Abstract
Orthopoxviruses code for numerous immunomodulatory proteins, the structure and function of which are clarified inadequately. Antibodies constitute a potent tool to study such proteins, enabling conclusions on protein location and time course of expression. However, common antibody production in mice or rabbits requires tedious protein expression and injection, as well as blood collection at regular intervals.
To simplify this procedure, IgY antibodies specific for poxviral proteins (F1L and p28) were generated by immunisation of chickens, because antibody retrieval from eggs allows the non-invasive generation of huge amounts of antibodies. The main intentions were (i) to decrease invasiveness, (ii) to immunise with native forms of proteins and (iii) to circumvent previous protein expression and purification. Therefore, chicken were immunised with DNA expression vectors coding for conserved domains of the selected proteins delivered for the first time by a gene gun. Four weeks after initial immunisation specific antibodies were found in the egg yolk as proven by immunofluorescence staining of poxvirus-infected cells. The specific IgY titre rose to 1:80,000 and was stable for more than 120 days. With this investigation we present an universal procedure for IgY design and production that can be applied for various issues in the future.
Keywords: IgY, Avian antibody, Gene gun, DNA immunisation, Poxvirus
1. Introduction
The research on viral pathogenicity mechanisms requires several qualified tools, like high-quality antibodies against relevant proteins which viruses can express. Such specific antibodies are often inaccessible and the only solution is in-house antibody production commonly conducted in laboratory mice and rabbits. Standard antibody production in mammals consists of in vitro expression of the antigen, its purification and administration and finally bleeding of the immunised animals for serum extraction. Depending on the amount of serum needed and on how bleeding is commenced up to the whole amount of the animals' blood must be collected. In such cases final bleeding is commenced and leads to animal sacrification.
A recent approach that promises the circumvention of these drawbacks is the generation of IgY antibodies. Since 1969 it has been known that IgG-like molecules are found in chicken serum (Leslie and Clem, 1969). Because of their predominance in the egg yolk they have been designated IgY. Until today IgY have been found in all bird species which offers new perspectives for antibody production. The necessity for antibodies specific for several poxviral proteins that are potentially involved in pathogenesis and modulation of apoptosis has led to the development of a straightforward procedure of producing IgY.
Compared to mammalian IgG (Sharma, 1997), avian antibodies offer many advantages. One of the most striking advantages is the fact that B-cell formation and differentiation in the chicken are performed completely differently from those in mammals (Reynaud et al., 1989, Pink et al., 1985). Because IgY antibodies recognise different antigens they can become valuable tools against difficult target epitopes. Moreover, birds can produce antibodies against proteins that are highly conserved in mammals and that may not be immunogenic in rabbits or mice (Tini et al., 2002). Finally, there is no cross-reactivity between mammal and avian antibodies, as well as no interference in immunological assays caused by the human complement system and rheumatoid factors. This is useful for complex immunocytochemistry applications with multiple antibodies (Larsson et al., 1993, Carlander et al., 1999). In cases where antibodies are used for virus diagnostics, IgY help avoiding false positive reactions in samples containing bacteria which could occur when IgG is used. This is founded by the fact that IgY do not bind bacterial surface proteins like protein A or protein G (Warr et al., 1995).
The amounts of antibody obtained from eggs argue for IgY technology as well; an egg yolk can contain IgY concentrations between 5 and 25 mg/ml (Rose et al., 1974, Schade et al., 2005). The yield of IgY per egg yolk can be as high as 100–150 mg and can therefore amount to 40 g of IgY per year and chicken, which is equivalent to the antibody amount obtained from 40 rabbits (Kovacs-Nolan and Mine, 2004). It is reported that the amount of IgY equal to the antibody content in 0.5 l of mammalian serum (in mice 0.1 ml blood can be taken once a week [Diehl et al., 2001]) can be delivered by a chicken in one month (Jensenius et al., 1981).
Moreover, the recovery of antibodies is less invasive, as no bleeding is necessary and recovery occurs by simple collection of eggs for antibody purification (Schade et al., 1994). This leads to a reduction of stress for the animals (Schade et al., 1991) along with the reduced frequency of immunisations because high antibody titres are reached after DNA applications (Gassmann et al., 1990). In general, DNA immunisation forms an effective alternative for antibody design and production in mice (Wolff et al., 1990, Wolff et al., 1992). By applying nucleic acids for immunisation, there is no longer a need for expensive expression, complex purification and renaturation of immunogenic epitopes. Furthermore, the exclusion of inactivated biological materials (like virus particles or bacterial cells) for immunisation increases security for animal holding facilities. By application of DNA there is no risk of contamination with inefficiently inactivated microorganisms especially when working with highly pathogenic agents.
The protein encoded by the DNA delivered is expressed directly in animal tissue under the control of a strong CMV promoter (Davis, 1997) and remains in native conformation which is beneficial for antibody generation. Furthermore, codon optimisation becomes possible for higher expression efficiency (avoiding the usage of low-level tRNA species) (Grosjean and Fiers, 1982), as well as selection of highly immunogenic domains of complex proteins for subunit immunisation. Recently, a DNA vaccine was used to produce antibodies in ducks specific for Hepadnavirus proteins (Abouzid et al., 2005). DNA was applied by injection and different expression vectors (pCI, pTriEX) were used to optimise protein expression. Also, recently IgY antibodies specific for SARS-Coronavirus were produced in chickens (Fu et al., 2006). However, whole inactivated virus particles were used in this approach, potentially reducing specificity to native virus particles, as immunogenicity may be modified following inactivation.
