Abstract
Background
Up to 80% of asthma exacerbations in white children are associated with viral upper respiratory infections. The relative importance of different respiratory pathogens and relevant microbiological data in Asian children are unclear. This study elucidated the epidemiology of respiratory infections in Hong Kong children with asthma exacerbation.
Methods
A total of 209 children aged 3-18 years with asthma exacerbations and 77 controls with stable asthma were recruited. The severity of asthma exacerbations was assessed according to Global Initiative for Asthma guideline, and subjects aged 6 years or older performed exhaled nitric oxide and spirometric measurements. Nested multiplex polymerase chain reaction was used to detect 20 different respiratory pathogens.
Results
Respiratory pathogens were detected in 105 (51.0%) subjects. The presence of any respiratory pathogen was associated with asthma exacerbation (odds ratio [OR], 2.77; 95% CI, 1.51–5.11; P < .001). Specifically, human rhinovirus (HRV) infection was more common among children with asthma exacerbation (OR, 2.38; 95% CI, 1.09–5.32; P = .018). All other pathogens or coinfections were not associated with asthmatic attacks. None of these respiratory infections was associated with the severity of asthma exacerbation (P > .15 for all). During peak HRV season in the winter of 2007 to 2008, this virus was detected in 46.4% of children with asthma exacerbations.
Conclusions
Respiratory viral infections are commonly found in children with asthma exacerbation, with HRV being the most important pathogen in our patients. Respiratory viral infection is a triggering factor for asthma exacerbation but does not correlate with its severity.
Abbreviations
- FeNO
fractional exhaled nitric oxide concentration
- HBoV
human bocavirus
- HCoV
human coronavirus
- HMPV
human metapneumovirus
- HRV
human rhinovirus
- ICS
inhaled corticosteroid
- NPA
nasopharyngeal aspirate
- OR
odds ratio
- PCR
polymerase chain reaction
- RSV
respiratory syncytial virus
- RTI
respiratory tract infection
Asthma is the most common chronic respiratory disorder in childhood, affecting about 10% of local children.1 Asthma exacerbation resulting in hospitalization accounts for a major fraction of the total cost of asthma care in society.2 Prospective epidemiologic studies show that up to 80% of childhood asthma exacerbations are associated with viral upper respiratory tract infections (RTIs).3 Among respiratory viruses, human rhinovirus (HRV) infection preceded as many as 50% of asthma exacerbations in children.4 Influenza viruses also accounted for substantial morbidity (eg, up to 200 annual outpatient visits per 1,000 children) in children with asthma and other chronic medical conditions.5 Respiratory syncytial virus (RSV) is another major pathogen causing asthma exacerbation.4 Respiratory viruses, such as human coronavirus (HCoV)-229E, HCoV-OC43, and human metapneumovirus (HMPV), are common causes of upper RTI,6, 7, 8 but their relation to asthma exacerbation remains unclear. Allander et al9 developed a system for large-scale molecular virus screening of clinical samples based on host DNA depletion, random polymerase chain reaction (PCR) amplification, large-scale sequencing, and bioinformatics. A more recently described parvovirus called human bocavirus (HBoV) was identified in children with lower RTIs,9 but its relation to asthma exacerbation is uncertain at present.
Asthma exacerbation may also be caused by atypical bacteria. Chlamydophila pneumoniae, an obligate intracellular respiratory pathogen, was linked to asthma exacerbation in children.10 Among children hospitalized for wheezing, Johnston11 detected respiratory viruses in 18% of patients aged less than 3 months and 58% in those aged more than 5 years. Asthma-related symptoms resolved or significantly improved in half of adult patients with asthma seropositive for C pneumoniae who were treated with macrolides or doxycycline.12 Mycoplasma pneumoniae is detected by PCR in more than 50% of patients with asthma,13 but it was also found in 11% of upper airway secretions from patients with stable asthma.14 It is important to understand the roles different respiratory pathogens play in precipitating asthmatic attacks in order to determine ways to reduce the health-care burden associated with asthma-related hospitalization. The objectives of this study were: (1) to investigate the importance of different respiratory pathogens in childhood asthma exacerbation, and (2) to delineate the epidemiology of respiratory pathogens causing asthma exacerbation in Hong Kong children.
Materials and Methods
Study Population
This study recruited children with asthma aged 3-18 years with disease exacerbation who received treatments either in pediatric wards or outpatient clinics of a university teaching hospital between January 2007 and February 2008. Inpatients hospitalized for asthma exacerbation were assessed within 48 h of hospitalization. Age- and sex-matched children with asthma who were free from RTIs for 4 weeks or longer and were seen in our allergy clinic within the same week as the above children with acute asthma were recruited as controls.
