Summary
Respiratory viruses that emerge in the human population may cause high morbidity and mortality, as well as concern about pandemic spread. Examples are severe acute respiratory syndrome coronavirus (SARS-CoV) and novel variants of influenza A virus, such as H5N1 and pandemic H1N1. Different animal models are used to develop therapeutic and preventive measures against such viruses, but it is not clear which are most suitable. Therefore, this review compares animal models of SARS and influenza, with an emphasis on non-human primates, ferrets and cats. Firstly, the pathology and pathogenesis of SARS and influenza are compared. Both diseases are similar in that they affect mainly the respiratory tract and cause inflammation and necrosis centred on the pulmonary alveoli and bronchioles. Important differences are the presence of multinucleated giant cells and intra-alveolar fibrosis in SARS and more fulminant necrotizing and haemorrhagic pneumonia in H5N1 influenza. Secondly, the pathology and pathogenesis of SARS and influenza in man and experimental animals are compared. Host species, host age, route of inoculation, location of sampling and timing of sampling are important to design an animal model that most closely mimics human disease. The design of appropriate animal models requires an accurate pathological description of human cases, as well as a good understanding of the effect of experimental variables on disease outcome.
Keywords: animal model, influenza, respiratory disease, SARS coronavirus
Introduction
In man, viral infections causing respiratory disease have been known for many years. Every now and then such viruses may cause epidemics involving large groups of people or even pandemics with worldwide spread. At the end of the last century and at the beginning of this century zoonotic viruses emerged that were of serious risk for the human population: severe acute respiratory syndrome (SARS) caused by SARS coronavirus (CoV), highly pathogenic avian influenza (HPAI) virus H5N1 and pandemic influenza virus A(H1N1)pdm09 (pH1N1). Both SARS-CoV and influenza A viruses cause respiratory disease that may lead to severe and even fatal cases of pneumonia. The course and outcome of these infections are related to their pathogenesis, which can be explored by describing and comparing pathology, virology and genomics. Understanding the pathogenesis of SARS and influenza is valuable for development of therapeutic and preventive strategies. Since the pathology of acute human fatal cases of SARS and influenza is rarely described, there is a need for animal models to provide information about the early stages of the disease. Additionally, pathological descriptions of human cases with uncomplicated viral pneumonia are sparse because patients have multiple therapeutic interventions and secondary co-infections that may alter the pathology. This review focuses on the pathology and pathogenesis of SARS-CoV and influenza A virus infections, not only comparing the two viruses, but also comparing the pathology of these virus infections in experimental animals to that in man (van den Brand, 2013).
Severe Acute Respiratory Syndrome Coronavirus
Background
In November 2002, there was an unusual epidemic of severe pneumonia of unknown cause in Guangdong province in southern China that spread rapidly across the world, peaked in the first half of 2003 and ended by July 2003 (Xu et al., 2004). A total number of 8,096 probable cases were reported by the World Health Organization (WHO), which resulted in 774 deaths, a fatality rate of almost 9.6% (WHO, 2003). A novel coronavirus, SARS-CoV, was proven to be the cause of the disease by fulfilling Koch's postulates (Drosten et al., 2003, Fouchier et al., 2003, Kuiken et al., 2003a).
SARS is a zoonotic disease and bats are believed to be the reservoir host of this virus (Li et al., 2005b). Masked palm civet cats and raccoon dogs, which are kept and sold at so-called Chinese wet-markets (markets selling live poultry, fish and exotic animals for human consumption), have provided transmission of the virus to man (Guan et al., 2003, Webster, 2004). In 2005, horseshoe bats were identified as a natural reservoir for a group of coronaviruses closely related to SARS-CoV with approximately 88–92% homology by genome sequences (Lau et al., 2005, Li et al., 2005b). SARS is spread by close person-to-person contact through droplet transmission or excretions (Peiris et al., 2003b), as was demonstrated in a hospital where virus spread due to over-crowding and poor ventilation (Wong et al., 2005), and later also by airborne transmission in a private residential complex in Hong Kong (Yu et al., 2005).
The Pathology of Severe Acute Respiratory Syndrome in Man
Gross pathology of the respiratory tract demonstrates a variable degree of consolidation, oedema, haemorrhage and congestion of the lungs, and pleural effusion in the thoracic cavity (Ding et al., 2003, Nicholls et al., 2003, Tse et al., 2004). The histopathology of SARS is characterized by diffuse alveolar damage (DAD). The stage of DAD is related to the duration of the illness and may be divided into an exudative phase, a proliferative phase and a fibrotic phase. Patients in the initial 10 days of the disease demonstrate an exudative phase, with necrosis of alveolar epithelial cells, intraluminal oedema, fibrin exudation, hyaline membrane formation, haemorrhage and infiltrates with inflammatory cells such as monocytes or macrophages, lymphocytes and neutrophils into the alveolar wall and lumina (Ding et al., 2003, Nicholls et al., 2003, Hsueh et al., 2004, Shieh et al., 2005). There is necrosis of the bronchiolar and bronchial epithelium with infiltration of monocytes, lymphocytes and neutrophils into the bronchial wall (Ding et al., 2003, Franks et al., 2003). In the proliferative phase, after 10–14 days, there is less epithelial damage with interstitial and alveolar fibrosis, bronchiolitis obliterans organizing pneumonia (BOOP) and regeneration that is characterized by type II pneumocyte hyperplasia (Franks et al., 2003, Cheung et al., 2004, Shieh et al., 2005, He et al., 2006). Large multinucleated cells composed of macrophages or pneumocytes are frequently observed and atypical enlarged pneumocytes with large nuclei, amphophilic granular cytoplasm and prominent nucleoli are seen (Franks et al., 2003, Nicholls et al., 2003, Tse et al., 2004). In the fibrotic phase after 14 days interstitial thickening is described with mild to moderate fibrosis and BOOP-like pattern and only few inflammatory cells (mainly histiocytes and lymphocytes) (Cheung et al., 2004, Tse et al., 2004). Other features are haemophagocytosis, squamous metaplasia (Nicholls et al., 2003) and fibrin thrombi in vessels (Ding et al., 2003).
Extrarespiratory changes are present that differ in severity of the pathology and vary with the duration of illness. The lymphoid system demonstrates haemophagocytic syndrome and lymphoid depletion or necrosis in lymph nodes and white pulp of the spleen (Ding et al., 2003, Gu et al., 2005). The pathology in other organs is characterized by acute tubular necrosis in the kidneys, oedema and degeneration of neurons in the central nervous system, myofibre necrosis and atrophy in skeletal muscles, necrosis and infiltration of lymphocytes and monocytes in the adrenal gland, destruction of follicular epithelial cells in the thyroid gland, germ cell destruction in the testes and oedema and atrophy of myocardial fibres in the heart (Ding et al., 2003). In the intestinal tract there is depletion of mucosal lymphoid tissue (Leung et al., 2003, Gu and Korteweg, 2007).
Animal Models for Human Severe Acute Respiratory Syndrome
Many different animal species are found to be susceptible to SARS-CoV and subsequently demonstrate viral replication and disease: non-human primates, cats, ferrets, mice, pigs, chickens, hamsters, guinea pigs and rats (Martina et al., 2003, Wentworth et al., 2004, Weingartl et al., 2004b, Liang et al., 2005, Roberts et al., 2006, Rockx et al., 2009). Experimental SARS-CoV infection in each of these species has been performed for a variety of purposes and resembles the clinical and pathological characteristics of SARS in man to a variable degree. Mice and hamsters have been used in studies of pathogenesis and screening for vaccines and antiviral drugs (Yang et al., 2004b, Roberts et al., 2005). Young mice show viral replication that is not accompanied by substantial inflammation in the lungs; in contrast, old BALB/c mice develop clinical illness with weight loss and histopathological changes characterized by pneumonitis and bronchiolitis (Wentworth et al., 2004, Roberts et al., 2005). Non-human primates, such as cynomolgus macaques, African green monkeys and rhesus macaques, have been used to evaluate treatment and for pathogenesis studies (Gao et al., 2003, Kuiken et al., 2003a, Bukreyev et al., 2004, McAuliffe et al., 2004, Rowe et al., 2004, Li et al., 2005a, Qin et al., 2006). Cats and ferrets have been used to evaluate the pathogenesis, while ferrets have also been used for vaccination studies (Martina et al., 2003, Weingartl et al., 2004a). Although different animal species are susceptible to SARS-CoV infection, no animal model has been established in which all aspects of the severe human disease are replicated accurately. In this review, SARS-CoV infection is described in non-human primates (cynomolgus macaques and African green monkeys [AGMs]) and carnivores (ferrets and cats) (Fouchier et al., 2003, Kuiken et al., 2003a, Martina et al., 2003, McAuliffe et al., 2004).
Non-Human Primates
In cynomolgus macaques the age of the animals influences the pathology after SARS-CoV infection. The severity of the lesions in aged cynomolgus macaques infected with SARS-CoV is more extensive than in young-adult macaques, including severe oedema with hyaline membrane formation and syncytia (Smits et al., 2010) (Fig. 1 ). Young-adult AGMs develop more severe lesions from SARS-CoV infection than young-adult cynomolgus macaques (Fouchier et al., 2003, Kuiken et al., 2003a, McAuliffe et al., 2004, Smits et al., 2011). By gross pathology, the percentage of affected lung tissue is higher in AGMs than in cynomolgus macaques and is characterized by multifocal consolidation. By histopathology, there is acute exudative DAD characterized by necrosis of alveolar epithelium, moderate multifocal hypertrophy and hyperplasia of type II pneumocytes, variable intraluminal oedema and exudate sometimes with hyaline membrane formation, mild multifocal necrosis and regeneration of bronchiolar epithelium, and sometimes intraluminal syncytial cells or mild multifocal tracheobronchoadenitis (inflammation of the submucosal glands of the trachea and bronchi). These pulmonary lesions are significantly more severe in young-adult AGMs than in young-adult cynomolgus macaques. However, the character of the pulmonary lesions is similar, except that young-adult AGMs show hyaline membranes and young-adult cynomolgus macaques do not (Table 1 ).
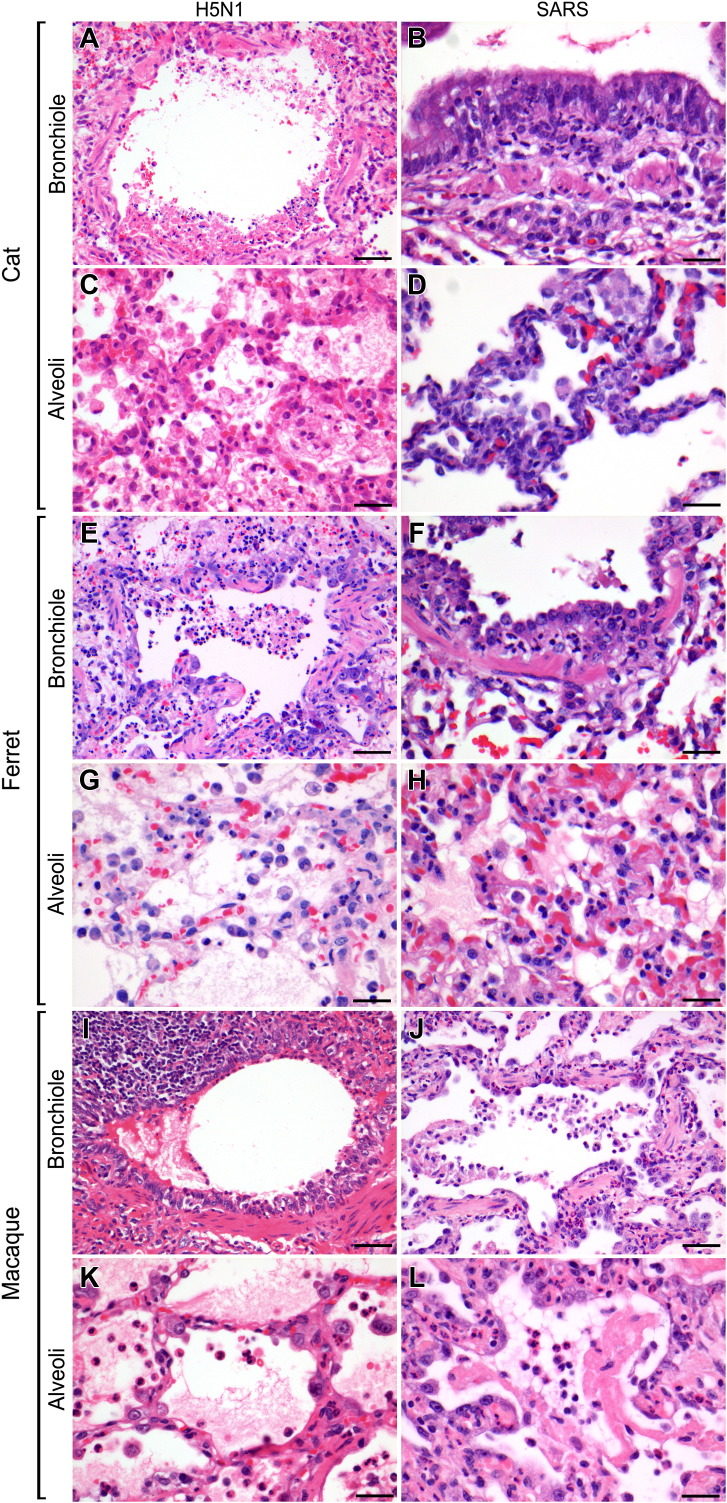
Fig. 1.
Lesions in the bronchioles and alveoli of cats, ferrets and cynomolgus macaques infected experimentally with H5N1 influenza virus and SARS-CoV are characterized by DAD and bronchiolitis. HE. Bars, 50 μm (A, E, I and J). Bars, 20 μm (B–D, F–H, K and L).
Table 1.
Histopathology, virus antigen expression and ACE2 antigen expression in different species infected with SARS-CoV
| Species | Age | Histological lesions |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alveoli |
Bronchioles |
Bronchi |
Interstitium |
Trachea |
|||||||||||
| Epithelial necrosis | Oedema | Hyaline membranes | Inflammation | Syncytial cells | Type II pneumocyte hyperplasia | Epithelial necrosis | Inflammation | Epithelial necrosis | Inflammation | Perivascular/peribronchiolar cuffing | Tracheobronchoadenitis | Epithelial necrosis | Inflammation | ||
| Human | + | + | + | + | + | + | + | + | − | − | − | − | − | ||
| Macaque∗ | Young-adult | + | + | − | + | − | + | + | + | − | − | + | + | − | − |
| Aged | + | + | + | + | + | + | + | + | − | − | + | − | − | − | |
| AGM† | Young-adult | + | + | + | + | + | + | + | + | − | − | + | + | − | − |
| Ferret | Young-adult | + | + | − | + | − | + | + | + | − | − | + | − | − | − |
| Cat | Young-adult | + | − | − | + | − | + | + | + | − | − | − | + | − | − |
| Species | Age | Virus antigen expression |
ACE2 antigen expression |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type I pneumocytes | Type II pneumocytes | Alveolar macrophages | Bronchiolar epithelial cells | Bronchial epithelial cells | Tracheal epithelial cells | Serous cells of submucosal glands | Type I pneumocytes | Type II pneumocytes | Alveolar macrophages | Bronchiolar epithelial cells | Bronchial epithelial cells | Pulmonary goblet cells | Tracheal epithelial cells | Serous cells of submucosal glands | ||
| Human | + | + | + | + | + | + | − | + | + | + | + | + | − | + | + | |
| Macaque∗ | Young-adult | + | + | + | + | + | − | − | − | + | + | + | N | N | N | N |
| Aged | + | + | + | + | + | − | − | − | + | + | + | N | N | N | N | |
| AGM† | Young-adult | + | + | + | + | − | + | − | − | + | N | N | N | N | N | N |
| Ferret | Young-adult | − | + | + | − | − | − | − | − | + | + | + | + | − | + | + |
| Cat | Young-adult | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
N, not done.
Cynomolgus macaque.
African green monkey.
Carnivores
Cats and ferrets infected with SARS-CoV develop similar lesions in the respiratory tract as people, macaques and AGMs (Table 1, Fig. 1). In the lungs of cats there is a multifocal, mild to moderate exudative DAD and multifocal mild to moderate tracheobronchoadenitis. In ferrets infected with SARS-CoV, the resultant DAD is more extensive and severe in ferrets than in cats, and includes alveolar oedema. Another difference is that cats develop tracheobronchoadenitis, while ferrets do not.
Comparative Pathology
When the pathological features of SARS in man and the above-mentioned laboratory animal species are compared, there are both similarities and differences in localization, character and severity (Table 1) (Ding et al., 2003, Franks et al., 2003, Gu et al., 2005, Shieh et al., 2005). Firstly, the localization of the lesions is similar among all species: lesions are centred on alveoli and bronchioles. Additionally, AGMs, young-adult macaques and cats have lesions in the submucosal glands of trachea and bronchi that are not seen in man. Secondly, the character of the lesions is similar among all species; these characteristics are epithelial necrosis, infiltration of inflammatory cells and type II pneumocyte hyperplasia. Additionally, syncytia and hyaline membranes are present in lesions of man, aged macaques and AGMs. Thirdly, the severity of the lesions among species can be divided into two groups. Man, aged macaques and AGMs have severe DAD, characterized by oedema, fibrin and hyaline membranes. Young-adult macaques, cats and ferrets have milder DAD demonstrating a more multifocal distribution and lacking the above-named features.