In order to shorten and simplify the prevailing immunisation processes and to reduce the cost of antigen preparation as well as to further decrease stress for immunised animals, we applied for the first time gene gun-based DNA immunisation of chickens to produce avian antibodies.
The micro projectile delivery system based on naked DNA coated on precious metal particles was first introduced in 1987 to transform onion cells (Klein et al., 1987). Sanford et al. adapted the process to transform animal tissue in situ (Sanford et al., 1987, Sanford, 1988) and finally in live animals (Williams et al., 1991). This procedure combines a non-invasive immunisation and the advantages of DNA vaccines.
The present study demonstrates a straightforward protocol for the gene gun-mediated DNA immunisation of chicken. As model targets two viral proteins (F1L and p28) involved in the modulation of apoptosis by poxviruses (Fischer et al., 2008, Huang et al., 2004, Nerenberg et al., 2005, Stewart et al., 2005) were selected, and the generated IgY proved to be valuable tools in studying their expression and function.
2. Materials and methods
2.1. Epitope selection
In order to select potentially immunogenic OPV-generic epitopes of F1L and p28 for DNA immunisation, nucleotide sequence alignments of the homologues from prominent OPV species (Variola virus [VARV], Vaccinia virus [VACV], Cowpox virus [CPXV], Camelpox virus [CMLV] and Ectromelia virus [ECTV]) were performed (BioEdit ver. 7.0.9). After choosing regions of high homology, nucleotide sequence stretches were selected that coded for protein epitopes with a high immunogenic index and surface detention probability, assisted by the Lasergene 7.0 Protean software (Fig. 1 ).
Fig. 1.
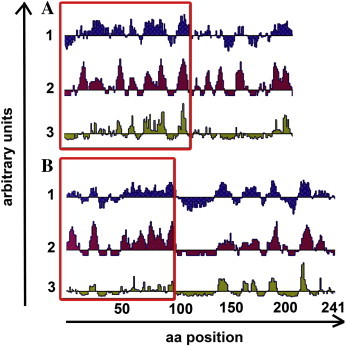
DNA-vaccine design. The structural characteristics of the protein sequences are shown according to Lasergene Protean software using standard settings: A: F1L and B: p28. A schematic representation of the protein that includes a Kyte-Doolittle hydrophilicity plot (1) (Kyte and Doolittle, 1982), a Jameson–Wolf antigenic index (2) (Jameson and Wolf, 1988) and an Emini surface probability plot (3) (Emini et al., 1985). The horizontal axis represents the corresponding amino acid position of F1L/p28, and the vertical axis of each plot represents arbitrary units. The amino acid residues that were used to select conserved DNA regions for immunisation, corresponding to a region of high hydrophilicity as well as high surface probability, are marked with a red frame. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.2. Vector preparation
Primers were designed to amplify gene regions coding for the chosen epitopes directly from genomic viral DNA. Primer sequences were selected to copy the epitope of choice in frame with the vector Kozak consensus sequence and to include a STOP-codon at the end of the PCR-product (Table 1 ). After conventional PCR (performed in 25 μl reaction containing 50 mM KCI, 20 mM Tris–HCl pH 8.4, 2 mM MgCl2, 0.1 mM of each dNTP, 100 ng template DNA, 0.5 U Platinum Taq DNA polymerase [Invitrogen, Karlsruhe, Germany]), amplification reactions were carried out in an Eppendorf Mastercycler using the following temperature profile: 95 °C for 5 min; 35× (95 °C for 30 s; 59 °C for 30 s; 74 °C for 1 min); 72 °C for 5 min. The products were purified by a PCR product purification kit (Invisorb Spin PCRapid Kit, Invitek, Berlin, Germany) and cloned into a pcDNA3.1/V5-His-TOPO vector (Invitrogen, Karlsruhe, Germany) according to the manufacturer's specifications. As positive controls Vivid Colors pcDNA6.2 YFP-GW/TOPO vectors from other experiments containing full-length homologues of F1L and p28 from CPXV were used for transfection. After chemical transformation plasmid DNA was purified by a PureLink™ HiPure Plasmid Filter Purification Kit (Invitrogen, Karlsruhe, Germany), implying low endotoxin levels, and finally eluted in water. Plasmids were checked for correct sequence by Sanger sequencing and stored at − 20 °C until gold ammunition coating.
Table 1.
Primers for cloning of F1L and p28 epitopes for DNA immunisation
| Primer name | Primer sequence |
|---|---|
| p28 pcDNA31 F | CCATggTAACAATATTACAATACATAgATgAACCAAATg |
| p28 pcDNA31S R | TTACgATTgAACAATCACATCggTTATCC |
| F1L pcDNA31 F | CCATggCgATgTTgTCgATgTTTATgTgTAATAATATCg |
| F1L pcDNA31S R | TTAATCATACATgATATTCATgTCCCTATTATAATCA |
2.3. Animals
Chickens were obtained from “Spreenhagener Vermehrungsbetrieb für Legehennen GmbH” in Bestensee, Germany, and kept under conditions constituted by local authorities of Berlin. Sixteen-week-old ISAR brown and Lohmann selected Leghorn were held in individual cages especially designed for egg-laying. The chickens laid up to one egg per day beginning around the 24th week of age — the time when the immunisation took place. The eggs were collected each day and stored at 4 °C until antibody preparation.
