Abstract
We studied the effects of human activity on concentrations of fungal and bacterial bioaerosols in indoor air environments. We conducted measurement experiments for concentrations of bioaerosols and aerosol particles in test chambers with people performing various activities inside. We found that the number of people and human activities had positive correlations with the concentrations of bacterial bioaerosols. However, the concentration of fungal bioaerosols was not influenced by human presence or activities. The findings regarding the concentrations of fungal and bacterial bioaerosols and the effects of human presence and activities will be useful for studying control methods against bioaerosols.
Keywords: Bioaerosol, Airborne bacteria, Airborne fungi, Anthropogenic bioaerosol, Human activity
Highlights
-
•
The number of people had correlations with the concentration of bacterial bioaerosols.
-
•
Concentrations of bacterial bioaerosols are strongly linked with human activities.
-
•
The concentration of fungal bioaerosols was not influenced by human presence.
1. Introduction
Bioaerosols are airborne biological particulate matter (Gόrny, Dutkiewicz & Krysinska-Traczyk, 1999). The diameter of bioaerosols ranges from 0.3 μm to 100 μm (Cox & Wathes, 1995), and in particular breathable bioaerosols (size range from 1 to 10 μm) are of primary concern (Heyder, Gebhart, Rudolf, Schiller & Stahlhofen, 1986). Bioaerosols are readily transported through the air and can remain in air environments because of their small size and light weight (Beggs (2003), Hinds, 1982). Bioaerosols, which can be inhaled or attached to human bodies in their airborne state, have been known to be etiological agents for respiratory and infectious human diseases (Cockcroft, Ruffin, Dolovich, & Hargreave (1977), Fiegel, Clarke, & Edwards (2006), Fung & Hughson (2003), Lacey & Crook (1988)). Several studies that have measured concentrations of bioaerosols in indoor and outdoor environments have shown that bioaerosols can significantly affect human health conditions (Ren, Jankun, & Leaderer (1999), Zhu et al. (2003)). Therefore, it is necessary to monitor and control bioaerosols in the air for occupational safety and public health purposes (Hwang et al. (2015), Lee et al. (2012), Mainelis et al. (2002), Nazaroff (2014)).
Several potential sources of bioaerosols have been proposed for indoor air environments, including air conditioning systems with contaminated filters, uncontrolled bathrooms, dirty kitchens, and outdoor airflows containing various outdoor microorganisms (Lee et al. (2012), Ren, Jankun, & Leaderer (1999), Zhu et al. (2003), Meadow et al. (2014), Nazaroff (2014)). In this study, we propose that, among potential sources, humans can be sources or strong factors for bioaerosols in indoor air environments.
Recent studies have shown that the activities of indoor occupants, such as walking, affect the concentration of ordinary aerosol particles (Batterman (2001), Ferro, Kopperud, & Hildemann (2004), He, Morawska, Hitchins, & Gilbert (2004), Raunemaa, Kulmala, Saari, Olin, & Kulmala (1989), Qian, Peccia, & Ferro (2014)). In particular, some studies have found that the activities of occupants increase the concentrations of coarser (aerosol diameter >1 μm) aerosol particulate matter (Batterman (2001), Raunemaa, Kulmala, Saari, Olin, & Kulmala (1989)) in indoor environments. Based on those works, in this study, we propose that human activity and human presence may affect the concentration of biological particles, as they do for ordinary aerosol particles. There have been a few previous studies on the correlation between human activities and bioaerosol concentrations in indoor air environments. It was found that human occupancy increased the bacterial genome concentration in indoor air environments such as in a classroom (Hospodsky et al. (2012), Qian, Hospodsky, Yamamoto, Nazaroff, & Peccia (2012)). One study found that residential activities had a noticeable effect on the concentrations of culturable fungal bioaerosols in indoor environments (Chen & Hildemann, 2009). It was suggested that human activities caused the resuspension of settled fungal particles into an airborne state (Buttner & Stetzenbach, 1993). The outdoor air flow, rather than human activities, was proposed to be the dominant factor responsible for the level of fungal bioaerosols in indoor air environments (Adams, Miletto, Taylor & Bruns, 2013).
In these previous studies, the measurements were conducted under limited conditions, such as on-off conditions (occupied or vacant), and only assessed genomic concentrations of bioaerosols. In addition, most of these experiments (Buttner & Stetzenbach (1993), Chen & Hildemann (2009), Hospodsky et al. (2012), Qian, Hospodsky, Yamamoto, Nazaroff, & Peccia (2012)) were conducted in North America. The sources of culturable bacterial and fungal bioaerosols in indoor environments with human presence were not explained, and no conclusions could be drawn regarding the possible regional variation outside of North America.
