Abstract
Luteolin, a plant flavonoid, has potent anti-inflammatory properties both in vitro and in vivo. However, the molecular mechanism of luteolin-mediated immune modulation has not been fully understood. In this study, we examined the effects of luteolin on the production of nitric oxide (NO) and prostaglandin E2 (PGE2), as well as the expression of inducible NO synthase (iNOS), cyclooxygenase-2 (COX-2), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6) in mouse alveolar macrophage MH-S and peripheral macrophage RAW 264.7 cells. Luteolin dose-dependently inhibited the expression and production of these inflammatory genes and mediators in macrophages stimulated with lipopolysaccharide (LPS). Semi-quantitative reverse-transcription polymerase chain reaction (RT-PCR) assay further confirmed the suppression of LPS-induced TNF- α, IL-6, iNOS and COX-2 gene expression by luteolin at a transcriptional level. Luteolin also reduced the DNA binding activity of nuclear factor-kappa B (NF-κB) in LPS-activated macrophages. Moreover, luteolin blocked the degradation of IκB-α and nuclear translocation of NF-κB p65 subunit. In addition, luteolin significantly inhibited the LPS-induced DNA binding activity of activating protein-1 (AP-1). We also found that luteolin attenuated the LPS-mediated protein kinase B (Akt) and IKK phosphorylation, as well as reactive oxygen species (ROS) production. In sum, these data suggest that, by blocking NF-κB and AP-1 activation, luteolin acts to suppress the LPS-elicited inflammatory events in mouse alveolar macrophages, and this effect was mediated, at least in part, by inhibiting the generation of reactive oxygen species. Our observations suggest a possible therapeutic application of this agent for treating inflammatory disorders in lung.
Keywords: Luteolin, LPS, NF-κB, AP-1, Alveolar macrophage, Anti-inflammation
Introduction
It is well documented that pulmonary oxidant stress and inflammatory reaction play important pathological roles in disease conditions, including acute lung injury/adult respiratory distress syndrome (ALI/ARDS), hyperoxia, ischemia–reperfusion, sepsis, radiation injury, lung transplantation, and chronic obstructive pulmonary disease (COPD). Reactive oxygen species (ROS), released from activated leukocytes and macrophage, damage the lungs and initiate cascades of pro-inflammatory reactions multiplying pulmonary and systemic stress (Christofidou-Solomidou and Muzykantov, 2006). Severe acute respiratory syndrome (SARS) is a novel global infectious disease induced by Corona virus. A clinical investigation has shown that pathological changes in SARS patients are similar to those of acute lung injury, as revealed by alveolar cell collapse, severe exudation, acute inflammatory reaction and hyaline membrane formation (Lew et al., 2003). Currently, no effective therapy is available for the management of ALI/ARDS/SARS and only supportive therapies exist. This has prompted us to search for novel compounds with therapeutic effect to reduce lung injuries and relieve symptoms (File and Tsang, 2005, Lai, 2005).
It is noteworthy that the use of medicinal plants or their crude extracts is increasingly becoming an attractive approach as a complement and an alternative for treating various inflammatory disorders. Lonicera japonica (Caprifoliaceae) has been known as an anti-inflammatory herb in traditional Chinese medicine for thousands of years and has been widely used for upper respiratory tract infections, diabetes mellitus and rheumatoid arthritis (Lee et al., 2001). This herbal medicine has also been employed for treatment of SARS in mainland China (Liu et al., 2006, Wu et al., 2004). Luteolin (3′,4′,5,7-tetra-hydroxyl-flavone), a flavonoid isolated from L. japonica, exhibits a strongly anti-inflammatory activity, can effectively inhibit the lipopolysaccharide (LPS)-induced tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and inducible nitric oxide (NO) production in vitro (Park et al., 2005, Xagorari et al., 2001), and also protect against LPS-induced lethal toxicity by inhibiting pro-inflammatory molecule expression in vivo and reducing leukocyte infiltration in tissues (Kotanidou et al., 2002). LPS from Gram-negative bacteria is well known to cause bacterial sepsis mediated through activation of monocytes, neutrophils and macrophages (Aderem and Ulevitch, 2000). Sometimes activation of these cells may induce hyper-secretion of various pro-inflammatory and toxicity mediating molecules such as TNF-α and IL-6, eicosonoids and nitric oxide (NO) (Nathan, 1987). The excessive inflammatory responses, in turn, may manifest respiratory failure and multiple organ dysfunction syndrome (MODS) i.e. loss of capillary integrity, distributive and septic shock, multiple organ dysfunction and mortality. Exposure of laboratory animals or cultured monocytes to bacterial LPS triggers the generation of ROS and gene induction; the inducible genes encode pro-inflammatory cytokines and enzymes such as TNF-α, IL-6, COX-2, and iNOS, which up-regulate the host defense systems but unfortunately also contribute to pathological conditions such as bacterial sepsis, ischemia/reperfusion injuries, chronic inflammatory disease, and the down-regulation of hepatic drug-metabolizing enzymes (Guha and Mackman, 2001, Guha et al., 2001). Recently, luteolin has been found to be able to inhibit the LPS-induced TNF-α release by inactivating the extracellular-regulated kinases (ERK) and p38 MAPK and blocking the transcriptional activation of nuclear factor-kappa B (NF-κB) (Xagorari et al., 2001, Xagorari et al., 2002). An in vivo study indicated that feeding of luteolin protects mice against LPS-induced toxicity (Kotanidou et al., 2002). Also, luteolin alleviates bronchoconstriction and airway hyperreactivity in ovalbumin-sensitized mice (Das et al., 2003) and decreases the acute Chlamydia pneumoniae infection load and inflammatory reactions in vivo (Tormakangas et al., 2005).
Alveolar macrophages play a vital role in the defense of the lung through their ability to scavenge inhaled particles, ingest and kill microorganisms, recruit and activate other inflammatory cells and elicit the immune response. They perform major roles in antigen presentation, production of a variety of biologically active substances (e.g. ROS and cytokines), and modulation and regulation of immune effector cells (Kobayashi et al., 1999, Matsunaga et al., 2001). Due to the lack of scientific evidence to address the effect of luteolin on the alveolar macrophages, in the present study, we examined the potential anti-inflammatory properties of luteolin in LPS-stimulated mouse alveolar macrophage MH-S and peripheral macrophage RAW 264.7 cell lines. In addition, we also investigate the molecular mechanism responsible for the inhibitory effect of luteolin. In this study, we demonstrate that luteolin inhibits LPS-induced inflammatory reactions in MH-S cells, apparently through blocking the NF-κB and activating the protein (AP-1) transcription activation pathways.
Materials and methods
Reagents and cell culture
Luteolin was obtained from Extrasynthese (Genay, France) and dissolved in EtOH:DMSO (1:1,v/v). Lipopolysaccharide (Escherichia coli 055:B5), 1, 7-bis (4-hydroxy-3-methoxy-phenyl)-1, 6-heptadiene-3, 5-dinone (curcumin), ammonium pyrryolidinedithio-carbamate (PDTC) and LY294002 were purchased from Sigma (St. Louis, MO, USA). Two different types of macrophages were used in this study. MH-S and RAW 264.7 cells were obtained from Culture Collection and Research Center (Hsinchu, Taiwan). MH-S were cultured in RPMI 1640 medium containing 10% fetal bovine serum (Hyclone, Logan, UT, USA), penicillin (100 units/ml), and streptomycin (100 μg/ml), 10 mM HEPES, 0.05 mM 2-mercaptoethanol. RAW 264.7 were cultured in Dulbecco's modified Eagle's medium supplemented with 10% FBS, penicillin (100 units/ml), and streptomycin (100 μg/ml). These cells were maintained at subconfluence in a 95% air, 5% CO2 humidified atmosphere at 37 °C.
Cell viability
Cell viability was assessed by the mitochondrial-dependent reduction of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) to purple formazan. Cells were incubated with MTT (0.5%) for 4 h at 37 °C. The medium was removed by aspiration, and formazan crystals were dissolved in DMSO. The extent of the reduction of MTT was quantified by measurement of A550.
Cytokine measurement
Cytokine concentrations in the supernatants were determined by ELISA kits that were specific against mouse cytokines. Levels of TNF-α and IL-6 were measured by using OptEIA Set from BD Biosciences. Assays were performed according to the manufacturer's instructions.
