Abstract
The nucleocapsid (N) protein of SARS coronavirus (SARS_CoV) is a major structural component of virions, which appears to be a multifunctional protein involved in viral RNA replication and translation. Heterogeneous nuclear ribonucleoprotein A1 (hnRNP A1) is related to the pre‐mRNA splicing in the nucleus and translation regulation in the cytoplasm. In this report, based on the relevant biophysical and biochemical assays, the nucleocapsid protein of SARS_CoV (SARS_N) was discovered to exhibit high binding affinity to human hnRNP A1. GST pull‐down results clearly demonstrated that SARS_N protein could directly and specifically bind to human hnRNP A1 in vitro. Yeast two‐hybrid assays further indicated in vivo that such binding relates to the fragment (aa 161–210) of SARS_N and the Gly‐rich domain (aa 203–320) of hnRNP A1. Moreover, kinetic analyses by surface plasmon resonance (SPR) technology revealed that SARS_N protein has a specific binding affinity against human hnRNP A1 with K D at 0.35 ± 0.02 μM (k on = 5.83 ± 0.42 × 103 M−1 s−1 and k off = 2.06 ± 0.12 × 10−3 s−1). It is suggested that both SARS_N and hnRNP A1 proteins are possibly within the SARS_CoV replication/transcription complex and SARS_N/human hnRNP A1 interaction might function in the regulation of SARS_CoV RNA synthesis. In addition, the determined results showed that SARS_N protein has only one binding domain for interacting with human hnRNP A1, which is different from the mouse hepatitis virus (MHV) binding case where the nucleocapsid protein of MHV (MHV_N) was found to have two binding domains involved in the MHV_N/hnRNP A1 interaction, thereby suggesting that SARS_N protein might carry out a different binding mode to bind to human hnRNP A1 for its further function performance in comparison with MHV_N.
Keywords: SARS_CoV, severe acute respiratory syndrome coronavirus, N protein, nucleocapsid protein, SARS_N, the nucleocapsid protein of SARS coronavirus, hnRNP A1, heterogeneous nuclear ribonucleoprotein A1, MHV, mouse hepatitis virus, SPR, surface plasmon resonance, IPTG, isopropyl-β-d-galactoside, SARS coronavirus, Nucleocapsid protein, Heterogeneous nuclear ribonucleoprotein A1, GST pull-down, Surface plasmon resonance, Yeast two-hybrid
1. Introduction
Between the end of the year 2002 and June of the year 2003, an atypical pneumonia, referred to as severe acute respiratory syndrome (SARS) broke out in China and more than 30 other countries. It has been known that SARS coronavirus (SARS_CoV) is responsible for SARS infection [1, 2, 3, 4], and further research showed that SARS_CoV belongs to one member of the Coronaviridae and is moderately related to the other known coronaviruses [5]. SARS_CoV has 11 open reading frames (ORFs) that encode 23 putative proteins including four major structural proteins: nucleocapsid (N), spike (S), membrane (M), and small envelope (E) proteins [5, 6]. The N protein of SARS_CoV (SARS_N) is a highly basic structural protein localized in the cytoplasm and the nucleolus of Trichoplusia ni BT1 Tn 5B1‐4 cells [7]. Previous studies have indicated that the N proteins of other coronaviruses are extensively phosphorylated and associated with viral RNA to form a helical ribonucleoprotein (RNP) that comprises the viral core structure [8]. Recently, we discovered the fact that SARS_N tightly binds to human cyclophilin A, which might provide a new hint in understanding the possible SARS_CoV infection pathway against human cell [9].
SARS_N is a 422 amino acid protein, which shares 20–30% homology with the N proteins of some other coronaviruses [5, 6]. It was reported that the nucleocapsid protein of MHV (MHV_N) binds to the tandemly repeated sequence (UCYAAC) of the viral genome according to the SRXX repeated region (aa 177–231) [10]. On the other hand, the genome sequence analysis for SARS_CoV has revealed that the transcription‐regulating sequences at 5′ end of each gene involve such partially conserved core sequence [5]. Structurally, SARS_N owns the SRXX repeated region that shares high similarity with that of MHV_N protein according to the sequence analysis. Therefore, SARS_N is predicted to interact with the viral RNA and be involved in the mRNA synthesis and transcription of SARS_CoV.
