Abstract
Liao H-F, Liu Y-C, Liu W-Y, Lin Y-T. Effectiveness of loaded sit-to-stand resistance exercise for children with mild spastic diplegia: a randomized clinical trial.
Objective
To investigate effectiveness of a functional strengthening program, the loaded sit-to-stand (STS) resistance exercise, for children with cerebral palsy (CP).
Design
A single-blind, randomized block design.
Setting
STS exercises were carried out at the children’s homes.
Participants
Twenty children (12 boys, 8 girls; age range, 5−12y) with spastic diplegia CP and classified by the Gross Motor Function Classification System as level I or II were stratified by their severity and age and randomly allocated into either the experimental or control group.
Intervention
Both groups received their regular physical therapy. The experimental group underwent loaded STS exercise 3 times a week for 6 weeks.
Main Outcome Measures
Goal dimension scores of the Gross Motor Function Measure (GMFM), gait speed, 1 repetition maximum (1-RM) of the loaded STS, isometric strength of knee extensor, and Physiological Cost Index (PCI). The outcome measures were conducted at the beginning and end of the 6-week study.
Results
After loaded STS exercise, the experimental group showed statistically significant differences in GMFM goal dimension scores, 1-RM STS, and PCI from the control group. The changes in gait speed and isometric strength of the knee extensor did not differ significantly between the 2 groups.
Conclusions
After the loaded STS exercise, children with mild spastic diplegia improved their basic motor abilities, functional muscle strength, and walking efficiency.
Key Words: Cerebral palsy, Exercise, Motor activity, Rehabilitation
CEREBRAL PALSY (CP) is described as “a group of disorders in the development of movement and posture, causing activity limitation, due to nonprogressive disturbances that occurred in the developing fetal or infant brain.”1 (p572) Children with spastic CP usually have problems of muscle weakness,2 which cause movement dysfunction.3 The muscle strength of lower limbs correlates with the motor activity function in children with CP.4, 5, 6, 7, 8 Most children with CP are of the spastic type,9 with mild CP and at the Gross Motor Function Classification System (GMFCS) levels I and II.10, 11 Although school age children with mild spastic CP can walk independently, their walking abilities are worse than their peers without disability,12 and may get worse with age,13, 14 resulting in a loss of their ability to walk.14 Therefore, an effective intervention for children with mild CP to preserve or improve their motor ability at school age is very important.
Activities of daily living are typically of the close-kinetic-chain and multiple-joint movement groups. Therefore, according to the task-oriented approach, a functional strengthening program which is a resistance exercise with a functional movement pattern (multijoint, close-kinetic-chain), has been proposed recently and has shown effectiveness in children with CP.15 However, the intervention program of the previous study included functional strengthening exercises as well as treadmill training and balance training.15 It is difficult to determine the effects of functional strengthening exercises according to the results of previous study. Besides, the resistance was provided solely by body weight,15 which may not fit in with the overload principles of the strengthening exercise.16 One of our previous studies17 showed the loaded sit-to-stand (STS) test was a reliable functional muscle strength test for children with CP with high test-retest reliability (intraclass correlation coefficient [ICC], .94). The maximum load obtained from the loaded STS test is significantly correlated with lean body mass (r=.79),18 walking velocity (r=.37),7 Physiological Cost Index (PCI) (r=.39),7 and gross motor function in children with CP (r=.80).8 In the present study, the loaded STS exercise was proposed. The loaded weight during the training was determined based on the previous results from the studies of the loaded STS test.7, 8, 17, 18 This study was designed to investigate the effectiveness of the loaded STS exercise on motor activity, muscle strength, and physiologic cost for children with mild spastic diplegia.
Methods
Participants
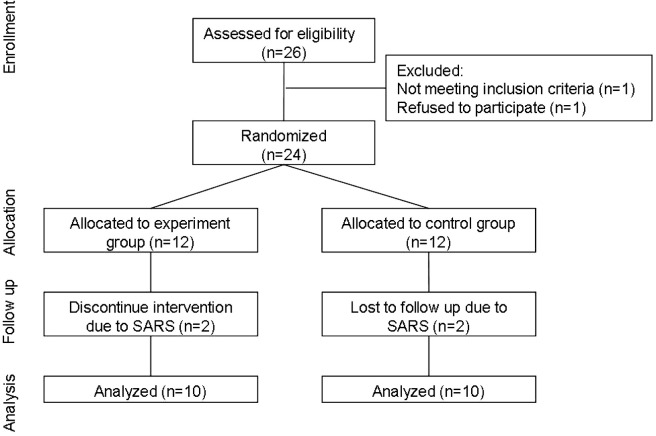
Based on a systematic review of strength training in children with CP,19 we calculated the sample size to be 9 children per group, 18 in total. The effect size was 1.20 and the power was 80%, with a 1-tailed significance level of .05.20 Before randomization, we asked the physical therapists, physicians, and special educators of 7 medical centers, teaching hospitals, and schools in northern Taipei to help recruit the children with spastic diplegia who met the inclusion criteria; 26 children (16 boys, 10 girls) were referred to us. The inclusion criteria of this study were as follows: (1) age between 5 and 12 years old; (2) spastic diplegia; (3) the GMFCS10 level I or II; (4) able to stand up from a chair independently and maintain standing for more than 5 seconds without falling; (5) able to follow verbal instructions; (6) without obvious limitation in the passive range of motion of lower extremities; (7) able to attend physical therapy (PT) treatment at least once a week before and during this study while keeping up with regular treatment programs; (8) had not received any strength-training program in the past 3 months before the study; and (9) parental commitment to allow participation without altering current therapy or activity. Exclusion criteria were: (1) have orthopedic intervention, selective dorsal rhizotomy, or botulinum toxin injection to the lower extremities within 6 months; and (2) orthopedic problems or medical conditions that prevented children from participating in the exercises. Of 26 children eligible for this study, 24 participated (fig 1). All parents of the participants signed consent forms that were approved by the Human Subjects Review Committee at National Taiwan University Hospital, Taiwan. The children were stratified by their GMFCS level (I or II) and age (≥8y or <8y) and then randomly allocated to either the experimental or the control group.
