Abstract
Acute peristome edema disease (APED) is a new disease that broke out in cultured sea cucumber along the Shangdong and Liaoning province coasts in China, PR, and has caused a great deal of death in Apostichopus japonicus (Selenka) since 2004. Here we report virus-like particles found in intestine epithelium of sea cucumbers reared in North China. It is the first time that sea cucumbers are reported to be infected by virus. Histological examinations showed that the viral inclusion bodies existed in intestine epithelium cells. Electron microscopic examinations show that the virions were spherical, 80–100 nm in diameter, and composed of a helical nucleocapsid within an envelope with surface projections. Detailed studies on the morphogenesis of these viruses found many characteristics previously described for coronaviruses. Virus particles always congregated, and formed a virus vesicle with an encircling membrane. The most obvious cellular pathologic feature is large granular areas of cytoplasm, relatively devoid of organelles. Tubular structures within virus-containing vesicles, nucleocapsid inclusions, and double-membrane vesicles are also found in the cytopathic cells. No rickettsia, chlamydia, bacteria, or other parasitic organisms were found.
Keywords: Ultra-pathology, Histopathology, Virus, APED
1. Introduction
In early 2004, Epidemic mortalities among cultured sea cucumber juveniles from a peristome edema disease with no identified agent were reported from Shangdong Province, China; they were followed by reports of sea cucumber adults with peristome edema disease and mortality of 10%. In early 2005, the disease broke out again, and the epidemic-stricken area spread to Liaoning Province, China. The mortalities of sea cucumber adults and juveniles were as high as 40% (Chang Y.Q., 20 March 2005, Key Lab Reports). A number of laboratories in China have undertaken the identification of the causative agent, and tried to prevent its spread or outbreak in 2006.
The diseased sea cucumbers first show edema in their peristomes. The tentacles cannot retract completely, and adhesion capacities for holding of tube feet on vertical surfaces weaken. Consequently most of them drop to the bottom of ponds. Compared to the normal ones, the grazing rate and activity of the diseased sea cucumbers decreased obviously, and 80% of individuals eviscerate. About 2–3 days later, small white lesions usually appear in some of the diseased sea cucumbers and gradually expand, with increased mucus secretion over large areas of the body wall. About 5–6 days later, the diseased sea cucumber will die, and mortality may be more than 90% (Wang et al., 2005). According to the typical symptom and state, the disease was designated acute peristome edema disease (APED), by the study groups.
After exclusion of environmental factors such as heavy metals, dissolved oxygen, salinity, pH, NH3–N content and so on (data not shown), biologic pathogens, including rickettsia, chlamydia, bacteria, virus, and other parasites were suspected.
Transmission electron microscopic examination showed virus-like particles in intestine epithelium of diseased sea cucumbers. In this paper, we present formal evidence for the existence of viral particles in APED affected sea cucumbers, in the first report of sea cucumbers infected by viruses. The virus assembly, the relevant pathology, and ultra-structure changes are analyzed, which offer valuable reference to subsequent work on the APED etiology and epidemiology.
2. Materials and methods
Five sea cucumber adults, averaged 420 g and five juvenile averaged 55 g, with typical APED symptoms, were taken stochastically, in March 2005, during the outbreak of sea cucumber APED. Tentacle (buccal podia), peristome, papillate podia, respiratory tress, hemal channel wall, and intestinal wall were selected for study.
The sea cucumbers were anesthetized by first being placed on moist cold filter papers in petri dishes for 10 min, and then into cold mineral water (fresh water, 4 °C) for 5 min. This ensured that the sea cucumbers were extended with muscles relaxed, allowing easy dissection. This was carried out on the dorsal side. The selected tissues were detached and arranged in two groups.
One group of tissue samples was prepared for ultra-thin sections and immersed in primary fixative (3% glutaraldehyde in 0.1 M phosphate buffer) (Sorour and Larink, 2001). After fixation for 3–4 h at 4 °C, the specimens were washed in the same buffer for 2.5 h at 4 °C. Postfixation in 1% osmium tetroxide in the phosphate buffer was carried out for 2 h at 4 °C, followed by dehydration in a graded series of ethanol. The specimens were embedded in Araldite mixture, and polymerized at 60 °C. Specimen blocks were sectioned with a Reichert Ultra-tome, 50 nm in thickness, and viewed with a JEOL JEM 1200EX electron microscope operating at 60 kV, after counterstaining with uranyl acetate and lead citrate (Becker et al., 2003).
