Graphical abstract
Abbreviations: APC, antigen-presenting cell; BMA, butyl methacrylate; C16, 2-(R/S)-hexadecanoic acid; (C18)2, N,N-dioctadecyl succinamic acid; CFA, complete Freund's adjuvant; CuAAC, copper-catalyzed azide-alkyne cycloaddition; DLS, dynamic light scattering; ELISA, enzyme-linked immunosorbent assay; FDA, Food and Drug Administration; GAS, group A streptococcus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HPV, human papilloma virus; IFA, incomplete Freund’s adjuvant; IgG, immunoglobulin G; LCP, lipid core peptide; OVA, ovalbumin; PADRE, pan DR epitope; Pam2Cys, dipalmitoyl-S-glyceryl cysteine; Pam3Cys, tripalmitoyl-S-glyceryl cysteine; PbCSP, Plasmodium berghei circumsporozoite protein; PBS, phosphate-buffered saline; PDSMA, pyridyl disulfide methacrylamide; PEG-PPS, poly(ethylene glycol)-stabilized poly(propylene sulfide) core nanoparticle; SAP, self-assembling polypeptide; SARS, severe acute respiratory syndrome; TEM, transmission electron microscopy; TLR2, toll-like receptor 2; TLR4, toll-like receptor 4; TLR9, toll-like receptor 9; T-VEC, talimogene laherparepvec; VLP, virus-like particle
Keywords: Self-assembly, Nanoparticle, Nanofiber, Vaccine, Conjugation, Polymer, Lipopeptide
Highlights
-
•
Smaller polymer-peptide conjugates-based nanoparticles are often more immunogenic.
-
•
Lipid-antigen conjugates-based nanoparticles can interact with immune receptors.
-
•
Peptides with β-sheet conformation usually form nanofibers.
-
•
α-Helical and random coil peptides tend to self-assemble into nanoparticles.
-
•
Peptide-based nanostructures are usually poorer inducers of immune responses.
Abstract
Peptide based-vaccines are becoming one of the most widely investigated prophylactic and therapeutic health care interventions against a variety of diseases, including cancer. However, the lack of a safe and highly efficient adjuvant (immune stimulant) is regarded as the biggest obstacle to vaccine development. The incorporation of a peptide antigen in a nanostructure-based delivery system was recently shown to overcome this obstacle. Nanostructures are often formed from antigens conjugated to molecules such as polymers, lipids, and peptide, with the help of self-assembly phenomenon. This review describes the application of self-assembly process for the production of peptide-based vaccine candidates and the ability of these nanostructures to stimulate humoral and cellular immune responses.
1. Introduction
A vaccine is an agent that can confer or improve the immunity of individuals to specific diseases. Vaccines are typically composed of attenuated or inactivated microorganisms or viruses [1], or their fragments [2], [3], [4], [5]. Since the development of the first vaccine two hundred years ago [6], vaccination has become one of the most popular interventions against a variety of infectious diseases. Moreover, a vaccine is the only available choice to combat infection where no effective drug treatment exists (e.g. Poliomyelitis) [7]. Compared to classical drugs, which require multiple doses, the long-term protection against specific pathogens or diseases makes vaccines a more cost and time effective choice. Additionally, in comparison to drugs, vaccines have typically lower toxicity, (especially as they are normally used as a single dose treatment) and mass vaccination can significantly control the spread of infectious diseases.
Classically, all vaccines were prophylactic; however, many modern vaccines are designed to eliminate existing disease (e.g. cancer) and thus are therapeutic. Prophylactic vaccines usually offer long-term protection against infectious diseases. In contrast to prophylactic vaccines, which usually induce humoral immune responses (antibody production), therapeutic vaccines are designed to stimulate a cytotoxic T lymphocyte response to eliminate cells that are already infected [8], [9], [10]. Therapeutic vaccines are a growing pharmaceutical sector. Sipuleucel-T (Provenge®) was approved by Food and Drug Administration (FDA) in 2010 for prostate cancer [11], [12] and talimogene laherparepvec (T-VEC) (Imlygic™) was introduced against metastatic melanoma in 2015 [13], [14], [15].
Despite their advantages, vaccines also have some drawbacks. Traditional vaccines based on attenuated and inactivated pathogens may revert to virulent form [16], [17], cause autoimmunity or strong allergic responses [18], [19]. Additionally, the production and stability of this type of vaccine is often challenging. Subunit vaccines contain a small fragment of the whole pathogenic organism, for instance a recombinant protein, peptide, carbohydrate [20], [21], [22], [23]. Recombinant protein-based vaccines cause fewer adverse effects, but the difficulty with protein extraction and possible contamination during production are significant disadvantages. Recombinant vaccine can also trigger undesired immune responses. To avoid these obstacles minimal fragment of protein caring immunological information (peptide epitope) can be used for vaccine design [3]. However, peptide-based vaccines still have their own Achilles’ heel. Peptides themselves are usually not immunogenic [3], [24]; therefore, the use of immunostimulating agents (adjuvants) are essential for the success of this vaccine formulation [25].
Adjuvants are compounds that are able to evoke or enhance the immune response against co-delivered antigens [26], [27], [28]. A number of adjuvants have already been developed, for example Freund’s adjuvant, lipid A, cholera toxin, aluminum salts, cytokines, liposomes, saponins, and CpG oligodeoxynucleotides [29], [30], [31]. However, the immunostimulating properties of these adjuvants are often poor or their toxicity is significant, therefore most of them are not suitable for human treatment. Thus, safer and more efficient adjuvants are urgently required [27].
Nanotechnology is one of the most quickly growing fields of science and engineering. It has been widely applied in materials, electronics, manufacturing, agricultural science, chemistry and bioscience [32], [33], [34], [35], [36], [37]. In bioscience, nanotechnology is used to create sensors, medical imaging and diagnostic tools, and for drug delivery. The most widely used nanostructures in this field are nanoparticles. Nanoparticles are often defined as particles with diameters ranging from 1 to 100 nm [24], [38], while particles with diameter within the range of 100 to 1000 nm are considered sub-micro particles. However, following common understanding of “nano-size” range, nanostructures in this article are defined as any nanometer-sized object ranging from 1 to 1000 nm [39], [40].
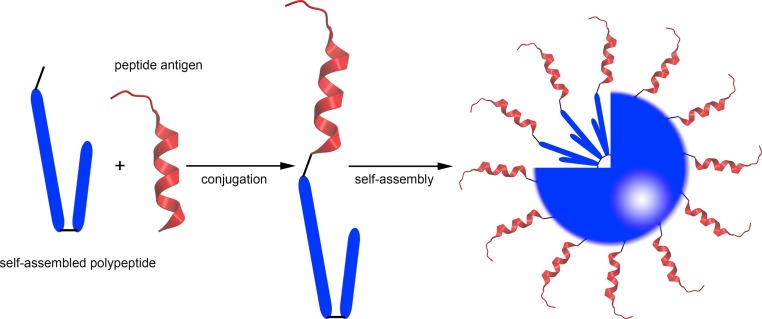
Nanoparticles are especially useful in vaccine design. Many pathogens are within the nanometer range, nanoparticles are usually taken up efficiently by antigen-presenting cells (APCs) [41]. APCs are the key element of the primary innate immune system [42] and are responsible for triggering further adaptive immune responses. Therefore nanostructures often have immunostimulating properties and help to trigger immune reactions against the antigens they delivered (Fig. 1 ) [24], [43], [44]. Moreover, the surface of nanostructures can be modified with a specific targeting moiety for better APC recognition and uptake [45], [46], [47].
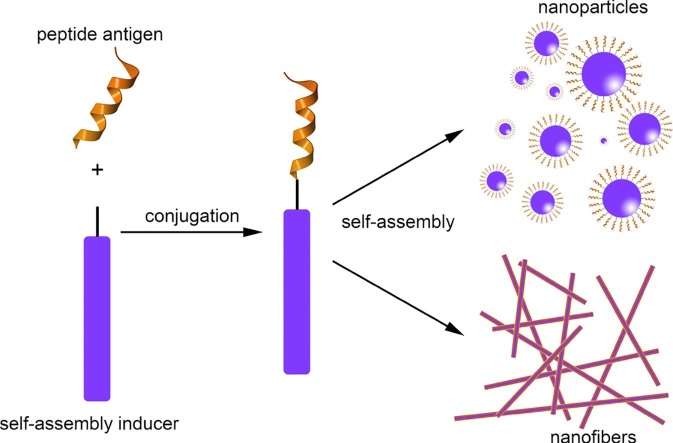
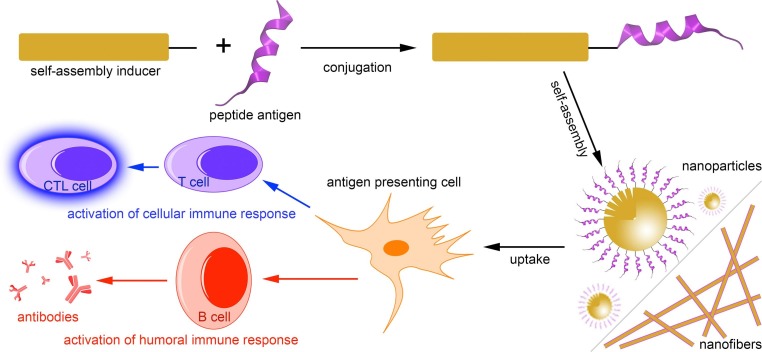
Fig. 1.
Schematic representation of the self-assembled peptide subunit vaccines and the immune responses induced upon immunization with these nanostructures.
