Highlights
-
•
Respiratory macrophages have dual functional roles that regulate CD4 T cell responses to recombinant adenovirus-based vaccination in a stage-dependent manner.
-
•
Respiratory macrophages suppress the initial CD4 T cell activation and the subsequent size of tissue-resident CD4 memory T cells.
-
•
Respiratory macrophages and potentially circulating monocytes are critically required for the development and fitness of long-term tissue-resident CD4 memory T cells.
Abbreviations: rAd, recombinant adenovirus-based vaccines; MΦ, respiratory macrophage; i.n., intranasal; i.p., intraperitoneal; i.m., intramuscular; MedLN, mediastinal lymph node; IngLN, inguinal lymph node; DLN, draining lymph node
Keywords: Respiratory macrophages, CD4 T cells, Memory response, Adenovirus-based vaccine, Intranasal immunization
Abstract
Respiratory immunization is an attractive way to generate systemic and mucosal protective memory responses that are required for preventing mucosally transmitted infections. However, the molecular and cellular mechanisms for controlling memory T cell responses remain incompletely understood. In this study, we investigated the role of respiratory macrophage (MΦ) in regulating CD4 T cell responses to recombinant adenovirus-based (rAd) vaccines. We demonstrated that rAd intranasal (i.n.) vaccination induced migration and accumulation of respiratory MΦ and circulatory monocytes in the mediastinal lymph nodes and lung parenchyma. Under the influence of respiratory MΦ CD4 T cells exhibited slow proliferation kinetics and an increased tendency of generating central memory, as opposed to effector memory, CD4 T cell responses in vitro and in vivo. Correspondingly, depletion of MΦ using clodronate-containing liposome prior to i.n. immunization significantly enhanced CD4 T cell proliferation and increased the frequency of CD4 memory T cells in the airway lumen, demonstrating that MΦ initially serve as a negative regulator in limiting generation of mucosal tissue-resident memory CD4 T cells. However, clodronate-containing liposome delivery following i.n. immunization markedly reduced the frequencies of memory CD4 T cells in the airway lumen and spleen, indicating that respiratory MΦ and potentially circulating monocytes are critically required for maintaining long-term memory CD4 T cells. Collectively, our data demonstrate that rAd-induced mucosal CD4 T memory responses are regulated by respiratory MΦ and/or monocytes at multiple stages.
1. Introduction
Mucosal surfaces of respiratory, genital and gastrointestinal tracts are the major gateways of infectious agents, and hence, the development of mucosal vaccines to stimulate protective humoral and/or cellular mucosal immunity has been one of the major focuses of vaccine research for decades [1], [2], [3]. However, the development of mucosal vaccines has proven to be a challenge and only a limited number of mucosal vaccines are licensed in humans [3]. It is widely established that induction of memory responses is the fundamental basis of vaccination and the development of mucosal vaccines against intracellular pathogens that rely on protective memory T cell responses requires greater understanding about molecular and cellular mechanisms in controlling vaccine-induced mucosal T cell responses.
According to CCR7 and CD62L (L-selectin) expression, memory CD4 and CD8 T cells are divided into two major subsets, the CCR7 +CD62Lhigh CD44highCD45RBlow central memory T (TCM) and CCR7−CD62LlowCD44highCD45RBlow effector memory T (TEM) cells [4]. In comparison, naive T cells have the phenotype of CCR7+CD62LhighCD44low CD45RBhigh [5], [6]. TCM cells predominantly circulate within secondary lymphoid organs, whereas TEM cells mainly circulate into the peripheral tissues where some are permanently retained in the tissue and become tissue-resident memory T (TRM) cells [7], [8]. Furthermore, TCM cells produce low levels of effector molecules such as IFN-γ and/or cytotoxic granules, but they have high proliferative capacity and are able to convert to TEM upon antigen restimulation. In contrast, TEM and TRM cells produce immediate effector molecules upon stimulation but have very poor proliferative potential [4], [9]. Compared to TCM cells that are preferentially localized in the systemic lymphoid organs, TEM and TRM cells located at mucosal sites confer immediate frontline immunity against mucosal pathogens [7], [8], [10]. Numerous factors such as the strength and duration of the T cell receptor (TCR) signal, the local inflammatory signals at the time of T cell priming, and other microenvironmental signals associated with the route and/or the immunization schedule all play a role in shaping memory T cell responses in terms of their quality, quantity and anatomic distributions [1], [4], [7], [8], [9], [10], [11]. However, most of our understanding has been obtained by studying CD8 memory T cells. CD4 and CD8 memory T cells are different in many aspects; they have different patterns of peripheral migration [12], different proliferation potential and different longevities [13]. Therefore, additional studies are required to understand the regulation of CD4 T memory responses.
Recombinant adenovirus-based (rAd) vaccines have strong abilities to stimulate CD4 and CD8 T cell responses and have been widely explored for developing vaccines against intracellular pathogens including, Mycobacterium tuberculosis (Mtb), human immunodeficiency virus, malaria and Chlamydia trachomatis [14], [15], [16], [17], [18], [19]. Using a rAd-based tuberculosis vaccine, we demonstrated previously that intranasal (i.n.) immunization with rAd induces accumulation of CD4 and CD8 TRM cells in the respiratory tract for a prolonged period of time and confers protection against pulmonary Mtb challenge via both CD4- and CD8-dependent immune mechanisms [15], [20]. In contrast, intramuscular (i.m.) immunization with rAd results in a predominant CD8 TCM cell response in the systemic lymphoid organs, which only translates into a transient partial protection from secondary Mtb challenge in the lung [15], [20]. While these studies highlight the importance of mucosal immunity in mediating vaccine efficacy against mucosal pathogens, the immune mechanisms that control mucosal CD4 memory T cell responses upon i.n. immunization with rAd are still unclear.
Antigen presenting cells (APCs) play key roles in the induction and regulation of pulmonary immune responses. In particular, respiratory macrophages (MΦs) are demonstrated to modulate respiratory immune responses via various modes of action [21], [22], [23]. For instance, respiratory MΦ can modulate immune responses via suppressing migration of dendritic cells (DCs) into the secondary lymphoid organs [21], [22], [24] or by promoting induction of FoxP3 regulatory T cells [25], [26]. Alternatively, respiratory MΦs are demonstrated to participate in respiratory immune responses through directly transporting pathogen/antigen into the draining lymph modes (DLNs) [27], [28]. Although respiratory MΦs are known to play essential roles during respiratory viral infections [29], [30], [31], it is unclear whether respiratory MΦs may modulate T cell memory responses upon rAd mucosal immunization. In this study, we specifically characterized OVA-specific CD4 T cell responses following i.n. immunization of rAd expressing OVA (AdOVA) and examined the role of respiratory MΦs in controlling CD4 memory T cell responses by depleting respiratory MΦs using clodronate-containing liposome. Our results indicate that respiratory MΦ populations have stage-dependent functional roles in shaping CD4 T memory responses. While respiratory MΦs limit the early stage of CD4 T cell activation and subsequent size of mucosal memory responses, they are critically required for maintaining long-term CD4 T memory responses at both mucosal and systemic compartments.
2. Materials and methods
2.1. Animals
Six to eight week-old female BALB/c mice (H-2d) were ordered from Charles River Laboratories (Senneville, Quebec, Canada). DO11.10 (H-2d) mice were originally from Jackson laboratory (Bar Harbor, ME, USA) and bred at the IWK Health Centre animal facility. All mice were housed under pathogen-free conditions and used according to the Canadian Council for Animal Care guidelines. Food and water were supplied ad libitum.
