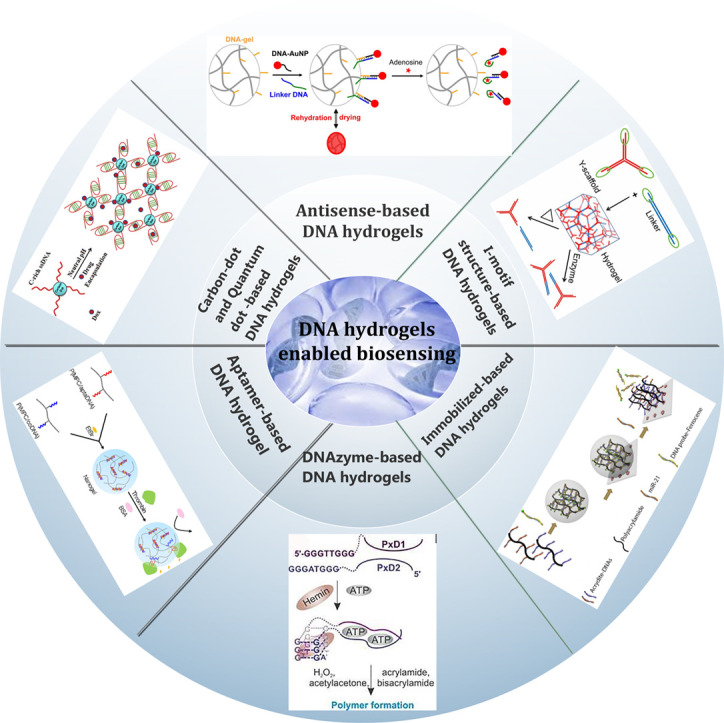
Abstract
DNA hydrogels as special members in the DNA nanotechnology have provided crucial prerequisites to create innovative gels owing to their sufficient stability, biocompatibility, biodegradability, and tunable multifunctionality. These properties have tailored DNA hydrogels for various applications in drug delivery, tissue engineering, sensors, and cancer therapy. Recently, DNA-based materials have attracted substantial consideration for the exploration of smart hydrogels, in which their properties can change in response to chemical or physical stimuli. In other words, these gels can undergo switchable gel-to-sol or sol-to-gel transitions upon application of different triggers. Moreover, various functional motifs like i-motif structures, antisense DNAs, DNAzymes, and aptamers can be inserted into the polymer network to offer a molecular recognition capability to the complex. In this manuscript, a comprehensive discussion will be endowed with the recognition capability of different kinds of DNA hydrogels and the alternation in physicochemical behaviors upon target introducing. Finally, we offer a vision into the future landscape of DNA based hydrogels in sensing applications.
Keywords: DNA hydrogels, Molecular diagnosis, Smart hydrogel, Sol to gel, Gel to sol
Graphical abstract
1. Introduction
Hydrogels are 3-D hydrophilic buildings covering nano to macro sizes with vast applications in medicine and industry. The hydrophilic nature enables them to swell in water up to several hundred folds of the gel dry mass. Before crosslinking, the polymers are easily dissolved in water but after crosslinking, they are in a gel state with a defined shape [1]. Hydrogels have gained immense consideration over the past years to be exploited as scaffolds in drug delivery carriers, tissue engineering, sensors, glues, and cancer therapy [2]. Thus far, innumerable hydrogels, composed of synthetic or natural crosslinked agents, have been discovered and engineered, however, due to biocompatibility demands, only a few synthetic polymers, such as polylactic-co-glycolic acid (PLGA) and polyethylene glycol (PEG), and natural polymers, such as polysaccharide, protein, and DNA have been utilized as the backbone [3,4]. Among various candidates, DNA is an excellent molecule due to its biocompatibility, precise molecular recognition capability, convenient programmability, and minimal toxicity [5]. DNA hydrogels can be fabricated through either chemical linkage of DNA molecules or physical entanglement between DNA chains. By chemical approaches, the polymers are bound together through covalent bonds, which endow environmental stability and intensive mechanical strength. In comparison, physical hydrogels rely on non-covalent interactions like hydrogen bonding, electrostatic interactions, and metal-ligand coordination [6]. In terms of composition, DNA hydrogels can be placed into two categories, named hybrid and pure DNA hydrogels [7]. Hybrid hydrogels are assembled through tethering of functional nucleic acids on synthetic or natural polymers. However, since multiple steps are necessary for modification of hybrid hydrogels, another material termed pure DNA hydrogel has been introduced to conquer the limitations of hybrid hydrogels. This type of gel is exclusively built from DNA molecules and assembled by (non) Watson-Crick interactions, enzymatic ligation, enzymatic polymerization, and specific binding of DNA motifs between their building blocks [8].
In particular, “smart hydrogels” which are equipped with a module with signal-triggered gel-to-sol transition capability or signal-stimulated gel stiffness controllability have achieved widespread applications in the expanding area of material science [9]. Physical cues such as pH, light, temperature, and redox reactions induce reversible nucleic acid structural switches by the separation of switch-integrated polymers or assembly of the switch counterparts [10]. Beyond the aforementioned stimulants, the hydrogel can be responsive to metal ions, nucleic acids, proteins, and metabolites, in which the input molecule is converted into biological or mechanical outputs. For this purpose, various functional DNA motifs with inherent molecular recognition properties (e.g., aptamers, DNAzymes, i-motif nanostructures, antisense DNAs, etc.) are embedded into the polymer network that noticeably expands the latitude of these materials for additional molecular recognition capabilities [11,12]. Moreover, thanks to the unique sequence-controlled functions of DNA, considerable attention has been dedicated toward the development of logic gate-based DNA gels and smart systems for logical biosensing applications [13]. Accordingly, DNA hydrogels have been suggested as an excellent platform for detecting a wide range of stimuli in several different ways. In the present review, we firstly demonstrate the recognition capability of DNA hydrogels for generating a detectable signal. Then, a summary and a vision into the future landscape of DNA hydrogels are given.
2. Exploration of smart DNA hydrogels for biosensing applications
2.1. Antisense-based DNA hydrogels
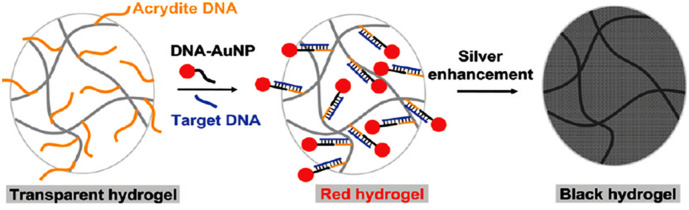
Highly sensitive nucleic acid detection has become increasingly important in various realms of research such as genomics, diagnosis, pathogen detection, and forensic sciences [14]. Up to now, different technologies have been developed for nucleic acid detection of which the majority is usually expensive and requires sophisticated equipment with low selectivity and sensitivity. Therefore, the development of simple and efficient methods to address these limitations is very important [15]. In a pioneering report, a DNA-sensitive gel was fabricated, in which the stiffness of gel was controlled by different DNA strands. Here, two grafted ssDNAs polymer chains were connected via a complementary linker DNA-strand to form a hydrogel network. When a fuel DNA strand complementary to the crosslinking strand was added, the gel stiffness increased. However, the gel was restored to the initial stiffness when the full complement was used [16]. In 2010, a colorimetric assay based on DNA-functionalized monolithic hydrogel and gold nanoparticles (AuNPs) was produced for DNA detection. As shown in Fig. 1 , in this assay one DNA probe was grafted to the gel network and the other probe was attached to AuNPs. By hybridization to the target DNA, the probe molecules were brought in close proximity. This led to the proximation of AuNPs to the transparent gel with a change of color to red as result. Moreover, to enhance the optical density, Ag+ ions were reduced in the presence of AuNPs so that the detection limit of the assay improved to 1 pM. By using a thermal treatment, the gels could be regenerated to restore their properties comparable to freshly prepared gels [17].
Fig. 1.
Mechanism of DNA detection by employing DNA-functionalized hydrogels and AuNPs accompanied by Ag+ reduction for signal amplification.
Adapted from ref. [17].
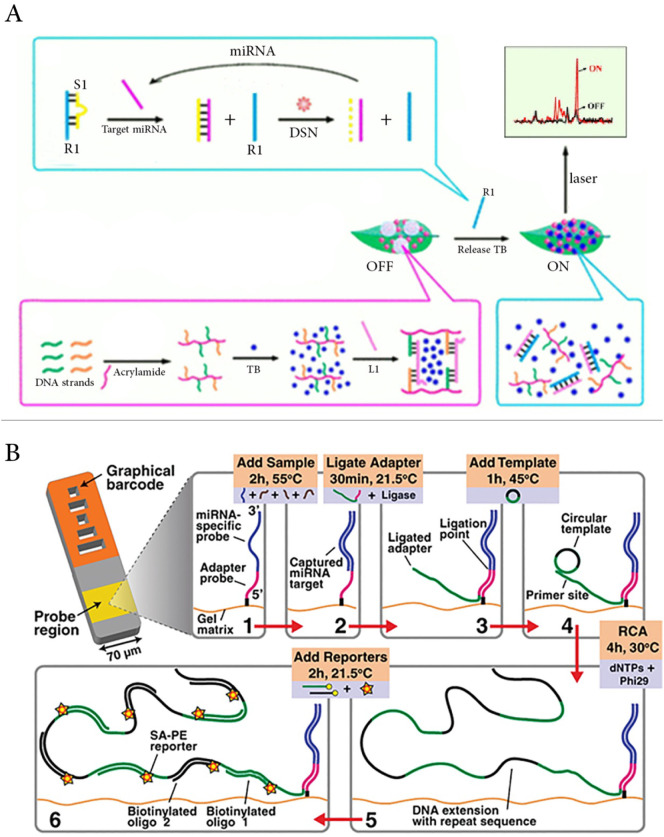
In 2017, Hi and colleagues designed a system to combine the advantages of Surface Enhancement Raman Spectrum (SERS) technology and switchable target-responsive DNA hydrogels (Fig. 2A). In this work, the Raman reporter toluidine blue (TB) was trapped inside a crosslinked DNA hydrogel gel that exhibited a blind Raman signal via an “OFF” situation. Here, the crosslinker DNA is entirely complementary with the product (R1) that is generated in a separate miRNA-155 target amplification reaction. In this target amplification reaction, ssDNA S1 sequesters R1 in absence of miRNA-155 by partial hybridization. Upon introduction of miRNA-155, the partial hybridization of R1-S1 is broken and S1 now fully base pairs with miRNA-155. The S1 DNA in this RNA-DNA hybrid is cleaved by a duplex-specific nuclease (DSN) to regenerate target miR-155 for performing the next cycle of multiplication. After addition of the reaction mixture containing the multiplied R1 DNA to the gel, hybridization of R1 with the crosslinker DNA (L1) occurred and the hydrogel collapsed. The TB molecules were released and produced a strong Raman signal via an “ON” situation that was in proportion to the amount of miRNA-155 [18].
