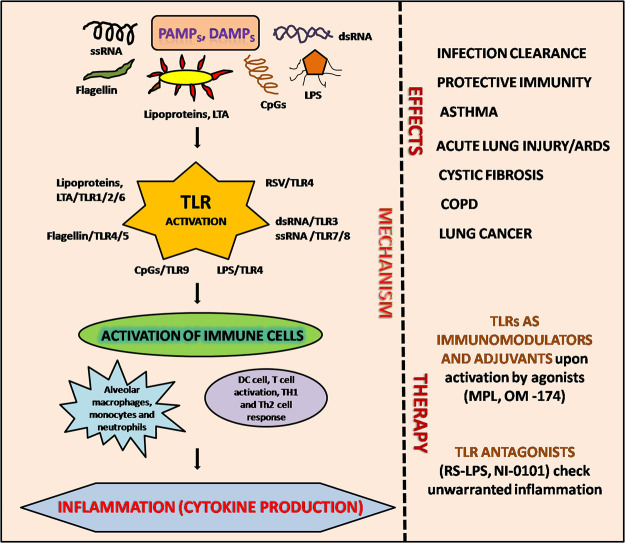
Abstract
Toll-like receptors (TLRs) comprise a clan of proteins involved in identification and triggering a suitable response against pathogenic attacks. As lung is steadily exposed to multiple infectious agents, antigens and host-derived danger signals, the inhabiting stromal and myeloid cells of the lung express an aggregate of TLRs which perceive the endogenously derived damage-associated molecular patterns (DAMPs) along with pathogen associated molecular patterns (PAMPs) and trigger the TLR-associated signalling events involved in host defence. Thus, they form an imperative component of host defence activation in case of microbial infections as well as non-infectious pulmonary disorders such as interstitial lung disease, acute lung injury and airways disease, such as COPD and asthma. They also play an equally important role in lung cancer. Targeting the TLR signalling network would pave ways to the design of more reliable and effective vaccines against infectious agents and control deadly infections, desensitize allergens and reduce inflammation. Moreover, TLR agonists may act as adjuvants by increasing the efficiency of cancer vaccines, thereby contributing their role in treatment of lung cancer too. Overall, TLRs present a compelling and expeditiously bolstered area of research and addressing their signalling events would be of significant use in pulmonary diseases.
Keywords: Toll-like receptors (TLRs), DAMPs, PAMPs, Asthma, COPD, Lung interstitial diseases
Graphical abstract
1. Introduction
Lungs are repeatedly divulged to several environmental stimuli including chemicals, dust, pollen along with numerous microorganisms. They are competent enough to oppose these environmental stimuli with the help of various host defences. Composite nasal passage geometry, airways branching pattern, secretion of mucus and soluble mediators like surfactant proteins, lysozyme, secretory IgA and antimicrobial peptides are the exemplifications of host defences. Furthermore, alveolar macrophages and dendritic cells provide a perpetual vigilance for pathogenic components. At the same time, they are also implicated in inhibiting T cell responses against non-pathogenic antigens [1]. These normal processes of host defences are trivial, but an inflammatory response is anticipated in case of a prolonged exposure to pathogenic antigens or highly virulent microbes. Prompt commencement of an inflammatory response is accomplished with the help of unique and conserved Pattern recognition receptors (PRRs) present on the surface of myeloid cells, lung epithelial cells and lymphoid tissue. PRRs comprise of Toll like receptors (TLRs), RIG-I like receptors (RLRs) and NOD like receptors (NLRs). These receptors are activated upon binding to their respective ligands, which trigger the release of cytokines and chemokines that calls for recruitment of antigen presenting cells and leukocytes at the site of infection and consequently induces the adaptive immunity [2].
Perpetual communication of respiratory tract with the environmental stimuli put a constraint on the host immune system to differentiate innoxious and pathogenic exposures in a robust manner. To accomplish this, TLRs selectively recognize unique microbial patterns called Pathogen associated molecular patterns (PAMPs) [3,4]. Lipopolysaccharide (LPS) in outer membrane of gram-negative bacteria, lipoteichoic acid and peptidoglycan in cell wall of gram-positive bacteria, flagellin of bacterial flagella, dsRNA and ssRNA of viruses etc. act as PAMPs [[5], [6], [7]]. PAMPs are recognized by the receptor called Pattern recognition receptors or PRRs present on the cells of the innate immunity, i.e. monocytes, macrophages, dendritic cells, neutrophils etc., non-immune cells like fibroblasts, endothelial cells, epithelial cells etc., and in various intercellular compartments like endosome and lysosome. Thus, PRRs can detect extracellular as well as intracellular pathogens. There are different types of PRRs and each type can recognize multiple pathogenic species that share a particular type of PRR and TLR is one among them [3]. TLRs are crucial components of the innate immune system, used to recognize and defend against invading pathogens. Toll is a gene in fruit fly and toll-like proteins have been found in animals and are known as TLRs. They are conserved and there are about 10 functional TLRs (TLR1 to TLR10) in humans and 12 (TLR1 to TLR9, TLR11 to TLR13) in mice. Human TLRs; TLR1, TLR2, TLR4, TLR5, TLR6 and TLR10 are expressed on cell surface, while TLR3, TLR7, TLR8 and TLR9 are expressed on the membrane of intracellular vesicles [[5], [6], [7], [8]].
Other than PAMPs, studies have reported the existence of Damage associated molecular patterns (DAMPs), which are known to be the ligands of TLRs. It was first suggested by studies on heat shock proteins [9]. These DAMPs originate from the host such as high mobility group box 1 (HMGB1), hyaluronan and heat shock proteins as they are released from damaged tissues [[5], [6], [7]], act as danger signals to alert the cells. In case of an injury, damaged tissues release certain intracellular components into the surroundings, some of which specifically activate TLRs. Detection of endogenous ligands may also lead to the incipience of autoimmune responses. For instance, HMGB1 proteins normally reside in the nucleus, but when released from apoptotic cells in a complex with nucleosomes can give rise to lupus like symptoms upon activation of TLR2 [10]. Breakdown of extracellular matrix also act as damage signals. Similarly, ischemic injury results from temporary blood flow loss and oxygen supply. Cell deaths during ischemia release damage signal and allow influx of WBCs, which instigate more detrimental and highly exaggerated inflammatory response [8].
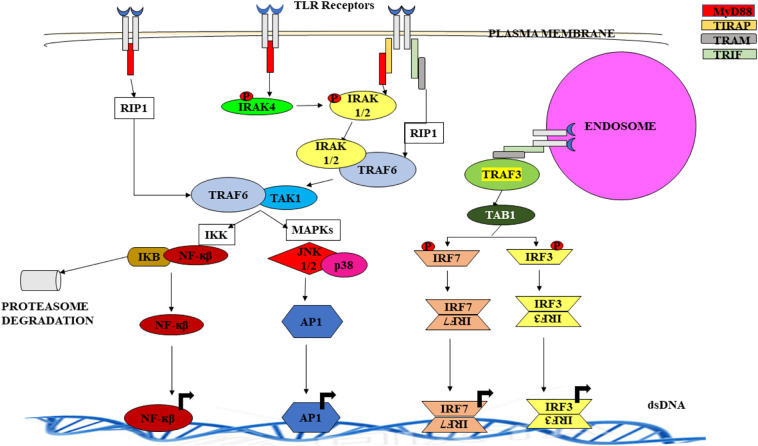
Each TLR is a horse shoe like structure with variable number of Leucine rich repeats (LRRs) at N-terminal facing the extracellular matrix, if present, on surface of the cell; while face lumen if present in intracellular vesicles. The intracellular or cytoplasmic domain is designated as Toll/IL-1R (TIR) as it shares similarity with Interleukin-1 receptor (IL-1R). TIR is involved in mediating the interaction between TLR and adaptor proteins and transducing signal while LRRs is believed to recognize ligands [11]. Signalling pathways activated downstream of the adaptor molecule interacting with TIR domain promote the expression of cytokines, chemokines, interferons, which assist in pathogen removal by recruitment of other immune cells and activation of specific adaptive immunity. Some TLRs work in dimer form, either homodimer or heterodimer [12]. It is also associated with other protein families like MD2 and CD14 to increase ligand diversity (Table 1 ) [13,14]. After the binding of ligand to the receptor, TIR interact with intracellular adaptor proteins like MyD88, TRIF (TICAM-1), TIRAP (MAL), TRAM (TICAM-2) and activate the downstream signalling pathways. MyD88 dependent pathway and TRIF dependent pathways are the two primarily involved signalling pathways, however, other adaptor proteins act as bridge molecules. MyD88 acquires three domains: an amino- (N-) terminal death domain (DD), a carboxy- (C-) terminal Toll/IL-1R (TIR) domain and a short linker sequence. One of its intermediate domain (ID) is in connection with IL-1R-associated kinases 4 (IRAK4) involved in TLR signalling [15,16]. All TLRs except TLR3 use MyD88-dependent signalling. On the other hand, TRIF (a large protein consisting of 712 amino acids in humans) binds to TLR3 directly and indirectly to TLR4 by means of an adaptor protein, TRIF related adaptor molecule (TRAM) [15]. It is known to possess a significant role in induction of inflammatory mediators, that consequently induce antiviral innate immune responses via TLR3 and TLR4 mediated signalling. This was confirmed upon defective expression of IFN-related genes, mediated by TLR3 and TLR4 in TRIF knockout mice. However, TLR4 mediated MyD88 pathway activation and early phase activation of NF-κβ was also observed. Thus, TLR4 is unique as it uses both pathways [3]. Activated TLRs initiate a cascade of events which involves variety of protein kinases that eventually leads to the activation of certain transcription factors like NF-κβ, MAPKs, IRFs, AP1 [4,12]. These factors enter the nucleus, binds DNA, transcribe and translate proteins that consequently enhance innate immune response and initiate adaptive immune responses against the pathogen (Fig. 1 ) [7,17,18]. Inappropriate or unregulated activation of TLR signalling can lead to chronic inflammation and autoimmune disorders.
Table 1.
Ligands and adaptors of TLRs.
| TLR | Localisation | Associated proteins | Ligand/agonist | Source/pathogen detected | Adaptor protein | Pathway |
|---|---|---|---|---|---|---|
| TLR-1/2 | Cell surface | CD14, CD36, DECTIN1 | Ac3LP, Glycolipids | Bacteria | MyD88, TIRAP | MyD88 |
| TLR-2 | Cell surface | CD36, CD14 | Lipoproteins | Multiple pathogens | MyD88, TIRAP | MyD88 |
| Peptidoglycan | Bacteria | |||||
| Porins | Bacteria | |||||
| Zymosan | Fungi | |||||
| β-Glycan | Fungi | |||||
| GPI-mucin | Protozoa | |||||
| Envelope glycoproteins | Viruses | |||||
| TLR-2/6 | Cell surface | CD14, CD38, DECTIN1 | Ac2LP, LTA, Zymosan | Bacteria | MyD88, TIRAP | MyD88 |
| TLR-2/10 | Cell surface | Ac3LP | Bacteria | MyD88, TIRAP | MyD88 | |
| TLR-3 | Intracellular | CD14, TRIL | dsRNA | Viruses | TRIF | TRIF |
| Poly(I:C) | Synthetic analogue of dsRNA | |||||
| TLR-4 | Cell surface | CD14, MD-2 | LPS, F-Prot | Bacteria | MyD88, TIRAF, TRIF, TRAM | MyD88, TRIF |
| RSV, VSV, Env-prot, MMTV others. | Viruses | |||||
| TLR-5 | Cell surface | Flagellin | Bacteria | MyD88 | MyD88 | |
| TLR-7 | Intracellular | CD14 | ssRNA | Viruses | MyD88 | MyD88 |
| TLR-8 | Intracellular | CD14 | ssRNA | Viruses | MyD88 | |
| TLR-9 | Intracellular | CD14, HMGB1 | Unmethylated CpG DNA | Bacteria, protozoa and viruses | MyD88 | MyD88 |
| Mitochondrial DNA | Endogenous |
Fig. 1.
TLR signalling - TLR1, TLR2, TLR4, TLR5, TLR6 and TLR10 are expressed on the surface of the cell whereas TLR3, TLR7, TLR8 and TLR9 in intracellular vesicles like endosome and lysosomes. All TLRs except TLR3 activates MyD88 pathway, TLR3 and TLR4 activate TRIF pathway. When ligand binds to the TLR receptors, adaptor molecules MyD88 and TRIF bind to the TIR domain either directly or indirectly via bridge molecules TIRAP and TRAM. MyD88 now recruits IRAK4 which in turn phosphorylate and activate IRAK1/2. The activated IRAKs now interact with TRAF6. This IRAK-TRAF6 complex recruits and activates TAK1. It also gets activated directly by MyD88 and RIP1. Activated TAK1 activates IKK complex and MAPKs. IKK complex phosphorylates and subsequently degrade Iκβ by proteasomes leading to release of transcription factor (TF) NF-κβ whereas activated MAPKs releases transcription factor AP1 by activating JNK1/2 and p38. These TFs translocate into the nucleus and bind to their corresponding TF binding segment on the DNA to induce transcription of cytokines, chemokines, interferons, interleukins etc. In TRIF pathway, TRIF binds to TIR domain via bridge molecule TRAM. TRIF binds TRAF3 and TRAF6. By binding to TRAF6 it normally activates MyD88 pathway whereas by binding with TRAF3 it activates TKB1 which further phosphorylates and activates IRF3 and IRF7. Their homodimers enter nucleus and binds to its binding segment on DNA and transcribe IFN-α and IFN-β.
TLRs are extensively expressed on resident as well as infiltrating myeloid and lymphoid cells in the lung tissue. TLR1 to TLR10 are expressed on primary bronchial epithelial cells, and consequently release CXCL8 (IL-8) upon activation of TLRs by several ligands [19]. Lower levels of TLR3, TLR5, TLR9 and higher levels of TLR1, TLR2, TLR4, TLR7 and TLR8 have been found to be expressed on the surface of human alveolar macrophages [20]. TLR4 is expressed on lung endothelial cells and is imperative for capillary concealment and neutrophil recruitment after systemic administration of LPS [21]. Recruited neutrophils also express TLR1, TLR2, TLR4, TLR5 and TLR9 [22]. These markers and anatomical locations can be used to distinguish different subsets of dendritic cells (DCs) [23]. Significantly high levels of TLR7 and TLR9 have been found to be expressed on lung plasmacytoid DCs (pDCs), which give rise to regulatory T cells and are also involved in suppressing allergic response [24].
TLR mediated responses are cell or tissue location dependent. This can be illustrated as - In the basal layers of airway epithelium, TLR2 and TLR4 are most relevant and maintained at low expression levels, but in inflamed cells and pathogenic infection, there is an upsurge in their expression levels [25,26]. Moreover, TLR4 is intracellular but can be willingly transferred to the surface of the cell for pathogen recognition [27]. It has been found that TLR4 is one of the host extracellular and cellular proteins, along with MD-2, HBD-2, CD14 and LBP (LPS binding protein); required for a precise response to several endotoxins. Human airway epithelial cells abundantly express TLR4 very little HBD-2 and little or no MD-2, thus they have to induced in these cells upon exposure to specific bacterial or host products in order to generate an appropriate response against the endotoxins [28]. In addition, TLR3 and TLR5 in the human airway epithelia also play important roles in ligand recognition [11,29,30].
Additionally, levels of TLR expression in cells cannot always be co-ordinated with the functional response [20,31]. This can be explained as enhanced secretion of TNF-α by alveolar macrophages, IL-6 and IL-10 by interstitial macrophages has been observed even when the levels of TLR mRNA are comparable [20]. Moreover, cellular activation of lung tissue can also be achieved via co-operative interactions with TLR expressing lymphoid cells. This was demonstrated by activation of airway smooth muscle cells, by IL-1β released by monocytes stimulated by LPS. Therefore, both intrinsic cellular mechanisms and co-operative interactions between resident and recruited cells play a significant role in determination of response to TLR ligands in the lung.
