Abstract
Respiratory tract infections (bovine respiratory disease) are a major concern in calf rearing. The objective of this study was to identify pathogen-specific risk factors associated with epidemic respiratory disease in calves. A cross-sectional study was conducted, involving 128 outbreaks (29 dairy, 58 dairy-mixed, and 41 beef) in Belgium (2016–2018). A semiquantitative PCR for 7 respiratory pathogens was done on a pooled nonendoscopic bronchoalveolar lavage sample for each herd. Potential risk factors were collected by questionnaire and derived from the national cattle registration databank. Most outbreaks occurred between October and March, and single and multiple viral infections were detected in 58.6% (75/128) and 13.3% (17/128), respectively. Bovine coronavirus (BCV) was the most frequently isolated virus (38.4%), followed by bovine respiratory syncytial virus (bRSV; 29.4%) and parainfluenzavirus type 3 (PI-3; 8.1%). Mycoplasma bovis, Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni were detected in 33.3, 41.2, 89.1, and 36.4% of the herds, respectively. Specific risk factors for BCV detection were detection of M. haemolytica [odds ratio (OR) = 2.8 (95% confidence interval = 1.1–7.5)], increasing herd size [OR = 1.3 (1.0–1.8) for each increase with 100 animals] and detection of BCV by antigen ELISA on feces in calves in the last year [OR = 3.6 (1.2–11.1)]. A seasonal effect was shown for bRSV only {more in winter compared with autumn [OR = 10.3 (2.8–37.5)]}. Other factors associated with bRSV were PI-3 detection [OR = 13.4 (2.1–86.0)], prevalence of calves with respiratory disease [OR = 1.02 (1.00–1.04) per 1% increase], and number of days with respiratory signs before sampling [OR = 0.99 (0.98–0.99) per day increase]. Next to its association with BCV, M. haemolytica was more frequently detected in herds with 5 to 10 animals per pen [OR = 8.0 (1.4–46.9)] compared with <5 animals, and in herds with sawdust as bedding [OR = 18.3 (1.8–191.6)]. Also, for H. somni, housing on sawdust was a risk factor [OR = 5.2 (1.2–23.0)]. Purchase of cattle [OR = 2.9 (1.0–8.0)] and housing of recently purchased animals in the same airspace [OR = 5.0 (1.5–16.5)] were risk factors for M. bovis. This study identified pathogen-specific risk factors that might be useful for the development of customized control and prevention and for the design of decision support tools to justify antimicrobial use by predicting the most likely pathogen before sampling results are available.
Key words: bovine respiratory disease, PCR, broncho-alveolar lavage, coronavirus
INTRODUCTION
Respiratory tract infections (RTI), and their clinical presentation as bovine respiratory disease (BRD), are a major economic issue in all cattle operations worldwide and a leading indication for antimicrobial use (Pardon et al., 2012a,b; Reiten et al., 2018; Karle et al., 2019). To reduce the selection pressure from antimicrobial use in the long run, better prevention and control of RTI is urgently needed (EMA/EFSA, 2017). Airway inflammation and bronchopneumonia result from a complex interplay between multiple viral and bacterial pathogens and environmental or host factors weakening defense mechanisms or stimulating pathogen spread (Griffin et al., 2010). A long-lasting concept is that viruses are primary pathogens, paving the way for bacterial infections (Griffin et al., 2010). Also, Mycoplasma bovis is increasingly recognized as a primary pathogen, although this remains controversial in the scientific community (Calcutt et al., 2018). Despite the importance of BRD, most available studies on pathogen identification in live animals are limited in number of herds and pathogens studied (Autio et al., 2007; Pardon et al., 2011; Murray et al., 2018). Also, most of them involve intensive systems such as feedlots or veal calves, which are confronted with respiratory disease year round at a predictable moment in the production cycle (Pardon et al., 2011; Timsit et al., 2017). In contrast, pathogens involved in the classic epidemic respiratory disease outbreaks in winter in the most frequent European farming system of family-owned medium-sized dairy and beef farms are hardly documented (O'Neill et al., 2014).
To justify antimicrobial use and customize prevention and control measures, sampling of the respiratory tract is recommended in more and more European countries (KNMVD, 2015; EMA/EFSA, 2017; AMCRA, 2019). Different sampling methods are available, of which deep nasopharyngeal swabs, transtracheal washes, and nonendoscopic broncho-alveolar lavage (nBAL) have found their way into practice (Doyle et al., 2017; Timsit et al., 2017; Van Driessche et al., 2017). Next to classic bacterial culture and susceptibility testing, PCR is increasingly popular (O'Neill et al., 2014; DGZ, 2016). According to practitioners, the main advantage is that many different pathogens, both viruses and bacteria, are tested and that the high sensitivity makes it possible to test pooled samples, which complies well with their desire to obtain a group diagnosis (O'Neill et al., 2014). Also, contamination of the sample does not interfere with the test result as much as with culture. Main disadvantages are the lack of antimicrobial susceptibility testing and difficulties in the interpretation of detection of opportunistic pathogens such as Pasteurellaceae (Fulton and Confer, 2012).
Many risk factors for RTI have been identified in multiple studies, but they all used a wide variety of case definitions, covered by the BRD concept. With the exception of M. bovis (Gille et al., 2018; Schibrowski et al., 2018), hardly any studies explored pathogen-specific risk factors for detection of the pathogen itself. In bovine mastitis, identification of pathogen-group-specific risk factors has led to targeted control and prevention, which is economically more efficient than a standard approach (Passchyn et al., 2014; Tolosa et al., 2015). Also, for respiratory pathogens, the eliciting risk factors might be different, enabling the possibility for customized advice instead of the current general approaches, conferring multiple and often expensive changes on farm. Additionally, being able to predict the most likely pathogen (virus, M. bovis, or Pasteurellaceae) involved at the time of the first visit based on easily measurable environmental or circumstantial factors would be of great practical value to justify antimicrobial use. Therefore, the objective of the present study was to identify at the herd level pathogen-specific risk factors for respiratory viruses and bacteria involved in epidemic outbreaks of respiratory disease.
MATERIALS AND METHODS
Study Population, Design, and Sample Size Calculation
A cross-sectional study was conducted. The study period was set to include 2 winter seasons, and therefore took from September 2016 to January 2018. This study used available data from the ‘Griepbarometer' initiative of the Flemish Animal Health Service and Ghent University (DGZ, 2016). This project aimed at developing an accessible monitoring tool for RTI in cattle, continuously available on a webpage to offer farmers and veterinarians a view on the prevalence and spread of respiratory pathogens in their surroundings. Collection and use of data were in compliance with local ethical requirements. To stimulate sample submission, the PCR analysis of the samples was at the expense of the Flemish Animal Health Service. The target population was all cattle herds from the northern part of Belgium (Flanders). The study population was conveniently selected based on willingness to participate at a first-come, first-serve basis until the budget was completed. The aim was to sample epidemic outbreaks of respiratory disease. Inclusion criteria were an acute outbreak of respiratory signs (cough, nasal discharge, increased breathing rate, fever, depression) affecting multiple animals (>5; minimum 15% ill animals) in multiple pens in the same barn/air space (compartment) of a farm in less than a week. The advice was given to sample the outbreak within 7 d after the index case to optimize the odds for viral detection. Financial support was only offered if the enquiry was filled in and returned. Communication was directed toward veterinarians, who could offer the sampling to their clients. This was done through the different communication channels (email, website) of the Flemish Animal Health Service.
