Ethylene controls post-germination starch degradation and seedling growth, but not radicle protrusion, in wheat through spatiotemporal alteration of the balance between abscisic acid and gibberellin.
Keywords: Coleoptile, embryo axis, gene expression, germination, plant hormones, root, seedling, starch degradation
Abstract
This study aimed to gain insights into the molecular mechanisms underlying the role of ethylene in regulating germination and seedling growth in wheat by combining pharmacological, molecular, and metabolomics approaches. Our study showed that ethylene does not affect radicle protrusion but controls post-germination endospermic starch degradation through transcriptional regulation of specific α-amylase and α-glucosidase genes, and this effect is mediated by alteration of endospermic bioactive gibberellin (GA) levels, and GA sensitivity via expression of the GA signaling gene, TaGAMYB. Our data implicated ethylene as a positive regulator of embryo axis and coleoptile growth through transcriptional regulation of specific TaEXPA genes. These effects were associated with modulation of GA levels and sensitivity, through expression of GA metabolism (TaGA20ox1, TaGA3ox2, and TaGA2ox6) and signaling (TaGAMYB) genes, respectively, and/or the abscisic acid (ABA) level and sensitivity, via expression of specific ABA metabolism (TaNCED2 or TaCYP707A1) and signaling (TaABI3) genes, respectively. Ethylene appeared to regulate the expression of TaEXPA3 and thereby root growth through its control of coleoptile ABA metabolism, and root ABA signaling via expression of TaABI3 and TaABI5. These results show that spatiotemporal modulation of ABA/GA balance mediates the role of ethylene in regulating post-germination storage starch degradation and seedling growth in wheat.
Introduction
Seed germination and seedling growth are complex physiological processes regulated by several plant hormones. Abscisic acid (ABA) and gibberellin (GA) are the major players in this regard; ABA inhibits seed germination and seedling growth while GA promotes these early developmental processes (Finkelstein et al., 2002; Sun, 2008; Shu et al., 2018; Tuan et al., 2018). These effects of ABA and GA are mediated partly by their levels, which are determined by the balance between their biosynthesis and catabolism. The level of ABA in plant tissues is regulated mainly by the actions of 9-cis-epoxycarotenoid dioxygenase (NCED) and ABA 8'-hydroxylase (ABA8′OH; encoded by CYP707A genes) that catalyze ABA biosynthesis and catabolism, respectively (Nambara et al., 2010), while the level of GA is controlled mainly by the actions of GA 20-oxidase (GA20ox) and GA 3-oxidase (GA3ox) (GA biosynthesis), and GA 2-oxidase (GA2ox) (GA catabolism) enzymes (Yamaguchi, 2008). The effects of ABA and GA are also mediated by tissue sensitivity to these hormones, which is regulated by their respective signaling pathways. ABA signaling involves several components, including the downstream transcriptional regulators ABI3 and ABI5 (Nambara et al., 2010), while the growth-repressing DELLA protein and downstream transcription factor GAMYB represent important components of GA signaling (Sun and Gubler, 2004). Genetic and mutational analyses of the ABA and GA metabolism and signaling genes have shown their importance in regulating seed germination and seedling growth (Finkelstein et al., 2002; Sun, 2008; Nambara et al., 2010; Matilla et al., 2015).
Other phytohormones such as ethylene (ET) also regulate seed germination and seedling growth. ET promotes germination in dicot species, while inhibition of ET synthesis is associated with repression of germination (Arc et al., 2013; Corbineau et al., 2014). The synthesis of ET in non-dormant seeds of both dicots and cereals starts with imbibition and peaks during radicle emergence (Lalonde and Saini, 1992; Petruzzelli et al., 1994; Kucera et al., 2005), and this is associated with the expression patterns of ET biosynthetic genes (Linkies and Leubner-Metzger, 2012). In contrast, neither inhibition of ET synthesis nor treatment with an ET precursor or ET-releasing compound affects the germination of non-dormant cereal seeds (Locke et al., 2000; Gianinetti et al., 2007). ET has also been implicated in regulating seedling growth; it inhibits root elongation in dicot seedlings (Ruzicka et al., 2007; Dong et al., 2011), and its effect on hypocotyl elongation depends on light as it exhibits inhibitory and stimulatory effects in the dark and light, respectively (Smalle et al., 1997). In cereal seedlings, ET promotes coleoptile elongation irrespective of light condition but inhibits root growth (Yin et al., 2017), while inhibition of ET synthesis represses the elongation of both tissues (Gianinetti et al., 2007).
The role of ET in regulating seed germination and seedling growth is mediated at least partly by its antagonism of ABA effects. For example, seed germination and seedling root growth in Arabidopsis mutants with enhanced ET synthesis and sensitivity such as ethylene overproducer1 (eto1) and eto3, and constitutive triple response1 (ctr1) are less sensitive to ABA (Beaudoin et al., 2000; Ghassemian et al., 2000). On the other hand, ET deficiency due to mutation in the ET biosynthetic gene ACS7 promotes seed sensitivity to ABA and thereby inhibition of seed germination and seedling growth (Dong et al., 2011). Furthermore, mutants with reduced ET sensitivity such as ethylene resistant1 (etr1) and ethylene insensitive2 (ein2) exhibit increased ABA content and sensitivity, leading to inhibition of germination (Arc et al., 2013). Seedling root growth in these mutants, however, is less sensitive to ABA (Ghassemian et al., 2000), suggesting the requirement for a functional ET signaling for inhibition of root growth by ABA. In cereal seedlings, ET-induced inhibition of root growth is reported to be mediated by an enhanced ABA level, while inhibition of coleoptile elongation by ABA is mediated by repression of the ET response (Ku et al., 1970; Ma et al., 2014).
Interplay between ET and GA has also been implicated in the regulation of germination and seedling growth. For example, inhibition of ET synthesis and signaling in imbibing dicot seeds alters the expression patterns of GA metabolism genes and thereby inhibits germination (Iglesias-Fernandez and Matilla, 2010), and such effects of a reduced ET level can be reversed by GA (Calvo et al., 2004b). Conversely, GA promotes ET synthesis and germination via up-regulating ET biosynthetic genes and enzymes, and ET can reverse the effects of decreased GA levels (Calvo et al., 2004a). Previous studies in Arabidopsis seedlings showed the requirement of GA for ET-mediated stimulation of apical hook formation that occurs through enhanced cell division and elongation (Vriezen et al., 2004) while inhibition of root growth by ET is DELLA dependent (Achard et al., 2003). Furthermore, the role of ET in promoting seedling elongation in rice appeared to be mediated through enhanced GA response (Furukawa et al., 1997). Consistently, GA promotes hypocotyl or coleoptile elongation (Cowling and Harberd, 1999; Konishi et al., 2005), and such an effect of GA is mediated by up-regulation of genes encoding expansins such as α-expansin (EXPA), proteins that act as major regulators of cell enlargement (Sampedro and Cosgrove, 2005). Despite these reports, the molecular links underlying the role of ET in regulating ABA/GA balance, and thereby germination and seedling growth, in cereals are poorly understood.
Post-germination seedling growth is also regulated by mobilization of seed storage reserves that serve as a primary source of energy, and mutations that compromise mobilization of seed storage reserves inhibit seedling establishment (Penfield et al., 2005). In seeds of cereals, endospermic starch serves as a major storage reserve, and its mobilization involves the actions of α-amylase (AMY), which directly acts on starch granules and produces branched and linear glucans that are further hydrolyzed into glucose by the combined actions of debranching enzyme limit-dextrinase, β-amylase (BAM), and α-glucosidase (AGL). Activation of these enzymes during imbibition is associated with reduction in the endospermic starch level and increases in the levels of soluble sugars such as glucose, fructose, and maltose (Palmiano and Juliano, 1972). It is well established that starch degradation during imbibition of cereal seeds is regulated antagonistically by ABA and GA; for example, the expression of starch-degrading genes such as AMY is enhanced by GA but repressed by ABA (Gubler et al., 1995; Gómez-Cadenas et al., 1999, 2001). Although earlier reports implicated ET in the control of amylase synthesis and release in the aleurone tissues of cereal seeds (Eastwell and Spencer, 1982; Varty et al., 1983), its role in regulating the degradation of endospermic starch and the underlying molecular bases remain to be elucidated.
Overall, previous studies that provided insights into the molecular mechanisms of ET interplay with ABA and GA during germination and seedling growth were focused mainly on dicot species; therefore, much less is known about this phenomenon in cereals, particularly in the polyploid wheat as the genomic/reverse genetic resources that are crucial in elucidating such mechanism are still very scarce. To this end, this study investigated the molecular bases underlying the role of ET in regulating spatiotemporal modulation of ABA/GA balance, and thereby germination, storage starch degradation, and seedling growth in wheat.
Materials and methods
Plant materials, germination, and seedling growth assays
Wheat genotype RL4452, which produces non-dormant seeds at maturity, was used for this study (Yamasaki et al., 2017). Mature seeds were harvested from plants grown under conditions described previously (Izydorczyk et al., 2018). Seed surface sterilization and germination assays were undertaken as described previously (Gao et al., 2013). To examine the effect of ET on germination and seedling growth, seeds were imbibed with 1 mM aminoethoxyvinylglycine (AVG), an ET biosynthesis inhibitor (Cayman Chemicals, Ann Arbor, MI, USA), 10 μM ethephon (a compound that releases ET), or a combination of 1 mM AVG and 10 μM ethephon. Three AVG concentrations (100 μM, 500 μM, and 1 mM) were tested initially, and 1 mM AVG was chosen as it was most effective in inhibiting seedling growth without any toxic effect; 10 μM ethephon was chosen as higher concentrations caused inhibition of root growth. To investigate if the effect of AVG on germination and seedling growth is mediated by changes in the levels of GA and ABA, both control and AVG-treated seeds were imbibed (25 seeds per plate per replicate, three replicates) with 50 µM GA3 (Sigma-Aldrich, St. Louis, MO, USA) or 50 μM fluridone (ABA biosynthesis inhibitor; Sigma-Aldrich) for 7 d.
Scoring of seed germination, which was defined by coleorhiza emergence through the seed coat, and measurement of the length of different seedling parts (embryo axis, coleoptile, primary root, and seminal root) were performed over a period of 7 d; the coleoptiles did not form any leaves during the study period. For gene expression and hormone (ABA, GA, and ET) level analyses, seeds were harvested (25 seeds per plate per replicate, three replicates) at 0, 1, 3, 5, and 7 days after imbibition (DAI) and separated into ‘endosperm’ (including aleurone and pericarp) and ‘embryo axis’ (including scutellum) at 0, 1, and 3 DAI, and into endosperm, coleoptile, and ‘root’ (including primary and seminal roots) at 5 and 7 DAI. The activity of starch-degrading enzymes, and the levels of starch and soluble sugars were also determined in the endosperm samples. To determine the origin of endospermic bioactive GA and its potential effect on the endospermic ABA level during imbibition, embryo axes were excised before the start of imbibition and the endospermic GA and ABA levels were determined at 5 and 7 DAI, when high GA accumulation was evident in the endosperm of control seeds (see below). All tissues were immediately frozen in liquid nitrogen after harvest and then stored at –80 °C until further use.
Ethylene measurement
The ET level was analyzed in the different tissues as described previously (Geisler-Lee et al., 2010) with minor modifications. Briefly, endosperm (1, 3, 5, and 7 DAI), embryo axis (1 and 3 DAI), and coleoptile and root (5 and 7 DAI) tissues were excised from imbibing seeds/seedlings and placed between two moist filter papers for 1 h to minimize the potential effects of wounding. Each sample was then transferred to a 10 ml glass tube containing a sterile 1 cm2 Whatman #1 filter paper moistened with 100 μl of sterile water to maintain hydration of the tissues, and the tubes were tightly closed with a rubber septum. After 3 h incubation, 3 ml of head space was removed from the tube with a gas-tight syringe and then injected onto a Bruker 450-GC gas chromatograph.
Identification of wheat starch-degrading and GAMYB genes and their specific primers
Available sequences of barley genes encoding starch-degrading enzymes (AMY, BAM, and AGL) and the GA signaling gene TaGAMYB were used to search the corresponding wheat homologs in the National Center for Biotechnology Information (NCBI) wheat UniGene database using the basic local alignment search tool (BLAST). The resulting UniGene sequences were then BLAST searched against the wheat genome sequence data in Ensembl Plants (http://plants.ensembl.org/Triticum_aestivum/) to identify the respective full-length sequences of the target genes and proteins. The resulting sequences of wheat starch-degrading and TaGAMYB genes were used as queries to blast search the respective orthologs in the NCBI database. Gene/homolog name assignment was based on their orthologs in barley and/or other cereal species (see Supplementary Table S1 at JXB online). Since wheat is a hexaploid, gene-specific primers (Supplementary Table S2) were designed from the conserved coding regions of the three homeologs of each target gene using Primer 3 software. Specificity of the primers and amplification efficiency of the PCRs (Supplementary Table S2) were evaluated as described previously (Mukherjee et al., 2015). Primer information for GA and ABA metabolism and signaling, and EXPA and Taβactin genes was described previously (Izydorczyk et al., 2018; Han et al., 2019).
