Abstract
Background
The goal of this study was to determine if IV magnesium, useful for severe pediatric asthma, reduces time to medical readiness for discharge in patients with bronchiolitis when added to supportive care.
Methods
We compared a single dose of 100 mg/kg of IV magnesium sulfate vs placebo for acute bronchiolitis. Patients received bronchodilator therapy, nebulized hypertonic saline, and 5 days of dexamethasone if there was eczema and/or a family history of asthma. Time to medical readiness for discharge was the primary efficacy outcome. Bronchiolitis severity scores and need for infirmary or hospital admission and for clinic revisits within 2 weeks were secondary outcomes. Cardiorespiratory instability onset was the safety outcome.
Results
A total of 162 previously healthy infants diagnosed with bronchiolitis aged 22 days to 17.6 months (median, 3.7 months) were enrolled. Approximately one-half of patients had eczema and/or a family history of asthma; 86.4% had positive findings on nasopharyngeal virus swabs. Geometric mean time until medical readiness for discharge was 24.1 h (95% CI, 20.0-29.1) for the 78 magnesium-treated patients and 25.3 h (95% CI, 20.3-31.5) for the 82 patients receiving placebo (ratio, 0.95 [95% CI, 0.52-1.80]; P = .91). Mean bronchiolitis severity scores over time were similar for the two groups. The frequency of clinic visits in the subsequent 2 weeks (33.8% and 27.2%, respectively) was also similar. Fifteen magnesium recipients (19.5%) vs five placebo recipients (6.2%) were readmitted to the infirmary or hospital within 2 weeks (P = .016). No acute cardiorespiratory side effects were reported.
Conclusions
IV magnesium did not provide benefit for patients with acute bronchiolitis and may be harmful.
Trial Registry
ClinicalTrials.gov; No.: NCT02145520; URL: www.clinicaltrials.gov.
Key Words: bronchiolitis, length of stay, magnesium sulfate, respiratory infections, respiratory syncytial virus
Despite many studies testing investigational therapies,1, 2, 3 the mainstay of bronchiolitis treatment remains supportive care, including supplemental oxygen and hydration.4, 5 A re-evaluation of nebulized hypertonic saline, previously suggested to favorably affect length of stay,6, 7, 8 has subsequently shown no benefit.9 However, a subset of infants with bronchiolitis with a history of eczema and/or family history of asthma in a first-degree relative seem to benefit from corticosteroid treatment, with reduced rates of admission, length of stay,10, 11 and time to readiness for discharge.11
During moderate to severe exacerbations of asthma in somewhat older children, IV magnesium can likewise decrease the admission rate and length of ED stay.12 We reasoned that IV magnesium, which to our knowledge has never previously been tested in a controlled trial in bronchiolitis, might be similarly beneficial, especially so for the subset with eczema and/or a strong family history of asthma.
Given the high clinical burden of bronchiolitis,4, 5, 6, 7, 8, 10, 13, 14 its increasing rate of requiring hospital admissions,15, 16, 17 and the absence of new inexpensive, readily available therapies, IV magnesium was tested in infants moderately to severely ill with bronchiolitis in a double-blind placebo-controlled study.
Methods
Setting and Participants
The study was conducted between October 2012 and May 2015 in the short stay unit of the Pediatric Emergency Center of Hamad General Hospital, the only pediatric emergency facility in the State of Qatar. The center serves an average of 280,000 patients annually and manages 52 beds in a short stay infirmary unit, to which patients are admitted if too ill to be sent home but do not require ICU or step-down care. The recent lengths of stay for bronchiolitis had ranged from 6 to 168 h.