2. TLR signalling in respiratory tract
A large number of commensal organisms are in continuous contact with the upper epithelium of respiratory tract which includes nasal cavity, pharynx, larynx [32]. In case of a respiratory disease or allergic conditions, production of mucus and anti-microbial substances is the primitive response of epithelial cells [11,17]. Mucins (protein component of mucus) expression is induced either directly or indirectly by TLR signalling [11,33]. In the lower respiratory tract; trachea, bronchi, and lungs express TLR3 on the luminal and basal side, whereas TLR2, TLR6 and TLR1 on basolateral side. TLR6 are present in high levels, lower levels of TLR2, TLR4, TLR5 and TLR10 along with TLR3, TLR7 and TLR9 are present on both cell surface as well as intracellular compartments [34]. In response to various TLR ligands, primary bronchial epithelial cells express TLR1 to TLR10 and secrete the chemokine CXCL8 (IL-8) [3,19]. TLR4 expressed in lung endothelial cells is crucial for neutrophil recruitment [22] and capillary sequestration [3,21]. Dendritic cells of lungs act as sentinels and bridge innate and adaptive immunity. TLR7 and TLR9 are highly expressed by lung pDCs (plasmacytoid DCs) that suppress the allergic response and regulatory T cells are raised by regulatory lung DCs [3,24]. It has been shown that alveolar macrophages express TLR3, TLR5 and TLR9 and higher levels of TLR1, TLR2, TLR4, TLR7 and TLR8 [3,20,31]. Thus, TLR signalling is implicated in secretion of cytokines, chemokines, interferons like TNF-α, IL-8, MIP-1α, MIP-1β, RANTES, GRO-α, -β and -γ, IL-6, IL-5 and TGF-β which are necessary for promoting influx of professional phagocytic/killing cells such as neutrophils, eosinophils, monocytes, NK cells, macrophages and DCs that are ultimately responsible for removal of pathogen [11,35,36]. In addition, TLR signalling is also involved in production of anti-microbial substances such as defensins, lysozyme, nitric oxide (NO) and IL-37 in lower respiratory tract [11]. Boron has been found to equilibrate the LPS-CD14/TLR4/MD2 complex in a study, leading to release of pro inflammatory cytokines by M1 macrophages [37].
3. TLRs in infectious diseases
Pathogen identification is a crucial component for induction of innate immune system in case of inflammation and is moderated through receptors termed PRRs, the pattern recognition receptors, which detect conserved molecular motifs on pathogens called PAMPs (pathogen associated molecular patterns) [38]. PRRs induce multitude of signalling pathways for accomplishment of early host response upon PAMP recognition, which is essential to kill infectious pathogens.
The first recognized PRRs were TLRs. TLRs identify broad array of PAMPs [13,[39], [40], [41]]. In humans, ten members of TLR family have been recognized and each one sense specific PAMPs derived from several pathogens such as bacteria, viruses, fungi, mycobacteria (Table 2 ) [4,39]. Identification of particular PAMPs by TLR initiate signalling cascade, upon recruitment of adaptor molecule (for example MyD88), leading to discharge of interferon, chemokines and host defence peptides; which initiate macrophage activation, stimulation of interferon-stimulated genes (ISGs) and recruitment of neutrophils causing infected microbe killing [4,42]. Certain TLRs are localized to intracellular vesicles while some are localized to the cell surface. TLR9, TLR8, TLR7 and TLR3 sense nucleic acids and are localized in intracellular vesicles. Cell surface TLRs such as TLR6, TLR5, TLR4, TLR2 and TLR1 sense microbial structures [43].
Table 2.
PAMPs detected by TLR.
| Pathogen | TLR | PAMPs detected by TLR |
|---|---|---|
| Fungus | TLR7 | RNA |
| TLR4, TLR2 | Mannan | |
| TLR6, TLR2 | β-Glucan, zymosan | |
| TLR9 | DNA | |
| Mycobacteria, bacteria | TLR7 | RNA |
| TLR4 | Lipopolysaccharide | |
| TLR9 | DNA | |
| TLR6, TLR2, TLR1 | Lipoproteins | |
| TLR5 | Flagellin | |
| Viruses | TLR8, TLR7, TLR3 | RNA |
| TLR9 | DNA | |
| TLR4, TLR2 | Structural protein |
3.1. TLRs in microbial infections
TLR-deficient mice have been used to study the role of TLRs in bacterial infections. The gram-positive bacterium Staphylococcus aureus comprises numerous TLR PAMPs. Lipoteichoic acid and lipoprotein are sensed by the TLR6-TLR2 heterodimer. After the infection with Staphylococcus aureus, there was decline in TLR2 deficient mice survival [44]. After sinus and eye infection, TLR2 deficient mice signify increase in disease severeness and bio burden [45] suggesting that the TLR2 mediated sensing of the bacterial lipoproteins stimulates an immune response against Staphylococcus aureus. But in case of bacterial brain abscess, both mice groups (control and TLR2 deficient) showed indistinguishable responses on bioburden and survival [46]. According to study of Miller et al. and Takeuchi et al 2000, in several mice infection groups, TLR2-deficient mice group was less vulnerable to bacterial infection than MyD88 deficient mice group, which proposed the role of another TLRs and IL-1R family members in stimulating immune responses [44,46]. Thus, both TLR2 signalling and IL-1R, in conjoint have been found to initiate immune responses against Staphylococcus aureus infection.
The gram-negative bacterium Salmonella typhimurium comprise numerous PAMPs sensed by TLRs. Cytidine-phosphate-guanosine (CpG) DNA sensed by TLR9, lipoprotein sensed by TLR2, flagellin sensed by TLR5 and LPS sensed by TLR4 [45]. After the infection with Salmonella typhimurium, TLR4-deficient mice indicated growth and Salmonella typhimurium accumulation inside Mesenteric lymphadenitis and was found to be more vulnerable to bacterial infection than control group. Weiss et al. reported that even during lack of functional TLR4, TLR2 efficiently respond to the infection as TLR4 and TLR2-deficient group was more vulnerable to the infection than TLR4 knock-out group [47].
Mycobacterium tuberculosis (M. tuberculosis), the causative pathogen of tuberculosis is present in aerosol particles. M. tuberculosis reproduce in the phagosome of dust cells (alveolar macrophages). TLR9, TLR4 and TLR2, expressed on the surface of alveolar macrophages recognize the primary Mycobacterium infection and initiate transcription of IL-6, TNF-α and IL-12 inflammatory cytokines in order to regulate infection [45,48]. According to some reports, M. tuberculosis inhibited the MyD88 dependent TLR2 signalling [4,49]. MyD88 deficient mice group infected with M. tuberculosis was highly vulnerable to the infection and exhibited high bioburden and reduced secretion of TNF-α and IL-12, suggesting that sensing of M. tuberculosis by TLR generated innate immunity against Mycobacterium infection [48]. According to several reports, high dosage of Mycobacterium infection into TLR9 and TLR2 deficient mice groups showed high vulnerability to infection but some reports suggested that low dosage of Mycobacterium infection into TLR9, TLR4 or TLR2 deficient mice group showed very less vulnerability to infection [48]. According to Holscher et al. [270], both control and TLR9-TLR4-TLR2 deficient mice groups infected with Mycobacterium exhibited controlled growth of M. tuberculosis in lung. Hence, TLRs sensing discrete M. tuberculosis PAMP are mostly superfluous in stimulating the innate immune response against low dose aerosol infection of M. tuberculosis.
Invasive candidiasis is an infection caused by fungus Candida and is a life-threatening infection for immunosuppressed individuals. Dendritic cells recognize the Candida infection and initiate the secretion of cytokines as well as the upregulation of molecules that cause naïve T cells differentiation in T helper 17 cell (Th17), T helper 2 cell (Th2), T helper 1 cell (Th1) and Regulatory T (Treg) cells. It has been reported that innate immunity is repressed by inducing a detrimental Treg and Th2 cells response in a mouse model infected with Candida albicans. However, Th17 and Th1 cell responses play a vital role in protection against Candida albicans infection. Candida species comprise numerous PAMPs such as mannan, nucleic acids, chitin and β-glucan and these PAMPs are sensed by TLR9, TLR7, TLR6, TLR4 and TLR2 [50,51].
PAMP such as mannans is expressed through Candida albicans (C. albicans) and Saccharomyces cerevisiae (S. cerevisiae) is sensed by TLR4 leading to the secretion of TNF-α [50]. According to the Netea and Marodi, TLR4 defective C3H/HeJ mice infected with C. albicans exhibit reduced secretion of cytokines and chemokines through macrophages and the group was more vulnerable to the infection than control mice. Moreover, TLR4 deficient mice group primarily infected with less virulent Candida yeast and re-infected with Candida hyphae exhibited vulnerability to the infection. But some reports also suggested that when TLR4-deficient mice are infected with Candida hyphae, they live more than control mice group [50].
MyD88 deficient mice infected with Candida albicans show high vulnerability to the infection. The role of IL-1R/TLR mediated responses has been owed to the high vulnerability of mice [52]. There are some conflicting studies on specific TLRs function to stimulate host immune responses against Candida spp. According to some reports, TLR2 plays redundant role against fungal infection in in vivo conditions. TLR2 deficient mice group is more vulnerable to the Candida fungal infection than the control mice group and secrete low level of chemokines and TNF-α. Furthermore, Both TLR2 deficient mice group and control mice group are defended against another challenge with virulent Candida albicans, even though, TLR2 deficient mice group exhibit reduced production of antibody against less virulent Candida species than control mice group [50]. Several reports suggested that, TLR2 mediated sensing of Candida species can be detrimental for the host species, as TLR2 deficient mice show reduced production of IL-10 and elevated secretion of IFN-γ and IL-12 and due to this, TLR2 deficient mice exhibit more resistance against Candida species infection than control group [53,54].
In viruses, nucleotides act as PAMPs and are sensed by several TLRs. Viral nucleic acids like DNA is sensed by TLR9, dsRNA is sensed by TLR3, and ssRNA is sensed by TLR8-TLR7 [7]. Viral DNA rich in CpG domains are sensed by TLR9 [39,43,55,56]. The TLR-mediated recognition of viral nucleotides stimulates the secretion of cytokines and IFN type-1 [57].
TLR4 and TLR2 are the cell surface TLRs which detect viruses. Respiratory syncytial virus (RSV) fusion proteins are sensed by TLR4 and initiate secretion of cytokines. TLR4 also senses envelop protein of mouse mammary tumor virus (MMTV). TLR4 deficient mice exhibit reduction in IL-12 secretion and reduced levels of mononuclear cells infiltration leading to the reduction in viral clearance [39]. Inactivated H5N1 avian influenza virus causes acute lung injury into mice and TLR4 deficient mice group is defended against the injury. Oxidative stress recognized by TLR4 also play role in inflammation [58]. Viral products like Herpes simplex virus 1 (HSV1), Human cytomegalovirus (CMV) and Measles virus hemagglutinin (MV-H) glycoproteins are recognized by TLR2 and produce inflammatory cytokines [2]. Moreover, TLR2 also initiate IFN type-1 production in cell type specific manner. After infection with inactivated vaccinia virus (VV), dendritic cells and macrophages produce inflammatory cytokines but not IFN type-1 via TLR2 whereas inflammatory monocytes induce IFN type-1 through TLR2 [59]. Furthermore, TLR2 mediated stimulation of IFN type-1 does not need nucleotides [60].
TLR3 induces secretion of IFN type-1 and inflammatory cytokines needed for virus elimination. However, there are conflicting reports regarding the demand of TLR3 in in vivo conditions for protection against viral infection. When TLR3 deficient mice group is infected with mouse cytomegalovirus (MCMV), it exhibits depletion in IFN type-1, IFN-γ and IL-12p40 secretion and depletion in activation of Natural Killer and Natural Killer T cells. Due to this, TLR3 deficient mice group is more vulnerable to viral infection than control mice group [61]. Negishi et al., reported that, in Coxsackie B3 virus (CVB3) infection, TLR3 dependent IFN-γ signalling is required for protection against the infection whereas IFN type-1 signalling is not required for protection [62]. Furthermore, TLR3 deficient mice group is more vulnerable to the virulent West Nile virus (WNV) infection than the control mice group [63]. Wang et al. [271] reported that TLR3 deficient group exhibits more survival rate against the WNV infection than the control group. Although, some reports suggested that, after infection with viruses like MCMV, Vesicular stomatitis virus (VSV), Reoviruses and Lymphocytic choriomeningitis virus (LCMV), deficiency of TLR3 does not influence CD8+ T and CD4+ T cells. Thus, it can be said that TLR3 is not required for protection against viruses [64].
4. TLRs in acute lung injury/acute respiratory distress syndrome (ARDS)
Acute lung injury (ALI) is a lethal disease described by an exaggerated host defence immune response in which influx of inflammatory cells, such as neutrophils and macrophages, into the lung tissue perpetuates a vicious cycle of inflammation that amplifies the accumulation of these cells [65]. A central pathogenic feature underlying ALI is the breakdown of lung epithelial-endothelial barrier function due to widespread cell death and disassembly of endothelial adherens junctions [[65], [66], [67]]. An intact barrier tightly controls lung vascular permeability [66] and leukocyte recruitment [68], whereas its disruption results in tissue oedema, neutrophil influx, activation of procoagulant pathways, and release of proinflammatory cytokines such as IL-1β [65]. ALI may be caused by bacterial or viral infection or could be a repercussion of non-infectious outrage including hyperoxia, trauma or environmental toxins such as ozone, heavy metals. Also, lungs are invariably exposed to circulating pathogens and bacterial endotoxins such as the LPS, which are known to induce inflammatory responses and are key for the pathogenesis of ALI [69]. TLRs conciliate ALI by identifying specific PAMPs such as LPS or endogenous DAMPs such as HMGB1, heat shock proteins, fibrinogen or hyaluronan [70,71], which, in turn activates inflammation [[72], [73], [74]]. The inflammatory response could be benign or adverse for host, depends upon the nature and severity of the instigating stimulus. Alterations in macrophage polarization may also influence the severity of acute inflammation in lung [75]. It could be beneficial for the preservation of tissue integrity and repair of host tissues but prolonged inflammatory responses may lead to aggravated fibrosis and accumulation of fluid in the lungs which could be detrimental for the lung tissue [58,76]. Severe bacterial pneumonia is one of the most common causative factor of ALI, ARDS and lung injury leading to enhanced pneumonia associated mortality and morbidity. TLR4 plays an important role in bacterial clearance in case of Pseudomonas pneumonia as well as Klebsiella pneumonia. This effect is mediated by GM-CSF (Granulocyte – macrophage colony stimulating factor), a pleiotropic growth factor that possess cytoprotective effects on alveolar epithelium [77]. GM-CSF is responsible for governing the intensity of inflammation by regulating the activation of Akt and Erk kinases, leading to activation of NF-κβ and AP-1transcription factor complex respectively. Moreover, suppression of GM-CSF has direct effects on activation of these kinases, generating anti – inflammatory effects, which could have clinical implications too in inflammatory diseases [78].
It has been found that Hemorrhagic shock (HS), customarily seen in trauma patients leads to excessive inflammatory responses resulting in development of ALI [79]. Studies based on mouse models of HS-induced ALI with LPS as the secondary stimulus have demonstrated that increased secretion of inflammatory mediator CXCL8, pulmonary oedema and enhanced neutrophils incursion are a result of cross talk between TLR2 and TLR4 [73,79,80]. Similarly, TLR4 is implicated in protection against hyperoxia-induced ALI by promoting the expression of Bcl-2 and Phospho Akt (anti apoptotic factors) [81]. On the other hand, TLR3 promotes ALI as evident from study demonstrating that TLR3 deficient mice show reduced neutrophil influx, increased expression of surfactant proteins and stimulation of pro-apoptotic factors which protects them from ALI [82]. Furthermore, TLR2 and TLR4 are also involved in mediating macrophage inflammatory responses to hyaluronan fragments in case of Bleomycin-induced lung injury [83].
TLR7 has been associated with production of IFN type-1, important for clearance of viruses such as the severe acute respiratory syndrome coronavirus (SARS CoV). TLR4 and MyD88 also possess a protective role in case of SARS infection [84,85]. TLR7 also plays a significant role in case of influenza infection. They are expressed on pDCs and mediate cell specific immune response via MyD88-dependent IFN induction. B-cell isotype switching is also observed in case of influenza infection and is mediated by MyD88 and IPS-1 (adaptor molecule involved in cytosolic RIG-I pathway) [86]. TLR3 is seen to be highly upregulated in human alveolar bronchial and epithelial cells during influenza infection. Moreover, RIG-I, NLRP3 and NOD-2 have also been found to be associated with immune response against influenza infections [[87], [88], [89], [90], [91]].
