Abstract
Study objectives
To assess the effect of ribavirin-induced anemia on the outcome of severe acute respiratory syndrome (SARS).
Design
A retrospective observational study.
Setting
Two medical centers in Taiwan.
Patients
Forty-four patients with SARS who received ribavirin and 7 patients with SARS who did not receive ribavirin.
Measurements and results
The mean peak C-reactive protein and lactate dehydrogenase levels were higher in SARS patients who were receiving ribavirin therapy than in SARS patients who were not receiving ribavirin therapy. The mortality was also higher, but the difference was not statistically significant. On multivariate analysis, hemoglobin level was an independent prognostic correlate of hypoxemia or mortality (odds ratio, 2.0; 95% confidence interval, 1.1 to 3.8; p = 0.03). The hemoglobin began decreasing in two thirds of SARS patients (32 of 44 patients; 73%) who were receiving ribavirin 3 days after therapy with the antiviral drug was started. Patients with a drop in hemoglobin level of > 2 g/dL had a significantly higher mortality rate than the other patients. Hypoxemia developed in one third of SARS patients (17 of 44 patients; 39%) who were receiving ribavirin, all of whom were anemic. Of the 17 hypoxemic patients, 11 (65%) had a drop in hemoglobin of > 2 g/dL, and 4 patients (24%) required a blood transfusion. The mean slope of the hemoglobin decrease was significantly steeper (p = 0.001) in hypoxemic patients with SARS who were receiving ribavirin than in the nonhypoxemic patients with SARS who were receiving ribavirin. Only one of seven SARS patients (14%) who was not receiving ribavirin became anemic, but this individual was not hypoxemic. Eventually, 5 of 17 hypoxemic and anemic SARS patients (29%) who were receiving ribavirin died. The combination of hypoxia with anemia was thus significantly associated with a higher mortality (p < 0.001).
Conclusions
Hypoxia combined with anemia increased the risk for death in SARS patients. Unless ribavirin can be shown to be effective against SARS-coronavirus, the risk of anemia posed by this drug argues against its use in SARS patients.
Keywords: anemia, hypoxemia, ribavirin, severe acute respiratory syndrome
Abbreviations: AE, adverse effect; CoV, coronavirus; FD, fever day; hospital C, Chang Gung Memorial Hospital; hospital M, Mackay Memorial Hospital; SARS, severe acute respiratory syndrome; S-R-H, severe acute respiratory syndrome-ribavirin-hypoxemic; S-R-NH, severe acute respiratory syndrome-ribavirin-nonhypoxemic
Severe acute respiratory syndrome (SARS) is caused by a novel coronavirus (CoV) infection.1 2 The disease emerged and spread rapidly early in the 21st century, causing great distress for patients, their families, and society, and raising fears of a global epidemic.3 This tremendous reaction occurred because of the rapid transmission of the disease, and the lack of effective diagnostic methods and treatment.4 5 As of December 31, 2003, a total of 8,096 probable cases had been reported from > 30 countries, with 774 deaths attributed to SARS.6
Faced with a rapidly spreading, novel infection that was lethal in a substantial number of patients, a variety of treatments were tried.7 8 Ribavirin, a water-soluble synthetic guanosine analog with broad-spectrum antiviral activity against both DNA and RNA viruses, has been indicated for the treatment of respiratory syncytial virus in infants, hepatitis C (in combination with interferon), hemorrhagic fever, and influenza A and B infections.9 10 11 12 The empiric use of ribavirin and corticosteroids was therefore initially recommended for the treatment of SARS by many hospitals who were managing the earliest wave of the SARS outbreak.13 14 However, the benefits of such therapy are still debated.15
Adverse effects (AEs) of ribavirin have been reported extensively in the literature, including dose-dependent anemia (with both hemolysis and bone marrow suppression), arrhythmia, elevated lactate and pyruvate levels, hypocalcemia, and hypomagnesemia.14 16 Other reported side effects include, for example, chest pain, dizziness, hyperuricemia, hyperbilirubinemia, interstitial pneumonitis, decreased WBC count, and thrombocytopenia.17 18 The most common AE is reversible, dose-dependent, time-dependent anemia, especially when the drug is given at doses of ≥ 1.2 g daily for > 10 days.16
The aim of this study was to evaluate the outcome of SARS in patients who were treated with ribavirin, particularly in relation to ribavirin-induced anemia, in order to provide some basis for assessing the risks and benefits of the drug.