Infants aged ≤ 18 months presenting for treatment of moderate to severe viral bronchiolitis were eligible for study. Moderate to severe bronchiolitis required having a prodromal history consistent with viral upper respiratory tract infection followed by wheezing and/or crackles on auscultation and a Wang bronchiolitis severity score ≥ 4 on presentation.18 The Wang bronchiolitis severity score ranges from 0 to 12 and has four variables, each receiving a score from 0 to 3; increasing scores denote worse status. Patients were excluded from the study if they had one or more of the following characteristics: preterm birth ≤ 34 weeks’ gestation, history of wheezing, steroid use within 48 h of presentation, obtundation and progressive respiratory failure requiring ICU admission, history of apnea within 24 h before presentation, oxygen saturation ≤ 85% on room air, history of a diagnosis of chronic lung disease, congenital heart disease, immunodeficiency, exposure to varicella within 21 days before enrollment, known magnesium or calcium metabolism disturbance, or known adverse reaction to magnesium sulfate. Written, informed consent, sought from one of the parents or legal guardians of consecutive eligible patients, was obtained for all participants. The study and consent forms were approved by the Hamad Medical Corporation institutional review board (number 12216/12).
Study Procedures
Patients were examined on presentation in the examination area of the center, and those requiring further treatment or observation were admitted to the short stay infirmary unit. Consecutive patients with bronchiolitis were assessed for study eligibility within 2 h of the initial physician assessment. Patients for whom written informed consent was obtained underwent plain chest radiography and nasopharyngeal swabs gathered for a rapid respiratory virus panel capable of identifying multiple respiratory viruses (multiplex real-time polymerase chain reaction assay on an ABI 7500 analyzer [Applied Biosystems]). Randomization was stratified into two groups: patients with a history of eczema and/or known to have a parent or full sibling with a prior physician diagnosis of asthma, and patients without. Block randomization was used for allocation. A sterile stock solution of 1 g/10 mL of magnesium sulfate or an identical volume of 0.9% saline placebo was used to prepare a fresh blinded syringe of 25 mg/mL and given at 4 mL/kg intravenously by using a syringe pump over 60 min; this design was according to a randomization table provided to the unblinded pharmacist, who withheld treatment assignment from all others. All patients were connected to a cardiorespiratory monitor immediately before the start of the infusion for 4 h to monitor safety.
Infants with a positive history of eczema and/or known to have a parent or a full sibling with a prior physician diagnosis of asthma were also started on oral dexamethasone at 1 mg/kg for the first day and then 0.6 mg/kg once daily for 4 days. All study patients received 5 mL of nebulized 5% hypertonic saline in 1 mL of 1:1000 epinephrine given at 4-h intervals throughout the infirmary stay (this use was protocolized treatment at the study site during the period the study was conducted); a 5% hypertonic saline solution was prepared daily in sterile conditions for the study patients. Inhaled therapies were delivered through a tight-fitted face mask by pressurized oxygen with the flow meter set at 10 L/min; nebulized epinephrine (0.5 mL/kg) at a minimum dose of 2.5 mL and a maximum dose of 5 mL was allowed, with 5 mL of 5% hypertonic saline at an hourly maximum frequency. Additional treatment (eg, supplemental oxygen, hydration, antibiotics) were given at the discretion of the treating physician. The magnesium infusion was to be withdrawn if clinical deterioration was determined to warrant ICU admission.
Patients were judged ready for discharge when the treating physician determined the patient did not need supplemental oxygen, was feeding adequately without IV fluids, and had minimal or absent wheezing, crackles, and chest retractions provided she or he had an oxygen saturation ≥ 94% and a Wang score < 4. At discharge, patients were sent home with salbutamol metered-dose inhalers with an appropriately-sized Aerochamber mask attachment (Forest Laboratories). Although there is no evidence base for the benefit of nebulized epinephrine or for discharge on salbutamol, these methods were commonly used elsewhere19 and at our center during the period the study was conducted. Telephone follow-up by a study nurse was mandatory daily for 2 weeks after discharge. The patient could return to the pediatric emergency center earlier if required.
Study Measurements and Outcomes
The primary outcome was time to medical readiness for discharge. The secondary outcomes were bronchiolitis severity score at 4, 8, 12, 24, 36, 48, 60, and 72 h. Also, for the 2 weeks after infirmary discharge, we recorded patients requiring hospital admission, or re-admission to the short stay infirmary unit (site of initial treatment), as well as patients visiting a clinic or revisiting the pediatric emergency center for the same illness but not requiring admission. Daily telephone calls for 2 weeks by the study nurse recorded information on general well-being, work of breathing, feeding tolerance, vomiting, diarrhea, and need for physician visits and hospitalization.
