Abstract
BACKGROUND
Noninvasive ventilation (NIV) via helmet or total facemask is an option for managing patients with respiratory infections in respiratory failure. However, the risk of nosocomial infection is unknown.
METHODS
We examined exhaled air dispersion during NIV using a human patient simulator reclined at 45° in a negative pressure room with 12 air changes/h by two different helmets via a ventilator and a total facemask via a bilevel positive airway pressure device. Exhaled air was marked by intrapulmonary smoke particles, illuminated by laser light sheet, and captured by a video camera for data analysis. Significant exposure was defined as where there was ≥ 20% of normalized smoke concentration.
RESULTS
During NIV via a helmet with the simulator programmed in mild lung injury, exhaled air leaked through the neck-helmet interface with a radial distance of 150 to 230 mm when inspiratory positive airway pressure was increased from 12 to 20 cm H2O, respectively, while keeping the expiratory pressure at 10 cm H2O. During NIV via a helmet with air cushion around the neck, there was negligible air leakage. During NIV via a total facemask for mild lung injury, air leaked through the exhalation port to 618 and 812 mm when inspiratory pressure was increased from 10 to 18 cm H2O, respectively, with the expiratory pressure at 5 cm H2O.
CONCLUSIONS
A helmet with a good seal around the neck is needed to prevent nosocomial infection during NIV for patients with respiratory infections.
ABBREVIATIONS: ACH, air changes per hour; A(H1N1), pandemic 2009 influenza A(H1N1); EPAP, expiratory positive airway pressure; HCW, health-care worker; HPS, human patient simulator; IPAP, inspiratory positive airway pressure; NIV, noninvasive ventilation; SARI, severe acute respiratory infection; SARS, severe acute respiratory syndrome
Respiratory failure is a major complication in patients hospitalized with severe acute respiratory infections (SARIs), such as severe acute respiratory syndrome (SARS),1, 2 pandemic 2009 influenza A(H1N1) (A[H1N1]),3 avian influenza (A[H5N1] or A[H7N9]),4, 5 and the Middle East respiratory syndrome.6, 7 Patients may progress rapidly to ARDS and multiorgan failure, requiring intensive care support.1, 2, 3, 4, 5, 6, 7, 8 Noninvasive ventilation (NIV) may play a supportive role in patients with early ARDS or acute lung injury due to SARI as a bridge to invasive mechanical ventilation.8, 9, 10 However, a systematic review has shown that mask ventilation, tracheal intubation, tracheotomy, and NIV may increase the risk of nosocomial transmission of respiratory infections to health-care workers (HCWs).11
Following the outbreak of SARS and emergence of the A(H1N1) infection, it has been recommended that when NIV is required for patients with acute hypoxemic respiratory failure due to SARI, infection control measures such as the use of helmets or full facemasks, double circuit tubes, and addition of viral-bacterial filters be considered.10, 12 However, whether these infection control measures are effective in minimizing exhaled air leakage has not been objectively evaluated. This study aimed to examine the dispersion of exhaled air during application of NIV via helmets and total facemask. Knowledge about the extent of exhaled air leakage from different masks will facilitate the development of preventive measures to reduce the risk of nosocomial transmission during application of NIV to high-risk patients hospitalized with SARI.
Materials and Methods
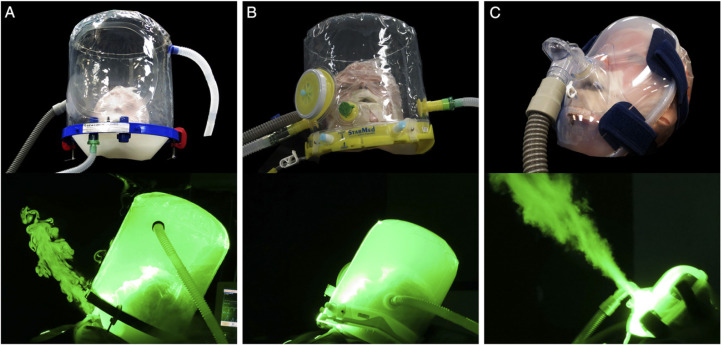
We examined the extent of exhaled air dispersion during application of NIV on a high-fidelity human patient simulator (HPS) (HPS 6.1; CAE Healthcare, Inc) via two different helmets (PN530L; Sea-Long Medical Systems Inc, and StarMed CaStar R; Intersurgical Ltd) using a SERVO-i ventilator (MAQUET) with double-limb circuit and filters. In addition, we studied the deliberate leakage from the exhalation port of a total facemask (Koninklijke Philips N.V.) during NIV via a bilevel positive airway pressure device and a single-limb circuit firmly attached to the HPS (Fig 1 ).