Materials and Methods
Patients
We retrospectively reviewed the records of patients with confirmed SARS infection who were being managed in two medical centers in Taiwan between April 24 and June 30, 2003. During the outbreak in Taiwan, patients who were suspected of having SARS were randomly sent to designated hospitals by the Department of Health. There was therefore no particular bias as to where the patients were seen. A standard treatment protocol including ribavirin, which was recommended by the Taiwan Center for Disease Control, was used in 44 patients at Mackay Memorial Hospital (hospital M). Another seven patients from Chang Gung Memorial Hospital (hospital C) received similar treatment with the exception of ribavirin, allowing a comparison of patients treated with and without ribavirin (Fig 1 ). All patients were confirmed to have SARS-CoV infection by reverse-transcriptase polymerase chain reaction or real-time polymerase chain reaction on throat or nasopharyngeal swabs on at least two different occasions. The study population thus consisted of 44 SARS patients who were receiving ribavirin and 7 SARS patients who were not receiving ribavirin.
Figure 1.
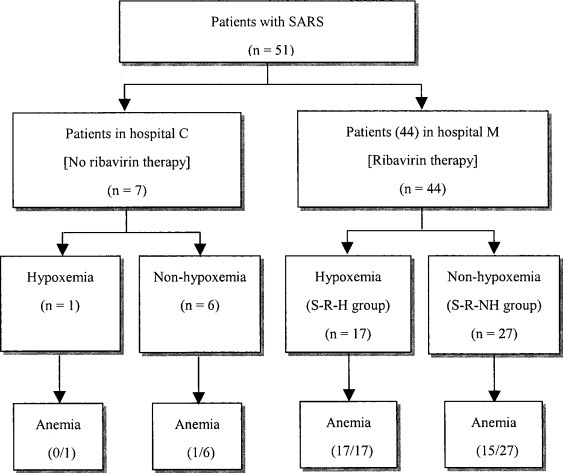
Distribution of 51 patients initially suspected of having SARS at hospital M and hospital C.
Clinical and Laboratory Investigations
Data collected from the records included symptoms, underlying diseases, physical findings, results of serial chest radiographs and O2 saturation monitoring, and laboratory data. Laboratory tests consisted of consecutive hematologic examinations, including hemoglobin concentration, absolute lymphocyte count, platelet count, and serum biochemistry, including the measurement of creatinine, aspartate aminotransferase, alanine aminotransferase, creatine phosphokinase, lactate dehydrogenase, and C-reactive protein levels. In order to define the chronologic progression of the disease, the first day of a documented temperature of > 38°C was designated as fever day (FD) 1.
The effectiveness of ribavirin was evaluated by assessing the patients’ response, including symptoms, signs, and chest radiograph patterns. All patients receiving at least 3 days of ribavirin therapy were evaluated for drug safety. Anemia was defined as a decrease in hemoglobin level of > 1.5 mg/L. Hypoxemia was defined as one of the following conditions: Pao 2 ≤ 60 mm Hg and arterial oxygen saturation ≤ 90%; ratio of Pao 2 to fraction of inspired oxygen of < 250 mm Hg; or the need for mechanical ventilation. In order to evaluate the relations among ribavirin, hypoxemia, and outcome, SARS patients were also assigned to one of the following two groups based on the presence of hypoxemia: SARS-ribavirin-nonhypoxemic (S-R-NH) [n = 27]; and SARS-ribavirin-hypoxemic (S-R-H) [n = 17]. The outcomes of both groups were compared (Fig 1).
Treatment Protocol
As soon as the diagnosis of pneumonia or SARS was considered, the patient was isolated and treated with an IV cephalosporin (eg, ceftriaxone, 2 g q12h, or cefepime, 2 g q12h) and an oral fluoroquinolone (eg, levofloxacin, 500 mg once daily, or moxifloxacin, 400 mg once daily). Once the clinical presentation fulfilled the case definition for SARS, the patient was admitted to a negative-pressure isolation room and the SARS treatment protocol was begun (Fig 2 ).
Figure 2.
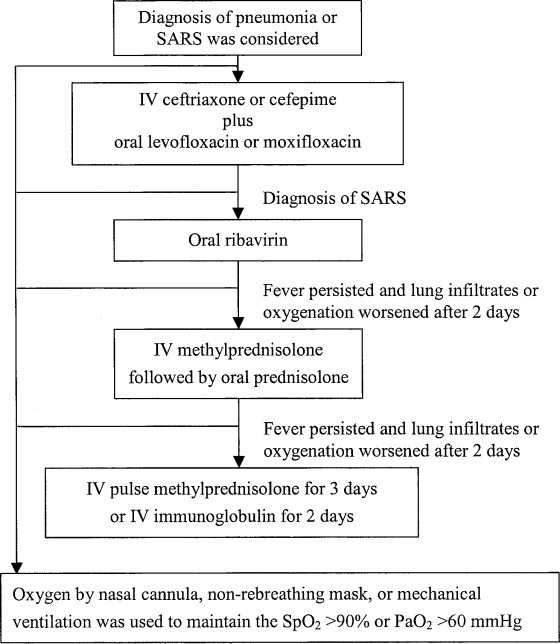
Treatment protocol for SARS. Spo2 = pulse oximetric saturation.
