Abstract
Most virus infections induce cycloxygenase-2 (COX-2) expression and subsequent prostaglandin E2 (PGE2) production in cells, an inflammatory response that might be detrimental to virus replication and pathogenesis. This response in dengue virus infection remains to be elucidated. Triptolide and tetrandrine, compounds derived from two commonly used Chinese herbs, both demonstrate anti-inflammatory and immunosuppressive effects partly through modulation of COX-2 expression and, hence, may have antiviral effects. In this study, we examined, firstly, the immune response to dengue virus infection with respect to COX-2 expression and PGE2 production in human lung cells (A549), liver cells (HepG2) and dendritic cells. Secondly, we assessed the potential antiviral effects of triptolide and tetrandrine on dengue virus infection vis-à-vis expression of COX-2, PGE2, transcription factors, as well as virus production. We found that dengue virus infection enhanced COX-2 expression and PGE2 production in A549 cells, similarly to the response in dendritic cells, but not in HepG2 cells. In dengue virus-infected A549 cells, nuclear factor κB (NF-κB) and activator protein 1 (AP-1) were also activated, and both were dose-dependently inhibited by triptolide (0.5–4 ng/ml). Tetrandrine (1–10 μM) had no similar immunosuppressive effects and, moreover, at higher concentrations, enhanced NF-κB and AP-1 activity, COX-2 expression and PGE2 production. However, unexpectedly, tetrandrine, but not triptolide, dose-dependently suppressed dengue virus production in A549 cells, independent of PGE2 level. Our findings imply that triptolide and tetrandrine may attenuate dengue virus infection in human lung cells, but through distinct pathways.
Keywords: Triptolide, Tetrandrine, Dengue virus, COX-2, PGE2, NF-κB, AP-1, A549 cell
1. Introduction
Dengue virus, a positive-sense single-stranded RNA virus belonging to the family Flaviviridae and mainly spread by the mosquito Aedes aegypti, is an important human infection pathogen in the tropics and subtropics. The clinical manifestations of dengue virus infection range from asymptomatic infection, undifferentiated fever and dengue fever (with agonizing limb pain and sobriquet of break-bone fever), to dengue hemorrhagic fever with plasma leakage and potentially life-threatening dengue shock syndrome. Thus, dengue virus infection remains an important public health burden requiring continuing attention. The diverse host responses against dengue virus infection correlate with the activation of not only humoral and cell-mediated immunity, but also autoimmunity (Lin et al., 2006).
Upon viral infection of a cell, multiple signaling pathways including those of cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS) and interferons are activated, and participate in the regulation of gene expression related to inflammation (Steer and Corbett, 2003). COX-2 is the rate-limiting enzyme for catalysis of arachidonic acid to prostaglandin G2, which by peroxidase is further reduced to prostaglandin H2 (PGH2), the precursor of various prostanoids, namely prostaglandins (including prostaglandin E2, PGE2), prostacyclins and thromboxanes (Tsatsanis et al., 2006). Overexpression of COX-2 leads to increased levels of PGE2, a central mediator of inflammation (Williams et al., 1999). PGE2 is one of the most important pro-inflammatory cytokines, associated with induction of inflammation, leukocyte chemoattraction, and pain perception. PGE2 modulates immune function in inflammatory reactions, regulates viral replication and virulence, and participates in a wide range of normal physiological processes (Phipps et al., 1991).
Expression of COX-2 is regulated by numerous transcription factors, including nuclear factor-kappaB (NF-κB), nuclear factor of activated T cells (NFAT)/activator protein-1 (AP-1), cyclic-AMP response element (CRE), PU.1, activating enhancer-binding protein-2 (AP-2), specificity protein-1 (SP-1), GATA box and CCAAT enhancer-binding protein (C/EBP), as well as the canonical TATA box at the promoter region (Chun and Surh, 2004, Park and Christman, 2006). In response to stimulation, activation of transcription factors NF-κB, AP-1 and/or other various transcription factors collaborate to induce transcription of numerous downstream genes as well as the COX-2 gene, which subsequently manipulates immunity (Zhong et al., 2006).
The cumulative data suggest that, in response to virus infection in most types of cells, COX-2 mRNA accumulation, protein expression, and PGE2 production are stimulated, however the opposite response has also been shown; in peripheral blood mononuclear cells infected with Epstein–Barr virus, the reactions seem to be virus- and cell type-dependent (Savard et al., 2000). Activated PGE2 may protect the cell from invasion but also be used by some viruses to favor viral production (Steer and Corbett, 2003). Induced COX-2 expression and resultant prostaglandins may also play an anti-inflammatory role (Lawrence et al., 2002). Although the COX-2 and PGE2 response to different viral infections in various types of cell has been widely investigated, little is known about this response to dengue virus infection.
Modulation of COX-2/PGE2 synthesis in stimulated cells by anti-inflammatory molecules could be a way to suppress viral replication and spread. Non-steroidal anti-inflammatory drugs (NSAIDs), potent non-selective COX inhibitors and pain relievers like aspirin, indomethacin and ibuprofen, all have been shown to exert antiviral effects or attenuate disease severity during infection by herpesvirus (Wachsman et al., 1990), cytomegalovirus (Speir et al., 1998), varicella-zoster virus (Primache et al., 1998), influenza virus (Liao et al., 2001), Japanese encephalitis virus (Chen et al., 2002), respiratory syncytial virus (Richardson et al., 2005), and mouse hepatitis coronavirus (Raaben et al., 2007). Selective COX-2 inhibitors, such as celecoxib and NS398, also have shown great potential for reducing virus infection by inhibiting viral protein synthesis in rotavirus-infected intestinal Caco-2 cells (Rossen et al., 2004), and by reducing viral yields of alphaherpes in rate embryonic fibroblasts (Ray et al., 2004, Reynolds and Enquist, 2006).
Anti-inflammatory compounds from herbs have been widely examined for their antiviral effects (Jassim and Naji, 2003, Martin and Ernst, 2003). Triptolide and tetrandrine, the major ingredients of Tripterygium wilfordii Hook F (Thunder God Vine) and Stephania tetrandra S. Moore (Han-Fang-Chi), respectively, are two commonly prescribed Chinese antirheumatic herbs, both of which have anti-inflammatory and immunomodulatory activities (Ho and Lai, 2004). Triptolide has been shown to suppress the induction of COX-2 activity and PGE2 production in lipopolysaccharide (LPS)-stimulated monocytes from human peripheral blood, fibroblasts from rheumatoid arthritis synovial tissue and human neonatal foreskin fibroblasts (Tao et al., 1998). Triptolide also impairs dendritic cell migration by inhibiting COX-2 and CCR7 expression through NF-κB and phosphatidylinositol-3 kinase (PI3-K)/Akt pathway (Liu et al., 2007). Tetrandrine also has been shown to be an effective inhibitor of COX-2 expression in human monocytic cells (Wu and Ng, 2007).
Accordingly, we hypothesized that the two compounds might possess antiviral effects via their anti-inflammatory and immunomodulatory properties. In the present study, we explored the effect of triptolide and tetrandrine on the immune response of COX-2/PGE2 to dengue virus infection in human lung cells (A549 cells). We found that triptolide, but not tetrandrine, was a dose-dependent suppressor of the COX-2/PGE2 activated by dengue virus infection, probably though inhibition of activation of NF-κB and AP-1. However, tetrandrine, but not triptolide, blocked dengue virus production in A549 cells.
2. Materials and methods
2.1. Preparation of tetrandrine and triptolide
The preparation of tetrandrine and its analogs has been described previously (Lai et al., 1999). In brief, tetrandrine powder (purity > 98%; Yichang Pharmaceutical Company, Hubei Province, PRC) was dissolved in 0.1 N HCl. For each experiment, the stock solution was further diluted with 0.1 N HCl to desired concentrations (1, 2.5, 5, 10 μM). Crystalline triptolide (PG490, molecular weight 360, purity 99%) was purchased from SIGMA. Triptolide was dissolved in dimethyl sulfoxide (DMSO) and stock solutions (20 mg/ml) stored at − 20 °C. Triptolide was freshly diluted to the indicated concentrations (0.5, 1, 2, 4 ng/ml) with culture medium before use in experiments. DMSO concentration in test conditions did not exceed 0.01%.
2.2. Culture medium and preparation of A549, HepG2 cells and human peripheral blood monocyte-derived dendritic cells
A549 cells (type II human lung alveolar epithelial cell carcinoma), obtained from American Type Culture Collection (ATCC), were maintained in Ham's F12K medium (GIBCO-BRL, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) (Biological Industries, Kibbutz Beit Haemek, Israel), 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen Life Techonologies). HepG2 cells (human hepatocellular carcinoma cell line) (ATCC) were cultured in Dulbecco's Modified Eagle Medium (Sigma, St Louis, MO) supplemented with 10% FBS (Gibco, Grand Island, NY). Human peripheral blood monocyte-derived dendritic cells were established from positively selected CD14+ monocytes using a MACS cell isolation column following manufacturer's instructions (Miltenyi Biotech) as described in our previous work (Ho et al., 2001a). The purity of dendritic cells, as determined by the positive staining of CD1a, was consistently higher than 90%. Baby hamster kidney (BHK-21) cells were maintained in DMEM containing 10% FBS.
2.3. Virus preparation and titer determination
The dengue virus preparation has been described with some modifications in our previous work (Ho et al., 2005). In short, dengue virus serotype 2 (DV2) New Guinea C (NGC) strain (ATCC) was propagated in C6/36 mosquito cells in RPMI-1640 containing 5% heat-inactivated FBS and maintained at 28 °C in a 5% CO2 atmosphere for 4–7 days. The supernatants were collected, from which virus titers were determined, then stored at − 80 °C until use.
Plaque assay was used to determine virus titers. In brief, serial dilutions of virus culture supernatants were placed into 6-well plates seeded with 80% confluent BHK-21 cells and incubated at 37 °C for 3 h. After adsorption, cells were washed by phosphate buffered saline (PBS) solution thrice and overlaid with RPMI-1640 (3 ml/well) containing 1% SeaPlaque agarose (FMC BioProducts) and 2% FBS. After incubation at 37 °C for 7 days, cells were fixed with 10% formaldehyde (> 1 h at room temperature) and stained with 0.5% crystal violet in normal saline solution containing 50% alcohol and 1.85% formaldehyde. The numbers of plaques were counted and corrected by individual dilution factors, and the results shown as plaque-forming units (PFU) per milliliter.
2.4. Virus infection
A549, HepG2 and dendritic cells cultured for 5 days were infected with mock or DV2 at a multiplicity of infection (m.o.i.) of 5 at 37 °C (Ho et al., 2001b), then supernatants and cells were collected, without removing unbound virus, at various time points for different analyses (see Results). In the studies of virus infection and drug treatment, A549 cells (1 × 106 ml− 1), incubated in Ham's F12K medium with 10% FBS, were pre-treated with various concentrations of triptolide for 16 h or tetrandrine for 2 h pre-infection, then transferred to Ham's F12 medium with 5% FBS and corresponding drug concentration, infected with dengue virus at m.o.i. 5, and finally harvested 48 h (triptolide groups) or 24 h (tetrandrine group) post-infection.
