Abstract
Accumulating evidence suggests that oxidative stress plays a major role in the pathogenesis of multiple sclerosis (MS). Reactive oxygen species (ROS), which if produced in excess lead to oxidative stress, have been implicated as mediators of demyelination and axonal damage in both MS and its animal models. One of the most studied cell populations in the context of ROS-mediated tissue damage in MS are macrophages and their CNS companion, microglia cells. However, and this aspect is less well appreciated, the extracellular and intracellular redox milieu is integral to many processes underlying T cell activation, proliferation and apoptosis. In this review article we discuss how oxidative stress affects central as well as peripheral aspects of MS and how manipulation of ROS pathways can potentially affect the course of the disease. It is our strong belief that the well-directed shaping of ROS pathways has the potential to ameliorate disease progression in MS.
Highlights
-
•
Reactive oxygen species play a major role in the pathogenesis of multiple sclerosis and contribute to demyelination in the CNS.
-
•
Redox states influence T cells, which are activated in the periphery and are strongly associated with MS pathogenesis.
-
•
We summarize central and peripheral mode of actions of ROS in MS.
-
•
We discuss treatment options, which target oxidative stress pathways, with regard to central and peripheral effects.
1. Introduction to the disease
1.1. General remarks
Multiple sclerosis (MS) is the most frequent neurological disease in young adults with a complex and still uncertain pathogenesis. The most widely accepted hypothesis is that auto-reactive T cells and B-cells induce myelin damage, neuroinflammation and neurodegeneration (Compston and Coles, 2008, Fletcher et al., 2010, Trapp and Nave, 2008). However, primary oligodendrocyte dysfunction has also been considered as a potential disease-promoting or disease-triggering factor (Barnett and Prineas, 2004). Whatever the trigger factors for lesion formation in MS are, we now know that both, central and peripheral cellular components are critically involved in the disease process. Despite being of unknown etiology, the (histo-) pathological hallmarks of MS lesions are well-defined. They include focal as well as diffuse demyelination, oligodendrocyte loss, activation of brain resident immune cells such as microglia and astrocytes, and damage of the neuro-axonal unit. Such cellular alterations can be found in various brain regions including diverse white and gray matter areas (Bo et al., 2006, Kipp and Amor, 2012). The fast activation of brain intrinsic cells, in particular microglia followed by the activation of astrocytes, is most frequently linked to the expression and release of oxidative-stress related molecules.
In this review article we first give a brief definition of “oxidative stress” and “reactive oxygen species (ROS)” and then describe the pathways and factors involved in this ubiquitous cellular state. Since MS pathogenesis is characterized by the interplay of central and peripheral cellular elements, we then go on to explain how both compartments are regulated by ROS. Finally, we will argue that currently approved treatment options, most importantly Fumaric acid esters (FAEs), interfere with oxidative stress pathways and by this mechanism exert their beneficial function. However, modulation of central and peripheral ROS pathways might result in side effects.
2. Reactive oxygen species and oxidative stress
2.1. Definition of oxidative stress and ROS
Oxygen is pivotal for multicellular life. At the same time, it is one of the most reactive and life-threatening agents known. However, at least for aerobic organisms, oxidation has become the main means of energy generation. To guard against the possible deleterious effects of oxygen, intracellular homeostasis is maintained by a balancing of oxidation and reduction (redox) reactions, the so-called “intracellular redox equilibrium”. In extreme cases, when metabolic processes or toxic insults lead to a situation where pro-oxidants outbalance the anti-oxidative counterparts, a state of “oxidative stress” is reached. This breakdown of cellular homeostasis results in oxidation-induced damage to lipids, proteins, carbohydrates and nucleic acids, eventually leading to cell death.
The agents inducing oxidative stress are chemical compounds classed as reactive oxygen species (ROS) or reactive nitrogen species (RNS). ROS/RNS are both instable, and mostly exist in a radical form, which means that they contain unpaired electrons on the outer orbital. The best-studied ROS/RNS include radicals of oxygen [superoxide anion (O2 −), hydroxyl radicals (OH.), and peroxyradicals (ROO•)] or nitrogen [nitric oxide (NO.)] as well as non-radical species, such as hydrogen peroxide (H2O2) and singlet oxygen. Nitric oxide, itself less reactive and generally non-damaging, can rapidly react with a superoxide anion to form peroxinitrate (ONOO−), one of the most deleterious ROS/RNS known. ROS and RNS have long been implicated in the pathogenesis of a plethora of diseases such as stroke, Parkinson's disease or Alzheimer's disease (Lin and Beal, 2006). On the other hand, and this aspect of ROS/RNS has been less well studied, low levels of ROS/RNS can act as second messengers for signal transduction/amplification and fulfill specific intracellular functions (Reth, 2002). Key transcription factors regulated by ROS include p53, AP-1 (c-Jun, c-Fos), NF-ĸB and, as discussed in this review article, the transcription factor Nrf2.
2.2. Cellular ROS-defense mechanisms
All cells are equipped with an intrinsic mechanism that neutralizes excess ROS and protects against oxidative injury. This so-called oxidative stress response is mainly, but not exclusively, controlled by the transcription factor nuclear factor (erythroid-derived 2)-like 2 (Nrf2). Nrf2 plays a vital role in maintaining cellular homeostasis, especially upon exposure of cells to chemical or oxidative stress, through its ability to regulate basal and inducible expression of a multitude of antioxidant proteins, detoxification enzymes and xenobiotic transporters (Kensler et al., 2007). In anti-oxidative stress responses, Nrf2 upregulates phase II detoxifying enzymes and antioxidant proteins. This Nrf2-induced enzymatic machinery includes enzymes mediating glutathione (GSH) synthesis, the thioredoxin (Trx) enzyme system and detoxifying enzymes like heme oxygenases (HO), or NAD(P)H: quinone oxidoreductase 1 (NQO1).
How do these components protect the cell? Reduced GSH acts by scavenging oxidative species such as superoxides, hydroxyl radicals, nitrogens, and ONOO (Forman et al., 2009). The crucial cysteine molecule is the key to the protection afforded by GSH. Its sulfur atom scavenges destructive molecules (peroxides and free radicals) converting them to harmless compounds, such as water. Trx plays an important role in maintaining a reduced environment in the cells through thiol-disulfide exchange reactions and, thus, protects cells and tissues from oxidative stress. NQO and HO are important as catalysts of heme and quinone degradation. Free heme is liberated under oxidative conditions and mediates ROS production. Quinones are highly redox active and also lead to formation of ROS. Since NQO and HO eliminate heme and quinone, they can exert an anti-oxidative function. Besides the induction of anti-oxidative factors, Nrf2 also contributes to different cellular functions such as differentiation, proliferation, inflammation and lipid synthesis, and there is increasing evidence of an association between aberrant expression and/or malfunctioning of Nrf2 and diverse pathologies including cancer, neurodegeneration or cardiovascular disease.
2.3. The Nrf2–Keap1–ARE pathway and its relevance for inflammation and degeneration
Having outlined the protective potential of the transcription-factor Nrf2, we will now describe its mode of action. The Nrf2 cell defense pathway is tightly regulated. Under quiescent conditions, Nrf2 is retained and degraded in the cytosol by Kelch ECH associating protein 1 (Keap1) (Zhang and Hannink, 2003) (Fig. 1 ). Various stress-associated stimuli, such as oxidative stress, induce conformational changes in Keap1 that result in the release of Nrf2 from its Keap1-binding. Subsequently, Nrf2 trans-locates into the nucleus where it trans-activates the expression of genes containing an antioxidant response element (ARE) in their promoter regions (Kensler et al., 2007). Although it is well established that Nrf2 activity is controlled, in part, by the cytosolic protein Keap1, the nature of this pathway and the mechanisms by which Keap1 acts to repress Nrf2 activity remain to be fully characterized (Nguyen et al., 2009).
Fig. 1.

Scheme of Nrf2 activation. Under basal conditions, Nrf2 interacts with Keap1, which results in degradation of Nrf2. In response to cellular stress, Nrf2 is liberated from its cytosolic inhibitor, trans-locates into the nucleus and binds to antioxidant response elements (AREs) in the promoters of target genes. Nrf2-regulated genes mainly include genes coding for antioxidative and detoxifying enzymes.
Whatever mechanisms are involved in Nrf2 liberation and subsequent ARE-binding, activation of this promoter region results in increased expression of cytoprotective genes. The first of these to be described under the regulation of ARE-activity were the two major detoxification enzymes, GSTA2 (glutathione S-transferase A2) and NQO1 (Favreau and Pickett, 1991, Friling et al., 1990). Thus, alteration of the cellular redox status due to elevated levels of ROS and electrophilic species and/or a reduced antioxidant capacity (e.g. GSH) appears to be an important signal for triggering the transcriptional response mediated by ARE. Besides its critical role in inducible gene expression, the ARE site is also pivotal for the low-level constitutive (or basal) expression of several genes under physiological conditions. Because ROS and other endogenous reactive molecules are continuously generated by normal (i.e. physiological) aerobic metabolism, ARE appears to be pivotal for the maintenance of cellular redox homeostasis under both stressed and non-stressed conditions.
Since inflammation is closely linked with oxidative stress, it is hardly surprising that Nrf2 deficient mice have a worse disease outcome in several inflammation-mediated animal models, including experimental asthma (Rangasamy et al., 2005), acute lung injury (Reddy et al., 2009), sepsis (Thimmulappa et al., 2006), T cell-mediated hepatitis (Osburn et al., 2008), or dextran sulfate sodium-induced colitis (Khor et al., 2006). Interestingly, late adult Nrf2−/− female mice are prone to develop autoimmune syndromes that closely resemble the human disorder systemic lupus erythematosus (Ma et al., 2006). T cells contribute to appearance and progression of inflammation and are associated with the above-mentioned disease models. The possible interaction of ROS–Nrf2 and T-cell priming and effector function will be discussed later in this review article.