Sample size was based on prevalence determination of respiratory pathogens in the study region. To determine the prevalence of the pathogens with 95% confidence, an expected prevalence of the pathogen of 50% (worst case scenario) and 10% accepted error, based on several herds in Flanders of 15,081 in 2016, 97 herds needed to be sampled (Winepiscope 2.0, Zaragoza, Spain; Thrusfield et al., 2001). The budget was set to sample 130 herds over 2 yr.
Sampling and Risk Factor Data Collection
Nonendoscopic broncho-alveolar lavage samples were taken as previously described using an instilled volume of saline of 0.5 to 1 mL/kg of BW (Van Driessche et al., 2017). Training sessions to perform the nBAL sampling had been organized for local practitioners by the Flemish Animal Health Service in collaboration with Ghent University all over the country in 2015 to 2017. Only ill animals were sampled. The advice to sample acute cases, which were not treated with antimicrobials in the last 14 d, was given. Animal selection was left at their discretion and consisted of a combination of the following signs: fever (>39°C), nasal discharge, ocular discharge, (induced or spontaneous) cough, increased respiratory rate (>45 breaths/min), adventitious lung sounds, and depression. For each farm 5 animals needed to be sampled. This sample size was based on the limitations of PCR to be used on pooled samples. Diagnostic performance of the PCR was identical to individual samples when the pool was limited to 5 samples (in-house validation). A separate sterile nBAL catheter was used for each animal. Lung lavage fluid was collected in sterile tubes and the 5 samples were sent to the laboratory as quickly as possible. All sampling techniques have been previously approved by the local ethical committee for use in routine sampling in practice (Ghent University EC 2014/189, EC 2016/20).
A questionnaire consisting of farm identification and 39 questions on management, housing, and clinical presentation was filled in by the practitioner using an oral interview of the farmer and local inspection of the environment. The questionnaire was pretested on 5 veterinarians and 5 farmers to ensure correct interpretation of the questions. Further data were collected from the national cattle identification, registration and movement database [SANITRACE, Animal Health Service Flanders (DGZ) and Federal Agency for the Safety of the Food Chain, Torhout/Brussels, Belgium]. This consisted of herd size and animal age categories, breed, and purchase history. All farmers gave informed consent for use of their data.
Laboratory Analysis
A pool was made from the 5 separate samples by taking 5 mL from each sample. A semiquantitative real-time PCR (Screening Pack Ruminant Respiratory Pathogens, Thermo Fisher Scientific, Waltham, MA) was conducted. Seven individual PCR reactions per sample were performed, each targeting a single respiratory pathogen: bovine respiratory syncytial virus (bRSV; N gene), bovine parainfluenzavirus type 3 (P gene), bovine coronavirus (N gene), M. bovis (target gene = polC), Histophilus somni (RpoB), Pasteurella multocida (16S), and Mannheimia haemolytica (plpE). The DNA and RNA were extracted from bronchial alveolar lavage fluid samples using the MagVet Universal Kit (Thermo Fisher Scientific) according to the manufacturer's instructions and using 200 µL for extraction. The real-time PCR was performed on the ABI7500 detection system (Thermo Fisher Scientific) with the following cycling conditions: 10 min incubation at 45°C, 10 min incubation at 95°C, and 45 cycles of 95°C for 15 s and 60°C for 1 min. The fluorescence data were collected during the 60°C, 1 min stage. The real-time PCR used TaqMan technology (Thermo Fisher Scientific, Waltham, MA). For each pathogen target, a duplex PCR was performed using a probe labeled with the FAM dye for the target gene and a probe labeled with the VIC dye for the internal positive control. This internal positive control is a bovine housekeeping gene whose detection is used to check for PCR inhibition and the presence of host genomic DNA. The PCR detection limits for all 7 pathogen targets range from 10 to 40 copies of nucleic acid per PCR reaction. The efficiency of the PCR reactions for the 7 pathogen targets comprised between 89.3 and 108.2%. The PCR results were classified according to the manufacturer's instructions as positive if the threshold cycle (Ct) value was <38. If no Ct value was obtained, samples were considered negative. Values ≥38 were considered not interpretable and not included in the analysis for that pathogen.
Statistical Analysis
Data were analyzed with SAS 9.4 (SAS Institute Inc., Cary, NC). The unit of analysis was the herd. Outcomes of interest were the 7 pathogens, as detected by PCR. Noninterpretable results were not included in the analysis. Risk factors for each of the 7 pathogens were identified using multivariable logistic regression. A generalized linear mixed model was used with binomial distribution and logit link function with Wald statistics for type 3 contrasts (PROC GLIMMIX). First, all predictors were tested univariably for their association with the outcome. A total of 45 predictors (Table 1 ) and the PCR results for the other 6 pathogens besides the outcome pathogen were considered for the analysis. Categorical predictors were regrouped if the number of observations within a group was lower than 8. All predictors with P < 0.2 were maintained for the multivariable model. Next, the multivariable model was built stepwise backward, gradually excluding nonsignificant variables. A variable was considered a confounder if it was not an intervening variable based on a causal diagram and induced changes >25% in the coefficient of another variable. For the final models, pairwise comparisons for categorical predictors were made using Bonferroni adjustments. All biologically relevant 2-way interactions of significant fixed effects were tested. Significance was set at P < 0.05 and P < 0.10 was considered a trend. Model fit was evaluated using the Hosmer-Lemeshow goodness-of-fit test for logistic models (Dohoo et al., 2009). Differences in management factors between dairy, dairy-mixed, and beef herds were determined by logistic regression (PROC GLIMMIX; as described above) for binary outcomes, and linear regression (PROC MIXED) for continuous outcomes. In the linear model, maximum likelihood was used, and Bonferroni corrections for multiple comparisons.
Table 1.
Overview of potential risk factors for respiratory pathogens, derived from a questionnaire and the national cattle registration database
| Subject | Description |
|---|---|
| Herd info | Production type (dairy, dairy-mixed, beef); total number of animals; ETEC history (y/n)1; BCV history (y/n)1; rotavirus history (y/n)1; Cryptosporidium parvum history (y/n)1; estimated annual neonatal diarrhea incidence (%); estimated annual respiratory disease incidence (%); endemic bovine respiratory disease problems (y/n); vaccination (RSV, PI-3, Mannheimia haemolytica, BVDv; y/n) |
| Outbreak data | Month of outbreak; season of outbreak (winter, spring, summer, autumn); duration of signs (d); animals with respiratory signs (%); adult animals affected (y/n); time passed since last outbreak (d) |
| Direct environment of the outbreak pen | Chronically ill animal in pen or adjacent pen (y/n); ventilation type (natural, mechanical, mixed); ammonia smell (y/n); spider webs present (y/n); air draft (y/n); ventilation audit in last year (y/n); adaptations made to stable ventilation in last year (y/n); floor type (full concrete, slatted floor); bedding material (straw, sawdust); adult cattle housed in same building as calves (y/n); stable type (open front, closed stable); group size (<5, 5–10, >10) |
| Calf management | Colostrum feeding (own farm only, other farm, or purchased); milk feeding (milk replacer, cow milk, suckler); age at first grouping (d); age at second grouping (d); automated milk feeding (y/n); shaving calves in winter (y/n); remaining >24 h with the dam after calving (y/n); nose-nose contact possible with other pens (y/n); individual housing type (igloo, pen indoors, pen outdoor, suckler cow) |
| Purchase management | Purchase (y/n); recent purchases in same stable as outbreak (y/n); quarantine stable (y/n); purchases in 2016–2017 (no./yr) |
Information derived from the question, “What pathogens were in the last year detected by on-farm antigen-ELISA (BCV, rotavirus, C. parvum, and ETEC)?” bRSV = bovine respiratory syncytial virus; ETEC = enterotoxic Escherichia coli; BCV = bovine coronavirus; PI-3 = parainfluenzavirus type 3; BVDv = bovine viral diarrhea virus; y/n = yes/no.
