5- and 6-ring PAHs.
Diagnostic ratios of PAH isomers in sediment may be used in a weight of evidence approach to identify potential sources[33, 34]. The ratio of fluoranthene/pyrene in B2 sediment pore water indicates a primarily petrogenic source consistent with petroleum or creosote and a primarily pyrogenic source at the other beaches[32–34]. However, a lack of consistent PAH detections across all beaches limits the number of usable diagnostic ratios and makes any type of conclusive statement of PAH sources overreaching.
Predictive modeling
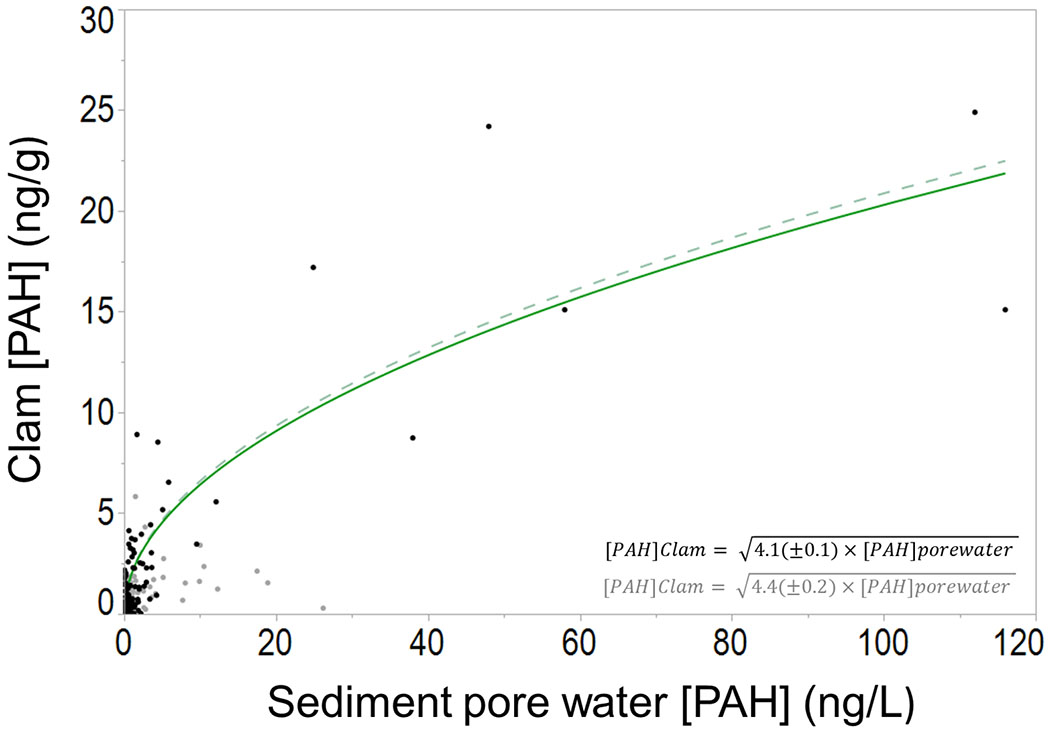
A linear regression model was generated to predict PAH levels in clams from Cfree in sediment pore water using the 42 detected PAHs (Figure 3). Limits of detection were substituted in the case of detection of a PAH in only 1 of a sediment pore water or clam paired sample. The line of best fit is shown in Equation 1 below.
Figure 3.
Regression of 42 PAHs detected in spatially paired sediment pore water passive samples (ng/L) and in clams (ng/g). Gray points indicate PAHs with less than 3-full -rings. Excluding these points, a second regression was constructed (dashed line). Clams were collected from 5 locations at each of 4 different beaches located on adjudicated usual and accustomed tribal fishing grounds and stations in the Puget Sound region of the Salish Sea in the Pacific Northwest, United States. The intercept was set to 0 (slope ± standard error).
| (Eq. 1) |
The root mean square error (RMSE) of this regression is 0.77. With Equation 1, PAH concentration in clams were predicted using only Cfree in sediment pore water measured with passive samplers. The square root function of this regression is such that as PAH concentration in sediment pore water increases, the rate of PAH accumulation in clams decreases. This attribute is fundamentally different than BSAFs which assume linear rates of bioaccumulation with increasing sediment concentrations. The non-linear nature of our predictive regression model may represent the induction of detoxifying enzymes, such as the cytochrome P450 enzymes which have been shown to be active in some species of clams[35–37]. Specifically, induction of these enzymes may result in a decreased rate of PAH accumulation in clams at higher PAH concentrations. As such, the regression model described in this study is an improvement over the linear nature of BSAFs which would over predict clam concentrations at elevated sediment pore water concentrations with this data set.