Fig 1.
Flow chart of participants’ enrollment, randomization, and data analysis. Abbreviation: SARS, severe acute respiratory syndrome.
Equipment
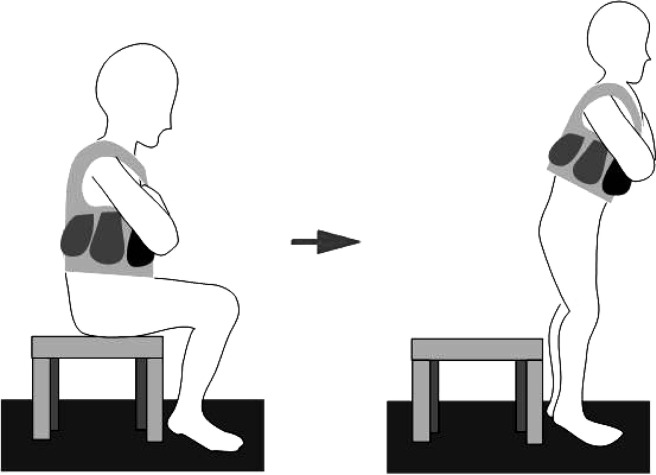
We used the Nicholas manual muscle testera to measure isometric strength of knee extensors. A Polar sports testerb was used to measure heart rate at resting and during walking. It recorded heart rate at intervals of 15 seconds. Body vests and lead weights were specially made for the loaded STS test and loaded STS exercise. Lead pieces weighed either 1 or 0.5kg. During the loaded STS test or loaded STS exercise, an appropriate amount of weight was put into the pockets of the body vest (fig 2).
Fig 2.
Loaded STS exercise conducted by a child with spastic diplegia.
Instruments
Gross Motor Function Measure
The Gross Motor Function Measure (GMFM-88) has high levels of validity, reliability, and responsiveness in evaluation of motor function and effects of intervention in children with CP.21, 22 Dimension D (13 items) and dimension E (24 items) of the GMFM-88, which measure motor activities in standing, walking, running, jumping, and hopping, were chosen as the outcome measures because they represented goal areas that many children with mild spastic diplegia have difficulty with,8 and they were activities that a lower-limb functional strengthening program was more likely to improve.
Gait speed
Self-selected gait speed has been used as the criterion standard to validate numerous outcome measures in different patient populations23 with high test-retest reliability (ICC=.85) for children with spastic CP.7 Gait speed in meters per minute was calculated using the time it took the child to walk the 10-m distance converted to meters per minute.12 Before the test, the tester had given the children instruction, such as “I’d like you to walk in the way you would normally do.” The average velocity of 3 separate trials was used as the self-selected speed.
Maximum knee extensor strength
Test-retest reliability of knee extensor strength was high (ICC=.93) for children with spastic diplegia.24 We tested each muscle 3 times. The left and right sides were tested alternatively to prevent muscle fatigue. The tester used the make test, so the instruction was, “hold, don’t let me push you down.” The average torque of 3 separate trials of both legs was used as the maximum knee extensor strength.