Fig. 2.
A) Mechanism of miRNA detection using SERS technology and target-responsive DNA hydrogel, adapted from ref. [18]. Schematic of miRNA detection using Encoded Gel microparticles in combination with RCA, adapted from ref. [20].
The next study is related to encoded gel microparticles which are mostly employed in miRNA diagnosis and profiling [19]. In this context, Chapin and coworkers exploited a system for the detection of rolling circle amplification (RCA) reaction products (Fig. 2B). After ligation of the target molecule to a universal adapter and extension by Phi29 DNA polymerase, a long DNA strand with repeated sequences was generated. Subsequently, every repeated region could be labeled with two different biotinylated oligonucleotides attached to streptavidin-phycoerythrin. Since each target molecule was labeled with multiple fluoroprotein reporters, it provided a highly sensitive detection platform for the respective target [20].
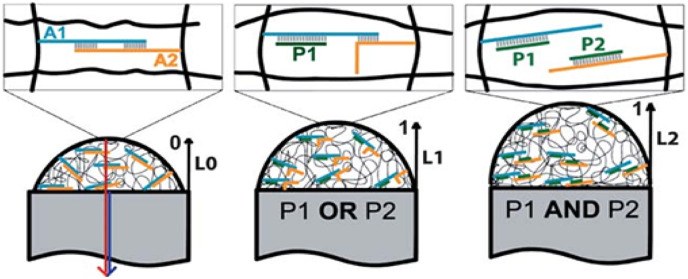
In another study, a new platform was reported for the detection of full-length mRNA sequences using polyethylene glycol diacrylate (PEGDA) hydrogel microparticles. Herein, acrydite-modified capture probes were incorporated into the synthesized particles that by hybridization via capture extenders could receive the mRNA targets. Then, the targets were hybridized with label extenders which consisted of a universal labeling region conjugated to multiple biotins. Finally, by introducing a streptavidin-conjugated fluorophor to the system, the target signal was amplified with a limit of detection of 6.4 amol [21,22]. In 2018, Choi and coworkers developed a multiplex assay for the detection of miRNAs (microRNA biomarkers for Alzheimer's disease; hsa-miR-342-3p, hsa-miR-18 bp, hsa-miR-30e-5p, hsa-miR-143-3p, and hsa-miR-424-5p) via hydrogel micropost-based qPCR with a detection limit of ~10 pg/μL. Here, five PEGDA hydrogel microposts, which were photochemically conjugated with target-specific forward primers, were immobilized in polycarbonate chips. Then, PCR products, which were entrapped in the hydrogel spot, were visualized by SYBR green [23]. Another research was introduced by Gawel et al., in which the swelling state of the gel was induced in response to various DNA inputs to simulate AND and OR logic gates. As demonstrated in Fig. 3 , A1 and A2 ssDNA strands copolymerized with polymers to give a hydrogel. Afterward, the addition of complementary DNA probes P1 and P2 resulted in the dissociation of the dsDNA crosslinkers. In this system, the swelling movement of the gel shifted in a Boolean expression [24,25].
Fig. 3.
Schematic of the responsive DNA hydrogel for the detection of different DNA inputs that induce a change of swelling behavior in a Boolean way that was monitored by the interferometric technique.
Adapted from ref. [24].
2.2. I-motif structure-based DNA hydrogels
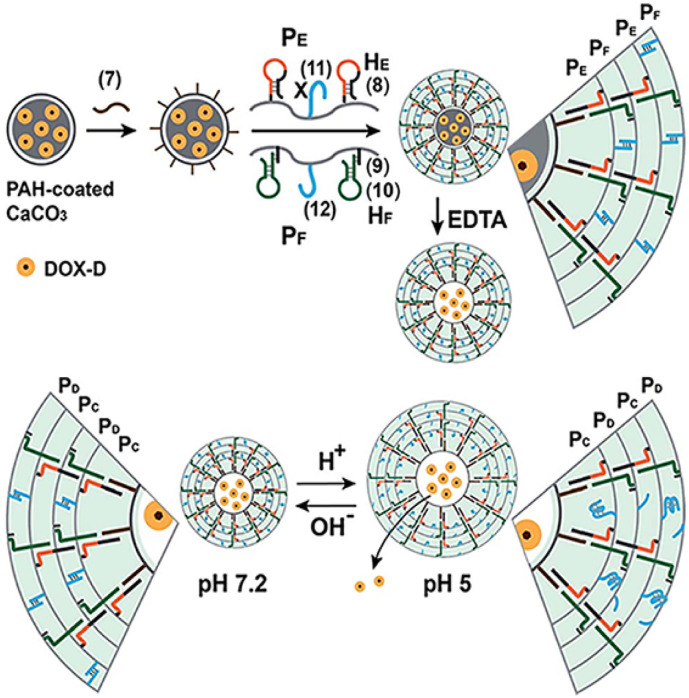
The self-assembly of quadruplex i-motif structures under acidic pH have offered more choices in fabrication of pH-sensitive DNA gels [26,27]. In such way, Y-shaped DNA nanostructures, which contained cytosine (C)-rich overhangs, were self-assembled through the formation of i-motif structures under acidic condition (pH 5), resulting in gel formation. In another study, these pH-responsive sequences were alternatively ordered in polymeric DNA chains during RCA-based DNA synthesis. The resulting products could form an intermolecular i-motif-based hydrogel in an acidic environment [28,29]. Recently, a new method was explored based on stimuli-responsive DNA hydrogel microcapsules. As depicted in Fig. 4 , the formation of the gel was based on the modification of two acrylamide copolymer chains (PE and PF) via two complementary hairpins (HE and HF) and a pH-sensitive ssDNA (strand 11) hybridized to its complementary tether (strand 12). Another ssDNA (strand 7) was attached to doxorubicin-modified dextran loaded poly(allylamine hydrochloride) coated CaCO3 microparticles and acted as an initiator strand to induce a hybridization chain reaction (HCR) between hairpins. Upon applying a pH of 7.2, the hydrogel was formed via HCR and the duplex formation between tethers (duplex 11/12). The CaCO3 core was dissolved using EDTA and under acidic pH, the tether units (duplex 11/12) were dissociated due to the formation of intramolecular i-motif structures (strand 11), leading to a decrease in the stiffness of the hydrogel shell. In this system, the anticancer drug doxorubicin (DOX) was loaded in these microcapsules, in order to sense and release the drug in the acidic environment around cancer cells [30].
Fig. 4.
Generation of pH-responsive hydrogel microcapsules driven by HCR for the release of a loaded drug through formation of intramolecular i-motif structures in single strand tether (11).
Adapted from ref. [28].
To extend the scope of these hydrogels, Guo and coworkers produced a dual-stimuli-responsive material that used thermosensitive poly-N-isopropylacrylamide (pNIPAM) polymer chains functionalized with C-rich tethers. In this study, the pNIPAM chains provided the reversible gel-sol transition in the hydrogel by performing heating-cooling cycles. Therefore, heating the gel to 45 °C generated a condensed solid state and cooling to 25 °C recovered the swollen state. Furthermore, the C-rich sequences provided a cyclic transition between gel-sol state by exploiting alternative pH under acidic (pH 5.2) and basic (pH 7.5) conditions [31]. To illustrate the generality of this design, ssDNAs were conjugated to pNIPAM chains for the specific detection of target molecules. Upon binding of the target DNA to the ssDNA, the volume phase transition temperature (VPTT) of the polymer changed proportional to the concentration of the target molecule [32]. In a pioneering research, Liu and coworkers designed a new type of pure DNA hydrogel through hybridization of the Y-shaped DNAs and linker strands. This hydrogel provided thermal and enzymatic responses by incorporating enzyme specific restriction sites in the linker strands [33]. Moreover, by inserting an i-motif sequence in the linker moiety, a swift conformational transition in the gel structure occurred in response to pH variations. The storage modulus – a measure of elastic response of materials – of the gel altered from 1000 to 250 Pa by switching the pH between 5 and 8 [34].