From another point of view, several research groups found that aerosol particles exhaled by humans are composed of small droplets of airway-lining fluid (Fiegel, Clarke, & Edwards (2006), Han, Weng, & Huang (2013)), and some of these exhaled droplets contain pathogens, such as influenza viruses (Fabian et al. (2008), Milton, Fabian, Cowling, Grantham, & McDevitt (2013)), tuberculosis bacteria (Turner & Bothamley, 2014), and severe acute respiratory syndrome viruses (World Health Organization (WHO), 2009). However, the concentrations of overall culturable bioaerosols in exhaled air from humans were not adequately measured in these reference studies.
In the current study, we investigate human impacts on the concentrations of culturable bacterial and fungal bioaerosols under various conditions. First, we measured the number of individuals as well as the concentrations of culturable bioaerosols in specific indoor environments to observe the effect of human presence (number of humans) on bioaerosols. Second, we monitored the concentration of bioaerosols with varied human activities such as standing, talking, and moving in small artificial indoor environments. Third, we directly measured the concentration of culturable bioaerosols from exhaled human breath under ordinary breathing patterns.
The aim of this study is to extend understanding of the effects of humans on concentrations of culturable bioaerosols. The findings can elucidate sources of culturable fungal and bacterial bioaerosols in indoor air environments.
2. Methods
2.1. Measurement methods for bioaerosols
In this study, culturable bioaerosols were targeted with a focus on the infectivity of the bioaerosols (Toivola, Nevalainen & Alm, 2004), which was consistent with the legal bioaerosol standard established by the Ministry of Environment of Korea (Ministry of Environment, Republic of Korea, 2014). During the measurement campaigns, a Bio-Culture device (Buck Bio-Culture, Model B30120, A.P. Buck, Inc., Orlando, Florida, U.S.) was used to sample bioaerosols (Heo et al., 2014). The Bio-Culture device is a multiple-jet impactor-type sampler for collecting airborne microorganisms. The sampling flow rate was 100 L/min and the sampling time was approximately 1 min per sample to prevent overcrowding of colonies. The measurement was replicated at least three times under individual sampling conditions.
Nutrient agar plates (beef extract 3%, peptone 5%, and agar 15%; Difco; 25 mL of agar per 90 mm by 15 mm petri dish) were used in the Bio-Culture device to sample bacterial bioaerosols (Hinds, 1998). Sampled bacterial aerosols were incubated at 37 °C for 24 h. For the measurement of fungal bioaerosols, malt extract agar (MEA: maltose 12.75%, dextrin 2.75%, glycerol 2.35%, peptone 0.75%, and agar 15%; Difco; 25 mL of agar per 90 mm by 15 mm petri dish) was used. The sampled fungal bioaerosols were incubated at 25 °C for 48 h. After the incubation of sampled bioaerosols, the number of colonies was enumerated and the concentration of culturable bioaerosols was determined. Data for the number of colonies were converted to the concentration of bioaerosols in the air, expressed as colony forming unit per unit volume (CFU/m3). A positive-hole correction table was used to adjust colony counts from a 400-hole impactor to allow the collection of multiple particles through a single hole (Macher, 1989).
2.2. Human presence and bioaerosol concentrations
To investigate the effect of the humans on bioaerosol concentrations, we first measured the concentration of total culturable bacterial and fungal bioaerosols at a student hall in Konkuk University, along with the number of students inside the hall. The floor area of the student hall is 562.5 m2 and the height is 3.4 m (total volume=1912.5 m3). The number of students inside the hall was highly varied. Sometimes hundreds of students were present in the hall, which we considered highly crowded, and sometimes the hall was almost empty. We simultaneously measured concentrations of bioaerosols and the number of people. We investigated the relationship between the two parameters.
Second, we measured the concentrations of bioaerosols with various human activities, under the condition of a single person inside a small indoor environment. One of the authors stayed inside the small glass chamber (chamber size=1.5 m×1.5 m×2 m; chamber volume=4.5 m3) and varied his motions, such as standing without motion, talking, and moving around inside the chamber. During the activities, the concentrations of fungal and bacterial aerosols were measured under individual conditions. Under the standing without motion condition, one person stayed in the chamber for 20 s without performing any action except breathing. Under the talking condition, one person talked to people outside of the glass chamber for 5 min. Under the moving condition, one person moved around inside the chamber at a speed of approximately 8 km/h for 20 s. We measured bacterial and fungal bioaerosols under individual conditions as well as the concentration of outside bioaerosols for comparison. In addition, before all experiments, we measured bioaerosols in the vacant chamber as a reference condition. Furthermore, we measured the concentrations of airborne particulate matter (PM) under individual conditions using the optical particle counting method (Portable Particle Counter, Model 3905, KANOMAX, Inc., New Jersey, USA).