The development of fibrosis in the late stages of human cases of SARS may be related to several factors. Firstly, there is irreversible damage to the pneumocytes, which therefore fail to re-epithelialize the alveolar walls. Instead, the denuded basement membrane is repaired by fibrosis. Secondly, there may be a specific epithelial sensitivity to interferon (IFN)-γ: lung epithelial cells are more responsive to IFN-γ-induced damage than fibroblasts during SARS-CoV infection. Thirdly, there may be a T-helper (Th)1-dominant immune-mediated cell death, which may favour the damage to infected alveolar epithelial cells over damage to non-infected fibroblasts, leaving the latter relatively intact. This would mean destruction of the epithelial layer, a basis for stimulation of fibroblasts for repair (Theron et al., 2005). Fourthly, there may be Fas-mediated apoptosis of human epithelial cells, while lung fibroblasts are protected and are also not infected by SARS-CoV (Coulter et al., 2002, Tanaka et al., 2002). The importance of the previously-mentioned factors for fibrosis in SARS is not clear and needs further investigation.
There are several differences that one has to take into account when comparing the lesions of SARS in man with those in experimentally infected laboratory animals (Table 1). These include differences in the route of entry of the virus, the dose of the virus on entry, the physiology of the respiratory tract, the susceptibility to SARS-CoV infection, the tropism of the virus and the immune response. Furthermore, one should realize that most pathological descriptions of human SARS involve people who have been hospitalized for an extended period and have undergone multiple interventions; there are only a limited number of descriptions of acute fatal human cases without intervention. In contrast, animals that have been infected experimentally with SARS-CoV usually do not undergo intervention and are killed before the endpoint of severe disease. Finally, one should consider that only about 10% of people with confirmed SARS-CoV infection die (Peiris et al., 2003b) and it is likely that many people who recovered from SARS had less severe respiratory tract lesions.
The Pathogenesis of Severe Acute Respiratory Syndrome Coronavirus Infection in Man
The pathological changes induced by SARS-CoV infection in man can be related to virus-specific factors and host-specific factors (Tables 3 and 4 ).
Table 3.
Similarities and differences in the pathology of SARS and H5N1 influenza
| Similarities | Differences |
|
|---|---|---|
| SARS | H5N1 Influenza | |
| Pneumocytes main target: resulting in DAD | Acute alveolar lesions | |
| DAD less fulminant with an acute and regenerative pattern | DAD more fulminant and necrotizing with marked haemorrhage | |
| Hyaline membranes in human cases | Typical histopathological features | |
| Multinucleated cells | No multinucleated cells | |
| Haemophagocytic syndrome and lymphoid depletion | Organizing phase | |
| Fibrocellular intra-alveolar organization with a BOOP-like pattern | Patchy and interstitial paucicellular fibrosis without BOOP-like pattern | |
| Hypoxia-related skeletal muscle and renal tubular necrosis | Development of severe disease | |
| Less rapid, 2nd week of illness | More rapid, end of 1st week of illness | |
| Pathology centred around the bronchioles for SARS and pH1N1 | Dissemination | |
| Respiratory tract, intestinal tract, liver, blood, urine and faeces | Respiratory tract, intestinal tract, brain, cerebrospinal fluid and blood | |
BOOP, bronchiolitis obliterans organizing pneumonia.
Based in part on Ng et al. (2006).
Table 4.
Similarities and differences between the pathogenesis of SARS and influenza
| Factors important for pathogenesis | Similarities | Differences |
|---|---|---|
| Virus-specific factors | ||
| Receptor specificity | – | Receptors: SARS: ACE2, DC-SIGN, L-SIGN Influenza: sialic acids |
| Direct cytopathic effect | – | SARS: not so important Influenza: important |
| Host-specific factors | ||
| Immune cells | Lymphopenia High neutrophil count |
– |
| Imbalanced cytokines | – | SARS: host specific Influenza: virus specific |
| Age | Old age is associated with fatal cases (SARS and seasonal influenza) | Not for pH1N1 and H5N1 |
| Co-morbidity | Co-morbidity is associated with fatal cases (SARS, seasonal influenza and pH1N1) | Not for H5N1 |
| Genetic factors | – | SARS: certain genetic factors are associated with severity of disease Influenza: not described |
| Species differences | Differences in pathological changes and disease outcome among man and animals are species related | – |
Virus-specific Factors: (1) Receptor Specificity
SARS-CoV enters the body via the respiratory system by droplet transmission and interacts with cellular receptors via the surface spike protein (S-protein) to infect target cells (Simmons et al., 2004). Several host cell receptors have been found to bind to the S-protein. One is metallopeptidase angiotensin-converting enzyme 2 (ACE2). Others are lectins: C-type lectin DC-SIGN (or CD209), human CD209L (or liver/lymph node specific (L)-SIGN and DC-SIGNR) and LSECtin (Li et al., 2003, Jeffers et al., 2004, Yang et al., 2004a, Gramberg et al., 2005). Binding of the S-protein to the main functional receptor ACE2 on the target cell leads to fusion between the virus envelope and the host cell membrane. SARS-CoV infection of ACE2-expressing cells seems to be dependent on the lysosomal proteolytic enzyme cathepsin L that is present in various cell types (Simmons et al., 2005, Huang et al., 2006b). ACE2 in man is present in type I and II pneumocytes, small intestinal enterocytes, the brush border of proximal tubular cells of the kidneys, endothelial cells of small and large arteries and veins, and arterial smooth muscle cells (Hamming et al., 2004). DC-SIGN (or CD209) is expressed on dendritic cells and macrophages. The low expression of cathepsin L in human endothelial cells might explain the low infection rate of these cells despite their high expression of ACE2 (Huang et al., 2006a). On the other hand, virus replication was observed in colonic epithelial cells and in hepatocytes without ACE2 expression, which may be explained by the presence of other receptors and co-receptors like dendritic cell-specific DC-SIGN and human CD209L (Lau and Peiris, 2005). Binding of SARS-CoV to this receptor did not lead to entry of virus into dendritic cells, but facilitated transfer of viruses to other susceptible cells (Yang et al., 2004a). CD209L acted in conjunction with LSECtin to enhance SARS-CoV infection (Jeffers et al., 2004).
The main target cells for the virus to infect are epithelial cells of the respiratory tract, which are the first cells the virus encounters after entering the body. The sites of viral replication correspond with the presence of ACE2. Immunohistochemistry (IHC) and in-situ hybridization (ISH) studies demonstrate virus antigen or viral nucleic acid in alveolar, bronchiolar, bronchial and tracheal epithelial cells, alveolar macrophages and multinucleated cells (To and Lo, 2004, Shieh et al., 2005). Co-labelling for cytokeratin and surfactant shows that the infected cells are mostly pneumocytes, predominantly type II pneumocytes (Shieh et al., 2005). Type II pneumocytes secrete surfactant, which is involved in reduction of the surface tension and integrity of the alveolar lumen, and they are important in tissue restitution and differentiation into type I pneumocytes. In extrarespiratory tissues, SARS-CoV RNA is detected in small and large intestine, lymph nodes, spleen, liver, heart, kidney, skeletal muscle, adrenal gland and cerebrum, suggesting that SARS has extra-pulmonary dissemination leading to virus excretion in respiratory secretions, stools, urine and possibly sweat (Leung et al., 2003, Ding et al., 2004, Farcas et al., 2005).
Virus-specific Factors: (2) Direct Cytopathic Effect
After attachment to and infection of the host cells, there is damage of those cells and surrounding cells with attraction of inflammatory cells. In the early stage, the severe oedema, fibrin deposits and haemorrhage, as is seen in the histopathology of human cases, is most likely due mainly to the damage to epithelial cells, with loss of epithelial lining resulting in vascular leakage. The epithelial damage can partly be explained by the direct cytopathic effect and apoptotic mechanisms due to viral infection and replication, resulting in lysis of the infected cells and inflammation in the infected tissue (Ng et al., 2003, Zhou et al., 2006). High titres of virus have been found in severely damaged organs (Farcas et al., 2005, Gu et al., 2005), with necrosis at the sites of virus particles (Nicholls et al., 2003). Fas-mediated apoptosis was demonstrated in human epithelial cells (Tanaka et al., 2002).
Host-specific Factors: (1) Immune and Inflammatory Cells
Infiltration of stimulated inflammatory cells induces the secretion of additional cytokines and enhances the inflammation (Yen et al., 2006). Immune and inflammatory cells such as lymphocytes, monocytes and neutrophils may play a role in the lesions caused by SARS-CoV infection. Lymphocytes such as cytotoxic T cells kill infected cells, monocytes, macrophages and neutrophils produce proinflammatory cytokines, and neutrophils release granules with enzymes that cause necrosis in surrounding cells and attract other inflammatory cells. Lymphopenia with a rapid decrease of CD4+ and CD8+ T cells and a high neutrophil count was seen in the blood of human patients in the acute phase of SARS-CoV infection, and was associated with an adverse outcome (Wong et al., 2003).
Host-specific Factors: (2) Induction of Cytokines
Next to damage due to virus replication, the severe pulmonary damage may be attributed to an excessive host immune response with the production of proinflammatory cytokines, as is demonstrated in cytokine and chemokine profiles (Nicholls et al., 2003, Peiris et al., 2003a, Wong et al., 2004, Zhang et al., 2004). In SARS patients, the results of cytokine and chemokine measurements are difficult to interpret due to many confounding factors. However, the levels of both cytokines and chemokines in the blood are elevated: interleukin (IL)-1, IL-6, IL-8, IL-12, IFN-γ, monocyte chemotactic protein (MCP)-1 (or CC-motif ligand 2, CCL2), monokine induced by IFN-γ (MIG), IFN-inducible protein (IP-10 or chemokine C-X-C motif ligand 10, CXCL10) and transforming growth factor (TGF)-β (Wong et al., 2004, Zhang et al., 2004, Huang et al., 2005, Jiang et al., 2005, Tang et al., 2005, Baas et al., 2008). Increased expression of chemokines and cytokines such as IP-10, MCP-1, IL-6 and IL-8 are important for chemotaxis and activation of neutrophils and monocytes (Ware and Matthay, 2000, Fan et al., 2001, Tsushima et al., 2009). Infiltration of these inflammatory cells corresponds with the severe pulmonary lesions observed in human cases (Huang et al., 2005, Jiang et al., 2005, Cameron et al., 2007, Smits et al., 2010).
The production of type I IFNs by the host after infection with a virus is an essential part of the antiviral innate immune system. SARS-CoV is suggested to cause inhibition of IFN production (Weber et al., 2004). In people with SARS, treatment with type I IFNs was associated with reduced disease-associated hypoxia and a more rapid resolution of radiographic lung abnormalities (Loutfy et al., 2003).
Host-specific Factors: (3) Risk Factors
The most important host-specific risk factors in people for more severe SARS-related disease and deaths due to acute respiratory distress syndrome (ARDS) are advanced age, sex and co-morbidities (Chan-Yeung and Xu, 2003, Donnelly et al., 2003, Lee et al., 2003, Manocha et al., 2003, Karlberg et al., 2004, Leung et al., 2004, Liu et al., 2006, Lau et al., 2010). Additionally, genetic factors such as certain human leucocyte antigen (HLA) haplotypes are associated with a higher susceptibility to SARS-CoV infection in man (Ng et al., 2004, Ip et al., 2005). In contrast, L-SIGN homozygote individuals have a lower susceptibility to SARS-CoV infection (Chan et al., 2006). Additionally, there may be another genetic factor involved in the protection against human SARS-CoV infection; genotypes producing low concentrations of mannose-binding lectin, a collectin in the serum that is able to bind the glycosylated S-protein, were associated with increased risk of developing SARS (Ip et al., 2005).
The Pathogenesis of Severe Acute Respiratory Syndrome Coronavirus Infection in Animal Models
Variation in disease severity in animal models can be explained by both virus-specific and host-specific factors.
Virus-specific Factors
In laboratory animals, cell type tropism of SARS-CoV is related to ACE2 expression (Table 1, Fig. 2 ). Similar to human ACE2, both ferret and feline ACE2 bind the attaching S-protein of SARS-CoV efficiently (Zamoto et al., 2006, Guo et al., 2008). However, cell tropism and ACE2 expression do not always correspond. For example, ferret bronchiolar epithelial cells express ACE2, but are apparently not infected by SARS-CoV (Table 1). This discrepancy may be explained by the necessity for other receptors besides ACE2 for virus attachment or for other factors (such as cathepsin L) for SARS-CoV replication in the host cell (Gramberg et al., 2005).
Fig. 2.
Cell type tropism in the respiratory tract of cats and ferrets infected experimentally with H5N1 influenza virus and SARS-CoV. The presence of receptors is demonstrated for SARS-CoV by the expression of ACE2 by IHC and for H5N1 by the expression of virus attachment by virus histochemistry. The presence of virus in similar cell types is demonstrated by virus antigen expression by IHC. Bars, 10 μm.
Another discrepancy between cell type tropism and ACE2 expression was observed in tracheobronchial submucosal glands. While the submucosal glands of both cats and ferrets expressed ACE2, only the submucosal glands of cats became infected and inflamed. As indicated above, this discrepancy may be due to species differences in expression of other co-factors necessary for viral replication. Alternatively, this discrepancy may also be due to species differences in the histological architecture of the tracheobronchial submucosal glands, which contain both serous and mucous cells. Ferrets have relatively more mucous cells than cats. This may have inhibited the attachment of SARS-CoV to serous cells, which was the main cell type infected in cats.
Most likely, direct cytopathic damage due to virus replication in SARS is not the most important factor for cellular and tissue damage in animal models, since differences in the severity of the pulmonary lesions between young-adult and aged macaques were observed, despite having similar virus loads.
Several genetic factors of both the virus and the host are known to modify the susceptibility and immune response to SARS-CoV infection. Genetic analyses of SARS-CoV demonstrate that genetic variation in the spike gene of SARS-CoV isolates from civet cats causes increased transmission and affinity of the virus for both civet and human ACE2 receptors (Tang et al., 2006, Rockx et al., 2009).
Host-specific Factors
SARS-CoV-infected young-adult and aged macaques demonstrated expression of various cytokines and chemokines such as IP-10, MCP-1, IL-6 and IL-8 in the lungs in a similar pattern as was seen in man (de Lang et al., 2007). The cytokine response in SARS is probably host specific since aged macaques had a stronger up-regulation of those chemokines and cytokines than young-adult macaques, despite similar virus replication (Smits et al., 2010). When up-regulated chemokines and cytokines of macaques and AGMs were compared, IP-10, MCP-1, CXCL-1, CXCL2, IL-6 and IL-8 were up-regulated in macaques, but not in AGMs. However, despite similar virus replication levels, the young-adult AGMs showed more severe lesions with hyaline membranes when compared with the young-adult macaques. Comparative gene expression analyses revealed induction of proinflammatory and antiviral pathways in both species. Cytokines important for ARDS or neutrophils attracting activity, such as CXCL-1, CXCL2, IL-6 and IL-8, were up-regulated in the macaques, but not in the AGMs. Other proinflammatory chemokines and cytokines such as SPP1 (osteopontin), CCL20 and CCL3 were up-regulated more in AGMs than in macaques. Additionally, osteopontin and CCL20 were significantly more up-regulated in AGMs and aged macaques than in young-adult macaques. Osteopontin is expressed predominantly by macrophages and is important in type 1 (Th1) cytokine expression and plays a role in development of lung fibrosis (O'Regan, 2003, Pardo et al., 2005). In AGMs, many macrophages, presumed to express osteopontin, were seen histologically, while in macaques there were both macrophages and neutrophils. The above-mentioned differences in the gene expression profile as well as the difference in the tropism, which also involves bronchiolar and tracheal epithelial cells in AGMs (Table 1), help to explain the more severe lesions in the AGMs.
When macaques experimentally infected with SARS-CoV were directly treated with IFN, there was also a protective effect, suggesting that supplementing IFN as a therapy can be beneficial (Haagmans et al., 2004). These results demonstrate that the inhibition of IFN production caused by the virus plays an important role in the induction of virus replication and associated severe lesions after SARS-CoV infection.
Genetic analyses of the host demonstrate that species-to-species variation in the sequence of the ACE2 gene affects the efficiency by which the virus can enter the cells (Li et al., 2005c). The differences in disease outcome between the species can be attributed to the differences in cell type tropism of SARS-CoV (Table 1) and the differences between cynomolgus macaques and AGMs in induction of cytokines (Smits et al., 2010). The cell type tropism as described in virus-specific factors by the distribution of ACE2 may result in differences in the severity of the disease between animal models and man. Additionally, the difference in the outcome of the disease between AGMs and cynomolgus macaques indicates that there may be more differences in the reaction of the immune response between the species. These differences may be related to evolutionary adaptations and differences in selection of major histocompatibility complexes, immune response, cytokine production and sensitivity to viruses. Co-morbidities and genetic factors that are associated with severe disease in man have not been investigated in laboratory animals and thus an insight into those factors from SARS animal models has not been obtained.