Figure 1 –
A-C, Application of noninvasive ventilation via the Sea-Long helmet (A), StarMed CaStar R helmet (B), and the Respironics total face mask (C) on the human patient simulator (HPS). The HPS represented a 70-kg adult man sitting on a 45°-inclined hospital bed and was programmed to mimic normal breathing, mild lung injury, and severe lung injury. Exhaled air, marked by the smoke particles, is illuminated by the laser light-sheet, with dispersion through the neck interface of the Sea-Long helmet (A) and through the exhalation port of the total face mask attached to the HPS (C). No significant leakage was noted with the StarMed CaStar R helmet.
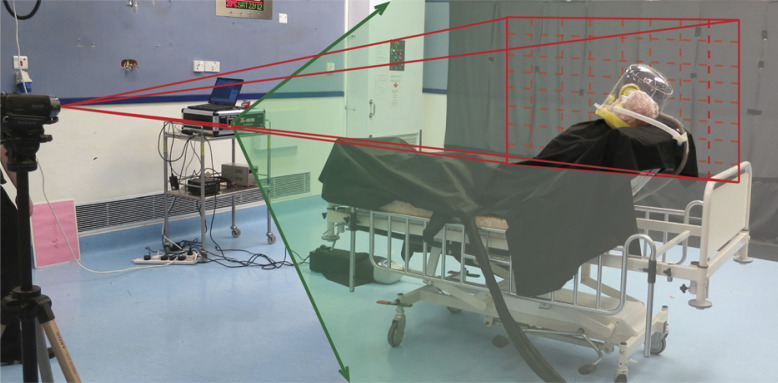
The experiments were conducted in a negative pressure room, with 12 air changes/h (ACH) (Fig 2 ). The experimental design and method of data analysis have been described in detail in our previous studies on exhaled air dispersion related to the application of NIV,12, 13 oxygen masks,14, 15 jet nebulizer,16 and mask ventilation.17
Figure 2 –
The room measured 6.1 (width) × 7.4 (depth) × 3.0 (height) m. The digital camera and the laser device were positioned along the coronal plane on the left side of the patient and along the sagittal plane of the patient at the end of the bed, respectively. Fresh air diffusers, as air inlet, were mounted on the ceiling. The negative pressure of the isolation room was produced by the air exhausts located near the floor.
NIV and Lung Model
The HPS represented a 70-kg adult man sitting on a 45°-inclined hospital bed. It was programmed to breathe spontaneously to mimic different degrees of lung injury (Table 1 ).12, 13, 14, 15, 16, 17, 18, 19 The HPS contains a realistic airway and a lung model that undergoes gas exchange by removing oxygen and adding CO2 to the system simultaneously. The lung compliance and airway resistance respond in a realistic manner to relevant respiratory challenges. In addition, the HPS produces an airflow pattern that is close to the in vivo situation and has been applied in previous studies to simulate human respiration.20, 21, 22, 23
TABLE 1.
| Settings | Normal Lung Condition | Mild Lung Injury | Severe Lung Injury |
|---|---|---|---|
| Oxygen consumption, mL/min | 200 | 300 | 500 |
| Lung compliance, mL/cm H2O | 70 | 35 | 10 |
| Respiratory rate,a breaths/min | 12 | 25 | 40 |
| Tidal volume,a mL | 700 | 300 | 150 |
HPS = human patient simulator.
The respiratory rate and tidal volume were adjusted by the HPS to achieve primarily the target oxygen consumption and lung compliance.
During examination of air leakage via the helmets, the inspiratory positive airway pressure (IPAP) was increased from 12 to 20 cm H2O, while keeping the expiratory positive airway pressure (EPAP) at 10 cm H2O. During examination of exhaled air dispersion from the total facemask, NIV was applied using a bilevel positive airway pressure device (ResMed VPAP III ST). The IPAP was initially set at 10 cm H2O and gradually increased to 14 and then 18 cm H2O, whereas the EPAP was maintained at 5 cm H2O throughout the study.12, 13 The EPAP was higher for the helmets than the total face mask, as higher pressure was required to prevent CO2 rebreathing in the helmets to overcome the larger anatomic dead space.