Asthma diagnosis was made according to British Thoracic Society criteria.15 Briefly, older patients were either hyperresponsive to methacholine or showed reversible airflow limitation, whereas young children with asthma had three or more episodes of cough, shortness of breath, and wheezing during the 12 months before the study. These young patients also showed good response to bronchodilator. This study selected only children older than 3 years with a definitive diagnosis of asthma because it may be difficult to differentiate acute bronchiolitis from asthma in the younger children.16 Patients who received antimicrobial agents (eg, neuraminidase inhibitors, ribavirin, and macrolides) within 2 weeks before study were also excluded. The severity of asthma exacerbation was classified according to Global Initiative for Asthma guidelines.17
Infection was defined as the detection of respiratory pathogens by our nested multiplex PCR assays. Our primary outcome was the difference in detection rate for any respiratory pathogen between children with asthma with acute exacerbation and controls (ie, stable asthma). Secondary outcomes consisted of differences in the clinical severity of asthma exacerbation, lung function parameters, and fractional exhaled nitric oxide concentration (FeNO) in relation to patients with different respiratory pathogens. The Clinical Research Ethics Committee of our university approved this study. Following informed written consent, subjects had clinical assessment followed by FeNO and spirometric measurements.
Exhaled Nitric Oxide and Spirometric Measurements
Subjects 6 years of age and older underwent online FeNO measurement using a chemiluminescence analyzer (Sievers; Boulder, CO) according to international guidelines.18 The mean FeNO of three NO plateau values was recorded. FeNO was measured within 48 h of hospitalization for children with acute asthma and at the clinic visit for stable patients. The former group was allowed to commence systemic corticosteroids as clinically indicated prior to FeNO because it would be unethical to withhold such treatment until this study. Following FeNO, they performed spirometry (Compact II; Vitalograph; Buckingham, UK) to measure FEV1, FVC, and FEV1/FVC.
Microbiological Investigations
In accordance with local Infection Control policy, nasopharyngeal aspirates (NPAs) were collected in negative-pressure isolation rooms. Deep nasal swabs were obtained as an alternative in situation where an isolation facility was unavailable.19 These specimens were put immediately in viral transport medium and kept at 4°C during transportation. Both viral RNA and DNA were extracted on the same day of collection by PureLink Viral RNA/DNA Mini Kit (Invitrogen; Carlsbad, CA). RNA extracted was converted to cDNA by reverse transcriptase (Superscript III Reverse Transcriptase; Invitrogen). All DNA and cDNA were used immediately for nested multiplex PCR for 20 respiratory pathogens as described previously.20 Tables 1a and 1b in the online supplement summarize sequences and amplicon sizes of the outer and inner sets of PCR primers. Briefly, each nested multiplex PCR assay detected four pathogens. Group 1 comprised influenza A and B group-specific and subtypes H1N1, H3N2, H5N1-specific primers; group 2 comprised parainfluenza viruses (PIV-1, PIV-2, PIV-3, PIV-4a, and PIV-4b); group 3 comprised RSV A and B, HRV, and enterovirus; group 4 comprised HCoV-OC43, HCoV-229E, SARS-CoV, and HMPV; and group 5 comprised M pneumoniae, C pneumoniae, HBoV, and adenovirus. Both the first and second rounds of PCR were conducted in 20-μl reaction mixtures using fast thermal cycler (Applied Biosystems; Foster City, CA). Two microliters of cDNA was used as the template for the first round of PCR for groups 1 to 4, whereas 8 μL of the extracted preparation was used for group 5. In the second round of PCR, a 0.2-μL aliquot of the first-round PCR product was used as a template. Table 1c in the online supplement summarizes the PCR conditions of five multiplex nested PCR assays. The PCR products were stained by SYBR Safe (Invitrogen) and visualized by electrophoresis in 1.5% agarose gels. Four corresponding positive controls and one negative control (sterile water) sample were included for each group simultaneously. In order to prevent PCR contamination, reagent preparation, sample processing, and nested PCR assays were performed in separate rooms away from where amplified products were analyzed. Aerosol-resistant pipette tips were used throughout the experiments.
Table 1.