Age is another host-specific factor that is related to more severe disease in man. Aged cynomolgus macaques (10–19 years old) infected with SARS-CoV had more severe lesions than young-adult animals (3–5 years old), even though viral replication levels were similar (Imai et al., 2008, Smits et al., 2010, Rockx et al., 2011). Additionally, aged mice showed more severe lesions than young-adult mice on infection with SARS-CoV and the transcription profile in aged mice generally indicated a stronger proinflammatory response than in young mice (Baas et al., 2008, Rockx et al., 2009). It is suggested that age-related accumulated oxidative damage and a weakened anti-oxidative defence system cause a disturbance in the redox balance, resulting in increased reacting oxygen species (Smits et al., 2010). Subsequently, redox-sensitive transcription factors, such as nuclear factor (NF)-κB, can be activated, which is followed by the induction of proinflammatory genes such as IL-1β, IL-6, TNF-α and adhesion molecules (Chung et al., 2006). Therefore, ageing is not only associated with alterations in the adaptive immune response, but also with a proinflammatory state in the host (Smits et al., 2010). Oxidative stress and Toll-like signalling, via NF-κB triggered by viral pathogens like SARS-CoV, may further amplify the host response, ultimately leading to acute lung injury (Imai et al., 2008).
Influenza A Virus
Background
Influenza was first described as epidemics of acute, rapidly spreading catarrhal fevers in man and the first epidemic was most probably described by Hippocrates in 412 B.C. (Beveridge, 1978, Cox and Subbarao, 2000). Epidemics or pandemics of influenza have occurred throughout the last 2,500 years of history. Today, human influenza occurs yearly as seasonal influenza, mainly in the winter months of temperate climates, every year as interpandemic epidemics, and, sporadically at an average of 35 year intervals, as more severe influenza (i.e. pandemics). Seasonal influenza and interpandemic epidemics occur as a result of mutations in influenza viruses that are circulating in the human population (antigenic drift). In contrast, influenza pandemics occur as a result of the introduction of an animal influenza virus or a human–animal influenza reassortant virus in which the surface glycoprotein haemagglutinin, with or without other virus proteins, substantially differs antigenically from those circulating in the human population (antigenic shift) (Cox and Subbarao, 2000).
Since 1900, there have been four pandemics of human influenza: 1918 H1N1 ‘Spanish’ influenza, 1957 H2N2 ‘Asian’ influenza, 1968 H3N2 ‘Hong Kong’ influenza and the 2009 H1N1 ‘Swine’ influenza (Cox and Subbarao, 2000, Morens et al., 2009). Of these, the 1918 ‘Spanish’ influenza pandemic was the worst, causing acute illness in 25–30% of the world population and the death of nearly 50 million people (Parker and Caywood, 1987, Jepson et al., 1997, Osterhaus, 2001). Most of the fatalities occurred among 15–34 year olds, with primary acute interstitial pneumonia, pulmonary haemorrhage and pulmonary oedema, often with secondary bacterial pneumonia (Parker and Caywood, 1987, Osterhaus, 2001).
Influenza A virus infections occur in both mammals and birds and are classified, based on surface glycoproteins, into 17 haemagglutinin (HA) (H1–H17) and nine neuraminidase (NA) (N1–N9) subtypes (Swayne and Halvorson, 2003, Tong et al., 2012). Avian influenza virus can be of high or low pathogenicity (HPAI or LPAI) based on the pathogenicity for chickens. During human infection, human influenza viruses use host trypsin-like proteases in the respiratory tract to cleave HA, while LPAI viruses replicate in both the respiratory and the digestive tracts of birds (WHO, 2004). HPAI viruses use a wide range of proteases allowing replication outside the respiratory tract in chickens (Horimoto and Kawaoka, 2001).
The HPAI virus H5N1 was initially present in poultry, but in 1997 it crossed the species barrier and infected men in China (Claas et al., 1998). H5N1 continues to circulate among poultry in many countries in Asia, Africa and Europe, and occasionally spreads to people, often with fatal consequences. H5N1 is the first avian influenza virus to cause significant numbers of human infections and deaths (i.e. 604 infections with 357 fatalities since January 2004) (Swayne and Halvorson, 2003, WHO, 2012).
At the start of April 2009, a novel H1N1 influenza A virus (pH1N1) was identified as the cause of acute respiratory disease of people in Mexico (Garten et al., 2009). This virus was a complex reassortant influenza A virus, which had not been previously reported in animals, but had gene segments related to North American classic H1N1 swine viruses (haemagglutinin, nucleoprotein and non-structural gene segments), North American avian viruses (polymerase A and B2 genes), human influenza A virus (polymerase B1 genes) and Eurasian H1N1 swine viruses (neuraminidase and matrix genes) (Garten et al., 2009). On 1 August 2010, at the end of the pandemic, more than 214 countries had reported laboratory confirmed cases of this pH1N1, including at least 18,449 deaths (WHO, 2010).
The Pathology of Influenza in Man
Uncomplicated influenza is a mild inflammation of the upper respiratory tract that consists mainly of rhinitis, paranasal sinusitis, pharyngitis and laryngitis. Histopathology demonstrates diffuse, superficial, necrotizing tracheobronchitis characterized by desquamation of epithelial cells, oedema and hyperaemia in the lamina propria and infiltration with lymphocytes and histiocytes. The inflammation is short lasting: epithelial regeneration is already visible within 2 days after onset of symptoms (Walsh et al., 1961).
Viral pneumonia is a complication of influenza virus infection. Gross examination of the lungs shows extensive consolidation with varying degrees of haemorrhage. Histopathology caused by influenza virus infection of the alveoli consists of DAD, which has the same general pathological features as in SARS, except that syncytia are not observed and in the fibrotic phase there is no bronchiolitis obliterans organizing pneumonia-like appearance (Ng et al., 2006, Taubenberger and Morens, 2008). Influenza viral pneumonia often occurs together with, or is followed by, bacterial pneumonia.
The pathology of pH1N1 infection in fatal human cases when compared with human cases of H5N1 infection showed similar DAD. However, fatal cases of pH1N1 infection showed more inflammation in the nose, trachea, bronchi and bronchioles, a feature that was also seen in fatal cases of seasonal influenza (Guarner et al., 2006, Guarner and Falcon-Escobedo, 2009). H5N1 virus infection has also been associated with extrarespiratory disease. In the lymphoreticular system there was marked histiocytic hyperplasia and reactive haemophagocytic syndrome. Other lesions included atrophy of white pulp in the spleen, centrilobular necrosis in the liver, acute tubular necrosis in the kidney, necrosis of skeletal muscle fibres and necrosis in the brain with microglial nodules (To et al., 2001, Peiris et al., 2004b, Chokephaibulkit et al., 2005, Uiprasertkul et al., 2005). Infection with pH1N1 rarely demonstrates haemophagocytosis or neuromuscular and cardiac complications (Rothberg et al., 2008). Infection with seasonal influenza virus primarily infects and causes disease in the respiratory tract and is associated, albeit to a lesser extent, with disease in extrarespiratory organs. These include influenza-associated acute encephalopathy (Studahl, 2003), myocarditis (Ray et al., 1989) and myopathy (Agyeman et al., 2004).
Animal Models for Human Influenza
Animal models help both to better understand influenza in man and to develop medical countermeasures against this disease. Forms of influenza in man for which animal models have been developed include uncomplicated influenza, influenza pneumonia, influenza-associated bacterial sepsis, influenza-associated neurological disease, influenza in immunocompromised hosts and virus transmission (Barnard, 2009). Specific goals for which animal models are designed are to determine transmissibility of different viruses, virulence of different viruses, pathogenesis of viral infection and efficacy of vaccines or antiviral drugs.
Experimental animal species used include the laboratory mouse, domestic ferret (Beigel et al., 2005), Syrian hamster (Friedewald and Hook, 1948), chinchilla (Giebink et al., 1980), domestic horse (Wattrang et al., 2003), laboratory rat (Rubin et al., 2004), domestic dog (Giese et al., 2008), domestic cat (Rimmelzwaan et al., 2006), cotton rat (Boukhvalova et al., 2009), domestic pig (Lipatov et al., 2008), guinea pig (Kwon et al., 2009) and non-human primates (e.g. squirrel monkeys, cynomolgus macaque and rhesus macaque) (Murphy et al., 1982, Kuiken et al., 2003b, Chen et al., 2009). Cynomolgus macaques have been used in pathogenesis studies and vaccination studies (Kreijtz et al., 2009). Ferrets have been used to model uncomplicated upper respiratory tract infection, to model viral pneumonia and to model influenza virus transmission among people (Munster et al., 2009, van den Brand et al., 2010a). Cats have been used to model systemic disease after infection with H5N1 influenza and to investigate the pathogenesis of H5N1 (Rimmelzwaan et al., 2006). The lesions of macaques, ferrets and cats infected with H5N1 match the clinical signs, virus replication and associated lesions in the respiratory tract, followed by death as is seen in man. However, there are differences between animal species and people infected with influenza virus. Therefore, the animal model used for severe disease in man needs proper consideration of all aspects of the model including animal species, inoculation route and inoculum dose. This review focuses on the pathological changes related to the pathogenesis of different influenza virus infections in macaques, ferrets and cats.
Non-Human Primates
The cynomolgus macaque is a non-human primate that is often used as an animal model for human disease caused by influenza virus infection (Kuiken et al., 2003b, Herfst et al., 2010). Experimental H5N1 infection in cynomolgus macaques causes both morbidity and mortality (Kuiken et al., 2010). Microscopical lesions are centred in the alveoli and bronchioles and consist of DAD that is more severe than for human influenza viruses (Table 2, Fig. 1) (Rimmelzwaan et al., 2001, Kuiken et al., 2003b, Kreijtz et al., 2009). Extrarespiratory tissues do not show histological lesions (Rimmelzwaan et al., 2001, Kuiken et al., 2003b, Baskin et al., 2009, Chen et al., 2009), although suppurative tonsillitis, lymphocytic necrosis in lymphoid organs, hepatic necrosis and renal tubular necrosis are seen rarely (Rimmelzwaan et al., 2001, Kuiken et al., 2003b). Experimental pH1N1 infection in cynomolgus macaques causes morbidity, but no mortality. Microscopical lesions consist of multifocal moderate DAD (Table 2). In addition, there is moderate bronchiolitis and mild bronchitis, tracheitis and rhinitis. The severity of pH1N1-induced pulmonary lesions is higher than those induced by seasonal influenza H1N1, but lower than those induced by H5N1. Experimental seasonal influenza virus infection in cynomolgus macaques also causes morbidity, but no mortality. Microscopical lesions consist of focal to multifocal mild DAD with mild necrosis and no oedema, hyaline membranes or lesions in the other parts of the respiratory tract (Herfst et al., 2010).
Table 2.
Histopathology, virus antigen expression and virus attachment in different species infected with different influenza viruses
| Virus and species | Histological lesions |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alveoli |
Bronchioles |
Bronchi |
Interstitium |
Trachea |
Nose |
||||||||||
| Epithelial necrosis | Oedema | Hyaline membranes | Inflammation | Type II pneumocyte hyperplasia | Epithelial necrosis | Inflammation | Epithelial necrosis | Inflammation | Perivascular/peribronchiolar cuffing | Tracheobronchoadenitis | Epithelial necrosis | Inflammation | Epithelial necrosis | Inflammation | |
| H5N1 | |||||||||||||||
| Human | + | + | + | + | + | + | − | − | − | − | − | − | − | − | |
| Macaque∗ | + | + | + | + | + | + | + | − | + | − | − | − | + | − | − |
| Ferret | + | + | − | + | + | + | + | + | + | + | + | − | + | + | + |
| Cat | + | + | − | + | + | + | + | − | + | + | − | − | − | − | − |
| pH1N1 | |||||||||||||||
| Human | + | + | + | + | + | + | + | + | + | + | + | + | + | N | N |
| Macaque | + | + | − | + | + | + | + | + | + | + | − | + | + | + | + |
| Ferret | + | + | − | + | + | + | + | + | + | + | + | + | + | + | + |
| Cat | + | + | − | + | + | + | + | − | + | + | − | − | − | − | − |
| Seasonal† | |||||||||||||||
| Human | + | + | + | + | + | + | + | + | + | − | + | + | + | + | + |
| Macaque | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − |
| Ferret | + | − | − | + | + | − | + | − | + | + | + | − | + | + | + |
| Cat | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| Virus and species | Virus antigen expression |
Virus attachment |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type I pneumocytes | Type II pneumocytes | Alveolar macrophages | Bronchiolar epithelial cells | Bronchial epithelial cells | Tracheal epithelial cells | Submucosal glandular epithelial cells | Nasal epithelial cells | Type I pneumocytes | Type II pneumocytes | Alveolar macrophages | Bronchiolar epithelial cells | Bronchial epithelial cells | Pulmonary goblet cells | Tracheal epithelial cells | Submucosal glandular epithelial cells | Nasal epithelial cells | |
| H5N1 | |||||||||||||||||
| Human | − | + | + | − | − | + | − | − | − | + | + | + | + | − | − | + | − |
| Macaque∗ | + | + | + | + | + | − | − | − | + | + | − | + | + | − | − | N | N |
| Ferret | + | + | + | + | + | − | − | + | − | + | − | − | − | − | − | N | N |
| Cat | + | + | + | + | + | − | − | − | − | + | + | + | − | − | − | N | N |
| pH1N1 | |||||||||||||||||
| Human | + | + | + | + | + | + | + | n | + | + | + | + | + | + | + | + | + |
| Macaque | + | + | − | − | − | − | − | + | N | N | N | N | N | N | N | N | N |
| Ferret | + | + | + | + | + | + | + | + | N | N | N | N | N | N | + | N | + |
| Cat | + | + | + | + | + | − | − | − | N | N | N | N | N | N | N | N | N |
| Seasonal† | |||||||||||||||||
| Human | − | − | − | − | + | − | + | N | + | − | − | + | + | + | + | − | + |
| Macaque | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | N |
| Ferret | − | + | − | − | − | − | − | + | + | − | − | + | + | − | + | + | N |
| Cat | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | N |
N, not done.
Cynomolgus macaque.
Seasonal human influenza virus H1N1 and H3N2.
Carnivores
A more frequently used animal species in influenza research is the ferret. Intratracheal inoculation of influenza virus into ferrets causes high morbidity and mortality for H5N1 (Rowe et al., 2003, Govorkova et al., 2005, Cameron et al., 2008), moderate morbidity and low mortality for pH1N1 (van den Brand et al., 2010a) and neither obvious morbidity nor mortality for seasonal influenza virus (Zitzow et al., 2002). This corresponds to differences in severity of pulmonary lesions (consisting of DAD), which is high for H5N1 (Fig. 1), intermediate for pH1N1 and low for seasonal H1N1. The extent and distribution of the lesions throughout the respiratory tract also differ between the viruses (Table 2). Extrarespiratory lesions are limited to H5N1 infection and consist of non-suppurative necrotizing encephalitis (Boltz et al., 2008), multifocal hepatitis, necrosis and hyperplasia of bile duct epithelium (Govorkova et al., 2005, Yen et al., 2007, Boltz et al., 2008, Kuiken et al., 2010).
When pH1N1 is inoculated intranasally into ferrets instead of intratracheally, lesions occur higher in the respiratory tract and consist of a mild to moderate necrotizing bronchiolitis, bronchitis, tracheitis and rhinitis (Munster et al., 2009). Intranasal inoculation of H5N1 in ferrets results primarily in encephalitis, compared with pneumonia after intratracheal inoculation (Bodewes et al., 2011, Schrauwen et al., 2012). This illustrates the effect of route of inoculation on the pathogenesis of experimental influenza virus infection (Bodewes et al., 2011).
The temporal and spatial dynamics after combined intranasal and intratracheal inoculation of influenza virus in ferrets differ between viruses (van den Brand et al., 2012a) (Fig. 3 ). H5N1 infection causes predominantly moderate DAD and bronchiolitis, starting at 12 h post infection (hpi), developing into severe DAD with oedema from 1 to 4 days post infection (dpi) and necrosis and inflammation in the bronchus, trachea and nose. pH1N1 infection causes mild lesions at 12 hpi, developing into moderate to severe lesions from 1 to 7 dpi, with more involvement of the nose when compared with ferrets infected with H5N1. At 14 dpi the lesions are again mild (van den Brand et al., 2012a). Human seasonal influenza virus infection does not consistently cause viral pneumonia, but is limited mainly to bronchiolitis, bronchitis, tracheitis and rhinitis (Svitek et al., 2008, Munster et al., 2009).
Fig. 3.
Virus antigen expression (IHC) and histopathology (HE) of respiratory tissue in ferrets infected with different influenza viruses; seasonal H3N2 (A–D), pH1N1 (E–H) and H5N1 (I–L). Bars, 50 μm (bronchiole). Bars, 25 μm (alveoli).