In human cells, it has been discovered that there are more than 20 heterogeneous nuclear ribonucleoproteins (hnRNPs), and hnRNP A1 is the best‐characterized protein that is most likely related to pre‐mRNA splicing and transport of cellular RNAs [11]. Research has indicated that hnRNP A1 behaves as a global regulator of alternative pre‐mRNA splicing by antagonizing the activities of several serine/arginine‐rich splicing factors (SR protein), thus resulting in the activation of alternative distal 5′ splicing sites and skipping of optional exons [12]. In structure, hnRNP A1 contains two RNA‐binding domains (RBDs) and a glycine‐rich domain that is responsible for protein–protein interaction. hnRNP A1 selectively interacts through its gly‐rich domain with different RNA‐binding proteins, especially some splicing factors (SR proteins) [13, 14]. It was proved that hnRNP A1 was involved in MHV RNA replication and transcription, certainly regulating RNA synthesis of a cytoplasmic virus [11]. A recently published paper demonstrated that the nucleocapsid protein of MHV could interact with hnRNP A1 [15]. Since SARS_N is able to bind to RNA [16] and also shares some homology with MHV_N, we think that during the SARS_CoV life cycle SARS_N might also possibly interact with human hnRNP A1 for mRNA synthesis and mature mRNA transporting and transcription.
In the current study, by use of GST pull‐down, yeast two‐hybrid and SPR techniques we have discovered that SARS_N could directly interact with human hnRNP A1 both in vitro and in vivo. The results indicated that SARS_N/human hnRNP A1 interaction involves the SR‐rich fragment (aa 161–210) of SARS_N and the Gly‐rich domain (aa 203–320) of hnRNP A1, which thereby suggests that both SARS_N and human hnRNP A1 proteins are possibly within the SARS_CoV replication/transcription complex, and their interaction might function in the regulation of SARS_CoV RNA synthesis similar to the MHV_N case. In addition, the fact that there is only one hnRNP A1‐binding domain for SARS_N has suggested that SARS_N protein binds to hnRNP A1 in SARS_CoV taking a different mode in comparison with MHV_N.
2. Materials and methods
2.1. Chemicals
The gluthatione S‐transferase (GST) affinity resin and the molecular weight marker were purchased from Amersham Pharmacia Biotech, Inc. IPTG was purchased from Promega. Yeast nitrogen base without amino acids, yeast synthetic drop‐out medium supplement without tryptophan, yeast synthetic drop‐out medium supplement without leucine and tryptophan, yeast synthetic drop‐out medium supplement without leucine, tryptophan and histidine were all purchased from Sigma Chemical Co. The 3‐amino‐1′,2′,4′‐triazole was from Sangon. All other chemicals were from Sigma in their analytical grade.
2.2. Cloning, expression and purification of GST‐tagged human hnRNP A1
The gene for human hnRNP A1 (Gene Bank Accession #http://BC070315) was amplified by PCR from a cDNA library of Hela cells. Primers for the amplification were 5′ ATGTCTAAGTCAGAGTCTCCTAAAGAGCCGAAC 3′ and 5′ TTAAAATCTTCTGCCACTGCCATAGCTACTGC 3′. The amplified products were cloned into pGEM®‐T Easy Vector (Promega) according to the standard Promega manual (No. 042) and sequenced. The gene in pGEM®‐T Easy Vector was amplified by PCR using the primers 5′ GGCTGGATCCATGTCTAAGTCAGAGTCTCCTA 3′ and 5′ GGTCGAATTCTTAAAATCTTCTGCCACTGCCATA 3′. The amplified product was digested with BamHI and EcoRI (underlined in the primer) then ligated with pGEX4T‐1 (Amersham Pharmacia Biotech) digested with the same restriction enzymes. The pGEX4T‐1‐hnRNP A1 vector was transformed into BL21 (DE3) Escherichia coli (Novagen). Overnight cultures of a single colony per 10 ml of LB medium containing 100 μg/ml ampicillin were diluted to 1:10 with the same medium. After 3 h when the OD600 reached 0.7, expression of the protein was induced by the addition of 0.5 mM IPTG, and cultures were incubated for 5 h at 22 °C with aeration. Cells were recovered by centrifugation, quick‐frozen, and stored overnight at −80 °C. Frozen cells were thawed, resuspended in 15 ml of the PBS buffer (140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 1.8 mM KH2PO4, pH 7.4) containing 5 mM EDTA and 5 mM β‐mercaptoethanol per gram of cells, and then lysed by sonication for 20 min in ice bath. The lysate was cleared by centrifugation, and the supernatant was loaded onto a glutathione‐column (Amersham Pharmacia) equilibrated with PBS buffer. The loaded column was washed by 200 ml PBS buffer at 4 °C. GST‐hnRNP A1 was eluted using 10 mM reduced glutathione freshly added to PBS buffer. The glutathione contained with the purified protein was removed by dialysis against PBS buffer. The purified protein was detected as a single band on SDS–PAGE gel, and protein concentration was measured by the absorbance at 280 nm using the extinction coefficient of 66990 l/mol/cm.
2.3. Expression and purification of His‐tagged SARS_N
The recombinant nucleocapsid protein of SARS coronavirus (SARS_N) was cloned, expressed and purified according to the published procedure reported by Luo et al. [17].
