Abstract
The spike (S) protein of severe acute respiratory syndrome coronavirus (SARS‐CoV) is an important viral structural protein. Based on bioinformatics analysis, 10 antigenic peptides derived from the S protein sequence were selected and synthesized. The antigenicity and immunoreactivity of all the peptides were tested in vivo and in vitro. Four peptides (P6, P8, P9 and P10) which contain B cell epitopes of the S protein were identified, and P8 peptide was confirmed in vivo to have a potential in serological diagnosis. By using a syncytia formation model, we tested the neutralization ability of all 10 peptides and their corresponding antibodies. It is interesting to find that P8 and P9 peptides inhibited syncytia formation, suggesting that the P8 and P9 spanning regions may provide a good target for anti‐SARS‐CoV drug design. Our data suggest that we have identified peptides derived from the S protein of SARS‐CoV, which are useful for SARS treatment and diagnosis.
Keywords: SARS-CoV, severe acute respiratory syndrome-associated coronavirus, Abs, antibodies, Severe acute respiratory syndrome coronavirus, Spike protein, Synthetic peptide, Syncytia formation and disease prevention
1. Introduction
Severe acute respiratory syndrome (SARS) is a serious epidemic infectious disease that occurred from November 2002 to September 2003 and reappeared in parts of China in 2004. This devastating disease has a relatively high mortality [1].
The SARS‐associated coronavirus, named SARS‐CoV, is the pathogen of this disaster [2]. This new kind of coronavirus is an enveloped and positive‐stranded RNA (+RNA) virus, with a single‐stranded genome, which contains four structural proteins, spike (S) protein, membrane (M) protein, small envelope protein and nucleocapsid (N) protein [3].
The SARS‐CoV spread quickly in China and many other countries. Since there is no effective method so far to prevent and treat this disease, an accurate diagnostic method and an effective clinical anti‐viral drug or vaccine are urgently needed. The S protein of SARS‐CoV is a good candidate for disease diagnosis and vaccine design.
The S protein (gi: 30173397) consists of 1255 amino acids and has several glycosylation sites. It is believed that the S protein has two domains, S1 and S2. S1 plays an important role in virus binding to virus‐receptor (ACE2) while S2 mediates the fusion between the viral particle and its target cell [4, 5]. According to our current work, we confirmed that S protein can be cleaved into two parts [5]. It is predicted that the S protein yields two fragments spanning residues from 1 to 667 (S1) and 668 to 1255 (S2). So 10 peptide sequences (with 18–30 residues in length) were selected from S protein (in which peptides P1‐8 are located in the S1 part and peptides P9‐10 are located in S2) based on a series of bioinformatics analysis [6, 7].
The peptides then were used to immunize rabbits. The specificity of antisera induced by peptides was analyzed by Western blot with recombinant expressed S, S1 and S2 proteins as well as the lysates of SARS‐CoV infected Vero E6 cells. SARS patients’ sera were used to confirm the specific immunoreactivity of the selected peptides. The syncytia formation model was used to evaluate the neutralization ability of the peptides and their corresponding antibodies (Abs).
The data demonstrated that all 10 peptides showed high antigenicity and immunoreactivity. The antisera from peptides (P6, P8, P9, and P10) immunized rabbits showed good specificity and high affinity to S protein. It was confirmed that the P8 peptide is the best candidate for usage in SARS serological diagnosis. After some optimization, the peptide PL8 derived from P8, revealed a specific response to SARS specific IgG in SARS patients’ sera, which gave a positive rate above 70% in all tested SARS patients.
Neutralization experiments using a syncytia formation model showed that the peptides P8 and P9 themselves can markedly inhibit syncytia formation, indicating that the corresponding regions spanning sequences (540–559) and (731–753) within the S protein may be new targets for efficient SARS drug development.
2. Materials and methods
2.1. Peptide design and synthesis
After analyzing the SARS spike protein (gi: 30173397) with software DNAstar, 10 peptides were selected from the whole sequence of the S protein based on their indices of antigenicity, hydrophilicity, surface probability, and flexible region. These peptides were synthesized using conventional solid‐phase chemical methodology and purified in GL Bio‐Chem (Shanghai) Ltd [8].