5. TLRs in cystic fibrosis
Cystic Fibrosis, an autosomal recessive genetic disorder is described by chronic inflammation, caused by prolonged bacterial colonization. Acute pneumonia or chronic lung disease with sharp, intermittent aggravations, as a result of Pseudomonas aeruginosa infection are the major causes of mortality and morbidity in cystic fibrosis [[92], [93], [94], [95]]. It has been observed that functional TLRs are expressed on both CF and non-CF lung epithelial cells but does not affect the susceptibility of chronic and gradual P. aeruginosa infection [96]. This has been confirmed by a study in which expression of surface TLR1 to TLR5 and TLR9 was observed in both human CFTE29o (CFTR) and 16HBE14o (bronchial non CF) cell lines [96]. TLR6 mRNA was found to be expressed in both cell lines but only CF cells retorted to MALP-2, a TLR2/TLR6 agonist [96]. Recently, it has been shown that bacterial flagellin and expression of TLR5 plays a significant role in enhanced inflammatory responses, observed upon stimulation of IB3-1 cells (CF airway epithelial cells) with clinical Pseudomonas isolates as compared to cell line expressing wild type CFTR gene. Moreover, peripheral blood mononuclear cells from CF patients also displayed aggravated inflammatory response upon stimulation with TLR ligands and P. aeruginosa in comparison to healthy controls. They also expressed higher levels of TLR5, suggesting the role of TLR5 in modulating host immune response via an undetermined mechanism. TLR5 was also selectively increased in airway but not circulating neutrophils from CF patients, but not in patients with Bronchiectasis and healthy controls [22]. TLR5 expression on neutrophils can be functionally related to chronic infection as they are implicated in phagocytosis, respiratory burst and flagellin dependent IL-8 secretion [22]. This association was confirmed by a study which showed abbreviated neutrophil recruitment, MCP-1 production and bacterial clearance, upon low dose stimulation with flagellated P. aeruginosa as compared to isotypic non flagellated strains [97]. In addition, TLR 4 detects LPS and TLR2/TLR4 detects ExoS toxin of P. aeruginosa [16,30,98]. Similarly, MyD88 also exhibit a momentous role during the early phase of inflammation as loss of MyD88 may confer hyper-susceptibility, enhanced bacterial load, reduced neutrophil influx while TLR deficiency does not collogue susceptibility to P. aeruginosa infection [97,99,100]. TLR2 and TLR4 signal via MyD88 dependent and independent pathways, whereas TLR5 signals via MyD88 exclusively. However, the complicated pathogenesis of P. aeruginosa infection leads to inconsistent results in studies for determination of relative contribution of TLR2, TLR4 and TLR5 [101].
Other than P. aeruginosa, Staphylococcus aureus and Burkholderia cenocepacia are also correlated with early and progressive lung disease associated with CF, respectively [102,103]. Severe pneumonia and sepsis in CF patients is a result of B. cenocepacia infection and stimulates damage to lung epithelial cells along with secretion of TNF-α [104,105]. Higher mortality has been observed due to flagellin mediated activation of TLR5 and MyD88 in case of B. cenocepacia infection [105]. MyD88 plays a very important role in inflammation associated with B. cenocepacia also, as demonstrated by a study which showed that loss of MyD88 decreased inflammation and mortality of mice despite higher loads of infection [106].
6. TLRs in chronic obstructive pulmonary disease (COPD)
COPD is a respiratory disorder designated by irreversible limited expiratory airflow and aberrant inflammation [4]. Smoking is the most provocative risk factor for COPD in humans and the disease prevalence is age associated [107]. Disease pathogenesis is not well appreciated but impaired function of alveolar macrophages, defective mucociliary clearance and aberrant expression of airway antimicrobial polypeptides are customarily observed in COPD [108]. Consequently, there is a gradual loss of lung function, due to an atrocious circle of inflammation generated upon establishment of pathogens in the lower respiratory tract [109]. Evidences have suggested that a typical innate immunity contributes to the disease pathogenesis [110]. Recent studies show that TLR4 perpetuates the lung oxidant/antioxidant balance by regulating the expression of NADPH oxidase 3 in structural cells. This was confirmed when increased oxidant stress, elastolysis and tissue necrosis (all features of age-related emphysema) were observed in TLR4 and MyD88 deficient mice. Thus, it was also hypothesized that free radicals and oxidants of tobacco smoke may supersede innate immunity, resulting in cell necrosis and damage to lung tissue [111]. A brief exposure of cigarette smoke leads to TLR4, MyD88 and IL-1R1 mediated neutrophilic airway inflammation in mice [112,113]. However, C3H/HeJ mice having naturally impaired TLR4 signalling establish less chronic inflammation after cigarette smoke exposure for 5 weeks [114]. Cigarette smoke exposure in for the long haul induced strain dependent emphysema in mice, while no association with TLRs was established [115]. There are several studies discussing the association of TLR function and expression in smokers, non-smokers and COPD patients. One of the studies has shown that the expression of TLR2 reduces in alveolar macrophages of smokers and COPD patients upon ex vivo ligand stimulation. However, the relative expression of TLR4 was insignificant. Other study reported the commensurate level of expression of TLR2, TLR4, CD14 or MD2 but stimulation of alveolar macrophages with ligands of both TLR2 or TLR4 decreases the expression of mRNA and protein of inflammatory cytokines (such as IL-6, TNF-α, IL-1β) and chemokines (such as RANTES and IL-8), exhibit defective NF-κβ activation, concealed IRAK-1 and p38 phosphorylation in smokers [116]. Therefore, it was suggested by authors that TLR2 and TLR4 mediated inflammatory response is dependent upon exposure time of LPS via cigarette smoking that selectively reprograms alveolar macrophages [117]. Another study reported decreased expression of TLR2 mRNA with no changes in TLR4 expression in nasal and tracheal epithelial cells of smokers in comparison to non-smokers [118]. However, expression of TLR4 and HBD-2 (an inducible antimicrobial peptide) is found to be increased in case of mild COPD. On the other hand, advanced COPD reduces their levels of expression [118]. Thus, several studies have been carried out to determine the inflection of TLR expression upon exposure to cigarette smoke and it was concluded that various factors such as degree of bacterial colonization, content of LPS in cigarette smoke and persistent inflammatory conditions, account for modulation of levels of TLR expression. Hence, a more powerful host response in case of mild COPD corresponds to enhanced TLR4 expression, and reduced expression in case of severe COPD can be correlated with impaired innate immunity [118].
Moreover, cigarette smoke gaseous and particulate phase contains 4500 or more components encompassing noxious particles, reactive chemicals and free radicals and an overly studied risk factor for COPD. The components of cigarette smoke instigate and augment immune cells such as the macrophages [119]. Damage to lung tissue occurs due to hypersecretion of mucus, mucociliary malfunction and inflammation. The cigarette smoke provocative response accumulates neutrophils, monocyte, dendritic cells, proinflammatory mediators, ROS, factors attracting T-lymphocyte and proteolytic enzymes. These responsive features are crucial players in COPD [120,121].
Toll like receptors associated to cigarette smoke-induced inflammation are TLR2, TLR4 and TLR9. An extensive research has been done on TLR4 function such as by Karimi et al., who showed TLR4 involvement in cigarette smoke-persuaded cytokine production [122]. Sarir et al. revealed that TLR4 surface manifestation is reduced upon brief exposure of cigarette smoke [123]. Pace et al. corroborated TLR4 involvement in cigarette smoke-inciting CXCL8-lung inflammation employing epithelial cells [124]. Maes et al. demonstrated that on sub-acute cigarette exposure neutrophils, lymphocyte levels in BALF were reduced in TLR4 knockout mice when compared with control wild type mice [115]. TLR4 role in COPD evidence comes from a population study which reported connotation between TLR4 polymorphism (TLRD299G, TLR2R753Q, TLR4T3991) and in 240 heavy smokers COPD development was investigated [125]. Deteriorated polymorphic TLR4T3991 contribute to COPD development in smokers, an exposition to upsurge susceptibility to infections whereas polymorphism of D299G TLR4 has been linked to reduce COPD severity due to LPS hypo reactivity. To fully explicate the function of TLR-polymorphism in COPD, more research is needed. Deficiency of TLR4 in mice results in pulmonary emphysema [111]. This shows TLR4 participation in healthy tissue homeostasis. Overall, above knowledge summarizes TLR4 mediated inflammation generated by components of environment as well as deregulated TLR4 in disease.
TLR2 altered function and expression, upon exposure of cigarette smoke has also been linked to COPD. When COPD patients and cigarette smokers were compared with wild type, a decreased expression of TLR2 on macrophages were observed [117]. Moreover, mRNA and protein expression of TLR2 was not elevated after triggering macrophages with LPS in smokers and COPD patients. Howbeit, Pons et al. reported increased TLR2 expression on monocytes from COPD patients [126]. This shows disagreement with the Droemann et al. findings [117], but Pons study was on peripheral blood mononuclear cells (PBMCs), signifying a dissimilarity in TLR2 expression in terms of alveolar and systemic effects.
Role of TLR9 in cigarette smoke-persuading signalling has been elaborated by a group who established in vitro, implication of TLR9 in cigarette-induced production of CXCL8 by neutrophils and plasmacytoid dendritic cells [127]. Evidence supporting role of TLR9 instigation in cigarette smoke-persuading inflammation invent from a study in which transfected cell line TLR9 human embryonic kidney (HEK) were used. TLR9 deficient cells showed decrease production of CXCL8. Synthetic CpG-oligodeoxynucleotides (ODNs), a TLR9 ligand targeting mice lung gave rise to infiltration of neutrophils, systemic inflammation along with increased pulmonary permeability [128,129]. This oligonucleotide inciting inflammation has been implied to be based on TNF-α. TLR9 was also found to be rodent specific due to increased transcription of TLR9 in rodent lung [130]. On the other hand, LPS treatment can upregulate TLR9 expression in lungs of horses. Henceforth, in non-rodent mammals, the expression of TLR9 and CpG-ODNs response might increase due to specific co-stimulatory patterns [131]. TLR9 stimulation induces various host defence genes expression, including IFN-α/β, which is required for antimicrobial immunity. Howbeit, on exposure of cigarette smoke extract, this effect is suppressed in vitro. Surprisingly cigarette smoke stimulates TLR9 mediated IL-8 expression and influx of neutrophils implying that ongoing smoking potentiates detrimental inflammation in airways in response to infection, in addition to impaired host airway defence.
Among viral ligands, TLR4 also interacts with viral pneumococci toxin pneumolysin [132] However, TLR4 interactions seems to be specific for nasopharynx as indicated by a study in which TLR4 deficient mice possessed very mild impairment of host immune response, upon direct infection of lower respiratory tract with pneumococcal toxin [133,134]. Pneumococcal infection also upregulates the expression of TLR2 and aggravates the host inflammatory response [135,136]. Other PRRs are also involved in case of Streptococcus pneumoniae infections as indicated by a study wherein there is clearance of high and low infectious doses of S. pneumoniae with a moderate reduction in production of inflammatory mediators in TLR2 deficient mice [134,137]. TLR4 recognizes pneumolysin, an intracellular toxin secreted by Streptococcus pneumonia. Despite TLR2 plays a decisive role in host defence against gram positive bacteria, nevertheless S. pneumoniae can be perceived by TLR4, even in the absence of TLR2 [138]. TLR9 also plays a role, as TLR9 deficiency increases the susceptibility to pneumococcal infection [139]. On the contrary, reduced production of inflammatory mediators (IL-6 and TNF-α), unconstrained systemic bacterial dissemination and airway pneumococcal growth has also been observed in case of rescission of MyD88 signalling [140]. The severity of response against S. pneumonia is enormously increased in case of MyD88 deficiency rather than single or combined deletion of TLR2 and TLR4 respectively [141].
COPD aggravations are also associated with non-typeable H. influenza (NTHi) infection. Although both TLR2 and TLR4 ligands are involved, but TLR4/MyD88 is the most assertive immune signalling pathway involved in in vitro and in vivo bacterial clearance. TLR3 also exhibit a role in production of inflammatory mediators but their bestowal in bacterial clearance is ambiguous [142].
7. TLRs in asthma
Asthma is a deadly disease that causes chronic airway inflammation described by discursive bronchoconstriction, hyperplasia of goblet cells, excessive secretion of mucus and tissue remodelling that eventuates in childhood. Host immune response against environmental stimuli of asthma i.e. pollen or dust particles constitutes the inhabitance of antigen specific Th2 cells secreting antigen specific IgE in lung [143,144]. Innate immunity plays a significant role in pathogenesis of disease as viral and bacterial infections can be correlated with stimulation or defence against asthma [145]. It has been reported by several epidemiological, human and animal studies that time and degree of LPS exposure and hence activation of TLR4 determines the type of response i.e. whether a defensive Th1 response or an acquiescent Th2 response is generated [146]. This can be illustrated as Th2 response develops upon intranasal administration of low dose of LPS in lung whereas a robust Th1 response is generated upon administration of LPS elsewhere in the body. Nonetheless, airway hyperresponsiveness, allergic sensitization and eosinophilic inflammation is repressed upon treatment with TLR agonists or microbes [[147], [148], [149]]. Recent studies have shown that intranasal administration of Acinetobacter lwoffii F78 into pregnant mice provides protection against ovalbumin-induced asthma in the progeny. The protective effect depends upon maternal pattern of TLR expression and recognition of microbes by maternal TLRs during pregnancy primes the foetal lung environment for a later Th1 response against these antigens [150]. TLR4 expressing lung resident cells recognize house dust mite (a pervasive home allergen) and induces Th2 cell response to produce IL-25, IL-33, granulocyte-macrophage colony stimulating factor and thymic stromal lymphopoietin. These cytokines leads to activation and polarization of ingenuous lymphocytes. Also, neurotoxin derived from eosinophil stimulates TLR2 activation, that eventually leads to enormous secretion of IL-6, IL-10 and Th2 polarization [152]. Similarly, basophils also lead to Th2 cell activation [153].
TLRs are also implicated in augmentation of allergic asthma as shown by numerous genetic association studies. For instance, TLR7 and TLR8 are correlated with progression of asthma whereas their ligands safeguard against airway remodelling in experimentally lured asthma [154]. The ligands for TLR10 are not defined yet, but their SNPs share correlation with asthma in two independent samples. TLR4 and TLR9 are associated with wheezing, while TLR4 is related to secretion of allergen specific IgE [155]. Hence, TLR9 ligands are in clinical trials for prevention or treatment of asthma at present [156]. Furthermore, bacterial infections such as Mycoplasma pneumonia, Chlamydia pneumoniae and Streptococcus pneumonia may worsen the disease conditions [157]. It was shown that MyD88 deficient mice infected with C. pneumonia exhibited reduced cytokine and chemokine expression, deferred recruitment of CD4+ and CD8+ cells, and inability to clear lung bacterium resulting in chronic inflammation and high mortality [158]. However, secretion of IL-1β, IFN-γ and other inflammatory mediators may be enhanced at later stages through MyD88 independent pathway but that are incompetent to preclude C. pneumonia associated mortality [159].
TLR2 and TLR4 either act in concert or other PRRs are also involved in C. pneumoniae infection as indicated by normal recovery of TLR2 and TLR4 deficient mice with proper bacterial clearance from C. pneumoniae infection [160]. M. pneumoniae infection leads to hypersecretion of airway mucin and upregulation of TLR2. However, TLR2 is inhibited during allergic inflammation caused by M. pneumoniae infection, along with reduced production of IL-6 and other mediators, needed for bacterial clearance [161]. Antibiotic treatments are highly effective in treating asthmatic patients with M. pneumoniae infections as they improve their pulmonary function and are suggestive of the contributions of bacterial interactions with the host immune system in asthma aggravations and mortality [162,163]. Other PRRs are also involved in case of Streptococcus pneumonia infections as indicated by a study wherein there is clearance of high and low infectious doses of S. pneumonia with a moderate reduction in production of inflammatory mediators in TLR2 deficient mice (Cite reference [118,121]). The severity of response against S. pneumonia is enormously increased in case of MyD88 deficiency rather than single or combined deletion of TLR2 and TLR4 respectively (cite reference [124]).
Viruses such as Respiratory Syncytial Virus (RSV) also contribute to development and aggravations associated with asthma. RSV, in particular causes acute bronchiolitis and wheezing in children, that subsequently progresses into asthma [164,165]. Wheezing due to serious infection with RSV in early years of life is associated with enhanced Th2 response, IL-10 secretion and eosinophilia [166,167]. As a result, TLRs are found to be extensively upregulated in human tracheal epithelial (9HTEo) cells. In the course of RSV infection, virus attaches to the lung epithelial cells via G protein, followed by fusion of viral envelope with host cell plasma membrane mediated by F protein [168,169]. TLR4 recognizes the viral F protein and stimulate NK cells [164,170]. Thus, TLR4 deficient mice possess elevated viral load and defective NK cells [171,172]. According to a study, inadequate TLR4 signalling is also associated with enhanced worsening in pre-term infants [173]. Additionally, dsRNA formed during RSV replication stimulates TLR3 mediated signalling in human lung fibroblasts and epithelial cells, resulting in exaggerated secretion of the chemokines RANTES and IP-10 [170]. Deficiency of TLR3 signalling leads to enhanced mucus secretion, airway eosinophilia and increased expression of IL-5 and IL-13. Recently, it has been demonstrated that expression of IFN-β induced by RIG-I, in the time of RSV infection, brings about TLR3 activation, suggesting the role of TLR3 in mediating secondary immune signalling pathway. Contrastingly, RSV viral clearance is totally mediated by TLR2/TLR6 heterodimers. Thus, allergic asthma can be mediated by infection and worsened by genetic predispositions. These infections may lead to protective inflammatory responses in some cases or acute allergic responses in other cases or may be permissive towards prolonged Th2 response.