2.4. Gold ammunition
Gold carriers (0.8–1.5 µm) were obtained from Alfa Aeser GmbH & Co KG (Karlsruhe, Germany). Coating with DNA was performed according to the manufacturer's specifications by using the Tubing Prep Station (Bio-Rad, München, Germany) and the pcDNA3.1/V5-His-TOPO constructs. The amount of expression plasmid per 75 cm length of gold-coated tubing was set to 35 µg. After the coating procedure the tubing was cut into cartridges of 1.25 cm in length and stored at 4 °C in a desiccator.
2.5. DNA immunisation
For immunisation an experienced animal keeper carefully secured the chickens horizontally in order to expose the breast (Fig. 2 A). The spacer mounted on the front of the gene gun barrel had a contact area with the animal's skin of approximately 2 cm2. The immunising gold particle delivery was placed on the Apteriae-area of the breast skin to avoid interference with feathers in the trajectory of the particle (Fig. 2B). The gold carrier ammunition was applied by a helium discharge with a pressure of 300 psi. Per chicken two non-overlapping shots were conducted per breast side, a total of four per animal. The total amount of DNA used for one animal was 2.8 µg per immunisation. Each Gene Gun-shot was accompanied by an acoustic shock induced by the helium pulse which obviously did not frighten the animals. It is recommended to conduct the immunisation in a room that allows outward sonic dispersion. The whole procedure of careful chicken retrieval, immunisation and reversion was kept as short as possible, not exceeding 5 min, and took place in a separate surgery to minimise stress for neighbouring subjects. Two booster immunisations were conducted on days 31 and 85 after initial immunisation.
Fig. 2.
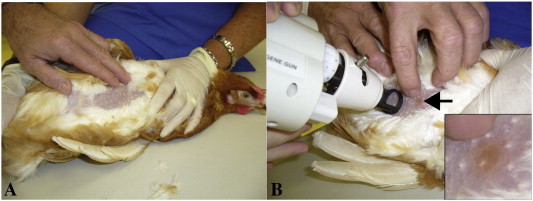
Gene gun immunisation of chicken. A: For DNA immunisation the chicken was fixed horizontally to assure Apteriae exposure. B: The feather-free area was sufficiently large to allow two non-overlapping applications per breast side. The arrow shows gold ammunition on subject's skin – a control for vaccine delivery. For better perceptibility a scale-up of the area can be seen in the lower right corner of panel B.
2.6. Antibody sampling and purification
Beginning with day 31 post immunisation, all eggs laid by the chickens were collected and stored at 4 °C until IgY purification. On average, one egg was laid per day. All eggs were collected and every third one was stored for purification and screening.
The IgY extraction was carried out by polyethylene-glycol (PEG) precipitation adapted from Polson (Polson et al., 1980). Briefly, the egg yolk was separated from the egg white by an egg spoon. The yolk was cut open and diluted 1:2 with PBS. Subsequently three precipitation steps with increasing amounts of PEG 6000 (3.5%, 8.5% and 12%) were performed, followed by a dialysis step at 4 °C overnight against PBS in a QuixSep device (Roth, Karlsruhe, Germany).
After purification the antibodies were stored at − 20 °C in ready-to-use aliquots in order to avoid freeze–thaw cycles. Glycerol was added to the aliquot in use in a ratio of 1:1 to prevent antibody damaging. The protein concentration in mg/ml was measured photometrically at 280 nm and calculated according to Lambert–Beer law with an extinction coefficient of 1.33 for IgY (Leslie and Clem, 1969). The antibodies could then be used in an immunofluorescence assay for monitoring specific titre devolution.
2.7. Immunofluorescence assay
Immunofluorescence staining of virus-infected cells was used to estimate the specific antibody titre. HEp-2 cells (ATCC#CCL-23) infected with the following orthopoxviruses were used for screening: VACV Western Reserve (ATCC#VR-1354/species Vaccinia Virus), VACV Lister Elstree (ATCC#VR-1549/species Vaccinia Virus), ECTV (ATCC#VR-1374/species Ectromelia Virus) and CPXV 81/02 (species Cowpox Virus, kindly provided by Prof. Hermann Meyer). The infections were conducted at a multiplicity of infection (MOI) of 1. For preparation the infected cells were seeded on sectioned microscope slides, incubated under standard cell culture conditions for 12–18 h and fixed with cold acetone for 20 min prior to usage. After fixation, microscope slides could be stored at − 20 °C for longer periods to ensure equal infection rates on all slides used for antibody titrations.
Purified IgYs that were pre-diluted 1:1 in glycerol were used for dilution series. The dilutions were performed tenfold by using antibody dilution buffer (containing PBS, BSA and NaN3).
For titration the microscope slides were blocked with 5% serum (secondary antibody species) for 20 min at room temperature. Subsequently, the antibody dilutions were applied to the microscope slides and incubated for 1 h at 37 °C. After incubation the slides were washed 3× (2× 1 min, 1× 10 min) with PBS and incubated with secondary antibody for 1 h at 37 °C. The second incubation step was followed by washing (as above) and mounting with mounting reagent (DAKO, Glostrup, Denmark) under a cover slip. The commercial antibodies used for immunofluorescence staining are listed in Table 2 . Microscopic analysis was carried out on a Carl Zeiss LSM510 Meta confocal laser scanning microscope.
Table 2.