The other group of samples was prepared for histopathology analyses that were performed on frozen sections immediately following microtomy. They were sectioned at 5 μm and stained with Mayer’s haematoxyin and eosin (H&E), followed by observation and photography.
3. Results
3.1. Histopathology
Histological examination of diseased sea cucumbers indicated that the intestine tissue was the primary target tissue. The lesions were characterized by tissue necrosis or histolysis accompanied with numbers of empty areas in tissues. Intestinal villi, with blurred limits, appeared shrunken, shortened, or even disappeared. The number of epithelium cells and the thickness of the epithelium layer both decreased, accompanied with nuclei arranged irregularly (Fig. 1 a). In the damaged epithelium, typically spherical viral inclusion bodies, 0.6–1.0 μm in diameter, were observed in cytoplasm, and were stained purple (Fig. 1b). No bacteria or other suspect pathogens (rickettsia, chlamydia, bacteria, protozoa, metazoa) were found. In the control samples, the limits of intestinal villis are clear, with thicker epithelium layers and the nuclei arranged regularly. No viral inclusion bodies were observed.
Fig. 1.
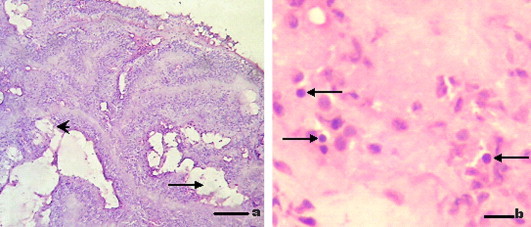
Histopathologic changes in intestine tissue of sea cucumbers with APED. (a) Histological examination of the intestine lesions in epithelium layers. Intestinal villi arranged irregularly and the epithelium layer obviously damaged or even disappeared (arrowhead) accompanied with empty areas for histolysis (arrow). Bar, 50 μm. (b) Viral inclusion bodies were observed in epithelium layer. The size of these obviously spherical inclusion bodies ranges between 0.6 and 1 μm, stained purple (arrow). Bar, 3 μm.
3.2. Ultra-pathology changes
The ultra-thin sections of tentacle (buccal podia), peristome, papillate podia, respiratory tress, hemal channel wall, and intestinal wall were observed by transmission electron microscopy (TEM). No rickettsia, chlamydia, bacteria, or other parasitic organisms were found; while only a kind of viral particles was prevalent in intestine epithelium cells.
The cell junctions in intestine epithelium are not tight, and large spaces exist between cytopathic cells. In the cytopathic cells, virus assembly was obviously going on, and numbers of vesicles, averaged 3–4 per cell, full of virus particles, were observed (Fig. 2 a). The nuclei of infected cells were of amorphous and hyper-stained, with no nucleoli observed. The endoplasmic reticulum attached to the karyotheca obviously swelled and formed a separation cisternae between the nucleus and the cytoplasm (Fig. 2b). Except for abnormally high mitochondrial densities, the cytoplasm of infected cells was nearly free of cellular structures, and composed of large areas of granular material and empty vesicles. The empty vesicles in cells differed: some were empty with clear membranes, some were empty but without clear edges (Fig. 2c), and some contained few virions along the encircling membranes.
Fig. 2.
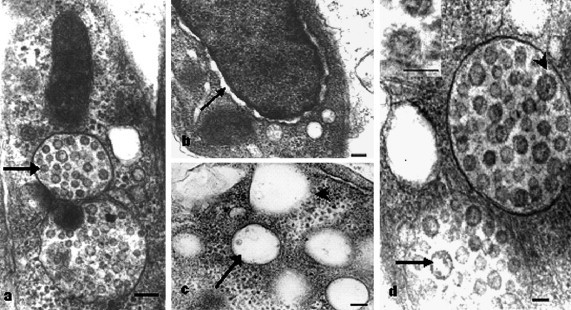
Pathologic changes in cells of intestine epithelium of sea cucumbers with APED. (a) A cytopathic cell with two vesicles full of virions (arrow). Bar, 200 nm. (b) A nucleus of virus-infected cell was of homogenous materials and hyper-stained, with no nucleoli observed, and the endoplasmic reticulum obviously swelled and formed a separation cisternae (arrow) between the nucleus and the cytoplasm. Bar, 200 nm. (c) Empty vesicles dispersed in granular areas of cytopathic cell of intestine epithelium, some with clear membrane (arrow), and some with blurry margins (arrowhead). Bar, 200 nm. (d) Virus-containing vesicles in cytopathic cells of intestine, in which some virus are light-colored in the center (arrow) as if they are hollow and some are over-colored with petal-like pattern (arrowhead). Orderly tubular structures can also be found around the vesicles. Bar, 50 nm. Inset: a typical virus particle, with an envelope and regular petal-like surface projections (arrow) surrounding the periphery of the particle. Bar, 100 nm.