RNA extraction and quantitative RT–PCR
Total RNA extraction, digestion of the RNA samples with DNase, cDNA synthesis and dilution, and subsequent quantitative PCR assays (in duplicate) were performed as described previously (Izydorczyk et al., 2018). The relative transcript levels of target genes were calculated, after normalization with the reference gene Taβactin, as described previously (Livak and Schmittgen, 2001). Analysis with NormFinder software (Andersen et al., 2004) revealed Taβactin as the most stably expressed reference gene out of three candidate reference genes (Taβactin, TaβTUB, and TaCDCP) analyzed.
Enzyme extractions and assays
Frozen endosperm samples were ground into fine powder in liquid nitrogen using a pre-chilled mortar and pestle. Extraction and assays for AMY and BAM were performed using the Ceralpha method (K-CERA kit, Megazyme International Ltd, Wicklow, Ireland) and the Betamyl-3 method (K-BETA3 kit, Megazyme International Ltd), respectively. Extraction and assay for α-glucosidase was performed as described previously (Sun and Henson, 1990) with minor modification. Briefly, the fine powder of each sample was homogenized with extraction buffer containing 50 mM sodium phosphate (pH 9.0), 1 M NaCl, and 1% Triton X-100. The homogenates were centrifuged at 6000 g for 10 min at room temperature. The extract (0.1 ml) was mixed with 1 ml of the substrate, which was prepared by dissolving 4-nitrophenyl α-d-glucopyranoside (Sigma-Aldrich) in 50 mM sodium acetate (pH 4.6), and then incubated in a water bath at 37 °C for 30 min. After stopping the reaction by adding 0.1 ml of 1 M NaOH, absorbance was determined at 420 nm. Assays for each sample were performed in duplicate.
Starch content measurements
Freeze-dried endosperm samples were ground to fine powder by a ball mill, and ~100 mg of the fine powder was mixed with 5 ml of ethanol (80%, v/v). The mixture was incubated in a water bath at ~80–85 °C for 5 min followed by addition of another 5 ml of ethanol (80%, v/v) and then centrifugation at 1800 g for 10 min. After removing the supernatant, the pellet was re-suspended in 10 ml of 80% ethanol and then centrifuged at 1800 g for 10 min. The pellet, after discarding the supernatant, was used for measuring total starch content using the amyloglucosidase–α-amylase method (K-TSTA kit, Megazyme International Ltd). Assays for each sample were performed in duplicate.
Analysis of sugar contents
Fine powder (~100 mg) of freeze-dried endosperm samples was mixed with 1 ml of 80% ethanol, and the mixture was immediately placed in a water bath at 70–75 °C for 10 min with constant mixing. After centrifugation at 3500 g for 10 min, the supernatant was transferred to a fresh tube. The pellet was re-suspended with 1 ml of 80% ethanol and incubated in a water bath at 70–75 °C for 10 min followed by centrifugation at 3500 g for 10 min, and these steps were repeated a second time. The supernatant from the three washes was pooled and completely dried under a stream of nitrogen gas. The residue was then mixed with 2 ml of deionized water followed by incubation in boiling water for 5 min. After filtering through a 0.45 µm GH Polypro (GHP) acrodisc syringe filter (Pall Corporation, Port Washington, NY, USA), the sample was analyzed with high-performance anion exchange chromatography (Dionex-ICS-5000; Dionex, Sunnyvale, CA, USA) equipped with a pulsed amperometric detector. Separation of soluble sugars (sucrose, maltose, glucose, and fructose) in the sample was performed with a CarboPac PA-1 column (4×250 mm i.d., Dionex) and a CarboPac PA-1 guard column (4×50 mm i.d., Dionex) at 30 °C using 100 mM NaOH as eluent A and 100 mM NaOH containing 400 mM sodium acetate as eluent B. A sample (10 µl) was injected and eluted at a flow rate of 1 ml min–1 with a linear gradient of 50% eluent B for 30 min followed by 100% eluent B for 1 min for washing and 5% eluent B for 15 min for equilibration. The sugars were identified and quantified relative to known standards. Data collection and peak analysis were performed using the Chromeleon 7.1 (Dionex) software.
Measurement of ABA and GA contents
Extraction of ABA and GA from the different tissues of dry and imbibing seeds and seedlings, and subsequent analysis of their levels by LC-ESI-MS/MS (Agilent 1260-6430; Agilent, Santa Clara, CA, USA) was performed exactly as described previously (Son et al., 2016; Izydorczyk et al., 2018).
Statistical analysis
Statistically significant differences between samples were determined using Student’s t-test at a probability value of P<0.05.
Results
Seed germination and seedling growth in response to inhibition of ethylene synthesis
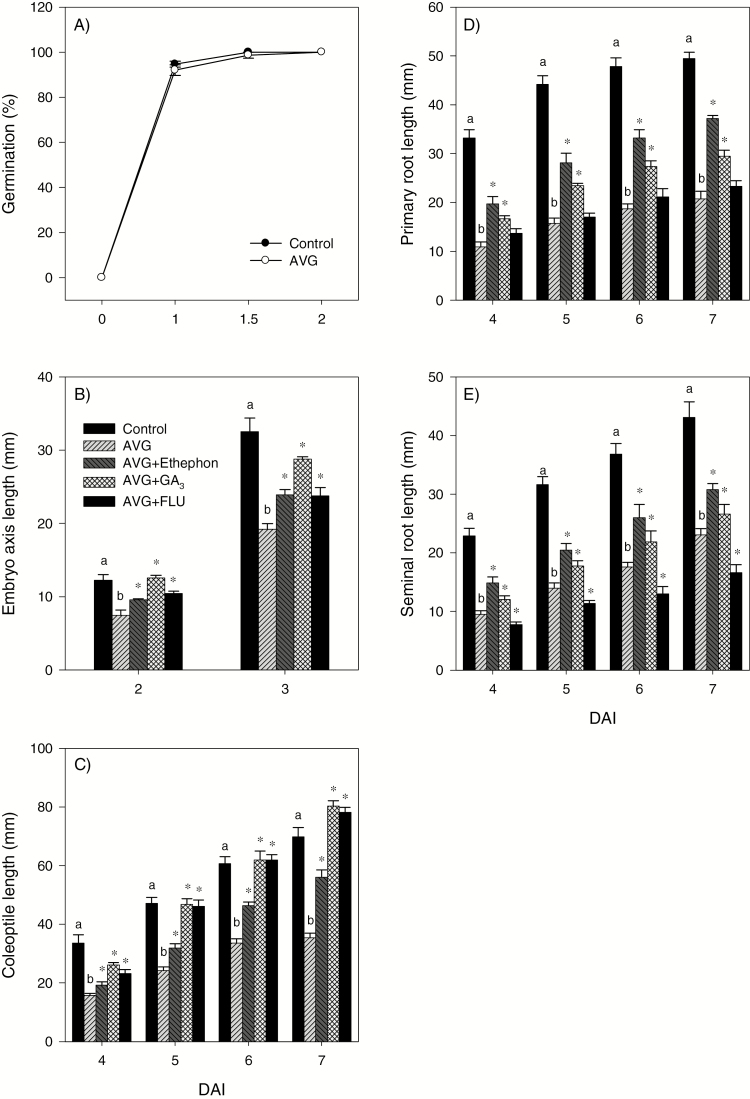
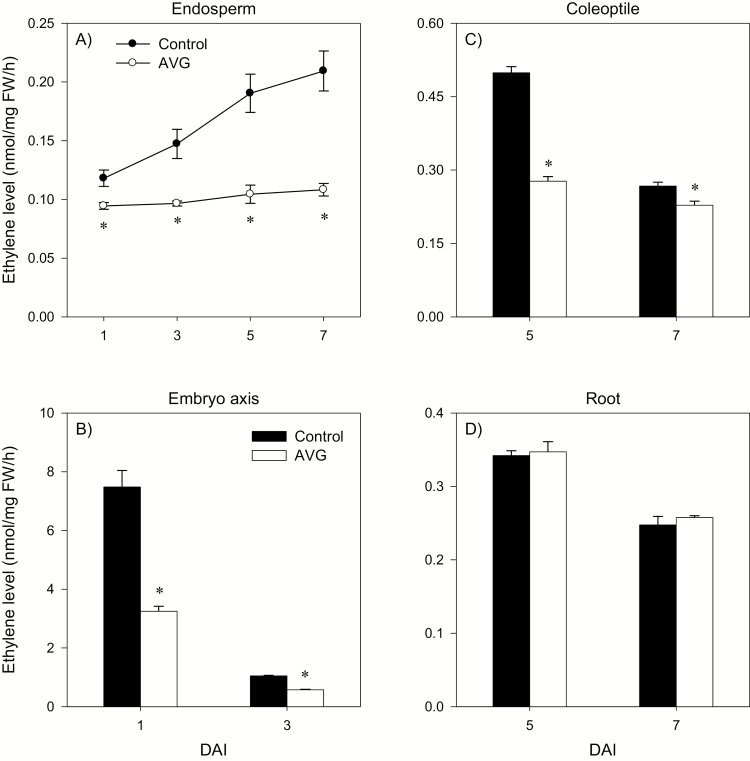
Treatment with AVG did not affect radicle protrusion, but reduced embryo axis, coleoptile, and primary and seminal root growth (Fig. 1). We detected ET production in all tissues studied; however, the embryo axis, especially at 1 DAI, produced much more ET than the other tissues (Fig. 2). AVG treatment reduced the ET level in all tissues with the exception of the root (Fig. 2). The inhibitory effect of AVG on embryo axis, coleoptile, and root growth was reversed by treatment with ethephon or GA3, or fluridone, except that the fluridone treatment either did not have any effect or caused further inhibition of primary and seminal root growth, respectively (Fig. 1B–E). The reversal of the AVG effect on growth by ethephon was partial as only 10 µM was used in order to avoid its inhibitory effect on root growth. Treatment of control seeds with GA3 increased embryo axis (2–3 DAI) and coleoptile (5–7 DAI) growth, but did not affect primary/seminal root growth except at 4 DAI (Supplementary Fig. S1). Fluridone also increased coleoptile growth (5–7 DAI) but repressed root growth. Treatment with ethephon (10 μM) did not affect growth in all tissues.
Fig. 1.
Effects of treatment with an ethylene biosynthesis inhibitor, ethephon, gibberellin, or an abscisic acid biosynthesis inhibitor on germination and seedling growth. Germination (A), and lengths of embryo axis (B), coleoptile (C), primary root (D), and seminal root (E) in response to seed imbibition with the ET biosynthesis inhibitor, aminoethoxyvinylglycine (AVG, 1 mM), AVG (1 mM)+ethephon (10 μM), AVG (1 mM)+GA3 (50 μM), or AVG (1 mM)+the ABA biosynthesis inhibitor, fluridone (FLU, 50 μM). Data are means ±SE, n=3, where n represents a batch of 20 seeds. Different letters indicate statistically significant differences between control and AVG-treated samples, while asterisks represent statistically significant differences between AVG treatment and treatment with AVG+ethephon, AVG+GA3, or AVG+FLU (P<0.05; Student’s t-test). DAI, day(s) after imbibition.
Fig. 2.
Ethylene levels in different seedling tissues. ET level in the endosperm (A), embryo axis (B), coleoptile (C), and root (D) in response to seed imbibition with AVG (1 mM). Data are means ±SE, n=3, where n represents a batch of eight tissues (for endosperm, embryo axis at 3 DAI, coleoptile, or root) or a batch of 20 tissues (for embryo axis at 1 DAI). Asterisks indicate statistically significant differences in ethylene levels between control and AVG-treated samples (P<0.05; Student’s t-test). DAI, day(s) after imbibition.
Transcriptional regulation of endospermic starch degradation
To determine if inhibition of ET production in the endosperm affects storage starch degradation, we examined the expression patterns of starch-degrading genes and activity of the corresponding enzymes, and starch and soluble sugars levels in the endosperm of imbibing seeds in response to AVG treatment.