Third, we directly measured the concentration of bioaerosols at the human mouth during inhalation and exhalation. We sampled bioaerosols from breathing air flow with two categories of inhalation and exhalation, and compared the concentrations of bioaerosols. Human subjects, including three men and three women, breathed naturally in an air-conditioned room and bioaerosols were sampled.
2.3. Statistical analyses
Correlation coefficients, linear regressions, and t-statistics for the experimental data were calculated using SPSS statistical software, version 22.0 (SPSS, Inc., Chicago, IL, USA), and Origin Pro statistical software, version 8.0 (OriginLab, Inc., Northampton, Ma, USA).
3. Results and discussion
3.1. The effect of the number of people on concentrations of bioaerosols
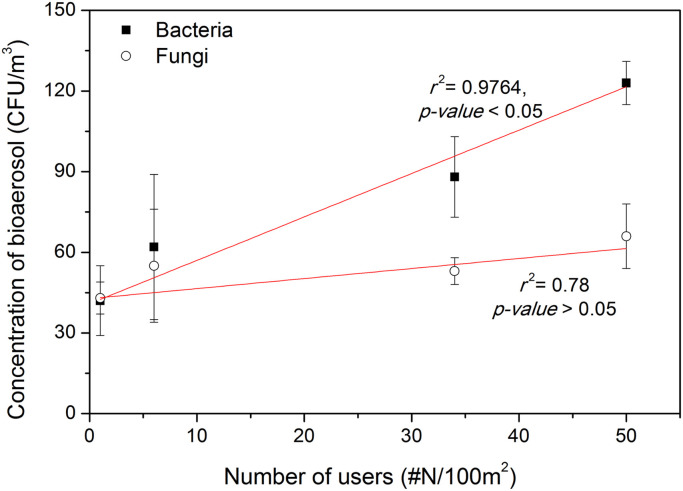
Fig. 1 shows the relationship between the number of people and concentration of bioaerosols in the student hall. The data show that all of the measured concentrations of bioaerosols in the test space were below the bioaerosol standard concentration established by the Ministry of Environment of the Republic of Korea for health care facilities (800 CFU/m3). The relationship in Fig. 1 shows that an increase in the number of people in the student hall results in increased concentrations of bacterial aerosols (y=1.62x+40.9, r 2=0.9764, t-test p-value<0.01). However, concentrations of fungal aerosols were not influenced by the number of occupants inside the test space (y=0.37x+42.8, r 2=0.78). The concentrations of fungal bioaerosols did not vary significantly regardless of the number of human occupants in the student hall (t-test p-value>0.05). Therefore, the experimental data in Fig. 1 support the hypotheses that humans increase the concentration of bacterial bioaerosols in indoor environments but that concentrations of fungal bioaerosols are not affected by the presence of humans. This result is in harmony with a previous study of underground subway stations (Heo & Lee, 2016). For fungal bioaerosols, this result agrees with previous findings that occupants were not a significant factor for fungal bioaerosols (Adams et al., 2013).
Fig. 1.
Relationship between the concentration of bioaerosols and the number of humans in the student hall.
3.2. The effect of human activities on the concentrations of bioaerosols
Under the condition of a single person inside the test chamber, we measured environmental parameters such as temperature and relative humidity, as well as concentrations of bioaerosols with various human activities. Table 1 shows median values, standard deviations, minimum values, and maximum values of temperature and relative humidity with the various human activities. The variation of temperature and relative humidity with human activities was compared to the variation of temperature and relative humidity of the environment outside of the test chamber. The variation of temperature and relative humidity due to human activities was not different from that of the outside environment (t-test p-value>0.05). Therefore, we could find that the presence of a single person was not a significant factor for temperature and humidity in the small test chamber.
Table 1.
Temperature and relative humidity in the test chamber.
| Temperature (°C) |
Humidity (%) |
|||||
|---|---|---|---|---|---|---|
| Average±S.D. | Min | Max | Average±S.D. | Min | Max | |
| Outdoor | 31.2±2.7 | 28.1 | 34.8 | 39.4±9.9 | 26.5 | 50.1 |
| Vacant | 29.7±4.2 | 26.1 | 39.4 | 43.7±11 | 21.3 | 54.9 |
| Standing | 30.3±4.4 | 27.2 | 40.4 | 45.7±12 | 25.4 | 67.1 |
| Talking | 30.1±3.4 | 27.5 | 36.3 | 43.7±11 | 24.8 | 54.2 |
| Moving | 30.2±3.4 | 27.5 | 36.1 | 43.7±10 | 25.7 | 54.9 |
S.D.: Standard deviation, Min: Minimum; Max: Maximum.