Maximum load of the loaded STS test
We used the 1 repetition maximum (1-RM) of the loaded STS test to represent maximum load of the loaded STS test and was defined as the maximal load a child is capable of carrying while standing up 1 time without falling from a sitting position.17 The standardized loaded STS test has high test-retest17 and interrater reliability (ICC=.94)7 for children with spastic diplegia. The testing procedures of the loaded STS test are described as follows. At the beginning, participants did general warm-up exercises for 5 minutes. Then the tester demonstrated STS movements. Chair height has been identified as 1 factor to influence STS performance in children with CP.25 Therefore, the starting position of each child was standardized with hip flexion at 90°, knee flexion at 105° (full extension was defined as 0°), feet parallel, ankle dorsiflexion at 15°, trunk erect, and hands on waist or crossing the chest. During testing, the child was asked to stand up 2 times at a comfortable speed and with symmetrical hip strategy to the defined standing position, which required the child’s trunk and lower extremities being fully extended. The hip strategy of the STS is that the child flexes his/her hip and moves the trunk forward until the shoulder is above the knee joint, and then the child stands up. The child was asked to sit down after standing for 2 seconds. The instruction was “Stand up without moving your feet and waving your arms at your comfortable speed, hold this standing position for 2 seconds, and then sit down slowly. No falling. If you try but cannot stand up, just let us know. It is OK.” Subsequent to tester’s demonstration, the child practiced STS 3 to 4 times from light load to moderate load at a comfortable speed. During the loaded STS test, the weights were added into the body vest initially at a load equaling 30% of the body weight of the subject being tested. Based on the ease of performing STS movements, the following trial’s load (increase or decrease by 0.5 to 4kg each trial) was determined. All children took a break of at least 2 minutes between test trials. The weight that the child could carry in the body vest and stand up only once was defined as the 1-RM STS of that child. The data were discarded if subjects were: (1) losing balance; (2) standing up with an obviously asymmetrical posture during test; (3) unable to maintain a standing position for 2 seconds after standing; (4) swaying their trunk back and forth several times to initiate the task of standing up; or (5) sitting down abruptly without good control. The interrater reliability of the loaded STS test for the tester was established before the beginning of the research (ICC=.94).7
Physiological Cost Index
We used the PCI to measure physiologic cost, calculated as the difference between the resting heart rate and walking heart rate and divided by the walking speed. The test-retest reliabilities (ICC range, .81−.94) and interrater reliability (ICC=.73) of PCI were high for children with CP.4, 7, 26 Concerning concurrent validity, Bowen et al27 found a moderate correlation coefficient of .50 between the PCI and oxygen cost. After a practice trial of walking at a self-selected free speed, each subject was asked to walk down a semicircular pathway, with a 15-m linear walkway, for 7 minutes after these verbal directions: “I’d like you to walk in the way you would normally do, all the way along the pathway without stopping till I ask you to stop.” The walking heart rate was defined as the average of the heart rate data collected during the last 4 minutes of walking with a constant walking speed. The higher the PCI, the higher the energy consumption required during walking.12
Design and Intervention
We used a single-blind and randomized block design for this study. At the beginning and end of this study, 1 blinded tester (Y-CL) who is a physical therapist with pediatric assessment experience (including GMFM-88, gait speed) for 6 years conducted the outcome measures and demographic data collection. The assessments for all the participants were conducted at about the same period of the day, so that all assessments would be performed in the morning for the same child, for example. At the end of a 6-week interval, the same blinded tester conducted outcome measures, including GMFM goal dimension scores, 1-RM STS, maximum isometric muscle strength of bilateral knee extensors, walking speed, and PCI. The order of outcome measures testing was first the walking tests (walking speed, PCI), then 1 muscle strength test (1-RM STS or knee extensor strength test) that was randomly chosen by coin, GMFM goal dimension scores, and last, the other muscle strength test. Participants were allowed to rest between trials of the strength test and the loaded STS test. All assessments took place in the laboratory.
For 6 weeks, the children of the experimental group executed an additional loaded STS exercise at home besides their regular PT, while the children of the control group only continued their regular PT. The regular PT programs in both groups included passive range of motion exercises, positioning, balance training, functional training, and neurodevelopment training.
For the experimental group, the trainer (Y-TL) was responsible to teach the children and their caregivers how to perform the loaded STS exercise, modify the loaded weight of the loaded STS exercise based on the loaded STS test during the every other week visit (at home or at the laboratory), and insure the compliance of loaded STS exercise during the training period via telephone interview.
For children in the experimental group, the trainer demonstrated and instructed the loaded STS exercise, and provided the body vest and weight to them and their caregivers. Children of the experimental group were asked to perform the loaded STS exercise 3 days a week, and 3 sets per day (see fig 2) under the supervision of the caregivers at home. The loaded STS exercise program started with 5 to 10 minute warm-up activities for each session. In addition to active movements of lower extremities, the warm-up included stretching of hip adductors, ankle plantarflexors, hamstring muscles, and lumbar extensors. After warming up, the child then performed STS 10 times with a body vest at 20% of 1-RM STS load. After a 1 to 2 minute rest, the child performed STS with the load at 50% of 1-RM STS repeatedly without stopping until fatigue. The height of the chair that the child sat in and performed the loaded STS exercise at home was similar to that used for the loaded STS test. Caregivers were asked to encourage the child to perform as many repetitions as possible. After the child rested for another 2 to 3 minutes, the child performed STS activities again for 10 times with 20% of 1-RM STS weight. Cooling down exercises, similar to warm-up exercises, were performed at the end of the loaded STS exercise. The progressive increasing of resistance weight was adjusted to 50% of 1-RM STS every 2 weeks according to the 1-RM STS results of the latest loaded STS test.17 An exercise diary was provided to the caregiver in the experimental group to document the child’s exercise date, weight, and repetition number of each exercise session. The trainer also educated caregivers to motivate child’s compliance for the exercise program. On average, each child took 20 to 30 minutes to complete 1 session at home.
