Thirty-nine GATA transcription factor genes were identified in the poplar genome. The GATA transcription factor PdGNC positively regulates photosynthesis and plant growth by promoting chloroplast development in poplar.
Keywords: CRISPR/Cas9, fast growth, GATA transcription factor, nitrogen, photosynthesis, poplar
Abstract
GATA transcription factors are involved in the regulation of diverse growth processes and environmental responses in Arabidopsis and rice. In this study, we conducted a comprehensive bioinformatic survey of the GATA family in the woody perennial Populus trichocarpa. Thirty-nine Populus GATA genes were classified into four subfamilies based on gene structure and phylogenetic relationships. Predicted cis-elements suggested potential roles of poplar GATA genes in light, phytohormone, development, and stress responses. A poplar GATA gene, PdGATA19/PdGNC (GATA nitrate-inducible carbon-metabolism-involved), was identified from a fast growing poplar clone. PdGNC expression was significantly up-regulated in leaves under both high (50 mM) and low (0.2 mM) nitrate concentrations. The CRISPR/Cas9-mediated mutant crispr-GNC showed severely retarded growth and enhanced secondary xylem differentiation. PdGNC-overexpressing transformants exhibited 25–30% faster growth, 20–28% higher biomass accumulation, and ~25% increase in chlorophyll content, photosynthetic rate, and plant height, compared with the wild type. Transcriptomic analysis showed that PdGNC was involved in photosynthetic electron transfer and carbon assimilation in the leaf, cell division and carbohydrate utilization in the stem, and nitrogen uptake in the root. These data indicated that PdGNC plays a crucial role in plant growth and is potentially useful in tree molecular breeding.
Introduction
Poplar is an important woody model plant that is distinguished from annual herbaceous model plants, such as Arabidopsis and rice (Oryza sativa), on account of its woody secondary growth and perennial habit. The rapid-growth characteristic of Populus spp. renders the wood commercially valued for paper, timber, construction materials, and biofuel production (Jansson and Douglas, 2007; Polle et al., 2013). Many factors affect the growth rate of trees, including photosynthetic efficiency, nutrient utilization, and hormone stimulation (Euring et al., 2014; Lu et al., 2015). Photosynthesis, as the most important process for carbon fixation and metabolism, converts light energy into chemical energy for plant development and growth (Yamori and Shikanai, 2016). Hence, chloroplast architecture and composition have a marked influence on photosynthetic capability, and a high chlorophyll content contributes to increased light-harvesting capacity. Efficient photosystems fix higher quantities of carbon and produce greater amounts of carbohydrates, which are requisite for plant growth and biomass accumulation.
Nitrogen (N) is an essential macronutrient for plant growth as a component of many vital molecules, such as nucleic acids, amino acids, proteins, and certain plant hormones. Sufficient N availability is necessary for plant growth and development, whereas N starvation or excess can expose plants to N stress (Yang et al., 2015). Nitrogen stress has marked effects on N uptake and metabolism (Luo et al., 2015), chlorophyll synthesis (An et al., 2014), lignin content (Rueda-López et al., 2015), and plant biomass accumulation (Hudson et al., 2013). Nitrogen-deficient plants exhibit low net photosynthetic rates and reduced biomass production (Bondada and Syvertsen, 2003; Nunes-Nesi et al., 2010). Carbon (C) and N are integral components of plants, and C and N metabolic processes are closely linked (Coruzzi and Bush, 2001). The N level in planta affects photosynthesis and C assimilation, whilst C metabolites contribute to N absorption and utilization. Thus, regulation of C and N interaction is crucial for modification of plant development and growth.
GATA proteins are evolutionarily conserved transcription factors that interact with the WGATAR (W=T or A; R=G or A) sequence motif and are ubiquitous in eukaryotes, including fungi, metazoans, and plants (Lowry and Atchley, 2000; Scazzocchio, 2000; Patient and McGhee, 2002; Teakle et al., 2002; Reyes et al., 2004; An et al., 2014). In Arabidopsis, a majority of the previously classified 30 GATA proteins contain only one zinc finger, C-X2-C-X18-C-X2-C, but a few proteins contain two zinc fingers, C-X2-C-X20-C-X2-C (Reyes et al., 2004; Bi et al., 2005). Previous studies have revealed that GATA transcription factors are widely involved in regulation of plant developmental and growth processes, such as seed germination (Liu et al., 2005), chloroplast development (Bi et al., 2005; Chiang et al., 2012; An et al., 2014, Zhang et al., 2015), flower development (Mara and Irish, 2008), response to light (Luo et al., 2010), and lateral root initiation identity (De Rybel et al., 2010). In addition, some studies indicate that GATA factors play roles in N metabolism (Reyes et al., 2004; Bi et al., 2005; Hudson et al., 2011; An et al., 2014). GATA-binding motifs have been detected in regulatory regions of many genes involved in N assimilation, such as nitrate reductase, nitrite reductase, and glutamine synthetase (Jarai et al., 1992; Oliveira and Coruzzi, 1999; Hudson et al., 2011). Thus, GATA transcription factors play a potential role in coordination of nutrition utility and vegetative growth.
The clustered regularly interspaced short palindromic repeats (CRISPR) system is a powerful tool in plant genome engineering and has been used successfully for genome editing of the woody plant Populus (Fan et al., 2015; Zhou et al., 2015; Wang et al., 2017; Xu et al., 2017). In this study, we conducted a genome-wide survey of Populus trichocarpa GATA-related sequences and explored their expression in different tissues in response to three nitrate concentrations. The poplar GATA family member, GNC (GATA Nitrate-inducible Carbon-metabolism-involved), was significantly induced by N and is an ortholog of Arabidopsis AtGNC (An et al., 2014). To extend our previous study of Populus GNC in Arabidopsis, we employed the CRISPR/Cas9 system to produce a GNC knockout mutant and overexpressed GNC in Populus to examine functions of GNC in the growth and development of a woody plant.
Materials and methods
Identification of GATA transcription factors in poplar
BLAST and keyword searches were utilized to compile a comprehensive and non-redundant data set of Populus trichocarpa proteins containing the conserved GATA domain. The query proteins and nucleotide sequences were obtained from different resources: chicken GATA1 (AAA49055), Aspergillus nidulans AreA (P17429), Neurospora crassa WC1 (Q01371), and all 30 documented GATA family genes of Arabidopsis (Reyes et al., 2004). A keyword search was performed in Phytozome (http://www.phytozome.net) for putative GATA factors by searching ontologies with the term of the GATA domain (PF00320). All sequences had e-values below 10−6. Sequences with a length of more than 100 amino acids were selected for further analysis. The candidate sequences were analysed with SMART (Letunic et al., 2012), InterPro (http://www.ebi.ac.uk/interpro/), and Pfam (http://pfam.xfam.org) software. The results were compared against predicted members of the GATA family in the PlantTFDB (Jin et al., 2014) and PlnTFDB databases (Pérez-Rodríguez et al., 2010). Paralogous pairs were explored using the Plant Genome Duplication Database (http://chibba.agtec.uga.edu/duplication/). A cis-acting element analysis of the promoter region (2 kb of genomic DNA sequence upstream from the translation start site) was conducted with the PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/).
Multiple sequence alignment and phylogenetic analysis
For exon–intron structural analysis, the genomic sequence and protein sequence for each putative poplar GATA gene were downloaded from Phytozome. The gene structure was analysed using the Gene Structure Display Server tool (Guo et al., 2007). Conserved amino acids were viewed using Logo (http://weblogo.berkeley.edu/logo.cgi). A phylogenetic tree derived from alignment of Populus GATA family amino acid sequences was constructed using Phylogeny (http://www.phylogeny.fr). The chromosomal location of the poplar GATA genes was determined using PopGenIE (http://www.popgenie.org/). An unrooted tree was constructed using MEGA 6 employing the neighbor-joining method and support for the tree topology was assessed by performing a bootstrap analysis with 1000 replicates (Tamura et al., 2013). Core consensus sequence logos of Arabidopsis and poplar GATA genes were created using Weblogo (http://weblogo.berkeley.edu/).
Plant materials and growth conditions
Cuttings of Populus clone NE-19 (P. nigra × (P. deltoides × P. nigra)) were cultivated following the description of Hao et al. (2011). Plants were pre-cultured in buckets containing sufficient nitrate medium (SN; 10 mM NO3−) for 1 week and then transplanted into buckets containing low nitrate medium (LN; 0.2 mM NO3−) or high nitrate medium (HN; 50 mM NO3−). Plants cultured in SN were considered to be the control. The hydroponic culture media were maintained at pH 5.8–6.0 and refreshed at weekly intervals. The growth chamber was set to 16 h white light (07.00–23.00 h) at 24 °C and 8 h darkness (23.00–07.00 h) at 20 °C, with 150 μmol m−2 s−1 irradiation.
RNA extraction and transcript analysis
Leaf, stem, and root samples taken from Populus clone NE-19 plants treated for 1 week with the three nitrate concentrations, and from different Populus GNC modified plants and then immediately frozen in liquid nitrogen and stored at −80 °C until RNA extraction. Three replications consisting of nine seedlings for each sampling time point were used. Total RNA was isolated using the RN38 EASYspin Plus Plant RNA Kit (Aidlab Biotech, Beijing, China). First-strand cDNA synthesis was performed using first-strand M-MLV Reverse Transcriptase and an oligo(dT) primer (Promega, Madison, WI, USA) following the manufacturer’s instructions. Samples were diluted 10 times (~100 ng μl−1) prior to quantitative real-time PCR (qPCR) analysis. The specific primers used to quantify Populus NE-19 GATA genes, PdGATA genes, and differentially expressed genes by qPCR were designed according to the corresponding gene sequences in the Populus trichocarpa reference genome (see Supplementary Tables S1, S2 at JXB online). The PCR mixture consisted of 1 μl sample, 0.6 μl (10 μM) forward primer, 0.6 μl (10 μM) reverse primer, 5.8 μl RNase-free ddH2O, 10 μl SuperReal PreMix Plus, and 2 μl ROX Reference Dye in a total volume of 20 μl. The reaction was amplified for 40 cycles at 95 °C for 10 s, 55 °C for 30 s, and 72 °C for 32 s.
PdGNC gene cloning and transformation
The Populus GATA gene PdGNC (GenBank accession KF541241), an ortholog of PtrGATA19 (Potri.006G229200), was identified and characterized from Populus clone NE-19. Extraction of total RNA and subsequent cDNA synthesis from leaves of Populus were performed using the aforementioned methods. The PdGNC cDNA sequence was amplified using the primers PdGNC-F (GGCCCTTTTAGCCTTGTTGTTTGT) and PdGNC-R (TCAGCTGTGAATAAAGCCACAAG). The 35S promoter-driven overexpression cassette 35S:PdGNC was constructed by introducing the PdGNC coding sequence into the pCAMBIA1301 expression vector and then transformed into Agrobacterium tumefaciens strain GV3101.