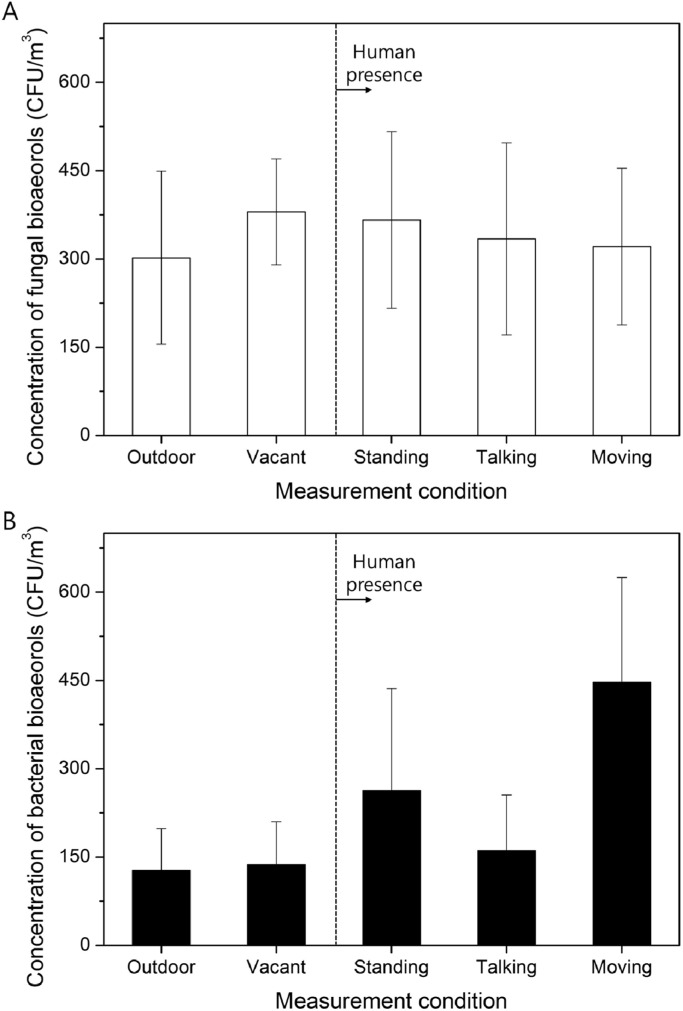
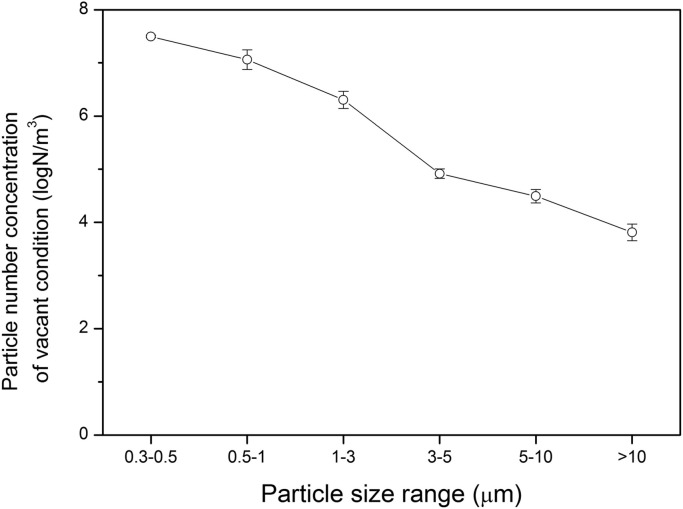
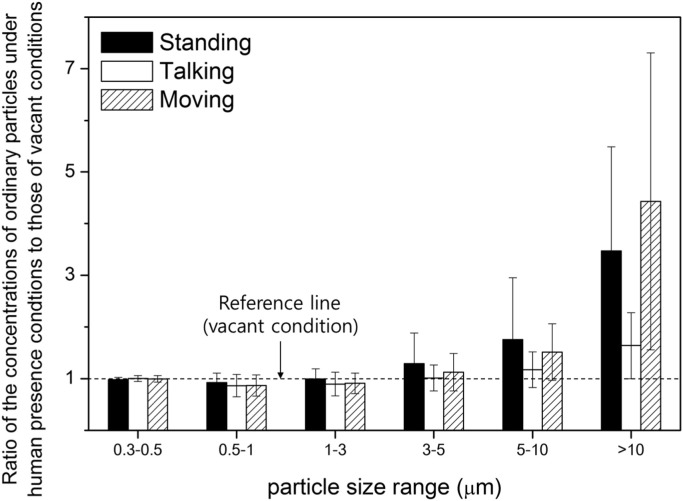
Fig. 2 shows the concentrations of fungal and bacterial bioaerosols in the outdoor and indoor environments inside the test chamber with a single person present under the four different conditions (vacant, single human standing, single human talking, single human moving). Fig. 3 shows the aerosol particle size distribution in the vacant chamber. Fig. 4 shows the ratio of ordinary particle concentrations with humans present to concentrations in the vacant chamber. Overall, the concentrations of bioaerosols show significant variations with various activities inside the test chamber in comparison with the variation of temperature and relative humidity. From the experimental results shown in Fig. 2, Fig. 4, we could obtain the following findings with various human activities.