2.5. MTT assay for cell viability
Cytotoxicity of triptolide and tetrandrine were studied by (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) (MTT) assay. A549 cells were incubated in 96-well plates at 1 × 104 cells per well containing 100 μl of RPMI-1640 medium and different concentrations of triptolide for 64 h or tetrandrine for 26 h. Cells were washed once before adding 50 μl FBS-free medium containing MTT (5 mg/ml). After 5 h of incubation at 37 °C, the medium was discarded and the formazan blue that formed in the cells was dissolved in DMSO (TECAN). The optical density was measured at 550 nm.
2.6. Enzyme-linked immunosorbent assay (ELISA)
PGE2 was measured in culture medium from A549 cells infected with mock or dengue virus and pre-treated with triptolide or tetrandrine at various time points using a commercial sandwich ELISA kit (Quantikine; R&D Systems, Minneapolis, MN). All tests were conducted with undiluted sera according to the manufacturer's protocols.
2.7. Nuclear extract preparation
Nuclear extracts were prepared according to our published work (Yang et al., 2003). In brief, A549 cells (1 × 106 cells in average in each treatment condition), after collection and a single wash with PBS solution, were left at 4 °C in 100 μl buffer A (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 1 mM dithiothreitol (DTT), 1 mM phenylmethylsulfonyl fluoride (PMSF), and 1 µg/ml aprotinin) for 50 min with occasional gentle vortexing. The swollen cells were subjected to centrifugation at 14,000 rpm for 3 min. After removal of the supernatants (cytoplasmic extracts), the pelleted nuclei were washed with 50 μl buffer A and cell pellets resuspended in 40 μl buffer C (20 mM HEPES, pH 7.9, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 25% glycerol, 1 mM DTT, 0.5 mM PMSF, and 1 μg/ml aprotinin), incubated at 4 °C for 30 min with occasional vigorous vortexing, followed by centrifugation of the mixtures at 15,000 rpm for 20 min; the supernatants were used as nuclear extracts.
2.8. Electrophoretic mobility shift assay (EMSA)
EMSAs were performed as detailed in our previous report (Yang et al., 2003). The oligonucleotides containing AP-1 (Santa Cruz), NF-κB (Santa Cruz) and CREB (Promega) sequences were purchased and used as DNA probes. The DNA probes were radiolabeled with [− 32P]ATP using T4 kinase according to the manufacturer's instructions (Promega). For the binding reaction, radiolabeled AP-1 and NF-κB probes were incubated with 4 µg nuclear extract. The binding buffer contained 10 mM Tris–HCl (pH 7.5), 50 mM NaCl, 0.5 mM EDTA, 1 mM DTT, 1 mM MgCl2, 4% glycerol, and 2 µg poly(dI–dC). This binding reaction was left at room temperature for 10 min. In order to verify DNA-binding specificity, in some reactions 100-fold molar excess of unlabeled competitive oligonucleotides (cold competitor) and unlabeled non-specific oligonucleotides (mutant competitor) were pre-incubated with nuclear extracts for 10 min before the addition of the radiolabeled probes. The final reaction mixture samples were loaded on a 6.6% non-denaturing polyacrylamide gel with 0.25 × Tris–borate–EDTA (TBE), and electrophoresis was continued at 250 V for 60 min. After electrophoresis, gels were dried and subjected to radiography.
2.9. Western blotting
ECL Western blotting (Amersham) was performed as described (Lai et al., 2001). Briefly, after extensive washing with PBS once, the cells (3 × 105) were pelleted and resuspended in 100 μl RIPA buffer (150 mM NaCl, 50 mM Tris–HCl (pH 7.5), 1 mM EDTA, 1% NP-40 (V/V), protease inhibitor (1 mM PMSF, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin), phosphatase inhibitor (1 mM Na3VO4, 1 mM NaF)). After periodic vortexing, the mixtures were subjected to centrifugation at 14,000 rpm for 20 min, the supernatant containing total cell lysate collected, and the protein concentration measured by Bio-Rad protein assay reagent. Equal amounts of protein (40 μg) from all samples were analyzed on 10% SDS-PAGE at 100 V for an appropriate time and transferred to nitrocellulose filters. For immunoblotting, the nitrocellulose filter was incubated with TBS-T (10 mM Tris–HCl (pH 8.0), 150 mM NaCl, 0.05% Tween-20) containing 5% nonfat milk (milk buffer) for 1 h, and then blotted with antisera against individual proteins (goat anti-COX-2 polyclonal Ab (sc-1745) from Santa Cruz, mouse anti-actin monoclonal antibody (MAB1501 from CHEMICON) for 2 h at room temperature. After washing with milk buffer thrice, the filter was incubated with secondary Ab (goat anti-mouse IgG from CHEMICON) conjugated to horseradish peroxidase (HRP) at a concentration of 1/5000 for 1 h at room temperature. The filter was then incubated with the ECL substrate (Amersham) and exposed to X-ray film.
2.10. Luciferase activity assay
A549 cells were seeded onto 6-well culture plates (5 × 105 cell/well) and transfected with 0.05 μg/well of herpes simplex thymidine kinase-driven Renilla luciferase reporter [pRL-TK] plasmid (Promega), as a transfection efficiency control, and 1 μg/well of NF-κB or AP-1 luciferase reporter plasmid, by using 3 μg Lipofectamine 2000 (Invitrogen) as specified by the manufacturer. The cells were incubated at 37 °C for 4 h then transferred to fresh culture medium and treated with various concentrations of tetrandrine for the next 24 h. The luciferase and Renilla activities of the A549 cells collected 24 h after tetrandrine treatment were measured using the Dual-Luciferases Reporter kit (Promega) according to the manufacturer's protocol. Luciferase activity was normalized to Renilla Luciferase activity and presented as luciferase units relative to control.
3. Results
3.1. Dengue virus-induced COX-2 expression and PGE2 production
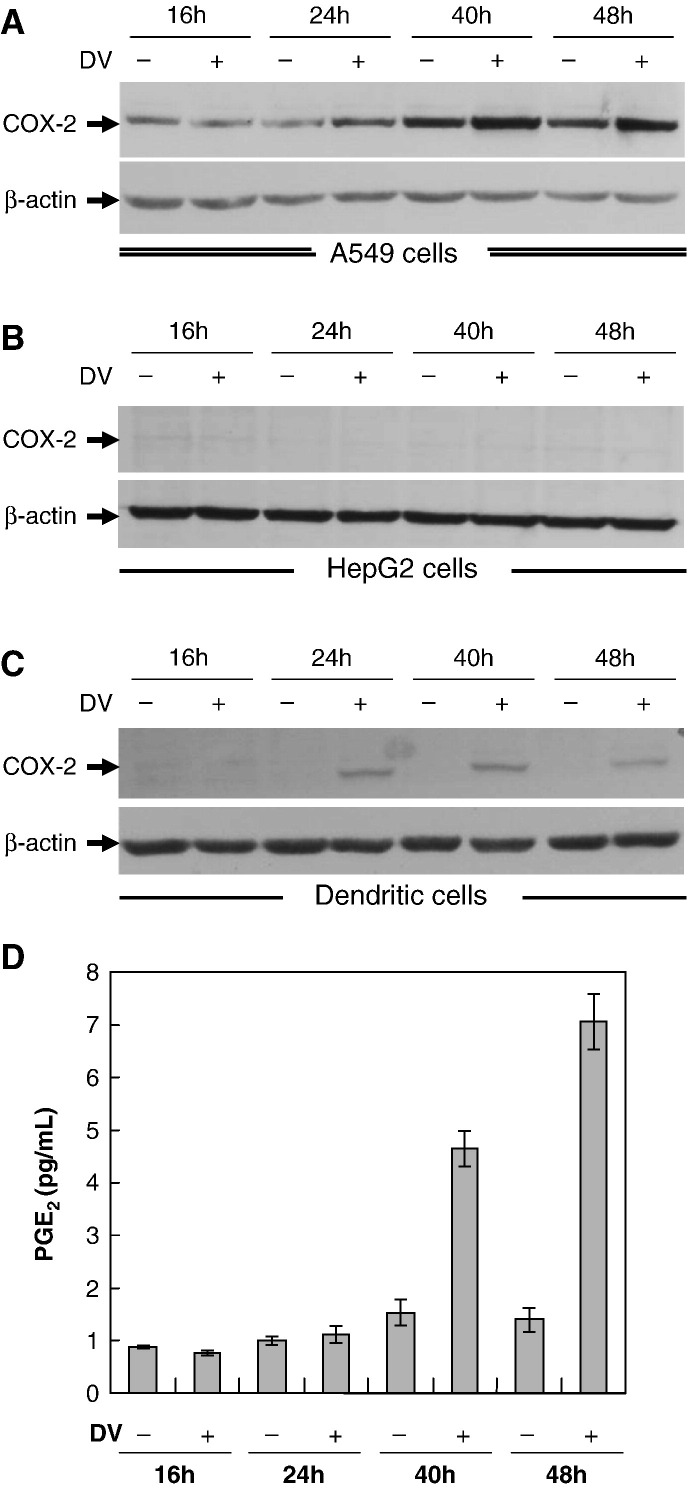
To determine whether dengue virus infection induces an inflammatory response, A549 and HepG2 cells, two commonly used cell lines for dengue virus infection study, and dendritic cells at 1 × 106 ml− 1 were infected with mock or dengue virus (NGS strain) at m.o.i. 5 and then collected at 16, 24, 40 and 48 h post-infection. In A549 cells, COX-2 expression, though constitutively expressed, was time-dependently enhanced by dengue virus infection, starting between 16 and 24 h post-infection in both A549 cells and dendritic cells (Fig. 1A, C), but not in HepG2 cells (Fig. 1B). PGE2 concentration was also prominently enhanced between 24 and 40 h post-infection in A549 cells (Fig. 1D) and dendritic cells (data not shown). The following studies were carried out using A549 cells with dengue virus infection.
Fig. 1.
Dengue virus stimulated COX-2 expression and PGE2 production. A549, HepG2 cells and human peripheral dendritic cells at 1 × 106 ml− 1 infected with mock or dengue virus (m.o.i. = 5) were collected at various time points post-infection. Total cell lysates were prepared and analyzed for COX-2 using Western blotting assay and PGE2 concentration using ELISA. (A) COX-2 expression at various time points in mock- or dengue virus-infected A549 cells. (B) COX-2 expression at various time points in mock- or dengue virus-infected HepG2 cells. (C) COX-2 expression at various time points in mock- or dengue virus-infected dendritic cells. (D) PGE2 level at various time points in mock- or dengue virus-infected A549 cells.
