Abstract
Objectives
To investigate the occurrence of respiratory pathogens in samples from children with and without respiratory symptoms and to identify whether age and/ or coinfections modify the impact of respiratory pathogens on symptoms.
Study design
In a prospective longitudinal study, 18 children were sampled biweekly for respiratory pathogens, irrespective of respiratory symptoms. Polymerase chain reaction was performed for 13 respiratory pathogens. Episodes were defined “asymptomatic” if no symptoms of any respiratory tract illness were present between 1 week before and 1 week after sampling.
Results
A total of 230 samples were collected. In 56% of the symptomatic episodes, a pathogen was detected, compared with 40% of the asymptomatic episodes (P = .03). Rhinovirus and coronaviruses were most prevalent in both symptomatic and asymptomatic episodes. In the youngest children, 9% of the pathogen-positive episodes were asymptomatic, compared with 36% in the oldest children (P = .01). Multiple pathogens were found in 17% of the symptomatic episodes and in 3% of the asymptomatic episodes (P = .02).
Conclusions
Respiratory pathogens are frequently detected in samples from children with no respiratory symptoms. Symptomatic cases occurred more often in younger children and with detections of more than 1 respiratory pathogen.
Abbreviations: PCR, Polymerase chain reaction; RSV, Respiratory syncytial virus
Respiratory tract infections occur frequently in early infancy and account for a major percentage of morbidity and mortality in childhood. Virus infections seem to be responsible for most of this burden. Since the introduction of molecular detection techniques, such as polymerase chain reaction (PCR), the percentage of pathogens found during respiratory tract illnesses in published studies has increased dramatically, up to 85%.1, 2, 3, 4
Although many studies have investigated the prevalence of respiratory pathogens during respiratory illnesses, little is known about the prevalence of pathogens in nonsymptomatic children. Whether pathogens are actually the cause of the respiratory symptoms or are simply colonizing the respiratory tract during symptomatic episodes remains unclear. It can be speculated that not every infection with a pathogen leads to respiratory symptoms and that pathogenicity might depend on host or environmental factors. In young children, the respiratory and immune systems are immature and may be more susceptible to respiratory pathogens.5 We hypothesized that infections with respiratory pathogens are likely to have the most serious effect on young children with developing respiratory and immunologic systems. Furthermore, we hypothesized that infection with multiple respiratory pathogens will more often lead to respiratory symptoms compared with infection with a single pathogen.6, 7, 8, 9
The aims of our study were to determine the prevalence of respiratory pathogens in the presence or absence of respiratory tract symptoms in prospectively sampled young children, and to identify whether age and coinfections modify the impact of a pathogen on illness. Sensitive PCR techniques were used to detect 13 common respiratory pathogens, 11 viruses, and 2 atypical bacteria.
Methods
Study Design and Subjects
A prospective longitudinal study was conducted during a 6-month winter season (November 2004 through April 2005). A total of 19 healthy children age 0 to 7 years were enrolled, 1 of which failed to complete the study. None of the children had a history of asthma or recurrent respiratory complaints. Parents were contacted twice a week by telephone or e-mail by 1 of the 2 study coordinators to determine the presence of any symptoms of respiratory tract illness. Respiratory tract symptoms were defined as symptoms of coryza (rhinorrhea or nasal congestion), sore throat, earache with or without ear discharge, cough, sputum production, or dyspnea, all with or without temperature above 38°C.
Samples were collected every 2 weeks regardless of the presence or absence of respiratory symptoms. The biweekly sampling frequency during the study resulted in about 13 subsequent observation episodes per child. An episode was defined as “asymptomatic” if there were no respiratory symptoms during a complete period of 1 week before to 1 week after sampling. An episode was defined as “symptomatic” if there were any respiratory symptoms during the period of 1 week before to 1 week after sampling.
The study design was approved by the local Medical Ethics Committee, and all parents gave written informed consent.