2.2. Immunization
Bovine serum albumin (BSA, Sigma) served as a carrier protein for conjugation with peptides. New Zealand white rabbits (Shanghai Laboratory Animal Center, Chinese Academy of Sciences) were immunized subcutaneously on the back of the rabbits with 200 μg of different BSA‐conjugated peptides emulsified (1/1 v/v) with complete Freund's adjuvant (CFA). The same amount of peptides dissolved in incomplete Freund's adjuvant (IFA) was used to boost immunization in the rabbits on days 21 and 42 following immunization. The antisera were collected on day 49 following first immunization.
2.3. Prokaryotic S protein expression
The recombinant intact S protein, S1 and S2 fragments were expressed by Escherichia coli and they were purified by Model 422 Electro‐Eluter (Bio‐rad) and subjected to SDS–PAGE analysis [5].
2.4. Preparation of virus lysates
Vero E6 cells were infected with SARS‐CoV for 24 h and collected as previously described [9]. Infected cells were lysed with a solution containing 40 mM Tris (pH 8.3) and 0.5% Nonident P‐40 at 22 °C for 5 min. The virus lysate was centrifuged at 8000 rpm for 5 min, and then the supernatant was collected and boiled for 5 min. All experiments using virus were carried out in a biosafety level 3 laboratory.
2.5. Western blot
The expressed whole length S protein, S1 and S2 fragments as well as the native S proteins extracted from the lysate of SARS‐CoV infected Vero E6 cells were used to detect their binding activities with peptide‐induced antisera. Binding Abs were then detected by the second Ab (HRP‐conjugated goat anti‐rabbit IgG). ECL‐detection reagents (Amersham Pharmacia Biotech) were used to develop the films.
2.6. Serum samples
58 sera of SARS patients and 40 sera of healthy individuals were used. All of the experiments using both of these sera were carried out in a biosafety level 3 laboratory. All the confirmed SARS patients’ sera were IgG positive when analyzed by ELISA using whole virus lysates.
2.7. ELISA procedure
The reactivity of the peptides to the SARS patients’ sera was also measured by ELISA. The 96‐well plates were coated with the BSA–peptide conjugates(5 μg/ml, 50 μl/well) in coating buffer (pH 9.4) at 4 °C overnight. Afterwards the wells were washed with PBS, and were blocked with BSA at 37 °C for 2 h and then the sera or the purified IgG from SARS patients’ sera was added as the first Ab. The sera dilution was 1:10 and the purified IgG dilution was 1:1000. Plates were stored at 37 °C for 30 min, then washed again with PBS. After that, HRP‐coupled goat anti‐human IgG (Bio‐rad) was added, and OD values were measured with a microplate autoreader (Biotek) at 450 nm.
2.8. Syncytia inhibition assay
Syncytia formation was used to mimic the SARS‐CoV infection procedure [10]. In our study, we modified this assay by introducing luciferase assay in the detecting system (Fig. 5 A and B).
Figure 1.
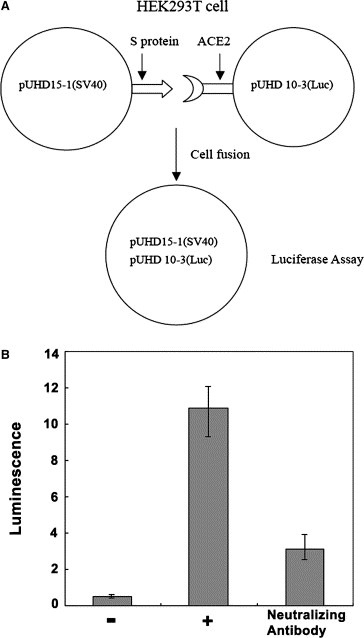
Spike protein‐ACE2‐mediated syncytia formation model. (A) HEK293T cells were transfected with SARS‐CoV spike protein gene and pUHD15‐1 (SV40); while at the same time, other HEK293T cells were transfected with ACE2 gene and pUHD10‐3. The two kinds of treated cells can fuse with each other and form syncytia, which contain pUHD15‐1 and pUHD10‐3 together and induce the reporter gene expression. (B) The model was tested by a known neutralizing monoclonal antibody, which was obtained from mice immunized with S protein integrated pseudo‐virus. (−) group: ACE2 is not transduced into the HEK293T cells, so no syncytia formation. (+) group: control IgG Ab was used in the test, resulting in the completed syncytia formation.