The Clinical and Objective Features of the Two Patient Groups
| Feature | Asthma Exacerbation (n = 209) | Stable Asthma (n = 77) |
|---|---|---|
| Age, ya | 7.6 (4.1)b | 11.1 (4.5) |
| Men | 68.4% | 75.3% |
| Duration of hospitalization, d | 3.6 (1.9) | NA |
| Domestic tobacco smoke exposure | 22.3%b | 13.0% |
| Received regular ICS treatment | 19.7% | 24.7% |
| Clinical status | ||
| Fever | 33.0% | NA |
| Shortness of breath on talking/at rest | 8.7% | NA |
| Only able to talk in phrases or words | 11.2% | NA |
| Altered consciousness (agitation or drowsiness) | 0 | NA |
| Duration of fever, d | 0.49 (0.86) | NA |
| Received supplemental oxygen | 23.0% | NA |
| GINA-defined severity of exacerbations | ||
| Mild | 5 (2.4%) | NA |
| Moderate | 101 (48.3%) | NA |
| Severe | 103 (49.3%) | NA |
| Imminent respiratory arrest | 0 | NA |
| Vital signs | ||
| Minimum Sao2, % | 94.1 (2.4) | NA |
| Maximum pulse rate, per min | 135 (22) | NA |
| Maximum respiratory rate, per min | 34 (9) | NA |
| Systolic blood pressure, mm Hg | 110 (15) | NA |
| Diastolic blood pressure, mm Hg | 69 (10) | NA |
| Laboratory results | ||
| FeNO, ppbc | 57.2 (43.0) | 77.2 (59.6) |
| FEV1, % predicted | 73.2 (21.7)b | 95.8 (15.2) |
| FVC, % predicted | 81.6 (32.5)d | 94.2 (18.8) |
| FEV1/FVC | 0.81 (0.25) | 0.86 (0.10) |
| PEF, L/min | 194 (79)b | 344 (132) |
| Outcomes | ||
| Received systemic corticosteroid | 75.4% | NA |
| ICU care | 3.5% | NA |
| Death | 0 | NA |
Results expressed in mean (SD) unless stated otherwise. FeNO = fractional exhaled nitric oxide concentration; GINA = Global INitiative for Asthma; ICS = inhaled corticosteroid; NA = not available or applicable; PEF = peak expiratory flow; Sao2 = arterial oxygen saturation.
Aged ≥ 6 y in 115 (55.0%) and 63 (81.8%) patients with asthma exacerbation and stable asthma, respectively (P < .001).
P < .001 for between-group comparisons.
Among children aged ≥ 6 y, FeNO was successfully measured in 35 (30.4%) patients with asthma exacerbation and 61 (96.8%) of those with stable asthma (P < .001).
P < .05 for between-group comparisons.
Statistical Analysis
As RTIs are age-dependent, we tried to match one control per patient with respect to their age and sex. However, we failed to recruit this target number of controls because many children with stable asthma had RTI symptoms during winter. The detection rates for respiratory pathogens between cases and controls were analyzed by χ2 or Fisher exact test. The severity of asthma exacerbation, FeNO, and spirometric parameters were analyzed between subgroups with different pathogens by χ2 or Student t test. Multivariate logistic regression was used to identify respiratory pathogens associated with asthma exacerbation, adjusted for age, inhaled corticosteroid (ICS) treatment, and domestic tobacco smoke exposure as covariates. All analyses were performed two-tailed using SPSS v.14 (SPSS Inc.; Chicago, IL), with the level of significance set at .05.
Results
Two hundred nine children with asthma exacerbation, including 203 patients hospitalized in our pediatric wards and six who attended our outpatient clinics, and 77 controls with stable asthma were recruited. Table 1 shows the characteristics of these patients. Children with asthma exacerbation were younger than the controls, mainly because of our inability to recruit one age-matched control for each child with asthma exacerbation. Similar proportions of patients in the two groups received regular ICS treatment.
Sufficient respiratory samples were collected from 206 (98.6%) cases and all controls; these consisted of 236 NPA samples and 47 nasal swabs. Respiratory pathogens were detected in 105 (51.0%) subjects. Table 2 summarizes the distributions of respiratory pathogens in two groups of patients. The presence of any virus with or without atypical bacteria was associated with asthma exacerbation (P < .001 for both). Specifically, HRV infection was more common among children with asthma exacerbation (odds ratio [OR], 2.38; 95% CI, 1.09–5.32; P = .018). On logistic regression, asthma exacerbation was associated with the detection of HRV (OR, 2.36; 95% CI, 1.11–5.00; P = .025), any respiratory virus (OR, 2.19; 95% CI, 1.17–4.08; P = .014), or any respiratory pathogen (OR, 2.15; 95% CI, 1.16–4.00; P = .015). None of the respiratory pathogens or their coinfection was associated with the severity of asthma exacerbation (P > .15 for all). Age, gender, and ICS treatment did not affect the detection of respiratory pathogens in patients with asthma exacerbation (P > .1 for all).