8. TLRs in lung cancer
Lung cancer is one of the foremost causes of cancer associated deaths all over the world. Inflammation is one of the hallmarks of cancer and tumor microenvironment also consists of cells that play highly significant roles in inflammation. These cells thus, actively participate in innate immune responses, wherein TLRs also play key roles. TLRs present on cells of the innate immune system are activated upon recognition of specific ligands and induce host adaptive immune responses consequently. Thus, TLRs act as a double edged sword where on one side identify cancer specific antigens and activate innate responses and on the other side, promote latent chronic inflammation by inducing persistent adaptive responses which promote the process of tumorigenesis [[174], [175], [176]]. Since TLRs are extensively expressed on resident as well as infiltrating myeloid and lymphoid cells (responsible for activation of adaptive responses), the maintenance of a fine equilibrium between stromal and haematopoietic cells become very crucial in case of cancer. TLR activation on both types of cells calls for recruitment of immune stimulating or tolerogenic cells, both of which participate in the process of oncogenesis. This can be explained as - activation of TLRs present on epithelial cells induce production of chemokines (such as IL-8, a strong neutrophils chemoattractant) and growth factors such as the Vascular endothelium-derived growth factor (VEGF) that is a key factor of angiogenesis [[177], [178], [179], [180]]. Similarly, TLR activation on innate immune cells helps in processing of antigens and their presentation, promotes the expression of CD80/86 (co-stimulatory molecules) which are involved in activation of T cells and finally secretes IL-6 that modulates the activity of Treg cells [181,182]. Activation of TLRs also promotes the production of IL-12p19 or IL-12p35 and IL-27, favouring Th1 or Th17 immunity, respectively [183]. Th1 exerts anti tumor immunity while Th17 can possess either pro-tumor or anti tumor activity, that is tissue or organ dependent [184]. One of the study has demonstrated the anti tumor activity of Th17 immunity in IL-17 knockout mice [185]. Thus, Th1 response generates a protective response, however, Th2 response bedews the host immune response, promoting latent chronic inflammation. This chronic inflammation can lead to the proliferation of transformed cells that can evade the immune system in cancer conditions. Interestingly, Treg cells are involved in these alterations of Th2 responses and hence play crucial roles in tumor immunology, determining the destiny of lung cancer cells. Moreover, several studies have displayed the role of TLRs in cancer development and progression [177]. Nonetheless, the debate on their protective role in immune system activation to fight against cancer and their cancer promoting role by facilitating chronic inflammation is still on [186].
As in other cases, TLRs expressed on dendritic cells play equally important roles in lung cancer too as upon TLR ligation and activation, they act as guards to look for antigens which are then presented and processed by T cells to generate adaptive immune responses. Thus, they act as a bridge between innate and adaptive immune system [187]. TLR 1 to TLR9 are expressed on both human and mouse myeloid dendritic cells, but their roles in lung carcinoma have not been elucidated yet. pDCs express TLR7/8 and TLR9 responsible for the production of IFN-α/β in MyD88 dependent manner [188,189]. IFN type-1 plays significant role in CD8+ T cell cytotoxicity, NK cell activation and proliferation, production of cytokines and TRAIL-mediated cell death [190], but their functions in lung carcinoma have not been defined yet. However, it is speculated that tumor microenvironment debilitates their anti tumor functions [191]. In addition, TLR2, TLR4, TLR7/8 and TLR9 are highly expressed in lung carcinoma tissues, and stimulate the production of IFN type-1 and IFN-γ, which in turn, promotes the production of IL-10, TGF-β (immunosuppressive cytokines) and tolerogenic enzyme indoleamine-2,3-dioxygenase (IDO) [189,192]. As a consequence, these cytokines and tolerogenic enzyme induces the production of adaptive and natural Tregs, involved in host immune alterations [193]. pDCs bind Treg through PD-1/PD-L1 (immune checkpoint regulators) while TGF-β (induced by TLR4, TLR7 and TLR9) stimulates production of Treg or Th17 [194,195]. TLR9 activation by CpG-ODN enhances lung metastasis in IL-17 knockout mice, however tumor burden reduces in other tumor models [185]. This discrepancy could be due to tissue specificity.
On the other hand, B cells express TLR7 and TLR9 in an enormous level. TLR9 binds to CpG and causes B-cell polarization to acquire B-1 phenotype, which promotes tumor regression in mice [189,196]. However, in other study B cells act as antigen presenting cells and immunoglobulin producing cells, leading to tumor regression in mouse model [197]. Moreover, roles of other TLRs in B-cell derived immunity needs further elucidation. Similarly, lung macrophages express high levels of TLR2, TLR3, TLR4 and TLR6 as compared to TLR7 and TLR9. Tumor macrophages are polarized to M2 phenotype, upon TLR stimulation. These macrophages are termed as Tumor associated macrophages (TAMs), expressing high levels of TLR2. Stimulation of TLR2 signalling pathway promotes lung metastasis [198]. Likewise, TLR4 signalling in TAMs also increases cancerous lesions [199]. CpG-ODN (type B) activation of TLR9, recruits an enormous amount of macrophages into the lungs of mice poised with tumor [200]. Deficiency of MyD88 reduces tumor growth owing to reduced incursion of macrophages to the tumor microenvironment [201]. MyD88 mediated signalling activates M2 phenotype while TRIF dependent signalling, strongly induced by TLR3 activation, stimulates M1 phenotype of macrophages. TLR activation on macrophages and myeloid-derived suppressor cells (MDSCs) repress immune surveillance of tumor cells [202]. It was confirmed in a recent study wherein TLR2 activation on MDSCs promotes tumor immune evasion, thereby enhancing tumor growth in lung adenocarcinoma, lymphoma and colon carcinoma [203]. Additionally, MDSCs are recruited to the lung upon administration of CpG-ODN (ligand of TLR9) into the mice bearing tumor [197]. Thus, MDSCs and Tregs, both have important contributions in progression of lung tumors and are swayed by activation of TLRs; elucidation of their roles in lung cancer would be therapeutically very beneficial.
NK cells, one of the leading effector cells against tumors are scarcely activated by MyD88 dependent pathway, but TRIF dependent pathway lures their cytotoxicity. It has been found that absence of TRIF mediates tumor progression upon administration of Poly I:C (a ligand of TLR3) [204]. However, in case of prostate cancer, Poly I:C administration regresses tumor growth as activation of TLR3 can also result in apoptotic cascade [205]. TLR7 and TLR9 signalling reduces upon viral infection [206], thereby reducing innate immune response in the course of tumorigenesis. NK cells are also activated upon activation of TLR2-MyD88 on mDCs. However, reduced lung metastases have been observed due to NK cell activation upon administration of ligands of TLR3, TLR7/8 [[207], [208], [209]]. Similarly, mast cells are also present in several tumor types and the activation of mast cell receptors and TLRs stimulate a number of growth stimulatory mediators and pro-angiogenic factors that enhance tumor proliferation. Contrastingly, TLR2 activation on mast cells reduced lung carcinoma growth upon subcutaneous implantation of LLC1 cells in mouse model [210]. Oldford et al. have demonstrated that TLR2 activation on mast cells facilitate release of IL-6 and CCL1, responsible for consequent increase in antitumor activities and recruitment of NK and CD8+ cytotoxic T cells [200,211]. Although lung cancer bears Th2 based pathology, the role of mast cells in tumor progression remains elusive.
Development and progression of lung cancer involves anti-inflammatory cytokines such as TGF-β [212], IL-10 and growth factors such as VEGF and Fibroblast growth factor 2 (FGF2), which are induced by activation of TLR2, TLR4 and TLR9. In lung epithelial cells, TGF- β1 has been recognized to facilitate apoptosis and inflammation [212]. However, TLR2 and TLR4 activation induces extracellular matrix remodelling and EGFR-mediated signalling, respectively, stimulating progression of lung carcinoma [213,214]. Exceptionally, TLR9 signalling along with cetuximab (an inhibitor of EGFR signalling) regresses tumor growth [215]. However, activation of TLR9 by CpG-ODN promotes the growth of human tumor epithelial lung cells [216]. Further, TLR9 activation induces release of VEGF, thereby promoting the growth of tumor cells [217]. The role of TLR3 and TLR7 signalling has not been yet explored in lung carcinoma. Although, TLR3 and TLR7 activation induces apoptosis and hence facilitate tumor regression in prostate and melanoma models of mouse as well as in in vitro studies conducted on human lung cancer cell line treated with imiquimod, a TLR7 ligand [218]. VEGF released from CpG-ODN induced lung carcinoma, acts as a pro-tumor factor, expediting the angiogenesis of cancer cells and recruitment of inflammatory cells, favouring anti-tumor activity [213,217,219]. Thus, VEGF may act as both pro-tumorigenic and anti-tumorigenic. More significantly, TLR2 and TLR4 activation also causes the extradition of extracellular matrix proteins such as fibrin and hyaluronan, which may act as DAMPs, exaggerating the inflammatory processes [213,220]. Pro-inflammatory cytokines such as IL-1, IL-6 and chemokines such as IL-8, and ICAM-1 are released upon activation of TLRs present on endothelial cells, and bolsters the recruitment of leukocytes to the lung [221]. Surprisingly, endothelial cells upregulate PD-1 and secrete high levels of IL-10, TGF-α, TGF-β and IFN-γ, thereby promoting recruitment of immunosuppressive cells such as pDCs which interacts with Tregs, to the lung, and hence enhance the immunosuppressive abeyants of activated Tregs [222]. Immunosuppressive effects could also arise from activation of MDSCs mediated by MyD88 signalling. These MDSCs are also associated with release of DAMPs such as HMGB1, vimentin, hsp72 into the tumor microenvironment. HMGB1 is recognized by TLR4 and TLR9, whereas hsp72, vimentin and HMGB1, all act as ligands for TLR2, which upon activation initiate a series of inflammatory processes associated with cancer development and progression [176,203].
9. TLRs as therapeutic targets
Extensive studies over the years have revealed a broad array of role played by TLRs in activation of innate immune response against various infectious agents and intrinsic molecules suggesting its potential as a therapeutic target. Dysregulation of TLR in induction of pro-inflammatory signalling cascade leads to continued cytokines and chemokines secretion. This makes TLRs a causative reason for several diseases such as auto-immune disorders, systemic lupus erythematosus (SLE), asthma, allergic rhinitis and ischemia reperfusion injury [223]. Consequently, TLRs are being targeted in a dual way - by development of small molecule ligands (agonists) which have the ability to bind with TLRs and augment host immune capabilities and also act as adjuvants in vaccine therapies and by inhibiting TLRs through specific antibodies, small molecule inhibitors, oligopeptides and by improving endogenous anti-TLR molecules. Also, negative regulation of signalling pathways involving TLRs are also gaining interest as a novel therapeutic strategy. Apart from infectious and inflammatory diseases, targeting TLRs have demonstrated promising effects in non-infectious diseases, including lung airway disease, ALI, and interstitial lung disease (ILD) [224].
9.1. TLR agonists
Emerging data have shown that TLRs like TLR2, TLR3, TLR4, TLR5, TLR7 and TLR9 are linked with various diseases and could be activated by agonists making them compelling immunomodulators and adjuvants. These agonists are catalogued either as cell surface or intracellular molecules on the basis of TLRs location. TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are cell surface TLRs whereas TLR3, TLR7, TLR8 and TLR9 are included in intracellular TLRs [225]. TLR4 is unique in itself as it can be active on the cell surface and also intracellularly in specific cells [225]. The microbial component, LPS, can activate TLR4 and its signalling by inducing the formation of a symmetric M-shaped TLR4–MD-2–LPS multimer which, in turn stimulates the production of TNF-α, as well as increase the production of inducible nitric oxide synthase (iNOS) in tumor cells, making it a possible therapeutic strategy for lung cancer treatment [226]. A TLR4 agonist MPL (monophosphoryl lipid A), derived from lipid A of Salmonella minnesota is an approved vaccine adjuvant in the Europe for hepatitis B and HPV virus [227]. Further, MPL works potently as an adjuvant in allergy vaccines and is used to treat asthma as it specifically activates the TRAM/TRIF pathway in TLR4 signalling and regulates CD80/86 complex which is vital for conferring MPL vaccine adjuvant ability [228]. MPL is also recently being used in combination with other molecules to synthesize novel vaccines such as Pollinex Quattro, which is currently in a Phase III clinical trial against seasonal airway allergies [229,230]. In addition, OM-174, a TLR4 agonist which is a purified form of lipid-A developed from E. coli has demonstrated anti-tumor activity in mice models by increasing IFN-γ production [231,232]. Few chemically synthesized TLR4 agonists, for example E6020 are vaccine adjuvants [233,234]. Apart from this, the aminoalkyl glucosaminide phosphates which are lipid A derivatives, are safe and effective adjuvants for influenza virus vaccine [235,236]. In a murine model of allergic type lung inflammation, poly-γ-glutamic acid (γ-PGA) which is a TLR4 agonist is seen to cause downregulation of Th2 cytokines, airway inflammation and eosinophilia while increasing the manifestation of co-stimulatory molecules, CD80, CD86 and CD40 on dendritic cells [237]. Recently, a synthetic TLR4 agonist (ER803022) has been developed which shows potential against asthma in mouse models in a TLR-dependent MyD88 activation and IL-12/IFN-γ production [238]. TLR2 is LPS receptor and identifies several ligands especially that of Gram-positive bacteria, a synthetic TLR2 agonist known as Pam3CSK4 shows anti-asthmatic properties by reducing Th2 cytokine release, airway inflammation and other symptom. Pam3CSK4 has two thioester linked lipid chains through which it binds to a TLR2 pocket and a small channel of TLR1, hence forming a bridge between the two molecules [239]. SMP-105 is a TLR2 agonist from Mycobacterium bovis that consists of mycolic acids, and peptidoglycans is an approved drug for bladder cancer [240]. This compound upregulates NF-κβ in a TLR2 dependent and TLR4 independent manner leading to decrease in TNF-α and IL-6 levels and can be used as an adjuvant as anti-inflammatory agent [240]. Another ligand, Macrophage activating lipopeptide-2 (MALP-2) binds to TLR2/TLR6 heterodimer and is effective against asthma. It is acquired from Mycoplasma fermentans and induces IFN-γ, CD80 (B7-1), CD86 (B7-2), MHC I and II in response to allergies [241]. A study showed that in a murine model of asthma, MALP-2 causes the decrease of AHR, eosinophilia and Th2 cytokines. In addition, it stimulates T cell, B cell and natural killer cell accumulation in lungs of adult mice [242]. Similarly, Lipoprotein 1 and Lipopeptide-CGP40774 are TLR2 agonists capable of controlling asthma and airway inflammation by suppressing IgE production, IL-4 and IL-13 production [243,244]. Accumulating evidence have indicated that activating TLR7 not only suppresses Th2-mediated airway inflammation but also decreases the manifestation of IgE, additionally it significantly inhibits IL-17 and IL-13 production through IL-10 mediated pathway [245]. Therefore, TLR7 is thought to recuperate airway diseases such as asthma and allergic rhinitis. Indeed, Imiquimod is a clinically approved TLR7 agonist for basal cell carcinoma, actinic keratosis and external genital wart [246,247]. This drug also works against asthma and viral induced lung and airway infection by increasing IFN-α via TLR7–MYD88 dependent pathway. Another compound Resiquimod (R-848), a TLR7 and TLR8 agonist displayed great tendency to induce IFN-α, IL-12 and TNF-α production while reducing lung eosinophilia and airway inflammation in in vivo models [248,249]. A synthetic TLR7 agonist SA-2 regulates T cell production through IFN-α, IL-27 and IL-10 and shows promising therapeutic potential [250]. ANA773 is a small molecule TLR7 agonist which is under phase I clinical trial for its anti-viral potential [251]. One of the most investigated class of compound developed for exacerbation of asthma known as CpG-ODN, is a TLR9 agonist [252]. It interacts with TLR9 present on leukocytes, B cells and T cells and stimulates the secretion of IL-6, IFN-γ, IL-12 and CD4 T lymphocytes, in turn eliciting a Th1 type of inflammatory response along with enhanced IL-12 and IL-10 production [253]. It also suppresses Th2 cytokine, airway eosinophilia, IgE levels, and bronchial hyperreactivity in in vitro and in vivo models [[254], [255], [256]]. CpG-ODN has shown encouraging results in clinical trials against asthma and allergic rhinitis as well [257]. Other TLR9 agonists like indoleamine 2, 3-dioxygenase, IMO-2055, MO-2125 and IMO-2134 are reported to have anti-inflammatory and anti-allergic responses [258,259]. A TLR3 agonist, Rintatolimod is also being experimented for treatment of severe acute respiratory syndrome, influenza, hepatitis infections and cancer. It is reported to modulate cellular RNase L enzyme levels in patients [260].