The protocol used at hospital M consisted of oral ribavirin (2,000 mg stat, then 1,200 mg daily (for patients who weighed ≥ 75 kg), or 1,000 mg (for patients who weighted < 75 kg) for 10 to 14 days. The doses were adjusted for renal dysfunction or a fall in hemoglobin. If the patient's condition did not improve following 2 days of therapy with ribavirin, corticosteroids were added, starting with IV methylprednisolone (1 mg/kg q8h for 5 days then q12h for 5 days), followed by oral prednisolone, which was tapered over 11 days. If fever persisted and lung infiltrates or oxygenation worsened after 2 days of therapy with methylprednisolone, the patient was given pulse methylprednisolone (500 mg twice daily for 3 days) and/or IV Ig (1 mg/kg for 2 days). Oxygen therapy administered by nasal cannula, nonrebreathing mask, or mechanical ventilation was used to maintain the pulse oximetric saturation at > 95% or Pao 2 at > 80 mm Hg. The protocol used at hospital C was similar except that ribavirin therapy was not included in the treatment.
Statistical Analysis
All data are expressed as the mean ± SD. Univariate analysis was conducted with the Pearson χ2 test for categoric variables, and the Mann-Whitney U test for continuous variables. The association between the duration of ribavirin therapy and a change in hemoglobin level was identified by linear regression analysis. Survival curves were obtained by Kaplan-Meier estimates and were compared by the log-rank test (two-sided p value). Multivariate logistic regression by backward stepwise analysis was performed to identify the independent risk factors associated with the development of hypoxemia or mortality. The trend of hemoglobin change was compared between the S-R-NH and S-R-H groups by Student t test. The results were considered to be statistically significant if the p value was < 0.05. The differences between groups were tabulated and analyzed with the use of a statistical software package (SPSS, version 10.0; SPSS; Chicago, IL).
Results
Patients’ Demographics and Initial Laboratory Indexes
The mean age of all patients was 38.0 ± 17.5 years. The male/female ratio was 1:2.9. SARS patients receiving ribavirin sought medical care at mean FD 3.4 ± 3.0, and SARS patients who were not receiving ribavirin sought medical care at mean FD 1.7 ± 1.5. Demographic data and initial laboratory results for each group are shown in Table 1 . Except for a higher aspartate aminotransferase level in SARS patients who were receiving ribavirin therapy compared with SARS patients who were not receiving ribavirin therapy (p = 0.018) and a slightly different lactate dehydrogenase level (p = 0.029), there were no significant differences in baseline characteristics between the two groups.
Table 1.
Demographic Data and Clinical Laboratory Indices of Patients Admitted to the Hospital With Suspected SARS*
| Characteristics |
SARS Patients |
p Value |
|
|---|---|---|---|
| With Ribavirin Therapy (n = 44) | Without Ribavirin Therapy (n = 7) | ||
| On hospital admission | |||
| Age, yr | 36.4 ± 15.7 | 49.8 ± 26.1 | NS |
| Gender | NS | ||
| Male | 11 | 2 | |
| Female | 33 | 5 | |
| Underlying illnesses, No. | 9 (20%)† | 2 (29%)‡ | NS |
| Initial Hb concentration, g/dL | 13.0 ± 1.5 | 13.1 ± 1.4 | NS |
| Initial lymphocyte count, ×109 cells/L | 8.7 ± 5.3 | 8.5 ± 2.2 | NS |
| Initial platelet count, ×103 cells/μL | 225.3 ± 149.9 | 206.3 ± 88.0 | NS |
| Initial creatinine level, mg/dL | 1.0 ± 1.0 | 0.8 ± 0.1 | NS |
| Initial AST level, IU/L | 37.1 ± 31.2 | 23.3 ± 7.4 | 0.018§ |
| Initial ALT level, IU/L | 35.7 ± 39.2 | 13.6 ± 5.8 | NS |
| Initial CPK level, IU/L | 190.4 ± 456.6 | 177.7 ± 219.4 | NS |
| Initial LDH level, IU/L | 213.2 ± 86.4 | 137.0 ± 86.7 | 0.029§ |
| During hospitalization | |||
| Nadir Hb level, g/dL | 11.1 ± 1.8 | 12.0 ± 0.6 | NS |
| Nadir absolute lymphocyte count, ×109 cells/L | 4.7 ± 2.1 | 5.1 ± 2.3 | NS |
| Peak LDH level, IU/L | 392.8 ± 307.5 | 162.5 ± 98.0 | 0.017§ |
| Peak CRP level, mg/dL | 10.3 ± 11.6 | 5.8 ± 6.2 | NS |
Values given as mean ± SD, unless otherwise indicated. AST = aspartate aminotransferase; ALT = alanine aminotransferase; CPK = creatinine phosphokinase; CRP = C-reactive protein; LDH = lactate dehydrogenase; Hb = hemoglobin; NS = not significant.