Furthermore, we observed that several of the 2- and 3-ring PAHs in our regression model were over-predicted in clams based on their sediment pore water concentrations. That is to say that these PAHs were at high concentrations in the sediment pore water, but did not appear to accumulate appreciably in the clams. This is likely because smaller PAHs are less hydrophobic (lower Kow values) and thus accumulate less in tissues compared to larger PAHs. To explore this finding, a second predictive model was constructed which included only PAHs with 3 full 6-membered rings and larger (dashed lines in Figure 3). In this model, the slope of the best fit equation increased slightly as expected but the overall accuracy remains largely unaffected with an RMSE of 0.78. Because of the minor differences between these best-fit lines, we would recommend using the original model that includes all PAHs.
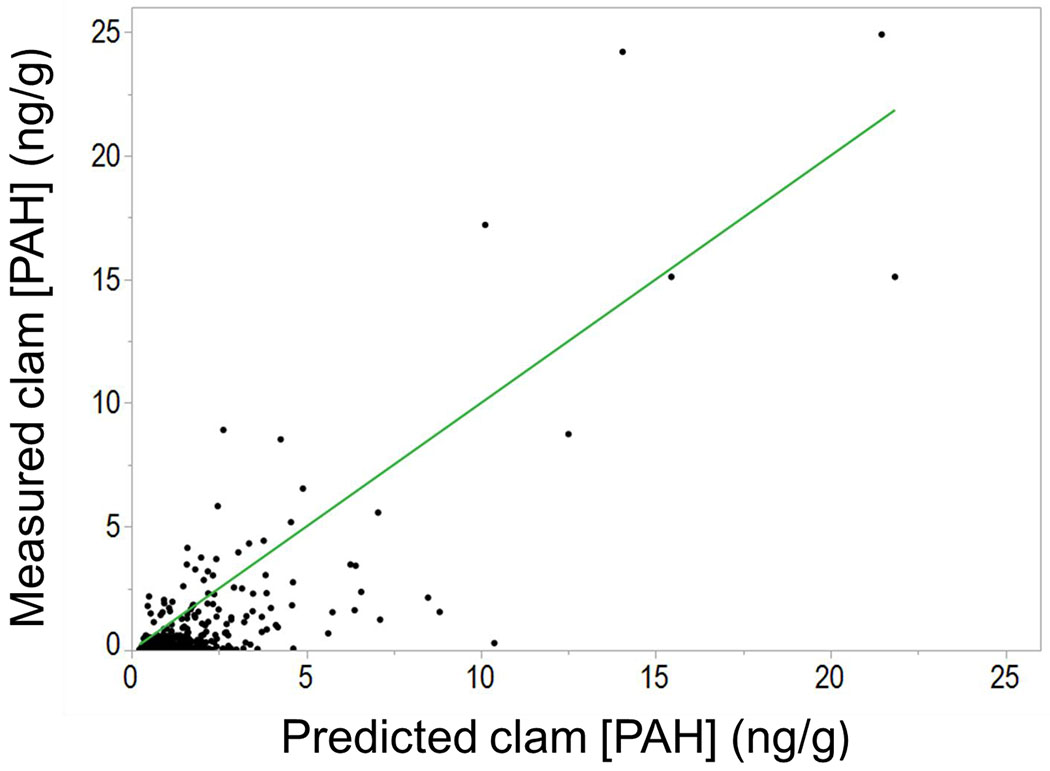
In order to assess model performance, predicted values from equation 1 were compared to measured values (Figure 4). Factors were calculated by taking a ratio of the predicted and measured values (Table S9). When calculating factors, it is possible that large factors may exist even when the absolute difference is negligible. For instance, if the predicted value was 1.0 ng/g and the actual value was 0.1 ng/g then the factor is 10 but the absolute difference is less than 1 ng/g. To increase the meaning of factor analysis, instances where either the predicted or measured clam concentration was less than 1 ng/g were excluded from calculation of factors. Refinement of these calculations resulted in 71 individual factor differences for 13 PAHs. Based on these values, the model predicted average concentrations in clams within a factor of 1.9 ± 0.2 of the measured values (0.2 indicates 2*SE). To illustrate that this process did not unduly lower the factors, when considering all factors, only 3.0% were greater than 3 when the absolute difference was also greater than 3.0 ng/g. All of these instances corresponded to 2- and 3-ring PAHs which were detected in sediment pore water and not in clams. It is possible that these small PAHs do not partition into clams because of their relatively low hydrophobicity compared to larger PAHs. Additionally, when considering all the points, the average absolute difference between predicted and measured clam concentrations was 1.1 ng/g.