9.2. TLR antagonists
Recent advances related to the structure and role of TLRs in normal physiology as well as in diseased conditions have led to their exploitation for developing novel therapeutic strategies. In this pursuit, TLR antagonists are being examined for treatment of various diseases mainly autoimmune disorders, although none of them have succeeded to be taken further than clinical trials. Antagonist treatment can reduce unwarranted inflammation by hindering the interaction between TLRs and pro-inflammatory ligands and their downstream signalling molecules. TLR2 and TLR4 antagonists are well studied and show great promise in preventing several diseases. Recently, a TLR4 antagonist, under acylated form of Rhodobacter sphaeroides LPS (RS-LPS) when inhaled, reduced the symptoms of asthma by inhibiting eosinophilia and lymphocytosis, also by reducing the levels of Th2 cytokines and lower airway hyperresponsiveness [261]. RS-LPS acts as a TLR4 antagonist by inhibiting the induction of IkBz (gene NFKBIZ, a member of the NF-kB family, playing a significant role in functioning of epithelial cells) in bronchial epithelial cells, consequently leading to inhibition of release of pro-inflammatory cytokines – IL6 and GMCSF in a co-culture based study of bronchial epithelial cells and monocytes stimulated with house dust mite mix (HDM) [262]. One of the few synthesized antibodies that target TLRs is NI-0101, which is specific to TLR4 and blocks TLR4 dimerization. It has the potency to reduce cytokine secretion and averts flu and its symptoms in ex vivo and in vivo models. This drug has successfully completed phase I clinical trial and is proven to be a safe and non-toxic drug at various concentrations [263]. 1A6, another anti-TLR4 monoclonal antibody is effective in reducing inflammatory disorders and has positive role in lung injury models [264]. Lipid A analogues specifically targets TLR4 signalling and has become potential therapeutic tool against airway diseases. Eritoran TM (E5564) is a TLR4 antagonist that affects TLR4/MD-2/LPS complex formation is the most advanced TLR agonist and is currently undergoing Phase III clinical trial for treatment of sepsis [265]. A fully humanized IgG4 monoclonal TLR2 specific antibody, OPN-305 blocks TLR2-mediated pro-inflammatory cytokine production in pre-clinical phase I studies [266]. Antagonists of TLR7 are also in focus for their anti-asthmatic effects along with their bronchodilatory mechanism which leads to protection from airway obstruction during viral infections. One such TLR7 antagonist is R837 that plays a critical role in relaxing the airway passage through production of nitric oxide in a TLR7 dependent manner in in vitro as well as in vivo models [267]. Recently, capsazepine and its analogues have been considered as TLR3 inhibitors and they have shown to repress the production of pro-inflammatory cytokines such as IL-8 and TNF-α in asthmatic patients [268]. Apart from synthesized compounds, a natural product known as Resveratrol that is found in grapes and peanuts down regulates TLR3 and one of its adapter molecule (TRIF) and provides a defensive mechanism against asthma development [269]. Though, antagonist therapy shows great potential, but their use can cause damage to the local immune defence and can result in development of other opportunistic disorders.
Despite the substantial amount of research going on for development of novel TLRs agonist or antagonist in the treatment of airway as well as other diseases only limited success have been achieved. The failure of these drugs at clinical trials is most probably a result of inappropriate dosage, toxicity, lack of proper time and route of administration. However, continuous effort and holistic approaches would open newer ways for utilizing these compounds as medicinal agents in the near future.
10. Conclusion
An extended exposure of lung to environmental aggravations and likely pathogens perpetuates the innate immune system to acquire a critical role in maintenance of lung tissue homeostasis. TLRs are principally involved in activation of innate immune system and an enormous amount of research has been carried out in recent years to explore the role of TLRs in host defence and tissue homeostasis. The acumens of this work have enabled us to predicate general principles associated with lung innate immunity. These principles involve development of acute pulmonary diseases (for instance – bronchiolitis and ALI) customarily lead to progression of chronic inflammatory conditions such as fibroproliferative ARDS or may lead to exposition of relapsing or remitting conditions as found in asthma. Secondly, infections (bacterial, fungal or viral) are the most prominent causes of prolonged inflammation in lung tissue, as represented by severe RSV infection preceding asthma development and progression of ARDS following severe influenza and pneumonia. Furthermore, aberrations in innate immune system lead to the evolution of chronic obstructive lung diseases. These disorders may be due to direct or indirect predilection of host to infection as in case of chronic P. aeruginosa infections leading to Cystic fibrosis, and S. pneumonia infections leading to subtle aggravations of COPD. Irrevocably, tissue remodelling, and repair are imperative for pathogenesis of lung inflammation along with host defence, and TLR mediated mechanisms reconcile these processes. Similarly, TLRs are also involved in lung cancer development and progression. Contemporary studies have demonstrated the tumor promoting as well as tumor suppressing roles of TLR signalling. Activation of TLRs on cells of tumor microenvironment can promote immune evasion by alterations of Tregs, thereby promoting tumor cell proliferation. On the other hand, they are also involved in activating immune system to generate anti-tumor immunity. Moreover, it has been reported that TLRs mediate activation of immune cells present in tumor microenvironment, which may result in anti-tumor as well as pro-tumor effects, but the role of TLRs in tumorigenic effects have not yet been fully elucidated. Although, TLR activation plays a critical role in progression of lung inflammatory diseases, there are still many questions that have to be answered. Besides this, using TLRs as therapeutic targets involve use of TLR agonists or antagonists. Few TLR agonists and antagonists have been used a therapeutic modality in case of airway diseases but success has not yet been achieved due to several reasons involving dosage toxicity and inappropriate time and route of administration. Thus, more studies are needed to explore their potential as a therapeutic strategy. Furthermore, a profound understanding of TLR associated mechanisms will help us elucidate the links between innate immune system and development of chronic inflammatory conditions in lung tissue. This knowledge will benefit us to identify TLRs as novel therapeutic targets that could limit the burden of pulmonary diseases.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
This work was supported by the Department of Science and Technology, India (No. SB/S21RJN-199/2014) and Senior Research Fellowship (Shweta Arora and Shaniya Ahmad) from Indian Council of Medical Research.
Author's contribution
MAS conceived the idea. SA, S. Ahmad, RI, YG, SR and NS performed the literature search and wrote the manuscript. MAS, KD, MH, S. Ali and AM provided inputs for the strategy and final edition of the article. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no conflict of interest associated with this manuscript.
Acknowledgements
We thank Jamia Millia Islamia for providing internet facility and access to journals. We acknowledge DST (Department of Science and Technology, India) for providing Ramanujan Fellowship. SA and S.Ahmad acknowledge ICMR (Indian council of Medical Research) for providing Senior Research Fellowship (SRF).note
References
- 1.Holt P.G., et al. Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol. 2008;8(2):142–152. doi: 10.1038/nri2236. [DOI] [PubMed] [Google Scholar]
- 2.Takeda K., Akira S. TLR signaling pathways. Semin. Immunol. 2004;16(1):3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Lafferty E.I., Qureshi S.T., Schnare M. The role of toll-like receptors in acute and chronic lung inflammation. J Inflamm (Lond) 2010;7:57. doi: 10.1186/1476-9255-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arora S., et al. Macrophages: their role, activation and polarization in pulmonary diseases. Immunobiology. 2018;223(4–5):383–396. doi: 10.1016/j.imbio.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto M., Takeda K. Current views of toll-like receptor signaling pathways. Gastroenterol. Res. Pract. 2010;2010 doi: 10.1155/2010/240365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L., Seki E. Toll-like receptors in liver fibrosis: cellular crosstalk and mechanisms. Front. Physiol. 2012;3:138. doi: 10.3389/fphys.2012.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christmas P. Toll-like receptors: sensors that detect infection. Nature Education. 2010;3(9):85. [Google Scholar]
- 9.Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? J. Leukoc. Biol. 2010;87(6):989–999. doi: 10.1189/jlb.1209775. [DOI] [PubMed] [Google Scholar]
- 10.Urbonaviciute V., et al. Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J. Exp. Med. 2008;205(13):3007–3018. doi: 10.1084/jem.20081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McClure R., Massari P. TLR-dependent human mucosal epithelial cell responses to microbial pathogens. Front. Immunol. 2014;5:386. doi: 10.3389/fimmu.2014.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basu S., Fenton M.J. Toll-like receptors: function and roles in lung disease. Am. J. Phys. Lung Cell. Mol. Phys. 2004;286(5):L887–L892. doi: 10.1152/ajplung.00323.2003. [DOI] [PubMed] [Google Scholar]
- 13.Beutler B.A. TLRs and innate immunity. Blood. 2009;113(7):1399–1407. doi: 10.1182/blood-2008-07-019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee C.C., Avalos A.M., Ploegh H.L. Accessory molecules for Toll-like receptors and their function. Nat. Rev. Immunol. 2012;12(3):168. doi: 10.1038/nri3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeuchi O., et al. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 2001;13(7):933–940. doi: 10.1093/intimm/13.7.933. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi F., et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410(6832):1099. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 17.Ryu J.-H., et al. Distinct TLR-mediated pathways regulate house dust mite–induced allergic disease in the upper and lower airways. J. Allergy Clin. Immunol. 2013;131(2):549–561. doi: 10.1016/j.jaci.2012.07.050. [DOI] [PubMed] [Google Scholar]
- 18.Zhao S., et al. Toll-like receptors and prostate cancer. Front. Immunol. 2014;5:352. doi: 10.3389/fimmu.2014.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sha Q., et al. Activation of airway epithelial cells by toll-like receptor agonists. Am. J. Respir. Cell Mol. Biol. 2004;31(3):358–364. doi: 10.1165/rcmb.2003-0388OC. [DOI] [PubMed] [Google Scholar]
- 20.Hoppstädter J., et al. Differential cell reaction upon Toll-like receptor 4 and 9 activation in human alveolar and lung interstitial macrophages. Respir. Res. 2010;11(1):124. doi: 10.1186/1465-9921-11-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andonegui G., et al. Endothelium-derived Toll-like receptor-4 is the key molecule in LPS-induced neutrophil sequestration into lungs. J. Clin. Invest. 2003;111(7):1011–1020. doi: 10.1172/JCI16510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koller B., et al. TLR expression on neutrophils at the pulmonary site of infection: TLR1/TLR2-mediated up-regulation of TLR5 expression in cystic fibrosis lung disease. J. Immunol. 2008;181(4):2753–2763. doi: 10.4049/jimmunol.181.4.2753. [DOI] [PubMed] [Google Scholar]
- 23.GeurtsvanKessel C., Lambrecht B. Division of labor between dendritic cell subsets of the lung. Mucosal Immunol. 2008;1(6):442. doi: 10.1038/mi.2008.39. [DOI] [PubMed] [Google Scholar]
- 24.Plantinga M., Hammad H., Lambrecht B.N. Origin and functional specializations of DC subsets in the lung. Eur. J. Immunol. 2010;40(8):2112–2118. doi: 10.1002/eji.201040562. [DOI] [PubMed] [Google Scholar]
- 25.Dong Z., Yang Z., Wang C. Expression of TLR2 and TLR4 messenger RNA in the epithelial cells of the nasal airway. Am. J. Rhinol. 2005;19(3):236–239. [PubMed] [Google Scholar]
- 26.Regueiro V., et al. Klebsiella pneumoniae increases the levels of Toll-like receptors 2 and 4 in human airway epithelial cells. Infect. Immun. 2009;77(2):714–724. doi: 10.1128/IAI.00852-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guillot L., et al. Response of human pulmonary epithelial cells to lipopolysaccharide involves toll-like receptor 4 (TLR4)-dependent signaling pathways: evidence for an intracellular compartmentalization of TLR4. J. Biol. Chem. 2004;279(4):2712–2718. doi: 10.1074/jbc.M305790200. [DOI] [PubMed] [Google Scholar]
- 28.Jia H.P., et al. Endotoxin responsiveness of human airway epithelia is limited by low expression of MD-2. Am J Physiol Lung Cell Mol Physiol. 2004;287(2):L428–L437. doi: 10.1152/ajplung.00377.2003. [DOI] [PubMed] [Google Scholar]
- 29.Bérubé J., et al. Distinct intracellular signaling pathways control the synthesis of IL-8 and RANTES in TLR1/TLR2, TLR3 or NOD1 activated human airway epithelial cells. Cell. Signal. 2009;21(3):448–456. doi: 10.1016/j.cellsig.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z., et al. Human airway epithelial cells sense Pseudomonas aeruginosa infection via recognition of flagellin by Toll-like receptor 5. Infect. Immun. 2005;73(11):7151–7160. doi: 10.1128/IAI.73.11.7151-7160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maris N., et al. Toll-like receptor mRNA levels in alveolar macrophages after inhalation of endotoxin. Eur. Respir. J. 2006;28(3):622–626. doi: 10.1183/09031936.06.00010806. [DOI] [PubMed] [Google Scholar]
- 32.Frank D.N., et al. The human nasal microbiota and Staphylococcus aureus carriage. PLoS One. 2010;5(5) doi: 10.1371/journal.pone.0010598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bautista M.V., et al. IL-8 regulates mucin gene expression at the posttranscriptional level in lung epithelial cells. J. Immunol. 2009;183(3):2159–2166. doi: 10.4049/jimmunol.0803022. (p. jimmunol. 0803022) [DOI] [PubMed] [Google Scholar]
- 34.Ioannidis I., et al. TLR expression and induction of type I and type III interferons in primary airway epithelial cells. J. Virol. 2013;87(6):3261–3270. doi: 10.1128/JVI.01956-12. (p. JVI. 01956-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohkuni T., et al. Poly (I: C) reduces expression of JAM-A and induces secretion of IL-8 and TNF-α via distinct NF-κB pathways in human nasal epithelial cells. Toxicol. Appl. Pharmacol. 2011;250(1):29–38. doi: 10.1016/j.taap.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 36.Wu C.A., et al. Bronchial epithelial cells produce IL-5: implications for local immune responses in the airways. Cell. Immunol. 2010;264(1):32–41. doi: 10.1016/j.cellimm.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Routray I., Ali S. Boron induces lymphocyte proliferation and modulates the priming effects of lipopolysaccharide on macrophages. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0150607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janeway C.A. Cold Spring Harbor Symposia on Quantitative Biology. Cold Spring Harbor Laboratory Press; 1989. Approaching the asymptote? Evolution and revolution in immunology. [DOI] [PubMed] [Google Scholar]
- 39.Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 40.Hoffmann J.A. The immune response of Drosophila. Nature. 2003;426(6962):33–38. doi: 10.1038/nature02021. [DOI] [PubMed] [Google Scholar]
- 41.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449(7164):819. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 42.Kawai T., Akira S. Innate immune recognition of viral infection. Nat. Immunol. 2006;7(2):131. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 43.Blasius A.L., Beutler B. Intracellular toll-like receptors. Immunity. 2010;32(3):305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 44.Takeuchi O., Hoshino K., Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 2000;165(10):5392–5396. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- 45.Gerold G., Zychlinsky A., Juana L. Seminars in Immunology. Elsevier; 2007. What is the role of Toll-like receptors in bacterial infections? [DOI] [PubMed] [Google Scholar]
