Abstract
Objectives:
We examined the serum levels of interferon-gamma-inducible protein 10 (IP-10), an inflammation-induced chemokine, in acute myocardial infarction (AMI).
Design and methods:
The subjects were 33 AMI patients, 20 stable angina pectoris patients (AP) and 20 normal subjects. In AMI patients, blood samples were collected before percutaneous coronary intervention (PCI) and on days 3, 7 and 28.
Results:
Patients with AMI showed significantly higher serum IP-10 levels (137.5 ± 79.8 pg/mL) than control subjects (91.2 ± 40.1 pg/mL) and patients with AP (93.3 ± 41.1 pg/mL). The serum IP-10 level before PCI was negatively correlated with infarct size, as indicated by cumulative release of creatine kinase (CK) and peak CK and its isoenzyme CK-MB. Stepwise multiple regression analysis revealed that the serum IP-10 level before PCI was an independent predictor of cumulative CK release.
Conclusions:
The serum IP-10 level was increased in AMI, and a higher level of serum IP-10 before PCI may be informative regarding infarct size.
Keywords: Coronary disease, Chemokines, Diagnosis, Immunoassay, Inflammation
Introduction
The prognosis after acute myocardial infarction (AMI) is determined by several parameters [1], [2]. Infarct size is one of the important factors that affects prognosis after MI [3]. The inflammatory response after AMI is known to correlate with infarct size [4]. All aspects of the inflammatory response, and subsequent healing processes, occur after AMI. The evaluation of inflammatory markers, including cytokines, by blood sampling in AMI patients is thus informative [5].
Chemokines are chemotactic cytokines that were initially characterized by their capacity to induce chemotaxis or migration of leucocytes, and that play important roles in the inflammatory response. In response to inflammation or injury, higher levels of chemokines permit the attraction and retention of specific populations of lymphocytes. Thus, chemokines may have additional effects in healing infarcts beyond their leukotactic properties. Chemokines regulate angiogenesis, collagen turnover, and apoptosis and have been reported to be dysregulated in the end-stage of heart failure [6]. Chemokines also play an important role in the pathophysiology of acute coronary syndromes, post-infarction left ventricular remodeling and chronic heart failure [7]. Interferon-gamma-inducible protein 10 kDa (IP-10, also referred to as CXCL10) is a highly inducible, anti-angiogenic protein that belongs to the C-X-C chemokine superfamily [8]. Increased serum IP-10 levels have been reported in atherosclerotic patients [9]. In an experimental myocardial infarction model, IP-10 protein was shown to be transiently induced in the reperfused myocardium [10]. However, whether the serum IP-10 levels are increased in AMI patients and whether the serum level of IP-10 is correlated with infarct size, as indicated by cumulative creatine kinase (CK) release, peak CK or CK isoenzyme MB (CK-MB), in AMI patients has never been examined. Accordingly, we examined the change of serum IP-10 level in AMI patients and compared it with cumulative CK and peak CK and CK-MB.
Methods
Patients
This study protocol conformed to the Declaration of Helsinki and was approved by the hospital ethics committee; written informed consent was obtained from all patients before the study. The eligible patients were first-AMI patients who received successful primary percutaneous coronary intervention (PCI) for the infarct-related coronary artery within 12 h from the onset. A total of 33 consecutive patients (26 men and 7 women; mean age, 61 ± 9 years) with AMI were admitted to the Cardiovascular Center, Sakakibara Hospital or Tsuyama Central Hospital (Okayama, Japan) from January 2000 to December 2001. The patients all had ST-segment elevated (0.2 mV) MI in more than two leads with chest pain sustained for longer than 30 min. Patients who had severe respiratory disease, liver disease, kidney disease, concomitant inflammatory diseases such as infections and autoimmune disorders, or malignancy were pre-excluded from this study. The extent of atherosclerotic regions in the vessels was diagnosed by X-ray coronary angiography (> 50% stenosis). The serum CK level and CK-MB level were determined every 4 h during the first and second days after admission and then once a day until the values returned to the normal range. All subjects with stable angina pectoris (AP) (n = 20, 16 men and 4 women; mean age, 63 ± 9 years) had stable effort angina lasting longer than 6 months. The diagnosis was confirmed by coronary angiography, and defined as > 75% narrowing of the luminal diameter of at least one diseased vessel. The control subjects in the study were 20 gender- and age-matched healthy blood donors. Informed consent for participation in the study was obtained from all individuals.
Blood sampling protocol
For collection of serum, blood was drawn into pyrogen-free tubes without additives. The tubes were immediately immersed in ice water, the blood was allowed to clot for 2 h, and centrifuged at 1000×g for 10 min, and serum was stored at − 80 °C until analyzed.