Figure 4.
PAH concentration measured in clam tissue (ng/g) versus PAH concentration predicted in clam tissue (ng/g) from freely dissolved concentrations of PAHs in sediment pore water. Predicted PAH concentrations were derived from Equation 1 from the linear regression model displayed in Figure 3. The reference line indicates where predicted/measured values = 1. On average, the predicted concentrations by the model were within a factor of 1.9 ± 0.2 of the measured values when considering only predicted and measured values greater than 1 ng/g.
Other researchers have used sediment PSDs to predict PAHs in other organisms including other clam species. For instance, Fernandez and Gschwend, 2015 predicted levels of phenanthrene, pyrene, and chrysene in soft-shell steamer clams (Mya arenaria) using low density polyethylene PSDs in sediment. The average ratios of predicted to measured values were 0.43, 3.7, and 1.1 for phenanthrene, pyrene, and chrysene respectively[10]. These numbers correspond to an average factor of 2.4 which is larger than the average factor of 1.9 for the 13 PAHs we reported in this study. Additionally, the standard deviation for the factors in our study (1.0) is less than shown by Fernandez and Gschwend, 2015 [10]. Using PSD derived sediment porewater concentrations, Muijs and Jonker, 2012 predicted concentrations of 13 PAHs in benthic worms generally within a factor of 4 [24]. The authors cite the relatively low capacity of SPME passive samplers and the use of generic BAF factors as reasons for higher than expected variability. Compared to the model presented by Fernandez and Gschwend to predict PAHs in steamer clams, our model is an improvement because of the incorporation of additional PAHs and the lower factor differences between measured and predicted concentrations. It’s possible that the lower factor differences result from the heterogeneity of sediment and the fact that PSDs were placed directly into the clam holes in this study as opposed to adjacent to the clam holes in the Fernandez and Gschwend study. An additional benefit, because we were concerned with the fraction of clam which would be eaten by Native American tribal members, our model considers the entire edible fraction as opposed to only the lipid and protein fraction. Taken together, our approach is preferable as a proxy method for monitoring PAH contamination in shellfish for determination of potential risk through ingestion.
In predictive models for bioaccumulation, over-prediction is preferable and false negatives should be kept at a minimum in order to be protective of health. When considering all data points, only 8.7% of individual PAHs were under predicted by the model. Importantly, individual PAHs were measured in clams but were below limits of detection in the sediment in only 2.5% of samples. In these cases, the model would fail to predict any concentration in clams. The majority of these were large PAHs including retene, coronene, and indeno[1,2,3-cd]pyrene and all the clam concentrations were less than 0.5ng/g. It is possible that these larger, more persistent PAHs reflect historical contamination or slow diffusion rates as butter clams can live up to 20 years. Based on the measured length of clams in this study (70-110mm) the corresponding ages are likely between 7-20 years [38].