Table 2.
Detection of Different Viral and Bacterial Pathogens in Subjects
| Asthma Exacerbation(n = 206a) | Stable Asthma(n = 77) | P Valueb | |
|---|---|---|---|
| Individual organism | |||
| Rhinovirus | 54 (26.2) | 10 (13.0) | .027c |
| Human metapneumovirus | 12 (5.8) | 2 (2.6) | .364 |
| Influenza A virus | 16 (7.8) | 4 (5.2) | .624 |
| Influenza B virus | 3 (1.5) | 0 | .565 |
| Parainfluenza viruses types 1-4 | 14 (6.8) | 2 (2.6) | .250 |
| Respiratory syncytial virus | 8 (3.9) | 1 (1.3) | .452 |
| Bocavirus | 5 (2.4) | 2 (2.6) | 1.000 |
| Adenovirus | 5 (2.4) | 0 | .328 |
| Human coronaviruses OC43 or 229E | 5 (2.4) | 0 | .328 |
| Enterovirus | 2 (1.0) | 0 | 1.000 |
| Mycoplasma pneumoniae | 2 (1.0) | 2 (2.6) | .299 |
| Chlamydophila pneumoniae | 4 (1.9) | 1 (1.3) | 1.000 |
| Presence of any virus | 103 (50.0) | 20 (26.0) | < .001c |
| M pneumoniae or C pneumoniae | 5 (2.4) | 1 (1.3) | 1.000 |
| Presence of any pathogen | 105 (51.0) | 21 (27.3) | < .001c |
| Coinfection by two or more pathogens | 22 (10.7) | 2 (2.6) | 0.053 |
Insufficient respiratory specimens were obtained from three patients.
Analyzed by χ2 (with Yates correction) or Fisher exact test as appropriate.
Odds ratios (95% CI) for asthma exacerbation were: 2.38 (1.09–5.32) for rhinovirus, 2.85 (1.54–5.30) for any virus, and 2.77 (1.51–5.11) for any pathogen.
Table 3 summarizes the clinical features of patients with asthma exacerbation in relation to HRV infections. FeNO was the only parameter that differed between patients with and without HRV, being significantly lower in the former group (P = .018). Ten controls were HRV positive, and mean (SD) FeNO of those with and without HRV were 38.6 (17.9) ppb and 82.2 (61.3) ppb, respectively (P < .001). Table 4 summarizes the relationship between age and different respiratory pathogens in patients with asthma exacerbation. Patients with asthma exacerbation caused by respiratory viruses were younger than those without identifiable viral infections (P < .05). This finding was attributed mainly to RSV (P < .005) and influenza A and HMPV infections (P < .05 for both). Age did not differ between the case and control groups with infections by other organisms, including HRV, or with coinfections.
Table 3.
Details of Asthma Exacerbations in Relation to Rhinovirus Infection
| Rhinovirus | No Rhinovirus | P Valuea | |
|---|---|---|---|
| Age, y | 7.3 (3.8) | 7.7 (4.1) | .462 |
| Duration of hospitalization, d | 3.4 (1.4) | 3.7 (2.0) | .349 |
| Duration of fever, d | 0.40 (0.63) | 0.53 (0.93) | .248 |
| Maximum temperature, °C | 38.5 (0.6) | 38.7 (0.7) | .266 |
| Vital signs | |||
| Minimum Sao2, % | 93.8 (2.8) | 94.3 (2.3) | .333 |
| Maximum pulse rate, per min | 139 (20) | 135 (22) | .181 |
| Maximum respiratory rate, per min | 34 (9) | 34 (9) | .645 |
| Systolic blood pressure, mm Hg | 108 (15) | 110 (15) | .426 |
| Diastolic blood pressure, mm Hg | 70 (11) | 69 (10) | .486 |
| Laboratory results | |||
| FeNO, ppb | 31.7 (20.3) | 62.4 (44.8) | .018 |
| FEV1, % predicted | 68.1 (28.1) | 74.3 (20.4) | .569 |
| FVC, % predicted | 70.7 (27.1) | 84.0 (33.4) | .251 |
| FEV1 to FVC ratio | 0.82 (0.11) | 0.81 (0.27) | .800 |
| PEF, L/min | 156 (57) | 201 (81) | .104 |
Results expressed in mean (SD). See Table 1 for expansion of abbreviations.
Analyzed by Student t test.
Table 4.
