Abstract
Severe acute respiratory syndrome (SARS) is a recently recognized febrile respiratory illness that first appeared in southern China in November 2002, has since spread to several countries, and has resulted in more than 8000 cases and more than 750 deaths. The disease has been etiologically linked to a novel coronavirus that has been named the SARS-associated coronavirus. It appears to be spread primarily by large droplet transmission. There is no specific therapy, and management consists of supportive care. This article summarizes currently available information regarding the epidemiology, clinical features, etiologic agent, and modes of transmission of the disease, as well as infection control measures appropriate to contain SARS.
Keywords: ARDS, acute respiratory distress syndrome; CDC, Centers for Disease Control and Prevention; CT, computed tomography; HCWs, health care workers; RT-PCR, reverse-transcriptase polymerase chain reaction; SARS, severe acute respiratory syndrome; SARS-CoV, SARS coronavirus
Severe acute respiratory syndrome (SARS) is the first important new infectious disease of the new millennium. It is now believed that the disease is caused by a novel coronavirus (SARS-CoV). SARS was first recognized as a distinct entity in February 2003 by Dr Carlo Urbani, an epidemiologist with the World Health Organization who was investigating the outbreak in Hanoi, Vietnam. Unfortunately, he himself contracted the disease and died. Since then and up to June 5, 2003, the disease has spread to affect more than 8000 worldwide.1 Although the medical community at large has been aware of this disease for only a few months, considerable progress has been made in understanding the epidemiology and clinical features, and the etiologic agent has been identified.
EPIDEMIOLOGY
A highly contagious atypical pneumonia first appeared in the Guangdong Province, People's Republic of China, in November 2002. This was not widely publicized, and the condition remained isolated to China for the next 3 months. On February 21, 2003, a Chinese physician from the Guangdong Province (patient A in Figure 1 ) who cared for patients with pneumonia and had himself developed symptoms traveled to Hong Kong to visit relatives. He stayed on the ninth floor of Hotel M for a day. On February 22, 2003, he was admitted to Hospital 2 with fever and respiratory symptoms and died of respiratory failure on March 4, 2003. He infected 2 of his family members and 4 health care workers (HCWs) in Hospital 2. In addition, 12 other hotel guests at Hotel M developed SARS, 10 of whom (patients B through K) were in the hotel the same day as patient A; the other 2 patients (patients L and M) stayed in the hotel during the time that 3 other symptomatic patients were guests at the hotel. Because of international travel and transmission to HCWs before the institution of protective measures, these patients were responsible for subsequent clusters of SARS around the globe. Patient B was the index patient for the outbreak in Hanoi involving HCWs and close contacts, including Dr Urbani. Patients C, D, and E were the index cases in Singapore. Patients F and G traveled back to Toronto, Canada, which resulted in the cluster of cases in Toronto. Patients H and J caused outbreaks among HCWs in other hospitals in Hong Kong. Patient L appears to have become infected during his stay at Hotel M, with subsequent transmission to his wife, patient M in the United States (Figure 1).
Figure 1.