Data Analysis
Item scores for each goal dimension of GMFM-88 (GMFM goal dimension score) were added together and converted to yield a percentage score for that dimension. The GMFM goal dimension score was derived by averaging the percentage scores for dimension D and E in this study.21 The outcome variables were GMFM goal dimension score, gait speed, 1-RM STS, knee extensor strength, and PCI. The normality of the outcome variables was examined first by the Shapiro-Wilks test. Except walking speed of the experimental group (Ws 10=.73, P=.002), the Ws 10 of all other outcome measures ranged from .86 to .97 (P range, .07−.98), which were not outside of the assumed normal distribution.
The relations between PT frequencies and the home exercise frequencies with the pre-post differences or post-training scores of the outcome measures were also examined by the Spearman correlation test, and we found no significant correlations. The post-training score of each outcome variable significantly correlated with the pretraining score (r range, .88−.90). Therefore, analysis of covariance (ANCOVA) on the post-training score using the pretraining score as a covariate was conducted for each outcome measure to test the training effects between the experimental group and the control group. The equality of error variances of the outcome measures were tested by Levene tests, and showed no significant differences between the 2 groups (F1,18 range, 0.10−2.82; P range, .11−.76). All statistical analysis was carried out using the SPSS.c We hypothesized that changes of the outcome measures in the experimental group and the control group were different and set at α equal to .05 (1-tailed). Effect sizes of various outcome measures were calculated according to the formula proposed by Guyatt et al.28 The effect size was the difference between the experimental group and the control group divided by the pooled standard deviation of change in the experimental group and the control group.
Results
There was a severe acute respiratory syndrome (SARS) epidemic in Taiwan near the end of the study. Of 24 children, 4 children (2 in the experimental group, 2 in the control group) withdrew before the study’s completion because parents were concerned about SARS and did not want their children to come to the laboratory, which was located inside a hospital, for a follow-up test.
Some of the demographic data of these children who withdrew differed from the participant children. Compared with the participant children, the children who withdrew were statistically significantly older (109.8±6.4mo), heavier (26.1±4.3kg), and taller (127.0±10.9cm). However, their outcome measure data for the preassessment were similar to the participant children in this study. For example, the GMFM goal scores of the children who withdrew were 81.5±7.4, and the 1-RM STS was 10.3±2.4. The values did not differ significantly from those of the participant group. Only 20 children with CP completed this study (follow-up rate, 83%). Their mean age, body weight, body height, and GMFCS levels are shown in table 1, and mean motor activities and muscle strength are in table 2. No statistical differences between demographic data and the 5 outcome measures of the 2 groups were found before training (t range, 0.29−1.19; independent t test, P range, .25−.78) (see Table 1, Table 2). At the beginning of the study, both the experimental and control groups had 2 children each that received PT twice a week and 8 children once a week. The PT service frequency was similar between the 2 groups.
Table 1.
Demographic Data at Study Entry of Participants Randomized to the Experimental and Control Groups
| Characteristics | Experimental Group | Control Group | t (P)⁎ |
|---|---|---|---|
| Mean age ± SD (mo) | 85.6±20.8 | 91.3±17.5 | −.66 (.52) |
| Mean body weight ± SD (kg) | 20±4.2 | 21.3±5.5 | −.59 (.56) |
| Mean height ± SD (m) | 1.15±0.09 | 1.12±0.09 | .82 (.42) |
| Sex (male/female) | 7/3 | 5/5 | NA |
| GMFCS level (I/II) | 4/6 | 6/4 | NA |
Abbreviations: NA, not applicable; SD, standard deviation.
Independent t test, experimental group vs control group.
Table 2.
ANCOVA on Post-Training Scores With the Pretraining Scores as the Covariate
| Variables | Actual Pretraining | Actual Post-Training | Adjusted Post-Training | Mean Square and F Values | P (1-tailed) |
|---|---|---|---|---|---|
| GMFM goal dimension score (%) | |||||
| Experimental | 76.6±4.4 | 79.8±4.1 | 82.7±0.7 | 21.82, F1,17=4.81 | .02⁎ |
| Control | 83.1±3.2 | 83.5±2.8 | 80.6±0.7 | ||
| Gait speed (m/min) | |||||
| Experimental | 56.9±5.1 | 58.4±5.0 | 61.3±1.7 | 24.56, F1,17=0.87 | .18 |
| Control | 63.8±3.0 | 62.0±2.6 | 59.0±1.7 | ||
| 1-RM STS (kg) | |||||
| Experimental | 9.6±1.6 | 13.5±1.9 | 14.4±0.5 | 47.81, F1,17=17.7 | .001⁎ |
| Control | 11.3±1.8 | 12.2±2.0 | 11.3±0.5 | ||
| Knee extensor strength (kg) | |||||
| Experimental | 5.3±0.8 | 6.0±0.7 | 6.1±0.4 | 0.06, F1,16=0.05 | .42 |
| Control | 5.7±1.1 | 6.4±0.8 | 6.2±0.4 | ||
| PCI (beats/min) | |||||
| Experimental | 1.14±0.14 | 1.01±0.14 | 0.96±0.04 | 0.12, F1,17=8.04 | .005⁎ |
| Control | 1.02±0.09 | 1.07±0.05 | 1.12±0.04 |
NOTE. Values are mean ± standard error.