RESULTS
Herd Characteristics
Between September 2016 and January 2018, samples from a total of 128 outbreaks arrived at the laboratory. Of the herds, 22.7% (29/128) were dairy (Holstein Friesian), 45.3% (58/128) were dairy type mixed farms, and 32.0% (41/128) were beef. Dairy herds were generally medium sized (25th and 75th percentile: 89–197 adult animals; range: 31–689 animals), family-owned farms, representative for the mainstream dairy production in Western Europe. Dairy-type mixed herds were herds with predominantly dairy cattle, but also a more limited number of beef cattle or dairy/beef crossbreds. Beef breeds were mainly medium sized (25th and 75th percentile: 51–142 adult animals; range: 15–345) family-owned operations, of which 31.7% (13/41) were suckler herds and 68.3% (28/41) separated calves from the dam, shortly after birth, to raise them further in individual housing first and group housing afterward on either cow milk or milk replacer. No specialized fattening units, such as veal facilities or feedlots, were included in the study. The mean number of adult animals (139.9 ± 118.8) in the study population was not significantly different from the target population (134; SANITRACE, national cattle registration databank; P = 0.58). Beef herds (85.8 ± 18.2) were significantly smaller than dairy herds (197.6 ± 20.8; P < 0.001) and dairy-mixed herds (146.6 ± 14.8; P = 0.03). In dairy herds, the second grouping of calves was done at a younger age than in beef herds (87.8 ± 12.2 d vs. 136.0 ± 11.4 d; P = 0.02). Other significant differences in the predictor variables are shown in Table 2 .
Table 2.
Overview of significant differences in predictor variables between production types
| Variable | Category | % (number) of herds |
|||
|---|---|---|---|---|---|
| Dairy | Mixed | Beef | Total | ||
| Remaining with the dam for >24 h after birth | Yes | 7.1a (2/28) | 11.3a (6/53) | 31.7b (13/41) | 17.2 (21/122) |
| Automated milk feeding system | Yes | 28.6a (8/28) | 7.1b (4/56) | 7.3b (3/41) | 12.0 (15/125) |
| Own farm colostrum only | Yes | 93.1a (27/29) | 81.5a (44/54) | 47.4b (18/38) | 73.6 (89/121) |
| Milk type | Cow milk (referent = milk replacer) | 31.0a (9/29) | 69.8b (37/53) | 41.1a (16/38) | 51.7 (62/120) |
| Presence of chronically ill animal in pen or adjacent pen | Yes | 62.1a (18/29) | 41.1a (23/56) | 31.7b (13/41) | 42.9 (54/126) |
| Bedding material | Sawdust (referent = straw) | 17.2a (5/29) | 6.9ab (4/58) | 0b (0/41) | 7.0 (9/128) |
| Shaving calves in winter | Yes | 28.6a (8/28) | 30.9a (17/55) | 73.2b (30/41) | 44.4 (55/124) |
| Vaccination against bRSV/PI-31 | Yes | 57.1a (16/28) | 47.4a (27/57) | 85.4b (35/41) | 61.9 (78/126) |
| Vaccination against Mannheimia haemolytica | Yes | 32.1a (9/28) | 33.3a (19/57) | 68.3b (28/41) | 44.4 (56/126) |
Percentages within a row with different superscripts are statistically different at P < 0.05.
bRSV = bovine respiratory syncytial virus; PI-3 = parainfluenzavirus type 3.
Description of Outbreaks and Pathogen Detection Rates
Of the study herds, 36.8% (46/125) reported epidemic respiratory disease only, whereas 63.2% (79/125) mentioned that the herd had suffered from endemic respiratory disease for a longer period as well. The average number of days respiratory signs had been present on the farm before sampling was 5.9 ± 4.4 (range = 0 −14) for the herds with only epidemic respiratory disease and 175.2 ± 165 (range = 15–600) for herds with also endemic respiratory disease. At the time of sampling the average percentage of calves showing respiratory signs was 44.0 ± 30.1%, ranging between 1 and 100%, and this did not differ between the epidemic only and endemic herds (P = 0.25).
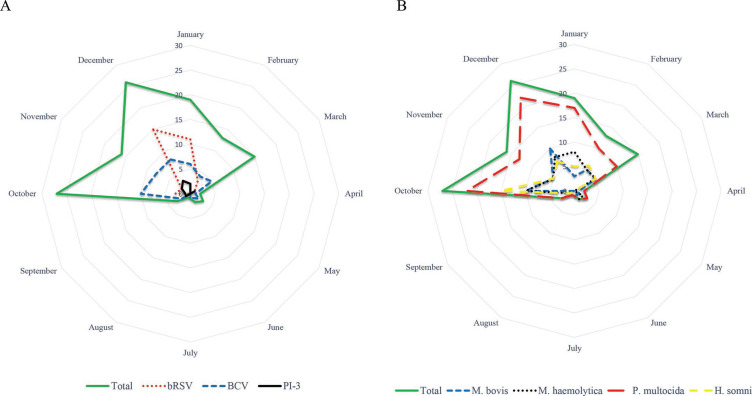
The vast majority of outbreaks occurred between October and March (Figure 1 ). Univariable analysis showed a significant seasonal influence for bRSV (P < 0.001) only. Viruses were detected in 58.6% (75/128) of the outbreaks: bRSV, PI-3, and bovine coronavirus (BCV) in 29.4% (35/119), 8.1% (10/124), and 38.4% (48/125), respectively. The prevalence of M. haemolytica, P. multocida, H. somni, and M. bovis was 41.2% (39/114), 89.1% (106/119), 36.4% (44/121), and 33.3% (41/123), respectively. In 13.3% (17/128) of the herds, multiple viruses were involved (Table 3 ). In 95.9% (71/74) of the herds with viral infection, also bacterial DNA was retrieved. Only in 4 herds was bRSV the single identified pathogen. Also, in 61.0% (25/41) of the M. bovis-positive outbreaks, a virus [72.0% (18/25) BCV, 16.0% (4/25) PI-3, and 12.0% (3/25) bRSV] was simultaneously detected. In 4.7% (6/128) of the herds, not a single one of the studied pathogens could be retrieved, and in 10.9% (14/128) only P. multocida DNA was found. No significant differences in detection rates between dairy, beef, or dairy-mixed herds were found for any of the studied pathogens. No differences were observed in pathogen detection rates between herds reporting endemic problems next to the epidemic and these with only epidemic disease, except for bRSV.
Figure 1.
Radar graph representing the monthly number of positive outbreaks for viral (A) and bacterial (B) pathogens. bRSV = bovine respiratory syncytial virus; BCV = bovine coronavirus; PI-3 = bovine parainfluenzavirus type 3.
Table 3.