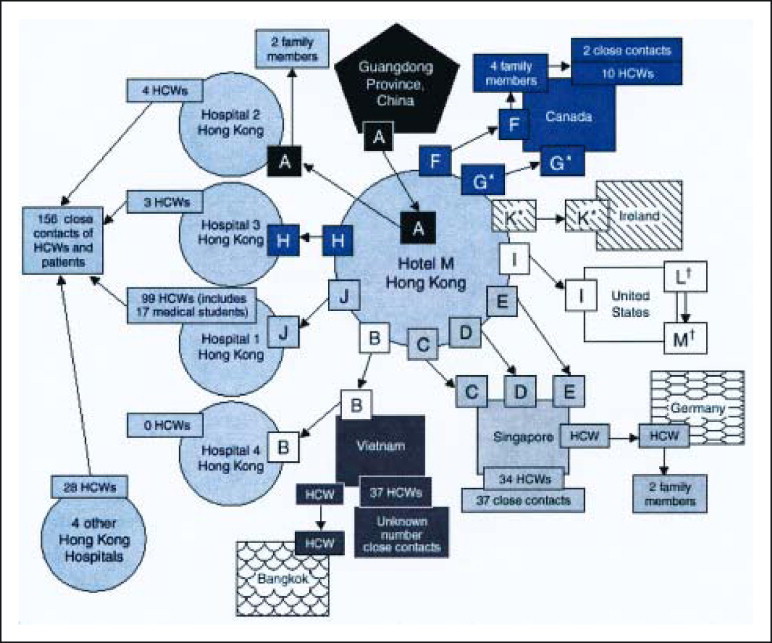
Chain of transmission of severe acute respiratory syndrome from the initial patient to other guests at Hotel M in Hong Kong in 2003.
*All guests except G and K stayed on the ninth floor of the hotel. Guest G stayed on the 14th floor, and guest K stayed on the 11th floor.
†Guests L and M (spouses) were not at Hotel M during the same time as index guest A but were at the hotel during the same times as guests G and H and were ill during this period. HCWs = health care workers. From the Centers for Disease Control and Prevention.2
Aggressive and unprecedented measures that included quarantine of thousands of people, travel restrictions, and temperature checks at airports have been successful to a large extent in containing the disease. Vietnam reported its last case more than 30 days ago. Singapore used its military forces to assist in contact tracing and enforcement of home quarantine. Through this and other measures, including screening of passengers at airports and seaports, concentration of patients in a single SARS-designated hospital, imposition of a no-visitors rule for all public hospitals, and use of a dedicated private ambulance service to transport all possible cases to the SARS-designated hospital, Singapore may have successfully controlled its outbreak and reported its last case on May 11, 2003. Hong Kong instituted a program of intensive contact tracing and home quarantine of all contacts and has reported a substantial decline in the number of new cases. Authorities in Toronto initially appeared to have controlled their outbreak, but on May 22, Health Canada began to report new clusters of cases of SARS, and since then 70 new cases linked to 4 Toronto hospitals have been reported. Mainland China and Taiwan continue to report new cases, and there is ongoing community transmission, although the daily number of reported new probable cases of SARS has declined from a mean of 166 cases during the first week of May to a mean of 2.5 cases in the first week of June.
Reported cases in all other countries have acquired infection through travel to endemic areas, and only limited local transmission through close contact has occurred. As of June 3, 2003, a total of 372 cases have been reported in the United States (303 suspect and 69 probable cases, no deaths), and almost all the patients acquired infection while traveling overseas.
CASE DEFINITION
Based on available data, the Centers for Disease Control and Prevention (CDC) defines a suspect case of SARS as a person with onset of fever (temperature >38°C [100.4°F]) and lower respiratory tract symptoms within 10 days of either travel to an area with documented transmission of SARS or close contact with a person believed to have SARS. A probable case is a suspect case who also has chest radiographic findings of pneumonia, acute respiratory distress syndrome (ARDS), or an unexplained respiratory illness resulting in death, with autopsy findings of ARDS without identifiable cause.3 Suspect and probable cases are further classified based on laboratory findings as laboratory positive, laboratory negative, or indeterminate (Table 1 ).
Table 1.
|
ARDS = acute respiratory distress syndrome; RT-PCR = reverse-transcriptase polymerase chain reaction; SARS = severe acute respiratory syndrome; SARS-CoV = SARS coronavirus.
Travel criteria for suspect or probable US cases of SARS. Last date of illness onset for inclusion as a reported case: China (mainland), Hong Kong, Taiwan, Toronto, ongoing; Hanoi, Vietnam, May 25, 2003; Singapore, June 4, 2003.