Besides being expressed in peripheral organ systems, Nrf2 is recognized as an important regulator of inflammation and cell death in the brain. Findings highlighting the relevance of the Nrf2–Keap1–ARE system for neurodegenerative and neuroinflammatory diseases include decreased nuclear Nrf2-levels in the hippocampus of Alzheimer's disease patients, increased Nrf2 nuclear translocation in Parkinson's disease (Ramsey et al., 2007), a prominent decrease in GSH levels in the substantia nigra of Parkinson's disease patients (Sian et al., 1994), and lower Nrf2 paralleled by higher Keap1 levels in amyotrophic lateral sclerosis vs. control samples (Sarlette et al., 2008). Taken together, although only a few studies have been conducted in humans, these do indicate that the Nrf2 system is dysregulated in brains of individuals suffering from neurodegenerative diseases and that this dysregulation may well contribute to chronic neuron degeneration in these disorders. Besides its likely impact on pathological progression in classical neurodegenerative disorders, the Nrf2–Keap1–ARE system appears at the same time to be a potent regulator of neuroinflammatory diseases. In Nrf2-deficient mice, the inflammatory response in the brain and magnitude of microglia activation in response to lipopolysaccharide is much more pronounced than in normal animals (Innamorato et al., 2008), and treatment with sulforaphane, a potent Nrf2-inducing agent, was found to be effective in reducing neurotoxicity associated with herpes simplex virus-stimulated microglial ROS production (Schachtele et al., 2012). Furthermore, Nrf2 deficient mice also show a more severe pathology in experimental autoimmune encephalomyelitis (EAE) (Johnson et al., 2010), the most widely used autoimmune-related MS animal model.
3. The current views of the contribution of ROS to MS lesion formation and progression
Although the pathogenesis of MS lesion development is complex and involves the activation of both central and peripheral elements of the immune system, adaptive immune-responses undoubtedly play an important role. Dysregulation of various different cell types of the adaptive immune system appears to contribute to lesion formation and progression, as shown for Th1 and Th2 (Hermans et al., 1997), Th17-cells (Tzartos et al., 2008), regulatory T (Treg) cells (Chen et al., 1994, Viglietta et al., 2004), B-cells (Sekizawa et al., 1974), and myeloid-derived suppressor cells (MDSCs) (Zhang et al., 2015). The later constitute a very heterogeneous and plastic cell population that consists of myeloid progenitor cells and immature myeloid cells.
The most popular concept of MS lesion formation is that acute demyelinating lesions are generated by phagocytes that internalize and degrade apparently normal myelin sheaths in the presence of infiltrating T cells. Immune cell recruitment is an early or even initial event in the formation of MS lesions in this model (Frischer et al., 2015, Lucchinetti et al., 2000). In sharp contrast to this idea, results of other studies suggest that extensive oligodendrocyte apoptosis with early, focal microglia activation is the major pathological feature in newly forming lesions, and immune cell recruitment is a response to this primary oligodendrocyte pathology (Barnett and Prineas, 2004, Stys et al., 2012). Whether or not the initial event of MS lesion formation is recruitment of auto-reactive T cells across the blood brain barrier into the brain parenchyma, without doubt the inflammatory process involves the activation and recruitment of T cells, macrophages and microglia to lesion sites. Once active demyelination is established, peripheral immune cells (such as lymphocyte, recruited monocytes, MDSC) and their central counterparts (astrocytes and microglia) contribute to progressive tissue damage in MS.
These inflammatory processes critically involve ROS-mediated tissue injury. Activated microglia and infiltrated macrophages are able to generate vast amounts of proinflammatory mediators and oxidizing radicals, such as superoxide, hydroxyl radicals, hydrogen peroxide and nitric oxide (Colton and Gilbert, 1993). Furthermore, the activation of immature myeloid cells (i.e. MDSCs) has been linked to the induction of NO and ROS production (Zhang et al., 2015).
Most studies addressing the relevance of oxidative stress for MS lesion formation and progression have focused on brain intrinsic cells and recruited monocytes. For example, it has been demonstrated that in white and gray matter lesions myeloperoxidase, a lysosomal enzyme which produces hypochlorous acid from hydrogen peroxide and chloride anion, is predominantly expressed by macrophages and/or activated microglia (Gray et al., 2008a, Gray et al., 2008b), emphasizing the key role of these myeloid cells in the generation of ROS. Further results from autopsy studies showed that in active lesions of the white matter and cerebral cortex, demyelination and neurodegeneration are closely associated with the presence of oxidized lipids (such as oxidized phospholipids and malondialdehyde) in myelin membranes, in apoptotic oligodendrocytes (Haider et al., 2011) and in the neuro-axonal compartment (Fischer et al., 2013, Haider et al., 2011). Furthermore, nuclei of dystrophic glia cells and neurons were found to contain oxidized DNA (Haider et al., 2011), and oxidative injury was associated with inflammation and oxidative burst in activated microglia and macrophages expressing p22phox, an essential subunit of NADPH oxidases (Fischer et al., 2012, Fischer et al., 2013). Although this situation appears to be only partly reflected in relevant MS animal models (Schuh et al., 2014), oxidative injury also takes place there. Macrophages and microglial cells, isolated from the CNS of Lewis rats with clinical signs of EAE, exhibited significantly elevated spontaneous and inducible ROS levels compared to similar cells isolated from healthy controls, or rats sacrificed before manifestation of clinical signs of EAE (Ruuls et al., 1995). From a functional point of view, treatment of EAE-rats with catalase, which scavenges the ROS H2O2, markedly suppressed the severity of the disease. Beyond that, the ROS protectant heme oxygenase-1 (HO-1) is expressed by monocytes in EAE (Schluesener and Seid, 2000), ROS aid phagocytosis of myelin by activated macrophages (van der Goes et al., 1998), and stabilization of mitochondria (which are a major source of ROS) ameliorates axonal damage in EAE (Forte et al., 2007, Qi et al., 2007). Although the EAE animal model is a heterogeneous group of experimental tolls to study MS pathogenesis (van der Star et al., 2012), these results foster the view that ROS are critically involved in autoimmune-mediated tissue damage in MS.
ROS accumulation is also evident in other in vivo demyelinating models such as in corona-virus-induced inflammatory demyelination (Schuh et al., 2014). Furthermore, our group observed strong induction of HO-1 after short-term cuprizone exposure (own observation, unpublished). Taken together, there is ample evidence that ROS actively contribute to tissue damage during MS lesion development and progression, and that the activation of the Nrf2 pathway might play a protective role in the pathogenesis of MS by operating on levels of certain enzymes. Such effects might include the induction of several antioxidant enzymes that can directly scavenge ROS, increasing levels of antioxidant enzymes that might reduce microglial activation and limit myelin phagocytosis and breakdown, and induction of antioxidant enzymes that might prevent oxidative damage to neurons and oligodendrocytes. However, as we will point out in the next chapter, ROS are also potent regulators of the adaptive immune-response. The functionality of ROS in MS patients should therefore be reflected in both compartments.
4. Molecular insight into the adaptive immune response
Cells belonging to the adaptive immune response, and in particular CD4+ T helper (Th) cells, play an important role in the pathogenesis of MS lesions. T cell activation relies on the binding between T cell receptors (TCRs) and antigens, which are typically short peptides presented by MHC molecules that are displayed on the surface of antigen-presenting cells (APCs), including macrophages, B cells and dendritic cells (DCs), as the most “professional” APC populations. In addition, T cell activation requires a second signal from co-stimulatory molecules (CD80/CD86). Stimulation of T cells through the TCR and co-receptor CD28 (CD80/86 ligand) induces transcriptional programs – including activation of NF-ĸB – which initiate the production of cytokines such as IL-2 that in turn are important for T cell proliferation and activation. Cytokines are also key regulators of T cell differentiation towards one of several Th cell subtypes, including Th1, Th2, Th17 and inducible Treg cells. Interleukin (IL)-12 and IFN-γ are two important cytokines for Th1 differentiation, while IL-4, IL-2, IL-7 and thymic stromal lymphopoietin drive Th2 differentiation. Transforming growth factor (TGF) β induces Th17 differentiation in the presence of IL-6, IL-21 and IL-23, while TGF-β in the presence of IL-2 induces Treg cells (rev. in Zhu and Paul (2010)). Once differentiated, Th cell subtypes are further defined by their pattern of cytokine production and by their distinct functions. Th1 cells produce interferon (IFN)-γ and are important for protective immune responses to intracellular viral and bacterial infections. In contrast, Th2 cells are critical for clearance of extracellular parasites, whereas Th17 cells play an important role in protection from bacterial and fungal infection. Beyond their central role in adaptive immune responses against pathogens, Th cells, especially through their autoreactive or exaggerated responses, are also involved in autoimmune reactions. Until recently Th1 cells were thought to be the main effector T cell in MS, but more recent studies have highlighted an additional pathogenic role for Th17 cells (Fletcher et al., 2010). Autoreactive T cells are mostly deleted in the thymus, but some of them escape this so-called central tolerance. Consequently, several mechanisms evolved to control autoreactive Th cells in the periphery (peripheral tolerance). Dominant tolerance by Treg cells is one strategy to prevent autoimmune disease and maintain immune homeostasis by suppressing autoreactive and exaggerated T cell responses (Campbell and Koch, 2011). Treg cells are categorized as thymus-derived (tTreg cells) or induced (iTreg cells). As the name implies, tTreg cells develop in the thymus, whereas iTreg cells differentiate from naive T-cell precursors in the periphery. Both Treg-cell types express FoxP3, the lineage-specific and most important transcription factor for maintenance of the Treg cell phenotype and suppressor function (Fontenot et al., 2003, Hori et al., 2003).