2.3. DNAzyme-based DNA hydrogels
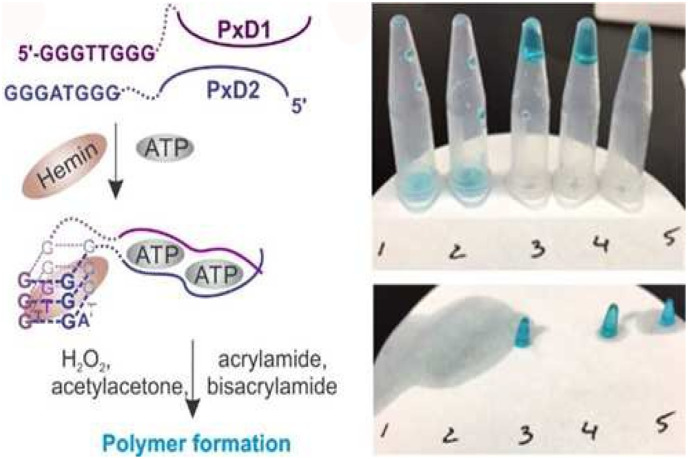
In recent years, DNAzymes have attracted considerable attention due to their high catalytic performance, low-cost synthesis, ease of modification, and versatility, for biosensing and biotechnological applications specialized in the hydrogel field [35]. The Hemin/G-quadruplex horseradish peroxidase (HRP)-mimicking DNAzyme is a type of non-natural catalytic nucleic acid that has catalytic capability similar to that of protein enzymes. HRP-mimicking DNAzyme is a hemin-intercalated G-quadruplex that catalyzes the oxidation of the slightly yellow HRP substrate ABTS2+ into the intensely turquoise colored product ABTS•− with the concomitant reduction of H2O2. In a similar way, Zhao et al. proposed a rapid colorimetric sensing hydrogel with a limit of detection (LOD) of 1.0 μM for H2O2. The working principle was based on the formation of active hemin-G-quadruplex DNAzymes embedded in a DNA-polyacrylamide hybrid hydrogel. The crosslinker DNAzyme with peroxidase activity was able to reduce the H2O2 in compensation of the TMB (3,3′,5,5′-tetramethylbenzidine) oxidation, resulting in a rapid color change of TMB [36]. In another work reported by Fedotova et al., the hydrogel formation was induced by employing a G-quadruplex DNAzyme. Here, peroxidase-like G-quadruplex DNAzymes (PxDs), which contained guanine (G)-rich DNA sequences, were used to form a stable G-quadruplex structure by adding analyte. As shown in Fig. 5 , the ATP-specific aptamer and the DNAzyme sequences cleaved into two parts (e.g. PxD1 and PxD2) that upon binding to ATP formed a full G-quadruplex. By adding the hemin cofactor, H2O2 catalyzed the oxidation of acetylacetone to acetylacetone-radical that initiated the polymerization of acrylamide into polyacrylamide accompanied a sol-to-gel transition [37].
Fig. 5.
Visual detection of ATP based on a polymerization process by liquid-to-gel transition.
Adapted from ref. [35].
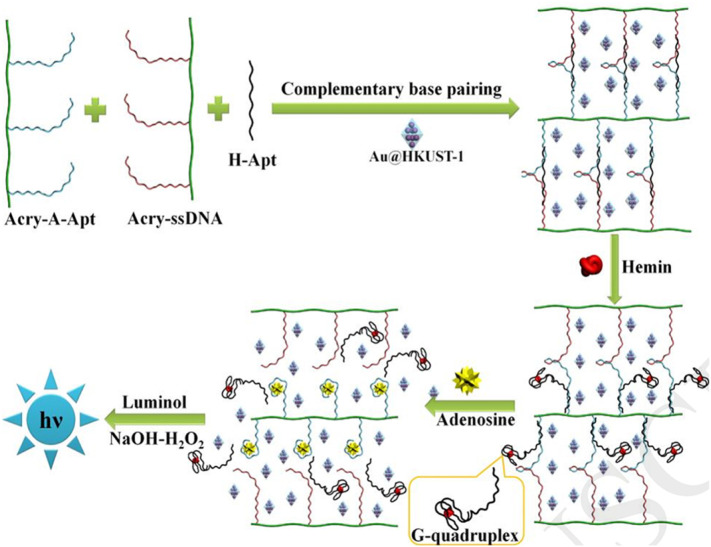
In 2019, Zhang and coworkers reported a DNAzyme-functionalized DNA hydrogel for visual detection of circulating tumor DNA (ctDNA). In this study, the ctDNA served as an initiator triggered rolling circle reaction (RCA) strategy employing Phi29 DNA polymerase to generate a G-quadruplex comprised hydrogel that could be observed by the naked eye. In addition, by using the DNAzyme activity of G-quadruplex/hemin, the hydrogel catalyzed the oxidation-reduction reaction of ABTS and hydrogen peroxide to yield turquoise oxidized ABTS*. This method exhibited great sensitivity for ctDNA with an LOD of 0.32 pM [38]. Recently, a chemiluminescent biosensor for the detection of adenosine was designed with a detection limit as low as 0.104 pM (Fig. 6 ). In this work, the adenosine aptamer and an ssDNA were independently grafted on linear polyacrylamide and designed to form a hydrogel by hybridization upon addition of the hemin aptamer. The hydrogel was loaded with gold nanoparticles that were coated into the cavities of the metal organic framework HKUST-1 which possesses a strong peroxidase-like activity. By binding hemin to the respective aptamer, the G-quadruplex/hemin structure formed and the DNA hydrogel remained in the gel state. Subsequently, adenosine was added which resulted in the complete dissolution of the DNA hydrogel. Then, G-quadruplex/hemin and Au@HKUST-1 were released that enabled the dual signal amplification of the biosensor [39].
Fig. 6.
Schematic of the chemiluminescent sensing platform for the detection of adenosine.
Adapted from ref. [37].
2.4. Aptamer-based DNA hydrogel
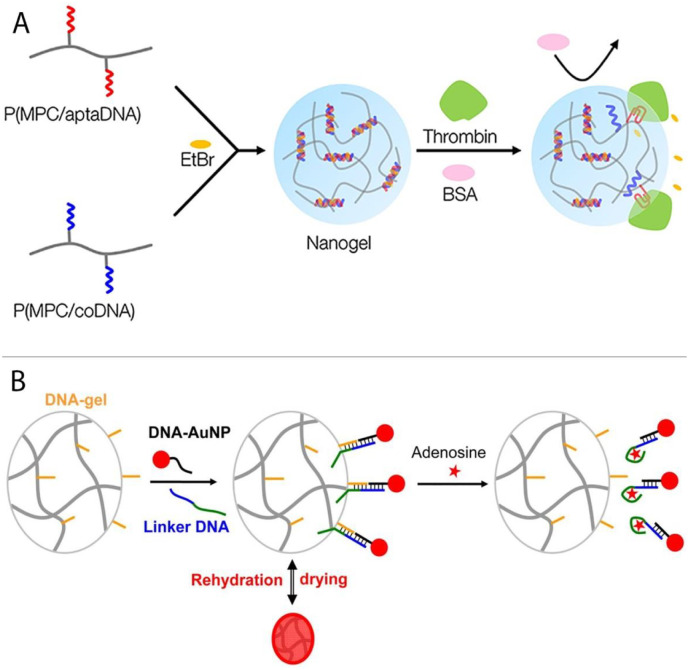
Nucleic acid aptamers are systematically evolved, engineered and/or natural functional nucleic acids that demonstrate a very high affinity for the detection of the targets they were raised against and/or bind to, e.g. ions, metabolites, drugs, proteins, and even whole cells [40]. In particular, aptamer-dependent gels have gained great attention in biosensing due to their unique properties, such as biocompatibility, chemical stability, and selective binding [41]. The first reported in this field was an aptamer-based gel for detection of adenosine. In this design, a DNA linker strand that contained an aptamer domain was linked to tethered DNA strands (S3 and S4) and caused the assembly of the gel. Addition of adenosine induced aptamer deformation and separated the crosslinks and disassembled the gel. To provide a colorimetric signal, AuNPs were entrapped inside the gel that were released in the presence of adenosine [42,43]. In a similar work, a thrombin-binding aptamer was incorporated in the gel. Upon addition of thrombin, a stable G-quadruplex structure in the aptamer structure emerged which behaved like a molecular switch between tight and relaxed states [44]. Moreover, the group of Iwasaki also detected thrombin by producing a nanogel structure (Fig. 7A). Herein, the gel was fabricated by crosslinking of two polymers of which one contained the thrombin-binding aptamer (MPC/aptaDNA) sequence and the other contained the complementary sequence. To acquire a label-free detection, ethidium bromide (EtBr) was physically embedded into the hybridized dsDNA linker to generate a relatively high fluorescent signal. Upon introduction of thrombin, the fluorescence of EtBr was diminished due to the disruption of the dsDNA helical structure [45]. In another study, Liu et al. developed a new assay to detect AMP and ATP metabolites by means of a linker aptamer (Fig. 7B). This aptamer was used as a connector to bring DNA-functionalized AuNPs and DNA-functionalized hydrogels in close proximity, resulting in a red colored solution. Upon binding of the aptamer to the target, the hybridization between the DNA-functionalized AuNPs and DNA-functionalized gels was disrupted which led to the release of the AuNPs followed by disappearance of the red color [46].
Fig. 7.
A) Mechanism of the thrombin responsive gel that incorporated EtBr to generate a fluorescent signal, adapted from ref. [43]. (B) Detection of AMP and ATP metabolites by disruption of the hybridization between DNA-functionalized gold nanoparticles and DNA-functionalized hydrogels followed by the release of gold nanoparticles resulting in the disappearance of the gel color, adapted from ref [44].
Moreover, a bioimprinting super-aptamer hydrogel, in which volume changes were used as the readout signal, was developed for the detection of proteins. In this system, two different methacrylamide-modified aptamers were combined with their target to generate aptamer-target-aptamer complexes. Then, by a copolymerization reaction, the complex was inserted into the hydrogel in which the thrombin served as the crosslinker imprinted. Removal of the proteins from the gel resulted in swelling and increasing the length of the hydrogel due to separation of the aptamer from the proteins. Re-addition of the proteins and re-binding to the aptamer resulted in shrinking that diminished the length of the hydrogel [47]. In 2017, an aptamer-based assay was designed to cloak and decloak circulating tumor cells (CTCs). Here, an aptamer-initiator bi-block and an ATP-binding aptamer were used to cloak and decloak the CTCs, respectively. By adding the MCF-7 cells, the aptamer-initiator bi-block was bound to the epithelial cell adhesion molecule (EpCAM) on the surface of the cell. This phenomenon induced the clamped hybridization chain reaction between two DNA hairpins (H1, H2) and a phase transition from sol to gel. In order to achieve a reversible state, an ATP-binding aptamer, which was incorporated in the H2 hairpin, was used to decloak the entrapped MCF-7 cells. The encapsulated AuNPs were exploited as the indicators of hydrogel formation via generating a red color at this state. Moreover, for real-time monitoring of DNA gelation, the rate of bacterial movement was considered as the criterion, which was decreased under gel formation [48].