Flow Visualization
Visualization of airflow around the helmets and the total facemask was facilitated by marking air with smoke particles produced by an M-6000 smoke generator (N19; Dick Smith Electronics Pty Limited) as described in our previous studies.12, 13, 14, 15, 16, 17 The oil-based smoke particles, measuring < 1 μm in diameter, are known to follow the airflow pattern precisely with negligible slip.24 The smoke was introduced continuously to the right main bronchus of the HPS. It mixed with alveolar gas and then exhaled through the airway. Sections through the leakage jet plume were then revealed by a thin laser light-sheet (Green, 532 nm wavelength, continuous-wave mode) created by a diode-pumped solid state laser (OEM UGH-800 mW, Lambdapro Technologies Ltd), with custom cylindrical optics for two-dimensional laser light-sheet generation.12, 13, 14, 15, 16, 17
The light-sheet was initially positioned in the median sagittal plane of the HPS and was subsequently shifted to the paramedian sagittal planes. This allowed us to investigate the regions directly above and lateral to the mask and the patient.12, 13, 14, 15, 16, 17
All leakage jet plume images revealed by the laser light-sheet were captured by the high-definition video camera (Sony high-definition digital video camcorder, HDR-SR8E ClearVid complementary metal oxide semiconductor Sensor, Carl Zeiss Vario-Sonnar T* Lens), with optical resolution of 1,440 × 1,080 pixels per video frame. Normalized smoke concentration in the plume was estimated from the light intensity scattered by smoke particles.12, 13, 14, 15, 16, 17
Image Analysis
We estimated normalized smoke concentration in the mask leakage air from the light scattered by the particles. The analysis was based on scattered light intensity being proportional to particle concentration under the special conditions of constant intensity laser light-sheet illumination and monodisperse, small (submicron) particles.24 In short, the thin laser light-sheet of near constant intensity illuminated smoke particle markers in the mask airflow leakage. Smoke particles scattered laser light perpendicular to the light-sheet, and this was collected and integrated by the video camera element and lens (Fig 2).12, 13, 14, 15, 16, 17
Image Capture and Frame Extraction
The motion video of breathing cycles for each NIV setting was captured and individual frames extracted as gray scale bitmaps for intensity analysis. Frames were extracted at times initiated from the beginning of each inspiration, to generate an ensemble average for the corresponding instance of the respiratory cycle.12, 13, 14, 15, 16, 17 The time at which the normalized concentration contours spread over the widest region from the NIV mask was chosen for the ensemble average to estimate the greatest dispersion distance. This was found to be approximately at the mid-respiratory cycle.12, 13
Intensity Averaging and Concentration Normalization
All gray scale frames were read into a program specifically developed for this study (MathCad 8.0),25 along with background intensity images taken with the laser switched off. The background intensity image was subtracted from each frame, pixel by pixel, to remove any stray background light, and the pixel intensity values were averaged over all frames to determine the ensemble averaged intensity. The resulting image was the total intensity of light scattered perpendicular to the light-sheet by the smoke particles and was directly proportional to smoke concentration under the conditions mentioned previously. The image was normalized against the highest intensity found within the leakage jet plume to generate normalized particle concentration contours.12, 13, 14, 15, 16, 17
As the smoke particles marked air that originated from the HPS airways, before leaking from the mask, the concentration contours effectively represent the probability of encountering air around the patient that has come from within the mask and/or the patient's respiratory system. The normalized concentration contours are made up of data collected from at least 20 breaths. A normalized concentration of 100% or a contour value of 1 indicates a region that consists entirely of air exhaled by the patient, where there is a very high chance of exposure to the exhaled air, such as at the mask exhaust vents. A normalized concentration of 0% or a contour value of 0 indicates no measurable air leakage in the region and a small chance of exposure to the exhaled air. Significant exposure was arbitrarily defined as where there was ≥ 20% of normalized smoke concentration.12, 13, 14, 15, 16, 17 The study received nonionizing radiation safety approval by the Chinese University of Hong Kong (N/DSCH/RFCID 2012).
Results
Results are presented with reference to the median sagittal plane.
