Abstract
Objectives
Lung remains the least-utilized solid organ for transplantation. Efforts to recover donor lungs with reversible injuries using ex vivo perfusion systems are limited to <24 hours of support. Here, we demonstrate the feasibility of extending normothermic extracorporeal lung support to 4 days using cross-circulation with conscious swine.
Methods
A swine behavioral training program and custom enclosure were developed to enable multiday cross-circulation between extracorporeal lungs and recipient swine. Lungs were ventilated and perfused in a normothermic chamber for 4 days. Longitudinal analyses of extracorporeal lungs (ie, functional assessments, multiscale imaging, cytokine quantification, and cellular assays) and recipient swine (eg, vital signs and blood and tissue analyses) were performed.
Results
Throughout 4 days of normothermic support, extracorporeal lung function was maintained (arterial oxygen tension/inspired oxygen fraction >400 mm Hg; compliance >20 mL/cm H2O), and recipient swine were hemodynamically stable (lactate <3 mmol/L; pH, 7.42 ± 0.05). Radiography revealed well-aerated lower lobes and consolidation in upper lobes of extracorporeal lungs, and bronchoscopy showed healthy airways without edema or secretions. In bronchoalveolar lavage fluid, granulocyte-macrophage colony-stimulating factor, interleukin (IL) 4, IL-6, and IL-10 levels increased less than 6-fold, whereas interferon gamma, IL-1α, IL-1β, IL-1ra, IL-2, IL-8, IL-12, IL-18, and tumor necrosis factor alpha levels decreased from baseline to day 4. Histologic evaluations confirmed an intact blood–gas barrier and outstanding preservation of airway and alveolar architecture. Cellular viability and metabolism in extracorporeal lungs were confirmed after 4 days.
Conclusions
We demonstrate feasibility of normothermic maintenance of extracorporeal lungs for 4 days by cross-circulation with conscious swine. Cross-circulation approaches could support the recovery of damaged lungs and enable organ bioengineering to improve transplant outcomes.
Key Words: acute lung injury, airway lavage, alveolar recruitment, bronchoalveolar lavage fluid, chimerism, cross-circulation, extracorporeal membrane oxygenation, ex vivo lung perfusion, infrared thermography, lung bioengineering, lung transplantation, medical thermography, normothermic organ perfusion, organ shortage, regenerative medicine, swine model, tissue engineering, transplantation, whole organ bioreactor
Abbreviations and Acronyms: BAL, bronchoalveolar lavage; EVLP, ex vivo lung perfusion; IFNγ, interferon gamma; IL, interleukin; TNFα, tumor necrosis factor alpha; XC, cross-circulation
Graphical abstract

Multiday normothermic support system for extracorporeal lungs using cross-circulation.
Central Message.
Normothermic support of extracorporeal lungs for 4 days is feasible using cross-circulation and could enhance recovery of donor lungs and enable methods to bioengineer lungs for transplantation.
Perspective.
Conventional ex vivo lung perfusion systems offer limited time for recovery and therapeutic intervention in extracorporeal lungs. We demonstrate that normothermic preservation of extracorporeal lung tissue structure and respiratory function can be maintained for 4 days using cross-circulation. This system could serve as a platform for lung bioengineering and organ recovery and regeneration.
See Commentaries on pages 1654 and 1656.
Lung transplantation, the only life-saving intervention for patients with end-stage lung disease, remains limited by the shortage of usable donor organs. Although the number of patients waiting to receive a lung transplant continues to rise, only 20% of donor lungs meet functional criteria for transplantation.1 This underuse of donor organs combined with the rising number of patients in need represents a major contributor to waitlist mortality.2 , 3
Many of the conditions that render donor lungs unacceptable for transplantation (eg, aspiration, infection, and pulmonary contusions) are potentially reversible, but conventional methods of donor lung preservation rely on nonphysiologic cold static ischemia and preclude endogenous repair and recovery.4 , 5 Ex vivo lung perfusion (EVLP) aims to address these limitations by providing initially unacceptable donor lungs with physiologic conditions—normothermia, perfusion, ventilation—to recover function outside the body to a level acceptable for transplantation.6 , 7 Since the introduction of EVLP by Steen and colleagues in 2001,8 EVLP platforms have demonstrated short-term support and recovery of marginal quality donor lungs in preclinical and clinical settings.8, 9, 10, 11 However, EVLP has been unable to recover the majority of unusable donor lungs, likely due to the inability of an isolated single-organ support system to provide an appropriate physiologic milieu that enables endogenous repair.
Despite efforts to address the shortage of transplantable lungs, physiologic constraints limit lung perfusion and preservation times, and thus restrict opportunities for donor organ recovery and bioengineering. To overcome these limitations, our group previously used a swine model of cross-circulation (XC) to establish a lung support system that extended normothermic extracorporeal support to 36 hours and enabled statistically significant ex vivo recovery of severely injured lungs.12 , 13
The use of an XC system to achieve ex vivo lung recovery mimics the clinical setting where in situ recovery of marginal quality lungs is achieved in patients placed on extracorporeal membrane oxygenation support for several days after transplantation.14, 15, 16 Motivated by this clinical practice, we hypothesize that extending the duration of extracorporeal support from hours to days could not only enable the recovery of damaged lungs not currently salvageable using EVLP systems but also enable investigation of bioengineering strategies to improve or personalize organs before transplantation.
In this proof-of-feasibility study, we developed an extracorporeal lung support system capable of maintaining lungs for 4 days using XC (Figure 1 ). A notable difference between this study and our previous studies, wherein swine recipients remained under anesthesia for the duration of extracorporeal support and recovery, is that we established a configuration in which swine recipients remained conscious throughout the procedure. Such an approach avoids the adverse effects of anesthetic agents and recipient immobility, provides access to nutrition ad libitum, and models a translational setup of XC between a patient and a donor organ. Throughout 4 days of normothermic support, all extracorporeal lungs and conscious swine recipients were subjected to longitudinal analyses to assess the safety and stability of the organ support system.
Figure 1.
Experimental overview of multiday extracorporeal lung support system. A, Lungs were explanted from donor swine and the pulmonary artery (PA) and pulmonary vein (PV) were cannulated. Extracorporeal lungs were maintained in a humidified, normothermic preservation chamber and connected to a mechanical ventilator (V) via endotracheal intubation (T). Recipient swine were cannulated with a dual-lumen cannula via the right internal jugular (RIJ) vein. After recovery from anesthesia, recipient swine were placed in a custom enclosure for the duration of the procedure. The extracorporeal circuit contained a centrifugal pump (P) that cross-circulated whole blood between the recipient swine and the extracorporeal lungs. In-line sensors (S) monitored the hemodynamic stability of the recipient swine and extracorporeal lungs during multiday normothermic support. B, Experimental timeline. Extracorporeal lungs were maintained by normothermic cross-circulation (XC) and periodically assessed for 4 days. BAL, Bronchoalveolar lavage.
Methods
Study Design
This study received approval from the Institutional Animal Care and Use Committee at Columbia University. In this proof-of-feasibility study, we investigated healthy swine lungs (n = 3) as a reproducible experimental input to assess the ability of the extracorporeal lung support system to maintain the structure, function, and integrity of extracorporeal lungs for 4 days, and to establish baseline values and methodologies. The mean total normothermic extracorporeal support time of all procedures was 100.7 ± 1.2 hours.
Animals
Six Yorkshire swine (3 donor–recipient pairs, aged 4-6 months) were used in this study. Swine lung donors had a mean weight of 43.3 kg (range, 35.7-57.0 kg), and swine recipients had a mean weight of 53.0 kg (range, 41.5-59.0 kg). No animals died in the course of this study.
Donor Lung Procurement
Swine lungs were procured in standard fashion as previously described.12 , 13 The mean duration of cold static lung preservation was 4.8 ± 0.6 hours.
Extracorporeal Lung Cannulation
A 20F cannula was secured within the pulmonary artery, and the trachea was intubated with a 7.5 mm cuffed endotracheal tube, as previously described.12 , 13 , 17 The aortic arch, serving as an endothelialized biobridge between the lungs and the extracorporeal circuit, was secured to the left atrial cuff with a running 6–0 polypropylene suture. A 36F venous drainage cannula was secured to the biobridge with a 2–0 braided polyester tie. Lungs were placed in the organ preservation chamber in prone position in a sterile, double-lined organ basin containing warm normal saline.
Recipient Swine Cannulation
Recipient swine underwent general anesthesia after intramuscular induction with tiletamine (5 mg/kg). Cefazolin (30 mg/kg) and enrofloxacin (5 mg/kg) were administered before skin incision and re-dosed every 8 and 24 hours, respectively. Immunosuppression was administered intravenously: tacrolimus (5 mg/kg), mycophenolate mofetil (500 mg), each re-dosed every 12 hours, and methylprednisolone (125 mg), re-dosed every 8 hours. After exposing the right internal jugular vein, a heparin bolus (15,000 U) was administered, and the vein was cannulated with a 19F to 23F dual-lumen cannula (Avalon Elite; Maquet Cardiopulmonary, Rastatt, Germany) (Figure 2 , A and B).
Figure 2.
Experimental setup of multiday extracorporeal lung support system using a swine recipient. A, A dual-lumen cannula was placed in the right internal jugular (RIJ) vein under fluoroscopic guidance. White arrows indicate cannula. B, The cannula was tunneled along the lateral aspect of the right neck and secured to the dorsum of recipient swine. C and D, Following initiation of cross-circulation, cannulated recipient swine were transferred to a Panepinto sling where they remained elevated from the enclosure floor for safe recovery from anesthesia and extubation. E, Once conscious, recipient swine were lowered into a custom enclosure where they remained for the duration of the procedure. SVC, Superior vena cava; R, right; IVC, inferior vena cava.
XC and Extracorporeal Lung Support
Calcium gluconate (1 g) was administered intravenously to recipient swine, and cross-circulation of blood between recipient swine and extracorporeal lungs was initiated, as previously described.12 , 13 The extracorporeal circuit contained a pump console (Jostra HL-20; Maquet Cardiopulmonary), disposable pump (Rotaflow Centrifugal Pump; Maquet Cardiopulmonary), and continuous monitoring software (VIPER; Spectrum Medical, Cheltenham, England). Circuit flow rate was maintained within a protective regime between 5% and 10% of the estimated cardiac output of recipient swine, with pulmonary artery pressures <20 mm Hg, and pulmonary vein pressures between 3 and 5 mm Hg (Figure E1, A).12 , 13 Extracorporeal lungs were ventilated (Oxylog 3000 plus; Dräger, Lübeck, Germany) (Video 1) with the following settings: respiratory rate, 6 to 8 bpm; tidal volume, 6 to 8 mL/kg; positive end-expiratory pressure, 5 cm H2O; inspired oxygen fraction, 40%; and maintained on XC for a mean duration of 1.5 ± 0.1 hours before initiating recovery of recipient swine from general anesthesia.
Video 1.
Normothermic maintenance of extracorporeal swine lungs with multiday lung support system. Video available at: https://www.jtcvs.org/article/S0022-5223(19)32146-4/fulltext.
Figure E1.
Maintenance of extracorporeal circuit parameters by height adjustments of extracorporeal lungs in response to changes in recipient swine position. A, Target ranges of extracorporeal circuit parameters. B, Representative photographs of recipient positions: upright and prone. C, Heights of circuit elements corresponding to recipient position. D, Extracorporeal circuit diagrams demonstrating changes in extracorporeal lung height corresponding to changes in recipient position in order to maintain target circuit parameters. PA, Pulmonary artery; PV, pulmonary vein.
