Abstract
GASTROENTROLOGY 2001;120:607-621
Abbreviations: AIDS, acquired immunodeficiency syndrome; CMV, cytomegalovirus; HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; IFN-α, interferon α; MAC, Mycobacterium avium complex; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor; RTI, reverse-transcriptase inhibitor; SIV, simian immunodeficiency virus; TMP-SMX, trimethoprim-sulfamethoxazole; ZDV, zidovudine
In the 2 decades since the first reports of the acquired immunodeficiency syndrome (AIDS), the human immunodeficiency virus (HIV)-1 pandemic has surpassed the European-centered bubonic plague of the 14th century in magnitude and number of deaths. Originating in chimpanzees in central Africa,1 AIDS reached epidemic proportions in the 1980s in the United States and Europe, where the vast majority of infections were acquired through homosexual contact. Subsequently, the epidemic spread to heterosexual populations, and by 1998, 35 million people were estimated to be infected with HIV-1 and 20 million to have died of the consequences of HIV-1 infection worldwide. In contrast to the initial focus of the epidemic in developed countries, today 70% of infected persons live in sub-Saharan Africa; in some countries, an astonishing 25% of the population is infected with HIV-1.2 Today, the virus is transmitted by heterosexual contact in 75%–85% of cases.3 As a consequence of heterosexual transmission, the passage of HIV-1 from mother to fetus, neonate, and nursing infant (vertical transmission) has emerged as a devastating public health problem, particularly in developing countries.
Coinciding with these changes in the epidemiology of HIV-1 infection, important new information has emerged regarding the role of the gastrointestinal tract in HIV-1 infection. This information is derived from an array of epidemiologic, basic, and clinical investigations. Here, we review the key findings from these investigations and present the emerging concepts regarding the role of the gastrointestinal tract mucosa in HIV-1 transmission and pathophysiology and the effect of highly active antiretroviral therapy (HAART) on the epidemiology, morbidity, and treatment of gastrointestinal infections.
Role of the gastrointestinal tract mucosa in HIV-1 transmission
Excluding infections acquired parenterally, virtually all HIV-1 infections are acquired via the mucosal surfaces of the gastrointestinal and genital tracts.4 For most heterosexual transmissions, the genital tract mucosa is the site of viral entry, whereas for vertical transmissions and infections resulting from oral–genital and anal–genital contact, the gastrointestinal tract mucosa is the portal of HIV-1 entry. During vertical transmission, the upper gastrointestinal tract mucosa is the site of viral entry as a consequence of the swallowing of infected amniotic fluid in utero, infected blood and cervical secretions intrapartum, and infected breast milk postpartum.5 In homosexual transmission, oral–genital contact has recently been shown to be more common than anal–genital contact,6, 7, 8 suggesting that the upper gastrointestinal tract mucosa is a more common site of HIV-1 entry than rectal mucosa. Indeed, up to a quarter of primary HIV-1 infections in one series in the United States resulted exclusively from receptive oral–genital exposure.8
Infectiousness
New information on the infectiousness of HIV-1 from persons who carry the virus is based largely on studies of heterosexual transmission. Nevertheless, many factors that promote transmission across the vaginal mucosa probably also enhance transmission across the gastrointestinal mucosa (Table 1).
Table 1.
Factors that increase HIV-1 transmission across the mucosa
| High viral load during primary HIV-1 infection in the index partner |
| High viral load during end-stage HIV-1 disease in the index partner |
| Mucosal trauma, inflammation, erosion, or ulcer in the recipient partner |
| Mucosal infection in the recipient partner |
| Increased frequency of sexual contacts |
| Unprotected sexual contact |
| Receptive anal intercourse |
| Absence of circumcision in a male index partner |
For example, infectiousness, defined as the probability of transmitting HIV-1 from one person to another, is greatest when the index partner has primary HIV-1 infection, which corresponds to the period of infection between exposure to the virus and development of circulating anti–HIV-1 antibodies.9 Primary infection is associated with the highest plasma levels of HIV-1.10, 11 Although the levels of HIV-1 in genital secretions during primary infection have not been reported, virus has been detected in genital secretions in all stages of disease. Thus, the presence of cell-free and cell-associated virus in genital secretions probably contributes to its increased infectiousness during this stage of the disease.12
Late-stage disease is also associated with increased levels of HIV-1 in the blood and semen of many12, 13, 14 but not all15, 16 infected men and is associated with an increased risk of HIV-1 transmission.17 The presence of reproductive tract inflammation and infections, such as urethritis, gonorrhea, and cytomegalovirus infection, also increase the infectiousness of the donor,18, 19, 20 likely because of increased HIV-1 shedding into semen.3 Genotypic differences in virus isolated from semen and blood in up to 40% of subjects argue for local production of virus in the genital tract of some infected men.21, 22 In summary, viral load in the blood is a key predictor of the risk of heterosexual transmission of HIV-1,23 and the blood viral load generally correlates with that of genital secretions. Thus, the amount of virus inoculated onto a mucosal surface, including the gastrointestinal mucosa, is an important factor in HIV-1 transmission.
Susceptibility
The susceptibility of the recipient partner is also an important factor in assessing the probability of HIV-1 transmission across the gastrointestinal tract mucosa. Normally, an intact mucosa prevents mucosal penetration of many luminal pathogens. In persons at risk for HIV-1 infection, trauma-induced disruption of the rectal mucosa provides inoculated virus direct access to the mucosal microcirculation and contributes to the high rate of HIV-1 transmission in receptive anal intercourse. Similarly, infection-associated mucosal inflammation and erosions probably provide virus access to lymphoid cells in the lamina propria. Supporting the latter notion are clinical observations that certain gastrointestinal tract infections that cause mucosal ulcers or deep erosions, including herpes simplex proctitis and syphilis, are associated with higher rates of HIV-1 transmission. In addition, certain mucosal infections enhance the susceptibility of lymphoid cells to HIV-1 infection. For example, Mycobacterium avium complex (MAC) enhances macrophage expression of CCR5, the chemokine coreceptor for macrophage-tropic HIV-1, and up-regulates HIV-1 expression by macrophages coinfected with MAC and HIV-1.24
Genetics also plays a role in susceptibility to HIV-1 infection in certain populations. Approximately 1% of white individuals have a 32-nucleotide deletion allele in the gene that encodes CCR5, the chemokine receptor that serves as a coreceptor for macrophage-tropic HIV-1 entry into CD4+ mononuclear cells.25, 26 The homozygous expression of this defective allele (Δ32/Δ32) leads to the absence of functional CCR5 coreceptor; consequently, mononuclear cells are resistant to infection by macrophage-tropic HIV-1.25, 26, 27, 28 Because the predominant transmitting strains of HIV-1 are macrophage-tropic, persons homozygous for the allele are not susceptible to infection with most HIV-1 isolates.
Finally, a spectrum of behavioral and sociologic factors, including increased frequency of sexual contacts,29 unprotected sexual contact,30 receptive anal intercourse, and the absence of circumcision in men31 are associated with increased HIV-1 transmission among persons in whom the gastrointestinal tract is the route of entry.
Role of the gastrointestinal tract mucosa in HIV-1 pathophysiology
HIV-1 entry
In the absence of mucosal disruption from trauma or infection, proposed routes of HIV-1 entry into the mononuclear cell–rich lamina propria include M cells, dendritic cells, and epithelial cells (Figure 1).
Fig. 1.
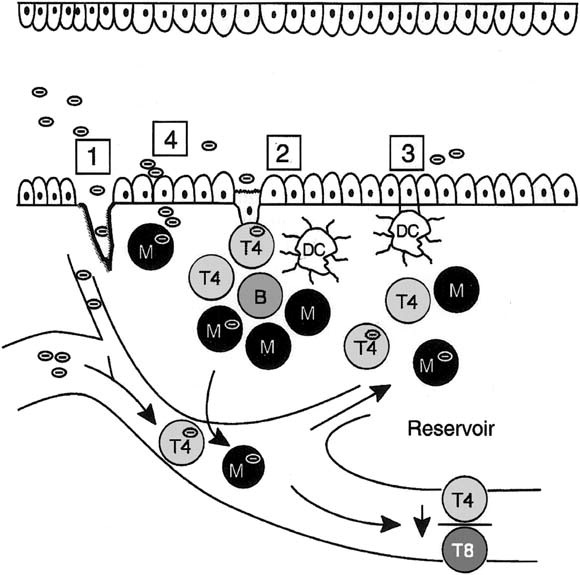
HIV-1 entry in the gastrointestinal mucosa. Potential sites for HIV-1 entry include (1) disrupted epithelium caused by trauma or infection, (2) M cells, (3) dendritic cells, and (4) epithelial cells. Modified and reprinted with permission.4
M cells are specialized epithelial cells present in the dome overlying Peyer's patches in the small intestine and lymphoid follicles in the rectum. M cells transport macromolecules and microorganisms, including viruses such as poliovirus and reovirus, by a nondegradative pathway to interdigitating mononuclear cells or possibly dendritic cells in the underlying lymphoid structure. In an ex vivo system, mouse and rabbit M cells are capable of transporting HIV-1 to mononuclear cells.32 In the simian immunodeficiency virus (SIV) model, atraumatic inoculation of SIV into the oral cavity leads to rapid infection of the tonsils, which are a rich source of M cells,33 suggesting M cell uptake and transport of the virus.34 However, human M cells have not yet been reported to take up and transport HIV-1, probably reflecting the rapidity of the transport process and the unavailability of tissue specimens from subjects shortly after virus inoculation. Nevertheless, the high prevalence of M cells in the rectum35 and tonsils strategically positions these cells for a potential role in HIV-1 transport in vertical and anal–genital transmission.
Dendritic cells are highly efficient antigen-presenting cells that express CD4. Dendritic cells, which assume a characteristic stellate morphology in the tissue, have been identified in rat small and large intestine,36 mouse Peyer's patches,37 macaque cervicovaginal mucosa and foreskin,38 and human tonsil and adenoid.39 Tonsilar dendritic cells form conjugates with T cells, resulting in high levels of viral replication by HIV-1–infected T cells in vitro.39 Recent evidence indicates that dendritic cells bind HIV-1 envelope gp120 through a C-type lectin,40 allowing HIV-1 capture (but not infection) for subsequent delivery to T cells and dissemination to secondary lymphoid organs.40, 41 Whether dendritic cells are involved in the pathogenesis of HIV-1 infection in the human small intestine is unclear because we have been unable to identify dendritic cells in human small intestinal lamina propria.42, 43 In these studies, lamina propria mononuclear cells isolated from normal jejunum did not express dendritic cell markers, including CD11b, CD11c, CD21, CD34, CD83, or CD123, or display ultrastructural characteristics of dendritic cells. In addition, treatment of purified lamina propria macrophages with optimal concentrations of glomerulocyte-macrophage colony–stimulating factor, tumor necrosis factor α, and interleukin 4 did not induce dendritic cells. However, cells thought to be dendritic cells have been identified morphologically in colon lamina propria (<1% of mononuclear cells)44; newly available dendritic cell–specific antibodies will facilitate confirmation of this observation. Whether dendritic cells in the colon participate in HIV-1 entry and pathogenesis awaits further study.
Epithelial cells are the most abundant cell type lining the mucosa of the intestine and colon and are a potentially important conduit for HIV-1 entry. A recent study of the mucosal translocation of HIV-1 using epithelial cell lines showed that the virus could be translocated across a tight epithelial cell monolayer by a transcytotic pathway.45 However, the phenotype of epithelial tumor cell lines differs from that of primary intestinal epithelial cells. Therefore, using a modification of the technique that we developed to isolate and purify human intestinal macrophages and lymphocytes,46 we purified human intestinal epithelial cells and studied their HIV-1 receptor and coreceptor expression.47 Primary intestinal epithelial cells expressed galactosyl ceramide (GalCer), an alternative primary receptor for HIV-1 on neural48 and epithelial lines.45, 49 In addition, the primary intestinal epithelial cells expressed CCR5, the coreceptor for macrophage-tropic HIV-1 isolates (referred to as R5 viruses because they use CCR5 for cell entry).47 In contrast to their expression of CCR5, primary intestinal epithelial cells did not express CXCR4, the chemokine coreceptor for lymphocyte-tropic HIV-1 isolates (referred to as X4 viruses because they use CXCR4 for entry), or CD4, the primary receptor for HIV-1 on mononuclear cells. This GalCer+CCR5+CXCR4− phenotype distinguishes primary intestinal epithelial cells from epithelial cell lines and indicates that cells equipped for the selection and uptake of R5 HIV-1 are present in the upper gastrointestinal tract mucosa. The phenotype of purified rectal epithelial cells has not yet been reported, although immunohistochemical staining of colon biopsy specimens suggests that CXCR4 may be present on rectal epithelium,50, 51 indicating that intestinal epithelium may differ from colonic epithelium in coreceptor expression.