P<.05, significant difference between experimental and control groups.
Although the investigatiors attempted to standardize the frequency and volume of the training, the children did not perform exactly as expected because of other activities. According to their exercise diary, children in the experimental group performed the loaded STS exercise 18.0±3.2 times (range, 12−21 times) during the 6-week training period. All children of the experimental group had loaded STS exercise at least twice a week, and 3 children exercised more than 3 times a week because the caregivers wanted more than we asked. Their average maximum repetitions of 50% of 1-RM STS varied from 20 to 100 each session during the training. With program progress, the mean 1-RM STS ± standard deviation increased from 9.6±5.2kg, to 11.0±5.2kg, to 12.6±6.0 kg, and to 13.5±5.9kg in the experiment group every 2 weeks. Therefore, the mean load used for loaded STS exercise during the first 2 weeks was 4.8kg (range, 1−9kg), the load for the following 2 weeks was 5.5kg (range, 2.3−9.5kg), and the load for the last 2 weeks was 6.3kg (range, 2.5−10.5kg). Children in both groups decreased or stopped PT services during this study because of the fear of the SARS epidemic in Taiwan. In the experimental group, 4 children received PT once a week, 2 children once every 2 weeks, and 4 children discontinued PT. In the control group, 1 child received PT twice a week, 5 once a week, 1 once every 2 weeks, and 3 received no PT at all. In general, children of the control group received PT more frequently during the study period.
Table 2 shows the actual pre- and post-training score means and the adjusted post-training means of the 5 outcome measures of the 2 groups, and the ANCOVA analysis on the post-training scores. Results indicated that, after a 6-week loaded STS exercise, there were significant differences in the GMFM goal dimension score (F1,17=4.81, P<.05), 1-RM STS (F1,17=17.7, P<.05), and PCI (F1,17=8.04, P<.05) between children in the experimental group and the control group. The effect size of the GMFM goal dimension score after the functional strengthening was 1.17 (95% confidence interval [CI], 0.18−2.07), of the 1-RM STS was 1.78 (95% CI, 0.68−2.73), and of the PCI was 1.34 (95% CI, 0.32−2.25). Nonetheless, there were no significant differences in gait speed and knee extensor strength between the 2 groups.
Discussion
The results of this study showed that a 6-week low-load STS exercise at home significantly promoted 1 motor activity parameter (GMFM goal dimension score) and 2 body function parameters (1-RM STS, PCI) in children with mild spastic diplegia CP. The compliance of this home strengthening exercise was high. The children could easily and correctly execute loaded STS exercise at home under their parents’ supervision and physical therapists’ regular monitoring. According to the classification of evidence levels for therapy studies proposed by Straus et al,29 this study belongs to level 2b.
Muscle-strengthening programs with single joint, open-kinetic-chained, and non-weight-bearing resistive exercises have been used for the treatment of children with CP,30, 31 and have shown evidence of improvement in the muscle strength of the target muscles19, 30, 32; however, their effectiveness on motor activities still remains controversial.19 The reasons for inconclusive effectiveness on motor activities may be due to the fact that single-joint and non-weight-bearing exercises are not task-oriented approaches and have limited transferability to actions that are weight bearing and that involve different and more complex patterns of muscle activation.33 That is the specificity of the strengthening program.15
Studies that have examined the effects of the functional strength training for children with CP, showed improvement in muscle strength, physical performance, and psychologic benefits.15, 34, 35 Dodd et al35 conducted a randomized clinical trial and found that a home-based, 6-week functional strength-training program could significantly increase the lower-limb strength, and might have beneficial effects on activities (GMFM) for children with spastic diplegia. The present study design was similar to Dodd’s study. Both studies were randomized clinical trials and home-based functional muscle strengthening programs for 6 weeks for children with spastic diplegia CP. However, the present study recruited younger children with milder CP than did Dodd’s study. Further study is needed to clarify the effectiveness of the loaded STS exercise for children with different ages and severities of CP.
This study showed that the loaded STS exercise could improve GMFM scores and muscle strength during STS activities, and decrease energy consumption during walking in some children with spastic diplegia CP. This functional strengthening program was highly effective in comparison to other strengthening programs because of the 4 main features of successful strengthening proposed by exercise specialists— specificity, intensity (overload), frequency, and volume,17, 36, 37 which were all well defined in this study. The issue of specificity was mentioned before. The variables of intensity included the load of resistance, repetition number per set, and the number of sets per session. In this study, the resisted load progressively increased every 2 weeks. The resisted load of 50% of 1-RM STS was chosen due to the fact that high-repetition moderate-load strength training was better than the low-repetition heavy-load in children.38, 39 For training frequency of resisted exercise, 2 days per week, 3 to 4 sets per session could effectively increase the muscle strength in children aged 8 to 12 years.36, 40
The present study showed that after training the GMFM goal scores significantly increased with an effect size of 1.17. After inspecting the raw data of the GMFM goal dimensions for every child of both groups, we found that the items that showed improvement after training in most children of the experimental group were activities related to jumping. Jumping is the fundamental movement that occurs when the body is projected into the air by the force generated in the extensor muscles of the lower legs and the body lands with flexed lower legs and an adjustment of the center of gravity of the whole body.41 Therefore, it requires both the high muscle strength of the lower legs and the balance control that the loaded STS exercise may be able to promote.