Management of Recipient Swine During Recovery From Anesthesia
Anesthetized recipient swine were transferred into a Panepinto sling suspended within a custom enclosure (Figure E2) and allowed to recover from anesthesia for a mean duration of 2.1 ± 1.3 hours (Figure 2, C and D). Following transfer to the Panepinto sling, recipient swine were weaned from general anesthesia and administered ketamine (0.5-3.0 mg/kg) and dexmedetomidine (0.1-0.4 mg/kg/h) as needed. When spontaneous breathing was achieved, recipient swine were extubated while suspended in the Panepinto sling, and subsequently lowered onto the floor of the custom enclosure, where they were allowed to fully recover from anesthesia (Video 2). Swine recipients were maintained in the custom enclosure for 4 days (Figure 2, E, and Video 3).
Video 2.
Transfer of swine recipient in Panepinto sling to custom enclosure. Video available at: https://www.jtcvs.org/article/S0022-5223(19)32146-4/fulltext.
Video 3.
Active enrichment of recipient swine in custom enclosure throughout multiday lung support. Video available at: https://www.jtcvs.org/article/S0022-5223(19)32146-4/fulltext.
Figure E2.
Custom enclosure for recipient swine. Stainless steel enclosure featured width-adjustable sidewall to ensure swine recipients remained comfortable and secure throughout multi-day procedures. The open top of the enclosure enabled easy access to recipient swine, cannula site, and circuit components. Immediately following recipient feeding, urination, and defecation, the excreta pan was removed, thoroughly cleaned, and replaced to minimize the presence of waste in the custom enclosure.
Analyses of Extracorporeal Lungs and Recipient Swine
Multiscale analyses of extracorporeal lungs were performed every 24 hours. Recipient swine were continuously monitored, and hemodynamic and biochemical parameters were recorded every 12 hours. Detailed methods are available in Appendix E1.
Results
Extracorporeal lungs were connected to the normothermic support system and periodically evaluated over 4 days (Figure 1). Recipient swine were continuously monitored through behavioral observation, vital signs, and blood samples analyses.
Extracorporeal Circuit Stability
All extracorporeal circuit parameters were maintained within target lung-protective ranges throughout 4 days of normothermic support. Pulmonary artery pressures remained below 25 mm Hg (Figure 3 , A); and the transpulmonary pressure gradient, the difference between pulmonary artery and vein pressures, was maintained within the target range of 5 to 15 mm Hg (Figure 3, C). Flows were maintained on average at 0.28 ± 0.03 L/min (8%-9% of estimated cardiac output18) (Figure 3, B), and the circuit had a mean temperature of 35.1°C ± 1.2°C throughout the 4 days (Figure 3, D). The pH of the perfusate stayed within the physiologic range of 7.42 ± 0.05 (Figure 3, E), and lactate remained below 2 mmol/L until day 4, when lactate increased slightly to 3.10 ± 0.14 mmol/L (Figure 3, F).
Figure 3.
Stability of circuit parameters during multiday extracorporeal lung support. A, Pressure. B, Flow. C, Transpulmonary pressure gradient (TPG), which is the difference between pulmonary artery and pulmonary vein pressures. D, Temperature. E, pH. F, Lactate. Dotted lines define target range. Values are presented as mean ± standard deviation.
Recipient Swine Safety and Stability
All recipient swine tolerated venous neck cannulation (Figure 2, B), the experimental custom enclosure (Figure 2, E), and exhibited normal food and water consumption and excretion throughout all procedures. Safety and stability were assessed by monitoring of recipient swine hemodynamic parameters, which were maintained within normal ranges (mean heart rate, 112 ± 18 bpm and mean systolic pressure, 120 ± 29 mm Hg), and by hemogas analysis (pH day 0, 7.36 ± 0.02; pH day 4, 7.37 ± 0.08) (Table E1). Hematocrit decreased gradually over 4 days of support (day 0, 27.2% ± 16.6%; day 4, 10.7% ± 2.3%) due to repeated blood sampling from both recipient swine and the extracorporeal circuit, and from minor transient bleeding from repeated lung tissue sampling. The inflammatory response of recipient swine was evaluated by quantification of serum inflammatory cytokine levels. From baseline to day 4, mean serum concentrations of granulocyte-macrophage colony-stimulating factor, interferon gamma (IFNγ), interleukin (IL) 1β, Il-1ra, IL-6, IL-10, and tumor necrosis factor alpha (TNFα) variably increased, with the largest increases in IL-1β (10.4-fold), IFNγ (19.2-fold), and IL-1ra (69-fold). Mean serum concentrations of IL-1α, IL-2, IL-4, IL-8, IL-12, and IL-18 decreased, with the largest decrease in IL-8 (14.8-fold) (Table E2). All serum cytokine concentrations after 4 days of normothermic support were within or below ranges reported in swine EVLP studies that provided a maximum of 12 hours of support (Table E3).
Functional Maintenance of Extracorporeal Lungs
Respiratory function of extracorporeal lungs was preserved over 4 days of normothermic support. Robust gas exchange with mean arterial oxygen tension/inspired oxygen fraction values above 430 mm Hg (day 0, 439.4 ± 227.1 mm Hg; day 4, 548.5 ± 176.9 mm Hg) (Figure 4 , A), and dynamic compliance with mean values above 20 mL/cm H2O (day 0, 22.2 ± 1.7 mL/cm H2O; day 4, 20.0 ± 1.0 mL/cm H2O) (Figure 4, B) were maintained consistently for the duration of all procedures. Mean peak inspiratory pressures increased slightly from day 0 to day 4 (day 0, 20.5 ± 0.7 cm H2O; day 4, 25.0 ± 4.2 cm H2O) (Figure 4, C), but always remained below 30 cm H2O for equivalent tidal volumes. All other functional parameters demonstrated minimal changes from baseline to day 4 (Table E4). Lung weight gradually increased over 4 days (day 0, 0.84 ± 0.17 kg; day 4, 1.18 ± 0.07 kg) (Figure 4, D), which was likely due to edema resulting from changes in hydrostatic pressure caused by variations in position of conscious swine recipients (eg, prone to standing) (Figure E1, B-D).
Figure 4.
Maintenance of extracorporeal lung function for 4 days using multiday lung support system. A, Arterial oxygen tension/inspired oxygen fraction. B, Dynamic compliance. C, Peak inspiratory pressure (PIP). D, Lung weight. Values are presented as mean ± standard deviation.
Multiscale Analyses of Extracorporeal Lungs
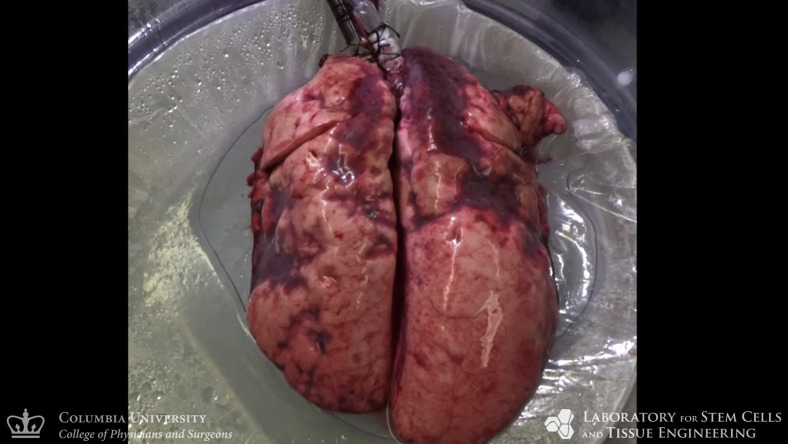
Gross photography of extracorporeal lungs showed normal appearance of the pleural surface with areas of localized consolidation periodically observed in upper lobes after day 2 (Figure 5 and Figure E3, A). Radiography confirmed that extracorporeal lungs remained aerated, with diffuse radiopacities in upper lobes after day 2 (Figure 5, C). Surface thermography revealed that lobes with lower surface temperatures were consistent with radiolucent areas (Figure 5, B), suggesting that consolidated regions in upper lobes had ventilation–perfusion mismatch with decreased ventilation leading to increased surface temperatures.19 , 20 Bronchoscopy confirmed normal appearance of large airways with no evidence of airway edema, erythema, or secretions after 4 days (Figure 5, D). Although histologic evaluations revealed edema in upper lobes, structural preservation of lung parenchyma, pulmonary airways, and the vascular tree was confirmed throughout the lungs (Figure 5, E and F, and Figure E3, B-D). Transmission electron microscopy confirmed preservation of the blood–gas barrier with intact alveolar epithelial lining and abundant type II pneumocytes with normal cuboidal morphology (Figure 5, G).
Figure 5.
Multiscale analyses of extracorporeal lung maintenance and integrity. A, Photographic appearance. B, Thermographic appearance. C, Radiographic appearance. D, Bronchoscopic evaluation of extracorporeal lungs throughout 4 days of normothermic support, including aerated regions (dotted lines), and areas of local consolidation (stars). Microscopic analyses of bilateral lower lobes by hematoxylin and eosin staining. E, Low magnification. F, High magnification. G, Transmission electron microscopy revealed type II pneumocytes (black arrows) with visible lamellar bodies containing surfactant, and intact alveolar epithelial barrier (white arrows) of type I pneumocytes.
Figure E3.
Histologic evaluation of the upper lobes of extracorporeal lungs throughout 4 days of normothermic support. A, Subpleural regions. B, Parenchyma. C, Pulmonary airways. D, vessels. Dotted lines outline surface of visceral pleura.
Inflammation and Histopathologic Assessment of Extracorporeal Lungs
Airway inflammation was assessed by quantification of inflammatory cytokines in bronchoalveolar lavage (BAL) fluid. From baseline to day 4, mean concentrations of granulocyte-macrophage colony-stimulating factor, IL-4, IL-6, and IL-10 trended upward but did not increase drastically, and mean concentrations of IFNγ, IL-1α, IL-1β, IL-1ra, IL-2, IL-8, IL-12, IL-18, and TNFα decreased in BAL fluid (Figure 6 , A-F, and Table E5 and E6). Notably, the largest increase of inflammatory cytokine concentrations in BAL fluid was IL-4 (5.5-fold), and the largest decrease was IFNγ (104.9-fold). To assess the degree of injury in extracorporeal lungs, tissue samples were subjected to blinded histopathologic review and assigned lung injury scores (Figure 6, G) according to an established injury scoring rubric (Figure E4, C). Polymorphonuclear cells, indicators of immune response to injury, remained low in airways but gradually increased in alveoli. Alveolar and interstitial edema increased slightly, which was consistent with the observed increase in lung weight. Nevertheless, the lack of significant increase in any lung injury category score from baseline to day 4 suggests that the extracorporeal lungs experienced minimal to no injury over 4 days of normothermic support.
Figure 6.
Quantification of airway cytokines and evaluation of lung injury during multiday extracorporeal lung support. Bronchoalveolar lavage fluid concentrations. A, Interferon gamma (IFNγ). B, Tumor necrosis factor alpha (TNFα). C, Interleukin (IL) 1β. D, IL-6. E, IL-8. F, IL-10. G, Lung injury scoring by blinded histopathologic review. Values are presented as mean ± standard deviation. PMN, Polymorphonuclear cells.