The cell fusion‐dependent reporter gene (luciferase) activation assay was adapted to our studies of spike protein‐mediated membrane fusion. In brief, effector cells were generated by cotransfection of HEK293 T cell monolayers (106 cells/60 mm dish) with plasmids pCDNA3.1‐ACE2 and pUHD 15‐1(SV40), while target cells (containing the luciferase reporter gene) were generated by cotransfection of HEK293 T cell with pCDNA3.1‐spike‐Ig and pUHD 10‐3. Twenty‐four hours after transfection, effector cells and target cells were trypsinized, resuspended in DMEM–10% FBS, then two kinds of cell suspensions were mixed (1:1 cell number) and repelleted.
All the antisera and peptides (20 μl of each antiserum and 125 μM of each peptide) were added into the cell suspension mixture to investigate their inhibitory capacity. Antiserum or peptide was incubated with cell suspensions at 23 °C for 1 h, and then re‐plated into a 48‐well culture plate at 37 °C for 24 h. Finally, media were removed and cells were lysed with 100 μl Passive Lysis Buffer (Promega). Luciferase intensity was measured in a luminometer TD‐20/20.
3. Results
3.1. Bioinformatics analysis and peptide selection
We predicted that the cleavage site of the SARS‐CoV spike protein containing 1255 residues is located between amino acid residues 667 and 668 [5]. The fragments of 1–667 and 668–1255 represent S1 and S2, respectively. All peptides are designated according to four characteristics: antigenicity, surface probability, hydrophilicity and flexible region. The peptides P1 to P8 were derived from the S1 fragment and the peptides P9 and P10 were from the S2 fragment (Fig. 1 and Table 1 ).
Figure 2.
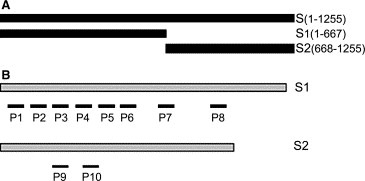
Map of peptides used for immunization. The spike protein is predicted to be cleaved at amino acid 667 and 668 according to bioinformatics analysis. (A) Full‐length S protein, S1 and S2 fragments were expressed by E. coli and served as target proteins for Western blot. (B) Eight peptide sequences were selected from the S1 fragment and two peptide sequences were selected from the S2 fragment.
Table 1.
Ten peptides were synthesized based on bioinformatics analysis
| No. | Position | Peptide sequence | Putative location | Antigenic index | Hydrophilicity plot | Surface probability plot | Flexible region |
|---|---|---|---|---|---|---|---|
| P1 | 34–54 | TSSMRGVYYPDEIFRSDTLYL | S1 | ++ | ++ | + | + |
| P2 | 94–113 | KSNVVRGWVFGSTMNNKSQS | S1 | ++ | + | + | + |
| P3 | 197–216 | YKGYQPIDVVRDLPSGFNTL | S1 | ++ | + | + | − |
| P4 | 273–293 | TDAVDCSQNPLAELKCSVKSF | S1 | ++ | + | − | + |
| P5 | 297–316 | KGIYQTSNFRVVPSGDVVRF | S1 | + | + | − | + |
| P6 | 332–351 | TKFPSVYAWERKKISNCVAD | S1 | ++ | + | + | + |
| P7 | 436–455 | YNYKYRYLRHGKLRPFERDI | S1 | +++ | +++ | ++ | + |
| P8 | 540–559 | PSSKRFQPFQQFGRDVSDFT | S1 | +++ | +++ | + | + |
| P9 | 731–753 | CANLLLQYGSFCTQLNRALSGIA | S2 | − | − | − | − |
| P10 | 758–780 | RNTREVFAQVKQMYKTPTLKYFG | S2 | + | ++ | ++ | + |
3.2. Antigenicities of synthesized peptides were evaluated by Western blot
In order to test the antigenicity of these synthetic peptides to immunize rabbits, all of them were coupled with BSA. The antisera with high titers were collected on day 49 following immunization. The binding activity and specificity of Abs were determined by Western blot with E. coli expressed S1 fragment, S2 fragment and full‐length S proteins as well as the native S protein isolated from the lysates of SARS‐CoV infected Vero‐E6 cells. The data demonstrated that anti‐P3, P5, P7 antisera bound to S1 and S2 fragments. They also showed weak and non‐specific binding activities to the lysates of virus infected Vero‐E6 cells, suggesting that the three Abs had poor binding specificity to the S Protein of SARS‐CoV. In contrast, anti‐P6, P8, P9 and P10 antisera had specific binding to the S fragments and S protein expressed in E. coli and SARS‐CoV infected cell lysates, suggesting that those Abs have a potential to recognize S protein of SARS‐CoV in its natural form (Fig. 2 and Table 2 ).