Nitrite measurement
The nitrite concentration in the medium was measured according to the Griess reaction, and the calculated concentration was taken as an indicator of NO production. The supernatant of cell cultures was mixed with an equal volume of Griess reagent (1% sulfanilamide in 5% phosphoric acid and 0.1% naphthylethylenediamine dihydrochloride in water). The optical density at 550 nm (A550) was measured and calculated against a sodium nitrite standard curve.
PGE2 measurements
PGE2 concentration in the supernatant of the culture medium was determined by using an EIA kit (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer's instructions.
Western blotting analysis
Confluent cells (1 × 106) in a 3.5-cm petri dish were pretreated without or with luteolin (25 μM, Extrasynthese, Genay, France) for 30 min and then treated with LPS (100 ng/ml) in the absence or presence of luteolin. At each point in time, cells were washed with PBS and then scraped into microcentrifuge tubes and pelleted. The cell pellets were resuspended in extraction lysis buffer (50 mM HEPES, pH 7.0, 250 mM NaCl, 5 mM EDTA, 0.1% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride, 0.5 mM dithiothreitol, 5 mM NaF, 5 mg/ml each of leupeptin and aprotinin) and incubated for 20 min at 4 °C. Cell debris was removed by microcentrifugation, followed by quick freezing of the supernatants. The protein concentration was determined by using the Bio-Rad protein assay reagent according to the manufacturer's instruction. Lysates (20 μg/lane) were separated by SDS-PAGE on polyacrylamide gels and transferred onto polyvinylidene difluoride membranes (PerkinElmer Life Sciences, Inc. Boston, MA, USA). The membranes were blocked in 5% milk in TBS-T solution for 1 h. Then membranes were subsequently probed with monoclonal anti-iNOS antibody (Sigma, St. Louis, MO, USA), anti-COX-2 and IκBα antibody (Santa Cruz Biochemicals, Santa Cruz, CA, USA), anti-phospho-Akt (Thr308) and phospho-Akt (Ser473), anti-IKKα/β, anti-phospho-IKKα/β antibody (Cell Signaling) overnight at 4 °C. The blots were washed with TBS-T three times and incubated with horseradish peroxidase-conjugated rabbit anti-mouse or anti-rabbit IgG (1:20,000; PerkinElmer Life Sciences, Inc. Boston, MA, USA) for 1 h at room temperature. Following three further washings in TBS-T, immunoreactive bands were visualized using the ECL-Plus detection system (PerkinElmer Life Sciences, Inc. Boston, MA, USA).
Semi-quantitative RT-PCR for TNF-α, IL-6, iNOS, COX-2, IL-10 and IL-1Ra
Total cellular RNA was extracted from both the controlled and treated cells in a 6-well plate by Trisolution Reagent Plus (GeneMark, Taiwan) in accordance with the manufacturer's instructions. Then RT-PCR analyses were performed by using the One-step RT-PCR kit (GeneMark, Taiwan). The sequences of the primers are presented in Table 1 . The first strand of cDNA synthesis was 1 cycle for reverse transcription (50 °C, 30 min) and 1 cycle for MMLV RTase inactivation (94 °C, 2 min). The second strand cDNA synthesis and PCR reaction were performed as follows: TNF-α, IL-6, IL-10 and IL-1Ra (94 °C for 1 min, 60 °C for 1 min, and 72 °C for 2 min), iNOS (94 °C for 45 s, 65 °C for 45 s, and 72 °C for 2 min) and COX-2 (94 °C for 45 s, 55 °C for 1 min, 72 °C for 2 min). For each combination of primers, the kinetics of PCR amplification was studied. The number of cycles corresponding to plateau was determined and PCR was performed at exponential range. PCRs were electrophoresed through a 1% agarose gel and visualized by ethidium bromide staining and UV irradiation.
Table 1.
Primers used in RT-PCR analysis
| Primer | Sequence |
|---|---|
| TNF-α | Forward 5′-ATGAGCACAGAAAGCATGATCCGC-3′ |
| (502 bp) | Reverse 5′-CTCAGGCCCGTCCAGATGAAACC-3′ |
| IL-6 | Forward 5′-ATGAAGTTCCTCTCTGCAAGAGACT-3′ |
| (247 bp) | Reverse 5′-CACTAGGTTTGCCGAGTAGATCTC-3′ |
| iNOS | Forward 5′-CAACCAGTATTATGGCTCCT-3′ |
| (835 bp) | Reverse 5′-GTGACAGCCCGGTCTTTCCA-3′ |
| COX-2 | Forward 5′-GGAGAGACTATCAAGATAGTGATC-3′ |
| (860 bp) | Reverse 5′-ATGGTCAGTAGACTTTTACAGCTC-3′ |
| IL-10 | Forward 5′-TGA ATT CCC TGG GTG AGA AG-3′ |
| (136 bp) | Reverse 5′-ACA CCT TGG TCT TGG AGC TT-3′ |
| IL-1Ra | Forward 5′-AAA TCT GCT GGG GAC CCT AC -3′ |
| (127 bp) | Reverse 5′-GGT CAA TAG GCA CCA TGT CT-3′ |
| GAPDH | Forward 5′-ACCACAGTCCATGCCATCAC-3′ |
| (451 bp) | Reverse 5′-TCCACCACCCTGTTGCTGTA-3′ |
Electrophoretic mobility shift assay
MH-S cells were plated in 3.5-cm dishes (1 × 106 cells). The cells were pretreated without or with luteolin (25 μM) for 30 min and then treated with LPS in the presence or absence of luteolin for 0–120 min. After treatment, cells were washed once with PBS, scraped into 1 ml of cold PBS, and pelleted by centrifugation. Nuclear fractions were extracted by buffer Ι (1 M Tris–HCl, 0.5 M EDTA, 100 mM EGTA) and Totex buffer. Twenty micrograms of nuclear protein was incubated with 100 pmol of 5′-biotinate double-stranded oligonucleotide probes containing a consensus binding sequence of NF-κB (5′-AGTTGAGGGGACTTTCCCAGGC-3′) or AP-1 (5′-CGCTTGATGAGTCAGCCGGAA-3′) for 30 min and resolved in 6% nondenaturing polyacrylamide gel. The protein–DNA–biotin complexes were blotted onto a nitrocellulose transfer membrane (PerkinElmer Life Science, Inc. Boston, MA, USA) followed by UV cross-linking. The complexes were revealed with streptavidin–horseradish peroxidase conjugate and SuperSignal chemiluminescent substrate then exposed to X-ray film.
Immunofluorescence
Cells were pretreated without or with luteolin (25 μM) for 30 min and then treated with LPS (100 ng/ml) in the presence or absence of luteolin, and then fixed with 2% paraformaldehyde for 20 min. The cells were incubated with 0.1% Triton X-100 for 30 min then blocked with 1% BSA for 30 min. Cells were probed with mouse anti-p65 antibody (Santa Cruz Biochemicals, Santa Cruz, CA, USA, 1:500) overnight at 4 °C, followed by FITC-conjugated goat anti-mouse IgG antibody (Sigma, St. Louis, MO, USA, diluted 1:200) 1 h at 37 °C, washed with PBS three times and then stained with propidium iodide for 15 min. NF-κB p65 subunit was observed with a laser scanning confocal microscope.
Reactive oxygen species (ROS) production
Intracellular oxidative stress was assayed by measuring intracellular oxidation of dichlorofluorescein (DCFH). The substrate was 2′, 7′-dichlorofluorescein diacetate (H2DCFDA) which easily diffuses into the cell and was next deacetylated by cellular esterases to the more hydrophilic, nonfluorescent DCFH. H2O2 produced in the cell oxidized DCFH to the fluorescent 2′, 7′-dichlorofluorescein (DCF). MH-S cells were pretreated with H2DCFDA (5 μM, Molecular Probes, Eugene, OR, USA) and luteolin (5–25 μM) for 30 min in a serum-free medium. Next, the cells (except for controls) were exposed to 100 ng/ml LPS at 37 °C for several times. Cells were detached and fluorescence was measured with a FACSCalibur flow cytometer using the Cell Quest software.
Statistical analysis
All results are expressed as means ± SEM of twelve replicates from three independent experiments. Figures shown in this article were obtained from at least three independent experiments with similar patterns. One-way ANOVA followed by a Scheffe post-hoc test and Student's t-test was used to determine the level of significance for the statistical analysis of data by using SPSS 10.0 statistical program. A p-value of less than 0.05 was considered significant.