Antibodies and markers used for immunocytochemistry
| Primary antibodies and markers | Host species | Source |
|---|---|---|
| Anti-F1L IgY | Chicken | This work |
| Anti-p28 IgY | Chicken | This work |
| Anti-OPV IgG | Human | Vaccinee serum |
| DAPI | Invitrogen, Karlsruhe, Germany | |
| Propidium iodide | Invitrogen, Karlsruhe, Germany | |
| Labelled secondary antibodies | Host species | Source |
| Anti-IgY-FITC IgG | Donkey | Dianova, Hamburg, Germany |
| Anti-IgY-Cy3 IgG | Donkey | Dianova, Hamburg, Germany |
| Anti-human IgG-Cy3 | Rabbit | Dianova, Hamburg, Germany |
| Anti-human IgG-FITC | Rabbit | Dianova, Hamburg, Germany |
3. Results
3.1. Laboratory animals
The animals used for immunisations did not show any abnormal responses like inflammation at the immunisation site. The short handling time of less than 5 min per chicken led to low stress levels and calm behaviour during DNA application. After vaccine application the egg production remained stable and comparable to the level of non-immunised animals. On average one egg per day was laid by each hen.
Long-term observation of up to 120 days after the first immunisation did not reveal exceptional behaviour among the test animals. After completion of all laboratory procedures, the chickens were kept in free-range for retirement without any obvious long-term consequences.
3.2. IgY extraction
The extraction of antibodies was monitored by SDS-gel electrophoresis while pre-immune and post-immune egg yolk was separated into heavy (60 kDa) and light (25 kDa) chains of IgY (supplemental Fig. 1) (Warr et al., 1995).
The total IgY concentration of one egg measured by photometry ranged from 15.7 mg/ml to 32.1 mg/ml with a mean value of 24.7 mg/ml. With 2 ml of antibody dilution collected per purification an average amount of 49.4 mg IgY was harvested from each egg.
Although each chicken laid one egg per day on average, every third egg was held back for antibody extraction. The samples were collected up to day 120 post immunisation, beginning with day 31.
3.3. Determination of specific IgY titre by immunocytochemistry
Cells which were transfected with Vivid Colors pcDNA6.2 YFP-GW/TOPO constructs, containing DNA coding for full-length protein homologues from CPXV expressing p28-YFP (yellow fluorescent protein) and F1L-YFP fusion proteins, were stained with anti-F1L-IgY and anti-p28-IgY antibodies. This staining was used as a control experiment to show specificity of the newly designed antibodies and exclude unspecific binding within the cells. Supplemental Fig. 2 shows IgY staining in red where it co-localises with the green fluorescence of the YFP fusion proteins.
As shown in Fig. 3 , the antibodies obtained did not bind to uninfected cells (negative control, Fig. 3A). Cells infected with poxvirus did not show any staining with secondary detection antibody only (secondary antibody control, Fig. 3B). Positive staining could be achieved for cells infected with VACV WR (Fig. 3B–C), CPXV (Fig. 3D–F) and ECTV (Fig. 3G–H) with anti-p28 IgY and anti-F1L IgY, respectively. Especially CPXV- and ECTV-infected preparations showed the typical virus inclusion bodies situated around cell nucleus, as can be seen in supplemental Fig. 2 in blue color due to a staining with anti-poxvirus-antibody-containing serum. VACV WR-infected specimens showed a more diffuse signal inside the cytoplasm. The staining of p28 inside of VACV LE-infected cells showed no positive signal (data not shown) and served as an additional negative control, as the VACV LE genome codes for a strongly truncated homologue of p28 which consists of the initial 44 amino acids at DNA level but is not known to be expressed. These 44 amino acids were part of the DNA used for immunisation; however, the IgY produced did not bind within VACV LE-infected cells. This fact confirms the assumption that p28 is not expressed in VACV LE.
Fig. 3.
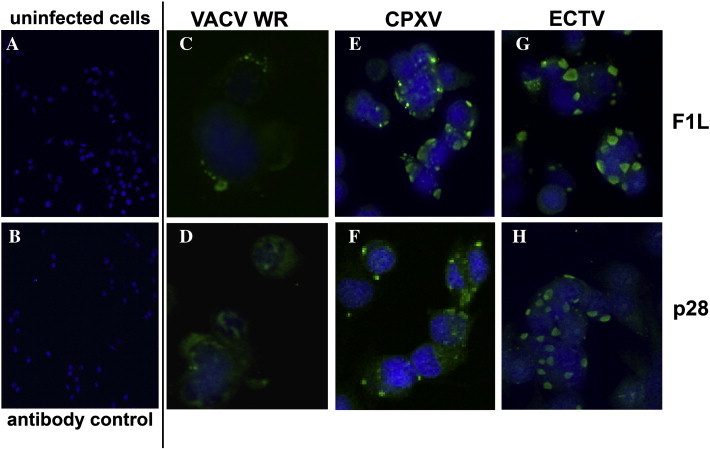
Staining of infected cells as a test for antibody specificity. OPV-infected HEp-2 cells were stained with DAPI (nucleus/blue) and IgY that were detected with anti-IgY secondary antibody (FITC/green). A: uninfected cell control staining with both IgY antibodies, B: CPXV-infected cells secondary antibody control, C: VACV WR-infected cells anti-F1L staining, D: VACV WR-infected cells anti-p28 staining, E: CPXV-infected cells anti-F1L staining, F: CPXV-infected cells anti-p28 staining, G: ECTV-infected cells anti-F1L staining, H: ECTV-infected cells anti-p28 staining. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Supplemental Fig. 3 also shows a Z-stack image of poxvirus-infected cells, consisting of six layers (0.4 µm each). The poxvirus staining (blue) was spread through the cells' cytoplasm around and above the nucleus (red). Circular structures of intensive blue staining illustrated viral factories situated horizontally around the nucleus. The green staining representing anti-p28 IgY was concentrated within the viral factories and co-localised with viral staining in these structures.
