Abstract
Background
Nonventilated hospital-acquired pneumonia (NVHAP) is a serious nosocomial infection that is increasingly attributed to antibiotic-resistant bacteria.
Methods
This is a retrospective case-control study comparing patients with and those without NVHAP from January 1, 2014 to December 31, 2014 at Barnes-Jewish Hospital, a 1,300-bed urban academic medical center in St. Louis, Missouri.
Results
One hundred seventy-four consecutive patients with NVHAP were enrolled. A random sample of 696 control patients matched by age, sex, race, and hospital admission date were selected from a total of 5,322 potential matched control subjects. NVHAP was pathogen-negative in 98 cases (56.3%). Respiratory viruses were identified in 42 patients (24.1%), gram-negative bacteria were seen in 25 patients (14.4%), and gram-positive bacteria were identified in 20 patients (11.5%). Individuals in whom NVHAP developed were more likely to die (15.5% vs 1.6%; P < .01), to require intensive care (56.3% vs 22.8%; P < .01) or mechanical ventilation (19.0% vs 3.9%; P < 0.01), and to have a longer hospital length of stay (15.9 days [range, 9.8-26.3 days] vs 4.4 days [range, 2.9-7.3 days]; P < 0.01). This case-control study identified a strong association between hospital mortality and NVHAP, with patients who acquired NVHAP having an 8.4 times greater odds of death (95% CI, 5.6-12.5).
Conclusions
The occurrence of NVHAP was associated with significant increases in mortality, the use of intensive care and mechanical ventilation, and hospital length of stay. We also found that respiratory viruses were an important cause of NVHAP. These findings suggest that efforts aimed at the successful prevention of NVHAP could improve patient outcomes and reduce health-care costs.
Key Words: antibiotic resistance, outcomes, pneumonia
Abbreviations: HAP, hospital-acquired pneumonia; MDR, multidrug-resistant; NVHAP, nonventilated hospital-acquired pneumonia; VAP, ventilator-associated pneumonia
FOR EDITORIAL COMMENT SEE PAGE 991
Hospital-acquired pneumonia (HAP) is a frequent and severe infection in hospitalized patients, with most reports focusing on HAP acquired in ICUs in the form of ventilator-associated pneumonia (VAP).1, 2, 3 Increasingly, antibiotic-resistant pathogens including extended-spectrum beta-lactamase-producing and carbapenem-resistant Enterobacteriaceae, methicillin-resistant Staphylococcus aureus, and multidrug-resistant (MDR) nonfermenting gram-negative bacilli (Pseudomonas aeruginosa, Acinetobacter baumannii, Stenotrophomonas maltophilia) are associated with HAP.4 Respiratory viruses have also recently been identified as potentially important causative pathogens for HAP.5 Antibiotic-resistant bacteria as well as respiratory viruses pose an ongoing challenge to hospitals, both in patient treatment and in the prevention of transmission of these pathogens from patient to patient. Unfortunately, most clinical studies assessing the impact of HAP on patient outcomes6, 7, 8 and guidelines for the prevention of HAP1, 9, 10, 11 are directed at VAP, with little attention focused on nonventilated HAP (NVHAP). This is likely the result of the greater severity of illness in patients in the ICU setting, as well as the ability to more precisely define the presence of true infection in ventilated patients with pneumonia using diagnostic techniques such as BAL with quantitative cultures.
Available studies suggest that NVHAP appears to have causative microorganisms and outcomes that are similar to those in VAP.12, 13, 14, 15 However, there is a lack of controlled studies focusing on NVHAP to quantitatively determine the impact of this nosocomial infection on patient outcomes. The availability of such data could influence hospitals and physicians to increase the efforts aimed at preventing NVHAP, as well as improve the treatment of this nosocomial infection. Therefore, we performed a case-control study to describe the causative pathogens associated with NVHAP and to determine the influence of NVHAP on patient outcomes.
Methods
Subjects and Study Design
This was a single-center retrospective case-control study of patients with NVHAP performed at Barnes-Jewish Hospital (a 1,300-bed urban academic medical center in St. Louis, Missouri) between January 1, 2014 and December 31, 2014. The study protocol was approved by the Washington University Institutional Review Board (IRB No. 201409001), and informed consent was waived. Adult patients (≥ 19 years of age) admitted to the hospital for more than 48 h were eligible for participation. Patients were excluded if they were transferred from an outside hospital.