Fig. 2.
Concentrations of (A) fungal and (B) bacterial bioaerosols in outdoor and indoor environments inside the test chamber, under four different conditions (vacant, standing, talking, and moving).
Fig. 3.
Aerosol particle size distribution at vacant condition in the test chamber.
Fig. 4.
Ratio of the concentrations of ordinary particles under human presence conditions to those of vacant conditions in the test chamber.
First, the concentrations of bioaerosols in the vacant test chamber were similar to outdoor bioaerosols concentrations, as shown in Fig. 2. These data indicate that the air quality in the vacant chamber was affected by the outdoor air conditions. The concentrations of total culturable bacterial bioaerosols in both environments are almost same. For the culturable fungal bioaerosol cases, the concentrations in the chamber were higher than those of outdoor environments, however the difference was not statistically significant (t-test p-value>0.05). This result is in accord with previous studies that performed genomic analyses of microorganisms wherein the main factor that affected concentrations of fungal bioaerosols was outdoor air flows (Adams et al., 2013). The concentrations of total culturable fungal bioaerosols were higher than the concentrations of total bacterial bioaerosols.
Second, when one human subject stood inside the chamber, the concentrations of bacterial bioaerosols increased two times in comparison with those under the vacant condition. The t-test p-values show that this variation was statistically significant (t-test p-value<0.05). This result agrees with the finding of a previous study in which the genomic analysis of bacteria revealed that elevated concentrations of genomes of bacterial bioaerosols were due to human occupancy (Hospodsky et al., 2012). In addition, one human presence inside the chamber increased the concentration of ordinary aerosol particles. As shown in Fig. 4, concentrations of large particles (optical particle diameter: dp>3 μm) in the standing condition were 1.2–3.5 times higher than those under the vacant condition. The results correspond to findings by Brauer, Hirtle, Lang, and Ott (2000) and Ferro et al. (2004), who measured ordinary particle concentrations with people present. The concentration ratios between the human standing and vacant conditions increased with increasing particle sizes in the current experiment. Furthermore, the correlation analysis between the concentration of large particles (optical particle diameter: dp>3 μm) and the concentrations of bacterial bioaerosols showed a positive relationship with statistical significance (t-test p-value<0.05). These analyses demonstrate that the investigated bacterial bioaerosols may form aggregates with large aerosol particles. Such conglomerations of aerosol particles with bacterial bioaerosols can provoke noxious respiratory effects as a result of synergistic toxic reactions with viable and inorganic compounds. The possibility of a combination of bacterial bioaerosols and ordinary large particles (optical particle diameter: dp>3 μm) can be studied in future work to elucidate the relationship between bioaerosols and humans.
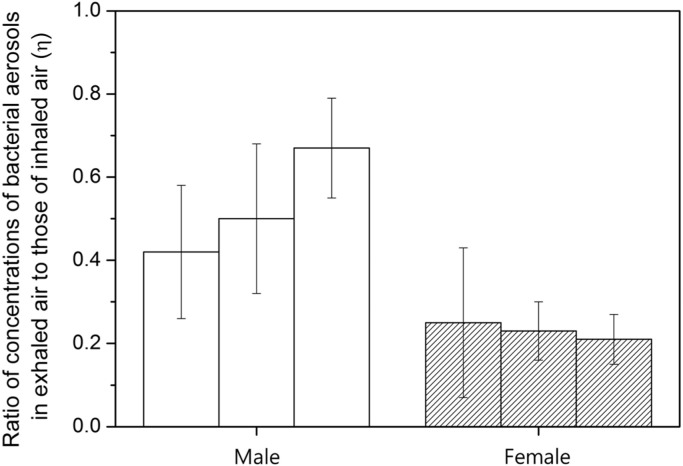
Third, the concentration of bacterial bioaerosols decreased from 263±173 CFU/m3 to 161±94 CFU/m3 when shifting from the standing condition to the talking condition in the test chamber. The variation of the bioaerosols concentration is statistically significant (t-test p-value<0.05). This result appears to be contradicted with the results of previous studies of bacterial bioaerosols in human breath (Edwards et al. (2004), Fabian et al. (2008), Qian, Hospodsky, Yamamoto, Nazaroff, & Peccia (2012)). Therefore, we designed and conducted additional experiments to elucidate this experimental result. We measured bioaerosols in inhalation and exhalation air flows in humans and compared the concentrations between both cases. We sampled bioaerosols from the breathing air flows of six volunteers, including the authors, while talking. Fig. 5 shows the ratio of concentrations of bacterial bioaerosols in exhaled air flow to those of the inhaled air flow, with the following parameter :
| (1) |
Fig. 5.