Several felid species are susceptible to severe or fatal disease from influenza virus infection, as suggested by natural cases of H5N1 and/or pH1N1 infection in cats, tigers and leopards (Keawcharoen et al., 2004, Lohr et al., 2010, Sponseller et al., 2010, van den Brand et al., 2010b). Intratracheal inoculation of influenza virus into cats causes morbidity and mortality for H5N1 (Rimmelzwaan et al., 2006), morbidity, but no mortality for pH1N1 and lack of infection for seasonal H3N2 (Rimmelzwaan et al., 2006, van den Brand et al., 2010b). This corresponds to more severe DAD for H5N1 than for pH1N1, as seen in ferrets (Table 2). Extrarespiratory lesions, characterized by inflammation and necrosis in the brain, heart, kidney, liver and adrenal gland, are limited to H5N1 infection (Rimmelzwaan et al., 2006), and are much more extensive than in ferrets.
Comparative Pathology
The pathological changes of influenza virus infection in fatal human cases and in laboratory animals show both differences and similarities (Table 2). These can be compared according to localization, character, severity and temporal changes in the lesions, and the degree of extrarespiratory spread. Firstly, the localization of the lesions in the respiratory tract, between different viruses, in general is similar for man and laboratory animals. In seasonal influenza infection, the upper respiratory tract is mostly affected, in pH1N1 infection, all parts of the respiratory tract are affected, and in H5N1 infection, the lower respiratory tract is mostly affected (Guarner et al., 2006, Shieh et al., 2010, van den Brand et al., 2012a). There are some differences in localization, since H5N1 infection in laboratory animals involves more of the air-conducting parts of the respiratory tract than in man, and vice versa for pH1N1 and seasonal influenza virus infections. Secondly, the character of the respiratory tract lesions is similar for man and laboratory animals. This includes the alveolar lesions after infection with H5N1 and pH1N1. These alveolar lesions result in DAD characterized by necrosis of epithelial cells, oedema, infiltration of inflammatory cells and epithelial regeneration. Thirdly, the severity of the respiratory tract lesions appears greater in man than in laboratory animals. For example, fatal human cases often show alveolar oedema and hyaline membranes, which are less common in laboratory animals. One factor may be that laboratory animals are killed before severe disease or death occurs. Fourthly, the temporal dynamics of influenza virus infection in ferrets may reflect the differences in the age of pathological changes in fatal human cases due to the duration of their illness before dying. Fifthly, the degree of extrarespiratory involvement of H5N1 infection in man is difficult to compare with that in laboratory animals because it is so poorly described in man. The involvement of the CNS in some human cases of H5N1 infection (de Jong et al., 2005) appears similar to that in ferrets, where extrarespiratory spread often is limited to the CNS. In contrast, extrarespiratory spread of H5N1 in man is probably not as common or as widespread as in cats (Rimmelzwaan et al., 2006). Overall, many features of the pathological changes in man and laboratory animals are similar. The choice of animal model, including laboratory animal species, route of virus inoculation and dose of inoculum, will depend on the specific feature of influenza in man that one wishes to study.
The Pathogenesis of Influenza Virus Infection in Man
As in SARS, the pathology of influenza in fatal human cases can be related to factors that have been proven important in influenza virus infection: virus-specific factors and host-specific factors (Tables 3 and 4).
Virus-specific Factors: (1) Receptor Specificity
As in SARS-CoV infection, the route of entry is airborne transmission into the respiratory system. Attachment of the viral haemagglutinin to its host cell receptor is the first step in the influenza virus replication cycle. The receptor on the surface of the host cell is a sialic acid (SA)-terminated glycan. Human-adapted influenza viruses prefer binding of an α-2,6 SA linkage, which is present throughout the human respiratory tract, while avian influenza viruses prefer binding by an α-2,3 SA linkage, which is abundantly present in the respiratory and intestinal tract of aquatic birds (Connor et al., 1994, Shinya et al., 2006). Because different influenza viruses use different SAs as their receptor, and because the expression of SAs differs both across the respiratory tract and across species, this step influences both the pattern of disease in the respiratory tract and the host range of virus infection.
In people, human-type receptors are predominantly expressed on the epithelium of the upper part of the respiratory tract (i.e. nose, trachea and bronchi), while avian-type receptors are mainly expressed in the epithelium of the lower part of the respiratory tract (i.e. bronchi, bronchioles and type II pneumocytes) (Shinya et al., 2006, Nicholls et al., 2007). Where virus attachment studies show the cells to which influenza viruses bind, virus antigen expression studies (using IHC) show in which cells influenza viruses actually replicate.
Virus-specific Factors: (2) Direct Cytopathic Effect
The severe damage to type I and type II pneumocytes allows fluid to flood into and accumulate in the alveolar lumina. This has severe consequences for the gas exchange function of the respiratory tract, resulting in severe and, in some cases, fatal respiratory dysfunction as is also seen in SARS-CoV infection (Ware and Matthay, 2000). Damage to the alveolar epithelium is in part due to the direct cytolytic effect of virus infection. The cytolytic effect could be due to necrosis or apoptosis as a result of replication of the virus. Direct cytopathic damage is suggested by high virus titres found in severely damaged lung tissue as well as in throat or nose swabs (Hien et al., 2004, Peiris et al., 2004b), while virus antigen expression in epithelial cells was associated with severe DAD (Uiprasertkul et al., 2005, Uiprasertkul et al., 2007, Kuiken et al., 2010).
Host-specific Factors: (1) Immune and Inflammatory Cells
As in SARS, immune cells such as lymphocytes, monocytes and neutrophils play a role in the lesions caused by influenza virus infection. In fatal human cases of H5N1 and pH1N1 infections, lymphopenia was associated with severe disease (Yuen et al., 1998, Perez-Padilla et al., 2009).
Host-specific Factors: (2) Cytokines
As in SARS, fatal infection with H5N1 is associated with elevated concentrations of serum cytokines (de Jong et al., 2006, Uiprasertkul et al., 2007). Chemokines and cytokines such as IP-10, MIG, MCP-1, IL-8, IL-10, IL-6, IL-1α and -β, and IFN-γ are elevated in the serum of patients infected with H5N1 and are particularly high in fatal cases. The cytokine levels correlate with the pharyngeal viral load, suggesting that the increased levels may reflect the viral replication (de Jong et al., 2006). Cytokine levels are not only elevated in the serum, but high expression of TNF-α also is detected in the lungs of fatal cases of H5N1 infection (Peiris et al., 2004b). The high viral load accompanied by high cytokine response may suggest a balanced response.
Host-specific Factors: (3) Risk Factors
Important host-specific risk factors for severe disease from human seasonal influenza virus infection are advanced age, co-morbidities such as pulmonary disease and cardiovascular disease, and pregnancy (Morens and Fauci, 2007, Taubenberger and Morens, 2008). Risk factors for severe disease from pH1N1 infection are in general similar to those for human seasonal influenza virus. In addition, they include diabetes, hypertension and obesity. Interestingly, advanced age does not appear to be a risk factor for severe disease from pH1N1 or H5N1 infections. Most patients with pH1N1 infection were (young) adults, with a median age of 36 years (Shieh et al., 2010), while most severe human cases of H5N1 infection were previously healthy, with a median age of 18 years (WHO, 2012). In addition, the difference in disease between species is another important host-specific factor.
Advanced age as a risk factor for severe disease from seasonal influenza virus infection may be related to increased host responses and decreased defence mechanisms against redox-induced damage, as is seen in SARS-CoV infection. The different age distribution of disease and fatality from H5N1 infection may reflect age-related patterns of exposure or risk behaviour such as close contact with sick poultry or age-related host resistance (Peiris et al., 2007). The different age distribution of fatality from pH1N1 infection is probably related to the presence of immunity from previous infections with H1N1 influenza viruses in older people. Because these viruses were antigenically related to pH1N1, the antibodies were cross-reactive and therefore protected from severe disease from pH1N1 infection (Hancock et al., 2009).
Other host-specific factors such as pre-existing morbidities and pregnancies, which are risk factors for seasonal and pH1N1 infection, may be related to a compromised immune system in chronically ill patients and pregnant women as well as aerodynamic ventilation problems in advanced pregnancy and obesity.
The Pathogenesis of Influenza Virus Infection in Animal Models
Virus-specific Factors
The receptor distribution differs between man and different animal species, as is determined by virus attachment studies (Table 2, Fig. 2) (van Riel et al., 2006). Those studies reveal that for H5N1 the virus attachment pattern in respiratory tissues of cats best resembles that of man. For seasonal influenza virus, the virus attachment pattern in respiratory tissues of ferrets best resembles that of man. For pH1N1, there are few data about virus attachment in animals.
Both the pattern of virus attachment and pattern of virus antigen expression are linked to the severity of disease and pattern of lesions in the respiratory tract in man and animals infected with influenza viruses (Table 2). Overall, the pattern of virus attachment and pattern of virus antigen expression corresponds with each other, but not always. A first reason for this discrepancy may be that, like SARS-CoV, influenza viruses also require other co-receptors or other factors for attachment and/or replication. A second reason may be the influence of surfactant proteins that protect against influenza in man and pigs and possibly in other animal species, explaining virus attachment without virus antigen expression (Benne et al., 1995).
To choose the most appropriate laboratory animal species for animal models of influenza in man, it is useful to compare the pattern of virus antigen expression among species (Fig. 1). For H5N1, the pattern of virus antigen expression shows more pulmonary epithelial cell types showing virus antigen expression in laboratory animals than in man. For pH1N1, the pattern of virus antigen expression in ferrets most closely resembles that in man. For seasonal influenza viruses, the pattern of virus antigen expression differs between man, cynomolgus macaques and ferrets. When comparing the results of virus antigen expression studies in experimentally infected animals and fatal human cases, the following factors need to be taken into account: route of virus entry, dose of virus inoculum, stage of disease at which tissue samples are taken, and immune status. The route of entry in animal models is mostly by intranasal or intratracheal inoculation, while in man it is by air through small or large droplets. The dose of the virus inoculum in animal experiments is usually much higher than in human infection. Tissue samples are often taken at an earlier, less severe stage of disease in laboratory animals than in man. Tissue sampling in laboratory animals is usually from non-fatal cases, because the animals are killed before they succumb to the infection. In contrast, tissue sampling in people is usually from fatal cases, where patients have died after protracted disease and multiple therapeutic interventions. Finally, the immune status is different: laboratory animals are usually naïve to influenza virus, while many people have specific immunity, which may alter the course of subsequent influenza virus infections.
In animal models, high virus titres in the lungs strongly suggest active viral replication in those tissues with subsequently more damage (Zitzow et al., 2002, Govorkova et al., 2005, Maines et al., 2005, Rimmelzwaan et al., 2006). Apoptotic damage is suggested by experiments with TNF-related apoptosis-inducing ligand (TRAIL)-expressing macrophages that induced epithelial cell apoptosis in influenza virus pneumonia (Takizawa et al., 1993, Lowy, 2003, Herold et al., 2008). Additionally, apoptosis may be induced by up-regulation of certain chemokines and cytokines (Uiprasertkul et al., 2007). These findings suggest that direct damage of the virus by cytopathic and apoptotic mechanisms is important in the development of pathological changes in influenza virus infections. However, damage due to other mechanisms, such as indirect damage of the host response, cannot be excluded.
The differences in genetic factors of the different influenza viruses have a substantial impact on the outcome of disease after infection. Influenza viruses change over time due to antigenic shift and drift. Avian influenza viruses are known to infect people and pigs and are endemic among poultry without overt disease. When such avian viruses combine gene segments and surface proteins from human or porcine influenza viruses due to reassortment, the reassortants may have altered receptor binding properties, leading to enhanced ability to infect and spread among people. The presence of cross-reactive antibodies against other subtypes may explain the relatively low mortality in older pH1N1 patients due to cross-reactive antibodies acquired during previous influenza infection. The differences in disease after infection with various subtypes indicate the importance of virus-specific genetic factors in the course of the disease.
Host-specific Factors
After H5N1 inoculation, the number of monocytes in the blood and alveoli of ferrets decreased and remained low, suggestive of exhaustion of the bone marrow (Tumpey et al., 2000). After pH1N1 inoculation there was an increase in blood monocytes a few days later (van den Brand et al., 2012a). Additionally, the neutrophil count in the blood of ferrets was lower with H5N1 infection than with pH1N1 infection, as was also seen in human cases (Hien et al., 2009). This decreased neutrophil count may be attributed to a higher demand of neutrophils than can be met by myelopoiesis. This is corroborated by the high number of immature neutrophils in the blood, again suggesting exhaustion of the myelopoietic component.
Host genetic factors that influence the outcome of the disease are not known for man or animals. Differences in histopathological changes, antigen expression and receptor specificity between people and laboratory animal species are species related, as was seen in SARS-CoV infection. Macaques infected with H5N1 displayed severe disease and activation of proinflammatory cytokine and chemokine responses (Baskin et al., 2009). In ferrets, H5N1 infection also induced severe disease associated with strong expression of interferon response genes, including the IFN-γ-induced chemokine CXCL10. When those ferrets were treated with an antagonist of the CXCL10 receptor (CXCR3), the severity of H5N1-related disease and the viral titres were reduced when compared with controls (Cameron et al., 2008). For pH1N1, the abundance of neutrophils and macrophages in pulmonary lesions corresponded with up-regulation of CCL2, CCL3, CCL8, CXC10, IL-8 and CXCL1, which are known chemoattractants for neutrophils and monocytes, as is seen in macaques. Compared with lungs of cynomolgus macaques infected with seasonal human H1N1 virus, concentrations of MCP-1, MIP-1α, IL-6 and IL-18 were higher. This is in line with the more severe pulmonary lesions in cynomolgus macaques infected with pH1N1 than with seasonal human influenza H1N1 virus (Itoh et al., 2009). On the contrary, alveolar macrophages infected with H5N1 did not induce excessive TNF-α. However, alveolar macrophages were infected more abundantly by H5N1 than by seasonal H1N1 or pH1N1 (van Riel et al., 2011). Therefore, the imbalance in the level of virus infection and resultant cytokine and chemokine production may at least in part contribute to the development of lesions after infection with H5N1 and pH1N1.
Comparing the Pathology and Pathogenesis of Severe Acute Respiratory Infection Syndrome and Influenza
When comparing SARS and influenza, there are both similarities and differences in pathology and pathogenesis (Table 3). In both diseases the respiratory tract is the main tissue affected and the changes are characterized by DAD and bronchiolitis, including necrosis, oedema and inflammation. The most obvious differences in histopathology include the multinucleated giant cells and the intra-alveolar fibrosis in SARS and the more fulminant necrotizing and hemorrhagic pneumonia in H5N1 influenza. However, there are many differences in the outcome of disease after infection with different influenza viruses.
When evaluating the relationship between the pathology and the pathogenesis of SARS and influenza in the alveoli, certain virus-specific and host-specific factors are important (Table 4). SARS and influenza show a similar character of lesions, typified by necrosis and inflammation. Firstly, the necrosis that is seen in the lungs with both SARS and influenza can in part be explained by a direct cytopathic effect or apoptotic mechanisms. These mechanisms lead to damage of epithelial cells, increased permeability of the alveolar epithelium, damage of endothelial cells and subsequent oedema and haemorrhage, followed by formation of hyaline membranes (Berthiaume and Matthay, 2007). Secondly, the necrosis and inflammation in SARS and influenza can additionally be attributed to the dysregulation of cytokine and chemokine production. Dysregulation of cytokines and chemokines in SARS-CoV and H5N1 virus infection correlates with high viral loads in pharyngeal swabs and more severe pathological changes in severe or fatal cases (de Jong et al., 2006, Cameron et al., 2007, de Lang et al., 2007). Proinflammatory cytokines such as IL-6 and IL-8, IP-10 and MCP-1 attract immune cells that constitute the inflammatory infiltrate, which leads to even more production of cytokines and chemokines (Theron et al., 2005, Berthiaume and Matthay, 2007, Thiel and Weber, 2008). Together with the activation of oxidative stress mechanisms that are induced by the up-regulated cytokines, there is further cellular damage and inflammation resulting in DAD (Tsushima et al., 2009). Additionally, the infiltration of neutrophils results in a release of lytic enzymes that cause further necrosis of epithelial cells and the infiltration of cytotoxic T cells that results in necrosis of infected epithelial cells.
The site of the lesions caused by SARS-CoV and influenza virus is related to the receptors, and although each virus uses different receptors, the role of the receptors in the development of disease is crucial in both infections. For infections with SARS-CoV and influenza viruses, there is a correlation between the distributions of the receptor, the tropism of the virus and the associated lesions (Tables 1 and 2). However, not all differences in the pathology between various animal species can be explained by the differences in receptor distribution (Tables 3 and 4). This implies that other receptors and factors also play a role in the attachment, replication, infectiousness and virulence of both viruses (Lau and Peiris, 2005, Nicholls et al., 2007).