For Agrobacterium-mediated 35S:PdGNC transformation of Populus clone 717, Agrobacterium-mediated 35S:PdGNC cells were collected and resuspended to OD600=0.3–0.4. The leaf discs were soaked for 1 h on a shaker with the resuspended cells at room temperature. The inoculated leaf discs were co-cultivated at 19–25 °C in the dark for 2–3 d. The leaf discs were washed with double-distilled water and cultured on medium for callus inducement supplemented with 500 mg l−1 cefotaxime and 50 mg l−1 kanamycin for 10–30 d in the dark. Shoot and root regeneration from the calli was induced on medium supplemented with 100 mg l−1 kanamycin for several weeks to months. The total period from co-cultivation to regeneration of a rooted transgenic plantlet varied widely among clones, ranging from about 4 to 8 months (Han et al., 2000). Transgenic lines were selected by kanamycin and identified by qPCR. The positive T0 transgenic plants were regenerated on antibiotic-free media that were also used for the controls to ensure their synchronization under the growth conditions for subsequent experimental analysis. Three transgenic lines chosen for further analysis showed higher expression levels of the target gene (see Supplementary Fig. S1).
CRISPR/Cas9-mediated targeted mutagenesis of Populus GNC
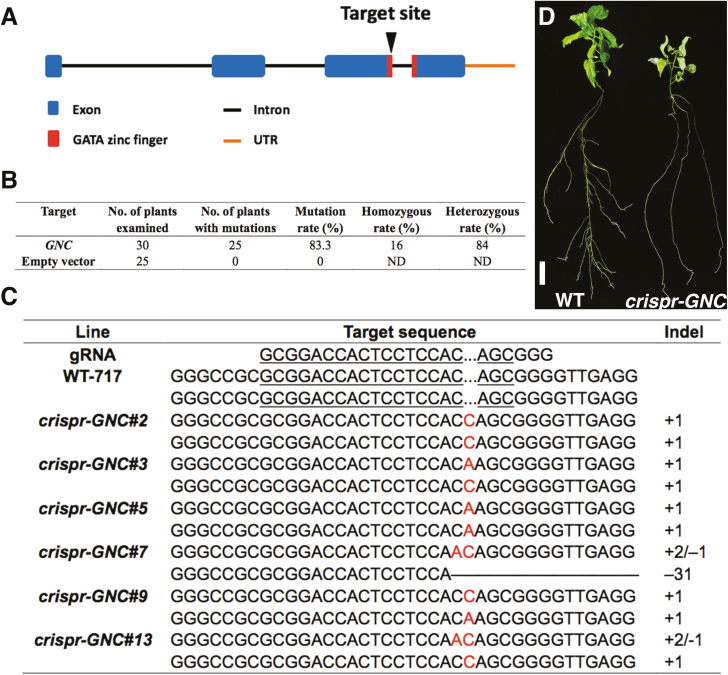
The single-guide RNA (sgRNA) sequence for Populus GNC of clone 717 was designed based on the SNP-bearing Populus 717 genomic database (Zhou et al., 2015). The target site of the designed sgRNA was confirmed by amplification and sequencing (the primers are listed in Supplementary Table S3). The designed sgRNA was assembled into the entry vector pEn-Chimera and then an expression construct was generated with the destination vector pDe-CAS9 (Fauser et al., 2014), using Gateway recombination cloning technology (Thermo Fisher Scientific, Waltham, MA, USA). The CRISPR/Cas9 construct was transformed into Populus clone 717 using the aforementioned method. For confirmation of positive transgenic plants, we used the primers GNC_Mut_F1 and GNC_Mut_R1 to amplify the genomic region flanking the target sites (see Supplementary Table S3). The forward primer was located 379 bp upstream of the target and the reverse primer was located 221 bp downstream. Individual amplicons from each transgenic event were visualized in agarose gel. Bands were excised using a clean razor and DNA was extracted using the TIANgel Midi Purification Kit (Tiangen, Beijing, China). Sanger DNA sequencing of PCR amplicons was used to evaluate the editing conditions of CRISPR/Cas9 transfection. Individual sequences were aligned to the wild type (WT) sequence using SnapGene to determine the severity of the mutation on the predicted final peptide sequence (see Results Fig. 3C and Supplementary Fig. S6).
Fig. 3.
CRISPR/Cas9-mediated mutagenesis of Populus GNC gene. (A) The selected target site in the Populus GNC locus. (B) Determination of mutation events in transgenic Populus plants. ND, not determined. (C) CRISPR/Cas9-induced mutagenesis at target sites of Populus GNC gene sequences. (D) Phenotypic comparison of the crispr-GNC mutant and wild-type Populus plants. Scale bar=1 cm.
Physiological measurements and histological analysis
Chlorophyll content was measured using leaf samples (~1 g) from 8-week-old plants. Leaves were immersed in 1 ml N,N-dimethylformamide overnight at 4 °C. Chlorophyll a and chlorophyll b contents were determined spectrophotometrically using published equations (Porra et al., 1989). The Li-6400 Portable Photosynthesis System (Li-Cor, Lincoln, NE, USA) was used to determine photosynthetic rates. Mature, fully expanded leaves of 8-week-old plants were used for measurements under an ambient CO2 concentration of 360 μmol mol−1, photosynthetic photon flux density of 600 μmol m−2 s−1, and chamber temperature of 24 °C at 10.00–11.00 h.
Preprocessing of samples for transmission electron microscopy (TEM) followed the method of An et al. (2014). The same position in the central portion of leaves was fixed by vacuum infiltration in 2.5% (v/v) glutaraldehyde and 0.2 M sodium phosphate buffer (pH 7.2). The samples were incubated in three changes of the fixative for 15 min each. Samples were post-fixed in 2% (w/v) OsO4 overnight. All TEM images were captured at 100 kV on a TEM 1010 apparatus (JEOL, Tokyo, Japan) equipped with an XR-41B AMT digital camera (Advanced Microscopy Techniques, Woburn, MA, USA). Transverse sections (50 μm thickness) were prepared using a Vibratome (Series 1000, Heath Company, USA), stained with toluidine blue, and visualized with a Leica microscope (Gerttula et al., 2015).
RNA-sequencing and gene co-expression network analysis
Leaf, stem, and root samples from WT, oxPdGNC, and crispr-GNC plants were harvested. Three biological replicates were included for each tissue and in total 27 samples were prepared for 150 bp paired-end sequencing using an Illumina HiSeq X Ten platform. Library construction and sequencing were performed following the manufacturer’s protocols. The data were trimmed and mapped to the Populus tremula × P. alba 717-1B4 reference genome (http://aspendb.uga.edu/index.php/databases/spta-717-genome). Differentially expressed genes were detected using Cufflinks software following the criteria (|log2(fold-change)| >1 and false discovery rate <0.05). Gene ontology (GO) enrichment analysis was performed using the R packages GOstats and GSEABase. The gene expression abundance was calculated and used for weighted gene co-expression network analysis (WGCNA; Langfelder and Horvath, 2008). The soft threshold power of the adjacency matrix for the co-expression relationship between genes was 8. Hierarchical clustering was performed with a minimum module size of 400 and a cut height of 0.994. Different colors were assigned to different modules. The RNA-sequencing data were deposited in the National Center of Biotechnology Information Sequence Read Archive database under BioProject PRJNA449161.
Results
Identification and analysis of poplar GATA transcription factors
We identified a total of 39 unique putative Populus GATA genes in the published Populus trichocarpa reference genome and consecutively designated the genes PtrGATA1 to PtrGATA39 based on their genomic location (Table 1). The GATA family genes were distributed on 15 of the 19 chromosomes at various densities except PtrGATA39, which was located in an as-yet-unattributed scaffold 694 (see Supplementary Fig. S2). The most recent Populus genome-wide duplication event contributed to gene duplication and expansion of gene families (Tuskan et al., 2006). We identified 10 paralogous pairs located in the segmental duplicated blocks (Supplementary Fig. S2), whereas 19 genes located in these blocks did not have a corresponding paired gene, which suggested that GATA family genes experienced diverse evolutionary processes after chromosome duplication.
Table 1.