Expression of α-amylase genes in the endosperm
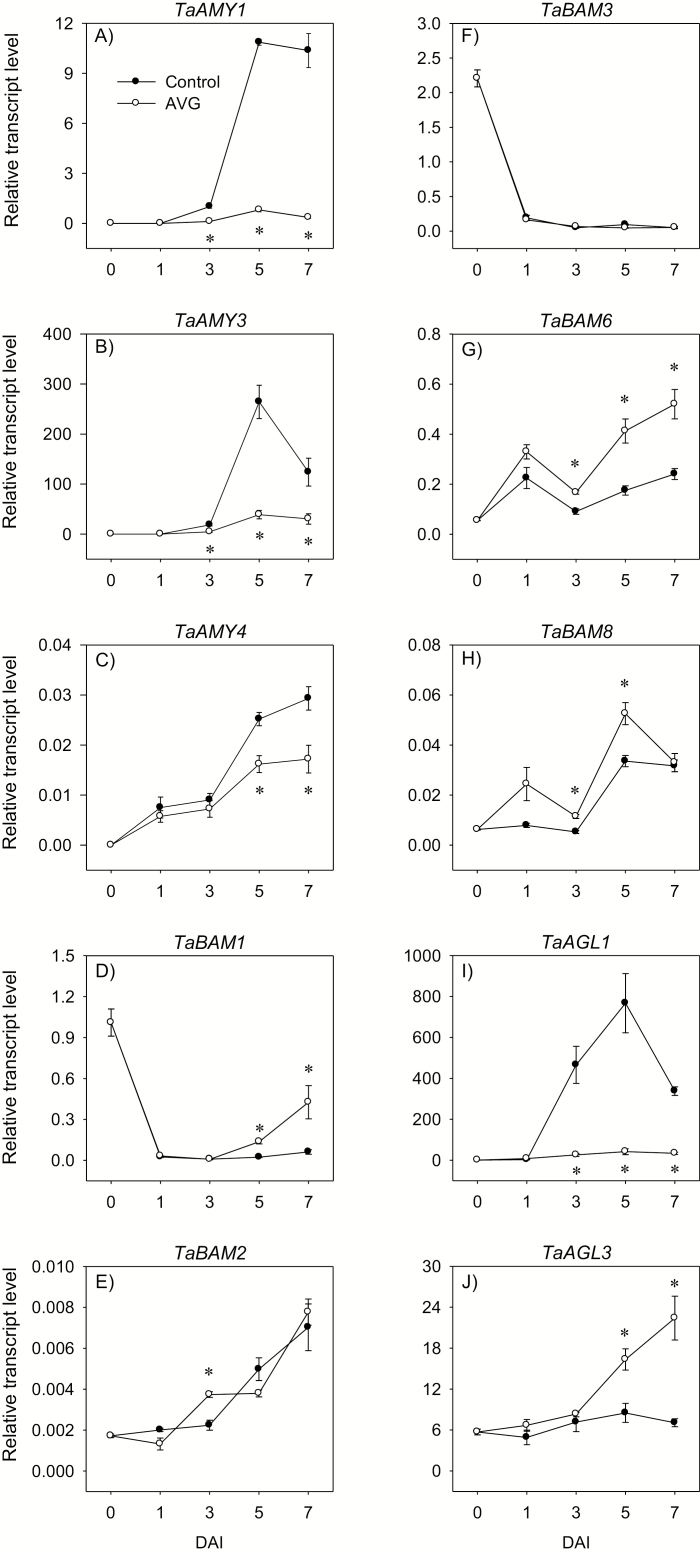
No or a minimal expression level of TaAMY genes was detected in the endosperm of dry seeds; however, their expression levels increased with imbibition in both control and AVG-treated seeds (Fig. 3A–C); TaAMY3 showed the highest magnitude of expression followed by TaAMY1 and then TaAMY4. However, AVG treatment decreased the expression levels of TaAMY genes; TaAMY1 (>9-fold) and TaAMY3 (>4-fold) following 3 DAI, and TaAMY4 (>1.6-fold) following 5 DAI.
Fig. 3.
Expression patterns of starch-degrading genes in the endosperm during imbibition. Relative transcript levels of TaAMY (A–C), TaBAM (D–H), and TaAGL (I, J) genes in AVG-treated samples and their respective controls. Transcript levels of TaAMY, TaBAM, and TaAGL genes were determined using Taβ-actin as a reference gene and expressed relative to the transcript levels of TaAMY1 in the control endosperm at 3 DAI, and the transcript levels of TaBAM1 and TaAGL1 in the control endosperm at 0 DAI, respectively, which were set to a value of 1. Data are means of three biological replicates ±SE. Asterisks indicate statistically significant differences in expression levels between control and AVG-treated samples (P<0.05; Student’s t-test). DAI, day(s) after imbibition.
Expression of β-amylase genes in the endosperm
The transcripts of all TaBAM genes were detected in the endosperm of dry seeds (Fig. 3D–H). High transcript levels of TaBAM1 and TaBAM3 were observed in dry seeds, and their transcript levels decreased substantially by 1 DAI in both AVG-treated and control samples, and remained at low levels afterwards. In contrast, the expression levels of TaBAM2, TaBAM6, and TaBAM8 exhibited gradual increases with imbibition in both AVG-treated and control seeds. AVG treatment did not affect the expression levels of TaBAM2 and TaBAM3 but up-regulated TaBAM1, TaBAM6, and TaBAM8 following 3 or 5 DAI (1.6- to 7-fold) (Fig. 3D, G, H).
Expression of α-glucosidase genes in the endosperm
The transcripts of TaAGL genes (TaAGL1 and TaAGL3) were detected in the endosperm of dry seeds (Fig. 3I, J). In the control samples, TaAGL1 expression showed a substantial increase with imbibition, resulting in a much higher level of expression than that observed for TaAGL3 (up to 90-fold). However, AVG treatment repressed TaAGL1 expression (>10-fold) following 3 DAI. Almost a similar level of TaAGL3 expression was maintained during the entire imbibition period, and AVG treatment enhanced its expression level (2- to 3-fold) following 5 DAI (Fig. 3J).
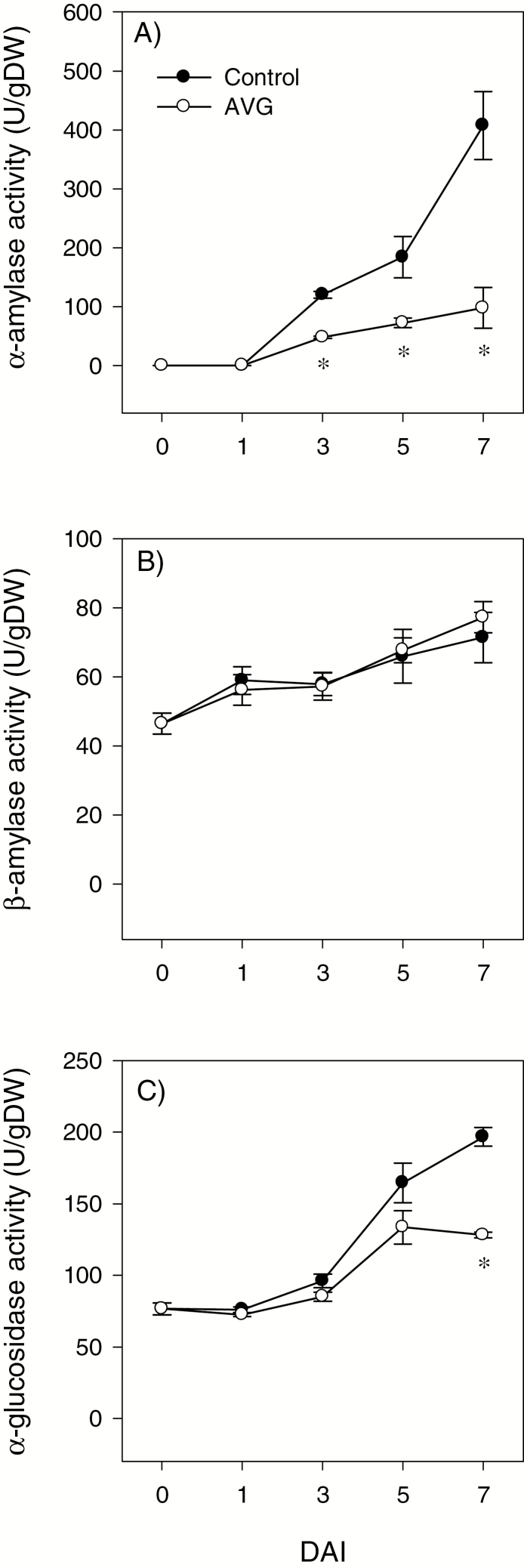
Activities of starch-degrading enzymes in the endosperm
The activities of AMY, BAM, and AGL were detected in the endosperm of dry seeds (Fig. 4). Although AMY activity increased with imbibition, it was inhibited by AVG treatment (>2-fold) following 1 DAI (Fig. 4A). The BAM activity detected in the endosperm of dry seeds showed a slight increase during the entire period studied, and it was not affected by treatment with AVG (Fig. 4B). The activity of AGL detected in the endosperm of dry seeds was also maintained at a similar level until 3 DAI, after which its activity increased (1.5- to 2-fold) in both samples through to the end of the period studied (Fig. 4C). However, AVG treatment repressed the AGL activity at 7 DAI.
Fig. 4.
Activities of starch-degrading enzymes in the endosperm during imbibition. Activities of α-amylase (A), β-amylase (B), and α-glucosidase (C) in AVG-treated samples and their respective controls. Data are means of three biological replicates ±SE. Asterisks indicate statistically significant differences in enzyme activities between control and AVG-treated samples (P<0.05; Student’s t-test). DAI, day(s) after imbibition.
Endospermic dry weight and starch and soluble sugar contents
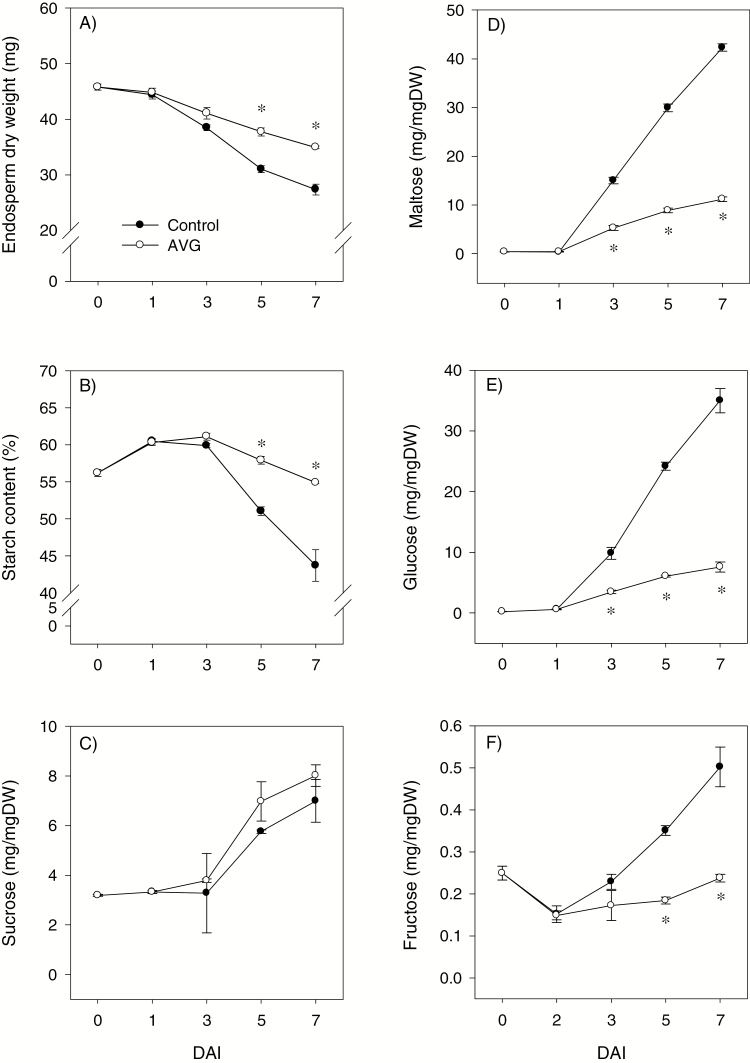
Endospermic dry weight declined with imbibition in both control and AVG-treated seeds; however, the AVG-treated seeds exhibited higher dry weight at 5 and 7 DAI than the control seeds (Fig. 5A). Consistent with this result, endospermic starch content exhibited a decrease with imbibition in both seed samples; however, its level was higher in AVG-treated seeds than in the respective controls at 5 and 7 DAI (Fig. 5B). Soluble sugars including maltose, glucose, and fructose were determined in the endosperm of dry seeds, and their level increased (2- to 157-fold) with imbibition (Fig. 5D–F). The levels of maltose and glucose were reduced by AVG treatment following 3 DAI (2.8- to 4.6-fold), while the reduction of fructose level (over ~2-fold) due to AVG treatment was evident only after 5 DAI. The endospermic sucrose level, however, showed a gradual increase with imbibition and it was not affected by AVG treatment (Fig. 5C).