Figure E4.
Randomized lung sampling and scoring rubric of lung injury score. A, Lung map used for randomized tissue sampling showing lungs were divided into 5 lobes. B, Tissue sample locations at each time point. Sample bias was avoided by predetermining tissue sampling location before the start of all experiments. C, Scoring rubric of lung injury scores. RUL, Right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe; PMN, polymorphonuclear cells; hpf, high-power field.
Cellular Integrity and Function of Extracorporeal Lungs
Pentachrome staining confirmed preservation of bronchial structures, including airway mucosa, smooth muscle, cartilage (Figure 7 , A), and bronchial epithelium with intact pseudostratified epithelium and airway cilia (Figure 7, B). In the respiratory zone, alveolar capillaries, and distal venules were well perfused (Figure 7, C), consistent with the outstanding respiratory performance at day 4. Immunohistochemical staining for vascular endothelial cadherin enabled visualization of the intact endothelial lining of pulmonary vessels (Figure 7, E). Following administration of nebulized methacholine on day 4, extracorporeal lungs demonstrated rapid bronchoresponsiveness (Figure 7, H) of airway smooth muscle (Figure 7, D). Cell viability in extracorporeal lungs was confirmed by uptake of viability marker carboxyfluorescein succinimidyl ester (Figure 7, F), and cellular metabolism in the parenchyma of extracorporeal lungs remained within range of the metabolic activity of lungs in vivo (Figure 7, G).
Figure 7.
Cellular integrity and function in extracorporeal lungs after 4 days of normothermic support. Pentachrome staining. A, Large airways at low magnification with preserved airway mucosa (AM), smooth muscle (SM), and cartilage plate (CP). B, Large airways at high magnification with outstanding preservation of airway cilia (AC), pseudostratified epithelium (PE), and basement membrane (BM). C, Alveoli with intact blood–gas barrier and perfused venule and alveolar capillaries (arrows). D, Immunohistochemical staining was used to confirm retention of alpha smooth muscle actin (αSMA) around small airways (stars). E, Immunohistochemical staining was used to confirm retention of vascular endothelial (VE)-cadherin by endothelial cells in large and small vessels throughout the pulmonary vascular tree. F, Cell viability throughout the lung parenchyma was confirmed by pervasive uptake of carboxyfluorescein succinimidyl ester (CFSE). Star indicates alveolar space. G, Metabolic activity of lung parenchyma. Dotted lines indicate normal range of metabolic activity of healthy swine lungs in vivo. H, Changes in peak inspiratory pressure after administration of nebulized methacholine (arrow). Values are presented as mean ± standard deviation. PIP, Peak inspiratory pressure.
Discussion
In this proof-of-feasibility study, we describe an extracorporeal organ support system capable of maintaining the structure, viability, and function of extracorporeal lungs for 4 days (Figure 8 ). This system extends the duration of normothermic extracorporeal lung support significantly beyond the capability of current ex vivo lung perfusion systems, from hours to multiple days. Our hypothesis is that homeostatic normothermic extracorporeal support for days to weeks could offer new opportunities for the assessment, recovery, and regeneration of donor lungs. Additionally, we established new methods to enable multiday XC of whole blood between extracorporeal lungs and a conscious large animal. Our results demonstrate feasibility that lungs can be maintained outside the body for 4 days with outstanding preservation of respiratory function, lung tissue structure, and cellular integrity and metabolism. Such a system offers a developmental platform for advanced therapeutic interventions such as gene or cell therapies for extracorporeal or intracorporeal organs.
Figure 8.
Multiday maintenance of extracorporeal lungs using cross-circulation with conscious swine: Experimental overview and results. A, Extracorporeal lungs were maintained using cross-circulation with conscious swine for a duration of 4 days. Lungs were placed in a normothermic organ preservation chamber and ventilated. Functional, biochemical, and multimodal imaging analyses were used to enable continuous monitoring of extracorporeal lungs and swine recipients. B, At day 4, lungs demonstrated maintenance of respiratory function, intact blood–gas barrier, cellular viability, and outstanding preservation of airway and alveolar architecture. PIP, Peak inspiratory pressure.
Three new methodologies were developed in this study: a swine behavioral training program (Table E7 and Figure E5) implemented 2 weeks before the start of XC to acclimate recipient swine to the custom enclosure used during the procedure, a technique to manage full recovery of anesthetized cannulated recipient swine to consciousness using a Panepinto sling and custom enclosure, and a novel extracorporeal circuit configuration with a single-site dual-lumen cannula to enable maintenance of circuit parameters within acceptable ranges. Altogether, these methods may prove useful in future translational studies involving the connection of conscious large animals or humans to extracorporeal circuits for organ support.
Figure E5.
Preprocedure swine behavior training program. A, Target training with clicker (red) to encourage swine to enter custom enclosure. B, Throughout the behavior training program, recipient swine comfort and familiarity were maintained while the width of the side wall (stars) of the custom enclosure was incrementally decreased, limiting the ability of recipient swine to rotate during the procedure. C, Active enrichment was provided throughout behavior training. D, Positive reinforcement was provided throughout behavior training.
The function of extracorporeal lungs in this study was robustly maintained throughout 4 days of normothermic support. In comparison to previous studies, wherein swine lungs were supported by EVLP systems for a total of 12 hours21 or 24 hours,22 lungs in this study at day 4 demonstrated superior mean arterial oxygen tension/inspired oxygen fraction (12 hours, ∼400 mm Hg; 24 hours, ∼400 mm Hg; XC 4 days, 548 mm Hg), equivalent mean compliance (12 hours, 20 mL/cm H2O; 24 hours, not reported; XC 4 days, 21 mL/cm H2O), and in-range mean peak inspiratory pressures (12 hours: 25 cm H2O; 24 hours, 15 cm H2O; XC 4 days, 25 cm H2O). IL-1β and IL-8 in BAL fluid decreased from baseline to day 4, and were only 1.4-fold and 2.2-fold higher, respectively, than in healthy swine lungs after 4 hours23 of EVLP support. Furthermore, airway concentrations of IL-6, TNFα, and IFNγ after 4 days were, respectively, 2.2-fold, 2.8-fold, and 1260-fold lower. Altogether, these data suggest that the overall function and condition of swine lungs after 4 days were superior to the condition of lungs placed on 12 to 24 hours of EVLP support.
An established lung injury scoring rubric12 , 13 was used to assess the extent of lung injury. A comparison of lung injury scores after 4 days of support to scores previously reported in established swine lung models of ischemia reperfusion injury12 and gastric aspiration injury13 revealed that lungs after 4 days showed less injury in all categories except for the mild interstitial edema reflected by increased lung weight. Overall, the composite injury score at day 4 in this study (4.6) was markedly lower than composite injury scores reported for ischemia reperfusion injury (6.8) and gastric aspiration (9.3) studies. When compared with recipient swine lungs at the conclusion of the procedure, extracorporeal lungs showed only a minimal increase in mean injury score (day 4, 4.6; recipient lungs, 3.8) (Figure E6).
Figure E6.
Histologic evaluation by hematoxylin and eosin stain of recipient swine lung after 4 days of normothermic support. A, Low-power microscopy of parenchyma. B, High-power microscopy of alveoli. C, Airway. D, Vessels. E, Comparison of lung injury scores at day 4 of multiday support to lung injury scores of recipient lung at the end of the procedure. PMN, Polymorphonuclear cells.
The maintenance of vascular pressures within physiological range is critical for the prevention of pulmonary edema and the preservation of extracorporeal lungs.24 In this study, the pressure at the pulmonary veins was dependent on the hydrostatic pressure difference between the extracorporeal lungs and recipient swine, and was regulated by adjusting the height difference between the lungs and recipient swine (target, 10 cm). In our previous studies, this height difference was fixed, as recipient swine were anesthetized and therefore immobile for the duration of all procedures. In this study, recipient swine were conscious and free to stand upright or lay prone at will, which resulted in intermittent changes in the height difference between the extracorporeal lungs and recipient swine. Adjustments to the height of the lungs were therefore necessary to maintain the target height difference of 10 cm. Controlled adjustments of lung height were performed manually using a hydraulic lift, but were technically challenging to perform in real time. Consequently, variabilities in pulmonary vein pressures resulting from changes in swine position, in conjunction with the persistent prone position of extracorporeal lungs, likely contributed to the development of dependent interstitial edema, most notably observed in the upper lobes.
Limitations
There are several limitations to the present study. This study involved a small number of procedures (n = 3). Although such a study size limits the opportunity for statistical analyses, the results demonstrated feasibility of normothermic support of extracorporeal lungs for 4 days. Future studies will investigate larger numbers of lungs, recipient swine, and a wider variety of experimental conditions including increased flow rates, recovery of damaged lungs, and therapeutic interventions. Further, because immunosuppression was used, immunological markers of injury may be different than in the absence of immunosuppression, which may account for differences observed between cytokine concentrations in BAL fluid and serum. Notably, several of the cytokines investigated in this study have been shown to have both pro- and anti-inflammatory roles,25, 26, 27 so their functions in this system are difficult to interpret. Future studies using a multiday extracorporeal support system could help elucidate the roles of cytokines in extracorporeal lung support systems. Several technical challenges remain to be resolved in the current system: specification and regulation of a long-term extracorporeal organ environment, controlled variability of extracorporeal organ orientation, and appropriate ventilation and perfusion management strategies. The inability to strictly regulate dynamic hydrostatic pressure changes led to the development of edema, necessitating future development of feedback-regulated pump controls and an automated organ height adjustment system capable of responding precisely in real time to dynamic changes in swine position, transpulmonary pressure gradient, and lung weight. Although this study did not investigate deposition of recipient cells or platelets in extracorporeal lungs, which could result in platelet-induced injury and chimerism, future studies of hematologic and immunologic interactions will be critical to assess the safety of clinical translation.
Despite these limitations, this study demonstrated that cross-circulation enables a quality and duration of extracorporeal lung support not previously shown by EVLP systems. The use of XC to recover damaged lungs ex situ could be applicable in clinical settings where patients receiving extracorporeal membrane oxygenation fail to match suitable donor lungs. In such patients, XC of lungs with reversible injuries for several days could enable functional assessment, lung-protective strategies, and graft recovery while avoiding the physiologic insult associated with major surgical intervention and severe primary graft dysfunction. Lungs recovered by XC would then be transplanted into the patient, thereby potentially decreasing the morbidity and mortality associated with transplantation of injured lungs. Future investigations using multiday extracorporeal organ support could also enable advanced interventions through immunomodulation,28 , 29 cell replacement,17 , 30, 31, 32, 33 or other bioengineering approaches,17 , 32 , 34, 35, 36 and ultimately serve as a platform to improve transplant outcomes.
Conclusions
In this study we demonstrate the longest duration of normothermic support of extracorporeal lungs reported to date (4 days), with outstanding maintenance of lung tissue and respiratory functions. We envision that this system could be applicable in clinical settings to recover and regenerate damaged donor organs, and in translational research settings as a platform to investigate new strategies for lung bioengineering.
Webcast
You can watch a Webcast of this AATS meeting presentation by going to: https://aats.blob.core.windows.net/media/19%20AM/Saturday_May4/203AC/203AC/S24%20-%20Mechanical%20Lung%20Support/S24_3_webcast_120637225.mp4.