Summary of Populus GATA transcription factors
| Name | Gene model ID | Pfam ID | gDNA | Transcript | CDS | Domains | AA | MM | GRAVY | pI | Homologs in Arabidopsis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PtrGATA1 | Potri.001G053500 | PF00320 | 2425 | 1801 | 1011 | 257–292 | 336 | 38.29 | −0.818 | 9.68 | At3g24050 (AtGATA1) |
| PtrGATA2 | Potri.001G151700 | PF00320 | 6921 | 2837 | 1659 | 15–50 | 552 | 61.30 | −0.655 | 6.96 | At4g17570 (AtGATA26) |
| PtrGATA3 | Potri.001G188500 | PF00320 | 1336 | 1000 | 753 | 166–201 | 250 | 28.01 | −0.617 | 5.41 | At4g32890 (AtGATA9) |
| PtrGATA4 | Potri.002G110800 | PF06200, PF06203, PF00320 | 2190 | 882 | 366 | 213–250 | 360 | 39.28 | −0.72 | 4.87 | At3g21175 (AtGATA24) |
| PtrGATA5 | Potri.002G110900 | PF06200, PF06203, PF00320 | 5321 | 1513 | 873 | 201–237 | 290 | 31.47 | −0.628 | 5.93 | At4g24470 (AtGATA25) |
| PtrGATA6 | Potri.002G142800 | PF00320 | 1357 | 1212 | 741 | 163–198 | 246 | 27.39 | −0.738 | 6.03 | At2g45050 (AtGATA2) |
| PtrGATA7 | Potri.002G199800 | PF00320 | 1112 | 894 | 444 | 30–65 | 147 | 16.6 | −0.879 | 9.4 | At5g49300 (AtGATA16) |
| PtrGATA8 | Potri.003G082800 | PF00320, PF04683 | 6299 | 2462 | 1623 | 7–42 | 540 | 60.24 | −0.672 | 6.58 | At4g17570 (AtGATA26) |
| PtrGATA9 | Potri.003G174800 | PF00320 | 2453 | 1564 | 780 | 179–213 | 259 | 29.02 | −0.797 | 9.17 | At3g24050 (AtGATA1) |
| PtrGATA10 | Potri.003G213300 | PF00320 | 1956 | 681 | 226 | 180–212 | 226 | 25.42 | −1.071 | 8.72 | At3g20750 (AtGATA29) |
| PtrGATA11 | Potri.004G161500 | PF00320 | 1957 | 1753 | 984 | 246–281 | 327 | 36.30 | −0.724 | 8.53 | At5g66320 (AtGATA5) |
| PtrGATA12 | Potri.004G211800 | PF00320 | 4420 | 1926 | 906 | 236–271 | 301 | 33.97 | −0.675 | 8.57 | At1g08010 (AtGATA11) |
| PtrGATA13 | Potri.005G020500 | PF00320 | 1853 | 1182 | 486 | 26–61 | 161 | 17.59 | −0.675 | 9.78 | At5g49300 (AtGATA16) |
| PtrGATA14 | Potri.005G066100 | PF00320 | 2822 | 771 | 771 | 190–225 | 256 | 29.32 | −0.559 | 8.75 | At4g36240 (AtGATA7) |
| PtrGATA15 | Potri.005G117600 | PF00320 | 1640 | 1403 | 1002 | 249–284 | 333 | 36.84 | −0.630 | 7.10 | At5g66320 (AtGATA5) |
| PtrGATA16 | Potri.005G122700 | PF00320 | 1176 | 1672 | 765 | 137–172 | 254 | 28.60 | −0.957 | 7.55 | At3g50870 (AtGATA18) |
| PtrGATA17 | Potri.005G152500 | PF06200, PF06203, PF00320 | 5157 | 1684 | 1098 | 218–255 | 365 | 39.71 | −0.626 | 5.13 | At1g51600 (AtGATA28) |
| PtrGATA18 | Potri.005G152800 | PF06200, PF06203, PF00320 | 12075 | 1150 | 867 | 200–237 | 288 | 31.46 | −0.698 | 5.67 | At4g24470 (AtGATA25) |
| PtrGATA19 | Potri.006G229200 | PF00320 | 2820 | 1396 | 1068 | 222–257 | 355 | 39.45 | −0.610 | 9.04 | At5g56560 (AtGATA21) |
| PtrGATA20 | Potri.006G237700 | PF00320 | 1914 | 1775 | 1122 | 262–297 | 373 | 41.40 | −0.791 | 6.04 | At5g25830 (AtGATA12) |
| PtrGATA21 | Potri.007G016600 | PF00320 | 1676 | 1453 | 1131 | 292–372 | 376 | 41.81 | −0.589 | 6.95 | At5g66320 (AtGATA5) |
| PtrGATA22 | Potri.007G024500 | PF00320 | 1438 | 1304 | 765 | 138–173 | 254 | 28.67 | −0.941 | 8.46 | At3g50870 (AtGATA18) |
| PtrGATA23 | Potri.007G116600 | PF06200, PF06203, PF00320 | 2108 | 1255 | 429 | 53–90 | 142 | 14.88 | −0.318 | 8.47 | At4g24470 (AtGATA25) |
| PtrGATA24 | Potri.007G116700 | PF00320 | 5875 | 1636 | 1155 | 218–255 | 384 | 43.22 | −0.852 | 4.90 | At3g21175 (AtGATA24) |
| PtrGATA25 | Potri.008G038900 | PF00320 | 3709 | 1909 | 1065 | 258–302 | 354 | 38.94 | −0.597 | 6.52 | At4g32890 (AtGATA9) |
| PtrGATA26 | Potri.008G213900 | PF00320 | 1290 | 1091 | 417 | 29–64 | 138 | 14.78 | −0.909 | 9.69 | At3g06740 (AtGATA15) |
| PtrGATA27 | Potri.009G123400 | PF00320 | 1898 | 1691 | 990 | 248–283 | 329 | 36.59 | −0.629 | 6.61 | At5g66320 (AtGATA5) |
| PtrGATA28 | Potri.010G001300 | PF00320 | 867 | 771 | 462 | 34–69 | 153 | 16.42 | −0.690 | 9.85 | At3g06740 (AtGATA15) |
| PtrGATA29 | Potri.010G223300 | PF00320 | 3408 | 1885 | 1341 | 350–385 | 446 | 49.07 | −0.641 | 5.93 | At4g32890 (AtGATA9) |
| PtrGATA30 | Potri.010G251600 | PF06200, PF06203, PF00320 | 3543 | 1641 | 924 | 231–268 | 307 | 33.37 | −0.893 | 7.80 | At1g51600 (AtGATA28) |
| PtrGATA31 | Potri.013G059600 | PF00320 | 1642 | 1288 | 888 | 227–262 | 295 | 32.67 | −0.746 | 6.53 | At5g25830 (AtGATA12) |
| PtrGATA32 | Potri.014G058600 | PF00320 | 1497 | 1397 | 756 | 165–200 | 251 | 27.96 | −0.780 | 6.42 | At2g45050 (AtGATA2) |
| PtrGATA33 | Potri.014G124400 | PF00320 | 1144 | 942 | 402 | 28–63 | 133 | 14.7 | −0.841 | 9.77 | At5g49300 (AtGATA16) |
| PtrGATA34 | Potri.017G042200 | PF06200, PF06203, PF00320 | 6316 | 1684 | 1221 | 251–288 | 406 | 46.03 | −0.756 | 5.36 | At3g21175 (AtGATA24) |
| PtrGATA35 | Potri.017G042300 | PF00320 | 356 | 356 | 348 | 61–95 | 115 | 12.87 | −0.111 | 9.94 | At1g51600 (AtGATA28) |
| PtrGATA36 | Potri.018G044900 | PF00320 | 1864 | 1342 | 912 | 173–206 | 380 | 42.11 | −0.809 | 6.22 | At5g25830 (AtGATA12) |
| PtrGATA37 | Potri.018G053600 | PF00320 | 1625 | 1496 | 1143 | 173–208 | 303 | 33.67 | −0.740 | 8.78 | At4g26150 (AtGATA22) |
| PtrGATA38 | Potri.019G033000 | PF00320 | 1808 | 1460 | 885 | 223–258 | 294 | 32.20 | −0.798 | 6.18 | At4g32890 (AtGATA9) |
| PtrGATA39 | Potri.T158300 | PF00320 | 1516 | 1308 | 1065 | 243–277 | 354 | 39.39 | −0.835 | 6.19 | At5g25830 (AtGATA12) |
AA, amino acid; CDS, coding sequence; GRAVY: grand average of hydropathicity; MM, molecular mass (kDa); pI: theoretical isoelectric point.
We assessed the zinc-finger conserved domain of Populus and Arabidopsis GATA proteins. The logo pattern from a multiple sequence alignment of 39 Populus zinc-finger sequences showed a close similarity with that from 30 Arabidopsis zinc-finger proteins (Supplementary Fig. S3A). Alignment of amino acid sequences for Populus and Arabidopsis GATA proteins revealed that zinc fingers of all GATA genes showed high sequence conservation in the α-helix and the four β-foldings (Supplementary Fig. S3B).
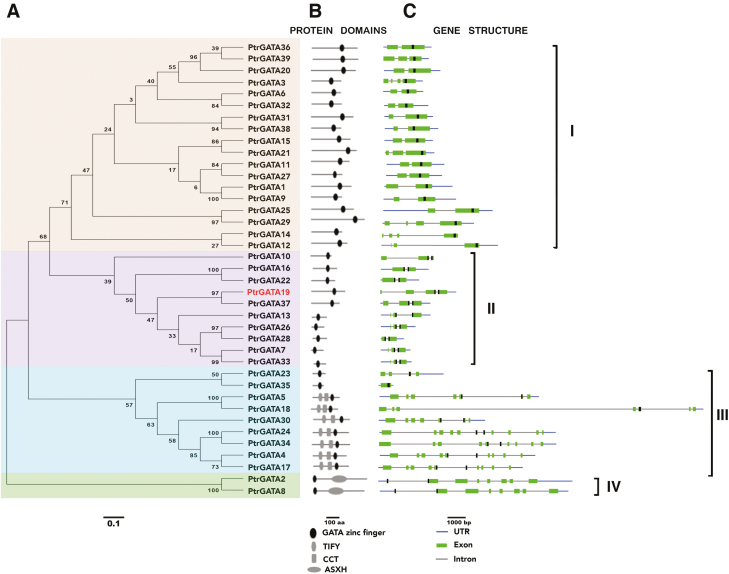
To examine phylogenetic relationships among Populus GATA family members, we generated an alignment of amino acid sequences for the 39 full-length GATA proteins. The phylogenetic tree of polypeptide sequences is displayed with their domains as well as the exon–intron organization of the corresponding genes (Fig. 1). The Populus GATA family was resolved into four subfamilies, with 30 members containing zinc-finger motifs with 18 residues C-X2-C-X18-C-X2-C (subfamilies I, II, and IV), and nine members with 20 residues C-X2-C-X20-C-X2-C (subfamily III). Subfamily I was composed of 18 genes containing two to four exons, of which the last exon encoded an entire zinc-finger motif and carboxy-terminal basic region. All proteins encoded by these genes exhibited a single zinc finger with 18 residues and an acidic amino-terminal region. Subfamily II was composed of 10 genes containing two to four exons, whose members also exhibited 18 residues in a zinc-finger loop, but the zinc-finger motif was located in the last two exons and separated by one intron. Subfamily III included nine genes containing 7–11 exons. All of the corresponding proteins exhibited a single zinc finger with 20 residues. Seven members displayed two additional conserved domains, namely CCT (CONSTANS, CONSTANS -like, and TIMING OF CAB EXPRESSION 1; Robson et al., 2001) and TIFY (Vanholme et al. 2007), in the central region of the gene sequence. Subfamily IV comprised two closely related genes that showed a non-homogeneous exon–intron composition and were characterized by one C-X2-C-X18-C-X2-C zinc-finger domain at the N-terminus, and an ASX homology domain (ASXH) at the C-terminus.
Fig. 1.
Phylogenetic analysis of GATA family genes in Populus. (A) Neighbor-joining tree constructed from full-length amino acid sequences from Populus GATA genes. (B) Protein domain distribution of PtrGATA members. (C) Gene structure analysis of PtrGATA genes. The position of nucleotide sequences coding for the GATA zinc finger is depicted in black.
To explore potential functions of the Populus GATA genes, the promoter regions of PtrGATA genes were analysed to identify potential cis-elements. Elements responsive to abiotic stress, phytohormone, and developmental processes, especially light-relevant factors, were identified (see Supplementary Fig. S4). The abiotic stress response elements included heat stress (HSE) and low temperature (LTR) responsive elements, MYB binding sites involved in drought inducibility (MBS), a defense and stress response element (TC-rich), and an anaerobic induction element (ARE). Phytohormone response elements, such as the ABA-responsive element (ABRE), ethylene-responsive element (ERE), SA-responsive element (TCA-element), and methyl jasmonate (MeJA)-responsive motifs (CGTCA motif and TGACG motif), were detected. Meristem expression (CAT box and CCGTCC box), zein metabolism regulation (O2 site), and endosperm expression (Skn-1 motif and GCN4 motif) elements were associated with developmental processes. Abundant light-responsive elements, such as BoxI, G-Box, circadian, and Box4, were detected.