With regard to MS and EAE, an imbalance of pro-inflammatory responses such as Th1 and Th17 and anti-inflammatory responses mediated by Treg or Th2 cells appears to be crucial for disease development and progression (Fletcher et al., 2010) (Fig. 2 ). Th1 and Th17 can be found in MS lesions (Lock et al., 2002, Tzartos et al., 2008) where they initiate and exacerbate an inflammatory cascade by the release of cytokines and recruitment of further inflammatory immune cells. Treg cells, which one would expect to control these exaggerated Th1 and Th17 responses, are characterized by several aberrancies in MS. Although there are no numerical deficits in Treg cells in MS, the suppressive function of Treg cells appears to be disturbed (Frisullo et al., 2009, Haas et al., 2005, Venken et al., 2008, Viglietta et al., 2004). Furthermore, Treg cells reveal an impaired capacity to proliferate in relapsing-remitting MS (RRMS), and express reduced levels of Foxp3, which is critical for maintaining function and lineage stability (Carbone et al., 2014, Huan et al., 2005). Recent reports also raise the question of whether defects in Treg function are caused by an enhanced plasticity of Tregs towards a proinflammatory, cytokine-producing effector phenotype. Patients with untreated RRMS have higher frequencies of Th1-like, IFN-γ-secreting Foxp3+ T cells, with a reduced suppressive capacity (Dominguez-Villar et al., 2011). A shift of Treg cells towards IL-17 producing cells is associated with psoriasis, autoimmune hepatitis and systemic sclerosis, but so far no direct association has been detected in MS.
Fig. 2.
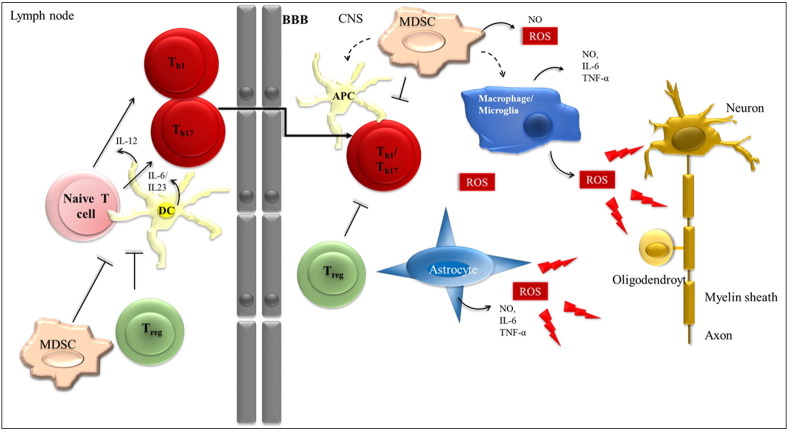
Treg cells fail to control effector T cells in the periphery and in the CNS under auto-immune conditions. In peripheral lymph nodes, DCs activate T cells and induce their differentiation towards inflammatory Th1 and Th17 cells by the release of cytokines, like IL-6 and IL23 (Th17) and IL-12 (Th1). In MS, Treg cells eventually fail to control this T cell activation process. Activated T cells migrate to and enter the CNS where they become reactivated by local APCs, and again are not adequately suppressed by Treg cells. Teff cells then expand and drive CNS inflammation. After having successfully entered the CNS, T cells are exposed to a completely novel oxidative milieu. There, ROS molecules are mainly produced by macrophages, microglia and astrocytes and lead to damage of neurons, axons, myelin and oligodendrocytes (indicated by arrows). MDSCs can suppress activated T cells, but can also differentiate into dendritic cells and macrophages in the CNS and influence the entire scenario.
In vivo studies from rodent EAE models demonstrate the central function of Treg cells in autoimmune neuroinflammation. Transfer of Treg cells ameliorates EAE symptoms (Kohm et al., 2002) and non-specific Treg cell ablation by anti-CD25 antibodies exacerbates EAE severity (Gartner et al., 2006). In addition, frequencies of Treg cell population within the CNS are elevated during the recovery phase of actively induced EAE (McGeachy et al., 2005).
Although the importance of Treg cells for MS disease development and progression is well appreciated, basic aspects of Treg cell biology remain unresolved. Whether Treg cells are suppressive at inflammatory sites or act predominantly to limit the priming of naive T cells in the lymph nodes (LNs) remains a controversial issue (Fig. 2). With respect to central effects, Treg cells isolated from the CNS during EAE were capable of suppressing Th1 but not Th17 cells (O'Connor et al., 2007) but were not able to suppress effector T cells directly isolated from the acutely inflamed CNS (Korn et al., 2007). With respect to peripheral effects, it has been shown that in the absence of Treg cells, there is an enhanced migration of effector T cells from the periphery (Lowther et al., 2013). Furthermore, Treg cells influence EAE course by affecting the priming and polarization of effector T cells (Kohm et al., 2002) and can also set a threshold for activation of autoreactive effector T cells by inhibiting their contacts with antigen-loaded dendritic cells (DCs) in the lymph nodes (Tadokoro et al., 2006). The interplay of local tissue inflammation and Treg cells is poorly understood but might be an important factor for the resolution of autoimmunity. In addition to Treg cells, innate immune cells are also capable to suppress T cell responses. For example, MDSCs have been described to suppress the activation of T cells, which makes them attractive targets for the treatment of autoimmune diseases. High accumulation of MDSCs in MS and EAE indicates that MDSCs play an important role (Zhang et al., 2015). However, beneficial (Moline-Velazquez et al., 2011) as well as pathogenic functions (Yi et al., 2012) of MDSCs in EAE are reported, therefore their exact role remains to be elucidated.
5. Relevance of ROS for lymphocyte polarization and effector function
Both, ROS and T-cells are important players in the cascade of MS pathogenesis. Do both interact, and if so, what is their site of “communication”?
Oxygen-derived reactive species have long been known to exert diverse effects on cultured mammalian cells, depending on the cell type considered, the oxidative stimulus, and the intensity and timing of stimulation. These effects are most frequently “positive”, or mitogenic, at low levels of oxidative stress and “negative”, i.e., toxic or growth-arresting, at greater oxidative burdens (Pani et al., 2000). In consequence, scavenging of endogenous oxygen species by either antioxidant enzymes or cell-permeant antioxidant and reducing agents would interfere with normal cell responses, attenuating or abolishing their effects. This general concept of ROS biology and pathology is also relevant to the immune system.
The concept that ROS are implicated in body defense is definitely not novel. The killing of bacteria through the generation of superoxide, H2O2, and hypochlorous acid by the coordinated action of NADPH oxidase and myeloperoxidase is a key event in the protective function of neutrophils and “professional” phagocytes (Pani et al., 2000). ROS have also been implicated in mechanisms of target cell killing by natural killer cells and in cytolysis by CD8+ lymphocytes. However, while well recognized as effectors of innate immunity, oxygen radicals are just starting to be considered as potential regulators of antigen-specific immunity and, by extension, of T-lymphocyte function.
Today we know that ROS and redox states are both central to immunity and T-cell function. First, and maybe most importantly, T-cells can sense redox stress (Secchi et al., 2015). Furthermore, redox stress is important for the functional outcome during experimentally-induced adaptive-immunity responses; e.g. in a graft-versus-host disease mouse model, free radical scavenger therapy with NecroX-7 attenuates disease severity, probably via the induction of Treg cells (Im et al., 2015) and oxidative damage regulates antigen-specific T cell responses in age-related macular degeneration (Cruz-Guilloty et al., 2014). In this context it is important to note that T-cell receptor activation results in intracellular ROS production (Devadas et al., 2002, Gutscher et al., 2008), and that the simultaneous action of the oxidative signal and Ca2+ influx is indispensable for T-cell activation-induced gene expression. Taken together, these findings illustrate that ROS and T-cell function are intimately linked.
Effects of ROS on adaptive immune responses probably depend on at least two variables, (i) lymphocyte subset and (ii) intra- or extracellular mode of actions. With respect to the first aspect it has been shown that distinct T-cell subsets have different redox statuses, and differential ROS susceptibility. Tregs, for example, are more resistant to oxidative stress-induced cell death compared to other T cell populations. This is paralleled by a greater secretion of redox proteins such as Trx. Furthermore, it has been shown that human Tregs express high levels of cell surface thiols, which are important reducing agents, and harbor an increased intracellular antioxidative capacity (Mougiakakos et al., 2009). Thus, ROS effects studied in one lymphocyte population do not necessarily exert the same effects in other ones (if applied at the same concentration).
Secondly, the intra- and extracellular modes of ROS action need to be studied separately. T cell receptor engagement triggers ROS production (Devadas et al., 2002) and these intracellularly produced ROS species might impact on T-cell function. Obviously, the intracellular redox state needs to be finely regulated, and a dysregulation of this dedicated network might impact on proper T-cell functioning. On a more detailed level, intracellular ROS play an important role in the immediate early events of T cell activation (Devadas et al., 2002, Los et al., 1995). However, under certain conditions the potentiation of oxidative stress by, for example, GSH depletion can result in impaired T cell activation (Rajah and Chow, 2014).
With regard to the extracellular mode of ROS action, ROS can act in mammalian cells as biochemical mediators involved in signal transduction from cell surface to the nucleus; implicated mechanisms are (i) induction of protein phosphorylation, and (ii) activation/inhibition of various redox-sensitive transcription factors, among them, Nrf2. The extracellular ROS milieu is indeed important for proper T-cell effector function, most notably by influencing the equilibrium between oxidized and reduced thiols on exofacial membrane proteins (Gelderman et al., 2006). Impaired functioning of the NADPH-oxidase complex, which results in lower ROS levels, is associated with an increased number of reduced thiol groups (− SH) on T cell membrane surfaces. This reducing extracellular milieu boosts T cell response and proliferation, thus showing that ROS production plays a key role in regulating surface redox levels on T cells and thereby suppresses autoreactivity. Among the APCs contributing to this reducing milieu, the action of DCs at the level of the immune synapse is of primary importance (Fig. 3C) (Angelini et al., 2002, Yan et al., 2009). In line with these findings, sustained pro-oxidant extracellular conditions have been shown to inhibit T cell activation (Matsue et al., 2003, Tse et al., 2007) and induce apoptosis in T cells (Hildeman et al., 1999, Tripathi and Hildeman, 2004) (Fig. 3B).
Fig. 3.
Redox regulation of T cell activation. (A) Induction of Treg cells by macrophages is dependent on ROS production (Kraaij et al., 2010). (B) Sustained pro-oxidant extracellular conditions inhibit T cell activation and induce apoptosis in T cells (Tripathi and Hildeman, 2004). (C) Interaction of DCs with T cells leads to cysteine accumulation in the extracellular space, which produces an extracellular redox potential promoting T cell proliferation (Angelini et al., 2002). (D) Treg cells inhibit dendritic cell induced extracellular reduced redox potential (Yan et al., 2010).