Results
Luteolin inhibited LPS-induced pro-inflammatory cytokine expression and NO and PGE2 production
Pro-inflammatory cytokines (including TNF-α and IL-6) and mediators (NO and PGE2) play important roles in the inflammatory process. To examine how luteolin regulates the production and expression of these cytokines and mediators, the levels of secreted TNF-α, IL-6, NO and PGE2 were measured. As demonstrated in Fig. 1A, treatment of mouse alveolar macrophage MH-S and peripheral macrophage RAW 264.7 cells with LPS (100 ng/ml) caused a substantial increase in the production of pro-inflammatory cytokines (TNF-α and IL-6) and mediators (NO and PGE2). When the cells were incubated with luteolin (5–25 μM) alone, the concentration of TNF-α, IL-6, NO and PGE2 maintained at a background level similar to that in the unstimulated culture (data not shown). To examine whether luteolin is cytotoxic to these two macrophages cell lines, MH-S and RAW 264.7 cells were incubated with 5–50 μM luteolin for 24 h. Within our tested concentrations, no cytotoxic effect of luteolin was observed in these two cells (Fig. 1B). However, treatment with luteolin for 1 h before being incubated with LPS resulted in a dose-dependent inhibition of the LPS-induced TNF-α, IL-6, NO and PGE2 production in both MH-S and RAW 264.7 cells (Fig. 1A).
Fig. 1.
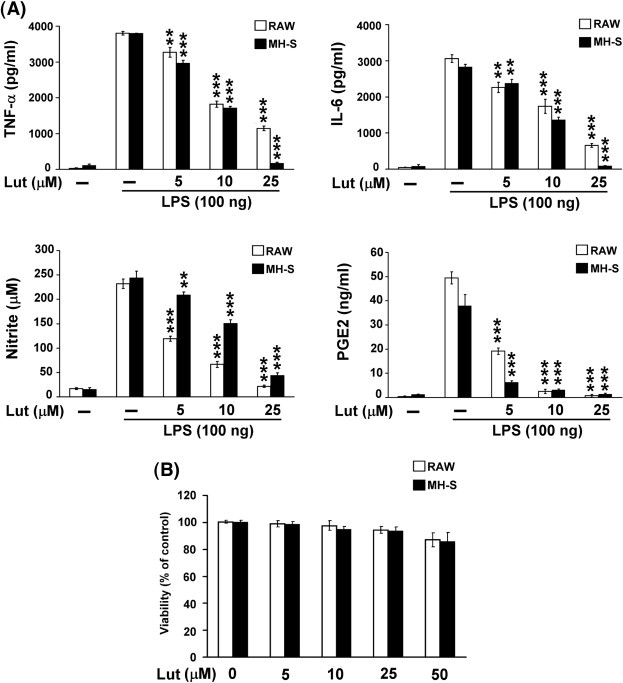
Luteolin inhibited LPS-induced pro-inflammatory cytokine and mediator production. MH-S and RAW 264.7 cells were pretreated with luteolin (5–25 μM) for 30 min, and then stimulated with LPS (100 ng/ml). (A) Culture media were collected at 8 h for TNF-α and IL-6 analysis, 16 h for NO and PGE2 analysis. (B) Viability in luteolin-treated cells was evaluated using the MTT assay. Cells were incubated with 5–50 μM luteolin for 24 h. The results are displayed in percentage of control samples. Data are presented as the mean ± SEM (n = 3) for three independent experiments; ⁎⁎p < 0.01, ⁎⁎⁎p < 0.001 as compared with the LPS treatment. Lut, luteolin.
Luteolin decreased the protein and mRNA levels of inflammation-associated genes and increased IL-1Ra mRNA level in LPS-stimulated macrophages
To determine if the inhibitory effect of luteolin on these inflammatory mediators (NO and PGE2) was related to the regulation of iNOS and COX-2, the levels of these two proteins were examined by Western blot analysis. As indicated in Fig. 2A, the protein levels of iNOS and COX-2 were markedly increased upon LPS treatment, and this induction was effectively inhibited by luteolin treatment.
Fig. 2.
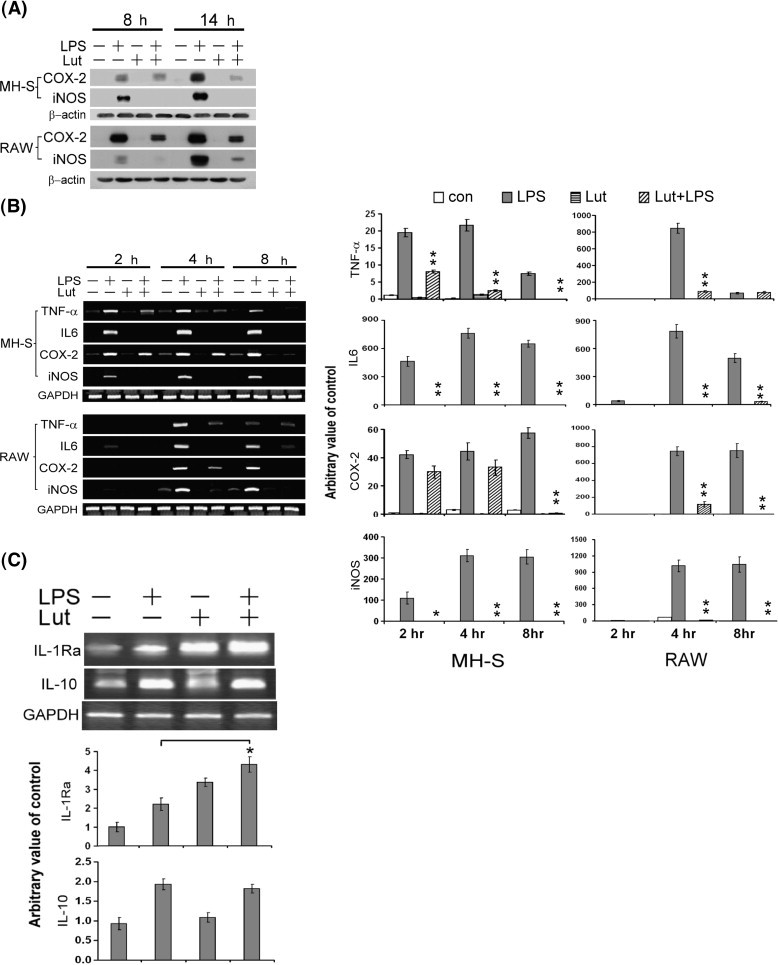
Luteolin decreased the protein and mRNA levels of inflammation-associated genes in LPS-stimulated macrophages. (A) MH-S and RAW 264.7 cells were pretreated with luteolin (25 μM) for 30 min, and then stimulated with LPS (100 ng/ml) for 8–14 h. Total protein extract was separated by SDS-PAGE, and then Western blot analysis with specific antibodies against COX-2 and iNOS. β-actin was used as an internal loading control. (B) RT-PCR analysis of TNF-α, IL-6, COX-2 and iNOS mRNA expression. Cells were pretreated with luteolin (25 μM) for 30 min, and then stimulated with LPS (100 ng/ml) for 2–8 h. Total RNA was analyzed by RT-PCR as described under Materials and methods. (C) RT-PCR analysis of IL-10 and IL-1Ra mRNA expression. MH-S cells were pretreated with luteolin (25 μM) for 30 min, and then stimulated with LPS (100 ng/ml) for 8 h. Total RNA was analyzed by RT-PCR. The figures were obtained from three independent experiments with similar patterns. Data are presented as the mean ± SEM (n = 3) for three independent experiments; ⁎p < 0.05, ⁎⁎p < 0.01 as compared with the LPS treatment.
To address the suppressive effect of luteolin on LPS-induced inflammatory molecules that might result from a transcriptional inhibition, MH-S and RAW 264.7 cells were stimulated with LPS in the presence or absence of luteolin at various indicated time points, and the mRNA levels of iNOS, COX-2, TNF-α and IL-6 were measured by RT-PCR. As presented in Fig. 2B, LPS-stimulated macrophages increased the mRNA levels of iNOS, COX-2, TNF-α, and IL-6. However, luteolin (25 μM) significantly attenuated the LPS-induced TNF-α, IL-6, COX-2 and iNOS mRNA expression in both MH-S and RAW 264.7 macrophages. These results summed up to indicate that the LPS-stimulated iNOS, COX-2 and pro-inflammatory cytokines (TNF-α and IL-6) expression would be suppressed by luteolin at the transcriptional level. Interleukin-10 (IL-10) and IL-1Ra are potent anti-inflammatory cytokines produced predominantly by activated macrophages. The effects of luteolin on the expression of IL-10 and IL-1Ra in LPS-activated MH-S macrophages were examined. As demonstrated in Fig. 2C, luteolin significantly increased the LPS-induced IL-1Ra mRNA level but had no observable effect on the expression of IL-10 mRNA in MH-S macrophages.