3.2. Dengue virus infection-enhanced DNA-binding activity of NF-κB and AP-1
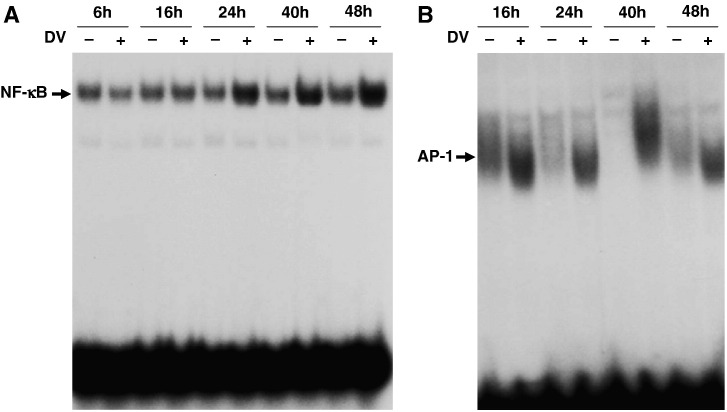
To explore the mechanism of dengue virus-induced COX-2 expression at the transcriptional level, the DNA-binding activities of NF-κB, AP-1 and CRE were evaluated using an EMSA of nuclear extracts from A549 cells sorted at 6, 16, 24, 40 and 48 h post-infection. The DNA-binding activity of both NF-κB and AP-1 were significantly increased at about 24 and 16 h post-infection, respectively (Fig. 2A, B), but CRE was not affected (data not shown). These results imply that dengue virus enhanced COX-2 expression in A549 cells partly through activation of NF-κB and AP-1 post-infection.
Fig. 2.
Dengue virus-activated NF-κB and AP-1. A549 cells at 1 × 106 ml− 1 infected with mock or dengue virus (m.o.i. = 5) were collected at various time points post-infection. The nuclear extracts were prepared and analyzed by EMSA. (A) NF-κB DNA-binding activity at various time points in mock- or dengue virus-infected A549 cells. (B) AP-1 DNA-binding activity at various time points in mock- or dengue virus-infected A549 cells.
3.3. Effect of triptolide and tetrandrine on COX-2 expression
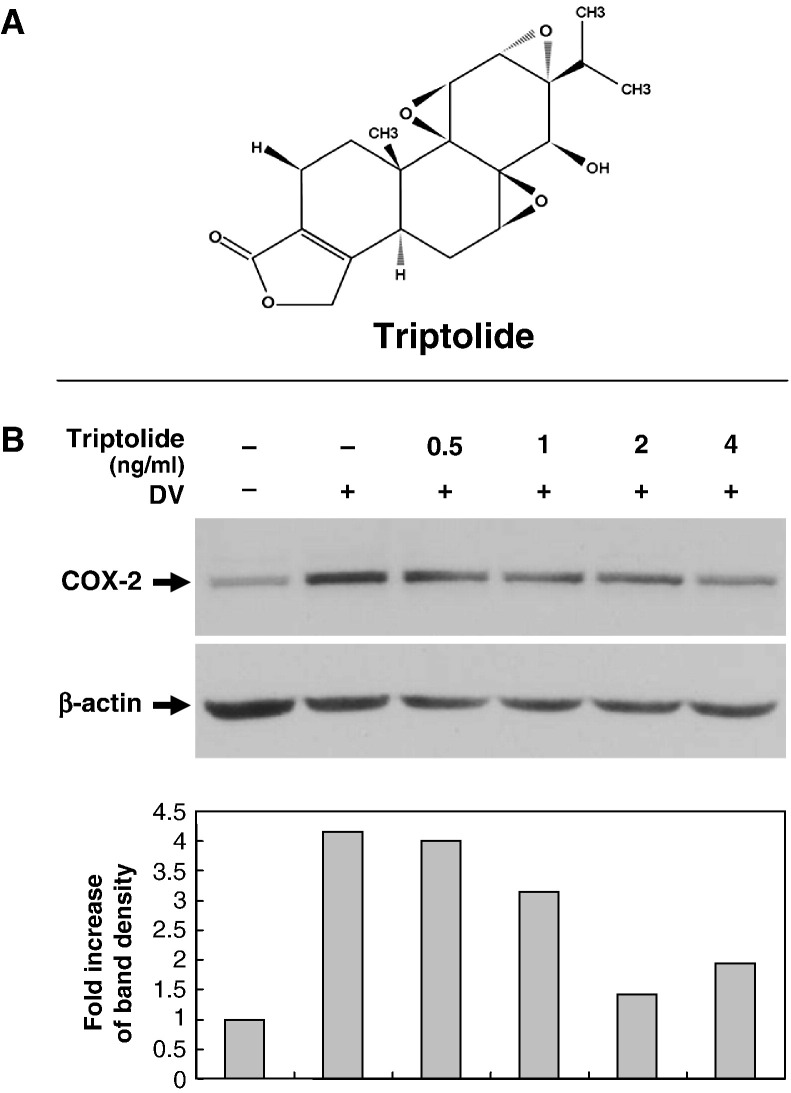
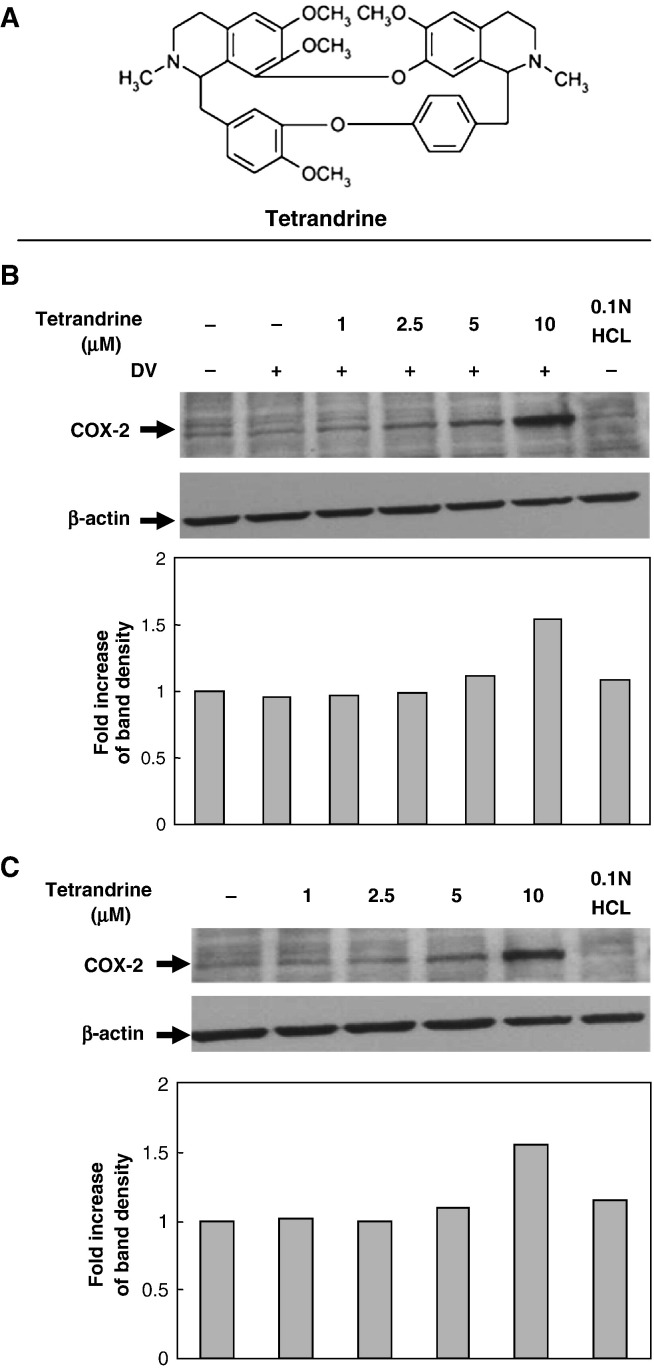
Triptolide and tetrandrine are the active compounds of two different Chinese herbs, both possessing immunosuppressive functions. To explore the antiviral properties of these two compounds, at first, we tested whether the dengue virus-induced COX-2 expression would be modulated by triptolide or tetrandrine using methods similar to our previous work (Ho et al., 2004). In the triptolide treatment group, A549 cells were pre-treated with various concentrations of triptolide (0.5, 1, 2, 4 ng/ml) for 16 h pre-infection, then collected 48 h post-infection with mock or dengue virus (m.o.i. = 5) (total drug treatment time 64 h). In the tetrandrine treatment group, A549 cells were pre-treated with various concentrations of tetrandrine (1, 2.5, 5, 10 μM) for 2 h pre-infection, then collected 24 h post-infection with mock or dengue virus (m.o.i. = 5) (total treatment time 26 h). Western blotting results showed that the dengue virus-induced COX-2 expression in A549 cells was apparently inhibited by triptolide in a dose-dependent manner (Fig. 3B). In the tetrandrine treatment group, the dengue virus-induced COX-2 expression at 24 h post-infection was not apparent in comparison with control cells but was significantly enhanced at a high concentration of tetrandrine (10 μM), not only in dengue virus-infected A549 cells (Fig. 4B), but also in non-infected A549 cells (Fig. 4C). There was no detectable cytotoxicity of triptolide and tetrandrine to A549 cells by MTT assays under the conditions tested (data not shown).
Fig. 3.
Dose-dependent triptolide inhibition of dengue virus-inducted COX-2 expression. A549 cells at 1 × 106 ml− 1 treated with various concentrations of triptolide (0.5, 1, 2 and 4 ng/ml) for 16 h pre-infection were collected at 48 h post-infection with mock or dengue virus (m.o.i. = 5) (total treatment time 64 h). (A) Chemical structure of triptolide (PG490). (B) Western blot showing COX-2 expression in dengue virus-infected A549 cells treated with triptolide. The fold induction was presented as a comparison with the intensity determined in the internal β-actin control.
Fig. 4.
Effect of tetrandrine on dengue virus-induced COX-2 expression. A549 cells at 1 × 106 ml− 1 treated with various concentration of tetrandrine (1, 2.5, 5 and 10 μM) for 2 h pre-infection were collected at 24 h post-infection with mock or dengue virus (m.o.i. = 5) (total treatment time 26 h). β-actin expression was used as internal control for normalization. The fold induction of COX-2 was presented as a comparison with the intensity of control cells determined by the internal β-actin control. (A) Chemical structure of tetrandrine. Western blot showing (B) COX-2 expression in dengue virus-infected A549 cells treated with tetrandrine and (C) COX-2 expression in uninfected A549 cells treated with tetrandrine.