2.4. GST pull‐down assay
GST pull‐down assay was performed using the ProFound™ Pull‐Down GST Protein: Protein Interaction Kit (Pierce). The purified GST‐hnRNP A1 was adsorbed onto 60 μl Glutathione–Sepharose beads, equilibrated with BupH™ TBS buffer (25 mM Tris–HCl and 0.15 M NaCl, pH 7.2) in a Handee™ Mini‐spin column, and served as bait proteins in the subsequent steps. After 4 h at 4 °C, the beads carrying GST fusion hnRNP A1 were resuspended in 400 μl 1:1 wash solution of TBS: ProFound™ lysis buffer for 4 times. After addition of 0.5 mg of the purified SARS_N as prey protein, the mixtures were gently shaken for 3 h at 4 °C and left for an additional 30 min without mixing. The rinsing was repeated for 5 times using 400 μl of wash solution as mentioned above. The bound proteins were eluted by 100 mM of glutathione elution buffer. The eluted samples were analyzed by SDS–PAGE.
2.5. Recombinant vectors construction for yeast two‐hybrid assay
The yeast vectors of pGADT7 and pGBKT7 were obtained from Clontech (Palo Alto, CA). The hnRNP A1 gene was amplified with the following primers: 5′ GGCGGAATTCATGTCTAAGTCAGAGTCTCCTA 3′ and 5′ GGTCGGATCCTTAAAATCTTCTGCCACTGCCATA 3′. The amplified products were digested with EcoRI and BamHI (NEB, USA) then cloned into the pGADT7 vector between the two sites. To confirm that the Gly‐rich domain of hnRNP A1 was involved in hnRNP A1/SARS_N interaction, the fragment 1–606 of hnRNP A1 was also cloned into pGADT7 vector by the same above‐mentioned method. The primers for this cloning were: 5′ GGCCGAATTCATGTCTAAGTCAGAGTCTCCTAA 3′ and 5′ GGCTGGATCCTTAAAAGTTTCCAGAACCACTTC 3′.
The SARS_N gene was amplified with the following primers: 5′ GGGTGAATTCATGTCTGATAATGGACCCCAAT 3′ and 5′ AATTGGATCCTTATGCCTGAGTTGAATCAGCAG 3′. The amplified products were digested with EcoRI and BamHI (NEB) then cloned into pGBKT7 vector. To identify the putative domain of amino acid sequence required for hnRNP A1/SARS_N interaction, different fragments of SARS_N gene were prepared by PCR. The anti‐sense primers for ΔN1, ΔN2, ΔN3 and ΔN4 and the sense primers for ΔN2′ and ΔN3′ were the same as those for full‐length SARS_N gene cloning as mentioned above. Other primers are listed in Table 1 .
Table Table 1.
Primers for the truncated fragments of SARS_N used in yeast two‐hybrid
| ΔN1 (sense) | GGTT GAATTC CCAGATGACCAAATTGGCTACTACC |
| ΔN2 (sense) | GGTT GAATTC CAACTTCCTCAAGGAACAACATTGCC |
| ΔN3(ΔN5) (sense) | AATT GAATTC ATGGCTAGCGGAGGTGGTGAAA |
| ΔN4 (sense) | GCGT GAATTC GACCTAATCAGACAAGGAACTGA |
| ΔN5 (anti‐sense) | GGCC GGATCC TTAGTTGTCTTTGAATTGTGGAT |
| ΔN2′ (anti‐sense) | GGAA GGATCC TTATAGCACGGTGGCAGCATTGT |
| ΔN3′ (anti‐sense) | GGAA GGATCC TTATCGAGCAGGAGAATTTCCCC |
2.6. Yeast transformation and culture
Competent cells of yeast strain AH109 were obtained from clontech (Plano Alto, CA). Transformations were performed according to the manufacturer's protocol. Briefly, 500 ng of plasmid DNA was added to 50 ml of competent cells and mixed again with 300 ml lithium acetate at 30 °C for 30 min followed by heat‐shock at 42 °C for 30 min and subsequently spread on a drop‐out‐agar plate in the absence of leucine and tryptophan. The plates were incubated at 30 °C for 48 h for yeast growth. PCR was used to confirm transformation with the target gene. A positive clone was inoculated in the SD medium lacking leucine, typtophan and histidine (SD‐LTH) and supplemented with 10 mM 3‐amino‐1′,2′,4′‐triazole (SD‐LTH + 3‐AT). The medium was shaken at 200 rpm at 30 °C for 96 h before 400 μl of the medium was filled into the well of a 96‐well microplate. The absorbance at 595 nm of the medium in the 96‐well microplate was measured in a TECAN reader (Switzerland). This is an alternative method based on the growth curve analysis for yeast culture that is amenable to microtiter plate format. The method used here is reproducible and of equal or greater sensitivity compared with the β‐galactosidase assay [18]. All the yeast media were prepared according to the standard Protocols Handbook (PT3024‐1, Clontech).