Expression profiles of poplar GATA genes in response to nitrogen
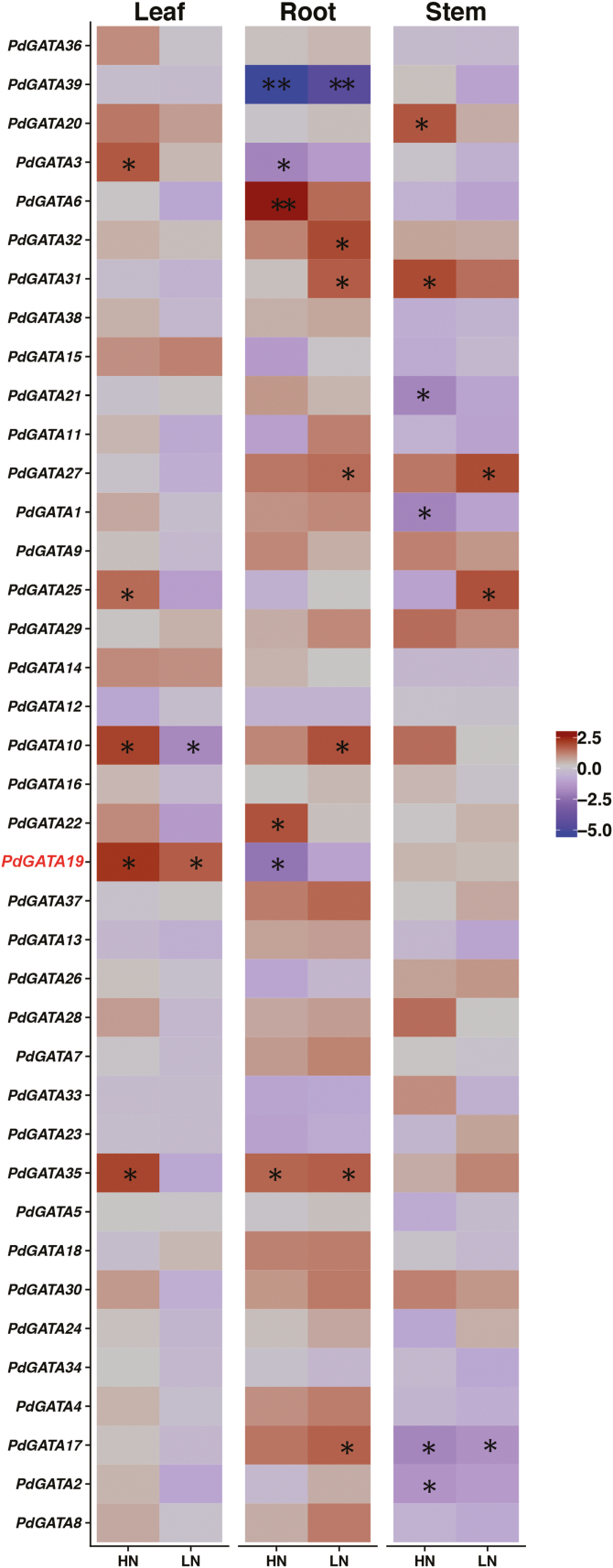
The first plant GATA gene identified, NTL1, was isolated as a homolog of the fungus GATA gene NIT2, which functions in N metabolism (Daniel-Vedele and Caboche, 1993). Previous studies have reported that plant GATA genes have functions in nitrate metabolism (Bi et al., 2005; Hudson et al., 2011). Given that the most abundant inorganic N source for plants in agricultural soils is nitrate, the majority of previous studies investigating plant N signaling have focused on this compound (Vidal et al., 2015). To determine potential roles of Populus GATA genes in response to different N conditions, expression abundance of the 39 homologous PdGATA genes was evaluated in the leaf, stem, and root of the rapid-growing Populus clone NE-19 treated with three nitrate concentrations for 1 week. The HN (50 mM NO3−) and LN (0.2 mM NO3−) treatments changed expression patterns of GATA genes (Fig. 2). In the leaf, 10 PdGATA genes were up-regulated under HN (fold change>2, P<0.05) and four genes were induced by LN. Four genes (PdGATA14, PdGATA15, PdGATA19, and PdGATA20) were induced by both HN and LN. In the root, 10 genes were significantly up-regulated by HN, 16 genes were induced by LN, and eight genes were induced by both HN and LN. In stem tissues, eight PdGATA genes were up-regulated by HN, five genes were induced by LN, and three genes were induced by both HN and LN. The majority of paralogous gene pairs showed distinct expression patterns, whereas a small number showed an almost identical expression pattern in different tissues, such as PdGATA4 and PdGATA17.
Fig. 2.
Expression profiles of GATA genes in the leaf, root, and stem of Populus NE-19 under high nitrate (HN), sufficient nitrate (SN), and low nitrate (LN) for 1 week. The expression levels of genes were determined using qPCR and normalized by log2 transformation. The data are the mean ±SD (n=3 experiments). Each experiment comprised three biological replicates. Asterisks indicate a significant difference (*P<0.05, **P<0.01; one-way ANOVA).
CRISPR/Cas9-mediated mutagenesis of poplar GATA19/GNC
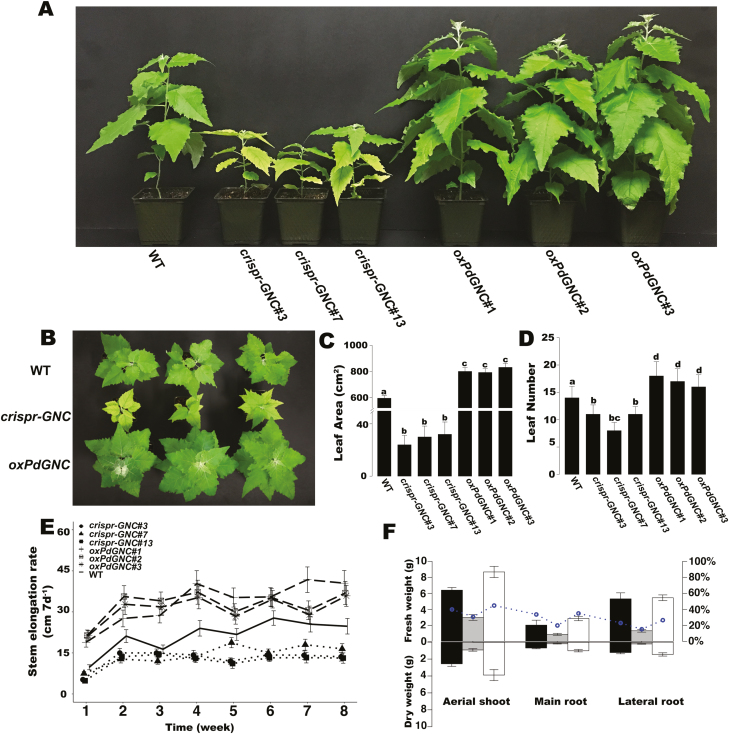
Expression profiles revealed that the Populus GATA gene GATA19/GNC was strongly up-regulated in Populus leaves in response to nitrate treatment. Analysis of phylogenetic relationships and multiple sequence alignment revealed that Populus GATA19 was phylogenetically closer to, and showed higher sequence similarity with, AtGNC (AT5G56860). The AtGNC mutant gnc exhibits yellow leaves. Our previous research showed that the Arabidopsis complementary lines gnc/PdGNC were restored to the WT chlorophyll levels (An et al., 2014), suggesting that Populus GATA19 and AtGNC were orthologous (see Supplementary Fig. S3B). AtGNC has been reported to participate in the correlation between N and C metabolism in Arabidopsis (Bi et al., 2005; An et al., 2014), but functional interpretation is not exhaustive especially in woody plants. The CRISPR/Cas9 system was employed to edit the Populus genomic sequence and disrupt GNC expression. The potential target site was located at the beginning of the coding sequence of the GATA zinc-finger domain so as to break the indispensable functional structure (Fig. 3A). Notably, nucleotide sequences of the GATA zinc-finger domain in all 39 genes were not identical and matched (Supplementary Fig. S5). The sequencing results also indicated that no off-target events happened within these sequences. With Agrobacterium-mediated transformation of the CRISPR/Cas9 module into Populus clone 717, 25 of 30 examined transgenic plants were successfully mutated (Fig. 3B) by editing the target site by insertion or deletion of a small number of nucleotides (Fig. 3C; Supplementary Fig. S6). The CRISPR/Cas9 system created mutation events in both of two Populus GNC allelic sequences, and several biallelic mutants were generated from different editing events. Nucleotide insertions/deletions led to a frameshift mutation and change in the translated amino acids, thereby disrupting expression of the Populus GNC protein. Loss-of-function crispr-GNC mutant plants showed visibly pale green leaves with significantly decreased chlorophyll content (Fig. 3D). In addition, crispr-GNC plants showed retarded growth compared with that of the controls (Fig. 4).
Fig. 4.
Phenotypic effects of PdGNC on growth and development of 8-week-old wild-type (WT), crispr-GNC mutant, and PdGNC-overexpression (oxPdGNC) plants. (A, E) Height. (B, C) Leaf area. (D) Leaf number. (F) Biomass accumulation. The parameters fresh weight (FW) and dry weight (DW) were compared among WT, crispr-GNC, and oxPdGNC plants. Circles represent the DW/FW values. The data are the mean ±SD (n=10). Bars with different letters indicate a significant difference (P<0.05; one-way ANOVA).