Prevention of LPS-induced NF-κB activation by luteolin
NF-κB is an important transcriptional regulator of inflammatory cytokines and plays a crucial role in immune responses (Aderem and Ulevitch, 2000). For the purpose of this study, we hypothesized that luteolin would inhibit the expression of these genes through the suppression of NF-κB activation. We examined the effect of luteolin on the binding activity of NF-κB to its consensus DNA oligonucleotide by employing the electrophoretic mobility shift assay (EMSA). As indicated in Fig. 3A, LPS treatment caused a significant increase in NF-κB–DNA binding activity within 60 min. This binding activity was decreased by pretreatment with luteolin, and the specific interaction between DNA and NF-κB was demonstrated by competitive inhibition with 100-fold excess of unlabeled oligonucleotide. LPS-mediated NF-κB–DNA binding activity was also inhibited by a NF-κB inhibitor, PDTC. Furthermore, we examined the regulatory effect of luteolin on LPS-induced nuclear translocation of the cytosolic NF-κB p65 subunit by immunostaining (Fig. 3B). As expected, luteolin treatment markedly suppressed the LPS-induced NF-κB p65 nuclear translocation. Since nuclear translocation of NF-κB was preceded by the phosphorylation, ubiquitination, and proteosome-dependent degradation of IκB (Ghosh and Baltimore, 1990, Hayden and Ghosh, 2004), we next examined the effect of luteolin on LPS-induced IκB degradation by Western blot analysis. As demonstrated in Fig. 3C, stimulation of MH-S or RAW 264.7 macrophages with 100 ng/ml LPS induced a rapid degradation of cytosolic IκB protein within 10 to 20 min; this effect was drastically blocked by treatment with luteolin (25 μM).
Fig. 3.
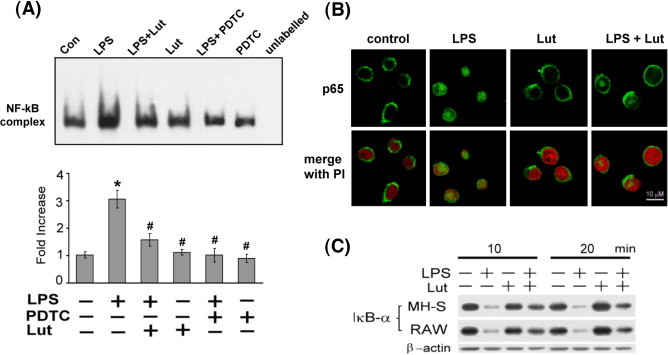
Attenuation of LPS-induced NF-κB activation by luteolin. (A) Effect of luteolin on LPS-induced NF-κB–DNA binding activity. MH-S cells were pretreated with luteolin (25 μM) or PDTC (200 μM) for 30 min, and then stimulated with or without LPS (100 ng/ml) for 60 min. Nuclear extracts were prepared and electrophoretic mobility shift assay was performed using biotin-labeled NF-κB consensus binding sequence. Competitive EMSA using an unlabeled NF-κB consensus sequence at 100-fold excess confirmed the specificity of NF-κB protein binding. Values were expressed as means ± SEM (n = 3). ⁎p < 0.05 as compared with the corresponding vehicle control; #p < 0.05 as compared with the LPS treatment. (B) Effects of luteolin on LPS-induced nuclear translocation of p65 subunit of NF-κB. MH-S macrophages were preincubated with luteolin (25 μM) for 30 min, and then treated with LPS (100 ng/ml) for 60 min. The subcellular localization of NF-κB p65 subunit was detected by immunofluorescence with antibody against p65 as described in Materials and methods. The same fields were counter stained with PI for location of nuclei. (C) Luteolin blocks LPS-induced IκBα degradation. MH-S and RAW 264.7 cells were pretreated with 25 μM luteolin for 30 min, and then stimulated with LPS (100 ng/ml) for 10 and 20 min. Protein extract was separated by SDS-PAGE followed by Western blot analysis that was performed with specific antibodies against IκBα. Equal loading of proteins was verified by actin immunoblotting. The figures were obtained from three independent experiments with similar patterns.
Prevention of LPS-induced AP-1 activation by luteolin
Fig. 3A shows that PDTC inhibited LPS-induced NF-κB–DNA binding activity. In order to determine whether this action is attributed to suppressing the expression of inflammatory genes, RT-PCR analysis was performed. LPS-mediated up-regulation of inflammatory genes (iNOS, COX-2, and IL-6) was significantly attenuated by PDTC except TNF-α (Fig. 4A). Based on these results, we hypothesize that there may be other routes participating in the inhibitory effect of luteolin on TNF-α expression. It has been reported that transcription factor activating protein-1 (AP-1) also appears to be activated by LPS and causing production of pro-inflammatory cytokines (Granger et al., 2000, Shaulian and Karin, 2002). Accordingly, subsequent study of LPS-induced AP-1 activation in MH-S cells was performed. Pretreatment of MH-S cells with 10 μM curcumin (an AP-1 inhibitor) resulted in a decrease of the LPS-stimulated TNF-α, IL-6, iNOS mRNA expression except COX-2 mRNA. The inhibitory effect of luteolin on inflammatory gene expression is stronger than that of combined treatment with curcumin and PDTC in LPS-stimulated macrophages (Fig. 4B). To assess the effect of luteolin and curcumin on AP-1–DNA binding activity, EMSA was done on MH-S cells. As indicated in Fig. 4C, treatment with LPS caused an increase in AP-1–DNA binding activity within 60 min; pretreatment with luteolin or curcumin caused a strong inhibition of LPS-induced AP-1–DNA binding activity. These results suggest that the inhibitory effect of luteolin on LPS-stimulated pro-inflammatory cytokines and mediator production might be regulated by both NF-κB and AP-1 transcriptional activation.
Fig. 4.
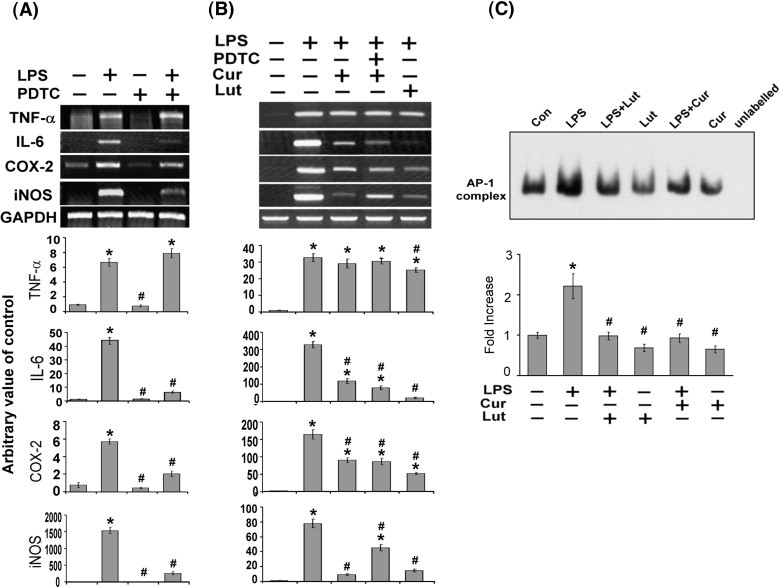
Suppression of LPS-induced AP-1 activation by luteolin. MH-S cells were pretreated with 200 μM PDTC (A) or combined treatment with PDTC and curcumin (B) for 30 min, and then stimulated with LPS (100 ng/ml) for 4 h. Effects of PDTC and curcumin on LPS-induced TNF-α, IL-6, COX-2 and iNOS mRNA expression were analyzed by RT-PCR as described under Materials and methods. (C) Effect of luteolin on LPS-induced AP-1–DNA binding activity. MH-S cells were pretreated with luteolin (25 μM) or curcumin (10 μM) for 30 min, and then stimulated with or without LPS (100 ng/ml) for 60 min. Nuclear extracts were prepared and EMSA was performed using biotin-labeled AP-1 consensus binding sequence. Competitive EMSA using an unlabeled AP-1 consensus sequence at 100-fold excess confirmed the specificity of AP-1 protein binding. Values are expressed as means ± SEM (n = 3). ⁎p < 0.05 as compared with the corresponding vehicle control; #p < 0.05 as compared with the LPS treatment. The figures were obtained from three independent experiments with similar patterns. Lut, luteolin; Cur, curcumin.