Impaired T-effector cell activation and reactivity in an oxidative environment might also be mediated by the suppressive action of Treg cells. For example, it has been shown that the induction of Treg cells by macrophages is dependent on production of ROS (Fig. 3A), and ROS deficiency may lead to decreased Treg induction and may hamper T-cell suppression (Kraaij et al., 2010). Furthermore, it was elegantly demonstrated that Treg cells disturb intracellular redox homeostasis in Teffs by interfering with extracellular redox remodeling ((Yan et al., 2010, Yan et al., 2009), Fig. 3D). In addition to that, MDSC-mediated suppression of T cell function involves ROS, NO and peroxynitrite, which induce post-translational modification of T-cell receptors and may thereby cause antigen-specific T-cell unresponsiveness (Gabrilovich and Nagaraj, 2009).
Notably, extracellular and intracellular ROS-related pathways are not completely separated. The antioxidant GSH serves as an important proliferative signal in T cells (Suthanthiran et al., 1990). Naive T cells require cysteine for GSH synthesis and activation, but are unable to import cystine, which can efficiently be converted to cysteine within the cell. They are thus, dependent on cysteine secretion by APCs. To make the situation even more complicated absolute ROS levels also appear to play an important role. Prolonged exposure to high ROS concentrations can inhibit T-cell proliferation and induce apoptosis, whereas low concentrations of ROS in T cells are a prerequisite for cell survival (Kesarwani et al., 2013).
In summary, ROS levels can impact on the acquired immune system via a variety of mechanisms and are thus critically involved in the development and progression of auto-immune disorders, including MS. Still, we are far away to completely understand this complicated scenario.
6. Impact of oxidative stress on MS — central and peripheral aspects
In the previous chapter we have seen that ROS levels and T cell functioning are intimately linked. Although somewhat neglected by the research community, it appears that oxidative stress not only regulates disease outcome in MS patients by acting within the CNS, but may impact on disease burden by shaping the immune response outside of the CNS.
Such a regulation might, for example, occur at the level of the endothelium. Endothelium cells, which are found at the interface between the CNS and periphery, are regulated in a MS-relevant manner by ROS. High ROS levels damage brain endothelium and affect blood–brain barrier (BBB) permeability (Imaizumi et al., 1996, Olesen, 1987). Thus, regulative effects of oxidative stress in MS probably include but are not limited to the CNS parenchyma. It may also regulate immune cell recruitment at the level of post-capillary venules.
An important question arising here is whether ROS can also regulate other peripheral aspects of the disease, namely, T effector cell polarization and proliferation. Results from other diseases strongly suggest that this is indeed the case. In many cases, mitochondrial disorders, which are closely linked to a disturbed oxidative environment, have hematological manifestations and result in recurrent infections. Complex I-deficient patients, in particular, present with a severely impaired immune response. They are prone to concomitant infections that inevitably lead to a worsening of symptoms or can even trigger primary occurrence of the disease. A close link between disturbed immune tolerance and mitochondrial ROS generation was found in systemic lupus erythematosus patients where T cells are characterized by a higher mitochondrial mass, persistently hyperpolarized mitochondria and elevated ROS levels (Gergely et al., 2002). In addition, T-cell mitochondria and presumably activation-induced mitochondrial ROS release play an important role in the pathology of the acquired-immunodeficiency syndrome (AIDS) (Kaminski et al., 2013). Thus, ROS and auto-immunity are indeed closely linked.
With respect to MS patients, a plethora of studies have analyzed changes in oxidative stress parameters. However, these studies were mainly conducted on serum and/or plasma samples (Fiorini et al., 2013), which somewhat limits their interpretation. In one study, urinary 8-iso-PGF2α levels, which reflect the degree of lipid peroxidation due to ongoing oxidative stress, were found to be significantly higher in MS subjects than in controls (Guan et al., 2015). Similar results have been reported for peripheral blood mononuclear cells (Wang et al., 2014). Other markers for oxidative stress that are found to be elevated in the serum and/or plasma of MS patients include a reduced serum ferroxidase activity, which can potentially lead to a rise in oxidative stress (Cervellati et al., 2014), lower antioxidant capacity (Karlik et al., 2015), and higher advanced oxidation protein products along with lower thiol group levels (Pasquali et al., 2015). Interestingly, MS patients with a favorable disease course show higher antioxidant factors, including CoQ10 and anti-OxLDL autoantibodies, which may shape the immune system in the periphery (Gironi et al., 2014). On the cellular level, ROS production by macrophages and lymphocytes was reported to be higher in MS vs. control patients, and decreased in peripheral blood mononuclear cells of IFNβ-1a-treated patients (Koch et al., 2008, Lucas et al., 2003). To conclude, these findings suggest a role of ROS in the peripheral component of MS lesion pathogenesis.
In summary, there is ample evidence for a critical function of redox homeostasis disruption in the pathogenesis of MS, and we suggest that this disrupted redox homeostasis is not limited to the brain but also occurs in the periphery in MS patients. The finding that peripheral immune cells withstand oxidative stress, due to upregulated cellular antioxidant defense mechanisms, suggests that ROS formation in MS is not necessarily deleterious but might form part of a finely tuned regulatory network. This delicate network could possibly be targeted by novel therapeutic interventions, as a supplement to established treatments.
7. Therapeutic manipulation of oxidative stress mechanisms in MS
A number of different therapeutic options are available to ameliorate disease activity in MS patients. More and more evidence suggests that currently approved drugs act both within the brain parenchyma and in the peripheral lymphoid organs. For example, the protective effects of the orally available sphingosine 1-phosphate (S1P) receptor modulator fingolimod (also known as FTY720 or Gilenya®), have for a long time been attributed to the hindrance of lymphocyte trafficking to target organs through lymphocyte sequestration in secondary lymphoid structures. This drug internalizes the S1P1-receptor subtype in T cells with subsequent degradation (i.e. acts as a functional antagonist at S1P1), preventing T-cell egress from lymphoid organs into the bloodstream, thus limiting the entry of pathogenic T cells into the CNS parenchyma. However, its efficacy in MS and related animal models may in part be due to additional, direct effects within the brain, and other S1P receptor subtypes appear to be involved (Kipp and Amor, 2012). In particular, it has been shown that due to its lipophilic character, fingolimod is able to access the CNS parenchyma through the blood–brain barrier. S1P receptors are expressed by neuroectodermal cell types such as oligodendrocytes, neurons or astrocytes, and may thus regulate neuron and/or glia cell morphology, migration, process extension, cell growth, differentiation, apoptosis and/or survival (Kipp and Amor, 2012). Furthermore, a direct neuroprotective effect of fingolimod has been demonstrated in recent preclinical studies (Slowik et al., 2015). Similarly, IFN-β has been shown to be a potent lymphocyte regulator (i.e. peripheral effect) but at the same time might interact with cells of the CNS (Esen et al., 2014, Vergara et al., 2010). Thus, the therapeutic efficacy of established interventions may originate in peripheral lymphoid organ but at the same time be manifested within the brain.
Dimethyl fumarate (DMF), marketed as Tecfidera® from Biogen Idec, has just recently been granted approval as an indicated treatment for MS by the US Food and Drug Administration (FDA). In the European Union, the medication received approval by the European Medicines Agency (EMA) in early 2013. DMF is a relatively simple molecule derived from fumaric acid. While fumaric acid itself is poorly absorbed by the gastrointestinal tract, its ester derivatives, monomethyl fumarate (MMF) and DMF, both proved beneficial in treating the skin disease psoriasis when administered either topically or orally (Altmeyer et al., 1994). In vitro studies have demonstrated that DMF is rapidly metabolized at the level of the gastrointestinal tract to its primary, active metabolite MMF by the abundant esterases present in the tissues.
The ultimate mechanism of action responsible for the positive treatment effects of FAEs is still under investigation. What has become clear so far is that, like other medications such as interferon or fingolimod, FAEs do not have a single mode of action but instead exert multitude biological effects. Immunomodulatory effects include a shift towards a Th2 response (de Jong et al., 1996, Litjens et al., 2004), and such a shift could result in decreased Th1 cell activation and tissue infiltration. This balance plays a key role in MS and a shift from Th1 towards a Th2 cytokine profile could have a beneficial effect on the clinical course of disease. However, it is not clear if these effects are indeed T cell intrinsic or mediated by APCs. FAEs also inhibit DC differentiation and reduce production of those cytokines that drive Th1 and Th17 cells (IL-12 and IL-6, IL-23) (Ghoreschi et al., 2011, Peng et al., 2012). With this regard, it might also be interesting to analyze if and how FAEs, which have been shown to inhibit dendritic cell maturation (Peng et al., 2012), regulate MDSC activation and/or differentiation into inflammatory APCs.
Furthermore, it has been reported that FAEs might enhance T cell apoptosis (Treumer et al., 2003), or might interfere with leucocyte migratory properties. In one study FAEs reduced the migratory activity of lymphocytes in vitro, most probably by changing their activation state, as no effect was seen on the expression of MMPs, chemokine receptors, and adhesion molecules (Dehmel et al., 2014). Furthermore, FAEs were shown to decrease the expression of adhesion molecules (CD25, HLA-DR) in lymphocytes in vitro (Rubant et al., 2008). Thus, FAE actions on lymphocytes are diverse.
With regard to oxidative stress in MS, another interesting FAE property emerging in preclinical trials was its ability to positively impact the natural anti-oxidative stress machinery of cells. As pointed out above, in resting states, Nrf2 is sequestered in the cytoplasm by Keap1. FAE has been shown to bind to Keap1 and enable the nuclear translocation of Nrf2, resulting in transcription of cytoprotective genes including hemoxygenase-1 (HMOX1) and NQO1 (Chen et al., 2014). In MOG-induced EAE, FAEs have been shown to destabilize the Nrf2-Keap1 complex, thus promoting anti-oxidative Nrf2 pathway activation (Linker et al., 2011). Interestingly, in vitro, MMF, the primary metabolite of DMF, protected cultured neurons and astrocytes from H2O2-induced cell death (Linker et al., 2011). Beyond, FAEs also suppressed nitrite production and inducible nitric oxide synthase (iNOS) levels in human astrocytes, where overproduction contributes to oligodendrocyte and neuronal damage (Lin et al., 2011). A decreased synthesis of the proinflammatory mediators iNOS, TNF-alpha, IL-1beta and IL-6 was also observed in activated FAE treated rat microglia and astrocytes in vitro (Wilms et al., 2010). These studies demonstrate that FAEs exert neuroprotective as well as anti-inflammatory effects. While the details of the interaction between FAEs and its target structures and cell types continue to be unveiled, further studies will be needed to show to what extent FAEs have a direct neuroprotective action, how immunosuppression is exerted, and whether Nrf2 is a critical link in these events.