Fig. 5.
Endosperm dry weight and starch and soluble sugar contents during imbibition. Dry weight (A), and contents of starch (B), sucrose (C), maltose (D), glucose (E), and fructose (F) in AVG-treated samples and their respective controls. Data are means of three biological replicates ±SE. Asterisks indicate statistically significant differences in dry weight and starch and soluble sugar contents between control and AVG-treated samples (P<0.05; Student’s t-test). DAI, day(s) after imbibition.
Transcriptional regulation of ABA/GA balance in the endosperm
To determine if the effects of inhibition of ET synthesis on endospermic starch degradation are associated with changes in ABA/GA balance, we investigated the expression patterns of ABA and GA metabolism and signaling genes, and measured ABA and GA levels in the endosperm of imbibing seeds in response to AVG treatment.
Transcriptional regulation of GA metabolism and signaling in the endosperm
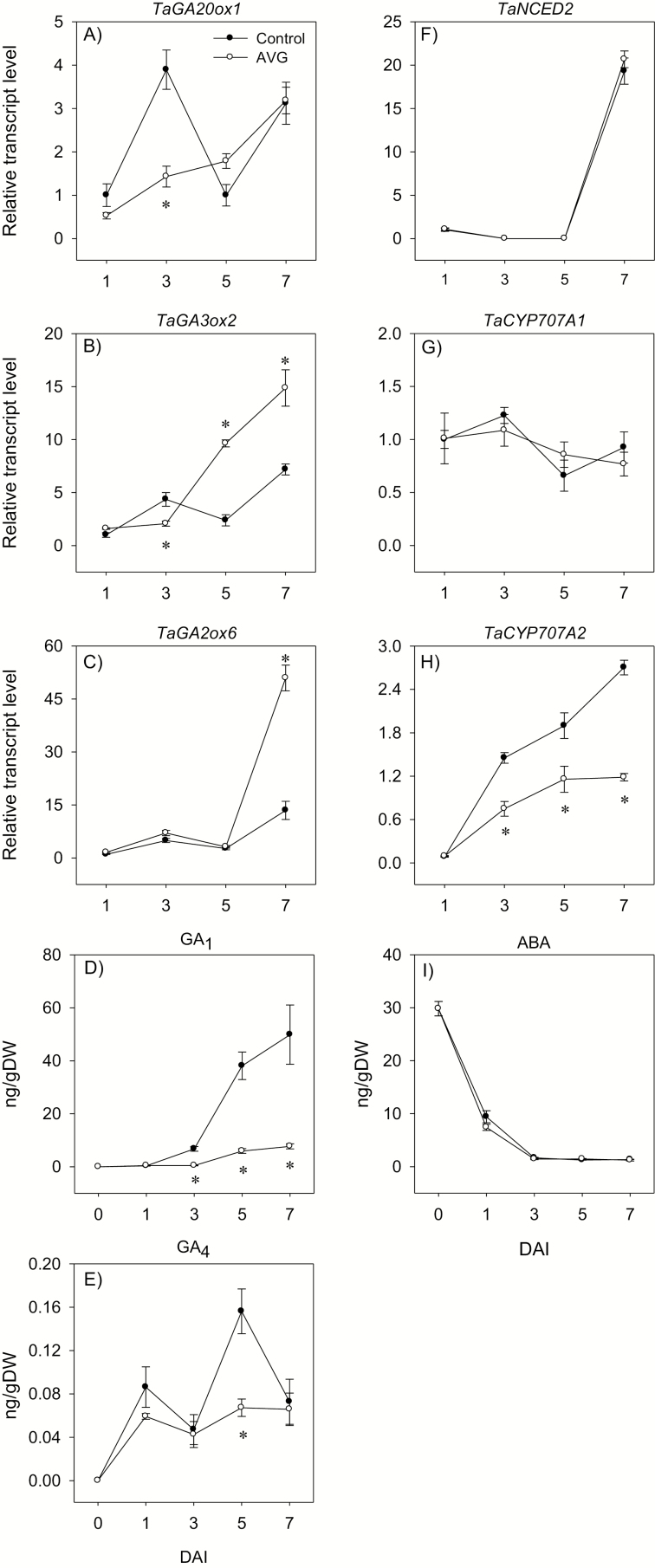
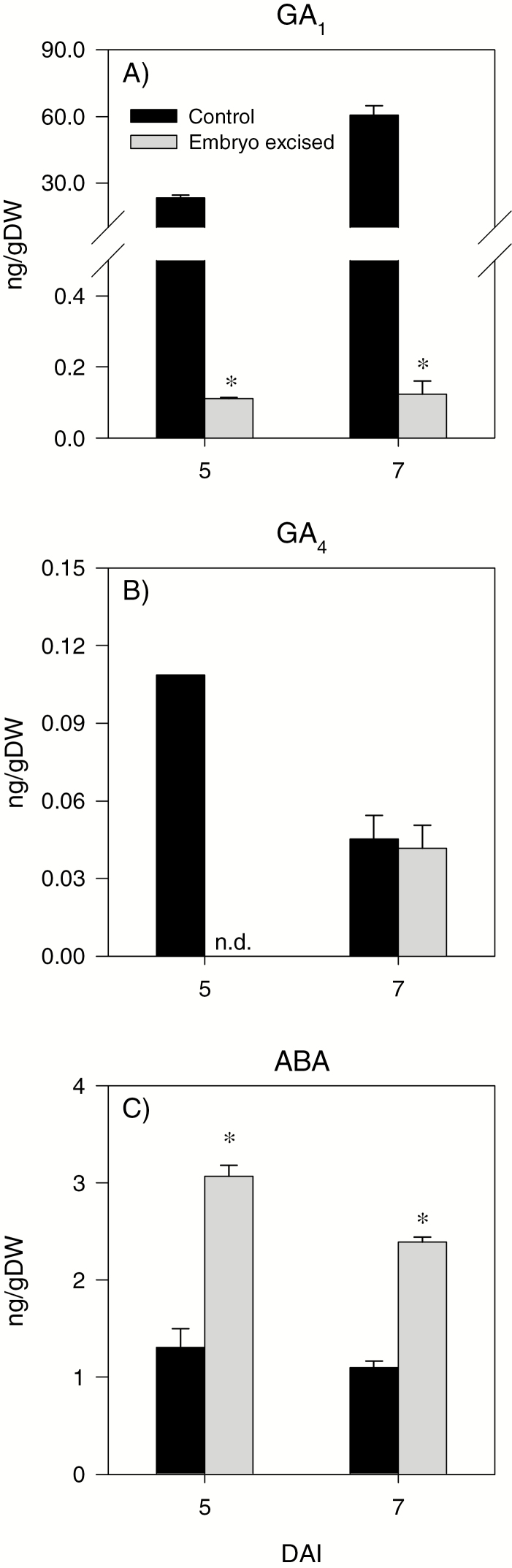
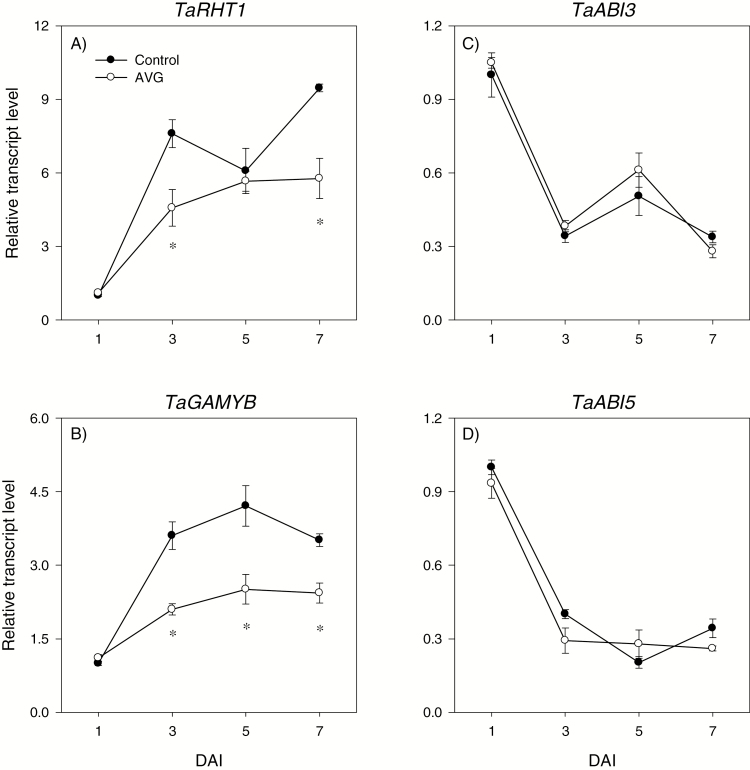
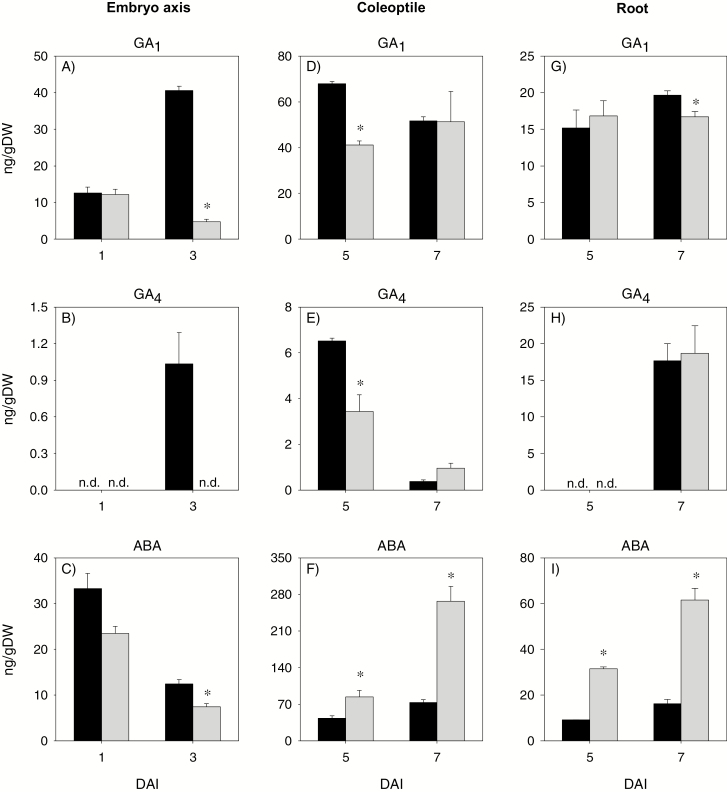
A similar expression level of the GA biosynthetic gene TaGA20ox1 was evident between the endosperms of control and AVG-treated seeds, except for the transient increase observed in the control samples at 3 DAI (Fig. 6A). The expression level of TaGA3ox2 increased with imbibition in both control and AVG-treated seeds; however, endosperms of AVG-treated seeds exhibited a higher expression level (>2-fold) following 5 DAI (Fig. 6B). Furthermore, AVG treatment led to an ~4-fold induction in the expression level of the GA catabolic gene GA2ox6 at 7 DAI (Fig. 6C). Bioactive GA1 and GA4 were detected in the endosperm of imbibed but not dry seeds, and their levels increased with imbibition (Fig. 6D, E). However, AVG treatment caused a substantial reduction in the endospermic GA1 level (6.5- to 15-fold) as compared with that observed in the control seeds following 3 DAI (Fig. 6D). The level of GA4 was also reduced by AVG treatment (>2-fold) at 5 DAI (Fig. 6E). Embryo axis excision before the start of imbibition led to a marked reduction in the accumulation of endospermic bioactive GA during the later phases of imbibition, at 5 and/or 7 DAI (Fig. 7A, B). The expression level of the endospermic GA signaling gene TaRHT1, which encodes the wheat DELLA protein, showed an increase with imbibition in both control and AVG-treated seeds; however, AVG treatment repressed its expression at 3 and 7 DAI (Fig. 8A). The expression level of the other GA signaling gene, TaGAMYB, also showed a gradual increase with imbibition in both seed samples (Fig. 8B), but AVG treatment repressed its expression following 3 DAI.
Fig. 6.