Statistical Analysis
Time to readiness for discharge was plotted by using univariate Kaplan-Meier survival analysis to depict the proportion of patients remaining in the infirmary in each group. The accelerated failure time model with log logistic function analysis was used to calculate and compare the geometric mean times to readiness for discharge for each treatment group according to their ratio. The mean bronchiolitis severity scores for the groups were plotted against different time intervals. Our previous study analysis11 regarding duration of infirmary stay for patients with bronchiolitis found a geometric mean length of stay of 27 h, and we selected that duration to estimate sample size, judging a reduction to 18 h (as seen in that study with dexamethasone) would be clinically significant. With a sample size of 76 per group, there would be 80% power to find a significant difference (P = .05, two-sided). To compensate for dropouts, we planned to recruit 200 patients but after three bronchiolitis seasons, noticing minimal dropout, we closed the study at 162 patients.
Categorical and continuous variables are expressed as frequency (percentage) and mean ± SD values. Descriptive statistics summarized baseline demographic and clinical characteristics. Quantitative variable means between the two independent groups were analyzed by using unpaired Student t test and Wilcoxon rank-sum tests. Associations between two or more qualitative/categorical variables were assessed by using the χ2 test. For small cell frequencies, the χ2 test with continuity correction was used. Significant values were reported with their corresponding 95% CIs. P values < .05 were considered to signify a threshold for statistical significance. Statistical analyses were performed by using SPSS version 19.0 (IBM SPSS Statistics, IBM Corporation). Data was transferred from the SPSS package to STATA SE 11.0 (StataCorp) for accelerated failure time model analysis.
Results
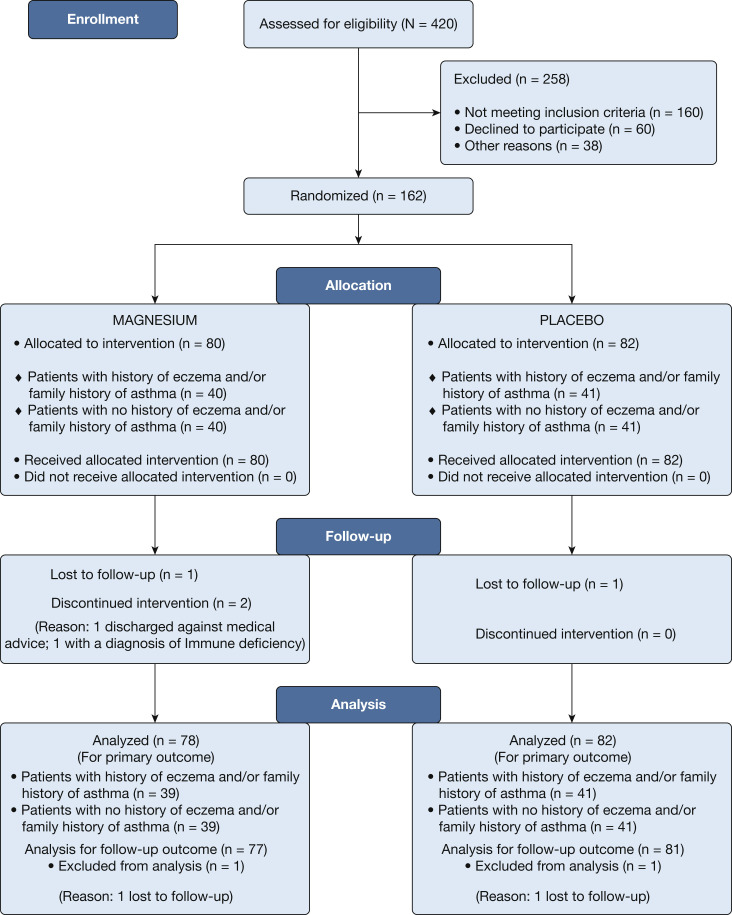
A total of 162 previously healthy infants diagnosed with viral bronchiolitis (median age, 3.7 months; range, 22 days-17.6 months) were enrolled in the study during three bronchiolitis seasons between October 2012 and May 2015 (patient flow summarized in Fig 1 ). Two enrolled infants could not be included in the analysis: one was diagnosed with an immunodeficiency, and one was discharged against medical advice. Of the 160 infant episodes remaining, 78 patients were randomized to receive IV magnesium, of whom 39 had a history of eczema and/or family history of asthma and 39 did not. A total of 82 patients received placebo, 41 who had a history of eczema and/or family history of asthma and 41 did not. Patients’ baseline characteristics were similar in the two treatment arms before enrollment (Table 1 ).