3.4. Triptolide inhibition of virus-induced COX-2 expression during post-infection
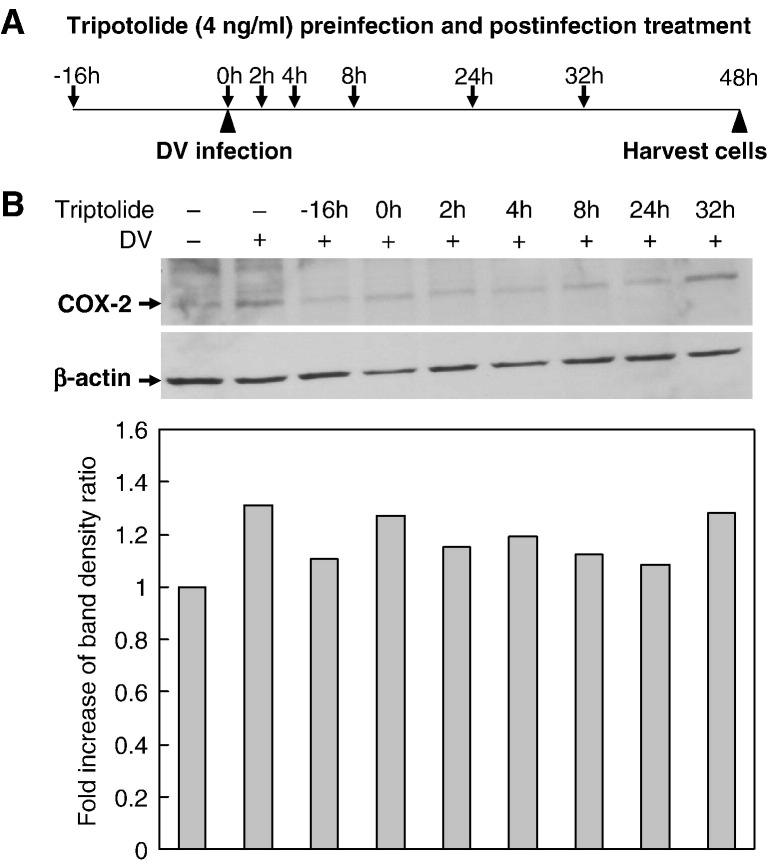
To examine the anti-inflammatory effect of triptolide in post-infection treatment, A549 cells were treated with 4 ng/ml triptolide at various time points before and after infection with dengue virus or mock (16 h pre-infection, 0, 2, 4, 8, 24 and 32 h post-infection) and COX-2 expression analyzed at 48 h post-infection. Compared with the COX-2 level in dengue virus-infected A549 cells without treatment, post-infection treatment with triptolide still inhibited COX-2 expression, even at 24 h and probably up until 32 h post-infection (Fig. 5 ).
Fig. 5.
Anti-inflammatory effect of triptolide in post-infection treatment. A549 cells were treated with 4 ng/ml of triptolide for various time periods before and after infection (16 h pre-infection, 0, 2, 4, 8, 24 and 32 h post-infection) with mock dengue virus or (m.o.i. = 5) and total cell lysates harvested at 48 h post-infection for analysis of COX-2 expression. (A) Illustration for the timeframe of drug treatment and dengue virus infection. (B) COX-2 level in mock- or dengue virus-infected A549 cells treated with triptolide at various time points. Protein band signals were analyzed by densitometry. The ratio of COX-2 expression between the treated cells and control was presented after using β-actin as internal control for normalization.
3.5. Opposite effects of triptolide and tetradrine on dengue virus-induced PGE2 production
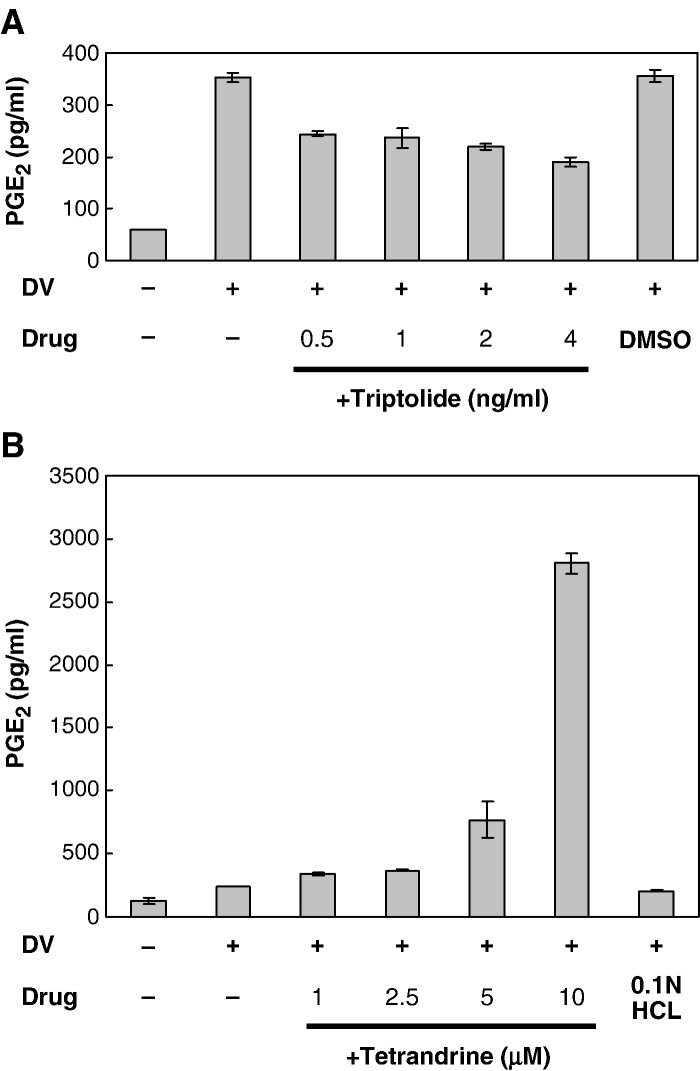
To determine whether the PGE2 levels paralleled the inhibition of COX-2, the PGE2 levels in the supernatants of A549 cells infected with dengue virus and treated with various concentrations of triptolide or tetrandrine at the same conditions and time points were detected by ELISA. Dengue virus-induced PGE2 production was inhibited by triptolide in a dose-dependent manner (Fig. 6A). Conversely, PGE2 production was dose-dependently activated by tetrandrine, markedly enhanced at a high tetrandrine concentration (10 μM), corresponding well to the activation of COX-2 at high concentration of tetrandrine (Fig. 4B).
Fig. 6.
Effects of triptolide and tetrandrine on dengue virus-induced PGE2 production. A549 cells were treated with various concentrations of triptolide for 16 h or tetrandrine for 2 h prior to infection with mock or dengue virus (m.o.i. = 5), and then collected at 48 and 24 h post-infection, respectively. ELISA showing (A) PGE2 level in dengue virus-infected A549 cells treated with triptolide and (B) PGE2 production in dengue virus infection A549 cells treated with tetrandrine.
3.6. Dose-dependent triptolide inhibition of NF-κB and AP-1 DNA-binding activity
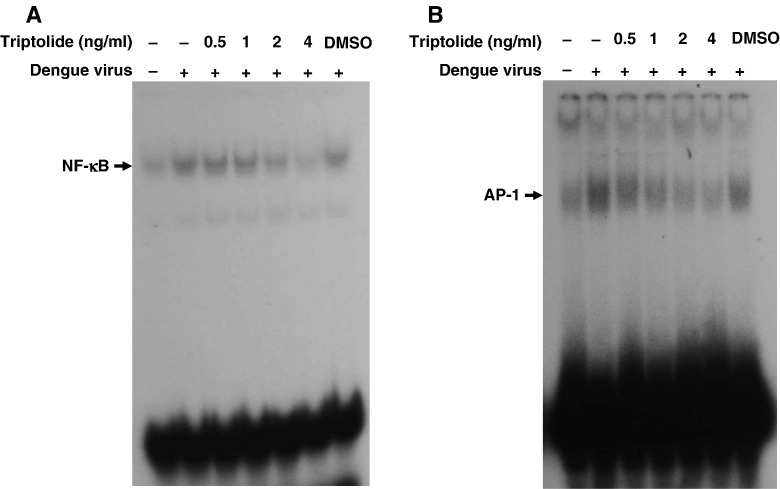
COX-2 gene expression is mainly regulated at the transcription level. We performed EMSA using specific binding oligonucleotides to further investigate the activity of NF-κB and AP-1 in the nucleus of dengue virus-infected A549 cells treated by various concentrations of triptolide 16 h pre-infection and harvested 48 h post-infection with mock or dengue virus (m.o.i. = 5). The DNA-binding activities of NF-κB and AP-1 induced by dengue virus infection in A549 cells were both inhibited by triptolides in a dose-dependent manner (Fig. 7A, B).
Fig. 7.
Effect of triptolide on dengue virus-induced NF-κB and AP-1 activation. A549 cells were treated with various concentrations of triptolide for 2 h prior to infection with mock or dengue virus (m.o.i. = 5) and collected 48 h post-infection. EMSA of nuclear extracts showing (A) NF-κB in dengue virus-infected A549 cells treated triptolide and (B) AP-1 DNA-binding activity in dengue virus-infected A549 cells treated with triptolide.