There are two main approaches to creating nanostructures: (1) mechanically breaking a large material into nanostructures, or (2) building up nanostructures from basic segments, often via a self-assembly process [48]. The first approach uses external forces like grinding, milling, or high pressure to break down the original material [49], [50], [51]. This process adds additional complexity to producing a nanostructure and the harsh conditions used to break down the initial component may damage the antigen. Nanostructures produced by this method typically have a low loading capacity and are often not biodegradable and thus may accumulate in the human tissues. Meanwhile, self-assembly-based strategies for production of nanoparticle vaccines can overcome these problems.
Here, we highlight how the use of carrier-antigen conjugates forming self-assembling nanostructures can be effectively combined with the rational vaccine design. The formation of self-assembled nanoparticles from carrier-antigen (peptide epitopes) conjugates, and their ability to generate humoral/cellular immune responses are discussed (Fig. 1). Extensive reviews on non-conjugated self-assembled antigen carrier, such as liposomes, can be found in the recent literature [52], [53], [54], [55]. Similarly, virus-like particles (VLPs), which are formed by self-assembly processes, have been reviewed extensively in the recent literature [56], [57], [58] and therefore are beyond the scope of this article.
2. Self-assembled nanostructures
Self-assembly refers to the phenomenon in which basic constituents arrange into a spontaneously ordered structure or pattern. Examples of self-assembly can be found in the macro-world (e.g. self-assembly of stars and galaxies [59]) as well as at micro and nano scale, many molecules were found to self-assemble into nano- or micro-structures [60]. Molecular self-assembly is defined as the spontaneous arrangement of molecules by non-covalent interactions [61], [62] directed by internal and external factors [63]. Internal factors are the properties of the nanostructure component molecules, for instance hydrophilic/hydrophobic ratio, charge, hydrogen bond formation, molecular weight, and molecular conformation [64], [65]. External factors are the environmental conditions surrounding the nanostructure such as pH, concentration, temperature, and solvent [66], [67].
Incorporating a self-assembly process in the production of drug or vaccine constructs offers several advantages. Firstly, the properties (for instance size, composition and charges) of the nanostructure itself can be precisely defined and have high batch-to-batch consistency [68]. Well-defined physical and chemical properties are crucial for any biomedical application of nanostructures. Secondly, the preparation of self-assembled nanostructure is more efficient and economical because the nanostructure is formed spontaneously. The minimal use of chemicals during self-assembly makes the process environmentally friendly. Thirdly, nanostructures formed by self-assembly process are expected to biodegrade easily because the basic units aren’t chemically cross-linked. Additionally, the well-defined properties of nanostructural material can make it easier to be approved for medical use by regulatory bodies such as the FDA.
Self-assembly acquires additional complexity when used in vaccine design. Normally, the structure (peptide sequence) of the antigen cannot be modified without affecting its immunological properties. Thus the self-assembly process is usually controlled by the carrier (assembly inducer) conjugated to the antigen (Fig. 1). Peptide antigens are often hydrophilic; therefore, hydrophobic moieties such as polymers, lipids and special peptides usually fill this role. This results in amphiphilic conjugates that can self-assemble into an ordered nanostructure.
3. Polymer-based self-assembled nanostructures
A polymer alone or conjugated to other moieties can exhibit amphiphilic properties and self-assemble into particles. These self-assembled particles can be used for vaccine delivery to protect the antigen from enzymatic degradation, enhance antigen uptake by APCs, and may have self-adjuvanting properties, removing the potential adverse effects associated with the use of classical adjuvants. The structure of polymers can easily be altered, thus the size, charge, mucoadhesion of the nanoparticle vaccines can be adjusted. Nanoparticle vaccines that self-assemble from polymer-antigen conjugates have only been developed recently [69].
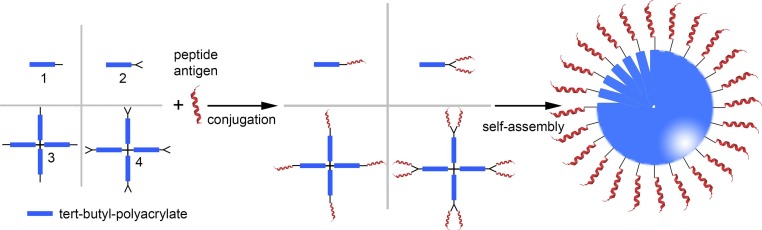
In 2010, Toth and colleagues had developed the first self-assembled tert-butyl polyacrylate peptide antigen conjugate-based vaccine [69]. Polyacrylate dendritic polymer 4 (Fig. 2 ) was obtained via successive atom-transfer radical polymerization. Multiple copies of hydrophilic B cell epitope (J14; KQAEDKVKASREAKKQVEKALEQLEDKVK) derived from a major GAS virulence factor (M protein) were attached to the terminal alkynes of hydrophobic dendrimer 4 via copper-catalyzed azide-alkyne cycloaddition (CuAAC) reaction [77], [78] to form amphiphilic conjugate 4-J14. Self-assembly under solvent exchange conditions resulted in 21 nm particles with a narrow size distribution. Subcutaneous immunization with these nanoparticles induced strong systemic humoral immune responses in mice. The titers of specific immunoglobulin G (IgG) secreted were similar to those induced by complete Freund's adjuvant (CFA). CFA is a powerful adjuvant often used as “gold standard” during vaccine development process; however, it is too toxic for human use [79]. The conjugation between polymer and peptide was crucial to induce immune responses as physical mixture of polymer and antigen did not induce antibody production. These nanoparticles were also able to induce high IgG titers after intranasal administration [70]. This antibody was able to partially opsonize one of the common GAS strains. Interestingly, when polymer 2 (Fig. 2) was conjugated to J14 epitope, the conjugate formed larger nanoparticles (∼500 nm) than their 4-J14 counterpart [71]. The larger particles induced significantly lower IgG titers than the CFA-adjuvanted control or 4-J14 (20 nm). Finally, a small library of polyacrylates 1–4 were investigated, which upon conjugation with peptide bearing J14 epitope and pan DR epitope (PADRE) universal T-helper (used to target the immunologically diverse “outbred” human population) formed large nanoparticles (100–1000 nm) [72]. All of them induced a strong humoral immune response, equipotent to that of CFA, despite the relatively large size of the particles. These antibodies efficiently killed clinical GAS isolates in opsonization experiments. Thus, the polyacrylate-polymer-based delivery system was an effective means of inducing a strong humoral immune response in both inbred and outbred mousel model.
Fig. 2.
Schematic representation of the application of polyacrylates in vaccine development. Linear and branched alkyne derivatives of polyacrylates (1–4) were conjugated to the chosen peptide antigen to produce polymer-based vaccine candidates. The conjugates were able to self-assemble into nanoparticles. This system has been used to deliver the following peptide antigens: B cell epitope derived from group A streptococcus M protein - J14 [69], [70], [71], [72]; Human Papilloma Virus (HPV)-16 E7 protein epitope 8Q, and its derivatives 8Qmin, 8QSer, 8QLys[73], [74], [75], [76].
Liu et al. applied a polyacrylate-based self-assembled nanoparticle delivery system to induce cellular immune responses and designed several therapeutic vaccine candidates against human papilloma virus (HPV)-related cancer (Fig. 2) [73], [74], [75], [76]. Initially, polyacrylate 3 was chosen as a polymeric core and 8Q (QAEPDRAHYNIVTFCCKCD; E744−62) epitope from human papilloma virus (HPV-16) E7 protein as an antigen. The 8Q epitope sequence contains cysteines that may form disulfide bonds and result in uncontrolled aggregation of conjugates. Therefore, three modified epitopes, 8QSer (QAEPDRAHYNIVTFSSKSD), 8QLys (QAEPDRAHYNIVTFSKKKK), and 8Qmin (QAEPDRAHYNIVTF) were designed and conjugated to the polymer 3. The polymer-peptide conjugates were self-assembled in water to form small nanoparticles (∼26 nm, 3–8QLys), big nanoparticles (∼530 nm, 3–8QSer) and microparticles (>1 μm, 3–8Qmin). However, all of the conjugates aggregate to form microparticles (∼10 μm) when formulated in phosphate-buffer saline (PBS) for the immunization study.
The microparticles were tested in an in vivo tumor challenge model in C57BL/6 mice. An emulsion of the 8Q epitope and incomplete Freunds adjuvant (IFA)-like adjuvant (Montanide IS A51) was used as the positive control [73]. Compound 3–8Qmin initiated stronger immune responses and tumor growth was slower in mice vaccinated with 3–8Qmin than with the positive control. The 8Qmin epitope was most effective in the tumor challenge study, thus it was conjugated to polyacrylate polymers that varied in their level of branching (1–4) [74]. All polymer-peptide vaccine candidates were able to self-assemble into microparticles with size range 12–17 μm, and a narrow size distribution (span 1.1–2.0). The dendrimer with highest level of branching induced the strongest antitumor responses in a tumor challenge study. The survival rate of mice vaccinated with 1–8Qmin, 2–8Qmin and 4–8Qmin was 90%, while only 40% of mice survived when immunized with the IFA-like adjuvant. Importantly, all mice immunized with 4–8Qmin which survived were tumor free at the end of the experiment. Conjugate 4–8Qmin also had the most efficient uptake by APCs and stimulated strong CD4+ and CD8+ T cell activation. In immune cell depletion experiment, it was confirmed that CD8+ T cells were crucial for vaccines anticancer activity [80].