Mixed viral infections in 128 outbreaks of epidemic respiratory disease in calves (2016–2017, Belgium)
| Pathogen(s)1 | Detection rate, % (no.) |
|---|---|
| Negative | 41.4 (53) |
| BCV | 28.9 (36) |
| bRSV | 16.4 (20) |
| BCV, bRSV | 7.8 (10) |
| bRSV, PI-3 | 3.9 (5) |
| BCV, PI-3 | 1.6 (2) |
| PI-3 | 0.8 (1) |
| BCV, PI-3, bRSV | 0.8 (1) |
bRSV = bovine respiratory syncytial virus; PI-3 = parainfluenzavirus type 3; BCV = bovine coronavirus.
Virus-Specific Risk Factors
Factors univariably associated with a positive bRSV PCR on pooled broncho-alveolar lavage (BAL) fluid samples were days with clinical signs before sampling (P = 0.02), percentage of calves with respiratory signs (P = 0.03), endemic respiratory disease (P < 0.01), vaccination against bRSV (P = 0.14), use of fresh colostrum (P = 0.15), H. somni-positive BAL PCR (P = 0.15), BCV-positive BAL PCR (P = 0.18), M. bovis-positive BAL PCR (P = 0.11), PI-3-positive BAL PCR (P = 0.03), month (P < 0.01), and season (P < 0.001). Month and season were associated, and therefore only season was added to the multivariable model building procedure. In winter (December to March), bRSV was significantly more frequent compared with summer/spring [odds ratio (OR) = 34.5 (95% CI = 3.0–392.9; P < 0.01)] or autumn [OR = 10.3 (95% CI = 2.8–37.5; P < 0.001)]. The final multivariable model consisted of PI-3-positive BAL PCR, season, number of days with signs, and prevalence of calves with respiratory disease (Table 4 ).
Table 4.
Final multivariable logistic regression model describing the association between risk factors and detection of bovine respiratory syncytial virus by PCR on pooled broncho-alveolar lavage samples from epidemic respiratory disease in calves1
| Independent variable | Category | n | % positive | b | SE | OR | 95% CI | P-value |
|---|---|---|---|---|---|---|---|---|
| Intercept | −1.3 | 0.5 | 0.01 | |||||
| PI-3 | Negative | 97 | 22.6 | Referent | ||||
| Positive | 10 | 60.0 | 2.6 | 0.9 | 13.4 | 2.1–86.0 | 0.02 | |
| Season | Autumn | 40 | 12.5 | Referent | <0.001 | |||
| Winter* | 45 | 48.9 | 2.3 | 0.7 | 10.3 | 2.8–37.5 | <0.001 | |
| Spring-summer | 22 | 4.5 | −1.2 | 1.3 | 0.30 | 0.03–3.5 | 0.33 | |
| Number of days with signs before sampling (per day) | 107 | −0.01 | 0.0 | 0.99 | 0.98–0.99 | 0.01 | ||
| Prevalence of calves with respiratory disease (per 1% increase) | 107 | 0.03 | 0.01 | 1.02 | 1.00–1.04 | <0.01 |
PI-3 = parainfluenzavirus type 3; b = regression coefficient; OR = odds ratio.
Winter was also significantly higher than spring-summer (P < 0.01).
For BCV, univariable associations were found for previous detection of coronavirus in diarrheic calves (P < 0.01), shaving calves for winter (P = 0.04), purchase of colostrum (P = 0.06), recent purchase of cattle (P = 0.05), detection of M. haemolytica (P = 0.02), herd size (P = 0.03), and year (P = 0.13). The final multivariable model is shown in Table 5 . The following factors were univariably associated with a positive PI-3 PCR: ill animals recently purchased (P = 0.08), use of an automatic straw disperser (P = 0.07), M. bovis-positive PCR (P = 0.11), bRSV-positive PCR (P = 0.03), and herd size (P = 0.10). The final multivariable model consisted of bRSV-positive PCR (OR = 4.6; 95% CI = 1.2–17.4; P = 0.03).
Table 5.
Final multivariable logistic regression model describing the association between risk factors and detection of bovine coronavirus by PCR on pooled broncho-alveolar lavage samples from epidemic respiratory disease in calves1
| Independent variable | Category | n | % positive | b | SE | OR | 95% CI | P-value |
|---|---|---|---|---|---|---|---|---|
| Intercept | −1.8 | 0.5 | <0.001 | |||||
| Mannheimia haemolytica | Negative | 50 | 34.0 | Referent | ||||
| Positive | 36 | 55.6 | 1.0 | 0.5 | 2.8 | 1.1–7.5 | 0.03 | |
| Detection of coronavirus in neonatal calves in the last year | No | 66 | 37.8 | Referent | ||||
| Yes | 20 | 60.0 | 1.3 | 0.6 | 3.6 | 1.2–11.1 | 0.03 | |
| Herd size (per increase of 100 animals) | 86 | 0.3 | 0.1 | 1.3 | 1.0–1.8 | 0.03 |
b = regression coefficient; OR = odds ratio.
Bacteria-Specific Risk Factors
For bacteria, univariable analysis showed associations between a positive PCR result for M. haemolytica and group size of the sampled pen (P = 0.01), presence of neonatal diarrhea in the calves (P = 0.06), presence of a shared airspace between ill calves and cattle over 1 yr old, age at first grouping (P = 0.08), suckler herd (P = 0.14), bedding type (P = 0.03), >24 h with the dam (P = 0.08), ventilation type (P = 0.15), BCV-positive PCR result (P = 0.02), and M. bovis-positive PCR result (P = 0.15). The final multivariable model consisted of number of animals per pen in the outbreak group, BCV-positive PCR result, and bedding type (Table 6 ).
Table 6.
Final multivariable logistic regression model describing the association between risk factors and detection of Mannhaemia haemolytica by PCR on pooled broncho-alveolar lavage samples from epidemic respiratory disease in calves (2016–2018, Belgium)1
| Independent variable | Category | n | % positive | b | SE | OR | 95% CI | P-value |
|---|---|---|---|---|---|---|---|---|
| Intercept | −2.6 | 1.0 | 0.07 | |||||
| Number of animals per pen in outbreak group | Individual | 13 | 23.1 | Referent | 0.03 | |||
| <5 per group | 22 | 18.2 | 0.02 | 1.0 | 0.98 | 0.13–7.3 | 0.98 | |
| 5–10 per group | 43 | 58.1 | 2.1 | 0.9 | 8.0 | 1.4–46.9 | 0.02 | |
| >10 per group | 30 | 46.7 | 1.5 | 0.9 | 4.5 | 0.7–27.4 | 0.10 | |
| BCV | Negative | 67 | 32.8 | Referent | ||||
| Positive | 44 | 54.5 | 1.4 | 0.5 | 3.8 | 1.5–9.8 | <0.01 | |
| Bedding type | Straw | 106 | 37.7 | Referent | ||||
| Sawdust | 8 | 87.5 | 2.9 | 1.2 | 18.3 | 1.8–191.6 | 0.02 |
BCV = bovine coronavirus; b = regression coefficient; OR = odds ratio.