2.7. Surface plasmon resonance technology based analysis
SPR technology based Biacore 3000 (Biacore AB) biosensor was used to perform the kinetic analysis of SARS_N/human hnRNP A1 interaction. CM5 research grade sensor chips and P20 were purchased from Biacore AB. N‐Hydroxysuccinimide, N‐ethy‐N′‐(3‐diethylaminopropyl)carbodiimide, and ethanolamine coupling regents were used to immobilize the ligand to the sensor surface using a standard amine‐coupling procedure. The running and sample buffers were 10 mM HEPES, pH 7.4, containing 150 mM NaCl, and all the buffers were filtered and degassed before use. The analyte was injected (120 s, 30 μl/s) followed by a dissociation phase (120 s). Each experiment was repeated at least twice, and the data were obtained at 25 °C. The association rate (k on), dissociation rate (k off), and equilibrium dissociation (K D, K D = k off/k on) constants were obtained from each sensorgram by fitting to 1:1 Langmuir binding model using a global fitting method from the BIAevaluation 3.1 software. The binding affinity of the synthesized peptide (ASSRSSSRSRGNSRN) to the human hnRNP A1 was calculated by steady‐state fitting mode in the BIAevaluation 3.1 software. In the inhibitory assays, the peptide with varied concentrations was mixed with 30 μM human hnRNP A1 for 2 h before injection to Biacore 3000.
3. Results
3.1. GST pull‐down assay directly revealed SARS_N/human hnRNP A1 interaction
In order to identify the interaction between hnRNP A1 and SARS_N, the recombinant human GST‐hnRNP A1 protein was over‐expressed in E. coli, followed by purification using Glutathione–Sepharose affinity beads. SARS_N/human hnRNP A1 interaction was then determined by GST pull‐down assay, in which the purified human GST‐hnRNP A1 (Fig. 1 , lane 3) protein was immobilized on the Glutathione–Sepharose beads as a bait protein according to the kit. The His‐tagged SARS_N protein as the prey was purified through a Ni affinity column (Fig. 1, lane 2). After the GST‐hnRNP A1 and the possible partner were eluted by glutathione, the samples were analyzed by SDS–PAGE. As indicated in lane 5 of Fig. 1, SARS_N protein could be also detected on the SDS–PAGE, suggesting that human GST‐hnRNP A1 was eluted by glutathione together with SARS_N protein. SARS_N binding to human hnRNP A1 could be considered to be specific because the bead itself did not pull down any SARS_N protein as shown in lane 4. All these results thereby determined that SARS_N protein could specifically bind to human hnRNP A1 in vitro.
Figure 1.
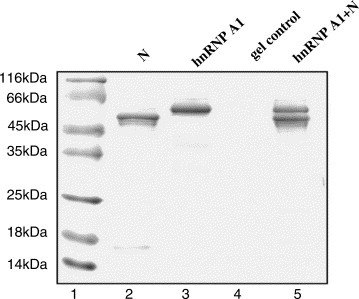
SARS_N/human hnRNP A1 interaction determined by GST pull‐down. Samples were analyzed on a 10% SDS–polyacrylamide gel, and the band was visualized with Coomassie brilliant blue. Components in each lane are shown at the top. Lane 1, molecular mass marker; lane 2, purified His‐tagged SARS_N; lane 3, purified GST‐tagged human hnRNP A1; lane 4, agarose gel control; lane 5, human hnRNP A1 and the pull‐down SARS_N.
3.2. Real‐time analysis of SARS_N/human hnRNP A1 interaction by Biacore 3000
We employed Biacore 3000 as an alternative approach to perform the kinetic analysis of SARS_N/human hnRNP A1 interaction. During the assay, SARS_N protein was immobilized on the CM5 sensor chip as ligand in 2000 RU according to the standard amine‐coupling wizard. The control flow cell was activated then blocked directly without immobilizing any protein to eliminate non‐specific interaction between hnRNP A1 and the sensor chip surface. The purified human hnRNP A1 protein was prepared in a series of concentrations as analyte then injected into Biacore 3000. The binding level (measured in RU) of human hnRNP A1 to the immobilized SARS_N rose and reached saturation with the increasing concentration (Fig. 2 A), which demonstrated again that human hnRNP A1 protein can directly bind to SARS_N in vitro. The association of SARS_N with hnRNP A1 could be described in a simple equilibrium:
The kinetic parameters (the equilibrium dissociation constant, K D; association rate constant, k on; and dissociation rate constant, k off) of the SARS_N/hnRNP A1 protein–protein interaction were determined by the 1:1 Langmuir binding model. As such, the evaluated curves were found to fit the experimental curves very well (Fig. 2A) and the fitting reliability was also reflected by the small chi 2 and residuals (Fig. 2B). The K D for SARS_N/hnRNP A1 interaction was thus simulated as 0.35 ± 0.02 μM with k on = 5.83 ± 0.42 × 103 M−1 s−1 and k off = 2.06 ± 0.12 × 10−3 s−1, which quantitatively revealed the high binding affinity of SARS_N against human hnRNP A1.