Figure 1.
Study flowchart of enrolled patients.
Table 1.
Baseline Characteristics of All Enrolled Infants
| Characteristic | Magnesium Group | Placebo Group |
|---|---|---|
| Total patients | n = 80 | n = 82 |
| With/without history of eczema and/or family history of asthma | n = 40/40 | n = 41/41 |
| Eczema in patient, No. (%) | 13 (33) | 18 (44) |
| Family history of asthma in first-degree relatives | 27 (68) | 31 (76) |
| Age, mean ± SD, mo | 4 ± 3 | 5 ± 4 |
| With/without history of eczema and/or family history of asthma | 4 ± 2/5 ± 4 | 5 ± 3/5 ± 4 |
| Duration of symptoms before enrollment, mean ± SD, d | 4 ± 3 | 4 ± 3 |
| With/without history of eczema and/or family history of asthma | 5 ± 3/4 ± 2 | 5 ± 3/4 ± 3 |
| Male:female | 58:22 | 48:34 |
| Male:female with/without history of eczema and/or family history of asthma | 30:10/28:12 | 20:21/28:13 |
| Baseline Wang bronchiolitis severity score, mean ± SD | 7 ± 1 | 7 ± 1 |
| With/without history of eczema and/or family history of asthma | 7 ± 1/7 ± 1 | 7 ± 1/7 ± 1 |
| Baseline oxygen saturation, mean ± SD, % | 97 ± 2 | 97 ± 2 |
| With/without history of eczema and/or family history of asthma | 97 ± 2/97 ± 2 | 96 ± 2/97 ± 2 |
| Baseline magnesium level, mean ± SD, mmol/L | 0.88 ± 0.06 | 0.89 ± 0.07 |
| With/without history of eczema and/or family history of asthma | 0.88 ± 0.05/0.88 ± 0.07 | 0.88 ± 0.07/0.90 ± 0.07 |
| Positive PCR in nasopharyngeal swab, No. (%) | 64 (80) | 76 (92.6) |
| RSV | 26 (32.5) | 30 (36.6) |
| Other virusesa | 18 (22.5) | 21 (25.6) |
| > 1 virus | 20 (25) | 25 (30.5) |
| With/without history of eczema and/or family history of asthma | 31 (77.5)/33 (82.5) | 37 (90.2)/39 (95.1) |
| RSV | 9 (22.5)/17 (42.5) | 12 (29.3)/18 (43.9) |
| Other virusesa | 10 (25)/8 (20) | 11 (26.8)/10 (24.4) |
| > 1 virus | 12 (30)/8 (20) | 14 (34.1)/11 (26.8) |
| Chest radiograph, No. (%) | ||
| Normal | 28 (35.4) | 39 (48.1) |
| Lobar consolidation/collapse | 21 (26.6) | 13 (16) |
| Lesser infiltrates | 28 (35.4) | 28 (34.6) |
| Pneumothorax/pleural effusion | 2 (2.5) | 1 (1.2) |
| With/without history of eczema and/or family history of asthma | ||
| Normal | 15 (38.5)/13 (32.5) | 21 (51.2)/18 (45) |
| Lobar consolidation/collapse | 11 (28.2)/10 (25) | 7 (17.1)/6 (15) |
| Lesser infiltrates | 13 (33.3)/15 (37.5) | 12 (29.3)/16 (40) |
| Pneumothorax and/or pleural effusion | 0/2 (5) | 1 (2.4)/0 |
| Patients needed antibiotics during hospitalization, No. (%) | 15 (18.8) | 16 (19.5) |
| With/without history of eczema and/or family history of asthma | 7 (17.5)/8 (20) | 8 (19.5)/8 (19.5) |
PCR = polymerase chain reaction; RSV = respiratory syncytial virus.