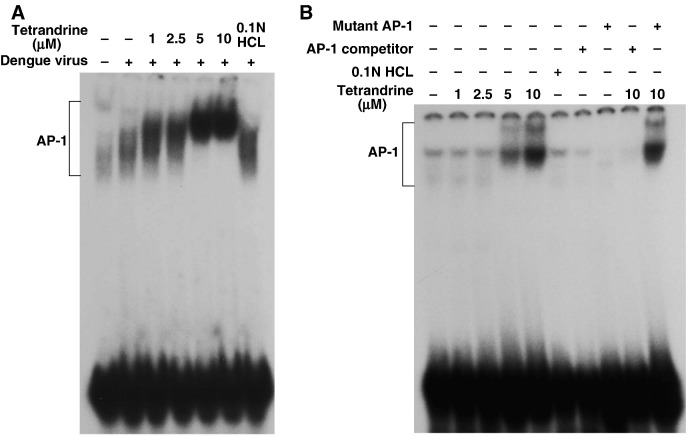
3.7. Viral infection-independent effects of tetrandrine on NF-κB and AP-1
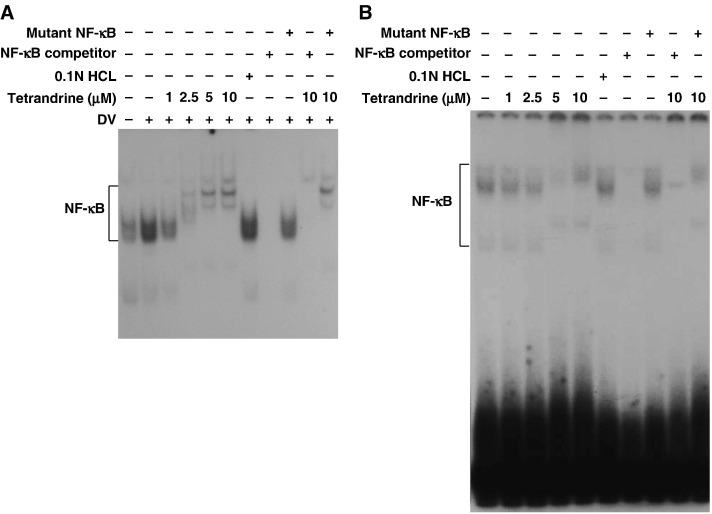
Our data showed that tetrandrine at high concentration activated COX-2 and PGE2. To determine whether this effect was due to modulation of transcription factors, the DNA-binding activities of NF-κB and AP-1 were evaluated using EMSA in the nuclear extracts of A549 cells treated with various concentrations of tetrandrine (1, 2.5, 5, 10 μM) for 2 h pre-infection and harvested 24 h post-infection with mock or dengue virus (m.o.i. = 5). Tetrandrine at low concentration (1 μM) inhibited the DNA-binding activity of NF-κB induced by dengue virus, but shifted the mobility and changed the pattern of the band indicating NF-κB at higher concentration (2.5, 5, 10 μM) (Fig. 8A). These findings imply that a high dose of tetrandrine changed the composition of NF-κB in A549 cells infected with dengue virus. In A549 cells without dengue virus infection, the bands of NF-κB were also shifted and patterns also changed at high concentration of tetrandrine (Fig. 8B). To confirm that the unusual bands appearing on the EMSA at higher concentration of tetrandrine were NF-κB specific, competition experiments were performed with a 100-fold molar excess of non-radioactive wild-type NF-κB with canonical sequence (NF-κB competitor), or a mutant NF-κB to ensure that the normal NF-κB band reappeared.
Fig. 8.
DNA-binding activity of NF-κB affected by tetrandrine. Nuclear extracts of A549 cells, treated with various concentrations of tetrandrine for 2 h prior to infection, infected with mock or dengue virus and harvested at 24 h post-infection, were collected and analyzed by EMSA to show DNA-binding activity of (A) NF-κB in dengue virus-infected A549 cells treated with tetrandrine and (B) NF-κB in uninfected A549 cells treated with tetrandrine. Some reactions included an unlabeled NF-κB competitor (oligonucleotide probe) (A, lanes 8 and 10; B, lanes 7 and 9) and an unlabeled mutant NF-κB (non-specific probe) (A, lanes 9 and 11; B, lanes 8 and 10).
Besides activating DNA binding of AP-1, tetrandrine at high concentration also decreased the AP-1 band mobility and change the band pattern on EMSA (Fig. 9A). In non-infected A549 cells, tetrandrine at higher concentrations also yielded similar results (Fig. 9B). The specificity of the AP-1 bands with mobility shift was also confirmed by competition analysis with an AP-1 competitor and mutant AP-1 probes (Fig. 9B, lanes 7–10).
Fig. 9.
DNA-binding activity of AP-1 affected by tetrandrine. The nuclear extracts of A549 cells, treated with various concentrations of tetrandrine for 2 h prior to infection, infected with mock or dengue virus and harvested at 24 h post-infection, were collected and analyzed by EMSA to show DNA-binding activity of AP-1 in (A) dengue virus-infected A549 cells treated with tetrandrine and (B) uninfected A549 cells treated with tetrandrine. In some reactions, an unlabeled AP-1 competitor (oligonucleotide probe) (B, lanes 7 and 9) and an unlabeled mutant AP-1 (non-specific probe) (A, lanes 8 and 10) were included.
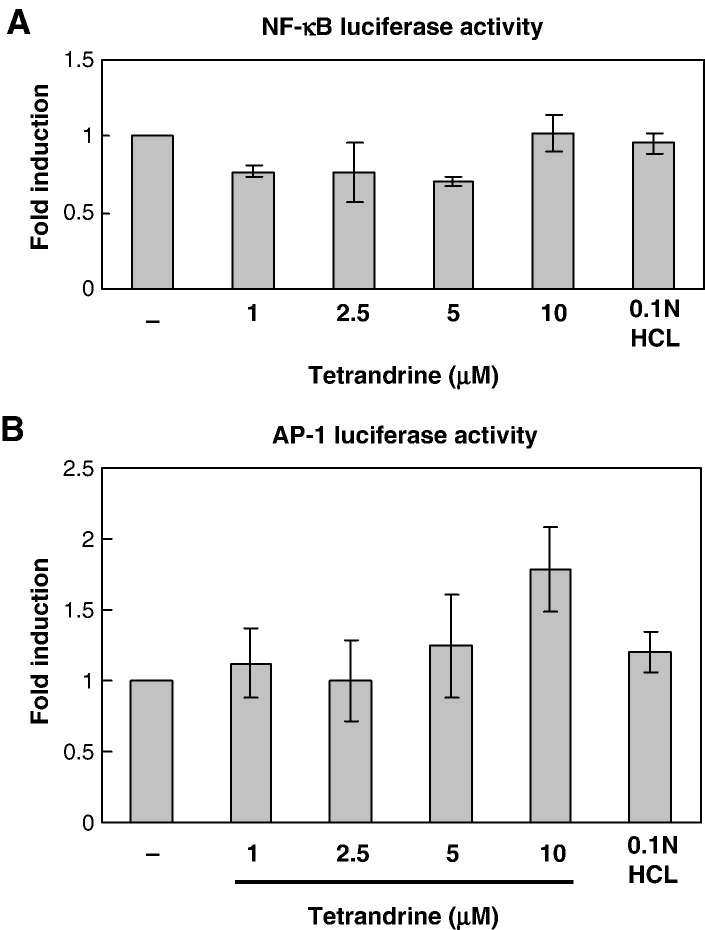
The DNA-binding activities of NF-κB and AP-1 were enhanced by high concentration of tetrandrine, but the compositions of these transcription factors seemed to be modified. To investigate the impact of the modification of NF-κB and AP-1 on gene expression, we transfected non-infected A549 cells with NF-κB and AP-1 luciferase constructs and then treated them with various concentrations of tetrandrine up to 24 h. We found that tetrandrine at lower concentrations (1, 2.5 and 5 μM) mildly inhibited the NF-κB luciferase activity, but at high concentration activated NF-κB to the control level (Fig. 10A). AP-1 activity was enhanced by tetrandrine, especially at high concentration (Fig. 10B). Higher concentration of tetrandrine also enhanced the DNA-binding activity of CRE in A549 cells without dengue virus infection (data not shown).
Fig. 10.
Lucifearse assay for NF-κB and AP-1 activities affected by tetrandrine. A549 cells were transfected with NF-κB or AP-1 luciferase reporter plasmid and pRL-TK plasmid and treated with various concentration of tetrandrine in fresh media for 24 h. The luciferase activities of NF-κB and AP-1 were normalized to pRL-TK luciferase activity and presented as fold induction relative to control. (A) NF-κB luciferase activity in A549 cells treated with tetrandrine. (B) AP-1 luciferase activity in A549 cells treated with tetrandrine at high concentration.
3.8. Dose-dependent suppression of virus production by tetrandrine
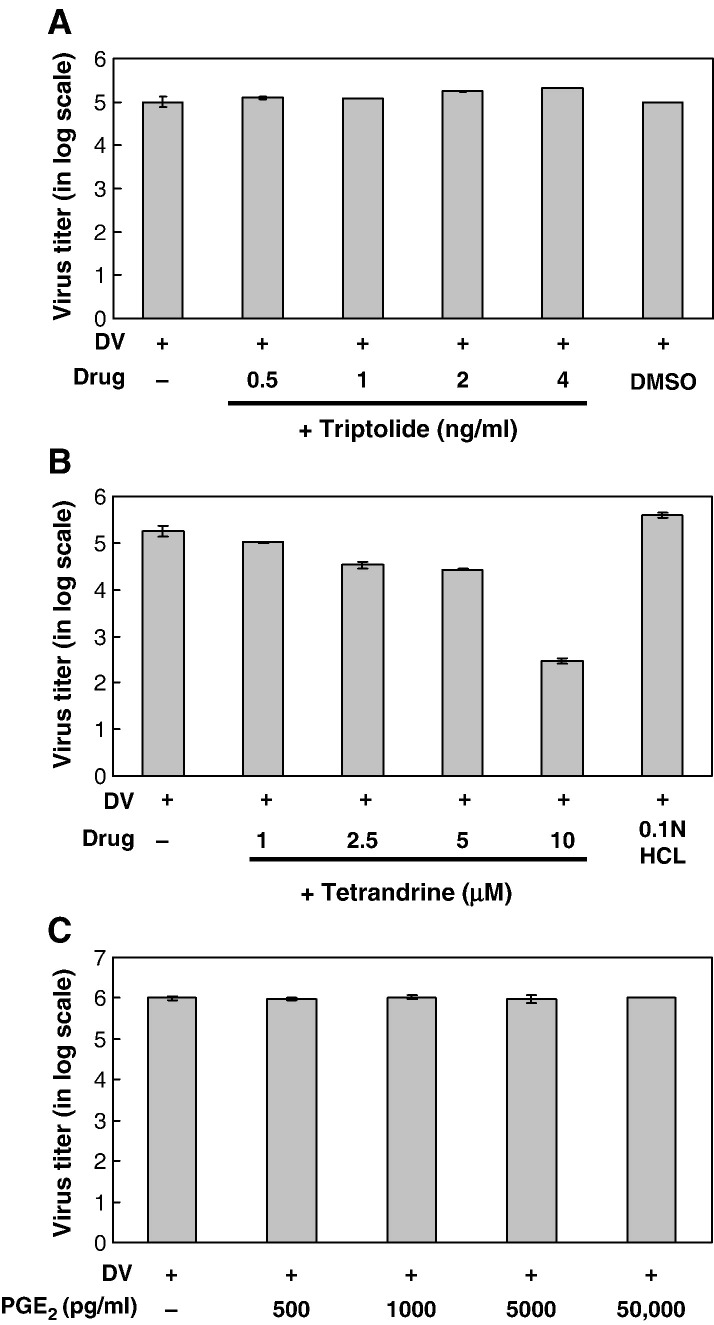
A number of studies support a role for PGE2 in the modulation of viral replication and virulence in a cell type- and virus-selective manner (Steer and Corbett, 2003). There is increasing evidence showing mutual influences between virus replication/production and the expression of COX-2, PGE2, NF-κB and other numerous factors (Santoro et al., 2003, Steer and Corbett, 2003). Modulation of these factors might be an antiviral strategy. The effects of triptolide and tetrandrine on virus production in dengue virus-infected A549 cells were investigated. A549 cells at 1 × 106 ml− 1 were pre-treated with various concentrations of triptolide for 16 h or tetrandrine for 2 h pre-infection, and then collected 48 and 24 h, respectively, post-infection with mock or dengue virus (m.o.i. = 5). Unexpectedly, tetrandrine dose-dependently inhibited viral production in dengue virus-infected A549 cell (Fig. 11B), but triptolide did not (Fig. 11A).