Univariable analysis for H. somni identified M. haemolytica vaccination (P = 0.04), bedding type (P = 0.06), herd size (P = 0.04), P. multocida PCR positive (P = 0.11), M. bovis PCR positive (P = 0.18), bRSV PCR positive (P = 0.15), ill animals recently purchased (P = 0.17), and season (P = 0.13) to be used in the multivariable analysis. In the final model, herds using sawdust bedding and vaccinating for M. haemolytica had higher odds for H. somni detection (Table 7 ). For P. multocida, a positive fecal ELISA result for enterotoxigenic Escherichia coli in the last year (P = 0.17), use of an automatic milk feeder (P = 0.02), purchase of colostrum (P = 0.15), availability of a quarantine stable (P = 0.16), H. somni positive PCR result (P = 0.10), and BCV-positive test result (P = 0.08) were selected for the multivariable model. In the final multivariable model herds with an automated milk feeder (compared with bucket or trough feeding) had lower odds of detecting P. multocida in BAL by PCR [69.2 vs. 92.0%; OR = 0.19 (0.05–0.75); P = 0.02].
Table 7.
Final multivariable logistic regression model describing the association between risk factors and detection of Histophilus somni by PCR on pooled broncho-alveolar lavage samples from epidemic respiratory disease in calves1
| Independent variable | Category | n | % positive | b | SE | OR | 95% CI | P-value |
|---|---|---|---|---|---|---|---|---|
| Intercept | −1.1 | 0.3 | <0.001 | |||||
| Bedding type | Straw | 103 | 35.0 | Referent | ||||
| Sawdust | 10 | 70.0 | 1.6 | 0.8 | 5.2 | 1.2–23.0 | 0.03 | |
| Vaccination against Mannheimia haemolytica | No | 61 | 29.5 | Referent | ||||
| Yes | 52 | 48.1 | 0.9 | 0.4 | 2.6 | 1.1–5.8 | 0.02 |
b = regression coefficient; OR = odds ratio.
Finally, for M. bovis the following factors were significant in the univariable analysis: respiratory signs in adults (P = 0.14), presence of a chronically ill animal in the pen (P = 0.14), age at first grouping (P = 0.06), recently purchased animals present in the same airspace (P < 0.01), H. somni-positive PCR (P = 0.17), M. haemolytica positive (P = 0.15), PI-3-positive PCR (P = 0.11), bRSV-positive PCR (P = 0.11), purchase of animals (P = 0.03), and herd size (P = 0.06). The final multivariable model consisted of purchase of animals and recently purchased animals in the same airspace (Table 8 ). For all viruses and bacteria detected, no significant effect of production type could be evidenced.
Table 8.
Final multivariable logistic regression model describing the association between risk factors and detection of Mycoplasma bovis by PCR on pooled broncho-alveolar lavage samples from epidemic respiratory disease in calves1
| Independent variable | Category | n | % positive | b | SE | OR | 95% CI | P-value |
|---|---|---|---|---|---|---|---|---|
| Intercept | −1.7 | 0.5 | <0.001 | |||||
| Recently purchased animals in the same airspace | No | 101 | 27.7 | Referent | ||||
| Yes | 15 | 66.7 | 1.6 | 0.6 | 5.0 | 1.5–16.5 | <0.01 | |
| Purchase of cattle | No | 26 | 15.4 | Referent | ||||
| Yes | 90 | 37.7 | 1.1 | 0.5 | 2.9 | 1.0–8.0 | 0.04 |
b = regression coefficient; OR = odds ratio.
DISCUSSION
This study provides a prevalence estimate of pathogens involved or potentially involved in epidemic respiratory disease in calves, based on a convenience sample from a Western European country. For correct interpretation of this work, some points need to be clarified to the reader first. We used PCR, which is increasingly popular and accessible, but has important drawbacks for its interpretation at the herd level (Parker et al., 2018). For what are considered primary pathogens (viruses and M. bovis), detection at the herd level likely represents involvement in the outbreak. For opportunistic pathogens, in this case the Pasteurellaceae family, no such conclusions can be drawn, because these species can be isolated from healthy animals as well (Van Driessche et al., 2017; Timsit et al., 2018). Also, PCR detects the smallest amount of even dead bacteria, pointing toward the importance of sample contamination by nasal passage with the nBAL technique. Therefore, in our opinion PCR results for Pasteurellaceae both at the herd and individual level can only be interpreted as presence of the pathogen in the herd/animal. We opted for the nBAL, even though deep nasopharyngeal swabs are easier to perform, because it has a higher sensitivity to detect bRSV (Kimman et al., 1986; Doyle et al., 2017). Many participating veterinarians also requested bacteriology to obtain an antibiogram, which is more straightforward to interpret on samples from the lower respiratory tract. The sample pooling procedure we used was evidenced in a previous study to increase the detection rate of respiratory viruses (O'Neill et al., 2014). Unfortunately, in many occasions the PCR returned noninterpretable results for some of the pathogens, reducing the power of the study. Most likely, noninterpretable results can be judged as negative, but we took the precaution not to include them in the analysis. Given the low number of positive and negative cases for PI-3 and P. multocida, respectively, results should be interpreted carefully. Detection of P. multocida on almost every farm, makes it different to interpret its role in BRD, when using PCR for diagnosis.
The study was subject to several sources of potential bias, as with many cross-sectional studies. First, using a convenience sampling holds the risk of selection bias. We could not demonstrate any significant differences in herd size between the study and target population, which is favorable in this regard. Also, working with many different veterinarians potentially having differing motivations (e.g., the attractiveness of a free analysis) holds the inherent risk that inclusion criteria were not respected to the same extent in every outbreak. We clearly targeted epidemic outbreaks using the inclusion criteria. However, in endemic BRD herds, a short-lived increase in disease prevalence can occur for various reasons (exposure to another risk factor or infection, increase in the amount of susceptible animals, and so on). Nevertheless, in this case the outbreak will also present as an epidemic according to our case definition. It is important to realize that when using more sensitive techniques such as thoracic ultrasonography, lung lesions are found in more than 40% of the calves in herds not reporting any issues with respiratory disease (van Leenen et al., 2019). Also, at the level of the calf, the use of clinical criteria for BRD diagnosis holds the risk of a substantial between-observer variation (Buczinski et al., 2016). The use of thoracic ultrasonography, currently the most accurate field diagnostic tool for pneumonia (Buczinski et al., 2014), would have improved our study. Unfortunately not enough veterinarians have mastered the technique in the study region. Also within the questionnaire, misclassification bias for the evaluated risk factors is possible because many different persons were interviewing. We also faced information bias because in some questionnaires not every question was answered, and sometimes handwriting was not legible.