The Relationship Between Different Respiratory Pathogens and Age Distributions of 206 Evaluable Patients With Asthma Exacerbations
| Infection (y) | No Infection (y) | |
|---|---|---|
| Individual organism | ||
| Rhinovirus | 7.3 (3.8) | 7.7 (4.1) |
| Human metapneumovirus | 5.8 (2.8)a | 7.7 (4.1) |
| Influenza A virus | 6.0 (3.0)a | 7.7 (4.1) |
| Parainfluenza viruses types 1-4 | 7.7 (4.9) | 7.6 (4.0) |
| Respiratory syncytial virus | 4.5 (2.1)b | 7.7 (4.1) |
| Bocavirus | 7.4 (2.8) | 7.6 (4.1) |
| Adenovirus | 6.2 (3.8) | 7.6 (4.1) |
| Human coronaviruses OC43 or 229E | 8.3 (4.3) | 7.6 (4.1) |
| Presence of any virus | 6.9 (3.5)a | 8.3 (4.4) |
| Mycoplasma pneumoniae or Chlamydophilia pneumoniae | 6.3 (4.0) | 7.6 (4.1) |
| Presence of any pathogen | 6.9 (3.5)c | 8.4 (4.4) |
| Coinfection by two or more pathogens | 6.5 (4.1) | 7.7 (4.0) |
Results expressed in mean (SD) and only included data for pathogens that were detected in five or more patients.
P < .05 for between-group comparisons.
P < .005 for between-group comparisons.
P < .01 for between-group comparisons.
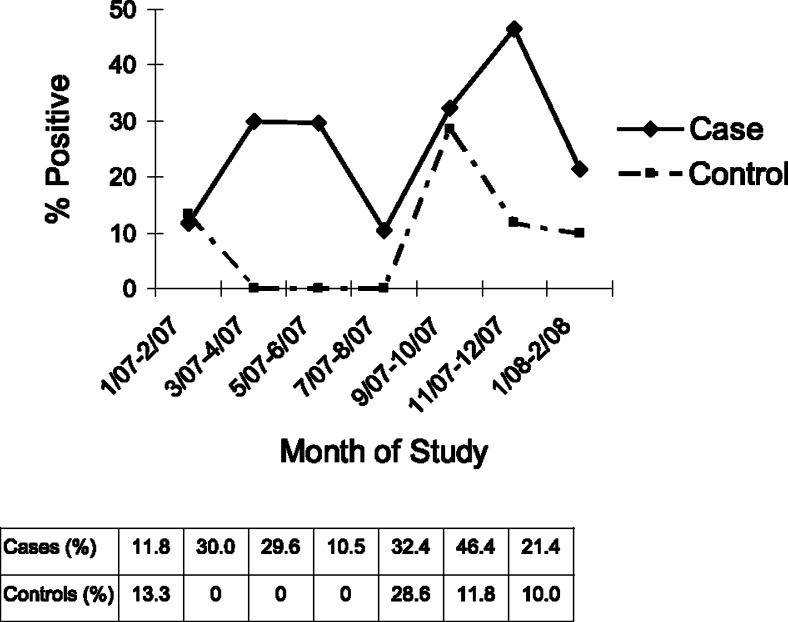
Figure 1 illustrates the seasonal pattern of HRV, which was found in patients throughout the study period (≥ 10%) and peaked in winter (November-December) of 2007 to 2008. Similarly, 28.6% of controls had HRV in autumn-winter (September-October), but none of them were HRV positive in spring-summer (March-August). The low positive rates for other respiratory pathogens in our subjects preclude our analysis of their seasonality patterns.
Figure 1.
The detection of human rhinovirus in subjects in relation to timing of this study.
Discussion
Our group performed multiplex nested PCR assays20 on 475 children hospitalized for acute RTIs from 2005 to 2006.21 Respiratory pathogens were detected in 47% of these patients, and HRV peaked in winter and early spring. These multiplex nested PCR assays were specific and 100- to 1,000-fold more sensitive than conventional methods in detecting the viruses. Our assays detected ≤ 10 nucleic acid copies for all viruses (except enteroviruses), which were comparable to those reported in widely quoted high-throughput multiplex PCR assays.21 Specifically, our nested PCR was able to detect one cDNA copy of HRV. The present study used the same method, except for Legionella pneumophila being replaced by HBoV, to investigate the infective causes of asthma exacerbation in Hong Kong children. HRV infections also peaked in winter of 2007/2008 in children with asthma exacerbation (Fig 1). Our detection rate (51%) was similar to previous local studies20, 22 but lower than those published in white populations.23 It is uncertain whether our low HRV detection rate was due to limitations of the PCR technique, which is less likely in view of our previously noted in vitro results, or a genuinely low incidence of HRV infection in Hong Kong children. Further studies in other Asian populations are needed to confirm our findings.