Many intact virus particles were found both inside and outside the cytopathic cells. Most of them were enveloped, with diameters between 80 and 100 nm. Lengths of the protrusions in envelope went between 10 and 20 nm (Fig. 2d, inset). Nucleocapsids were always round in shape, with diameters between 45 and 70 nm. Similar to what has been reported previously for coronaviruses, in cytopathic cells of APED, virus particles always aggregated, and formed a virus vesicle with an encircling membrane. Within such vesicles, diameters of virus particles were about 50 nm. Staining of the virus differed: some were electron lucent in the center, as if hollow; some were electron-dense with petal-like pattern (Fig. 2d).
In normal intestine epithelium cells of healthy sea cucumbers, the nuclear envelope as usual (Ferreira and Manker, 1965) is formed by two membranes with pores, and the nucleoprotein consists of granular and filamentous components randomly distributed. In each nucleus one or two nucleoli are regularly seen. In the cytoplasm, the Golgi complex is generally apparent in a peri-nuclear position. Mitochondria, ribosomes, rough membranes of the endoplasmic reticulum, and occasionally small lipid inclusions are diffusedly scattered through the cytoplasm.
3.3. Ultrastructural Characteristics of virus assembly
The morphologic features of the virus particles in sea cucumber were similar to those of the family Coronaviridae.
Large, ill-defined areas of cytoplasm, containing ribosome-like structures and devoid of other organelles, were noted in some virus-infected cells (Fig. 3 a). On the other hand, in some virus-containing vesicles, dense, granular material was seen interspersed between the virions. Cytoplasmic inclusions of electron-dense viral nucleocapsids were mostly found in association with virus-containing vesicles, or empty vesicles (Fig. 3b).
Fig. 3.
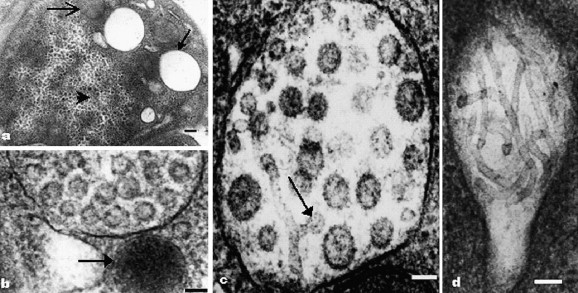
Ultrastructural characteristics of virus assembly in cytopathic cells of APED. (a) A cytopathic cell of intestine epithelium, with a large area of granular material (arrowhead) and empty vesicles (arrow), devoid of organelles, and only mitochondria sometimes seen (open arrow). Bar, 200 nm. (b) Nucleocapsid inclusion (arrow), hyper-stained, in association with virus-containing vesicles or double-membrane vesicles. Bar, 100 nm. (c) Tubular structures, averaging 25 nm in diameter, within some virus-containing vesicles; some ends of the tubular structures swell and are similar to the spherical virions (arrow). Bar, 100 nm. (d) Tubular structures within an enclosed vesicle in cytopathic cell of intestine. Bar, 100 nm.
Tubular structures averaging 25 nm in diameter were seen within some virus-containing vesicles. Some ends of the tubular structures swell and are similar to the spherical virions (Fig. 3c). Tubular structures within nonvirus-containing vesicles, whose size and morphology are similar to those in virus-containing vesicles, are also found (Fig. 3d). Outside the virus-containing vesicles, but always adjacent to the vesicles, there occurred another kind of tubular structures, averaging 15 nm in diameter. Some of these linear structures were arrayed at low density (Fig. 4 a) and some of them were dense compactly, and orderly (Fig. 4b).
Fig. 4.