There are two pathological features that are remarkable in both SARS-CoV and influenza virus infections: pulmonary fibrosis and tracheobronchoadenitis. First, in severe human cases of both SARS and influenza there is marked loss of alveolar epithelial lining, which may lead to re-epithelialization and recovery, to death or to pulmonary fibrosis. Late stages of fatal human cases of SARS were characterized by intra-alveolar and intrabronchiolar fibrosis, and in approximately 62% of non-fatal SARS cases there was evidence for fibrosis in thin section computed tomography (CT) 1 month later (Franks et al., 2003). In contrast, interstitial fibrosis has been described in only a few fatal cases of H5N1 influenza, including in two case descriptions with radiological follow-up showing fibrosis-related changes (To et al., 2001, Antonio et al., 2003). For pH1N1 influenza, pulmonary fibrosis was also seen in only a few cases by follow-up CT and the fibrosis often disappeared after 1 month (Bai et al., 2011, Mineo et al., 2012). The importance of the previously mentioned factors for fibrosis in SARS is not clear. Unfortunately, fibrosis is not a prominent feature of SARS-CoV or influenza virus infection in laboratory animals. This may be explained in part by the less severe lesions and the early time point at which the animals are killed. Therefore, this phenomenon is difficult to study in animal models. The reason for the severe fibrosis in fatal human cases of SARS remains unclear and needs further investigation. Secondly, tracheobronchoadenitis was demonstrated in SARS-CoV infection in cats and young macaques (van den Brand et al., 2008, Smits et al., 2010) and in pH1N1 virus infection in ferrets and was clearly associated with expression of virus antigen (van den Brand et al., 2012a). This finding has potentially important implications for the excretion of those viruses. Excretion of virus might increase due to infection of the tracheobronchial submucosal glands and a diminished efficient defence by the mucociliary system, and virus secreted by these glands into the trachea and bronchi is more likely to be expectorated than virus produced lower in the respiratory tract.
Ageing is an important host factor associated with increased morbidity and mortality from infections with SARS-CoV and human seasonal influenza virus (Meyer, 2001, Peiris et al., 2004a). This phenomenon is also seen in a variety of other viral infections, such as West Nile virus and norovirus infections, probably because the elderly respond poorly to new antigens compared with younger people due to immunosenescence (Plackett et al., 2004, Licastro et al., 2005, Meyer, 2005, Murasko and Jiang, 2005, Salvioli et al., 2006). Immunosenescence is a multifactorial process that is associated with thymic involution, chronic antigen stimulation due to persistent infections, signal transduction changes in immune cells and protein–energy malnutrition (Fulop et al., 2005). Although all components of immunity are affected with ageing, the T cells are the most susceptible and the increased susceptibility to lower respiratory tract viral infections is particularly related to defective T cell responses (Fulop et al., 2005, Holt et al., 2005, Johnston, 2007). Additionally, advanced age causes a general increase in the levels of proinflammatory cytokines in plasma, resulting in an age-related increase of inflammation (Salvioli et al., 2006). Increased amounts of proinflammatory cytokines, such as IL-1β and IL-8, were produced upon stimulation of leucocytes in the elderly, while induction of antiviral type I IFNs was decreased compared with young adults (Rink et al., 1998, Yoon et al., 2004, Kong et al., 2008). Moreover, ageing results in less protection against the oxidative stress that is induced by virus infections and overreacting proinflammatory responses (Chung et al., 2006).
Other factors related to the severity of disease in SARS, seasonal influenza and pH1N1 infection are pre-existing co-morbidities (e.g. diabetes mellitus and cardiopulmonary disease), pregnancy and sex. Pre-existing co-morbidities and pregnancy are associated predominantly with suppression of the immune response, as is seen in older patients. In SARS, male sex is correlated with more severe disease, while in H5N1 infection females appear to have a worse outcome than males, although not significantly (Table 4) (Karlberg et al., 2004, Louie et al., 2011, WHO, 2012). Interestingly, a sex difference in the pathology of immune responses after viral infection is suggested, since influenza virus infection of mice results in greater neutrophil influx and more severe lesions in females than in males (Karnam et al., 2012).
When comparing the influenza virus infections in man, it is remarkable that H5N1 appears more likely to spread to extrarespiratory tissues than seasonal influenza virus (de Jong et al., 2006). In patients infected with H5N1, diarrhoea was associated with the detection of H5N1 RNA in faeces; it was suggested that the virus may infect the gastrointestinal tract directly or after subsequent dissemination via blood, since H5N1 RNA was also detected in plasma (Beigel et al., 2005, Uiprasertkul et al., 2005, Chutinimitkul et al., 2006, de Jong et al., 2006). The hypothesis that H5N1 could enter the human host via the gastrointestinal tract is supported by the results of experimental H5N1 infections in cats. Intestinal H5N1 inoculation resulted in viral replication in the capillary endothelium of the intestinal mucosa, which was not seen in cats infected intratracheally (Rimmelzwaan et al., 2006, Reperant et al., 2012). Like H5N1 influenza, SARS in man is a respiratory disease with extrarespiratory virus dissemination, as demonstrated by antigen expression in several organs and excretion of virus via respiratory secretions, stool, urine and possibly sweat (Ding et al., 2004, Farcas et al., 2005). Additionally, diarrhoea was seen in SARS, with active viral replication in enterocytes but minimal disruption of the intestinal architecture or cellular infiltration. Up-regulation of the potent immunosuppressive cytokine TGF-β (Cheng et al., 2004) and an anti-apoptotic host cellular response in the intestinal epithelial cells (Peiris et al., 2004a) may be a cause for the diarrhoea. Spread to other extrarespiratory tissues, such as the CNS, has been noted in H5N1 infection of mammals such as mice, ferrets and felids (Lipatov et al., 2003, Tanaka et al., 2003, Keawcharoen et al., 2004), but has been recorded rarely in human H5N1 infections (Morishima et al., 2002, Sugaya, 2002, de Jong et al., 2005, Gu et al., 2007). For SARS in man, no involvement of the CNS has been seen.
Concluding Remarks
Points to Consider when Using Animal Models
SARS-CoV and influenza virus infections in experimental animals demonstrate different pathways that result in similar disease overall, which can be compared with the situation in fatal human cases (Tables 3 and 4). Samples taken from fatal human cases of SARS-CoV and influenza virus infections often do not represent the full range of the different temporal stages of a disease. Instead, they are more likely to represent late-stage disease. In addition, the lesions caused by the viral infection may be complicated by lesions caused by clinical treatments and concurrent pre-existing disease. Therefore, to be able to study the course of the disease caused by these viruses, good animal models are necessary for SARS-CoV and influenza virus infections in man. Good animal models for SARS and influenza in man should ideally show virus replication dynamics, clinical illness and pathological changes that resemble those of human cases. The choice and design of an animal model used in an experiment is crucial for the outcome of the investigation and should be considered carefully. Time course experiments provide information about the temporal and spatial dynamics that help to design such animal models, including which time points and which samples are best to gain the most useful information (van den Brand et al., 2012a). Not only the best time points and analyses of the most informative samples should be considered carefully, but also the animal species, the inoculation route and the age of the animals are important, as is shown by studies of H5N1 influenza in ferrets and of SARS in aged mice and macaques (Rockx et al., 2009, Smits et al., 2010, Bodewes et al., 2011). Taken together, animal models should be designed specifically to address the aspect of SARS or influenza in man that one is interested in, since no one animal model can hope to address all the variation that is inherent in these viral diseases.
Although current animal models do not fully mimic the disease in fatal human cases of SARS, they can be useful in pathogenesis and vaccination studies. For pathogenesis studies, SARS-CoV need not cause mortality in the animal model, since the vast majority of human SARS cases are not fatal. For vaccination studies, virus replication, virus excretion, antibody titres and pathology scores are useful parameters for assessing the level of protection against infection and disease.
In designing animal models for SARS, important factors are the species and age of the laboratory animal used. Non-human primates, especially aged cynomolgus macaques and AGMs, most closely reflect the pathology in fatal human SARS by demonstrating typical pathological features such as hyaline membranes and syncytial cells on SARS-CoV infection (Smits et al., 2011). However, the severity of the clinical signs and lesions, level of mortality and extent of antigen expression in non-human primates is less (Table 1) (Nicholls et al., 2006). The variation in pathological changes and cytokine profiles among different non-human primate species, as well as among age groups within each species, may reflect the variation in severity of disease among people. For example, the mild degree of pneumonia in SARS-CoV-infected young-adult cynomolgus macaques may resemble the milder cases of SARS in man (Kuiken et al., 2004, Qin et al., 2005, Lawler et al., 2006, Smits et al., 2010). While aged cynomolgus macaques are good animal models for pathogenesis studies, vaccination studies and intervention studies of fatal SARS in man, large disadvantages are ethical, cost-related and housing problems. Ferrets infected with SARS-CoV only partly resemble fatal human SARS in terms of clinical illness, mortality and typical histopathological features (Table 1) (van den Brand et al., 2008). Moreover, methods for measuring gene expression profiles of ferrets are not yet fully developed. Nevertheless, ferrets can be used for pathogenesis and vaccination studies because they show SARS-CoV-associated pulmonary lesions and develop humoral immunity associated with reduced virus replication on vaccination. Cats infected with SARS-CoV show fewer similarities with the human situation and have greater ethical, cost-related and housing problems. Therefore, they are used less as animal models for human SARS (van den Brand et al., 2008). Although not discussed here, the use of aged BALB/c mice as an animal model for fatal human SARS may be an alternative, since there are many difficulties in the use of aged macaques (Subbarao and Roberts, 2006). Recombinant zoonotic and early phase SARS-CoV infection of these mice results in clinical signs, virus replication, pulmonary lesions and associated virus antigen expression in type II-resembling pneumocytes, bronchiolar and bronchial epithelial cells that partly resemble the features seen in fatal human SARS (Rockx et al., 2009). Therefore, aged BALB/c mice may be used for pathogenesis, antiviral and vaccination studies. A disadvantage may be age-related disease or mortality not related to SARS-CoV infection, which may influence the results.
In designing animal models for influenza, an important factor is the route of inoculation, because it can markedly affect the outcome of infection. Therefore, route of inoculation often depends on the purpose of the experiment. Transmission studies (at least in ferrets) may well use intranasal inoculation, which favours replication in the nose rather than in the lungs. This may correlate with virus excretion from the nose and minor pulmonary lesions. Additionally, intranasal inoculation in ferrets results in encephalitis, sometimes without pneumonia (Bodewes et al., 2011), and therefore this is not a good route for inducing viral pneumonia. In contrast, intratracheal inoculation favours replication in the lungs and is more likely to result in severe pneumonia, which makes this route of inoculation useful for pathogenesis and vaccination studies for viral pneumonia (van den Brand et al., 2010a).
Another important factor in designing an animal model for influenza, as in SARS, is the species of laboratory animal used. In cynomolgus macaques, the outcome of infection differs markedly according to the influenza virus used (Table 2) (Herfst et al., 2010). Therefore, cynomolgus macaques are probably not the best species to use for pathogenesis studies of fatal human disease from pH1N1 or seasonal influenza virus infections. However, because these viruses do replicate and cause virus-associated lesions, cynomolgus macaques may be useful for vaccination studies. In contrast, cynomolgus macaques infected with H5N1 show similar features as fatal human H5N1 infection and therefore can be used for pathogenesis, vaccination or therapeutics studies of H5N1 infection. Ferrets infected with different influenza viruses show similar virus replication dynamics, clinical illness, virus associated pathological changes and virus attachment patterns as in man (Table 2), and therefore are often used in animal models for various purposes. Cats infected with H5N1 show similar pathology of the respiratory tract, but additionally demonstrate a more widespread systemic infection than is seen in human cases. The virus antigen expression and virus attachment patterns in cats show differences and similarities similar to the case in ferrets. The systemic dissemination makes the cat less useful for animal models of fatal human H5N1 pneumonia or vaccination studies, since extrarespiratory replication and inflammation can influence the results. Taken together, ferrets are a good animal species to model fatal human influenza; however, there are species differences between man and ferrets, as well as differences between laboratory and field conditions. Therefore, the conclusions from ferret studies need thorough assessment to be able to make full use of the results without over-interpreting them.
Recently-emerging Respiratory Viruses
The importance of thorough pathological examination and development of good animal models for severe disease from respiratory viruses is demonstrated by the occurrence of recently-emerging viruses. Only very recently and ongoing there are two outbreaks of mild to severe respiratory illness with clinical disease and pathological features comparable to those in SARS-CoV and influenza viruses. In 2012, there was an outbreak of Middle East respiratory syndrome (MERS) caused by the MERS-CoV in the Middle East (van Boheemen et al., 2012). People from Europe and Africa have acquired MERS-CoV while travelling or staying in the Middle East, with to date more than 139 cases with 60 fatal cases (CDC, 2013). Additionally, there is limited human to human transmission, as is demonstrated by reports on 12 clusters, which occurred among close contacts or in healthcare settings (CDC, 2013). Only one autopsy of a MERS patient is described so far and this revealed severe DAD comparable with that seen in SARS (unpublished data). Experiments with rhesus and cynomolgus macaques and ferrets have demonstrated that the character of the lesions is comparable with those in macaques infected with SARS-CoV (unpublished data) (Munster et al., 2013). As in SARS-CoV infection in cynomolgus macaques, the severity and extent of the lesions in macaques is less than seen in human patients. Ferrets did not show a productive infection in contrast to what is seen in ferrets infected with SARS-CoV. As in SARS, a good animal model for severe pneumonia in human MERS has not yet been established and more research needs to be done.
At the beginning of 2013 an outbreak of LPAI virus H7N9 caused severe and fatal pneumonia in people in China. To date there are 135 reported cases, including 45 fatal cases (WHO, 2013). The first experiments with ferrets show disease more comparable with that of HPAI H5N1 in ferrets, so ferrets may be a good animal model for H7N9 infection in man (Belser et al., 2013, Richard et al., 2013, Watanabe et al., 2013). The outbreaks of MERS and H7N9 demonstrate the need for good animal models for severe pneumonia in people infected with respiratory viruses. These models are needed to develop preventive and therapeutic measures and to gain insight into the pathogenesis of such virus infections.
Future Perspectives
Many steps in the development of severe and fatal disease after SARS-CoV and influenza virus infection remain unknown. In the future, new or improved techniques such as genomics, proteomics and stem cell research may improve our knowledge of disease and recovery after disease and may be helpful in the development of effective interventions against severe disease. For seasonal influenza, aged animals may be an alternative for the inoculation of high doses of virus in ferrets as is seen in SARS-CoV infection in aged macaques and mice and recently for pH1N1 in aged macaques (Rockx et al., 2009, Smits et al., 2010, Josset et al., 2012). For all respiratory viruses that cause severe damage of alveolar and bronchiolar epithelium, treatment with stem cells may prevent severely ill patients from dying (Kumar et al., 2011). For both SARS and influenza, experiments with immunosuppressed animals (e.g. by using methods that are also used in organ transplantation) may help find better animal models that more accurately mimic the clinical situation (van der Vries et al., 2013).
Animal models are important not only for research on the known virus strains and subtypes of SARS-CoV and influenza A viruses. All kinds of viruses are ubiquitous among (wild) animals (Kuiken et al., 2005, van den Brand et al., 2012b); for example, bats harbour viruses that are similar to the epidemic strain of SARS-CoV and MERS-CoV (Li et al., 2005b, van Boheemen et al., 2012). Such viruses may be the source of future emerging infections. The travelling behaviour of people, as well as transport of animals and animal products, can result in easy spread of such infections, as was seen in the recent SARS and pH1N1 pandemics.
In conclusion, at the time of an outbreak of severe fatal disease in man, all steps from the discovery of the cause of the disease to the development and testing of appropriate therapeutic and preventive strategies should be carefully considered and pursued. These actions should take into account all of the lessons learned from previous outbreaks and be performed as a joint effort with all relevant disciplines, as part of the concept of ‘One Health’, to meet the many serious challenges to the health of people, domestic animals and wildlife and to the integrity of ecosystems.
Funding
This study was funded in part by the European Union FP7 project ANTIGONE (Grant agreement number 278976).
Acknowledgements
We thank F. van der Panne for figure preparation.