2.2. Recombinant replication-deficient adenoviral vectors (rAd vectors)
The recombinant human type 5 adenoviral vectors encoding chicken ovalbumin (AdOVA) and empty control vector Addl70-3 have been described before [14], [32]. Viral vectors were amplified, purified and titrated according to the protocols previously described [15].
2.3. Animal model of DO11.10:BALB/c mice and sample preparations
DO11.10:BALB/c mice were used for monitoring OVA-specific CD4 T cell responses. To do this, naïve CD4+CD62L+ T lymphocytes were isolated from pooled peripheral lymph nodes (LN) of DO11.10 mice using mouse naïve CD4 T cell isolation kit (Order No. 130-104-453, Miltenyi Biotec Inc, Auburn, CA, USA) and adoptively transferred into BALB/c mice via tail vein injection (∼2 × 106 cells/mouse) 24 h prior to immunization.
To monitor CD4 T cell proliferation, purified naïve DO11.10 CD4 T cells were first labeled with carboxyfluorescein succinimidyl ester (CFSE) dye (Life Science, Oakville, ON, Canada) prior to transfer. Naïve CD4+CD62L+ T cells were resuspended in PBS/0.1% BSA (pre-warmed at 37 °C), mixed with CFSE at final 10 μM concentration and incubated 10 min at 37 °C. Five volumes of ice-cold PBS/10% FBS were used to stop the reaction. Cells were then washed twice with RPMI/5% FBS medium. Twenty-four hours following cell transfer, DO11.10:BALB/c mice were i.n. or i.m. immunized with 5 × 108 or 1 × 109 plaque-forming units (PFU) of AdOVA or empty control virus Addl70-3 as described previously [14], [15], [33]. At days 0, 3, 5 and 7, single cell suspensions were prepared from peripheral LNs including mediastinal (MedLN), inguinal (IngLN) and axillary LN, bronchoalveolar lavage (BAL), and spleen of each mouse, and stained with DO11.10 TCR-specific monoclonal antibody KJ1-26 (eBioscience, San Diego, CA, USA). OVA-specific CD4 T cell proliferation was analyzed by flow cytometry.
In some experiments, cells isolated from BAL, LN, or spleen were seeded in 96-well plates (1 × 106/well) and restimulated with or without OVA323-339 peptide (5 μg/mL) for 6, 12, 24 or 48 h at 37 °C with 5% CO2. At the end of each time point, culture supernatants were collected and the concentrations of IFN-γ, IL-13, IL-17A, TNF-α, IL-6 and IL-10 were analyzed by ELISA. OVA-specific cytokine measurements in Addl-70-3-immunized mice were only detected at the background levels. Samples without OVA323-339 peptide stimulation had no detectable cytokine production. For the purpose of simplicity, these control groups are not included in data presentation.
2.4. In vivo respiratory macrophage migration assay
To examine whether respiratory MΦs migrate into the MedLN following i.n. immunization with rAd, mice were first instilled with 50 μl of PBS containing 2 mM CFSE via i.n. route and then inoculated with 50 μl of PBS containing AdOVA (1 × 109 PFU/mouse) or PBS alone at 6 h post CFSE delivery. Mice were sacrificed at 40 h post AdOVA immunization and single cell suspensions were prepared from the MedLN and lung tissue of CFSE-labeled mice (CFSE/Ad and CFSE/PBS) and surface labeled with antibodies recognizing MHC II, CD11c, F4/80, and B220 for flow cytometry analyses.
2.5. In vitro co-culture of CD4 T cells, DCs and MΦs
CD11c+ cells were purified by MACS MicroBeads (Miltenyi Biotec Inc) or sorted by flowcytometry from lung, MedLN and IngLN of mice that were pre-immunized with 1 × 109 PFU of AdOVA for 3–5 days. CD11c+ DCs were co-cultured with proliferation dye (CFSE or eFluro647)-labeled naïve DO11.10 CD4+CD62L+ T cells (1:5 ratio of DC:CD4) in complete RPMI medium in presence or absence of OVA323-339 peptide (5 μg/mL) plus IL-2 (10 U/mL) for 3–4 days. In some experiments, CD11c+F4/80+ and CD11c-F4/80+ MΦs were sorted from lungs of AdOVA-immunized mice and added to the co-culture at a ratio of 1:5:1 (DC:CD4:MΦ). CD4 T cells were stained with antibodies recognizing CD44, CD62L, CCR7, CD45RB, and KJ1-26 in different combinations. CD4 T cell proliferation and the activation phenotype in each culture condition were analyzed by flow cytometry.
2.6. In vivo depletion of MΦs
Clodronate-containing liposomes (2 mg clodronate per 20 g body weight) were used to deplete MΦs via intraperitoneal (i.p.) injection as described before [34]. Empty liposomes were used as controls. To examine the effects of depletion, mononuclear cells were isolated from lung and spleen 3 days post liposome delivery and stained with antibodies recognizing MHCII, CD11c, F4/80, B220 and CD3. To determine the impact of MΦ depletion on CD4 memory T cell responses, clodronate-containing liposomes or empty liposomes were injected at 3 days prior to AdOVA immunization, or after AdOVA immunization with three consecutive deliveries at days 7, 14 and 21. Mice were sacrificed at day 28 post immunization. OVA-specific memory CD4 T cells were monitored in the BAL and spleen.
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
The levels of cytokines in the supernatants were measured by ELISA using antibody pairs specific for mouse IFN-γ, IL-13, IL-17A, TNF-α, IL-6 and IL-10 (eBioscience).
2.8. Statistical analyses
Tests for statistical significance were performed using GraphPad Prism 5 software. The specific analysis is indicated in each figure. P values ⩽0.05 were considered statistically significant.
3. Results
3.1. Intranasal and intramuscular immunization with AdOVA induces distinct OVA-specific memory CD4 T cell responses
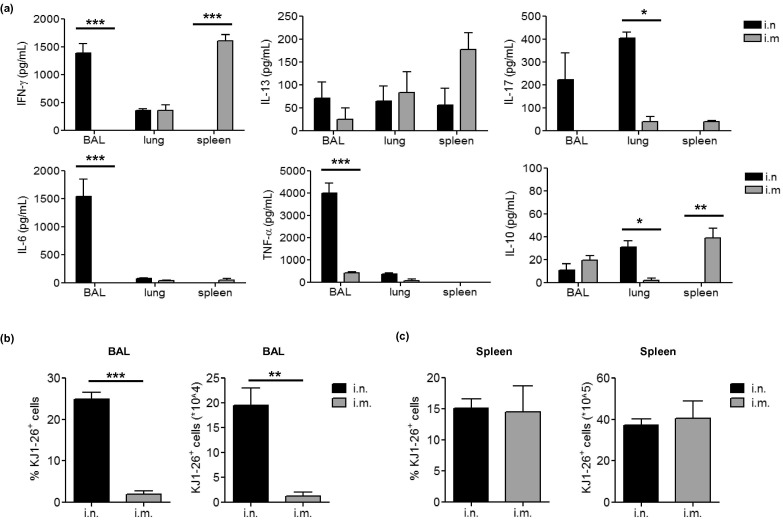
To study CD4 T cell responses to immunization with rAd, a model vaccine encoding OVA (AdOVA) was used to immunize DO11.10:BALB/c mice via i.m. or i.n. route. The antigen-specific CD4 memory T cell recall responses at 4 weeks post-immunization were examined by stimulating single cell suspensions derived from BAL, lung, or spleen with OVA323-339 peptides. Notably, AdOVA i.n. immunization induced a mixed Th1 and Th17 immune recall profile in the BAL- and lung-derived cell cultures, but a very low level in the splenocyte cultures. In contrast, i.m. immunization induced a Th1-dominant immune response that was predominantly distributed in the spleen, but very low in the respiratory tract (Fig. 1 a). Furthermore, OVA323-339-specific CD4 T cells activated by i.n. but not i.m. immunization also produced high levels of proinflammatory cytokines IL-6 and TNF-α (Fig. 1a). Corresponding to the cytokine profile in the BAL, approximately 25% of BAL cells from i.n. immunized mice were OVA-specific DO11.10 cells. In comparison, only ∼1.5% of total BAL cells from i.m. immunized mice were OVA-specific DO11.10 cells and the total number of DO11.10 cells in the BAL of i.m. immunized mice was significantly reduced compared to i.n. immunized mice (Fig. 1b). Of interest, the frequencies of DO11.10 cells in the spleen of i.n. and i.m. immunized mice were comparable despite a significantly different cytokine profile in splenocyte cultures (Fig. 1a/c). These results are consistent with our previous studies using rAd vaccines encoding bacterial antigens [14], [15], indicating a common phenomenon for rAd-based vaccination that different routes of immunization with rAd stimulate CD4 memory T cell responses with different quantity, quality and differential anatomic distributions.