Adenovirus, bocavirus, coronavirus, human metapneumovirus, influenza and parainfluenza viruses, and rhinovirus.
Efficacy
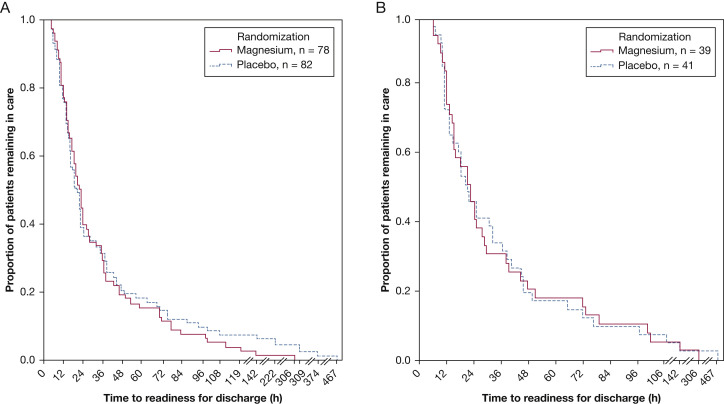
Among the 78 magnesium recipients, the geometric mean time to readiness for discharge was 24.1 h (95% CI, 20.0-29.1), whereas among 82 control patients, it was 25.3 h (95% CI, 20.3-31.5) with a ratio of 0.95 (95% CI, 0.52-1.80; P = .91) (Fig 2 A). For the patient subset with a history of eczema and/or family history of asthma, geometric mean durations until readiness for discharge were 23.3 h (95% CI, 17.2-31.5 h) and 23.6 h (95% CI, 17.5-31.8 h) for magnesium and placebo, respectively, with a ratio of 0.98 (0.39–2.46; P = 0.95) (Fig 2B). Four patients receiving placebo and one magnesium-treated patient required ICU admission from the infirmary (P = .50).
Figure 2.
A, Bronchiolitis discharge for all enrolled patients. B, Bronchiolitis discharge for patients with eczema and/or family history of asthma.
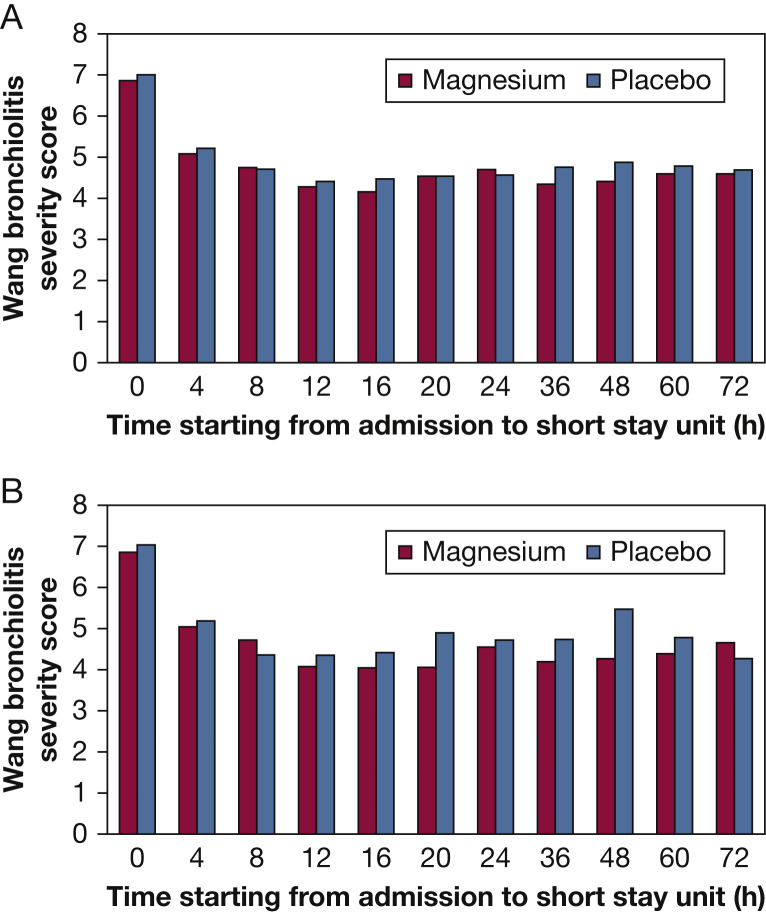
Mean bronchiolitis severity scores from baseline to discharge were similar for both arms in the overall patient group and in patients with a history of eczema and/or family history of asthma (Figs 3A, 3B). For the other secondary outcomes (Table 2 ), in the 14 days after discharge, we considered need for readmission to infirmary or hospital as most important and found 15 such readmissions (11 infirmary, four hospital) among magnesium-treated patients (19.5% [95% CI, 11.3-30.1]) and five admissions (four infirmary and one hospital) among patients receiving placebo (6.2% [95% CI, 0.02-13.8]; P = .016). These readmissions were significantly more frequent among the eczema/family history of asthma subsets: 26.3% (95% CI, 13.4-43.1) for magnesium vs 7.5% (95% CI, 1.6-20.4) for placebo (P = .034). Visits to clinic or the emergency center were of similar frequency: 26 (33.8%) visits for magnesium recipients and 22 (27.2%) visits for placebo recipients.
Figure 3.
A, Mean bronchiolitis severity score assessments over time in all enrolled patients. B, Mean bronchiolitis severity score assessments over time in patients with eczema and/or family history of asthma.
Table 2.
Secondary Outcomes
| Outcome | Magnesium Group | Placebo Group | P |
|---|---|---|---|
| Total patients | n = 77 | n = 81 | |
| With/without history of eczema and/or family history of asthma | n = 38/39 | n = 40/41 | |