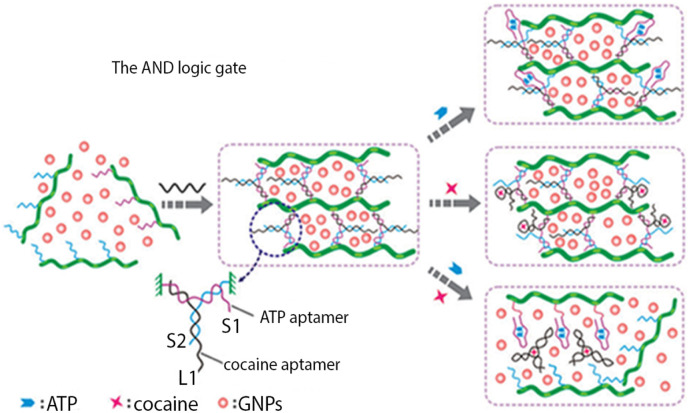
In recent years, logic gate based systems have attracted remarkable attention due to their potency to supply intelligent responses to the external stimuli and convert input signals into a certain output signal [49]. In particular, hydrogel-based logic systems have obtained substantial interest in biosensing research [50]. In this respect, Tan et al. exploited “AND” and “OR” logic gates with cocaine- and ATP-binding crosslinker aptamers to assemble a hydrogel structure. The hydrogel served as an “AND” logic gate, whereby in the proximity of both targets, it was dissolved and led to the release of entrapped AuNPs (Fig. 8 ). For the “OR” gate, either cocaine or ATP collapsed the gel and released the AuNPs [51].
Fig. 8.
The “AND” logic gate system for cocaine and ATP detecting.
Adapted from ref. [49].
2.5. Carbon-dot and quantum dot–based DNA hydrogels
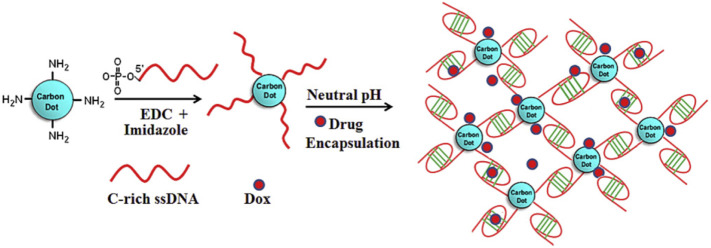
Carbon dots are small and luminescent carbon nanoparticles that in recent years have gained a lot of attention in the family of carbon nanomaterials due to their photostability, water solubility, biocompatibility, and excellent fluorescent properties. These nanomaterials have displayed tremendous applications in medicine, bioimaging, drug delivery, and biosensing [52,53]. In this way, Singh and coworkers encapsulated doxorubicin in a DNA-carbon dot (CD) hybrid that could be release in acidic pH (relevant to tumor microenvironment) through creating i-motif structures (Fig. 9 ). Here, CD acted as a crosslinker for network formation and also participated in encapsulating the drug by electrostatic interaction along with DNA. Release of the drug was observed under acidic conditions and was tracked using the fluorescent properties of the CDs [54].
Fig. 9.
Schematic of the encapsulated doxorubicin that can be released around the acidic microenvironment of tumor cells by creating i-motif structures.
Adapted from ref. [52].
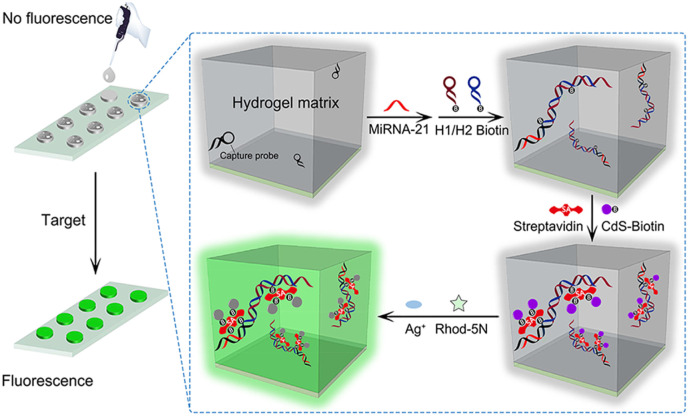
Quantum dots (QDs) are small semiconductor particles possessing optical and electronic properties that make them a central topic in the DNA nanotechnology [55]. In 2010, a DNA hydrogel suspension array coupled with quantum-dot-encoded technology was developed for multiplex label-free DNA detection. In this study, a quantum-dot tagged DNA hydrogel was incorporated into photonic beads that condensed the gel structure by binding to the target DNA. The switch to the shrunken state could be detected as a corresponding blue shift in the Bragg diffraction peak position of the beads [56]. Moreover, to perform a fluorescent analysis, Li and coworkers introduced a strategy for the detection of avian influenza virus (AIV). Herein, the two ends of a virus-specific aptamer sequence were partially complementary to two single-stranded DNAs. Here, a QD reporter was attached to the 5′-terminal of one of the ssDNAs and a quencher was conjugated to the 3′-terminal of the aptamer so that the QDs were quenched in the absence of the virus. Treatment of gel with the virus led to the swollen state of the gel and the dissociation of the aptamer from the ssDNAs. The QD fluorescence reporter was thereby separated from the quencher, resulting in the generation of a detectable fluorescent signal [57]. In 2019, a fluorescence PEG hydrogel assay was developed for the ultrasensitive detection of microRNAs based on DNA hybridization chain reaction (HCR) and interfacial cation exchange amplification (Fig. 10 ). Herein, the PEG hydrogel was constructed on a glass slide and modified with a microRNA capture probe. Upon binding the target microRNA to the capture probe, HCR was initiated in hydrogel through the addition of biotinylated hairpin strands (H1 and H2). The DNA polymer chains produced in the hydrogel provided positions for the binding of biotinylated CdS QDs conjugated to a streptavidin bridge. Subsequently, the interfacial cation exchange reaction was performed by introducing Ag+ and Rhod-5N dye mixture solution, followed by the release of thousands of Cd2+ from CdS. The released Cd2+ was bound to Rhod-5N and produced a strong fluorescence signal for target identification with a detection limit of 0.835 f. [58]. Another fluorescence amplification platform was introduced for the detection of miR-141 based on a DNA hydrogel formation employing DNA walking and HCR. In the presence of the target, a large number of ssDNA was generated by walking amplification. Then, the released strands bound to a hairpin H1 DNA on the SiO2 microsphere, followed by HCR of hairpins (H2 and H3) that formed the DNA hydrogel. Therefore, by constructing the gel, large amounts of SYBR Green (SG) I dyes or quantum dots (QDs) were loaded onto the hydrogel that amplified the fluorescence signal [59].
Fig. 10.
Illustration of a fluorescence (PEG) hydrogel assay for ultrasensitive detection of the microRNA.
Adapted from ref. [56].
2.6. Immobilized-based DNA hydrogels
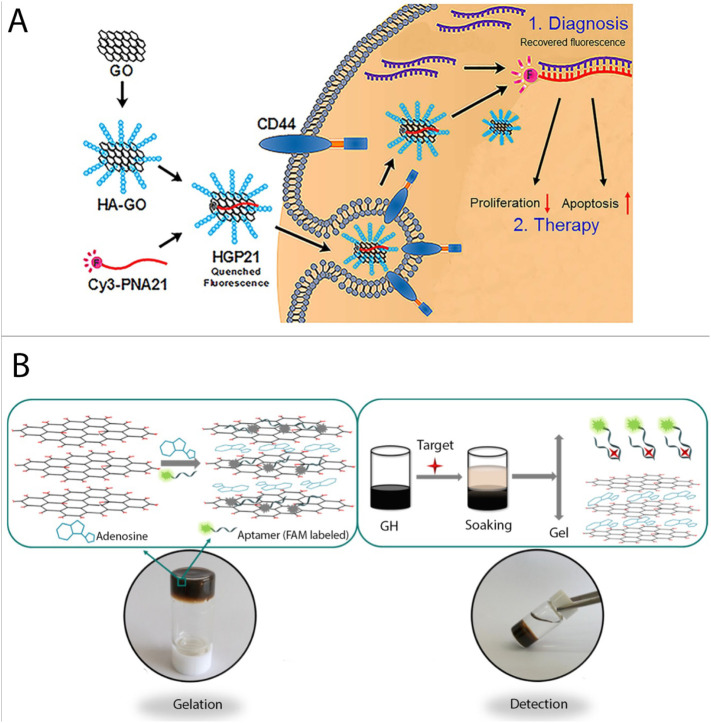
The field of DNA hydrogels has been further extended by the insertion of different functional properties, to achieve additional diversity and functionality. In 2010, Xu et al. reported a hybrid DNA gel in which ssDNA chains were spread on the surface of graphene oxide (GO) sheets via stacking interactions. In this report, dsDNA was mixed with GO dispersions and upon heating, the formed ssDNA was adsorbed to the GO sheets via strong noncovalent forces, such as π-π stacking interactions [60]. By using this method, an ultrasensitive method for the detection of mitochondrial DNA mutations was developed by an impedimetric approach [61]. An ultrasensitive detection platform for a K-ras point mutation was developed by the assistance of DNA-carrying hydrogel microspheres. In this sandwich-format assay, peptide nucleic acid (PNA) probes immobilized on the sensor surface acted as the analyte capture molecule. After hybridization of the analyte to the PNA probe, the acrylamide-DNA microspheres were bound to the target DNA which could be observed an increase in dielectric constant. By employing these microspheres, the sensitivity improved about 100 fold in comparison to the non-amplified detection state [62,63]. In addition, Hwang and coworkers designed a fluorescence nanoplatform using hyaluronic acid conjugated graphene oxide (HA-GO) for sensing the oncogenic microRNA. Herein, antisense miR-21 peptide nucleic acid (PNA) was labeled with the fluorescent dye Cyanine3 and was loaded onto HA-GO via an “off” fluorescent signal (Fig. 11A). In the presence of CD44-positive cells and endogenous miR-21, the fluorescence signal recovered [64]. More recently, a fluorescent sensing method based on GO hydrogel was developed for the detection of the antibiotic oxytetracycline (OTC), in which adenosine and antibiotic-binding aptamer served as co-crosslinkers of the GO sheets (Fig. 11B). Here, the aptamers were labeled with FAM of which the fluorescence was quenched by GO sheets, due to the fluorescence resonance energy transfer (FRET) phenomenon Upon binding of the aptamer to the antibiotic, the complex was released from the GO surface and the fluorescence signal of the dye was restored. This approach could detect OTC with a limit of quantitation (LOQ) of 25 μg/L. This method was further extended to other functional components [65,66].