Ratio of concentrations of bacterial aerosols in exhaled air to those of inhaled air. Temperature and humidity in the test chamber were 25.1±1.2 °C and 62.1±7.8%, respectively.
In this experiment, the concentrations of the bioaerosols in inhalation air flows of humans were considered to be same as the concentration of bioaerosols in nearby air environments.
As shown in Fig. 5, the concentrations of bacterial bioaerosols decreased after being exhaled by humans. The concentrations of exhaled breath bacterial bioaerosols were 13% to 75% of the concentrations of inhaled bacterial bioaerosols. Therefore, a maximum of 87% of bacterial bioaerosols were removed from the air during the talking activity. This result demonstrates that strong mouth breathing by humans can decrease the concentrations of bacterial bioaerosols in surrounding air environments. A decrease in concentrations of ordinary aerosol concentrations after the talking activity can also be observed inside the chamber. For large aerosol particles (optical particle diameter: dp>3 μm), concentrations decreased 20% to 50% after switching from the standing to the talking activities inside the test chamber. The decrease was statistically significant (t-test p-value<0.05). As shown in Fig. 4, for the largest tested particles (optical particle diameter: dp>10 μm), half of the particles were removed from the air by human mouth breathing. There are several hypotheses that may explain this phenomenon. Human respiratory systems, including oral parts, larynx, and the trachea, may capture a portion of bacterial bioaerosols and ordinary particles, thereby decreasing their concentrations. In addition, talking activity may increase moisture in the surrounding air, which may attach to and increase the diameter of hygroscopic aerosol particles, increasing the high gravitational sedimentation rate.
Fourth, the moving activity increased concentrations of bacterial bioaerosols in the test chamber. The concentration of bacterial bioaerosols was two to three times higher under the moving activity than under other conditions (t-test p-value<0.05). The average concentration of ordinary large aerosol particles (optical particle diameter: dp>3 μm) under the moving condition was 1.2 to 4.5 times higher than those under the vacant condition. The experimental results are in harmony with the results from Brauer et al. (2000), in which the aerosols from personal clouds were more pronounced when the human subject was walking than when the human subject was sedentary.
Under all of the above conditions, highly different patterns of concentrations were observed for fungal bioaerosols. The variation of conditions in the test chamber (vacant, standing, talking, and moving) did not affect the concentration of fungal bioaerosols. Under all conditions, including outdoor conditions, the concentration of fungal bioaerosols was approximately 300 CFU/m3 without significant variations (t-test p-value>0.05). This result differs from one previous result of a study involving a classroom experiment with the genomic analysis of fungal bioaerosols (Qian et al., 2012) in which more fungal genomic particles were detected when the classroom was occupied than when the classroom was vacant. However, in that previous study, the difference in bacterial genomic particles between the occupied condition and vacant condition was much larger than the difference in fungal particles between the occupied condition and vacant condition. Therefore, it can be concluded that in the previous study, the bacterial particles were more influenced by human presence than the fungal particles (Qian et al., 2012). This issue regarding the difference between culturability and genomic data on bioaerosols remains to be addressed in future studies.
In summary, Fig. 1, Fig. 2 clearly show that humans affected the concentration of bacterial bioaerosols. Generally, the presence of humans and moving activities increased the concentration of bacterial bioaerosols. However, talking activities decreased the concentration of bacterial bioaerosols in the test chamber, as shown in Fig. 2, Fig. 5. The reduction of bacterial bioaerosols during strong respiratory activity is suspected as an explanation of this phenomenon. Under all conditions, culturable fungal bioaerosols showed robust concentrations. Overall, this experimental result demonstrates that bacterial bioaerosols are highly connected with human activities and the source of bacterial bioaerosols may be strongly linked with humans. However, the result for fungal bioaerosols suggests that sources of fungal bioaerosols are not connected with humans. The elucidation of the detailed mechanisms of these findings can be future work. An example of possible explanation is that bacterial particles on human skin particles may contribute to the relationship between human presence and bacterial bioaerosols (Hospodsky et al., 2012). There are several limitations in this study. All experiments were conducted in Seoul, Republic of Korea, with a focus on the culturability of bioaerosols. In this study, it was not covered to sample various areas and detect non-culturable bioaerosols. Although there were at least three replications for all tested conditions, this study covered only a limited number of experimental variables, such as three activities (standing, talking, and moving).
4. Conclusions
In this study, we examined the relationship between human activities and the concentration of culturable bioaerosols. Concentrations of bacterial bioaerosols are strongly linked with human activities. Human presence and moving activities increased the concentration of bacterial bioaerosols. Talking activities, including heavy inhalation and exhalation, decreased the concentration of bacterial bioaerosols in the confined indoor space. The effects of humans on fungal bioaerosol concentrations were negligible. For ordinary aerosol particles, the concentration of large particles (optical particle diameter: dp>3 μm) in the test chamber increased with human presence and moving activities but decreased with the talking activity. Detailed mechanisms for the relationship between bacterial bioaerosol concentrations and human activities can be studied in future work. The elucidation of the relationship between humans and bioaerosols will be an important foundation for performing future air infection studies and developing air cleaning technologies.