The study clearly shows that epidemic respiratory disease peaks in winter months and is primarily of viral origin. We found a much higher prevalence of viruses than the only other PCR study so far, available from Ireland (O'Neill et al., 2014). Possibly, more strict case definitions and sampling earlier in the disease course in our study explains these differences. However, we still suspect an underestimation of the viral component, as viruses can only be detected for a limited number of days (Saif, 2010), and part of the samplings were later than a week after the start of the outbreak. Also, not including bovine herpesvirus-1 and bovine viral diarrhea virus in our analysis might have contributed to an underestimation. However, Belgium has official eradication programs for both diseases and outbreaks of these viruses have become scarce. Also, not including bovine adenovirus type 3 can be considered a flaw, given the high seroconversion rates in earlier work in this region (Pardon et al., 2011). However, the role of adenoviruses in BRD is still not clear (Caldow et al., 1988; Giusti et al., 1998). As seen in Ireland, BCV was the most prevalent virus, followed by bRSV (O'Neill et al., 2014). In recent years, although still under debate, a primary role of BCV in BRD is more and more recognized (Ellis, 2019). Bovine coronavirus has been linked as a single pathogen to lethal outbreaks of respiratory disease and diarrhea in adult dairy cattle (Decaro et al., 2008; Saif, 2010), but within the BRD complex this virus is basically still considered an initiator of secondary infections (Ellis, 2019). The association between coronavirus and M. haemolytica in our study might be pointing in this direction, as was previously observed for H. somni as well (Workman et al., 2019). The question remains whether BCV or other viruses are a necessary initiator for these Pasteurellaceae to cause pneumonia. Regardless, with this large prevalence of respiratory BCV in Western European countries (Ohlson et al., 2010; Pardon et al., 2011; Toftaker et al., 2016), it deserves better control and prevention. In contrast to North America, to our knowledge no licensed respiratory coronavirus vaccines are available within the European Union, leaving only biosecurity options accessible. Indeed, biosecurity is the core of the recently initiated BCV and bRSV eradication campaign in Norway (Toftaker et al., 2018). Larger herds were at increased risk for a BCV outbreak in our study, as was shown in a serological study in Norway before (Toftaker et al., 2016). In contrast to that study, purchase of animals was only univariably associated with BCV detection in our study. The routine use of antigen-ELISA in cases of neonatal diarrhea might be useful to identify herds at risk in time, as the pathogen is not seasonal and potentially persists on farm.
Despite relatively high vaccination rates in the study population, bRSV was still frequently detected in outbreaks on these farms. Given the use of intranasal vaccines in Belgium, false positives cannot be totally excluded (Timsit et al., 2009). In contrast to BCV, bRSV showed a strictly seasonal distribution between November and April with a peak in December as is well known from human RSV, but sometimes neglected in veterinary medicine (Leecaster et al., 2011). The multivariable model only consisted of typical characteristics of an RSV outbreak such as seasonality, many diseased animals, and recent occurrence of disease. These factors might be suitable for building predictive models for RSV outbreaks, distinguishing them from other pathogens. The PI-3 was not frequently detected in the present study and almost always together with RSV. This might signify that PI-3 as a single agent only induces mild clinical signs instead of more severe outbreaks as studied here.
Also, M. bovis, mostly known as a chronic pathogen, was frequently detected in these acute outbreaks of respiratory disease. Its involvement in epidemic outbreaks is well known from the veal industry, where the incidence reaches 100% (Pardon et al., 2011; Soehnlen et al., 2012). Detection of M. bovis in one-third of the outbreaks on dairy and beef farms is very worrisome, but in line with a recent prevalence study on bulk tank milk in the studied region (Gille et al., 2018). The detection likely represents primary involvement in the outbreak, rather than persistent infection, as it was not detected more frequently on farms reporting endemic respiratory disease besides the outbreak. The M. bovis-specific risk factors identified in our model all relate to purchase activities, which are the major risk factors for this pathogen (Maunsell et al., 2011).
Pasteurella multocida was ubiquitous, whereas this was not the case for M. haemolytica and H. somni, each being present in approximately one-third of the herds. For H. somni, the prevalence was higher than usually detected by culture methods, likely because the bacteria require specific growth conditions and are easily overgrown (Pardon et al., 2011; Timsit et al., 2017). For M. haemolytica, next to simultaneous BCV detection 2 interesting risk factors were identified. First, the bacteria appears to be more prevalent in herds with larger groups of calves (>5 per pen). Group housing and increased number of animals per pen are well-known risk factors for respiratory disease (Buczinski et al., 2018), and perhaps this is related to M. haemolytica. Second, housing on sawdust appeared to be a risk factor for both the presence of M. haemolytica and H. somni. Sawdust bedding is a known risk factor for mastitis associated with Klebsiella spp. (Schukken et al., 2012). Possibly, also the gram-negative Pasteurellaceae thrive well in this environment, facilitating spread or infection. The use of sawdust might also signify a supplemental dust challenge to the airways and wet bedding might worsen cold stress. Sawdust also clustered within dairy herds, so potential confounding by another, unmeasured factor typical for dairy herds cannot be excluded.
Finally, in a way it could have been expected that vaccinated herds would have lower odds for PCR detection of the respective pathogen, compared with nonvaccinated herds for that pathogen. In our study we could not confirm this hypothesis for any of the studied pathogens. Questioning the efficacy of the vaccines would be one option (Theurer et al., 2015). However, a more likely explanation is that most vaccines do not prevent infection, but rather reduce shedding and clinical signs (Theurer et al., 2015). Anyway, it does illustrate that vaccine efficacy is hard to judge based on PCR analysis. The observation that herds that vaccinated for M. haemolytica faced higher odds for H. somni is difficult to explain. Cross-protection or more H. somni instead of M. haemolytica in herds vaccinating for the latter is possible, or the association can be explained by the cross-sectional nature of the study.
CONCLUSIONS
Viral infections play an important role in epidemic outbreaks of respiratory disease, and a strict winter seasonality is especially present for bRSV. Bovine coronavirus was most prevalent and significantly associated with M. haemolytica, suggesting a potential interplay between both pathogens. Highly different risk factors could be identified for the specific pathogens, which might help practitioners on the one hand to remove specific risk factors and on the other hand to make a better estimation of the most likely pathogen to target with control and therapy before sampling results are available.
ACKNOWLEDGMENTS
The authors thank the financial and technical support of ‘veepeiler rund' (Animal Health Service Flanders; Federal Agency for Safety of the Food Chain) and Boehringer Ingelheim Belgium, which made the Griepbarometer initiative (Griepbarometer), which sourced the data of this study, possible. All collaborating farmers and veterinarians are greatly acknowledged for their active cooperation. BP has received honoraria for acting as speaker or consultant for pharmaceutical (Zoetis, Zaventem, Belgium; MSD, Brussels, Belgium; Vetoquinol, Aartselaar, Belgium; Dopharma, Raamdonskveer, the Netherlands; Boehringer Ingelheim, Brussels, Belgium; Dechra, Northwich, UK; Hipra, Amer, Spain; Ceva, Brussels, Belgium; Merial, Brussels, Belgium; and Elanco, Antwerp, Belgium) and agricultural (Boerenbond, Leuven, Belgium; Algoet Nutrition, Zulte, Belgium) companies. JM is an employee of Boehringer Ingelheim. JC has received speaker or consultant honoraria from pharmaceutical (Boehringer Ingelheim, Hipra, and Elanco) and agricultural (Algoet Nutrition) companies. All authors declare that these relationships have not influenced the independent analysis of the current study.