Measurement of creatine kinase
The CK activity was measured by the modified Rosalski method [11]. The control range for CK was obtained for 100 age- and gender-matched volunteers. The upper limit of the range (+ 2 SD above the mean) for the controls for CK activity was 155 IU/L. The activity of CK-MB was measured using the reagents of an immune-inhibition assay kit (Diatron, Tokyo, Japan), according to established methods [12], [13]. The upper limit of the range for the controls for CK-MB activity was 20 IU/L. The cumulative CK release was calculated according to the modified method reported by Norris et al. [14] and Shell et al. [15].
Serum IP-10 level
Blood samples were drawn immediately on the day of admission (before PCI) and on days 3, 7, and 28 in AMI patients. Enzyme linked immunoassay (ELISA) was performed as previously reported [16], [17], [18], [19]. Serum IP-10 levels were measured using a one-step sandwich ELISA kit for human IP-10 (R&D Systems, Minneapolis, MN, USA). This ELISA kit has been widely used for detecting serum IP-10 levels in a variety of diseases, and the measurement method has been well established in both normal subjects and diseased patients elsewhere [9], [20]. Briefly, a monoclonal antibody to human IP-10 is used for capture, and a biotinylated polyclonal antibody is used for detection. Recombinant human IP-10 served as a standard. The lower limit of sensitivity was 20 pg/mL, and the intra-assay and inter-assay coefficients of variation were < 5% and < 7%, respectively. The investigators were blinded to the IP-10 levels during the determination of the cumulative CK release and peak CK and CK-MB.
Left ventriculography
Left ventriculography was performed with informed consent in 22 patients approximately 4 weeks after the onset of AMI. The left ventriculogram was analyzed using a digitizer and a computer (SONY Graphtec Digitizer KD4030B, Tokyo, Japan) as previously reported [21], [22], [23]. Briefly, the end-diastolic volume (LV EDV) was determined using the electrocardiogram recorded simultaneously on cine film as the frame nearest the peak of the R wave of the electrocardiogram (ECG). The frame with the smallest left ventricular (LV) volume was taken to show the end-systolic volume (LV ESV), and LV volume was calculated using a modification of Dodge's formula [24]. LV volume indices were available in 13 patients and were compared with serum IP-10 levels and indexed for body surface area.
Statistical analysis
Data were expressed as mean ± SD. Differences in characteristics across quartiles were compared by ANOVA followed by Bonferroni's post hoc test for continuous variables and the chi-square test for dichotomous variables. Correlations between serum IP-10 levels and cumulative CK release were assessed by using Pearson's correlation coefficients. Because serum IP-10 level and cumulative CK release were not normally distributed, log-transformed values for these variables were used. Stepwise multiple regression analysis was used to assess the possible determinants of cumulative CK release. For the analysis, adjustments were performed with the following variables: age, gender, use of angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker, calcium blocker, or aspirin, time to reperfusion, culprit lesion, thrombolysis in myocardial infarction (TIMI) flow grade before primary PCI, collateral flow before primary PCI, TIMI flow grade after PCI, and serum IP-10 level before PCI (pg/mL). All analyses were performed using SPSS 11.0 for Windows (SPSS Inc., Chicago, IL) or StatView 5.0 (SAS Institute, San Francisco, CA). A p value of < 0.05 was considered significant.
Results
Serum IP-10 levels in AMI patients
Patients with AMI had significantly higher IP-10 levels before PCI (137.5 ± 79.8 pg/mL) than control subjects and patients with AP (91.2 ± 40.1 and 93.3 ± 41.1 pg/mL, p = 0.010 and 0.013, respectively) (Fig. 1 ). The time courses of the serum IP-10 level of 23 patients with AMI who satisfied the inclusion criteria of the present study were investigated, and revealed that the mean level of serum IP-10 slightly decreased at 3 days (111.6 ± 42.9 pg/mL), was elevated at 7 days (161.8 ± 81.2 pg/mL), and continued to increase at 28 days (248.4 ± 182.5 pg/mL), as shown in Fig. 2a. The serum IP-10 levels before PCI were significantly higher in the group of patients with multiple vessel disease (Fig. 2b). When the patients were classified into tertiles according to their serum IP-10 level before PCI, only the patients classified into the highest tertile showed a transient decrease of serum IP-10 level on day 3 (Fig. 2c). Accordingly, we determined the difference of serum IP-10 level between admission day and 3 days (calculated delta 3d IP-10 as serum IP-10ad level − serum IP-103d level). The delta 3d IP-10 was significantly lower only in the patients classified into the highest tertile (data not shown).