Fig. 1.
Intranasal and intramuscular immunization with recombinant adenovirus-based vaccine expressing OVA induces distinct OVA-specific memory CD4 T cells. 2 × 106 LN cells from naïve DO11.10 mice were transferred i.v. to naïve BALB/c mice. Recipient mice were immunized with 5 × 108 pfu AdOVA i.n. or i.m. 24 h post-adoptive transfer (5 mice per group). Four weeks post immunization, single cell suspensions were prepared from the alveolar space, lung parenchyma and spleen and restimulated with OVA323-339 peptide for 72 h. (a) The concentrations of IFN-γ, IL-13, IL-17, IL-6, TNF-α and IL-10 in culture supernatants were determined by ELISA. The differences between groups were analyzed using two-way ANOVA test (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001). (b/c) The frequencies (b) and total number (c) of DO11.10 CD4 T cells in single cell suspensions were determined by flow cytometry.
3.2. Characterization of CD4 T cell proliferation and dissemination in the local draining lymph nodes and spleen following i.n. and i.m. immunization
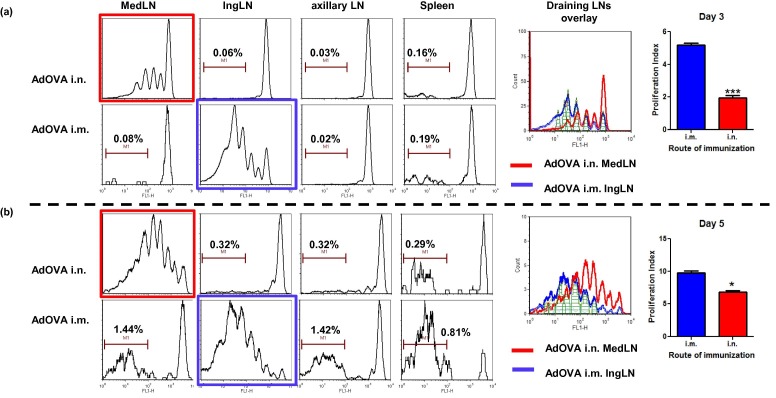
Memory CD4 T cells are very heterogeneous and generated through divergent pathways from antigen-activated precursors [35], [36], [37]. The fate of T cells becoming stable memory T cells may be determined during the first division of T cell activation [35], [38]. In order to understand how different routes of immunization lead to different CD4 memory T cell responses, we examined CD4 T cell proliferation, an early event of T cell activation, in the lymphoid organs of i.n. and i.m. immunized mice. Based on the characteristic CFSE dilution profile, a clear DO11.10 CD4 T cell proliferation profile in the DLNs (in the MedLN of i.n. immunized mice and in IngLN of i.m. immunized mice) was evident at days 3 and 5 post immunization (Fig. 2 ). Notably, CD4 T cell proliferation kinetics induced by i.n. and i.m. immunization differed drastically at day 3 post immunization (see overlay). The frequencies of non-proliferating OVA-specific CD4 T cells in the IngLN and MedLN were ∼10% and ∼30%, respectively. Correspondingly, more DO11.10 CD4 T cells progressed to later cell cycles (cycle 4 and 5) in the IngLN of the i.m. group than that in the MedLN of i.n. immunized mice (Fig. 2a). However, the difference in proliferation kinetics appeared to be reduced by day 5 (Fig. 2b). As expected, no characteristic CD4 T cell proliferation was observed in the non-draining axillary LN or spleen in any immunization group at both time points. Instead, DO11.10 cells in the axillary LN and spleen exhibited distinct CFSE intensities that were indicative of either non-proliferating cells (the initial transferred DO11.10 T cells) or highly divided T cells (the disseminated DO11.10 cells from the DLNs) (Fig. 2a/b). Associated with the T cell proliferation kinetics in the DLNs, we observed a slow dissemination of activated CD4 T cells in the non-draining axillary LN and spleen of i.n. immunized mice compared to i.m. immunized mice. For instance, the frequencies of disseminated DO11.10 cells in the spleen of i.n. and i.m. immunized mice were 0.29% and 0.81%, respectively, at day 5 post immunization (Fig. 2a/b). Similarly, the frequencies of disseminated DO11.10 cells in the axillary LN were 0.32% (i.n.) versus 1.4% (i.m.) at day 5 post immunization (Fig. 2a/b). The frequencies of disseminated DO11.10 cells in the axillary LN and spleen of i.n. and i.m. immunized mice were comparable by day 7 post immunization (data not shown).
Fig. 2.
The routes of immunization have potent effects on the kinetics of CD4 T cell proliferation and dissemination. DO11.10:BALB/c mice were immunized with 5 × 108 pfu AdOVA i.n. or i.m. 24 h after naïve DO11.10 CD4 T cell transfer. OVA-specific CD4 T cell proliferation in the MedLN, IngLN, Axiilary LN and spleen at day 3(a) and day 5(b) post immunization was determined by flow cytometry. At each time point, an overlay plot with the DO11.10 T cell proliferation profile in the draining LNs (MedLN for i.n. group and IngLN for i.m. group) is displayed. The proliferation index in i.n. and i.m. immunized mice were compared using unpaired studentt-test. *p < 0.05, ***p < 0.001. The percentage of disseminated DO11.10 CD4 T cells in distal lymphoid organs is indicated in corresponding plots. The results are representative of two independent experiments with 4 mice per group.