Fig. 11.
(A) Schematic of the miR-21 detection using hyaluronic acid conjugated graphene oxide (HA-GO), adapted from ref. [62]. (B) The mechanism of oxytetracycline (OTC) fluorescent detection based on target-responsive graphene oxide hydrogel, adapted from ref. [63].
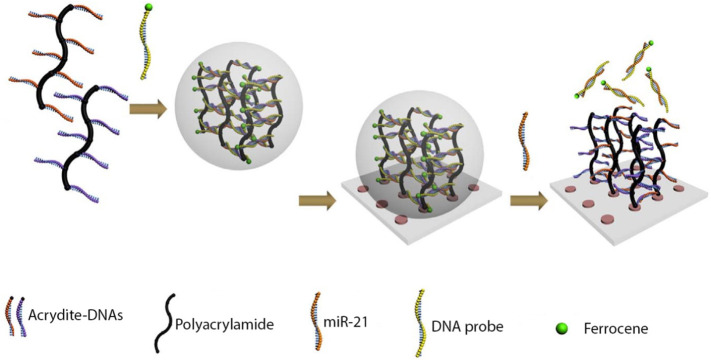
Indium-tin-oxide/polyethylene terephthalate (ITO/PET) is another type of functional material, which has been used for the detection of microRNA, protein, and small cues. In a typical study, grafted DNA strands immobilized on the surface of ITO/PET electrode and also bound to ferrocene-tagged DNAs (recognition probes) (Fig. 12 ). By addition of miR-21, the recognition probe hybridized to the miR-21 that subsequently released the duplexes from the ITO surface. After that, the hydrogel dissolved and the ferrocene tags were released and induced a reduction in the electrode current which was monitored by cyclic voltammetry and differential pulse voltammetry [67]. Moreover, another surface immobilized DNA hydrogel was synthesized on the ITO electrode through a surficial primer-induced strategy. In this work, the scaffold hydrogel was constructed by linear rolling circle amplification (LRCA) and multi-primed rolling circle amplification (MRCA), respectively and enzymes were captured in the hydrogel structure. Bilirubin oxidase (BOD) was captured in the gel for the detection of bilirubin and, the decrease in absorbance was linearly related to the concentration of bilirubin. Additionally, sensing of hydrogen peroxide was performed through the horseradish peroxidase-catalyzed redox reaction using the maximum light absorption at 414 nm [68].
Fig. 12.
Illustration of an electrochemical based DNA hydrogel assay to detect target miR-21.
Adapted from ref. [65].
In recent years, microfabricated cantilevers have emerged as a substantial analytical instrument for chemical and biological detections. Accordingly, this tool has considerable implications for nucleic acid sensing. To achieve this goal, a stimuli-responsive chitosan film was deposited onto the cantilever surface for the sensing of nucleic acids (cantilever arrays). Herein, after binding the target to a DNA probe-conjugated chitosan hydrogel, the extent of binding of the cantilever was monitored by optical laser detectors or capacitance analyzers [69,70]. Moreover, an enzyme-free microRNA detection system was reported by a strand displacement reaction (SDR) amplification strategy with ferrocene (Fc) as a signal molecule. Herein, probe DNAs were deposited on N-carboxymethyl chitosan/molybdenum carbide nanocomposite material. In the presence of miR-21, two hairpin DNAs (HDNA1 and Fc-modified HDNA2) created a duplex structure by SDR with a single stranded overhang which was captured by the probe DNA. The Fc group of the modified HDNA2 is now in close proximity to the electrode surface resulting in an enhancement of the electrochemical signal. By using this method, the target miRNA could be determined with a detection limit of 0.34 f. and the detection of breast cancer-related miR-21 was performed [71]. In another study, a sensing platform was designed for the detection of the ssrA gene in Listeria monocytogenes. For this purpose, an ssDNA probe against the ssrA gene was immobilized on a carboxymethylated dextran layer that was grafted onto a graphite electrode. By introducing the biotin-labeled target and the glucose oxidase-avidin conjugate to the system, hybridization of the immobilized probe with the target occurred accompanied by the oxidation of glucose. The generated current was measured using cyclic voltammetry with a LOD of 0.2 nmol [72].
2.7. DNA hydrogels for point-of-care (POC) applications
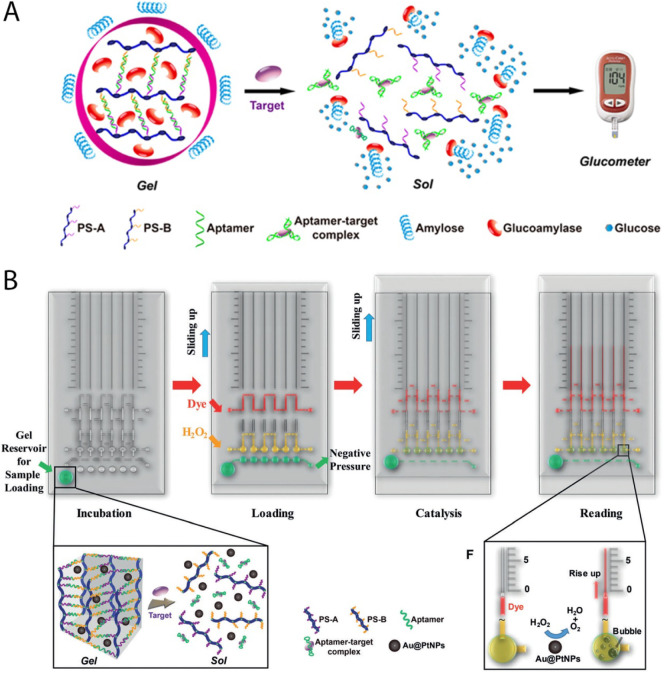
In recent years, substantial efforts have been directed toward the development of point-of-care testing (POCT) devices that meet the requirements of stability, portability, ease of storage, and low cost. Accordingly, DNA crosslinked hydrogels have been shown to be ideal signal transduction strategies for POCT [73,74]. In this pursuit, Zhu et al. designed a target-responsive gel via a personal glucose meter (PGM) to monitor non-glucose targets such as cocaine by applying the respective aptamers. As exhibited in Fig. 13A, two short DNAs grafted on linear polyacrylamide (PS-A and PS-B) were complementary to the adjacent regions of an aptamer sequence. By assembly of the gel, glucoamylase was trapped inside of the gel and separated it from its substrate on the outside of the gel (amylose). Binding of the target to the aptamer resulted in the collapse of the gel and released glucoamylase that hydrolyzed the amylose to glucose for quantification by the PGM. Herein, there was a quantitative relationship between the target concentration and PGM readout [75].
Fig. 13.
A) Schematic of the target-responsive hydrogel combined with a PGM, adapted from ref. [74]. B) Schematic of the Au@Pt NP-encapsulated hydrogel for visual detection, adapted from ref. [75].
Since the incorporation of a hydrogel into a PGM cannot be simply established, the same group introduced a novel quantitative assay to address this problem. As exhibited in Fig. 13B, this approach used an Au core/Pt shell nanoparticle (Au@PtNP), which was entrapped in a hydrogel, and a volumetric bar-chart chip (HV-Chip) as a visual quantitative readout. Upon target introduction and formation of the aptamer-target complexes, the gel was broken down and resulted in the release of the entrapped Au@PtNPs. Then by applying a negative pressure and sliding up, the supernatant was brought into contact with H2O2, which resulted in the H2O2 decomposition into O2. Subsequently red ink was pushed upwards into the top channel by the O2 bubbles that the migrated distance of the ink was in proportion with the target concentration. Using this method, cocaine could be detected at a concentration lower than 1 μM [76] and was additionally used for the detection of other targets such as ochratoxin A [77] and Aflatoxin B1 in beer, with a limit of detection of 1.77 nm [78]. Furthermore, in other similar work, the detection of uranium and lead were reported by utilizing a DNAzyme-substrate complex instead of the aptamer structure [79,80].