Figure 3.
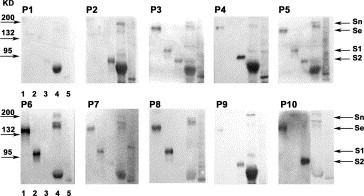
Specificity of the peptide‐induced antisera as indicated by Western blot analysis. Lane 1: recombinant SARS‐CoV Spike protein expressed in E. coli; lane 2: S1 fragment expressed in E. coli; lane 3: S2 fragment; lane 4: SARS‐CoV infected Vero E6 cell lysate; lane 5: non‐infected Vero E6 cell lysate. Details are seen in Table 2. Sn, native spike protein; Se, recombinant S protein expressed in E. coli; S1, recombinant S1 fragment of S protein expressed in E. coli; S2, recombinant S2 fragment.
Table 2.
Western blot analysis: the interactions between peptide‐specific antibodies and different kinds of S antigen
| Anti‐P1 | Anti‐P2 | Anti‐P3 | Anti‐P4 | Anti‐P5 | Anti‐P6 | Anti‐P7 | Anti‐P8 | Anti‐P9 | Anti‐P10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| S protein | − | − | + | + | + | + | + | + | + | + |
| S1 fragment | − | − | + | − | + | + | + | + | − | − |
| S2 fragment | − | + | + | + | + | − | − | − | + | + |
| Native S protein | − | + | + | − | + | + | + | + | + | + |
| Negative control | − | − | − | + | + | − | + | +/− | − | + |
| Specificity | − | − | − | − | − | + | − | + | + | + |
3.3. Immunoreactivity of synthesized peptides were evaluated with IgG purified from SARS patients’ sera
To study the immunogenicity of peptides derived from S protein of SARS‐CoV in vivo, the S protein‐specific IgG was purified from 10 SARS patients’ sera and the mixture containing IgG served as a template to determine its reaction with the peptides. Ten peptides showed different immunoreactivities to the purified IgG, in which the P5, P8 and P9 peptides revealed relatively higher responses than other peptides (Fig. 3 ). The P8 peptide with the highest immunoreactivity may contain a potential dominant B cell epitope on the S protein of SARS‐CoV.
Figure 4.
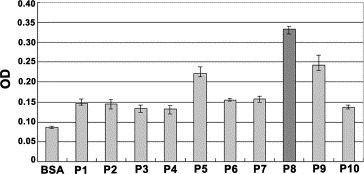
Immunoreactivities of all peptides (P1–P10). The purified IgG from SARS patients’ sera served as S‐protein specific Ab. BSA was used as a negative control. The binding activities of the IgG to all 10 peptides derived from S protein were detected by ELISA. The purified human IgG of SARS patients were 1:500 dilution and HRP‐coupled anti‐human IgG served as second Ab.
3.4. The application of P8 peptide in SARS serological diagnosis
Since P8 peptide can react with purified IgG from SARS patients’ sera, we ask whether the P8 peptide could be used as an antigen for SARS serological diagnosis. To initially address this possibility, the reactions between P8 and sera from SARS patients as well as normal individuals were tested by ELISA. The peptide derived from murine IL‐12β2‐receptor (IL‐12R: CNRLDLGINLSPDLAESPRFI) served as a negative control [11]. As we expected, P8 had a good positive response to the sera from SARS patients (positive in 9/10 SARS patients). However, it also had a false positive response to the sera of normal individuals (positive in 3/6 cases), whereas the control peptide showed a negative response to sera both from SARS patients and from uninfected normal individuals (Table 3 ).
Table 3.
The immunoreactivity of P8 peptide to the IgG of SARS patients’ sera was determined by ELISA
| Group | P8 peptide | IL‐12R peptide | ||||
|---|---|---|---|---|---|---|
| No. | P | % | No. | P | % | |
| SARS patients | 10 | 9 | 90 | 10 | 0 | 0 |
| Health control | 6 | 3 | 50 | 6 | 0 | 0 |
It was likely that a short peptide (about 20 amino acid residues in length) was not sufficient to mimic a specific B cell epitope within the S protein. In addition to linear B cell epitopes, the conformational structure in the synthetic peptide might also be helpful to form the specific binding site recognized by its corresponding Ab in vivo.