- 46.Miller L.S., et al. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006;24(1):79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 47.Weiss D.S., et al. Toll-like receptors are temporally involved in host defense. J. Immunol. 2004;172(7):4463–4469. doi: 10.4049/jimmunol.172.7.4463. [DOI] [PubMed] [Google Scholar]
- 48.Saiga H., Shimada Y., Takeda K. Innate immune effectors in mycobacterial infection. Clin. Dev. Immunol. 2011;2011 doi: 10.1155/2011/347594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pathak S.K., et al. Direct extracellular interaction between the early secreted antigen ESAT-6 of Mycobacterium tuberculosis and TLR2 inhibits TLR signaling in macrophages. Nat. Immunol. 2007;8(6):610. doi: 10.1038/ni1468. [DOI] [PubMed] [Google Scholar]
- 50.Netea M.G., Maródi L. Innate immune mechanisms for recognition and uptake of Candida species. Trends Immunol. 2010;31(9):346–353. doi: 10.1016/j.it.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 51.Romani L. Immunity to fungal infections. Nat. Rev. Immunol. 2004;4(1):11. doi: 10.1038/nri1255. [DOI] [PubMed] [Google Scholar]
- 52.Bellocchio S., et al. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J. Immunol. 2004;172(5):3059–3069. doi: 10.4049/jimmunol.172.5.3059. [DOI] [PubMed] [Google Scholar]
- 53.Netea M.G., et al. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J. Immunol. 2004;172(6):3712–3718. doi: 10.4049/jimmunol.172.6.3712. [DOI] [PubMed] [Google Scholar]
- 54.Sutmuller R.P., et al. Toll-like receptor 2 controls expansion and function of regulatory T cells. J. Clin. Invest. 2006;116(2):485–494. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gilliet M., Cao W., Liu Y.-J. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 2008;8(8):594. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 56.Reizis B., et al. Plasmacytoid dendritic cells: recent progress and open questions. Annu. Rev. Immunol. 2011;29:163–183. doi: 10.1146/annurev-immunol-031210-101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kawai T., Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 58.Imai Y., et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133(2):235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barbalat R., et al. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat. Immunol. 2009;10(11):1200. doi: 10.1038/ni.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi Z., et al. A novel Toll-like receptor that recognizes vesicular stomatitis virus. J. Biol. Chem. 2011;286(6):4517–4524. doi: 10.1074/jbc.M110.159590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tabeta K., et al. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc. Natl. Acad. Sci. 2004;101(10):3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Negishi H., et al. A critical link between Toll-like receptor 3 and type II interferon signaling pathways in antiviral innate immunity. Proc. Natl. Acad. Sci. 2008;105(51):20446–20451. doi: 10.1073/pnas.0810372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Daffis S., et al. Toll-like receptor 3 has a protective role against West Nile virus infection. J. Virol. 2008;82(21):10349–10358. doi: 10.1128/JVI.00935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edelmann K.H., et al. Does Toll-like receptor 3 play a biological role in virus infections? Virology. 2004;322(2):231–238. doi: 10.1016/j.virol.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 65.Ware L.B., Matthay M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 66.Mehta D., Malik A.B. Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 2006;86(1):279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 67.Gong H., et al. HIF2alpha signaling inhibits adherens junctional disruption in acute lung injury. J. Clin. Invest. 2015;125(2):652–664. doi: 10.1172/JCI77701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nourshargh S., Alon R. Leukocyte migration into inflamed tissues. Immunity. 2014;41(5):694–707. doi: 10.1016/j.immuni.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 69.Maniatis N.A., Orfanos S.E. The endothelium in acute lung injury/acute respiratory distress syndrome. Curr. Opin. Crit. Care. 2008;14(1):22–30. doi: 10.1097/MCC.0b013e3282f269b9. [DOI] [PubMed] [Google Scholar]
- 70.Park J.S., et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 2004;279(9):7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 71.Tsung A., et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J. Exp. Med. 2005;201(7):1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaczorowski D.J., et al. Early events in the recognition of danger signals after tissue injury. J. Leukoc. Biol. 2008;83(3):546–552. doi: 10.1189/jlb.0607374. [DOI] [PubMed] [Google Scholar]
- 73.Xiang M., Fan J. Pattern recognition receptor-dependent mechanisms of acute lung injury. Mol. Med. 2010;16(1–2):69–82. doi: 10.2119/molmed.2009.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Opitz B., et al. Innate immune recognition in infectious and noninfectious diseases of the lung. Am. J. Respir. Crit. Care Med. 2010;181(12):1294–1309. doi: 10.1164/rccm.200909-1427SO. [DOI] [PubMed] [Google Scholar]
- 75.Syed M.A., Bhandari V. Hyperoxia exacerbates postnatal inflammation-induced lung injury in neonatal BRP-39 null mutant mice promoting the M1 macrophage phenotype. Mediat. Inflamm. 2013;2013 doi: 10.1155/2013/457189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hollingsworth J.W., et al. The role of Toll-like receptor 4 in environmental airway injury in mice. Am. J. Respir. Crit. Care Med. 2004;170(2):126–132. doi: 10.1164/rccm.200311-1499OC. [DOI] [PubMed] [Google Scholar]
- 77.Standiford L.R., et al. TLR4-dependent GM-CSF protects against lung injury in Gram-negative bacterial pneumonia. Am J Physiol Lung Cell Mol Physiol. 2012;302(5):L447–L454. doi: 10.1152/ajplung.00415.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bozinovski S., et al. Granulocyte/macrophage-colony-stimulating factor (GM-CSF) regulates lung innate immunity to lipopolysaccharide through Akt/Erk activation of NFkappa B and AP-1 in vivo. J. Biol. Chem. 2002;277(45):42808–42814. doi: 10.1074/jbc.M207840200. [DOI] [PubMed] [Google Scholar]
- 79.Fan J., et al. Hemorrhagic shock-activated neutrophils augment TLR4 signaling-induced TLR2 upregulation in alveolar macrophages: role in hemorrhage-primed lung inflammation. Am. J. Phys. Lung Cell. Mol. Phys. 2006;290(4):L738–L746. doi: 10.1152/ajplung.00280.2005. [DOI] [PubMed] [Google Scholar]
- 80.Fan J. TLR cross-talk mechanism of hemorrhagic shock-primed pulmonary neutrophil infiltration. The open critical care medicine journal. 2010;2:1. doi: 10.2174/1874828700902010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vaneker M., et al. Low-tidal-volume mechanical ventilation induces a Toll-like receptor 4–dependent inflammatory response in healthy mice. Anesthesiology: The Journal of the American Society of Anesthesiologists. 2008;109(3):465–472. doi: 10.1097/ALN.0b013e318182aef1. [DOI] [PubMed] [Google Scholar]
- 82.Murray L.A., et al. Deleterious role of TLR3 during hyperoxia-induced acute lung injury. Am. J. Respir. Crit. Care Med. 2008;178(12):1227–1237. doi: 10.1164/rccm.200807-1020OC. [DOI] [PubMed] [Google Scholar]
- 83.Jiang D., et al. The role of Toll-like receptors in non-infectious lung injury. Cell Res. 2006;16(8):693. doi: 10.1038/sj.cr.7310085. [DOI] [PubMed] [Google Scholar]
- 84.Roberts A., et al. Animal models and vaccines for SARS-CoV infection. Virus Res. 2008;133(1):20–32. doi: 10.1016/j.virusres.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sheahan T., et al. MyD88 is required for protection from lethal infection with a mouse-adapted SARS-CoV. PLoS Pathog. 2008;4(12) doi: 10.1371/journal.ppat.1000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koyama S., et al. Differential role of TLR-and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J. Immunol. 2007;179(7):4711–4720. doi: 10.4049/jimmunol.179.7.4711. [DOI] [PubMed] [Google Scholar]
- 87.Heer A.K., et al. TLR signaling fine-tunes anti-influenza B cell responses without regulating effector T cell responses. J. Immunol. 2007;178(4):2182–2191. doi: 10.4049/jimmunol.178.4.2182. [DOI] [PubMed] [Google Scholar]
- 88.Allen I.C., et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30(4):556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ichinohe T., et al. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J. Exp. Med. 2009;206(1):79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rehwinkel J., et al. RIG-I detects viral genomic RNA during negative-strand RNA virus infection. Cell. 2010;140(3):397–408. doi: 10.1016/j.cell.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 91.Sabbah A., et al. Activation of innate immune antiviral responses by Nod2. Nat. Immunol. 2009;10(10):1073. doi: 10.1038/ni.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gibson R.L., Burns J.L., Ramsey B.W. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003;168(8):918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 93.Faure K., et al. TLR4 signaling is essential for survival in acute lung injury induced by virulent Pseudomonas aeruginosa secreting type III secretory toxins. Respir. Res. 2004;5(1):1. doi: 10.1186/1465-9921-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Campodonico V.L., et al. Airway epithelial control of Pseudomonas aeruginosa infection in cystic fibrosis. Trends Mol. Med. 2008;14(3):120–133. doi: 10.1016/j.molmed.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fu P., et al. Role of nicotinamide adenine dinucleotide phosphate–reduced oxidase proteins in Pseudomonas aeruginosa–induced lung inflammation and permeability. Am. J. Respir. Cell Mol. Biol. 2013;48(4):477–488. doi: 10.1165/rcmb.2012-0242OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Greene C.M., et al. TLR-induced inflammation in cystic fibrosis and non-cystic fibrosis airway epithelial cells. J. Immunol. 2005;174(3):1638–1646. doi: 10.4049/jimmunol.174.3.1638. [DOI] [PubMed] [Google Scholar]
- 97.Morris A.E., et al. Role of Toll-like receptor 5 in the innate immune response to acute P. aeruginosa pneumonia. Am. J. Phys. Lung Cell. Mol. Phys. 2009;297(6):L1112–L1119. doi: 10.1152/ajplung.00155.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Epelman S., et al. Different domains of Pseudomonas aeruginosa exoenzyme S activate distinct TLRs. J. Immunol. 2004;173(3):2031–2040. doi: 10.4049/jimmunol.173.3.2031. [DOI] [PubMed] [Google Scholar]
- 99.Balloy V., et al. The role of flagellin versus motility in acute lung disease caused by Pseudomonas aeruginosa. J. Infect. Dis. 2007;196(2):289–296. doi: 10.1086/518610. [DOI] [PubMed] [Google Scholar]
- 100.Skerrett S.J., et al. Redundant Toll-like receptor signaling in the pulmonary host response to Pseudomonas aeruginosa. Am. J. Phys. Lung Cell. Mol. Phys. 2007;292(1):L312–L322. doi: 10.1152/ajplung.00250.2006. [DOI] [PubMed] [Google Scholar]
- 101.Power M., et al. The myeloid differentiation factor 88 is dispensable for the development of a delayed host response to Pseudomonas aeruginosa lung infection in mice. Clinical & Experimental Immunology. 2006;146(2):323–329. doi: 10.1111/j.1365-2249.2006.03210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mahenthiralingam E., Urban T.A., Goldberg J.B. The multifarious, multireplicon Burkholderia cepacia complex. Nat. Rev. Microbiol. 2005;3(2):144. doi: 10.1038/nrmicro1085. [DOI] [PubMed] [Google Scholar]
- 103.Speert D.P. Advances in Burkholderia cepacia complex. Paediatr. Respir. Rev. 2002;3(3):230–235. doi: 10.1016/s1526-0542(02)00185-9. [DOI] [PubMed] [Google Scholar]
- 104.De C. Ventura G.M., et al. TLR 5, but neither TLR2 nor TLR4, is involved in lung epithelial cell response to Burkholderia cenocepacia. FEMS Immunology & Medical Microbiology. 2008;54(1):37–44. doi: 10.1111/j.1574-695X.2008.00453.x. [DOI] [PubMed] [Google Scholar]
- 105.Urban T.A., et al. Contribution of Burkholderia cenocepacia flagella to infectivity and inflammation. Infect. Immun. 2004;72(9):5126–5134. doi: 10.1128/IAI.72.9.5126-5134.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ventura G.M., et al. Lack of MyD88 protects the immunodeficient host against fatal lung inflammation triggered by the opportunistic bacteria Burkholderia cenocepacia. J. Immunol. 2009;183(1):670–676. doi: 10.4049/jimmunol.0801497. (p. jimmunol. 0801497) [DOI] [PubMed] [Google Scholar]
- 107.Lopez A., et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur. Respir. J. 2006;27(2):397–412. doi: 10.1183/09031936.06.00025805. [DOI] [PubMed] [Google Scholar]
- 108.Sethi S., Mallia P., Johnston S.L. New paradigms in the pathogenesis of chronic obstructive pulmonary disease II. Proc. Am. Thorac. Soc. 2009;6(6):532–534. doi: 10.1513/pats.200905-025DS. [DOI] [PubMed] [Google Scholar]
- 109.Sethi S., Murphy T.F. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N. Engl. J. Med. 2008;359(22):2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 110.Schleimer R.P. Innate immune responses and chronic obstructive pulmonary disease: “terminator” or “terminator 2”? Proc. Am. Thorac. Soc. 2005;2(4):342–346. doi: 10.1513/pats.200504-030SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang X., et al. Toll-like receptor 4 deficiency causes pulmonary emphysema. J. Clin. Invest. 2006;116(11):3050–3059. doi: 10.1172/JCI28139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cosio M.G., Saetta M., Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N. Engl. J. Med. 2009;360(23):2445–2454. doi: 10.1056/NEJMra0804752. [DOI] [PubMed] [Google Scholar]
- 113.Sopori M. Effects of cigarette smoke on the immune system. Nat. Rev. Immunol. 2002;2(5):372. doi: 10.1038/nri803. [DOI] [PubMed] [Google Scholar]
- 114.Doz E., et al. Cigarette smoke-induced pulmonary inflammation is TLR4/MyD88 and IL-1R1/MyD88 signaling dependent. J. Immunol. 2008;180(2):1169–1178. doi: 10.4049/jimmunol.180.2.1169. [DOI] [PubMed] [Google Scholar]
- 115.Maes T., et al. Murine TLR4 is implicated in cigarette smoke-induced pulmonary inflammation. Int. Arch. Allergy Immunol. 2006;141(4):354–368. doi: 10.1159/000095462. [DOI] [PubMed] [Google Scholar]
- 116.Guerassimov A., et al. The development of emphysema in cigarette smoke-exposed mice is strain dependent. Am. J. Respir. Crit. Care Med. 2004;170(9):974–980. doi: 10.1164/rccm.200309-1270OC. [DOI] [PubMed] [Google Scholar]
- 117.Droemann D., et al. Toll-like receptor 2 expression is decreased on alveolar macrophages in cigarette smokers and COPD patients. Respir. Res. 2005;6(1):68. doi: 10.1186/1465-9921-6-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen H., et al. Tobacco smoking inhibits expression of proinflammatory cytokines and activation of IL-1R-associated kinase, p38, and NF-κB in alveolar macrophages stimulated with TLR2 and TLR4 agonists. J. Immunol. 2007;179(9):6097–6106. doi: 10.4049/jimmunol.179.9.6097. [DOI] [PubMed] [Google Scholar]
- 119.Sarir H., et al. IL-8 production by macrophages is synergistically enhanced when cigarette smoke is combined with TNF-α. Biochem. Pharmacol. 2010;79(5):698–705. doi: 10.1016/j.bcp.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 120.Reynolds P.R., Cosio M.G., Hoidal J.R. Cigarette smoke–induced Egr-1 upregulates proinflammatory cytokines in pulmonary epithelial cells. Am. J. Respir. Cell Mol. Biol. 2006;35(3):314–319. doi: 10.1165/rcmb.2005-0428OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu X., et al. Plasminogen activator inhibitor-1 promotes inflammatory process induced by cigarette smoke extraction or lipopolysaccharides in alveolar epithelial cells. Exp. Lung Res. 2009;35(9):795–805. doi: 10.3109/01902140902912519. [DOI] [PubMed] [Google Scholar]
- 122.Karimi K., et al. Toll-like receptor-4 mediates cigarette smoke-induced cytokine production by human macrophages. Respir. Res. 2006;7(1):66. doi: 10.1186/1465-9921-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sarir H., et al. Cigarette smoke regulates the expression of TLR4 and IL-8 production by human macrophages. J. Inflamm. 2009;6(1):12. doi: 10.1186/1476-9255-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pace E., et al. Cigarette smoke increases Toll-like receptor 4 and modifies lipopolysaccharide-mediated responses in airway epithelial cells. Immunology. 2008;124(3):401–411. doi: 10.1111/j.1365-2567.2007.02788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Speletas M., et al. Association of TLR4-T399I polymorphism with chronic obstructive pulmonary disease in smokers. Clin. Dev. Immunol. 2009;2010 doi: 10.1155/2009/260286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pons J., et al. Expression of Toll-like receptor 2 is up-regulated in monocytes from patients with chronic obstructive pulmonary disease. Respir. Res. 2006;7(1):64. doi: 10.1186/1465-9921-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mortaz E., et al. Cigarette smoke induces CXCL8 production by human neutrophils via activation of TLR9 receptor. Eur. Respir. J. 2010;36(5):1143–1154. doi: 10.1183/09031936.00062209. [DOI] [PubMed] [Google Scholar]