References
- Agyeman P., Duppenthaler A., Heininger U., Aebi C. Influenza-associated myositis in children. Infection. 2004;32:199–203. doi: 10.1007/s15010-004-4003-2. [DOI] [PubMed] [Google Scholar]
- Antonio G.E., Wong K.T., Chu W.C., Hui D.S., Cheng F.W. Imaging in severe acute respiratory syndrome (SARS) Clinical Radiology. 2003;58:825–832. doi: 10.1016/S0009-9260(03)00308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas T., Roberts A., Teal T.H., Vogel L., Chen J. Genomic analysis reveals age-dependent innate immune responses to severe acute respiratory syndrome coronavirus. Journal of Virology. 2008;82:9465–9476. doi: 10.1128/JVI.00489-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L., Gu L., Cao B., Zhai X.L., Lu M. Clinical features of pneumonia caused by 2009 influenza A(H1N1) virus in Beijing, China. Chest. 2011;139:1156–1164. doi: 10.1378/chest.10-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard D.L. Animal models for the study of influenza pathogenesis and therapy. Antiviral Research. 2009;82:A110–A122. doi: 10.1016/j.antiviral.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin C.R., Bielefeldt-Ohmann H., Tumpey T.M., Sabourin P.J., Long J.P. Early and sustained innate immune response defines pathology and death in non-human primates infected by highly pathogenic influenza virus. Proceedings of the National Academy of Sciences of the USA. 2009;106:3455–3460. doi: 10.1073/pnas.0813234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel J.H., Farrar J., Han A.M., Hayden F.G., Hyer R. Avian influenza A (H5N1) infection in humans. New England Journal of Medicine. 2005;353:1374–1385. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- Belser J.A., Gustin K.M., Pearce M.B., Maines T.R., Zeng H. Pathogenesis and transmission of avian influenza A (H7N9) virus in ferrets and mice. Nature. 2013;501:556–559. doi: 10.1038/nature12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benne C.A., Kraaijeveld C.A., van Strijp J.A., Brouwer E., Harmsen M. Interactions of surfactant protein A with influenza A viruses: binding and neutralization. Journal of Infectious Diseases. 1995;171:335–341. doi: 10.1093/infdis/171.2.335. [DOI] [PubMed] [Google Scholar]
- Berthiaume Y., Matthay M.A. Alveolar edema fluid clearance and acute lung injury. Respiratory Physiological & Neurobiology. 2007;159:350–359. doi: 10.1016/j.resp.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge W.I.B. Prodist; New York: 1978. Influenza: the Last Great Plague. [Google Scholar]
- Bodewes R., Kreijtz J.H., van Amerongen G., Fouchier R.A., Osterhaus A.D. Pathogenesis of influenza A/H5N1 virus infection in ferrets differs between intranasal and intratracheal routes of inoculation. American Journal of Pathology. 2011;179:30–36. doi: 10.1016/j.ajpath.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boltz D.A., Rehg J.E., McClaren J., Webster R.G., Govorkova E.A. Oseltamivir prophylactic regimens prevent H5N1 influenza morbidity and mortality in a ferret model. Journal of Infectious Diseases. 2008;197:1315–1323. doi: 10.1086/586711. [DOI] [PubMed] [Google Scholar]
- Boukhvalova M.S., Prince G.A., Blanco J.C. The cotton rat model of respiratory viral infections. Biologicals. 2009;37:152–159. doi: 10.1016/j.biologicals.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukreyev A., Lamirande E.W., Buchholz U.J., Vogel L.N., Elkins W.R. Mucosal immunisation of African green monkeys (Cercopithecus aethiops) with an attenuated parainfluenza virus expressing the SARS coronavirus spike protein for the prevention of SARS. Lancet. 2004;363:2122–2127. doi: 10.1016/S0140-6736(04)16501-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron C.M., Cameron M.J., Bermejo-Martin J.F., Ran L., Xu L. Gene expression analysis of host innate immune responses during lethal H5N1 infection in ferrets. Journal of Virology. 2008;82:11308–11317. doi: 10.1128/JVI.00691-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron M.J., Ran L., Xu L., Danesh A., Bermejo-Martin J.F. Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. Journal of Virology. 2007;81:8692–8706. doi: 10.1128/JVI.00527-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . 2013. Middle East Respiratory Syndrome (MERS)http://www.cdc.gov/coronavirus/mers/ Accessed 21-10-2013. [Google Scholar]
- Chan V.S., Chan K.Y., Chen Y., Poon L.L., Cheung A.N. Homozygous L-SIGN (CLEC4M) plays a protective role in SARS coronavirus infection. Nature Genetics. 2006;38:38–46. doi: 10.1038/ng1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Yeung M., Xu R.H. SARS: epidemiology. Respirology. 2003;8(Suppl. S1):S9–S14. doi: 10.1046/j.1440-1843.2003.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Deng W., Jia C., Dai X., Zhu H. Pathological lesions and viral localization of influenza A (H5N1) virus in experimentally infected Chinese rhesus macaques: implications for pathogenesis and viral transmission. Archives of Virology. 2009;154:227–233. doi: 10.1007/s00705-008-0277-5. [DOI] [PubMed] [Google Scholar]
- Cheng V.C., Hung I.F., Tang B.S., Chu C.M., Wong M.M. Viral replication in the nasopharynx is associated with diarrhea in patients with severe acute respiratory syndrome. Clinical Infectious Diseases. 2004;38:467–475. doi: 10.1086/382681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung O.Y., Chan J.W., Ng C.K., Koo C.K. The spectrum of pathological changes in severe acute respiratory syndrome (SARS) Histopathology. 2004;45:119–124. doi: 10.1111/j.1365-2559.2004.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chokephaibulkit K., Uiprasertkul M., Puthavathana P., Chearskul P., Auewarakul P. A child with avian influenza A (H5N1) infection. Pediatric Infectious Disease Journal. 2005;24:162–166. doi: 10.1097/01.inf.0000151037.25237.1e. [DOI] [PubMed] [Google Scholar]
- Chung H.Y., Sung B., Jung K.J., Zou Y., Yu B.P. The molecular inflammatory process in aging. Antioxidants and Redox Signaling. 2006;8:572–581. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]
- Chutinimitkul S., Bhattarakosol P., Srisuratanon S., Eiamudomkan A., Kongsomboon K. H5N1 influenza A virus and infected human plasma. Emerging Infectious Diseases. 2006;12:1041–1043. doi: 10.3201/eid1206.060227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claas E.C.J., Osterhaus A.D.M.E., van Beek R., de Jong J.C., Rimmelzwaan G.F. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351:472–477. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- Connor R.J., Kawaoka Y., Webster R.G., Paulson J.C. Receptor specificity in human, avian and equine H2 and H3 influenza virus isolates. Virology. 1994;205:17–23. doi: 10.1006/viro.1994.1615. [DOI] [PubMed] [Google Scholar]
- Coulter K.R., Doseff A., Sweeney P., Wang Y., Marsh C.B. Opposing effect by cytokines on Fas-mediated apoptosis in A549 lung epithelial cells. American Journal of Respiratory Cell and Molecular Biology. 2002;26:58–66. doi: 10.1165/ajrcmb.26.1.4285. [DOI] [PubMed] [Google Scholar]
- Cox N.J., Subbarao K. Global epidemiology of influenza: past and present. Annual Review of Medicine. 2000;51:407–421. doi: 10.1146/annurev.med.51.1.407. [DOI] [PubMed] [Google Scholar]
- de Jong M.D., Bach V.C., Phan T.Q., Vo M.H., Tran T.T. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. New England Journal of Medicine. 2005;352:686–691. doi: 10.1056/NEJMoa044307. [DOI] [PubMed] [Google Scholar]
- de Jong M.D., Simmons C.P., Tran T.T., Hien V.M., Smith G.J. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nature Medicine. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lang A., Baas T., Teal T., Leijten L.M., Rain B. Functional genomics highlights differential induction of antiviral pathways in the lungs of SARS-CoV-infected macaques. PLoS Pathogens. 2007;3:e112. doi: 10.1371/journal.ppat.0030112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., He L., Zhang Q., Huang Z., Che X. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. Journal of Pathology. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Wang H., Shen H., Li Z., Geng J. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. Journal of Pathology. 2003;200:282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly C.A., Ghani A.C., Leung G.M., Hedley A.J., Fraser C. Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. Lancet. 2003;361:1761–1766. doi: 10.1016/S0140-6736(03)13410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Günther S., Preiser W., van der Werf S., Brodt H.R. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. New England Journal of Medicine. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Fan J., Ye R.D., Malik A.B. Transcriptional mechanisms of acute lung injury. American Journal of Physiology: Lung Cellular and Molecular Physiology. 2001;281:L1037–L1050. doi: 10.1152/ajplung.2001.281.5.L1037. [DOI] [PubMed] [Google Scholar]
- Farcas G.A., Poutanen S.M., Mazzulli T., Willey B.M., Butany J. Fatal severe acute respiratory syndrome is associated with multiorgan involvement by coronavirus. Journal of Infectious Diseases. 2005;191:193–197. doi: 10.1086/426870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouchier R.A., Kuiken T., Schutten M., van Amerongen G., Van Doornum G.J. Aetiology: Koch's postulates fulfilled for SARS virus. Nature. 2003;423:240. doi: 10.1038/423240a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks T.J., Chong P.Y., Chui P., Galvin J.R., Lourens R.M. Lung pathology of severe acute respiratory syndrome (SARS): a study of 8 autopsy cases from Singapore. Human Pathology. 2003;34:743–748. doi: 10.1016/S0046-8177(03)00367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedewald W.F., Hook E.W., Jr. Influenza virus infection in the hamster: a study of inapparent virus infection and virus adaptation. Journal of Experimental Medicine. 1948;88:343–353. doi: 10.1084/jem.88.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T., Larbi A., Wikby A., Mocchegiani E., Hirokawa K. Dysregulation of T-cell function in the elderly: scientific basis and clinical implications. Drugs and Aging. 2005;22:589–603. doi: 10.2165/00002512-200522070-00005. [DOI] [PubMed] [Google Scholar]
- Gao W., Tamin A., Soloff A., D'Aiuto L., Nwanegbo E. Effects of a SARS-associated coronavirus vaccine in monkeys. Lancet. 2003;362:1895–1896. doi: 10.1016/S0140-6736(03)14962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten R.J., Davis C.T., Russell C.A., Shu B., Lindstrom S. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325:197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebink G.S., Berzins I.K., Marker S.C., Schiffman G. Experimental otitis media after nasal inoculation of Streptococcus pneumoniae and influenza A virus in chinchillas. Infectious Immunology. 1980;30:445–450. doi: 10.1128/iai.30.2.445-450.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese M., Harder T.C., Teifke J.P., Klopfleisch R., Breithaupt A. Experimental infection and natural contact exposure of dogs with avian influenza virus (H5N1) Emerging Infectious Diseases. 2008;14:308–310. doi: 10.3201/eid1402.070864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorkova E.A., Rehg J.E., Krauss S., Yen H.L., Guan Y. Lethality to ferrets of H5N1 influenza viruses isolated from humans and poultry in 2004. Journal of Virology. 2005;79:2191–2198. doi: 10.1128/JVI.79.4.2191-2198.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramberg T., Hofmann H., Moller P., Lalor P.F., Marzi A. LSECtin interacts with filovirus glycoproteins and the spike protein of SARS coronavirus. Virology. 2005;340:224–236. doi: 10.1016/j.virol.2005.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Gong E., Zhang B., Zheng J., Gao Z. Multiple organ infection and the pathogenesis of SARS. Journal of Experimental Medicine. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. American Journal of Pathology. 2007;170:1136–1147. doi: 10.2353/ajpath.2007.061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Xie Z., Gao Z., Liu J., Korteweg C. H5N1 infection of the respiratory tract and beyond: a molecular pathology study. Lancet. 2007;370:1137–1145. doi: 10.1016/S0140-6736(07)61515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y., Zheng B.J., He Y.Q., Liu X.L., Zhuang Z.X. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- Guarner J., Falcon-Escobedo R. Comparison of the pathology caused by H1N1, H5N1, and H3N2 influenza viruses. Archives of Medical Research. 2009;40:655–661. doi: 10.1016/j.arcmed.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Guarner J., Paddock C.D., Shieh W.J., Packard M.M., Patel M. Histopathologic and immunohistochemical features of fatal influenza virus infection in children during the 2003–2004 season. Clinical Infectious Diseases. 2006;43:132–140. doi: 10.1086/505122. [DOI] [PubMed] [Google Scholar]
- Guo H., Guo A., Wang C., Yan B., Lu H. Expression of feline angiotensin converting enzyme 2 and its interaction with SARS-CoV S1 protein. Research in Veterinary Science. 2008;84:494–496. doi: 10.1016/j.rvsc.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagmans B.L., Kuiken T., Martina B.E., Fouchier R.A., Rimmelzwaan G.F. Pegylated interferon-alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nature Medicine. 2004;10:290–293. doi: 10.1038/nm1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G.J. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. Journal of Pathology. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock K., Veguilla V., Lu X., Zhong W., Butler E.N. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. New England Journal of Medicine. 2009;361:1945–1952. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- He L., Ding Y., Zhang Q., Che X., He Y. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. Journal of Pathology. 2006;210:288–297. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herfst S., van den Brand J.M., Schrauwen E.J., de Wit E., Munster V.J. Pandemic 2009 H1N1 influenza virus causes diffuse alveolar damage in cynomolgus macaques. Veterinary Pathology. 2010;47:1040–1047. doi: 10.1177/0300985810374836. [DOI] [PubMed] [Google Scholar]
- Herold S., Steinmueller M., von Wulffen W., Cakarova L., Pinto R. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. Journal of Experimental Medicine. 2008;205:3065–3077. doi: 10.1084/jem.20080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien N.D., Ha N.H., Van N.T., Ha N.T., Lien T.T. Human infection with highly pathogenic avian influenza virus (H5N1) in northern Vietnam, 2004–2005. Emerging Infectious Diseases. 2009;15:19–23. doi: 10.3201/eid1501.080073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien T.T., de Jong M., Farrar J. Avian influenza – a challenge to global health care structures. New England Journal of Medicine. 2004;351:2363–2365. doi: 10.1056/NEJMp048267. [DOI] [PubMed] [Google Scholar]
- Holt P.G., Upham J.W., Sly P.D. Contemporaneous maturation of immunologic and respiratory functions during early childhood: implications for development of asthma prevention strategies. Journal of Allergy and Clinical Immunology. 2005;116:16–24. doi: 10.1016/j.jaci.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Horimoto T., Kawaoka Y. Pandemic threat posed by avian influenza A viruses. Clinical Microbiology Reviews. 2001;14:129–149. doi: 10.1128/CMR.14.1.129-149.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh P.R., Chen P.J., Hsiao C.H., Yeh S.H., Cheng W.C. Patient data, early SARS epidemic, Taiwan. Emerging Infectious Diseases. 2004;10:489–493. doi: 10.3201/eid1003.030571. [DOI] [PubMed] [Google Scholar]
- Huang I.C., Bosch B.J., Li F., Li W., Lee K.H. SARS coronavirus, but not human coronavirus NL63, utilizes cathepsin L to infect ACE2-expressing cells. Journal of Biological Chemistry. 2006;281:3198–3203. doi: 10.1074/jbc.M508381200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang I.C., Bosch B.J., Li W., Farzan M., Rottier P.M. SARS-CoV, but not HCoV-NL63, utilizes cathepsins to infect cells: viral entry. Advances in Experimental Medicine and Biology. 2006;581:335–338. doi: 10.1007/978-0-387-33012-9_60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J.L., Lin H.T., Wang Y.M., Yeh Y.C., Peck K. Rapid and sensitive detection of multiple genes from the SARS-coronavirus using quantitative RT-PCR with dual systems. Journal of Medical Virology. 2005;77:151–158. doi: 10.1002/jmv.20432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Kuba K., Neely G.G., Yaghubian-Malhami R., Perkmann T. Identification of oxidative stress and Toll-like receptor 4 signalling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip W.K., Chan K.H., Law H.K., Tso G.H., Kong E.K. Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. Journal of Infectious Diseases. 2005;191:1697–1704. doi: 10.1086/429631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y., Shinya K., Kiso M., Watanabe T., Sakoda Y. In-vitro and in-vivo characterization of new swine-origin H1N1 influenza viruses. Nature. 2009;460:1021–1025. doi: 10.1038/nature08260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers S.A., Tusell S.M., Gillim-Ross L., Hemmila E.M., Achenbach J.E. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proceedings of the National Academy of Sciences of the USA. 2004;101:15748–15753. doi: 10.1073/pnas.0403812101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepson P.D., Brew S., MacMillan A.P., Baker J.R., Barnett J. Antibodies to Brucella in marine mammals around the coast of England and Wales. Veterinary Record. 1997;141:513–515. doi: 10.1136/vr.141.20.513. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Xu J., Zhou C., Wu Z., Zhong S. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. American Journal of Respiratory and Critical Care Medicine. 2005;171:850–857. doi: 10.1164/rccm.200407-857OC. [DOI] [PubMed] [Google Scholar]
- Johnston S.L. Innate immunity in the pathogenesis of virus-induced asthma exacerbations. Proceedings of the American Thoracic Society. 2007;4:267–270. doi: 10.1513/pats.200701-030AW. [DOI] [PubMed] [Google Scholar]