As an inducer of Nrf2, FAEs clearly have the potential to become a classical neuroprotective compound. Several earlier studies elaborated on the possible neuroprotective effects of other inducers of Nrf2, including ceftriaxone (Lewerenz et al., 2009), sulforaphane (Danilov et al., 2009), and chitosan (Khodagholi et al., 2010). Furthermore, Nrf2-pathway activation by FAEs might manipulate the oxidative network in lymphocytes by favoring Th2 or Treg activation. Anti-inflammatory effects of Nrf2 activation and the proinflammatory effects of Nrf2 deletion have been demonstrated in a large number of studies with Nrf2 deficient (Nrf2−/−) mice, which also included T cell dependent models (as explained in detail above). Furthermore, T cell studies demonstrate that Nrf2 activation inhibits Th1 cytokine production, while concurrently promoting Th2 cytokine production (Rockwell et al., 2012). The role of Nrf2 signaling in Treg cells is less clear. The fact that Treg cells, for example, are more resistant to oxidative stress-induced cell death than conventional T cells (Mougiakakos et al., 2009), and that this increased resistance is associated with a greater expression of Nrf2 mediated genes (Mougiakakos et al., 2009, Pae et al., 2003), suggest that Nrf2 signaling critically influences Treg cells. In conjunction with these studies and the present data, we propose a dual mechanism of action for FAEs in MS: targeting immune cells on the one hand and CNS cells on the other (as summarized in Fig. 4 ). Further exploration of the mechanisms operating during immune effector cell priming will require the use of conditional, tissue-specific knock-out animals.
Fig. 4.
Immunomodulatory and neuroprotective effects of FAEs. FAEs activate the cellular stress response by activating Nrf2 and thereby protect neurons and oligodendroytes from oxidative injury (Linker et al., 2011). In addition, FAEs reduce secretion of proinflammatory cytokines by astrocytes and macrophages (Lin et al., 2011, Wilms et al., 2010). FAEs inhibit leukocyte migration and thereby infiltration of immune cells in the CNS (Dehmel et al., 2014, Rubant et al., 2008). In the periphery FAEs reduce DC maturation and release of DC cytokines driving a Th1/Th17 response (de Jong et al., 1996). FAEs might also directly mediate a shift towards Th2 instead of Th1 differentiation (Litjens et al., 2004). How FAEs affect MDSC function and/or differentiation towards inflammatory APCs remains to be elucidated.
8. Concluding remarks
As pointed out above, there is substantial evidence that ROS production is important for the regulation of surface redox levels of T cells and thereby suppresses auto-reactivity and auto-immune disease development. Thus, ROS can exert anti-inflammatory effects! In contrast, boosting of Nrf2-activity, which antagonizes ROS-mediated effects – at least in part – can as well ameliorate inflammation. It thus appears that ROS-mediated effects depend on the fine-tuning of a multi-cellular and multi-cascade network, and we are just beginning to understand these finely tuned events. Fumaric acid esters (i.e. DMF/MMF), which are prescribed for the treatment of psoriasis and MS, are considered to have a favorable risk profile. However, treatment-related progressive multifocal leukoencephalopathy (PML) has been reported in association with long-lasting, severe lymphocytopenia (Ermis et al., 2013, Nieuwkamp et al., 2015, Rosenkranz et al., 2015). Since the number of patients who are being treated with DMF has been rapidly increasing since approval of delayed-release DMF (Tecfidera) as a first-line treatment for RRMS, a better understanding is needed of the relevance of ROS to immune-cell function. There is no doubt that ROS-mediated pathways and cellular effects are involved in immune cell priming in the peripheral lymphoid organs. To exert their deleterious effects, Teff as well as Tmem cells travel through the circulation into the brain parenchyma where they are re-activated in MS by local APC such as microglia or macrophages. Since the oxidative brain environment is altered in MS patients, T-cell reactivation might be affected depending on the redox status in these tissues. For future studies, we recommend detailed examination of the interaction of recruited immune cells with brain resident cells such as microglia and astrocytes, as this would help to establish the circumstances under which T-cells can promote inflammatory cascades within the brain parenchyma and thus regulate the formation and progression of new MS lesions.
References
- Altmeyer P.J., Matthes U., Pawlak F., Hoffmann K., Frosch P.J., Ruppert P., Wassilew S.W., Horn T., Kreysel H.W., Lutz G. Antipsoriatic effect of fumaric acid derivatives. Results of a multicenter double-blind study in 100 patients. J. Am. Acad. Dermatol. 1994;30:977–981. doi: 10.1016/s0190-9622(94)70121-0. [DOI] [PubMed] [Google Scholar]
- Angelini G., Gardella S., Ardy M., Ciriolo M.R., Filomeni G., Di Trapani G., Clarke F., Sitia R., Rubartelli A. Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc. Natl. Acad. Sci. U. S. A. 2002;99:1491–1496. doi: 10.1073/pnas.022630299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett M.H., Prineas J.W. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann. Neurol. 2004;55:458–468. doi: 10.1002/ana.20016. [DOI] [PubMed] [Google Scholar]
- Bo L., Geurts J.J., Mork S.J., van der Valk P. Grey matter pathology in multiple sclerosis. Acta Neurol. Scand. Suppl. 2006;183:48–50. doi: 10.1111/j.1600-0404.2006.00615.x. [DOI] [PubMed] [Google Scholar]
- Campbell D.J., Koch M.A. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat. Rev. Immunol. 2011;11:119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone F., De Rosa V., Carrieri P.B., Montella S., Bruzzese D., Porcellini A., Procaccini C., La Cava A., Matarese G. Regulatory T cell proliferative potential is impaired in human autoimmune disease. Nat. Med. 2014;20:69–74. doi: 10.1038/nm.3411. [DOI] [PubMed] [Google Scholar]
- Cervellati C., Romani A., Fainardi E., Trentini A., Squerzanti M., Baldi E., Caniatti M.L., Granieri E., Bellini T., Castellazzi M. Serum ferroxidase activity in patients with multiple sclerosis: a pilot study. In Vivo. 2014;28:1197–1200. [PubMed] [Google Scholar]
- Chen Y., Kuchroo V.K., Inobe J., Hafler D.A., Weiner H.L. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science (New York, N.Y.) 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- Chen H., Assmann J.C., Krenz A., Rahman M., Grimm M., Karsten C.M., Kohl J., Offermanns S., Wettschureck N., Schwaninger M. Hydroxycarboxylic acid receptor 2 mediates dimethyl fumarate's protective effect in EAE. J. Clin. Invest. 2014;124:2188–2192. doi: 10.1172/JCI72151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton C.A., Gilbert D.L. Microglia, an in vivo source of reactive oxygen species in the brain. Adv. Neurol. 1993;59:321–326. [PubMed] [Google Scholar]
- Compston A., Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- Cruz-Guilloty F., Saeed A.M., Duffort S., Cano M., Ebrahimi K.B., Ballmick A., Tan Y., Wang H., Laird J.M., Salomon R.G., Handa J.T., Perez V.L. T cells and macrophages responding to oxidative damage cooperate in pathogenesis of a mouse model of age-related macular degeneration. PLoS One. 2014;9 doi: 10.1371/journal.pone.0088201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilov C.A., Chandrasekaran K., Racz J., Soane L., Zielke C., Fiskum G. Sulforaphane protects astrocytes against oxidative stress and delayed death caused by oxygen and glucose deprivation. Glia. 2009;57:645–656. doi: 10.1002/glia.20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong R., Bezemer A.C., Zomerdijk T.P., van de Pouw-Kraan T., Ottenhoff T.H., Nibbering P.H. Selective stimulation of T helper 2 cytokine responses by the anti-psoriasis agent monomethylfumarate. Eur. J. Immunol. 1996;26:2067–2074. doi: 10.1002/eji.1830260916. [DOI] [PubMed] [Google Scholar]
- Dehmel T., Dobert M., Pankratz S., Leussink V.I., Hartung H.P., Wiendl H., Kieseier B.C. Monomethylfumarate reduces in vitro migration of mononuclear cells. Neurol. Sci. 2014;35:1121–1125. doi: 10.1007/s10072-014-1663-2. [DOI] [PubMed] [Google Scholar]
- Devadas S., Zaritskaya L., Rhee S.G., Oberley L., Williams M.S. Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: selective regulation of mitogen-activated protein kinase activation and fas ligand expression. J. Exp. Med. 2002;195:59–70. doi: 10.1084/jem.20010659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Villar M., Baecher-Allan C.M., Hafler D.A. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat. Med. 2011;17:673–675. doi: 10.1038/nm.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermis U., Weis J., Schulz J.B. PML in a patient treated with fumaric acid. N. Engl. J. Med. 2013;368:1657–1658. doi: 10.1056/NEJMc1211805. [DOI] [PubMed] [Google Scholar]
- Esen N., Rainey-Barger E.K., Huber A.K., Blakely P.K., Irani D.N. Type-I interferons suppress microglial production of the lymphoid chemokine, CXCL13. Glia. 2014;62:1452–1462. doi: 10.1002/glia.22692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favreau L.V., Pickett C.B. Transcriptional regulation of the rat NAD(P)H:quinone reductase gene. Identification of regulatory elements controlling basal level expression and inducible expression by planar aromatic compounds and phenolic antioxidants. J. Biol. Chem. 1991;266:4556–4561. [PubMed] [Google Scholar]