Additionally, it is possible that the presence of these larger PAHs represents the ingestion of particle-bound PAHs by the clams. Clams, like other bivalves, are filter feeders and therefore may be exposed to both dissolved and particulate-bound PAHs in both the sediment pore water and the water column[39, 40]. However, studies have demonstrated that even for organisms whose exposure to contaminants is expected to be through the ingestion of sediment, bulk sediment concentrations often do not correlate well with bioaccumulation [19]. Here, the fact the passive sampler generally measured the same PAHs in the clams seems to indicate that the majority of exposure for clams occurs through PAHs in the freely dissolved phase. This observation is further enforced by the similarity between the PAH profiles in sediment pore water and clams. The passive sampling model presented here enables accurate prediction of PAH concentrations in clams and has applicability for risk assessors and land managers.
Human health risk assessment
A quantitative human health risk assessment was conducted to assess the carcinogenic risk from PAHs associated with ingestion of clams using the relative potency factor approach as described by the U.S. EPA[30]. Cancer risk is expressed as the excess lifetime cancer risk (ECLR) which is defined as a probability of the number of potential cancer cases above background. Table 1 shows ELCR estimates for each of the 4 beaches. The acceptable risk level is generally defined by the U.S. EPA as an ECLR of between 10−6 (1 in a million) and 10−4 (1 in 10,000) [41].
The estimated lifetime cancer risk from consuming shellfish in this study was more than 20 fold higher at beach B2 than at the other 3 locations, and more than 20 fold higher for subsistence consumers compared to the general population. The highest risk was for subsistence consumers at B2 where the ECLR was 1.3 in 10,000 based on the average clam concentration at that site. At B1, carcinogenic risk was lower, but remained above 1 in a million while at B3 and B4 risk levels were below 1 in million. For the general population, the ECLR at B2 was 6 in a million and below 1 in a million at the other locations.
This data demonstrates the importance of site-specific monitoring for making recommendations regarding shellfish consumption even within a small geographical region. It is important to note that the excess cancer risk calculated is based only on PAH concentrations. Additionally, cancer target levels set by the U.S. EPA or state agencies may not be representative of the target cancer levels of individual Native American tribal communities. Each Native American tribe may identify a different level of risk that is acceptable to them. Furthermore, it is essential to balance the risk of consuming potentially contaminated clams with both health and cultural benefits[42].
There are several important limitations to this cancer risk assessment. First, the risk assessment assumes that clams with the same PAH concentrations will be consumed daily for 70 years. Second, it is possible that clam concentrations may change over that time period altering the risk. The risk assessment is also limited by the relatively small numbers of clams which were collected from four locations and which were not chosen by random sample generation. In addition, this risk assessment considers only ingestion exposure to PAHs through the consumption of clams. In reality, total lifetime exposure to PAHs may occur through separate inhalation or dermal routes. This risk assessment only considers PAHs when in reality many other chemicals may contribute to risk. Finally, uncertainty exists in every PAH risk assessment due to interspecies extrapolations which are inherently part of the RPFs and because of the lack of toxicity data for the vast majority of PAHs[30].
Conclusions
This study showed that PAHs in butter clams collected in the Salish Sea vary considerably. It is possible that clams from certain locations within adjudicated usual and accustomed tribal fishing grounds and stations in the Pacific Northwest contain levels of PAH that are harmful to human health particularly for Native Americans who have higher traditional consumption rates. To improve monitoring of contaminant levels in clams and other shellfish while addressing cultural norms and limiting damage to an already fragile ecosystem, it is important to consider alternative proxy measurements from passive sampling devices. The predictive model developed in this study that was based on data collected using a sediment pore water passive sampling device provides a low impact, simple approach to predict PAH levels in butter clams within a factor of 2. As anthropogenic impacts on ecosystems continue to increase, the importance of accurately and noninvasively measuring contaminant accumulation in organisms will grow in importance.
Supplementary Material
Acknowledgments
This work was funded by the National Institute of Environmental Health Sciences grants P42-ES016465, P30ES000210 and T32ES007060-32. We would like to thank the Native American tribes in this study, who wish to remain anonymous, for their assistance in all aspects of this study including study design, sample location identification, sample collection, sample processing, and analysis. Additionally, we are grateful for the analytical expertise and assistance in the field provided by Glenn Wilson, Gary Points, Holly Dixon, Carey Donald, Alan Bergmann, and Jorge Padilla. The authors of this study declare no conflicts of interest.