It is important to understand the meaning of close contact. It is defined as having cared for or lived with a person known to have SARS or unprotected contact with body secretions from such a person. This includes kissing, embracing, sharing utensils or bedding, performing a physical examination, or other direct physical contact between persons. It does not include sitting across a waiting room for a brief period, walking by a person with SARS, or other casual contact.
CLINICAL FEATURES
The incubation period is 2 to 10 days. Early manifestations include influenza-like symptoms, such as fever, myalgias, and headache. Fever occurs in virtually all patients and is often the presenting symptom. Often, fever is high and sometimes associated with chills and rigors. Fever may occasionally be absent in elderly persons or may have resolved by the time respiratory symptoms occur. Typically, rash and neurologic findings are absent. Diarrhea has been reported in up to 25% of patients.5, 6, 7 The respiratory phase starts within 2 to 4 days of onset of fever with a dry, nonproductive cough. This may progress to shortness of breath, usually in the second week of the illness, and might be accompanied by or progress to hypoxemia. In 10% to 20% of patients, the respiratory illness is severe enough to require tracheal intubation and mechanical ventilation. The case fatality rate is approximately 3% to 12% overall. The mortality rate may be as high as 45% in patients older than 60 years, particularly those with preexisting comorbidity (eg, diabetes, renal failure, and other chronic medical conditions).8 A concerning feature of this disease has been that young, previously healthy persons, many of them health care professionals, have also died of SARS. The reason for this is unclear but may be due to exposure to patients with higher viral loads or due to their host response. In contrast, SARS has affected relatively few children and appears to be milder in the pediatric age group.9
A biphasic course has been described in many patients, with an initial illness followed by improvement and then subsequent deterioration. This worsening can present as recurrent fever 4 to 7 days after initial defervescence, new chest infiltrates, respiratory failure, or watery diarrhea. In a cohort of 75 patients in Hong Kong, 85% had recurrent symptoms after initial improvement.8 The authors described a triphasic course with fever, myalgia early in week 1, and recurrent fever, hypoxemia, diarrhea, and shifting chest infiltrates in week 2. Twenty percent of patients progressed to ARDS during the third week of the illness. Quantitative reverse-transcriptase polymerase chain reaction (RT-PCR) of nasopharyngeal aspirate in 14 patients with relapse showed peak viral loads occurred at day 10 after onset of symptoms, suggesting that the late deterioration may be due to the host immune response rather than to uncontrolled viral replication.
Radiology
Chest radiographs of patients with SARS show patchy focal infiltrates or consolidation often with a peripheral distribution, which may progress to diffuse infiltrates. Pleural effusions have not been reported. Early findings may be subtle, and initial findings on chest radiographs may be normal in up to 25% of patients.6 Computed tomography (CT) may be more sensitive than plain films. Highresolution CT scans have shown abnormalities in patients with suspected SARS who have normal findings on plain films.10 The characteristic CT finding is bilateral peripheral airspaceground-glass consolidation mimicking that seen in bronchiolitis obliterans with organizing pneumonia.
Laboratory
Laboratory findings in patients with SARS include thrombocytopenia and leukopenia (in particular lymphopenia). Elevated creatine kinase, lactate dehydrogenase, and transaminase levels have been noted. A high peak lactate dehydrogenase level and an initial elevated white blood cell count may carry a poor prognosis.7
ETIOLOGY
The etiologic link of a coronavirus with the SARS epidemic was established by Peiris et al11 in Hong Kong. Several diagnostic laboratory methods were used to initially recover and then characterize coronavirus infection in various specimens obtained from patients with SARS. These included inoculation and recovery of the virus in cell cultures, characterization of morphologic features by electron microscopy, serologic antibody determination, and molecular amplification and sequencing of the target RNA of the agent. Overall, 45 of the 50 patients these investigators studied had 1 or more laboratory tests (serology, 32; RT-PCR, 22; culture, 2) that supported a coronavirus etiology. The results of this initial report were quickly confirmed and expanded by collaborative studies in several major public health centers and medical institutions throughout the world coordinated by the World Health Organization.12, 13 Molecular sequencing analyses have indicated that the virus is only distantly related to previously sequenced coronaviruses. Based on serologic studies, it appears that this virus has not previously circulated in humans and is now referred to as SARS-CoV.14, 15
Coronaviruses are enveloped RNA viruses that cause disease in humans and animals. The previously known human coronaviruses are a major cause of the common cold and can occasionally cause pneumonia. Research teams in Hong Kong and Shenzhen, China, recently detected several coronaviruses closely related genetically to the SARS-CoV in 2 animal species (masked palm civet and raccoon-dog) and antibodies against the SARS-CoV in 1 additional species (Chinese ferret badger). These and other wild animals are traditionally considered delicacies and are sold for human consumption in markets throughout southern China.