Definitions
We defined NVHAP cases in accordance with the American Thoracic Society's position statement on nosocomial pneumonia.1 All patients with a respiratory culture specimen obtained during the study period were screened for study entry. NVHAP was defined as a new or progressive radiographic infiltrate developing more than 48 h after hospital admission plus at least two of the following clinical features: fever > 38°C, leukocytosis (> 10 × 109 cells/L), leukopenia (≤ 4 × 109 cells/L), or purulent secretions. The Charlson comorbidity index was used as a summative score of underlying disease states.16 The presence of a new or progressive radiographic infiltrate was based on the interpretation of the chest radiograph by board-certified radiologists blinded to the study. All patient charts identified as having new or progressive infiltrates were reviewed by one of the investigators (M. H. K.) to confirm the radiographic findings and by two other investigators (S. T. M., B. C.) to identify patients meeting the case definition for NVHAP. Pneumonia was classified as pathogen-negative if all respiratory culture results and applied molecular techniques failed to identify a pathogen. Pathogen-positive pneumonia was defined as growth of a pathogenic organism from sputum, tracheal aspirate, or bronchoscopic or blind BAL fluid when tracheal aspirates and bronchoscopic or blind BAL fluid were obtained in patients with NVHAP within 24 h after respiratory failure developed. Additionally, a positive urinary antigen test result for Legionella qualified as a positive culture result, as did positive qualitative nucleic acid multiplex test results for respiratory viruses, Bordetella pertussis, Chlamydophila pneumoniae, and Mycoplasma pneumoniae (FilmArray Respiratory Panel, BioFire Diagnostics).
Population Control Subjects
We selected control subjects by using a risk set sampling scheme. Four control subjects were selected for each case, matched on age, sex, race, and hospital admission date within 1 month of the case patient admission date. A random sample of matched control patients was selected for each case of NVHAP using a random number generator.
End Points
The main end point evaluated was hospital mortality. Secondary measures included ICU admission, mechanical ventilation, length of stay, and 30-day readmission after the index hospitalization.
Statistical Analysis
The primary data analysis compared patients with NVHAP to those without NVHAP. Categorical variables were compared using the χ2 or Fisher exact test as appropriate. Continuous variables were compared using the Mann-Whitney U test. Data from the matched case-control study were analyzed using conditional fixed-effect logistic analysis. Model goodness of fit was assessed by the Hosmer-Lemeshow test. All tests were two-tailed, and P values < .05 were considered significant. All statistical analyses were performed using IBM SPSS Statistics, version 22.0 (SPSS).