Luteolin inhibited the LPS-mediated ROS generation
It has been reported that LPS rapidly induces the generation of ROS especially H2O2 in macrophages (Hsu and Wen, 2002, Lu and Wahl, 2005). In order to investigate the effect of luteolin on LPS-induced ROS production, H2DCFDA was applied in order to detect cell oxygen burst. As illustrated in Fig. 5A, treatment of MH-S cells with LPS rapidly increased intracellular ROS level, especially H2O2 and •OH. Preincubation with luteolin (5, 10 and 25 μM) for 30 min attenuated the ROS generation in a dose-dependent manner in the LPS-activated macrophages (Fig. 5B).
Fig. 5.
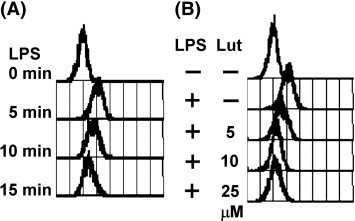
Effect of luteolin on LPS-induced ROS generation. (A) MH-S macrophages were pretreated with H2DCFDA (5 μM) for 30 min in serum-free medium and then exposed to LPS (100 ng/ml) at 37 °C for 0, 5, 10, 20 min. (B) MH-S cells were pretreated with H2DCFDA (5 μM) and luteolin (5, 10, and 25 μM) for 30 min, and then exposed to LPS (100 ng/ml) at 37 °C for another 5 min. Cells were collected and fluorescence was measured with a flow cytometer using Cell Quest software. The figure was obtained from three independent experiments with similar patterns.
Luteolin inhibited the LPS-induced Akt and IKK phosphorylation
Previous study indicated that transcriptional activation of NF-κB can be regulated through IKK or Akt signaling pathway. To further address the upstream signaling molecule in luteolin-mediated NF-κB inactivation, MH-S cells were pretreated with luteolin for 30 min and then stimulated with 100 ng/ml LPS for 10 min, the effect of luteolin on LPS-induced Akt and IKK activation was examined. Pretreatment with luteolin significantly reduced the LPS-elicited Akt (Thr308 and Ser473) and IKKα/β (Ser176/180) phosphorylation. Similarly, pretreatment with a PI3-k selective inhibitor LY294002 (10 μM) resulted in a dramatic reduction of Akt and IKKα/β phosphorylation (Fig. 6 ). These data suggested that luteolin inhibited NF-κB activation via suppression of Akt and IKK phosphorylated activation.
Fig. 6.
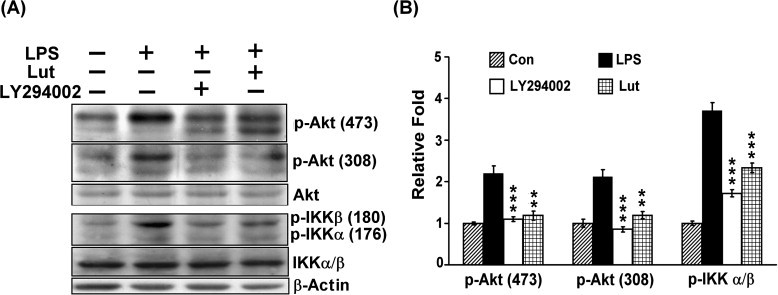
Luteolin decreased the LPS-induced Akt and IKKα/β phosphorylation. MH-S cells were pretreated with luteolin (25 μM) or LY294002 (10 μM) for 30 min, and then stimulated with LPS (100 ng/ml) for 10 min. Fifty micrograms of each protein was separated by SDS-PAGE, and then Western blot analysis with specific antibodies against phospho-Akt (Thr308), phospho-Akt (Ser473) and phosphor-IKKα/β (Ser176/180) was performed. β-actin was used as a control. Results are expressed as the mean±SEM for three independent experiments; ⁎⁎p< 0.01, ⁎⁎⁎p < 0.001 vs. LPS treatment. Lut, luteolin.
Discussion
Flavonoids are ubiquitous plant secondary metabolites and have a variety of biological effects, including antioxidant, anti-tumor, anti-microbial, anti-allergic, and anti-angiogenic properties (Shimada et al., 2006). Some natural flavonoids have anti-inflammatory effects in mammalian cells, which may be due to their ability to suppress the production of pro-inflammatory cytokines and mediators (Yuan et al., 2006). Accumulated evidence indicates that dysregulation of cytokines, such as TNF-α and IL-6, plays an essential role in many inflammatory conditions such as septic shock, hemorrhagic shock, rheumatoid arthritis and atherosclerosis. Inhibition of inflammatory cytokine and mediator production or function serves as a key mechanism in the control of inflammation, and agents that suppress the expression of these inflammation-associated genes have therapeutic potential for treatment of inflammatory diseases. Therefore, natural flavonoids have attracted interest as potential therapeutic agents for the treatment of inflammation. The plant flavonoid luteolin exhibits anti-inflammatory effects by inhibiting NF-κB-elicited gene expression and pro-inflammatory cytokine production in vitro (Xagorari et al., 2001), and histologically suppresses the inflammation in bacteria-infected lung tissue in C57BL/6J mice (Tormakangas et al., 2005). On the other hand, alveolar macrophages especially are thought to be the preferential immunoregulatory cell in lung tissue and targeted by bacterial endotoxins such as LPS. In this study, we found that luteolin significantly inhibits LPS-induced TNF-α, IL-6, cyclooxygenase-2, and iNOS expression at the transcriptional level in murine alveolar macrophage MH-S and peripheral macrophage RAW 264.7 cell lines.
Macrophages/monocytes, derived from a bone marrow pluripotent stem cell, are specialized hematopoietic cells distributed throughout different tissues of the body where they play a central role in homeostasis and tissue remodeling. Generation of an immune response may be considered another aspect of macrophage function. When monocytes leave the bone marrow, they do not appear to be programmed for any particular tissue. Rather, they undergo tissue-specific maturation in response to factors in tissues. Different macrophage populations may arise from differing local conditions in the tissue; these populations differ in morphology and functional activity (cytotoxicity, migration, oxygen radical production, pinocytosis and tissue factor production). In the lung, alveolar macrophage function can be influenced by surfactant and other components of lung lining fluid that can be obtained by lung lavage (Lambrecht, 2006). The alveolar macrophages play a key role in inflammatory and immune responses in the lung. However, the alveolar macrophage is thought to be a relatively poor antigen-presenting cell, compared with interstitial macrophages and monocytes. It has been reported that LPS-inducible genes were differentially regulated in macrophages derived from different tissues (Feng et al., 1999). To our knowledge, this is the first report implicating the inhibition of LPS-stimulated inflammatory molecule gene expression by luteolin in both alveolar and peripheral macrophage cell lines. Our observations are consistent with the findings of previous reports demonstrating that luteolin significantly suppressed the LPS-increased expression of TNF-α and iNOS genes in murine primary bone marrow-derived macrophages (Comalada et al., 2006), and of IL-6 and COX-2 in peripheral macrophage cell line RAW 264.7 (Xagorari et al., 2001, Harris et al., 2006). However, our findings show that the different populations of macrophages exhibit differential kinetics of activation in response to LPS. For example, while the activation of MH-S macrophages by LPS is relatively rapid (detectable at 2 h, Fig. 2B). LPS stimulation of RAW 264.7 macrophage activation is more slow (detectable at 4 h, Fig. 2B). Interestingly, the inhibitory effect of luteolin on LPS-induced gene expression (TNF-α, IL-6, COX-2 and iNOS) is stronger in MH-S macrophages than in peripheral macrophages (Fig. 2B). These results imply that alveolar macrophages are more sensitive to tested agents (LPS and luteolin) than peripheral macrophages. The reason for such differences may be due to functional heterogeneity among tissue macrophages as regards their cytokine production and signal transducing properties.