Other polymers have been used to form self-assembled nanoparticle systems. Perrier and colleagues conjugated two different length epitopes (GSTA and GVTSAPDTRPAPGSTAPPAH) from extracellular variable number tandem repeat region of cancer-associated glycoprotein MUC1 to poly(n-isopropyl acrylamide) by CuAAC reaction, respectively, to produce vaccine candidates [81]. The vaccine candidates were able to self-assemble into 20 nm and 130 nm nanoparticles, respectively. However, the efficacy of these nanoparticles was not confirmed in an immunization experiment. Hubbell and coworker developed a poly(ethylene glycol)-stabilized poly(propylene sulfide) core nanoparticle (PEG-PPS) polymer that self-assembled to form 25 nm nanoparticles [82]. When ovalbumin (OVA) antigen was conjugated to the PEG-PPS nanoparticles via a disulfide bond, the particle size increased to 35 nm. Upon the intradermal immunization, this vaccine candidate induced higher antibody titres but lower cytokine production than soluble antigen alone. Keller et al. also conjugated OVA to self-assembled amphiphilic diblock copolymers poly(HPMA-co-pyridyl disulfide methacrylamide (PDSMA))-b-(PAA-co-poly(dimethylaminoethyl methacrylate)-co-butyl methacrylate(BMA)) to form 30 nm (pH = 7.4) nanoparticles [83]. In this case, the polymer was allowed to self-assemble before OVA protein was conjugated to the particle via a disulfide bond. The conjugate was more efficiently taken up by APCs and, after subcutaneous immunization, induced stronger interferon gamma (IFN-γ) production (a correlate of T cell activity) than a physical mixture of OVA protein and carrier.
Although polymer based self-assembled nanostructures are very effective at stimulating immune responses, they are not fully defined. The lengths of polymers cannot be precisely controlled during the polymerization reaction. This is a disadvantage from a regulatory perspective because it may influence the size of the vaccine particles. Indeed, relationship between degree of polymerization and size of self-assembled polymer nanoparticle was reported [84]. Additionally, the conjugation between the polymer and specific epitopes often produce products with variable degree of substitution. Although non-biodegradable polymers can form stable nanoparticles, the potential in vivo toxicity of this type of material must be considered. In the case of biodegradable polymers, the properties (for example size, charge, etc.) of nanovaccines may change before the vaccine can reach its target in the body, and thus such vaccine may not induce the desired immune response [3]. Consequently, other materials have been investigated for the development of peptide-based subunit vaccines.
4. Lipid-based self-assembled nanostructure
Lipids are one of the most important biomolecules in the nature. They are involved in energy supply; are the main components of biological membranes; act as steroidal hormones, enzyme activators, growth factors, signaling molecules in the neural system, and antioxidants in living organisms. In the immune system they often play role of “danger signal” via activation of the toll like receptors (TLRs) such as TLR2 and TLR4. These receptors are important membrane proteins present on the surface of APCs that can activate the immune system in response to foreign substances such as lipopolysaccharides or lipopeptides present on the membranes of microbial pathogens [31], [85], [86]. With the exception of aluminum- and virus like particle (VLPs)-based adjuvants, all other adjuvants in commercial vaccines are lipid-based formulations (i.e. AF03, MF59, AS03, AS04 and liposomes).
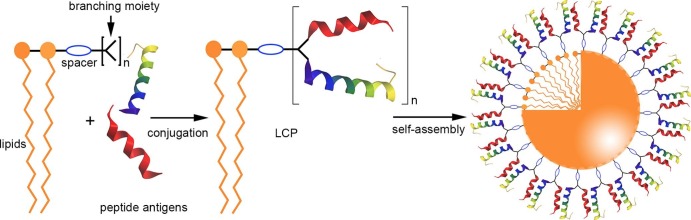
Several lipidic self-assembled nanoparticle vaccine candidates have been developed using the amphiphilic properties of lipid-peptide conjugates. One of most intensively studied lipidic delivery systems, the lipid-core peptide (LCP), was first proposed in 1993 by Toth et al. (Fig. 3 ) [87]. This system incorporated peptide epitopes, a branching moiety, and lipids (e.g. 5) linked together by covalent bonds to produce a single-molecule vaccine candidate. Similar to other amphiphilic conjugates, LCP-based vaccine candidates could self-assemble into nano- or microparticles under aqueous conditions [88], [89], [90] (Fig. 3). Moreover, the lipidic moiety in LCP is recognized by TLR2, thus has self-adjuvanting properties [91], is preferentially taken up by APCs, and induced strong immune responses against the incorporated antigens [92], [93]. For example, Fuaad et al. synthesized an LCP-based vaccine candidate against schistosomiasis carrying an epitope (p2, KQAEDKVKAGPTDEIQKINAKQLEDKVK) derived from cathepsin D hemoglobinase [94], [95]. The conjugate self-assembled into nanoparticles with diameters of 30–80 nm. The p2-LCP induced high IgG titers after subcutaneous injection, similar to those triggered by the positive control: a CFA-adjuvanted p2 epitope. Moreover, the anti-p2 antibodies neutralized the enzymatic activity of the Schistosoma cathepsin D.
Fig. 3.
Schematic representation of LCP vaccine synthesis and self-assemble.
An LCP-based system was also applied in hookworm vaccine development [96], [97]. Protease Na-APR-1 was chosen as the target antigen because of its involvement in the hookworm digestive system. A neutralizing B cell epitope (A291Y, AGPKAQVEAIQKY) from this protein was chosen as the peptide epitope for conjugation into the LCP system. Several LCP vaccine candidates were synthesized with varying levels of branching. The A291Y epitope was modified to adopt helical or β-sheet conformations. All compounds were able to self-assemble into nanoparticles with diameters ranging from 12 nm to 43 nm. Interestingly, only the epitope that retained its β-sheet conformation when conjugated to the LCP was able to induce production of antibodies that recognized the parent protein. This result indicated the importance of epitope conformation for peptide subunit vaccine development. Importantly, both series of vaccine candidates that targeted hookworm and Schistosoma did not induce any hemolytic effect and were not toxic in vitro even at a high concentration (100 μM) [95], [97].
Vaccine candidates based on the LCP system against Streptococcus pyogenes have been also investigated [88], [89], [98]. For example, two types of epitopes were incorporated into LCP: B cell epitopes FNBR-B (VETEDTKEPGVLMGGQSESVEFTKDTQTGM) or FNBR-BT (EFTKDTQTGMSGQTTPQVETEDTKEPGVLM) from highly conserved fibronectin-binding repeat region (FNBR) of SfbI protein and J14 from the helical C repeat region of M protein [98]. Three monoepitopic vaccine candidates have also been synthesized (LCP bearing FNBR-B, FNBR-BT or J14). All the conjugates were able to self-assemble into particles with very distinct sizes, ranging from a few nanometers to submicron/microparticles. LCPs that bore hydrophilic epitopes formed small nanoparticles, while the incorporation of more hydrophobic peptides into LCP triggered the formation of larger particles. Thus balance between hydrophilic and hydrophobic properties of LCP component was crucial to controlling the size of the resultant nanoparticle.
Variations in the length, number, and position of lipids in the conjugate can significantly influence the particles self-assembly. For example, lipopeptide that carried short lipids adopted random coil secondary structure and self-assembled in nanoparticles while longer lipids induced β-sheet formation and the production of fibrils [99]. These structural differences may influence the immunological properties of particle-based vaccines. For instance, size was found to play an important role when LCP-based vaccines were administered intranasally. Smaller nanoparticles (5–10 nm) were taken up more efficiently by APCs and strongly enhanced APC-maturation to generate robust immune responses, in comparison to bigger particles (∼100 nm) [100]. Furthermore, lipopeptides that carried longer lipids induced stronger immune responses than shorter analogues [101].
The LCP system has also been applied to the delivery of multiepitope-based polypeptides [102]. A semisynthetic vaccine against GAS was produced by maleimide conjugation of LCP lipidic moiety 5 (see Fig. 4 ) obtained using simple chemical synthesis and recombinant polypeptide, expressed in Escherichia coli. The recombinant polypeptide was designed as a linear combination of multiple epitopes (eight B cell epitopes and one T helper epitope) derived from M protein. The lipopolypeptide self-assembled into 39–43 nm diameter particles. Although the vaccine candidate stimulated an immune response against the recombinant polypeptide after subcutaneously injection in C57BL/6 mice, the IgG titers against several epitopes contained within the lipopolypeptide were weaker than those induced by the CFA-adjuvanted polypeptide. The disulfide bond between cysteines, present in the polypeptide, may have changed the conformation of those epitopes, reducing the epitope-specific humoral immune response.
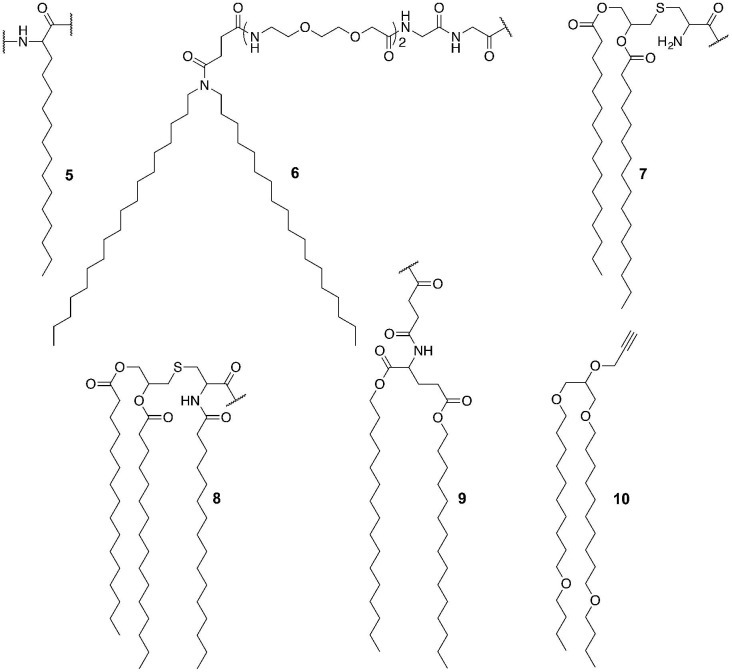
Fig. 4.