Conflict of Interest Statement
Drs Guenthart, O'Neill, Vunjak-Novakovic, Bacchetta, and Mr Fung have a pending patent for a cross-circulation platform for recovery, regeneration, and maintenance of extracorporeal organs. All other authors have nothing to disclose with regard to commercial support.
Acknowledgments
The authors thank the Institute of Comparative Medicine veterinary staff, including A. Romanov, S. Robertson, R. Ober, A. McLuckie, G. Geist, N. Herndon, S. Hastings, D. Ordanes, and A. Rivas for supporting animal studies; Weill Cornell Microscopy and Image Analysis Core Facility staff, including L. Cohen-Gould and J. P. Jimenez for transmission electron microscopy imaging services. The authors also thank the Herbert Irving Comprehensive Cancer Center Molecular Pathology Shared Resources, including T. Wu, D. Sun, and R. Chen for histology services; E. Lopes, G. Pierre, and I. Fedoriv for support with technical analytics; and S. Pistilli and S. Halligan for administrative support.
Footnotes
Supported by the National Institutes of Health (grant Nos. HL120046, HL134760, HL007854, HL143733, and EB027062), the Richard Bartlett Foundation, the Blavatnik Foundation, and the Mikati Foundation.
Contributor Information
Gordana Vunjak-Novakovic, Email: gv2131@columbia.edu.
Matthew Bacchetta, Email: matthew.bacchetta@vumc.org.
Supplementary Data
Normothermic maintenance of extracorporeal swine lungs with multiday lung support system. Video available at: https://www.jtcvs.org/article/S0022-5223(19)32146-4/fulltext.
Transfer of swine recipient in Panepinto sling to custom enclosure. Video available at: https://www.jtcvs.org/article/S0022-5223(19)32146-4/fulltext.
Active enrichment of recipient swine in custom enclosure throughout multiday lung support. Video available at: https://www.jtcvs.org/article/S0022-5223(19)32146-4/fulltext.
Appendix E1. Details of preprocedure and procedure methods
Preprocedure Behavior Training of Recipient Swine
Before initiation of cross-circulation, a swine behavior training program (Table E7) was implemented to acclimate recipient swine to the custom enclosure (Figure E2). Target training (ie, conditioning swine to touch nose to target when the trainer activated an audible clicker) was initiated at least 14 days before the start of cross-circulation and was used to encourage recipient swine to enter the custom enclosure by day 4 of training (Figure E5, A). Initially, swine were maintained in the custom enclosure for up to 1 hour. Through days 5 to 14 of training, recipient swine were maintained in the custom enclosure for at least 2 hours per day to increase comfort and familiarity with the experimental environment. The width of the custom enclosure was incrementally reduced to limit rotational movements (Figure E5, B). Behavior training was essential to minimize stress and maximize comfort of recipient swine, and thus decrease risk of decannulation during cross-circulation procedures. Throughout the preprocedure behavior training program, recipient swine experienced enrichment with manipulata (Figure E5, C), social interactions with procedure personnel (Figure E5, D), and standard and high-quality edible treats.
Management and Monitoring of Recipient Swine
During cannulation of the right internal jugular vein, physiologic parameters of anesthetized recipient swine, including heart rate, electrocardiogram, blood pressure (cuff and arterial line), oxygen saturation, end-tidal carbon dioxide, temperature, and respiratory rate were continuously monitored and recorded. Following recovery from anesthesia and throughout the duration of procedures, recipient swine were continuously monitored. Physical examinations to monitor and record respiratory signs (ie, dyspnea, tachypnea, coughing, and hypoxia), change in appetite, abnormal attitude or mentation, and signs of pain or discomfort were conducted by a large-animal veterinarian and animal behavior specialist at least twice daily. Throughout the duration of cross-circulation, recipient swine were maintained on a continuous intravenous heparin infusion (initial rate, 25 U/kg/h). Activated clotting time was measured and recorded hourly, and heparin infusion rates were titrated accordingly to remain within the target activated clotting time range of 200 to 300 seconds. Recipient swine were allowed to eat, drink, sleep, and play freely in the custom enclosure. Twice daily, recipient swine received food (Laboratory Mini-Pig Grower Diet; LabDiet) and water ad libitum. To prevent interference with recipient swine activities, extracorporeal circuit tubing was secured at the top of the custom enclosure. One unit of whole blood was collected from each donor swine and was stored at 4°C. A transfusion was given to the recipient swine if hemoglobin decreased below 4 g/dL.
Blood Analysis
Before recovery of recipient swine from anesthesia, blood samples were collected from an auricular arterial line. Following recovery from anesthesia, blood samples were collected every 12 hours from a central venous line placed in the left external jugular vein. Hemogas analysis was performed using a point-of-care blood analysis system (epoc; Siemens Healthineers). Complete blood counts, basic metabolic panels, liver function tests, and coagulation panels were performed by a diagnostic laboratory service (Antech Diagnostics). Mycophenolate levels were not measured, but FK506 (tacrolimus) levels were measured (Architect System; Abbott). Inflammatory cytokines (granulocyte-macrophage colony-stimulating factor, interferon gamma, interleukin (IL) 1α, IL-1β, IL-1ra, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-18, and tumor necrosis factor alpha) in recipient swine serum were quantified in triplicate by multiplex cytokine array (Discovery Assay Pig Cytokine Array; Eve Technologies).
Extracorporeal Lung Monitoring
Blood samples were collected from sample ports at the pulmonary artery and pulmonary veins every 12 hours throughout the duration of normothermic support. Arterial oxygen tension/inspired oxygen fraction and dynamic compliance (Cdyn = tidal volume/(peak inspiratory pressure − positive end-expiratory pressure)) were calculated at baseline and every 24 hours. Lung weight was obtained every 24 hours using a scale (Denver Instrument Company) housed inside the organ chamber. Gross photographs of extracorporeal lungs were acquired using a high-definition camera (Hero 5; GoPro), and radiographs were acquired using a portable unit (PXP-16HF; United Radiology Systems) with images captured at 2.2 mAs and 90 kVp. Thermographs of extracorporeal lungs were acquired using an infrared camera (T430sc; FLIR).
Bronchoscopy and Bronchoalveolar Lavage Fluid Analysis
Bronchoscopic assessment of the airways was performed, and bronchographs were obtained at baseline and every 24 hours. Bronchoalveolar (BAL) fluid samples were collected by introducing a 3.8 mm flexible bronchoscope (aScope 3; Ambu) into subsegmental bronchi of the left and right lower lobes of extracorporeal lungs and injecting sterile normal saline (5 mL) with subsequent aspiration and collection of BAL fluid in a sterile specimen trap. BAL fluid samples were centrifuged at 3500 rpm for 10 minutes at 4°C. Supernatants were snap frozen in liquid nitrogen and stored at –80°C until further processing. Inflammatory cytokines (granulocyte-macrophage colony-stimulating factor, interferon gamma, IL-1α, IL-1β, IL-1ra, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-18, and tumor necrosis factor alpha) in BAL fluid of extracorporeal lungs were quantified in triplicate by multiplex cytokine array (Discovery Assay Pig Cytokine Array; Eve Technologies).
Extracorporeal Lung Tissue Sampling
A surgical stapler (GIA Auto Suture; Covidien) was used to collect tissue samples from extracorporeal lungs at baseline and every 24 hours. Tissue sampling locations were predetermined using randomization software (randomizer.org) (Figure E4, A and B). Tissue samples were immediately fixed in cold phosphate-buffered 4% paraformaldehyde for 24 to 48 hours, embedded in paraffin, and sectioned at 5-μm thickness. All sections were stained for hematoxylin and eosin and Movat's pentachrome by histology services in the Department of Molecular Pathology at Columbia University Medical Center. All sections were examined under light microscopy and imaged with a slide scanner (SCN400; Leica).
Recipient Lung Tissue Sampling
After each experiment the recipient lungs were procured via a median sternotomy, and a surgical stapler (GIA Auto Suture) was used to collect tissue samples from recipient lungs from all 5 lobes. Tissue samples were processed in a similar manner to extracorporeal lungs described above.
Transmission electron microscopy
Lung tissue samples were fixed with 2.5% glutaraldehyde, 4% paraformaldehyde, and 0.02% picric acid in 0.1M Na-cacodylate buffer (pH 7.2). Samples were then postfixed with 1% osmium tetroxide in Sorenson's buffer for 1 hour, dehydrated, and embedded in Lx-112 (Ladd Research Industries). Sections (thickness, 60 nm) were prepared using an ultramicrotome, stained with uranyl acetate and lead citrate, and examined with an electron microscope (JEM-1200 EXII; JEOL). Images were captured with a digital camera (ORCA-HR; Hamamatsu Photonics).
Histopathologic review
An experienced lung transplant pathologist blinded to the study protocol performed all histopathologic review that included evaluation of lung tissue samples from upper, middle, and lower lobes. A previously reported lung injury scoring rubric (Figure E4, C) was used to assess the degree of lung injury.E1, E2
Immunohistochemical staining
Following deparaffinization, lung sections were subjected to boiling citrate buffer (pH 6.0) for antigen retrieval and blocked with 10% normal donkey serum in phosphate-buffered saline for 2 hours at room temperature. Next, primary antibodies (VE-cadherin: Abcam, ab232880; alpha smooth muscle actin: Abcam, ab5694) were diluted 1:200 and incubated for 2 hours. Secondary antibody (Abcam, ab150077) was diluted 1:300 and incubated for 2 hours at room temperature. Sections were subsequently mounted, and images were obtained using a fluorescent microscope (FSX100; Olympus).
Bronchoresponsiveness
Airway smooth muscle tone in extracorporeal lungs was assessed after 4 days of normothermic support by intratracheal delivery of nebulized methacholine chloride (4 mg). Changes in peak inspiratory pressures were recorded for 1 minute before and 5 minutes after delivery of methacholine chloride.
Cell viability assay
To assess cell viability in the parenchyma of extracorporeal lungs, carboxyfluorescein succinimidyl ester (eBioscience) was reconstituted in dimethyl sulfoxide at a concentration of 1.06 M. After 4 days of normothermic support, carboxyfluorescein succinimidyl ester was delivered via flexible bronchoscope into distal regions of left and right lower lobes of extracorporeal lungs and incubated for 15 minutes. Lung tissue samples were then collected, washed 5 times with phosphate-buffered saline, fixed in cold phosphate-buffered 4% paraformaldehyde for 48 hours, embedded, deparaffinized, mounted, and imaged using a fluorescence microscope (FSX100).
Metabolic activity assay
To assess cellular metabolism in extracorporeal lungs, tissue samples were collected from the parenchyma at baseline, day 2, and day 4 of normothermic support. Tissue samples (volume: 250 μL; triplicates) were finely minced, gently homogenized, and placed in a 96-well plate. Cell metabolism assay reagent (Alamar Blue; ThermoFisher) was diluted 1:10 in DMEM supplemented with 10% fetal bovine serum, and 100 μL Alamar Blue reagent was added to wells containing lung sample homogenates.E3 Alamar Blue reagent alone (100 μL) was added to wells containing no lung homogenate (negative controls). The multiwell plate was protected from light and incubated at 37°C with gentle shaking for 2 hours. Following incubation, absorbance was measured at 570 nm and normalized to absorbance at 600 nm. To obtain a benchmark comparison of metabolic activity to healthy swine lungs in vivo, fresh lung tissues were collected from healthy swine immediately following thoracotomy, and metabolic activity assays were performed on both extracorporeal swine lung tissue samples and fresh healthy swine lung tissue samples.