Detection of Respiratory Pathogens
Respiratory pathogens were detected by PCR. After receiving precise instruction at the beginning of the study, parents collected the samples by rubbing 1 of the child's nostrils and the posterior oropharynx using separate cotton-tipped swabs. After sampling, the 2 swabs were collected into a single vial containing GLY medium with 0.1 mg/mL of pimaricine as a viral transport medium and sent to our laboratory by regular mail. Samples were stored at −20°C until analysis. Sampling of respiratory pathogens by parents using nose and throat swabs has proven feasible and reliable. Both the sampling frequency and the viral recovery rate in parental samples are higher compared with sampling by a dedicated research nurse.10, 11
PCR was performed at the National Institute of Public Health and the Environment, Bilthoven, The Netherlands. The respiratory pathogens human rhinovirus and enterovirus, human metapneumovirus, human coronaviruses OC43 and 229E, Chlamydophila pneumoniae, and Mycoplasma pneumoniae were analyzed by conventional PCR, essentially as described previously.3
The PCR for adenovirus consisted of 40 cycles of 1 minute at 94°C, 1.5 minute at 45°C, 1 minute at 72°C with a final extension of 10 minutes at 72°C (PE 9700) with the following primers: forward, 5′-GCCGCAGTGGTCTTACATGCACAT-3′; reverse, 5′-ARCACICCICGRATGTCAAAG-3′ and 5′-CAGCACGCCGCGGATGTCAAAGT-3′ targeting the hexon gene. Amplicons were analyzed by gel electrophoresis.
Real-time PCR for human coronavirus NL63, influenza viruses A and B, and respiratory syncytial virus (RSV) A and B was performed using the Lightcycler 2.0 format with Lightcycler Taqman Mastermix (Roche, Germany). A separate reverse-transcription step with avian myeloblastosis virus reverse transcriptase was used for 60 min at 42°C for NL63 and for 60 minutes at 50°C for influenza and RSV.
The reaction for NL63 consisted of 1 cycle of 10 minutes at 95°C and 45 cycles of 5 seconds at 50°C and 10 seconds at 72°C, with primers 5′-AACCTAATAAGCCTCTTTCTC-3′ and 5′-TTTGGCATCACCATTCTG-3′ and probe 5′-6FAM-AGTGCTTTGGTCCTCGTG-Tamra-3′ targeting the nucleocapsid gene, as provided by L. van der Hoek.12
The reaction for influenza consisted of 1 cycle of 10 minutes at 95°C, and 45 cycles of 10 seconds at 95°C, 20 seconds at 50°C, and 10 seconds at 72°C, with primers 5′-AAGACCAATCCTGTCACCTCTGA-3′ and 5′-CAAAGCGTCTACGCTGCAGTCC-3′ with probe 5′-6Fam-TTTGTGTTCACGCTCACCGTGCC-BHQ1-3′ for influenza A, targeting the M pneumoniae gene, and 5′-TGAAGGACATTCAAAGC-3′ and 5′-ACCAGTCTAATTGTCTC-3′ with probe 5′-YY-AGCACCGATTACACCAG-BHQ1-3′ for influenza B, targeting the NS gene.
The reaction for RSV consisted of I cycle of 10 minutes at 95°C and 45 cycles of 15 seconds at 95°C and 47 seconds at 60°C with primers 5′-TGAACAACCCAAAAGCATCA-3′ and 5′-CCTAGGCCAGCA GCATTG-3′ with probe 5′-6Fam-AATTTCCTCACTTCTCCAGTGTAGTATTAGG-BHQ1-3′ for RSV A and 5′-TGTCAATATTATCTCCTGTACTACGTTGAA-3′ and 5-GATGGCTCTTAGCAAAGTCAAGTTAA-3′ with probe 5′-YY-TGATACATTAAATAAGGATCAGCTGCTGTCATCCA-BHQ1-3′ for RSV B, both targeting the nucleocapsid gene.
Each sample was spiked with the equine arteritis virus as an internal control to detect inhibition of RNA isolation and PCR.13
Statistical Analysis
All statistical analyses were done using SPSS version 12.0 (SPSS Inc, Chicago, Illinois). A 2-tailed χ2 test was used to compare differences between groups. Differences were considered statistically significant at a P value ≤ .05.
Results
During a period of 460 child-weeks, respiratory symptoms in were recorded in 18 children. There were 2 sibling pairs; all other children were unrelated. Other characteristics of the study group are presented in Table I.
Table I.