NIV Applied via the Sea-Long Oxygen Head Tent
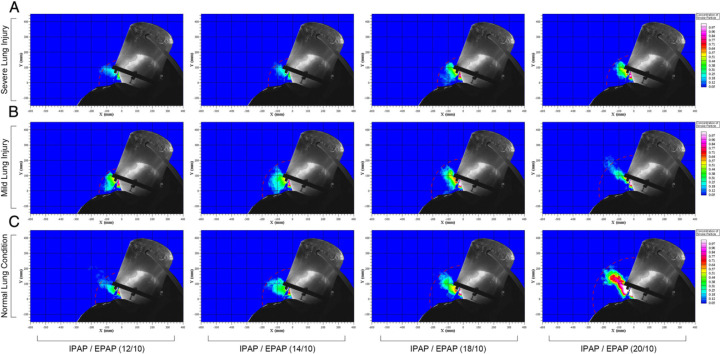
During application of NIV via the Sea-Long oxygen head tent, exhaled air was observed to leak through the neck-tent interface (Fig 1A). Using a normalized smoke concentration of 20% as a cutoff, the radial dispersion distance was 170 mm in normal lung condition with IPAP of 12 cm H2O, and the dispersion distance was 150 mm when the HPS was programmed in either mild or severe lung injury. When IPAP was increased gradually from 12 to 20 cm H2O, the dispersion distance increased to 270 mm, 230 mm, and 180 mm in normal condition, mild lung injury, and severe lung injury, respectively (Fig 3 , e-Fig 1).
Figure 3 –
A-C, Exhaled air dispersions through the neck interface during application of noninvasive ventilation via a servoventilator with double limb circuit and filters to the HPS using the Sea-Long head tent. EPAP was maintained at 10 cm H2O, and IPAP was increased from 12 to 14, 18, and 20 cm H2O gradually in four experiment settings. With normal lung condition, the mean (± SD) exhaled air dispersion distances with 20% normalized smoke concentration were 170 ± 39 mm, 200 ± 23 mm, 219 ± 32 mm, and 270 ± 20 mm, respectively. With mild lung injury, the exhaled air dispersion distances were 150 ± 12 mm, 200 ± 17 mm, 210 ± 28 mm, and 230 ± 37 mm, respectively. With severe lung injury, the corresponding values were 150 ± 7 mm, 160 ± 17 mm, 170 ± 21 mm, and 180 ± 22 mm, respectively. EPAP = expiratory positive airway pressure; IPAP = inspiratory positive airway pressure. See Figure 1 legend for expansion of other abbreviation.
NIV Applied via the StarMed CaStar R Helmet
The StarMed CaStar R helmet had a tight air cushion around the neck-helmet interface. Therefore, negligible air leak was noted during application of NIV when IPAP was increased from 12 to 20 cm H2O (Fig 1B).
NIV Applied via the Respironics Total Facemask
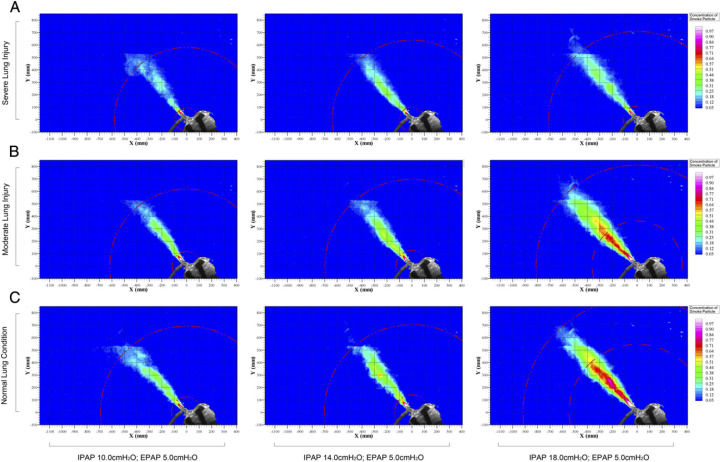
Figure 4 shows the dispersion distance of exhaled smoke during application of NIV using the Respironics total facemask in different lung conditions. It was observed that exhaled air jet leaked through the mask exhalation port (Fig 1C) to 693, 618, and 580 mm when the HPS was programmed in normal lung condition, mild lung injury, and severe lung injury, respectively, while receiving IPAP of 10 cm H2O and EPAP of 5 cm H2O. The dispersion distance increased by 31% when IPAP was increased from 10 to 18 cm H2O. As the severity of lung injury worsened, there was a decrease in the dispersion distance (Fig 4, e-Fig 2).