- Fiorini A., Koudriavtseva T., Bucaj E., Coccia R., Foppoli C., Giorgi A., Schinina M.E., Di Domenico F., De Marco F., Perluigi M. Involvement of oxidative stress in occurrence of relapses in multiple sclerosis: the spectrum of oxidatively modified serum proteins detected by proteomics and redox proteomics analysis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0065184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M.T., Sharma R., Lim J.L., Haider L., Frischer J.M., Drexhage J., Mahad D., Bradl M., van Horssen J., Lassmann H. NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain. 2012;135:886–899. doi: 10.1093/brain/aws012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M.T., Wimmer I., Hoftberger R., Gerlach S., Haider L., Zrzavy T., Hametner S., Mahad D., Binder C.J., Krumbholz M., Bauer J., Bradl M., Lassmann H. Disease-specific molecular events in cortical multiple sclerosis lesions. Brain. 2013;136:1799–1815. doi: 10.1093/brain/awt110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher J.M., Lalor S.J., Sweeney C.M., Tubridy N., Mills K.H. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 2010;162:1–11. doi: 10.1111/j.1365-2249.2010.04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot J.D., Gavin M.A., Rudensky A.Y. Foxp3 programs the development and function of CD4 + CD25 + regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Forman H.J., Zhang H., Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009;30:1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte M., Gold B.G., Marracci G., Chaudhary P., Basso E., Johnsen D., Yu X., Fowlkes J., Rahder M., Stem K., Bernardi P., Bourdette D. Cyclophilin D inactivation protects axons in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. Proc. Natl. Acad. Sci. U. S. A. 2007;104:7558–7563. doi: 10.1073/pnas.0702228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friling R.S., Bensimon A., Tichauer Y., Daniel V. Xenobiotic-inducible expression of murine glutathione S-transferase Ya subunit gene is controlled by an electrophile-responsive element. Proc. Natl. Acad. Sci. U. S. A. 1990;87:6258–6262. doi: 10.1073/pnas.87.16.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischer J.M., Weigand S.D., Guo Y., Kale N., Parisi J.E., Pirko I., Mandrekar J., Bramow S., Metz I., Bruck W., Lassmann H., Lucchinetti C.F. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann. Neurol. 2015 doi: 10.1002/ana.24497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisullo G., Nociti V., Iorio R., Patanella A.K., Caggiula M., Marti A., Sancricca C., Angelucci F., Mirabella M., Tonali P.A., Batocchi A.P. Regulatory T cells fail to suppress CD4T +-bet + T cells in relapsing multiple sclerosis patients. Immunology. 2009;127:418–428. doi: 10.1111/j.1365-2567.2008.02963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich D.I., Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner D., Hoff H., Gimsa U., Burmester G.R., Brunner-Weinzierl M.C. CD25 regulatory T cells determine secondary but not primary remission in EAE: impact on long-term disease progression. J. Neuroimmunol. 2006;172:73–84. doi: 10.1016/j.jneuroim.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Gelderman K.A., Hultqvist M., Holmberg J., Olofsson P., Holmdahl R. T cell surface redox levels determine T cell reactivity and arthritis susceptibility. Proc. Natl. Acad. Sci. U. S. A. 2006;103:12831–12836. doi: 10.1073/pnas.0604571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergely P., Jr., Niland B., Gonchoroff N., Pullmann R., Jr., Phillips P.E., Perl A. Persistent mitochondrial hyperpolarization, increased reactive oxygen intermediate production, and cytoplasmic alkalinization characterize altered IL-10 signaling in patients with systemic lupus erythematosus. J. Immunol. 2002;169:1092–1101. doi: 10.4049/jimmunol.169.2.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoreschi K., Bruck J., Kellerer C., Deng C., Peng H., Rothfuss O., Hussain R.Z., Gocke A.R., Respa A., Glocova I., Valtcheva N., Alexander E., Feil S., Feil R., Schulze-Osthoff K., Rupec R.A., Lovett-Racke A.E., Dringen R., Racke M.K., Rocken M. Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J. Exp. Med. 2011;208:2291–2303. doi: 10.1084/jem.20100977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gironi M., Borgiani B., Mariani E., Cursano C., Mendozzi L., Cavarretta R., Saresella M., Clerici M., Comi G., Rovaris M., Furlan R. Oxidative stress is differentially present in multiple sclerosis courses, early evident, and unrelated to treatment. J. Immunol. Res. 2014;2014:961863. doi: 10.1155/2014/961863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray E., Thomas T.L., Betmouni S., Scolding N., Love S. Elevated activity and microglial expression of myeloperoxidase in demyelinated cerebral cortex in multiple sclerosis. Brain Pathol. 2008;18:86–95. doi: 10.1111/j.1750-3639.2007.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray E., Thomas T.L., Betmouni S., Scolding N., Love S. Elevated myeloperoxidase activity in white matter in multiple sclerosis. Neurosci. Lett. 2008;444:195–198. doi: 10.1016/j.neulet.2008.08.035. [DOI] [PubMed] [Google Scholar]
- Guan J.Z., Guan W.P., Maeda T., Guoqing X., GuangZhi W., Makino N. Patients with multiple sclerosis show increased oxidative stress markers and somatic telomere length shortening. Mol. Cell. Biochem. 2015;400:183–187. doi: 10.1007/s11010-014-2274-1. [DOI] [PubMed] [Google Scholar]
- Gutscher M., Pauleau A.L., Marty L., Brach T., Wabnitz G.H., Samstag Y., Meyer A.J., Dick T.P. Real-time imaging of the intracellular glutathione redox potential. Nat. Methods. 2008;5:553–559. doi: 10.1038/nmeth.1212. [DOI] [PubMed] [Google Scholar]
- Haas J., Hug A., Viehover A., Fritzsching B., Falk C.S., Filser A., Vetter T., Milkova L., Korporal M., Fritz B., Storch-Hagenlocher B., Krammer P.H., Suri-Payer E., Wildemann B. Reduced suppressive effect of CD4 + CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur. J. Immunol. 2005;35:3343–3352. doi: 10.1002/eji.200526065. [DOI] [PubMed] [Google Scholar]
- Haider L., Fischer M.T., Frischer J.M., Bauer J., Hoftberger R., Botond G., Esterbauer H., Binder C.J., Witztum J.L., Lassmann H. Oxidative damage in multiple sclerosis lesions. Brain. 2011;134:1914–1924. doi: 10.1093/brain/awr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans G., Stinissen P., Hauben L., Van den Berg-Loonen E., Raus J., Zhang J. Cytokine profile of myelin basic protein-reactive T cells in multiple sclerosis and healthy individuals. Ann. Neurol. 1997;42:18–27. doi: 10.1002/ana.410420106. [DOI] [PubMed] [Google Scholar]
- Hildeman D.A., Mitchell T., Teague T.K., Henson P., Day B.J., Kappler J., Marrack P.C. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 1999;10:735–744. doi: 10.1016/s1074-7613(00)80072-2. [DOI] [PubMed] [Google Scholar]
- Hori S., Nomura T., Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science (New York, N.Y.) 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Huan J., Culbertson N., Spencer L., Bartholomew R., Burrows G.G., Chou Y.K., Bourdette D., Ziegler S.F., Offner H., Vandenbark A.A. Decreased FOXP3 levels in multiple sclerosis patients. J. Neurosci. Res. 2005;81:45–52. doi: 10.1002/jnr.20522. [DOI] [PubMed] [Google Scholar]
- Im K.I., Kim N., Lim J.Y., Nam Y.S., Lee E.S., Kim E.J., Kim H.J., Kim S.H., Cho S.G. The free radical scavenger NecroX-7 attenuates acute graft-versus-host disease via reciprocal regulation of Th1/regulatory T cells and inhibition of HMGB1 release. J. Immunol. 2015 doi: 10.4049/jimmunol.1402609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi S., Kondo T., Deli M.A., Gobbel G., Joo F., Epstein C.J., Yoshimoto T., Chan P.H. The influence of oxygen free radicals on the permeability of the monolayer of cultured brain endothelial cells. Neurochem. Int. 1996;29:205–211. doi: 10.1016/0197-0186(95)00120-4. [DOI] [PubMed] [Google Scholar]
- Innamorato N.G., Rojo A.I., Garcia-Yague A.J., Yamamoto M., de Ceballos M.L., Cuadrado A. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J. Immunol. 2008;181:680–689. doi: 10.4049/jimmunol.181.1.680. [DOI] [PubMed] [Google Scholar]
- Johnson D.A., Amirahmadi S., Ward C., Fabry Z., Johnson J.A. The absence of the pro-antioxidant transcription factor Nrf2 exacerbates experimental autoimmune encephalomyelitis. Toxicol. Sci. 2010;114:237–246. doi: 10.1093/toxsci/kfp274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski M.M., Roth D., Krammer P.H., Gulow K. Mitochondria as oxidative signaling organelles in T-cell activation: physiological role and pathological implications. Arch. Immunol. Ther. Exp. 2013;61:367–384. doi: 10.1007/s00005-013-0235-0. [DOI] [PubMed] [Google Scholar]
- Karlik M., Valkovic P., Hancinova V., Krizova L., Tothova L., Celec P. Markers of oxidative stress in plasma and saliva in patients with multiple sclerosis. Clin. Biochem. 2015;48:24–28. doi: 10.1016/j.clinbiochem.2014.09.023. [DOI] [PubMed] [Google Scholar]