HRV infection was associated with asthma exacerbation in the children, which is consistent with published data about the importance of HRV in white populations. In the Childhood Origins of Asthma birth cohort, a total of 259 children were followed prospectively from birth to 6 years of age.24 HRV-associated wheezing in years 1 and 3 were the strongest predictor for asthma diagnosis at the age of 6 years. Nearly 90% of children who wheezed with HRV in year 3 subsequently developed asthma. Two other studies found HRV to be the most important microbiological risk factors for asthma diagnosis and disease exacerbation.25, 26, 27 More recently, Miller et al28 reported that childhood asthma exacerbations were associated with the novel group C of HRV rather than the two previously known phylogenetic groups A and B. On the other hand, HRV infection was detected in 13% of our subjects with stable asthma who did not experience any symptom or sign of disease exacerbation. In a longitudinal study of healthy children, 20.6% of all HRV infections were asymptomatic.29 Future studies should delineate the pathogenic linkage between HRV and worsened asthma.
During the past few years, there has been impressive advance in our understanding of the interactions between HRV and host immunity.30 HRV infects human cells via ligation with its major group receptor intercellular adhesion molecule 1. Infected respiratory epithelial cells, and possibly macrophages, produce a variety of proinflammatory cytokines, chemokines, and leukotrienes. These mediators in turn attract different inflammatory cells to the airway, resulting in worsened immunopathology and increased bronchial hyperresponsiveness observed in patients with asthma. In addition, airway epithelium from patients with asthma is deficient in mounting adequate antiviral responses to HRV.31, 32
HBoV was detected in 5.0% of 1,906 local children hospitalized for acute RTIs,33 and seasonal distribution was noted from September to February. Despite this, the detection of HBoV in 2.4% of cases and 2.6% of controls was not associated with asthma exacerbation in the present study. This finding might be explained by our exclusion of infants and young children, who were at increased risk of HBoV infection.9, 33
Three-fifths of adults with asthma exacerbation had M pneumoniae and/or C pneumoniae,34 and telithromycin was shown to be a useful treatment in these patients. M pneumoniae was detected in more than half of patients with asthma,11 and also in upper airway secretions from 11% of patients with chronic stable asthma.14 Hahn12 claimed oral macrolides to be efficacious for patients with acute asthma. On the other hand, Cunningham et al10 failed to show any relation between M pneumoniae and childhood asthma exacerbation. M pneumoniae and C pneumoniae were detected only in 2.4% of our children with asthma exacerbations, which was similar to that observed in patients with stable asthma. Our findings do not support atypical bacteria to be important pathogens for asthma exacerbations in children or the usefulness of macrolides in treating these patients.
The major limitation of this project relates to its study power. The number of controls was much lower than that of our recruited cases, mainly because case-control matching within 1 week was not possible on many occasions when stable and especially younger patients also complained of nonspecific upper respiratory symptoms (eg, rhinorrhea, blocked nose, sore throat) during change of weather. Our sample size had a power of 97% for detecting any difference in the detection of any virus between cases and controls, but had a marginal power of 70% for HRV infection (GraphPad StatMate; San Diego, CA) and < 50% for the detection of other respiratory pathogens because of their rarity. Thus, larger studies are needed to delineate the possible association between asthma exacerbation and RTIs by these organisms. The lack of standardization on the methodology of HRV detection would also pose a problem. A recent study revealed that HRVs consist of > 100 distinct serotypes.35 In view of this degree of phylogenetic heterogeneity, the PCR primers designed for our multiplex assays were not able to detect all HRVs. Despite the use of sensitive multiplex assays as discussed previously, our molecular approach for detecting viruses would miss some HRVs that were not covered by our PCR primers. Future studies need to adopt multiple PCR primers that specifically target as many HRV serotypes as possible. Another weakness is that NPA samples were collected from 236 (83.4%) subjects, whereas nasal swabs were collected from the remaining subjects. As we previously reported, the overall sensitivity of detecting influenza, parainfluenza, RSV, and adenovirus in NPA was higher than that obtained by nasal swabs in local children.36 On the other hand, we did not have relevant data for HRV. Although more than 80% of subjects had NPA samples, it is possible that we might have missed some organisms in those with only nasal swabs. We also observed that only 30% of patients hospitalized for asthma exacerbation had successfully performed FeNO measurement according to guideline (Table 1).18 As patients with severe bronchospasm were probably too breathless for the procedure, only patients with milder attacks would contribute to FeNO readings in this group. In addition, a substantial proportion of these patients were treated with systemic corticosteroids prior to FeNO. These reasons explain the lower FeNO in these patients when compared with patients with stable asthma.