Fig. 1.
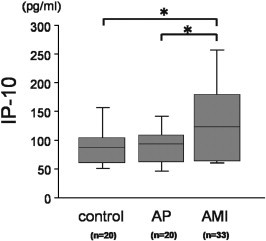
Serum IP-10 levels in AMI patients before PCI. Box plot illustrating serum IP-10 levels in control subjects and patients with stable angina pectoris (AP) or acute myocardial infarction before PCI (AMI). Patients with AMI had higher serum IP-10 levels than control subjects and patients with AP. The boxes represent the 25th, 50th, and 75th percentiles, and the whiskers indicate the 10th and 90th percentiles. ⁎p < 0.05.
Fig. 2.
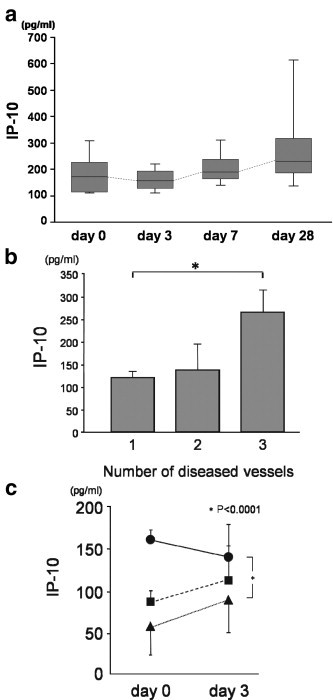
Time course of serum IP-10 level in AMI patients. (a) The alteration of serum IP-10 level was determined in 22 AMI patients. The boxes represent the 25th, 50th, and 75th percentiles, and the whiskers indicate the 10th and 90th percentiles. (b) The serum IP-10 level and extent of atherosclerosis. The serum IP-10 level on admission day was compared with the extent of coronary atherosclerosis as determined by coronary angiography. Patients with atherosclerosis in three vessels showed a higher level of serum IP-10 level before PCI. ⁎p < 0.05 vs. single diseased vessel. (c) The alteration of serum IP-10 levels between admission day (day 0) and day 3. The closed circles indicate the highest tertile for serum IP-10 level before PCI, closed boxes indicate the middle tertile, and closed triangles indicate the lowest tertile, respectively. Note that only the highest tertile for serum IP-10 level before PCI showed a decrease of serum IP-10 level at 3 days, while others increased at 3 days. ⁎p < 0.0001 vs. lowest tertile.
Clinical and angiographic characteristics
Table 1 shows the clinical characteristics according to the tertile of serum IP-10 level before PCI. There was no difference in age, gender, incidence of coronary risk factors or in five categories of medications taken. The time to reperfusion of patients classified into the highest tertile was longer than that of patients classified into the other tertiles. Angiographic findings were comparable among the tertiles (Table 2 ).
Table 1.
Patient characteristics
| Total (N = 33) | IP-10 on admission (pg/mL) |
p value | |||
|---|---|---|---|---|---|
| T1 (30.7–76.3) (N = 11) | T2 (85.8–158.3) (N = 11) | T3 (165.3–341.1) (N = 11) | |||
| Age (years) | 61 ± 9 | 60 ± 8 | 59 ± 9 | 63 ± 10 | NS |
| Male, n (%) | 26 (79) | 9 (82) | 10 (91) | 7 (64) | NS |
| Hypertension, n (%) | 22 (67) | 7 (64) | 7 (64) | 8 (73) | NS |
| Hyperlipidemia, n (%) | 21 (64) | 6 (55) | 9 (82) | 6 (55) | NS |
| Diabetes, n (%) | 8 (24) | 3 (27) | 3 (27) | 2 (18) | NS |
| Current smoking, n (%) | 15 (45) | 5 (46) | 5 (46) | 5 (46) | NS |
| Creatinine (mg/dL) | 0.96 ± 0.38 | 0.99 ± 0.23 | 1.1 ± 0.58 | 0.81 ± 0.19 | NS |
| Medication, n (%) | |||||
| ACEI/ARB | 10 (30) | 5 (46) | 3 (27) | 2 (18) | NS |
| Calcium blocker | 5 (15) | 2 (18) | 3 (27) | 0 (0) | NS |
| β blocker | 4 (12) | 2 (18) | 2 (18) | 0 (0) | NS |
| Nitrate | 10 (30) | 5 (46) | 3 (27) | 2 (18) | NS |
| Aspirin | 13 (39) | 7 (64) | 4 (36) | 2 (18) | NS |
| Time to reperfusion (h) | 4.9 ± 2.6 | 4.1 ± 1.7 | 3.8 ± 2.3 | 6.8 ± 2.6 | 0.007 |
Table 2.