Examples of lipid moieties (5–10) present in lipid-based self-assembled nanostructures.
Other lipid moieties have also been used in self-assembled peptide vaccine development. For example, Accardo et al. conjugated lipid 6 to epitopes gB498−505 (SSIEFARL) and gD301−309 (SALLEDPVG) from HSV envelope glycoprotein B (gB) and glycoprotein D (gD), respectively, to develop a self-assembled vaccine candidate [103]. The conjugates formed 50–80 nm nanoparticles. The vaccine candidates induced more cytokine production (e.g. IL-6, IL-8, TNF-α and IL-23) than positive control lipopolysaccharide.
Dipalmitoyl-S-glyceryl cysteine (Pam2Cys, 7) and tripalmitoyl-S-glyceryl cysteine (Pam3Cys, 8) are popular TLR2 ligands in vaccine development (see Fig. 4). For example, Jackson and coworkers developed a hepatitis C virus (HCV) vaccine candidate by conjugating CD4+ T-helper and CD8+ T cell epitopes from HCV NS5B protein (NS5B2594–2602; ALYDVVTKL) to 7 [104]. The conjugate induced strong epitope-specific cellular immune responses measured through the production of IFN-γ. Wilkinson et al. conjugated 7 to tumor-associated MUC1 epitopes (peptide or glycopeptide) to produce vaccine candidates against breast cancer [105]. These compounds self-assembled into 12–20 nm particles and stimulated the production of anti-MUC1 antibodies after intradermal immunization. However, the strength of humoral immune responses was not compared to classical adjuvanting system, and therefore the efficacy of the delivery system could not be appraised.
Tirrell and coworkers incorporated a lipid building block (9) to cytotoxic T cell epitope OVA253–266 (EQLESIINFEKLTE) to develop a self-assembling and self-adjuvanting vaccine delivery system [106]. The conjugate formed 5–11 nm diameter and 50 nm–300 nm fiber-like cylindrical nanoparticles. The vaccine candidate (9-OVA253–266) induced a strong cellular immune response in a tumor challenge experiment. Mice treated with the conjugate developed smaller tumors and had significantly higher survival rates than those immunized with an emulsion of IFA and OVA253–266. Lipid 9 was also conjugated to GAS B cell epitopes J8 (QAEDKVKQSREAKKQVEKALKQLEDKVQ) [107]. This conjugate formed 5–15 nm diameter and 200 nm–2 µm long cylindrical nanoparticles. The IgG antibody titer induced by this vaccine candidate was similar to those triggered by epitopes adjuvanted with IFA. The authors suggested that despite similarity of lipid structure to a typical TLR2 ligand (i.e. Pam2Cys) the immune responses induced by the lipopeptide were not mediated by TLR2.
Recently, Hussein conjugated different lipidic moieties (7 and 10) to HPV cytotoxic T lymphocyte (CTL) epitopes E643–57 (QLLRREVYDFAFRDL) and 8Qmin to produce self-assembled nanovaccines (see Fig. 4) [108]. 10-(8Qmin)E643–57 formed 350–750 nm nanoparticles, while 7-(8Qmin)E643–57 formed much larger particles (>5 μm). The nanoparticles induced a stronger cellular immune response than the microparticles. Interestingly, a vaccine candidate that bore the well-known TLR2 receptor agonist Pam2Cys (7) did not induce a significant cellular immune response. The 7-(8Qmin)E643–57 formed rather large aggregates than particles; therefore, it was suggested that the conjugate poor aqueous solubility was the key factor influencing their vaccine candidate performance. Significantly, a vast number of lipopeptides have been reported as vaccine candidates to date [92], [109]; however, their ability to form particles has not been commonly explored.
Although lipid-peptide conjugates are able to induce a strong immune response, the potential toxicity of lipopeptides needs to be taken into account. Therefore, self-assembled peptide nanovaccines that only consist of peptide components are an important alternative.
5. Peptide-based self-assembled nanostructures
In contrast to classical polymers described above, peptide-based self-assembled nanostructures are fully defined structures. Peptides are heteropolymers composed of amino acids (typically 2–50 units), while polypeptides, similar to proteins, usually have sequences longer than 50 amino acids. Peptides that are longer than a few amino acids can form secondary structures like α-helix or β-sheet, which further may self-assemble to form nanostructures. With the development of modern chemistry, including solid phase synthesis, practically any peptide can be obtained synthetically with high purity.
When a peptide carrier with self-assembling properties is conjugated to a peptide epitope, the resulting conjugate may retain self-assembly properties of carrier, and therefore form nanostructures under aqueous conditions. Lustig, Burkhard and coworkers used in silico modeling to design a helical self-assembling polypeptide (SAP) (DEMLRELQETNAALQDVRELLRQQVKQITFLKCLLMGGRLLCRLEELERRLEELERRLEELERR) that self-assembled into regular polyhedral nanoparticles (Fig. 5 ) [110], [111]. Several vaccine candidates have been produced based on this peptide carrier and its derivatives. For example, vaccine candidate P6HRC1 was obtained by conjugating a peptide carrier to a B cell epitope from severe acute respiratory syndrome (SARS) B HRC1 spike protein and self-assembled by dialysis against the refolding buffer [112]. The diameter of the P6HRC1 nanoparticle was approximately 25 nm, while SAP alone formed smaller nanoparticles (16 nm). Mice immunized with P6HRC1 produced antibodies with high binding affinity for the native protein, confirming that the delivery system presented the epitopes in the desired conformation.
Fig. 5.
Schematic representation of a self-assembling peptide (SAP)-based vaccine.
The SAP strategy was also used to develop a malaria vaccine [113]. A tandem repeat of the B cell immunodominant repeat epitope (DPPPPNPN)2D from malaria Plasmodium berghei circumsporozoite protein (PbCSP) gene was incorporated to the SAP gene, and the vaccine candidate called P4c-Mal was produced by expression in Escherichia coli. This vaccine candidate induced a strong immune response against the incorporated B cell epitope and 60% of mice survived a double challenge with live sporozoites after receiving three doses of P4c-Mal.
The SAP derivatives that self-assembled into nanoparticles were also applied to the design of a vaccine against human immunodeficiency virus (HIV) [114]. A self-assembling polypeptide that consisted of two covalently linked peptide domains was derived from SAP. This carrier peptide was conjugated to the membrane proximal external region of HIV-1 gp41 protein. The conjugate formed 25–40 nm nanoparticles. Both intraperitoneal immunization of the conjugate with IFA and intradermal vaccination without adjuvant were then performed on outbred Sprague–Dawley rats. The SAP analogue stimulated humoral immunity against the incorporated antigen; however, these antibodies were not able to neutralize the HIV-1 gp41 protein.
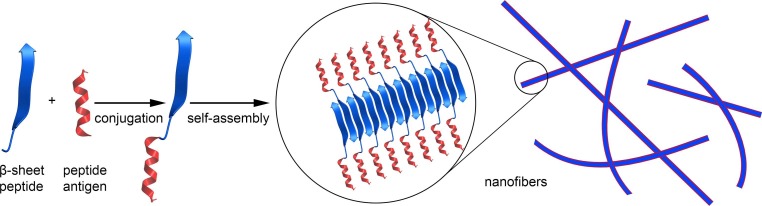
The ability of peptides that adopt β-sheet conformation to form fibers is a well-known natural phenomenon. For example, β-sheet amyloid peptides involved in Alzheimer disease spontaneously form fibrils in the human brain [115]. Waku et al. attempted to develop a novel vaccine candidate based on self-assembled nanofibers (Fig. 6 ) [116]. A peptide that forms a β-sheet (FVIFLD) was conjugated with a peptide epitope OVA257–264 (SIINFEKL) and a highly hydrophilic cell-penetrating peptide TAT (RKKRRQRRR) to form a linear oligopeptide. When incubated in 150 mM saline, this compound formed nanofibers that were a few micrometres long, but was unable to self-assemble when incubated in water. These fibers were trimmed into 240 ± 100 nm lengths by extrusion through a membrane filter with a 0.45 μm pore diameter. The width and height of the nanofibers, estimated by TEM and atomic-force microscopy (AFM), were ∼7 nm and ∼4 nm, respectively. The nanofibers were taken up more efficiently by murine macrophage-like cells (RAW264) than OVA257–264-TAT or OVA257–264 peptides. However, it was not reported whether this system could to induce an immune response in vivo.
Fig. 6.
Schematic representation of self-assembled nanofiber vaccine.
Collier and coworkers examined another fibrillizing peptide (Q11; QQKFQFQFEQQ) for its ability to serve as carrier for OVA323–339 (ISQAVHAAHAEINEAGR) [117]. Q11 peptide was conjugated with the OVA323–339 epitope by a Ser-Gly-Ser-Gly spacer, and the conjugate self-assembled into nanofibers in aqueous environments that contained salt. The unbranched nanofibers were approximately 15 nm wide and micron scale in length. The titers of IgG induced by the nanofiber vaccine and the emulsion of CFA with OVA323–339 were comparable, while the unconjugated physical mixture of the peptides did not induce antibody production. However, the dose of vaccine candidate (around 0.3 mg per mouse per injection) used in this immunization study was about 10-fold higher than typical doses used for other self-assembled vaccine delivery systems (0.03 mg per mouse per injection). The nanofiber forming peptide (Q11) did not induce cytotoxicity or inflammation, in contrast to Alum adjuvant (Imject Alum), when examined with a macrophage cell line (J774.1 cells) [118]. Upon immunization with Q11-OVA, the titers of OVA specific antibody were as high as induced by the emulsion of OVA epitopes and CFA, and significantly higher than the alum adjuvanted OVA epitopes.