Included diabetes mellitus (four patients), seizures (one patient), rheumatoid arthritis (one patient), hepatitis B infection (one patient), congestive heart failure (one patient), and bacteremia (one patient).
Included two patients with hypertension.
p < 0.05 was considered to be significant.
Clinical and Laboratory Investigations
A fall in hemoglobin level was common in SARS patients (32 of 44 patients; 73%). Only one of seven SARS patients (14%) who were not receiving ribavirin became anemic, which was significantly fewer than among those patients receiving ribavirin (Fig 1).
On hospital admission, 27 SARS patients who were receiving ribavirin (27 of 44 patients; 61%) and 6 SARS patients who were not receiving ribavirin (6 of 7 patients; 86%) had an abnormal chest radiograph finding. In SARS patients receiving ribavirin, 10 patients already had multifocal infiltrates at hospital admission. Of the other 34 patients for whom the time course of radiograph abnormalities could be ascertained, 24 patients (71%) had unifocal airspace infiltrates and 10 patients (39%) had multifocal infiltrates appearing on FD 3 to 6. In SARS patients who were not receiving ribavirin, four of six patients (67%) had unifocal infiltrates and two of six patients (33%) had multifocal infiltrates, appearing on FD 2 to 6, respectively. Eventually, all SARS patients studied had pulmonary infiltrates, mostly diffuse, that progressed from FD 9 to 14.
Hypoxemia developed in 39% of SARS patients (17 of 44 patients) who were receiving ribavirin at a mean of FD 10.4 ± 1.6. Only one of seven patients (14%) with SARS who were not receiving ribavirin had hypoxemia, developing on FD 9 (p = 0.398) [Fig 1].
The mean peak C-reactive protein and lactate dehydrogenase levels were higher in SARS patients who were receiving ribavirin therapy than in SARS patients who were not receiving ribavirin therapy (Table 1). The mortality was also higher, but the difference was not statistically significant (Fig 3 ).
Figure 3.
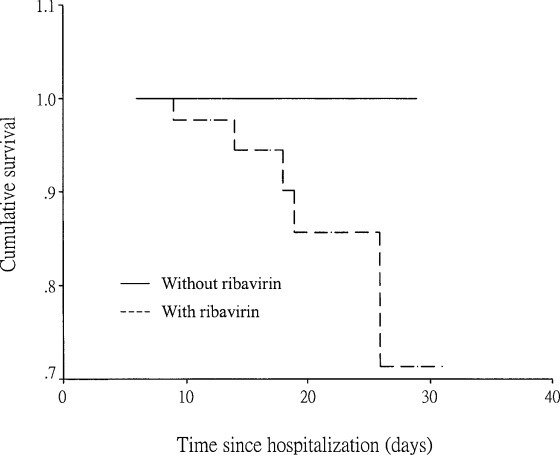
Survival curves in patients treated with or without ribavirin.
In SARS patients who were receiving ribavirin, we first looked for variables associated with hypoxemia or mortality by univariate analysis (Table 2 ). If we found an association with a p value of < 0.1 by univariate analysis, we further analyzed the association by using multivariate logistic regression. On multivariate analysis, only the hemoglobin level was significantly correlated with hypoxemia or death. The odds ratio was 2.0 (95% confidence interval, 1.1 to 3.8; p = 0.03).
Table 2.
Factors Associated With Hypoxemia and Clinical Outcome*
| Factors | S-R-H Group (n = 17) | S-R-NH Group (n = 27) | p Value |
|---|---|---|---|
| Age, yr | 46.2 ± 12.0 | 30.9 ± 12.0 | 0.002† |
| Hb level, g/dL | 12.4 ± 1.6 | 13.3 ± 1.3 | 0.091 |
| Absolute lymphocyte count, ×109 cells/L | 7.5 ± 5.2 | 9.5 ± 5.4 | 0.23 |
| Initial platelet count, ×103 cells/μL | 175.7 ± 71.5 | 222.1 ± 101.0 | 0.144 |
| Initial creatinine level, mg/dL | 1.3 ± 1.5 | 0.8 ± 0.2 | 0.446 |
| Initial AST level, IU/L | 52.1 ± 45.0 | 27.5 ± 10.7 | 0.035† |
| Initial ALT level, IU/L | 56.9 ± 58.1 | 28.0 ± 25.7 | 0.446 |
| Initial CPK level, IU/L | 350.1 ± 754.8 | 112.2 ± 188.3 | 0.651 |
| LDH level, IU/L | 265.7 ± 92.0 | 199.2 ± 56.2 | 0.016† |
| Mean CRP level, mg/dL | 4.7 ± 4.4 | 2.2 ± 3.8 | 0.051 |
| Mortality, No. | 5 (29%) | 0 (0%) | < 0.001† |
Values given as mean ± SD, unless otherwise indicated. See Table 1 for abbreviations not used in the text.
p < 0.05 was considered to be significant.