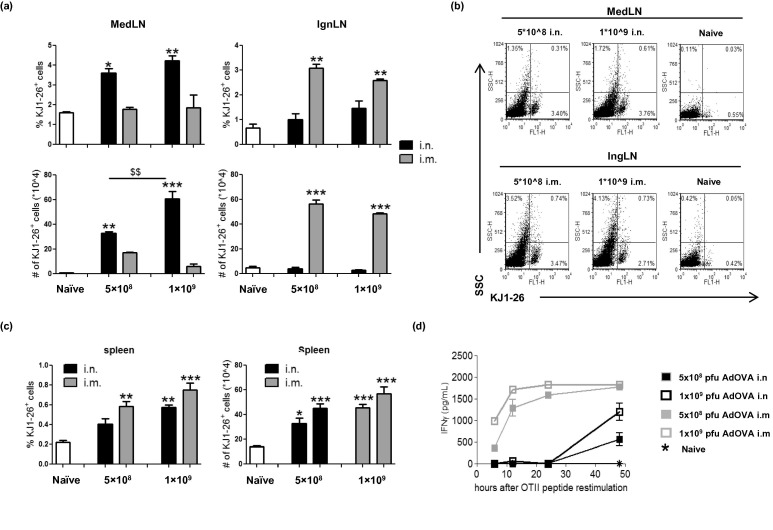
In order to examine whether the potential differences in antigen uptake during i.n. and i.m. immunization contribute to differential CD4 T cell proliferation kinetics, two doses of AdOVA were included in our comparison. While a dose-dependent KJ1-26+ T cell expansion was observed in the MedLN following i.n. immunization of AdOVA, the dose-dependent reaction was not observed in the IngLN of i.m. immunized mice (Fig. 3a/b), which may attribute to the concurrent events of CD4 T cell proliferation and active T cell emigration. In comparison, the number of KJ1-26+ T cells in the spleen displayed a clear trend of dose-dependent responses in both i.n. and i.m. immunized mice. Consistent with the proliferation profile, the overall levels of KJ1-26+ T cell expansion in the spleen of i.n. immunized mice were lower compared to i.m. immunized mice at both doses (Fig. 3 c), suggesting that the model of antigen uptake in i.n. and i.m. immunization has a role in regulating CD4 T cell responses. Remarkably, the kinetics of OVA323-339-specific IFN-γ response in the splenocyte cultures was characteristically affected by the route of immunization (Fig. 3d). OVA peptide stimulated a rapid IFN-γ response, a typical TEM response, in i.m. immunized mice which was readily detected as early as 6 h and peaked at 12–24 h post stimulation. In contrast, the IFN-γ response in i.n. immunized mice was not detectable until 48 h post stimulation. Notably, an increased dose of AdOVA in both i.n. and i.m. immunization groups only affected the level, but not the kinetics, of IFN-γ recall responses (Fig. 3d), demonstrating that other factors, in addition to the model of antigen uptake, also play a role in controlling the CD4 T cell responses. Together, our results demonstrate that the impact of route of immunization on CD4 T cell responses starts at the very beginning of the immune induction phase via controlling CD4 T cell proliferation in the local DLNs. Not only does the distinct T cell proliferation kinetics result in different numbers of activated CD4 T cells, but also it affects the quality of activated CD4 T cells.
Fig. 3.
The route of immunization has potent effects on the quality of CD4 T cells. DO11.10:BALB/c mice were immunized with 5 × 108 pfu or 1 × 109 pfu of AdOVA i.n. or i.m. for 5 days (3 mice per group). (a) The percentage and the number of KJ1-26+ cells in the MedLN and IngLN of naïve or immunized DO11.10:BALB/c mice were determined by flow cytometry. (b) The representative dot plots show the frequency of DO11.10 cells. (c) The percentage and the number of KJ1-26+ cells in the spleen of naïve or immunized DO11.10:BALB/c mice. (d) Splenocytes were stimulated with 5 μg/mL OVA peptide for 6, 12, 24 and 48 h. IFN-γ concentration was determined by ELISA. The data are expressed as the mean ± SEM (n = 3) and the differences between groups were analyzed using two-way ANOVA test. $$p < 0.01 between indicated groups, ∗p < 0.05, ∗∗p < 0.01 compared to naïve mice.
3.3. Respiratory macrophages inhibit CD4 T cell activation and proliferation in vitro and ex vivo
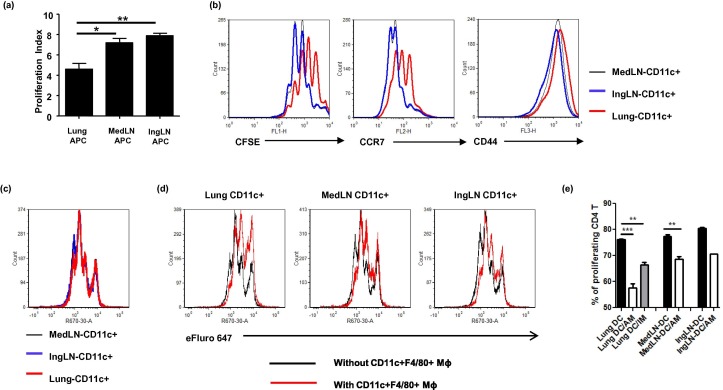
Since APCs play an instrumental role in CD4 T cell activation, we then questioned whether distinct OVA-specific CD4 T cell responses induced by i.n. or i.m. immunization were controlled by distinct APC populations. To this end, mice were first immunized with AdOVA to prime APC populations in vivo. Lung APCs and MedLN APCs were purified from AdOVA i.n. immunized mice and IngLN APCs were isolated from AdOVA i.m. immunized mice using anti-CD11c-conjugated MACS beads. Purified CD11c+ cells were used to stimulate OVA-specific CD4 T cells ex vivo. Notably, a significantly reduced T cell proliferation was observed in the lung APC-containing cultures compared to those with APCs from MedLN and IngLN (Fig. 4 a/b). In parallel with a slower proliferation kinetics, more CD4 T cells activated by lung-derived APCs displayed higher levels of CCR7 compared to CD4 T cells activated by LN-derived APCs. In comparison, CD4 T cells in all culture conditions displayed comparable levels of CD44 (Fig. 4b). These results suggest that CD4 T cells activated by lung-derived APCs and LN-derived APCs may have an increased tendency to develop into CCR7+ TCM and CCR7- TEM CD4 T cells, respectively.
Fig. 4.
Respiratory macrophages inhibit CD4+ T cell activation and proliferation ex vivo. (a/b) DO11.10:BALB/c mice were immunized with 5 × 108 pfu AdOVA i.n. or i.m. 24 h after adoptive T cell transfer (3 mice per group). APC populations were magnetic bead-purified from AdOVA-immunized mice and co-cultured with naïve DO11.10 CD4 T cells in the presence of OVA peptide. T cell proliferation was measured by FACS analysis based on CFSE dilution. The data are representative of two experiments and presented as the mean ± SD of triplicate wells. The differences between groups were analyzed using one-way ANOVA test (∗p < 0.05, ∗∗p < 0.01). Representative FACS plots in panel b are showing CFSE, CCR7 and CD44 expression on CD4 T cells. (c/d) CD11c+ DCs were sorted by flowcytometry from the lungs or lymph nodes of AdOVA-immunized mice and co-cultured with proliferation dye-labeled naïve CD4 T cells in the presence of OVA peptides for 3 days. CD11c+F4/80+ AMs and CD11c-F4/80+ IMs were also sorted from lung tissue and added into some wells. CD4 T cell proliferation was measured by FACS analysis. (e) The percentages of proliferating CD4 T cells in each culture condition are presented as the mean ± SD of 2–3 wells. The inhibitory effects of AMs or IMs on CD4 T cell proliferation were analyzed using one-way ANOVA test by comparing to corresponding DC controls (∗∗p < 0.01, ∗∗∗p < 0.001).