Footnotes
Data availability
Data are available in the supplemental data and are available on request form the corresponding author (kim.anderson@oregonstate.edu).
References
- [1].Swinomish. 2006. Bioaccumulative Toxics in Subsistence-Harvested Shellfish- Contaminant Results and Risk Assessment. In Office of Planning and Community Development WRP, ed. [Google Scholar]
- [2].Coast Salish Gathering. www.coastsalishgathering.com.
- [3].Donatuto J, Campbell L, Gregory R. 2016. Developing Responsive Indicators of Indigenous Community Health. Int J Environ Res Public Health 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].U.S.EPA. 2014. A Decade of Tribal Environmental Health Research: Results and Impacts from EPA’s Extramural Grants and Fellowships Programs.
- [5].Bayen S, ter Laak TL, Buffle J, Hermens JLM. 2009. Dynamic Exposure of Organisms and Passive Samplers to Hydrophobic Chemicals. Environmental Science & Technology 43:2206–2215. [DOI] [PubMed] [Google Scholar]
- [6].Arnot JA, Gobas FAPC. 2006. A Review of Bioconcentration Factor (BCF) and Bioaccumulation Factor (BAF) Assessments for Organic Chemicals in Aquatic Organisms. Environmental Reviews 14:257–297. [Google Scholar]
- [7].Boese BL, Lee Ii H, Specht DT, Randall R, Pelletier J. 1996. Evaluation of PCB and Hexachlorobenzene Biota-Sediment Accumulation Factors Based on Ingested Sediment in a Deposit-Feeding Clam. Environmental Toxicology and Chemistry 15:1584–1589. [Google Scholar]
- [8].Cornelissen G, Gustafsson Ö, Bucheli TD, Jonker MTO, Koelmans AA, van Noort PCM. 2005. Extensive Sorption of Organic Compounds to Black Carbon, Coal, and Kerogen in Sediments and Soils: Mechanisms and Consequences for Distribution, Bioaccumulation, and Biodegradation. Environmental Science & Technology 39:6881–6895. [DOI] [PubMed] [Google Scholar]
- [9].Hauck M, Huijbregts MAJ, Koelmans AA, Moermond CTA, Van den Heuvel-Greve MJ, Veltman K, Hendriks AJ, Vethaak AD. 2007. Including Sorption to Black Carbon in Modeling Bioaccumulation of Polycyclic Aromatic Hydrocarbons: Uncertainty Analysis and Comparison to Field Data. Environmental Science & Technology 41:2738–2744. [DOI] [PubMed] [Google Scholar]
- [10].Fernandez LA, Gschwend PM. 2015. Predicting Bioaccumulation of Polycyclic Aromatic Hydrocarbons in Soft-Shelled Clams (Mya arenaria) Using Field Deployments of Polyethylene Passive Samplers. Environmental Toxicology and Chemistry 34:993–1000. [DOI] [PubMed] [Google Scholar]
- [11].Oen AMP, Schaanning M, Ruus A, Cornelissen G, Källqvist T, Breedveld GD. 2006. Predicting Low Biota to Sediment Accumulation Factors of PAHs by Using Infinite-Sink and Equilibrium Extraction Methods as well as BC-Inclusive Modeling. Chemosphere 64:1412–1420. [DOI] [PubMed] [Google Scholar]
- [12].Heijden SAvd Jonker MTO. 2009. PAH Bioavailability in Field Sediments: Comparing Different Methods for Predicting in Situ Bioaccumulation. Environmental Science & Technology 43:3757–3763. [DOI] [PubMed] [Google Scholar]
- [13].Mayer P, Parkerton TF, Adams RG, Cargill JG, Gan J, Gouin T, Gschwend PM, Hawthorne SB, Helm P, Witt G, You J, Escher BI. 2014. Passive Sampling Methods for Contaminated Sediments: Scientific Rationale Supporting use of Freely Dissolved Concentrations. Integr Environ Assess Manag 10:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hawthorne SB, Azzolina NA, Neuhauser EF, Kreitinger JP. 2007. Predicting Bioavailability of Sediment Polycyclic Aromatic Hydrocarbons to Hyalella azteca using Equilibrium Partitioning, Supercritical Fluid Extraction, and Pore Water Concentrations. Environmental Science & Technology 41:6297–6304. [DOI] [PubMed] [Google Scholar]
- [15].Huckins JN, Petty JD, Booij K. 2006. Monitors of the Organic Chemicals in the Environment Monitors of the Organic Chemicals in the Environment. Springer, New York, NY, p. 223. [Google Scholar]