TNF-α, a cytotoxic cytokine, is known to play a key role in inflammatory processes (Nathan, 1987). As LPS stimulation induces a large increase in macrophage TNF-α production (Guha and Mackman, 2001), this production of TNF-α is also known to be responsible for induction of other pro-inflammatory mediators (Chandel et al., 2000). Our results, similar to those of a previous report (Xagorari et al., 2001), have shown an inhibitory action of luteolin on the LPS-induced production of TNF-α mRNA expression in LPS-activated MH-S macrophages. Moreover, both luteolin and curcumin cause a strong inhibition of LPS-induced AP-1–DNA binding activity (Fig. 4). These observations suggest that luteolin-suppressed TNF-α production most likely occurs via an AP-1-dependent pathway in LPS-stimulated MH-S macrophages.
Interleukin (IL)-6 is a typical pleiotropic cytokine which plays an important role in the homeostasis of the immune system and hematopoietic system, in addition to its physiological functions upon the nervous system, endocrine system and bone metabolism (Kamimura et al., 2003). However, IL-6 production is rapidly increased in acute inflammatory responses associated with infection, injury, trauma and other stress. As such, a dysregulated, high-level production of IL-6 could induce an undesirable inflammatory condition in many organs. With this study we show that luteolin not only inhibits IL-6 production (Fig. 1) but also reduces its mRNA transcript in LPS-stimulated MH-S and RAW 264.7 macrophages, indicating that this compound could transcriptionally down-regulate IL-6 expression. The NF-κB transcription factor has been proved to play a key role in the transcriptional up-regulation of the LPS-inducible IL-6 gene (Guha and Mackman, 2001). We found that although the LPS-induced NF-κB and AP-1–DNA binding activities are inhibited by luteolin (Figs. 3A and 4C), blocking NF-κB activity with PDTC significantly attenuates IL-6 mRNA expression. However, blocking AP-1 activity with curcumin slightly reduced LPS-induced IL-6 expression (Fig. 4). These results suggest that luteolin-mediated NF-κB inactivation is the most important pathway involved in IL-6 down-regulation in LPS-stimulated macrophages.
NO is a free radical playing a pivotal role as a vasodilator, neurotransmitter and immune regulator in a variety of tissues at physiological concentrations. Currently, at least three distinct isoforms of NOS have been isolated and cloned: eNOS (endothelial NOS), iNOS (inducible NOS) and nNOS (neuronal NOS). Many cell types can express iNOS for their function in the host defense against microbial and viral pathogens (Bogdan, 2001), and this leads to the formation of NO radicals or S-nitrosothiols or ONOO− in the host cell or in the microbe itself. On the other hand, iNOS expression in macrophages is activating by particular inducers and participating in the pathology of inflammatory diseases including atherosclerosis, rheumatoid arthritis, diabetes, septic shock, transplant rejection, and multiple sclerosis, leading to cell death (Buttery et al., 1994). Therefore, the regulation of NO production or iNOS expression levels might be an important target in the treatment of inflammatory disorders.
Luteolin has been shown to inhibit the NO production and iNOS expression in LPS-stimulated BV-2 microglial cells (Kim et al., 2006), in primary bone marrow-derived macrophages (Comalada et al., 2006) and in human gingival fibroblasts (Gutierrez-Venegas et al., 2006). Consistently, we found that suppression of iNOS expression by luteolin is in parallel with the comparable inhibition of NO production in LPS-activated MH-S and RAW 264.7 macrophages. Both NF-κB and AP-1 binding sites have been identified on the murine iNOS promoter and play a role in LPS-mediated induction of iNOS in macrophages (Aktan, 2004, Cho et al., 2002). Our results indicate that in addition to the inhibition of the NF-κB activation by luteolin, a slight decrease in the AP-1 activation may also contribute to the suppression of iNOS gene expression.
Cyclooxygenase (COX)-2, an enzyme in the conversion of arachidonic acid to prostaglandins (PGs), prostacyclin, and thromboxane A2, clearly represents an important step towards relieving the inflammatory process in macrophages (Wadleigh et al., 2000). Thus, selective inhibitors of COX-2 have been considered therapeutically effective for preventing inflammatory reaction and disease. The production of PGs by LPS in macrophages is primarily because of the transcriptional activation of the COX-2 gene. Luteolin has been found to inhibit inflammation-associated COX-2 expression and PGE2 formation in RAW 264.7 cells (Harris et al., 2006). We demonstrate here that luteolin could suppress PGE2 production and COX-2 gene expression via inactivation of NF-κB in both MH-S and RAW 264.7 macrophages. Our data also revealed that luteolin might not affect COX-1 enzymatic activity, because this compound did not alter basal PGE2 production in these two macrophage cell lines (data not shown).
It has been reported that endogenous IL-1Ra produced by both macrophages and infiltrating neutrophils, exhibits an important anti-inflammatory effect in LPS-induced lung-injury model (Ulich et al., 1992). Expression of IL-1Ra may attenuate bronchial hyperreactivity and inflammation in both antigen-induced disease and in allergen-induced late asthmatic reactions in guinea pigs (Okada et al., 1995). Our observations showed that luteolin could up-regulate IL-1Ra mRNA expression in LPS-stimulated MH-S macrophages. These results revealed that the anti-inflammatory effect of luteolin may not only down-regulate the pro-inflammatory cytokines but also up-regulate the anti-inflammatory cytokines.
The intracellular redox (reduction–oxidation) state is physiologically important in maintaining cellular homeostasis and is vital for proper cellular functions. Oxidative stress occurs when this balance is disrupted by excessive production of ROS. ROS are commonly multiplied during inflammatory processes that involve signal transduction and gene activation and can contribute to host cell and organ damage. Accumulating evidence indicates that generation of ROS is involved in regulating the activation status of a variety of transcription factors, including NF-κB and AP-1 (Pham et al., 2004). Intracellular redox changes induced by ROS augment NF-κB activation through the phosphorylation and degradation of IκB by increasing IKK or Akt kinase activity (Gloire et al., 2006). Several agents that activate NF-κB are found to have led to increased intracellular production of ROS. Previous studies proved that antioxidants inhibit NF-κB activation and block the expression of inflammatory cytokines as well as the production of NO and PGE2. Also, it has been reported that ectopic overexpression of catalase attenuates ROS production and subsequently inactivates AP-1 in endothelial cells (Lin et al., 2004). Structurally distinct antioxidants have been shown to inhibit the expression of NF-κB- and AP-1-dependent cytokines, iNOS and COX-2 and, thus, reduce inflammation (Comalada et al., 2006, Ma et al., 2003). Therefore, it is suggested that the suppressive effects of these antioxidant compounds on the production of the associated inflammatory mediators are related with their antioxidant activities.
Luteolin exhibits obvious antioxidative and has been shown to be able to scavenge ROS (Harris et al., 2006). In this study, we found the LPS-induced ROS produced within the initial 5 min, degradation of IκBα would occur within 10–20 min, and the NF-κB and AP-1 DNA binding activities would increase within 30 min. It is likely that the LPS induced NF-κB activation via the generation of ROS and possible that luteolin, through scavenging of ROS, may mediate suppression of NF-κB and/or AP-1 signaling, thus reducing the level of downstream products (including TNF-α, IL-6, iNOS and COX-2) in alveolar MH-S macrophages. These results suggest that this pharmacological property of luteolin may have therapeutic potential with respect to modulating redox-sensitive alveolar macrophage functions causally implicated in acute lung injury and other inflammatory disorders within the alveolar compartment during evolving microbial sepsis.
LPS signaling in macrophages involves a series of phosphorylation events leading to transcription factor activation and cytokine production. Stimulation of RAW 264.7 with LPS leads to a time dependent phosphorylation of tyrosine residues of several proteins that is inhibited by luteolin (Xagorari et al., 2001). It has been reported that LPS stimulated NF-κB activation through the phosphorylation and degradation of IκB by increasing IKK or Akt kinase activity (Ardeshna et al., 2000). Studies by Ozes et al. and Madrid et al. showed that a signaling pathway culminating in phosphorylation both IKKα and β by Akt are necessary for activation of NF-κB (Madrid et al., 2001, Ozes et al., 1999). Our results show that luteolin significantly decreases the phosphorylation of Akt on Ser473 and Thr308 and IKKα/β on Ser176/180 (Fig. 6) similarly to results obtained with LY294002, and this inhibitory event is accompanied by the suppression of IκB degradation and NF-κB p65 subunit nuclear translocation in alveolar MH-S macrophages, indicating that Akt and IKK inactivation might be involved in luteolin-mediated anti-inflammatory actions. Experiments are underway to further dissect the molecular mechanism that involves kinase in the inhibitory effects of luteolin on the LPS-induced cytokine and mediator production in MH-S cells.