3.4. IgY titre progression
The respective titre of an IgY preparation was determined by immunofluorescence staining of orthopoxvirus-infected cells. Uninfected cells were used as negative controls for signal strength and localisation. Significant and strong fluorescence signals localised within the cytoplasm were counted as positive. Fig. 4 shows a representative example of a dilution series where a 1:2000 dilution of IgY was defined as negative.
Fig. 4.
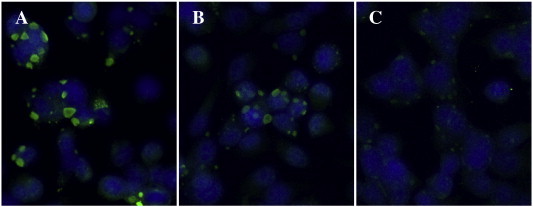
Example of anti-p28-IgY dilution series for titre determination. An IgY titration performed by immunocytochemistry. ECTV-infected cells stained by anti-p28-IgY (green) and DAPI (nucleus/blue). The following IgY dilutions were used: A: 1:20 (positive), B: 1:200 (positive), C: 1:2000 (negative). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 5 summarizes the titre progression after immunisation. Sample collection started 31 days after initial DNA application with the first boost vaccination. At this point an anti-F1L-IgY titre of 1:20 was already detectable. Forty-one days after the immunisation the titre began to rise and reached a level of 1:2000 after 60 days post immunisation. The second boost on day 90 led to maximum titres of 1:80,000 after 100 days. Last samples taken for anti-F1L-IgY after 120 days still showed a titre of 1:40,000. Immunisation with the p28 expression vector resulted in a similar titre progression up to day 60, but, in contrast to F1L, began to decrease after 70 days. IgY specific for p28 became undetectable after 87 days and did not rise after the second boost. The titre of anti-F1L continued to increase up to a value of 1:20,000 and after the third immunisation reached a maximum of 1:80,000.
Fig. 5.
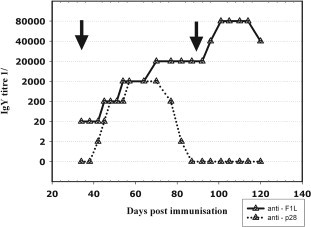
Titre progression of IgY antibodies in eggs after DNA immunisation. The plot shows the titre for specific anti-F1L (solid) and anti-p28 (dotted) IgY in protein fraction of collected chicken egg yolks. The black arrows mark the time points of the 2nd and the 3rd immunisation.
4. Discussion
The wide field of protein research often requires antibodies that are not available commercially. The usage of antibodies for medical diagnostics, therapy and research is indispensable (Carlander et al., 1999). In particular, antibodies for newly discovered proteins or biologically hazardous targets, which may come into scope, have to be produced quickly in an uncomplicated manner.
In the present work the benefits of IgY technology and DNA immunisation were successfully fused in an animal-friendly manner by using a gene gun for immunisation.
Since subject-friendly antibody generation is a major intention in future antibody generation projects, the first goal was to decrease invasiveness and the related stress for laboratory animals. The combination of gene gun delivery and egg yolk antibody extraction reduced the stress for the chickens, which was further minimized by short handling times (5 min). Finally the laboratory animals in this study did not show any complications or abnormal behaviour. The immunisation procedure and the DNA-vaccine itself did not have any detectable side effects on egg laying capacity. As the production of eggs is an indicator for the chickens' state of health it can be assumed that no negative side effects have been induced by the immunisation.
The second goal was to immunise against the native forms of the proteins in scope. The application of virus-derived DNA sequences as templates and the direct transfection into the antibody-producing animals led to expression of native protein as immunogenic agent.
The third goal was to circumvent previous protein expression and purification. This was achieved by using DNA as vaccine. The straight-forward procedure applied in this work enables rapid vaccine design on a simple bioinformatics basis. Standard procedures available in molecular biology laboratories, like PCR, cloning and plasmid purification, are sufficient for DNA vaccine preparation.
Beyond this, the average total amount of IgY of almost 50 mg/egg is a very efficient yield compared to rabbit or mouse immunisation (Jensenius et al., 1981, Kovacs-Nolan and Mine, 2004). When collecting all samples with a specific titre of ≥ 1:2000, there are approximately as much as 35 g of antibody harvested per chicken within 70 days for anti-F1L-IgY and even more if collected after day 120 post immunisation; for anti-p28 IgY there is still a yield of 10 g of antibody. Assuming that up to 10% of the total IgY can be specific (Schade et al., 1991), there can be as much as 3.5 g of specific anti-F1L antibody and 1.0 g of specific anti-p28 antibody, respectively.
The antibodies produced bound all F1L and p28 homologues tested in infected cells (except for the VACV LE-truncated p28 version) as a result of subunit immunisation with potentially highly conserved and immunogenic epitopes and so offer a great tool for protein research (Sakhatskyy et al., 2008).