- [16].Mayer P, Vaes WHJ, Wijnker F, Legierse KCHM, Kraaij R, Tolls J, Hermens JLM. 2000. Sensing Dissolved Sediment Porewater Concentrations of Persistent and Bioaccumulative Pollutants Using Disposable Solid-Phase Microextraction Fibers. Environmental Science & Technology 34:5177–5183. [Google Scholar]
- [17].Fernandez LA, Harvey CF, Gschwend PM. 2009. Using Performance Reference Compounds in Polyethylene Passive Samplers to Deduce Sediment Porewater Concentrations for Numerous Target Chemicals. Environmental Science & Technology 43:8888–8894. [DOI] [PubMed] [Google Scholar]
- [18].Oen AMP, Janssen EML, Cornelissen G, Breedveld GD, Eek E, Luthy RG. 2011. In Situ Measurement of PCB Pore Water Concentration Profiles in Activated Carbon-Amended Sediment Using Passive Samplers. Environmental Science & Technology 45:4053–4059. [DOI] [PubMed] [Google Scholar]
- [19].Lu X, Skwarski A, Drake B, Reible DD. 2011. Predicting Bioavailability of PAHs and PCBs with Porewater Concentrations Measured by Solid-Phase Microextraction Fibers. Environmental Toxicology and Chemistry 30:1109–1116. [DOI] [PubMed] [Google Scholar]
- [20].Paulik LB, Smith BW, Bergmann AJ, Sower GJ, Forsberg ND, Teeguarden JG, Anderson KA. 2015. Passive Samplers Accurately Predict PAH Levels in Resident Crayfish. Sci Total Environ 544:782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Forsberg ND, Smith BW, Sower GJ, Anderson KA. 2014. Predicting Polycyclic Aromatic Hydrocarbon Concentrations in Resident Aquatic Organisms Using Passive Samplers and Partial Least-Squares Calibration. Environmental Science & Technology 48:6291–6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Joyce AS, Pirogovsky MS, Adams RG, Lao W, Tsukada D, Cash CL, Haw JF, Maruya KA. 2015. Using Performance Reference Compound-Corrected Polyethylene Passive Samplers and Caged Bivalves to Measure Hydrophobic Contaminants of Concern in Urban Coastal Seawaters. Chemosphere 127:10–17. [DOI] [PubMed] [Google Scholar]
- [23].Vinturella AE, Burgess RM, Coull BA, Thompson KM, Shine JP. 2004. Use of Passive Samplers To Mimic Uptake of Polycyclic Aromatic Hydrocarbons by Benthic Polychaetes. Environmental Science & Technology 38:1154–1160. [DOI] [PubMed] [Google Scholar]
- [24].Muijs B, Jonker MT. 2012. Does Equilibrium Passive Sampling Reflect Actual in Situ Bioaccumulation of PAHs and Petroleum Hydrocarbon Mixtures in Aquatic Worms? Environ Sci Technol 46:937–944. [DOI] [PubMed] [Google Scholar]
- [25].Minick DJ, Anderson KA. 2017. Diffusive Flux of PAHs Across Sediment-Water and Water-Air Interfaces at Urban Superfund Sites. Environmental Toxicology & Chemistry In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Donald CE, Elie MR, Smith BW, Hoffman PD, Anderson KA. 2016. Transport Stability of Pesticides and PAHs Sequestered in Polyethylene Passive Sampling Devices. Environmental Science and Pollution Research 23:12392–12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Anderson KA, Sethajintanin D, Sower G, Quarles L. 2008. Field Trial and Modeling of Uptake Rates of In Situ Lipid-Free Polyethylene Membrane Passive Sampler. Environmental Science & Technology 42:4486–4493. [DOI] [PubMed] [Google Scholar]
- [28].Allan SE, Smith BW, Anderson KA. 2012. Impact of the Deepwater Horizon Oil Spill on Bioavailable Polycyclic Aromatic Hydrocarbons in Gulf of Mexico Coastal Waters. Environmental Science & Technology 46:2033–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Anderson KA, Szelewski MJ, Wilson G, Quimby BD, Hoffman PD. 2015. Modified Ion Source Triple Quadrupole Mass Spectrometer Gas Chromatograph for Polycyclic Aromatic Hydrocarbon Analyses. Journal of Chromatography A 1419:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].U.S.EPA. 2010. Development of a Relative Potency Factor (RPF) Approach for Polycyclic Aromatic Hydrocarbon (PAH) Mixtures.