We assumed that extending the peptide longer may be helpful to create a specific binding site. The P8 peptide was extended to 30 amino acids (Sequence: PSSKRFQPFQQFGRDVSDFTDSVRDPKTSE and referred to PL8). To confirm our hypothesis, S protein‐specific IgG was purified from the sera of SARS patients. The binding affinity of PL8 and P8 to the IgG was compared by ELISA. As we expected, PL8 showed higher affinity than that of P8 (Fig. 4 A). The results supported our hypothesis.
Figure 5.
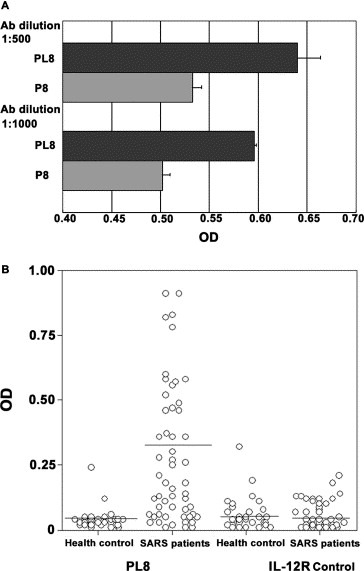
The extended PL‐8 peptide showed higher immunoreactivity and higher specificity than P8. (A) The PL‐8 had a higher affinity to the IgG purified from the sera of SARS patients than that of P8. (B) The specificity of peptides to SARS sera was compared between PL8 and IL‐12R peptides. PL8 showed a high specificity and affinity to SARS sera, but it did not response to the sera from health control.
To further confirm whether PL8 could be suitable for diagnosis of SARS, the sera were obtained from 58 diagnosed SARS patients and 40 uninfected normal individuals. The binding specificity of PL8 to SARS‐specific IgG was measured by ELISA. In the parallel experiments, 70% of 58 SARS patients tested were positive. In contrast, only 5% of the normal healthy individuals tested were positive. (Fig. 4B). The control peptides, IL‐12R, had a very low rate of binding to both sera from the patients and the uninfected people. It was likely that the 30 amino acids length peptide might create a conformational structure within the S protein recognized S protein‐specific Ab in vivo.
3.5. Synthetic peptides suppress interaction between ACE2 and S protein
A syncytia formation model has been widely used as an assay to detect neutralizing activities of Abs and peptides [10].
Initially, to make sure whether our syncytia formation model works well, we tested the model by using a neutralizing monoclonal Ab, which was generated in our lab. It is specific to S protein (S310–510 fragment) of SARS‐CoV and its neutralizing activity had been confirmed in SARS‐CoV infected Vero E6 cells (data not shown). The data demonstrated that the syncytia formation was inhibited by this Ab (Fig. 5A and B). The results indicate that the model is suitable for testing the neutralizing activities of Abs or peptides.
Then a potential prevention of SARS infectivity by those peptides or their corresponding Abs was evaluated with this model. It is interesting to note that, compared with control antisera, only a few of the antisera had a minor influence on syncytia formation, such as anti‐P6, P8, P10 antisera (Fig. 6 A). The results showed that all peptide‐induced polyclonal Abs were unable to inhibit syncytia formation, suggesting that the tested Abs did not have neutralizing activities. When PL8‐induced antiserum was tested with the model, it also did not show an inhibition (data not shown).
Figure 6.