Even though both DNA vaccines could induce antibody formation in chickens, it is unsatisfactory that anti-p28 IgY did not reach the same high titre levels as anti-F1L IgY. A reason for this could be the fact that the p28 subunit chosen for immunisation showed a low relative surface probability compared to the F1L subunit according to Fig. 1. Due to its putative function and importance within p28, the C-terminal part (RING domain) of this protein seems more adequate for immunisation. However, this part is lacking in p28 homologues of several VACV strains and should not be used for antibody production when generic detection is required. An expansion of the subunit used for immunisation in 3′-direction would presumably have not increased immunogenicity as hydrophilicity, antigenic index and surface probability downstream amino acid residue 100 are extremely low. The implementation of an earlier third immunisation step before day 60 post immunisation could prevent a titre reduction and lead to a further boost (Wang et al., 2008).
For immunisation DNA sequences of conserved regions of both genes have been used to guarantee immunogenicity for many protein homologues. A software-based analysis of the codon usage in OPV compared to the chicken codon table showed that over 10% of the codon species used for immunisation are not optimal (data not shown). The OPV codon usage is more effective in mice as rodents represent a possible host for VACV and a natural host for CPXV. Codon optimisation during DNA vaccine design could achieve higher immunogenicity caused by a more effective expression of antigen in the chicken and lead to higher titres, particularly with regard to p28 (Garmory et al., 2003, Liu et al., 2001). A drawback of codon engineering is the necessity of gene synthesis or a complex PCR strategy; however, future projects will evaluate this approach.
The staining of cells expressing p28- and F1L-YFP fusions after transfection (supplemental material Fig. 3) showed co-localisation of YFP signal and anti-IgY antibody where YFP appeared to be more intense. The presence of a strong YFP fluorescence in comparison to the signal delivered by the anti-IgY antibody can be explained by the N-terminal fusion of YFP. The fluorescent tag is expressed before the transfected protein and appears even if the expression remains incomplete.
Finally a well-reproducible method for DNA vaccine production became generally available without the need for complex preliminary work. The necessity of chicken keeping remains, however, these animals do not need elaborate facilities (Scharmann, 1996).
The tasks following this work should be to find out the best possible immunisation strategy regarding the combination of chicken as IgY source and gene gun application. The question of number and frequency of booster applications as well as an optimal DNA amount must be elucidated.
Recently, the procedure presented could be applied to generate further IgY antibodies directed specifically against another Orthopoxvirus protein of interest (data not shown).
Summing up, the gene gun-supported DNA immunisation of chickens performed in this work could generate large amounts of antibodies against OPV F1L and p28 within 40 days. This timely economic method saved resources that are usually needed for protein expression and purification. The work presented here describes universally applicable instructions for efficient and elaborate design and production of DNA-vaccination-based IgY antibodies — an all-purpose procedure reproducible and adaptable to several research situations. For the first time chickens were immunised with a DNA vaccine via gene gun in order to produce IgY antibodies for diagnostic and research applications.
Acknowledgements
We are very grateful to Beate Diemar and Sandra Kühn for excellent technical assistance, as well as Kazimierz Madela for his advice in confocal microscopy and Ursula Erikli for copy-editing.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jim.2008.11.008.
Appendix A. Supplementary data
Supplemental Fig. 1.
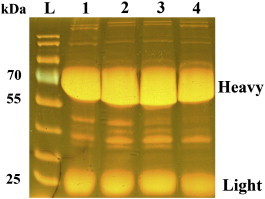
Protein composition in the egg yolk after gel electrophoresis. The Coomassie-stained SDS gel shows the overall protein composition of purified egg yolks. L marks the protein ladder lane (Fermentas, PageRuler prestained), 1–4 are the sample lanes, where lanes 1–2 show samples for anti-F1L IgY on days 35 and 110. Lanes 3–4 show samples for anti-p28 IgY on days 35 and 65 post immunisation. The two main bands in all samples show the heavy and light chains of purified IgY (designated in the picture). As the overall amount of IgY does not change significantly, it must be the composition of IgY (ratio of specific vs. unspecific antibodies) that changes after immunisation.
Supplemental Fig. 2.
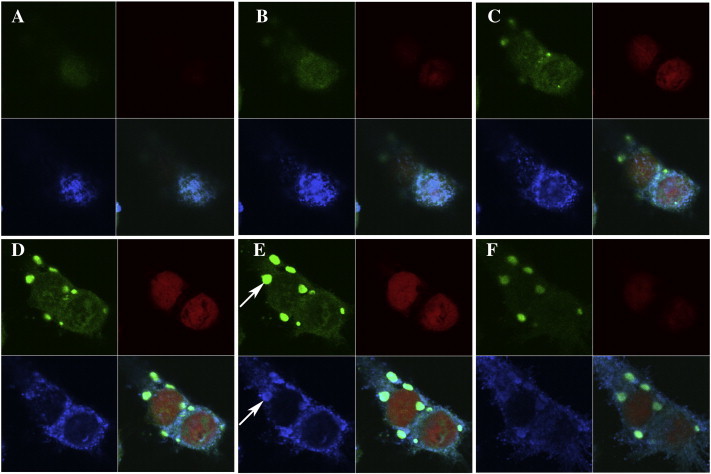
Visualisation of viral inclusion bodies in a Z-Stack micrograph of CPXV-infected cells. The picture series shows a Z-stack of CPXV-infected cells stained with anti-p28 antibody (green/upper left), PI (nucleus/red/upper right), polyclonal anti-poxvirus antibody (blue/lower left). The series starts with picture A and continues sequentially to F, showing six layers of the sample (0.4 µm each). The white arrows show virus factories stained by anti-poxvirus and anti-p28 antibodies — it becomes obvious that p28 localises particularly there.