Angiographic characteristics
| T1 (N = 11) | T2 (N = 11) | T3 (N = 11) | p value | |
|---|---|---|---|---|
| Culprit lesion (LAD/Cx/RCA) | 8/1/2 | 7/1/3 | 6/1/4 | NS |
| TIMI grade before PCI (0/1) | 11/0 | 11/0 | 9/2 | NS |
| Collateral grade (0/1/2/3) | 3/6/0/2 | 6/3/1/1 | 6/3/1/1 | NS |
| TIMI grade after PCI (2/3) | 2/9 | 2/9 | 0/11 | NS |
CK and CK-MB levels and LV function
As shown in Figs. 3a and b, both the cumulative CK release and peak CK level in the highest tertile of serum IP-10 level before PCI were significantly lower than those in the lowest tertile (p < 0.05). Peak CK-MB level, which was examined in 31 patients in this study, also showed the same trend (Fig. 3c). There was a significant negative correlation between the log serum IP-10 level before PCI and log cumulative CK release, peak CK and peak CK-MB in the patients enrolled in the present study (Figs. 3d–f). Fig. 4a shows that the LV EF (4 weeks after the onset of AMI) of patients classified into the lowest IP-10 tertile was worse than that of patients classified into the middle tertile. Serum IP-10 level before PCI was significantly negatively correlated with LV ESVI and LV EDVI (Figs. 4b and c).
Fig. 3.
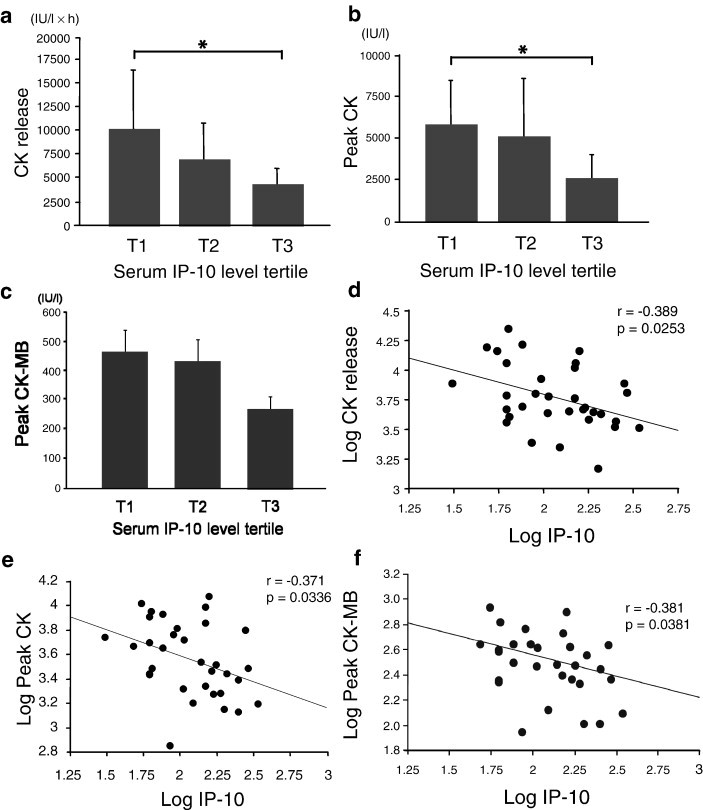
Relationship of serum IP-10 level to CK release and peak CK and CK-MB in AMI patients. Relationship between tertile of serum IP-10 level (before PCI) to CK release and peak CK and CK-MB: CK release (a), peak CK (b), and peak CK-MB (c). ⁎p < 0.05. Relationship of log serum IP-10 level (before PCI) to CK release and peak CK and CK-MB: log CK release (d), log peak CK (e), and log peak CK-MB (f). The correlation coefficient and the p value for each relationship are indicated.
Fig. 4.
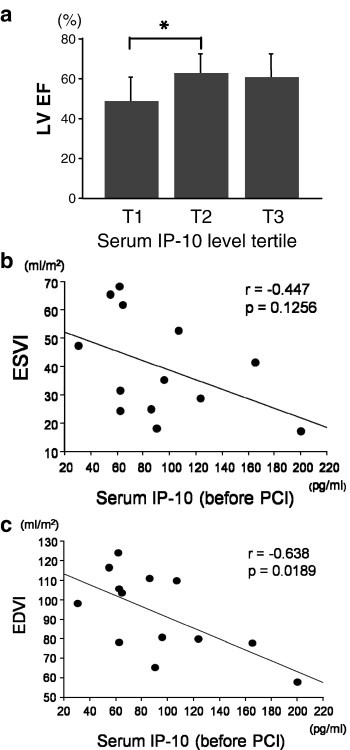
Relationship of serum IP-10 levels before PCI to LV function in AMI patients. Relationship between tertile of serum IP-10 level (before PCI) and LV EF at 4 weeks after AMI (a). ⁎p < 0.05. Relationship of serum IP-10 level (before PCI) to LV ESVI (b) and EDVI (c). The correlation coefficient and the p value for each relationship are indicated.