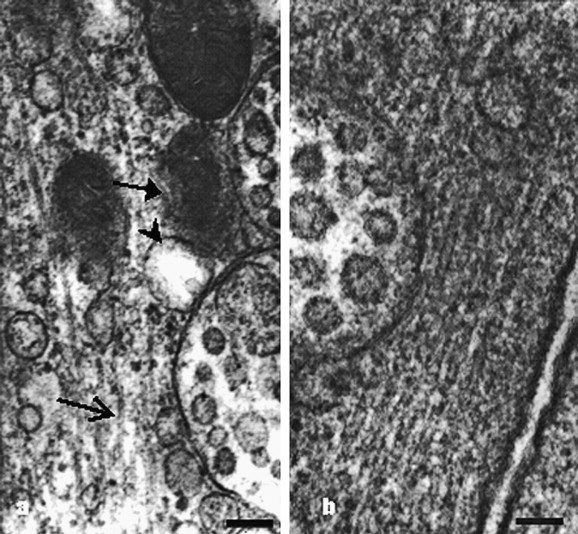
Tubular structures outside the virus-containing vesicles. (a) Tubular structures (open arrow) arrayed with large space but in the same direction; nucleocapsid inclusions (arrow), empty vesicle (arrowhead) and virus-containing vesicles dispersed among them. Bar, 100 nm. (b) Tubular structures arranged orderly around the virus-containing vesicles in cytopathic cells. Bar, 100 nm.
Additional cytoplasmic structures associated with virus infections were double-membrane vesicles, which have been similarly noted in coronavirus-infected cells (Dubois et al., 1982, Gosert et al., 2002). In contrast, double-membrane vesicles in cytopthic cells of sea cucumber with APED, typically were composed of accumulations of multiple single-membrane vesicles enclosed within an outer membrane (Fig. 5 a), and virus particles were sometimes located between the two membranes. On the other hand, vesicles with tight double-membranes were also found in cytopathic cells of sea cucumber with APED, and their formation seems probably associated with the varicose rough endoplasmic reticula (Fig. 5b).
Fig. 5.
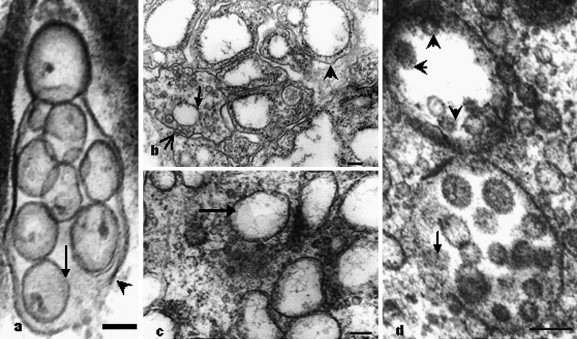
Double-membrane vesicles and the assembly of virus particles. (a) Double-membrane vesicles in cytopthic cells of intestine, typically composed of accumulations of multiple single-membrane (arrow) vesicles enclosed within an outer membrane (arrowhead). Bar, 100 nm. (b) Double-membranes vesicles (arrowhead), probably coming from rough endoplasmic reticula, and a double-membrane vesicle with a large space between the inner (arrow) and outer (open arrow) membranes of the vesicle, virus particles located between the membranes. Bar, 200 nm. (c) Some vesicles with dark blurry edge areas around clear membrane (arrow) are also found in the cytopathic cells. Bar, 200 nm. (d) The virions (arrowheads) always exist in the dark blurry margins around the membrane, if the vesicles are not full of virions; virions acquired an envelope by budding into the cisternae (arrows) and formed mostly spherical, sometimes pleomorphic, particles, which suggest these vesicles seem to be the site of the viral assembly. Bar, 200 nm.
Some vesicles with dark, blurry margins around clear membrane were also found in the cytopathic cells (Fig. 5c). The virions always occurred in the dark blurry margins near membranes, if the vesicles were not full of virions. Virions acquired an envelope by budding into the cisternae, and formed mostly spherical, sometimes pleomorphic, particles (Fig. 5d), suggesting that these vesicles may be the site of viral assembly.
4. Discussion
Frozen section technology is widely applied in clinical diagnostics of humans, and can display the histological changes accurately and rapidly. In our study, viral inclusion bodies in affected cells and tissues is one of obvious characters compared with the normal ones and they can be found easily in the diseased samples by frozen section and H&E stain. Therefore the frozen section technology was used for the fast detection of these viral infections in our epidemiology and diagnostic study. As to the apply of TEM to discover agents, with SARS, as with previous investigations of outbreaks involving such viruses as Ebola, Hendra, Nipah, and more recently, monkey pox, TEM played an essential role (Goldsmith et al., 2004). The epidemic viral diseases of aquatic organisms, especially those whose cells cannot be cultured yet, can be studied uniquely with this powerful tool.