Drug Therapy
At hospital M, ribavirin was administered beginning at a mean of FD 6.0 ± 2.6 for a total mean duration of 11.5 ± 3.0 days. The majority of SARS patients (41 of 44 patients of patients receiving ribavirin [93%]; 3 of 7 of patients not receiving ribavirin [43%]) had persistent or progressive symptoms and signs, and went on to receive corticosteroids during hospitalization. A smaller number of patients (22 of 44 patients receiving ribavirin [50%]; 2 of 7 patients not receiving ribavirin [29%]) also received pulse methylprednisolone and/or IV Ig.
AEs of Ribavirin
Anemia (32 of 44 patients; 72.7%) was the most common AE observed in SARS patients at hospital M. Sixteen of 32 patients (50%) had a drop in hemoglobin level of > 2 g/dL. These patients had a significantly higher mortality than did patients who did not have as great a drop in hemoglobin. Figure 4 shows the survival curves in SARS patients with or without a drop in hemoglobin > 2 g/dL.
Figure 4.
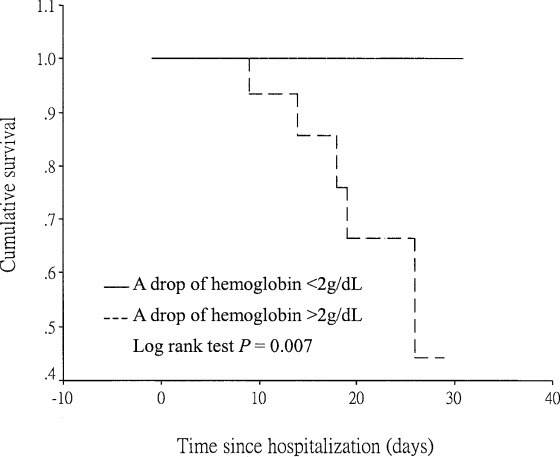
Survival curves in SARS patient according to the fall in hemoglobin level.
To evaluate the association between ribavirin administration and the fall in hemoglobin levels and hypoxemia, the change in hemoglobin level was recorded (Fig 5 ). Before ribavirin therapy was begun, the mean hemoglobin concentrations in both the S-R-H and S-R-NH groups were normal, and the mean hemoglobin concentration in the S-R-H group was not significantly lower than that in the other group. In the S-R-H group, hemoglobin levels began decreasing 3 days after the start of ribavirin therapy, reached a nadir 16 days after starting therapy, and returned to normal 2 to 4 weeks after discontinuing the drug. The mean decrease in hemoglobin level was 2.4 ± 0.9 g/dL. All 17 patients in the S-R-H group became anemic according to our definition, and 4 patients (24%) required a blood transfusion. There was a linear statistical correlation between hemoglobin and survival in this group of patients (r = 0.67; p = 0.001) [Fig 6 ]. In the S-R-NH group, hemoglobin levels dropped somewhat, but the mean remained above the lower limit of normal. Of the 27 patients in this group, 15 became anemic according to our definition. In the S-R-H group, the mean slope of the hemoglobin decrease was significantly steeper (−0.221 ± 0.141 vs −0.009 ± 0.209; p = 0.001) [Fig 5] and anemia was significantly more common (p < 0.001) [Fig 1] than in the S-R-NH group. Interestingly, 9 of 19 patients who had initially been treated with ribavirin but were subsequently excluded from having SARS also developed anemia. Moreover, there was no difference in the incidence of anemia between these non-SARS and SARS patients (32 of 44 patients vs 9 of 19 patients; p = 0.083), linking an even stronger relationship between the use of ribavirin and the development of anemia.
Figure 5.