Figure 4 –
A-C, Exhaled air dispersions during application of noninvasive ventilation using a bilevel positive airway pressure device with a single circuit to the HPS via the Respironics total facemask. IPAP was increased in three experiment settings from 10 to 14 and 18 cm H2O, respectively, while maintaining EPAP at 5 cm H2O. With normal lung condition, the mean (± SD) exhaled air dispersion distances with 20% normalized smoke concentration were 693 ± 83 mm, 704 ± 57 mm, and 916 ± 35 mm, respectively. With mild lung injury, the exhaled air dispersion distances were 618 ± 67 mm, 698 ± 48 mm, and 812 ± 65 mm, respectively. With severe lung injury, the corresponding values were 580 ± 72 mm, 638 ± 53 mm, and 710 ± 103 mm, respectively. Se Figure 1 and 3 legends for expansion of abbreviations.
Discussion
Because of the lack of any reliable and safe marker that can be introduced into human lungs for study, we have examined the maximum distribution of exhaled air, marked by fine smoke particles, from the HPS during application of NIV using two different helmets and a total facemask. We have shown that leakage of exhaled air was negligible when NIV was applied to the HPS via a helmet with double limb circuit, filters and a good seal at the neck-helmet interface, whereas leakage at the neck interface could reach a maximum radial distance of 270 mm through another helmet without a tight seal in the interface. In addition, exhaled air jet through the exhalation port could reach a distance of 916 mm when NIV was applied to the HPS via the total face mask using single circuit.
In addition to reporting the exhaled air dispersion during manual ventilation with and without addition of a viral-bacterial filter on the HPS model,17 we have previously examined the maximum exhaled air distances from the HPS receiving NIV via several orofacial masks using a single limb circuit.12, 13 The ResMed mirage mask could leak through its exhalation port up to 500 mm when the HPS was programmed in mild lung injury,12 whereas leakage from the Respironics ComfortFull 2 mask would increase from 650 to 850 mm through the exhalation diffuser at a direction perpendicular to the head of the HPS along the sagittal plane, when IPAP was increased from 10 to 18 cm H2O, respectively.13 In contrast, even when a low IPAP of 10 cm H2O was applied to the HPS via the Respironics Image 3 mask connected to the whisper swivel exhalation port, the exhaled air leaked far more diffusely than via the ComfortFull 2 mask, dispersing a low normalized concentration to 950 mm along the median sagittal plane of the HPS, whereas higher IPAP resulted in wider spread of a higher normalized concentration of smoke around the HPS in the isolation room with negative pressure.13
NIV is effective in the treatment of patients with respiratory failure due to COPD, acute cardiogenic pulmonary edema, and pneumonia in immunocompromised patients, but the evidence supporting its use in hypoxic respiratory failure due to SARI is limited.26 NIV was applied successfully to some patients with SARS in a hospital in Hong Kong, with reduced needs for tracheal intubation and decreased mortality without causing any clinical and serological evidence of nosocomial transmission among the HCWs.27 The hospital involved managed to install exhaust ventilation fans in the windows of the treatment rooms to create a negative pressure environment with > 8 ACH, and the HCWs involved complied well with airborne precautions.27 However, other reports suggested that NIV might have led to nosocomial transmission of SARS involving HCWs,28, 29 whereas NIV was shown to be an independent risk factor of super-spreading events in the hospital setting in a large case-controlled study.30 Indeed, a systematic review has shown that NIV is one of the factors that may lead to increased risk of nosocomial infection.11
In immunocompromised patients with lung infiltrates and acute hypoxic respiratory failure, NIV delivered via helmet appeared as effective as NIV via face mask in avoiding tracheal intubation and improving gaseous exchange, whereas fewer NIV discontinuations and fewer complications were observed in the helmet group.31 NIV has been applied to some patients with severe H7N9 infection5 and Middle East respiratory syndrome,32 but the majority of patients required invasive mechanical ventilation. Several groups had applied NIV to patients hospitalized with influenza A(H1N1) and acute hypoxemic respiratory failure with variable success.33, 34, 35
Influenza A viruses may spread between humans through contact, large respiratory droplets, and small particle droplet nuclei (aerosols).36, 37 There was evidence of possible aerosol transmission in a nosocomial outbreak of seasonal influenza temporally related to the use of NIV in an index patient with acute exacerbation of COPD due to influenza A(H3N2) in our acute hospital medical ward with an imbalanced indoor airflow.38
Patients with mild ARDS due to SARI may be considered for a trial of NIV if there is sufficient local experience.10, 39 However, NIV is generally regarded as one of the aerosol-generating procedures in which there is possibly increased risk of respiratory pathogen transmission.11, 40 Thus, it is advisable to apply NIV carefully with airborne precautions in an adequately ventilated room (with 6-12 ACH) when caring for patients with SARI of infectious nature.10, 12, 13, 40, 41 Based on our study findings, NIV via the helmet with double limb circuit and a good seal at the neck-helmet interface would be a safe option for managing infectious patients with hypoxemic respiratory failure due to SARI.