This study provides the first indication that the SARS-CoV exists outside a human host. Studies are needed to determine how widespread the SARS virus might be in animals in Guangdong and elsewhere and if these animals can excrete the virus in an amount sufficient to infect humans and through what route such transmission occurs.
TRANSMISSION
The SARS-CoV appears to be transmitted primarily by large droplet spread, although surface contamination and possibly airborne spread may play a role. Recent data suggest that the virus may remain viable for considerable periods on a dry surface (up to 24 hours); hence, transmission through fomites may occur. The outbreak in an apartment complex in Hong Kong that accounted for more than 300 cases has been attributed to fecal spread. A patient with SARS who had diarrhea stayed with his brother in this building. It is thought that infection spread from him to other residents in the building through a leaking sewage drain, which allowed aerosolization of virus-containing material. Sewage also backed into bathroom floor drains in some apartments and may have accounted for some of the transmission. The SARS-CoV is stable in feces (and urine) at room temperature for at least 1 to 2 days. The virus is more stable (up to 4 days) in stool from patients with diarrhea (which has a higher pH than normal stool).16
It is presently unclear at what stage of the disease viral shedding occurs or whether someone who is infected but asymptomatic can infect others. As our knowledge of SARS and the etiologic coronavirus evolves, we will be able to address these important issues.
Some close contacts have reported a mild febrile illness without respiratory signs or symptoms, suggesting the illness might not always progress to the respiratory phase; others have not become ill at all. In contrast, “super spreaders” have been described who have infected 10 or more contacts, including HCWs, family and social contacts, or visitors to the health care facilities where patients were hospitalized. A similar phenomenon has been described with some other diseases, such as rubella, laryngeal tuberculosis, and Ebola virus, and might be the result of a combination of host, environment, and virus interactions. In Singapore, 5 super spreaders were responsible for a total of 170 suspect and probable cases of SARS.17 Additional data on the natural history of infection are needed to understand factors that might be associated with this phenomenon. Regardless of whether it is the result of other transmission routes, inadequate infection-control measures, or more viral shedding by certain patients, the fact remains that transmission of the SARS virus is highly efficient in some circumstances.
DIAGNOSIS
The initial diagnosis of SARS is one of exclusion. Hence, common causes of respiratory illnesses should be sought. Initial diagnostic testing for patients with suspected SARS should include chest radiography, pulse oximetry, blood cultures, sputum Gram stain and culture, and testing for other respiratory pathogens, notably influenza A and B, respiratory syncytial virus, and Legionella (Table 2 ). Clinicians should save any available clinical specimens (respiratory, blood, serum, and stool) for additional testing until a specific diagnosis is made.
Table 2.
Diagnostic Approach to Patients With Possible SARS*
|
SARS = severe acute respiratory syndrome; SARS-CoV = SARS corona-virus.
Acute and convalescent (>21 days after onset of symptoms) serum samples should be collected from each patient who meets the SARS case definition. Paired sera and other clinical specimens can be forwarded through state and local health departments for testing at the CDC. Specific instructions for collecting specimens from suspected SARS patients are available from the CDC.18 Laboratory diagnostic tests used at the CDC to test clinical specimens for evidence of SARS-CoV include serology, PCR testing, and viral cultures.