Stepwise multiple regression analysis
The serum IP-10 level as well as culprit lesion (LAD; left anterior descending artery) was an independent predictor of cumulative CK release (Table 3 ). The time to reperfusion was not an independent predictor of the cumulative CK release.
Table 3.
Stepwise multiple regression analysis of variables associated with cumulative CK release
| Dependent variable | Explanatory variable | Standardized regression coefficient | t | p value |
|---|---|---|---|---|
| Cumulative CK release | Culprit lesion (LAD) | 0.20 | 2.371 | 0.026 |
| Serum IP-10 levels (before PCI) | − 0.399 | − 2.229 | 0.035 |
Stepwise multiple regression analysis was performed using the following variables: age, gender, use of ACE/ARB, Ca blocker or aspirin, time to reperfusion, culprit lesion, thrombolysis in myocardial infarction (TIMI) flow grade before primary PCI, collateral flow before primary PCI, TIMI flow grade after PCI, and serum IP-10 level on admission (pg/mL).
Discussion
In this study, (1) the serum IP-10 level was found to be increased in AMI patients on admission (2) the time course of IP-10 release after AMI was determined and (3) the serum IP-10 level on admission was shown to be negatively correlated with infarct size, as indicated by cumulative CK release and peak CK and CK-MB.
The present method used for determining the serum IP-10 level is well established, and the intra- and inter-assay coefficients of variation are sufficiently small [25]. According to previous reports, the serum IP-10 levels in normal subjects range from 20 pg/mL to 105 pg/mL [26], [27], [28]. In this study, we investigated serum IP-10 levels in 20 gender- and age-matched healthy blood donors. The serum-IP-10 levels observed in this study were consistent with the levels reported for previous studies using the same ELISA assay kit [26], [27], [28], [29], indicating that the present assay methods were appropriate and the results were valid.
Our data demonstrated that the serum IP-10 level was significantly increased in AMI patients prior to PCI compared to healthy controls. Recently accumulated evidence has demonstrated an elevated serum level of IP-10 in various diseases, including inflammatory disease, and shown its utility as a clinically informative marker [26], [27], [28], [29]. For instance, Tang et al. reported that early, enhanced induction of IP-10 is an independent predictor of outcome for severe acute respiratory syndrome (SARS) patients [30]. In myocardial infarction, Frangogiannis et al. found that IP-10 is primarily synthesized by ischemic microvascular endothelial cells and that its enhanced production declined within 24 h after reperfusion in an animal ischemia–reperfusion model [10]. Rothenbacher et al. reported positive correlations of serum IP-10 levels with several acute-phase proteins or inflammation-associated cytokines in coronary heart disease patients [9]. Aukrust et al. recently reviewed the usefulness of serum or plasma chemokine levels to predict the risk of cardiovascular events, and suggested an important pathogenic role of chemokines in atherogenesis and plaque destabilization [31]. Our data showing elevated serum IP-10 levels in AMI were in line with these reports. In addition, serum IP-10 levels were rarely increased in stable angina patients in this study. Unstable angina patients were not enrolled because serum IP-10 levels were reported to be correlated with inflammation-associated cytokines, including IL-8 [9]. Therefore, it is postulated that the lack of increase in serum IP-10 level in AP patients in this study may have been related to their plaque stability.
This study demonstrated for the first time that serum IP-10 levels changed with time after AMI. The mean value of serum IP-10 in AMI patients decreased at 3 days. It should be noted that only the patients classified into the highest tertile for serum IP-10 level before PCI showed a decrease of serum IP-10 level at 3 days. This indicates that serum IP-10 level can increase remarkably before PCI under certain conditions in AMI patients and return to regular infarct healing process levels at 3 days after AMI. The increase of serum IP-10 level prior to PCI may be related to some beneficial conditions/situations for the ischemic heart (e.g., ischemic preconditioning or transient recanalization before arriving at the hospital) which could not be identified from this study, and that may be why the AMI patients who had higher serum IP-10 levels had a smaller infarct size. In addition, serum IP-10 level showed an increase of the mean level with more variance at 28 days after AMI. Little is currently known about the regulation of IP-10 after AMI, and it is possible that serum IP-10 levels increased in accordance with the development of heart failure, because elevated chemokine expression in patients with heart failure has been reported [6]. This may be, at least in part, an explanation for the wide variability of serum IP-10 levels on day 28 after AMI in this study. Therefore, further clinical investigations in patients with various cardiovascular diseases are needed, including monitoring of serum IP-10 levels in acute and chronic heart failure.