Expression patterns of gibberellin and abscisic acid metabolism genes, and endogenous gibberellin and abscisic acid levels in the endosperm during imbibition. Relative transcript levels of TaGA20ox1 (A), TaGA3ox2 (B), TaGA2ox6 (C), TaNCED2 (F), and TaCYP707A genes (G and H) in AVG-treated samples and their respective controls. Transcript levels were determined exactly as described in Fig. 3 and expressed relative to the transcript levels of TaGA20ox1, TaGA3ox2, TaGA2ox6, TaNCED2, and TaCYP707A1 in the control endosperm at 0 DAI, which were set to a value of 1. Endospermic GA1 (D), GA4 (E), and ABA (I) levels in AVG-treated samples and their respective controls. Data are means of three biological replicates ±SE except that the GA1 data for the control samples at 5 DAI are means of five biological replicates ±SE. Asterisks indicate statistically significant differences in expression levels or hormone contents between control and AVG-treated samples (P<0.05; Student’s t-test). DAI, day(s) after imbibition.
Fig. 7.
Endospermic gibberellin and abscisic acid levels in response to embryo excision before the start of imbibition. Endospermic GA1 (A), GA4 (B), and ABA (C) levels of seeds imbibed for 5 d and 7 d with embryo axis (control) and no embryo axis (embryo excised). Data are means of three biological replicates ±SE. Asterisks indicate statistically significant differences in hormone contents between endosperm samples imbibed with and with no embryo (P<0.05; Student’s t-test). n.d., not detected.
Fig. 8.
Expression patterns of gibberellin and abscisic acid signaling genes in the endosperm during imbibition. Transcript levels of TaRHT1 (A), TaGAMYB (B), TaABI3 (C), and TaABI5 (D) were determined in AVG-treated samples and their respective controls exactly as described in Fig. 3 and expressed relative to their respective transcript levels in the control endosperm at 0 DAI, which were set to a value of 1. Data descriptions are as shown in Fig. 3. DAI, day(s) after imbibition.
Transcriptional regulation of ABA metabolism and signaling in the endosperm
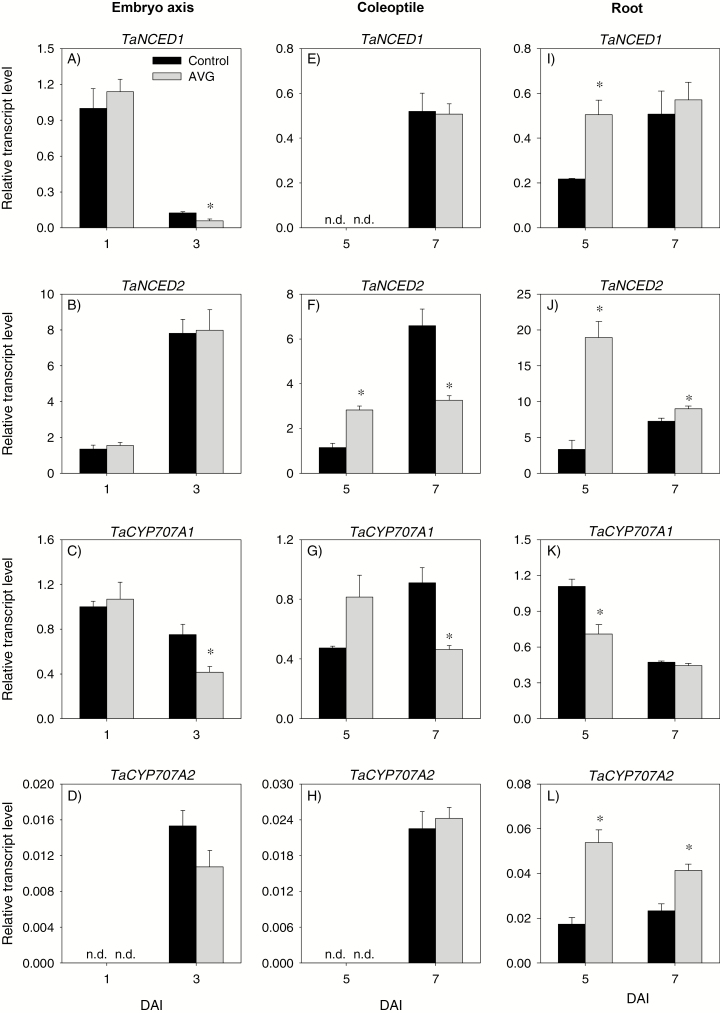
Expression of the ABA biosynthetic gene TaNCED1 was below the detectable level in the endosperm of imbibing control and AVG-treated seeds (data not shown). Similarly, no or a minimal expression level of TaNCED2 was detected in both seed samples except that drastic increases in its expression level were observed at 7 DAI (Fig. 6F). The expression level of one of the ABA catabolic genes, TaCYP707A1, remained constant during imbibition, and it was not affected by AVG treatment, while that of TaCYP707A2 increased with imbibition in both seed samples and its expression level was decreased by AVG treatment following 3 DAI (Fig. 6G, H). A high level of ABA was detected in the endosperm of dry seeds, but it decreased to a very low level in both control and AVG-treated seed samples as imbibition progressed, and no effect of AVG treatment was evident (Fig. 6I). Embryo axis excision led to a substantial increase in endospermic ABA levels during the later phases of imbibition, at 5 and 7 DAI (Fig. 7C). The expression levels of endospermic genes encoding the downstream ABA signaling transcription factors, TaABI3 and TaABI5, decreased with imbibition, and there was no apparent effect of AVG on their expression levels (Fig. 8C, D).
Transcriptional regulation of ABA/GA balance in post-germination seedlings
To determine if the effects of inhibition of ET synthesis on seedling growth are associated with changes in ABA/GA balance in non-nurturing tissues, we investigated the expression patterns of ABA and GA metabolism and signaling genes, and measured ABA and GA levels in the embryo axis, coleoptile, and root tissues in response to AVG treatment.
Transcriptional regulation of GA and ABA metabolism and signaling in the embryo axis
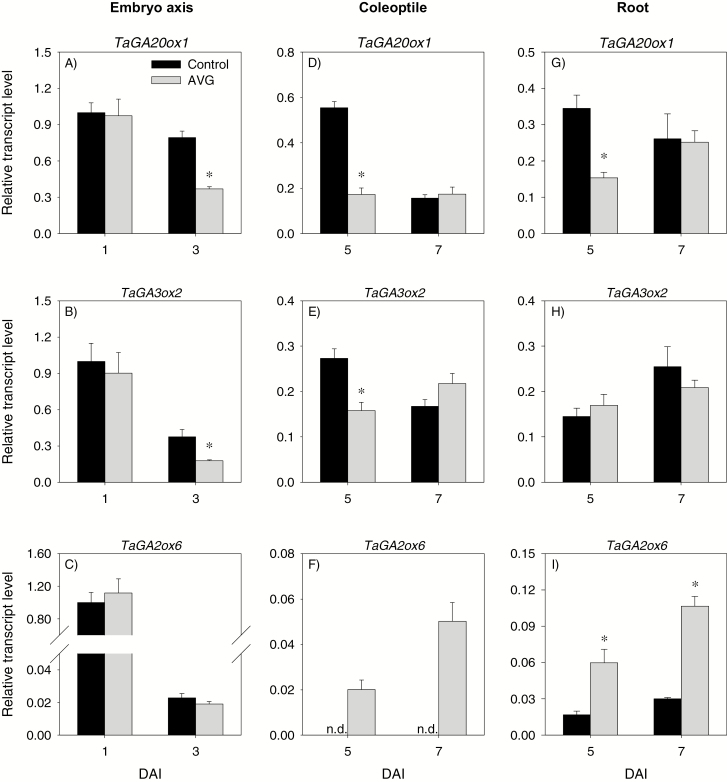
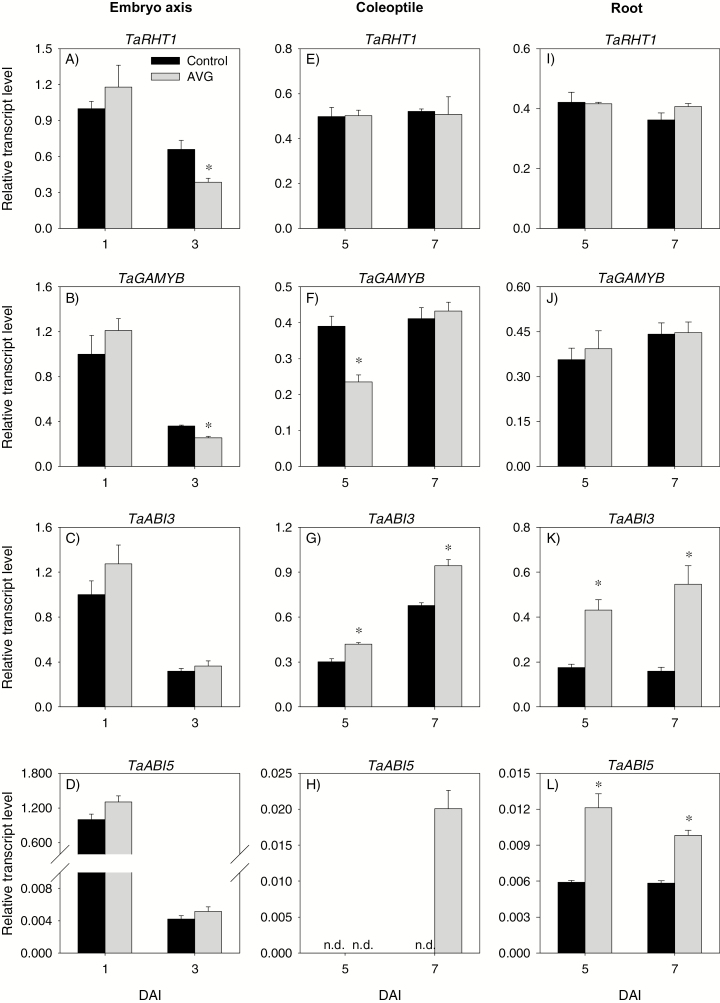
There was no differential expression of GA biosynthetic (TaGA20ox1 and TaGA3ox2) and catabolic (TaGA2ox6) genes between the control and AVG-treated embryo axis at 1 DAI (Fig. 9A–C). However, AVG treatment decreased (>2-fold) the expression levels of TaGA20ox1 and TaGA3ox2 in the embryo axis at 3 DAI with no effect on that of TaGA2ox6. Consistently, the level of GA1 in 1 DAI embryo axis was not affected by AVG treatment, while its level in the 3 DAI embryo axis exhibited a substantial decrease (8.5-fold) (Fig. 10A). The other bioactive GA, GA4, was also detected in 3 DAI embryo axis, but its level decreased to an undetectable level due to AVG treatment (Fig. 10B). No effect of AVG treatment was evident in the expression levels of TaRHT1 and TaGAMYB in 1 DAI embryo axis; however, AVG treatment decreased the expression levels of both genes in the 3 DAI embryo axis tissue (Fig. 11A, B).
Fig. 9.
Expression patterns of gibberellin metabolism genes in the embryo axis, coleoptile, and root of post-germination seedlings. Relative transcript levels of TaGA20ox1 (A, D, and G), TaGA3ox2 (B, E, and H), and TaGA2ox6 (C, F, and I) in AVG-treated samples and their respective controls. Transcript levels of each gene were determined exactly as described in Fig. 3 and expressed relative to their respective transcript level in the control embryo axis at 1 DAI, which was set to a value of 1. Data descriptions are as shown in Fig. 3. DAI, day(s) after imbibition; n.d., not detected.
Fig. 10.
Gibberellin and abscisic acid levels in the embryo axis, coleoptile, and root of post-germination seedlings. GA1 (A, D, and G), GA4 (B, E, and H), and ABA (C, F, and I) in AVG-treated samples and their respective controls. Data are means of three biological replicates ±SE. Asterisks indicate statistically significant differences in hormone contents between control and AVG-treated samples (P<0.05; Student’s t-test). DAI, day(s) after imbibition; n.d., not detected.
Fig. 11.
Expression patterns of gibberellin and abscisic acid signaling genes in the embryo axis, coleoptile, and root of post-germination seedlings. Transcript levels of TaRHT1 (A, E, and I), TaGAMYB (B, F, and J), TaABI3 (C, G, and K), and TaABI5 (D, H, and L) were determined in AVG-treated samples and their respective controls exactly as described in Fig. 3 and expressed relative to their respective transcript levels in the control embryo axis at 1 DAI, which were set to a value of 1. Data descriptions are as shown in Fig. 3. DAI, day(s) after imbibition; n.d., not detected.