Fig. 11.
Virus production in dengue virus-infected A549 cells treated with triptolide, tetrandrine and exogenous PGE2. A549 cells at 1 × 106 ml− 1 were treated with various concentrations of triptolide for 16 h pre-infection and 48 h post-infection, or treated with various concentrations of tetrandrine for 2 h pre-infection and 24 h post-infection with mock or dengue virus (m.o.i. = 5). Virus titers are expressed in log scale. (A) Virus titers in A549 cells treated with triptolide. (B) Virus titers in A549 cells treated with tetrandrine. (C) A549 cells at 1 × 106 ml− 1 were treated with different concentrations of exogenous PGE2 for 2 h pre-infection and 24 h post-infection with mock or dengue virus (m.o.i. = 5). The virus titers in supernatants were analyzed using plaque assay.
Tetrandrine at high concentration enhanced PGE2 production but suppressed viral production in dengue virus-infected A549 cells. To clarify the relationship between PGE2 level and virus production, A549 cells were treated with various concentrations of exogenous PGE2 (500–50,000 pg/ml) for 2 h pre-infection and collected 24 h post-infection for virus titer measurement. We found that the viral titer in dengue virus-infected A549 cells was not affected by exogenous PGE2 at all, even with a concentration up to 50,000 pg/ml, 17 times more than the PGE2 level induced by high concentration (10 μM) of tetrandrine (Fig. 11C).
4. Discussion
In most mammalian tissues, COX-2 expression remains undetectable under basal conditions but is induced by various extracellular signals and stimuli, including oncogenes, mitogens, cytokines, growth factors, inflammatory molecules, endotoxins and tumor promoters, as well as viral infection (Williams et al., 1999, Steer and Corbett, 2003). In the present study, we demonstrate for the first time that dengue virus infection can induce overexpression of COX-2 and significant PGE2 release in dendritic cells, which are the first target of dengue virus after infection by mosquito-feeding. The so-called break-bone pain is probably related to the overproduction of PGE2 after systemic dengue virus infection.
Recently, it has been proven that dengue virus is able to infect and replicate in human primary lung epithelium and various lung cancer cell lines, including A549 (Lee et al., 2007). The results of this study further verify the vulnerability of human lung tissue to dengue virus infection by showing activated COX-2 expression and PGE2 production in A549 cells following viral infection, similar to the response in dendritic cells. However, the absence of COX-2 expression in HepG2 cells, which are usually infected by dengue virus as well, implies that the immune response to virus infection is cell-specific.
It is now known that apart from its constitutive expression in a few organs, including the central nervous system, kidneys and the gonads, COX-2 is overexpressed in many types of human cancer, such as colon, breast, prostate, esophagus, pancreas and lung, and appears to be an excellent target for chemoprevention or cancer treatment (de Moraes et al., 2007, Sarkar et al., 2007). In agreement with other studies, our results also showed that A549, a human airway type II alveolar epithelial carcinoma cell line, overexpresses COX-2 at the basal state (Watkins et al., 1999, Marcet et al., 2007).
The induction of COX-2 is mediated by a variety of transcription factors including NF-κB, AP-1, CRE binding protein (CREB), NFAT and nuclear factor interleukin-6 (NF-IL6) (Iniguez et al., 2000, Lara-Pezzi et al., 2002). Activation of NF-κB is a hallmark of most viral infections (Santoro et al., 2003). Cytokines stimulated by NF-κB, such as TNF-α and IL-1β, are also potent NF-κB inducers, hence establishing a positive autoregulatory loop that can amplify the inflammatory response and lead to chronic infection. It is now clear that viruses can directly activate NF-κB and utilize it in different ways to their own advantage (Hiscott et al., 2006, Santoro et al., 2003). Thus, inhibitors of NF-κB are of interest as potential anti-inflammatory drugs.
Until recently, AP-1 was considered a transcription factor expressed in most tissues to regulate cellular and even viral genes (Herrlich, 2001, Karin et al., 1997, Sadowska et al., 2003). Various stimuli have now been shown to induce activity of AP-1 family members, including physiological agents, cellular stress, inflammatory cytokines and pharmacological compounds, as well as bacterial and viral infections (De Bosscher et al., 2003).
As our EMSA results showed (Fig. 2), DNA-binding activity of NF-κB and AP-1 in dengue virus-infected A549 cells was significantly activated at about 24 and 16 h post-infection, respectively, and lasted more than 48 h post-infection. The dengue virus-induced activation of AP-1 reached a peak at 40 h post-infection, but NF-κB might reach its peak later. The dengue virus-induced COX-2 expression in both A549 and dendritic cells occurred earlier than 24 h post-infection (Fig. 1A, C), but the dengue virus-induced PGE2 production was not obvious until 40 h post-infection (Fig. 1D). Once activated, the time course between COX-2 mRNA expression and COX-2 protein production generally correlated well. COX-2 expression is induced within 2–6 h in several types of cells including fibroblasts, endothelial cells and monocytes in response to bacterial endotoxins, cytokines, hormones or growth factors (Tsatsanis et al., 2006). In our study of A549 cells infected with dengue virus, the AP-1 activation seemed to occur earlier than NF-κB but with a shorter duration. To our knowledge, this is the first demonstration that dengue virus infection in human lung cells can activate NF-κB and AP-1, both transcription factors of the COX-2 gene. It suggests that dengue virus infection in A549 cells might induce numerous intracellular signaling cascades converging with the activation of NF-κB, AP-1 and possibly other transcription factors, which act independently or coordinately to regulate expression of cox-2 and subsequent PGE2 release.
The release of PGE2 through activation of COX-2 is a common host inflammatory response to various stimuli. PGE2, induced by viral infection as well as other stimuli, has important effects on the replication and infectivity of a virus (Steer and Corbett, 2003, Tsatsanis et al., 2006). Modulation of COX-2 has been showed to be promising target for controlling not only inflammation, pain and autoimmune disease, but also viral infection (Rocca and FitzGerald, 2002, Steer and Corbett, 2003). Here, we investigated the anti-inflammatory and antiviral potentials of two compounds extracted from Chinese herbs known to possess inhibition of COX-2 expression function.
Triptolide, a diterpenoid epoxide sometimes referred to as PG490 (Fig. 3A), is believed to be the major therapeutic extract of TWHf (Tao et al., 1998) with anti-inflammatory and immunosuppressive activities. It has been shown to inhibit lymphocyte proliferation, and synthesis and secretion of pro-inflammatory cytokines (Qiu et al., 1999, Krakauer et al., 2005). The medicinal chemistry and pharmacology of triptolide have been reviewed recently (Brinker et al., 2007). In this study, we showed that triptolide not only dose-dependently inhibited the COX-2 expression and PGE2 production induced by dengue virus, but also dose-dependently inhibited the activities of NF-κB and AP-1. These results mirror the findings in other studies showing that triptolide suppresses transactivation of NF-κB and AP-1 in human gastric cancer cells (Jiang et al., 2001) and inhibits COX-2 expression via NF-κB pathway in astrocytes (Dai et al., 2006) and dendritic cells (Liu et al., 2006).
Tetrandrine, a bis-benzylisoquinoline alkaloid, is purified from the tuberous roots of creeper S. tetrandrae S. Moore. In China, it is traditionally prescribed to treat hypertension, rheumatoid arthritis, inflammation and silicosis and might possess anticancer, immunosuppressive and free radical scavenging activities (Ho and Lai, 2004). Tetrandrine has been shown to inhibit NF-κB activation in human peripheral blood T cells induced by various stimuli through downregulation of the IκBα kinases-IκBα-NF-κB signaling pathway (Ho et al., 2004) and in LPS-stimulated pancreatic acinar cells of rat (Zhang et al., 2006). However, in our study, tetrandrine at high concentration further activated COX-2 and markedly increased PGE2 production, more than 17 times over control, corresponding to the activation of NF-κB and AP-1. High-concentration tetrandrine also activated COX-2 expression, and NF-κB and AP-1 activity in the absence of dengue virus infection. An early study exploring the cytotoxicity of tetrandrine on mouse peritoneal macrophages found the dose-dependent loss of cell viability was accompanied by the generation of PGE2 to levels 285–877% of control (Pang and Hoult, 1997), suggesting evidence of COX-2 activation by tetrandrine at high concentration.
Some viruses activate, incorporate into, and make use of the NF-κB signaling pathway in favor of blocking apoptosis, prolonging survival of the host cell to gain time for replication and increase virus production (Hiscott et al., 2006). In two early studies, it was shown that aspirin at high concentration not only inhibited COX but also directly inhibited the activity of NF-κB in Jurkat cells and AP-1 in JB6 cells (Dong et al., 1997, Kopp and Ghosh, 1994), implying that aspirin might inhibit NF-κB and AP-1 in a cell-specific fashion. It was also shown that salicylates restricted flavivirus replication independent of the NF-κB pathway (Liao et al., 2001), implying that NF-κB might not be the target for antiviral therapy; i.e. NSAIDs might yield their antiviral effect though the COX/PGE2 pathway instead. In our study, the activation of NF-κB and AP-1 in dengue virus-infected A549 cells could be via either defensive or defeated pathway, but an inflammatory response to dengue virus infection was indicated by the corresponding activation of COX-2 and PGE2. However, triptolide, while it dose-dependently inhibited the dengue virus-activated COX-2/PGE2/NF-κB/AP-1, did not affect viral production at all. This implies that dengue virus did not usurp NF-κB and AP-1 pathway for replication. Nevertheless, the post-infection anti-inflammatory effect of triptolide through blockage of COX-2/PGE2 still holds treatment potential via attenuation of the overwhelming inflammation and agonizing bone pain in dengue fever. On the contrary, the potential antiviral effect of tetrandrine might act by way of activating NF-κB and AP-1, rather than COX-2/PGE2, although both were enhanced by tetrandrine at high concentration.
While PGE2 is mainly considered to promote inflammation, it has complex effects on immunity depending on the pathogen and surrounding milieu (Gualde and Harizi, 2004) hence regulates viral replication and virulence. For instance, PGE2 inhibits replication of some viruses (Liu et al., 2005, Steer and Corbett, 2003, Waris and Siddiqui, 2005), but enhances replication of others (Khyatti and Menezes, 1990, Moriuchi et al., 2001, Carey et al., 2005, Hooks et al., 2006, Sharma-Walia et al., 2006). Our study showed that PGE2, while activated by high-concentration tetrandrine, is not the mechanism though which tetrandrine suppressed virus production, confirmed by the unchanged virus titer in A549 cells treated with exogenous PGE2.