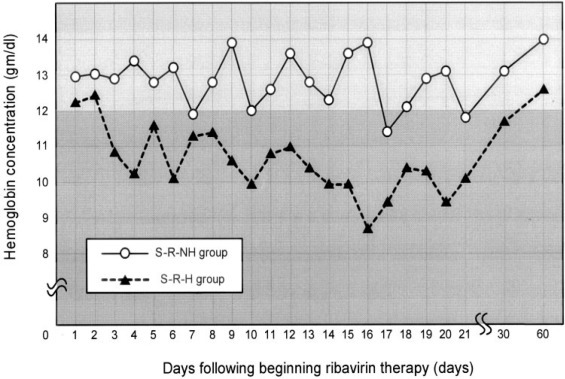
Changes in hemoglobin levels during ribavirin therapy in patients with SARS. Before ribavirin was begun, the mean hemoglobin concentrations in both the S-R-H and S-R-NH groups were normal, and the mean in the S-R-H group was not significantly lower than that in the S-R-NH group. In the S-R-H group, hemoglobin levels began decreasing 3 days after the start of ribavirin therapy, reached a nadir 16 days after starting therapy, and returned to normal 2 to 4 weeks after discontinuing therapy.
Figure 6.
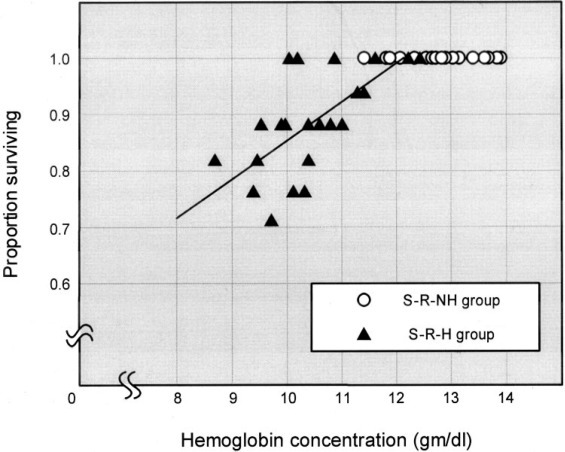
Proportion of patients surviving as related to hemoglobin concentration. There was a linear statistical correlation between hemoglobin level and survival in the S-R-H group (r = 0.67; p = 0.001). A decrease in hemoglobin level in patients superimposed on hypoxemia secondary to SARS-CoV-induced lung pathology had a higher risk of death.
Discussion
We have described a relatively poor outcome in SARS patients who were receiving ribavirin who had both anemia and hypoxemia. A fall in hemoglobin level is a common side effect of ribavirin, but it has also been observed in patients with a variety of severe diseases, including SARS.19 20 We found that 73% of SARS patients (32 of 44 patients) who were receiving ribavirin developed a decrease in hemoglobin level, compared with only 14% of SARS patients (1 of 7 patients) who were not receiving ribavirin (p = 0.006). We also found that, of patients who initially had been suspected of having SARS and therefore treated with ribavirin but who later proved to have other causes of pneumonia, approximately half (9 of 19 patients) became anemic during hospitalization. Hypoxemia was less common, with 17 of 44 patients (39%) who were receiving ribavirin and 1 of 7 patients (14%) who were not receiving ribavirin becoming hypoxemic during hospitalization. Perhaps the most striking finding among the patients who were receiving ribavirin was the number of deaths (5 of 17 patients; 29%) in hypoxemic, anemic patients, compared with that among patients who were not hypoxemic (0 of 27 patients). About half of patients in the latter group (15 of 27 patients) were anemic, although the mean hemoglobin level for the nonhypoxemic group as a whole remained normal or near-normal throughout the hospitalization. Only one of the seven patients who were not receiving ribavirin became hypoxemic, but this patient was not anemic (Fig 1). It is not possible to prove to what extent the anemia observed in this series was caused by ribavirin. However, it is reasonable to assume that a drug known to cause anemia may have contributed in part to the drop in hemoglobin.
Symptoms of SARS developed sequentially, beginning with fever, then cough, diarrhea, and eventually dyspnea.21 22 Pulmonary infiltrates appeared on chest radiographs in our patients at an average of FD 3 to 6 and were accompanied by respiratory symptoms. Ribavirin therapy was begun at a mean of FD 6.0 ± 2.6 and was given for a total of 11.5 ± 3.0 days. Over two thirds of patients (32 of 44 patients) with SARS had a decrease in hemoglobin beginning 3 days after ribavirin was started, or about FD 9. This coincided with the progression in some patients to diffuse pulmonary infiltrates and hypoxemia at a mean of FD 10.4 ± 1.6, which is presumably part of the natural course of SARS. Thus, reduced oxygen-carrying capacity, most likely related at least in part to treatment with ribavirin, was superimposed on hypoxemia secondary to SARS-CoV-induced lung pathology. This was a dangerous combination that was associated with a higher risk of death.