Acknowledgments
This paper was supported by Konkuk University in 2016.
References
- Adams R.I., Miletto M., Taylor J.W., Bruns T.D. Dispersal in microbes: Fungi in indoor air are dominated by outdoor air and show dispersal limitation at short distances. The ISME Journal. 2013;7(7):1262–1273. doi: 10.1038/ismej.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterman S.A. Characterization of particulate emissions from occupant activities in offices. Indoor Air. 2001;11(1):35–48. doi: 10.1034/j.1600-0668.2001.011001035.x. [DOI] [PubMed] [Google Scholar]
- Beggs C. The airborne transmission of infection in hospital buildings: Fact or fiction? Indoor and Built Environment. 2003;12(1–2):9–18. [Google Scholar]
- Brauer M., Hirtle R., Lang B., Ott W. Assessment of indoor fine aerosol contributions from environmental tobacco smoke and cooking with a portable nephelometer. Journal of Exposure Analysis and Environmental Epidemiology. 2000;10:136–144. doi: 10.1038/sj.jea.7500076. [DOI] [PubMed] [Google Scholar]
- Buttner M.P., Stetzenbach L.D. Monitoring airborne fungal spores in an experimental indoor environment to evaluate sampling methods and the effects of human activity on air sampling. Applied and Environmental Microbiology. 1993;59(1):219–226. doi: 10.1128/aem.59.1.219-226.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Hildemann L.M. The effects of human activities on exposure to particulate matter and bioaerosols in residential homes. Environmental Science Technology. 2009;43(13):4641–4646. doi: 10.1021/es802296j. [DOI] [PubMed] [Google Scholar]
- Cockcroft D., Ruffin R., Dolovich J., Hargreave F. Allergen‐induced increase in non‐allergic bronchial reactivity. Clinical Experimental Allergy. 1977;7(6):503–513. doi: 10.1111/j.1365-2222.1977.tb01481.x. [DOI] [PubMed] [Google Scholar]
- Cox C.S., Wathes C.M. CRC Press; Boca Raton, Florida: 1995. Bioaerosols handbook. [Google Scholar]
- Edwards D.A., Man J.C., Brand P., Katstra J.P., Sommerer K., Stone H.A., Scheuch G. Inhaling to mitigate exhaled bioaerosols. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(50):17383–17388. doi: 10.1073/pnas.0408159101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian P., McDevitt J.J., DeHaan W.H., Fung R., Cowling B.J., Chan K.H., Milton D.K. Influenza virus in human exhaled breath: An observational study. PloS one. 2008;3(7):e2691. doi: 10.1371/journal.pone.0002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro A.R., Kopperud R.J., Hildemann L.M. Elevated personal exposure to particulate matter from human activities in a residence. Journal of Exposure Science and Environmental Epidemiology. 2004;14:S34–S40. doi: 10.1038/sj.jea.7500356. [DOI] [PubMed] [Google Scholar]
- Fiegel J., Clarke R., Edwards D.A. Airborne infectious disease and the suppression of pulmonary bioaerosols. Drug Discovery Today. 2006;11(1):51–57. doi: 10.1016/S1359-6446(05)03687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung F., Hughson W.G. Health effects of indoor fungal bioaerosol exposure. Applied Occupational and Environmental Hygiene. 2003;18(7):535–544. doi: 10.1080/10473220301451. [DOI] [PubMed] [Google Scholar]
- Gόrny R.L., Dutkiewicz J., Krysinska-Traczyk E. Size distribution of bacterial and fungal bioaerosols in indoor air. Annals of Agricultural and Environmental Medicine. 1999;6:105–113. [PubMed] [Google Scholar]
- Han Z., Weng W., Huang Q. Characterizations of particle size distribution of the droplets exhaled by sneeze. Journal of The Royal Society Interface. 2013;10(88):20130560. doi: 10.1098/rsif.2013.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C., Morawska L., Hitchins J., Gilbert D. Contribution from indoor sources to particle number and mass concentrations in residential houses. Atmospheric Environment. 2004;38(21):3405–3415. [Google Scholar]
- Heo K.J., Lee B.U. Seasonal variation in the concentrations of culturable bacterial and fungal aerosols in underground subway systems. Journal of Aerosol Science. 2016;92:122–129. [Google Scholar]
- Heyder J., Gebhart J., Rudolf G., Schiller C.F., Stahlhofen W. Deposition of particles in the human respiratory tract in the size range 0.005–15 μm. Journal of Aerosol Science. 1986;17(5):811–825. [Google Scholar]