REFERENCES
- AMCRA Classification of antimicrobials: Methods. 2019. https://formularium.amcra.be/classification.php
- Autio T., Pohjanvirta T., Holopainen R., Rikula U., Pentikainen J., Huovilainen A., Rusanen H., Soveri T., Sihvonen L., Pelkonen S. Etiology of respiratory disease in non-vaccinated, non-medicated calves in rearing herds. Vet. Microbiol. 2007;119:256–265. doi: 10.1016/j.vetmic.2006.10.001. 17084565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczinski S., Borris M.E., Dubuc J. Herd-level prevalence of the ultrasonographic lung lesions associated with bovine respiratory disease and related environmental risk factors. J. Dairy Sci. 2018;101:2423–2432. doi: 10.3168/jds.2017-13459. 29290447. [DOI] [PubMed] [Google Scholar]
- Buczinski S., Faure C., Jolivet S., Abdallah A. Evaluation of inter-observer agreement when using a clinical respiratory scoring system in preweaned dairy calves. N. Z. Vet. J. 2016;64:243–247. doi: 10.1080/00480169.2016.1153439. 26878417. [DOI] [PubMed] [Google Scholar]
- Buczinski S., Forté G., Francoz D., Bélanger A.M. Comparison of thoracic auscultation, clinical score, and ultrasonography as indicators of bovine respiratory disease in preweaned dairy calves. J. Vet. Intern. Med. 2014;28:234–242. doi: 10.1111/jvim.12251. 24236441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcutt M.J., Lysnyansky I., Sachse K., Fox L.K., Nicholas R.A.J., Ayling R.D. Gap analysis of Mycoplasma bovis disease, diagnosis and control: An aid to identify future development requirements. Transbound. Emerg. Dis. 2018;65:91–109. doi: 10.1111/tbed.12860. 29582590. [DOI] [PubMed] [Google Scholar]
- Caldow G.L., Edwards S., Nixon P., Peters A.R. Associations between viral infection and respiratory disease in young beef bulls. Vet. Rec. 1988;122:529–531. doi: 10.1136/vr.122.22.529. 2842921. [DOI] [PubMed] [Google Scholar]
- Decaro N., Mari V., Desario C., Campolo M., Elia G., Martella V., Greco G., Cirone F., Colaianni M.L., Cordioli P., Buonavoglia C. Severe outbreak of bovine coronavirus infection in dairy cattle during the warmer season. Vet. Microbiol. 2008;126:30–39. doi: 10.1016/j.vetmic.2007.06.024. 17669602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohoo I.R., Martin W., Stryhn H. 2 ed. VER Inc.; Charlottetown, Prince Edward Island, Canada: 2009. Veterinary Epidemiological Research; p. 865. [Google Scholar]
- Doyle D., Credille B., Lehenbauer T.W., Berghaus R., Aly S.S., Champagne J., Blanchard P., Crossley B., Berghaus L., Cochran S., Woolums A. Agreement among 4 sampling methods to identify respiratory pathogens in dairy calves with acute bovine respiratory disease. J. Vet. Intern. Med. 2017;31:954–959. doi: 10.1111/jvim.14683. 28295570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. What is the evidence that bovine coronavirus is a biologically significant respiratory pathogen in cattle? Can. Vet. J. 2019;60:147–152. 30705449. [PMC free article] [PubMed] [Google Scholar]
- EMA/EFSA EMA and EFSA Joint Scientific Opinion on measures to reduce the need to use antimicrobial agents in animal husbandry in the European Union, and the resulting impacts on food safety (RONAFA) 2017. https://www.ema.europa.eu/en/documents/report/ema-efsa-joint-scientific-opinion-measures-reduce-need-use-antimicrobial-agents-animal-husbandry_en.pdf [DOI] [PMC free article] [PubMed]
- Fulton R.W., Confer A.W. Laboratory test descriptions for bovine respiratory disease diagnosis and their strengths and weaknesses: Gold standards for diagnosis, do they exist? Can. Vet. J. 2012;53:754–761. 23277642. [PMC free article] [PubMed] [Google Scholar]
- Gille L., Callens J., Supre K., Boyen F., Haesebrouck F., Van Driessche L., van Leenen K., Deprez P., Pardon B. Use of a breeding bull and absence of a calving pen as risk factors for the presence of Mycoplasma bovis in dairy herds. J. Dairy Sci. 2018;101:8284–8290. doi: 10.3168/jds.2018-14940. 30126602. [DOI] [PubMed] [Google Scholar]
- Giusti A.M., Luini M., Benko M., Scanziani E. Pathological and in situ hybridisation findings in calves experimentally infected with bovine adenovirus type 4. Dtsch. Tierarztl. Wochenschr. 1998;105:142–144. 9618984. [PubMed] [Google Scholar]
- DGZ Griepbarometer. 2016. https://www.dgz.be/griepbarometer-volgt-de-griepsituatie-op-de-voet
- Griffin D., Chengappa M.M., Kuszak J., McVey D.S. Bacterial pathogens of the bovine respiratory disease complex. Vet. Clin. North Am. Food Anim. Pract. 2010;26:381–394. doi: 10.1016/j.cvfa.2010.04.004. 20619191. [DOI] [PubMed] [Google Scholar]
- Karle B.M., Maier G.U., Love W.J., Dubrovsky S.A., Williams D.R., Anderson R.J., Van Eenennaam A.L., Lehenbauer T.W., Aly S.S. Regional management practices and prevalence of bovine respiratory disease in California's preweaned dairy calves. J. Dairy Sci. 2019;102:7583–7596. doi: 10.3168/jds.2018-14775. 30527977. [DOI] [PubMed] [Google Scholar]
- Kimman T.G., Zimmer G.M., Straver P.J., de Leeuw P.W. Diagnosis of bovine respiratory syncytial virus infections improved by virus detection in lung lavage samples. Am. J. Vet. Res. 1986;47:143–147. 3511803. [PubMed] [Google Scholar]
- KNMVD Procedures for the development of formularies for responsible antimicrobial use. 2015. https://www.knmvd.nl/app/uploads/sites/4/2018/09/150209-procedure-opstellen-formularia-definitief.pdf
- Leecaster M., Gesteland P., Greene T., Walton N., Gundlapalli A., Rolfs R., Byington C., Samore M. Modeling the variations in pediatric respiratory syncytial virus seasonal epidemics. BMC Infect. Dis. 2011;11:105. doi: 10.1186/1471-2334-11-105. 21510889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsell F.P., Woolums A.R., Francoz D., Rosenbusch R.F., Step D.L., Wilson D.J., Janzen E.D. Mycoplasma bovis infections in cattle. J. Vet. Intern. Med. 2011;25:772–783. doi: 10.1111/j.1939-1676.2011.0750.x. 21745245. [DOI] [PubMed] [Google Scholar]
- Murray G.M., More S.J., Clegg T.A., Earley B., O'Neill R.G., Johnston D., Gilmore J., Nosov M., McElroy M.C., Inzana T.J., Cassidy J.P. Risk factors associated with exposure to bovine respiratory disease pathogens during the peri-weaning period in dairy bull calves. BMC Vet. Res. 2018;14:53. doi: 10.1186/s12917-018-1372-9. 29482563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill R., Mooney J., Connaghan E., Furphy C., Graham D.A. Patterns of detection of respiratory viruses in nasal swabs from calves in Ireland: A retrospective study. Vet. Rec. 2014;175:351. doi: 10.1136/vr.102574. 25037889. [DOI] [PubMed] [Google Scholar]
- Ohlson A., Heuer C., Lockhart C., Traven M., Emanuelson U., Alenius S. Risk factors for seropositivity to bovine coronavirus and bovine respiratory syncytial virus in dairy herds. Vet. Rec. 2010;167:201–207. doi: 10.1136/vr.c4119. 20693503. [DOI] [PubMed] [Google Scholar]