Detailed studies described here on the morphogenesis of a sea cucumbers virus by thin-section TEM found many characteristics previously described for cornaviruses (Becker et al., 1967, Oshiro et al., 1971, Siddell et al., 1983, Zhang et al., 2003). Virions accumulated in dilated cytoplasmic vesicles that appeared to migrate to the cell surface where the virus particles were released. Virus particles formed upon membranes of an apparent “budding compartment,” a term used to describe the continuous membrane system from the rough endoplasmic reticulum to the Golgi complex (Dubois et al., 1982, Tooze et al., 1984). Additional cytoplasmic structures associated with coronavirus infections included nucleocapsid inclusions and double-membrane vesicles, which have been proposed as the replication complex for coronaviruses and arteriviruses (Gosert et al., 2002, Pedersen et al., 1999).
As has been reported for coronavirus, additional cytoplasmic features were associated with virus-infected sea cucumbers cells. Tubular structures were occasionally seen within virus-containing vesicles. Some ends of the tubular structures swell and are similar to the spherical virions; but the actual relation between the spherical virions and the tubular structures are not certain (Goldsmith et al., 2004). The tubular structures outside vesicles are not the same as the tubulorecticular structures, which were reported for coronavirus (Ferreira and Manker, 1965, Goldsmith et al., 2004). Their high linearity suggest that they maybe microtubules (Wu and Yuan, 2003) and function as a transport or organizer for the migration of all kinds of vesicles for the virus assembly, including virus-containing vesicles, empty vesicles, and nucleocapsid inclusions.
Though all findings proved that the intestine epithelium cells were infected by these virus particles, that such viruses had not been detected, in normal controls, it still cannot be concluded that these viruses are the agent of sea cucumbers APED, without infection experiments. As has been reported, the cells of invertebrates cannot be cultured, and the viruses of invertebrates cannot be replicated in vitro (Deuff and Renault, 1999). Only minimal intestine tissue can be obtained as material when the disease occurs, because of the sea cucumbers’ peculiar evisceration. Therefore it is difficult to purify viruses from infected tissues. We have tried to purify the viral particles by sucrose gradient centrifugation of homogenates from intestine tissues. Unfortunately, to date, we have not obtained the viruses pure enough for infection experiments, nor for sequencing the viral genome. Currently primary culture of intestine epithelium are being tried to obtain enough viruses for future research.
Another question is the origin of these viruses in sea cucumbers, which have never been reported as hosts for any viral infection or out break of epidemic disease. From the severe traumas among virus-infected cells, it can be concluded that these sea cucumbers are not the natural hosts, but suffer as victims infected from other hosts. Even worse is that these viruses were also observed in the diseased larvae of sea cucumbers (1 mm), which were just 30 days old. In June of 2006, epidemic disease broke out during the seed cultivation in North China and caused 80% mortality. Similar viruses were observed replicated at high numbers in cytopathic cells, and causing severe pathological changes. That observation suggests that the viruses may now be transmitted vertically from the parents, even though the first infection and origin have not been confirmed yet. Now it seems less important for us to find the origin of these viruses, but to select uninfected parent sea cucumbers for brood stock cultivation.
The emergence of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) highlights the importance of virus surveillance in wild animals (Drosten et al., 2003, Ksiazek et al., 2003, Peiris et al., 2003, Susanna et al., 2005). Now a corona-like virus is reported in diseased sea cucumbers reared in North China, during epidemic mortalities. Would these viruses infect other cultured creatures in same ponds? Will it be safe for us to eat and touch these aquatics? A series of questions emerge, but one re-assuring option can be concluded! To date, all the staff involved with this study remains healthy.
Acknowledgments
This work was supported by Chinese National Programs for Science and Technology Development (2006BAD09A01) and National 863 Project (2006AA10A411).We are most grateful to C.S. Goldsmith from Centers for Disease Control and Prevention, for advice on the pathological ultrastructures and for providing the correlative references. We also thank C.Z. Gao, Dalian Medical University, for helping with the electron microscopy.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jip.2007.03.001.