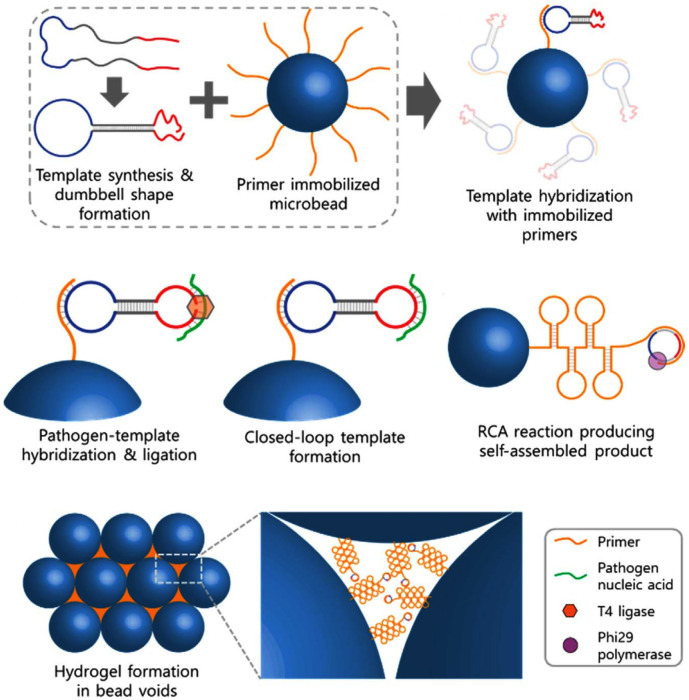
Employing microfluidic systems, Lee and coworkers developed a diagnosis platform using isothermal an amplification process to diagnose Ebola and Bacillus Anthracis concurrently. In their approach, a template strand consisting of three different regions was designed. These regions included a primer binding site for hybridization to an immobilized primer, a pathogen-binding site, and a self-assembly sequence to make a dumbbell shape structure. When the template strand was hybridized to the respective target, a closed dumbbell shape structure was generated. The structure was amplified via rolling circle amplification (RCA) to produce a hydrogel amenable to block the microfluidic flow channels [81]. Despite the high sensitivity and selectivity of this method due to the limited surface area, the whole process took 2 h to completely block the flow within the channel and also required a large amount of DNA strands to assemble the gel. To overcome this drawback, several improvements have been made that were based on the same principle. For instance, Jung et al. described a platform in which the introduction of short DNA primers complementary to the DNA products, initiated a DNA polymerization reaction that resulted in a quick shrinkage of the hydrogel construction. By means of this assay, a robust and fast diagnosis of Middle East respiratory syndrome coronavirus (MERS-CoV) became possible with a 100-fold enhancement in sensitivity after 30 min of reaction time [82]. More recently, Na and coworkers presented a system in which the primers were immobilized on microbeads instead of channel surface immobilization (Fig. 14 ). Accordingly, the detection time of this assay was reduced to less than 15 min and had a limit of detection (LOD) of 10–100 times lower than the previously reported systems [83].
Fig. 14.
Schematic of the detection of pathogenic viruses driven by DNA hydrogel formation through RCA of primer immobilized microbeads.
Adapted from ref. [82].
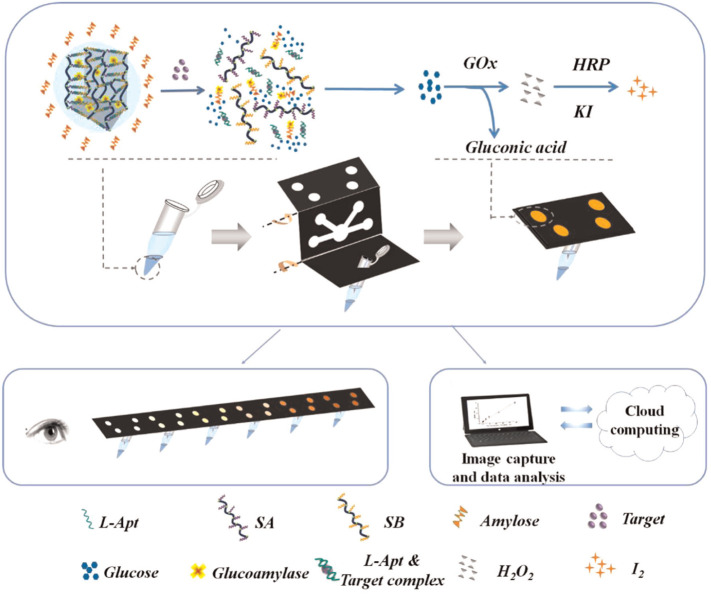
In 2015, a versatile approach was described in which a microfluidic paper-based analytic device (μPAD) integrated with a DNA was used for the diagnosis of several cues including adenosine, cocaine, and Pb2+. Upon binding of the target, the hydrogel structure collapsed which resulted in the flow of a solution that carried a colored dye molecule [63]. The same group introduced a novel method based on integrating a glucoamylase-trapped hydrogel with a μPAD and cascaded enzymatic reactions. As shown in Fig. 15 , exposure of the gel to the target resulted in the collapse of the hydrogel and hydrolysis of amylose to glucose by the released glucoamylase. The formed glucose diffused to the test-zone and was oxidized by glucose oxidase (GOx), which resulted in the formation of H2O2 that was in turn reduced by horseradish peroxidase (HRP). This led to the oxidization of the colorless iodide to brown iodine, in which the color change in the testing wells was observed by the naked eye. This platform was used for the semi-quantitative detection of various targets such as adenosine and cocaine [84].
Fig. 15.
Illustration of the colorimetric detection of adenosine and cocaine employing a hydrogel–μPAD system.
Adapted from ref. [83].
3. Conclusions and perspectives
In this contribution, we offered a comprehensive review that describes that how DNA-based hydrogels have been applied in biosensing accompanied by recent applications that illustrate the versatility of these materials. Despite substantial progress in this field, several challenges must be addressed to take this field forward. First, despite the simple and direct visual sensing of various cues, sensitivity and quantification obstacles have limited the usage of DNA hydrogels. Therefore, designing intelligent gels that quickly respond to macromolecular input is a major challenge. Fortunately, by taking advantage of signal amplification mechanisms such as the amylase/iodide system and cascade reactions, the sensitivity can be significantly enhanced. Another issue that should be considered is the diffusion time of the cargo that severely depends on the cargo size. Slow diffusion can disturb the sensibility and severely lengthen the monitoring time. To solve this problem, the introduction of novel ways to enhance the response rate is required. Overall, the aforementioned challenges can be a prospective route for designing improved hydrogel-based sensing systems. It should be noted that this class of polymers will support more advanced applications in biosensor development, controlled drug delivery, in vivo sensing, mechanical actuators, and novel photodynamic materials. In addition, DNA hydrogels may lay the foundation for the generation of soft robots or cell-free synthetic biology. Although these studies are still at the conceptual level, they represent promising potential usages of DNA hydrogels.
Declaration of Competing Interest
The authors report no relationships that could be construed as a conflict of interest.
Acknowledgments
This work was supported by Shahid Bahonar University of Kerman, Kerman, Iran.
References
- 1.Ahmed E.M. Hydrogel: preparation, characterization, and applications: a review. J Adv Res. 2015;6:105–121. doi: 10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willner I. ACS Publications; 2017. Stimuli-controlled hydrogels and their applications. [DOI] [PubMed] [Google Scholar]
- 3.Kahn J.S., Hu Y., Willner I. Stimuli-responsive DNA-based hydrogels: from basic principles to applications. Acc Chem Res. 2017;50:680–690. doi: 10.1021/acs.accounts.6b00542. [DOI] [PubMed] [Google Scholar]
- 4.Xiong X., Wu C., Zhou C., Zhu G., Chen Z., Tan W. Responsive DNA-based hydrogels and their applications. Macromol Rapid Commun. 2013;34:1271–1283. doi: 10.1002/marc.201300411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D., Hu Y., Liu P., Luo D. Bioresponsive DNA hydrogels: beyond the conventional stimuli responsiveness. Acc Chem Res. 2017;50:733–739. doi: 10.1021/acs.accounts.6b00581. [DOI] [PubMed] [Google Scholar]
- 6.Khimji I., Kelly E.Y., Helwa Y., Hoang M., Liu J. Visual optical biosensors based on DNA-functionalized polyacrylamide hydrogels. Methods. 2013;64:292–298. doi: 10.1016/j.ymeth.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Topuz F., Okay O. Rheological behavior of responsive DNA hydrogels. Macromolecules. 2008;41:8847–8854. [Google Scholar]
- 8.Li J., Mo L., Lu C.-H., Fu T., Yang H.-H., Tan W. Functional nucleic acid-based hydrogels for bioanalytical and biomedical applications. Chem Soc Rev. 2016;45:1410–1431. doi: 10.1039/c5cs00586h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Culver H.R., Clegg J.R., Peppas N.A. Analyte-responsive hydrogels: intelligent materials for biosensing and drug delivery. Acc Chem Res. 2017;50:170–178. doi: 10.1021/acs.accounts.6b00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J. Oligonucleotide-functionalized hydrogels as stimuli responsive materials and biosensors. Soft Matter. 2011;7:6757–6767. [Google Scholar]
- 11.Kashanian S., Rafipour R., Tarighat F., Ravan H. Immobilisation of cobaltferritin onto gold electrode based on self-assembled monolayers. IET Nanobiotechnol. 2012;6:102–109. doi: 10.1049/iet-nbt.2011.0042. [DOI] [PubMed] [Google Scholar]
- 12.Ebrahimi A., Ravan H., Khajouei S. DNA nanotechnology and bioassay development. TrAC Trends Anal Chem. 2019;114:126–142. [Google Scholar]
- 13.Kashanian S., Ravan H., Ghobadi S., Omidfar K., Askari S. Structural and functional study of rabbit polyclonal antibody for immunoassay purposes. Hybridoma. 2008;27:48–53. doi: 10.1089/hyb.2007.0547. [DOI] [PubMed] [Google Scholar]
- 14.Sharifzadeh G., Hosseinkhani H. Biomolecule-responsive hydrogels in medicine. Adv Healthc Mater. 2017;6 doi: 10.1002/adhm.201700801. (1700801) [DOI] [PubMed] [Google Scholar]
- 15.Ravan H., Yazdanparast R. Loop region-specific oligonucleotide probes for loop-mediated isothermal amplification–enzyme-linked immunosorbent assay truly minimize the instrument needed for detection process. Anal Biochem. 2013;439:102–108. doi: 10.1016/j.ab.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Sader J.E., Larson I., Mulvaney P., White L.R. Method for the calibration of atomic force microscope cantilevers. Rev Sci Instrum. 1995;66:3789–3798. [Google Scholar]