Supplemental Fig. 3.
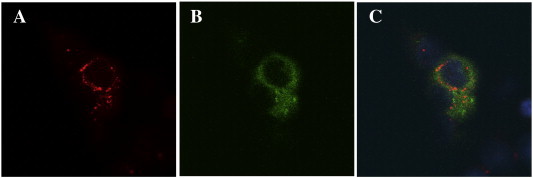
Antigenic specificity of IgY. Binding of anti-p28 IgY to heterologously p28 expressed as YFP fusion protein in transfected HEK-293 cells. A: anti-p28 staining (red), B: YFP fluorescence (green), C: merge.
References
- Abouzid K., Ndeboko B., Durantel S., Jamard C., Zoulim F., Buronfosse T., Cova L. Genetic vaccination for production of DNA-designed antibodies specific to Hepadnavirus envelope proteins. Vaccine. 2005;24(21):4615. doi: 10.1016/j.vaccine.2005.08.085. [DOI] [PubMed] [Google Scholar]
- Carlander D., Stålberg J., Larsson A. Chicken antibodies: a clinical chemistry perspective. Ups. J. Med. Sci. 1999;104:179. doi: 10.3109/03009739909178961. [DOI] [PubMed] [Google Scholar]
- Davis H.L. Plasmid DNA expression systems for the purpose of immunization. Curr. Opin. Biotechnol. 1997;8:635. doi: 10.1016/s0958-1669(97)80041-9. [DOI] [PubMed] [Google Scholar]
- Diehl K.H., Hull R., Morton D., Pfister R., Rabemampianina Y., Smith D., Vidal J.M., van de Vorstenbosch C. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J. Appl. Toxicol. 2001;21:15. doi: 10.1002/jat.727. [DOI] [PubMed] [Google Scholar]
- Emini E.A., Hughes J.V., Perlow D.S., Boger J. Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J. Virol. 1985;55:836. doi: 10.1128/jvi.55.3.836-839.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S.F., Ludwig H., Holzapfel J., Kvansakul M., Chen L., Huang D.C., Sutter G., Knese M., Hacker G. Modified vaccinia virus Ankara protein F1L is a novel BH3-domain-binding protein and acts together with the early viral protein E3L to block virus-associated apoptosis. Cell Death Differ. 2008;15:1532. doi: 10.1038/sj.cdd.4401718. [DOI] [PubMed] [Google Scholar]
- Fu C.Y., Huang H., Wang X.M., Liu Y.G., Wang Z.G., Cui S.J., Gao H.L., Li Z., Li J.P., Kong X.G. Preparation and evaluation of anti-SARS coronavirus IgY from yolks of immunized SPF chickens. J. Virol. Methods. 2006;133:112. doi: 10.1016/j.jviromet.2005.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmory H.S., Brown K.A., Titball R.W. DNA vaccines: improving expression of antigens. Genet. Vaccines Ther. 2003;1:2. doi: 10.1186/1479-0556-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann M., Thommes P., Weiser T., Hubscher U. Efficient production of chicken egg yolk antibodies against a conserved mammalian protein. FASEB J. 1990;4:2528. doi: 10.1096/fasebj.4.8.1970792. [DOI] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982;18:199. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Huang J., Huang Q., Zhou X., Shen M.M., Yen A., Yu S.X., Dong G., Qu K., Huang P., Anderson E.M., Daniel-Issakani S., Buller R.M., Payan D.G., Lu H.H. The poxvirus p28 virulence factor is an E3 ubiquitin ligase. J. Biol. Chem. 2004;279:54110. doi: 10.1074/jbc.M410583200. [DOI] [PubMed] [Google Scholar]
- Jameson B.A., Wolf H. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput. Appl. Biosci. 1988;4:181. doi: 10.1093/bioinformatics/4.1.181. [DOI] [PubMed] [Google Scholar]
- Jensenius J.C., Andersen I., Hau J., Crone M., Koch C. Eggs: conveniently packaged antibodies. Methods for purification of yolk IgG. J. Immunol. Methods. 1981;46:63. doi: 10.1016/0022-1759(81)90333-1. [DOI] [PubMed] [Google Scholar]
- Klein T.M., Wolf E.D., Wu R., Sanford J.C. High-velocity microprojectiles for delivering nucleic acids into living cells. Nature. 1987;327:70. [PubMed] [Google Scholar]
- Kovacs-Nolan J., Mine Y. Avian egg antibodies: basic and potential applications. Avian Poult. Biol. Rev. 2004;15:25. [Google Scholar]
- Kyte J., Doolittle R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982;157:105. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Larsson A., Balow R.M., Lindahl T.L., Forsberg P.O. Chicken antibodies: taking advantage of evolution—a review. Poult. Sci. 1993;72:1807. doi: 10.3382/ps.0721807. [DOI] [PubMed] [Google Scholar]
- Leslie G.A., Clem L.W. Phylogeny of immunoglobulin structure and function. 3. Immunoglobulins of the chicken. J. Exp. Med. 1969;130:1337. doi: 10.1084/jem.130.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.J., Zhao K.N., Gao F.G., Leggatt G.R., Fernando G.J., Frazer I.H. Polynucleotide viral vaccines: codon optimisation and ubiquitin conjugation enhances prophylactic and therapeutic efficacy. Vaccine. 2001;20:862. doi: 10.1016/s0264-410x(01)00406-6. [DOI] [PubMed] [Google Scholar]