Characteristics of the study group (n =18)
| Sex, M/F | 3/15 |
| Age, years | 3.67 (0-7) |
| 0-2 | 8 |
| 3-4 | 6 |
| 5-7 | 4 |
| Number of siblings/child | 1.50 (0-4) |
| Number of symptomatic episodes/child | 9.50 (4-15) |
| Number of asymptomatic infections/child | 3.00 (0-9) |
Data are expressed as median and range in parentheses.
A total of 230 biweekly samples from both symptomatic and asymptomatic episodes were tested for the presence of respiratory pathogens; 119 samples (52%) were positive for at least 1 respiratory pathogen. Table II gives the results of the PCR testing of samples obtained during symptomatic and asymptomatic episodes. Significantly greater numbers of pathogens were found in symptomatic episodes compared with the asymptomatic episodes. A pathogen was detected in 56% of the symptomatic episodes, compared with 40% of the asymptomatic episodes (P = .03). Rhinovirus was the most prevalent virus, found in 23% of the symptomatic episodes and in 22% of the asymptomatic episodes; this difference was not significant (P = .88). The second most prevalent pathogens were the coronaviruses, which also were detected in comparable numbers in symptomatic and asymptomatic episodes (P = .38). Human metapneumovirus and M pneumoniae were never detected as a monoinfection, but only as a coinfection with another pathogen (Table III; available at www.jpeds.com).
Table II.
Respiratory pathogens detected in asymptomatic and symptomatic episodes in young children
| Asymptomatic samples (n = 65) | Symptomatic samples (n = 165) | P value | |
|---|---|---|---|
| Any pathogen | 26 (40) | 93 (56) | .03 |
| Human rhinovirus | 14 (22) | 38 (23) | .88 |
| Enterovirus | 2 (3) | 7 (4) | NA |
| Coronaviruses (total) | 5 (8) | 15 (9) | .38 |
| HCoV OC43 | 1 (2) | 6 (4) | |
| HCoV 229E | 4 (6) | 6 (4) | |
| HCoV NL63 | 0 | 3 (2) | |
| RSV B | 0 | 1 (1) | NA |
| Influenza virus A | 0 | 1 (1) | NA |
| Human metapneumovirus | 0 | 0 | NA |
| Adenovirus | 0 | 1 (1) | NA |
| Chlamydophila pneumoniae | 3 (5) | 2 (1) | NA |
| Mycoplasma pneumoniae | 0 | 0 | NA |
| Multiple pathogens | 2 (3) | 28 (17) | .02 |
NA, not applicable; HcoV, human coronavirus.
Data are presented as numbers with percentage of the samples in parentheses. P values are calculated with the 2-tailed χ2 test.
Table III.
Combinations of respiratory pathogens found in samples with multiple pathogens detected
| Asymptomatic episodes (n = 2) | Symptomatic episodes (n = 28) | |
|---|---|---|
| HRV + CP | 1 | 3 |
| HRV + HCoV OC43 | 1 | 3 |
| HRV + MP | 4 | |
| HRV + HCoV 229E | 5 | |
| HRV +IVB | 1 | |
| HRV + RSVB | 1 | |
| HRV + CP + MP | 1 | |
| HRV + CP + RSVB | 1 | |
| HRV + MP + HCoV OC43 | 1 | |
| HCoV 229E + HCoV OC43 | 1 | |
| HCoV 229E + HCoV NL63 | 1 | |
| HCoV 229E + CP | 1 | |
| HCoV 229E + hMPV + EV | 1 | |
| HCoV 229E + HCoV OC43 + hMPV | 1 | |
| HCoV 229E + HCoV OC43 + EV | 1 | |
| HCoV OC43 + RSVA | 1 | |
| HCoV OC43 + RSVB | 1 |
HRV, human rhinovirus; CP, Chlamydophila pneumoniae; HcoV, human coronavirus; MP, Mycoplasma pneumoniae; IVB, influenza virus B; hMPV, human metapneumovirus; EV, enterovirus.