Selective transfer of R5 HIV-1 by intestinal epithelial cells
HIV-1 isolated from acutely infected persons is predominantly R5.52, 53, 54, 55, 56 Over time, mutations lead to the emergence of X4 viruses, resulting in the presence of a mixture of R5 and X4 viruses in most chronically infected persons. Because most HIV-1 infections are acquired across a mucosal surface, mucosal cells could conceivably select the R5 viruses that are initially acquired by the recipient from the mixture of HIV-1 viruses inoculated onto the mucosa by a chronically infected donor. Having shown that intestinal epithelial cells express CCR5, but not CXCR4, we investigated whether the cells could selectively transfer R5 HIV-1 to target cells. We found that intestinal epithelial cells selected and transferred R5, but not X4, HIV-1 to CCR5+ target cells, which replicated and amplified virus expression.47 In addition, our studies supported Bomsel's observation in cell lines that HIV-1 transfer was accomplished by microtubule-dependent transcytosis. Our finding that CCR5+ intestinal epithelial cells select and transfer exclusively R5 viruses offers a mechanism for the selective transmission of R5 HIV-1 in primary infection acquired through the upper gastrointestinal tract (Figure 2).
Once HIV-1 has crossed the epithelium and entered the lamina propria, the virus encounters the largest reservoir of macrophages and lymphocytes in the body. In studies of the biologic parameters of HIV-1 infection of these cells, we showed that lamina propria macrophages surprisingly do not express CCR5 and are not permissive to R5 HIV-1 in vitro.42, 43, 57, 58 This observation may explain the very low prevalence of HIV-1–infected macrophages in the upper gastrointestinal tract mucosa in vivo59 and indicates that intestinal macrophages probably do not participate in the selection of R5 viruses in primary infection. Intestinal lymphocytes, on the other hand, express both CCR5 and CXCR4 and support replication by both R5 and X4 HIV-1 in vitro,43 indicating that intestinal lymphocytes also probably do not participate in the selective acquisition of R5 viruses during primary infection (Figure 2). Thus, epithelial cells, rather than resident macrophages or lymphocytes, appear to be the mucosal cell capable of selecting R5 HIV-1 in primary infection.
Fig. 2.
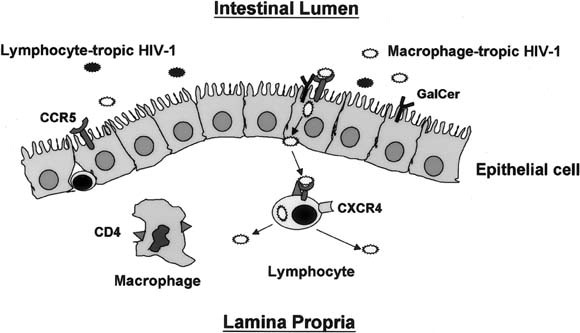
Schematic representation of HIV-1 translocation across the intestinal epithelium. Although both lymphocyte-tropic and macrophage-tropic HIV-1 are inoculated onto the mucosa, CCR5-mediated endocytosis, possibly in association with GalCer, initiates the transcytosis of macrophage-tropic virus from the apical surface of the epithelial cell to the basal surface. Released macrophage-tropic HIV-1 then infects CCR5+ lamina propria lymphocytes but not HIV-1. During early infection, virus replicates predominantly, if not exclusively, in lamina propria lymphocytes.
Local HIV-1 replication and depletion of lamina propria CD4+ T Cells
A series of insightful in vivo studies of HIV-1 infection in humans60, 61 and SIV infection in rhesus macaques62, 63 have sought to dissect the early events in mucosal HIV-1 infection. Consistent with our finding that human intestinal lymphocytes, not macrophages, are a target cell for HIV-1 infection in vitro, Veazey et al.62 showed that during the first few days of SIV infection of macaques, infected macrophages were rare, and more virus-infected T cells were present in the intestinal mucosa than in the blood. Because the virus replicates more efficiently in activated T cells, the greater abundance of activated CD4+ T cells in the lamina propria than in the blood64, 65 probably accounts for the initial higher frequency of virus-infected lymphocytes in the gastrointestinal mucosa than in the blood.
Coincident with the early local accumulation of SIV-infected lymphocytes is the presence of a high viral load in the intestinal mucosa.63 Importantly, the early and high viral load in the intestinal mucosa in SIV-infected macaques is associated with villous atrophy and malabsorption, consistent with SIV-induced enteropathy.63 However, after 7–14 days of infection, CD4+ T cells in the intestinal and colonic mucosa became rapidly and profoundly depleted, probably as a consequence of cell lysis and apoptosis. This profound depletion of mucosal CD4+ T cells, occurring predominantly in the lamina propria, preceeds the depletion of such cells in the blood in both humans and macaques.60, 61, 62, 63
Sequence of mucosal events in early HIV-1 infection
Taken together, the studies discussed above suggest the following sequence of events in the intestinal mucosa during early HIV-1 infection acquired by vertical transmission or homosexual contact. A mixture of R5 and X4 viruses, typically present in the blood of chronically infected donors, are released into the milk or semen and then enter the recipient through inoculation into the oral cavity. In the oral cavity, virus that reaches the tonsils encounters M cells in the overlying epithelium, and these cells deliver the virus to the dendritic cell–rich lymphoid tissue of the tonsils. Although dendritic cells alone do not support HIV-1 replication, dendritic cell–T cell conjugates support high levels of viral replication. Dendritic cells, which normally migrate through the lymphatics to secondary lymphoid organs, contain a C-type lectin that traps virus and thus may participate in the dissemination of HIV-1 from the gastrointestinal tract to lymphoid tissues.
In the oral cavity, virus mixes with saliva, which contains antiviral factors, including secretory leukocyte protease, a potent inhibitor of HIV-1 activity.66, 67 These factors may prevent local (tonsilar) infection, allowing swallowed virus to pass through the oral cavity. Passage through the stomach would be facilitated by the absence of gastric acid production in the fetus and neonate. In the adult, gastric acid could limit the amount of viable virus that reaches the intestine, but this issue requires further study. Thus, a mixture of inoculated viruses reaches the recipient's upper gastrointestinal tract mucosa. There, GalCer+CCR5+ CXCR4− epithelial cells bind and then selectively transfer R5 viruses from the cells' apical to basolateral surface, probably by microtubule-dependent, nondegradative transcytosis. Virus translocated across the epithelium encounters lamina propria macrophages and T cells, but only the lymphocytes express CCR5, the coreceptor that mediates R5 HIV-1 entry. Because the lamina propria contains the largest reservoir of activated T cells in the body and because HIV-1 replicates most efficiently in activated T cells, the lamina propria becomes the initial major site of HIV-1 replication and amplification. Virus that infects activated T cells in the organized lymphoid structures, possibly via M cell uptake and transport, could then disseminate HIV-1 to distant mucosal sites through normal receptor-mediated homing. Soon, HIV-1–induced T cell death by cell lysis, apoptosis, and cytotoxic lymphocyte-induced killing, leads to rapid depletion of lamina propria CD4+ T cells long before the decrease in circulating CD4+ T cells.
Eventually, local immunosuppression induced by T cell depletion predisposes the gastrointestinal tract mucosa to an array of opportunistic infections, leading to mucosal inflammation and the release of chemoattractant peptides. These peptides recruit to the mucosa CCR5+ and CXCR4+ monocytes and lymphocytes, which serve as new target cells for the perpetuation of local HIV-1 replication. As a consequence of this inflammation or possibly local HIV infection, mucosal function is compromised and malabsorption ensues, even before opportunistic infections induce clinical symptoms. Thus, besides serving as a site of opportunistic infections in late-stage HIV-1 disease, the gastrointestinal tract also plays a fundamental role in early HIV-1 disease by serving as the site for virus entry, the initial site of virus replication and amplification, and the initial site of CD4+ T cell depletion. As Veazey et al.62 aptly suggested, the gastrointestinal tract mucosa, not the peripheral lymphoid tissue, is the initial tissue site of HIV-1 disease.
Sequelae of mucosal HIV-1 infection
After these initial events and as a consequence of such extensive infection of intestinal T cells, HIV-1–infected patients show a rapid, dramatic, and persistent decrease in the numbers of CD4+ cells in the lamina propria, which precedes that in peripheral sites.61, 68, 69, 70 These immunologic insults to the mucosae are associated with a range of physiologic and morphologic perturbations that lead to remarkably increased rates of oral, esophageal, and gastrointestinal symptoms.71, 72, 73, 74, 75, 76, 77 Rates of secondary opportunistic infections correlate with the number of peripheral CD4+ T cells and presumably with those in the mucosa.78, 79, 80 Prominent among these syndromes is chronic diarrhea.
Chronic diarrhea affects most HIV-1–infected adults in resource-poor nations and, until relatively recently, 25%–50% of those in the United States and Western Europe.72, 81, 82, 83, 84 A number of excellent primary investigations and reviews have revealed that with a sequential and comprehensive approach, a specific organism can be identified in 50%–85% of HIV-1–infected patients with diarrhea, and most of these infections can be effectively treated or suppressed (Table 2).72, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98
Table 2.
Potential causes of diarrhea in HIV-1–infected patients
| Noninfectious | Antiretroviral therapya | ||
| Other medications | |||
| Malignancies (intestinal lymphoma,b Kaposi's sarcoma) | |||
| Idiopathic (“AIDS enteropathy”)a | |||
| Inflammatory bowel disease | |||
| Infectious | Viral | Bacterial | Parasitic |
| Cytomegalovirusa,b,c | Salmonellae spp.b | Cryptosporidium parvuma,b,c | |
| HIV-1 (especially acute infection)b,c | Campylobacter spp.b | Isospora bellib | |
| Adenovirus | Shigella spp.b | Microsporidia | |
| Enterocytozoon bieneusic | |||
| Septata intestinalisb | |||
| Herpes simplex virusb | Clostridium difficileb | Giardia lambliab | |
| Listeria monocytogenesb | Cyclospora cayetanensisb | ||
| Rotavirus, calcivirus, astrovirus, coronavirus, picobirnavirus | Enteroaggregative E. colib | Entamoeba histolyticab | |
| Mycobacterium avium complexb,c | Blastocystis hominisb | ||
| Aeromonas spp.b | Other parasites | ||
| Fungal | |||
| Histoplasma capsulatumb | |||
| aRelatively common. bTreatment available. cDocumented resolution or decreased incidence of infection in the presence of HAART. | |||
The diagnostic algorithms and specifics of therapy are now conveniently outlined.99, 100, 101 However, perhaps the most effective approach to prevention and treatment of mucosal infections and many opportunistic infections among patients with HIV-1 disease has been the recent introduction of HAART.
Role of antiretroviral therapy
HAART
Results of therapy with the first antiretroviral agent available, zidovudine (ZDV; AZT), showed in 1987 for the first time that chemotherapy could retard the progression of HIV-1 disease and lower the incidence of opportunistic infections and HIV-1–associated death.102 Subsequent analyses of these and other results indicate that the duration of single-agent antiviral activity was limited because of the development of resistance and inadequate control of viral replication. Thus, similar to successful control of tuberculosis, multiple antiretroviral agents were developed to suppress HIV-1 replication over long periods, decrease HIV-1 disease progression, and prevent or reverse HIV-1–induced immunologic incompetence.103, 104, 105, 106, 107 This powerful “cocktail” of agents improves the natural history of HIV-1 infection by combining inhibitors of HIV-1 reverse transcriptase and protease to provide HAART.