The present study showed that the loaded STS exercise could not significantly improve the gait speed and knee extensor strength when compared with the control group. The effects of the muscle strengthening program on the walking speed were controversial in the previous studies.5, 6, 31 Buchner et al42 proposed a theory that there was a curvilinear relationship between gait speed and muscle strength. The benefit of a muscle strengthening program on gait speed depends on the target group. Only those whose muscle strength was less than the demand of the walking task can have benefits from the strengthening program. The children in the present study had mild CP, and their muscle weakness problem may not have been severe enough to impair the gait speed. Therefore, the gait speed did not improve significantly after the loaded STS exercise. The loaded STS exercise is an isotonic contraction, but the maximum knee extensor strength was tested by isometric contraction in this study. Specificity of the strengthening program may be able to explain the ineffectiveness of the loaded STS exercise on the knee extensor strength. The isotonic muscle strengthening of knee extensors increased mainly the isotonic muscle strength.43
The possible adverse effects of the loaded STS exercise were similar to that reported by McBurney et al,34 such as equipment, parental psychologic and physical assistance, and the need of 20 to 30 minutes of spare time to finish this home exercise program. Most children (80%) reported pressure on the shoulders from the body vest during the loaded STS exercise; however, no pain or injury was reported due to the training. In the present study, the withdrawn children were older than the participant children. The heavier homework load at the senior class of the elementary school may be one of the reasons to cause the interruption of a follow-up or intervention program. However, the age of the children did not significantly correlate with the home-exercise frequency and scores of the outcome measures and the motor severities of the withdrawn children were similar to the participant children. Therefore, the results of this study might be generalized to the children with mild spastic CP.
There are some limitations in this study. First, inconsistency of regular treatment programs occurred during the study. However, in general, children of the control group received PT services more frequently than the experiment group. The treatment number and the effects of PT services would more likely have represented an advantage in favor of the children in the control group. Therefore, the favorable results in the experimental group could not be explained by this attribute. Second, the duration of the follow-up was not sufficient. The immediate effect was positive. It would be interesting to see if the positive effects observed in this study are maintained longitudinally. Third, this is a single-blind study, and children and their parents were not kept blind to the treatment condition. Therefore, children of the experiment group may be psychologically encouraged to perform better in the post-training test. Finally, only children with limited severity and diagnosis participated in this study. Therefore, future studies are needed to investigate the long-term effects of the loaded STS exercise for children with various severities of CP or with other disabilities.
Conclusions
Results of this randomized clinical trial support that a 6-week home-based functional strengthening program can improve basic motor abilities, functional muscle strength, and walking efficiency in children with mild spastic diplegia CP aged 5 to 12 years. The loaded STS exercise program had high compliance and could be implemented by parents at home with simple equipment (body vest, weights, and chair of appropriate height) and with regular follow-ups by clinicians (1 time/2wk). Further studies are needed to develop effectiveness of the loaded STS exercise for children with different severities of CP.
Supplier
Footnotes
Supported by the National Science Council, Taiwan (grant no. NSC90-2314-B-002-315).
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the author(s) or upon any organization with which the author(s) is/are associated.
Model 01160; Lafayette Instrument Co, 3700 Sagamore Pkwy N, PO Box 5729, IN 47903.
Polar Electro Oy, Professorintie 5, FIN-90440 Kempele, Finland.
Version 10.0; SPSS Inc, 233 S Wacker Dr, 11th Fl, Chicago, IL 60606-6307.