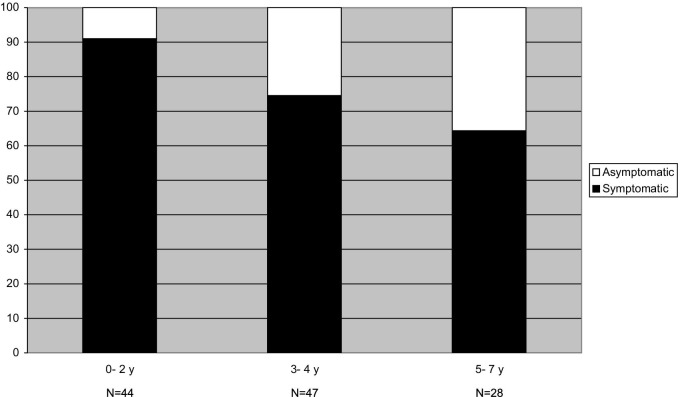
We analyzed the influence of age on the occurrence of symptoms during an episode. For this reason, we selected the episodes in which at least 1 pathogen was detected and divided them into 3 age categories. Figure 1 shows the distribution of symptomatic and asymptomatic episodes in the different age categories. With increasing age, pathogen-positive children are increasingly asymptomatic. Only 9% of the pathogen-positive episodes were asymptomatic in children age 0 to 2 years, compared with 26% in those age 3 to 4 years and 36% in those age 5 to 7 years (P = .04 and .01, respectively; χ2 test).
Figure 1.
A selection of pathogen- positive episodes. The proportion of symptomatic and asymptomatic episodes was studied in 3 different age categories.
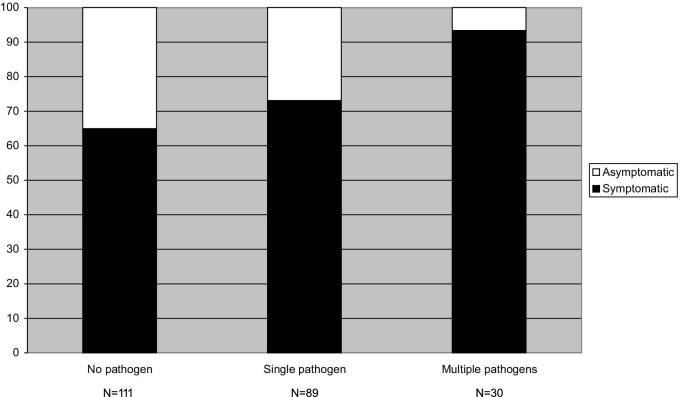
Multiple pathogens were seen in 17% of the symptomatic episodes, compared with 3% of the asymptomatic episodes (P = .01). Figure 2 shows the distribution of symptomatic and asymptomatic episodes when no pathogen, a single pathogen, or multiple pathogens were detected. If no pathogen was found, then 35% of the episodes were asymptomatic, compared with 7% of the episodes with multiple pathogens (P < .01).
Figure 2.
Percentages of the symptomatic and asymptomatic samples related to the number of pathogens detected.
Discussion
Our data indicate that respiratory pathogens are frequently found in samples from children with no respiratory symptoms (∼40%). Rhinovirus and coronaviruses were found in most of the symptomatic cases and asymptomatic cases. Symptomatic cases were more often associated with detection of more than 1 respiratory pathogen.
Two limitations of this study are the small sample size and the fact that we sampled only during the winter season. These aspects limit the generalizability of our results. Nevertheless, the longitudinal sampling of healthy children at home and the use of a broad panel of respiratory pathogens for detection gives a detailed picture of the prevalence of respiratory pathogens during a winter season in young children.
The most prevalent virus in our study was rhinovirus, found in both symptomatic and asymptomatic episodes. The fact that rhinovirus is often found in asymptomatic children is not surprising, because it is generally a relatively mild pathogen that can colonize the nasal mucosa without causing symptoms.4 On the other hand, recent studies attribute a more important role to rhinovirus in both upper and lower respiratory tract infections.2, 14 Presumably host and environmental determinants play roles in the pathogenicity of this virus.
Coronaviruses were the second most prevalent single virus in both asymptomatic and symptomatic children. This is in keeping with studies in which coronaviruses accounted for approximately 1/3 of common colds in children.15 Coronaviruses often were found in multiple infections,15, 16, 17 which suggest a relatively mild pathogenicity of coronaviruses. The recovery of both RSV and influenza virus was remarkably low in our study, possibly due to the small number and the varying ages (0 to 7 years) of the children enrolled in this study.