In conclusion, respiratory viruses and atypical bacteria are detected in more than half of Hong Kong children with asthma exacerbation. HRV infection is the most important risk factor for asthma exacerbation in these patients. Nonetheless, none of these pathogens is associated with severity of asthma exacerbation.
Acknowledgments
Author contributions: Dr Leung: contributed to the development of the study design, collected study data, performed statistical analysis, and wrote the manuscript.
Mr To: contributed to performing clinical and virologic investigations.
Mr Yeung: contributed to performing clinical and virologic investigations.
Mr Y. S. Wong: contributed to performing clinical and virologic investigations.
Dr G. W. K. Wong: contributed to subject recruitment and manuscript preparation.
Dr Chan: contributed to designing and supervising virologic investigations and participated in manuscript preparation.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Footnotes
Funding/Support: This study was supported by the Research Fund for the Control of Infectious Diseases [project no. 05050202], Food and Health Bureau of Hong Kong SAR.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/site/misc/reprints.xhtml).
Supplementary Material
References
- 1.Wong GW, Hui DS, Chan HH. Prevalence of respiratory and atopic disorders in Chinese schoolchildren. Clin Exp Allergy. 2001;31(8):1225–1231. doi: 10.1046/j.1365-2222.2001.01140.x. [DOI] [PubMed] [Google Scholar]
- 2.Nelson EA, Tam JS, Yu LM, Li AM, Chan PK, Sung RY. Assessing disease burden of respiratory disorders in Hong Kong children with hospital discharge data and linked laboratory data. Hong Kong Med J. 2007;13(2):114–121. [PubMed] [Google Scholar]
- 3.Johnston SL, Pattemore PK, Sanderson G. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995;310(6989):1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thumerelle C, Deschildre A, Bouquillon C. Role of viruses and atypical bacteria in exacerbations of asthma in hospitalized children: a prospective study in the Nord-Pas de Calais region (France) Pediatr Pulmonol. 2003;35(2):75–82. doi: 10.1002/ppul.10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neuzil KM, Wright PF, Mitchel EF, Jr, Griffin MR. The burden of influenza illness in children with asthma and other chronic medical conditions. J Pediatr. 2000;137(6):856–864. doi: 10.1067/mpd.2000.110445. [DOI] [PubMed] [Google Scholar]
- 6.Moës E, Vijgen L, Keyaerts E. A novel pancoronavirus RT-PCR assay: frequent detection of human coronavirus NL63 in children hospitalized with respiratory tract infections in Belgium. BMC Infect Dis. 2005;5(1):6. doi: 10.1186/1471-2334-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan PK, Tam JS, Lam CW. Human metapneumovirus detection in patients with severe acute respiratory syndrome. Emerg Infect Dis. 2003;9(9):1058–1063. doi: 10.3201/eid0909.030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peiris JS, Tang WH, Chan KH. Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerg Infect Dis. 2003;9(6):628–633. doi: 10.3201/eid0906.030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA. 2005;102(36):12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham AF, Johnston SL, Julious SA, Lampe FC, Ward ME. Chronic Chlamydia pneumoniae infection and asthma exacerbations in children. Eur Respir J. 1998;11(2):345–349. doi: 10.1183/09031936.98.11020345. [DOI] [PubMed] [Google Scholar]
- 11.Johnston SL. Influence of viral and bacterial respiratory infections on exacerbations and symptom severity in childhood asthma. Pediatr Pulmonol Suppl. 1997;16:88–89. doi: 10.1002/ppul.1950230851. [DOI] [PubMed] [Google Scholar]
- 12.Hahn DL. Treatment of Chlamydia pneumoniae infection in adult asthma: a before-after trial. J Fam Pract. 1995;41(4):345–351. [PubMed] [Google Scholar]
- 13.Kraft M, Cassell GH, Henson JE. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am J Respir Crit Care Med. 1998;158(3):998–1001. doi: 10.1164/ajrccm.158.3.9711092. [DOI] [PubMed] [Google Scholar]
- 14.Martin RJ, Kraft M, Chu HW, Berns EA, Cassell GH. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107(4):595–601. doi: 10.1067/mai.2001.113563. [DOI] [PubMed] [Google Scholar]
- 15.British Thoracic Society, Scottish Intercollegiate Guidelines Network (SIGN) British guideline on the management of asthma. Thorax. 2003;58(suppl 1):1–94. doi: 10.1136/thorax.58.suppl_1.1i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ, The Group Health Medical Associates Asthma and wheezing in the first six years of life. N Engl J Med. 1995;332(3):133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 17.National Institutes of Health, National Heart, Lung, and Blood Institute . Global Initiative for Asthma. National Heart, Lung, and Blood Institute; Bethesda, MD: 2002. NIH Publication No. 02-3659. [Google Scholar]