- 17.Baeissa A., Dave N., Smith B.D., Liu J. DNA-functionalized monolithic hydrogels and gold nanoparticles for colorimetric DNA detection. ACS Appl Mater Interfaces. 2010;2:3594–3600. doi: 10.1021/am100780d. [DOI] [PubMed] [Google Scholar]
- 18.He Y., Yang X., Yuan R., Chai Y. Switchable target-responsive 3D DNA hydrogels as a signal amplification strategy combining with SERS technique for ultrasensitive detection of miRNA 155. Anal Chem. 2017;89:8538–8544. doi: 10.1021/acs.analchem.7b02321. [DOI] [PubMed] [Google Scholar]
- 19.Ravan H. Translating nucleic-acid hybridization into universal DNA-reporter sequences. TrAC Trends Anal Chem. 2015;65:97–106. [Google Scholar]
- 20.Chapin S.C., Doyle P.S. Ultrasensitive multiplexed microRNA quantification on encoded gel microparticles using rolling circle amplification. Anal Chem. 2011;83:7179–7185. doi: 10.1021/ac201618k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi N.W., Kim J., Chapin S.C., Duong T., Donohue E., Pandey P., et al. Multiplexed detection of mRNA using porosity-tuned hydrogel microparticles. Anal Chem. 2012;84:9370–9378. doi: 10.1021/ac302128u. [DOI] [PubMed] [Google Scholar]
- 22.Lee J., Bisso P.W., Srinivas R.L., Kim J.J., Swiston A.J., Doyle P.S. Universal process-inert encoding architecture for polymer microparticles. Nat Mater. 2014;13:524. doi: 10.1038/nmat3938. [DOI] [PubMed] [Google Scholar]
- 23.Choi W., Yeom S.Y., Kim J., Jung S., Jung S., Shim T.S., et al. Hydrogel micropost-based qPCR for multiplex detection of miRNAs associated with Alzheimer's disease. Biosens Bioelectron. 2018;101:235–244. doi: 10.1016/j.bios.2017.10.039. [DOI] [PubMed] [Google Scholar]
- 24.Gawel K., Stokke B.T. Logic swelling response of DNA–polymer hybrid hydrogel. Soft Matter. 2011;7:4615–4618. [Google Scholar]
- 25.Pu F., Ren J., Qu X. Nucleic acids and smart materials: advanced building blocks for logic systems. Adv Mater. 2014;26:5742–5757. doi: 10.1002/adma.201401617. [DOI] [PubMed] [Google Scholar]
- 26.Wang F., Liu X., Willner I. DNA switches: from principles to applications. Angew Chem Int Ed. 2015;54:1098–1129. doi: 10.1002/anie.201404652. [DOI] [PubMed] [Google Scholar]
- 27.Dong Y., Yang Z., Liu D. DNA nanotechnology based on i-motif structures. Acc Chem Res. 2014;47:1853–1860. doi: 10.1021/ar500073a. [DOI] [PubMed] [Google Scholar]
- 28.Cheng E., Xing Y., Chen P., Yang Y., Sun Y., Zhou D., et al. A pH-triggered, fast-responding DNA hydrogel. Angew Chem Int Ed. 2009;48:7660–7663. doi: 10.1002/anie.200902538. [DOI] [PubMed] [Google Scholar]
- 29.Xu W., Huang Y., Zhao H., Li P., Liu G., Li J., et al. DNA hydrogel with tunable pH-responsive properties produced by rolling circle amplification. Chemistry. 2017;23:18276–18281. doi: 10.1002/chem.201704390. [DOI] [PubMed] [Google Scholar]
- 30.Liao W.-C., Lilienthal S., Kahn J.S., Riutin M., Sohn Y.S., Nechushtai R., et al. pH-and ligand-induced release of loads from DNA–acrylamide hydrogel microcapsules. Chem Sci. 2017;8:3362–3373. doi: 10.1039/c6sc04770j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo W., Lu C.H., Qi X.J., Orbach R., Fadeev M., Yang H.H., et al. Switchable bifunctional stimuli-triggered poly-N-isopropylacrylamide/DNA hydrogels. Angew Chem Int Ed. 2014;53:10134–10138. doi: 10.1002/anie.201405692. [DOI] [PubMed] [Google Scholar]
- 32.Kaniewska K., Kowalczyk A., Karbarz M., Nowicka A.M. Changes in the volume phase transition temperature of hydrogels for detection of the DNA hybridization process. Analyst. 2016;141:5815–5821. doi: 10.1039/c6an00523c. [DOI] [PubMed] [Google Scholar]
- 33.Xing Y., Cheng E., Yang Y., Chen P., Zhang T., Sun Y., et al. Self-assembled DNA hydrogels with designable thermal and enzymatic responsiveness. Adv Mater. 2011;23:1117–1121. doi: 10.1002/adma.201003343. [DOI] [PubMed] [Google Scholar]
- 34.Zhou X., Li C., Shao Y., Chen C., Yang Z., Liu D. Reversibly tuning the mechanical properties of a DNA hydrogel by a DNA nanomotor. Chem Commun. 2016;52:10668–10671. doi: 10.1039/c6cc04724f. [DOI] [PubMed] [Google Scholar]
- 35.Fozooni T., Ravan H., Sasan H. Signal amplification technologies for the detection of nucleic acids: from cell-free analysis to live-cell imaging. Appl Biochem Biotechnol. 2017;183:1224–1253. doi: 10.1007/s12010-017-2494-4. [DOI] [PubMed] [Google Scholar]
- 36.Zhao H., Jiang G., Weng J., Ma Q., Zhang H., Ito Y., et al. A signal-accumulating DNAzyme-crosslinked hydrogel for colorimetric sensing of hydrogen peroxide. J Mater Chem B. 2016;4:4648–4651. doi: 10.1039/c6tb00825a. [DOI] [PubMed] [Google Scholar]
- 37.Fedotova T.A., Kolpashchikov D.M. Liquid-to-gel transition for visual and tactile detection of biological analytes. Chem Commun. 2017;53:12622–12625. doi: 10.1039/c7cc07035g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mao X., Pan S., Zhou D., He X., Zhang Y. Fabrication of DNAzyme-functionalized hydrogel and its application for visible detection of circulating tumor DNA. Sens Actuators B. 2019;285:385–390. [Google Scholar]
- 39.Lin Y., Wang X., Sun Y., Dai Y., Sun W., Zhu X., et al. A chemiluminescent biosensor for ultrasensitive detection of adenosine based on target-responsive DNA hydrogel with Au@ HKUST-1 encapsulation. Sens Actuators B. 2019;289:56–64. [Google Scholar]
- 40.Srinivas R.L., Chapin S.C., Doyle P.S. Aptamer-functionalized microgel particles for protein detection. Anal Chem. 2011;83:9138–9145. doi: 10.1021/ac202335u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu C.-H., Qi X.-J., Li J., Yang H.-H. Aptamers Selected by Cell-SELEX for Theranostics. Springer; 2015. Aptamer-based Hydrogels and Their Applications; pp. 163–195. [Google Scholar]
- 42.Yang H., Liu H., Kang H., Tan W. Engineering target-responsive hydrogels based on aptamer− target interactions. J Am Chem Soc. 2008;130:6320–6321. doi: 10.1021/ja801339w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma Y., Mao Y., An Y., Tian T., Zhang H., Yan J., et al. Target-responsive DNA hydrogel for non-enzymatic and visual detection of glucose. Analyst. 2018;143:1679–1684. doi: 10.1039/c8an00010g. [DOI] [PubMed] [Google Scholar]
- 44.Deshpande S.R., Hammink R., Nelissen F.H., Rowan A.E., Heus H.A. Biomimetic stress sensitive hydrogel controlled by DNA nanoswitches. Biomacromolecules. 2017;18:3310–3317. doi: 10.1021/acs.biomac.7b00964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iwasaki Y., Kondo J.-i., Kuzuya A., Moriyama R. Crosslinked duplex DNA nanogels that target specified proteins. Sci Technol Adv Mater. 2016;17:285–292. doi: 10.1080/14686996.2016.1189798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El-Hamed F., Dave N., Liu J. Stimuli-responsive releasing of gold nanoparticles and liposomes from aptamer-functionalized hydrogels. Nanotechnology. 2011;22 doi: 10.1088/0957-4484/22/49/494011. (494011) [DOI] [PubMed] [Google Scholar]
- 47.Bai W., Gariano N.A., Spivak D.A. Macromolecular amplification of binding response in superaptamer hydrogels. J Am Chem Soc. 2013;135:6977–6984. doi: 10.1021/ja400576p. [DOI] [PubMed] [Google Scholar]
- 48.Song P., Ye D., Zuo X., Li J., Wang J., Liu H., et al. DNA hydrogel with aptamer-toehold-based recognition, cloaking, and decloaking of circulating tumor cells for live cell analysis. Nano Lett. 2017;17:5193–5198. doi: 10.1021/acs.nanolett.7b01006. [DOI] [PubMed] [Google Scholar]
- 49.Seelig G., Soloveichik D., Zhang D.Y., Winfree E. Enzyme-free nucleic acid logic circuits. Science. 2006;314:1585–1588. doi: 10.1126/science.1132493. [DOI] [PubMed] [Google Scholar]
- 50.Komatsu H., Matsumoto S., Tamaru S.-i., Kaneko K., Ikeda M., Hamachi I. Supramolecular hydrogel exhibiting four basic logic gate functions to fine-tune substance release. J Am Chem Soc. 2009;131:5580–5585. doi: 10.1021/ja8098239. [DOI] [PubMed] [Google Scholar]
- 51.Yin B.-C., Ye B.-C., Wang H., Zhu Z., Tan W. Colorimetric logic gates based on aptamer-crosslinked hydrogels. Chem Commun. 2012;48:1248–1250. doi: 10.1039/c1cc15639j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin F., Bao Y.-W., Wu F.-G. Carbon dots for sensing and killing microorganisms. C. 2019;5:33. [Google Scholar]
- 53.Sun X., Li G., Yin Y., Zhang Y., Li H. Carbon quantum dot-based fluorescent vesicles and chiral hydrogels with biosurfactant and biocompatible small molecule. Soft Matter. 2018;14:6983–6993. doi: 10.1039/c8sm01155a. [DOI] [PubMed] [Google Scholar]