A portion of respiratory MΦ also express CD11c [39] and represent a fraction of CD11c+ cells in lung-derived APCs. Since respiratory MΦ are reported to have immune suppressive activity under various conditions [40], [41], the difference between lung-derived APCs and LN-derived APCs (MedLN and IngLN) on CD4 T cell activation kinetics might be due to a decreased number of DCs in lung-derived APCs and the contaminated respiratory MΦ may suppress CD4 T cell activation. Since Alveolar MΦ(AM), interstitial MΦ(IM) and respiratory DCs are distinguishable based on characteristic F4/80 and CD11c expression [24], we repeated co-cultured experiments by using pure CD11c+ DCs that were sorted from the lung and LNs of AdOVA-immunized mice by FACS. As demonstrated in Fig. 4c, sorted-CD11c+ DCs from lung and MedLN as well as IngLN stimulated CD4 T cell proliferation at comparable rates although lung DCs remained to be somewhat weaker compared to LN-derived DCs (Fig. 4c/e). CD11c+F4/80+ AMs and CD11c-F4/80+ IMs were also sorted from the lungs of AdOVA-immunized mice. Adding purified CD11c+F4/80+ AMs significantly reduced CD4 T cell proliferation in all co-cultures (Fig. 4d/e). CD11c-F4/80+ IMs also showed inhibitory effect on CD4 T cell proliferation in lung-DC cultures although they appeared to be less inhibitory (Fig. 4e). Notably, alveolar MΦ isolated from naïve mice had even stronger inhibitory effect (data not shown). Together, our data suggest that respiratory MΦ are capable of limiting CD4 T cell activation and proliferation.
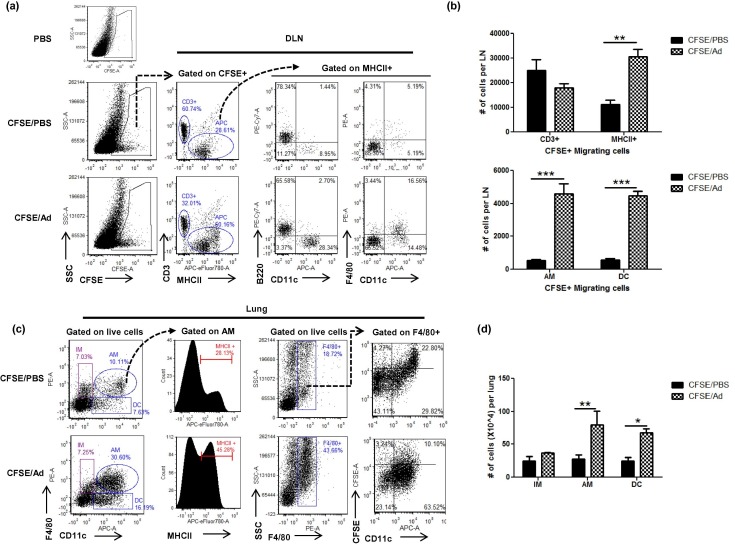
3.4. AdOVA i.n. immunization induces respiratory MΦ and monocytes migration and accumulation in the draining LN and lung parenchyma
Subsequently, we examined whether respiratory MΦ actually migrate into the DLN and regulate CD4 T cell responses in vivo. To this end, we instilled CFSE to the airway to label the respiratory cells 6 h prior to AdOVA i.n. immunization and analyzed the frequency and phenotype of CFSE-positive MΦ in the DLN and lung by flow cytometry at 40 h post immunization. As shown in Fig. 5 , the vast majority of CFSE+ migrating cells in mice treated with CFSE/PBS were CD3+ and B220+, indicating a homeostatic migration of T cells and B cells from the airway to the DLN. Remarkably, AdOVA immunization significantly increased the frequency and total number of CFSE+ DCs (MHCII+CD11c+) and MΦ (MHCII+CD11c+F4/80+) in the DLNs compared to the CFSE/PBS control group (Fig. 5a/b). Corresponding to the changes in the DLN, AdOVA immunization significantly increased the frequency and number of AMs and DCs in the lung (Fig. 5c/d). These results indicate that AdOVA i.n. immunization induces concurrent migration and/or expansion of respiratory DCs and MΦ populations in the DLNs and lung parenchyma. Notably, AMs in Ad-immunized mice displayed an increased frequency of MHC II-positive (45% in CFSE/Ad group versus 28% in CFSE/PBS group). In addition, the F4/80+ cells observed in the lung of AdOVA-immunized mice were predominantly CFSE-negative and CD11c+ (Fig. 5b), indicating that accumulation of AMs in Ad-immunized mice is largely due to the recruitment of circulating monocyte-derived MΦ. Together, our results demonstrate a dynamic involvement of respiratory MΦ populations upon i.n. vaccination with rAd.
Fig. 5.
AdOVA immunization induces respiratory macrophage migration and accumulation in the MedLN and lung parenchyma. CFSE-labeled BALB/c mice were immunized with AdOVA i.n or PBS for 40 h. Single cell suspensions were prepared from the MedLN and lung and stained for flow cytometry. (a/c) Representative dot plots to show the gating strategy and the characteristics of CSFE-positive cells in the DLN (a) or lung (c). (b) The number of CFSE-positive migrating cells in the MedLNs of AdOVA immunized and PBS-treated mice. (d) The number of respiratory macrophage populations and DCs in the lung of AdOVA immunized and PBS-treated mice. The results are representative of two independent experiments with 4 mice per group. The differences between groups were analyzed using two-way ANOVA test (∗∗p < 0.01, ∗∗∗p < 0.001).
3.5. Macrophages regulate CD4 T cell activation and CD4 T memory formation
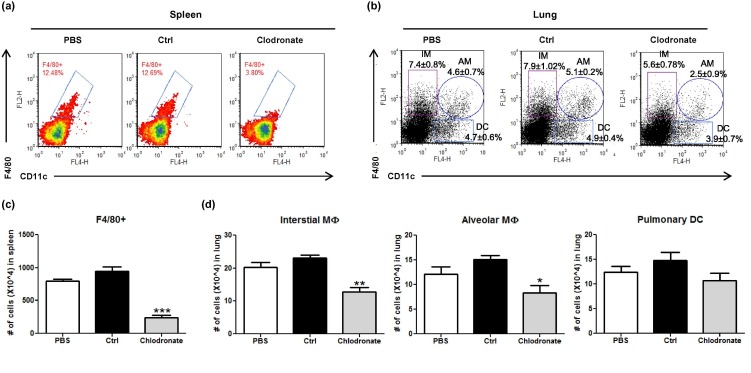
In order to understand the biological role of respiratory MΦ in regulating CD4 T cell responses in vivo, we utilized clodronate-containing liposomes to deplete MΦ in vivo [42]. The impact of liposome delivery on F4/80+ macrophages, CD11c+ DCs and other CD11b+ myeloid cells in the spleen and lung was first determined by flow cytometry. Remarkably, the frequency of F4/80-positive macrophages in the spleen was markedly reduced from ∼13% in PBS-treated or control liposome-treated mice to ∼3% in clodronate-containing liposome-treated mice (Fig. 6 a/b). Consistent with a previous report [34], i.p. injection of clodronate-containing liposome also significantly depleted respiratory macrophages (both CD11c- IMs and CD11c+ AMs) (Fig. 6c/d). In sharp contrast to a potent depletion effect on macrophages, no significant depletion was observed on F4/80-/CD11c+ pulmonary DCs in the lung (Fig. 6c/d).
Fig. 6.