- Kensler T.W., Wakabayashi N., Biswal S. Cell survival responses to environmental stresses via the Keap1–Nrf2–ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Kesarwani P., Murali A.K., Al-Khami A.A., Mehrotra S. Redox regulation of T-cell function: from molecular mechanisms to significance in human health and disease. Antioxid. Redox Signal. 2013;18:1497–1534. doi: 10.1089/ars.2011.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodagholi F., Eftekharzadeh B., Maghsoudi N., Rezaei P.F. Chitosan prevents oxidative stress-induced amyloid beta formation and cytotoxicity in NT2 neurons: involvement of transcription factors Nrf2 and NF-kappaB. Mol. Cell. Biochem. 2010;337:39–51. doi: 10.1007/s11010-009-0284-1. [DOI] [PubMed] [Google Scholar]
- Khor T.O., Huang M.T., Kwon K.H., Chan J.Y., Reddy B.S., Kong A.N. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res. 2006;66:11580–11584. doi: 10.1158/0008-5472.CAN-06-3562. [DOI] [PubMed] [Google Scholar]
- Kipp M., Amor S. FTY720 on the way from the base camp to the summit of the mountain: relevance for remyelination. Mult. Scler. 2012;18:258–263. doi: 10.1177/1352458512438723. [DOI] [PubMed] [Google Scholar]
- Koch M., Mostert J., Arutjunyan A., Stepanov M., Teelken A., Heersema D., De Keyser J. Peripheral blood leukocyte NO production and oxidative stress in multiple sclerosis. Mult. Scler. 2008;14:159–165. doi: 10.1177/1352458507082075. [DOI] [PubMed] [Google Scholar]
- Kohm A.P., Carpentier P.A., Anger H.A., Miller S.D. Cutting edge: CD4 + CD25 + regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J. Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- Korn T., Reddy J., Gao W., Bettelli E., Awasthi A., Petersen T.R., Backstrom B.T., Sobel R.A., Wucherpfennig K.W., Strom T.B., Oukka M., Kuchroo V.K. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat. Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraaij M.D., Savage N.D., van der Kooij S.W., Koekkoek K., Wang J., van den Berg J.M., Ottenhoff T.H., Kuijpers T.W., Holmdahl R., van Kooten C., Gelderman K.A. Induction of regulatory T cells by macrophages is dependent on production of reactive oxygen species. Proc. Natl. Acad. Sci. U. S. A. 2010;107:17686–17691. doi: 10.1073/pnas.1012016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewerenz J., Albrecht P., Tien M.L., Henke N., Karumbayaram S., Kornblum H.I., Wiedau-Pazos M., Schubert D., Maher P., Methner A. Induction of Nrf2 and xCT are involved in the action of the neuroprotective antibiotic ceftriaxone in vitro. J. Neurochem. 2009;111:332–343. doi: 10.1111/j.1471-4159.2009.06347.x. [DOI] [PubMed] [Google Scholar]
- Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Lin S.X., Lisi L., Dello Russo C., Polak P.E., Sharp A., Weinberg G., Kalinin S., Feinstein D.L. The anti-inflammatory effects of dimethyl fumarate in astrocytes involve glutathione and haem oxygenase-1. ASN Neuro. 2011;3 doi: 10.1042/AN20100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker R.A., Lee D.H., Ryan S., van Dam A.M., Conrad R., Bista P., Zeng W., Hronowsky X., Buko A., Chollate S., Ellrichmann G., Bruck W., Dawson K., Goelz S., Wiese S., Scannevin R.H., Lukashev M., Gold R. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011;134:678–692. doi: 10.1093/brain/awq386. [DOI] [PubMed] [Google Scholar]
- Litjens N.H., Rademaker M., Ravensbergen B., Rea D., van der Plas M.J., Thio B., Walding A., van Dissel J.T., Nibbering P.H. Monomethylfumarate affects polarization of monocyte-derived dendritic cells resulting in down-regulated Th1 lymphocyte responses. Eur. J. Immunol. 2004;34:565–575. doi: 10.1002/eji.200324174. [DOI] [PubMed] [Google Scholar]
- Lock C., Hermans G., Pedotti R., Brendolan A., Schadt E., Garren H., Langer-Gould A., Strober S., Cannella B., Allard J., Klonowski P., Austin A., Lad N., Kaminski N., Galli S.J., Oksenberg J.R., Raine C.S., Heller R., Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- Los M., Schenk H., Hexel K., Baeuerle P.A., Droge W., Schulze-Osthoff K. IL-2 gene expression and NF-kappa B activation through CD28 requires reactive oxygen production by 5-lipoxygenase. EMBO J. 1995;14:3731–3740. doi: 10.1002/j.1460-2075.1995.tb00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowther D.E., Chong D.L., Ascough S., Ettorre A., Ingram R.J., Boyton R.J., Altmann D.M. Th1 not Th17 cells drive spontaneous MS-like disease despite a functional regulatory T cell response. Acta Neuropathol. 2013;126:501–515. doi: 10.1007/s00401-013-1159-9. [DOI] [PubMed] [Google Scholar]
- Lucas M., Rodriguez M.C., Gata J.M., Zayas M.D., Solano F., Izquierdo G. Regulation by interferon beta-1a of reactive oxygen metabolites production by lymphocytes and monocytes and serum sulfhydryls in relapsing multiple sclerosis patients. Neurochem. Int. 2003;42:67–71. doi: 10.1016/s0197-0186(02)00057-8. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C., Bruck W., Parisi J., Scheithauer B., Rodriguez M., Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann. Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Ma Q., Battelli L., Hubbs A.F. Multiorgan autoimmune inflammation, enhanced lymphoproliferation, and impaired homeostasis of reactive oxygen species in mice lacking the antioxidant-activated transcription factor Nrf2. Am. J. Pathol. 2006;168:1960–1974. doi: 10.2353/ajpath.2006.051113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsue H., Edelbaum D., Shalhevet D., Mizumoto N., Yang C., Mummert M.E., Oeda J., Masayasu H., Takashima A. Generation and function of reactive oxygen species in dendritic cells during antigen presentation. J. Immunol. 2003;171:3010–3018. doi: 10.4049/jimmunol.171.6.3010. [DOI] [PubMed] [Google Scholar]
- McGeachy M.J., Stephens L.A., Anderton S.M. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4 + CD25 + regulatory cells within the central nervous system. J. Immunol. 2005;175:3025–3032. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- Moline-Velazquez V., Cuervo H., Vila-Del Sol V., Ortega M.C., Clemente D., de Castro F. Myeloid-derived suppressor cells limit the inflammation by promoting T lymphocyte apoptosis in the spinal cord of a murine model of multiple sclerosis. Brain Pathol. 2011;21:678–691. doi: 10.1111/j.1750-3639.2011.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mougiakakos D., Johansson C.C., Kiessling R. Naturally occurring regulatory T cells show reduced sensitivity toward oxidative stress-induced cell death. Blood. 2009;113:3542–3545. doi: 10.1182/blood-2008-09-181040. [DOI] [PubMed] [Google Scholar]
- Nguyen T., Nioi P., Pickett C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkamp D.J., Murk J.L., van Oosten B.W., Cremers C.H., Killestein J., Viveen M.C., Van Hecke W., Frijlink D.W., Wattjes M.P. PML in a patient without severe lymphocytopenia receiving dimethyl fumarate. N. Engl. J. Med. 2015;372:1474–1476. doi: 10.1056/NEJMc1413724. [DOI] [PubMed] [Google Scholar]
- O'Connor R.A., Malpass K.H., Anderton S.M. The inflamed central nervous system drives the activation and rapid proliferation of Foxp3 + regulatory T cells. J. Immunol. 2007;179:958–966. doi: 10.4049/jimmunol.179.2.958. [DOI] [PubMed] [Google Scholar]
- Olesen S.P. Free oxygen radicals decrease electrical resistance of microvascular endothelium in brain. Acta Physiol. Scand. 1987;129:181–187. doi: 10.1111/j.1748-1716.1987.tb08057.x. [DOI] [PubMed] [Google Scholar]
- Osburn W.O., Yates M.S., Dolan P.D., Chen S., Liby K.T., Sporn M.B., Taguchi K., Yamamoto M., Kensler T.W. Genetic or pharmacologic amplification of nrf2 signaling inhibits acute inflammatory liver injury in mice. Toxicol. Sci. 2008;104:218–227. doi: 10.1093/toxsci/kfn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pae H.O., Oh G.S., Choi B.M., Chae S.C., Chung H.T. Differential expressions of heme oxygenase-1 gene in CD25- and CD25 + subsets of human CD4 + T cells. Biochem. Biophys. Res. Commun. 2003;306:701–705. doi: 10.1016/s0006-291x(03)01037-4. [DOI] [PubMed] [Google Scholar]
- Pani G., Colavitti R., Borrello S., Galeotti T. Redox regulation of lymphocyte signaling. IUBMB Life. 2000;49:381–389. doi: 10.1080/152165400410227. [DOI] [PubMed] [Google Scholar]
- Pasquali L., Pecori C., Lucchesi C., LoGerfo A., Iudice A., Siciliano G., Bonuccelli U. Plasmatic oxidative stress biomarkers in multiple sclerosis: relation with clinical and demographic characteristics. Clin. Biochem. 2015;48:19–23. doi: 10.1016/j.clinbiochem.2014.09.024. [DOI] [PubMed] [Google Scholar]
- Peng H., Guerau-de-Arellano M., Mehta V.B., Yang Y., Huss D.J., Papenfuss T.L., Lovett-Racke A.E., Racke M.K. Dimethyl fumarate inhibits dendritic cell maturation via nuclear factor kappaB (NF-kappaB) and extracellular signal-regulated kinase 1 and 2 (ERK1/2) and mitogen stress-activated kinase 1 (MSK1) signaling. J. Biol. Chem. 2012;287:28017–28026. doi: 10.1074/jbc.M112.383380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X., Lewin A.S., Sun L., Hauswirth W.W., Guy J. Suppression of mitochondrial oxidative stress provides long-term neuroprotection in experimental optic neuritis. Invest. Ophthalmol. Vis. Sci. 2007;48:681–691. doi: 10.1167/iovs.06-0553. [DOI] [PubMed] [Google Scholar]
- Rajah T., Chow S.C. The inhibition of human T cell proliferation by the caspase inhibitor z-VAD-FMK is mediated through oxidative stress. Toxicol. Appl. Pharmacol. 2014;278:100–106. doi: 10.1016/j.taap.2014.04.014. [DOI] [PubMed] [Google Scholar]