The expression levels of all ABA metabolism genes in 1 DAI embryo axis were unaffected by AVG treatment (Fig. 12A–D), leading to no change in ABA level (Fig. 10C). In contrast, AVG treatment led to reductions in the expression levels of TaNCED1 and TaCYP707A1 genes, and the ABA level in 3 DAI embryo axis. AVG treatment did not affect the expression levels of ABA signaling genes, TaABI3 and TaABI5, in the embryo axis at either time point (Fig. 11C, D).
Fig. 12.
Expression patterns of abscisic acid metabolism genes in the embryo axis, coleoptile, and root of post-germination seedlings. Relative transcript levels of TaNCED1 (A, E, and I), TaNCED2 (B, F, and J), TaCYP707A1 (C, G, and K), and TaCYP707A2 (D, H, and L) in AVG-treated samples and their respective controls. Transcript levels of NCED and CYP707A genes were determined exactly as described in Fig. 3 and expressed relative to TaNCED1 and TaCYP707A1 transcript levels in the control embryo axis at 1 DAI, respectively, which were set to a value of 1. Data descriptions are as shown in Fig. 3. DAI, day(s) after imbibition; n.d., not detected.
Transcriptional regulation of GA and ABA metabolism and signaling in the coleoptile
Treatment with AVG repressed the expression levels of TaGA20ox1 and TaGA3ox1 in the coleoptile at 5 DAI but increased that of TaGA2ox6, leading to reduction of GA1 and GA4 levels (Figs 9D–F, 10D, E). However, no effect of AVG treatment was evident on the expression levels of TaGA20ox1 and TaGA3ox1, and bioactive GA levels in the coleoptile at 7 DAI although up-regulation of TaGA2ox6 was evident. AVG treatment did not affect the expression levels of TaRHT1 in the coleoptile at either time point; however, it repressed that of TaGAMYB at 5 DAI (Fig. 11E, F).
With respect to ABA metabolism genes, treatment with AVG enhanced the expression level of TaNCED2 in the coleoptile at 5 DAI, with no effect on the expression levels of the other genes (Fig. 12E–H). Consistently, AVG treatment led to an increase in ABA level (Fig. 10F). The AVG treatment, however, caused reduction in the expression levels of TaNCED2 (2-fold) and TaCYP707A1 (2-fold) in the coleoptile at 7 DAI (Fig. 12F, G), and this effect was associated with an increase in ABA level (>3-fold) (Fig. 10F). Treatment with AVG led to an enhanced expression level of TaABI3 at both time points and of TaABI5 at 7 DAI (Fig. 11G, H).
Transcriptional regulation of GA and ABA metabolism and signaling in the root
The expression levels of TaGA20ox1 and TaGA3ox1 in the root were not affected by AVG treatment except that the expression of TaGA20ox1 was repressed at 5 DAI (Fig. 9G, H). The treatment, however, enhanced the expression level of TaGA2ox6 (>3-fold) at 5 and 7 DAI (Fig. 9I). Despite these results, AVG treatment did not affect root GA1 or GA4 levels except the slight reduction observed in GA1 level at 7 DAI (Fig. 10G, H). Treatment with AVG did not have an effect on the expression levels of root TaRHT1 and TaGAMYB at either time point (Fig. 11I, J).
Treatment with AVG enhanced the expression levels of root TaNCED1 at 5 DAI (>2-fold), and of TaNCED2 (1.2- to 5.7-fold) and TaCYP707A2 (1.8- to 3.1-fold) at both 5 and 7 DAI, but repressed that of TaCYP707A1 at 5 DAI (Fig. 12I–L). Root ABA level at 5 and 7 DAI exhibited an increase (>3-fold) in response to AVG treatment (Fig. 10I). The AVG treatment also enhanced the expression levels of root TaABI3 and TaABI5 genes (1.7- to 3.4-fold) at both 5 and 7 DAI (Fig. 11K, L).
Expression analysis of cell elongation genes
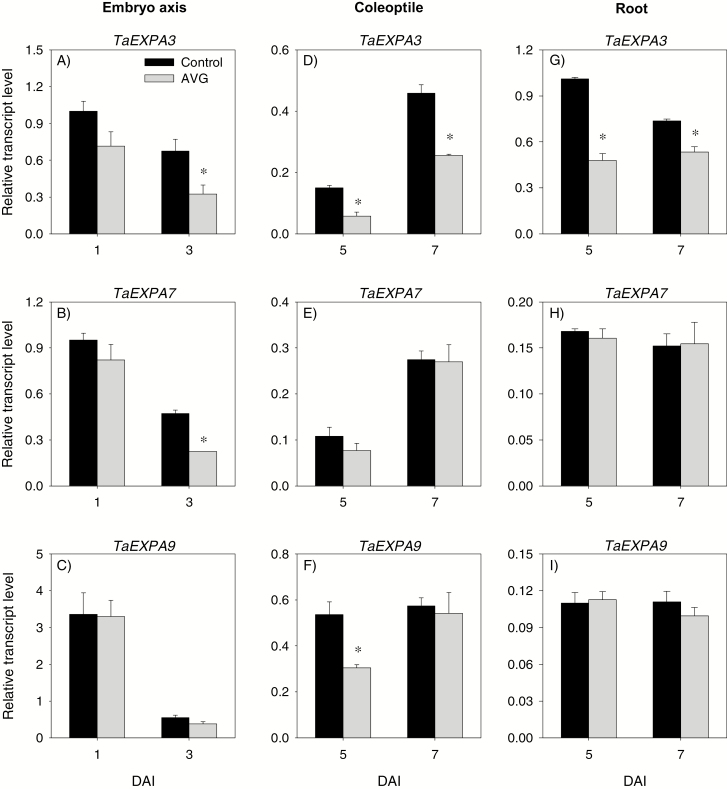
To determine if the effect of the reduced ET level on seedling growth is mediated by transcriptional regulation of cell elongation, we monitored the expression patterns of TaEXPA genes. Our analysis showed reduction in the expression level of TaEXPA3 in the embryo axis at 3 DAI, and in the coleoptile and root tissues at both 5 and 7 DAI in response to AVG treatment (Fig. 13A, D, G). The other two TaEXPA genes, TaEXPA7 and TaEXPA9, showed AVG-mediated down-regulation only in 3 DAI embryo axis and 5 DAI coleoptile, respectively (Fig. 13B, F).
Fig. 13.
Expression patterns of α-expansin genes in the embryo axis, coleoptile, and root of post-germination seedlings. Transcript levels of TaEXPA3 (A, D, and G), TaEXPA7 (B, E, and H), and TaEXPA9 (C, F, and I) were determined in AVG-treated samples and their respective controls exactly as described in Fig. 3 and expressed relative to the transcript level of TaEXPA3 in the control embryo axis at 1 DAI, which was set to a value of 1. Data descriptions are as shown in Fig. 3. DAI, day(s) after imbibition.
Discussion
Seed germination and seedling growth are regulated mainly by GA and ABA; however, other plant hormones such as ET also control these early developmental processes. This study investigated the molecular mechanisms underlying the role of ET in regulating ABA/GA balance, and thereby seed germination, and starch degradation and seedling growth in wheat. Although ET promotes seed germination in dicots (Petruzzelli et al., 2000; Matilla and Matilla-Vázquez, 2008; Linkies et al., 2009), inhibition of its synthesis did not affect radicle protrusion in wheat (Fig. 1A) as observed in other monocots such as barley and rice (Locke et al., 2000; Gianinetti et al., 2007). Mobilization of endospermic starch is required to provide energy for seedling growth in cereals (Zeeman et al., 2010), and the expression of starch-degrading genes such as AMYs and activity of the corresponding enzymes are reported to increase with seed imbibition (Perata et al., 1992; Guglielminetti et al., 1995; Sun et al., 2018). Our data revealed that inhibition of ET synthesis during imbibition represses the expression of endospermic TaAMY genes (TaAMY1, TaAMY3, and TaAMY4) and the TaAGL1 gene, together with the activity of AMY and AGL, leading to inhibition of storage starch degradation and reduction in the levels of soluble sugars including maltose, glucose, and fructose (Figs 3–5). Since previous studies implicated ET in the regulation of seedling growth (Matilla 2000; Yin et al., 2017), the association of these effects of reduced endospermic ET level with decreases in embryo axis, coleoptile, and root growth (Fig. 1B–E) indicate that ET regulates seedling growth in wheat partly through transcriptional control of storage starch degradation. Inhibition of ET synthesis; however, did not alter BAM activity despite causing up-regulation of specific TaBAM genes (TaBAM1, TaBAM6, and TaBAM8) (Figs 3, 4), indicating that these genes are regulated post-transcriptionally. The concomitant gradual increases in the levels of their expression during imbibition with that of BAM activity are indicative of the involvement of TaBAM6 and TaBAM8, and to a much lesser extent TaBAM2, in storage starch degradation. However, the activity of BAM during imbibition was markedly lower than that of AMY, indicating its minor role in storage starch degradation (Fig. 4A, B). Consistently, mutant seeds deficient in BAM activity can complete their germination and produce normal seedlings (Kihara et al., 1999; Kaneko et al., 2000).
GA and ABA act as primary regulators of starch degradation in cereal seeds through their antagonistic action (Gubler et al., 1995; Gómez-Cadenas et al., 1999, 2001), and ET has been reported to interact with GA and ABA in other plant systems (Kende et al., 1998; Choi, 2007). In accordance with our recent report (Izydorczyk et al., 2018), no bioactive GA but a high level of ABA was detected in the endosperm of dry seeds, and the level of ABA decreased to a very low level during germination (Fig. 6D, E, I). Such changes in GA and ABA levels have also been reported either in the embryo axis or at the whole-seed level during seed germination in wheat and other species (Ogawa et al., 2003; Gubler et al., 2008; Izydorczyk et al., 2018). Endospermic bioactive GAs were detected only after completion of germination, which was determined by the emergence of coleorhiza through the seed coat, and their levels increased with imbibition (Fig. 6D, E). Inhibition of ET synthesis during imbibition did not affect either the endospermic ABA level or the expression levels of ABA signaling genes TaABI3 and TaABI5 (Figs 6I, 8C, D); however, it decreased bioactive GA levels and GA sensitivity, as evidenced by down-regulation of TaGAMYB (Figs 6, 8B). These results along with the promotion of embryo axis and/or coleoptile growth, which is fueled through mobilization of storage starch, by GA3, or by fluridone (Supplementary Fig. S1) indicate the significance of endospermic GA level and sensitivity in mediating ET-induced modulation of the ABA/GA balance and thereby transcriptional regulation of starch degradation. In agreement with this, inhibition of ET synthesis alters the expression patterns of GA biosynthetic genes, TaGA20ox2 and TaGA3ox2, and thereby represses seed germination (Iglesias-Fernández and Matilla, 2010). The up-regulation of TaGA3ox2 in the endosperm where bioactive GA levels were reduced due to inhibition of ET synthesis (Fig. 6B) shows its negative feedback regulation by GA as observed for specific GA3ox genes in Arabidopsis (Matsushita et al., 2007), while the repression of endospermic TaRHT1 (Fig. 8A), which encodes wheat DELLA protein that acts as a negative regulator of GA signaling, suggests either its post-transcriptional regulation or the occurrence of GA signaling through a DELLA-independent pathway (Cao et al., 2006).
To gain insights into the origins of ABA and GA detected in the endosperm, we performed experiments involving embryo axis excision prior to imbibition. Detection of GA1 and GA4 in the endosperm samples imbibed with no embryo axis (Fig. 7A, B) is indicative of their synthesis in the aleurone, while the substantial reduction of their levels, especially that of GA1, as compared with the control (imbibed with embryos) implicates that GA transport from non-endospermic tissues accounts for the majority of the bioactive GA detected in the endosperm, and thereby regulation of storage starch degradation. Our results are consistent with a report that implicated the scutellum/embryo axis as the main site of GA synthesis in germinating wheat seeds (Appleford and Lenton, 1997). In contrast, Kaneko et al. (2003) suggested that no GA synthesis occurs in the aleurone of germinating rice seeds. However, their conclusion was merely based on expression analysis of GA biosynthesis genes. Embryo axis excision prior to imbibition, on the other hand, increased the endospermic ABA level (Fig. 7C), showing that most of the ABA accumulated in the endosperm is synthesized in situ, and this induction of the ABA level is consistent with a reduction in the levels of GA, which regulates ABA synthesis negatively (Seo et al., 2009).