Figure 2.
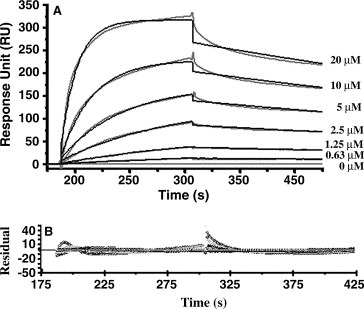
Sensorgrams of human hnRNP A1 binding to the immobilized SARS_N. The binding curves were fitted to 1:1 Langmuir binding model. Superposition of fitting curves (in black) to original curves (in grey) (A) and the small residuals (B) demonstrates the goodness of the fitting.
3.3. SARS_N/hnRNP A1 interaction validation and interaction region mapping by yeast two‐hybrid assay
To further validate the SARS_N/hnRNP A1 interaction, the yeast two‐hybrid assay technology was performed. The full‐length SARS_N protein and human hnRNP A1 were cloned in‐frame into pGBKT7 (pGBKT7‐N) and pGADT7 (pGADT7‐A1) vectors. Upon co‐transforming the both plasmid constructs into AH109, a phenotype was observed on high stringency nutrition medium (SD‐LTH + 10 mM 3‐AT) (Fig. 3 B) even on higher stringency (30 mM 3‐AT) (data not shown), which therefore indicated the possible presence of SARS_N/hnRNP A1 interaction in vivo. Moreover, the fact that the control with empty plasmid did not show cell growth (Fig. 3B) further suggests that the SARS_N/hnRNP A1 interaction might be relatively specific in yeast.
Figure 3.
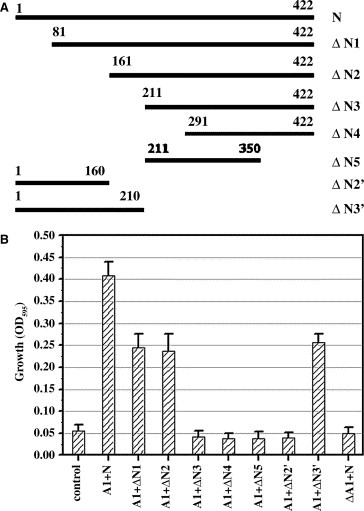
Mapping the interaction domain of SARS_N. Schematic description of the truncated fragments (A) and the yeast two‐hybrid assay results for SARS_N/human hnRNP A1 interactions in their truncated and non‐truncated forms (B). The empty vectors pGBKT7 and pGADT7 co‐transformed were used as the negative control.
To map the involved regions of SARS_N within the SARS_N/hnRNP A1 interaction, a series of 5 nested fragments of SARS_N were generated (Fig. 3A). These fragments, designated from ΔN1 to ΔN5, were cloned into pGBKT7 vector, and then co‐transformed respectively with pGADT7‐hnRNP A1 into the AH109 yeast cell. After 96‐h growth in high stringency nutrition medium (SD‐LTH + 10 mM 3‐AT) at 30 °C with shaking, 300 μl cells were transferred into the 96‐well microplate. The growth curves of these cells were then evaluated by measuring their absorbance at 595 nm in the 96‐well microplate. As indicated in Fig. 3B, ΔN1 (aa 81–422) and ΔN2 (aa 161–422) showed viability compared to the empty control, thus suggesting that these two segments of SARS_N protein have binding affinities to human hnRNP A1. It is noted that deletion of the fragment from aa 1 to 211 of SARS_N resulted in the loss of its binding activity to human hnRNP A1 as indicated by the fact that no viability was detected for ΔN3 (aa 211–422), ΔN4 (aa 291–422) and ΔN5 (aa 211–350). This therefore suggested that the interacting fragment of SARS_N protein involved in the SARS_N/human hnRNP A1 interaction is most probably within the segment of aa 1–211. In addition, it is found that the growths of both ΔN1 (aa 81–422) and ΔN2 (aa 161–422) are close to each other but a little less than that of the full‐length N, it seems that the N terminal 80 amino acid residues of SARS_N might be involved in SARS_N/hnRNP A1 interaction. To validate this possibility, we made two truncated fragments for SARS_N, ΔN2′ (aa 1–160) and ΔN3′ (aa 1–210). As indicated in Fig. 3B, ΔN2′ had no binding affinity to human hnRNP A1, while ΔN3′ could bind to human hnRNP A1, which thereby indicates that the N terminal 80aa fragment does not participate in the SARS_N/human hnRNP A1 interaction and the segment of aa 161–210 of SARS_N is indispensable for this protein–protein interaction.