Reverse-transcriptase inhibitors (RTIs), both nucleoside analogues (NRTIs) such as ZDV and nonnucleoside analogues (NNRTIs), primarily inhibit the ability of HIV-1 RNA to encode viral DNA, which can integrate into the host genome. After attachment of HIV-1 to the cell membrane, release from its envelope, and entry into the cytoplasm, the retroviral RNA generates a double-stranded DNA provirus through reverse transcriptase. This proviral DNA can then enter the nucleus and integrate into the host cell genomic DNA, and viral replication is initiated, particularly in activated cells. However, during prolonged RTI therapy, site-specific mutations in the pol gene, which encodes reverse transcriptase, confer progressive resistance to RTI therapy, necessitating the use of additional agents to limit viral replication and the development of resistance. Protease inhibitors (PIs), a second major class of antiretroviral agents, induce dose-dependent inhibition of HIV-1 aspartic protease, which is required for production of mature infectious virions.99, 108 By blocking the cleavage of HIV-1 Gag-Pol polyprotein precursor into requisite structural core proteins and enzymes (reverse transcriptase, integrase, and protease), PIs cause the production of abnormal viral proteins and noninfectious virions. However, the development of selective mutations in the protease during therapy leads to resistance, and resistance to one PI affects the susceptibility of HIV-1 to other PIs.99, 108 Thus, virtually all current HAART regimens include 3 or more agents (typically a PI and 2 NRTIs) to diminish or ablate viral replication (reflected in a reduction in viral load), prevent the development of resistance, enhance recovery of immunologic competence (resulting in an increase in CD4+ T cell number), and improve clinical status (based on CDC stage).99, 104, 108, 109, 110 Clinically, the goals of therapy are to prevent HIV-1–associated illness and prolong survival.
Impact on mortality
HAART has dramatically reduced the death rate among patients with HIV-1 infection from the period before its widespread use (before 1996) to the present. Among US patients with advanced HIV-1 disease (CD4+ T cell count < 100 cells/μL; normal, ≈750–1200 cells/μL), mortality decreased between 1995 and 1997 from 29.4 to 8.8 per 100 person-years.107 Concomitantly, the incidence of the three most common HIV-1–associated opportunistic infections (Pneumocystis carinii pneumonia, MAC disease, and cytomegalovirus {CMV} retinitis) decreased by more than 75% (Figure 3A and B).This decrease was proportional to the intensity of antiretroviral therapy and was most prominent among persons on combination and PI-containing regimens. Similar results have been reported from Western Europe between 1994 and 1998 for AIDS-defining illnesses (Figure 3B) and for these and other infections in the United States.29, 78, 106, 111 These decreased rates were significant for a number of complications involving the alimentary tract, including esophageal candidiasis, MAC, CMV, cryptosporidiosis, and herpes simplex virus, as well as Kaposi's sarcoma and non-Hodgkin's lymphoma. Thus, combination antiretroviral therapy, particularly HAART, has had a significant impact on the natural history of HIV-1 infection and has substantially reduced the incidence of HIV-1–related infectious and neoplastic diseases, including those of the gastrointestinal tract, as well as death.
Fig. 3.
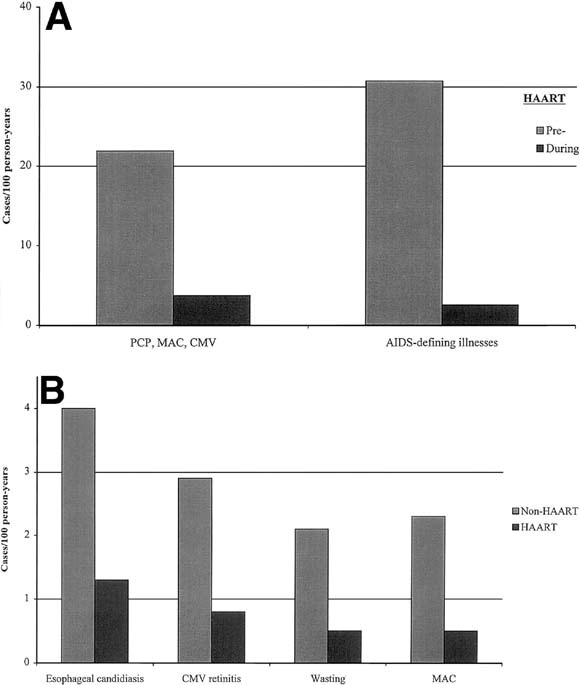
Effect of HAART on rates of major opportunistic infections in the populations. The pre-HAART and post-HAART dates are (A) 1995 and 1997, respectively,107 and (B) 1994 and 1998, respectively.106
The dramatic effects of HAART on mortality and HIV-1–related infections are most likely caused by the inhibition of HIV-1 replication and the consequent recovery of immune function because most antiretroviral agents have little activity against other viruses or pathogens. Decreases in mortality and rates of infection before the introduction of PI and HAART were associated with earlier recognition of HIV-1 infection and treatment with ZDV, more rapid diagnosis and treatment of specific opportunistic infections, and prophylactic therapy with trimethoprim-sulfamethoxazole (TMP-SMX) for Pneumocystis carinii pneumonia (and toxoplasmosis). The widespread introduction of ZDV and TMP-SMX, each of which has activity against gram-negative bacteria, also was associated with a dramatic decrease in HIV-1–associated non-typhi Salmonellae infections in the United States.112, 113 Before this time, Salmonellae species were common causes of enteritis and bacteremia in these patients,114, 115, 116, 117, 118, 119 as they remain in African patients with HIV-1 disease today. Other than the activity of the PI aprenavir against Giardia lamblia 99 and of adefovir dipivoxil against DNA viruses (herpes simplex virus, CMV, and hepatitis B virus), most other antiretroviral agents have shown little activity against viral, bacterial, protozoan, or fungal pathogens. Thus, recovery of immune function probably underlies much of the clinical benefit that accompanies viral suppression with antiretroviral therapy.
Substantial immunologic benefits result from reductions in the levels of HIV-1 in both blood and lymphoid tissue following HAART. These benefits include increases in the number of CD4+ T cells, decreases in T and B cell activation with subsequent enhancement of specific proliferative responses, and restoration of the functional architecture of lymph nodes.120, 121, 122, 123 However, functional immunologic recovery occurs over many months. Consequently, rates of HIV-1–related infections decrease most prominently after 2 months of potent antiretroviral therapy.124 Thus, data supporting recommendations to discontinue prophylactic therapy against opportunistic infections after initiating HAART confirm the safety of this recommendation in patients whose CD4+ T cell levels have increased to 200 cells/μL (or 14% of total lymphocytes) for more than 12 weeks.125, 126, 127, 128, 129
HAART has 3 potential effects on the natural history of HIV-1–associated infections, including enteric infections. First, effective control of HIV-1 results in a decreased incidence of secondary infections and decreased rates of reactivation of latent infections. Second, an indirect benefit of the decreased rates of such infections in patients receiving HAART is the discontinuation of long-time prophylactic antimicrobial therapy, leading to a decrease in the rate of antibiotic-associated enteric infections with Clostridium difficile,130, 131 as well as lower rates of drug reactions. A third consequence of HAART is the resolution of ongoing infections, probably because of repopulation of the peripheral T cells. Recent reports indicate that as the viral load decreases in the intestinal mucosa, CD4+ T cells repopulate the mucosa,132, 133 and that on HAART this repopulation occurs more rapidly in the intestinal mucosa than in the blood.134
Earlier reports have suggested that heretofore untreatable or inadequately treatable enteric infections, such as Cryptosporidium, could improve clinically and microbiologically with ZDV.80, 135, 136 However, the natural history of cryptosporidiosis is variable.80, 137, 138, 139 More recently, a case-control study showed that the use of antiretroviral therapy was associated with significant, but not absolute, protection against infection with Cryptosporidium.137 In an open randomized trial among patients with previously diagnosed infections and persistent diarrhea, symptoms resolved and pathogens were undetectable in stools from 3 of 6 patients with microsporidia (Enterocytozoon bieneusi) and 2 patients with concomitant Cryptosporidium 140 after addition of the PI indinavir to NRTI.140 Similarly, among 9 patients with chronic diarrhea associated with microsporidiosis (n = 5), cryptosporidiosis (n = 3), or both (n = 1), in 8 of whom antiparasitic therapy had failed, HAART induced complete resolution of symptoms and weight gain after a median of 6 weeks of therapy.141 Organisms were not detected in the 8 patients evaluated, and marked histologic improvement was observed in those in whom biopsies were performed. However, diarrhea recurred in 4 patients at a median of 11 months (range, 7–13 months) when the patients failed to respond to antiretroviral therapy (increase in plasma HIV-1 load and decrease in CD4+ T cell number). In an HIV-1–infected patient evaluated over time, persistent Cryptosporidium parvum infection resolved with HAART in association with decreases in viral RNA and dramatic and sustained increases in CD4+ T cell number in blood and intestinal mucosa.134
Given these promising results, it can be speculated that HAART will also improve one of the most difficult and challenging complications of HIV-1 infection, chronic diarrhea of unknown etiology, often referred to “AIDS enteropathy.” As awareness of the potential pathogens has increased among clinicians and pathologists and as diagnostic techniques have become more sophisticated and sensitive (including stains, diagnostic antibodies, cultures, electron microscopy, and polymerase chain reaction), the number of patients with this complication has decreased.91, 92, 99 Microsporidiosis, viral infections (including cytomegalovirus and adenovirus), and intestinal malignancies (Kaposi's sarcoma and non-Hodgkin's small bowel lymphoma) assume increasing importance among patients with AIDS enteropathy. Nevertheless, 10% to 50% of HIV-1–infected patients have diarrhea without an identifiable pathogen, and some potential pathogens are not treatable.91, 92, 96 Thus, the report that HAART induced sustained clinical improvement in 8 such patients with chronic diarrhea of unknown etiology is extremely encouraging140 for patients and frustrated physicians.
Hepatitis C
Hepatitis C virus is the leading cause of chronic hepatitis among HIV-1–infected patients in the United States. Intravenous drug users and patients with hemophilia make up the majority of these coinfected patients; 40% of HCV-infected hemophiliac men are coinfected with HIV-1.142 The natural history of patients with dual infections differs from that in HIV-1–seronegative adults. Compared with patients with HCV alone, those with concomitant HIV-1 infection have higher HCV RNA levels in blood and liver tissue, more rapid progression of liver disease, and possibly increased rates of death related to hepatic disease.143, 144, 145, 146, 147 HCV viral loads are typically increased with advancing immunosuppression and thus are inversely proportional to CD4+ T cell number.143, 146, 147 Despite the accelerated course of HCV replication and associated liver disease, coinfection with HCV does not usually appear to promote progression of HIV-1 disease or to increase mortality.148 However, an increased risk of death and new AIDS-defining illnesses, as well as delayed recovery of CD4+ T cells with antiretroviral therapy, were recently reported among HIV-1–infected Swiss patients with HCV.149 These increased risks were primarily associated with intravenous drug use and unrelated to more progressive liver disease.
Responses of HCV to antiviral therapy with interferon α (IFN-α) among patients with HIV-1 coinfection appear to be similar to those among HIV-1–seronegative subjects; approximately a quarter of patients have sustained virologic responses 12 months after they discontinue therapy.150, 151, 152 Most responders had high CD4+ T cell counts (>500 cells/μL). Preliminary data suggest that the effects of IFN-α therapy with ribavirin may be greater than with IFN-α alone among both HIV-1–infected and –seronegative patients,153, 154 although anemia is an important complication. Another option for therapy in this group is HAART. Among HIV-1–infected patients with hepatitis B, initiation of potent antiretroviral therapy may lead to resolution of chronic infection with the development of antibodies to the hepatitis B surface antigen. In several series that monitored HCV status in patients before and during HAART, levels of plasma HCV and transaminases showed transient and clinically inapparent increases in HCV (0%–90%) and transaminases (0%–33%).142, 155, 156, 157, 158, 159 However, HCV RNA decreased to below the limits of detection in a small minority, as is observed in up to 15% of otherwise healthy patients after initial infection. Although both severe and clinically inapparent hepatitis can occur in patients upon initiation of HAART,160, 161, 162, 163 the presence of coinfection with HCV and HIV-1 should not serve as a primary determinant of the decision to begin effective antiretroviral therapy. The relatively infrequent “immune restoration” syndrome, in which deleterious inflammatory responses or even reactivation of infections (e.g., CMV and MAC) follow HAART,164, 165, 166 does not appear to affect HCV infections to any consistent degree. In patients with HIV-1 disease, efforts to limit the impact of HCV infection should include limiting alcohol use, administering hepatitis A vaccine if the patient is seronegative, monitoring hepatic transaminase levels, and considering specific therapy for HCV to eradicate infection or limit progressive liver injury in those with significant hepatic pathology.