ET acts as a positive regulator of shoot growth in cereal seedlings (Furukawa et al., 1997; Locke et al., 2000; Yin et al., 2017). Consistently, inhibition of ET synthesis caused decreases in embryo axis and coleoptile growth, and these effects were reversed by treatments with ethephon or exogenous GA or fluridone (Fig. 1B, C). While treatment with GA3 or fluridone fully reversed coleoptile growth, ethephon caused only partial reversal and this result, along with the absence of any effect of ethephon on coleoptile growth in the control samples (Supplementary Fig. S1), indicates that the ethephon concentration we used was not sufficient to increase ET to the level where it promotes further coleoptile growth. It is well documented that GA promotes growth via degrading the growth-repressing DELLA proteins (Olszewski et al., 2002; Achard et al., 2009) and thereby activating expansins (Cho and Cosgrove, 2004), whereas ABA inhibits growth via repressing cell wall extensibility (da Silva et al., 2008). Previous studies in other plant systems have shown that ET regulates growth via modulating the balance between these two hormones (Kende et al., 1998; Choi, 2007). In the present study, inhibition of ET synthesis did not affect the expression levels of ABA and GA metabolism and signaling genes, and ABA and GA levels in the embryo axis at the early stage (1 DAI) (Figs 9–12). However, it caused reductions in the ABA level, potentially through repression of TaNCED1, with no effect on ABA sensitivity, as evidenced by the expression patterns of ABI3 and ABI5, at the later stage (3 DAI) (Figs 10–12). Despite these results, repression of TaEXPA3 and TaEXPA7 and inhibition of embryo axis growth (Figs 1B, 13A, B) were evident. These results, along with the prevalence of reduced bioactive GA levels and sensitivity, mainly through repressions of TaGA20ox1 and TaGA3ox2, and TaGAMYB, respectively (Figs 9–11), highlight that ET-mediated transcriptional regulation of GA biosynthesis and signaling modulates ABA/GA balance, and thereby cell wall expansion and embryo axis growth.
With respect to the coleoptiles, reduction of the ET level at 5 DAI caused a decrease in bioactive GA levels and sensitivity via altering the expression levels of GA metabolism genes and repressing TaGAMYB while enhancing ABA content and sensitivity, mainly through up-regulation of TaNCED2 and TaABI3, respectively (Figs 9–12). These results together with the repression of TaEXPA3 and TaEXPA9 and reduction of coleoptile growth (Figs 1C, 13D, F) demonstrate that transcriptional control of GA and ABA metabolism and signaling by ET acts as a primary regulator of ABA/GA balance, and thereby cell wall expansion and coleoptile growth. However, as the coleoptile grew further, inhibition of ET synthesis increased the ABA level and sensitivity, mainly through down-regulating TaCYP707A1 and up-regulating TaABI3 and TaABI5, respectively, with no effects on bioactive GA levels and expression of GA signaling genes. These observations along with repression of TaEXPA3 indicate the significance of transcriptional regulation of ABA catabolism and signaling in mediating ET-induced modulation of ABA/GA balance, and thereby cell wall expansion and coleoptile elongation at the later seedling growth stage.
Both exogenous ET and inhibition of ET synthesis have been reported to repress radicle/root growth in rice seedlings (Gianinetti et al., 2007; Ma et al., 2014). Likewise, inhibition of ET synthesis caused reduction in root growth (Fig. 1D, E). This effect was reversed by treatment with ethephon or GA but not by fluridone, which reportedly represses root elongation (Supplementary Fig. S1, Spollen et al., 2000; Han et al., 2004). The detection of a higher ABA level in AVG-treated roots despite the absence of any change in root ET level (Figs 2D, 10I) indicates that the root ABA level is regulated by ET released from the embryo axis and/or a portion of the ABA detected in the root is transported from the coleoptile, where ABA accumulation was evident (Fig. 10F). The enhancement of the ABA level in AVG-treated roots along with up-regulation of TaABI3 and TaABI5 also explains the transcriptional induction of TaNCED genes and TaCYP707A2 as these genes are under positive feedback and feedforward regulation by ABA, respectively (Xiong and Zhu, 2003; Saito et al., 2004). ABA accumulation in AVG-treated roots at the later stage of seedling growth was associated with up-regulation of TaGA2ox6 and reduction of the GA1 level (Figs 9I, 10G), reflecting the negative regulation of the GA level by ABA (Seo et al., 2009). However, AVG treatment did not alter the expression levels of TaRHT1 and TaGAMYB in the root despite the repression of TaEXPA3 (Figs 11, 13). This result, along with the absence of any effect of exogenous GA on root growth in the control samples (Supplementary Fig. S1), indicates that ET-mediated regulation of ABA metabolism in the coleoptile and ABA signaling in the root determine the ABA/GA balance and thereby cell wall expansion and root growth.
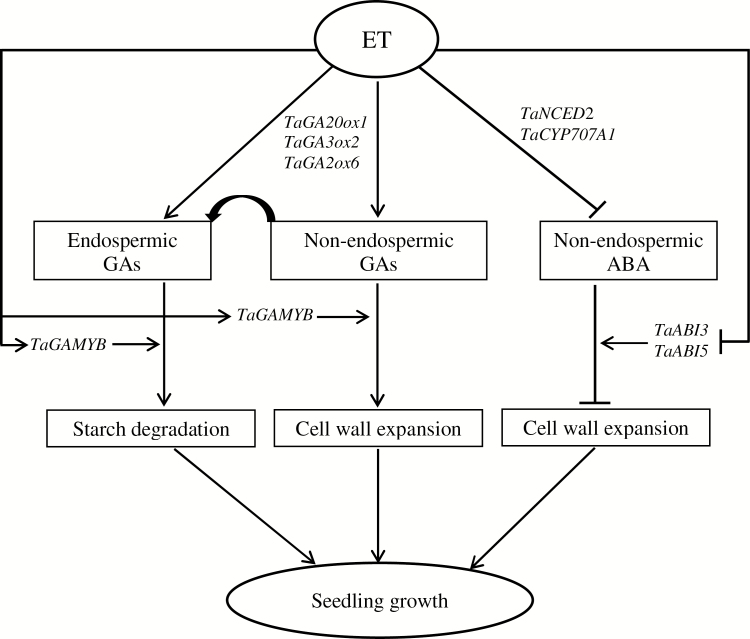
In summary, our study showed that ET controls storage starch degradation and seedling growth in wheat via spatiotemporal regulation of ABA and GA metabolism and signaling, and therefore the ABA/GA balance as depicted in Fig 14.
Fig. 14.
Schematic depiction of the role of ethylene (ET) in regulating abscisic acid (ABA)/gibberellin (GA) balance and seedling growth in wheat. ET enhances seedling growth by inducing endospermic and non-endospermic (embryo axis, coleoptile, and root) GA level and sensitivity through expression of GA biosynthetic (TaGA20ox1 and TaGA3ox2) and/or catabolic (TaGA2ox6), and GA signaling (TaGAMYB) genes. Endospermic bioactive GA, which mainly comprises GA transported from non-endospermic tissues, induces storage starch degradation through enhancing the expression levels of specific α-amylase (TaAMY1, TaAMY3, and TaAMY4) and α-glucosidase (TaAGL1) genes and the activity of α-amylase and α-glucosidase, while GAs in non-endospermic tissues induce cell wall expansion via expression of specific α-expansin (TaEXPA3, and/or TaEXPA7, and/or TaEXPA9) genes. Furthermore, ET represses the ABA level and signaling in non-endospermic tissues via expression of ABA biosynthetic (TaNCED2), catabolic (TaCYP707A1), and ABA signaling (TaABI3 and/or TaABI5) genes, contributing to the induction cell wall expansion via expression of TaEXPA genes. ET does not affect radicle protrusion in wheat seeds.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Effects of treatment with ethephon, gibberellin, or an abscisic acid biosynthesis inhibitor on seedling growth.
Table S1. Sequence similarity of starch-degrading and GAMYB genes with their orthologs.
Table S2. Gene-specific primers used for expression analysis.
Table S3. ABRE motifs in the promoters of TaNCED1 and TaNCED2 genes.
Acknowledgements
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada, Manitoba Wheat and Barley Growers Association, and Ag Action Manitoba to BTA. The authors would like to thank Sharon Bazin and Tricia McMillan for their technical assistance. The authors declare no conflict of interest
Glossary
Abbreviations
- ABA
abscisic acid
- AGL
α-glucosidase
- AMY
α-amylase
- AVG
aminoethoxyvinylglycine
- BAM
β-amylase
- ET
ethylene
- EXPA
α-expansin
- GA
gibberellin
References
- Achard P, Gusti A, Cheminant S, Alioua M, Dhondt S, Coppens F, Beemster GT, Genschik P. 2009. Gibberellin signaling controls cell proliferation rate in Arabidopsis. Current Biology 19, 1188–1193. [DOI] [PubMed] [Google Scholar]
- Achard P, Vriezen WH, Van Der Straeten D, Harberd NP. 2003. Ethylene regulates arabidopsis development via the modulation of DELLA protein growth repressor function. The Plant Cell 15, 2816–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen CL, Jensen JL, Ørntoft TF. 2004. Normalization of real-time quantitative reverse transcription–PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Research 64, 5245–5250. [DOI] [PubMed] [Google Scholar]
- Appleford NEJ, Lenton JR. 1997. Hormonal regulation of α-amylase gene expression in germinating wheat (Triticum aestivum) grains. Physiologia Plantarum 100, 534–542. [Google Scholar]
- Arc E, Sechet J, Corbineau F, Rajjou L, Marion-Poll A. 2013. ABA crosstalk with ethylene and nitric oxide in seed dormancy and germination. Frontiers in Plant Science 4, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin N, Serizet C, Gosti F, Giraudat J. 2000. Interactions between abscisic acid and ethylene signaling cascades. The Plant Cell 12, 1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo AP, Nicolás C, Lorenzo O, Nicolás G, Rodríguez D. 2004a Evidence for positive regulation by gibberellins and ethylene of ACC oxidase expression and activity during transition from dormancy to germination in Fagus sylvatica L. seeds. Journal of Plant Growth Regulation 23, 44–53. [Google Scholar]
- Calvo AP, Nicolás C, Nicolás G, Rodríguez D. 2004b Evidence of a cross-talk regulation of a GA 20-oxidase (FsGA20ox1) by gibberellins and ethylene during the breaking of dormancy in Fagus sylvatica seeds. Physiologia Plantarum 120, 623–630. [DOI] [PubMed] [Google Scholar]
- Cao D, Cheng H, Wu W, Soo HM, Peng J. 2006. Gibberellin mobilizes distinct DELLA-dependent transcriptomes to regulate seed germination and floral development in Arabidopsis. Plant Physiology 142, 509–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H-T, Cosgrove DJ. 2004. Expansins as agents in hormone action. In: Davies PJ, ed. Plant hormones: biosynthesis, signal transduction, action! Dordrecht: Springer Netherlands, 262–281. [Google Scholar]
- Choi D. 2007. Ethylene-induced stem growth of deepwater rice is correlated with expression of gibberellin- and abscisic acid-biosynthetic genes. Journal of Plant Biology 50, 595–599. [Google Scholar]
- Corbineau F, Xia Q, Bailly C, El-Maarouf-Bouteau H. 2014. Ethylene, a key factor in the regulation of seed dormancy. Frontiers in Plant Science 5, 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowling RJ, Harberd NP. 1999. Gibberellins control Arabidopsis hypocotyl growth via regulation of cellular elongation. Journal of Experimental Botany 50, 1351–1357. [Google Scholar]
- da Silva EA, Toorop PE, Van Lammeren AA, Hilhorst HW. 2008. ABA inhibits embryo cell expansion and early cell division events during coffee (Coffea arabica ‘Rubi’) seed germination. Annals of Botany 102, 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Zhen Z, Peng J, Chang L, Gong Q, Wang NN. 2011. Loss of ACS7 confers abiotic stress tolerance by modulating ABA sensitivity and accumulation in Arabidopsis. Journal of Experimental Botany 62, 4875–4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwell KC, Spencer MS. 1982. Modes of ethylene action in the release of amylase from barley aleurone layers. Plant Physiology 69, 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Gampala SS, Rock CD. 2002. Abscisic acid signaling in seeds and seedlings. The Plant Cell 14(Suppl), S15–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K, Yang YY, Honda I, Yanagisawa T, Sakurai A, Takahashi N, Kamiya Y. 1997. Effects of ethylene and gibberellins on the elongation of rice seedlings (Oryza sativa L.). Bioscience, Biotechnology, and Biochemistry 61, 864–869. [DOI] [PubMed] [Google Scholar]
- Gao F, Rampitsch C, Chitnis VR, Humphreys GD, Jordan MC, Ayele BT. 2013. Integrated analysis of seed proteome and mRNA oxidation reveals distinct post-transcriptional features regulating dormancy in wheat (Triticum aestivum L.). Plant Biotechnology Journal 11, 921–932. [DOI] [PubMed] [Google Scholar]