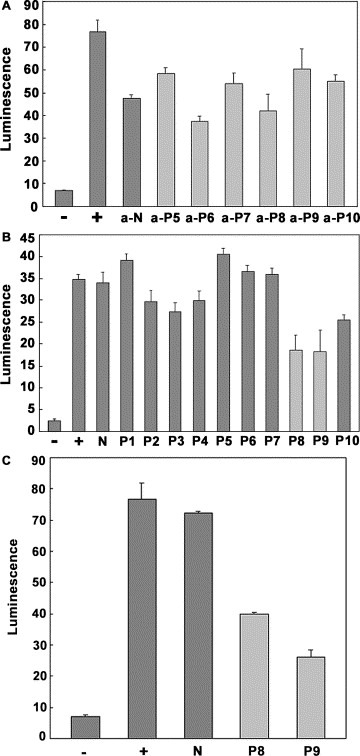
Neutralization test on S‐ACE2 syncytia formation model. (A) Six antisera from P5 to P10, which have good reaction with S proteins, were selected to test their inhibition. Only anti‐P6 and anti‐P8 Abs showed minor inhibition. (−) group: ACE2 is not transduced into the HEK293T cells, so no syncytia formation. (+) group: no Ab was used in the test, result in the completed syncytia formation. a‐N group: antibodies against an irrelative peptide N1 (from N protein of SARS‐CoV) was used in the test as a control. (B) All of the peptides were used in the test, and peptides P8 and P9 can inhibit the syncytia formation to nearly 50%. (−) group: ACE2 was not transduced into the HEK293T cells, so no syncytia formation; (+) group: no peptide was used in the test, resulting in complete syncytia formation; (N) group: an irrelevant peptide N1 (from N protein of SARS‐CoV) was used in the test as a control; no inhibition was observed. (C) Only P8 and P9 peptides were selected to do the test again, and the data demonstrate the inhibition remarkably. All of the experiments were repeated several times. (−) group: ACE2 was not transduced into the HEK293T cells, so no syncytia formation. (+) group: no peptide was used in the test, resulting in complete syncytia formation. (N) group: an irrelevant peptide N1 was used in the test, no inhibition was observed.
Recently, there are many reports demonstrating that peptides themselves are a good candidate for anti‐virus drug design [12]. In this study, we also tested all peptide‐mediated inhibition by using the model. It was obvious that the P8 and P9 peptides could significantly inhibit syncytia formation (Fig. 6B and C). The data indicated that the regions spanning P8 and P9 peptide sequences within the S protein might represent a new target for drug development.
4. Discussion
SARS‐CoV spike protein is an essential structural protein in the virus’ life span. It has an important function in SARS pathogenesis [13]. It not only plays an important role in viral infection, but it also is a potential drug target. In this study, we probed for the B cell epitopes on the S protein by bioinformatics analysis and peptide immunization in vivo, and explored their potential usage in disease diagnosis and treatment.
Although most peptides showed a high antigenic index (AI) and epitope prediction score, according to bioinformatics analysis, only a few of them could induce a strong and specific immune response. Our data suggested that the AI for peptides was not a crucial factor, in fact, the flexible region, hydrophilicity and surface probability were also important factors for epitope prediction.
In order to test the bioinformatics analysis results, a series of experiments were performed. To confirm that these peptides contain relevant B cell epitopes within the S protein of the SARS‐CoV, the native S protein from the lysates of SARS‐CoV infected Vero E6 cells and purified S protein‐specific IgG from SARS patients were used to determine their antigenicity and immunoreactivity. The data demonstrate that the P8 spanning region contains a good B cell epitope on S protein and that it has the potential for application in SARS diagnosis.
Unexpectedly, the P8 peptide was highly antigenic, but had some cross reactivity with sera obtained from uninfected normal individuals. Its sequence analysis showed that small fragments of 6–8aa in length of the P8 peptide (PSSKRFQPFQQFGRDVSDFT) shared homology with other pathogens and human tissue antigens. One report demonstrated that a bootscan recombination analysis of the spike gene revealed high nucleotide identity between the SARS virus and a feline infectious peritonitis virus throughout the gene, except for a 200‐base‐pair region of high identity to an avian sequence, indicating a mammalian‐avian mosaic origin for the host‐determining spike protein [14]. Furthermore, in our experiments, P8 also had a higher cross reactivity with sera from autoimmune disease patients, such as SLE (data not shown). Our results suggest that the P8 peptide has homologies either to normal tissue antigens or to other pathogens which were contacted by uninfected normal individuals during their lifetime. This may account for the cross reaction.
Then we attempted to optimize the P8 peptide by extending its length by adding a 10aa length fragment at its C terminal. The obtained peptide PL8 had a specific response rate of 70% in SARS patients whereas the false positive rate was below 5% in uninfected normal individuals (2/40). It is possible that the longer PL8 can form a conformational B cell epitope, which is more specific and immunogenic than the shorter P8. Our data confirm this hypothesis.