Table E1.
Safety and stability of recipient swine during multiday extracorporeal lung support. Analysis of recipient swine vitals, hemogas, biochemistry, coagulation, and electrolytes throughout 4 days of cross-circulation
| Parameter | Time (d) |
||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Vitals | |||||
| Heart rate (bpm) | 88 ± 9 | 111 ± 8 | 113 ± 51 | 110 ± 35 | 140 ± 42 |
| Systolic BP (mm Hg) | 97 ± 14 | 106 ± 17 | 155 ± 9 | 133 ± 45 | 89 ± 37 |
| Temperature (°F) | 97.3 ± 1.9 | 97.0 ± 1.7 | 96.2 ± 2.8 | 98.3 ± 2.3 | 98.5 ± 1.0 |
| Hemogas | |||||
| pH | 7.36 ± 0.02 | 7.46 ± 0.02 | 7.45 ± 0.04 | 7.46 ± 0.05 | 7.37 ± 0.08 |
| Oxygen tension (mm Hg)∗ | 274 ± 34 | 45 ± 23 | 39 ± 16 | 30 ± 2 | 42 ± 3 |
| Carbon dioxide tension (mm Hg) | 57 ± 3.4 | 46 ± 3.3 | 50 ± 8.5 | 47 ± 8.7 | 42 ± 5.8 |
| Bicarbonate (mmol/L) | 32 ± 1.2 | 33 ± 1.7 | 39 ± 15.9 | 34 ± 3.8 | 30 ± 3.0 |
| Lactate (mmol/L) | 1.42 ± 0.49 | 1.58 ± 0.38 | 1.08 ± 0.27 | 0.91 ± 0.31 | 3.1 ± 0.14 |
| Glucose (mg/dL) | 112 ± 77 | 199 ± 49 | 127 ± 33 | 177 ± 55 | 159 ± 38 |
| Biochemical analysis | |||||
| WBC (109/L) | 26.1 ± 19.6 | 18.3 ± 4.2 | 12.3 ± 6.4 | 14.3 ± 1.9 | 9.5 ± 5.2 |
| % Neutrophils | 47 ± 11 | 67 ± 5 | 53 ± 14 | 65 ± 19 | 68 ± 12 |
| % Reticulocytes | 1 ± 1 | 1 ± 1 | 1 ± 1 | 1 ± 1 | 3 ± 1 |
| Platelets (109/L) | 423 ± 222.4 | 307 ± 47.7 | 203 ± 97.1 | 118 ± 53.8 | 94 ± 61.6 |
| Hgb (g/dL) | 8.1 ± 4.9 | 7.6 ± 0.8 | 7.9 ± 3.3 | 4.9 ± 0.6 | 3.1 ± 0.7 |
| Hct (%) | 27.2 ± 16.6 | 25.7 ± 3.8 | 20.8 ± 3.9 | 16.5 ± 2.5 | 10.7 ± 2.3 |
| AST (U/L) | 47 ± 16.9 | 54 ± 13.6 | 49 ± 13.4 | 28 ± 19.0 | 37 ± 3.5 |
| ALT (U/L) | 67 ± 12.3 | 61 ± 23.2 | 51 ± 23.4 | 40 ± 1.2 | 29 ± 3.6 |
| Creatinine (mg/dL) | 1.33 ± 0.26 | 1.15 ± 0.13 | 1.01 ± 0.14 | 1.05 ± 0.06 | 1.74 ± 0.58 |
| Tacrolimus (ng/mL) | 0.93 ± 1.04 | 13.37 ± 9.06 | 20.23 ± 8.93 | 22.37 ± 8.89 | 15.21 ± 3.83 |
| Activated clotting time (s) | 296 ± 83 | 222 ± 35 | 225 ± 65 | 250 ± 66 | 239 ± 47 |
| Electrolytes and other | |||||
| Sodium (mmol/L) | 136 ± 3.8 | 141 ± 2.4 | 136 ± 1.0 | 136 ± 1.0 | 140 ± 2.8 |
| Potassium (mmol/L) | 7.9 ± 2.9 | 6.3 ± 2.5 | 5.6 ± 1.1 | 5.6 ± 1.1 | 5.0 ± 0.5 |
| Calcium (mg/dL) | 7.8 ± 0.8 | 7.2 ± 1.1 | 8.1 ± 0.8 | 8.1 ± 0.8 | 6.4 ± 1.4 |
| Phosphate (mmol/L) | 11.4 ± 2.0 | 9.5 ± 0.8 | 9.2 ± 0.6 | 9.2 ± 0.6 | 10.9 ± 0.6 |
| PTT (s) | 78.6 ± 47.1 | 78.1 ± 40.3 | 87.2 ± 61.5 | 64.1 ± 56.0 | 75.5 ± 63.8 |
| PT (s) | 14.9 ± 1.2 | 14.5 ± 0.8 | 17.0 ± 5.1 | 10.5 ± 7.0 | 10.4 ± 5.4 |
Values are presented as mean ± standard deviation. BP, Blood pressure; WBC, white blood cells; Hgb, hemoglobin; Hct, hematocrit; AST, aspartate transaminase; ALT, alanine transaminase; PTT, partial thromboplastin time; PT, prothrombin time.
Oxygen tension values at day 0 were obtained while anesthetized swine were intubated and mechanically ventilated with inspired oxygen fraction of 100%. Oxygen tension values at days 1 to 4 were obtained from a central venous catheter while conscious swine were breathing room air.
Table E2.
Quantification of inflammatory cytokines in recipient swine serum
| Inflammatory cytokine (pg/mL) | Time (d) |
Fold change∗ | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| GM-CSF | –† | –† | 8.48 ± 6.5 | 32.68 ± 57.2 | 9.6 ± 8.3 | +9.6 |
| IFNγ | 20.6 ± 28.8 | 15.3 ± 30.5 | 52.3 ± 55.2 | 736.1 ± 1010.9 | 396.3 ± 477.5 | +19.2 |
| IL-1α | 2.6 ± 2.3 | 1.4 ± 2.0 | 2.4 ± 4.0 | 2.5 ± 4.1 | 1.9 ± 2.0 | –1.3 |
| IL-1β | 45.0 ± 31.7 | 37.7 ± 35.3 | 67.8 ± 52.3 | 169.7 ± 182.7 | 471.6 ± 649.8 | +10.4 |
| IL-1ra | 930.0 ± 932.7 | 437.7 ± 250.9 | 294.1 ± 229.9 | 694.7 ± 523.7 | 65024.5 ± 52388.8 | +69 |
| IL-2 | 8.5 ± 13.0 | 6.0 ± 12.1 | 16.1 ± 29.6 | 16.7 ± 30.3 | 0.9 ± 1.6 | –7.6 |
| IL-4 | 55.3 ± 65.8 | 46.2 ± 78.6 | 38.8 ± 63.2 | 51.7 ± 92.6 | 13.4 ± 11.6 | –4.1 |
| IL-6 | 59.6 ± 110.7 | 59.8 ± 93.9 | 76.4 ± 124.6 | 82.9 ± 131.3 | 184.4 ± 126.1 | +3.0 |
| IL-8 | 56.3 ± 47.5 | –† | 9.5 ± 11.6 | 1.0 ± 2.0 | 3.8 ± 3.4 | –14.8 |
| IL-10 | 46.4 ± 40.7 | 47.5 ± 36.3 | 57.8 ± 55.8 | 54.8 ± 45.2 | 129.9 ± 33.9 | +2.8 |
| IL-12 | 672.2 ± 257.4 | 246.4 ± 87.7 | 375.7 ± 149.0 | 386.0 ± 171.0 | 625.4 ± 107.2 | –1.1 |
| IL-18 | 673.3 ± 665.6 | 237.4 ± 191.7 | 178.0 ± 187.2 | 280.6 ± 128.2 | 320.7 ± 84.6 | –2.1 |
| TNFα | 12.9 ± 21.9 | 5.0 ± 6.6 | 8.1 ± 6.4 | 18.8 ± 30.6 | 22.9 ± 14.7 | +1.77 |
Values are presented as mean ± standard deviation. GM-CSF, Granulocyte-macrophage colony-stimulating factor; IFNγ, interferon-gamma; IL, interleukin; TNFα, tumor necrosis factor alpha.
Fold change (– or +) represents the change in cytokine concentration from day 0 to day 4.
Dash indicates cytokine value below detectable assay range.
Table E3.