Our study was limited by the use of smoke particles as markers for exhaled air. However, evaporation of water content in some droplets during NIV may produce droplet nuclei suspended in air, whereas the larger droplets will fall to the ground in a trajectory pathway.42 As the smoke particles in this study mark the continuous air phase, our data contours are referring to exhaled air. Our results would therefore represent the “upper bound” estimates for the dispersion of droplets, which would be expected to follow a shorter trajectory than the air jet because of gravitational effects, but not fully reflect the risk of droplet transmission.12, 13, 14, 15, 16, 17
In summary, we have shown that leakage of exhaled air was negligible when NIV was applied to the HPS via a helmet with double limb circuit, filters, and a good seal at the neck interface, whereas leakage at the neck interface could reach a maximum radial distance of 270 mm through another helmet without a tight neck seal. In addition, leakage of exhaled air jet through the exhalation port could reach a distance of 916 mm when NIV was applied via the total face mask and a single circuit. HCWs should take adequate precautions when providing NIV support to patients with SARI complicated by respiratory failure.
Acknowledgments
Author contributions: D. S. H. is the guarantor of the content of the manuscript, including the data and analysis. D. S. H., B. K. C., and M. T. V. C. contributed to the study design, data interpretation, and writing the manuscript and T. L., S. S. N., F. W. K., and T. G. contributed technical support for the study and revision of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor approved the study design but played no role in development of the research and manuscript.
Other contributions: We thank the Health and Medical Research Fund #12110392 (Food and Health Bureau, Government of the Hong Kong Special Administrative Region) for supporting this study.
Additional information: The e-Figures can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This study was funded by the Health and Medical Research Fund [Grant 12110392], Food and Health Bureau, Government of the Hong Kong Special Administrative Region.
This is an open access article distributed under the terms of the Creative Commons Attribution-Noncommercial License (http://creativecommons.org/licenses/by-nc/3.0/), which permits unrestricted use, distribution, and reproduction to noncommercial entities, provided the original work is properly cited. Information for reuse by commercial entities is available online.
Supplementary Material
References
- 1.Lee N, Hui D, Wu A. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 2.Hui DS, Sung JJ. Treatment of severe acute respiratory syndrome. Chest. 2004;126(3):670–674. doi: 10.1378/chest.126.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hui DS, Lee N, Chan PK. Clinical management of pandemic 2009 influenza A(H1N1) infection. Chest. 2010;137(4):916–925. doi: 10.1378/chest.09-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdel-Ghafar AN, Chotpitayasunondh T, Gao Z. Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med. 2008;358(3):261–273. doi: 10.1056/NEJMra0707279. Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza A (H5N1) Virus. [DOI] [PubMed] [Google Scholar]
- 5.Gao HN, Lu HZ, Cao B. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med. 2013;368(24):2277–2285. doi: 10.1056/NEJMoa1305584. [DOI] [PubMed] [Google Scholar]
- 6.State of knowledge and data gaps of Middle East respiratory syndrome coronavirus (MERS-CoV) in humans. PLoS Curr. 2013 doi: 10.1371/currents.outbreaks.0bf719e352e7478f8ad85fa30127ddb8. Nov 12:5 International Union Against Cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hui DS, Memish ZA, Zumla A. Severe acute respiratory syndrome vs. the Middle East respiratory syndrome. Curr Opin Pulm Med. 2014;20(3):233–241. doi: 10.1097/MCP.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 8.Arabi Y, Gomersall CD, Ahmed QA, Boynton BR, Memish ZA. The critically ill avian influenza A (H5N1) patient. Crit Care Med. 2007;35(5):1397–1403. doi: 10.1097/01.CCM.0000262940.34596.4B. [DOI] [PubMed] [Google Scholar]
- 9.Hui DS. Influenza A/H5N1 infection: other treatment options and issues. Respirology. 2008;13(suppl 1):S22–S26. doi: 10.1111/j.1440-1843.2008.01250.x. [DOI] [PubMed] [Google Scholar]
- 10.Conti G, Larrsson A, Nava S, Navalesi P. http://dev.ersnet.org/uploads/Document/63/WEB_CHEMIN_5410_1258624143.pdf On the role of non-invasive ventilation (NIV) to treat patients during the H1N1 influenza pandemic. ERS & ESICM Published November 2009. Accessed April 25, 2014.