Intraperitoneal injection of clodronate-containing liposomes systemically depletes F4/80+ macrophages in the spleen and lung. Single cell suspensions were isolated from spleen and lung of mice that received i.p injection of 200 μl PBS, or control liposomes, or clodronate-containing liposomes (2 mg/20 g BW). Flow cytometry analysis was conducted by analyzing CD11c and F4/80 expression in the spleen (a) and lung (b). The number of F4/80+ macrophages in the spleen (c) and the number of interstitial macrophages, alveolar macrophages and respiratory DCs in the lung (d) following different treatment are presented as mean ± SEM. The results are representative of two independent experiments with 4 mice per group. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 compared to control liposome group using one-way ANOVA test.
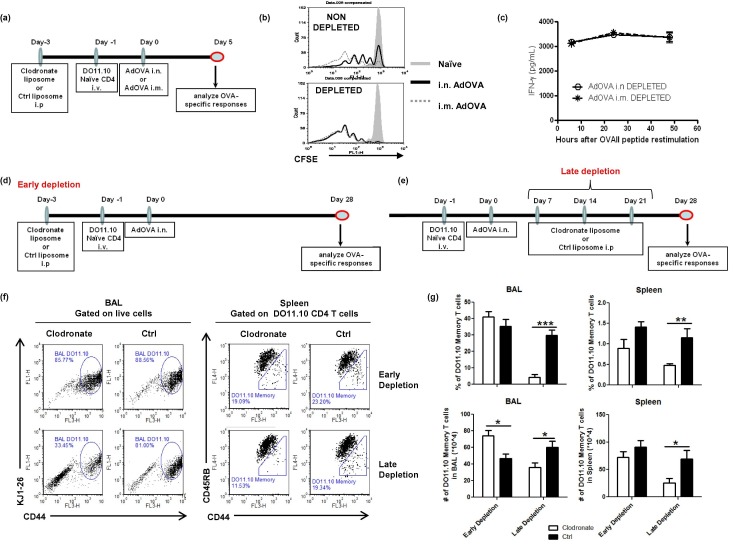
We subsequently conducted experiments using clodronate-containing liposomes or control liposomes in early or late depletion regimens in which liposomes were given 3 days prior to immunization or after immunization, respectively (Fig. 7 a/d/e). Notably, MΦ depletion prior to immunization completely eliminated the differences in i.n. and i.m. immunized mice at the CD4 T cell activation phase (Fig. 7a/b/c). The T cell proliferation kinetics in the draining LNs (Fig. 7b) and OVA peptide-induced IFN-γ responses in splenocyte cultures (Fig. 7c) became indistinguishable among i.n. and i.m. immunized mice upon MΦ depletion, highlighting a role of macrophages in suppressing CD4 T cell activation. We also examined the frequency of CD4+CD44highCD45RBlow memory T cells in the BAL and spleen of early depleted versus non-depleted mice at 4 weeks post immunization. As demonstrated in Fig. 7f/g, MΦ-depletion prior to immunization significantly increased the number of CD4 memory T cells in the BAL compared to the control liposome-treated mice. No apparent impact on memory T cells in the spleen was observed. In sharp contrast to the early depletion regimen, MΦ-depletion following AdOVA i.n. immunization resulted in a markedly reduced frequency and number of DO11.10 CD4 memory T cells in the BAL and spleen ( Fig. 7f/g), demonstrating a functional role of respiratory MΦ for maintaining long-term CD4 memory T cell responses.
Fig. 7.
Macrophage depletion after immunization, but not prior to immunization, markedly reduces the number of tissue resident and systemic CD4 memory T cells. (a/d/e) Diagrams of macrophage depletion regimens used in the study. (b) Representative FACS plots showing CD4 T cell proliferation in the DLNs of i.n. immunized versus i.m. immunized mice with or without MΦ-depletion at day 3 post immunization. (c) IFN-γ recall responses in the splenocyte cultures of MΦ-depleted mice at day 5 post i.n or i.m. immunization. (f/g) DO11.10:BALB/c mice were treated with clodronate-containing liposomes or control liposomes at 3 days prior to AdOVA immunization or at 7, 18 and 24 days post immunization. CD4 memory T cells in BAL and spleen were determined by FACS at 4 weeks post immunization. The percentage and the number of DO11.10 CD4 memory T cells (CD44highCD45RBlow) are presented as the mean ± SEM of 5–8 mice per group pooled from two experiments. The differences between groups were analyzed using two-way ANOVA test (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001).
4. Discussion
In this study, the immune mechanisms in controlling CD4 T cell responses to mucosal immunization with rAd-based vaccines were investigated. By characterizing early events of CD4 T cell activation following i.n. and i.m. immunization, we have demonstrated that the differential CD4 T cell responses associated with the route of immunization are controlled by multiple factors including the model of antigen uptake and the intrinsic properties of APCs. By tracking respiratory macrophages upon i.n. immunization, we have revealed a dynamic process of respiratory macrophages induced by mucosal immunization and demonstrated that respiratory MΦ and circulating monocytes migrate into the local draining LNs and accumulate in the lung parenchyma. By depleting macrophages using clodronate-containing liposomes, we have further demonstrated divergent roles for respiratory MΦ populations in controlling CD4 T memory responses to rAd immunization in a stage-dependent manner. While respiratory MΦ populations act as a negative regulator to limit the early stage of CD4 T cell activation and proliferation and the quality of T cells, they are critically required for maintaining long-term memory T cells, both the tissue-resident and systemic memory CD4 T cells.
As the most abundant professional phagocytes in the airway, MΦs play a predominant role for capturing soluble antigens and cellular debris under steady conditions and microorganisms upon respiratory infections [43], [44]. However, pulmonary MΦ function poorly as professional APCs but possess immune suppressive activities to dampen the induction of adaptive immune response [21], [23], [25], [26], [40], [43]. Under resting conditions, MΦ-mediated immune suppression is essential in maintaining respiratory homeostasis and for preventing the induction of aberrant immune responses against inert antigens and environmental particles [43]. Upon respiratory infections, production of pro-inflammatory mediators is required for orchestrating innate immune cells such as DCs to counteract the inhibitory effect of pulmonary MΦs. It has been demonstrated that MΦ-mediated immune suppression accounts for poor T cell responses and severe disease in mice during respiratory coronavirus infection [31]. Depletion of MΦ in vivo with liposome delivery results in a significant increase in pulmonary CD4 and CD8 T cell responses against influenza and coronavirus infections [30], [31]. Consistent with these reports, we have also observed immune suppressive activity of pulmonary MΦ in controlling the induction of CD4 T cell response and subsequent CD4 memory response during rAd i.n. immunization. Respiratory MΦ may simply suppress induction of T cell responses via producing soluble molecules like prostaglandins, TGF-β and IL-10 [41], [45].