- 128.Knuefermann P., et al. CpG oligonucleotide activates Toll-like receptor 9 and causes lung inflammation in vivo. Respir. Res. 2007;8(1):72. doi: 10.1186/1465-9921-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tasaka S., et al. Intratracheal synthetic CpG oligodeoxynucleotide causes acute lung injury with systemic inflammatory response. Respir. Res. 2009;10(1):84. doi: 10.1186/1465-9921-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Campbell J.D., et al. CpG-containing immunostimulatory DNA sequences elicit TNF-α–dependent toxicity in rodents but not in humans. J. Clin. Invest. 2009;119(9):2564–2576. doi: 10.1172/JCI38294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schneberger D., et al. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology: Advances in Integrative Anatomy and Evolutionary Biology. 292(7) 2009. Expression of toll-like receptor 9 in horse lungs; pp. 1068–1077. [DOI] [PubMed] [Google Scholar]
- 132.Cockeran R., et al. Pneumolysin activates the synthesis and release of interleukin-8 by human neutrophils in vitro. J. Infect. Dis. 2002;186(4):562–565. doi: 10.1086/341563. [DOI] [PubMed] [Google Scholar]
- 133.Malley R., et al. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. 2003;100(4):1966–1971. doi: 10.1073/pnas.0435928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Branger J., et al. Role of Toll-like receptor 4 in gram-positive and gram-negative pneumonia in mice. Infect. Immun. 2004;72(2):788–794. doi: 10.1128/IAI.72.2.788-794.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schwandner R., et al. Peptidoglycan-and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J. Biol. Chem. 1999;274(25):17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 136.Yoshimura A., et al. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J. Immunol. 1999;163(1):1–5. [PubMed] [Google Scholar]
- 137.Knapp S., et al. Toll-like receptor 2 plays a role in the early inflammatory response to murine pneumococcal pneumonia but does not contribute to antibacterial defense. J. Immunol. 2004;172(5):3132–3138. doi: 10.4049/jimmunol.172.5.3132. [DOI] [PubMed] [Google Scholar]
- 138.Dessing M.C., et al. Toll-like receptor 2 contributes to antibacterial defence against pneumolysin-deficient pneumococci. Cell. Microbiol. 2008;10(1):237–246. doi: 10.1111/j.1462-5822.2007.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Albiger B., et al. Toll-like receptor 9 acts at an early stage in host defence against pneumococcal infection. Cell. Microbiol. 2007;9(3):633–644. doi: 10.1111/j.1462-5822.2006.00814.x. [DOI] [PubMed] [Google Scholar]
- 140.Albiger B., et al. Myeloid differentiation factor 88-dependent signalling controls bacterial growth during colonization and systemic pneumococcal disease in mice. Cell. Microbiol. 2005;7(11):1603–1615. doi: 10.1111/j.1462-5822.2005.00578.x. [DOI] [PubMed] [Google Scholar]
- 141.Khan A.Q., et al. Both innate immunity and type 1 humoral immunity to Streptococcus pneumoniae are mediated by MyD88 but differ in their relative levels of dependence on toll-like receptor 2. Infect. Immun. 2005;73(1):298–307. doi: 10.1128/IAI.73.1.298-307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Teng F., et al. Toll-like receptor 3 is involved in airway epithelial cell response to nontypeable Haemophilus influenzae. Cell. Immunol. 2010;260(2):98–104. doi: 10.1016/j.cellimm.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 143.Renz H., Bradley K., Gelfand E.W. Production of interleukin-4 and interferon-gamma by TCR-V beta-expressing T-cell subsets in allergen-sensitized mice. Am. J. Respir. Cell Mol. Biol. 1996;14(1):36–43. doi: 10.1165/ajrcmb.14.1.8534484. [DOI] [PubMed] [Google Scholar]
- 144.Robinson D., et al. Activation of CD4+ T cells, increased TH2-type cytokine mRNA expression, and eosinophil recruitment in bronchoalveolar lavage after allergen inhalation challenge in patients with atopic asthma. J. Allergy Clin. Immunol. 1993;92(2):313–324. doi: 10.1016/0091-6749(93)90175-f. [DOI] [PubMed] [Google Scholar]
- 145.Tulic M.K., et al. TLR4 polymorphisms mediate impaired responses to respiratory syncytial virus and lipopolysaccharide. J. Immunol. 2007;179(1):132–140. doi: 10.4049/jimmunol.179.1.132. [DOI] [PubMed] [Google Scholar]
- 146.Chaudhuri N., et al. Toll-like receptors and chronic lung disease. Clin. Sci. 2005;109(2):125–133. doi: 10.1042/CS20050044. [DOI] [PubMed] [Google Scholar]
- 147.Erb K.J., et al. Infection of mice with Mycobacterium bovis–bacillus Calmette-Guerin (BCG) suppresses allergen-induced airway eosinophilia. J. Exp. Med. 1998;187(4):561–569. doi: 10.1084/jem.187.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Broide D., et al. Immunostimulatory DNA sequences inhibit IL-5, eosinophilic inflammation, and airway hyperresponsiveness in mice. J. Immunol. 1998;161(12):7054–7062. [PubMed] [Google Scholar]
- 149.Kline J.N., et al. Cutting edge: modulation of airway inflammation by CpG oligodeoxynucleotides in a murine model of asthma. J. Immunol. 1998;160(6):2555–2559. [PubMed] [Google Scholar]
- 150.Conrad M.L., et al. Maternal TLR signaling is required for prenatal asthma protection by the nonpathogenic microbe Acinetobacter lwoffii F78. J. Exp. Med. 2009;206(13):2869–2877. doi: 10.1084/jem.20090845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yang D., et al. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2–MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J. Exp. Med. 2008;205(1):79–90. doi: 10.1084/jem.20062027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sokol C.L., et al. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat. Immunol. 2009;10(7):713. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Møller-Larsen S., et al. Association analysis identifies TLR7 and TLR8 as novel risk genes in asthma and related disorders. Thorax. 2008;63(12):1064–1069. doi: 10.1136/thx.2007.094128. [DOI] [PubMed] [Google Scholar]
- 155.Genuneit J., et al. A multi-centre study of candidate genes for wheeze and allergy: the International Study of Asthma and Allergies in Childhood Phase 2. Clin Exp Allergy. 2009;39(12):1875–1888. doi: 10.1111/j.1365-2222.2009.03364.x. [DOI] [PubMed] [Google Scholar]
- 156.Kline J.N., Krieg A.M. Toll-like receptor 9 activation with CpG oligodeoxynucleotides for asthma therapy. Drug News Perspect. 2008;21(8):434–439. doi: 10.1358/dnp.2008.21.8.1272133. [DOI] [PubMed] [Google Scholar]
- 157.Allegra L., et al. Acute exacerbations of asthma in adults: role of Chlamydia pneumoniae infection. Eur. Respir. J. 1994;7(12):2165–2168. doi: 10.1183/09031936.94.07122165. [DOI] [PubMed] [Google Scholar]
- 158.Naiki Y., et al. MyD88 is pivotal for the early inflammatory response and subsequent bacterial clearance and survival in a mouse model of Chlamydia pneumoniae pneumonia. J. Biol. Chem. 2005;280(32):29242–29249. doi: 10.1074/jbc.M503225200. [DOI] [PubMed] [Google Scholar]
- 159.Rodriguez N., et al. Differential involvement of TLR2 and TLR4 in host survival during pulmonary infection with Chlamydia pneumoniae. Eur. J. Immunol. 2006;36(5):1145–1155. doi: 10.1002/eji.200535152. [DOI] [PubMed] [Google Scholar]
- 160.Kraft M., et al. Mycoplasma pneumoniae induces airway epithelial cell expression of MUC5AC in asthma. Eur. Respir. J. 2008;31(1):43–46. doi: 10.1183/09031936.00103307. [DOI] [PubMed] [Google Scholar]
- 161.Wu Q., et al. Toll-like receptor 2 down-regulation in established mouse allergic lungs contributes to decreased mycoplasma clearance. Am. J. Respir. Crit. Care Med. 2008;177(7):720–729. doi: 10.1164/rccm.200709-1387OC. [DOI] [PubMed] [Google Scholar]
- 162.Andersen P. Pathogenesis of lower respiratory tract infections due to Chlamydia, Mycoplasma, Legionella and viruses. Thorax. 1998;53(4):302–307. doi: 10.1136/thx.53.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kraft M., et al. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest. 2002;121(6):1782–1788. doi: 10.1378/chest.121.6.1782. [DOI] [PubMed] [Google Scholar]
- 164.Tan W.C. Viruses in asthma exacerbations. Curr. Opin. Pulm. Med. 2005;11(1):21–26. doi: 10.1097/01.mcp.0000146781.11092.0d. [DOI] [PubMed] [Google Scholar]
- 165.Thompson W.W., et al. Mortality associated with influenza and respiratory syncytial virus in the United States. Jama. 2003;289(2):179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 166.Bont L., et al. Monocyte IL-10 production during respiratory syncytial virus bronchiolitis is associated with recurrent wheezing in a one-year follow-up study. Am. J. Respir. Crit. Care Med. 2000;161(5):1518–1523. doi: 10.1164/ajrccm.161.5.9904078. [DOI] [PubMed] [Google Scholar]
- 167.Ehlenfield D.R., Cameron K., Welliver R.C. Eosinophilia at the time of respiratory syncytial virus bronchiolitis predicts childhood reactive airway disease. Pediatrics. 2000;105(1):79–83. doi: 10.1542/peds.105.1.79. [DOI] [PubMed] [Google Scholar]
- 168.Meyer G., Deplanche M., Schelcher F. Human and bovine respiratory syncytial virus vaccine research and development. Comp. Immunol. Microbiol. Infect. Dis. 2008;31(2–3):191–225. doi: 10.1016/j.cimid.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 169.Xie X.-H., et al. Lipopolysaccharide induces IL-6 production in respiratory syncytial virus-infected airway epithelial cells through the toll-like receptor 4 signaling pathway. Pediatr. Res. 2009;65(2):156. doi: 10.1203/PDR.0b013e318191f5c6. [DOI] [PubMed] [Google Scholar]
- 170.Kurt-Jones E.A., et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 2000;1(5):398. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 171.Haynes L.M., et al. Involvement of toll-like receptor 4 in innate immunity to respiratory syncytial virus. J. Virol. 2001;75(22):10730–10737. doi: 10.1128/JVI.75.22.10730-10737.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Haeberle H.A., et al. Respiratory syncytial virus–induced activation of nuclear factor–κB in the lung involves alveolar macrophages and toll-like receptor 4–dependent pathways. J. Infect. Dis. 2002;186(9):1199–1206. doi: 10.1086/344644. [DOI] [PubMed] [Google Scholar]
- 173.Awomoyi A.A., et al. Association of TLR4 polymorphisms with symptomatic respiratory syncytial virus infection in high-risk infants and young children. J. Immunol. 2007;179(5):3171–3177. doi: 10.4049/jimmunol.179.5.3171. [DOI] [PubMed] [Google Scholar]
- 174.Jahrsdörfer B., Weiner G.J. CpG oligodeoxynucleotides as immunotherapy in cancer. Update on cancer therapeutics. 2008;3(1):27–32. doi: 10.1016/j.uct.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Igney F.H., Krammer P.H. Immune escape of tumors: apoptosis resistance and tumor counterattack. J. Leukoc. Biol. 2002;71(6):907–920. [PubMed] [Google Scholar]
- 176.Colotta F., et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 177.Kluwe J., Mencin A., Schwabe R.F. Toll-like receptors, wound healing, and carcinogenesis. J. Mol. Med. 2009;87(2):125. doi: 10.1007/s00109-008-0426-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sorrentino R., et al. Pattern recognition receptors and interleukin-8 mediate effects of Gram-positive and Gram-negative bacteria on lung epithelial cell function. Br. J. Pharmacol. 2008;154(4):864–871. doi: 10.1038/bjp.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yu L., Chen S. Toll-like receptors expressed in tumor cells: targets for therapy. Cancer Immunol. Immunother. 2008;57(9):1271–1278. doi: 10.1007/s00262-008-0459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Koff J.L., et al. Multiple TLRs activate EGFR via a signaling cascade to produce innate immune responses in airway epithelium. Am. J. Phys. Lung Cell. Mol. Phys. 2008;294(6):L1068–L1075. doi: 10.1152/ajplung.00025.2008. [DOI] [PubMed] [Google Scholar]
- 181.Lechmann M., et al. Role of CD83 in the immunomodulation of dendritic cells. Int. Arch. Allergy Immunol. 2002;129(2):113–118. doi: 10.1159/000065883. [DOI] [PubMed] [Google Scholar]
- 182.Doganci A., Eigenbrod T., Krug N., De Sanctis G.T., Hausding M., Erpenbeck V.J., Haddad el B., Lehr H.A., Schmitt E., Bopp T., Kallen K.J., Herz U., Schmitt S., Luft C., Hecht O., Hohlfeld J.M., Ito H., Nishimoto N., Yoshizaki K., Kishimoto T., Rose-John S., Renz H., Neurath M.F., Galle P.R., Finotto S. The IL-6R alpha chain controls lung CD4+ CD25+ Treg development and function during allergic airway inflammation in vivo. J. Clin. Invest. 2005;115:313–325. doi: 10.1172/JCI22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Reiner S.L., Sallusto F., Lanzavecchia A. Division of labor with a workforce of one: challenges in specifying effector and memory T cell fate. Science. 2007;317(5838):622–625. doi: 10.1126/science.1143775. [DOI] [PubMed] [Google Scholar]
- 184.Wang L., et al. IL-17 can promote tumor growth through an IL-6–Stat3 signaling pathway. J. Exp. Med. 2009;206(7):1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Martin-Orozco N., et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31(5):787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rakoff-Nahoum S., Medzhitov R. Toll-like receptors and cancer. Nat. Rev. Cancer. 2009;9(1):57. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 187.Lambrecht B.N., Hammad H. The role of dendritic and epithelial cells as master regulators of allergic airway inflammation. Lancet. 2010;376(9743):835–843. doi: 10.1016/S0140-6736(10)61226-3. [DOI] [PubMed] [Google Scholar]
- 188.Jankowska O., et al. Phenotype of dendritic cells generated in the presence of non-small cell lung cancer antigens-preliminary report. Folia Histochem. Cytobiol. 2008;46(4):465–470. doi: 10.2478/v10042-008-0078-4. [DOI] [PubMed] [Google Scholar]
- 189.Xu H., et al. IDO: a double-edged sword for TH1/TH2 regulation. Immunol. Lett. 2008;121(1):1–6. doi: 10.1016/j.imlet.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Smyth M.J. Type I interferon and cancer immunoediting. Nat. Immunol. 2005;6(7):646. doi: 10.1038/ni0705-646. [DOI] [PubMed] [Google Scholar]
- 191.Fabricius D., et al. Prostaglandin E2 inhibits IFN-α secretion and Th1 costimulation by human plasmacytoid dendritic cells via E-prostanoid 2 and E-prostanoid 4 receptor engagement. J. Immunol. 2010;184(2):677–684. doi: 10.4049/jimmunol.0902028. [DOI] [PubMed] [Google Scholar]
- 192.Smit J.J., et al. The balance between plasmacytoid DC versus conventional DC determines pulmonary immunity to virus infections. PLoS One. 2008;3(3) doi: 10.1371/journal.pone.0001720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gregori S., et al. in Immunological Tolerance. Springer; 2007. Isolation, expansion, and characterization of human natural and adaptive regulatory T cells; pp. 83–105. [DOI] [PubMed] [Google Scholar]
- 194.Sharma M.D., et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2, 3-dioxygenase. J. Clin. Invest. 2007;117(9):2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Karanikas V., et al. Indoleamine 2, 3-dioxygenase (IDO) expression in lung cancer. Cancer biology & therapy. 2007;6(8) doi: 10.4161/cbt.6.8.4446. (p. 1269-1268) [DOI] [PubMed] [Google Scholar]
- 196.Inoue S., et al. Inhibitory effects of B cells on antitumor immunity. Cancer Res. 2006;66(15):7741–7747. doi: 10.1158/0008-5472.CAN-05-3766. [DOI] [PubMed] [Google Scholar]
- 197.Sorrentino R., et al. B cells contribute to the antitumor activity of CpG-oligodeoxynucleotide in a mouse model of metastatic lung carcinoma. Am. J. Respir. Crit. Care Med. 2011;183(10):1369–1379. doi: 10.1164/rccm.201010-1738OC. [DOI] [PubMed] [Google Scholar]
- 198.Mantovani A., Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr. Opin. Immunol. 2010;22(2):231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 199.Kalluri R., Zeisberg M. Fibroblasts in cancer. Nat. Rev. Cancer. 2006;6(5):392. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 200.Sorrentino R., et al. Plasmacytoid dendritic cells alter the antitumor activity of CpG-oligodeoxynucleotides in a mouse model of lung carcinoma. J. Immunol. 2010;185(8):4641–4650. doi: 10.4049/jimmunol.1000881. 1000881. [DOI] [PubMed] [Google Scholar]