Complications of HAART
Despite the remarkable ability of HAART and other measures to dramatically decrease the level of HIV-1 in plasma and the rates of opportunistic infections, antiretroviral therapy has not been sufficient to eradicate HIV-1 infection in any patient. Moreover, undetectable HIV-1 RNA levels are achieved in only about a third to half of patients on HAART in nonstudy settings.167, 168 Impaired access to care, particularly among blacks, Latinos, the uninsured or underinsured, women, and certain risk groups,169 also reduces the impact of HAART in the community. In addition, the high cost and the inability of some patients to tolerate 1–3 dozen pills per day, even among those with access to regular skilled care, significantly affects the response to HAART.
Gastroenterologists may not routinely serve as the primary care physician for patients with HIV-1 infection, but they are often consulted to evaluate gastrointestinal symptoms in patients receiving HAART. Consequently, they should be aware of the toxicities associated with antiretroviral and support drugs (Table 3).In general, gastrointestinal symptoms, such as nausea, vomiting, abdominal pain, and diarrhea, often occur early in the course of therapy and may abate over time. Gastrointestinal toxicity significantly limits the initial tolerance to protease inhibitors, often requiring dose escalation and sequential addition of other medications, such as nucleoside analogue RTI. Hepatic toxicity is more often associated with long-term therapy and is typically mild, although severe hepatic toxicity, particularly with the protease inhibitor ritonavir, may require discontinuation of the medication.162 Pancreatitis is a more specific complication of individual drugs. As in other immunocompromised hosts treated with multiple medications, such as organ and marrow transplant patients, rates of toxic reactions ascribed to each drug may be confounded by concomitant administration of 2 or more drugs. Increased rates of adverse events, such as pancreatitis or hepatic toxicity, also may result from simultaneous administration of medications with overlapping toxicities. Moreover, differentiating the symptoms of drug toxicities from those of opportunistic infections may require careful evaluation of the timing of events and presence of comorbid findings.
Primary toxicities
Adverse effects of antiretroviral therapy may severely compromise effective patient care, particularly the ability to consistently reduce HIV-1 viral load, because of poor adherence with drug regimens. Such events may be more common among patients with advanced disease (CD4+ T cell <200/μL).170 Rates of specific intestinal complications for each agent are summarized in Table 3. Among patients taking NRTI, gastrointestinal symptoms, including nausea, vomiting, abdominal pain, and diarrhea, occur in more than a quarter of patients. The inhibition of cellular DNA polymerases, including mitochondrial polymerase γ, with long-term therapy may result in mitochondrial toxicity and associated myopathic, neuropathic, hepatic, pancreatic, and hematologic complications.171, 172, 173 Among those taking NNRTI, gastrointestinal and hepatic toxicities are not common. Mild rashes are the predominant side effect. Drug interactions with NNRTIs are related primarily to their effects as inducers of and substrates for the cytochrome P450 system. The most frequent dose-limiting toxicities with PIs are diarrhea, nausea, and abdominal discomfort. Hepatic toxicity is most often associated with ritonavir. The presence of chronic hepatitis B or hepatitis C may increase the rate but not necessarily the severity of hepatic effects162; these infections should not limit the initiation of HAART.
Table 3.
Gastrointestinal complications of antiretroviral therapy and potential interactions with other medications used in gastrointestinal medicine
| Type of Medication | Medication | Trade Name | Primary GI Toxicitya | GI-associated Drug Interactions |
|---|---|---|---|---|
| Antiretroviral NRTI | Zidovudine (AZT, ZDV) | Retrovir | Anorexia; nausea (4%–26%); vomiting (3%–8%)(rare hepatitis, steatosis, lactic acidosis) | Activity may be inhibited by ribavirin |
| Didanosine (ddI) | Videx | Pancreatitis (4%–8%); diarrhea (16%); ↑ALT/AST(6%–20%) | Potential increased rate of pancreatitis with pentamidine, azathioprine); buffers associated with ↓absorption of itraconazole, ketoconazole, dapsone; tetracycline, ciprofloxacin; ddI levels ↑with rantidine, ganciclovir | |
| Zalcitabine (ddC) | HIVID | Stomatitis (self-limited) (2%–17%); pancreatitis (0.5%–9%) | Few; possible ↑neuropathy with metronidazole, disulfuram | |
| Stavudine (d4T) | Zerit | Diarrhea (33%), nausea/vomiting (26%), abdominal pain (23%); ↑ALT/AST (65%); significant GI symptoms and hepatic abnormalities in 4%–10% | Few; possible ↑ neuropathy with ethanol; ribavirin may increase activity (in vitro data) | |
| Lamivudine (3TC) | Epivir | Limited; mild and transient diarrhea, nausea, abdominal pain | Few | |
| Abacavir (ABC) | Ziagen | Nausea (45%), diarrhea (25%), vomiting (15%) and abdominal pain (15%) may decrease over weeks of therapy; hypersensitivity reaction with fever ± rash, malaise, nausea, vomiting, ↑ALT/AST in 2%–5% within 1 month | None reported | |
| Nucleotide analog RTI | Adefovir dipivoxil | Preveon | Usually mild nausea, diarrhea, occasional vomiting(1%–8%); ↑ALT/AST (4%); (renal toxicity 38%) | None reported |
| ZDV-3TC | Combivir | As above | ||
| NNRTI | Nevirapine (NVP) | Viramune | Nausea; ↑ALT/AST (1%); isolated ↑GGT common; (rash most common) | |
| Delavirdine (DLV) | Rescriptor | Nausea (7%); diarrhea (4%); ↑ALT/AST (≥5%);(rash most common; usually transient) | Level ↓with rifampin, rifabutin (P-450 system) and antacids (↑gastric pH); level ↑with clarithromycin, rifabutin, cisapride | |
| Efavirenz (EFV) | Sustiva | (Transient rash and CNS symptoms of headache, dizziness, impaired concentration) | Avoid cisapride; should not be taken within 2 hours of antacids; not recommended with clarithromycin; (interacts with other PI) | |
| PIb | Saquinivir (SQC); Saquinavir-SGC (soft gel capsule) | Invirase; Fortovase | Nausea, diarrhea, abdominal pain, dyspepsia (5%–10%) | Cisapride contraindicated; SQV level ↑ with ketoconazole, clarithromycin; SQV level ↓ with rifampin, rifabutin; Clarithromycin level ↑ with SQV; (Interacts with other PI) |
| Ritonavir (RTV) | Norvir | Nausea, diarrhea, vomiting, anorexia, abdominal pain (20%–40%), especially in first few weeks of therapy; taste perversion (10%); increased triglycercides (60%, but >1500 mg/dL in 2%–8%); ↑ALT/AST (10%–15%), especially with NRTI | Cisapride contraindicated; caution with dronabinol, ondansetron, cimetidine, promethazine, corticosteroids; RTV level ↑ with ketoconazole, itraconazole; RTV level ↓ with rifampin; clarithromycin, rifampin; clarithromycin, erythromycin, rifampin, rifabutin levels increased with RTV;(interacts with other PI) | |
| Indinavir (IDV) | Crixivan | Nausea, vomiting, diarrhea, abdominal pain (4%–15%); increased indirect bilirubin (10%); (nephrolithiasis 5–10%) | Cisapride contraindicated; IDV level ↓ with rifabutin; IDV level ↑ with ketoconazole; rifabutin level ↑ with IDV; (interacts with other PI) | |
| Nelfinavir (NLF) | Viracept | Diarrhea (usually mild) (2%–19%) | Cisapride and rifabutin contraindicated; NLF levels ↓ with rifampin; rifabutin levels ↑ with NLF;(interacts with other PI) | |
| Amprenavir | Agenerase | Diarrhea, nausea, vomiting (7%–33%); rash (18%) | Cisapride and rifampin contraindicated; amprenavir levels ↓ with rifabutin; rifabutin levels ↑ with amprenavir; (interacts with other PI) | |
| ABT 378 (Lopinavir-Ritonavir) | Kaletra | Diarrhea (10%–20%); ↑ ALT/AST (8%) | Similar to other PI | |
| Other | Interleukin 2 | Proleukin | Diarrhea, abdominal pain, stomatitis (7%); nausea; vomiting; isolated ↑ bilirubin (8%); ↑ ALT/AST; acalculous cholecystitis; constitutional flu-like symptoms (fever, chills, muscle/joint pain) (45%) | |
| Hydroxyurea | Hydrea | Nausea (12%); ↑ ALT/AST (2%); stomatitis (8%); diarrhea; occasional anorexia, vomiting, diarrhea, constipation (myelosuppression; rash) | ||
| Alternative | Vitamin C | |||
| Allicin (garlic extract) Malaleuca (tea tree extract) N-acetylcysteine (mucomyst) | ||||
| aRates may vary among studies. bAs with other inhibitors of cytochrome p450 system, specifically CYP 3A4, protease inhibitors should not be given with cisapride (Propulsid), astemizole (Hismanal), terfenadine (Seldane), midazolam (Versed), triazolam (Halcion), or ergot derivatives. | ||||
ALT/AST, alanine/aspartate transaminase elevation >2.5–5 fold above normal values; GGT, γ-glutamyl transpeptidase; GI, gastrointestinal; CNS, central nervous system. Symptoms listed in parenthesis represent nongastrointestinal but prominent symptoms.
Recent attention has focused on a number of metabolic complications, particularly with any PI-containing regimen and, less often, with NRTIs. These abnormalities include (1) glucose intolerance, (2) elevations in triglyceride and cholesterol levels, and (3) an enigmatic and disturbing complication referred to as “lipodystrophy syndrome.” Patients with this last syndrome have increased abdominal fat; loss of peripheral subcutaneous fat, including that in the face, arms, legs, and buttocks; and the presence or absence of a “buffalo hump.” Women on therapy may experience breast enlargement. These conditions may occur concomitantly or separately. Rates of observed fat redistribution are 17%–68% with PIs and 16% with dual NRTIs.141, 171, 174 Mitochondrial dysfunction has been invoked as one potential unifying mechanism underlying these syndromes in patients taking PI-containing combination therapy.171, 173 The first 2 conditions may be treated with standard medical management, but whether discontinuation of medications will reverse the potentially disfiguring effects of the lipodystrophy syndrome is currently unclear.
Management of the adverse events with antiretroviral therapy may be complicated by the difficulties of identifying a specific inciting agent in a multidrug regimen. Because many of the toxic effects of antiretroviral medications may be worse early in the course of treatment and are dose dependent, lowering the dose in this period may allow therapy to continue. However, optimal antiretroviral activity is usually dose dependent, particularly with PIs, and development of resistance of HIV-1 is a very real danger, so monitoring of both clinical and virologic status is important. Changing antiretroviral agents may be preferable to lowering doses. Evaluation of the overlapping toxicities of different medications and their effects on drug metabolism may allow successful continuation of the most critical agents. In this regard, patients should be asked about other unrecorded or nontraditional agents they may be taking. Severe or prolonged complications require discontinuation of individual agents, as does the development of hypersensitivity reactions with abacavir. Gastrointestinal symptoms are common and may be quite difficult to resolve. Antidiarrheal agents, such as loperamide or diphenoxylate atropine, may be useful for drug-related diarrhea, as may antiemetics for vomiting. Taking medications with or after eating, when appropriate, may limit nausea. Histamine receptor blockers and antacids, although commonly prescribed, may affect absorption of some drugs and are usually ineffective.
Drug interactions
The majority of observed and proposed drug interactions associated with PI is related to its high affinity for, and thus inhibition of, several -450 isoenzymes. In addition to the medications shown in Table 3, a more complete list and discussion of drug interactions with PI is available from the manufacturer and in articles by Dolan et al.99 and Burger et al.175 Cisapride has potential life-threatening cardiotoxic effects in the presence of protease inhibitors, azole antifungals, and macrolides because of the ability of these agents to inhibit the hepatic metabolism of cisapride. Clinically significant interactions of PIs with rifampin/rifabutin, macrolides (erythromycin, clarithromycin, azithromycin), and azole antifungals (ketoconazole, fluconazole, and itraconazole) are highlighted in Table 3. The chelating effects of didanosine, antacids, iron and calcium products, and sucralfate limit the absorption and levels of quinolone antibiotics, which are most commonly used for empiric and directed treatment of acute bacterial diarrhea.
In summary, the clinical care of HIV-1–infected patients has improved dramatically over the last decade because of greater understanding of the pathogenesis of the virus, more rapid clinical diagnosis of HIV-1 disease and its complications, and the availability of potent antimicrobial and antiretroviral agents to resolve and prevent opportunistic infections. Each advance brings new hope as well as new challenges, such as providing affordable therapy to all who need it and managing immunologic and clinical complications of HAART, particularly its enteric toxicity. The greatest challenge is to use newly gained insights into the pathogenesis of primary HIV-1 infection at mucosal sites to provide protection against the first exposure to the virus and against the subsequent irreversible decline in mucosal defense mechanisms.