Serologic testing for coronavirus antibody consists of indirect fluorescent antibody testing and enzyme-linked immunosorbent assays that are specific for antibody produced after infection. Patients seem to seroconvert at a mean of 10 days after onset of symptoms. The CDC has made reagents for SARS antibody testing available to state public health laboratories.
An RT-PCR test specific for RNA from the SARS-CoV has been positive within the first 10 days after fever onset in respiratory specimens from most patients considered probable cases of SARS who have been tested and in stool samples in the second week of illness. The duration of detectable viremia or viral shedding needs further study.12, 13
Despite the fact that several thousand specimens from patients with SARS have been processed in laboratories worldwide, to date there have been no reported clusters of illness in laboratory workers. Nevertheless, reasonable precautions should be taken in handling these specimens. The CDC recommends that specimens from patients with SARS be labeled accordingly and that the laboratory be alerted before the samples are sent. Laboratory workers who handle these specimens should use standard precautions in a Biosafety Level 2 laboratory. Any procedure with the potential to generate fine particulate aerosols should be performed in a biological safety cabinet.19
TREATMENT
There is no specific treatment currently available for SARS. Management consists of supportive care and appropriate infection control measures to prevent spread. Because the diagnosis is uncertain, empirical therapy for community-acquired pneumonia should be administered by using antibiotics with activity against both typical and atypical respiratory pathogens including influenza when appropriate. In all series of SARS cases described to date, therapy has involved broad-spectrum antibiotics, including a fluoroquinolone or macrolide. The antiviral drug ribavirin has been used in most patients treated in Hong Kong and in Toronto, without evidence of efficacy.7, 10 The adverse effects of ribavirin are significant, particularly hemolytic anemia and electrolyte disturbances such as hypokalemia and hypomagnesemia; hence, empirical therapy with ribavirin is not warranted.
Anecdotal evidence suggests that corticosteroids may be beneficial, particularly in patients with progressive pulmonary infiltrates and hypoxemia. Various regimens have been used in different centers, with dosages of methylpred-nisolone ranging from 40 mg twice daily (similar to therapy for Pneumocystis carinii pneumonia) to 2 mg/kg per day (similar to late-phase therapy for ARDS) to pulse doses of 500 mg intravenously per day.20 Because corticosteroids have potential adverse effects, clinicians should carefully assess the risk vs the benefit on a case-by-case basis.
INFECTION CONTROL
Early recognition and isolation of patients with SARS is key to limiting spread of the disease. Based on our current understanding of disease transmission, infection control measures for suspected SARS cases should include the following.
Screening and Triage
Early identification of patients with SARS in health care facilities and appropriate isolation are essential in preventing large outbreaks. The CDC has recommended that in health care settings, patients should be screened with targeted questions as soon as possible after arrival or while the patient is on the telephone making an appointment. We have been using a simple screening tool (Figure 2 ) to screen all patients in our facility. Patients who have positive findings on the initial screen are given a mask, separated from other patients, placed in an examination room (a negative-air room if available), and evaluated further by a physician with airborne (N-95 respirator) and contact (gown, gloves) precautions. If, during this evaluation, the patient fits the CDC case definition for SARS and needs admission, the patient is admitted to a negative-air room. If admission is unnecessary but further testing is required, the patient is escorted to appropriate areas (for laboratory tests, radiological studies, etc) and testing expedited so that exposure of other patients is minimized.
Figure 2.