References
- 1.Bax M., Goldstein M., Rosenbaum P., Executive Committee for the Definition of Cerebral Palsy Proposed definition and classification of cerebral palsy. Dev Med Child Neurol. 2005;47:571–576. doi: 10.1017/s001216220500112x. [DOI] [PubMed] [Google Scholar]
- 2.Wiley M.E., Damiano D.L. Lower-extremity strength profiles in spastic cerebral palsy. Dev Med Child Neurol. 1998;40:100–107. doi: 10.1111/j.1469-8749.1998.tb15369.x. [DOI] [PubMed] [Google Scholar]
- 3.Fetters L. Measurement and treatment in cerebral palsy: an argument for a new approach. Phys Ther. 1991;71:244–274. doi: 10.1093/ptj/71.3.244. [DOI] [PubMed] [Google Scholar]
- 4.Kramer J.F., MacPhail H.E. Relationship among measures of walking efficiency, gross motor ability, and isokinetic strength in adolescents with cerebral palsy. Pediatr Phys Ther. 1994;6:3–8. [Google Scholar]
- 5.MacPhail H.E., Kramer J.F. Effect of isokinetic strength-training on functional ability and walking efficiency in adolescents with cerebral palsy. Dev Med Child Neurol. 1995;37:763–775. doi: 10.1111/j.1469-8749.1995.tb12060.x. [DOI] [PubMed] [Google Scholar]
- 6.Damiano D.L., Abel M.F. Functional outcome of strength training in spastic cerebral palsy. Arch Phys Med Rehabil. 1998;79:119–125. doi: 10.1016/s0003-9993(98)90287-8. [DOI] [PubMed] [Google Scholar]
- 7.Liu C.C., Liao H.F., Lin K.H. The relations between the sit-to-stand functional muscle strength and walking capacity in children with mild spastic diplegia. Formos J Phys Ther. 2004;29:176–183. [Google Scholar]
- 8.Liao H.F., Huang W.B., Hsu A.T., Wong A.M. Loaded sit-to-stand capacity and gross motor function, muscle strength of lower extremities in children with spastic diplegia. Formos J Phys Ther. 2005;30:207–216. [Google Scholar]
- 9.Nordmark E., Hagglund G., Lagergren J. Cerebral palsy in southern Sweden: II. Gross motor function and disabilities. Acta Paediatr. 2001;90:1277–1282. doi: 10.1080/080352501317130326. [DOI] [PubMed] [Google Scholar]
- 10.Palisano R.J., Rosenbaum P.L., Walter S.D., Russell D.J., Wood E.P., Galuppi B.E. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 11.Beckung E., Hagberg G. Neuroimpairments, activity limitations, and participation restrictions in children with cerebral palsy. Dev Med Child Neurol. 2002;44:309–316. doi: 10.1017/s0012162201002134. [DOI] [PubMed] [Google Scholar]
- 12.Liao H.F., Jeng S.F., Lai J.S., Cheng C.K., Hu M.H. The relation between standing balance and walking function in children with spastic diplegic cerebral palsy. Dev Med Child Neurol. 1997;39:106–112. doi: 10.1111/j.1469-8749.1997.tb07392.x. [DOI] [PubMed] [Google Scholar]
- 13.Bell K.J., Ounpuu S., Deluca P.A., Romness M.J. Natural progression of gait in children with cerebral palsy. J Pediatr Orthop. 2002;22:677–682. [PubMed] [Google Scholar]
- 14.Bottos M., Feliciangeli A., Sciuto L., Bologna A., Gericke C., Vianello A. Functional status of adult with cerebral palsy and implications for treatment of children. Dev Med Child Neurol. 2001;43:516–528. doi: 10.1017/s0012162201000950. [DOI] [PubMed] [Google Scholar]
- 15.Blundell S.W., Shepherd R.B., Dean C.M., Adams R.D., Cahill B.M. Functional strength training in cerebral palsy: a pilot study of a group circuit training class for children aged 4-8 years. Clin Rehabil. 2003;17:48–57. doi: 10.1191/0269215503cr584oa. [DOI] [PubMed] [Google Scholar]
- 16.Faigenbaum A.D., Kraemer W.J., Cahill B. Youth resistance training: position statement paper and literature review. Strength Cond. 1996;18:62–75. [Google Scholar]
- 17.Gan S.M., Liao H.F. The reliability study and comparison of sit-to-stand repetitive maximum capacity in children with cerebral palsy and children without disability. Formos J Phys Ther. 2002;27:292–302. [Google Scholar]
- 18.Liao H.F., Liu Y.C., Liu C.C. The relations between body composition and muscle strength of lower limb in independent walker with spastic diplegia and non-disabled children. Formos J Phys Ther. 2002;27:273–282. [Google Scholar]
- 19.Dodd K.J., Taylor N.F., Damiano D.L. A systematic review of the effectiveness of strength-training programs for people with cerebral palsy. Arch Phys Med Rehabil. 2002;83:1157–1164. doi: 10.1053/apmr.2002.34286. [DOI] [PubMed] [Google Scholar]
- 20.Portney L.G., Watkins M.P. Foundations of clinical research: applications to practice. 2nd ed. Prentice Hall; Upper Saddle River: 2000. [Google Scholar]
- 21.Russell D., Rosenbaum P., Avery L.M., Lane M. Gross Motor Function Measure (GMFM-66 & GMFM-88) user’s manual. Mac Keith Pr; London: 2002. [Google Scholar]