Footnotes
Address requests for reprints to: Phillip D. Smith, M.D., Division of Gastroenterology, University of Alabama at Birmingham, 703 19th Street S., ZRB 633, Birmingham, Alabama 35294-0007. Fax: (205) 934-8493.
Funded in part by the Veterans Affairs Research Service, the Center for Mucosal and Vaccine Biology, Minneapolis Veterans Affairs Medical Center, and National Institutes of Health grants AI-39445, DE-72621, DK-47322, HL-57880, and AI-41530.
References
- 1.Gao F, Bailes E, Robertson DL. Origin of HIV-1 in the chimpanzee Pan troglodytes. Nature. 1999;397:436–441. doi: 10.1038/17130. [DOI] [PubMed] [Google Scholar]
- 2.UNAIDS and WHO . UNAIDS/WHO; Geneva: 1998. Report on the global HIV/AIDS epidemic–June 1998; pp. 48–50. [Google Scholar]
- 3.Royce RA, Sena A, Cates W, Jr, Cohen MS. Sexual transmission of HIV. N Engl J Med. 1997;336:1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 4.Smith PD, Wahl SM. Immunobiology of mucosal HIV-1 infection. In: Ogra PH, Mestecky J, Lamm ME, Strober W, Biennenstock J, McGhee JR, editors. 2nd ed. Academic; San Diego: 1999. (Mucosal immunology). [Google Scholar]
- 5.Newell M-L. Mechanisms and timing of mother-to-child transmission of HIV-1. AIDS. 1998;12:831–837. doi: 10.1097/00002030-199808000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Maayan S, Soskoine V, Engelhard D, Moses A, Rahav G, Raveh D. Sexual behavior of homosexual and bisexual man attending an HIV testing clinic in Jerusalem. Isr J Psychiatry Rel Sci. 1993;30:150–154. [PubMed] [Google Scholar]
- 7.Schwarcz SK, Kellogg TA, Kohn RP, Katz MH, Lemp GF, Golan GA. Temporal trends in human immunodeficiency virus seroprevalence and sexual behavior at the San Francisco municipal sexually transmitted disease clinic. Am J Epidemiol. 1995;142:314–322. doi: 10.1093/oxfordjournals.aje.a117637. [DOI] [PubMed] [Google Scholar]
- 8.Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257–264. doi: 10.7326/0003-4819-125-4-199608150-00001. [DOI] [PubMed] [Google Scholar]
- 9.Jacquez JA, Koopman SJ, Simon CP, Longini IM. Role of the primary infection in epidemics of HIV infection in gay cohorts. J Acquir Immune Defic Syndr. 1994;7:1169–1184. [PubMed] [Google Scholar]
- 10.Kinloch-de Loes S, Hirschel BJ, Hoen B. A controlled trial of zidovudine in primary human immunodeficiency virus infection. N Engl J Med. 1995;333:408–413. doi: 10.1056/NEJM199508173330702. [DOI] [PubMed] [Google Scholar]
- 11.Daar ES, Moudgil T, Meyer RD, Ho DD. Transient high levels of viremia in patients with primary human immunodeficiency virus type 1 infection. N Engl J Med. 1991;324:961–964. doi: 10.1056/NEJM199104043241405. [DOI] [PubMed] [Google Scholar]
- 12.Vernazza PL, Eron JJ, Cohen MS, van de Horst CM, Troiani L, Fiscus SA. Detection and biologic characterization of infectious HIV-1 in semen of seropositive men. AIDS. 1994;8:1325–1329. doi: 10.1097/00002030-199409000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Mellors J, Rinaldo CR, Jr, Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 14.Anderson DJ, O'Brien TR, Politch JA. Effects of disease stage and zidovudine therapy on the detection of human immunodeficiency virus type 1 in semen. JAMA. 1992;267:2769–2774. [PubMed] [Google Scholar]
- 15.Krieger JN, Coombs RW, Collier AC. Recovery of human immunodeficiency virus type 1 from semen: minimal impact of stage of infection and current antiviral therapy. J Infect Dis. 1991;163:386–388. doi: 10.1093/infdis/163.2.386. [DOI] [PubMed] [Google Scholar]
- 16.Krieger JN, Combs RW, Collier AC, Ross SO, Speck C, Corey L. Seminal shedding of human immunodeficiency virus type 1 and human cytomegalovirus: evidence for different immunologic controls. J Infect Dis. 1995;171:1018–1022. doi: 10.1093/infdis/171.4.1018. [DOI] [PubMed] [Google Scholar]
- 17.Nelson KE, Rungruengthanakit K, Margolick J. High rates of transmission of subtype E human immunodeficiency virus type 1 among heterosexual couples in northern Thailand: role of sexually transmitted diseases and immune compromise. J Infect Dis. 1999;180:337–343. doi: 10.1086/314882. [DOI] [PubMed] [Google Scholar]
- 18.Clemetson DB, Moss GB, Willerford DM. Detection of HIV DNA in cervical and vaginal secretions: prevalence and correlates among women in Nairobi, Kenya. JAMA. 1993;269:2860–2864. [PubMed] [Google Scholar]
- 19.Cohen MS, Hoffman IF, Royce RA. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1868–1873. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 20.Fleming DT, Wasserheit JN. From epidemiologic synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu T, Wang N, Carr D. Genetic characterization of human immunodeficiency virus type 1 in blood and genital secretions: evidence for viral compartmentalization and selection during sexual transmission. J Virol. 1996;70:3098–3107. doi: 10.1128/jvi.70.5.3098-3107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ping LH, Cohen MS, Hoffman I. Effects of genital tract inflammation on human immunodeficiency virus type 1 V3 populations in blood and semen. J Virol. 2000;74:8946–8952. doi: 10.1128/jvi.74.19.8946-8952.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quinn TC, Wawer MJ, Sewankambo N. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 24.Orenstein JM, Fox C, Wahl SM. Macrophages as a source of HIV during opportunistic infections. Science. 1997;276:1857–1861. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- 25.Huang Y, Paxton WA, Wolinsky SM. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 26.Dean M, Carrington M, Winkler C. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 27.Liu R, Paxton WA, Choe S. Homozygous defect in HIV-1 coreceptor accounts for resistence of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 28.Samson M, Libert F, Doranz BJ. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 29.Vittinghoff E, Scheer S, O'Malley P, Colfax G, Holmberg SD, Buchbinder SP. Combination antiretroviral therapy and recent declines in AIDS incidence and mortality. J Infect Dis. 1999;179:717–720. doi: 10.1086/314623. [DOI] [PubMed] [Google Scholar]
- 30.Downs AM, De Vincenzi I., European Study Group in Heterosexual Transmission of HIV Probability of heterosexual transmission of HIV: relationship to the number of unprotected sexual contacts. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;11:388–395. doi: 10.1097/00042560-199604010-00010. [DOI] [PubMed] [Google Scholar]
- 31.Halperin DT, Bailey RC. Male circumcision and HIV infection: 10 years and counting. Lancet. 1999;354:1813–1815. doi: 10.1016/S0140-6736(99)03421-2. [DOI] [PubMed] [Google Scholar]
- 32.Amerongen HM, Weltzin R, Farnet CM, Michetti P, Haseltine WA, Neutra MR. Transepithelial transport of HIV-1 by intestinal M cells: a mechanism for transmission of AIDS. J Acquir Immune Defic Syndr. 1991;4:760–765. [PubMed] [Google Scholar]
- 33.Baba TW, Trichel AM, An L. Infection and AIDS in adult macaques after nontraumatic oral exposure to cell-free SIV. Science. 1996;272:1486–1489. doi: 10.1126/science.272.5267.1486. [DOI] [PubMed] [Google Scholar]
- 34.Stahl-Hennig C, Steinman RM, Tenner-Racz K. Rapid infection of oral mucosal-associated lymphoid tissue with simian immunodeficiency virus. Science. 1999;285:126–129. doi: 10.1126/science.285.5431.1261. [DOI] [PubMed] [Google Scholar]
- 35.O'Leary AD, Sweeney EC. Lymphoglandular complexes of the co-lon: structure and distribution. Histopathology. 1986;10:267–283. doi: 10.1111/j.1365-2559.1986.tb02481.x. [DOI] [PubMed] [Google Scholar]
- 36.Maric I, Holt PG, Perdue MH, Bienenstock J. Class II MHC antigen (Ia)-bearing dendritic cells in the epithelium of the rat intestine. J Immunol. 1996;156:1408–1414. [PubMed] [Google Scholar]
- 37.Kelsall BL, Strober W. Distinct populations of dendritic cells are present in the subepithelial dome and T cell regions of the murine Peyer's patch. J Exp Med. 1996;183:237–247. doi: 10.1084/jem.183.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hussain LA, Lehner T. Comparative investigation of Langerhans' cells and potential receptors for HIV in oral, genitourinary and rectal epithelia. Immunology. 1995;85:475–484. [PMC free article] [PubMed] [Google Scholar]
- 39.Frankel SS, Wenig BM, Burke AP. Replication of HIV-1 in dendritic cell-derived syncytia at the mucosal surface of the adenoid. Science. 1996;272:115–117. doi: 10.1126/science.272.5258.115. [DOI] [PubMed] [Google Scholar]
- 40.Geijtenbeek TBH, Kwon DS, Torensma R. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances transinfection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 41.Cameron PU, Freudenthal PS, Barker JM, Gezelter S, Inaba K, Steinman RM. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science. 1992;257:383–387. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- 42.Smith PD, Meng G, Sellers MT, Rogers T, Shaw GM. Biological parameters of HIV-1 infection in primary intestinal lymphocytes and macrophages. J Leukoc Biol. 2000;68:360–365. [PubMed] [Google Scholar]
- 43.Meng G, Sellers MT, Mosteller-Barnu M, Rogers TS, Shaw GM, Smith PD. Lamina propria lymphocytes, not macrophages, express CCR5 and CXCR4 and are the likely target cell for human immunodeficiency virus type 1 in the intestinal mucosa. J Infect Dis. 2000;182:785–791. doi: 10.1086/315790. [DOI] [PubMed] [Google Scholar]
- 44.Pavli L, Hume D, Van de Pol E, Doe W. Dendritic cells, the major antigen-presenting cells of the human colonic lamina propria. Immunology. 1993;78:132–141. [PMC free article] [PubMed] [Google Scholar]
- 45.Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat Med. 1997;3:42–47. doi: 10.1038/nm0197-42. [DOI] [PubMed] [Google Scholar]
- 46.Smith PD, Janoff EN, Mosteller-Barnum M. Isolation and purification of CD14-negative mucosal macrophages from normal human small intestine. J Immunol Meth. 1997;202:1–11. doi: 10.1016/s0022-1759(96)00204-9. [DOI] [PubMed] [Google Scholar]
- 47.Meng G, Wu X, Wei X, et al. Intestinal epithelial cells express CCR5 and selectively transfer R5 HIV-1 to CCR5+ cells (submitted for publication). [DOI] [PubMed]
- 48.Harouse JM, Bhat S, Spitalnik SL. Inhibition of entry of HIV-1 in neural cell lines by antibodies against galactosyl ceramide. Science. 1991;253:320–323. doi: 10.1126/science.1857969. [DOI] [PubMed] [Google Scholar]
- 49.Fantini J, Cook DG, Nathanson N, Spitalnik SL, Gonzalez-Scarano F. Infection of colonic epithelial cell lines by type 1 human immunodeficiency virus is associated with cell surface expression of galactosylceramide, a potential alternative gp 120 receptor. Proc Natl Acad Sci U S A. 1993;90:2700–2704. doi: 10.1073/pnas.90.7.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dwinell MB, Eckmann L, Leopard JD, Varki NM, Kagnoff MF. Chemokine receptor expression by human intestinal epithelial cells. Gastroenterology. 1999;117:359–367. doi: 10.1053/gast.1999.0029900359. [DOI] [PubMed] [Google Scholar]