| Patients needing clinic visits but not infirmary in 2 wk after discharge | 26 (33.8) | 22 (27.2) | .36 |
| Eczema and/or family history of asthma | 17 (44.7) | 14 (35) | .38 |
| No history of eczema and/or family history of asthma | 9 (23.1) | 8 (19.5) | .69 |
| Patients needing infirmary care but not hospital admission in the 2 wk after discharge | 11 (14.2) | 4 (4.9) | .05 |
| Eczema and/or family history of asthma | 8/38 (21.0) | 3/40 (7.5) | .19 |
| No history of eczema and/or family history of asthma | 3/39 (7.6) | 1/41 (2.4) | .35 |
| Patients needing hospital admission in the 2 wk after discharge | 4 (5.2) | 1 (1.2) | .20 |
| Eczema and/or family history of asthma | 2 (5.3) | 0 (0.0) | .23 |
| No history of eczema and/or family history of asthma | 2 (5.1) | 1 (2.4) | .61 |
Data are presented as No. (%) unless otherwise indicated.
Safety
No patient was withdrawn from the study because of apnea, cyanosis, or hemodynamic instability. Blood pressure and heart rate were similar between the magnesium and placebo groups throughout the infirmary stay.
Discussion
The present study found no benefit in adding IV magnesium to treatment for infant bronchiolitis, even in patients characterized to be at a higher risk for asthma.20 To our knowledge, this trial is the first randomized study to investigate the effect of IV magnesium in a population with bronchiolitis. Nebulized magnesium has been tested in bronchiolitis; in a previous multicenter study report, nebulized magnesium sulfate with epinephrine was compared with nebulized epinephrine in infants with moderate to severe bronchiolitis, but patients with previous repeated courses of steroids or bronchodilators and/or family history of asthma were excluded.21 Although there was significant improvement in respiratory distress assessment scores in the second and third day after admission favoring nebulized magnesium, no difference was found in the length of stay or need for oxygen. In a second small trial that enrolled patients with moderate bronchiolitis and compared nebulized magnesium, nebulized salbutamol, and nebulized magnesium sulfate/salbutamol combined, the only significant difference was the Wang severity score at 4 h, which favored the combined aerosol group compared with the nebulized magnesium alone group (P < .05).22 Given the uncertainties about clear-cut efficacy for—as well as the amount of magnesium delivered by—nebulizing magnesium for the treatment of asthma,23 we chose to use IV magnesium for the present study.