- 54.Singh S., Mishra A., Kumari R., Sinha K.K., Singh M.K., Das P. Carbon dots assisted formation of DNA hydrogel for sustained release of drug. Carbon. 2017;114:169–176. [Google Scholar]
- 55.Norouzi A., Ravan H., Mohammadi A., Hosseinzadeh E., Norouzi M., Fozooni T. Aptamer–integrated DNA nanoassembly: a simple and sensitive DNA framework to detect cancer cells. Anal Chim Acta. 2018;1017:26–33. doi: 10.1016/j.aca.2018.02.037. [DOI] [PubMed] [Google Scholar]
- 56.Zhao Y., Zhao X., Tang B., Xu W., Li J., Hu J., et al. Quantum-dot-tagged bioresponsive hydrogel suspension array for multiplex label-free DNA detection. Adv Funct Mater. 2010;20:976–982. [Google Scholar]
- 57.Xu L., Wang R., Kelso L.C., Ying Y., Li Y. A target-responsive and size-dependent hydrogel aptasensor embedded with QD fluorescent reporters for rapid detection of avian influenza virus H5N1. Sens Actuators B. 2016;234:98–108. [Google Scholar]
- 58.Wu L., Wang Y., He R., Zhang Y., He Y., Wang C., et al. Fluorescence hydrogel array based on interfacial cation exchange amplification for highly sensitive microRNA detection. Anal Chim Acta. 2019;1080:206–214. doi: 10.1016/j.aca.2019.07.024. [DOI] [PubMed] [Google Scholar]
- 59.Li C., Li H., Ge J., Jie G. Versatile fluorescence detection of microRNA based on novel DNA hydrogel-amplified signal probes coupled with DNA walker amplification. Chem Commun. 2019;55:3919–3922. doi: 10.1039/c9cc00565j. [DOI] [PubMed] [Google Scholar]
- 60.Xu Y., Wu Q., Sun Y., Bai H., Shi G. Three-dimensional self-assembly of graphene oxide and DNA into multifunctional hydrogels. ACS Nano. 2010;4:7358–7362. doi: 10.1021/nn1027104. [DOI] [PubMed] [Google Scholar]
- 61.Sun L., Hu N., Peng J., Chen L., Weng J. Ultrasensitive detection of mitochondrial DNA mutation by graphene oxide/DNA hydrogel electrode. Adv Funct Mater. 2014;24:6905–6913. [Google Scholar]
- 62.Sato Y., Ikegaki S., Suzuki K., Kawaguchi H. Hydrogel-microsphere-enhanced surface plasmon resonance for the detection of a K-ras point mutation employing peptide nucleic acid. J Biomater Sci Polym Ed. 2003;14:803–820. doi: 10.1163/156856203768366530. [DOI] [PubMed] [Google Scholar]
- 63.Okumura A., Sato Y., Kyo M., Kawaguchi H. Point mutation detection with the sandwich method employing hydrogel nanospheres by the surface plasmon resonance imaging technique. Anal Biochem. 2005;339:328–337. doi: 10.1016/j.ab.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 64.Kim H.Y., Li F., Park J.Y., Kim D., Park J.H., Han H.S., et al. In vivo visualization of endogenous miR-21 using hyaluronic acid-coated graphene oxide for targeted cancer therapy. Biomaterials. 2017;121:144–154. doi: 10.1016/j.biomaterials.2016.12.028. [DOI] [PubMed] [Google Scholar]
- 65.Tan B., Zhao H., Du L., Gan X., Quan X. A versatile fluorescent biosensor based on target-responsive graphene oxide hydrogel for antibiotic detection. Biosens Bioelectron. 2016;83:267–273. doi: 10.1016/j.bios.2016.04.065. [DOI] [PubMed] [Google Scholar]
- 66.Cheng E., Li Y., Yang Z., Deng Z., Liu D. DNA-SWNT hybrid hydrogel. Chem Commun. 2011;47:5545–5547. doi: 10.1039/c1cc11028d. [DOI] [PubMed] [Google Scholar]
- 67.Liu S., Su W., Li Y., Zhang L., Ding X. Manufacturing of an electrochemical biosensing platform based on hybrid DNA hydrogel: taking lung cancer-specific miR-21 as an example. Biosens Bioelectron. 2018;103:1–5. doi: 10.1016/j.bios.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 68.Mao X., Chen G., Wang Z., Zhang Y., Zhu X., Li G. Surface-immobilized and self-shaped DNA hydrogels and their application in biosensing. Chem Sci. 2018;9:811–818. doi: 10.1039/c7sc03716c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koev S.T., Powers M.A., Yi H., Wu L.-Q., Bentley W.E., Rubloff G.W., et al. Mechano-transduction of DNA hybridization and dopamine oxidation through electrodeposited chitosan network. Lab Chip. 2007;7:103–111. doi: 10.1039/b609149k. [DOI] [PubMed] [Google Scholar]
- 70.Seong G.H., Zhan W., Crooks R.M. Fabrication of microchambers defined by photopolymerized hydrogels and weirs within microfluidic systems: application to DNA hybridization. Anal Chem. 2002;74:3372–3377. doi: 10.1021/ac020069k. [DOI] [PubMed] [Google Scholar]
- 71.Tian L., Qi J., Ma X., Wang X., Yao C., Song W., et al. A facile DNA strand displacement reaction sensing strategy of electrochemical biosensor based on N-carboxymethyl chitosan/molybdenum carbide nanocomposite for microRNA-21 detection. Biosens Bioelectron. 2018;122:43–50. doi: 10.1016/j.bios.2018.09.037. [DOI] [PubMed] [Google Scholar]
- 72.Hajdukiewicz J., Boland S., Kavanagh P., Leech D. An enzyme-amplified amperometric DNA hybridisation assay using DNA immobilised in a carboxymethylated dextran film anchored to a graphite surface. Biosens Bioelectron. 2010;25:1037–1042. doi: 10.1016/j.bios.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 73.Ravan H., Amandadi M. Analysis of yeh fimbrial gene cluster in Escherichia coli O157: H7 in order to find a genetic marker for this serotype. Curr Microbiol. 2015;71:274–282. doi: 10.1007/s00284-015-0842-6. [DOI] [PubMed] [Google Scholar]
- 74.Ramroodi N., Khani M., Ganjali Z., Javan M.R., Sanadgol N., Khalseh R., et al. Prophylactic effect of BIO-1211 small-molecule antagonist of VLA-4 in the EAE mouse model of multiple sclerosis. Immunol Invest. 2015;44:694–712. doi: 10.3109/08820139.2015.1085391. [DOI] [PubMed] [Google Scholar]
- 75.Yan L., Zhu Z., Zou Y., Huang Y., Liu D., Jia S., et al. Target-responsive “sweet” hydrogel with glucometer readout for portable and quantitative detection of non-glucose targets. J Am Chem Soc. 2013;135:3748–3751. doi: 10.1021/ja3114714. [DOI] [PubMed] [Google Scholar]
- 76.Zhu Z., Guan Z., Jia S., Lei Z., Lin S., Zhang H., et al. Au@ Pt nanoparticle encapsulated target-responsive hydrogel with volumetric bar-chart chip readout for quantitative point-of-care testing. Angew Chem Int Ed. 2014;53:12503–12507. doi: 10.1002/anie.201405995. [DOI] [PubMed] [Google Scholar]
- 77.Liu R., Huang Y., Ma Y., Jia S., Gao M., Li J., et al. Design and synthesis of target-responsive aptamer-cross-linked hydrogel for visual quantitative detection of Ochratoxin A. ACS Appl Mater Interfaces. 2015;7:6982–6990. doi: 10.1021/acsami.5b01120. [DOI] [PubMed] [Google Scholar]
- 78.Ma Y., Mao Y., Huang D., He Z., Yan J., Tian T., et al. Portable visual quantitative detection of aflatoxin B 1 using a target-responsive hydrogel and a distance-readout microfluidic chip. Lab Chip. 2016;16:3097–3104. doi: 10.1039/c6lc00474a. [DOI] [PubMed] [Google Scholar]
- 79.Huang Y., Fang L., Zhu Z., Ma Y., Zhou L., Chen X., et al. Design and synthesis of target-responsive hydrogel for portable visual quantitative detection of uranium with a microfluidic distance-based readout device. Biosens Bioelectron. 2016;85:496–502. doi: 10.1016/j.bios.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 80.Ravan H., Yazdanparast R. Development of a new loop-mediated isothermal amplification assay for prt (rfbS) gene to improve the identification of Salmonella serogroup D. World J Microbiol Biotechnol. 2012;28:2101–2106. doi: 10.1007/s11274-012-1014-5. [DOI] [PubMed] [Google Scholar]
- 81.Lee H.Y., Jeong H., Jung I.Y., Jang B., Seo Y.C., Lee H., et al. DhITACT: DNA hydrogel formation by isothermal amplification of complementary target in fluidic channels. Adv Mater. 2015;27:3513–3517. doi: 10.1002/adma.201500414. [DOI] [PubMed] [Google Scholar]
- 82.Jung I.Y., You J.B., Choi B.R., Kim J.S., Lee H.K., Jang B., et al. A highly sensitive molecular detection platform for robust and facile diagnosis of Middle East respiratory syndrome (MERS) corona virus. Adv Healthc Mater. 2016;5:2168–2173. doi: 10.1002/adhm.201600334. [DOI] [PubMed] [Google Scholar]
- 83.Na W., Nam D., Lee H., Shin S. Rapid molecular diagnosis of infectious viruses in microfluidics using DNA hydrogel formation. Biosens Bioelectron. 2018;108:9–13. doi: 10.1016/j.bios.2018.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tian T., Wei X., Jia S., Zhang R., Li J., Zhu Z., et al. Integration of target responsive hydrogel with cascaded enzymatic reactions and microfluidic paper-based analytic devices (μPADs) for point-of-care testing (POCT) Biosens Bioelectron. 2016;77:537–542. doi: 10.1016/j.bios.2015.09.049. [DOI] [PubMed] [Google Scholar]