In this study, we found a significant negative correlation of serum IP-10 level before PCI with infarct size, as indicated by cumulative CK release and peak CK and CK-MB. There are many reports of attempts to estimate myocardial ischemic damage by measuring biomarkers in AMI patients. Ahumada et al. reviewed studies showing that CK-MB is a highly sensitive and specific biomarker of myocardial injury [32]. Recently, novel biomarkers, including troponin T (TnT) and troponin I (TnI), have been found and these factors have been evaluated for estimating infarct size. For instance, Licka et al. reported that serum TnT level (after 72 h after AMI) and peak CK were both significantly correlated with infarct size, as estimated by the uptake score of thallium-201 myocardial single photon emission computed tomography (SPECT) [17]. Younger et al. reported that TnI level 72 h after AMI was similarly useful to serial CK measurements for the estimation of infarct size using late gadolinium hyper-enhancement cardiac magnetic resonance (LGE-CMR) [33]. Tanaka et al. reported that the peak TnT and TnI levels were correlated with peak CK level and cumulative CK release in successful reperfusion AMI patients [16]. Di Chiara et al. reported that peak CK was the best predictor of infarct size in first-AMI patients [18]. These studies indicate the usefulness of CK release and peak CK and CK-MB for estimating the infarct size. Thus, cumulative CK release and peak CK and CK-MB could be used to examine the relationship of the serum IP-10 level to the infarct size.
There were several limitations in this study. First, the present study included a small number of patients because we carefully chose patients with a first AMI following successful reperfusion by primary PCI without any disease complications. Nevertheless, serum IP-10 level was significantly correlated with both cumulative CK release and peak CK, implying that our findings shed light on the role of IP-10 as an informative biomarker in AMI patients. A further study with a random sample of a larger population will of course be required to draw more definite conclusions. Second, we cannot exclude the possibility that the initial high serum IP-10 levels as well as the alteration of serum IP-10 levels in the patients in the present study were an epiphenomenon reflecting some unknown mechanisms that reduced the infarct size. There have been no reports of investigations of serum IP-10 levels in patients with severe heart disease such as acute heart failure and myocarditis. As discussed in the previous section, further clinical investigations of the serum level of IP-10 and its alterations in various cardiovascular disease will be required.
Conclusion
In conclusion, we detected a significant increase of the serum IP-10 level in AMI patients. The measurement of this chemokine may provide some information regarding infarct size in AMI patients.
Acknowledgments
The authors would like to thank Dr. Keizo Yamamoto, Dr. Takashi Murakami, Dr. Kazuyoshi Hina, Dr. Hiroshi Kawamura, Dr. Koichiro Iwasaki and Dr. Issei Komatsubara for stimulating discussions. This work was supported in part by funding from a Grant-in-Aid for Scientific Research from the Japan Society (grant 18390416 to S.H.).