In sharp contrast to a negative role of MΦ over CD4 T cell responses upon i.n. immunization with rAd, MΦ-depletion after immunization markedly reduced the frequencies of CD4 memory T cells in the BAL and spleen (Fig. 7f/g). Therefore, our study has extended our understanding for a role of respiratory MΦ populations in maintaining long-term memory CD4 T cells. At the moment, the molecular mechanisms underlying the observation remain unclear. Recent fate-mapping studies indicate that the tissue-resident MΦ population is derived from the yolk sac and fetal liver during development [46], [47], and mixed with monocyte-derived MΦs recruited from the bone marrow after tissue injury and infection [48]. While the yolk-sac-derived MΦ population is capable of self-renewing in the steady state, its role in lung infection/immunization remains unclear. In our system, massive expansion of pulmonary MΦ population after rAd immunization was observed (Fig. 5c/d). Although the vast majority of pulmonary MΦ population appeared to be recruited from circulating monocytes, we cannot rule of the possibility that a portion of them are proliferating yolk-sac-derived MΦ population. In addition, there might be an enhanced secondary monocyte recruitment in the lung following repeated clodronate-containing liposome delivery (Sup. Fig. 1). These monocytes are likely mobilized from the spleen as a result of heightened inflammatory responses in the lung [49]. However, investigating the precise role of these monocytes, monocyte-derived macrophages and different pulmonary MΦ populations in maintaining long-term CD4 memory T cells requires a more sophisticated system. Nevertheless, it has been suggested in a recent study that immune regulatory macrophages differentiated from monocytes by M-CSF and IL-34 are able to switch non-committed human CD4 memory T cells into Th17 cells [50]. Conversely, the pro-inflammatory macrophages induced by GM-CSF promote Th1 cells [50]. Furthermore, a recent study using intra-vaginal immunization with an attenuated herpes simplex virus 2 demonstrates that tissue resident CD4 memory T cells are maintained by chemokines, particularly CCL5, secreted by local macrophages [51]. Therefore, the accumulation of respiratory TEM CD4 T cells in i.n immunized mice is unlikely only controlled at the initial phase of CD4 T cell activation in the DLNs. Rather, we believe that the initial TEM CD4 T cells disseminated from the DLNs may have undergone additional expansion, modification and fitness within the respiratory microenvironment and this process is largely dependent on the presence of respiratory MΦ populations and the soluble mediators released by MΦ.
Long-term memory CD8 T cells in the airway lumen by mucosal immunization with rAd undergo antigen-driven proliferation in situ [52]. Therefore, respiratory MΦ may maintain CD4 memory T cells via presenting antigen to activated CD4 T cells at the immunization site. Viral-infected alveolar MΦ are known to undergo apoptosis in order to control intracellular viral infection [34], [45]. Potentially, those apoptotic MΦ and cellular debris are taken up by neighboring/recruiting MΦ for maintaining normal respiratory function [29], [34], [45]. Under this condition, alveolar MΦ may acquire viral antigens through active internalization of viral-infected dying alveolar MΦ and present antigen to activated CD4 T cells in the airway lumen for a prolonged period of time. Further investigations are needed to delineate the involvement of respiratory MΦ in protecting tissue-resident CD4 memory T cells.
In summary, we have demonstrated that respiratory MΦ populations have dual functional roles in controlling the quality and quantity of CD4 T cell responses at both immune induction and memory phases. Improved mucosal vaccination strategies aiming at enhancing long-term CD4 T cell responses for intracellular pathogens at mucosal surfaces may be developed by modulating respiratory MΦs.
Author contributions
EAR performed the experiments and data analyses and drafted the manuscript. CT, RMK and MRT contributed to the experiments examining macrophage migration in vivo, macrophage depletion using liposomes and sorted-macrophage suppression assays. RAS, ZX and SAH provided reagents and contributed to editing the manuscript. JW designed the experiments, contributed to writing the manuscript and provided overall direction.
Conflict of interest disclosure
All authors declare no commercial or financial conflict of interest.
Acknowledgments
This study was supported by funds from the IWK Health Centre, the Nova Scotia Health Research Foundation (NSHRF), the Canadian Institutes of Health Research (CIHR), and the Canadian Foundation for Innovation (CFI). EAR was supported by an IWK Fellowship Award.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cellimm.2016.07.006.
Appendix A. Supplementary data
This document contains supplementary Fig. 1.
References
- 1.Holmgren J., Czerkinsky C. Mucosal immunity and vaccines. Nat. Med. 2005;11(4 Suppl.):S45–S53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 2.Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat. Rev. Immunol. 2012;12(8):592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- 3.Woodrow K.A., Bennett K.M., Lo D.D. Mucosal vaccine design and delivery. Annu. Rev. Biomed. Eng. 2012;14:17–46. doi: 10.1146/annurev-bioeng-071811-150054. [DOI] [PubMed] [Google Scholar]
- 4.Sallusto F., Geginat J., Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 5.Sallusto F., Lenig D., Forster R., Lipp M., Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 6.Wherry E.J., Teichgraber V., Becker T.C. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 2003;4(3):225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 7.Woodland D.L., Kohlmeier J.E. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat. Rev. Immunol. 2009;9(3):153–161. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- 8.Mueller S.N., Gebhardt T., Carbone F.R., Heath W.R. Memory T cell subsets, migration patterns, and tissue residence. Annu. Rev. Immunol. 2013;31:137–161. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- 9.Sallusto F., Lanzavecchia A. Heterogeneity of CD4+ memory T cells: Functional modules for tailored immunity. Eur. J. Immunol. 2009;39(8):2076–2082. doi: 10.1002/eji.200939722. [DOI] [PubMed] [Google Scholar]
- 10.Wang J., Xing Z. Tuberculosis vaccines: the past, present and future. Expert Rev. Vaccines. 2002 Oct;1(3):341–354. doi: 10.1586/14760584.1.3.341. [DOI] [PubMed] [Google Scholar]
- 11.Sheridan B.S., Lefrancois L. Regional and mucosal memory T cells. Nat. Immunol. 2011;12(6):485–491. doi: 10.1038/ni.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gebhardt T., Whitney P.G., Zaid A. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477(7363):216–219. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 13.Homann D., Teyton L., Oldstone M.B. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat. Med. 2001;7(8):913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- 14.Brown T.H., David J., Acosta-Ramirez E. Comparison of immune responses and protective efficacy of intranasal prime-boost immunization regimens using adenovirus-based and CpG/HH2 adjuvanted-subunit vaccines against genital Chlamydia muridarum infection. Vaccine. 2012;30(2):350–360. doi: 10.1016/j.vaccine.2011.10.086. [DOI] [PubMed] [Google Scholar]
- 15.Wang J., Thorson L., Stokes R.W. Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J. Immunol. 2004;173(10):6357–6365. doi: 10.4049/jimmunol.173.10.6357. [DOI] [PubMed] [Google Scholar]
- 16.Liniger M., Zuniga A., Naim H.Y. Use of viral vectors for the development of vaccines. Expert Rev. Vaccines. 2007;6(2):255–266. doi: 10.1586/14760584.6.2.255. [DOI] [PubMed] [Google Scholar]
- 17.Li S., Locke E., Bruder J. Viral vectors for malaria vaccine development. Vaccine. 2007;25(14):2567–2574. doi: 10.1016/j.vaccine.2006.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchbinder S.P., Mehrotra D.V., Duerr A. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the step study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008 Nov 29;372(9653):1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smaill F., Jeyanathan M., Smieja M. A human type 5 adenovirus-based tuberculosis vaccine induces robust T cell responses in humans despite preexisting anti-adenovirus immunity. Sci. Transl. Med. 2013;5(205) doi: 10.1126/scitranslmed.3006843. 205ra134. [DOI] [PubMed] [Google Scholar]