- Geisler-Lee J, Caldwell C, Gallie DR. 2010. Expression of the ethylene biosynthetic machinery in maize roots is regulated in response to hypoxia. Journal of Experimental Botany 61, 857–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P. 2000. Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. The Plant Cell 12, 1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianinetti A, Laarhoven LJ, Persijn ST, Harren FJ, Petruzzelli L. 2007. Ethylene production is associated with germination but not seed dormancy in red rice. Annals of Botany 99, 735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Cadenas A, Verhey SD, Holappa LD, Shen Q, Ho TH, Walker-Simmons MK. 1999. An abscisic acid-induced protein kinase, PKABA1, mediates abscisic acid-suppressed gene expression in barley aleurone layers. Proceedings of the National Academy of Sciences, USA 96, 1767–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Cadenas A, Zentella R, Walker-Simmons MK, Ho TH. 2001. Gibberellin/abscisic acid antagonism in barley aleurone cells: site of action of the protein kinase PKABA1 in relation to gibberellin signaling molecules. The Plant Cell 13, 667–679. [PMC free article] [PubMed] [Google Scholar]
- Gubler F, Hughes T, Waterhouse P, Jacobsen J. 2008. Regulation of dormancy in barley by blue light and after-ripening: effects on abscisic acid and gibberellin metabolism. Plant Physiology 147, 886–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler F, Kalla R, Roberts JK, Jacobsen JV. 1995. Gibberellin-regulated expression of a myb gene in barley aleurone cells: evidence for Myb transactivation of a high-pI alpha-amylase gene promoter. The Plant Cell 7, 1879–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielminetti L, Yamaguchi J, Perata P, Alpi A. 1995. Amylolytic activities in cereal seeds under aerobic and anaerobic conditions. Plant Physiology 109, 1069–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SY, Kitahata N, Sekimata K, Saito T, Kobayashi M, Nakashima K, Yamaguchi-Shinozaki K, Shinozaki K, Yoshida S, Asami T. 2004. A novel inhibitor of 9-cis-epoxycarotenoid dioxygenase in abscisic acid biosynthesis in higher plants. Plant Physiology 135, 1574–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Liu Y, Deng X, Liu D, Liu Y, Hu Y, Yan Y. 2019. Genome-wide identification and expression analysis of expansin gene family in common wheat (Triticum aestivum L.). BMC Genomics 20, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias-Fernández R, Matilla AJ. 2010. Genes involved in ethylene and gibberellins metabolism are required for endosperm-limited germination of Sisymbrium officinale L. seeds: germination in Sisymbrium officinale L. seeds. Planta 231, 653–664. [DOI] [PubMed] [Google Scholar]
- Izydorczyk C, Nguyen TN, Jo S, Son S, Tuan PA, Ayele BT. 2018. Spatiotemporal modulation of abscisic acid and gibberellin metabolism and signalling mediates the effects of suboptimal and supraoptimal temperatures on seed germination in wheat (Triticum aestivum L.). Plant, Cell & Environment 41, 1022–1037. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Itoh H, Inukai Y, Sakamoto T, Ueguchi-Tanaka M, Ashikari M, Matsuoka M. 2003. Where do gibberellin biosynthesis and gibberellin signaling occur in rice plants? The Plant Journal 35, 104–115. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Kihara M, Ito K, Takeda K. 2000. Molecular and chemical analysis of β-amylase-less mutant barley in Tibet. Plant Breeding 119, 383–387. [Google Scholar]
- Kende H, van der Knaap E, Cho HT. 1998. Deepwater rice: a model plant to study stem elongation. Plant Physiology 118, 1105–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara M, Kaneko T, Ito K, Aida Y, Takeda K. 1999. Geographical variation of β-amylase thermostability among varieties of barley (Hordeum vulgare) and β-amylase deficiency. Plant Breeding 118, 453–455. [Google Scholar]
- Konishi H, Kitano H, Komatsu S. 2005. Identification of rice root proteins regulated by gibberellin using proteome analysis. Plant, Cell & Environment 28, 328–339. [Google Scholar]
- Ku HS, Suge H, Rappaport L, Pratt HK. 1970. Stimulation of rice coleoptile growth by ethylene. Planta 90, 333–339. [DOI] [PubMed] [Google Scholar]
- Kucera B, Cohn MA, Leubner-Metzger G. 2005. Plant hormone interactions during seed dormancy release and germination. Seed Science Research 15, 281–307. [Google Scholar]
- Lalonde S, Saini HS. 1992. Comparative requirement for endogenous ethylene during seed germination. Annals of Botany 69, 423–428. [Google Scholar]
- Linkies A, Leubner-Metzger G. 2012. Beyond gibberellins and abscisic acid: how ethylene and jasmonates control seed germination. Plant Cell Reports 31, 253–270. [DOI] [PubMed] [Google Scholar]
- Linkies A, Müller K, Morris K, et al. . 2009. Ethylene interacts with abscisic acid to regulate endosperm rupture during germination: a comparative approach using Lepidium sativum and Arabidopsis thaliana. The Plant Cell 21, 3803–3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Locke JM, Bryce JH, Morris PC. 2000. Contrasting effects of ethylene perception and biosynthesis inhibitors on germination and seedling growth of barley (Hordeum vulgare L.). Journal of Experimental Botany 51, 1843–1849. [DOI] [PubMed] [Google Scholar]
- Ma B, Yin CC, He SJ, Lu X, Zhang WK, Lu TG, Chen SY, Zhang JS. 2014. Ethylene-induced inhibition of root growth requires abscisic acid function in rice (Oryza sativa L.) seedlings. PLoS Genetics 10, e1004701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matilla AJ. 2000. Ethylene in seed formation and germination. Seed Science Research 10, 111–126. [Google Scholar]
- Matilla AJ, Carrillo-Barral N, Rodríguez-Gacio MdC. 2015. An update on the role of NCED and CYP707A ABA metabolism genes in seed dormancy induction and the response to after-ripening and nitrate. Journal of Plant Growth Regulation 34, 274–293. [Google Scholar]
- Matilla AJ, Matilla-Vázquez MA. 2008. Involvement of ethylene in seed physiology. Plant Science 175, 87–97. [Google Scholar]
- Matsushita A, Furumoto T, Ishida S, Takahashi Y. 2007. AGF1, an AT-hook protein, is necessary for the negative feedback of AtGA3ox1 encoding GA 3-oxidase. Plant Physiology 143, 1152–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Liu A, Deol KK, Kulichikhin K, Stasolla C, Brûlé-Babel A, Ayele BT. 2015. Transcriptional coordination and abscisic acid mediated regulation of sucrose transport and sucrose-to-starch metabolism related genes during grain filling in wheat (Triticum aestivum L.). Plant Science 240, 143–160. [DOI] [PubMed] [Google Scholar]
- Nambara E, Okamoto M, Tatematsu K, Yano R, Seo M, Kamiya Y. 2010. Abscisic acid and the control of seed dormancy and germination. Seed Science Research 20, 55–67. [Google Scholar]
- Ogawa M, Hanada A, Yamauchi Y, Kuwahara A, Kamiya Y, Yamaguchi S. 2003. Gibberellin biosynthesis and response during Arabidopsis seed germination. The Plant Cell 15, 1591–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski N, Sun TP, Gubler F. 2002. Gibberellin signaling: biosynthesis, catabolism, and response pathways. The Plant Cell 14(Suppl), S61–S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiano EP, Juliano BO. 1972. Biochemical changes in the rice grain during germination. Plant Physiology 49, 751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield S, Graham S, Graham IA. 2005. Storage reserve mobilization in germinating oilseeds: Arabidopsis as a model system. Biochemical Society Transactions 33, 380–383. [DOI] [PubMed] [Google Scholar]
- Perata P, Pozueta-Romero J, Akazawa T, Yamaguchi J. 1992. Effect of anoxia on starch breakdown in rice and wheat seeds. Planta 188, 611–618. [DOI] [PubMed] [Google Scholar]
- Petruzzelli L, Coraggio I, Leubner-Metzger G. 2000. Ethylene promotes ethylene biosynthesis during pea seed germination by positive feedback regulation of 1-aminocyclo-propane-1-carboxylic acid oxidase. Planta 211, 144–149. [DOI] [PubMed] [Google Scholar]
- Petruzzelli L, Harren F, Reuss J. 1994. Patterns of C2H4 production during germination and seedling growth of pea and wheat as indicated by a laser-driven photoacoustic system. Environmental and Experimental Botany 34, 55–61. [Google Scholar]
- Ruzicka K, Ljung K, Vanneste S, Podhorská R, Beeckman T, Friml J, Benková E. 2007. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. The Plant Cell 19, 2197–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Hirai N, Matsumoto C, Ohigashi H, Ohta D, Sakata K, Mizutani M. 2004. Arabidopsis CYP707As encode (+)-abscisic acid 8'-hydroxylase, a key enzyme in the oxidative catabolism of abscisic acid. Plant Physiology 134, 1439–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampedro J, Cosgrove DJ. 2005. The expansin superfamily. Genome Biology 6, 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M, Nambara E, Choi G, Yamaguchi S. 2009. Interaction of light and hormone signals in germinating seeds. Plant Molecular Biology 69, 463–472. [DOI] [PubMed] [Google Scholar]
- Shu K, Zhou W, Chen F, Luo X, Yang W. 2018. Abscisic acid and gibberellins antagonistically mediate plant development and abiotic stress responses. Frontiers in Plant Science 9, 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalle J, Haegman M, Kurepa J, Van Montagu M, Straeten DV. 1997. Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proceedings of the National Academy of Sciences, USA 94, 2756–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son S, Chitnis VR, Liu A, Gao F, Nguyen TN, Ayele BT. 2016. Abscisic acid metabolic genes of wheat (Triticum aestivum L.): identification and insights into their functionality in seed dormancy and dehydration tolerance. Planta 244, 429–447. [DOI] [PubMed] [Google Scholar]
- Spollen WG, LeNoble ME, Samuels TD, Bernstein N, Sharp RE. 2000. Abscisic acid accumulation maintains maize primary root elongation at low water potentials by restricting ethylene production. Plant Physiology 122, 967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Yamasaki Y, Ayele BT. 2018. Comparative expression analysis of starch degrading genes between dormant and non-dormant wheat seeds. Plant Signaling & Behavior 13, e1411449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun TP. 2008. Gibberellin metabolism, perception and signaling pathways in Arabidopsis. The arabidopsis book 6, e0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun TP, Gubler F. 2004. Molecular mechanism of gibberellin signaling in plants. Annual Review of Plant Biology 55, 197–223. [DOI] [PubMed] [Google Scholar]
- Sun Z, Henson CA. 1990. Degradation of native starch granules by barley alpha-glucosidases. Plant Physiology 94, 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan PA, Kumar R, Rehal PK, Toora PK, Ayele BT. 2018. Molecular mechanisms underlying abscisic acid/gibberellin balance in the control of seed dormancy and germination in cereals. Frontiers in Plant Science 9, 668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varty K, Arreguín LB, Lopez TPJ, Sanchez FCJ, Garcia HE, Gomez LMA. 1983. The role of ethylene in the induction of amylase activity in wheat aleurone tissue. Physiologia Plantarum 58, 318–324. [Google Scholar]
- Vriezen WH, Achard P, Harberd NP, Van Der Straeten D. 2004. Ethylene-mediated enhancement of apical hook formation in etiolated Arabidopsis thaliana seedlings is gibberellin dependent. The Plant Journal 37, 505–516. [DOI] [PubMed] [Google Scholar]
- Xiong L, Zhu JK. 2003. Regulation of abscisic acid biosynthesis. Plant Physiology 133, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S. 2008. Gibberellin metabolism and its regulation. Annual Review of Plant Biology 59, 225–251. [DOI] [PubMed] [Google Scholar]
- Yamasaki Y, Gao F, Jordan MC, Ayele BT. 2017. Seed maturation associated transcriptional programs and regulatory networks underlying genotypic difference in seed dormancy and size/weight in wheat (Triticum aestivum L.). BMC Plant Biology 17, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin CC, Zhao H, Ma B, Chen SY, Zhang JS. 2017. Diverse roles of ethylene in regulating agronomic traits in rice. Frontiers in Plant Science 8, 1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeman SC, Kossmann J, Smith AM. 2010. Starch: its metabolism, evolution, and biotechnological modification in plants. Annual Review of Plant Biology 61, 209–234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.