To explore the possible binding fragment of human hnRNP A1 within the SARS_N/hnRNP A1 interaction, a C‐terminus‐deleted fragment, ΔA1 (aa 1–202) of human hnRNP A1 was studied. This fragment was cloned into pGADT7 plasmid (pGADT7‐ΔA1) then co‐transformed with pGBKT7‐N into AH109. As shown in Fig. 3B, the yeast cell containing pGADT7‐ΔA1 and pGBKT7‐N showed no viability (lane ΔA1 + N), suggesting that ΔA1 (aa 1–202) of hnRNP A1 might not interact with SARS_N. Since a lot of facts have confirmed that hnRNP A1 protein interacts selectively through its C‐terminal Gly‐rich domain with different RNA‐binding proteins [12], together with our result, it is suggested that the C‐terminal Gly‐rich domain of human hnRNP A1 may be also involved in the SARS_N/human hnRNP A1 interaction.
3.4. SRXX‐repeat domain of SARS_N contributes to SARS_N/hnRNP A1 interaction
It is noticed that there is an SRXX‐repeat section within the fragment aa 161–210 of SARS_N, and this SRXX‐repeat segment seems to be a common region of hnRNP A1 binding proteins [12, 13]. It has been reported that this SRXX‐repeat fragment of MHV_N is within the MHV_N/hnRNP A1 interaction parts [15]. Therefore, encouraged by these facts, we supposed that the SRXX‐repeat fragment of SARS_N might be also involved in the SARS_N/hnRNP A1 interaction. To validate this hypothesis, we synthesized the peptide (ASSRSSSRSRGNSRN) according to the fragment aa 183–197 of SARS_N and applied it to Biacore assay. The curves of this peptide binding to the immobilized GST‐hnRNP A1 (flow cell was immobilized with GST as control) were shown in Fig. 4 A. The binding affinity of this peptide to the human hnRNP A1 protein was thus evaluated as K D at 148 ± 12 μM by the steady‐state fit model. To further confirm this specific binding of this SARS_N SRXX‐repeat domain to hnRNP A1, we also performed an inhibitory experiment by using this peptide to inhibit hnRNP A1 interaction with the immobilized SARS_N protein through Biacore 3000. As shown in Fig. 4B, 100 and 625 μM of this peptide did perturb 10 μM hnRNP A1 binding to SARS_N protein, which thereby suggested that the SRXX‐repeat fragment of SARS_N does contribute to the SARS_N/human hnRNP A1 interaction.
Figure 4.
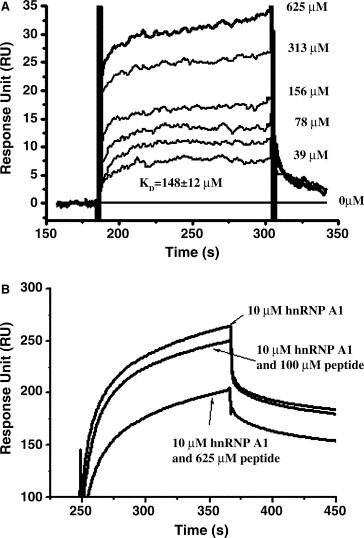
Interaction of the peptide (ASSRSSSRSRGNSRN) with human hnRNP A1 (A), and the peptide inhibition assay against the human hnRNP A1/SARS_N interaction (B).
4. Discussion
In the present study, by employing a series of biochemical and biophysical methods, we have firstly reported that SARS_N protein has a specific binding affinity to human hnRNP A1, and the further yeast two‐hybrid assay demonstrated that the C‐terminus of human hnRNP A1 and the fragment aa 161–210 of SARS_N probably contribute to the SARS_N/human hnRNP A1 interaction. Since both human hnRNP A1 and SARS_N proteins bind to RNA and implement their functions in RNA replication and transcription [11], we thus tentatively proposed that, together with the bound RNA, SARS_N might probably interact with the human hnRNP A1 to form a complex in the regulation of the transcription and replication of SARS_CoV according to the reported model for MHV [11]. Therefore, it seems that the cross talk between human hnRNP A1 and SARS_N protein might be essential for SARS_CoV replication and transcription. Additionally, SARS_N protein binds to the C‐terminus of human hnRNP A1 and cooperates with hnRNP A1 to recruit more proteins in forming the transcription or replication complex. As such, the C‐terminal‐deletion mutant of human hnRNP does not interact with SARS_N and fails to bring it into the initiation complex, possibly resulting in the failure of SARS‐CoV RNA replication and transcription.