Pharmacotherapy for SARS during the outbreak was diverse and based largely on anecdotal evidence.18 In addition to ribavirin, oseltamivir, and ritonavir/lopinavir (Kaletra; Abbott Laboratories; Abbott Park, IL) were tried.2 17 23 Some physicians in Hong Kong13 15 recommended IV ribavirin, 1,200 mg daily for at least 3 days, followed by oral treatment with 2,400 mg daily, or continued IV ribavirin therapy at a dose of 8 mg/kg every 8 h for 7 to 10 days. In Canada, patients received a loading dose of 2 g IV, followed by 1 g IV every 6 h for 4 days, then 500 mg every 8 h for 3 to 6 days.15 However, over the course of the outbreak there, patients who had been treated with ribavirin appeared to have a worse outcome.19 Because of concern about its side effects and the lack of evidence for efficacy against SARS-CoV either in vitro or anecdotally, Health Canada stopped providing ribavirin for the treatment of SARS several months into the outbreak.24 We used a lower dose of ribavirin in our treatment protocol. However, most of our patients had clinical progression of SARS after ribavirin therapy was begun, so they were given additional therapy such as steroids or IV Ig, according to the protocol. We observed no evidence in our patients to support a role for ribavirin in treating SARS.
Ribavirin-induced anemia appears to result from the inhibition of the late stages of erythrocyte maturation in bone marrow and from the hemolysis of erythrocytes.16 There is also evidence that ribavirin-induced oxidative damage to the erythrocyte membrane may cause erythrophagocytic extravascular destruction. Ribavirin has been shown to lower levels of adenosine triphosphate and glucose 6-phosphate in erythrocytes,25 which might be an additional factor affecting their oxygen-carrying capacity.
The reasons for poor outcome in SARS patients appears to be multifactorial. The risk factors proposed by various investigators include genetic polymorphism, initial viral load, older age, and comorbidity (especially diabetes mellitus and heart disease).26 27 28 Research in critical care has identified anemia and hypoxia as important risk factors for mortality. Anemic patients with pulmonary disease develop dyspnea, tachypnea, and severe hypoxia more easily than patients with a normal hemoglobin level. Global tissue hypoxia is an independent risk factor for microcirculatory failure, organ dysfunction, poor outcome, and death.29 Early goal-directed therapy carried out by maintaining central venous oxygen saturation at ≥ 70% with blood transfusion and providing O2 supplementation has been shown to decrease mortality significantly in critically ill patients.29 In our S-R-H patients, hypoxemia developed coincidentally with a fall in hemoglobin levels, so it is perhaps not surprising that they had a worse outcome than others in this series. These limited data suggest that patients with SARS require more intensive O2 therapy and possibly transfusion of RBCs to maintain the hemoglobin level at ≥ 12.0 mg/dL.
Obvious limitations of our study include the small number of patients and the uncontrolled, retrospective design. For better or worse, this is the case with most of the literature on SARS. Faced with a previously unknown disease with substantial mortality, there was no opportunity to perform prospective randomized trials. The best that could be done was to try to understand the pathology induced by the virus and then design therapy that might theoretically help. If SARS does not reemerge, we will probably never be able to develop enough evidence to give clear guidance on treatment. The best we can do at this point is to work in retrospect with the limited data we have.
Our study raises a serious question as to whether the use of ribavirin in SARS patients is wise. SARS infection clearly leads to hypoxemia in a substantial number of patients. Regardless of its cause, anemia increases the risk of death in hypoxemic patients. Given the well-established AE of anemia in some patients who have been treated with ribavirin, the drug should be used only if a considerable benefit of the treatment outweighs the risk posed by drug-induced anemia. Such a benefit has not yet been demonstrated in patients who have been infected with SARS-CoV. Until it is, it might be better to forgo the use of ribavirin in SARS patients.
Footnotes
This study was supported by the Taiwan National Science Council (grant No. NSC92–2751-B-195–001-Y) and National SARS Research Program (SCLI01).
References
- 1.Ruan Y, Wei CL, Ee LA. Comparative full-length genome sequence analysis of 14 SARS coronavirus isolates and common mutations associated with putative origins of infection. Lancet. 2003;361:1779–1785. doi: 10.1016/S0140-6736(03)13414-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drosten C, Gunther S, Preiser W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 3.Maunder R, Hunter J, Vincent L. The immediate psychological and occupational impact of the 2003 SARS outbreak in teaching hospital. Can Med Assoc J. 2003;168:1245–1251. [PMC free article] [PubMed] [Google Scholar]
- 4.Hon KLE, Li AM, Cheng FWT. Personal view of SARS: confusing definition, confusing diagnoses. Lancet. 2003;361:1984–1985. doi: 10.1016/S0140-6736(03)13556-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peiris JS, Yuen KY, Osterhaus AD. The severe acute respiratory syndrome. N Engl J Med. 2003;349:2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003: revised 21 April 2004. Available at: http://www.who.int/crs/sars/contry/table2004%2004%2021/en/ Accessed June 20, 2005.