The same fibrillizing peptide (Q11) was conjugated to the malaria B cell epitope (NANP)3 (NANPNANPNANP) derived from the circumsporozoite protein of Plasmodium falciparum. The (NANP)3-Q11 conjugate self-assembled into nanofibers. The conjugate induced humoral immune responses against Plasmodium falciparum circumsporozoite protein when delivered subcutaneously in a mouse model [119]. Another peptide that spontaneously assembles into nanofibers (FKFEFKFE; KFE8) was also investigated in combination with OVA323–339 peptide and compared with the OVA-Q11 conjugate. No significant differences in immune responses were observed, suggesting that nanofiber formation is responsible for adjuvanting activity, not the specific carrier-peptide sequence [120].
Azmi et al. also tried to develop peptide-based self-assembling nanofiber vaccines against GAS infection [121]. J14 GAS epitope was covalently linked to Q11 via a spacer. However, the resultant compound formed nanoparticles instead of the expected nanofibers and did not induce significant IgG production in mice when compared with PBS. The poor immunogenicity of the construct might be related to the low dose used for the immunization study (30 μg in comparison to 300 μg typically use for Q11 conjugates) [117], [119]). Interestingly, when two copies of lipid moiety 5 were conjugated to Q11-J14 (or J14 alone), the formed nanoparticles (at 30 μg dose) induced high antibody titers, similar to those triggered by J14 emulsified with CFA. Therefore, the fibrillizing peptide’s self-adjuvanting ability was weaker than that of the lipids. Recently, poly-β-branched amino acids were also suggested as delivery platform for vaccines [122]. It was demonstrated that B cell epitopes derived from GAS M protein formed water soluble molecules when conjugated to valine-rich isopeptides (depsipeptides). However, upon change in pH from acidic to neutral, the isopeptides undergo an O-N acyl migration reaction to form the linear “parent” peptide. This peptide adopted a β-sheet conformation and promptly aggregated into fibrils. The immunogenic properties of these fibrils have not been reported yet.
In summary, β-sheets are one of the most prevalent secondary structures in protein conformation, thus the nanofibers approach seems to be more “natural” and safe than other self-assembly strategies. Thus, further investigation into the role of nanofibres in vaccine development is an area of growing interest.
6. Conclusion
Self-assembly is becoming a popular method for producing nanostructure-based vaccines. Nanoparticles built from polymers or lipids are the most widely used for antigen delivery. However, the potential use of nanofibers as antigen carriers cannot be neglected. The low toxicity and high biocompatibility of most self-assembled materials makes them more valuable for vaccine formulation than classical adjuvants. Self-assembly inducers are often recognized by receptors on immune cells and therefore nanostructures display self-adjuvanting properties, removing the need to use toxic adjuvants. Moreover, self-assembled antigen-carrier conjugates usually have well-defined chemical structures.
Despite these advantages, self-assembled vaccines have some drawbacks, which may influence their chance for commercialization. In the case of polymer-based self-assembled vaccines, polymerization reactions used in their production do not form a single product with a defined molecular weight. Some polymer-based particles also have poor biodegradability, thus careful polymer selection is required to avoid adverse effects associated with polymer accumulation in the tissues. The optimal balance between biodegradability and stability must be found. Polymers that degrade too quickly may cause the nanovaccine to change its properties (e.g. size) before it can reach its destination (lymph nodes, APCs, etc.).
Lipid-based self-assembled nanostructures also face obstacles, particularly toxicity, membrane disruption, and poor induction of cellular immunity. One of the most efficient lipidic adjuvant, Lipid A, is highly toxic. Therefore, the hemolytic and toxicity properties of lipopeptides must be examined. Fortunately, several lipopeptides are highly immunogenic and non-toxic (e.g. LCP system). Although lipopeptides rarely induce a strong cellular immune response, one possible solution is to incorporate APC-targeting moieties into the self-assembly system to allow better antigen cross-presentation. In addition, some lipids are normally racemic (e.g. Pam2Cys) and therefore form a diastereomeric mixture when conjugated to the peptide. Each diastereoisomer may have a different safety and activity profile.
The disadvantage of peptide-based carriers is the low immunogenicity of the formed conjugates. Thus high dose is required to stimulate desired immune response which might be a potential obstacle in development of peptide-carrier-based self-assembled nanostructure.
Limited understanding of the interaction between the nanostructure vaccine and immune cell receptors hinders the development of self-assembled nanostructure vaccines. Investigation of conjugates’ stability under different conditions, APC uptake and targeting, T cell activation, DC maturation, cytokine profiling, and the recognition of the conjugate by receptors on human and animal tissue is a task for the future. However, once these areas are understood, self-assembled nanostructure vaccines can be developed to exploit these opportunities.
The majority of self-assembled conjugates are monovalent, carrying a single epitope/antigen, and therefore can only induce immune responses against one targeted subtype of pathogen. The self-assembly process offers a critical advantage: several different building blocks, each bearing a different epitope, can be mixed to self-assemble into a single multiantigenic nanostructure.
Overall, the application of a self-assembled strategy to the development of peptide-based vaccines has demonstrated that polymer-, lipid- and peptide-based delivery systems can overcome the low immunogenicity of peptide antigens without causing adverse effects. Thus, the use of self-adjuvanting particles could eliminate the requirement for toxic adjuvants in vaccine design. Although no peptide subunit vaccines are commercially available, it is expected that in the near future, multivalent self-assembled nanoparticles with higher immune efficacy and lower toxicity will appear on the market.
Acknowledgements
This work was supported by the National Health and Medical Research Council, Australia [NHMRC Program Grant 496600]. We thank Miss Thalia Guerin for her critical review of the manuscript.
References
- 1.Burke C.J., Hsu T.A., Volkin D.B. Formulation, stability, and delivery of live attenuated vaccines for human use. Crit. Rev. Ther. Drug Carr. Syst. 1999;16(1):1–83. [PubMed] [Google Scholar]
- 2.Masignani V., Rappuoli R., Pizza M. Reverse vaccinology: a genome-based approach for vaccine development. Expert Opin. Biol. Ther. 2002;2(8):895–905. doi: 10.1517/14712598.2.8.895. [DOI] [PubMed] [Google Scholar]
- 3.Skwarczynski M., Toth I. Peptide-based synthetic vaccines. Chem. Sci. 2016;7(2):842–854. doi: 10.1039/c5sc03892h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moyle P.M., Toth I. Modern subunit vaccines: development, components, and research opportunities. ChemMedChem. 2013;8(3):360–376. doi: 10.1002/cmdc.201200487. [DOI] [PubMed] [Google Scholar]
- 5.Rocha C.D., Caetano B.C., Machado A.V., Bruna-Romero O. Recombinant viruses as tools to induce protective cellular immunity against infectious diseases. Int. Microbiol. 2004;7(2):83–94. [PubMed] [Google Scholar]
- 6.Baxby D. Edward Jenner's Inquiry; a bicentenary analysis. Vaccine. 1999;17(4):301–307. doi: 10.1016/s0264-410x(98)00207-2. [DOI] [PubMed] [Google Scholar]
- 7.O.M. Kew, R.W. Sutter, EM. de Gourville, W.R. Dowdle, M.A. Pallansch, Vaccine-derived polioviruses and the endgame strategy for global polio eradication, Annual Review of Microbiology. Palo Alto: Annual Reviews, 2005, p. 587–635. [DOI] [PubMed]
- 8.Guo C., Manjili M.H., Subjeck J.R., Sarkar D., Fisher P.B., Wang X.-Y. Therapeutic cancer vaccines: past, present and future. Adv. Cancer Res. 2013;119:421–475. doi: 10.1016/B978-0-12-407190-2.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou W.P. Regulatory T cells, tumour immunity and immunotherapy. Nat. Rev. Immunol. 2006;6(4):295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 10.Emens L.A. Cancer vaccines: on the threshold of success. Expert Opin. Emerg. Drugs. 2008;13(2):295–308. doi: 10.1517/14728214.13.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei X.X., Fong L., Small E.J. Prostate cancer immunotherapy with sipuleucel-T: current standards and future directions. Expert Rev. Vaccines. 2015;14(12):1529–1541. doi: 10.1586/14760584.2015.1099437. [DOI] [PubMed] [Google Scholar]
- 12.Higano C.S., Schellhammer P.F., Small E.J., Burch P.A., Nemunaitis J., Yuh L. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115(16):3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 13.Andtbacka R.H.I., Kaufman H.L., Collichio F., Amatruda T., Senzer N., Chesney J. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 2015;33(25):2780–U2798. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 14.Nouri N., Garbe C. Intralesional immunotherapy as a strategy to treat melanoma. Expert Opin. Biol. Ther. 2016;16(5):619–626. doi: 10.1517/14712598.2016.1157161. [DOI] [PubMed] [Google Scholar]
- 15.Guo C.Q., Manjili M.H., Subjeck J.R., Sarkar D., Fisher P.B., Wang X.Y. Therapeutic cancer vaccines: past, present, and future. In: Tew K.D., Fisher P.B., editors. vol. 119. Elsevier Academic Press Inc.; San Diego: 2013. pp. 421–475. (Advances in Cancer Research). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Josefsberg J.O., Buckland B. Vaccine process technology. Biotechnol. Bioeng. 2012;109(6):1443–1460. doi: 10.1002/bit.24493. [DOI] [PubMed] [Google Scholar]
- 17.Lambert P.H., Laurent P.E. Intradermal vaccine delivery: Will new delivery systems transform vaccine administration? Vaccine. 2008;26(26):3197–3208. doi: 10.1016/j.vaccine.2008.03.095. [DOI] [PubMed] [Google Scholar]
- 18.Blaney J.E., Wirblich C., Papaneri A.B., Johnson R.F., Myers C.J., Juelich T.L. Inactivated or live-attenuated bivalent vaccines that confer protection against rabies and ebola viruses. J. Virol. 2011;85(20):10605–10616. doi: 10.1128/JVI.00558-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang Y.H., Seong B.L. Cross-protective immune responses elicited by live attenuated influenza vaccines. Yonsei Med. J. 2013;54(2):271–282. doi: 10.3349/ymj.2013.54.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liljeqvist S., Stahl S. Production of recombinant subunit vaccines: protein immunogens, live delivery systems and nucleic acid vaccines. J. Biotechnol. 1999;73(1):1–33. doi: 10.1016/s0168-1656(99)00107-8. [DOI] [PubMed] [Google Scholar]
- 21.Kang T.H., Monie A., Wu L.S.F., Pang X.W., Hung C.F., Wu T.C. Enhancement of protein vaccine potency by in vivo electroporation mediated intramuscular injection. Vaccine. 2011;29(5):1082–1089. doi: 10.1016/j.vaccine.2010.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naz R.K., Dabir P. Peptide vaccines against cancer, infectious diseases, and conception. Front. Biosci. 2007;12:1833–1843. doi: 10.2741/2191. [DOI] [PubMed] [Google Scholar]
- 23.Skwarczynski M., Toth I. Peptide-based subunit nanovaccines. Curr. Drug Delivery. 2011;8(3):282–289. doi: 10.2174/156720111795256192. [DOI] [PubMed] [Google Scholar]
- 24.Skwarczynski M., Toth I. Recent advances in peptide-based subunit nanovaccines. Nanomedicine. 2014;9(17):2657–2669. doi: 10.2217/nnm.14.187. [DOI] [PubMed] [Google Scholar]
- 25.Azmi F., Fuaad A.A.A., Skwarczynski M., Toth I. Recent progress in adjuvant discovery for peptide-based subunit vaccines. Human Vaccines Immunotherapeutics. 2014;10(3):778–796. doi: 10.4161/hv.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coffman R.L., Sher A., Seder R.A. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33(4):492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reed S.G., Orr M.T., Fox C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013;19(12):1597–1608. doi: 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- 28.Oleszycka E., Lavelle E.C. Immunomodulatory properties of the vaccine adjuvant alum. Curr. Opin. Immunol. 2014;28:1–5. doi: 10.1016/j.coi.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Montomoli E., Piccirella S., Khadang B., Mennitto E., Camerini R., De Rosa A. Current adjuvants and new perspectives in vaccine formulation. Expert Rev. Vaccines. 2011;10(7):1053–1061. doi: 10.1586/erv.11.48. [DOI] [PubMed] [Google Scholar]
- 30.C.R. Alving, Vaccine adjuvants, Vaccines for Biodefense and Emerging and Neglected Diseases, 2009, p. 115–129.