- 11.Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS ONE. 2012;7(4):e35797. doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hui DS, Hall SD, Chan MT. Noninvasive positive-pressure ventilation: An experimental model to assess air and particle dispersion. Chest. 2006;130(3):730–740. doi: 10.1378/chest.130.3.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hui DS, Chow BK, Ng SS. Exhaled air dispersion distances during noninvasive ventilation via different Respironics face masks. Chest. 2009;136(4):998–1005. doi: 10.1378/chest.09-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hui DS, Ip M, Tang JW. Airflows around oxygen masks: a potential source of infection? Chest. 2006;130(3):822–826. doi: 10.1378/chest.130.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hui DS, Hall SD, Chan MT. Exhaled air dispersion during oxygen delivery via a simple oxygen mask. Chest. 2007;132(2):540–546. doi: 10.1378/chest.07-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hui DS, Chow BK, Chu LC. Exhaled air and aerosolized droplet dispersion during application of a jet nebulizer. Chest. 2009;135(3):648–654. doi: 10.1378/chest.08-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan MT, Chow BK, Chu L, Hui DS. Mask ventilation and dispersion of exhaled air. Am J Respir Crit Care Med. 2013;187(7):e12–e14. doi: 10.1164/rccm.201201-0137im. [DOI] [PubMed] [Google Scholar]
- 18.Kuhlen R, Max M, Dembinski R, Terbeck S, Jürgens E, Rossaint R. Breathing pattern and workload during automatic tube compensation, pressure support and T-piece trials in weaning patients. Eur J Anaesthesiol. 2003;20(1):10–16. doi: 10.1017/s0265021503000024. [DOI] [PubMed] [Google Scholar]
- 19.Light RB. Pulmonary pathophysiology of pneumococcal pneumonia. Semin Respir Infect. 1999;14(3):218–226. [PubMed] [Google Scholar]
- 20.Meka VV, van Oostrom JH. Bellows-less lung system for the human patient simulator. Med Biol Eng Comput. 2004;42(3):413–418. doi: 10.1007/BF02344718. [DOI] [PubMed] [Google Scholar]
- 21.So CY, Gomersall CD, Chui PT, Chan MT. Performance of an oxygen delivery device for weaning potentially infectious critically ill patients. Anaesthesia. 2004;59(7):710–714. doi: 10.1111/j.1365-2044.2004.03802.x. [DOI] [PubMed] [Google Scholar]
- 22.Goodwin JA, van Meurs WL, Sá Couto CD, Beneken JE, Graves SA. A model for educational simulation of infant cardiovascular physiology. Anesth Analg. 2004;99(6):1655–1664. doi: 10.1213/01.ANE.0000134797.52793.AF. [DOI] [PubMed] [Google Scholar]
- 23.Lampotang S, Lizdas DE, Gravenstein N, Robicsek S. An audible indication of exhalation increases delivered tidal volume during bag valve mask ventilation of a patient simulator. Anesth Analg. 2006;102(1):168–171. doi: 10.1213/01.ANE.0000181833.23904.4E. [DOI] [PubMed] [Google Scholar]
- 24.Soo SL. Blaisdell Publishing Company; Toronto, Canada: 1967. (Fluid Dynamics of Multiphase Systems). [Google Scholar]
- 25.MathSoft Inc; Cambridge, MA: 2000. (Mathcad 8.0 for Windows, Users Guide). International Union Against Cancer. [Google Scholar]
- 26.Ambrosino N, Vagheggini G. Noninvasive positive pressure ventilation in the acute care setting: where are we? Eur Respir J. 2008;31(4):874–886. doi: 10.1183/09031936.00143507. [DOI] [PubMed] [Google Scholar]
- 27.Cheung TM, Yam LY, So LK. Effectiveness of noninvasive positive pressure ventilation in the treatment of acute respiratory failure in severe acute respiratory syndrome. Chest. 2004;126(3):845–850. doi: 10.1378/chest.126.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fowler RA, Guest CB, Lapinsky SE. Transmission of severe acute respiratory syndrome during intubation and mechanical ventilation. Am J Respir Crit Care Med. 2004;169(11):1198–1202. doi: 10.1164/rccm.200305-715OC. [DOI] [PubMed] [Google Scholar]