Our secondary outcome analysis found that IV magnesium treatment might increase the risk of symptomatic relapse among the patient group as a whole and especially in those patients with eczema and/or a family history of asthma. These may have been chance findings, but the significant P values cause us concern that magnesium treatment during infirmary care in this subset might have masked worse bronchiolitis in patients who were therefore discharged too soon or may somehow prolong disease course in these patients. It is also possible that the bronchiolitis treatment practice in our center of using 5% hypertonic saline nebulization in all patients and systemic dexamethasone in selected patients6, 7, 11 might have led to a disadvantageous drug interaction in the magnesium intervention group.
As with other “negative studies,” we may have failed to identify a benefit from IV magnesium in a patient subgroup because of the limited sample size. However, we believe these findings are generalizable to a similarly heterogeneous group of patients presenting for bronchiolitis care in a busy urban ED.
Conclusions
IV magnesium did not provide benefit for patients with acute bronchiolitis and may be harmful.
Acknowledgments
Author contributions: K. A. conceptualized and designed the study, and drafted the initial manuscript and is the guarantor of this manuscript. B. L. D. helped design the study, conducted the initial analyses, and reviewed and revised the manuscript. R. S., S. A. J., and M. G. designed the data collection instruments, coordinated and supervised data collection, and critically reviewed the manuscript. All authors approved the final manuscript as submitted.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor provided the facility and funded all physicians (except B. L. D.) as well as the study nurses.
Other contributions: We thank the patients and their families, our study and clinical staff, and the consulting biostatistician, Rajvir Singh, PhD(Biostatistics), for his thoughtful guidance in analysis. Dr Singh is funded solely by Hamad Medical Corporation and has no competing interest.
Footnotes
FUNDING/SUPPORT: This study was hospital-sponsored by Hamad Medical Corporation.
References
- 1.Hall C.B., McBride J.T., Walsh E.E. Aerosolized ribavirin treatment of infants with respiratory syncytial viral infection—a randomized double-blind study. N Engl J Med. 1983;308(24):1443–1447. doi: 10.1056/NEJM198306163082403. [DOI] [PubMed] [Google Scholar]
- 2.Lozano J.M., Wang E. Bronchiolitis. Clin Evid. 2002;8:291–303. [PubMed] [Google Scholar]
- 3.McCallum G.B., Morris P.S., Grimwood K. Three-weekly doses of azithromycin for indigenous infants hospitalized with bronchiolitis: a multicentre, randomized, placebo-controlled trial. Front Pediatr. 2015;3(32):1–9. doi: 10.3389/fped.2015.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wainwright C. Acute viral bronchiolitis in children—a very common condition with few therapeutic options. Paediatr Respir Rev. 2010;11(1):39–45. doi: 10.1016/j.prrv.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis Diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774–1793. doi: 10.1542/peds.2006-2223. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L., Mendoza-Sassi R.A., Klassen T.P., Wainwright C. Nebulized hypertonic saline for acute bronchiolitis: a systematic review. Pediatrics. 2015;136(4):687–701. doi: 10.1542/peds.2015-1914. [DOI] [PubMed] [Google Scholar]
- 7.Al-Ansari K., Sakran M., Davidson B.L., El Sayyed R., Mahjoub H., Ibrahim K. Nebulized 5% or 3% hypertonic or 0.9% saline for treating acute bronchiolitis in infants. J Pediatr. 2010;157(4):630–634. doi: 10.1016/j.jpeds.2010.04.074. [DOI] [PubMed] [Google Scholar]