References
- 1.Sobel B.E., Bresnahan G.F., Shell W.E., Yoder R.D. Estimation of infarct size in man and its relation to prognosis. Circulation. 1972;46:640–648. doi: 10.1161/01.cir.46.4.640. [DOI] [PubMed] [Google Scholar]
- 2.White H.D., Norris R.M., Brown M.A., Brandt P.W., Whitlock R.M., Wild C.J. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 3.Wei J.Y., Markis J.E., Malagold M., Grossman W. Time course of serum cardiac enzymes after intracoronary thrombolytic therapy. Creatine kinase, creatine kinase MB isozyme, lactate dehydrogenase, and serum glutamic–oxaloacetic transaminase. Arch Intern Med. 1985;145:1596–1600. [PubMed] [Google Scholar]
- 4.Hirschl M.M., Gwechenberger M., Binder T. Assessment of myocardial injury by serum tumour necrosis factor alpha measurements in acute myocardial infarction. Eur Heart J. 1996;17:1852–1859. doi: 10.1093/oxfordjournals.eurheartj.a014803. [DOI] [PubMed] [Google Scholar]
- 5.Theroux P., Armstrong P.W., Mahaffey K.W. Prognostic significance of blood markers of inflammation in patients with ST-segment elevation myocardial infarction undergoing primary angioplasty and effects of pexelizumab, a C5 inhibitor: a substudy of the COMMA trial. Eur Heart J. 2005;26:1964–1970. doi: 10.1093/eurheartj/ehi292. [DOI] [PubMed] [Google Scholar]
- 6.Damas J.K., Eiken H.G., Oie E. Myocardial expression of CC- and CXC-chemokines and their receptors in human end-stage heart failure. Cardiovasc Res. 2000;47:778–787. doi: 10.1016/s0008-6363(00)00142-5. [DOI] [PubMed] [Google Scholar]
- 7.Filippatos G., Parissis J.T., Adamopoulos S., Kardaras F. Chemokines in cardiovascular remodeling: clinical and therapeutic implications. Curr Mol Med. 2003;3:139–147. doi: 10.2174/1566524033361546. [DOI] [PubMed] [Google Scholar]
- 8.Neville L.F., Mathiak G., Bagasra O. The immunobiology of interferon-gamma inducible protein 10 kD (IP-10): a novel, pleiotropic member of the C-X-C chemokine superfamily. Cytokine Growth Factor Rev. 1997;8:207–219. doi: 10.1016/s1359-6101(97)00015-4. [DOI] [PubMed] [Google Scholar]
- 9.Rothenbacher D., Muller-Scholze S., Herder C., Koenig W., Kolb H. Differential expression of chemokines, risk of stable coronary heart disease, and correlation with established cardiovascular risk markers. Arterioscler Thromb Vasc Biol. 2006;26:194–199. doi: 10.1161/01.ATV.0000191633.52585.14. [DOI] [PubMed] [Google Scholar]
- 10.Frangogiannis N.G., Mendoza L.H., Lewallen M., Michael L.H., Smith C.W., Entman M.L. Induction and suppression of interferon-inducible protein 10 in reperfused myocardial infarcts may regulate angiogenesis. FASEB J. 2001;15:1428–1430. doi: 10.1096/fj.00-0745fje. [DOI] [PubMed] [Google Scholar]
- 11.Rosalki S.B. An improved procedure for serum creatine phosphokinase determination. J Lab Clin Med. 1967;69:696–705. [PubMed] [Google Scholar]
- 12.Horder M., Elser R.C., Gerhardt W., Mathieu M., Sampson E.J. International Federation of Clinical Chemistry. Scientific Division, Committee on Enzymes: approved recommendation on IFCC methods for the measurement of catalytic concentration of enzymes: Part 7. IFCC method for creatine kinase (ATP: creatine N-phosphotransferase, EC 2.7.3.2) Eur J Clin Chem Clin Biochem. 1991;29:435–456. [PubMed] [Google Scholar]
- 13.Murakami M., Iwasaki K., Kusachi S. Nicorandil reduces the incidence of minor cardiac marker elevation after coronary stenting. Int J Cardiol. 2006;107:48–53. doi: 10.1016/j.ijcard.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 14.Norris R.M., Whitlock R.M., Barratt-Boyes C., Small C.W. Clinical measurement of myocardial infarct size. Modification of a method for the estimation of total creatine phosphokinase release after myocardial infarction. Circulation. 1975;51:614–620. doi: 10.1161/01.cir.51.4.614. [DOI] [PubMed] [Google Scholar]
- 15.Shell W.E., Kjekshus J.K., Sobel B.E. Quantitative assessment of the extent of myocardial infarction in the conscious dog by means of analysis of serial changes in serum creatine phosphokinase activity. J Clin Invest. 1971;50:2614–2625. doi: 10.1172/JCI106762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka H., Abe S., Yamashita T. Serum levels of cardiac troponin I and troponin T in estimating myocardial infarct size soon after reperfusion. Coron Artery Dis. 1997;8:433–439. doi: 10.1097/00019501-199707000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Licka M., Zimmermann R., Zehelein J., Dengler T.J., Katus H.A., Kubler W. Troponin T concentrations 72 hours after myocardial infarction as a serological estimate of infarct size. Heart. 2002;87:520–524. doi: 10.1136/heart.87.6.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Chiara A., Plewka M., Werren M., Badano L.P., Fresco C., Fioretti P.M. Estimation of infarct size by single measurements of creatine kinase levels in patients with a first myocardial infarction. J Cardiovasc Med (Hagerstown) 2006;7:340–346. doi: 10.2459/01.JCM.0000223256.01439.1b. [DOI] [PubMed] [Google Scholar]