- 22.Bjornson K.F., Graubert C.S., Buford V.L., McLaughlin J. Validity of the Gross Motor Function Measure. Pediatr Phys Ther. 1998;10:43–47. [PubMed] [Google Scholar]
- 23.Finch E., Brooks D., Stratford P.W., Mayo N.E. Physical rehabilitation outcome measures: A guide to enhanced clinical decision making. 2nd ed. Canadian Physiotherapy Association; Hamilton: 2002. [Google Scholar]
- 24.Hwang A.W., Liao H.F., Hsu A.T., Gan S.M., Lee C.R. Reliability of Nicholas hand-held dynamometer of muscle strength measurement in non-disabled children and children with cerebral palsy. Formos J Phys Ther. 2002;27:69–82. [Google Scholar]
- 25.Hennington G., Johnson J., Penrose J., Barr K., McMulkin M.L., Vander Linden D.W. Effect of bench height on sit-to-stand in children without disabilities and children with cerebral palsy. Arch Phys Med Rehabil. 2004;85:70–76. doi: 10.1016/s0003-9993(03)00407-6. [DOI] [PubMed] [Google Scholar]
- 26.Izeman M.J., Nene A.V. Feasibility of the physiological cost index as an outcome measure of the assessment of energy expenditure during walking. Arch Phys Med Rehabil. 2002;83:1777–1782. doi: 10.1053/apmr.2002.35655. [DOI] [PubMed] [Google Scholar]
- 27.Bowen T.R., Lennon N., Castagno M.S., Miller F., Richard J. Variability of energy-consumption measures in children with cerebral palsy. J Pediatr Orthop. 1998;18:738–742. [PubMed] [Google Scholar]
- 28.Guyatt H., King D.R., Feeny D.H., Stubbing D. Generic and specific measurement of health-related quality of life in a clinical trial of respiratory rehabilitation. J Clin Epidemiol. 1999;52:187–192. doi: 10.1016/s0895-4356(98)00157-7. [DOI] [PubMed] [Google Scholar]
- 29.Straus S.E., Richardson W.S., Glasziou P., Haynes R.B. Evidence-based medicine: how to practice and teach EBM. 3rd ed. Churchill Livingstone; New York: 2005. [Google Scholar]
- 30.Damiano D.L., Vaughan C.L., Abel M.F. Muscle response to heavy resistance exercise in children with spastic cerebral palsy. Dev Med Child Neurol. 1995;37:737–739. doi: 10.1111/j.1469-8749.1995.tb15019.x. [DOI] [PubMed] [Google Scholar]
- 31.Damiano D.L., Kelly L.E., Vaughn C.L. Effects of quadriceps femoris muscle strengthening on crouch gait in children with spastic diplegia. Phys Ther. 1995;75:658–671. doi: 10.1093/ptj/75.8.658. [DOI] [PubMed] [Google Scholar]
- 32.Morton J.F., Brownlee M., McFadyen A.K. The effects of progressive resistance training for children with cerebral palsy. Clin Rehabil. 2005;19:283–289. doi: 10.1191/0269215505cr804oa. [DOI] [PubMed] [Google Scholar]
- 33.Carr J.H., Shephard R.B. Neurological rehabilitation: optimizing motor performance. Butterworth-Heinemann; Oxford: 1999. [Google Scholar]
- 34.McBurney H., Taylor N.F., Dodd K.J., Graham H.K. A qualitative analysis of the benefits of strength training for young people with cerebral palsy. Dev Med Child Neurol. 2003;45:658–663. doi: 10.1017/s0012162203001233. [DOI] [PubMed] [Google Scholar]
- 35.Dodd K.J., Taylor N.F., Graham H.K. A randomized clinical trial of strength training in young people with cerebral palsy. Dev Med Child Neurol. 2003;45:652–657. doi: 10.1017/s0012162203001221. [DOI] [PubMed] [Google Scholar]
- 36.Faigenbaum A.D., Zaichkowsky L.D., Westcott W.L., Micheli L.J. The effects of a twice-week strength training program on children. Pediatr Exerc Science. 1993;5:339–346. [Google Scholar]
- 37.Zatsiosky V.M. Science and practice of strength training. Human Kinetics; Champaign: 1995. [Google Scholar]
- 38.Faigenbaum A.D., Westcott W.L., Loud R.L., Long C. The effects of different resistance training protocols on muscular strength and endurance development in children. Pediatrics. 1999;104:e5. doi: 10.1542/peds.104.1.e5. [DOI] [PubMed] [Google Scholar]
- 39.Faigenbaum A.D. Strength training for children and adolescents. Clin Sports Med. 2000;19:593–619. doi: 10.1016/s0278-5919(05)70228-3. [DOI] [PubMed] [Google Scholar]
- 40.Webb D.R. Strength training in children and adolescents. Sports Med. 1990;37:1187–1210. doi: 10.1016/s0031-3955(16)36983-8. [DOI] [PubMed] [Google Scholar]
- 41.Payne V.G., Isaacs L.D. Human motor development: a life approach. 2nd ed. Mayfield; Mountain View: 1995. pp. 247–256. [Google Scholar]
- 42.Buchner D.M., Beresford S.A., Larson E.B. Effect of physical activity on health status in older adults: II: intervention studies. Annu Rev Public Health. 1992;13:469–488. doi: 10.1146/annurev.pu.13.050192.002345. [DOI] [PubMed] [Google Scholar]
- 43.Rutherford O.M., Jones D.A. The role of learning and coordination in strength training. Eur J Appl Physiol. 1986;55:100–105. doi: 10.1007/BF00422902. [DOI] [PubMed] [Google Scholar]