- 201.Rakoff-Nahoum S., Medzhitov R. Role of toll-like receptors in tissue repair and tumorigenesis. Biochem. Mosc. 2008;73(5):555–561. doi: 10.1134/s0006297908050088. [DOI] [PubMed] [Google Scholar]
- 202.Luo J.-L., et al. Inhibition of NF-κB in cancer cells converts inflammation-induced tumor growth mediated by TNFα to TRAIL-mediated tumor regression. Cancer Cell. 2004;6(3):297–305. doi: 10.1016/j.ccr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 203.Li X., Jiang S., Tapping R.I. Toll-like receptor signaling in cell proliferation and survival. Cytokine. 2010;49(1):1–9. doi: 10.1016/j.cyto.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Akazawa T., et al. Antitumor NK activation induced by the Toll-like receptor 3-TICAM-1 (TRIF) pathway in myeloid dendritic cells. Proc. Natl. Acad. Sci. 2007;104(1):252–257. doi: 10.1073/pnas.0605978104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chin A.I., et al. Toll-like receptor 3–mediated suppression of TRAMP prostate cancer shows the critical role of type I interferons in tumor immune surveillance. Cancer Res. 2010:0008–5472. doi: 10.1158/0008-5472.CAN-09-1162. (CAN-09-1162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hirsch I., et al. Impaired Toll-like receptor 7 and 9 signaling: from chronic viral infections to cancer. Trends Immunol. 2010;31(10):391–397. doi: 10.1016/j.it.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 207.Ma F., et al. The TLR7 agonists imiquimod and gardiquimod improve DC-based immunotherapy for melanoma in mice. Cellular & molecular immunology. 2010;7(5):381. doi: 10.1038/cmi.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zanoni I., et al. TLR-dependent activation stimuli associated with Th1 responses confer NK cell stimulatory capacity to mouse dendritic cells. J. Immunol. 2005;175(1):286–292. doi: 10.4049/jimmunol.175.1.286. [DOI] [PubMed] [Google Scholar]
- 209.Hamm S., et al. Cancer immunotherapeutic potential of novel small molecule TLR7 and TLR8 agonists. J. Immunotoxicol. 2009;6(4):257–265. doi: 10.3109/15476910903286733. [DOI] [PubMed] [Google Scholar]
- 210.Welsh T.J., et al. Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non–small-cell lung cancer. J. Clin. Oncol. 2005;23(35):8959–8967. doi: 10.1200/JCO.2005.01.4910. [DOI] [PubMed] [Google Scholar]
- 211.Oldford S.A., et al. A critical role for mast cells and mast cell-derived IL-6 in TLR2-mediated inhibition of tumor growth. J. Immunol. 2010;185(11):7067–7076. doi: 10.4049/jimmunol.1001137. 1001137. [DOI] [PubMed] [Google Scholar]
- 212.Sureshbabu A., et al. Conditional overexpression of TGFβ1 promotes pulmonary inflammation, apoptosis and mortality via TGFβR2 in the developing mouse lung. Respir. Res. 2015;16(1):4. doi: 10.1186/s12931-014-0162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Damiano V., et al. TLR9 agonist acts by different mechanisms synergizing with bevacizumab in sensitive and cetuximab-resistant colon cancer xenografts. Proc. Natl. Acad. Sci. 2007;104(30):12468–12473. doi: 10.1073/pnas.0705226104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Droemann D., et al. Human lung cancer cells express functionally active Toll-like receptor 9. Respir. Res. 2005;6(1):1. doi: 10.1186/1465-9921-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Johnson B., et al. Physiology and therapeutics of vascular endothelial growth factor in tumor immunosuppression. Curr. Mol. Med. 2009;9(6):702–707. doi: 10.2174/156652409788970634. [DOI] [PubMed] [Google Scholar]
- 216.Leung D.W., et al. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246(4935):1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 217.Sorrentino R., et al. CpG-ODN increases the release of VEGF in a mouse model of lung carcinoma. Int. J. Cancer. 2011;128(12):2815–2822. doi: 10.1002/ijc.25626. [DOI] [PubMed] [Google Scholar]
- 218.Cherfils-Vicini J., et al. Triggering of TLR7 and TLR8 expressed by human lung cancer cells induces cell survival and chemoresistance. J. Clin. Invest. 2010;120(4):1285–1297. doi: 10.1172/JCI36551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Chapoval S.P., et al. Lung vascular endothelial growth factor expression induces local myeloid dendritic cell activation. Clin. Immunol. 2009;132(3):371–384. doi: 10.1016/j.clim.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Elenbaas B., Weinberg R.A. Heterotypic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Exp. Cell Res. 2001;264(1):169–184. doi: 10.1006/excr.2000.5133. [DOI] [PubMed] [Google Scholar]
- 221.Li J., et al. CpG DNA-mediated immune response in pulmonary endothelial cells. Am. J. Phys. Lung Cell. Mol. Phys. 2004;287(3):L552–L558. doi: 10.1152/ajplung.00436.2003. [DOI] [PubMed] [Google Scholar]
- 222.Bedke T., et al. Endothelial cells augment the suppressive function of CD4+ CD25+ Foxp3+ regulatory T cells: involvement of programmed death-1 and IL-10. J. Immunol. 2010;184(10):5562–5570. doi: 10.4049/jimmunol.0902458. (p. ji_0902458) [DOI] [PubMed] [Google Scholar]
- 223.Ferreiro-Neira I., et al. Opposed independent effects and epistasis in the complex association of IRF5 to SLE. Genes Immun. 2007;8(5):429–438. doi: 10.1038/sj.gene.6364407. [DOI] [PubMed] [Google Scholar]
- 224.Jezierska A., Kolosova I.A., Verin A.D. Toll like receptors signaling pathways as a target for therapeutic interventions. Current Signal Transduction Therapy. 2011;6(3):428–440. doi: 10.2174/157436211797483930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Aryan Z., et al. A new era of targeting the ancient gatekeepers of the immune system: Toll-like agonists in the treatment of allergic rhinitis and asthma. Int. Arch. Allergy Immunol. 2014;164(1):46–63. doi: 10.1159/000362553. [DOI] [PubMed] [Google Scholar]
- 226.Park B.S., et al. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458(7242):1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 227.Qureshi N., Takayama K., Ribi E. Purification and structural determination of nontoxic lipid A obtained from the lipopolysaccharide of Salmonella typhimurium. J. Biol. Chem. 1982;257(19):11808–11815. [PubMed] [Google Scholar]
- 228.Mata-Haro V., et al. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316(5831):1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 229.Baldrick P., et al. Pollinex Quattro Ragweed: safety evaluation of a new allergy vaccine adjuvanted with monophosphoryl lipid A (MPL) for the treatment of ragweed pollen allergy. J. Appl. Toxicol. 2007;27(4):399–409. doi: 10.1002/jat.1223. [DOI] [PubMed] [Google Scholar]
- 230.Patel P., et al. Efficacy of a short course of specific immunotherapy in patients with allergic rhinoconjunctivitis to ragweed pollen. J. Allergy Clin. Immunol. 2014;133(1):121–129.e2. doi: 10.1016/j.jaci.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 231.De Ridder M., et al. The radiosensitizing effect of immunoadjuvant OM-174 requires cooperation between immune and tumor cells through interferon-gamma and inducible nitric oxide synthase. Int. J. Radiat. Oncol. Biol. Phys. 2006;66(5):1473–1480. doi: 10.1016/j.ijrobp.2006.07.1381. [DOI] [PubMed] [Google Scholar]
- 232.D’Agostini C., et al. Antitumour effect of OM-174 and cyclophosphamide on murine B16 melanoma in different experimental conditions. Int. Immunopharmacol. 2005;5(7–8):1205–1212. doi: 10.1016/j.intimp.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 233.Przetak M., et al. Novel synthetic LPS receptor agonists boost systemic and mucosal antibody responses in mice. Vaccine. 2003;21(9–10):961–970. doi: 10.1016/s0264-410x(02)00737-5. [DOI] [PubMed] [Google Scholar]
- 234.Ishizaka S.T., Hawkins L.D. E6020: a synthetic Toll-like receptor 4 agonist as a vaccine adjuvant. Expert Rev Vaccines. 2007;6(5):773–784. doi: 10.1586/14760584.6.5.773. [DOI] [PubMed] [Google Scholar]
- 235.G Stöver A., et al. Vol. 279. 2004. Structure-Activity Relationship of Synthetic Toll-Like Receptor 4 Agonists; pp. 4440–4449. [DOI] [PubMed] [Google Scholar]
- 236.Cluff C.W., et al. Synthetic toll-like receptor 4 agonists stimulate innate resistance to infectious challenge. Infect. Immun. 2005;73(5):3044–3052. doi: 10.1128/IAI.73.5.3044-3052.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lee K., et al. Bacillus-derived poly-gamma-glutamic acid attenuates allergic airway inflammation through a Toll-like receptor-4-dependent pathway in a murine model of asthma. Clin. Exp. Allergy. 2011;41(8):1143–1156. doi: 10.1111/j.1365-2222.2011.03792.x. [DOI] [PubMed] [Google Scholar]
- 238.Bortolatto J., et al. Toll-like receptor 4 agonists adsorbed to aluminium hydroxide adjuvant attenuate ovalbumin-specific allergic airway disease: role of MyD88 adaptor molecule and interleukin-12/interferon-gamma axis. Clin. Exp. Allergy. 2008;38(10):1668–1679. doi: 10.1111/j.1365-2222.2008.03036.x. [DOI] [PubMed] [Google Scholar]
- 239.Zhou C., Kang X.D., Chen Z. A synthetic Toll-like receptor 2 ligand decreases allergic immune responses in a mouse rhinitis model sensitized to mite allergen. J Zhejiang Univ Sci B. 2008;9(4):279–285. doi: 10.1631/jzus.B0730029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Murata M., et al. SMP-105, cell-wall skeleton purified from Mycobacterium bovis BCG Tokyo 172, activates innate immunity through TLR2/MyD88 pathway and prevents tumor metastasis into draining lymph nodes, Cancer Research. Cancer Res. 2007;67(9 Supplement):3554. [Google Scholar]
- 241.Weigt H., et al. The Toll-like receptor-2/6 agonist macrophage-activating lipopeptide-2 cooperates with IFN-gamma to reverse the Th2 skew in an in vitro allergy model. J. Immunol. 2004;172(10):6080–6086. doi: 10.4049/jimmunol.172.10.6080. [DOI] [PubMed] [Google Scholar]
- 242.Weigt H., et al. Efficacy of macrophage-activating lipopeptide-2 combined with interferon-gamma in a murine asthma model. Am. J. Respir. Crit. Care Med. 2005;172(5):566–572. doi: 10.1164/rccm.200411-1490OC. [DOI] [PubMed] [Google Scholar]
- 243.Aumeunier A., et al. Systemic Toll-like receptor stimulation suppresses experimental allergic asthma and autoimmune diabetes in NOD mice. PLoS One. 2010;5(7) doi: 10.1371/journal.pone.0011484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Revets H., et al. Lipoprotein I, a TLR2/4 ligand modulates Th2-driven allergic immune responses. J. Immunol. 2005;174(2):1097–1103. doi: 10.4049/jimmunol.174.2.1097. [DOI] [PubMed] [Google Scholar]
- 245.Drake M.G., et al. The therapeutic potential of Toll-like receptor 7 stimulation in asthma. Inflammation & allergy drug targets. 2012;11(6):484–491. doi: 10.2174/187152812803589967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.https://clinicaltrials.gov/ct2/show/NCT01453179. Long-term effects of Imiquimod and diclofenac in actinic keratoses (LEIDA 2) (LEIDA 2). 2017
- 247.Gkoulioni V., et al. The efficacy of imiquimod on dysplastic lesions of the oral mucosa: an experimental model. Anticancer Res. 2010;30(7):2891–2896. [PubMed] [Google Scholar]
- 248.Gorden K.B., et al. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J. Immunol. 2005;174(3):1259–1268. doi: 10.4049/jimmunol.174.3.1259. [DOI] [PubMed] [Google Scholar]
- 249.Peng G., et al. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309(5739):1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 250.Isobe Y., et al. Synthesis and structure-activity relationships of 2-substituted-8-hydroxyadenine derivatives as orally available interferon inducers without emetic side effects. Bioorg. Med. Chem. 2003;11(17):3641–3647. doi: 10.1016/s0968-0896(03)00369-9. [DOI] [PubMed] [Google Scholar]
- 251.Bergmann J.F., et al. Randomised clinical trial: anti-viral activity of ANA773, an oral inducer of endogenous interferons acting via TLR7, in chronic HCV. Aliment. Pharmacol. Ther. 2011;34(4):443–453. doi: 10.1111/j.1365-2036.2011.04745.x. [DOI] [PubMed] [Google Scholar]
- 252.Vollmer J., Krieg A.M. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv. Drug Deliv. Rev. 2009;61(3):195–204. doi: 10.1016/j.addr.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 253.Gupta G.K., Agrawal D.K. CpG oligodeoxynucleotides as TLR9 agonists: therapeutic application in allergy and asthma. BioDrugs. 2010;24(4):225–235. doi: 10.2165/11536140-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 254.Serebrisky D., et al. CpG oligodeoxynucleotides can reverse Th2-associated allergic airway responses and alter the B7.1/B7.2 expression in a murine model of asthma. J. Immunol. 2000;165(10):5906–5912. doi: 10.4049/jimmunol.165.10.5906. [DOI] [PubMed] [Google Scholar]
- 255.Banerjee B., et al. Modulation of airway inflammation by immunostimulatory CpG oligodeoxynucleotides in a murine model of allergic aspergillosis. Infect. Immun. 2004;72(10):6087–6094. doi: 10.1128/IAI.72.10.6087-6094.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Chiang D.J., et al. Ribavirin or CpG DNA sequence-modulated dendritic cells decrease the IgE level and airway inflammation. Am. J. Respir. Crit. Care Med. 2003;168(5):575–580. doi: 10.1164/rccm.2205005. [DOI] [PubMed] [Google Scholar]
- 257.Senti G., et al. Use of A-type CpG oligodeoxynucleotides as an adjuvant in allergen-specific immunotherapy in humans: a phase I/IIa clinical trial. Clin. Exp. Allergy. 2009;39(4):562–570. doi: 10.1111/j.1365-2222.2008.03191.x. [DOI] [PubMed] [Google Scholar]
- 258.Agrawal S., Kandimalla E.R. Synthetic agonists of Toll-like receptors 7, 8 and 9. Biochem. Soc. Trans. 2007;35(Pt 6):1461–1467. doi: 10.1042/BST0351461. [DOI] [PubMed] [Google Scholar]
- 259.Panter G., Kuznik A., Jerala R. Therapeutic applications of nucleic acids as ligands for Toll-like receptors. Curr. Opin. Mol. Ther. 2009;11(2):133–145. [PubMed] [Google Scholar]
- 260.Overton E.T., et al. Intranasal seasonal influenza vaccine and a TLR-3 agonist, rintatolimod, induced cross-reactive IgA antibody formation against avian H5N1 and H7N9 influenza HA in humans. Vaccine. 2014;32(42):5490–5495. doi: 10.1016/j.vaccine.2014.07.078. [DOI] [PubMed] [Google Scholar]
- 261.Hammad H., et al. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 2009;15(4):410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Sundaram K., et al. House dust mite allergens and the induction of monocyte interleukin 1beta production that triggers an IkappaBzeta-dependent granulocyte macrophage colony-stimulating factor release from human lung epithelial cells. Am. J. Respir. Cell Mol. Biol. 2015;53(3):400–411. doi: 10.1165/rcmb.2014-0370OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Monnet E., et al. Evidence of NI-0101 pharmacological activity, an anti-TLR4 antibody, in a randomized phase I dose escalation study in healthy volunteers receiving LPS. Clin. Pharmacol. Ther. 2017;101(2):200–208. doi: 10.1002/cpt.522. [DOI] [PubMed] [Google Scholar]
- 264.Ungaro R., et al. A novel Toll-like receptor 4 antagonist antibody ameliorates inflammation but impairs mucosal healing in murine colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296(6):G1167–G1179. doi: 10.1152/ajpgi.90496.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Savov J.D., et al. Toll-like receptor 4 antagonist (E5564) prevents the chronic airway response to inhaled lipopolysaccharide. Am J Physiol Lung Cell Mol Physiol. 2005;289(2):L329–L337. doi: 10.1152/ajplung.00014.2005. [DOI] [PubMed] [Google Scholar]
- 266.Hennessy E., Parker A.E., O’Neill L. Vol. 9. 2010. Targeting Toll-like Receptors: Emerging Therapeutics? pp. 293–307. [DOI] [PubMed] [Google Scholar]
- 267.Hemmi H., et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 2002;3(2):196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 268.Mahmutovic-Persson I., et al. Capacity of capsazepinoids to relax human small airways and inhibit TLR3-induced TSLP and IFNbeta production in diseased bronchial epithelial cells. Int. Immunopharmacol. 2012;13(3):292–300. doi: 10.1016/j.intimp.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 269.Zang N., et al. Resveratrol-mediated gamma interferon reduction prevents airway inflammation and airway hyperresponsiveness in respiratory syncytial virus-infected immunocompromised mice. J. Virol. 2011;85(24):13061–13068. doi: 10.1128/JVI.05869-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Hölscher C., et al. Containment of aerogenic Mycobacterium tuberculosis infection in mice does not require MyD88 adaptor function for TLR2, -4 and -9. European Journal of Immunology. 2008;vol. 38(3):680–694. doi: 10.1002/eji.200736458. [DOI] [PubMed] [Google Scholar]
- 271.Wang T., Town T., Alexopoulou L., Anderson J.F., Fikrig E., Flavell R.A. Toll-like Receptor 3 Mediates West Nile Virus Entry into the Brain Causing Lethal Encephalitis. Nat. Med. 2004;10(12):1366. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.