- Nerenberg B.T., Taylor J., Bartee E., Gouveia K., Barry M., Fruh K. The poxviral RING protein p28 is a ubiquitin ligase that targets ubiquitin to viral replication factories. J. Virol. 2005;79:597. doi: 10.1128/JVI.79.1.597-601.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pink J.R., Vainio O., Rijnbeek A.M. Clones of B lymphocytes in individual follicles of the bursa of Fabricius. Eur. J. Immunol. 1985;15:83. doi: 10.1002/eji.1830150116. [DOI] [PubMed] [Google Scholar]
- Polson A., von Wechmar M.B., van Regenmortel M.H. Isolation of viral IgY antibodies from yolks of immunized hens. Immunol. Commun. 1980;9:475. doi: 10.3109/08820138009066010. [DOI] [PubMed] [Google Scholar]
- Reynaud C.A., Dahan A., Anquez V., Weill J.C. Somatic hyperconversion diversifies the single Vh gene of the chicken with a high incidence in the D region. Cell. 1989;59:171. doi: 10.1016/0092-8674(89)90879-9. [DOI] [PubMed] [Google Scholar]
- Rose M.E., Orlans E., Buttress N. Immunoglobulin classes in the hen's egg: their segregation in yolk and white. Eur. J. Immunol. 1974;4:521. doi: 10.1002/eji.1830040715. [DOI] [PubMed] [Google Scholar]
- Sakhatskyy P., Wang S., Zhang C., Chou T.H., Kishko M., Lu S. Immunogenicity and protection efficacy of subunit-based smallpox vaccines using variola major antigens. Virology. 2008;371:98. doi: 10.1016/j.virol.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford J.C. The biolistic process. Trends Biotechnol. 1988;6:299. [Google Scholar]
- Sanford J.C., Klein T.M., Wolf E.D., Allen N. Delivery of substances into cells and tissues using a particle bombardment process. J. Part. Sci. Technol. 1987;5:27. [Google Scholar]
- Schade R., Pfister C., Halatsch R., Henklein P. Polyclonal IgY antibodies from chicken egg yolk — an alternative to the production of mammalian IgG type antibodies in rabbits. ATLA. 1991;19:403. [Google Scholar]
- Schade R., Burger W., Schoneberg T., Schniering A., Schwarzkopf C., Hlinak A., Kobilke H. Avian egg yolk antibodies. The egg laying capacity of hens following immunisation with antigens of different kind and origin and the efficiency of egg yolk antibodies in comparison to mammalian antibodies. ALTEX. 1994;11:75. [PubMed] [Google Scholar]
- Schade R., Calzado E.G., Sarmiento R., Chacana P.A., Porankiewicz-Asplund J., Terzolo H.R. Chicken egg yolk antibodies (IgY-technology): a review of progress in production and use in research and human and veterinary medicine. Altern. Lab. Anim. 2005;33:129. doi: 10.1177/026119290503300208. [DOI] [PubMed] [Google Scholar]
- Scharmann W. Accommodation of laying hens in the laboratory in accordance with animal welfare requirements. ALTEX. 1996;13:136. [PubMed] [Google Scholar]
- Sharma J.M. The structure and function of the avian immune system. Acta Vet. Hung. 1997;45:229. [PubMed] [Google Scholar]
- Stewart T.L., Wasilenko S.T., Barry M. Vaccinia virus F1L protein is a tail-anchored protein that functions at the mitochondria to inhibit apoptosis. J. Virol. 2005;79:1084. doi: 10.1128/JVI.79.2.1084-1098.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tini M., Jewell U.R., Camenisch G., Chilov D., Gassmann M. Generation and application of chicken egg-yolk antibodies. Comp. Biochem. Physiol., Part A Mol. Integr. Physiol. 2002;131:569. doi: 10.1016/s1095-6433(01)00508-6. [DOI] [PubMed] [Google Scholar]
- Wang S., Zhang C., Zhang L., Li J., Huang Z., Lu S. The relative immunogenicity of DNA vaccines delivered by the intramuscular needle injection, electroporation and gene gun methods. Vaccine. 2008;26:2100. doi: 10.1016/j.vaccine.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr G.W., Magor K.E., Higgins D.A. IgY: clues to the origins of modern antibodies. Immunol. Today. 1995;16:392. doi: 10.1016/0167-5699(95)80008-5. [DOI] [PubMed] [Google Scholar]
- Williams R.S., Johnston S.A., Riedy M., DeVit M.J., McElligott S.G., Sanford J.C. Introduction of foreign genes into tissues of living mice by DNA-coated microprojectiles. Proc. Natl. Acad. Sci. U. S. A. 1991;88:2726. doi: 10.1073/pnas.88.7.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J.A., Malone R.W., Williams P., Chong W., Acsadi G., Jani A., Felgner P.L. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- Wolff J.A., Ludtke J.J., Acsadi G., Williams P., Jani A. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle. Hum. Mol. Genet. 1992;1:363. doi: 10.1093/hmg/1.6.363. [DOI] [PubMed] [Google Scholar]