In addition, the yeast two‐hybrid result has clearly indicated that only one fragment of SARS_N (aa 161–211) is required for SARS_N/human hnRNP A1 interaction, which is different from the MHV_N case where MHV_N has two domains, aa 1–292 and aa 392–455 that are involved in its binding to hnRNP A1 [15] (Fig. 5 ), suggesting that MHV_N/hnRNP A1 interaction seems to be stronger in comparison with SARS_N/hnRNP A1. As we know, the fragment aa 161–211 contains SRXX‐repeat domain that is multifunctional and conserved in N protein of coronaviruses [19]. In this work, the fact that the synthesized SRXX‐repeat peptide has weak binding affinity (K D = 148 ± 12 μM) to human hnRNP A1 (Fig. 2A) suggests that the SRXX‐repeat segment of SARS_Nis involved in but does not dominate the SARS_N/human hnRNP A1 interaction, which is also within the similar binding feature of this corresponding fragment in MHV_N (Fig. 5). Interestingly, it has been recently reported that SRXX‐repeat fragment is indispensable for SARS_N multimerization [20] and for SARS_N interaction with SARS_CoV membrane protein [19]. All these facts have indicated that SRXX‐repeat fragment of SARS_N protein might play a potent role in SARS_CoV infection, even though more studies should be addressed in exploring how SARS_N regulates itself to adapt its interaction with the different partners.
Figure 5.
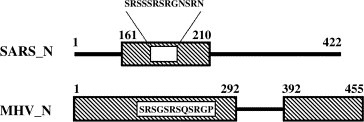
Comparison of the human hnRNP A1‐binding fragments (the part in frame) in SARS_N with MHV_N.
In conclusion, our data have shown for the first time both in vitro and in vivo that the nucleocapsid protein of SARS coronavirus has a high binding affinity to human hnRNP A1, and such a protein–protein interaction involves the region aa 161–210 of SARS_N and the C‐terminus aa 203–320 of human hnRNP A1. Based on the analytical results for MHV, it is suggested that both SARS_N and human hnRNP A1 proteins might be among the SARS_CoV replication/transcription complex and their interaction may be involved in the regulation of SARS_CoV RNA synthesis. The fact that there is only one instead of two human hnRNP A1‐binding domain in SARS_N indicates that the SARS_N protein interaction with human hnRNP A1 for SARS_CoV might take a different binding type compared with that for MHV, which is possibly due to the low sequence homology for SARS_CoV relative to other coronaviruses.
Acknowledgement
This work was supported by the State Key Program of Basic Research of China (Grants 2002CB512802, 2002CB512807 and 2004CB518905), the National Natural Science Foundation of China (Grants 20372069 and 20472095), Shanghai Basic Research Project from the Shanghai Science and Technology Commission (Grants 02DJ14070, 03DZ19228 and 03DZ19212), the 863 Hi‐Tech Program (Grants 2002AA104270 and 2002AA233011), Sino‐European Project on SARS Diagnostics and Antivirals (Proposal/Contract No.: 003831).
Luo Haibin,Chen Qing,Chen Jing,Chen Kaixian,Shen Xu and Jiang Hualiang(2005), The nucleocapsid protein of SARS coronavirus has a high binding affinity to the human cellular heterogeneous nuclear ribonucleoprotein A1, FEBS Letters, 579, doi: 10.1016/j.febslet.2005.03.080
Contributor Information
Xu Shen, Email: xshen@mail.shcnc.ac.cn.
Hualiang Jiang, Email: hljiang@mail.shcnc.ac.cn.
References
- 1. Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L., Law K.I., Tang B.S., Hon T.Y., Chan C.S., Chan K.H., Ng J.S., Zheng B.J., Ng W.L., Lai R.W., Guan Y., Yuen K.Y., Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet, 361, (2003), 1767– 1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peiris J.S., Lai S.T., Poon L.L., Guan Y., Yam L.Y., Lim W., Nicholls J., Yee W.K., Yan W.W., Cheung M.T., Cheng V.C., Chan K.H., Tsang D.N., Yung R.W., Ng T.K., Yuen K.Y., Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet, 361, (2003), 1319– 1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Drosten C., Gunther S., Preiser W., Van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Muller S., Rickerts V., Sturmer M., Vieth S., Osterhaus A.D., Schmitz H., Doerr H.W., Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med., 348, (2003), 1967– 1976. [DOI] [PubMed] [Google Scholar]
- 4. Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med., 348, (2003), 1953– 1966. [DOI] [PubMed] [Google Scholar]