- 31.Hussein W.M., Liu T.Y., Skwarczynski M., Toth I. Toll-like receptor agonists: a patent review (2011–2013) Expert Opin. Therapeutic Patents. 2014;24(4):453–470. doi: 10.1517/13543776.2014.880691. [DOI] [PubMed] [Google Scholar]
- 32.Tour J.M. Transition to organic materials science. Passive, active, and hybrid nanotechnologies. J. Org. Chem. 2007;72(20):7477–7496. doi: 10.1021/jo070543s. [DOI] [PubMed] [Google Scholar]
- 33.Derycke V., Auvray S., Borghetti J., Chung C.L., Lefevre R., Lopez-Bezanilla A. Carbon nanotube chemistry and assembly for electronic devices. C R Phys. 2009;10(4):330–347. [Google Scholar]
- 34.Su H.N., Lee P.C., Tsai M.H., Chien K.M. Current situation and industrialization of Taiwan nanotechnology. J. Nanopart Res. 2007;9(6):965–975. [Google Scholar]
- 35.Ferreira L., Karp J.M., Nobre L., Langer R. New opportunities: the use of nanotechnologies to manipulate and track stem cells. Cell Stem Cell. 2008;3(2):136–146. doi: 10.1016/j.stem.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 36.Subramanian K.S., Tarafdar J.C. Prospects of nanotechnology in Indian farming. Indian J. Agric. Sci. 2011;81(10):887–893. [Google Scholar]
- 37.Shi W.Q., Yuan L.Y., Li Z.J., Lan J.H., Zhao Y.L., Chai Z.F. Nanomaterials and nanotechnologies in nuclear energy chemistry. Radiochim. Acta. 2012;100(8–9):727–736. [Google Scholar]
- 38.Davis M.E., Chen Z., Shin D.M. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008;7(9):771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 39.Zhao L., Seth A., Wibowo N., Zhao C.X., Mitter N., Yu C.Z. Nanoparticle vaccines. Vaccine. 2014;32(3):327–337. doi: 10.1016/j.vaccine.2013.11.069. [DOI] [PubMed] [Google Scholar]
- 40.Peek L.J., Middaugh C.R., Berkland C. Nanotechnology in vaccine delivery. Adv. Drug Delivery Rev. 2008;60(8):915–928. doi: 10.1016/j.addr.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mamo T., Poland G.A. Nanovaccinology: the next generation of vaccines meets 21st century materials science and engineering. Vaccine. 2012;30(47):6609–6611. doi: 10.1016/j.vaccine.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 42.Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y.T. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000;18:767. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 43.Oyewumi M.O., Kumar A., Cui Z.R. Nano-microparticles as immune adjuvants: correlating particle sizes and the resultant immune responses. Expert Rev. Vaccines. 2010;9(9):1095–1107. doi: 10.1586/erv.10.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marasini N., Skwarczynski M., Toth I. Oral delivery of nanoparticle-based vaccines. Expert Rev. Vaccines. 2014;13(11):1361–1376. doi: 10.1586/14760584.2014.936852. [DOI] [PubMed] [Google Scholar]
- 45.Demento S.L., Cui W., Criscione J.M., Stern E., Tulipan J., Kaech S.M. Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials. 2012;33(19):4957–4964. doi: 10.1016/j.biomaterials.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Demento S.L., Siefert A.L., Bandyopadhyay A., Sharp F.A., Fahmy T.M. Pathogen-associated molecular patterns on biomaterials: a paradigm for engineering new vaccines. Trends Biotechnol. 2011;29(6):294–306. doi: 10.1016/j.tibtech.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bandyopadhyay A., Fine R.L., Demento S., Bockenstedt L.K., Fahmy T.M. The impact of nanoparticle ligand density on dendritic-cell targeted vaccines. Biomaterials. 2011;32(11):3094–3105. doi: 10.1016/j.biomaterials.2010.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Acosta E. Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Curr. Opin. Colloid Interface Sci. 2009;14(1):3–15. [Google Scholar]
- 49.Hamley I.W. Nanotechnology with soft materials. Angew. Chem. – Int. Edit. 2003;42(15):1692–1712. doi: 10.1002/anie.200200546. [DOI] [PubMed] [Google Scholar]
- 50.Kipp J.E. The role of solid nanoparticle technology in the parenteral delivery of poorly water-soluble drugs. Int. J. Pharmaceutics. 2004;284(1–2):109–122. doi: 10.1016/j.ijpharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 51.Sanguansri P., Augustin M.A. Nanoscale materials development – a food industry perspective. Trends Food Sci. Technol. 2006;17(10):547–556. [Google Scholar]
- 52.Ghaffar K.A., Giddam A.K., Zaman M., Skwarczynski M., Toth I. Liposomes as nanovaccine delivery systems. Curr. Top Med. Chem. 2014;14(9):1194–1208. doi: 10.2174/1568026614666140329232757. [DOI] [PubMed] [Google Scholar]
- 53.Romero E.L., Morilla M.J. Topical and mucosal liposomes for vaccine delivery. Wiley Interdiscip Rev. – Nanomed. Nanobiotechnol. 2011;3(4):356–375. doi: 10.1002/wnan.131. [DOI] [PubMed] [Google Scholar]
- 54.Morachis J.M., Mahmoud E.A., Almutairi A. Physical and chemical strategies for therapeutic delivery by using polymeric nanoparticles. Pharmacol. Rev. 2012;64(3):505–519. doi: 10.1124/pr.111.005363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tyrrell Z.L., Shen Y.Q., Radosz M. Fabrication of micellar nanoparticles for drug delivery through the self-assembly of block copolymers. Prog. Polym. Sci. 2010;35(9):1128–1143. [Google Scholar]
- 56.Roldao A., Mellado M.C.M., Castilho L.R., Carrondo M.J.T., Alves P.M. Virus-like particles in vaccine development. Expert Rev. Vaccines. 2010;9(10):1149–1176. doi: 10.1586/erv.10.115. [DOI] [PubMed] [Google Scholar]
- 57.Lua L.H.L., Connors N.K., Sainsbury F., Chuan Y.P., Wibowo N., Middelberg A.P.J. Bioengineering virus-like particles as vaccines. Biotechnol. Bioeng. 2014;111(3):425–440. doi: 10.1002/bit.25159. [DOI] [PubMed] [Google Scholar]
- 58.Zhao Q.J., Li S.W., Yu H., Xia N.S., Modis Y. Virus-like particle-based human vaccines: quality assessment based on structural and functional properties. Trends Biotechnol. 2013;31(11):654–663. doi: 10.1016/j.tibtech.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 59.Drouart A., Dubrulle B., Gautier D., Robert F. Structure and transport in the solar nebula from constraints on deuterium enrichment and giant planets formation. Icarus. 1999;140(1):129–155. [Google Scholar]
- 60.Brun Y., Science UoSCC. University of Southern California; 2008. Self-Assembly for Discreet, Fault-Tolerant, and Scalable Computation on Internet-Sized Distributed Networks. [Google Scholar]
- 61.Luo D., Yan C., Wang T. Interparticle forces underlying nanoparticle self-assemblies. Small. 2015;11(45):5984–6008. doi: 10.1002/smll.201501783. [DOI] [PubMed] [Google Scholar]
- 62.Bishop K.J.M., Wilmer C.E., Soh S., Grzybowski B.A. Nanoscale forces and their uses in self-assembly. Small. 2009;5(14):1600–1630. doi: 10.1002/smll.200900358. [DOI] [PubMed] [Google Scholar]
- 63.Min Y.J., Akbulut M., Kristiansen K., Golan Y., Israelachvili J. The role of interparticle and external forces in nanoparticle assembly. Nat. Mater. 2008;7(7):527–538. doi: 10.1038/nmat2206. [DOI] [PubMed] [Google Scholar]
- 64.Liu R.C.W., Pallier A., Brestaz M., Pantoustier N., Tribet C. Impact of polymer micro structure on the self-assembly of amphiphilic polymers in aqueous solutions. Macromolecules. 2007;40(12):4276–4286. [Google Scholar]