- 51.Jordan NJ, Kolios G, Abbot SE. Expression of functional CXCR4 chemokine receptors on human colonic epithelial cells. J Clin Invest. 1999;104:1061–1069. doi: 10.1172/JCI6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McNearey T, Hornickova Z, Markham R. Relationship of human immunodeficiency virus type 1 sequence heterogeneity to stage of disease. Proc Natl Acad Sci U S A. 1992;89:10247–10251. doi: 10.1073/pnas.89.21.10247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolinsky SM, Wike CM, Korber BTM. Selective transmission of human immunodeficiency virus type-1 variants from mothers to infants. Science. 1992;255:1134–1137. doi: 10.1126/science.1546316. [DOI] [PubMed] [Google Scholar]
- 54.Zhu T, Mo H, Wang N. Genotypic and phenotypic characterization of HIV-1 in patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 55.Dragic T, Litwin V, Allaway GP. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 56.Lu Z-H, Chen Y-H, Turner JD. Evolution of HIV-1 coreceptor usage through interactions with distinct CCR5 and CXCR4 domains. Proc Natl Acad Sci U S A. 1997;94:6426–6431. doi: 10.1073/pnas.94.12.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith PD, Meng G, Shaw GM, Li L. Infection of gastrointestinal macrophages by HIV-1. J Leukoc Biol. 1997;621:72–77. doi: 10.1002/jlb.62.1.72. [DOI] [PubMed] [Google Scholar]
- 58.Li L, Meng G, Graham MF, Shaw GM, Smith PD. Intestinal macrophages display reduced permissiveness to human immunodeficiency virus 1 and decreased surface CCR5. Gastroenterology. 1999;116:1043–1053. doi: 10.1016/s0016-5085(99)70007-7. [DOI] [PubMed] [Google Scholar]
- 59.Smith PD, Fox CH, Masur H, Winter HS, Alling DW. Quantitative analysis of mononuclear cells expressing HIV-1 RNA in esophageal mucosa. J Exp Med. 1994;180:1541–1546. doi: 10.1084/jem.180.4.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schneider T, Jahn HU, Schmidt W. Loss of CD4 T lymphocytes in patients infected with immunodeficiency virus type 1 is more pronounced in the duodenal mucosa than in the peripheral blood. Gut. 1995;37:524–529. doi: 10.1136/gut.37.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clayton F, Snow G, Reka S, Kotler DP. Selective depletion of rectal lamina propria rather than lymphoid aggregate CD4 lymphocytes in HIV infection. Clin Exp Immunol. 1997;107:288–292. doi: 10.1111/j.1365-2249.1997.236-ce1111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Veazey RS, DeMaria M, Chalifoux LV. Gastrointestinal tract as a major site of CD4 1 T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 63.Kewenig S, Schneider T, Hohloch K. Rapid mucosal CD4(+) T-cell depletion and enteropathy in simian immunodeficiency virus-infected rhesus macaques. Gastroenterology. 1999;116:1115–1123. doi: 10.1016/s0016-5085(99)70014-4. [DOI] [PubMed] [Google Scholar]
- 64.Zeitz M, Greene WC, Peffer NJ, James SP. Lymphocytes isolated from the intestinal lamina propria of normal nonhuman primates have increased expression of genes associated with T-cell activation. Gastroenterology. 1988;94:647–655. doi: 10.1016/0016-5085(88)90235-1. [DOI] [PubMed] [Google Scholar]
- 65.Schieferdecker HL, Ullrich R, Hirseland H, Zeitz M. T cell differentiation antigens on lymphocytes in the human intestinal lamina propria. J Immunol. 1992;149:2816–2822. [PubMed] [Google Scholar]
- 66.McNeely TB, Dealy M, Dripps DJ, Orenstein JM, Eisenberg SP, Wahl SM. Secretory leukocyte protease inhibitor: a human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. J Clin Invest. 1995;96:456–464. doi: 10.1172/JCI118056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McNeely TB, Shugars DC, Rosendahl M, Tucker C, Eisenberg SP, Wahl SM. Inhibition of HIV-1 infectivity by secretory leukocyte protease inhibitor occurs prior to viral reverse transcription. Blood. 1997;90:1141–1149. [PubMed] [Google Scholar]
- 68.Lim SG, Condez A, Lee CA, Johnson MA, Elia C, Poulter LW. Loss of mucosal CD4 lymphocytes is an early feature of HIV infection. Clin Exp Immunol. 1993;92:448–454. doi: 10.1111/j.1365-2249.1993.tb03419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rodgers VD, Fassett R, Kagnoff MF. Abnormalities in intestinal mucosal T cells in homosexual populations including those with the lymphadenopathy syndrome and acquired immunodeficiency syndrome. Gastroenterology. 1986;90:552–558. doi: 10.1016/0016-5085(86)91108-x. [DOI] [PubMed] [Google Scholar]
- 70.Snijders F, Meenan J, van den Blink B, van Deventer SJ, ten Kate FJ. Duodenal intraepithelial and lamina propria T lymphocytes in human immunodeficiency virus–infected patients with and without diarrhoea. Scand J Gastroenterol. 1996;31:1176–1181. doi: 10.3109/00365529609036907. [DOI] [PubMed] [Google Scholar]
- 71.Greenspan D. Oral manifestations of HIV infection. In: Robertson PB, Greenspan JS, editors. PSG Publishing; Littleton, MA: 1988. (Oral manifestations of AIDS). [Google Scholar]
- 72.Smith PD, Quinn TC, Strober W, Janoff EN, Masur H. Gastrointestinal infections in AIDS. Ann Intern Med. 1992;116:63–77. doi: 10.7326/0003-4819-116-1-63. [DOI] [PubMed] [Google Scholar]
- 73.Ullrich R, Zeitz M, Heise W, L'age M, Höffken G, Riecken EO. Small intestinal structure and function in patients infected with human immunodeficiency virus (HIV): evidence for HIV-induced enteropathy. Ann Intern Med. 1989;111:15–21. doi: 10.7326/0003-4819-111-1-15. [DOI] [PubMed] [Google Scholar]
- 74.Riecken EO, Zeitz M, Ullrich R. Non-opportunistic causes of diarrhoea in HIV infection. Baillieres Clin Gastroenterol. 1990;4:385–403. doi: 10.1016/0950-3528(90)90008-5. [DOI] [PubMed] [Google Scholar]
- 75.Ullrich R, Zeitz M, Heise W. Mucosal atrophy is associated with loss of activated T cells in the duodenal mucosa of human immunodeficiency virus (HIV)-infected patients. Digestion. 1990;46(suppl 2):302–307. doi: 10.1159/000200401. [DOI] [PubMed] [Google Scholar]
- 76.Ullrich R, Riecken E-O, Zeitz M. Human immunodeficiency virusinduced enteropathy. Immunol Res. 1991;10:456–464. doi: 10.1007/BF02919742. [DOI] [PubMed] [Google Scholar]
- 77.Wilcox CM, Mönkemüller KE. Diagnosis and management of esophageal disease in the acquired immunodeficiency syndrome. South Med J. 1998;91:1002–1008. doi: 10.1097/00007611-199811000-00002. [DOI] [PubMed] [Google Scholar]
- 78.Forrest DM, Seminari E, Hogg RS. The incidence and spectrum of AIDS-defining illnesses in persons treated with antiretroviral drugs. Clin Infect Dis. 1998;27:1379–1385. doi: 10.1086/515030. [DOI] [PubMed] [Google Scholar]
- 79.Rabeneck L, Crane MM, Risser JMH, Lacke CE, Wray NP. Effect of HIV transmission category and CD4 count on the occurrence of diarrhea in HIV-infected patients. Am J Gastroenterol. 1993;88:1720–1723. [PubMed] [Google Scholar]
- 80.Flanigan T, Whalen C, Turner J. Cryptosporidium infection and CD4 counts. Ann Intern Med. 1992;116:840–842. doi: 10.7326/0003-4819-116-10-840. [DOI] [PubMed] [Google Scholar]
- 81.Colebunders R, Francis H, Mann JM. Persistent diarrhea, strongly associated with HIV infection in Kinshasa, Zaire. Am J Gastroenterol. 1987;82:859–864. [PubMed] [Google Scholar]
- 82.Malebranche R, Arnoux E, Guérin JM. Acquired immunodeficiency syndrome with severe gastrointestinal manifestations in Haiti. Lancet. 1983;2:873–878. doi: 10.1016/s0140-6736(83)90868-1. [DOI] [PubMed] [Google Scholar]
- 83.Serwadda D, Mugerwa RD, Sewankambo NK. Slim disease: a new disease in Uganda and its association with HTLV-III infection. Lancet. 1985;1:849–852. doi: 10.1016/S0140-6736(85)90122-9. [DOI] [PubMed] [Google Scholar]
- 84.Janoff EN. Diarrheal disease with viral enteric infections in immunocompromised patients. In: Owen RL, Surawicz C, editors. Saunders; Philadelphia: 1995. pp. 93–120. (Gastrointestinal and hepatic infections). [Google Scholar]
- 85.Smith PD, Wilcox CM. Gastrointestinal complications of the acquired immunodeficiency syndrome. In: Yamada T, Alpers DH, Laine L, Owyang C, Powell DW, editors. Lippincott Williams & Wilkins; Philadelphia: 1999. pp. 2400–2414. (Textbook of gastroenterology). [Google Scholar]
- 86.Wilcox CM, Mönkemüller KE. Gastrointestinal disease. In: Dolin R, Masur H, Saag MS, editors. Churchill Livingstone; Philadelphia: 1999. pp. 752–765. (AIDS Therapy). [Google Scholar]
- 87.Bellosillo NA, Gorback SL. Diarrhea and HIV infection. Infect Dis Clin Prac. 1998;7:213–219. [Google Scholar]
- 88.Flanigan TP. Cryptosporidium, Isospora, and Cyclospora infections. In: Dolin R, Masur H, Saag MS, editors. Churchill Livingstone; Philadelphia: 1999. pp. 328–335. (AIDS therapy). [Google Scholar]
- 89.Laughon BE, Druckman DA, Vernon A. Prevalence of enteric pathogens in homosexual men with and without acquired immunodeficiency syndrome. Gastroenterology. 1988;94:984–993. doi: 10.1016/0016-5085(88)90557-4. [DOI] [PubMed] [Google Scholar]
- 90.Smith PD, Lane C, Gill VJ. Intestinal infections in patients with the acquired immunodeficiency syndrome (AIDS): etiology and response to therapy. Ann Intern Med. 1988;108:328–333. doi: 10.7326/0003-4819-108-3-328. [DOI] [PubMed] [Google Scholar]
- 91.Blanshard C, Gazzard BG. Natural history and prognosis of diarrhoea of unknown cause in patients with acquired immunodeficiency syndrome (AIDS) Gut. 1995;36:283–286. doi: 10.1136/gut.36.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blanshard C, Francis N, Gazzard BG. Investigation of chronic diarrhoea in acquired immunodeficiency syndrome: a prospective study of 155 patients. Gut. 1996;39:824–832. doi: 10.1136/gut.39.6.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Crotty B, Smallwood RA. Investigating diarrhea in patients with acquired immunodeficiency syndrome. Gastroenterology. 1996;110:296–298. doi: 10.1053/gast.1996.v110.agast960296. [DOI] [PubMed] [Google Scholar]
- 94.Johanson JF. To scope or not to scope: the role of endoscopy in the evaluation of AIDS-related diarrhea. Am J Gastroenterol. 1996;91:2261–2262. [PubMed] [Google Scholar]
- 95.Rinder H, Janitschke K, Aspöck H. A blinded, externally controlled multicenter evaluation for the detection of microsporidia by light microscopy and PCR. J Clin Microbiol. 1998;36:1814–1818. doi: 10.1128/jcm.36.6.1814-1818.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weber R, Ledergerber B, Zbinden R. Enteric infections and diarrhea in human immunodeficiency virus-infected persons. Arch Intern Med. 1999;159:1473–1480. doi: 10.1001/archinte.159.13.1473. [DOI] [PubMed] [Google Scholar]
- 97.Thea DM, St. Louis ME, Atido U. A prospective study of diarrhea and HIV-1 infection among 429 Zairian infants. N Engl J Med. 1993;329:1696–1702. doi: 10.1056/NEJM199312023292304. [DOI] [PubMed] [Google Scholar]