Luteolin belongs to the flavonoid family of compounds. Flavonoids are composed of two aromatic rings linked through three carbon atoms that form an oxygenated heterocycle. Variations in the basic structure of flavonoids yield different classes of flavonoids. The structure variations may mediate the differences in the bioactivity of these related compounds. Xagorari and co-workers used RAW 264.7 macrophages to evaluate the effect of flavonoids on macrophage physiology. Their results showed that among the tested flavonoids, quercetin and luteolin are the most active compounds in reducing the action of LPS on pro-inflammatory cytokine release in macrophage and luteolin showed a lower IC50 than quercetin (Xagorari et al., 2001). These data are in line with the findings of Comalada et al. (2006) who showed that luteolin and quercetin effectively inhibited LPS-stimulated TNF-α release in primary bone marrow-derived macrophages. Analysis of the structure–activity relationship showed that four hydroxylations at positions 5, 7, 3′ and 4′, together with the double bond at C2–C3 and the position of the B ring at 2, seem to be necessary for the highest anti-inflammatory effect. The major determinants of antioxidative capacity have been classically considered to be the presence of a catechol group in the B ring, the presence of a 3-OH group, and the C2–C3 double bond (Silva et al., 2002). Consistent with this, our preliminary study also showed that luteolin is more potent than baicalein in suppression of LPS-induced macrophage activation. The anti-inflammatory capacity of flavonoids has long been put to use in Chinese medicine as crude plant extracts. Some flavonoids have been found to inhibit chronic inflammation in animal models (Camuesco et al., 2004, Kawaguchi et al., 2004). Current clinical approaches showed that flavonoids are promising anti-inflammatory drugs. However, the pharmacokinetic characteristics of flavonoids have to be considered before these agents may be used successfully in vivo. Most flavonoids are found glycosylated in plants and cannot be absorbed by the intestinal epithelium in that form. Hydrolysis of the glycosides by the intestinal flora yields free flavonoids that are absorbed efficiently. Comalada et al. suggested that flavonoids must be administered orally in glycosidic form in order to be applied as anti-inflammatory agents (Comalada et al., 2006).
In conclusion, our observations suggest that luteolin has a strong antioxidant capacity to inhibit inflammation-associated gene expression (TNF-α, IL-6, iNOS, and COX-2) by suppressing redox-dependent NF-κB and AP-1 activation, resulting in its anti-inflammatory effects, and imply that luteolin may be a potential drug for treating inflammatory diseases. Our observations also demonstrate that the anti-inflammatory property of L. japonica may be partly due to the luteolin, providing support for the traditional use of this plant in Chinese medicine against alveolar inflammatory disorders. However, further in vivo investigation of this activity is necessary to qualify its mechanism and medicinal potential.
Acknowledgements
This work was supported by grants from the National Science Council (NSC-94-2320-B-075A-004) and Taichung Veterans General Hospital (TCVGH-957314C), Taiwan, Republic of China.
References
- Aderem A., Ulevitch R.J. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sciences. 2004;75:639–653. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- Ardeshna K.M., Pizzey A.R., Devereux S., Khwaja A. The PI3 kinase, p38 SAP kinase, and NF-kappaB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood. 2000;96:1039–1046. [PubMed] [Google Scholar]
- Bogdan C. Nitric oxide and the immune response. Nature Immunology. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- Buttery L.D., Evans T.J., Springall D.R., Carpenter A., Cohen J., Polak J.M. Immunochemical localization of inducible nitric oxide synthase in endotoxin-treated rats. Laboratory Investigation. 1994;71:755–764. [PubMed] [Google Scholar]
- Camuesco D., Comalada M., Rodriguez-Cabezas M.E., Nieto A., Lorente M.D., Concha A., Zarzuelo A., Galvez J. The intestinal anti-inflammatory effect of quercitrin is associated with an inhibition in iNOS expression. British Journal of Pharmacology. 2004;143:908–918. doi: 10.1038/sj.bjp.0705941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel N.S., Trzyna W.C., McClintock D.S., Schumacker P.T. Role of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. Journal of Immunology. 2000;165:1013–1021. doi: 10.4049/jimmunol.165.2.1013. [DOI] [PubMed] [Google Scholar]
- Cho M.K., Suh S.H., Kim S.G. JunB/AP-1 and NF-kappa B-mediated induction of nitric oxide synthase by bovine type I collagen in serum-stimulated murine macrophages. Nitric Oxide. 2002;6:319–332. doi: 10.1006/niox.2001.0415. [DOI] [PubMed] [Google Scholar]
- Christofidou-Solomidou M., Muzykantov V.R. Antioxidant strategies in respiratory medicine. Treatments in Respiratory Medicine. 2006;5:47–78. doi: 10.2165/00151829-200605010-00004. [DOI] [PubMed] [Google Scholar]
- Comalada M., Ballester I., Bailon E., Sierra S., Xaus J., Galvez J., de Medina F.S., Zarzuelo A. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: analysis of the structure–activity relationship. Biochemical Pharmacology. 2006;72:1010–1021. doi: 10.1016/j.bcp.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Das M., Ram A., Ghosh B. Luteolin alleviates bronchoconstriction and airway hyperreactivity in ovalbumin sensitized mice. Inflammation Research. 2003;52:101–106. doi: 10.1007/s000110300021. [DOI] [PubMed] [Google Scholar]
- Feng G.J., Goodridge H.S., Harnett M.M., Wei X.Q., Nikolaev A.V., Higson A.P., Liew F.Y. Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. Journal of Immunology. 1999;163:6403–6412. [PubMed] [Google Scholar]
- File T.M., Jr., Tsang K.W. Severe acute respiratory syndrome: pertinent clinical characteristics and therapy. Treatments in Respiratory Medicine. 2005;4:95–106. doi: 10.2165/00151829-200504020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Baltimore D. Activation in vitro of NF-kappa B by phosphorylation of its inhibitor I kappa B. Nature. 1990;344:678–682. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- Gloire G., Legrand-Poels S., Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochemical Pharmacology. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Granger R.L., Hughes T.R., Ramji D.P. Gene, stimulus and cell-type specific regulation of activator protein-1 in mesangial cells by lipopolysaccharide and cytokines. Biochimica et Biophysica Acta. 2000;1492:100–107. doi: 10.1016/s0167-4781(00)00089-0. [DOI] [PubMed] [Google Scholar]
- Guha M., Mackman N. LPS induction of gene expression in human monocytes. Cellular Signalling. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- Guha M., O'Connell M.A., Pawlinski R., Hollis A., McGovern P., Yan S.F., Stern D., Mackman N. Lipopolysaccharide activation of the MEK–ERK1/2 pathway in human monocytic cells mediates tissue factor and tumor necrosis factor alpha expression by inducing Elk-1 phosphorylation and Egr-1 expression. Blood. 2001;98:1429–1439. doi: 10.1182/blood.v98.5.1429. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Venegas G., Kawasaki-Cardenas P., Arroyo-Cruz S.R., Maldonado-Frias S. Luteolin inhibits lipopolysaccharide actions on human gingival fibroblasts. European Journal of Pharmacology. 2006;541:95–105. doi: 10.1016/j.ejphar.2006.03.069. [DOI] [PubMed] [Google Scholar]
- Harris G.K., Qian Y., Leonard S.S., Sbarra D.C., Shi X. Luteolin and chrysin differentially inhibit cyclooxygenase-2 expression and scavenge reactive oxygen species but similarly inhibit prostaglandin-E2 formation in RAW 264.7 cells. The Journal of Nutrition. 2006;136:1517–1521. doi: 10.1093/jn/136.6.1517. [DOI] [PubMed] [Google Scholar]
- Hayden M.S., Ghosh S. Signaling to NF-kappaB. Genes and Development. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- Hsu H.Y., Wen M.H. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. The Journal of Biological Chemistry. 2002;277:22131–22139. doi: 10.1074/jbc.M111883200. [DOI] [PubMed] [Google Scholar]