Expression modification of PdGNC reveals GNC-associated regulation of vegetative growth, chlorophyll content, and vascular development
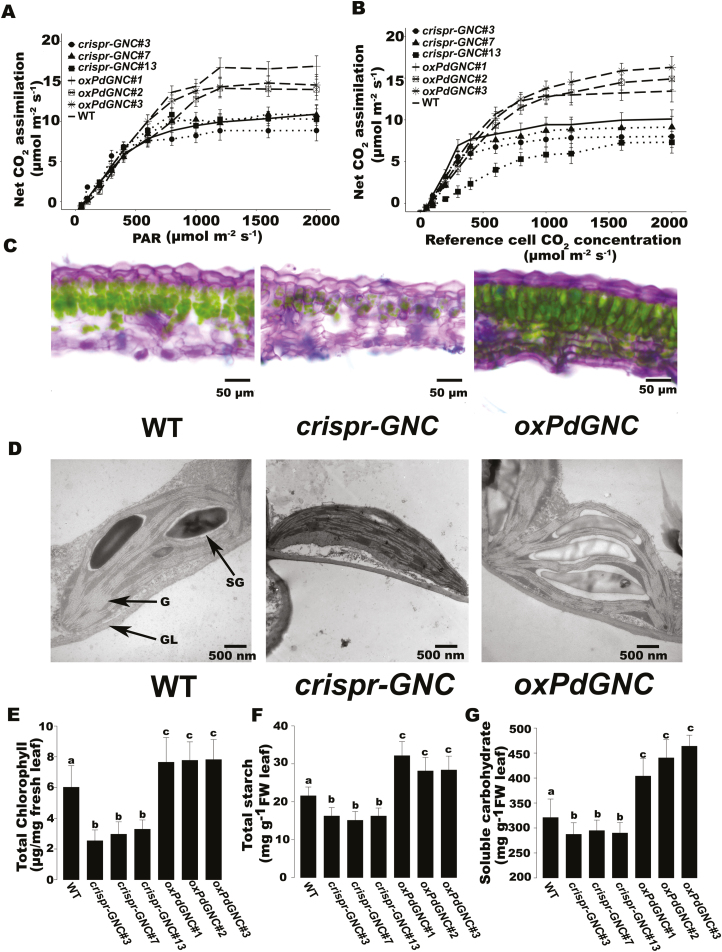
To further investigate the role of Populus GNC in plant growth, we cloned PdGATA19/PdGNC from Populus clone NE-19 and transformed the gene into Populus clone 717. Transgenic overexpression plants (oxPdGNC) exhibited darker green leaves and faster growth than the control WT under the normal condition. Analysis of relative growth rates measured at weekly intervals revealed that oxPdGNC plants showed a faster growth rate than the WT and crispr-GNC mutant (Fig. 4A, E). The oxPdGNC plants also showed increased leaf area (Fig. 4B, C) and leaf number (Fig. 4B, D), as well as higher photosynthetic rates, which were 20–28% and 100–110% higher than those of the WT and crispr-GNC mutant, respectively. Photosynthesis–light and photosynthesis–CO2 response curves indicated that the oxPdGNC plants showed higher photosynthetic capability than the WT and crispr-GNC mutant (Fig. 5A, B). Photosynthetic rates per unit leaf volume of oxPdGNC plants were 20–28% and 30–60% higher than those of the WT and crispr-GNC mutant, respectively (see Supplementary Fig. S7). Higher photosynthetic efficiency contributed to greater biomass accumulation. The fresh weight (FW) of aboveground biomass of overexpression lines increased by ~35.5% compared with the WT and ~190% compared with the crispr-GNC mutant; the dry weight (DW) increased by ~51% compared with that of the WT and ~320% compared with the crispr-GNC mutant. The DW/FW values of the above- and belowground biomass of overexpression lines were all larger than those of the WT and crispr-GNC lines. The crispr-GNC mutant showed the slowest growth rate and lowest biomass accumulation (Fig. 4F).
Fig. 5.
Photosynthesis and chlorophyll content modulated by PdGNC. (A) A–Ci curve. (B) A–light response curve. (C) Transverse sections of the fifth functional leaf. Scale bar=50 μm. (D) Chloroplast ultrastructure. Scale bar=500 nm. G, grana; GL, grana lamellae; SG, starch granules. The data are the mean ±SD (n=10). Bars with different letters indicate a significant difference (P<0.05; one-way ANOVA). (E) Total chlorophyll content of leaves. (F) Total starch content of leaves. (G) Soluble carbohydrate content of leaves.
Coincident with higher photosynthesis rates, oxPdGNC plants displayed higher chlorophyll content. The total chlorophyll content of oxPdGNC plants was 25–30% and 105–130% higher than those of the WT and crispr-GNC mutant, respectively (Fig. 5C, E). Transverse sections of 2-month-old fully expanded leaves showed the absence of palisade parenchyma and spongy mesophyll in the crispr-GNC mutant. In contrast, chloroplast biogenesis in the leaf mesophyll was enhanced by PdGNC overexpression (Fig. 5E). Further analysis of cellular organelles by TEM indicated that in the WT chloroplasts were distributed uniformly and thylakoids were tightly stacked. In contrast, in crispr-GNC plants, the thylakoids were slightly scattered and very few or no visible starch granules were observed (Fig. 5D). The number of thylakoids in oxPdGNC and the WT differed slightly, but the grana number in oxPdGNC was 2.2-fold higher than that in crispr-GNC plants. The total starch content of overexpression lines increased ~36.9% compared with the WT and ~68.5% compared with crispr-GNC plants (Fig. 5F). Soluble carbohydrate content increased ~35.8% compared with the WT and ~55.4% compared with the crispr-GNC mutant (Fig. 5G).
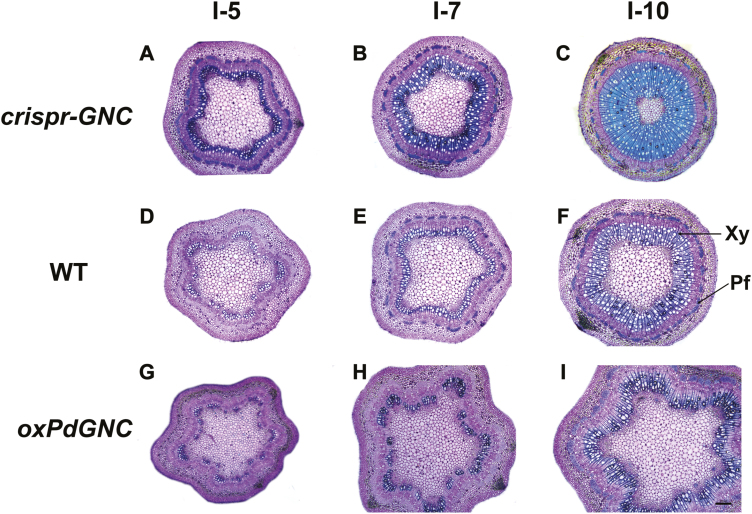
The dwarf phenotype of the crispr-GNC mutant encouraged us to explore variation in stem structure. Transverse sections of the stem from the shoot apex to the base showed a gradual transition from primary to secondary growth in the stem, and that stems of crispr-GNC plants produced a higher number of secondary xylem cells than WT and oxPdGNC plants at the same developmental stage. The crispr-GNC mutant formed a mature xylem ring around the cambium at the fifth internode (I-5), whereas at the same internode WT and oxPdGNC plants produced very few secondary growth cells. At the seventh internode (I-7), crispr-GNC plants had produced 2- or 3-fold more secondary xylem cell layers compared with I-5, whereas WT plants had just formed the xylem ring and in oxPdGNC plants only a small number of mature xylem cells had differentiated. At the tenth node (I-10), oxPdGNC plants had just begun to develop the lignified xylem ring (Fig. 6).
Fig. 6.
Transverse sections of stem from 2-month-old crispr-GNC mutant, wild type (WT), and PdGNC-overexpression (oxPdGNC) plants. (A, D, G) Magnified view of the fifth internode of crispr-GNC, WT, and oxPdGNC stem during primary growth. (B, E, H) Magnified view of the seventh internode of crispr-GNC, WT, and oxPdGNC stem during the transition to secondary growth. (C, F, I) Magnified view of the 10th internode of crispr-GNC, WT, and oxPdGNC stem. Pf, phloem fiber; Xy, xylem. Scale bar=100 μm. Each experiment comprised three biological replicates.
Transcriptional model modification by poplar GNC overexpression or suppression
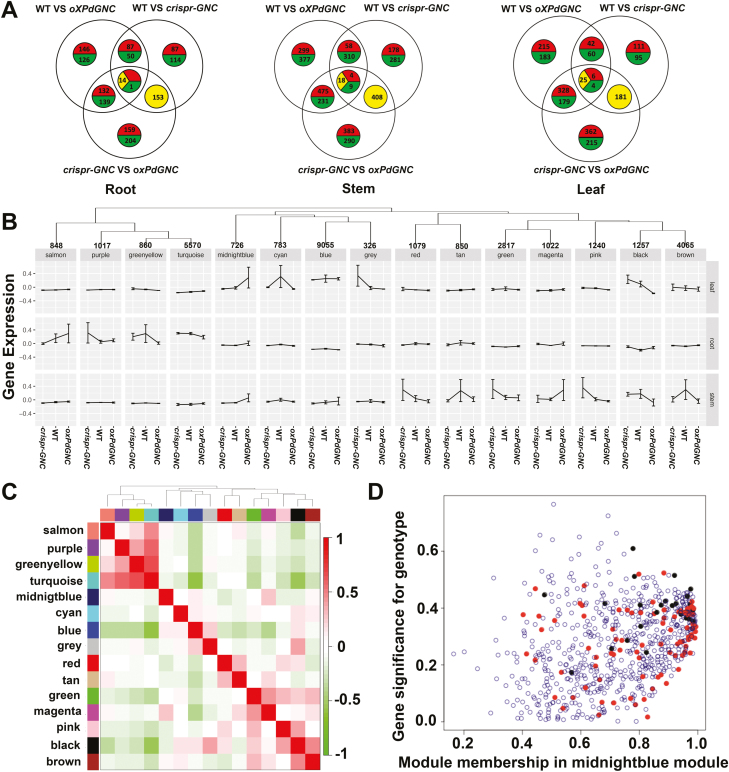
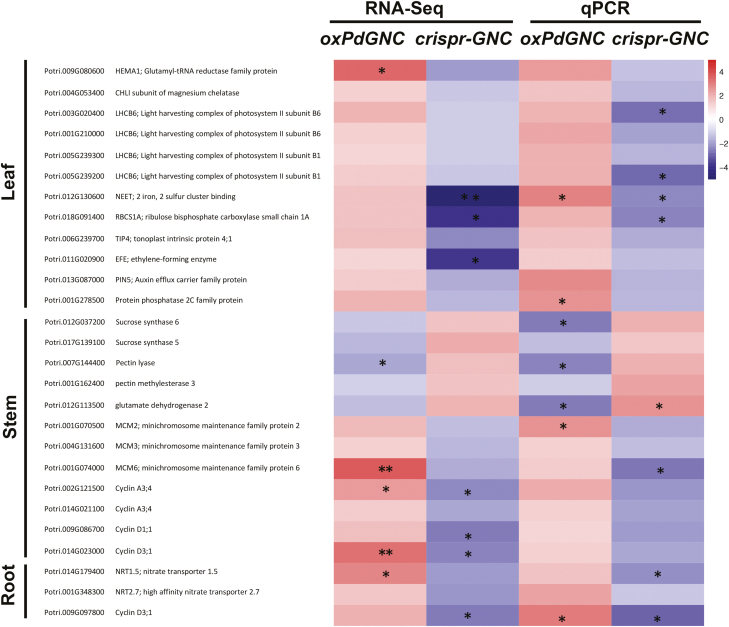
The phenotypic variation motivated us to further explore transcriptional changes in the leaf, stem, and root induced by Populus GNC overexpression and suppression. Differential gene expression analysis showed that modification of the GNC transcript level resulted in different transcriptional reprogramming between genotypes and tissues (Fig. 7A). In leaves, the hormone response, such as ethylene, auxin, ABA, and jasmonic acid, clustered around many up-regulated genes induced by PdGNC overexpression (see Supplementary Dataset S1). The ethylene synthesis gene Ethylene-Forming Enzyme (EFE) and the auxin efflux carrier gene PIN5 were up-regulated in oxPdGNC plants and down-regulated in crispr-GNC lines. The nitrogen transporter gene Tonoplast Intrinsic Protein 4 (TIP4) exhibited the same expression profile (Fig. 8). In stems, several genes involved in cell wall biogenesis and carbohydrate metabolism were up-regulated in overexpression lines. Many genes associated with the cell cycle and cell division were down-regulated in crispr-GNC plants. In roots, many genes associated with cell wall biosynthesis, the cell cycle, and nitrate transport were up-regulated in overexpression plants (Supplementary Dataset S1).