- Josset L., Engelmann F., Haberthur K., Kelly S., Park B. Increased viral loads and exacerbated innate host response in aged macaques infected with 2009 pandemic H1N1 influenza A virus. Journal of Virology. 2012;86:11115–11127. doi: 10.1128/JVI.01571-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlberg J., Chong D.S., Lai W.Y. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? American Journal of Epidemiology. 2004;159:229–231. doi: 10.1093/aje/kwh056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnam G., Rygiel T.P., Raaben M., Grinwis G.C., Coenjaerts F.E. CD200 receptor controls sex-specific TLR7 responses to viral infection. PLoS Pathogens. 2012;8:e1002710. doi: 10.1371/journal.ppat.1002710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keawcharoen J., Oraveerakul K., Kuiken T., Fouchier R.A., Amonsin A. Avian influenza H5N1 in tigers and leopards. Emerging Infectious Diseases. 2004;10:2189–2191. doi: 10.3201/eid1012.040759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong K.F., Delroux K., Wang X., Qian F., Arjona A. Dysregulation of TLR3 impairs the innate immune response to West Nile virus in the elderly. Journal of Virology. 2008;82:7613–7623. doi: 10.1128/JVI.00618-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreijtz J.H., Suezer Y., de Mutsert G., van den Brand J.M., van Amerongen G. Recombinant modified vaccinia virus Ankara expressing the hemagglutinin gene confers protection against homologous and heterologous H5N1 influenza virus infections in macaques. Journal of Infectious Diseases. 2009;199:405–413. doi: 10.1086/595984. [DOI] [PubMed] [Google Scholar]
- Kuiken T., Fouchier R.A., Schutten M., Rimmelzwaan G.F., van Amerongen G. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiken T., Leighton F.A., Fouchier R.A., LeDuc J.W., Peiris J.S. Public health. Pathogen surveillance in animals. Science. 2005;309:1680–1681. doi: 10.1126/science.1113310. [DOI] [PubMed] [Google Scholar]
- Kuiken T., Rimmelzwaan G.F., van Amerongen G., Osterhaus A.D. Pathology of human influenza A (H5N1) virus disease in cynomolgus macaques (Macaca fascicularis) Veterinary Pathology. 2003;40:304–310. doi: 10.1354/vp.40-3-304. [DOI] [PubMed] [Google Scholar]
- Kuiken T., van den Brand J.M., van Riel D., Pantin-Jackwood M., Swayne D.E. Comparative pathology of select agent influenza A virus infections. Veterinary Pathology. 2010;47:893–914. doi: 10.1177/0300985810378651. [DOI] [PubMed] [Google Scholar]
- Kuiken T., van den Hoogen B.G., van Riel D.A., Laman J.D., van Amerongen G. Experimental human metapneumovirus infection of cynomolgus macaques (Macaca fascicularis) results in virus replication in ciliated epithelial cells and pneumocytes with associated lesions throughout the respiratory tract. American Journal of Pathology. 2004;164:1893–1900. doi: 10.1016/S0002-9440(10)63750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P.A., Hu Y., Yamamoto Y., Hoe N.B., Wei T.S. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y.K., Lipatov A.S., Swayne D.E. Bronchointerstitial pneumonia in guinea pigs following inoculation with H5N1 high pathogenicity avian influenza virus. Veterinary Pathology. 2009;46:138–141. doi: 10.1354/vp.46-1-138. [DOI] [PubMed] [Google Scholar]
- Lau E.H., Hsiung C.A., Cowling B.J., Chen C.H., Ho L.M. A comparative epidemiologic analysis of SARS in Hong Kong, Beijing and Taiwan. BMC Infectious Diseases. 2010;10:50. doi: 10.1186/1471-2334-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S.K., Woo P.C., Li K.S., Huang Y., Tsoi H.W. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proceedings of the National Academy of Sciences of the USA. 2005;102:14040–14045. doi: 10.1073/pnas.0506735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau Y.L., Peiris J.S. Pathogenesis of severe acute respiratory syndrome. Current Opinion in Immunology. 2005;17:404–410. doi: 10.1016/j.coi.2005.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J.V., Endy T.P., Hensley L.E., Garrison A., Fritz E.A. Cynomolgus macaque as an animal model for severe acute respiratory syndrome. PLoS Medicine. 2006;3:e149. doi: 10.1371/journal.pmed.0030149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., Hui D., Wu A., Chan P., Cameron P. A major outbreak of severe acute respiratory syndrome in Hong Kong. New England Journal of Medicine. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- Leung G.M., Hedley A.J., Ho L.M., Chau P., Wong I.O. The epidemiology of severe acute respiratory syndrome in the 2003 Hong Kong epidemic: an analysis of all 1755 patients. Annals of Internal Medicine. 2004;141:662–673. doi: 10.7326/0003-4819-141-9-200411020-00006. [DOI] [PubMed] [Google Scholar]
- Leung W.K., To K.F., Chan P.K., Chan H.L., Wu A.K. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011–1017. doi: 10.1016/j.gastro.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B.J., Tang Q., Cheng D., Qin C., Xie F.Y. Using siRNA in prophylactic and therapeutic regimens against SARS coronavirus in Rhesus macaque. Nature Medicine. 2005;11:944–951. doi: 10.1038/nm1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Shi Z., Yu M., Ren W., Smith C. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- Li W., Zhang C., Sui J., Kuhn J.H., Moore M.J. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO Journal. 2005;24:1634–1643. doi: 10.1038/sj.emboj.7600640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L., He C., Lei M., Li S., Hao Y. Pathology of guinea pigs experimentally infected with a novel reovirus and coronavirus isolated from SARS patients. DNA and Cell Biology. 2005;24:485–490. doi: 10.1089/dna.2005.24.485. [DOI] [PubMed] [Google Scholar]
- Licastro F., Candore G., Lio D., Porcellini E., Colonna-Romano G. Innate immunity and inflammation in ageing: a key for understanding age-related diseases. Immunity and Ageing. 2005;2:8. doi: 10.1186/1742-4933-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipatov A.S., Krauss S., Guan Y., Peiris M., Rehg J.E. Neurovirulence in mice of H5N1 influenza virus genotypes isolated from Hong Kong poultry in 2001. Journal of Virology. 2003;77:3816–3823. doi: 10.1128/JVI.77.6.3816-3823.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipatov A.S., Kwon Y.K., Sarmento L.V., Lager K.M., Spackman E. Domestic pigs have low susceptibility to H5N1 highly pathogenic avian influenza viruses. PLoS Pathogens. 2008;4:e1000102. doi: 10.1371/journal.ppat.1000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Liang W.N., Chen Q., Xie X.Q., Wu J. Risk factors for SARS-related deaths in 2003, Beijing. Biomedical and Environmental Sciences. 2006;19:336–339. [PubMed] [Google Scholar]
- Lohr C.V., DeBess E.E., Baker R.J., Hiett S.L., Hoffman K.A. Pathology and viral antigen distribution of lethal pneumonia in domestic cats due to pandemic (H1N1) 2009 influenza A virus. Veterinary Pathology. 2010;47:378–386. doi: 10.1177/0300985810368393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie J.K., Jean C., Acosta M., Samuel M.C., Matyas B.T. A review of adult mortality due to 2009 pandemic (H1N1) influenza A in California. PLoS One. 2011;6:e18221. doi: 10.1371/journal.pone.0018221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loutfy M.R., Blatt L.M., Siminovitch K.A., Ward S., Wolff B. Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. Journal of the American Medical Association. 2003;290:3222–3228. doi: 10.1001/jama.290.24.3222. [DOI] [PubMed] [Google Scholar]
- Lowy R.J. Influenza virus induction of apoptosis by intrinsic and extrinsic mechanisms. International Reviews of Immunology. 2003;22:425–449. doi: 10.1080/08830180305216. [DOI] [PubMed] [Google Scholar]
- Maines T.R., Lu X.H., Erb S.M., Edwards L., Guarner J. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. Journal of Virology. 2005;79:11788–11800. doi: 10.1128/JVI.79.18.11788-11800.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manocha S., Walley K.R., Russell J.A. Severe acute respiratory distress syndrome (SARS): a critical care perspective. Critical Care Medicine. 2003;31:2684–2692. doi: 10.1097/01.CCM.0000091929.51288.5F. [DOI] [PubMed] [Google Scholar]
- Martina B.E., Haagmans B.L., Kuiken T., Fouchier R.A., Rimmelzwaan G.F. Virology: SARS virus infection of cats and ferrets. Nature. 2003;425:915. doi: 10.1038/425915a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuliffe J., Vogel L., Roberts A., Fahle G., Fischer S. Replication of SARS coronavirus administered into the respiratory tract of African green, rhesus and cynomolgus monkeys. Virology. 2004;330:8–15. doi: 10.1016/j.virol.2004.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K.C. The role of immunity in susceptibility to respiratory infection in the aging lung. Respiration Physiology. 2001;128:23–31. doi: 10.1016/S0034-5687(01)00261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K.C. Aging. Proceedings of the American Thoracic Society. 2005;2:433–439. doi: 10.1513/pats.200508-081JS. [DOI] [PubMed] [Google Scholar]
- Mineo G., Ciccarese F., Modolon C., Landini M.P., Valentino M. Post-ARDS pulmonary fibrosis in patients with H1N1 pneumonia: role of follow-up CT. Radiologia Medica. 2012;117:185–200. doi: 10.1007/s11547-011-0740-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morens D.M., Fauci A.S. The 1918 influenza pandemic: insights for the 21st century. Journal of Infectious Diseases. 2007;195:1018–1028. doi: 10.1086/511989. [DOI] [PubMed] [Google Scholar]
- Morens D.M., Taubenberger J.K., Fauci A.S. The persistent legacy of the 1918 influenza virus. New England Journal of Medicine. 2009;361:225–229. doi: 10.1056/NEJMp0904819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima T., Togashi T., Yokota S., Okuno Y., Miyazaki C. Encephalitis and encephalopathy associated with an influenza epidemic in Japan. Clinical Infectious Diseases. 2002;35:512–517. doi: 10.1086/341407. [DOI] [PubMed] [Google Scholar]
- Munster V.J., de Wit E., Feldmann H. Pneumonia from human coronavirus in a macaque model. New England Journal of Medicine. 2013;368:1560–1562. doi: 10.1056/NEJMc1215691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster V.J., de Wit E., van den Brand J.M., Herfst S., Schrauwen E.J. Pathogenesis and transmission of swine-origin 2009 A(H1N1) influenza virus in ferrets. Science. 2009;325:481–483. doi: 10.1126/science.1177127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murasko D.M., Jiang J. Response of aged mice to primary virus infections. Immunology Reviews. 2005;205:285–296. doi: 10.1111/j.0105-2896.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- Murphy B.R., Hinshaw V.S., Sly D.L., London W.T., Hosier N.T. Virulence of avian influenza A viruses for squirrel monkeys. Infectious Immunology. 1982;37:1119–1126. doi: 10.1128/iai.37.3.1119-1126.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng M.H., Lau K.M., Li L., Cheng S.H., Chan W.Y. Association of human leukocyte antigen class I (B*0703) and class II (DRB1*0301) genotypes with susceptibility and resistance to the development of severe acute respiratory syndrome. Journal of Infectious Diseases. 2004;190:515–518. doi: 10.1086/421523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng M.L., Tan S.H., See E.E., Ooi E.E., Ling A.E. Proliferative growth of SARS coronavirus in Vero E6 cells. Journal of General Virology. 2003;84:3291–3303. doi: 10.1099/vir.0.19505-0. [DOI] [PubMed] [Google Scholar]
- Ng W.F., To K.F., Lam W.W., Ng T.K., Lee K.C. The comparative pathology of severe acute respiratory syndrome and avian influenza A subtype H5N1 – a review. Human Pathology. 2006;37:381–390. doi: 10.1016/j.humpath.2006.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls J.M., Butany J., Poon L.L., Chan K.H., Beh S.L. Time course and cellular localization of SARS-CoV nucleoprotein and RNA in lungs from fatal cases of SARS. PLoS Medicine. 2006;3:e27. doi: 10.1371/journal.pmed.0030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls J.M., Chan M.C.W., Chan W.Y., Wong H.K., Cheung C.Y. Tropism of avian influenza A (H5N1) in the upper and lower respiratory tract. Nature Medicine. 2007;13:147–149. doi: 10.1038/nm1529. [DOI] [PubMed] [Google Scholar]
- Nicholls J.M., Poon L.L.M., Lee K.C., Ng W.F., Lai S.T. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:1773–1778. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Regan A. The role of osteopontin in lung disease. Cytokine & Growth Factor Reviews. 2003;14:479–488. doi: 10.1016/s1359-6101(03)00055-8. [DOI] [PubMed] [Google Scholar]
- Osterhaus A.D.M.E. Catastrophes after crossing species barriers. Philosophical Transactions of the Royal Society of London. 2001;356:1–3. doi: 10.1098/rstb.2001.0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo A., Gibson K., Cisneros J., Richards T.J., Yang Y. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Medicine. 2005;2:e251. doi: 10.1371/journal.pmed.0020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker N.R., Caywood D.D. Surgical diseases of the esophagus. Veterinary Clinics of North America: Small Animal Practice. 1987;17:333–358. doi: 10.1016/s0195-5616(87)50030-4. [DOI] [PubMed] [Google Scholar]
- Peiris J.S., de Jong M.D., Guan Y. Avian influenza virus (H5N1): a threat to human health. Clinical Microbiology Reviews. 2007;20:243–267. doi: 10.1128/CMR.00037-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S., Guan Y., Yuen K.Y. Severe acute respiratory syndrome. Nature Medicine. 2004;10:S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S., Lai S.T., Poon L.L., Guan Y., Yam L.Y. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S., Yu W.C., Leung C.W., Cheung C.Y., Ng W.F. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet. 2004;363:617–619. doi: 10.1016/S0140-6736(04)15595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S., Yuen K.Y., Osterhaus A.D., Stöhr K. The severe acute respiratory syndrome. New England Journal of Medicine. 2003;349:2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- Perez-Padilla R., Rosa-Zamboni D., Ponce D.L., Hernandez M., Quinones-Falconi F. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. New England Journal of Medicine. 2009;361:680–689. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]
- Plackett T.P., Boehmer E.D., Faunce D.E., Kovacs E.J. Aging and innate immune cells. Journal of Leukocyte Biology. 2004;76:291–299. doi: 10.1189/jlb.1103592. [DOI] [PubMed] [Google Scholar]
- Qin C., Wang J., Wei Q., She M., Marasco W.A. An animal model of SARS produced by infection of Macaca mulatta with SARS coronavirus. Journal of Pathology. 2005;206:251–259. doi: 10.1002/path.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin E., Shi H., Tang L., Wang C., Chang G. Immunogenicity and protective efficacy in monkeys of purified inactivated Vero-cell SARS vaccine. Vaccine. 2006;24:1028–1034. doi: 10.1016/j.vaccine.2005.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray C.G., Icenogle T.B., Minnich L.L., Copeland J.G., Grogan T.M. The use of intravenous ribavirin to treat influenza virus-associated acute myocarditis. Journal of Infectious Diseases. 1989;159:829–836. doi: 10.1093/infdis/159.5.829. [DOI] [PubMed] [Google Scholar]
- Reperant L.A., van de Bildt M.W., van Amerongen G., Leijten L.M., Watson S. Marked endotheliotropism of highly pathogenic avian influenza virus H5N1 following intestinal inoculation in cats. Journal of Virology. 2012;86:1158–1165. doi: 10.1128/JVI.06375-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard M., Schrauwen E.J., De Graaf M., Bestebroer T.M., Spronken M.I. Limited airborne transmission of H7N9 influenza A virus between ferrets. Nature. 2013;501:560–563. doi: 10.1038/nature12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmelzwaan G., van Riel D., Baars M., Bestebroer T.M., van Amerongen G. Influenza A virus (H5N1) infection in cats causes systemic disease with potential novel routes of virus spread within and between hosts. American Journal of Pathology. 2006;168:176–183. doi: 10.2353/ajpath.2006.050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmelzwaan G.F., Kuiken T., van Amerongen G., Bestebroer T.M., Fouchier R.A.M. Pathogenesis of influenza A (H5N1) virus infection in a primate model. Journal of Virology. 2001;75:6687–6691. doi: 10.1128/JVI.75.14.6687-6691.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink L., Cakman I., Kirchner H. Altered cytokine production in the elderly. Mechanisms of Ageing and Development. 1998;102:199–209. doi: 10.1016/s0047-6374(97)00153-x. [DOI] [PubMed] [Google Scholar]
- Roberts A., Paddock C., Vogel L., Butler E., Zaki S. Aged BALB/c mice as a model for increased severity of severe acute respiratory syndrome in elderly humans. Journal of Virology. 2005;79:5833–5838. doi: 10.1128/JVI.79.9.5833-5838.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A., Thomas W.D., Guarner J., Lamirande E.W., Babcock G.J. Therapy with a severe acute respiratory syndrome-associated coronavirus-neutralizing human monoclonal antibody reduces disease severity and viral burden in golden Syrian hamsters. Journal of Infectious Diseases. 2006;193:685–692. doi: 10.1086/500143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockx B., Baas T., Zornetzer G.A., Haagmans B., Sheahan T. Early upregulation of acute respiratory distress syndrome-associated cytokines promotes lethal disease in an aged-mouse model of severe acute respiratory syndrome coronavirus infection. Journal of Virology. 2009;83:7062–7074. doi: 10.1128/JVI.00127-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockx B., Feldmann F., Brining D., Gardner D., LaCasse R. Comparative pathogenesis of three human and zoonotic SARS-CoV strains in cynomolgus macaques. PLoS One. 2011;6:e18558. doi: 10.1371/journal.pone.0018558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg M.B., Haessler S.D., Brown R.B. Complications of viral influenza. American Journal of Medicine. 2008;121:258–264. doi: 10.1016/j.amjmed.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe T., Cho D.S., Bright R.A., Zitzow L.A., Katz J.M. Neurological manifestations of avian influenza viruses in mammals. Avian Diseases. 2003;47:1122–1126. doi: 10.1637/0005-2086-47.s3.1122. [DOI] [PubMed] [Google Scholar]