- 20.Santosuosso M., Zhang X., McCormick S., Wang J., Hitt M., Xing Z. Mechanisms of mucosal and parenteral tuberculosis vaccinations: adenoviral-based mucosal immunization preferentially elicits sustained accumulation of immune protective CD4 and CD8 T cells within the airway lumen. J. Immunol. 2005;174(12):7986–7994. doi: 10.4049/jimmunol.174.12.7986. [DOI] [PubMed] [Google Scholar]
- 21.Jakubzick C., Tacke F., Llodra J., van Rooijen N., Randolph G.J. Modulation of dendritic cell trafficking to and from the airways. J. Immunol. 2006;176(6):3578–3584. doi: 10.4049/jimmunol.176.6.3578. [DOI] [PubMed] [Google Scholar]
- 22.Hardy C.L., LeMasurier J.S., Mohamud R. Differential uptake of nanoparticles and microparticles by pulmonary APC subsets induces discrete immunological imprints. J. Immunol. 2013;191(10):5278–5290. doi: 10.4049/jimmunol.1203131. [DOI] [PubMed] [Google Scholar]
- 23.Thepen T., Hoeben K., Breve J., Kraal G. Alveolar macrophages down-regulate local pulmonary immune responses against intratracheally administered T-cell-dependent, but not T-cell-independent antigens. Immunology. 1992;76(1):60–64. [PMC free article] [PubMed] [Google Scholar]
- 24.Bedoret D., Wallemacq H., Marichal T. Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. J. Clin. Invest. 2009;119(12):3723–3738. doi: 10.1172/JCI39717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coleman M.M., Ruane D., Moran B., Dunne P.J., Keane J., Mills K.H. Alveolar macrophages contribute to respiratory tolerance by inducing FoxP3 expression in naive T cells. Am. J. Respir. Cell Mol. Biol. 2013;48(6):773–780. doi: 10.1165/rcmb.2012-0263OC. [DOI] [PubMed] [Google Scholar]
- 26.Soroosh P., Doherty T.A., Duan W. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J. Exp. Med. 2013;210(4):775–788. doi: 10.1084/jem.20121849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirby A.C., Coles M.C., Kaye P.M. Alveolar macrophages transport pathogens to lung draining lymph nodes. J. Immunol. 2009;183(3):1983–1989. doi: 10.4049/jimmunol.0901089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thepen T., Claassen E., Hoeben K., Breve J., Kraal G. Migration of alveolar macrophages from alveolar space to paracortical T cell area of the draining lymph node. Adv. Exp. Med. Biol. 1993;329:305–310. doi: 10.1007/978-1-4615-2930-9_51. [DOI] [PubMed] [Google Scholar]
- 29.Schneider C., Nobs S.P., Heer A.K. Alveolar macrophages are essential for protection from respiratory failure and associated morbidity following influenza virus infection. PLoS Pathog. 2014;10(4):e1004053. doi: 10.1371/journal.ppat.1004053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wijburg O.L., DiNatale S., Vadolas J., Van R.N., Strugnell R.A. Alveolar macrophages regulate the induction of primary cytotoxic T-lymphocyte responses during influenza virus infection. J. Virol. 1997;71(12):9450–9457. doi: 10.1128/jvi.71.12.9450-9457.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao J., Zhao J., Van R.N., Perlman S. Evasion by stealth: inefficient immune activation underlies poor T cell response and severe disease in SARS-CoV-infected mice. PLoS Pathog. 2009;5(10):e1000636. doi: 10.1371/journal.ppat.1000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Damjanovic D., Zhang X., Mu J., Fe Medina M., Xing Z. Organ distribution of transgene expression following intranasal mucosal delivery of recombinant replication-defective adenovirus gene transfer vector. Genet. Vaccines Ther. 2008;6(1):5. doi: 10.1186/1479-0556-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J., Palmer K., Lotvall J. Circulating, but not local lung, IL-5 is required for the development of antigen-induced airways eosinophilia. J. Clin. Invest. 1998;102(6):1132–1141. doi: 10.1172/JCI2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tyner J.W., Uchida O., Kajiwara N. CCL5-CCR5 interaction provides antiapoptotic signals for macrophage survival during viral infection. Nat. Med. 2005;11(11):1180–1187. doi: 10.1038/nm1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moulton V.R., Bushar N.D., Leeser D.B., Patke D.S., Farber D.L. Divergent generation of heterogeneous memory CD4 T cells. J. Immunol. 2006;177(2):869–876. doi: 10.4049/jimmunol.177.2.869. [DOI] [PubMed] [Google Scholar]
- 36.Kaech S.M., Wherry E.J., Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2002;2(4):251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 37.McKinstry K.K., Strutt T.M., Bautista B. Effector CD4 T-cell transition to memory requires late cognate interactions that induce autocrine IL-2. Nat. Commun. 2014;5:5377. doi: 10.1038/ncomms6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kinjyo I., Qin J., Tan S.Y. Real-time tracking of cell cycle progression during CD8+ effector and memory T-cell differentiation. Nat. Commun. 2015;6:6301. doi: 10.1038/ncomms7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franke-Ullmann G., Pfortner C., Walter P., Steinmuller C., Lohmann-Matthes M.L., Kobzik L. Characterization of murine lung interstitial macrophages in comparison with alveolar macrophages in vitro. J. Immunol. 1996;157(7):3097–3104. [PubMed] [Google Scholar]
- 40.Holt P.G., Oliver J., Bilyk N. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J. Exp. Med. 1993;177(2):397–407. doi: 10.1084/jem.177.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roth M.D., Golub S.H. Human pulmonary macrophages utilize prostaglandins and transforming growth factor beta 1 to suppress lymphocyte activation. J. Leukoc. Biol. 1993;53(4):366–371. doi: 10.1002/jlb.53.4.366. [DOI] [PubMed] [Google Scholar]
- 42.Van R.N., Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods. 1994;174(1–2):83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 43.Holt P.G., Strickland D.H., Wikstrom M.E., Jahnsen F.L. Regulation of immunological homeostasis in the respiratory tract. Nat. Rev. Immunol. 2008;8(2):142–152. doi: 10.1038/nri2236. [DOI] [PubMed] [Google Scholar]
- 44.Gordon S.B., Read R.C. Macrophage defences against respiratory tract infections. Br. Med. Bull. 2002;61:45–61. doi: 10.1093/bmb/61.1.45. [DOI] [PubMed] [Google Scholar]
- 45.Coulombe F., Jaworska J., Verway M. Targeted prostaglandin E2 inhibition enhances antiviral immunity through induction of type I interferon and apoptosis in macrophages. Immunity. 2014;40(4):554–568. doi: 10.1016/j.immuni.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 46.Ginhoux F., Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity. 2016;44(3):439–449. doi: 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 47.Guilliams M, De K.I., Henri S. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J. Exp. Med. 2013;210(10):1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wynn T.A., Vannella K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44(3):450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swirski F.K., Nahrendorf M., Etzrodt M. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325(5940):612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Foucher E.D., Blanchard S., Preisser L. IL-34- and M-CSF-induced macrophages switch memory T cells into Th17 cells via membrane IL-1alpha. Eur. J. Immunol. 2015;45(4):1092–1102. doi: 10.1002/eji.201444606. [DOI] [PubMed] [Google Scholar]
- 51.Iijima N., Iwasaki A. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science. 2014;346(6205):93–98. doi: 10.1126/science.1257530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeyanathan M., Mu J., McCormick S. Murine airway luminal antituberculosis memory CD8 T cells by mucosal immunization are maintained via antigen-driven in situ proliferation, independent of peripheral T cell recruitment. Am. J. Respir. Crit. Care Med. 2010;181(8):862–872. doi: 10.1164/rccm.200910-1583OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This document contains supplementary Fig. 1.