Fig. 7.
Gene differential expression and co-expression network of Populus GNC modified plants. (A) Gene differential expression in the root, stem, and leaf of crispr-GNC, wild type, and PdGNC-overexpression (oxPdGNC) plants. The red part represents the genes up-regulated in all comparisons, the green represents the genes down-regulated in all comparisons, and the yellow part represents the genes down-regulated or up-regulated in different comparisons. (B) Eigengene expression profiles of modules. (C) Correlation between modules. Each row and column corresponds to a module eigengene. (D) Scatterplot of gene significance for genotype versus module membership of all genes in the module midnightblue. Red points are transcription factor genes, black points are ethylene response-related transcription factors.
Fig. 8.
Heatmap of expression profiles of differentially expressed genes between PdGNC-overexpression (oxPdGNC), crispr-GNC, and wild type plants. qPCR was used to analyse expression levels of genes differentially expressed in the leaf, stem, and root. Asterisks indicate a significant difference (*P<0.05, **P<0.01; one-way ANOVA).
Additionally, WGCNA was employed to define different co-expressed modules of genes that were expressed across different tissues of WT, PdGNC overexpression, and CRISPR-induced plants. A total of 15 modules were determined by the gene co-expression relationship (Fig. 7C). The plot of eigengene values in each module revealed the tissue-specific variation in gene expression in different PdGNC-modified plants and in different gene co-expression modules (Fig. 7B). All modules were also annotated with GO enrichment terms to shed additional light on functional properties of the modules.
Eigengene expression in module midnightblue was up-regulated in all tissues, and GO annotation showed that the module was enriched in the regulatory processes responses to hormones and nitrogen metabolism. Sixty transcription factors, including members of the ERF, WRKY, C2H2, GRAS, MYB, and NAC transcription factor families, were differentially expressed in this module (Fig. 7D; Supplementary Dataset S2). Twenty ERF and five MYB genes were up-regulated in different tissues of oxPdGNC lines in the ethylene response process. For the ABA response process, except transcription factors of the ERF, C2H2, and MYB, Protein Phosphatase 2C (PP2C), and Plasma membrane Intrinsic Protein 3 (PIP3) were up-regulated in the leaves. With regard to N metabolism processes, Asparaginase B1 (ASPGB1) and Phenylalanine Ammonia Lyase 1 (PAL1) were induced in the leaves. In addition, two glutamyl-tRNA reductase (HEMA1) genes were up-regulated in the leaves. The latter protein functions as a crucial rate-limiting enzyme in the early steps of chlorophyll biosynthesis.
The module blue showed significant tissue-specific expression profiling and genes within this module were mainly expressed in the leaves. GO annotation revealed enrichment in a variety of biological processes and molecular structures involved in photosynthesis, such as chloroplast organization, photosynthetic electron transport chain, and chlorophyll binding. Differentially expressed genes within this module mostly participated in the chloroplast envelope and stroma, and ion homeostasis. Two light harvesting complex B1 subunit protein (LHCB1) genes and two B6 subunit (LHCB6) genes were up-regulated in oxPdGNC and down-regulated in crispr-GNC plants. These proteins are components of PSII antenna proteins and responsible for light harvesting and electron transport rate limitation (de Bianchi et al., 2008). In addition, two Mg chelatase subunit I (CHLI) genes and the subunit H gene CHLH, and a NEET gene functioning as a Fe–S cluster donor for ferredoxins primarily in the photosynthetic electron transport chain also displayed parallel expression patterns (Nechushtai et al., 2012). The biological processes that were enriched in these modules demonstrated the important roles of Populus GNC genes in photosynthesis.
Discussion
Evolution and divergence of genes encoding GATA transcription factors
Transcription factors regulate expression of genes that mediate diverse biological processes in cells and organisms, and are employed as a principal source of the diversity and change that underlie evolution (Riechmann and Ratcliffe, 2000). Investigation of the Populus genome revealed 39 Populus GATA transcription factor genes, which indicated that the woody tree Populus has a higher number of GATA family members than the 30 and 28 GATA genes identified in Arabidopsis (AtGATAs) and rice (OsGATAs), respectively (Reyes et al., 2004; Bi et al., 2005). Variation in GATA family gene number may be attributed to gene or genome duplication, which has been considered to be a primary source of genetic novelty and progress in plant evolution (Yang et al., 2006). Previous studies revealed that GATA genes can be classified into seven subfamilies (I–VII) based on their phylogenetic relationships (Reyes et al., 2004). Populus and Arabidopsis lack the rice-specific subfamilies V, VI, and VII (see Supplementary Table S4). Concerning the GATA subfamily classification in Populus, Arabidopsis, and rice, we inferred that both woody and herbaceous eudicotyledons harbor GATA subfamilies I, II, III, and IV, but subfamily IV is absent in monocotyledons. This result further verifies the hypothesis that subfamilies I, II, and III arose before divergence of monocotyledons and eudicotyledons, whereas subfamilies IV, V, VI, and VII may have been lost or arose after divergence of monocotyledons and eudicotyledons (Reyes et al., 2004). Evolutionary variation in monocotyledon and eudicotyledon plants may have contributed to functional divergence of GATA genes in subfamilies IV, V, VI, and VII, but currently little information is available on functional differences among these subfamilies.
Functional variance of GATA transcription factors in poplar
Mapping the reported functions of characterized GATA genes to the phylogenetic tree revealed that similar gene functions were clustered in the same subfamily. The Arabidopsis homologous genes in subfamily I are mainly involved in light signaling pathways (Manfield et al., 2007; Luo et al., 2010). Although Populus genes in subfamily I have not been reported until now, cis-element analysis of the promoter region of Populus genes revealed abundant elements associated with light responses, implying that these genes have potential roles in light responses.
Subfamily II contains many genes that function in flowering regulation. Recent studies reveal that subfamily II could be subdivided into two classes respectively characterized by an N-terminal HAN (HANABA TARANU) and a C-terminal LLM (leucine–leucine–methionine) domain. GATA genes that contain the HAN domain play roles in floral development (Zhang et al., 2013), whereas genes that contain the LLM domain, such as GNC and CGA1/GNL (Richter et al., 2013; Behringer and Schwechheimer, 2015; Ranftl et al., 2016), function in the control of flowering time, as well as chloroplast development and vegetative growth. These studies show that different gene structures combined with the GATA core domain may contribute to neofunctionalization of GATA genes.
Atypical architectures of the CCT and TIFY domains identified in the Populus GATA subfamily III members (Fig. 1) would develop additional functions, such as stress and phytohormone response (Shikata et al., 2004; Vanholme et al., 2007; Ye et al., 2009; Chung et al., 2009; Kazan and Manners, 2012; Pérez et al., 2014). The CCT domain is mainly present in subfamily III in both Populus and Arabidopsis, which is suggestive of conserved functions for this subfamily in woody and herbaceous plants. Members of the subfamily are an important regulator and a central component of the photoperiodic floral progress and circadian regulation (Hayama and Coupland, 2003). Although several homologs of the genes that govern flowering in Arabidopsis are present in trees (Böhlenius et al., 2006; Mohamed et al., 2010; Hsu et al., 2011), it will be necessary to investigate the tree genes in tree-specific processes. The GATA-CCT-dependent pathway would provide insights to elucidate such tree-specific processes for flowering and circadian regulation. Accompanied by the CCT domain, the TIFY domain is also present in subfamily III. The TIFY domain was first identified in Arabidopsis AtGATA25 (At4g24470), which contains the CCT domain in addition to TIFY (Nishii et al., 2000). Several screenings have revealed a link between TIFY proteins and the jasmonic acid-related (JA) response (Ye et al., 2009). Three Arabidopsis GATA subfamily III genes, namely AtGATA24/ZML1 (At3g21175), AtGATA25/ZIM (At4g24470), and AtGATA28/ZML2 (At1g51600), contain CCT and TIFY domains and are involved in JA responses (Vanholme et al., 2007; Kazan and Manners, 2012; Pérez et al., 2014). The Populus GATA subfamily III genes PtrGATA5, PtrGATA30, and PtrGATA34, which are homologs of AtGATA24, AtGATA25, and AtGATA28, respectively, have the JA-related CGTCA motif and TGACG motif in the promoter sequences, in addition to the CCT and TIFY domains, which implies that these GATA genes may also participate in the JA response. Coupled with the presence of stress-related elements, we suggest that Populus GATA genes have potential roles in response to additional heat/low temperature, drought, and other external signals. They may contribute to the evolution of a greater number of traits adaptive to environmental stress conditions for perennial woody plants, compared with annual herbaceous plants.
Conserved roles of poplar GATA transcription factors in chlorophyll biosynthesis and photosynthesis
The phenotypic traits associated with GNC in Arabidopsis (Hudson et al., 2011) and Populus indicate that plant GNC genes show conserved functions in chlorophyll biosynthesis and starch accumulation in both herbaceous and woody plants (Fig. 3D; Fig. 5). The chlorophyll content reflects the N status and increase in chlorophyll content provides an improved capacity to convert light energy to chemical energy and enhanced carbohydrate accumulation (Dordas and Sioulas, 2008). Chlorophyll is a type of tetrapyrrole and the PdGNC-induced HEMA gene encodes an enzyme that participates in biosynthesis of 5-aminolevulinic acid, which is a universal precursor of tetrapyrrole synthesis (Jahn et al., 1992). This important rate-limiting step, which is boosted by PdGNC overexpression, would expedite chlorophyll biosynthesis and increase chlorophyll content. In addition, up-regulation of Mg-chelatase genes further stimulates photosynthetic pigment biosynthesis. The other integral LHCB protein genes induced by PdGNC also contribute to the robustness of the PSII light harvesting complex, which increases the capability and efficiency of light absorption (de Bianchi et al., 2008). The harvested energy is transferred by the photosynthetic electron transfer chain, which is promoted by PdGNC-regulated iron–sulfur cluster metabolism. Proteins containing iron–sulfur clusters include ferredoxins and NADH dehydrogenase, which participate in the oxidation–reduction reactions in photosynthesis. The elevated chlorophyll content and photosynthetic efficiency induced by PdGNC overexpression will lead to production of a larger C source for plant growth, resulting in accelerated growth and enhanced biomass accumulation.