Currently there are many diagnostic methods for SARS detection, such as real time RT‐PCR and ELISA with virus lysates as an antigen. RT‐PCR is too expensive to be applied in small hospitals, and its use was limited in a clinical setting due to difficulties in sample collection and low levels of accuracy [15]. ELISA is a simple and convenient method to detect SARS, but a diagnostic kit for SARS‐CoV is not available. Currently, some methods, such as those employing lysates of virus infected Vero E6 cells as antigen, could be used for SARS diagnosis, but their specificity is not adequate and safety is a concern, too. The peptide‐based SARS ELISA test has some advantages. This test is much cheaper and quicker than others, such as RT‐PCR. The peptide is easy and safe to prepare as compared to whole virus lysates. It is conceivable that the test can be optimized by using more than one peptide which often will improve the sensitivity and reliability of a diagnostic test. In this study, we demonstrated that PL8 is a good candidate for usage in serological diagnosis of SARS.
Synthetic peptides are also useful to develop efficient SARS therapeutic drugs. For example, peptide immunization can generate specific Abs [16, 17, 18]. If a peptide containing a neutralizing epitope, which can inhibit the interaction between virus spike protein and its ACE2 receptor. The epitope can be used for generating an effective neutralizing Ab or for vaccination. The peptide itself can also be used as an agent to inhibit virus infection, if it can provide a spatial block between S protein and ACE2 [12].
The spike protein‐ACE2 syncytia formation model mimics the SARS‐CoV infection processes and this model can be safely used in a laboratory. Some antisera, peptides or small chemical molecules can be selected to screen their neutralizing ability by using this model.
In this study, we successfully identified four B cell epitopes on the spike protein of SARS‐CoV, but they did not show a neutralizing activity. However, when all the peptides were tested for their neutralization ability, P8 and P9 peptides significantly inhibited syncytia formation. When their sequences were compared, P8 (540 PSSKRFQPFQQFGRDVSDFT 559) and P9 (731CANLLLQYGSFCTQLNRA LSGIA 753) did not show any special characteristics. The two peptides spanning sequences within the S protein are different from the ACE2 binding site (from S318 to 510) or the fusion motif (S889‐972 and S1142‐1185) [12, 19]. Our data indicate that the two regions (P8 and P9) from S protein may contain a crucial new drug target, but the actual mechanism of the inhibition is unclear.
In summary, bioinformatics analysis and peptide immunization in vivo are essential to identify B cell epitopes on a given antigen, such as SARS‐CoV spike protein. PL8 represents an immunogenic peptide sequence from S protein, which has a potential to be used in SARS diagnosis. The neutralization ability of P8 and P9 peptides indicates that the respective regions of the S protein may contain a new target for anti‐SARS drug development.
Acknowledgments
We thank Dr. Peter Reinach (from Columbia University) for critical reading of the manuscript. This work was supported by Grants of National Natural Science Foundation #30340031, Chinese Academy of Sciences #KSCX2‐SW‐225, National Key Basic Research Program of China #2003CB514106, Science and Technology Commission of Shanghai Municipality #03DZ19119 to J.R. Wu; the Grants of Technology Commission of Shanghai Municipality (03DZ19113, 04DZ19108), National Key Basic Research Program of China (2001CB510006), National Natural Science Foundations of China (30170888 and 30340032), 973 Project (G1999053907), SARS 973 Grant (2003CB514117), Grant of Sino‐Germany center on SARS project (GZ238(202/11)), the Outstanding Young Scientist Fund by The National Natural Science Foundation of China (30228016 and 30325018) and Grant from E‐institutes of Shanghai Universities Immunology Division and Grant of 04DZ14902 to B. Sun.
Lu Wei,Wu Xiao-Dong,Shi Mu De,Yang Rui Fu,He You Yu,Bian Chao,Shi Tie Liu,Yang Sheng,Zhu Xue-Liang,Jiang Wei-Hong,Li Yi Xue,Yan Lin-Chen,Ji Yong Yong,Lin Ying,Lin Guo-Mei,Tian Lin,Wang Jin,Wang Hong Xia,Xie You Hua,Pei Gang,Wu Jia Rui and Sun Bing(2005), Synthetic peptides derived from SARS coronavirus S protein with diagnostic and therapeutic potential, FEBS Letters, 579, doi: 10.1016/j.febslet.2005.02.070
Contributor Information
Jia Rui Wu, Email: wujr@sibs.ac.cn.
Bing Sun, Email: bsun@sibs.ac.cn.