- 29.Xiao Z, Li Y, Chen R, Li S, Zhong S, Zhong N. A retrospective study of 78 patients with severe acute respiratory syndrome. Chin Med J (Engl) 2003;116(6):805–810. [PubMed] [Google Scholar]
- 30.Yu IT, Xie ZH, Tsoi KK. Why did outbreaks of severe acute respiratory syndrome occur in some hospital wards but not in others? Clin Infect Dis. 2007;44(8):1017–1025. doi: 10.1086/512819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rocco M, Dell'Utri D, Morelli A. Noninvasive ventilation by helmet or face mask in immunocompromised patients: a case-control study. Chest. 2004;126(5):1508–1515. doi: 10.1378/chest.126.5.1508. [DOI] [PubMed] [Google Scholar]
- 32.Arabi YM, Arifi AA, Balkhy HH. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160(6):389–397. doi: 10.7326/M13-2486. [DOI] [PubMed] [Google Scholar]
- 33.Masclans JR, Pérez M, Almirall J. Early non-invasive ventilation treatment for severe influenza pneumonia. Clin Microbiol Infect. 2013;19(3):249–256. doi: 10.1111/j.1469-0691.2012.03797.x. H1N1 GTEI/SEMICYUC Investigators. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brink M, Hagberg L, Larsson A, Gedeborg R. Respiratory support during the influenza A (H1N1) pandemic flu in Sweden. Acta Anaesthesiol Scand. 2012;56(8):976–986. doi: 10.1111/j.1399-6576.2012.02727.x. [DOI] [PubMed] [Google Scholar]
- 35.Nicolini A, Tonveronachi E, Navalesi P. Effectiveness and predictors of success of noninvasive ventilation during H1N1 pandemics: a multicenter study. Minerva Anestesiol. 2012;78(12):1333–1340. [PubMed] [Google Scholar]
- 36.Fabian P, McDevitt JJ, DeHaan WH. Influenza virus in human exhaled breath: an observational study. PLoS ONE. 2008;3(7):e2691. doi: 10.1371/journal.pone.0002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cowling BJ, Ip DK, Fang VJ. Aerosol transmission is an important mode of influenza A virus spread. Nat Commun. 2013;4:1935. doi: 10.1038/ncomms2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong BC, Lee N, Li Y. Possible role of aerosol transmission in a hospital outbreak of influenza. Clin Infect Dis. 2010;51(10):1176–1183. doi: 10.1086/656743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.http://www.who.int/csr/disease/coronavirus_infections/InterimGuidance_ClinicalManagement_NovelCoronavirus_11Feb13u.pdf?ua=1 Interim guidance document on clinical management of severe acute respiratory infections when novel coronavirus is suspected: what to do and what not to do. World Health Organization website Accessed March 24, 2014.
- 40.http://www.who.int/csr/bioriskreduction/infection_control/publication/en/ Infection prevention and control of epidemic- and pandemic-prone acute respiratory diseases in health care. Geneva, World Health Organization, Global Alert and Response, April 2014. World Health Organization website Accessed July 6, 2014 International Union Against Cancer.
- 41.Zumla A, Hui DS. Infection control and MERS-CoV in health-care workers. Lancet. 2014;383(9932):1869–1871. doi: 10.1016/S0140-6736(14)60852-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie X, Li Y, Chwang AT, Ho PL, Seto WH. How far droplets can move in indoor environments—revisiting the Wells evaporation-falling curve. Indoor Air. 2007;17(3):211–225. doi: 10.1111/j.1600-0668.2007.00469.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.