Divergent roles of poplar GATA transcription factor in plant growth
In contrast to the notable differences in growth and morphology of oxPdGNC plants and the crispr-GNC mutant (Fig. 4), neither the Arabidopsis GNC (AtGNC) overexpressing transformant nor the gnc mutant show notable phenotypic variation with regard to overall growth (Hudson et al., 2011). The difference in impacts of GNC gene modification between Arabidopsis and Populus may be attributable to the divergent functions of GNC genes in different growth regulatory systems between herbaceous and woody plants. AtGNC does not have significant effects on plant growth, except for chlorophyll content and starch production. Arabidopsis has a short life cycle, and vegetative growth strongly impacts on reproduction. Greater material reserves and energy accumulation are utilized for reproductive growth, and proportionally less for vegetative growth. However, the perennial woody tree Populus has a longer life cycle and requires substantially greater quantities of nutrients and energy for vegetative growth. Thus, PdGNC, the counterpart of AtGNC in a woody tree system, is involved in not only chlorophyll biosynthesis and starch accumulation, but also promotion of plant growth and maintenance of overall plant architecture. Transcriptomic data indicated that a large number of cyclin genes (CYCAs, CYCBs, and CYCDs) and mini-chromosome maintenance protein genes (MCMs) were down-regulated in the crispr-GNC lines (Fig. 8). Decreased expression levels of these important regulators of DNA replication and the cell cycle may be responsible for the retarded growth of crispr-GNC plants (Tuteja et al., 2011). In stems of the crispr-GNC plants, pectin methylesterase genes (PMEs) were up-regulated. The proteins encoded by these genes demethylesterify homogalacturonan, which is the main component of pectic polysaccharides in the cell wall. These low-methylesterified homogalacturonans are degraded by pectin lyases and polygalacturonases to form oligogalacturonides, which can loosen the cell wall and eventually lead to vertical growth inhibition (Ridley et al., 2001). In addition, sucrose synthase genes (SUSs) were up-regulated. These genes control C flow during cell wall biosynthesis (Fujii et al., 2010). Higher quantities of carbohydrates are incorporated in the secondary cell wall of woody cells but are not necessarily assigned to other normal plant vegetative growth processes, such as increment in shoot height and leaf area. The genes associated with carbohydrate transport and the electron transport needed for sugar transport were down-regulated in the crispr-GNC mutant. Therefore, we infer that Populus GNC genes participate in the cell cycle and C distribution. Thus, PdGNC positively regulates C fixation and distributes C metabolites for plant vegetative growth to attain greater biomass accumulation.
The study of Populus GATA genes enables clarification of the functional conservation and divergence of GATA genes in herbaceous and woody plants. Although the majority of Populus GATA genes are of unknown function, the present comparative phylogenetic and expression analysis provides a basis for future functional studies of the GATA transcription factor family in Populus and other woody plants. In particular, the responses of GNC genes to N stress will be a focus of future research.
Supplementary data
Supplementary data are available at JXB online.
Dataset S1. Gene ontology (GO) annotation and enrichment analysis of differentially expressed genes in the leaf, stem, and root of oxPdGNC, wild type, and crispr-GNC poplar plants.
Dataset S2. Summary of transcription factors differentially expressed in module midnightblue.
Fig. S1. PdGNC gene expression levels in different overexpression lines.
Fig. S2. Location of GATA gene family members on Populus chromosomes.
Fig. S3. Conserved core sequence logos and amino acid sequence alignment of Populus and Arabidopsis GATA zinc-finger motifs.
Fig. S4. Overview of predicted cis-acting elements in the promoter region of PtrGATA genes.
Fig. S5. Alignment of nucleotide sequences of the GATA zinc-finger domain in all 39 Populus GATA genes.
Fig. S6. Representative Sanger sequencing chromatograms at the target site.
Fig. S7. Comparison of net photosynthetic rate per unit leaf volume.
Table S1. The qPCR primers used for expression profiles of Populus GATA genes in response to nitrogen.
Table S2. Primers used for qPCR analysis of genes differentially expressed in the leaf, stem, and root of oxPdGNC, crispr-GNC, and wild-type Populus plants.
Table S3. Primers used in CRISPR/Cas9-mediated mutagenesis of Populus GNC gene.
Table S4. Summary of GATA subfamilies in Populus, Arabidopsis, and rice.
Acknowledgements
We are very grateful to Andrew Groover (USDA Forest Service and University of California, Davis, CA, USA) and Alan Bennett (University of California, Davis, CA, USA) for helpful comments and technical assistance. We thank Robert McKenzie, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript. This research are supported by the National Natural Science Foundation of China (31770649, 31570308, and 31600484), the 111 Project (B13007), and the China Scholarship Council.
References
- An Y, Han X, Tang S, Xia X, Yin W. 2014. Poplar GATA transcription factor PdGNC is capable of regulating chloroplast ultrastructure, photosynthesis, and vegetative growth in Arabidopsis under varying nitrogen levels. Plant Cell, Tissue and Organ Culture 119, 313–327. [Google Scholar]
- Behringer C, Schwechheimer C. 2015. B-GATA transcription factors – insights into their structure, regulation, and role in plant development. Frontiers in Plant Science 6, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi YM, Zhang Y, Signorelli T, Zhao R, Zhu T, Rothstein S. 2005. Genetic analysis of Arabidopsis GATA transcription factor gene family reveals a nitrate-inducible member important for chlorophyll synthesis and glucose sensitivity. The Plant Journal 44, 680–692. [DOI] [PubMed] [Google Scholar]
- Böhlenius H, Huang T, Charbonnel-Campaa L, Brunner AM, Jansson S, Strauss SH, Nilsson O. 2006. CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 312, 1040–1043. [DOI] [PubMed] [Google Scholar]
- Bondada BR, Syvertsen JP. 2003. Leaf chlorophyll, net gas exchange and chloroplast ultrastructure in citrus leaves of different nitrogen status. Tree Physiology 23, 553–559. [DOI] [PubMed] [Google Scholar]
- Chiang YH, Zubo YO, Tapken W, Kim HJ, Lavanway AM, Howard L, Pilon M, Kieber JJ, Schaller GE. 2012. Functional characterization of the GATA transcription factors GNC and CGA1 reveals their key role in chloroplast development, growth, and division in Arabidopsis. Plant Physiology 160, 332–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HS, Niu Y, Browse J, Howe GA. 2009. Top hits in contemporary JAZ: an update on jasmonate signaling. Phytochemistry 70, 1547–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coruzzi G, Bush DR. 2001. Nitrogen and carbon nutrient and metabolite signaling in plants. Plant Physiology 125, 61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel-Vedele F, Caboche M. 1993. A tobacco cDNA clone encoding a GATA-1 zinc finger protein homologous to regulators of nitrogen metabolism in fungi. Molecular & General Genetics 240, 365–373. [DOI] [PubMed] [Google Scholar]
- de Bianchi S, Dall’Osto L, Tognon G, Morosinotto T, Bassi R. 2008. Minor antenna proteins CP24 and CP26 affect the interactions between photosystem II subunits and the electron transport rate in grana membranes of Arabidopsis. The Plant Cell 20, 1012–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rybel B, Vassileva V, Parizot B, et al. 2010. A novel aux/IAA28 signaling cascade activates GATA23-dependent specification of lateral root founder cell identity. Current Biology 20, 1697–1706. [DOI] [PubMed] [Google Scholar]
- Dordas CA, Sioulas C. 2008. Safflower yield, chlorophyll content, photosynthesis, and water use efficiency response to nitrogen fertilization under rainfed conditions. Industrial Crops and Products 27, 75–85. [Google Scholar]
- Euring D, Bai H, Janz D, Polle A. 2014. Nitrogen-driven stem elongation in poplar is linked with wood modification and gene clusters for stress, photosynthesis and cell wall formation. BMC Plant Biology 14, 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D, Liu T, Li C, Jiao B, Li S, Hou Y, Luo K. 2015. Efficient CRISPR/Cas9-mediated targeted mutagenesis in Populus in the first generation. Scientific Reports 5, 12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauser F, Schiml S, Puchta H. 2014. Both CRISPR/Cas-based nucleases and nickases can be used efficiently for genome engineering in Arabidopsis thaliana. The Plant Journal 79, 348–359. [DOI] [PubMed] [Google Scholar]
- Fujii S, Hayashi T, Mizuno K. 2010. Sucrose synthase is an integral component of the cellulose synthesis machinery. Plant & Cell Physiology 51, 294–301. [DOI] [PubMed] [Google Scholar]
- Gerttula S, Zinkgraf M, Muday GK, Lewis DR, Ibatullin FM, Brumer H, Hart F, Mansfield SD, Filkov V, Groover A. 2015. Transcriptional and hormonal regulation of gravitropism of woody stems in Populus. The Plant Cell 27, 2800–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo AY, Zhu QH, Chen X, Luo JC. 2007. GSDS: a gene structure display server. Yi chuan = Hereditas 29, 1023–1026. [PubMed] [Google Scholar]
- Han KH, Meilan R, Ma C, Strauss SH. 2000. An Agrobacterium tumefaciens transformation protocol effective on a variety of cottonwood hybrids (genus Populus). Plant Cell Reports 19, 315–320. [DOI] [PubMed] [Google Scholar]
- Hao S, Zhao T, Xia X, Yin W. 2011. Genome-wide comparison of two poplar genotypes with different growth rates. Plant Molecular Biology 76, 575–591. [DOI] [PubMed] [Google Scholar]
- Hayama R, Coupland G. 2003. Shedding light on the circadian clock and the photoperiodic control of flowering. Current Opinion in Plant Biology 6, 13–19. [DOI] [PubMed] [Google Scholar]
- Hudson D, Guevara DR, Hand AJ, Xu Z, Hao L, Chen X, Zhu T, Bi YM, Rothstein SJ. 2013. Rice cytokinin GATA Transcription Factor1 regulates chloroplast development and plant architecture. Plant Physiology 162, 132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson D, Guevara D, Yaish MW, Hannam C, Long N, Clarke JD, Bi YM, Rothstein SJ. 2011. GNC and CGA1 modulate chlorophyll biosynthesis and glutamate synthase (GLU1/Fd-GOGAT) expression in Arabidopsis. PLoS One 6, e26765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn D, Verkamp E, Söll D. 1992. Glutamyl-transfer RNA: a precursor of heme and chlorophyll biosynthesis. Trends in Biochemical Sciences 17, 215–218. [DOI] [PubMed] [Google Scholar]