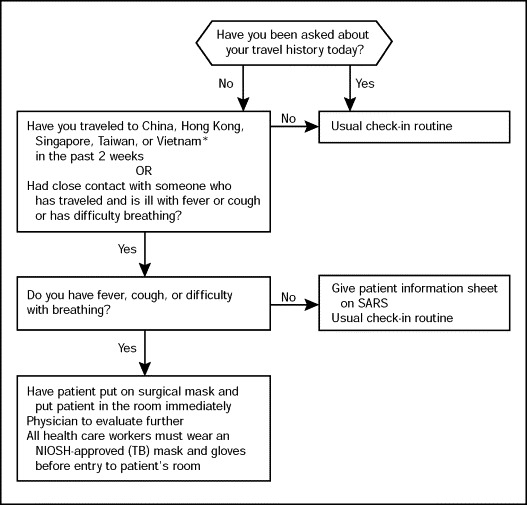
Screening algorithm used to triage patients at first point of contact.
*List of affected countries may change; the evaluating physician should check the Centers for Disease Control and Prevention Web site on severe acute respiratory syndrome (SARS) for the most current epidemiological case definition. NIOSH = National Institute of Occupational Safety and Health; TB = tuberculosis.
Outpatient Setting
Diagnosis of SARS does not automatically necessitate admission. Patients who do not otherwise need to be admitted to a hospital should be managed as outpatients as long as they can limit interactions outside the home.
Persons being managed as outpatients should be instructed to wear a surgical mask when in the presence of household members, and they should contain respiratory secretions in facial tissue and place these in lined containers for disposal with household waste. They should wash their hands frequently with soap and water or an alcohol-based hand sanitizer, especially after touching respiratory secretions and other body fluids. Sharing of eating utensils, towels, and bedding between patients with SARS and others should be avoided until these items have been washed with soap and hot water. Such patients should stay away from work, school, or other activities in a public place for 10 days after the resolution of fever provided that cough and other respiratory symptoms have resolved or have improved. A plan will need to be formulated to decide how these outpatients will obtain food and other supplies such as surgical masks during the period of isolation and how arrangements will be made for travel to and from necessary medical appointments.
Household members or other close contacts of patients with SARS should be advised to wear disposable gloves for any direct contact with body fluids from the patient and to practice good hand hygiene. Such people should be vigilant for the development of fever or respiratory symptoms, and if these occur, they should seek health care evaluation. They should inform their health care provider in advance of arriving for the evaluation that they have had close contact with a patient with SARS so that arrangements can be made, as necessary, to prevent transmission to others in the health care setting. In the absence of fever or respiratory symptoms, they need not limit their activities outside the home.
Hospital Setting
Patients who require hospitalization should be admitted to a negative pressure, specially ventilated room. Visitors should be discouraged; the number of HCWs involved in the patient's care should be limited. All HCWs should use personal protective equipment appropriate for standard, contact, and airborne precautions (ie, hand hygiene, gown, gloves, and N-95 respirator) in addition to eye protection while caring for these patients. Procedures that result in coughing and aerosolization of respiratory secretions should be avoided. If bronchoscopy is considered essential for patient care, it should be performed by experienced personnel using maximum infection control precautions (Table 3 ).
Table 3.
Infection Control Precautions for Patients Hospitalized With Suspected/Probable SARS*
|
BiPAP = biphasic positive airway pressure; CPAP = continuous positive airway pressure; EPA = Environmental Protection Agency; HEPA = high-efficiency particulate air; SARS = severe acute respiratory syndrome.
MANAGING HCW EXPOSURE
An important feature of the SARS outbreaks in Canada, Singapore, and Hong Kong has been the fact that several HCWs have developed SARS after caring for patients with SARS. Transmission to HCWs appears to have occurred after close, unprotected contact with symptomatic individuals. This has placed additional strain on health care systems that have already been stressed by outbreaks of SARS.
Active surveillance for fever and respiratory symptoms (eg, daily screening) should be conducted in HCWs with unprotected exposure for 10 days after the exposure, including checking the temperature when the employee reports to work and taking a history of respiratory symptoms. Workers with unprotected exposure who develop fever and/or respiratory symptoms should not come to work; they should stay home and report symptoms to infection control or the employee health service immediately. Health care workers with unprotected exposure during aerosolgenerating procedures (intubation, suctioning, bronchos-copy, etc) should be quarantined for a 10-day period at home because these procedures pose a higher risk of disease transmission.