Appendix A. Supplementary data
References
- Becker P., Gillan D., Lanterbecq D., Jangoux M., Rasolofonirina R., Rakotovao J., Eeckhaut I. The skin ulceration disease in cultivated juveniles of Holothuria scabra (Holothuroidea, Echinodermata) Aquaculture. 2003 [Google Scholar]
- Becker W.B., McIntosh K., Dess J.H., Chanock R.M. Morphogenesis of avian infectious bronchitis virus and a related human virus (strain 229E) J. Virol. 1967;1:1019–1027. doi: 10.1128/jvi.1.5.1019-1027.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuff R.M.L., Renault T. Purification and partial genome characterization of a herpers-like virus infecting the Japanese oyster, Crassostrea gigas. J. Gen. Virol. 1999;80:1317–1322. doi: 10.1099/0022-1317-80-5-1317. [DOI] [PubMed] [Google Scholar]
- Drosten C., Gunther S., Preiser W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Dubois D.M.E., Doller W.W., Haspel M.V., Holes K.V. Cell tropism and expression of mouse hepatitis viruses (MHV) in mouse spinal cord cultures. Virology. 1982;119:317–331. doi: 10.1016/0042-6822(82)90092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira J.F.D., Manker R.A. An electron microscope study of the development of a mouse hepatitis virus in tissue culture cells. J. Cell. Biol. 1965;24:57–78. doi: 10.1083/jcb.24.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith C.S., Tatti K.M., Ksiazek T.G. Ultrastrucutral characterization of SARS coronavirus. Emerg. Infect. Dis. 2004;10:320–326. doi: 10.3201/eid1002.030913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosert R., Kanjanahaluethai A., Egger D., Bienz K., Baker S.C. RNA replication of mouse hepatitis virus takes place at double-membrane vesicles. J. Virol. 2002;76:3697–3708. doi: 10.1128/JVI.76.8.3697-3708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Godsmith C.S. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Oshiro L.S., Schieble J.H., Lennette E.H. Electron microscopic studies of coronavirus. J. Gen. Virol. 1971;12:161–168. doi: 10.1099/0022-1317-12-2-161. [DOI] [PubMed] [Google Scholar]
- Pedersen K.W., Van D.M.Y., Roos N., Snijder E.J. Open reading frame la-encoded subunits of the artervirus replicase induce endoplasmic reticulum-derived double-membrane vesicles which carry the virual replication complex. J. Virol. 1999;73:2016–2026. doi: 10.1128/jvi.73.3.2016-2026.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris, J.S.M., Lai, S.T., Poon, L.L.M., et al., 8 April 2003. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. [Online.] http://image.thelancet.com/extras/03art3477web.pdf.
- Siddell S., Wege H., Ter M.V. The biology of coronaviruses. J. Gen. Virol. 1983;64:761–776. doi: 10.1099/0022-1317-64-4-761. [DOI] [PubMed] [Google Scholar]
- Sorour J., Larink O. Toxic effects of benomyl on the ultrastructure during spermatogenesis of the earthworm Eisenia fetid. Ecotoxicol, Environ, Saf. 2001;50:180–188. doi: 10.1006/eesa.2001.2067. [DOI] [PubMed] [Google Scholar]
- Susanna K.P.L., Patrick C.Y.W., Kenneth S.M.L. Severe acute respiratory syndrome coronavirus-like virus in chinese horseshoe bats. Proc. Natl. Acad. Sci. USA. 2005;102:14040–14045. doi: 10.1073/pnas.0506735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J., Tooze S., Warren G. Replication of coronavirus MHV-A59 in sac-cells: determination of the first site of budding of progeny virions. Eur. J. Cell. Biol. 1984;33:281–293. [PubMed] [Google Scholar]
- Wang P.H., Chang Y.Q., Xu G.R., Song L.S. Isolation and ultrastructure of an enveloped virus in cultured sea cucumber. J. Fish. Sci. Sin. 2005;12:766–771. [Google Scholar]
- Wu, Z.B., Yuan, Y.B., 2003. Cytoskeletal System. Chapter IV.10. In: Ultrastructural Pathology. First ed., Wu, Z. B., Zhou, X. J., et al. (Eds.), Shang Hai: Shanghai Science and Technology Press, pp.110--119.
- Zhang Q.F., Cui J.M., Huang X.J. Morphology and morphogenesis of severe acute respiratory syndrome (SARS)—associated virus. Acta. Biochim. Biophys. Sin. 2003;35:587–591. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