In our EMSA study, it was incidentally found that high concentration of tetrandrine induced mobility shift and band pattern changes in NF-κB and AP-1 in A549 cells, probably by activation of non-specific protein binding. Because both NF-κB and AP-1 are implicated in the expression of various genes, tetrandrine-modified NF-κB and AP-1 may collaborate with other transcription factors or enhancers, and thus activate other genes involved in inflammation or immune response in addition to COX-2. This might explain our finding that high-concentration tetrandrine suppressed virus production in dengue virus-infected A549 cells.
In conclusion, herein we demonstrated that dengue virus infection induced COX-2/PGE2 expression in human lung cells, similarly to dendritic cells, presumably via activation of NF-κB and AP-1. Triptolide suppressed these viral-induced inflammatory responses in a dose-dependent manner, but failed to affect virus production. Conversely, tetrandrine at high concentration enhanced COX-2/PGE2, activated and modified NF-κB and AP-1 in A549 cells, regardless of infection status. Surprisingly, tetrandrine, not triptolide, suppressed virus production in A549 cells. Our data demonstrating the differential effects of triptolide and tetrandrine shed light on the immune response to dengue virus infection via COX-2 and its transcription factors, and suggest the potential for anti-inflammatory and antiviral therapy with herbal medicines. Additional gene or transcription factor targets of modulation by triptolide or tetrandrine deserve further investigation.
Acknowledgments
We thank all colleagues in our laboratories for their diligent efforts and kind help. This work was supported by grants NSC95-2314-B-016-051-MY3 and NHRI-EX95-9208SI from National Science Council and National Health Research Institute, respectively, Taiwan, Republic of China.
Contributor Information
Jun-Ting Liou, Email: ljtmail@gmail.com.
Zih-Yan Chen, Email: smileooo7161@yahoo.com.tw.
Ling-Jun Ho, Email: lingjunho@nhri.org.tw.
Jenn-Haung Lai, Email: haungben@tpts5.seed.net.tw.
References
- Brinker A.M., Ma J., Lipsky P.E., Raskin I. Medicinal chemistry and pharmacology of genus Tripterygium (Celastraceae) Phytochemistry. 2007;68:732–766. doi: 10.1016/j.phytochem.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey M.A., Bradbury J.A., Seubert J.M., Langenbach R., Zeldin D.C., Germolec D.R. Contrasting effects of cyclooxygenase-1 (COX-1) and COX-2 deficiency on the host response to influenza A viral infection. J. Immunol. 2005;175:6878–6884. doi: 10.4049/jimmunol.175.10.6878. [DOI] [PubMed] [Google Scholar]
- Chen C.J., Raung S.L., Kuo M.D., Wang Y.M. Suppression of Japanese encephalitis virus infection by non-steroidal anti-inflammatory drugs. J. Gen. Virol. 2002;83:1897–1905. doi: 10.1099/0022-1317-83-8-1897. [DOI] [PubMed] [Google Scholar]
- Chun K.S., Surh Y.J. Signal transduction pathways regulating cyclooxygenase-2 expression: potential molecular targets for chemoprevention. Biochem. Pharmacol. 2004;68:1089–1100. doi: 10.1016/j.bcp.2004.05.031. [DOI] [PubMed] [Google Scholar]
- Dai Y.Q., Jin D.Z., Zhu X.Z., Lei D.L. Triptolide inhibits COX-2 expression via NF-kappa B pathway in astrocytes. Neurosci. Res. 2006;55:154–160. doi: 10.1016/j.neures.2006.02.013. [DOI] [PubMed] [Google Scholar]
- De Bosscher K., Vanden Berghe W., Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-kappaB or activator protein-1: molecular mechanisms for gene repression. Endocr. Rev. 2003;24:488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- de Moraes E., Dar N.A., de Moura Gallo C.V., Hainaut P. Cross-talks between cyclooxygenase-2 and tumor suppressor protein p53: balancing life and death during inflammatory stress and carcinogenesis. Int. J. Cancer. 2007;121:929–937. doi: 10.1002/ijc.22899. [DOI] [PubMed] [Google Scholar]
- Dong Z., Huang C., Brown R.E., Ma W.Y. Inhibition of activator protein 1 activity and neoplastic transformation by aspirin. J. Biol. Chem. 1997;272:9962–9970. doi: 10.1074/jbc.272.15.9962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualde N., Harizi H. Prostanoids and their receptors that modulate dendritic cell-mediated immunity. Immunol. Cell Biol. 2004;82:353–360. doi: 10.1111/j.0818-9641.2004.01251.x. [DOI] [PubMed] [Google Scholar]
- Herrlich P. Cross-talk between glucocorticoid receptor and AP-1. Oncogene. 2001;20:2465–2475. doi: 10.1038/sj.onc.1204388. [DOI] [PubMed] [Google Scholar]
- Hiscott J., Nguyen T.L., Arguello M., Nakhaei P., Paz S. Manipulation of the nuclear factor-kappaB pathway and the innate immune response by viruses. Oncogene. 2006;25:6844–6867. doi: 10.1038/sj.onc.1209941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L.J., Lai J.H. Chinese herbs as immunomodulators and potential disease-modifying antirheumatic drugs in autoimmune disorders. Curr. Drug Metab. 2004;5:181–192. doi: 10.2174/1389200043489081. [DOI] [PubMed] [Google Scholar]
- Ho L.J., Chang D.M., Shiau H.Y., Chen C.H., Hsieh T.Y., Hsu Y.L., Wong C.S., Lai J.H. Aspirin differentially regulates endotoxin-induced IL-12 and TNF-alpha production in human dendritic cells. Scand. J. Rheumatol. 2001;30:346–352. doi: 10.1080/030097401317148543. [DOI] [PubMed] [Google Scholar]
- Ho L.J., Wang J.J., Shaio M.F., Kao C.L., Chang D.M., Han S.W., Lai J.H. Infection of human dendritic cells by dengue virus causes cell maturation and cytokine production. J. Immunol. 2001;166:1499–1506. doi: 10.4049/jimmunol.166.3.1499. [DOI] [PubMed] [Google Scholar]
- Ho L.J., Juan T.Y., Chao P., Wu W.L., Chang D.M., Chang S.Y., Lai J.H. Plant alkaloid tetrandrine downregulates IkappaBalpha kinases-IkappaBalpha-NF-kappaB signaling pathway in human peripheral blood T cell. Br. J. Pharmacol. 2004;143:919–927. doi: 10.1038/sj.bjp.0706000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L.J., Hung L.F., Weng C.Y., Wu W.L., Chou P., Lin Y.L., Chang D.M., Tai T.Y., Lai J.H. Dengue virus type 2 antagonizes IFN-alpha but not IFN-gamma antiviral effect via down-regulating Tyk2-STAT signaling in the human dendritic cell. J. Immunol. 2005;174:8163–8172. doi: 10.4049/jimmunol.174.12.8163. [DOI] [PubMed] [Google Scholar]
- Hooks J.J., Chin M.S., Srinivasan K., Momma Y., Hooper L.C., Nagineni C.N., Chan C.C., Detrick B. Human cytomegalovirus induced cyclooxygenase-2 in human retinal pigment epithelial cells augments viral replication through a prostaglandin pathway. Microbes Infect. 2006;8:2236–2244. doi: 10.1016/j.micinf.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Iniguez M.A., Martinez-Martinez S., Punzon C., Redondo J.M., Fresno M. An essential role of the nuclear factor of activated T cells in the regulation of the expression of the cyclooxygenase-2 gene in human T lymphocytes. J. Biol. Chem. 2000;275:23627–23635. doi: 10.1074/jbc.M001381200. [DOI] [PubMed] [Google Scholar]
- Jassim S.A., Naji M.A. Novel antiviral agents: a medicinal plant perspective. J. Appl. Microbiol. 2003;95:412–427. doi: 10.1046/j.1365-2672.2003.02026.x. [DOI] [PubMed] [Google Scholar]
- Jiang X.H., Wong B.C., Lin M.C., Zhu G.H., Kung H.F., Jiang S.H., Yang D., Lam S.K. Functional p53 is required for triptolide-induced apoptosis and AP-1 and nuclear factor-kappaB activation in gastric cancer cells. Oncogene. 2001;20:8009–8018. doi: 10.1038/sj.onc.1204981. [DOI] [PubMed] [Google Scholar]
- Karin M., Liu Z., Zandi E. AP-1 function and regulation. Curr. Opin. Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- Khyatti M., Menezes J. The effect of indomethacin, prostaglandin E2 and interferon on the multiplication of herpes simplex virus type 1 in human lymphoid cells. Antivir. Res. 1990;14:161–172. doi: 10.1016/0166-3542(90)90032-3. [DOI] [PubMed] [Google Scholar]
- Kopp E., Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- Krakauer T., Chen X., Howard O.M., Young H.A. Triptolide attenuates endotoxin- and staphylococcal exotoxin-induced T-cell proliferation and production of cytokines and chemokines. Immunopharmacol. Immunotoxicol. 2005;27:53–66. doi: 10.1081/iph-51294. [DOI] [PubMed] [Google Scholar]
- Lai J.H., Ho L.J., Kwan C.Y., Chang D.M., Lee T.C. Plant alkaloid tetrandrine and its analog block CD28-costimulated activities of human peripheral blood T cells: potential immunosuppressants in transplantation immunology. Transplantation. 1999;68:1383–1392. doi: 10.1097/00007890-199911150-00027. [DOI] [PubMed] [Google Scholar]
- Lai J.H., Ho L.J., Lu K.C., Chang D.M., Shaio M.F., Han S.H. Western and Chinese antirheumatic drug-induced T cell apoptotic DNA damage uses different caspase cascades and is independent of Fas/Fas ligand interaction. J. Immunol. 2001;166:6914–6924. doi: 10.4049/jimmunol.166.11.6914. [DOI] [PubMed] [Google Scholar]
- Lara-Pezzi E., Gomez-Gaviro M.V., Galvez B.G., Mira E., Iniguez M.A., Fresno M., Martinez A.C., Arroyo A.G., Lopez-Cabrera M. The hepatitis B virus X protein promotes tumor cell invasion by inducing membrane-type matrix metalloproteinase-1 and cyclooxygenase-2 expression. J. Clin. Invest. 2002;110:1831–1838. doi: 10.1172/JCI200215887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence T., Willoughby D.A., Gilroy D.W. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat. Rev., Immunol. 2002;2:787–795. doi: 10.1038/nri915. [DOI] [PubMed] [Google Scholar]