Of the asymptomatic children, 40% carried 1 or more pathogens. In the literature, the prevalence of respiratory pathogens in samples from asymptomatic children ranges from 5% to 68%.3, 18, 19, 20, 21 This wide range may be explained by differences in study populations, definitions of symptoms, and sampling and virus detection methods. Most of these studies were performed in older children, usually hospitalized for elective surgery. Furthermore, most of the studies are of a cross-sectional design comparing single symptomatic and asymptomatic episodes in different subjects. Such a design disregards the natural variation of virus colonization in an individual during a certain period. Winther et al20 also sampled longitudinally and found a 9% prevalence of picornavirus in asymptomatic children. This discrepancy with our findings may be explained by the fact that those authors used a period of 4 weeks around the onset of respiratory illness to define symptomatic episodes.
In our study, the youngest children were more often symptomatic when a pathogen was detected compared with older children. This finding is in agreement with the results of a recently published Finnish study on the transmission of rhinovirus within families.11 In that study, most rhinovirus infections in young children were symptomatic, and nearly half of the infections in older children and adults were asymptomatic. Presumably, older children have developed immunity against most respiratory pathogens.
We found a greater prevalence of multiple pathogens in samples from symptomatic children compared with samples from asymptomatic children. There is some debate regarding the association between infections with multiple pathogens and disease severity. Some have argued that multiple pathogens cause more severe disease,6, 7, 8, 9 whereas others have reported no difference between single and multiple infections in terms of disease severity.8, 22, 23 Our findings support the former assertion and indicate an association between multiple infections and illness severity. During our 6-month study period, the children had a median of 9.5 (range, 4 to 15) symptomatic episodes, supporting the fact that apparently normal children can appear “chronically or repeatedly” infected during the respiratory season.
Acknowledgments
We thank all of the parents and children who participated in our study. We also thank B. Zwan and T. Yimam (National Institute of Public Health and the Environment, Bilthoven, The Netherlands) for laboratory assistance.
Footnotes
Supported by a MD/PhD grant from the University Medical Center Utrecht, The Netherlands (to M.vdZ.) and a fellowship from the Wilhelmina Children's Hospital Research Fund (to B.vE.). None of the authors reports any conflict of interest.
References
- 1.Johnston S.L., Pattemore P.K., Sanderson G., Smith S., Lampe F., Josephs L. Community study of role of viral infections in exacerbations of asthma in 9- to 11-year-old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kusel M.M., de Klerk N.H., Holt P.G., Kebadze T., Johnston S.L., Sly P.D. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr Infect Dis J. 2006;25:680–686. doi: 10.1097/01.inf.0000226912.88900.a3. [DOI] [PubMed] [Google Scholar]
- 3.van Gageldonk-Lafeber A.B., Heijnen M.L., Bartelds A.I., Peters M.F., van der Plas S.M., Wilbrink B. A case-control study of acute respiratory tract infection in general practice patients in The Netherlands. Clin Infect Dis. 2005;41:490–497. doi: 10.1086/431982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Benten I., Koopman L., Niesters B., Hop W., van Middelkoop B., de Waal L. Predominance of rhinovirus in the nose of symptomatic and asymptomatic infants. Pediatr Allergy Immunol. 2003;14:363–370. doi: 10.1034/j.1399-3038.2003.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh J.W. Respiratory viral infections and early asthma in childhood. Allergol Int. 2006;55:369–372. doi: 10.2332/allergolint.55.369. [DOI] [PubMed] [Google Scholar]
- 6.Aberle J.H., Aberle S.W., Pracher E., Hutter H.P., Kundi M., Popow-Kraupp T. Single versus dual respiratory virus infections in hospitalized infants: impact on clinical course of disease and interferon-gamma response. Pediatr Infect Dis J. 2005;24:605–610. doi: 10.1097/01.inf.0000168741.59747.2d. [DOI] [PubMed] [Google Scholar]