- [31].U.S.EPA. 2014. Estimated Fish Consumption Rates for the U.S. Population and Selected Subpopulations (NHANES 2003-2010). In EPA U, ed. [Google Scholar]
- [32].Mueller JG, Chapman PJ, Pritchard PH. 1989. Creosote-Contaminated Sites. Their Potential for Bioremediation. Environmental Science & Technology 23:1197–1201. [Google Scholar]
- [33].Tobiszewski M, Namiesnik J. 2012. PAH Diagnostic Ratios for the Identification of Pollution Emission Sources. Environ Pollut 162:110–119. [DOI] [PubMed] [Google Scholar]
- [34].Yunker MB, Macdonald RW, Vingarzan R, Mitchell RH, Goyette D, Sylvestre S. 2002. PAHs in the Fraser River Basin: A Critical Appraisal of PAH Ratios as Indicators of PAH Source and Composition. Organic Geochemistry 33:489–515. [Google Scholar]
- [35].Simpson CD, Cullen WR, He TYT, Ikonomou M, Reimer KJ. 2002. Metabolism of Pyrene by Two Clam Species, Mya Arenaria and Protothaca Staminea. Chemosphere 49:315–322. [DOI] [PubMed] [Google Scholar]
- [36].Bebianno MJ, Barreira LA. 2009. Polycyclic Aromatic Hydrocarbons Concentrations and Biomarker Responses in the Clam Ruditapes Decussatus Transplanted in the Ria Formosa Lagoon. Ecotoxicology and Environmental Safety 72:1849–1860. [DOI] [PubMed] [Google Scholar]
- [37].Liu D, Pan L, Li Z, Cai Y, Miao J. 2014. Metabolites Analysis, Metabolic Enzyme Activities and Bioaccumulation in the Clam Ruditapes Philippinarum Exposed to Benzo[a]pyrene. Ecotoxicology and Environmental Safety 107:251–259. [DOI] [PubMed] [Google Scholar]
- [38].Goong SA, Chew KK. 2001. Growth of Butter Clams, Saxidomus Giganteus Deshayes, on Selected Beaches in the State of Washington. Journal of Shellfish Research 20:6. [Google Scholar]
- [39].Boehm PD, Page DS, Brown JS, Neff JM, Edward Bence A. 2005. Comparison of Mussels and sSemi-Permeable Membrane Devices as Intertidal Monitors of Polycyclic Aromatic Hydrocarbons at Oil Spill Sites. Marine Pollution Bulletin 50:740–750. [DOI] [PubMed] [Google Scholar]
- [40].Lohmann R, Burgess RM, Cantwell MG, Ryba SA, MacFarlane JK, Gschwend PM. 2004. Dependency of Polychlorinated Biphenyl and Polycyclic Aromatic Hydrocarbon Bioaccumulation in Mya Arenaria on Both Water Column and Sediment Bed Chemical Activities. Environmental Toxicology and Chemistry 23:2551–2562. [DOI] [PubMed] [Google Scholar]
- [41].Cachada A, Ferreira da Silva E, Duarte AC, Pereira R. 2016. Risk Assessment of Urban Soils Contamination: The Particular Case of Polycyclic Aromatic Hydrocarbons. Science of The Total Environment 551:271–284. [DOI] [PubMed] [Google Scholar]
- [42].Donatuto JL, Satterfield TA, Gregory R. 2011. Poisoning the Body to Nourish the Soul: Prioritising Health Risks and Impacts in a Native American Community. Health, Risk & Society 13:103–127. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.