- Pardon B., Catry B., Dewulf J., Persoons D., Hostens M., De Bleecker K., Deprez P. Prospective study on quantitative and qualitative antimicrobial and anti-inflammatory drug use in white veal calves. J. Antimicrob. Chemother. 2012;67:1027–1038. doi: 10.1093/jac/dkr570. 22262796. [DOI] [PubMed] [Google Scholar]
- Pardon B., De Bleecker K., Dewulf J., Callens J., Boyen F., Catry B., Deprez P. Prevalence of respiratory pathogens in diseased, non-vaccinated, routinely medicated veal calves. Vet. Rec. 2011;169:278. doi: 10.1136/vr.d4406. 21831999. [DOI] [PubMed] [Google Scholar]
- Pardon B., De Bleecker K., Hostens M., Callens J., Dewulf J., Deprez P. Longitudinal study on morbidity and mortality in white veal calves in Belgium. BMC Vet. Res. 2012;8:26. doi: 10.1186/1746-6148-8-26. 22414223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker A.M., Sheehy P.A., Hazelton M.S., Bosward K.L., House J.K. A review of mycoplasma diagnostics in cattle. J. Vet. Intern. Med. 2018;32:1241–1252. doi: 10.1111/jvim.15135. 29671903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passchyn P., Piepers S., De Vliegher S. Pathogen group-specific risk factors for intramammary infection in treated and untreated dairy heifers participating in a prepartum antimicrobial treatment trial. J. Dairy Sci. 2014;97:6260–6270. doi: 10.3168/jds.2014-8119. 25108863. [DOI] [PubMed] [Google Scholar]
- Reiten M., Rousing T., Thomsen P.T., Otten N.D., Forkman B., Houe H., Sorensen J.T., Kirchner M.K. Mortality, diarrhea and respiratory disease in Danish dairy heifer calves: Effect of production system and season. Prev. Vet. Med. 2018;155:21–26. doi: 10.1016/j.prevetmed.2018.04.007. 29786521. [DOI] [PubMed] [Google Scholar]
- Saif L.J. Bovine respiratory coronavirus. Vet. Clin. North Am. Food Anim. Pract. 2010;26:349–364. doi: 10.1016/j.cvfa.2010.04.005. 20619189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibrowski M.L., Gibson J.S., Hay K.E., Mahony T.J., Barnes T.S. Mycoplasma bovis and bovine respiratory disease: A risk factor study in Australian feeder cattle. Prev. Vet. Med. 2018;157:152–161. doi: 10.1016/j.prevetmed.2018.06.005. 30086843. [DOI] [PubMed] [Google Scholar]
- Schukken Y., Chuff M., Moroni P., Gurjar A., Santisteban C., Welcome F., Zadoks R. The “other” gram-negative bacteria in mastitis: Klebsiella, Serratia, and more. Vet. Clin. North Am. Food Anim. Pract. 2012;28:239–256. doi: 10.1016/j.cvfa.2012.04.001. 22664206. [DOI] [PubMed] [Google Scholar]
- Soehnlen M.K., Aydin A., Murthy K.S., Lengerich E.J., Hattel A.L., Houser B.A., Fenton G.D., Lysczek H.R., Burns C.M., Townsend A.M., Brooks J.W., Wolfgang D.R., Jayarao B.M. Epidemiology of Mycoplasma bovis in Pennsylvania veal calves. J. Dairy Sci. 2012;95:247–254. doi: 10.3168/jds.2011-4309. 22192204. [DOI] [PubMed] [Google Scholar]
- Theurer M.E., Larson R.L., White B.J. Systematic review and meta-analysis of the effectiveness of commercially available vaccines against bovine herpesvirus, bovine viral diarrhea virus, bovine respiratory syncytial virus, and parainfluenza type 3 virus for mitigation of bovine respiratory disease complex in cattle. J. Am. Vet. Med. Assoc. 2015;246:126–142. doi: 10.2460/javma.246.1.126. 25517335. [DOI] [PubMed] [Google Scholar]
- Thrusfield M., Ortega C., de Blas I., Noordhuizen J.P., Frankena K. Win Episcope 2.0.: Improved epidemiological software for veterinary medicine. Vet. Rec. 2001;148:567–572. doi: 10.1136/vr.148.18.567. [DOI] [PubMed] [Google Scholar]
- Timsit E., Hallewell J., Booker C., Tison N., Amat S., Alexander T.W. Prevalence and antimicrobial susceptibility of Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni isolated from the lower respiratory tract of healthy feedlot cattle and those diagnosed with bovine respiratory disease. Vet. Microbiol. 2017;208:118–125. doi: 10.1016/j.vetmic.2017.07.013. 28888626. [DOI] [PubMed] [Google Scholar]
- Timsit E., Le Dréan E., Maingourd C., Belloc C., Guattéo R., Bareille N., Seegers H., Douart A., Sellal E., Assié S. Detection by real-time RT-PCR of bovine respiratory syncytial virus vaccine in calves vaccinated intranasally. Vet. Rec. 2009;165:230–233. doi: 10.1136/vr.165.8.230. 19700783. [DOI] [PubMed] [Google Scholar]
- Timsit E., Workentine M., van der Meer F., Alexander T. Distinct bacterial metacommunities inhabit the upper and lower respiratory tracts of healthy feedlot cattle and those diagnosed with bronchopneumonia. Vet. Microbiol. 2018;221:105–113. doi: 10.1016/j.vetmic.2018.06.007. 29981695. [DOI] [PubMed] [Google Scholar]
- Toftaker I., Agren E., Stokstad M., Nodtvedt A., Frossling J. Herd level estimation of probability of disease freedom applied on the Norwegian control program for bovine respiratory syncytial virus and bovine coronavirus. Prev. Vet. Med. 2018 doi: 10.1016/j.prevetmed.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toftaker I., Sanchez J., Stokstad M., Nodtvedt A. Bovine respiratory syncytial virus and bovine coronavirus antibodies in bulk tank milk - risk factors and spatial analysis. Prev. Vet. Med. 2016;133:73–83. doi: 10.1016/j.prevetmed.2016.09.003. 27720029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolosa T., Verbeke J., Ayana Z., Piepers S., Supre K., De Vliegher S. Pathogen group specific risk factors for clinical mastitis, intramammary infection and blind quarters at the herd, cow and quarter level in smallholder dairy farms in Jimma, Ethiopia. Prev. Vet. Med. 2015;120:306–312. doi: 10.1016/j.prevetmed.2015.05.001. 26008577. [DOI] [PubMed] [Google Scholar]
- Van Driessche L., Valgaeren B.R., Gille L., Boyen F., Ducatelle R., Haesebrouck F., Deprez P., Pardon B. A deep nasopharyngeal swab versus nonendoscopic bronchoalveolar lavage for isolation of bacterial pathogens from preweaned calves with respiratory disease. J. Vet. Intern. Med. 2017;31:946–953. doi: 10.1111/jvim.14668. 28425146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leenen K., Van Driessche L., De Cremer L., Gille L., Masmeijer C., Boyen F., Deprez P., Pardon B. Factors associated with lung cytology as obtained by non-endoscopic broncho-alveolar lavage in group-housed calves. BMC Vet. Res. 2019;15:167. doi: 10.1186/s12917-019-1921-x. 31126282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman A.M., Kuehn L.A., McDaneld T.G., Clawson M.L., Loy J.D. Longitudinal study of humoral immunity to bovine coronavirus, virus shedding, and treatment for bovine respiratory disease in pre-weaned beef calves. BMC Vet. Res. 2019;15:161. doi: 10.1186/s12917-019-1887-8. 31118011. [DOI] [PMC free article] [PubMed] [Google Scholar]