- 5. Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S., Khattra J., Asano J.K., Barber S.A., Chan S.Y., Cloutier A., Coughlin S.M., Freeman D., Girn N., Griffith O.L., Leach S.R., Mayo M., McDonald H., Montgomery S.B., Pandoh P.K., Petrescu A.S., Robertson A.G., Schein J.E., Siddiqui A., Smailus D.E., Stott J.M., Yang G.S., Plummer F., Andonov A., Artsob H., Bastien N., Bernard K., Booth T.F., Bowness D., Czub M., Drebot M., Fernando L., Flick R., Garbutt M., Gray M., Grolla A., Jones S., Feldmann H., Meyers A., Kabani A., Li Y., Normand S., Stroher U., Tipples G.A., Tyler S., Vogrig R., Ward D., Watson B., Brunham R.C., Krajden M., Petric M., Skowronski D.M., Upton C., Roper R.L., The Genome sequence of the SARS-associated coronavirus. Science, 300, (2003), 1399– 1404. [DOI] [PubMed] [Google Scholar]
- 6. Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P., Penaranda S., Bankamp B., Maher K., Chen M.H., Tong S., Tamin A., Lowe L., Frace M., DeRisi J.L., Chen Q., Wang D., Erdman D.D., Peret T.C., Burns C., Ksiazek T.G., Rollin P.E., Sanchez A., Liffick S., Holloway B., Limor J., McCaustland K., Olsen_Rasmussen M., Fouchier R., Gunther S., Osterhaus A.D., Drosten C., Pallansch M.A., Anderson L.J., Bellini W.J., Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science, 300, (2003), 1394– 1399. [DOI] [PubMed] [Google Scholar]
- 7. Ren A.X., Xie Y.H., Kong Y.Y., Yang G.Z., Zhang Y.Z., Wang Y., Wu X.F., Expression, purification and sublocalization of SARS-CoV nucleocapsid protein in insect cells. Acta. Biochim. Biophys. Sin, 36, (2004), 754– 758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Macneughton M.R., Davies H.A., Ribonucleoprotein-like structures from coronavirus particles. J. Gen. Virol., 39, (1978), 545– 549. [DOI] [PubMed] [Google Scholar]
- 9. Luo C., Luo H., Zheng S., Gui C., Yue L., Yu C., Sun T., He P., Chen J., Shen J., Luo X., Li Y., Liu H., Bai D., Shen J., Yang Y., Li F., Zuo J., Hilgenfeld R., Pei G., Chen K., Shen X., Jiang H., Nucleocapsid protein of SARS coronavirus tightly binds to human cyclophilin A. Biochem. Biophys. Res. Commun., 321, (2004), 557– 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nelson G.W., Stohlman S.A., Tahara S.M., High affinity interaction between nucleocapsid protein and leader/intergenic sequence of mouse hepatitis virus RNA. J. Gen. Virol., 81, (2000), 181– 188. [DOI] [PubMed] [Google Scholar]
- 11. Shi S.T., Huang P., Li H.P., Lai M.M., Heterogeneous nuclear ribonucleoprotein A1 regulates RNA synthesis of a cytoplasmic virus. EMBO J., 19, (2000), 4701– 4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu R.M., Jokhan L., Cheng X., Mayeda A., Krainer A.R., Crystal structure of human UP1, the domain of hnRNP A1 that contains two RNA-recognition motifs. Structure, 5, (1997), 559– 570. [DOI] [PubMed] [Google Scholar]
- 13. Cartegni L., Maconi M., Morandi E., Cobianchi F., Riva S., Biamonti G., hnRNP A1 selectively interacts through its Gly-rich domain with different RNA-binding proteins. J. Mol. Biol., 259, (1996), 337– 348. [DOI] [PubMed] [Google Scholar]
- 14. Fu X.D., The superfamily of arginine/serine-rich splicing factors. RNA, 1, (1995), 663– 680. [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Y., Zhang X., The nucleocapsid protein of coronavirus mouse hepatitis virus interacts with the cellular heterogeneous nuclear ribonucleoprotein A1 in vitro and in vivo. Virology, 265, (1999), 96– 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang Q., Yu L., Petros A.M., Gunasekera A., Liu Z., Xu N., Hajduk P., Mack J., Fesik S.W., Olejniczak E.T., Structure of the N-terminal RNA-binding domain of the SARS CoV nucleocapsid protein. Biochemistry, 43, (2004), 6059– 6063. [DOI] [PubMed] [Google Scholar]
- 17. Luo H., Ye F., Sun T., Yue L., Peng S., Chen J., Li G., Du Y., Xie Y., Yang Y., Shen J., Wang Y., Shen X., Jiang H., In vitro biochemical and thermodynamic characterization of nucleocapsid protein of SARS. Biophys. Chem., 112, (2004), 15– 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Diaz-Camino C., Risseeuw E.P., Liu E., Crosby W.L., A high-throughput system for two-hybrid screening based on growth curve analysis in microtiter plates. Anal. Biochem., 316, (2003), 171– 174. [DOI] [PubMed] [Google Scholar]
- 19. He R., Leeson A., Ballantine M., Andonov A., Baker L., Dobie F., Li Y., Bastien N., Feldmann H., Strocher U., Theriault S., Cutts T., Cao J., Booth T.F., Plummer F.A., Tyler S., Li X., Characterization of protein–protein interactions between the nucleocapsid protein and membrane protein of the SARS coronavirus. Virus Res., 105, (2004), 121– 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. He R., Dobie F., Ballantine M., Leeson A., Li Y., Bastien N., Cutts T., Andonov A., Cao J., Booth T.F., Plummer F.A., Tyler S., Baker L., Li X., Analysis of multimerization of the SARS coronavirus nucleocapsid protein. Biochem. Biophys. Res. Commun., 316, (2004), 476– 483. [DOI] [PMC free article] [PubMed] [Google Scholar]