Comparison of reported values of serum inflammatory cytokines in swine lung and ex vivo lung perfusion (EVLP) studies
| Serum cytokine | Reported value (pg/mL) | Experimental parameter | Reference |
|---|---|---|---|
| IFNγ |
0 900 ± 1500 1300 ± 1900 300 to 625 Not detected Not detected 67.3 ± 105.6 2201.8 ± 1746.9 5941 ± 5868.4 |
4 h EVLP, STEEN perfusate 4 h EVLP, STEEN + blood perfusate 4 h EVLP, Papworth–blood perfusate Control swine not exposed to PRCV 36 h XC 36 h XC, after 18 h cold static ischemia Baseline, before gastric aspiration 6 h after gastric aspiration 36 h XC, after gastric aspiration; Interventional treatments |
Roman and colleaguesE4 Jung and colleaguesE5 O'Neill and colleaguesE1 Guenthart and colleaguesE2 |
| IL-1β |
200 ± 300 2800 ± 4900 2300 ± 4000 46 ± 3.2 4.3 ± 1.3 0 to 100 4.7 × baseline 4200 5000 3800 1800 67.18 ± 8.39 248.69 ± 26.69 228.5 ± 67.8 230.5 ± 58.5 296.2 ± 183.8 3000 |
4 h EVLP, STEEN perfusate 4 h EVLP, STEEN + blood perfusate 4 h EVLP, Papworth–blood perfusate 4 h EVLP, after HCl aspiration; control (no treatment) 4 h EVLP, after HCl aspiration; Surfactant treatment Control swine Swine with PRRSV infection 6 h EVLP, after gastric aspiration; control (no treatment) 6 h EVLP, after gastric aspiration; aurfactant treatment 6 h EVLP, after gastric aspiration; lavage treatment 6 h EVLP, after gastric aspiration; surfactant + lavage treatment 36 h XC 36 h XC, after 18 h cold static ischemia Baseline, before gastric aspiration 6 h after gastric aspiration 36 h XC, after gastric aspiration; Interventional treatments 12 h EVLP |
Roman and colleaguesE4 Inci and colleaguesE6 Guo and colleaguesE7 Nakajima and colleaguesE8 O'Neill and colleaguesE1 Guenthart and colleaguesE2 Iskender and colleaguesE9 |
| IL-6 |
1400 ± 1500 4000 ± 4100 2100 ± 2100 1142 ± 556.3 616 ± 188.4 7 (0 to 50) 1160 (745 to 2766) 10 to125 0.2 × baseline 49 ± 91 680 ± 711 0 to 60 75 to 320 2400 2800 2200 1500 36.98 ± 2.21 140.96 ± 10.47 25.1 ± 32.2 64.5 ± 26.3 101.5 ± 75.5 5000 4000 9000 |
4 h EVLP, STEEN perfusate 4 h EVLP, STEEN + blood perfusate 4 h EVLP, Papworth–blood perfusate 4 h EVLP, after HCl aspiration; control (no treatment) 4 h EVLP, after HCl aspiration; surfactant treatment Baseline, before EVLP 6 h EVLP Control swine Swine with PRRSV infection Control swine Swine with ventilator-induced lung injury Control swine Swine with PRCV infection 6 h EVLP, after gastric aspiration; control (no treatment) 6 h EVLP, after gastric aspiration; Sufactant treatment 6 h EVLP, after gastric aspiration; lavage treatment 6 h EVLP, after gastric aspiration; surfactant + lavage treatment 36 h XC 36 h XC, after 18 h cold static ischemia Baseline, before gastric aspiration 6 h after gastric aspiration 36 h XC, after gastric aspiration; interventional treatments 12 h EVLP, cellular perfusate 12 h EVLP, acellular perfusate 12 h EVLP |
Roman and colleaguesE4 Inci and colleaguesE6 Adrian and colleaguesE10 Guo and colleaguesE7 Protti and colleaguesE11 Renukaradhya and colleaguesE12 Nakajima and colleaguesE8 O'Neill and colleaguesE1 Guenthart and colleaguesE2 Aboelnazar and colleaguesE13 Iskender and colleaguesE9 |
| IL-8 |
8700 ± 1110 5100 ± 3400 5600 ± 6000 6000 12 to 18 0.9 × baseline 169 ± 96 149 ± 240 25,000 30,000 18,000 8000 5.45 ± 1.05 35.52 ± 14.56 166.2 ± 108.2 330.1 ± 301.6 309.6 ± 241.8 1400 3000 12,000 |
4 h EVLP, STEEN perfusate 4 h EVLP, STEEN + blood perfusate 4 h EVLP, Papworth–blood perfusate 6 h EVLP Control swine Swine with PRRSV infection Control swine Swine with ventilator-induced lung injury 6 h EVLP, after gastric aspiration; control (no treatment) 6 h EVLP, after gastric aspiration; surfactant treatment 6 h EVLP, after gastric aspiration; lavage treatment 6 h EVLP, after gastric aspiration; surfactant + lavage treatment 36 h XC 36 h XC, after 18 h cold static ischemia Baseline, before gastric aspiration 6 h after gastric aspiration 36 h XC, after gastric aspiration; interventional treatments 12 h EVLP, cellular perfusate 12 h EVLP, acellular perfusate 12 h EVLP |
Roman and colleaguesE4 Kakishita and colleaguesE14 Guo and colleaguesE7 Protti and colleaguesE11 Nakajima and colleaguesE8 O'Neill and colleaguesE1 Guenthart and colleaguesE2 Aboelnazar and colleaguesE13 Iskender and colleaguesE9 |
| IL-10 |
100 ± 100 600 ± 500 400 ± 5000 31 (29 to 49) 51 (33 to 91) <4 <4 30 to 60 75 to 160 9.62 ± 0.97 13.14 ± 1.33 142.8 ± 84.2 149.1 ± 84.3 137.5 ± 138.3 450 |
4 h EVLP, STEEN perfusate 4 h EVLP, STEEN + blood perfusate 4 h EVLP, Papworth–blood perfusate Baseline, before EVLP 6 h EVLP Control swine Swine with ventilator-induced lung injury Control swine Swine with PRCV infection 36 h XC 36 h XC, after 18 h cold static ischemia Baseline, before gastric aspiration 6 h after gastric aspiration 36 h XC, after gastric aspiration; Interventional treatments 12 h EVLP |
Roman and colleaguesE4 Adrian and colleaguesE10 Protti and colleaguesE11 Renukaradhya and colleaguesE12 O'Neill and colleaguesE1 Guenthart and colleaguesE2 Iskender and colleaguesE9 |
| TNFα |
500 ± 700 2400 ± 2600 1600 ± 1600 3800 12 to 20 3 × baseline 113 ± 47 83 ± 59 7.67 ± 1.12 84.76 ± 14.41 26.6 ± 16.9 62.2 ± 57.5 180.7 ± 124.1 2200 2200 200 |
4 h EVLP, STEEN perfusate 4 h EVLP, STEEN + blood perfusate 4 h EVLP, Papworth–blood perfusate 6 h EVLP Control swine Swine with PRRSV infection Control swine Swine with ventilator-induced lung injury 36 h XC 36 h XC, after 18 h cold static ischemia Baseline, before gastric aspiration 6 h after gastric aspiration 36 h XC, after gastric aspiration; Interventional treatments 12 h EVLP, cellular perfusate 12 h EVLP, acellular perfusate 12 h EVLP |
Roman and colleaguesE4 Kakishita and colleaguesE14 Guo and colleaguesE7 Protti and colleaguesE11 O'Neill and colleaguesE1 Guenthart and colleaguesE2 Aboelnazar and colleaguesE13 Iskender and colleaguesE9 |
IFNγ, Interferon gamma; EVLP, ex vivo lung perfusion; PRCV, porcine respiratory coronavirus; XC, cross-circulation; IL, interleukin; HCl, hydrochloric acid; PRRSV, porcine reproductive and respiratory syndrome virus; TNFα, tumor necrosis factor alpha.
Table E4.
Quantification of functional parameters of extracorporeal lungs over 4 days of normothermic support
| Parameter | Result |
|---|---|
| Arterial oxygen tension/Inspired oxygen fraction (mm Hg) | |
| Baseline | 439.4 ± 227 |
| Day 4 | 548.5 ± 176 |
| Change | +109.1 ± 51 |
| Compliance (mL/cmH2O) | |
| Baseline | 22.3 ± 1.6 |
| Day 4 | 20.0 ± 1.0 |
| Change | –1.7 ± 0.6 |
| Peak inspiratory pressure (mm Hg) | |
| Baseline | 20.5 ± 0.7 |
| Day 4 | 25.0 ± 4.2 |
| Change | –4.5 ± 3.5 |
| Lactate (mmol) | |
| Baseline | 1.4 ± 0.5 |
| Day 4 | 3.1 ± 0.2 |
| Change | +1.7 ± 0.3 |
| pH | |
| Baseline | 7.36 ± 0.02 |
| Day 4 | 7.37 ± 0.08 |
| Change | +0.01 ± 0.06 |
Values are presented as mean ± standard deviation.
Table E5.
Quantification of inflammatory cytokines in bronchoalveolar lavage (BAL) fluid of extracorporeal lungs
| Inflammatory cytokine (pg/mL) | Time (d) |
Fold change∗ | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| GM-CSF | 11.1 ± 10.9 | 5.9 ± 8.2 | 2.1 ± 4.6 | 13.9 ± 17.0 | 28.1 ± 25.1 | +2.5 |
| IFNγ | 104.9 ± 192.5 | 7.19 ± 16.1 | 2.1 ± 4.7 | 3.64 ± 8.1 | –† | –104.9 |
| IL-1α | 614.6 ± 670.6 | 35.4 ± 16.1 | 399.8 ± 480.2 | 115.0 ± 144.0 | 69.7 ± 91.0 | –8.8 |
| IL-1β | 4831.3 ± 5898.4 | 1142.6 ± 1065.1 | 5772.9 ± 5662.4 | 3377.6 ± 4347.7 | 2161.5 ± 2457.7 | –2.2 |
| IL-1ra | 4372.3 ± 6104.8 | 725.9 ± 664.2 | 5367.5 ± 6231.2 | 4096.5 ± 4210.2 | 3455.6 ± 2516.0 | –1.2 |
| IL-2 | 4105.6 ± 6311.4 | 420.1 ± 746.6 | 4866.6 ± 6665.0 | 3057.3 ± 4591.0 | 1653.1 ± 2858.3 | –2.4 |
| IL-4 | 4.1 ± 9.2 | –† | 4.4 ± 9.8 | 32.9 ± 43.9 | 22.7 ± 39.4 | +5.5 |
| IL-6 | 94.7 ± 117.8 | 466.2 ± 513.3 | 719.6 ± 859.3 | 684.7 ± 789.6 | 472.1 ± 817.7 | +4.9 |
| IL-8 | 17797.1 ± 18704.1 | 8382.1 ± 6745.5 | 17069.0 ± 12276.2 | 12260.3 ± 12016.8 | 10917.3 ± 12690.8 | –1.6 |
| IL-10 | 6.1 ± 1.7 | 4.4 ± 3.1 | 4.0 ± 3.6 | 23.4 ± 25.1 | 21.7 ± 27.8 | +3.5 |
| IL-12 | 66.6 ± 92.7 | 16.8 ± 15.9 | 24.5 ± 32.9 | 63.2 ± 78.2 | 53.9 ± 83.7 | –1.2 |
| IL-18 | 710.4 ± 959.6 | 146.2 ± 186.0 | 581.2 ± 779.8 | 186.1 ± 198.6 | 130.0 ± 215.5 | –5.4 |
| TNFα | 704.1 ± 965.4 | 126.2 ± 198.5 | 573.2 ± 786.9 | 158.2 ± 217.0 | 126.3 ± 218.7 | –5.5 |
Values are presented as mean ± standard deviation of cytokine concentrations in BAL fluid collected from both left and right lungs. GM-CSF, Granulocyte-macrophage colony-stimulating factor; IFNγ, interferon-gamma; IL, interleukin; TNFα, tumor necrosis factor alpha.
Fold change represents the change (– or +) in cytokine concentration from day 0 to day 4.
Cytokine level below detectable assay range.
Table E6.