- 19.Hedstrom E., Astrom-Olsson K., Ohlin H. Peak CKMB and cTnT accurately estimates myocardial infarct size after reperfusion. Scand Cardiovasc J. 2007;41:44–50. doi: 10.1080/14017430601071849. [DOI] [PubMed] [Google Scholar]
- 20.Rotondi M., Falorni A., De Bellis A. Elevated serum interferon-gamma-inducible chemokine-10/CXC chemokine ligand-10 in autoimmune primary adrenal insufficiency and in vitro expression in human adrenal cells primary cultures after stimulation with proinflammatory cytokines. J Clin Endocrinol Metab. 2005;90:2357–2363. doi: 10.1210/jc.2004-1062. [DOI] [PubMed] [Google Scholar]
- 21.Hirohata S., Kusachi S., Murakami M. Time dependent alterations of serum matrix metalloproteinase-1 and metalloproteinase-1 tissue inhibitor after successful reperfusion of acute myocardial infarction. Heart. 1997;78:278–284. doi: 10.1136/hrt.78.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murakami T., Kusachi S., Murakami M. Time-dependent changes of serum carboxy-terminal peptide of type I procollagen and carboxy-terminal telopeptide of type I collagen concentrations in patients with acute myocardial infarction after successful reperfusion: correlation with left ventricular volume indices. Clin Chem. 1998;44:2453–2461. [PubMed] [Google Scholar]
- 23.Suezawa C., Kusachi S., Murakami T. Time-dependent changes in plasma osteopontin levels in patients with anterior-wall acute myocardial infarction after successful reperfusion: correlation with left-ventricular volume and function. J Lab Clin Med. 2005;145:33–40. doi: 10.1016/j.lab.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Greene D.G., Carlisle R., Grant C., Bunnell I.L. Estimation of left ventricular volume by one-plane cine angiography. Circulation. 1967;35:61–69. doi: 10.1161/01.cir.35.1.61. [DOI] [PubMed] [Google Scholar]
- 25.Diago M., Castellano G., Garcia-Samaniego J. Association of pretreatment serum interferon gamma inducible protein 10 levels with sustained virological response to peginterferon plus ribavirin therapy in genotype 1 infected patients with chronic hepatitis C. Gut. 2006;55:374–379. doi: 10.1136/gut.2005.074062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franciotta D., Martino G., Zardini E. Serum and CSF levels of MCP-1 and IP-10 in multiple sclerosis patients with acute and stable disease and undergoing immunomodulatory therapies. J Neuroimmunol. 2001;115:192–198. doi: 10.1016/s0165-5728(01)00261-2. [DOI] [PubMed] [Google Scholar]
- 27.Nicoletti F., Conget I., Di Mauro M. Serum concentrations of the interferon-gamma-inducible chemokine IP-10/CXCL10 are augmented in both newly diagnosed Type I diabetes mellitus patients and subjects at risk of developing the disease. Diabetologia. 2002;45:1107–1110. doi: 10.1007/s00125-002-0879-5. [DOI] [PubMed] [Google Scholar]
- 28.Scarpini E., Galimberti D., Baron P. IP-10 and MCP-1 levels in CSF and serum from multiple sclerosis patients with different clinical subtypes of the disease. J Neurol Sci. 2002;195:41–46. doi: 10.1016/s0022-510x(01)00680-3. [DOI] [PubMed] [Google Scholar]
- 29.Leung T.F., Ma K.C., Hon K.L. Serum concentration of macrophage-derived chemokine may be a useful inflammatory marker for assessing severity of atopic dermatitis in infants and young children. Pediatr Allergy Immunol. 2003;14:296–301. doi: 10.1034/j.1399-3038.2003.00052.x. [DOI] [PubMed] [Google Scholar]
- 30.Tang N.L., Chan P.K., Wong C.K. Early enhanced expression of interferon-inducible protein-10 (CXCL-10) and other chemokines predicts adverse outcome in severe acute respiratory syndrome. Clin Chem. 2005;51:2333–2340. doi: 10.1373/clinchem.2005.054460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aukrust P., Yndestad A., Smith C., Ueland T., Gullestad L., Damas J.K. Chemokines in cardiovascular risk prediction. Thromb Haemost. 2007;97:748–754. [PubMed] [Google Scholar]
- 32.Ahumada G., Roberts R., Sobel B.E. Evaluation of myocardial infarction with enzymatic indices. Prog Cardiovasc Dis. 1976;18:405–420. doi: 10.1016/0033-0620(76)90004-9. [DOI] [PubMed] [Google Scholar]
- 33.Younger JF, Plein S, Barth J, Ridgway JP, Ball SG Greenwood JP. Troponin-I concentration 72 hours after myocardial infarction correlates with infarct size and presence of microvascular obstruction. Heart in press.doi:10.1136/hrt.2006.109249. [DOI] [PMC free article] [PubMed]