- 18.Baraldi E, de Jongste JC, European Respiratory Society, American Thoracic Society Measurement of exhaled nitric oxide in children, 2001. Eur Respir J. 2002;20(1):223–237. doi: 10.1183/09031936.02.00293102. [DOI] [PubMed] [Google Scholar]
- 19.Heikkinen T, Salmi AA, Ruuskanen O. Comparative study of nasopharyngeal aspirate and nasal swab specimens for detection of influenza. BMJ. 2001;322(7279):138. doi: 10.1136/bmj.322.7279.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam WY, Yeung AC, Tang JW. Rapid multiplex nested PCR for detection of respiratory viruses. J Clin Microbiol. 2007;45(11):3631–3640. doi: 10.1128/JCM.00280-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee W-M, Grindle K, Pappas T. High-throughput, sensitive, and accurate multiplex PCR-microsphere flow cytometry system for large-scale comprehensive detection of respiratory viruses. J Clin Microbiol. 2007;45(8):2626–2634. doi: 10.1128/JCM.02501-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sung RY, Chan PK, Tsen T. Identification of viral and atypical bacterial pathogens in children hospitalized with acute respiratory infections in Hong Kong by multiplex PCR assays. J Med Virol. 2009;81(1):153–159. doi: 10.1002/jmv.21364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh AM, Busse WW. Asthma exacerbations. 2: aetiology. Thorax. 2006;61(9):809–816. doi: 10.1136/thx.2005.045179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson DJ, Gangnon RE, Evans MD. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178(7):667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khetsuriani N, Kazerouni NN, Erdman DD. Prevalence of viral respiratory tract infections in children with asthma. J Allergy Clin Immunol. 2007;119(2):314–321. doi: 10.1016/j.jaci.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gern JE, Calhoun W, Swenson C, Shen G, Busse WW. Rhinovirus infection preferentially increases lower airway responsiveness in allergic subjects. Am J Respir Crit Care Med. 1997;155(6):1872–1876. doi: 10.1164/ajrccm.155.6.9196088. [DOI] [PubMed] [Google Scholar]
- 27.Venarske DL, Busse WW, Griffin MR. The relationship of rhinovirus-associated asthma hospitalizations with inhaled corticosteroids and smoking. J Infect Dis. 2006;193(11):1536–1543. doi: 10.1086/503809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller EK, Edwards KM, Weinberg GA, New Vaccine Surveillance Network A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol. 2009;123(1):98–104. doi: 10.1016/j.jaci.2008.10.007. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winther B, Hayden FG, Hendley JO. Picornavirus infections in children diagnosed by RT-PCR during longitudinal surveillance with weekly sampling: Association with symptomatic illness and effect of season. J Med Virol. 2006;78(5):644–650. doi: 10.1002/jmv.20588. [DOI] [PubMed] [Google Scholar]
- 30.Kelly JT, Busse WW. Host immune responses to rhinovirus: mechanisms in asthma. J Allergy Clin Immunol. 2008;122(4):671–682. doi: 10.1016/j.jaci.2008.08.013. quiz 683-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wark PA, Johnston SL, Bucchieri F. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201(6):937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Contoli M, Message SD, Laza-Stanca V. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12(9):1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 33.Ip M, Nelson EA, Cheuk ES, Leung E, Sung R, Chan PK. Pediatric hospitalization of acute respiratory tract infections with human bocavirus in Hong Kong. J Clin Virol. 2008;42(1):72–74. doi: 10.1016/j.jcv.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 34.Johnston SL, Blasi F, Black PN, Martin RJ, Farrell DJ, Nieman RB, TELICAST Investigators The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354(15):1589–1600. doi: 10.1056/NEJMoa044080. [DOI] [PubMed] [Google Scholar]
- 35.Palmenberg AC, Spiro D, Kuzmickas R. Sequencing and analyses of all known human rhinovirus genomes reveal structure and evolution. Science. 2009;324(5923):55–59. doi: 10.1126/science.1165557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sung RY, Chan PK, Choi KC. Comparative study of nasopharyngeal aspirate and nasal swab specimens for diagnosis of acute viral respiratory infection. J Clin Microbiol. 2008;46(9):3073–3076. doi: 10.1128/JCM.01209-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.