- 8.Zorc J.J., Hall C.B. Bronchiolitis: recent evidence on diagnosis and management. Pediatrics. 2010;125(2) doi: 10.1542/peds.2009-2092. 342-339. [DOI] [PubMed] [Google Scholar]
- 9.Brooks C.G., Harrison W.N., Ralston S.L. Association between hypertonic saline and hospital length of stay in acute viral bronchiolitis: a reanalysis of 2 meta-analyses. JAMA Pediatr. 2016;170(6):577–584. doi: 10.1001/jamapediatrics.2016.0079. [DOI] [PubMed] [Google Scholar]
- 10.Schuh S., Coates A.L., Binnie R. Efficacy of oral dexamethasone in outpatients with acute bronchiolitis. J Pediatr. 2002;140(1):27–32. doi: 10.1067/mpd.2002.120271. [DOI] [PubMed] [Google Scholar]
- 11.Alansari K., Sakran M., Davidson B.L., Ibrahim K., Alrefai M., Zakaria I. Oral dexamethasone for bronchiolitis: a randomized trial. Pediatrics. 2013;132(4):e810–e816. doi: 10.1542/peds.2012-3746. [DOI] [PubMed] [Google Scholar]
- 12.Cheuk D.K., Chau T.C., Lee S.L. A meta-analysis on intravenous magnesium sulphate for treating acute asthma. Arch Dis Child. 2005;90(1):74–77. doi: 10.1136/adc.2004.050005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coffin S.E. Bronchiolitis: in-patient focus. Pediatr Clin North Am. 2005;52(14):1047–1057. doi: 10.1016/j.pcl.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Hall CB. Respiratory syncytial virus. Textbook of Pediatric Infectious Diseases. 3rd ed. Philadelphia, PA: Saunders; 1991:1633-1656.
- 15.Martinez F.D. Respiratory syncytial virus bronchiolitis and the pathogenesis of childhood asthma. Pediatr Infect Dis J. 2003;22(2):76–82. doi: 10.1097/01.inf.0000053889.39392.a7. [DOI] [PubMed] [Google Scholar]
- 16.Oymar K., Skjerven H.O., Mikalsen I.B. Acute bronchiolitis in infants, a review. Scand J Trauma Resusc Emerg Med. 2014;22:23. doi: 10.1186/1757-7241-22-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansbach J.M., Emond J.A., Camargo C.A., Jr. Bronchiolitis in US emergency departments 1992 to 2000: epidemiology and practice variation. Pediatr Emerg Care. 2005;21(4):242–247. doi: 10.1097/01.pec.0000161469.19841.86. [DOI] [PubMed] [Google Scholar]
- 18.Wang E.E., Milner R.A., Navas L., Maj H. Observer agreement for respiratory signs and oximetry in infants hospitalized with lower respiratory infections. Am Rev Respir Dis. 1992;145(1):106–109. doi: 10.1164/ajrccm/145.1.106. [DOI] [PubMed] [Google Scholar]
- 19.Plint A.C., Taljaard M., McGahern C. Management of bronchiolitis in community hospitals in Ontario: a multicentre cohort study. CJEM. 2016;18(6):443–452. doi: 10.1017/cem.2016.7. [DOI] [PubMed] [Google Scholar]
- 20.Tang E.A., Matsui E., Wiesch D.G., Samet J.M. 7th ed. Elsevier; Philadelphia, PA: 2009. Middleton’s Allergy Principles and Practice; pp. 735–738. [Google Scholar]
- 21.Modaresi M.R., Faghihinia J., Kelishadi R. Nebulized magnesium sulfate in acute bronchiolitis: a randomized controlled trial. Indian J Pediatr. 2015;82(9):794–798. doi: 10.1007/s12098-015-1729-z. [DOI] [PubMed] [Google Scholar]
- 22.Kose M., Ozturk M.A., Poyrazoglu H. The efficacy of nebulized salbutamol, magnesium sulfate, and salbutamol/magnesium sulfate combination in moderate bronchiolitis. Eur J Pediatr. 2014;173(9):1157–1160. doi: 10.1007/s00431-014-2309-3. [DOI] [PubMed] [Google Scholar]
- 23.Alansari K., Ahmed W., Davidson B.L., Alamri M., Zakaria I., Alrifaai M. Nebulized magnesium for moderate and severe pediatric asthma: a randomized trial. Pediatr Pulmonol. 2015;50(12):1191–1199. doi: 10.1002/ppul.23158. [DOI] [PMC free article] [PubMed] [Google Scholar]