- Jansson S, Douglas CJ. 2007. Populus: a model system for plant biology. Annual Review of Plant Biology 58, 435–458. [DOI] [PubMed] [Google Scholar]
- Jarai G, Truong HN, Daniel-Vedele F, Marzluf GA. 1992. NIT2, the nitrogen regulatory protein of Neurospora crassa, binds upstream of nia, the tomato nitrate reductase gene, in vitro. Current Genetics 21, 37–41. [DOI] [PubMed] [Google Scholar]
- Jin J, Zhang H, Kong L, Gao G, Luo J. 2014. PlantTFDB 3.0: a portal for the functional and evolutionary study of plant transcription factors. Nucleic Acids Research 42, D1182–D1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazan K, Manners JM. 2012. JAZ repressors and the orchestration of phytohormone crosstalk. Trends in Plant Science 17, 22–31. [DOI] [PubMed] [Google Scholar]
- Langfelder P, Horvath S. 2008. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9, 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I, Doerks T, Bork P. 2012. SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Research 40, D302–D305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PP, Koizuka N, Martin RC, Nonogaki H. 2005. The BME3 (Blue Micropylar End 3) GATA zinc finger transcription factor is a positive regulator of Arabidopsis seed germination. The Plant Journal 44, 960–971. [DOI] [PubMed] [Google Scholar]
- Lowry JA, Atchley WR. 2000. Molecular evolution of the GATA family of transcription factors: conservation within the DNA-binding domain. Journal of Molecular Evolution 50, 103–115. [DOI] [PubMed] [Google Scholar]
- Lu H, Viswanath V, Ma C, Etherington E, Dharmawardhana P, Shevchenko O, Strauss SH, Pearce DW, Rood SB, Busov V. 2015. Recombinant DNA modification of gibberellin metabolism alters growth rate and biomass allocation in Populus. Tree Genetics & Genomes 11, 127. [Google Scholar]
- Luo J, Zhou J, Li H, Shi W, Polle A, Lu M, Sun X, Luo ZB. 2015. Global poplar root and leaf transcriptomes reveal links between growth and stress responses under nitrogen starvation and excess. Tree Physiology 35, 1283–1302. [DOI] [PubMed] [Google Scholar]
- Luo XM, Lin WH, Zhu S, et al. 2010. Integration of light- and brassinosteroid-signaling pathways by a GATA transcription factor in Arabidopsis. Developmental Cell 19, 872–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfield IW, Devlin PF, Jen CH, Westhead DR, Gilmartin PM. 2007. Conservation, convergence, and divergence of light-responsive, circadian-regulated, and tissue-specific expression patterns during evolution of the Arabidopsis GATA gene family. Plant Physiology 143, 941–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mara CD, Irish VF. 2008. Two GATA transcription factors are downstream effectors of floral homeotic gene action in Arabidopsis. Plant Physiology 147, 707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed R, Wang CT, Ma C, et al. 2010. Populus CEN/TFL1 regulates first onset of flowering, axillary meristem identity and dormancy release in Populus. The Plant Journal 62, 674–688. [DOI] [PubMed] [Google Scholar]
- Nechushtai R, Conlan AR, Harir Y, et al. 2012. Characterization of Arabidopsis NEET reveals an ancient role for NEET proteins in iron metabolism. The Plant Cell 24, 2139–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishii A, Takemura M, Fujita H, Shikata M, Yokota A, Kohchi T. 2000. Characterization of a novel gene encoding a putative single zinc-finger protein, ZIM, expressed during the reproductive phase in Arabidopsis thaliana. Bioscience, Biotechnology, and Biochemistry 64, 1402–1409. [DOI] [PubMed] [Google Scholar]
- Nunes-Nesi A, Fernie AR, Stitt M. 2010. Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Molecular Plant 3, 973–996. [DOI] [PubMed] [Google Scholar]
- Oliveira IC, Coruzzi GM. 1999. Carbon and amino acids reciprocally modulate the expression of glutamine synthetase in Arabidopsis. Plant Physiology 121, 301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patient RK, McGhee JD. 2002. The GATA family (vertebrates and invertebrates). Current Opinion in Genetics & Development 12, 416–422. [DOI] [PubMed] [Google Scholar]
- Pérez AC, Durand AN, Bossche RV, De Clercq R, Persiau G, Van Wees SC, Pieterse CM, Gevaert K, De Jaeger G, Goossens A. 2014. The non-JAZ TIFY protein TIFY8 from Arabidopsis thaliana is a transcriptional repressor. PLoS One 9, e84891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Rodríguez P, Riaño-Pachón DM, Corrêa LG, Rensing SA, Kersten B, Mueller-Roeber B. 2010. PlnTFDB: updated content and new features of the plant transcription factor database. Nucleic Acids Research 38, D822–D827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polle A, Janz D, Teichmann T, Lipka V. 2013. Poplar genetic engineering: promoting desirable wood characteristics and pest resistance. Applied Microbiology and Biotechnology 97, 5669–5679. [DOI] [PubMed] [Google Scholar]
- Porra R, Thompson W, Kriedemann P. 1989. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochimica et Biophysica Acta 975, 384–394. [Google Scholar]
- Ranftl QL, Bastakis E, Klermund C, Schwechheimer C. 2016. LLM-domain containing B-GATA factors control different aspects of cytokinin-regulated development in Arabidopsis thaliana. Plant Physiology 170, 2295–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes JC, Muro-Pastor MI, Florencio FJ. 2004. The GATA family of transcription factors in Arabidopsis and rice. Plant Physiology 134, 1718–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter R, Behringer C, Zourelidou M, Schwechheimer C. 2013. Convergence of auxin and gibberellin signaling on the regulation of the GATA transcription factors GNC and GNL in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA 110, 13192–13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley BL, O’Neill MA, Mohnen D. 2001. Pectins: structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 57, 929–967. [DOI] [PubMed] [Google Scholar]
- Riechmann JL, Ratcliffe OJ. 2000. A genomic perspective on plant transcription factors. Current Opinion in Plant Biology 3, 423–434. [DOI] [PubMed] [Google Scholar]
- Robson F, Costa MM, Hepworth SR, Vizir I, Piñeiro M, Reeves PH, Putterill J, Coupland G. 2001. Functional importance of conserved domains in the flowering-time gene CONSTANS demonstrated by analysis of mutant alleles and transgenic plants. The Plant Journal 28, 619–631. [DOI] [PubMed] [Google Scholar]
- Rueda-López M, Cañas RA, Canales J, Cánovas FM, Ávila C. 2015. The overexpression of the pine transcription factor PpDof5 in Arabidopsis leads to increased lignin content and affects carbon and nitrogen metabolism. Physiologia Plantarum 155, 369–383. [DOI] [PubMed] [Google Scholar]
- Scazzocchio C. 2000. The fungal GATA factors. Current Opinion in Microbiology 3, 126–131. [DOI] [PubMed] [Google Scholar]
- Shikata M, Matsuda Y, Ando K, Nishii A, Takemura M, Yokota A, Kohchi T. 2004. Characterization of Arabidopsis ZIM, a member of a novel plant-specific GATA factor gene family. Journal of Experimental Botany 55, 631–639. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution 30, 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teakle GR, Manfield IW, Graham JF, Gilmartin PM. 2002. Arabidopsis thaliana GATA factors: organisation, expression and DNA-binding characteristics. Plant Molecular Biology 50, 43–57. [DOI] [PubMed] [Google Scholar]
- Tuskan GA, Difazio S, Jansson S, et al. 2006. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 313, 1596–1604. [DOI] [PubMed] [Google Scholar]
- Tuteja N, Tran NQ, Dang HQ, Tuteja R. 2011. Plant MCM proteins: role in DNA replication and beyond. Plant Molecular Biology 77, 537–545. [DOI] [PubMed] [Google Scholar]
- Vanholme B, Grunewald W, Bateman A, Kohchi T, Gheysen G. 2007. The tify family previously known as ZIM. Trends in Plant Science 12, 239–244. [DOI] [PubMed] [Google Scholar]
- Vidal EA, Álvarez JM, Moyano TC, Gutiérrez RA. 2015. Transcriptional networks in the nitrate response of Arabidopsis thaliana. Current Opinion in Plant Biology 27, 125–132. [DOI] [PubMed] [Google Scholar]
- Wang L, Ran L, Hou Y, Tian Q, Li C, Liu R, Fan D, Luo K. 2017. The transcription factor MYB115 contributes to the regulation of proanthocyanidin biosynthesis and enhances fungal resistance in poplar. New Phytologist 215, 351–367. [DOI] [PubMed] [Google Scholar]
- Xu C, Fu X, Liu R, Guo L, Ran L, Li C, Tian Q, Jiao B, Wang B, Luo K. 2017. PtoMYB170 positively regulates lignin deposition during wood formation in poplar and confers drought tolerance in transgenic Arabidopsis. Tree Physiology 37, 1713–1726. [DOI] [PubMed] [Google Scholar]
- Yamori W, Shikanai T. 2016. Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth. Annual Review of Plant Biology 67, 81–106. [DOI] [PubMed] [Google Scholar]
- Yang W, Yoon J, Choi H, Fan Y, Chen R, An G. 2015. Transcriptome analysis of nitrogen-starvation-responsive genes in rice. BMC Plant Biology 15, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Tuskan GA, Cheng MZ. 2006. Divergence of the Dof gene families in poplar, Arabidopsis, and rice suggests multiple modes of gene evolution after duplication. Plant Physiology 142, 820–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H, Du H, Tang N, Li X, Xiong L. 2009. Identification and expression profiling analysis of TIFY family genes involved in stress and phytohormone responses in rice. Plant Molecular Biology 71, 291–305. [DOI] [PubMed] [Google Scholar]
- Zhang C, Hou Y, Hao Q, et al. 2015. Genome-wide survey of the soybean GATA transcription factor gene family and expression analysis under low nitrogen stress. PLoS One 10, e0125174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhou Y, Ding L, Wu Z, Liu R, Meyerowitz EM. 2013. Transcription repressor HANABA TARANU controls flower development by integrating the actions of multiple hormones, floral organ specification genes, and GATA3 family genes in Arabidopsis. The Plant Cell 25, 83–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Jacobs TB, Xue LJ, Harding SA, Tsai CJ. 2015. Exploiting SNPs for biallelic CRISPR mutations in the outcrossing woody perennial Populus reveals 4-coumarate:CoA ligase specificity and redundancy. New Phytologist 208, 298–301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.