- 65.Mahmoud Z.N., Grundy D.J., Channon K.J., Woolfson D.N. The non-covalent decoration of self-assembling protein fibers. Biomaterials. 2010;31(29):7468–7474. doi: 10.1016/j.biomaterials.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 66.Li M., Li G.L., Zhang Z.G., Li J., Neoh K.G., Kang E.T. Self-assembly of pH-responsive and fluorescent comb-like amphiphilic copolymers in aqueous media. Polymer. 2010;51(15):3377–3386. [Google Scholar]
- 67.Boucenna I., Guedeau-Boudeville M.A., Lapp A., Colinart P., Proag A., Royon L. Temperature directed-assembly of coated-laponite nanoparticles in pluronic micellar solutions. Soft Matter. 2013;9(1):170–176. [Google Scholar]
- 68.Tran P.H.L., Tran T.T.D., Vo T.V., Vo C.L.N., Lee B.J. Novel multifunctional biocompatible gelatin-oleic acid conjugate: self-assembled nanoparticles for drug delivery. J. Biomed. Nanotechnol. 2013;9(8):1416–1431. doi: 10.1166/jbn.2013.1621. [DOI] [PubMed] [Google Scholar]
- 69.Skwarczynski M., Zaman M., Urbani C.N., Lin I.C., Jia Z.F., Batzloff M.R. Polyacrylate DENDRIMER NANOPARTICLES: A SELF-ADJUVANTING VACCINE DELIVERY SYSTEM. Angew. Chem.– Int. Edit. 2010;49(33):5742–5745. doi: 10.1002/anie.201002221. [DOI] [PubMed] [Google Scholar]
- 70.Zaman M., Skwarczynski M., Malcolm J.M., Urbani C.N., Jia Z. Self-adjuvanting polyacrylic nanoparticulate delivery system for group A streptococcus (GAS) vaccine. Nanomed.: Nanotechnol. Biol. Med. 2011;7(2):168–173. doi: 10.1016/j.nano.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 71.Fuaad A., Jia Z.F., Zaman M., Hartas J., Ziora Z.M., Lin I.C. Polymer-peptide hybrids as a highly immunogenic single-dose nanovaccine. Nanomedicine. 2014;9(1):35–43. doi: 10.2217/nnm.13.7. [DOI] [PubMed] [Google Scholar]
- 72.Chandrudu S., Bartlett S., Khalil Z.G., Jia Z., Hussein W.M., Capon R.J. Linear and branched polyacrylates as a delivery platform for peptide-based vaccines. Therapeutic Delivery. 2016;7(9):601–609. doi: 10.4155/tde-2016-0037. [DOI] [PubMed] [Google Scholar]
- 73.Liu T.Y., Hussein W.M., Jia Z.F., Ziora Z.M., McMillan N.A.J., Monteiro M.J. Self-adjuvanting polymer-peptide conjugates as therapeutic vaccine candidates against cervical cancer. Biomacromolecules. 2013;14(8):2798–2806. doi: 10.1021/bm400626w. [DOI] [PubMed] [Google Scholar]
- 74.Liu T.Y., Hussein W.M., Giddam A.K., Jia Z.F., Reiman J.M., Zaman M. Polyacrylate-based delivery system for self-adjuvanting anticancer peptide vaccine. J. Med. Chem. 2015;58(2):888–896. doi: 10.1021/jm501514h. [DOI] [PubMed] [Google Scholar]
- 75.Hussein W.M., Liu T.Y., Jia Z.F., McMillan N.A.J., Monteiro M.J., Toth I. Multiantigenic peptide-polymer conjugates as therapeutic vaccines against cervical cancer. Bioorganic Med. Chem. 2016;24(18):4372–4380. doi: 10.1016/j.bmc.2016.07.036. [DOI] [PubMed] [Google Scholar]
- 76.Liu T.Y., Giddam A.K., Hussein W.M., Jia Z.F., McMillan N.A.J., Monteiro M.J. Self-adjuvanting therapeutic peptide-based vaccine induce CD8(+) Cytotoxic T lymphocyte responses in a murine human papillomavirus tumor model. Curr. Drug Deliv. 2015;12(1):3–8. doi: 10.2174/1567201811666141001155729. [DOI] [PubMed] [Google Scholar]
- 77.Urbani C.N., Bell C.A., Lonsdale D., Whittaker M.R., Monteiro M.J. Self-assembly of amphiphilic polymeric dendrimers synthesized with selective degradable linkages. Macromolecules. 2008;41(1):76–86. [Google Scholar]
- 78.Hein J.E., Fokin V.V. Copper-catalyzed azide-alkyne cycloaddition (CuAAC) and beyond: new reactivity of copper(I) acetylides. Chem. Soc. Rev. 2010;39(4):1302–1315. doi: 10.1039/b904091a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stills H.F. Adjuvants and antibody production: dispelling the myths associated with Freund's complete and other adjuvants. Ilar J. 2005;46(3):280–293. doi: 10.1093/ilar.46.3.280. [DOI] [PubMed] [Google Scholar]
- 80.Liu T.Y., Giddam A.K., Hussein W.M., Jia Z., McMillan N.A., Monteiro M.J. Self-adjuvanting therapeutic peptide-based vaccine Induce CD8+ Cytotoxic T lymphocyte responses in a murine human papillomavirus tumor model. Curr. Drug Deliv. 2015;12(1):3–8. doi: 10.2174/1567201811666141001155729. [DOI] [PubMed] [Google Scholar]
- 81.Kakwere H., Chun C.K.Y., Jolliffe K.A., Payne R.J., Perrier S. Polymer-peptide chimeras for the multivalent display of immunogenic peptides. Chem. Commun. 2010;46(13):2188–2190. doi: 10.1039/b924112d. [DOI] [PubMed] [Google Scholar]
- 82.Eby J.K., Dane K.Y., O'Neil C.P., Hirosue S., Swartz M.A., Hubbell J.A. Polymer micelles with pyridyl disulfide-coupled antigen travel through lymphatics and show enhanced cellular responses following immunization. Acta Biomater. 2012;8(9):3210–3217. doi: 10.1016/j.actbio.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 83.Keller S., Wilson J.T., Patilea G.I., Kern H.B., Convertine A.J., Stayton P.S. Neutral polymer micelle carriers with pH-responsive, endosome-releasing activity modulate antigen trafficking to enhance CD8(+) T cell responses. J. Controlled Release. 2014;191:24–33. doi: 10.1016/j.jconrel.2014.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Foster E.J., Berda E.B., Meijer E.W. Tuning the size of supramolecular single-chain polymer nanoparticles. J. Polym. Sci. Pol. Chem. 2011;49(1):118–126. [Google Scholar]
- 85.Phillipps K.S.M., Wykes M.N., Liu X.Q., Brown M., Blanchfield J., Toth I. A novel synthetic adjuvant enhances dendritic cell function. Immunology. 2009;128(1pt2):e582–e588. doi: 10.1111/j.1365-2567.2008.03038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miyake K. Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin. Immunol. 2007;19(1):3–10. doi: 10.1016/j.smim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 87.Toth I., Danton M., Flinn N., Gibbons W.A. A combined adjuvant and carrier system for enhancing synthetic peptides immunogenicity utilizing lipidic amino-acids. Tetrahedron Lett. 1993;34(24):3925–3928. [Google Scholar]
- 88.Skwarczynski M., AH Ahmad Fuaad A., Rustanti L., M Ziora Z., Aqil M., R Batzloff M. Group A streptococcal vaccine candidates based on the conserved conformational epitope from M protein. Drug Delivery Lett. 2011;1(1):2–8. [Google Scholar]
- 89.Skwarczynski M., Kamaruzaman K.A., Srinivasan S., Zaman M., Lin I.C., Batzloff M.R. M-protein-derived conformational peptide epitope vaccine candidate against Group A streptococcus. Curr. Drug Deliv. 2013;10(1):39–45. doi: 10.2174/1567201811310010007. [DOI] [PubMed] [Google Scholar]
- 90.Zaman M., Abdel-Aal A.B.M., Fujita Y., Ziora Z.M., Batzloff M.R., Good M.F. Structure-activity relationship for the development of a self-adjuvanting mucosally active lipopeptide vaccine against streptococcus pyogenes. J. Med. Chem. 2012;55(19):8515–8523. doi: 10.1021/jm301074n. [DOI] [PubMed] [Google Scholar]
- 91.Zaman M., Abdel-Aal A.M., Phillipps K.S.M., Fujita Y., Good M.F., Toth I. Structure-activity relationship of lipopeptide Group A streptococcus (GAS) vaccine candidates on toll-like receptor 2. Vaccine. 2010;28(10):2243–2248. doi: 10.1016/j.vaccine.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 92.Moyle P.M., Toth I. Self-adjuvanting lipopeptide vaccines. Curr. Med. Chem. 2008;15(5):506–516. doi: 10.2174/092986708783503249. [DOI] [PubMed] [Google Scholar]
- 93.M. Skwarczynski, M. Zaman, I. Toth, Chapter 78 - Lipo-Peptides/Saccharides for Peptide Vaccine Delivery A2 - Kastin, Abba J. Handbook of Biologically Active Peptides, second ed., Academic Press, Boston, 2013, p. 571–579.