- 98.Thea DM, Glass R, Grohmann GS. Prevalence of enteric viruses among hospital patients with AIDS in Kinshasa, Zaire. Trans R Soc Trop Med Hyg. 1993;87:263–266. doi: 10.1016/0035-9203(93)90119-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dolin R, Masur H, Saag MS. Churchill Livingstone; Philadelphia, Pennsylvania: 1999. AIDS therapy. [Google Scholar]
- 100.Gilbert DN, Moellering J, Sande MA. 30th ed. Antimicrobial Therapy; Hyde Park, VT: 2000. Sanford guide to antimicrobial therapy. [Google Scholar]
- 101.Bartlett JG, Gallant JE. Port City; Baltimore, MD: 2000. Medical management of HIV infection. [Google Scholar]
- 102.Fischl MA, Richman DD, Grieco MH. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. N Engl J Med. 1987;317:185–191. doi: 10.1056/NEJM198707233170401. [DOI] [PubMed] [Google Scholar]
- 103.Gulick RM, Mellors JW, Havlir D. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 104.Gulick RM. Assessing the benefits of antiretroviral therapy (editorial) Ann Intern Med. 2000;133:471–473. doi: 10.7326/0003-4819-133-6-200009190-00016. [DOI] [PubMed] [Google Scholar]
- 105.Hammer SM, Squires KE, Hughes MD. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 106.Mocroft A, Katlama C, Johnson AM. AIDS across Europe, 1994-98: the EuroSIDA Study. Lancet. 2000;356:291–296. doi: 10.1016/s0140-6736(00)02504-6. [DOI] [PubMed] [Google Scholar]
- 107.Palella FJ, Jr, Delaney KM, Moorman AC. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 108.Deeks SG, Smith M, Holodniy M, Kahn JO. HIV-1 protease inhibitors. A review for clinicians. JAMA. 1997;277:145–153. [PubMed] [Google Scholar]
- 109.Centers for Disease Control Revision of the CDC surveillance case definition for acquired immunodeficiency syndrome. MMWR. 1987;36(suppl 1S):1S–15S. [PubMed] [Google Scholar]
- 110.Mellors JW, Kingsley LA, Rinaldo CRJ. Quantitation of HIV-1 RNA in plasma predicts outcome after seroconversion. Ann Intern Med. 1995;122:573–579. doi: 10.7326/0003-4819-122-8-199504150-00003. [DOI] [PubMed] [Google Scholar]
- 111.Kaplan JE, Hanson D, Dworkin MS. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin Infect Dis. 2000;30:S5–S14. doi: 10.1086/313843. [DOI] [PubMed] [Google Scholar]
- 112.Salmon D, Detruchis P, Leport C. Efficacy of zidovudine in preventing relapses of salmonella bacteremia in AIDS. J Infect Dis. 1991;163:415–416. doi: 10.1093/infdis/163.2.415. [DOI] [PubMed] [Google Scholar]
- 113.Levine WC, Buehler JW, Bean NH, Tauxe RV. Epidemiology of nontyphoidal Salmonella bacteremia during the human immunodeficiency virus epidemic. J Infect Dis. 1991;164:81–87. doi: 10.1093/infdis/164.1.81. [DOI] [PubMed] [Google Scholar]
- 114.Fischl MA, Dickinson GM, Sinave C, Pitchenik, Cleary TJ. Salmonella bacteremia as manifestation of acquired immunodeficiency syndrome. Arch Intern Med. 1986;146:113–115. [PubMed] [Google Scholar]
- 115.Glaser JB, Morton-Kute L, Berger SR. Recurrent Salmonella typhimurium bacteremia associated with the acquired immunodeficiency syndrome. Ann Intern Med. 1985;102:189–193. doi: 10.7326/0003-4819-102-2-189. [DOI] [PubMed] [Google Scholar]
- 116.Jacobs JL, Gold JWM, Murray HW, Roberts RB, Armstrong D. Salmonella infections in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1985;102:186–188. doi: 10.7326/0003-4819-102-2-186. [DOI] [PubMed] [Google Scholar]
- 117.Jacobson MA, Hahn SM, Gerberding JL, Lee B, Sande MA. Ciprofloxacin for salmonella bacteremia in the acquired immunodeficiency syndrome (AIDS) Ann Intern Med. 1989;110:1027–1029. doi: 10.7326/0003-4819-110-12-1027. [DOI] [PubMed] [Google Scholar]
- 118.Nelson MR, Shanson DC, Hawkins DA, Gazzard BG. Salmonella, Campylobacter and Shigella in HIV-seropositive patients. AIDS. 1992;6:1495–1498. doi: 10.1097/00002030-199212000-00012. [DOI] [PubMed] [Google Scholar]
- 119.Smith PD, Macher AM, Bookman MA. Salmonella typhimurium enteritis and bacteremia in the acquired immunodeficiency syndrome. Ann Intern Med. 1985;102:207–209. doi: 10.7326/0003-4819-102-2-207. [DOI] [PubMed] [Google Scholar]
- 120.Autran B, Carcelain G, Li TS. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 121.Cavert W, Notermans DW, Staskus K. Kinetics of response in lymphoid tissues to antiretroviral therapy of HIV-1 infection. Science. 1997;276:960–964. doi: 10.1126/science.276.5314.960. [DOI] [PubMed] [Google Scholar]
- 122.Pontesilli O, Kerkhof-Garde S, Notermans DW. Functional T cell reconstitution and human immunodeficiency virus-1–specific cell-mediated immunity during highly active antiretroviral therapy. J Infect Dis. 1999;180:76–86. doi: 10.1086/314837. [DOI] [PubMed] [Google Scholar]
- 123.Zhang ZQ, Notermans DW, Sedgewick G. Kinetics of CD4+ T cell repopulation of lymphoid tissues after treatment of HIV-1 infection. Proc Natl Acad Sci U S A. 1998;95:1154–1159. doi: 10.1073/pnas.95.3.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Michelet C, Arvieux C, Francois C. Opportunistic infections occurring during highly active antiretroviral treatment. AIDS. 1998;12:1815–1822. doi: 10.1097/00002030-199814000-00013. [DOI] [PubMed] [Google Scholar]
- 125.Centers for Disease Control and Prevention USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus. MMWR Morb Mortal Wkly Rep. 1999;48:RR-106. [Google Scholar]
- 126.Currier JS, Williams PL, Koletar SL. Discontinuation of Mycobacterium avium complex prophylaxis in patients with antiretroviral therapy-induced increases in CD4+ cell count. Ann Intern Med. 2000;133:493–503. doi: 10.7326/0003-4819-133-7-200010030-00008. [DOI] [PubMed] [Google Scholar]
- 127.Furrer H, Egger M, Opravil M. Discontinuation of primary prophylaxis against Pneumocystis carinii pneumonia in HIV-1-infected adults treated with combination antiretroviral therapy. N Engl J Med. 1999;340:1301–1306. doi: 10.1056/NEJM199904293401701. [DOI] [PubMed] [Google Scholar]
- 128.Furrer H, Opravil M, Bernasconi E, Telenti A, Egger M, for the Swiss HIV Cohort Study Stopping primary prophylaxis in HIV-1-infected patients at high risk of toxoplasma encephalitis. Lancet. 2000;355:2217–2218. doi: 10.1016/s0140-6736(00)02407-7. [DOI] [PubMed] [Google Scholar]
- 129.Mussini C, Pezzotti P, Govoni A. Discontinuation of primary prophylaxis for Pneumocystis carinii pneumonia and toxoplasmic encephalitis in human immunodeficiency virus type 1-infected patients: the changes in opportunistic prophylaxis study. J Infect Dis. 2000;181:1635–1642. doi: 10.1086/315471. [DOI] [PubMed] [Google Scholar]
- 130.Hutin Y, Molina J-M, Casin I. Risk factors for Clostridium difficile–associated diarrhoea in HIV-infected patients. AIDS. 1993;7:1441–1447. doi: 10.1097/00002030-199311000-00006. [DOI] [PubMed] [Google Scholar]
- 131.Tacconelli E, Tumbarello M, de Gaetano Donati K, Leone F, Mazzella P, Cauda R. Clostridium difficile–associated diarrhea in human immunodeficiency virus infection–a changing scenario (letter) Clin Infect Dis. 1999;28:936–937. doi: 10.1086/517233. [DOI] [PubMed] [Google Scholar]
- 132.Mattapallil JJ, Smit-McBride Z, Dailey P, Dandekar S. Activated memory CD4(+) T helper cells repopulate the intestine early following antiretroviral therapy of simian immunodeficiency virus-infected rhesus macaques but exhibit a decreased potential to produce interleukin-2. J Virol. 1999;73:6661–6669. doi: 10.1128/jvi.73.8.6661-6669.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kotler DP, Shimada T, Snow G. Effect of combination antiretroviral therapy upon rectal mucosal HIV RNA burden and mononuclear cell apoptosis. AIDS. 1998;12:597–604. doi: 10.1097/00002030-199806000-00008. [DOI] [PubMed] [Google Scholar]
- 134.Schmidt W, Wahnschaffe U, Schäfer M. Rapid increase of mucosal CD4 T-cells followed by clearance of intestinal cryptosporidiosis in an AIDS patient receiving highly active antiretroviral therapy indicating effective mucosal immune reconstitution. Gastroenterology. 2000 doi: 10.1053/gast.2001.22557. (in press) [DOI] [PubMed] [Google Scholar]
- 135.Greenberg RE, Mir R, Bank S, Siegal FP. Resolution of intestinal cryptosporidiosis after treatment of AIDS with AZT. Gastroenterology. 1989;97:1327–1330. doi: 10.1016/0016-5085(89)91708-3. [DOI] [PubMed] [Google Scholar]
- 136.Chandrasekar PH. “Cure” of chronic cryptosporidiosis during treatment with azidothymidine in a patient with the acquired immune deficiency syndrome. Am J Med. 1987;83:187. doi: 10.1016/0002-9343(87)90524-9. [DOI] [PubMed] [Google Scholar]
- 137.Manabe YC, Clark DP, Moore RD. Cryptosporidiosis in patients with AIDS: correlates of disease and survival. Clin Infect Dis. 1998;27:536–542. doi: 10.1086/514701. [DOI] [PubMed] [Google Scholar]
- 138.Janoff EN, Limas C, Gebhard RL, Penley KA. Cryptosporidial carriage without symptoms in the acquired immunodeficiency syndrome (AIDS) Ann Intern Med. 1990;112:75–76. doi: 10.7326/0003-4819-112-1-75. [DOI] [PubMed] [Google Scholar]
- 139.Zar F, Geiseler PJ, Brown VA. Asymptomatic carriage of Cryptosporidium in the stool of a patient with acquired immunodeficiency syndrome. J Infect Dis. 1985;151:195. doi: 10.1093/infdis/151.1.195. [DOI] [PubMed] [Google Scholar]
- 140.Foudraine NA, Weverling GJ, van Gool T. Improvement of chronic diarrhoea in patients with advanced HIV-1 infection during potent antiretroviral therapy. AIDS. 1998;12:35–41. doi: 10.1097/00002030-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 141.Carr A, Marriott D, Field A, Vasak E, Cooper DA. Treatment of HIV-1-associated microsporidiosis and cryptosporidiosis with combination antiretroviral therapy. Lancet. 1998;351:256–261. doi: 10.1016/S0140-6736(97)07529-6. [DOI] [PubMed] [Google Scholar]
- 142.Ragni MV, Bontempo FA. Increase in hepatitis C virus load in hemophiliacs during treatment with highly active antiretroviral therapy. J Infect Dis. 1999;180:2027–2029. doi: 10.1086/315143. [DOI] [PubMed] [Google Scholar]
- 143.Beld M, Penning M, Lukashov V. Evidence that both HIV and HIV-induced immunodeficiency enhance HCV replication among HCV seroconverters. Virology. 1998;244:504–512. doi: 10.1006/viro.1998.9130. [DOI] [PubMed] [Google Scholar]
- 144.Bonacini M, Govindarajan S, Blatt LM, Schmid P, Conrad A, Lindsay KL. Patients co-infected with human immunodeficiency virus and hepatitis C virus demonstrate higher levels of hepatic HCV RNA. J Virol Hep. 1999;6:203–208. doi: 10.1046/j.1365-2893.1999.00153.x. [DOI] [PubMed] [Google Scholar]
- 145.Darby SC, Ewart DW, Giangrande PL. Mortality from liver cancer and liver disease in haemophilic men and boys in UK given blood products contaminated with hepatitis C. UK Haemophilia Centre Directors' Organisation. Lancet. 1997;350:1425–1431. doi: 10.1016/s0140-6736(97)05413-5. [DOI] [PubMed] [Google Scholar]