- Hinds W.C. Vol. 1982. Wiley-Interscience; New York: 1982. Aerosol technology: properties, behavior, and measurement of airborne particles; p. 1. [Google Scholar]
- Hospodsky D., Qian J., Nazaroff W.W., Yamamoto N., Bibby K., Rismani-Yazdi H., Peccia J. Human occupancy as a source of indoor airborne bacteria. PloS One. 2012;7(4):e34867. doi: 10.1371/journal.pone.0034867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang G.B., Heo K.J., Yun J.H., Lee J.E., Lee H.J., Nho C.W., Jung J.H. Antimicrobial air filters using natural Euscaphis japonica nanoparticles. PloS One. 2015;10(5):e0126481. doi: 10.1371/journal.pone.0126481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey J., Crook B. Fungal and actinomycete spores as pollutants of the workplace and occupational allergens. Annals of Occupational Hygiene. 1988;32(4):515–533. doi: 10.1093/annhyg/32.4.515. [DOI] [PubMed] [Google Scholar]
- Lee B.U., Hong I.G., Lee D.H., Chong E.-S., Jung J.H., Lee J.H., Lee I.-S. Bacterial bioaerosol concentrations in public restroom environments. Aerosol and Air Quality Research. 2012;12:251. [Google Scholar]
- Macher J.M. Positive-hole correction of multiple-jet impactors for collecting viable microorganisms. The American Industrial Hygiene Association Journal. 1989;50(11):561–568. doi: 10.1080/15298668991375164. [DOI] [PubMed] [Google Scholar]
- Mainelis G., Górny R.L., Reponen T., Trunov M., Grinshpun S.A., Baron P., Willeke K. Effect of electrical charges and fields on injury and viability of airborne bacteria. Biotechnology and Bioengineering. 2002;79(2):229–241. doi: 10.1002/bit.10290. [DOI] [PubMed] [Google Scholar]
- Meadow J.F., Altrichter A.E., Kembel S.W., Moriyama M., O’Connor T.K., Womack A.M.…Bohannan B.J. Bacterial communities on classroom surfaces vary with human contact. Microbiome. 2014;2(1):1. doi: 10.1186/2049-2618-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton D.K., Fabian M.P., Cowling B.J., Grantham M.L., McDevitt J.J. Influenza virus aerosols in human exhaled breath: Particle size, culturability, and effect of surgical masks. PLoS Pathogens. 2013;9(3):e1003205. doi: 10.1371/journal.ppat.1003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Environment, Republic of Korea (2014). Indoor air quality control in public-use facilities, etc. act. [Act No.12216, 07. Jan, 2014].
- Nazaroff W.W. Indoor bioaerosol dynamics. Indoor Air. 2014 doi: 10.1111/ina.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J., Peccia J., Ferro A.R. Walking-induced particle resuspension in indoor environments. Atmospheric Environment. 2014;89:464–481. [Google Scholar]
- Qian J., Hospodsky D., Yamamoto N., Nazaroff W.W., Peccia J. Size‐resolved emission rates of airborne bacteria and fungi in an occupied classroom. Indoor Air. 2012;22(4):339–351. doi: 10.1111/j.1600-0668.2012.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raunemaa T., Kulmala M., Saari H., Olin M., Kulmala M.H. Indoor air aerosol model: Transport indoors and deposition of fine and coarse particles. Aerosol Science and Technology. 1989;11(1):11–25. [Google Scholar]
- Ren P., Jankun T.M., Leaderer B.P. Comparisons of seasonal fungal prevalence in indoor and outdoor air and in house dusts of dwellings in one Northeast American county. Journal of Exposure Science and Environmental Epidemiology. 1999;9(6):560–568. doi: 10.1038/sj.jea.7500061. [DOI] [PubMed] [Google Scholar]
- Toivola M., Nevalainen A., Alm S. Personal exposures to particles and microbes in relation to microenvironmental concentrations. Indoor Air. 2004;14(5):351–359. doi: 10.1111/j.1600-0668.2004.00258.x. [DOI] [PubMed] [Google Scholar]
- Turner R.D., Bothamley G.H. Cough and the transmission of tuberculosis. Journal of Infectious Diseases. 2014 doi: 10.1093/infdis/jiu625. (jiu625) [DOI] [PubMed] [Google Scholar]
- WHO (2009). WHO guidelines for indoor air quality: dampness and mould. [PubMed]
- Zhu H., Phelan P.E., Duan T., Raupp G.B., Fernando H.J., Che F. Experimental study of indoor and outdoor airborne bacterial concentrations in Tempe, Arizona, USA. Aerobiologia. 2003;19(3–4):201–211. [Google Scholar]