- 94.Ahmad Fuaad A.A.H., Roubille R., Pearson M.S., Pickering D.A., Loukas A.C., Skwarczynski M. The use of a conformational cathepsin D-derived epitope for vaccine development against Schistosoma mansoni. Bioorgan. Med. Chem. 2015;23(6):1307–1312. doi: 10.1016/j.bmc.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 95.Dougall A.M., Skwarczynski M., Khoshnejad M., Chandrudu S., Daly N.L., Toth I. Lipid core peptide targeting the cathepsin D hemoglobinase of Schistosoma mansoni as a component of a schistosomiasis vaccine. Human Vaccines Immunother. 2014;10(2):399–409. doi: 10.4161/hv.27057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fuaad A., Pearson M.S., Pickering D.A., Becker L., Zhao G.Z., Loukas A.C. Lipopeptide nanoparticles: development of vaccines against hookworm parasite. Chemmedchem. 2015;10(10):1647–1654. doi: 10.1002/cmdc.201500227. [DOI] [PubMed] [Google Scholar]
- 97.Skwarczynski M., Dougall A.M., Khoshnejad M., Chandrudu S., Pearson M.S., Loukas A. Peptide-based subunit vaccine against hookworm infection. PLoS One. 2012;7(10):7. doi: 10.1371/journal.pone.0046870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Skwarczynski M., Parhiz B.H., Soltani F., Srinivasan S., Kamaruzaman K.A., Lin I.C. Lipid peptide core nanoparticles as multivalent vaccine candidates against streptococcus pyogenes. Aust. J. Chem. 2012;65(1):35–39. [Google Scholar]
- 99.Eskandari S., Stephenson R.J., Fuaad A.A., Apte S.H., Doolan D.L., Toth I. Synthesis and characterisation of self-assembled and self-adjuvanting asymmetric multi-epitope lipopeptides of ovalbumin. Chem. – Eur. J. 2015;21(3):1251–1261. doi: 10.1002/chem.201404997. [DOI] [PubMed] [Google Scholar]
- 100.Zaman M., Chandrudu S., Giddam A.K., Reiman J., Skwarczynski M., McPhun V. Group A Streptococcal vaccine candidate: contribution of epitope to size, antigen presenting cell interaction and immunogenicity. Nanomedicine. 2014;9(17):2613–2624. doi: 10.2217/nnm.14.190. [DOI] [PubMed] [Google Scholar]
- 101.Chan A., Hussein W.M., Ghaffar K.A., Marasini N., Mostafa A., Eskandari S. Structure-activity relationship of lipid core peptide-based Group A Streptococcus vaccine candidates. Bioorg. Med. Chem. 2016;24(14):3095–3101. doi: 10.1016/j.bmc.2016.03.063. [DOI] [PubMed] [Google Scholar]
- 102.Moyle P.M., Hartas J., Henningham A., Batzloff M.R., Good M.F., Toth I. An efficient, chemically-defined semisynthetic lipid-adjuvanted nanoparticulate vaccine development system. Nanomed. – Nanotechnol. Biol. Med. 2013;9(7):935–944. doi: 10.1016/j.nano.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 103.Accardo A., Vitiello M., Tesauro D., Galdiero M., Finamore E., Martora F. Self-assembled or mixed peptide amphiphile micelles from Herpes simplex virus glycoproteins as potential immunomodulatory treatment. Int. J. Nanomed. 2014;9:2137–2148. doi: 10.2147/IJN.S57656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tan A.C.L., Eriksson E.M.Y., Kedzierska K., Deliyannis G., Valkenburg S.A., Zeng W.G. Polyfunctional CD8(+) T cells are associated with the vaccination-induced control of a novel recombinant influenza virus expressing an HCV epitope. Antiviral Res. 2012;94(2):168–178. doi: 10.1016/j.antiviral.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 105.Wilkinson B.L., Day S., Chapman R., Perrier S., Apostolopoulos V., Payne R.J. Synthesis and immunological evaluation of self-assembling and self-adjuvanting tricomponent glycopeptide cancer-vaccine candidates. Chem. – Eur. J. 2012;18(51):16540–16548. doi: 10.1002/chem.201202629. [DOI] [PubMed] [Google Scholar]
- 106.Black M., Trent A., Kostenko Y., Lee J.S., Olive C., Tirrell M. Self-assembled peptide amphiphile micelles containing a Cytotoxic T-Cell epitope promote a protective immune response in vivo. Adv. Mater. 2012;24(28):3845–3849. doi: 10.1002/adma.201200209. [DOI] [PubMed] [Google Scholar]
- 107.Trent A., Ulery B.D., Black M.J., Barrett J.C., Liang S., Kostenko Y. Peptide amphiphile micelles self-adjuvant Group A streptococcal vaccination. Aaps J. 2015;17(2):380–388. doi: 10.1208/s12248-014-9707-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hussein W.M., Liu T.Y., Maruthayanar P., Mukaida S., Moyle P.M., Wells J.W. Double conjugation strategy to incorporate lipid adjuvants into multiantigenic vaccines. Chem. Sci. 2016;7(3):2308–2321. doi: 10.1039/c5sc03859f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Eriksson E.M.Y., Jackson D.C. Recent advances with TLR2-Targeting lipopeptide-based Vaccines. Curr. Protein Pept. Sci. 2007;8(4):412–417. doi: 10.2174/138920307781369436. [DOI] [PubMed] [Google Scholar]
- 110.Raman S., Machaidze G., Lustig A., Aebi U., Burkhard P. Structure-based design of peptides that self-assemble into regular polyhedral nanoparticles. Nanomed.: Nanotechnol., Biol. Med. 2006;2(2):95–102. doi: 10.1016/j.nano.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 111.Burkhard P., Meier M., Lustig A. Design of a minimal protein oligomerization domain by a structural approach. Protein Sci. 2000;9(12):2294–2301. doi: 10.1110/ps.9.12.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pimentel T., Yan Z., Jeffers S.A., Holmes K.V., Hodges R.S., Burkhard P. Peptide nanoparticles as novel immunogens: design and analysis of a prototypic severe acute respiratory syndrome vaccine. Chem. Biol. Drug Des. 2009;73(1):53–61. doi: 10.1111/j.1747-0285.2008.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kaba S.A., Brando C., Guo Q., Mittelholzer C., Raman S., Tropel D. A nonadjuvanted polypeptide nanoparticle vaccine confers long-lasting protection against rodent malaria. J. Immunol. (Baltimore, Md : 1950) 2009;183(11):7268–7277. doi: 10.4049/jimmunol.0901957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wahome N., Pfeiffer T., Ambiel I., Yang Y.K., Keppler O.T., Bosch V. Conformation-specific Display of 4E10 and 2F5 epitopes on self-assembling protein nanoparticles as a potential HIV vaccine. Chem. Biol. Drug Des. 2012;80(3):349–357. doi: 10.1111/j.1747-0285.2012.01423.x. [DOI] [PubMed] [Google Scholar]
- 115.Haass C., Selkoe D.J. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007;8(2):101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 116.Waku T., Kitagawa Y., Kawabata K., Nishigaki S., Kunugi S., Tanaka N. Self-assembled beta-sheet peptide nanofibers for efficient antigen delivery. Chem. Lett. 2013;42(11):1441–1443. [Google Scholar]
- 117.Rudra J.S., Tian Y.F., Jung J.P., Collier J.H. A self-assembling peptide acting as an immune adjuvant. Proc. Natl. Acad. Sci. USA. 2010;107(2):622–627. doi: 10.1073/pnas.0912124107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen J.J., Pompano R.R., Santiago F.W., Maillat L., Sciammas R., Sun T. The use of self-adjuvanting nanofiber vaccines to elicit high-affinity B cell responses to peptide antigens without inflammation. Biomaterials. 2013;34(34):8776–8785. doi: 10.1016/j.biomaterials.2013.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rudra J.S., Mishra S., Chong A.S., Mitchell R.A., Nardin E.H., Nussenzweig V. Self-assembled peptide nanofibers raising durable antibody responses against a malaria epitope. Biomaterials. 2012;33(27):6476–6484. doi: 10.1016/j.biomaterials.2012.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rudra J.S., Sun T., Bird K.C., Daniels M.D., Gasiorowski J.Z., Chong A.S. Modulating adaptive immune responses to peptide self-assemblies. Acs Nano. 2012;6(2):1557–1564. doi: 10.1021/nn204530r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Azmi F., Fuaad A.A.A., Giddam A.K., Batzloff M.R., Good M.F., Skwarczynski M. Self-adjuvanting vaccine against group A streptococcus: application of fibrillized peptide and immunostimulatory lipid as adjuvant. Bioorg. Med. Chem. 2014;22(22):6401–6408. doi: 10.1016/j.bmc.2014.09.042. [DOI] [PubMed] [Google Scholar]
- 122.Skwarczynski M., Kowapradit J., Ziora Z.M., Toth I. PH-triggered peptide self-assembly into fibrils: a potential peptide-based subunit vaccine delivery platform. Biochem. Compounds. 2013;1(1) [Google Scholar]