CONCLUSION
Case definitions of SARS are currently based on the presence of epidemiological risk factors (close contact with patients with SARS or travel to SARS-affected areas) and a combination of fever and respiratory symptoms, with or without chest radiographic changes. However, if SARS spreads into the general population, our ability to distinguish it from other community-acquired pneumonias based on such epidemiological linkages will fail. If this happens, SARS will need to be considered in the differential diagnosis of any community-acquired or nosocomial pneumonia. A “typical” history, suggestive laboratory values, and failure to respond to conventional antibiotics should raise suspicion. Diagnostic tests will be crucial in the future both to ensure that patients are isolated rapidly and to provide appropriate therapy.
SARS has resulted in important challenges for the medical community. In affected areas, policies are changing daily as more information about the virus and the disease is obtained.
The resurgence of cases in Toronto in early May 2003 shows the difficulty of maintaining control over a new disease characterized by many puzzling epidemiological and clinical features. The continued alertness of Singapore, which most recently broke the chain of SARS transmission, shows the importance of maintaining a high level of vigilance and preparedness to ensure that a single imported case does not reignite an outbreak. Currently, control depends on prompt detection and isolation of cases, good infection control in hospitals, and the tracing and quarantine of contacts.
Footnotes
A question-and-answer section appears at the end of this article.
Questions About SARS
-
1.In which one of the following countries did the first cases of SARS seem to occur?
-
a.United States
-
b.Vietnam
-
c.China
-
d.Thailand
-
e.Singapore
-
a.
-
2.Which one of the following is the etiologic agent of SARS?
-
a.A novel coronavirus
-
b.Paramyxovirus
-
c.Influenza
-
d.Avian influenza
-
e.Resistant pneumococcus
-
a.
-
3.Which one of the following patients would fit the CDC case definition for probable SARS?
-
a.A 45-year-old executive with a fever of 39°C (102°F), myalgias, dry cough, and a patchy left lower lobe infiltrate 5 days after returning from a business trip to Beijing
-
b.A 50-year-old male smoker with severe chronic obstructive pulmonary disease who presents with cough, greenish sputum, and left lower lobe consolidation 5 days after returning from a 2-week vacation with his grandchildren at Walt Disney World in Florida
-
c.A 35-year-old woman with a fever of 39°C, myalgias, dry cough, and normal findings on a chest x-ray film who returned from China 5 days ago with her adopted infant
-
d.A 40-year-old recent immigrant to the United States who presents with a 2-month history of cough productive of blood-streaked sputum, low-grade fever (maximum 37.8°C [100°F]), and progressive weight loss
-
e.A 60-year-old man with an ejection fraction of 20% who presents with shortness of breath, orthopnea, bilateral pulmonary infiltrates, and no fever 7 days after returning from Singapore
-
a.
-
4.Which one of the following infection control precautions is not recommended by the CDC for possible cases of SARS in the hospital setting?
-
a.Airborne isolation
-
b.Droplet isolation
-
c.Use of gloves, gowns, good hand hygiene, and eye protection by health care personnel
-
d.Quarantine of the physician who performed bronchoscopy on the patient before the diagnosis of SARS was considered and infection control measures instituted
-
e.Mandatory quarantine of the patient's family even though they have no symptoms
-
a.
-
5.Which one of the following statements regarding SARS is false?
-
a.A fever higher than 38°C (100.4°F) is necessary to fit the current CDC case definition
-
b.Nonproductive cough is frequently a presenting symptom
-
c.All patients who may have SARS must be admitted to a hospital
-
d.SARS may be less severe in children
-
e.Some patients with SARS may have a biphasic or triphasic illness
-
a.