- 7.Greensill J., McNamara P.S., Dove W., Flanagan B., Smyth R.L., Hart C.A. Human metapneumovirus in severe respiratory syncytial virus bronchiolitis. Emerg Infect Dis. 2003;9:372–375. doi: 10.3201/eid0903.020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papadopoulos N.G., Moustaki M., Tsolia M., Bossios A., Astra E., Prezerakou A. Association of rhinovirus infection with increased disease severity in acute bronchiolitis. Am J Respir Crit Care Med. 2002;165:1285–1289. doi: 10.1164/rccm.200112-118BC. [DOI] [PubMed] [Google Scholar]
- 9.Semple M.G., Cowell A., Dove W., Greensill J., McNamara P.S., Halfhide C. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J Infect Dis. 2005;191:382–386. doi: 10.1086/426457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Zalm M.M., Uiterwaal C.S., de Jong B.M., Wilbrink B., van der Ent C.K. Viral specimen collection by parents increases response rate in population-based virus studies. J Allergy Clin Immunol. 2006;117:955–956. doi: 10.1016/j.jaci.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Peltola V., Waris M., Osterback R., Susi P., Ruuskanen O., Hyypia T. Rhinovirus transmission within families with children: incidence of symptomatic and asymptomatic infections. J Infect Dis. 2008;197:382–389. doi: 10.1086/525542. [DOI] [PubMed] [Google Scholar]
- 12.van der Hoek L., Pyrc K., Jebbink M.F., Vermeulen-Oost W., Berkhout R.J., Wolthers K.C. Identification of a new human coronavirus. Nat Med. 2004;10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheltinga S.A., Templeton K.E., Beersma M.F., Claas E.C. Diagnosis of human metapneumovirus and rhinovirus in patients with respiratory tract infections by an internally controlled multiplex real-time RNA PCR. J Clin Virol. 2005;33:306–311. doi: 10.1016/j.jcv.2004.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemanske R.F., Jr, Jackson D.J., Gangnon R.E., Evans M.D., Li Z., Shult P.A. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 15.Kuypers J., Martin E.T., Heugel J., Wright N., Morrow R., Englund J.A. Clinical disease in children associated with newly described coronavirus subtypes. Pediatrics. 2006;119:70–76. doi: 10.1542/peds.2006-1406. [DOI] [PubMed] [Google Scholar]
- 16.Arden K.E., Nissen M.D., Sloots T.P., Mackay I.M. New human coronavirus, HCoV-NL63, associated with severe lower respiratory tract disease in Australia. J Med Virol. 2005;75:455–462. doi: 10.1002/jmv.20288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boivin G., Baz M., Cote S., Gilca R., Deffrasnes C., Leblanc E. Infections by human coronavirus-NL in hospitalized children. Pediatr Infect Dis J. 2005;24:1045–1048. doi: 10.1097/01.inf.0000183743.68569.c7. [DOI] [PubMed] [Google Scholar]
- 18.Nokso-Koivisto J., Kinnari T.J., Lindahl P., Hovi T., Pitkaranta A. Human picornavirus and coronavirus RNA in nasopharynx of children without concurrent respiratory symptoms. J Med Virol. 2002;66:417–420. doi: 10.1002/jmv.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rakes G.P., Arruda E., Ingram J.M., Hoover G.E., Zambrano J.C., Hayden F.G. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care: IgE and eosinophil analyses. Am J Respir Crit Care Med. 1999;159:785–790. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 20.Winther B., Hayden F.G., Hendley J.O. Picornavirus infections in children diagnosed by RT-PCR during longitudinal surveillance with weekly sampling: association with symptomatic illness and effect of season. J Med Virol. 2006;78:644–650. doi: 10.1002/jmv.20588. [DOI] [PubMed] [Google Scholar]
- 21.Jartti T., Lehtinen P., Vuorinen T., Koskenvuo M., Ruuskanen O. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J Med Virol. 2004;72:695–699. doi: 10.1002/jmv.20027. [DOI] [PubMed] [Google Scholar]
- 22.Simon A., Wilkesmann A., Muller A., Schildgen O. HMPV infections are frequently accompanied by co-infections. Pediatr Pulmonol. 2007;42:98. doi: 10.1002/ppul.20540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkesmann A., Schildgen O., Eis-Hubinger A.M., Geikowski T., Glatzel T., Lentze M.J. Human metapneumovirus infections cause similar symptoms and clinical severity as respiratory syncytial virus infections. Eur J Pediatr. 2006;165:467–475. doi: 10.1007/s00431-006-0105-4. [DOI] [PubMed] [Google Scholar]