- Rowe T., Gao G., Hogan R.J., Crystal R.G., Voss T.G. Macaque model for severe acute respiratory syndrome. Journal of Virology. 2004;78:11401–11404. doi: 10.1128/JVI.78.20.11401-11404.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin S., Liu D., Pletnikov M., McCullers J., Ye Z. Wild-type and attenuated influenza virus infection of the neonatal rat brain. Journal of Neurovirology. 2004;10:305–314. doi: 10.1080/13550280490499579. [DOI] [PubMed] [Google Scholar]
- Salvioli S., Capri M., Valensin S., Tieri P., Monti D. Inflamm-aging, cytokines and aging: state of the art, new hypotheses on the role of mitochondria and new perspectives from systems biology. Current Pharmaceutical Design. 2006;12:3161–3171. doi: 10.2174/138161206777947470. [DOI] [PubMed] [Google Scholar]
- Schrauwen E.J., Herfst S., Leijten L.M., van Run P., Bestebroer T.M. The multibasic cleavage site in H5N1 virus is critical for systemic spread along the olfactory and hematogenous routes in ferrets. Journal of Virology. 2012;86:3975–3984. doi: 10.1128/JVI.06828-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh W.J., Blau D.M., Denison A.M., Deleon-Carnes M., Adem P. 2009 pandemic influenza A (H1N1): pathology and pathogenesis of 100 fatal cases in the United States. American Journal of Pathology. 2010;177:166–175. doi: 10.2353/ajpath.2010.100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh W.J., Hsiao C.H., Paddock C.D., Guarner J., Goldsmith C.S. Immunohistochemical, in-situ hybridization, and ultrastructural localization of SARS-associated coronavirus in lung of a fatal case of severe acute respiratory syndrome in Taiwan. Human Pathology. 2005;36:303–309. doi: 10.1016/j.humpath.2004.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinya K., Ebina M., Yamada S., Ono M., Kasai N. Influenza virus receptors in the human airway. Nature. 2006;440:435–436. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- Simmons G., Gosalia D.N., Rennekamp A.J., Reeves J.D., Diamond S.L. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proceedings of the National Academy of Sciences of the USA. 2005;102:11876–11881. doi: 10.1073/pnas.0505577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G., Reeves J.D., Rennekamp A.J., Amberg S.M., Piefer A.J. Characterization of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) spike glycoprotein-mediated viral entry. Proceedings of the National Academy of Sciences of the USA. 2004;101:4240–4245. doi: 10.1073/pnas.0306446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits S.L., de Lang A., van den Brand J.M., Leijten L.M., van Ijcken W.F. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathogens. 2010;6:e1000756. doi: 10.1371/journal.ppat.1000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits S.L., van den Brand J.M., de Lang A., Leijten L.M., van Ijcken W.F. Distinct severe acute respiratory syndrome coronavirus-induced acute lung injury pathways in two different nonhuman primate species. Journal of Virology. 2011;85:4234–4245. doi: 10.1128/JVI.02395-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponseller B.A., Strait E., Jergens A., Trujillo J., Harmon K. Influenza A pandemic (H1N1) 2009 virus infection in domestic cat. Emerging Infectious Diseases. 2010;16:534–537. doi: 10.3201/eid1603.091737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studahl M. Influenza virus and CNS manifestations. Journal of Clinical Virology. 2003;28:225–232. doi: 10.1016/s1386-6532(03)00119-7. [DOI] [PubMed] [Google Scholar]
- Subbarao K., Roberts A. Is there an ideal animal model for SARS? Trends in Microbiology. 2006;14:299–303. doi: 10.1016/j.tim.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugaya N. Influenza-associated encephalopathy in Japan. Seminars in Pediatric Infectious Diseases. 2002;13:79–84. doi: 10.1053/spid.2002.122993. [DOI] [PubMed] [Google Scholar]
- Svitek N., Rudd P.A., Obojes K., Pillet S., von M.V. Severe seasonal influenza in ferrets correlates with reduced interferon and increased IL-6 induction. Virology. 2008;376:53–59. doi: 10.1016/j.virol.2008.02.035. [DOI] [PubMed] [Google Scholar]
- Swayne D.E., Halvorson D.A. Influenza. In: Saif Y.M., Barnes H.J., Glisson J.R., Fadly A.M., McDougald L.R., editors. Diseases of Poultry. 11th Edit. Iowa State University Press; Ames: 2003. pp. 135–160. [Google Scholar]
- Takizawa T., Matsukawa S., Higuchi Y., Nakamura S., Nakanishi Y. Induction of programmed cell death (apoptosis) by influenza virus infection in tissue culture cells. Journal of General Virology. 1993;74:2347–2355. doi: 10.1099/0022-1317-74-11-2347. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Park C.H., Ninomiya A., Ozaki H., Takada A. Neurotropism of the 1997 Hong Kong H5N1 influenza virus in mice. Veterinary Microbiology. 2003;95:1–13. doi: 10.1016/s0378-1135(03)00132-9. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Yoshimi M., Maeyama T., Hagimoto N., Kuwano K. Resistance to Fas-mediated apoptosis in human lung fibroblast. European Respiratory Journal. 2002;20:359–368. doi: 10.1183/09031936.02.00252602. [DOI] [PubMed] [Google Scholar]
- Tang N.L., Chan P.K., Wong C.K., To K.F., Wu A.K. Early enhanced expression of interferon-inducible protein-10 (CXCL-10) and other chemokines predicts adverse outcome in severe acute respiratory syndrome. Clinical Chemistry. 2005;51:2333–2340. doi: 10.1373/clinchem.2005.054460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X.C., Zhang J.X., Zhang S.Y., Wang P., Fan X.H. Prevalence and genetic diversity of coronaviruses in bats from China. Journal of Virology. 2006;80:7481–7490. doi: 10.1128/JVI.00697-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenberger J.K., Morens D.M. The pathology of influenza virus infections. Annual Review of Pathology. 2008;3:499–522. doi: 10.1146/annurev.pathmechdis.3.121806.154316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theron M., Huang K.J., Chen Y.W., Liu C.C., Lei H.Y. A probable role for IFN-γ in the development of a lung immunopathology in SARS. Cytokine. 2005;32:30–38. doi: 10.1016/j.cyto.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel V., Weber F. Interferon and cytokine responses to SARS-coronavirus infection. Cytokine and Growth Factor Reviews. 2008;19:121–132. doi: 10.1016/j.cytogfr.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K.F., Chan P.K.S., Chan K.F., Lee W.K., Lam W.Y. Pathology of fatal human infection associated with avian influenza A H5N1 virus. Journal of Medical Virology. 2001;63:242–246. doi: 10.1002/1096-9071(200103)63:3<242::aid-jmv1007>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- To K.F., Lo A.W. Exploring the pathogenesis of severe acute respiratory syndrome (SARS): the tissue distribution of the coronavirus (SARS-CoV) and its putative receptor, angiotensin-converting enzyme 2 (ACE2) Journal of Pathology. 2004;203:740–743. doi: 10.1002/path.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S., Li Y., Rivailler P., Conrardy C., Castillo D.A. A distinct lineage of influenza A virus from bats. Proceedings of the National Academy of Sciences of the USA. 2012;109:4269–4274. doi: 10.1073/pnas.1116200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse G.M., To K.F., Chan P.K., Lo A.W., Ng K.C. Pulmonary pathological features in coronavirus associated severe acute respiratory syndrome (SARS) Journal of Clinical Pathology. 2004;57:260–265. doi: 10.1136/jcp.2003.013276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsushima K., King L.S., Aggarwal N.R., De Gorordo A., D'Alessio F.R. Acute lung injury review. Internal Medicine. 2009;48:621–630. doi: 10.2169/internalmedicine.48.1741. [DOI] [PubMed] [Google Scholar]
- Tumpey T.M., Lu X., Morken T., Zaki S.R., Katz J.M. Depletion of lymphocytes and diminished cytokine production in mice infected with a highly virulent influenza A (H5N1) virus isolated from humans. Journal of Virology. 2000;74:6105–6116. doi: 10.1128/jvi.74.13.6105-6116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uiprasertkul M., Kitphati R., Puthavathana P., Kriwong R., Kongchanagul A. Apoptosis and pathogenesis of avian influenza A (H5N1) virus in humans. Emerging Infectious Diseases. 2007;13:708–712. doi: 10.3201/eid1305.060572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uiprasertkul M., Puthavathana P., Sangsiriwut K., Pooruk P., Srisook K. Influenza A H5N1 replication sites in humans. Emerging Infectious Diseases. 2005;11:1036–1041. doi: 10.3201/eid1107.041313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boheemen S., De Graaf M., Lauber C., Bestebroer T.M., Raj V.S. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio. 2012;3 doi: 10.1128/mBio.00473-12. e00473–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brand J.M., Haagmans B.L., Leijten L., van Riel D., Martina B.E. Pathology of experimental SARS coronavirus infection in cats and ferrets. Veterinary Pathology. 2008;45:551–562. doi: 10.1354/vp.45-4-551. [DOI] [PubMed] [Google Scholar]
- van den Brand J.M., Stittelaar K.J., van Amerongen G., Reperant L., de Waal L. Comparison of temporal and spatial dynamics of seasonal H3N2, pandemic H1N1 and highly pathogenic avian influenza H5N1 virus infections in ferrets. PLoS One. 2012;7:e42343. doi: 10.1371/journal.pone.0042343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brand J.M., Stittelaar K.J., van Amerongen G., Rimmelzwaan G.F., Simon J. Severity of pneumonia due to new H1N1 influenza virus in ferrets is intermediate between that due to seasonal H1N1 virus and highly pathogenic avian influenza H5N1 virus. Journal of Infectious Diseases. 2010;201:993–999. doi: 10.1086/651132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brand J.M., Stittelaar K.J., van Amerongen G., van de Bildt M.W., Leijten L.M. Experimental pandemic (H1N1) 2009 virus infection of cats. Emerging Infectious Diseases. 2010;16:1745–1747. doi: 10.3201/eid1611.100845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brand J.M., van Leeuwen M., Schapendonk C.M., Simon J.H., Haagmans B.L. Metagenomic analysis of the viral flora of pine marten and European badger feces. Journal of Virology. 2012;86:2360–2365. doi: 10.1128/JVI.06373-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brand J.M. Erasmus University; Rotterdam: 2013. Experimental SARS and Influenza: Similar Disease, Different Pathways. PhD Thesis. [Google Scholar]
- van der Vries E., Stittelaar K.J., van Amerongen G., Veldhuis Kroeze E.J., de Waal L. Prolonged influenza virus shedding and emergence of antiviral resistance in immunocompromised patients and ferrets. PLoS Pathogens. 2013;9:e1003343. doi: 10.1371/journal.ppat.1003343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Riel D., Leijten L.M., van der E.M., Hoogsteden H.C., Boven L.A. Highly pathogenic avian influenza virus H5N1 infects alveolar macrophages without virus production or excessive TNF-alpha induction. PLoS Pathogens. 2011;7:e1002099. doi: 10.1371/journal.ppat.1002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Riel D., Munster V.J., de Wit E., Rimmelzwaan G.F., Fouchier R.A.M. H5N1 virus attachment to lower respiratory tract. Science. 2006;311:399. doi: 10.1126/science.1125548. [DOI] [PubMed] [Google Scholar]
- Walsh J.J., Dietlein L.F., Low F.N., Burch G.E., Mogabgab W.J. Bronchotracheal response in human influenza: type A, Asian strain, as studied by light and electron microscopic examination of bronchoscopic biopsies. Archives of Internal Medicine. 1961;108:376–388. doi: 10.1001/archinte.1961.03620090048006. [DOI] [PubMed] [Google Scholar]
- Ware L.B., Matthay M.A. The acute respiratory distress syndrome. New England Journal of Medicine. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Kiso M., Fukuyama S., Nakajima N., Imai M. Characterization of H7N9 influenza A viruses isolated from humans. Nature. 2013;501:551–555. doi: 10.1038/nature12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattrang E., Jessett D.M., Yates P., Fuxler L., Hannant D. Experimental infection of ponies with equine influenza A2 (H3N8) virus strains of different pathogenicity elicits varying interferon and interleukin-6 responses. Viral Immunology. 2003;16:57–67. doi: 10.1089/088282403763635456. [DOI] [PubMed] [Google Scholar]
- Weber F., Kochs G., Haller O. Inverse interference: how viruses fight the interferon system. Viral Immunology. 2004;17:498–515. doi: 10.1089/vim.2004.17.498. [DOI] [PubMed] [Google Scholar]
- Webster R.G. Wet markets – a continuing source of severe acute respiratory syndrome and influenza? Lancet. 2004;363:234–236. doi: 10.1016/S0140-6736(03)15329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingartl H., Czub M., Czub S., Neufeld J., Marszal P. Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. Journal of Virology. 2004;78:12672–12676. doi: 10.1128/JVI.78.22.12672-12676.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingartl H.M., Copps J., Drebot M.A., Marszal P., Smith G. Susceptibility of pigs and chickens to SARS coronavirus. Emerging Infectious Diseases. 2004;10:179–184. doi: 10.3201/eid1002.030677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth D.E., Gillim-Ross L., Espina N., Bernard K.A. Mice susceptible to SARS coronavirus. Emerging Infectious Diseases. 2004;10:1293–1296. doi: 10.3201/eid1007.031119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2003. Summary of Probable SARS Cases with Onset of Illness from 1 November 2002 to 31 July 2003.http://www.who.int/csr/sars/country/table2004_04_21/en/index.html Accessed 27-07-2012. [Google Scholar]
- WHO . WHO; Geneva: 2004. Manual on Animal Influenza Diagnosis and Surveillance; pp. 62–63. [Google Scholar]
- WHO . 2010. Pandemic (H1N1) 2009 – Update 112.http://www.who.int/csr/don/2010_08_06/en/index.html Accessed 27-06-2012. [Google Scholar]
- WHO . 2012. Cumulative Number of Confirmed Human Cases for Avian Influenza A (H5N1) Reported to WHO, 2003–2012.http://www.who.int/influenza/human_animal_interface/EN_GIP_20120529CumulativeNumberH5N1cases.pdf Accessed 27-06-2012. [Google Scholar]
- WHO . 2013. Number of Confirmed Cases of Human Cases of Avian Influenza A (H7N9) Reported to the WHO.http://www.who.int/influenza/human_animal_interface/influenza_h7n9/09_ReportWebH7N9Number.pdf Accessed 18-09-2013. [Google Scholar]
- Wong C.K., Lam C.W., Wu A.K., Ip W.K., Lee N.L. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clinical and Experimental Immunology. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R.S., Wu A., To K.F., Lee N., Lam C.W. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. British Medical Journal. 2003;326:1358–1362. doi: 10.1136/bmj.326.7403.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T., Wallington T., McDonald L.C., Abbas Z., Christian M. Late recognition of SARS in nosocomial outbreak, Toronto. Emerging Infectious Diseases. 2005;11:322–325. doi: 10.3201/eid1102.040607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R.H., He J.F., Evans M.R., Peng G.W., Field H.E. Epidemiologic clues to SARS origin in China. Emerging Infectious Diseases. 2004;10:1030–1037. doi: 10.3201/eid1006.030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.Y., Huang Y., Ganesh L., Leung K., Kong W.P. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. Journal of Virology. 2004;78:5642–5650. doi: 10.1128/JVI.78.11.5642-5650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.Y., Kong W.P., Huang Y., Roberts A., Murphy B.R. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428:561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen H.L., Lipatov A.S., Ilyushina N.A., Govorkova E.A., Franks J. Inefficient transmission of H5N1 influenza viruses in a ferret contact model. Journal of Virology. 2007;81:6890–6898. doi: 10.1128/JVI.00170-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen Y.T., Liao F., Hsiao C.H., Kao C.L., Chen Y.C. Modeling the early events of severe acute respiratory syndrome coronavirus infection in vitro. Journal of Virology. 2006;80:2684–2693. doi: 10.1128/JVI.80.6.2684-2693.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon P., Keylock K.T., Hartman M.E., Freund G.G., Woods J.A. Macrophage hyporesponsiveness to interferon-gamma in aged mice is associated with impaired signaling through Jak-STAT. Mechanisms of Ageing and Development. 2004;125:137–143. doi: 10.1016/j.mad.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Yu I.T., Wong T.W., Chiu Y.L., Lee N., Li Y. Temporal–spatial analysis of severe acute respiratory syndrome among hospital inpatients. Clinical Infectious Diseases. 2005;40:1237–1243. doi: 10.1086/428735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen K.Y., Chan P.K., Peiris M., Tsang D.N., Que T.L. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351:467–471. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- Zamoto A., Taguchi F., Fukushi S., Morikawa S., Yamada Y.K. Identification of ferret ACE2 and its receptor function for SARS-coronavirus. Advances in Experimental Medicine and Biology. 2006;581:519–522. doi: 10.1007/978-0-387-33012-9_93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Li J., Zhan Y., Wu L., Yu X. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infection and Immunity. 2004;72:4410–4415. doi: 10.1128/IAI.72.8.4410-4415.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Law H.K., Cheung C.Y., Ng I.H., Peiris J.S. Functional tumor necrosis factor-related apoptosis-inducing ligand production by avian influenza virus-infected macrophages. Journal of Infectious Diseases. 2006;193:945–953. doi: 10.1086/500954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitzow L.A., Rowe T., Morken T., Shieh W.J., Zaki S. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. Journal of Virology. 2002;76:4420–4429. doi: 10.1128/JVI.76.9.4420-4429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]