- Lee Y.R., Su C.Y., Chow N.H., Lai W.W., Lei H.Y., Chang C.L., Chang T.Y., Chen S.H., Lin Y.S., Yeh T.M., Liu H.S. Dengue viruses can infect human primary lung epithelia as well as lung carcinoma cells, and can also induce the secretion of IL-6 and RANTES. Virus Res. 2007;126:216–225. doi: 10.1016/j.virusres.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Liao C.L., Lin Y.L., Wu B.C., Tsao C.H., Wang M.C., Liu C.I., Huang Y.L., Chen J.H., Wang J.P., Chen L.K. Salicylates inhibit flavivirus replication independently of blocking nuclear factor kappa B activation. J. Virol. 2001;75:7828–7839. doi: 10.1128/JVI.75.17.7828-7839.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.F., Wan S.W., Cheng H.J., Lei H.Y., Lin Y.S. Autoimmune pathogenesis in dengue virus infection. Viral Immunol. 2006;19:127–132. doi: 10.1089/vim.2006.19.127. [DOI] [PubMed] [Google Scholar]
- Liu T., Zaman W., Kaphalia B.S., Ansari G.A., Garofalo R.P., Casola A. RSV-induced prostaglandin E2 production occurs via cPLA2 activation: role in viral replication. Virology. 2005;343:12–24. doi: 10.1016/j.virol.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Liu Q., Chen T., Chen G., Li N., Wang J., Ma P., Cao X. Immunosuppressant triptolide inhibits dendritic cell-mediated chemoattraction of neutrophils and T cells through inhibiting Stat3 phosphorylation and NF-kappaB activation. Biochem. Biophys. Res. Commun. 2006;345:1122–1130. doi: 10.1016/j.bbrc.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Liu Q., Chen T., Chen G., Shu X., Sun A., Ma P., Lu L., Cao X. Triptolide impairs dendritic cell migration by inhibiting CCR7 and COX-2 expression through PI3-K/Akt and NF-kappaB pathways. Mol. Immunol. 2007;44:2686–2696. doi: 10.1016/j.molimm.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Marcet B., Libert F., Boeynaems J.M., Communi D. Extracellular nucleotides induce COX-2 up-regulation and prostaglandin E2 production in human A549 alveolar type II epithelial cells. Eur. J. Pharmacol. 2007;566:167–171. doi: 10.1016/j.ejphar.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Martin K.W., Ernst E. Antiviral agents from plants and herbs: a systematic review. Antivir. Ther. 2003;8:77–90. [PubMed] [Google Scholar]
- Moriuchi M., Inoue H., Moriuchi H. Reciprocal interactions between human T-lymphotropic virus type 1 and prostaglandins: implications for viral transmission. J. Virol. 2001;75:192–198. doi: 10.1128/JVI.75.1.192-198.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang L., Hoult J.R. Cytotoxicity to macrophages of tetrandrine, an antisilicosis alkaloid, accompanied by an overproduction of prostaglandins. Biochem. Pharmacol. 1997;53:773–782. doi: 10.1016/s0006-2952(96)00817-9. [DOI] [PubMed] [Google Scholar]
- Park G.Y., Christman J.W. Involvement of cyclooxygenase-2 and prostaglandins in the molecular pathogenesis of inflammatory lung diseases. Am. J. Physiol., Lung Cell. Mol. Physiol. 2006;290:L797–L805. doi: 10.1152/ajplung.00513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps R.P., Stein S.H., Roper R.L. A new view of prostaglandin E regulation of the immune response. Immunol. Today. 1991;12:349–352. doi: 10.1016/0167-5699(91)90064-Z. [DOI] [PubMed] [Google Scholar]
- Primache V., Binda S., De Benedittis G., Barbi M. In vitro activity of acetylsalicylic acid on replication of varicella-zoster virus. New Microbiol. 1998;21:397–401. [PubMed] [Google Scholar]
- Qiu D., Zhao G., Aoki Y., Shi L., Uyei A., Nazarian S., Ng J.C., Kao P.N. Immunosuppressant PG490 (triptolide) inhibits T-cell interleukin-2 expression at the level of purine-box/nuclear factor of activated T-cells and NF-kappaB transcriptional activation. J. Biol. Chem. 1999;274:13443–13450. doi: 10.1074/jbc.274.19.13443. [DOI] [PubMed] [Google Scholar]
- Raaben M., Einerhand A.W., Taminiau L.J., van Houdt M., Bouma J., Raatgeep R.H., Buller H.A., de Haan C.A., Rossen J.W. Cyclooxygenase activity is important for efficient replication of mouse hepatitis virus at an early stage of infection. Virol. J. 2007;4:55. doi: 10.1186/1743-422X-4-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray N., Bisher M.E., Enquist L.W. Cyclooxygenase-1 and -2 are required for production of infectious pseudorabies virus. J. Virol. 2004;78:12964–12974. doi: 10.1128/JVI.78.23.12964-12974.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A.E., Enquist L.W. Biological interactions between herpesviruses and cyclooxygenase enzymes. Rev. Med. Virol. 2006;16:393–403. doi: 10.1002/rmv.519. [DOI] [PubMed] [Google Scholar]
- Richardson J.Y., Ottolini M.G., Pletneva L., Boukhvalova M., Zhang S., Vogel S.N., Prince G.A., Blanco J.C. Respiratory syncytial virus (RSV) infection induces cyclooxygenase 2: a potential target for RSV therapy. J. Immunol. 2005;174:4356–4364. doi: 10.4049/jimmunol.174.7.4356. [DOI] [PubMed] [Google Scholar]
- Rocca B., FitzGerald G.A. Cyclooxygenases and prostaglandins: shaping up the immune response. Int. Immunopharmacol. 2002;2:603–630. doi: 10.1016/s1567-5769(01)00204-1. [DOI] [PubMed] [Google Scholar]
- Rossen J.W., Bouma J., Raatgeep R.H., Buller H.A., Einerhand A.W. Inhibition of cyclooxygenase activity reduces rotavirus infection at a postbinding step. J. Virol. 2004;78:9721–9730. doi: 10.1128/JVI.78.18.9721-9730.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowska B., Barrucco R., Khalili K., Safak M. Regulation of human polyomavirus JC virus gene transcription by AP-1 in glial cells. J. Virol. 2003;77:665–672. doi: 10.1128/JVI.77.1.665-672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro M.G., Rossi A., Amici C. NF-kappaB and virus infection: who controls whom. EMBO J. 2003;22:2552–2560. doi: 10.1093/emboj/cdg267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar F.H., Adsule S., Li Y., Padhye S. Back to the future: COX-2 inhibitors for chemoprevention and cancer therapy. Mini Rev. Med. Chem. 2007;7:599–608. doi: 10.2174/138955707780859431. [DOI] [PubMed] [Google Scholar]
- Savard M., Belanger C., Tremblay M.J., Dumais N., Flamand L., Borgeat P., Gosselin J. EBV suppresses prostaglandin E2 biosynthesis in human monocytes. J. Immunol. 2000;164:6467–6473. doi: 10.4049/jimmunol.164.12.6467. [DOI] [PubMed] [Google Scholar]
- Sharma-Walia N., Raghu H., Sadagopan S., Sivakumar R., Veettil M.V., Naranatt P.P., Smith M.M., Chandran B. Cyclooxygenase 2 induced by Kaposi's sarcoma-associated herpesvirus early during in vitro infection of target cells plays a role in the maintenance of latent viral gene expression. J. Virol. 2006;80:6534–6552. doi: 10.1128/JVI.00231-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speir E., Yu Z.X., Ferrans V.J., Huang E.S., Epstein S.E. Aspirin attenuates cytomegalovirus infectivity and gene expression mediated by cyclooxygenase-2 in coronary artery smooth muscle cells. Circ. Res. 1998;83:210–216. doi: 10.1161/01.res.83.2.210. [DOI] [PubMed] [Google Scholar]
- Steer S.A., Corbett J.A. The role and regulation of COX-2 during viral infection. Viral Immunol. 2003;16:447–460. doi: 10.1089/088282403771926283. [DOI] [PubMed] [Google Scholar]
- Tao X., Schulze-Koops H., Ma L., Cai J., Mao Y., Lipsky P.E. Effects of Tripterygium wilfordii hook F extracts on induction of cyclooxygenase 2 activity and prostaglandin E2 production. Arthritis Rheum. 1998;41:130–138. doi: 10.1002/1529-0131(199801)41:1<130::AID-ART16>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Tsatsanis C., Androulidaki A., Venihaki M., Margioris A.N. Signalling networks regulating cyclooxygenase-2. Int. J. Biochem. Cell Biol. 2006;38:1654–1661. doi: 10.1016/j.biocel.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Wachsman M., Aurelian L., Burnett J.W. The prophylactic use of cyclooxygenase inhibitors in recurrent herpes simplex infections. Br. J. Dermatol. 1990;123:375–380. doi: 10.1111/j.1365-2133.1990.tb06298.x. [DOI] [PubMed] [Google Scholar]
- Waris G., Siddiqui A. Hepatitis C virus stimulates the expression of cyclooxygenase-2 via oxidative stress: role of prostaglandin E2 in RNA replication. J. Virol. 2005;79:9725–9734. doi: 10.1128/JVI.79.15.9725-9734.2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Watkins D.N., Peroni D.J., Lenzo J.C., Knight D.A., Garlepp M.J., Thompson P.J. Expression and localization of COX-2 in human airways and cultured airway epithelial cells. Eur. Respir. J. 1999;13:999–1007. doi: 10.1034/j.1399-3003.1999.13e12.x. [DOI] [PubMed] [Google Scholar]
- Williams C.S., Mann M., DuBois R.N. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18:7908–7916. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- Wu S.J., Ng L.T. Tetrandrine inhibits proinflammatory cytokines, iNOS and COX-2 expression in human monocytic cells. Biol. Pharm. Bull. 2007;30:59–62. doi: 10.1248/bpb.30.59. [DOI] [PubMed] [Google Scholar]
- Yang S.P., Ho L.J., Lin Y.L., Cheng S.M., Tsao T.P., Chang D.M., Hsu Y.L., Shih C.Y., Juan T.Y., Lai J.H. Carvedilol, a new antioxidative beta-blocker, blocks in vitro human peripheral blood T cell activation by downregulating NF-kappaB activity. Cardiovasc. Res. 2003;59:776–787. doi: 10.1016/s0008-6363(03)00459-0. [DOI] [PubMed] [Google Scholar]
- Zhang H., Li Y.Y., Wu X.Z. Effect of tetrandrine on LPS-induced NF-kappaB activation in isolated pancreatic acinar cells of rat. World J. Gastroenterol. 2006;12:4232–4236. doi: 10.3748/wjg.v12.i26.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong B., Tien P., Shu H.B. Innate immune responses: crosstalk of signaling and regulation of gene transcription. Virology. 2006;352:14–21. doi: 10.1016/j.virol.2006.04.029. [DOI] [PubMed] [Google Scholar]