Comparison of reported values of bronchoalveolar lavage fluid (BALF) inflammatory cytokines in swine lung and ex vivo lung perfusion (EVLP) studies
| BALF cytokine | Reported value (pg/mL) | Experimental parameter | Reference |
|---|---|---|---|
| IFNγ |
0 1260 ± 520 970 ± 110 5.1 ± 0.7 248.2 ± 87 500.4 ± 13.9 1347.3 ± 397.5 |
4 h EVLP, STEEN perfusate 4 h EVLP, STEEN + blood perfusate 4 h EVLP, Papworth–blood perfusate Thoracotomy only Transplantation, after 6 h cold static ischemia 36 h XC, control (no injury) 36 h XC, after gastric aspiration; Interventional treatments |
Roman and colleaguesE4 LaPar and colleaguesE15 Guenthart and colleaguesE2 |
| IL-1β |
2520 ± 60 2380 ± 1460 1030 ± 700 323.2 ± 117.9 56.1 ± 34.2 851.1 ± 262.8 259.5 ± 87.3 28.6 ± 1.2 265.1 ± 43.7 50 4.4 × baseline 44.3 ± 14.4 to 831.7 ± 136.6 844.2 ± 98.31 to 1251.9 ± 244.61500 100 1800 6142.5 ± 7626.7 40,170.7 ± 10,783 |
4 h EVLP, STEEN perfusate 4 h EVLP, STEEN + blood perfusate 4 h EVLP, Papworth–blood perfusate 4 h EVLP, after HCl aspiration; control (no treatment) 4 h EVLP, after HCl aspiration; surfactant treatment 4 h cold static ischemia Transplantation, after 4 h cold static ischemia and 4 h EVLP Thoracotomy only Transplantation, after 6 h cold static ischemia Control swine Swine with PRRSV infection 36 h XC 36 h XC, after 18 h cold static ischemia 24 h after gastric aspiration, control (no treatment) 4 h EVLP, control (no injury) 4 h EVLP, after gastric aspiration 36 h XC, control (no injury) 36 h XC, after gastric aspiration; Interventional treatments |
Roman and colleaguesE4 Inci and colleaguesE6 Mulloy and colleaguesE16 LaPar and colleaguesE15 Guo and colleaguesE7 O'Neill and colleaguesE1 Khalifé-Hocquemiller and colleaguesE17 Guenthart and colleaguesE2 |
| IL-6 |
1580 ± 2150 1050 ± 1390 280 ± 160 1640 ± 305.1 636.6 ± 169.4 2 to 3.5 4 × baseline 211 ± 364 2198 ± 1685 71.2 ± 14.3 to 109.8 ± 33.6 308.0 ± 67.0 to 447.2 ± 26.9 75 25 100 617.8 ± 20.3 2358.4 ± 1915.2 |
4 h EVLP, STEEN perfusate 4 h EVLP, STEEN + blood perfusate 4 h EVLP, Papworth–blood perfusate 4 h EVLP, after HCl aspiration; Control (no treatment) 4 h EVLP, after HCl aspiration; Surfactant treatment Control swine Swine with PRRSV infection Control swine Swine with ventilator-induced lung injury 36 h XC 36 h XC, after 18 h cold static ischemia 24 h after gastric aspiration, control (no treatment) 4 h EVLP, control (no injury) 4 h EVLP, after gastric aspiration 36 h XC, control (no injury) 36 h XC, after gastric aspiration; Interventional treatments |
Roman and colleaguesE4 Inci and colleaguesE6 Guo and colleaguesE7 Protti and colleagueE11 O'Neill and colleaguesE1 Khalifé-Hocquemiller and colleaguesE17 Guenthart and colleaguesE2 |
| IL-8 |
16,400 ± 16,700 4860 ± 4580 2080 ± 220 1795.5 ± 164.9 659.5 ± 166.5 531.5 ± 331.4 112.1 ± 74.8 51.3 ± 16.7 222.4 ± 41.4 25 to 35 7.6 × baseline 1569 ± 1475 725 ± 1092 0.1 ± 0.1 to 3.0 ± 1.0 4.0 ± 0.8 to 5.47 ± 1.8 10 0.4 11 2529.6 ± 77.3 3188.4 ± 169.2 336.8 ± 447.2 to 3847.7 ± 796.1 |
4 h EVLP, STEEN perfusate 4 h EVLP, STEEN + blood perfusate 4 h EVLP, Papworth–blood perfusate 4 h EVLP, after HCl aspiration; Control (no treatment) 4 h EVLP, after HCl aspiration; Surfactant treatment 4 h cold static ischemia Transplantation, after 4 h cold static ischemia and 4 h EVLP Thoracotomy only Transplantation, after 6 h cold static ischemia Control swine Swine with PRRSV infection Control swine Swine with ventilator-induced lung injury 36 h XC 36 h XC, after 18 h cold static ischemia 24 h after gastric aspiration, control (no treatment) 4 h EVLP, control (no injury) 4 h EVLP, after gastric aspiration 36 h XC, control (no injury) 36 h XC, after gastric aspiration; Interventional treatments 2 h EVLP, after 24 h cold static ischemia (control) |
Roman and colleaguesE4 Inci and colleaguesE6 Mulloy and colleaguesE16 LaPar and colleaguesE15 Guo and colleaguesE7 Protti and colleaguesE11 O'Neill and colleaguesE1 Khalifé-Hocquemiller and colleaguesE17 Guenthart and colleaguesE2 Yamada and colleaguesE18 |
| IL-10 |
120 ± 160 90 ± 120 10 ± 2 2.7 ± 0.8 63.1 ± 13.1 <4 <4 3.4 ± 1.7 to 19.0 ± 6.6 11.8 ± 4.6 to 14.1 ± 1.4 11.1 ± 9.7 to 46.9 ± 49.7 11.1 ± 9.7 to 127.6 ± 72.2 |
4 h EVLP, STEEN perfusate 4 h EVLP, STEEN + blood perfusate 4 h EVLP, Papworth–blood perfusate Thoracotomy only Transplantation, after 6 h cold static ischemia Control swine Swine with ventilator-induced lung injury 36 h XC 36 h XC, after 18 h cold static ischemia 36 h XC, control (no injury) 36 h XC, after gastric aspiration; Interventional treatments |
Roman and colleaguesE4 LaPar and colleaguesE15 Protti and colleaguesE11 O'Neill and colleaguesE1 Guenthart and colleaguesE2 |
| TNFα |
170 ± 220 360 ± 510 80 ± 50 1050.2 ± 353.8 233.2 ± 84.9 2.5 ± 0.6 25.9 ± 4.6 0.5 to 1.5 20.6 × baseline 202 ± 252 46 ± 71 0.8 ± 0.5 to 2.7 ± 0.8 53.8 ± 13.3 to 96.6 ± 10.5 5.1 ± 8.9 to 32.5 ± 16.2 5.1 ± 8.9 to 61.7 ± 20.8 |
4 h EVLP, STEEN perfusate 4 h EVLP, STEEN + blood perfusate 4 h EVLP, Papworth–blood perfusate 4 h cold static ischemia Transplantation, after 4 h cold static ischemia and 4 h EVLP Thoracotomy only Transplantation, after 6 h cold static ischemia Control swine Swine with PRRSV infection Control swine Swine with ventilator-induced lung injury 36 h XC 36 h XC, after 18 h cold static ischemia 36 h XC, control (no injury) 36 h XC, after gastric aspiration; Interventional treatments |
Roman and colleaguesE4 Mulloy and colleaguesE16 LaPar and colleaguesE15 Guo and colleaguesE7 Protti and colleaguesE11 O'Neill and colleaguesE1 Guenthart and colleaguesE2 |
IFNγ, Interferon gamma; EVLP, ex vivo lung perfusion; XC, cross-circulation; IL, interleukin; HCl, hydrochloric acid; PRRSV, porcine reproductive and respiratory syndrome virus; TNFα, tumor necrosis factor alpha.
Table E7.
Preprocedure training program for recipient swine. Before initiation of cross-circulation between extracorporeal lungs and recipient swine, preprocedure behavior training was conducted for 13 days. On day 14, cross-circulation was initiated and continued through day 18
| Day | Activity |
|---|---|
| 0 | Arrival at animal housing facility where recipient swine were placed into standard housing enclosure. |
| 1-2 | Initiation of target training in swine housing enclosure. Swine conditioned to touch nose to target when trainer used clicker. Behavior reinforced with standard quality edible treats. Duration: 1 h daily. |
| 3 | Continuation of target training in housing enclosure. Introduction of custom enclosure within the standard housing enclosure area. Duration: 1 h. |
| 4 | Progression of target training to encourage swine to enter custom enclosure. Duration: 1 h. Swine placed into custom enclosure and transported to operating room. Duration: 1 h. |
| 5-13 | Continuation of daily decreases in width of custom enclosure to inhibit swine from rotating within the cage while remaining comfortable and allowing forward, backward, upward, and downward movements. Swine encouraged to remain within the enclosure using high quality edible treats. Duration: 2 h daily. |
| 14 | Transportation to operating room where swine were anesthetized, cross-circulation was initiated, and swine were placed into custom enclosure. |
| 14-18 | Active enrichment during multiday extracorporeal organ support studies using manipulata (ie, toys), social interactions with procedure personnel, and standard and high quality edible treats. |
References
- 1.Ware L.B., Wang Y., Fang X., Warnock M., Sakuma T., Hall T.S. Assessment of lungs rejected for transplantation and implications for donor selection. Lancet. 2002;360:619–620. doi: 10.1016/s0140-6736(02)09774-x. [DOI] [PubMed] [Google Scholar]
- 2.Singer J., Chen J., Blanc P.D., Leard L.E., Kukreja J., Chen H. A thematic analysis of quality of life in lung transplant: the existing evidence and implications for future directions. Am J Transplant. 2013;13:839–850. doi: 10.1111/ajt.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kugler C., Gottlieb J., Warnecke G., Schwarz A., Weissenborn K., Barg-Hock H. Health-related quality of life after solid organ transplantation: a prospective, multiorgan cohort study. Transplant J. 2013;96:316–323. doi: 10.1097/TP.0b013e31829853eb. [DOI] [PubMed] [Google Scholar]
- 4.Pinezich M., Vunjak-Novakovic G. Bioengineering approaches to organ preservation ex vivo. Exp Biol Med. 2019;244:630–645. doi: 10.1177/1535370219834498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guibert E.E., Petrenko A.Y., Balaban C.L., Somov A.Y., Rodriguez J.V., Fuller B.J. Organ preservation: current concepts and new strategies for the next decade. Transfus Med Hemotherapy. 2011;38:125–142. doi: 10.1159/000327033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makdisi G., Makdisi T., Jarmi T., Caldeira C.C. Ex vivo lung perfusion review of a revolutionary technology. Ann Transl Med. 2017;5:343. doi: 10.21037/atm.2017.07.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tane S., Noda K., Shigemura N. Ex vivo lung perfusion: a key tool for translational science in the lungs. Chest. 2017;151:1220–1228. doi: 10.1016/j.chest.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 8.Steen S., Sjöberg T., Pierre L., Liao Q., Eriksson L., Algotsson L. Transplantation of lungs from a non-heart-beating donor. Lancet. 2001;357:825–829. doi: 10.1016/S0140-6736(00)04195-7. [DOI] [PubMed] [Google Scholar]
- 9.Cypel M., Yeung J.C., Liu M., Anraku M., Chen F., Karolak W. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med. 2011;364:1431–1440. doi: 10.1056/NEJMoa1014597. [DOI] [PubMed] [Google Scholar]
- 10.Warnecke G., Van Raemdonck D., Smith M.A., Massard G., Kukreja J., Rea F. Normothermic ex-vivo preservation with the portable Organ Care System Lung device for bilateral lung transplantation (INSPIRE): a randomised, open-label, non-inferiority, phase 3 study. Lancet Respir Med. 2018;6:357–367. doi: 10.1016/S2213-2600(18)30136-X. [DOI] [PubMed] [Google Scholar]
- 11.Cypel M., Yeung J.C., Machuca T., Chen M., Singer L.G., Yasufuku K. Experience with the first 50 ex vivo lung perfusions in clinical transplantation. J Thorac Cardiovasc Surg. 2012;144:1200–1207. doi: 10.1016/j.jtcvs.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 12.O'Neill J.D., Guenthart B.A., Kim J., Chicotka S., Queen D., Fung K. Cross-circulation for extracorporeal support and recovery of the lung. Nat Biomed Eng. 2017;1:0037. [Google Scholar]