- Ramsey C.P., Glass C.A., Montgomery M.B., Lindl K.A., Ritson G.P., Chia L.A., Hamilton R.L., Chu C.T., Jordan-Sciutto K.L. Expression of Nrf2 in neurodegenerative diseases. J. Neuropathol. Exp. Neurol. 2007;66:75–85. doi: 10.1097/nen.0b013e31802d6da9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy T., Guo J., Mitzner W.A., Roman J., Singh A., Fryer A.D., Yamamoto M., Kensler T.W., Tuder R.M., Georas S.N., Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J. Exp. Med. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy N.M., Kleeberger S.R., Kensler T.W., Yamamoto M., Hassoun P.M., Reddy S.P. Disruption of Nrf2 impairs the resolution of hyperoxia-induced acute lung injury and inflammation in mice. J. Immunol. 2009;182:7264–7271. doi: 10.4049/jimmunol.0804248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat. Immunol. 2002;3:1129–1134. doi: 10.1038/ni1202-1129. [DOI] [PubMed] [Google Scholar]
- Rockwell C.E., Zhang M., Fields P.E., Klaassen C.D. Th2 skewing by activation of Nrf2 in CD4(+) T cells. J. Immunol. 2012;188:1630–1637. doi: 10.4049/jimmunol.1101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz T., Novas M., Terborg C. PML in a patient with lymphocytopenia treated with dimethyl fumarate. N. Engl. J. Med. 2015;372:1476–1478. doi: 10.1056/NEJMc1415408. [DOI] [PubMed] [Google Scholar]
- Rubant S.A., Ludwig R.J., Diehl S., Hardt K., Kaufmann R., Pfeilschifter J.M., Boehncke W.H. Dimethylfumarate reduces leukocyte rolling in vivo through modulation of adhesion molecule expression. J. Invest. Dermatol. 2008;128:326–331. doi: 10.1038/sj.jid.5700996. [DOI] [PubMed] [Google Scholar]
- Ruuls S.R., Bauer J., Sontrop K., Huitinga I., t Hart B.A., Dijkstra C.D. Reactive oxygen species are involved in the pathogenesis of experimental allergic encephalomyelitis in Lewis rats. J. Neuroimmunol. 1995;56:207–217. doi: 10.1016/0165-5728(94)00154-g. [DOI] [PubMed] [Google Scholar]
- Sarlette A., Krampfl K., Grothe C., Neuhoff N., Dengler R., Petri S. Nuclear erythroid 2-related factor 2-antioxidative response element signaling pathway in motor cortex and spinal cord in amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 2008;67:1055–1062. doi: 10.1097/NEN.0b013e31818b4906. [DOI] [PubMed] [Google Scholar]
- Schachtele S.J., Hu S., Lokensgard J.R. Modulation of experimental herpes encephalitis-associated neurotoxicity through sulforaphane treatment. PLoS One. 2012;7 doi: 10.1371/journal.pone.0036216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluesener H.J., Seid K. Heme oxygenase-1 in lesions of rat experimental autoimmune encephalomyelitis and neuritis. J. Neuroimmunol. 2000;110:114–120. doi: 10.1016/s0165-5728(00)00352-0. [DOI] [PubMed] [Google Scholar]
- Schuh C., Wimmer I., Hametner S., Haider L., Van Dam A.M., Liblau R.S., Smith K.J., Probert L., Binder C.J., Bauer J., Bradl M., Mahad D., Lassmann H. Oxidative tissue injury in multiple sclerosis is only partly reflected in experimental disease models. Acta Neuropathol. 2014;128:247–266. doi: 10.1007/s00401-014-1263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secchi C., Carta M., Crescio C., Spano A., Arras M., Caocci G., Galimi F., La Nasa G., Pippia P., Turrini F., Pantaleo A. T cell tyrosine phosphorylation response to transient redox stress. Cell. Signal. 2015;27:777–788. doi: 10.1016/j.cellsig.2014.12.014. [DOI] [PubMed] [Google Scholar]
- Sekizawa T., Sasaki M., Kumagai K., Takase S., Nakamura S. B lymphocytes bearing immunoglobulins in multiple sclerosis. Tohoku J. Exp. Med. 1974;114:395–397. doi: 10.1620/tjem.114.395. [DOI] [PubMed] [Google Scholar]
- Sian J., Dexter D.T., Lees A.J., Daniel S., Agid Y., Javoy-Agid F., Jenner P., Marsden C.D. Alterations in glutathione levels in Parkinson's disease and other neurodegenerative disorders affecting basal ganglia. Ann. Neurol. 1994;36:348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- Slowik A., Schmidt T., Beyer C., Amor S., Clarner T., Kipp M. The sphingosine 1-phosphate receptor agonist FTY720 is neuroprotective after cuprizone-induced CNS demyelination. Br. J. Pharmacol. 2015;172:80–92. doi: 10.1111/bph.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stys P.K., Zamponi G.W., van Minnen J., Geurts J.J. Will the real multiple sclerosis please stand up? Nature reviews. Neuroscience. 2012;13:507–514. doi: 10.1038/nrn3275. [DOI] [PubMed] [Google Scholar]
- Suthanthiran M., Anderson M.E., Sharma V.K., Meister A. Glutathione regulates activation-dependent DNA synthesis in highly purified normal human T lymphocytes stimulated via the CD2 and CD3 antigens. Proc. Natl. Acad. Sci. U. S. A. 1990;87:3343–3347. doi: 10.1073/pnas.87.9.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro C.E., Shakhar G., Shen S., Ding Y., Lino A.C., Maraver A., Lafaille J.J., Dustin M.L. Regulatory T cells inhibit stable contacts between CD4 + T cells and dendritic cells in vivo. J. Exp. Med. 2006;203:505–511. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmulappa R.K., Scollick C., Traore K., Yates M., Trush M.A., Liby K.T., Sporn M.B., Yamamoto M., Kensler T.W., Biswal S. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem. Biophys. Res. Commun. 2006;351:883–889. doi: 10.1016/j.bbrc.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp B.D., Nave K.A. Multiple sclerosis: an immune or neurodegenerative disorder? Annu. Rev. Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- Treumer F., Zhu K., Glaser R., Mrowietz U. Dimethylfumarate is a potent inducer of apoptosis in human T cells. J. Invest. Dermatol. 2003;121:1383–1388. doi: 10.1111/j.1523-1747.2003.12605.x. [DOI] [PubMed] [Google Scholar]
- Tripathi P., Hildeman D. Sensitization of T cells to apoptosis—a role for ROS? Apoptosis. 2004;9:515–523. doi: 10.1023/B:APPT.0000038033.14925.02. [DOI] [PubMed] [Google Scholar]
- Tse H.M., Milton M.J., Schreiner S., Profozich J.L., Trucco M., Piganelli J.D. Disruption of innate-mediated proinflammatory cytokine and reactive oxygen species third signal leads to antigen-specific hyporesponsiveness. J. Immunol. 2007;178:908–917. doi: 10.4049/jimmunol.178.2.908. [DOI] [PubMed] [Google Scholar]
- Tzartos J.S., Friese M.A., Craner M.J., Palace J., Newcombe J., Esiri M.M., Fugger L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Goes A., Brouwer J., Hoekstra K., Roos D., van den Berg T.K., Dijkstra C.D. Reactive oxygen species are required for the phagocytosis of myelin by macrophages. J. Neuroimmunol. 1998;92:67–75. doi: 10.1016/s0165-5728(98)00175-1. [DOI] [PubMed] [Google Scholar]
- van der Star B.J., Vogel D.Y., Kipp M., Puentes F., Baker D., Amor S. In vitro and in vivo models of multiple sclerosis. CNS Neurol. Disord. Drug Targets. 2012;11:570–588. doi: 10.2174/187152712801661284. [DOI] [PubMed] [Google Scholar]
- Venken K., Hellings N., Broekmans T., Hensen K., Rummens J.L., Stinissen P. Natural naive CD4 + CD25 + CD127low regulatory T cell (Treg) development and function are disturbed in multiple sclerosis patients: recovery of memory Treg homeostasis during disease progression. J. Immunol. 2008;180:6411–6420. doi: 10.4049/jimmunol.180.9.6411. [DOI] [PubMed] [Google Scholar]
- Vergara D., Martignago R., Bonsegna S., De Nuccio F., Santino A., Nicolardi G., Maffia M. IFN-beta reverses the lipopolysaccharide-induced proteome modifications in treated astrocytes. J. Neuroimmunol. 2010;221:115–120. doi: 10.1016/j.jneuroim.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Viglietta V., Baecher-Allan C., Weiner H.L., Hafler D.A. Loss of functional suppression by CD4 + CD25 + regulatory T cells in patients with multiple sclerosis. J. Exp. Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Xie K., Wang C., Bi J. Oxidative stress induced by lipid peroxidation is related with inflammation of demyelination and neurodegeneration in multiple sclerosis. Eur. Neurol. 2014;72:249–254. doi: 10.1159/000363515. [DOI] [PubMed] [Google Scholar]
- Wilms H., Sievers J., Rickert U., Rostami-Yazdi M., Mrowietz U., Lucius R. Dimethylfumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL-1beta, TNF-alpha and IL-6 in an in-vitro model of brain inflammation. J. Neuroinflammation. 2010;7:30. doi: 10.1186/1742-2094-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z., Garg S.K., Kipnis J., Banerjee R. Extracellular redox modulation by regulatory T cells. Nat. Chem. Biol. 2009;5:721–723. doi: 10.1038/nchembio.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z., Garg S.K., Banerjee R. Regulatory T cells interfere with glutathione metabolism in dendritic cells and T cells. J. Biol. Chem. 2010;285:41525–41532. doi: 10.1074/jbc.M110.189944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H., Guo C., Yu X., Zuo D., Wang X.Y. Mouse CD11b + Gr-1 + myeloid cells can promote Th17 cell differentiation and experimental autoimmune encephalomyelitis. J. Immunol. 2012;189:4295–4304. doi: 10.4049/jimmunol.1200086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.D., Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell. Biol. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Fujino M., Xu J., Li X.K. The role and potential therapeutic application of myeloid-derived suppressor cells in allo- and autoimmunity. Mediat. Inflamm. 2015;2015:421927. doi: 10.1155/2015/421927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Paul W.E. Peripheral CD4 + T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol. Rev. 2010;238:247–262. doi: 10.1111/j.1600-065X.2010.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]