Correct answers:
-
1.
c,
-
2.
a,
-
3.
a,
-
4.
e,
-
5.
c
REFERENCES
- 1.World Health Organization Cumulative number of reported probable cases of severe acute respiratory syndrome (SARS) Available at: www.who.int/csr/sarscountry/2003_04_23/en Accessibility verified June 3, 2003.
- 2.Centers for Disease Control and Prevention Preliminary clinical description of severe acute respiratory syndrome. MMWR Morb Mortal Wkly Rep. 2003;52:255–256. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Updated interim U.S. case definition of severe acute respiratory syndrome (SARS) Available at: www.cdc.gov/ncidod/sars/casedefinition.htm Accessibility verified June 5, 2003.
- 4.Centers for Disease Control and Prevention Updated interim surveillance case definition for severe acute respiratory syndrome (SARS)—United States, April 29, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:391–393. [PubMed] [Google Scholar]
- 5.Poutanen SM, Low DE, Henry B. Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;348:1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- 6.Hsu L-Y, Lee C-C, Green JA. Severe Acute Respiratory Syndrome (SARS) in Singapore: clinical features of index patient and initial contacts. Emerg Infect Dis [serial online] June 2003. Available at: www.cdc.gov/ncidod/EID/vol9no6/03-0264.htm Accessibility verified June 4, 2003. [DOI] [PMC free article] [PubMed]
- 7.Booth CM, Matukas LM, Tomlinson GA. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. Available at: http://jama.ama-assn.org/cgi/search?fulltext=greater+toronto+area Accessibility verified June 4, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Peiris JSM, Chu CM, Cheng VCC, HKU/UCH SARS Study Group Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet [serial online] May 2003. Available at: http://image.thelancet.com/extras/03art4432web.pdf Accessibility verified June 5, 2003. [DOI] [PMC free article] [PubMed]
- 9.Hon KLE, Leung CW, Cheng WTF. Clinical presentations and outcome of severe acute respiratory syndrome in children. Lancet [serial online] April 2003. Available at: http://image.thelancet.com/extras/03let4127web.pdf Accessibility verified June 4, 2003. [DOI] [PMC free article] [PubMed]
- 10.Lee N, Hui D, Wu A. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 11.Peiris JSM, Lai ST, Poon LL. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drosten C, Günther S, Preiser W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 13.Ksiazek TG, Erdman D, Goldsmith CS. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 14.Marra MA, Jones SJM, Astell CR. The genome sequence of the SARS-associated coronavirus. Science Mag [serial on-line] 2003;300:1399–1404. doi: 10.1126/science.1085953. Available at: www.sciencemag.org/cgi/content/abstract/1085953v1 Accessibility verified June 4, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Rota PA, Oberste MS, Monroe SS. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science Mag [serial online] 2003;300:1394–1399. doi: 10.1126/science.1085952. Available at: www.sciencemag.org/cgi/content/abstract/1085952v1 Accessibility verified June 4, 2003. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization First data on stability and resistance of SARS coronavirus compiled by members of WHO laboratory network. Available at: www.who.int/csr/sars/survival_2003_05_04/en/ Accessibility verified June 4, 2003.
- 17.Centers for Disease Control and Prevention Severe acute respiratory syndrome—Singapore, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:405–411. [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention Guidelines for collection of specimens from potential cases of SARS. Available at: www.cdc.gov/ncidod/sars/specimen_collection_sars2.htm Accessibility verified June 3, 2003.
- 19.Centers for Disease Control and Prevention Interim laboratory biosafety guidelines for handling and processing specimens associated with severe acute respiratory syndrome (SARS) Available at: www.cdc.gov/ncidod/sars/sarslabguide.htm Accessibility verified June 3, 2003.
- 20.So LK, Lau AC, Yam LY. Development of a standard treatment protocol for severe acute respiratory syndrome. Lancet. 2003;361:1615–1617. doi: 10.1016/S0140-6736(03)13265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