- 146.Eyster ME, Fried MW, Di Bisceglie AM, Goedert JJ. Increasing hepatitis C virus RNA levels in hemophiliacs: relationship to human immunodeficiency virus infection and liver disease. Multicenter Hemophilia Cohort Study. Blood. 1994;84:1020–1023. [PubMed] [Google Scholar]
- 147.Sulkowski MS, Mast EE, Seeff LB, Thomas DL. Hepatitis C virus infection as an opportunistic disease in persons infected with human immunodeficiency virus. Clin Infect Dis. 2000;30(suppl 1):S77–S84. doi: 10.1086/313842. [DOI] [PubMed] [Google Scholar]
- 148.Macias J, Pineda JA, Leal M. Influence of hepatitis C virus infection on the mortality of antiretroviral-treated patients with HIV disease. Eur J Clin Microbiol Infect Dis. 1998;17:167–170. doi: 10.1007/BF01691112. [DOI] [PubMed] [Google Scholar]
- 149.Greub G, Ledergerber B, Battegay M. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet. 2000;356:1800–1805. doi: 10.1016/s0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- 150.Soriano V, Rodriguez-Rosado R, Garcia-Samaniego J. Management of chronic hepatitis C in HIV-infected patients. AIDS. 1999;13:539–546. doi: 10.1097/00002030-199904010-00002. [DOI] [PubMed] [Google Scholar]
- 151.Dieterich DT, Purow JM, Rajapaksa R. Activity of combination therapy with interferon alfa-2b plus ribavirin in chronic hepatitis C patients co-infected with HIV. Semin Liver Dis. 1999;19:87–94. [PubMed] [Google Scholar]
- 152.Mauss S, Klinker H, Ulmer A. Response to treatment of chronic hepatitis C with interferon alpha in patients infected with HIV-1 is associated with higher CD4+ cell count. Infection. 1998;26:16–19. doi: 10.1007/BF02768746. [DOI] [PubMed] [Google Scholar]
- 153.Sulkowski M. The treatment of chronic HCV infection in HIV-infected persons (abstr S11). 7th Conference on Retroviruses and Opportunistic Infections, San Francisco, CA; 2000. [Google Scholar]
- 154.Poynard T, Marcellin P, Lee SS. Randomised trial of interferon alpha2b plus ribavirin 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis International Therapy Group. Lancet. 1998;352:1426–1432. doi: 10.1016/s0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- 155.Fialaire P, Payan C, Vitour D. Sustained disappearance of hepatitis C viremia in patients receiving protease inhibitor treatment for human immunodeficiency virus infection. J Infect Dis. 1999;180:574–575. doi: 10.1086/314910. [DOI] [PubMed] [Google Scholar]
- 156.Puoti M, Gargiulo F, Roldan EQ. Liver damage and kinetics of hepatitis C virus and human immunodeficiency virus replication during the early phases of combination antiretroviral treatment. J Infect Dis. 2000;181:2033–2036. doi: 10.1086/315529. [DOI] [PubMed] [Google Scholar]
- 157.Rutschmann OT, Negro F, Hirschel B, Hadengue A, Anwar D, Perrin LH. Impact of treatment with human immunodeficiency virus (HIV) protease inhibitors on hepatitis C viremia in patients coinfected with HIV. J Infect Dis. 1998;177:783–785. doi: 10.1086/517808. [DOI] [PubMed] [Google Scholar]
- 158.Zylberberg H, Chaix M-L, Rabian C. Tritherapy for human immunodeficiency virus infection does not modify replication of hepatitis C virus in coinfected subjects. Clin Infect Dis. 1998;26:1104–1106. doi: 10.1086/520281. [DOI] [PubMed] [Google Scholar]
- 159.Perez-Olmeda M, Garcia-Samaniego J, Soriano V. Hepatitis C viraemia in HIV-HCV co-infected patients having immune restoration with highly active antiretroviral therapy (letter) AIDS. 2000;14:212. doi: 10.1097/00002030-200001280-00023. [DOI] [PubMed] [Google Scholar]
- 160.Arribas JR, Ibanez C, Ruiz-Antoran B. Acute hepatitis in HIV-infected patients during ritonavir treatment. AIDS. 1998;12:722–723. [PubMed] [Google Scholar]
- 161.John M, Flexman J, French MA. Hepatitis C virus-associated hepatitis following treatment of HIV- infected patients with HIV protease inhibitors: an immune restoration disease? AIDS. 1998;12:2289–2293. doi: 10.1097/00002030-199817000-00010. [DOI] [PubMed] [Google Scholar]
- 162.Sulkowski MS, Thomas DL, Chaisson RE, Moore RD. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA. 2000;283:74–80. doi: 10.1001/jama.283.1.74. [DOI] [PubMed] [Google Scholar]
- 163.Bräu N, Leaf HL, Wieczorek RL, Margolis DM. Severe hepatitis in three AIDS patients treated with indinavir (letter) Lancet. 1997;349:924. doi: 10.1016/S0140-6736(05)62700-6. [DOI] [PubMed] [Google Scholar]
- 164.French MAH. Antiretroviral therapy immune restoration disease in HIV-infected patients with HAART. AIDS Reader. 1999;9:548–562. [PubMed] [Google Scholar]
- 165.DeSimone JA, Pomerantz RJ, Babinchak TJ. Inflammatory reactions in HIV-1-infected persons after initiation of highly active antiretroviral therapy. Ann Intern Med. 2000;133:447–454. doi: 10.7326/0003-4819-133-6-200009190-00013. [DOI] [PubMed] [Google Scholar]
- 166.Cinti SK, Kaul DR, Sax PE, Crane LR, Kazanjian PH. Recurrence of Mycobacterium avium infection in patients receiving highly active antiretroviral therapy and antimycobacterial agents. Clin Infect Dis. 2000;30:511–514. doi: 10.1086/313705. [DOI] [PubMed] [Google Scholar]
- 167.Lucas GM, Chaisson RE, Moore RD. Highly active antiretroviral therapy in a large urban clinic: risk factors for virologic failure and adverse drug reactions. Ann Intern Med. 1999;131:81–87. doi: 10.7326/0003-4819-131-2-199907200-00002. [DOI] [PubMed] [Google Scholar]
- 168.Volberding PA, Deeks SG. Antiretroviral therapy for HIV infection: promises and problems. JAMA. 1998;279:1343–1344. doi: 10.1001/jama.279.17.1343. [DOI] [PubMed] [Google Scholar]
- 169.Shapiro MF, Morton SC, McCaffrey DF. Variations in the care of HIV-infected adults in the United States: results from the HIV Cost and Services Utilization Study. JAMA. 1999;281:2305–2315. doi: 10.1001/jama.281.24.2305. [DOI] [PubMed] [Google Scholar]
- 170.Jones JL, Hanson DL, Dworkin MS. Surveillance for AIDS-defining opportunistic illnesses. 1992-1997. CDC Surveillance Summaries. MMWR Morb Mortal Wkly Rep. 1999;48:1–22. [PubMed] [Google Scholar]
- 171.Max B, Sherer R. Management of the adverse effects of antiretroviral therapy and medication adherence. Clin Infect Dis. 2000;30(suppl 2):S96–S116. doi: 10.1086/313859. [DOI] [PubMed] [Google Scholar]
- 172.Brinkman K, ter Hofstede HJ, Burger DM, Smeitink JA, Koopmans PP. Adverse effects of reverse transcriptase inhibitors: mitochondrialtoxicity ascommonpathway. AIDS. 1998;12:1735–1744. doi: 10.1097/00002030-199814000-00004. [DOI] [PubMed] [Google Scholar]
- 173.Brinkman K, Smeitink JA, Romijn JA, Reiss P. Mitochondrial toxicity induced by nucleoside-analogue reverse-transcriptase inhibitors is a key factor in the pathogenesis of antiretroviral-therapy-related lipodystrophy. Lancet. 1999;354:1112–1115. doi: 10.1016/S0140-6736(99)06102-4. [DOI] [PubMed] [Google Scholar]
- 174.Carr A, Samaras K, Chisholm DJ, Cooper DA. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet. 1998;351:1881–1883. doi: 10.1016/S0140-6736(98)03391-1. [DOI] [PubMed] [Google Scholar]
- 175.Burger DM, Hoetelmans RMW, Koopman PP. Clinically relevant drug interactions with antiretroviral agents. Antiviral Ther. 1997;2:149. [PubMed] [Google Scholar]
- 176.Janoff EN, Smith PD. Perspectives on gastrointestinal infections in AIDS. Gastroenterol Clin North Am. 1988;17:451–463. [PubMed] [Google Scholar]
- 177.Janoff EN, Orenstein JM, Manischewitz JF, Smith PD. Adenovirus colitis in the acquired immunodeficiency syndrome. Gastroenterology. 1991;100:976–979. doi: 10.1016/0016-5085(91)90272-m. [DOI] [PubMed] [Google Scholar]
- 178.Wanke CA, Mayer H, Weber R, Zbinden R, Watson DA, Acheson D. Enteroaggregative Escherichia coli as a potential cause of diarrheal disease in adults infected with human immunodeficiency virus. J Infect Dis. 1998;178:185–190. doi: 10.1086/515595. [DOI] [PubMed] [Google Scholar]
- 179.Perlman DM, Ampel NM, Schifman RB. Persistent Campylobacter jejuni infections in patients infected with human immunodeficiency virus (HIV) Ann Intern Med. 1988;108:540–546. doi: 10.7326/0003-4819-108-4-540. [DOI] [PubMed] [Google Scholar]
- 180.Gurtman A, Szabo S, Rose D, Mayer H, Sacks H. Comparison of Clostridium difficile diarrhea (CdD) in hospitalized HIV seropositive (HIV+) and HIV seronegative (HIV−) patients. The 32nd Interscience Conference on Antimicrobial Agents and Chemotherapy. Anaheim, CA; October 11-14, 1992. [Google Scholar]
- 181.Grohmann GS, Glass RI, Pereira HG. Enteric viruses and diarrhea in HIV-infected patients. N Engl J Med. 1993;329:14–20. doi: 10.1056/NEJM199307013290103. [DOI] [PubMed] [Google Scholar]
- 182.Cheung ANY, Ng IOL. Cytomegalovirus infection of the gastrointestinal tract in non-AIDS patients. Am J Gastroenterol. 1993;88:1882–1886. [PubMed] [Google Scholar]
- 183.Schmidt W, Schneider T, Heise W, Berlin Diarrhea/Wasting Syndrome Study Group Stool viruses, coinfections, and diarrhea in HIV-infected patients. J Acquir Immune Defic Syndr. 1996;13:33–38. doi: 10.1097/00042560-199609000-00006. [DOI] [PubMed] [Google Scholar]
- 184.Kartalija M, Sande MA. Diarrhea and AIDS in the era of highly active antiretroviral therapy. Clin Infect Dis. 1999;28:701–707. doi: 10.1086/515191. [DOI] [PubMed] [Google Scholar]
- 185.Didier ES. Microsporidiosis. Clin Infect Dis. 1998;27:1–8. doi: 10.1086/514607. [DOI] [PubMed] [Google Scholar]
- 186.Dore GJ, Marriott DJ, Hing MC, Harkness JL, Field AS. Disseminated microsporidiosis due to Septata intestinalis in nine patients infected with the human immunodeficiency virus: response to therapy with albendazole. Clin Infect Dis. 1995;21:70–76. doi: 10.1093/clinids/21.1.70. [DOI] [PubMed] [Google Scholar]
- 187.Wilcox CM, Chalasani N, Lazenby A, Schwartz DA. Cytomegalovirus colitis in acquired immunodeficiency syndrome: a clinical and endoscopic study. Gastrointest Endosc. 1998;48:39–43. doi: 10.1016/s0016-5107(98)70126-9. [DOI] [PubMed] [Google Scholar]
- 188.Goguel J, Katlama C, Sarfati C, Maslo C, Leport C, Molina JM. Remission of AIDS-associated intestinal microsporidiosis with highly active antiretroviral therapy (letter) AIDS. 1997;11:1658–1659. [PubMed] [Google Scholar]
- 189.Beach JW. Chemotherapeutic agents for human immunodeficiency virus infection: mechanism of action, pharmacokinetics, metabolism, and adverse reactions. Clin Ther. 1998;20:2–25. doi: 10.1016/s0149-2918(98)80031-3. [DOI] [PubMed] [Google Scholar]
- 190.Moore RD, Fortgang I, Keruly J, Chaisson RE. Adverse events from drug therapy for human immunodeficiency virus disease. Am J Med. 1996;101:34–40. doi: 10.1016/s0002-9343(96)00077-0. [DOI] [PubMed] [Google Scholar]
- 191.Carr A, Cooper DA. Adverse effects of antiretroviral therapy. Lancet. 2000;356:1423–1430. doi: 10.1016/S0140-6736(00)02854-3. [DOI] [PubMed] [Google Scholar]