- Kamimura D., Ishihara K., Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Reviews of Physiology, Biochemistry and Pharmacology. 2003;149:1–38. doi: 10.1007/s10254-003-0012-2. [DOI] [PubMed] [Google Scholar]
- Kawaguchi K., Kikuchi S., Hasunuma R., Maruyama H., Yoshikawa T., Kumazawa Y. A citrus flavonoid hesperidin suppresses infection-induced endotoxin shock in mice. Biological and Pharmaceutical Bulletin. 2004;27:679–683. doi: 10.1248/bpb.27.679. [DOI] [PubMed] [Google Scholar]
- Kim J.S., Lee H.J., Lee M.H., Kim J., Jin C., Ryu J.H. Luteolin inhibits LPS-stimulated inducible nitric oxide synthase expression in BV-2 microglial cells. Planta Medica. 2006;72:65–68. doi: 10.1055/s-2005-873145. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Kobayashi M., Heming T.A., Bidani A., Pollard R.B., Suzuki F. Cytokine production by rabbit alveolar macrophages: differences between activated and suppressor cell phenotypes. Immunology Letters. 1999;69:339–346. doi: 10.1016/s0165-2478(99)00114-5. [DOI] [PubMed] [Google Scholar]
- Kotanidou A., Xagorari A., Bagli E., Kitsanta P., Fotsis T., Papapetropoulos A., Roussos C. Luteolin reduces lipopolysaccharide-induced lethal toxicity and expression of proinflammatory molecules in mice. American Journal of Respiratory and Critical Care Medicine. 2002;165:818–823. doi: 10.1164/ajrccm.165.6.2101049. [DOI] [PubMed] [Google Scholar]
- Lai S.T. Treatment of severe acute respiratory syndrome. European Journal of Clinical Microbiology & Infectious Diseases. 2005;24:583–591. doi: 10.1007/s10096-005-0004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht B.N. Alveolar macrophage in the driver's seat. Immunity. 2006;24:366–368. doi: 10.1016/j.immuni.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Ko W.S., Kim Y.H., Kang H.S., Kim H.D., Choi B.T. Anti-inflammatory effect of the aqueous extract from Lonicera japonica flower is related to inhibition of NF-kappaB activation through reducing I-kappaBalpha degradation in rat liver. International Journal of Molecular Medicine. 2001;7:79–83. [PubMed] [Google Scholar]
- Lew T.W., Kwek T.K., Tai D., Earnest A., Loo S., Singh K., Kwan K.M., Chan Y., Yim C.F., Bek S.L., Kor A.C., Yap W.S., Chelliah Y.R., Lai Y.C., Goh S.K. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA: The Journal of the American Medical Association. 2003;290:374–380. doi: 10.1001/jama.290.3.374. [DOI] [PubMed] [Google Scholar]
- Lin S.J., Shyue S.K., Liu P.L., Chen Y.H., Ku H.H., Chen J.W., Tam K.B., Chen Y.L. Adenovirus-mediated overexpression of catalase attenuates oxLDL-induced apoptosis in human aortic endothelial cells via AP-1 and C-Jun N-terminal kinase/extracellular signal-regulated kinase mitogen-activated protein kinase pathways. Journal of Molecular and Cellular Cardiology. 2004;36:129–139. doi: 10.1016/j.yjmcc.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Liu X., Zhang M., He L., Li Y.P., Kang Y.K. Cochrane Database of Systematic Reviews, CD004882. 2006. Chinese herbs combined with Western medicine for severe acute respiratory syndrome (SARS) [DOI] [PubMed] [Google Scholar]
- Lu Y., Wahl L.M. Oxidative stress augments the production of matrix metalloproteinase-1, cyclooxygenase-2, and prostaglandin E2 through enhancement of NF-kappa B activity in lipopolysaccharide-activated human primary monocytes. Journal of Immunology. 2005;175:5423–5429. doi: 10.4049/jimmunol.175.8.5423. [DOI] [PubMed] [Google Scholar]
- Ma Q., Kinneer K., Ye J., Chen B.J. Inhibition of nuclear factor kappaB by phenolic antioxidants: interplay between antioxidant signaling and inflammatory cytokine expression. Molecular Pharmacology. 2003;64:211–219. doi: 10.1124/mol.64.2.211. [DOI] [PubMed] [Google Scholar]
- Madrid L.V., Mayo M.W., Reuther J.Y., Baldwin A.S., Jr. Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. The Journal of Biological Chemistry. 2001;276:18934–18940. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- Matsunaga K., Klein T.W., Friedman H., Yamamoto Y. Alveolar macrophage cell line MH-S is valuable as an in vitro model for Legionella pneumophila infection. American Journal of Respiratory Cell and Molecular Biology. 2001;24:326–331. doi: 10.1165/ajrcmb.24.3.4359. [DOI] [PubMed] [Google Scholar]
- Nathan C.F. Secretory products of macrophages. The Journal of Clinical Investigation. 1987;79:319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S., Inoue H., Yamauchi K., Iijima H., Ohkawara Y., Takishima T., Shirato K. Potential role of interleukin-1 in allergen-induced late asthmatic reactions in guinea pigs: suppressive effect of interleukin-1 receptor antagonist on late asthmatic reaction. Journal of Allergy and Clinical Immunology. 1995;95:1236–1245. doi: 10.1016/s0091-6749(95)70081-1. [DOI] [PubMed] [Google Scholar]
- Ozes O.N., Mayo L.D., Gustin J.A., Pfeffer S.R., Pfeffer L.M., Donner D.B. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- Park E., Kum S., Wang C., Park S.Y., Kim B.S., Schuller-Levis G. Anti-inflammatory activity of herbal medicines: inhibition of nitric oxide production and tumor necrosis factor-alpha secretion in an activated macrophage-like cell line. The American Journal of Chinese Medicine. 2005;33:415–424. doi: 10.1142/S0192415X05003028. [DOI] [PubMed] [Google Scholar]
- Pham C.G., Bubici C., Zazzeroni F., Papa S., Jones J., Alvarez K., Jayawardena S., De Smaele E., Cong R., Beaumont C., Torti F.M., Torti S.V., Franzoso G. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell. 2004;119:529–542. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Shaulian E., Karin M. AP-1 as a regulator of cell life and death. Nature Cell Biology. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- Shimada H., Miura K., Imamura Y. Characteristics and inhibition by flavonoids of 20alpha-hydroxysteroid dehydrogenase activity in mouse tissues. Life Sciences. 2006;78:2931–2936. doi: 10.1016/j.lfs.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Silva M.M., Santos M.R., Caroco G., Rocha R., Justino G., Mira L. Structure–antioxidant activity relationships of flavonoids: a re-examination. Free Radical Research. 2002;36:1219–1227. doi: 10.1080/198-1071576021000016472. [DOI] [PubMed] [Google Scholar]
- Tormakangas L., Vuorela P., Saario E., Leinonen M., Saikku P., Vuorela H. In vivo treatment of acute Chlamydia pneumoniae infection with the flavonoids quercetin and luteolin and an alkyl gallate, octyl gallate, in a mouse model. Biochemical Pharmacology. 2005;70:1222–1230. doi: 10.1016/j.bcp.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Ulich T.R., Guo K., Yin S., del Castillo J., Yi E.S., Thompson R.C., Eisenberg S.P. Endotoxin-induced cytokine gene expression in vivo. IV. Expression of interleukin-1 alpha/beta and interleukin-1 receptor antagonist mRNA during endotoxemia and during endotoxin-initiated local acute inflammation . American Journal of Pathology. 1992;141:61–68. [PMC free article] [PubMed] [Google Scholar]
- Wadleigh D.J., Reddy S.T., Kopp E., Ghosh S., Herschman H.R. Transcriptional activation of the cyclooxygenase-2 gene in endotoxin-treated RAW 264.7 macrophages. The Journal of Biological Chemistry. 2000;275:6259–6266. doi: 10.1074/jbc.275.9.6259. [DOI] [PubMed] [Google Scholar]
- Wu C.Y., Jan J.T., Ma S.H., Kuo C.J., Juan H.F., Cheng Y.S., Hsu H.H., Huang H.C., Wu D., Brik A., Liang F.S., Liu R.S., Fang J.M., Chen S.T., Liang P.H., Wong C.H. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10012–10017. doi: 10.1073/pnas.0403596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xagorari A., Papapetropoulos A., Mauromatis A., Economou M., Fotsis T., Roussos C. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. The Journal of Pharmacology and Experimental Therapeutics. 2001;296:181–187. [PubMed] [Google Scholar]
- Xagorari A., Roussos C., Papapetropoulos A. Inhibition of LPS-stimulated pathways in macrophages by the flavonoid luteolin. British Journal of Pharmacology. 2002;136:1058–1064. doi: 10.1038/sj.bjp.0704803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan G., Wahlqvist M.L., He G., Yang M., Li D. Natural products and anti-inflammatory activity. Asia Pacific Journal of Clinical Nutrition. 2006;15:143–152. [PubMed] [Google Scholar]