References
- 1. Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med., 348, (2003), 1967– 1976. [DOI] [PubMed] [Google Scholar]
- 2. Ksiazek T.G., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med., 348, 20 (2003), 1947– 1959. [DOI] [PubMed] [Google Scholar]
- 3. Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S., The genome sequence of the SARS associated coronavirus. Science, 300, (2003), 1399– 1404. [DOI] [PubMed] [Google Scholar]
- 4. Wenhui Li, Moore Michael J., Natalya Vasilieva, Jianhua Sui, Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature, 426, (2003), 450– 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu Xiao-Dong, Shang Bo, Yang Rui Fu, Yu Hao, Ma Zhi-Hai, Sun Bing, The spike protein of severe acute respiratory syndrome (SARS) is cleaved in virus infected Vero-E6 cells. Cell Res., 14, 5 (2004), 400– 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qin E.D., Zhu Q.Y., Yu M., Fan B.C., Chang G.H., Si B.Y., A complete sequence and comparative analysis of a SARS-associated virus (Isolate BJ01). Chin. Sci. Bull., 48, (2003), 941– 948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xiao X., Chakraborti S., Dimitrov A.S., Gramatikoff K., Dimitrov D.S., The SARS-CoV S glycoprotein: expression and functional characterization. Biochem. Biophys. Res. Commun., 312, 4 (2003), 1159– 1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin Ying, Shen Xu, Yang Rui Fu, Li Yi Xue, Ji Yong Yong, He You Yu, Identification of an epitope of SARS-coronavirus nucleocapsid protein. Cell Res., 13, 3 (2003), 141– 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zeng R., Yang R.F., Shi M.D., Jiang M.R., Characterization of the 3a protein of SARS-associated coronavirus in infected vero E6 cells and SARS patients. J. Mol. Biol., 341, 1 (2004), 271– 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sui Jianhua, Li Wenhui, Murakami Akikazu, Tamin Azaibi, Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proc. Natl. Acad. Sci. USA, 101, (2004), 2536– 2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu Yadi, Hou Wanqiu, Wang Yangping, Sun Bing, IFN-γ up-regulation of IL-12β2 receptor expression was involved in the susceptibility to experimental autoimmune uveitis. J. Neuroimmunol., 137, (2003), 145– 153. [DOI] [PubMed] [Google Scholar]
- 12. Bosch Berend Jan, Martina Byron E.E., van der Zee Ruurd, Lepault Jean, Severe acute respiratory syndrome coronavirus (SARS-CoV) infection inhibition using spike protein heptad repeat-derived peptides. Proc. Natl. Acad. Sci. USA, 101, (2004), 8455– 8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ho T.Y., Wu S.L., Cheng S.E., Wei Y.C., Huang S.P., Hsiang C.Y., Antigenicity and receptor-binding ability of recombinant SARS coronavirus spike protein. Biochem. Biophys. Res. Commun., 313, 4 (2004), 938– 947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stavrinides J., Guttman D.S., Mosaic evolution of the severe acute respiratory syndrome coronavirus. J. Virol., 78, 1 (2004), 76– 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Poon Leo L.M., Chan Kwok Hung, Wong On Kei, Yam Wing Cheong, Yuen Kwok Yang, Guan Yi, Early diagnosis of SARS coronavirus infection by real time RT-PCR. J. Clin. Virol., 28, (2003), 233– 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ijaz M.K., Alkami T.O., El-Mekki A.W., Priming and induction of anti-rotavirus antibody response by synthetic peptides derived from VP7 and VP4. Vaccine, 13, (1995), 331– 338. [DOI] [PubMed] [Google Scholar]
- 17. Taouji Saïd, Nomura Izumi, Giguère Steeve, Tomomitsu Seiji, Immunogenecity of synthetic peptides representing linear B-cell epitopes of VapA of Rhodococcus equi. Vaccine, 22, (2004), 1114– 1123. [DOI] [PubMed] [Google Scholar]
- 18. Wang Jingqiang, Wen Jie, Li Jing Xiang, Yin Jianning, Assessment of immunoreactive synthetic peptides from the structural proteins of severe acute respiratory syndrome coronavirus. Clin. Chem., 49, 12 (2004), 1989– 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wong S.K., Li W., Moore M.J., Choe H., Farzan M.A., 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J. Biol. Chem., 279, 5 (2004), 3197– 3201. [DOI] [PMC free article] [PubMed] [Google Scholar]