- 13.Guenthart B.A., O'Neill J.D., Kim J., Queen D., Chicotka S., Fung K. Regeneration of severely damaged lungs using an interventional cross-circulation platform. Nat Commun. 2019;10:1985. doi: 10.1038/s41467-019-09908-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Raemdonck D., Hartwig M.G., Hertz M.I., Davis R.D., Cypel M., Hayes D., Jr. Report of the ISHLT working group on primary lung graft dysfunction part IV: prevention and treatment: a 2016 consensus group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017;36:1121–1136. doi: 10.1016/j.healun.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Song J.H., Park J.E., Lee J.G., Lee C.Y., Nam K.S., Suh J.W. Outcomes of perioperative extracorporeal membrane oxygenation use in patients undergoing lung transplantation. J Thorac Dis. 2017;9:5075–5084. doi: 10.21037/jtd.2017.10.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoetzenecker K., Schwarz S., Muckenhuber M., Benazzo A., Frommlet F., Schweiger T. Intraoperative extracorporeal membrane oxygenation and the possibility of postoperative prolongation improve survival in bilateral lung transplantation. J Thorac Cardiovasc Surg. 2018;155:2193–2206.e3. doi: 10.1016/j.jtcvs.2017.10.144. [DOI] [PubMed] [Google Scholar]
- 17.Guenthart B.A., O'Neill J.D., Kim J., Fung K., Vunjak-Novakovic G., Bacchetta M. Cell replacement in human lung bioengineering. J Heart Lung Transplant. 2019;38:215–224. doi: 10.1016/j.healun.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin H.-Y., Freed D., Lee T.W., Arora R.C., Ali A., Almoustadi W. Quantitative assessment of cardiac output and left ventricular function by noninvasive phase-contrast and cine MRI: validation study with invasive pressure-volume loop analysis in a swine model. J Magn Reson Imaging. 2011;34:203–210. doi: 10.1002/jmri.22587. [DOI] [PubMed] [Google Scholar]
- 19.West J.B. How well designed is the human lung? Am J Respir Crit Care Med. 2006;173:583–584. doi: 10.1164/rccm.200510-1682OE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motoyama H., Chen F., Hijiya K., Kondo T., Ohata K., Takahashi M. Novel thermographic detection of regional malperfusion caused by a thrombosis during ex vivo lung perfusion. Interact Cardiovasc Thorac Surg. 2015;20:242–247. doi: 10.1093/icvts/ivu386. [DOI] [PubMed] [Google Scholar]
- 21.Aboelnazar N.S., Himmat S., Hatami S., White C.W., Burhani M.S., Dromparis P. Negative pressure ventilation decreases inflammation and lung edema during normothermic ex-vivo lung perfusion. J Heart Lung Transplant. 2018;37:520–530. doi: 10.1016/j.healun.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Sommer W., Salman J., Avsar M., Hoeffler K., Jansson K., Siemeni T.N. Prediction of transplant outcome after 24-hour ex vivo lung perfusion using the organ care system in a porcine lung transplantation model. Am J Transplant. 2019;19:345–355. doi: 10.1111/ajt.15075. [DOI] [PubMed] [Google Scholar]
- 23.Roman M., Gjorgjimajkoska O., Neil D., Nair S., Colah S., Parmar J. Comparison between cellular and acellular perfusates for ex vivo lung perfusion in a porcine model. J Heart Lung Transplant. 2015;34:978–987. doi: 10.1016/j.healun.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 24.Linacre V., Cypel M., Machuca T., Nakajima D., Hashimoto K., Zamel R. Importance of left atrial pressure during ex vivo lung perfusion. J Heart Lung Transplant. 2016;35:808–814. doi: 10.1016/j.healun.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 26.Arend W.P., Malyak M., Guthridge C.J., Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- 27.Cavaillon J.M. Pro- versus anti-inflammatory cytokines: myth or reality. Cell Mol Biol. 2001;47:695–702. [PubMed] [Google Scholar]
- 28.Cypel M., Liu M., Rubacha M., Yeung J.C., Hirayama S., Anraku M. Functional repair of human donor lungs by IL-10 gene therapy. Sci Transl Med. 2009;1:4ra9. doi: 10.1126/scitranslmed.3000266. [DOI] [PubMed] [Google Scholar]
- 29.Mordant P., Nakajima D., Kalaf R., Iskender I., Maahs L., Behrens P. Mesenchymal stem cell treatment is associated with decreased perfusate concentration of interleukin-8 during ex vivo perfusion of donor lungs after 18-hour preservation. J Heart Lung Transplant. 2016;35:1245–1254. doi: 10.1016/j.healun.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 30.Gennai S., Monsel A., Hao Q., Park J., Matthay M.A., Lee J.W. Microvesicles derived from human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. Am J Transplant. 2015;15:2404–2412. doi: 10.1111/ajt.13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.La Francesca S., Ting A.E., Sakamoto J., Rhudy J., Bonenfant N.R., Borg Z.D. Multipotent adult progenitor cells decrease cold ischemic injury in ex vivo perfused human lungs: an initial pilot and feasibility study. Transplant Res. 2014;3:19. doi: 10.1186/2047-1440-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinezich M., Vunjak-Novakovic G. Bioengineering approaches to organ preservation ex vivo. Exp Biol Med. 2019;244:630–645. doi: 10.1177/1535370219834498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mao A.S., Mooney D.J. Regenerative medicine: current therapies and future directions. Proc Natl Acad Sci U S A. 2015;112:14452–14459. doi: 10.1073/pnas.1508520112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dorrello N.V., Guenthart B.A., O'Neill J.D., Kim J., Cunningham K., Chen Y.W. Functional vascularized lung grafts for lung bioengineering. Sci Adv. 2017;3:e1700521. doi: 10.1126/sciadv.1700521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilpin S.E., Charest J.M., Ren X., Ott H.C. Bioengineering lungs for transplantation. Thorac Surg Clin. 2016;26:163–171. doi: 10.1016/j.thorsurg.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Ott H.C., Clippinger B., Conrad C., Schuetz C., Pomerantseva I., Ikonomou L. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
E-References
- O'Neill J.D., Guenthart B.A., Kim J., Chicotka S., Queen D., Fung K. Cross-circulation for extracorporeal support and recovery of the lung. Nat Biomed Eng. 2017;1:0037. [Google Scholar]
- Guenthart B.A., O'Neill J.D., Kim J., Queen D., Chicotka S., Fung K. Regeneration of severely damaged lungs using an interventional cross-circulation platform. Nat Commun. 2019;10:1985. doi: 10.1038/s41467-019-09908-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampersad S.N. Multiple applications of alamar blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors. 2012;12:12347. doi: 10.3390/s120912347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman M., Gjorgjimajkoska O., Neil D., Nair S., Colah S., Parmar J. Comparison between cellular and acellular perfusates for ex vivo lung perfusion in a porcine model. J Heart Lung Transplant. 2015;34:978–987. doi: 10.1016/j.healun.2015.03.023. [DOI] [PubMed] [Google Scholar]
- Jung K., Renukaradhya G.J., Alekseev K.P., Fang Y., Tang Y., Saif L.J. Porcine reproductive and respiratory syndrome virus modifies innate immunity and alters disease outcome in pigs subsequently infected with porcine respiratory coronavirus: implications for respiratory viral co-infections. J Gen Virol. 2009;90(Pt 11):2713–2723. doi: 10.1099/vir.0.014001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inci I., Ampollini L., Arni S., Jungraithmayr W., Inci D., Hillinger S. Ex vivo reconditioning of marginal donor lungs injured by acid aspiration. J Heart Lung Transplant. 2008;27:1229–1236. doi: 10.1016/j.healun.2008.07.027. [DOI] [PubMed] [Google Scholar]
- Guo B., Lager K.M., Henningson J.N., Miller L.C., Schlink S.N., Kappes M.A. Experimental infection of United States swine with a Chinese highly pathogenic strain of porcine reproductive and respiratory syndrome virus. Virology. 2013;435:372–384. doi: 10.1016/j.virol.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima D., Liu M., Ohsumi A., Kalaf R., Iskender I., Hsin M. Lung lavage and surfactant replacement during ex vivo lung perfusion for treatment of gastric acid aspiration–induced donor lung injury. J Heart Lung Transplant. 2017;36:577–585. doi: 10.1016/j.healun.2016.11.010. [DOI] [PubMed] [Google Scholar]
- Iskender I., Cosgun T., Arni S., Trinkwitz M., Fehlings S., Yamada Y. Cytokine filtration modulates pulmonary metabolism and edema formation during ex vivo lung perfusion. J Heart Lung Transplant. 2018;37:283–291. doi: 10.1016/j.healun.2017.05.021. [DOI] [PubMed] [Google Scholar]
- Adrian K., Skogby M., Gatzinsky V., Friberg L.-G., Mellgren K. Procedure-induced inflammation and endothelial cell activation in an artificially ventilated and circulated porcine double-lung model. Artif Organs. 2006;30:922–928. doi: 10.1111/j.1525-1594.2006.00325.x. [DOI] [PubMed] [Google Scholar]
- Protti A., Maraffi T., Milesi M., Votta E., Santini A., Pugni P. Role of strain rate in the pathogenesis of ventilator-induced lung edema. Crit Care Med. 2016;44:e838–e845. doi: 10.1097/CCM.0000000000001718. [DOI] [PubMed] [Google Scholar]
- Renukaradhya G.J., Alekseev K., Jung K., Fang Y., Saif L.J. Porcine reproductive and respiratory syndrome virus–induced immunosuppression exacerbates the inflammatory response to porcine respiratory coronavirus in pigs. Viral Immunol. 2010;23:457–466. doi: 10.1089/vim.2010.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboelnazar N.S., Himmat S., Hatami S., White C.W., Burhani M.S., Dromparis P. Negative pressure ventilation decreases inflammation and lung edema during normothermic ex-vivo lung perfusion. J Heart Lung Transplant. 2018;37:520–530. doi: 10.1016/j.healun.2017.09.007. [DOI] [PubMed] [Google Scholar]
- Kakishita T., Oto T., Hori S., Miyoshi K., Otani S., Yamamoto S. Suppression of inflammatory cytokines during ex vivo lung perfusion with an adsorbent membrane. Ann Thorac Surg. 2010;89:1773–1779. doi: 10.1016/j.athoracsur.2010.02.077. [DOI] [PubMed] [Google Scholar]
- LaPar D.J., Laubach V.E., Emaminia A., Crosby I.K., Hajzus V.A., Sharma A.K. Pretreatment strategy with adenosine A2A receptor agonist attenuates reperfusion injury in a preclinical porcine lung transplantation model. J Thorac Cardiovasc Surg. 2011;142:887–894. doi: 10.1016/j.jtcvs.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulloy D.P., Stone M.L., Crosby I.K., Lapar D.J., Sharma A.K., Webb D.V. Ex vivo rehabilitation of non–heart-beating donor lungs in preclinical porcine model: delayed perfusion results in superior lung function. J Thorac Cardiovasc Surg. 2012;144:1208–1216. doi: 10.1016/j.jtcvs.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalifé-Hocquemiller T., Sage E., Dorfmuller P., Mussot S., Le Houérou D., Eddahibi S. Exogenous surfactant attenuates lung injury from gastric-acid aspiration during ex vivo reconditioning in pigs. Transplant J. 2014;97:413–418. doi: 10.1097/01.TP.0000441320.10787.c5. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Iskender I., Arni S., Hillinger S., Cosgun T., Yu K. Ex vivo treatment with inhaled N-acetylcysteine in porcine lung transplantation. J Surg Res. 2017;218:341–347. doi: 10.1016/j.jss.2017.06.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Normothermic maintenance of extracorporeal swine lungs with multiday lung support system. Video available at: https://www.jtcvs.org/article/S0022-5223(19)32146-4/fulltext.
Transfer of swine recipient in Panepinto sling to custom enclosure. Video available at: https://www.jtcvs.org/article/S0022-5223(19)32146-4/fulltext.
Active enrichment of recipient swine in custom enclosure throughout multiday lung support. Video available at: https://www.jtcvs.org/article/S0022-5223(19)32146-4/fulltext.