- 7.Kontoyiannis DP, Pasqualini R, Arap W. Aminopeptidase N inhibitors and SARS [letter] Lancet. 2003;361:1558. doi: 10.1016/S0140-6736(03)13186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cinatl J, Morgenstern B, Bauer G. Treatment of SARS with human interferons. Lancet. 2003;362:1158–1159. doi: 10.1016/S0140-6736(03)13973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herzog KD, Long SS, McGuigan M. Impact of treatment guidelines on use of ribavirin. Am J Dis Child. 1990;144:1001–1004. doi: 10.1001/archpedi.1990.02150330061022. [DOI] [PubMed] [Google Scholar]
- 10.Lam NP. Hepatitis C. Natural history, diagnosis, and management. Am J Health Syst Pharm. 1999;56:961–976. doi: 10.1093/ajhp/56.10.961. [DOI] [PubMed] [Google Scholar]
- 11.Borio L, Inglesby T, Peters CJ. Hemorrhagic fever viruses as biological weapons: medical and public health management. JAMA. 2002;287:2391–2405. doi: 10.1001/jama.287.18.2391. [DOI] [PubMed] [Google Scholar]
- 12.Stein DS, Creticos CM, Jackson GG. Oral ribavirin treatment of influenza A and B. Antimicrob Agents Chemother. 1987;31:1285–1287. doi: 10.1128/aac.31.8.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.So LKY, Lau ACW, Yam LYC. Development of a standard treatment protocol for severe acute respiratory syndrome. Lancet. 2003;361:1615–1617. doi: 10.1016/S0140-6736(03)13265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koren G, King S, Knowles S. Ribavirin in the treatment of SARS: a new trick for an old drugs? Can Med Assoc J. 2003;168:1289–1292. [PMC free article] [PubMed] [Google Scholar]
- 15.Zhaori G. Antiviral treatment of SARS: can we draw any conclusions? Can Med Assoc J. 2003;169:1165–1166. [PMC free article] [PubMed] [Google Scholar]
- 16.American Society of Health-System Pharmacists . Anti-infective agents. In: Gerald K, editor. American hospital formulary service drug information. American Society of Health-System Pharmacists; Bethesda, MD: 2003. pp. 578–590. [Google Scholar]
- 17.Tsang K, Zhong NS. SARS: pharmacotherapy. Respirology. 2003;8:S25–S30. doi: 10.1046/j.1440-1843.2003.00525.x. [DOI] [PubMed] [Google Scholar]
- 18.Karim A, Ahmed S, Khan A. Interstitial pneumonitis in a patients treated with α-interferon and ribavirin for hepatitis C infection. Am J Med Sci. 2001;322:233–235. doi: 10.1097/00000441-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Booth CM, Matukas LM, Tomlinson GA. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 20.Knowles SR, Phillips EJ, Dresser L. Common adverse events associated with the use of ribavirin for severe acute respiratory syndrome in Canada. Clin Infect Dis. 2003;37:1139–1142. doi: 10.1086/378304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu CL, Lu YT, Peng MJ. Clinical and laboratory features of severe acute respiratory syndrome vis-à-vis onset of fever. Chest. 2004;126:509–517. doi: 10.1378/chest.126.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen SY, Chiang WC, Ma MHM. Sequential symptomatic analysis in probable severe acute respiratory syndrome cases. Ann Emerg Med. 2004;43:27–33. doi: 10.1016/j.annemergmed.2003.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chu CM, Cheng VCC, Hung IFN. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wenzel RP, Edmond MB. Managing SARS amidst uncertainty. N Engl J Med. 2003;348:1947–1948. doi: 10.1056/NEJMp030072. [DOI] [PubMed] [Google Scholar]
- 25.Franceschi LD, Fattovich G, Turrini F. Hemolytic anemia induced by ribavirin therapy in patients with chronic hepatitis C virus infection: role of membrane oxidative damage. Hepatology. 2000;31:997–1004. doi: 10.1053/he.2000.5789. [DOI] [PubMed] [Google Scholar]
- 26.Lin M, Tseng HK, Trejaut JA. Association of HLA class I with severe acute respiratory syndrome coronavirus infection. BMC Med Genet. 2003;4:9–15. doi: 10.1186/1471-2350-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peiris JS, Chu CM, Cheng VC. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan JWM, Ng CK, Chan YH. Short term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS) Thorax. 2003;58:686–689. doi: 10.1136/thorax.58.8.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rivers E, Nguyen B, Havstas S. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]