Results
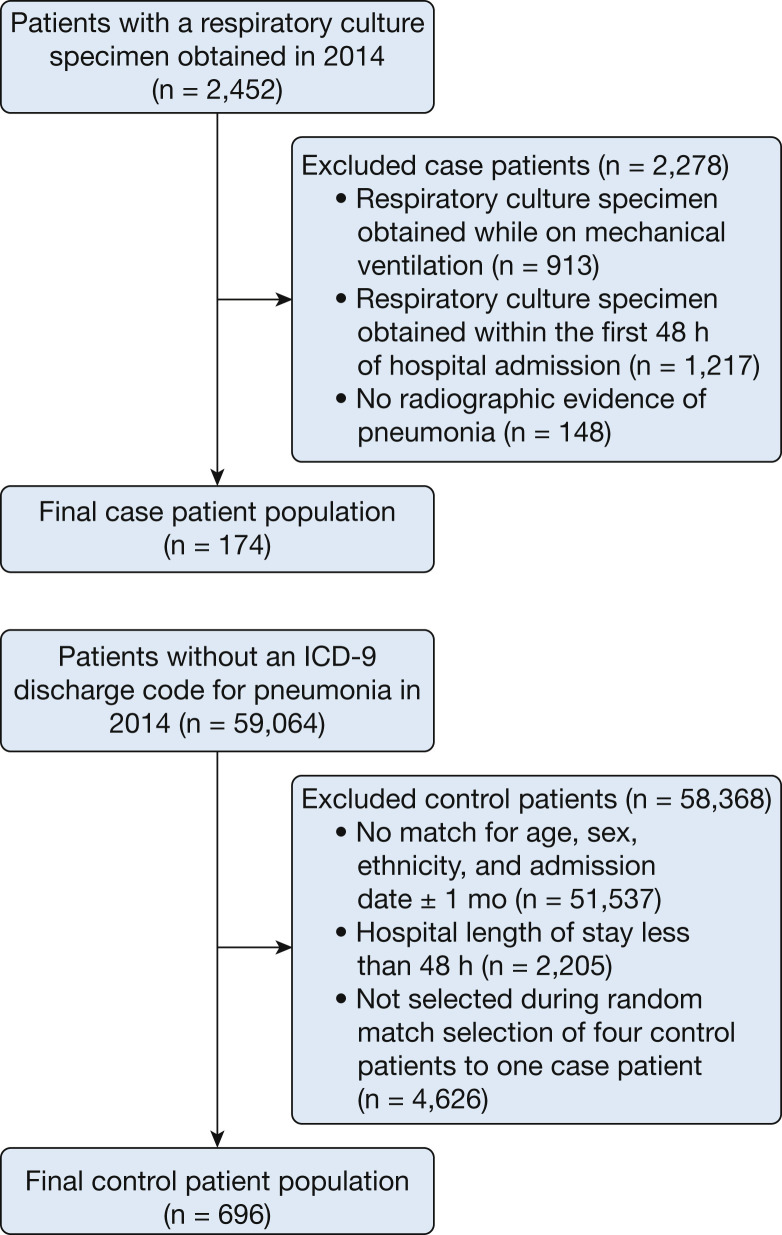
A total of 174 cases of NVHAP were identified, and 696 control subjects were selected (Fig 1 ). Among the 174 patients with NVHAP, 148 (85.1%) had blood culture samples obtained (8 of 148 being positive) and 174 (100%) had at least one respiratory tract culture specimen obtained (sputum, 45.4%; tracheal aspirate, 23.6%; BAL fluid, 31.0%) (40 of 174 being positive). Nucleic acid multiplex tests were performed on respiratory samples from 92 patients (52.9%), with NVHAP (42 of 92 being positive). There were 98 pathogen-negative (56.3%) cases of NVHAP. Viruses were identified in 42 patients (24.1%) (19 rhinovirus, seven influenza A, six parainfluenza virus, five coronavirus, four human metapneumovirus, and four respiratory syncytial virus), gram-negative bacteria were isolated in 25 patients (14.4%) (nine P aeruginosa, four Escherichia coli, four Haemophilus species, three Klebsiella pneumoniae, two Enterobacter species, two Stenotrophomonas maltophilia, and 1 each for Moraxella catarrhalis, Citrobacter koseri, and Achromobacter xylosoxidans), and gram-positive bacteria were found in 20 patients (11.5%) (17 S aureus, two beta-hemolytic streptococci group F, and 1 Streptococcus pneumoniae). Among S aureus isolates, nine were methicillin resistant (52.9%), whereas 12 of the gram-negative isolates (48.0%) were resistant to ceftriaxone (representing an antibiotic typically prescribed for pneumonia in patients without risk factors for antibiotic resistance).
Figure 1.
Study flow diagram. Case and control patients were selected from the Barnes-Jewish Hospital Informatics Repository. ICD-9 = International Classification of Diseases, Ninth Revision.
The mean duration to occurrence of NVHAP was on hospital day 4.2 ± 3.8. Characteristics of case patients and control subjects are listed in Table 1 . Patients with NVHAP were more likely to have higher baseline comorbidity on admission based on the Charlson comorbidity index and to have chronic obstructive pulmonary disease. Patient outcomes are shown in Table 2 . Patients with NVHAP were statistically more likely to die during their hospital stay compared with patients without NVHAP (15.5% vs 1.6%; P < .01). Similarly, patients with NVHAP were more likely to require transfer to an ICU (56.3% vs 22.8%; P < .01) and mechanical ventilation (19.0% vs 3.9%; P < .01) and to have a longer hospital length of stay (median, 15.9 vs 4.4 days; P < .01). Thirty-day hospital readmission rates were similar between the two study groups.
Table 1.
Baseline Demographic and Clinical Characteristics
| Characteristic | Cases With NVHAP (n = 174) | Control Subjects Without NVHAP (n = 696) | P Value |
|---|---|---|---|
| Age, y | 57.5 ± 15.0 | 57.5 ± 14.9 | 1.0 |
| Male sex, No. (%) | 95 (54.6) | 380 (54.6) | 1.0 |
| White, No. (%) | 124 (71.3) | 523 (75.4) | .27 |
| African American, No. (%) | 37 (21.3) | 167 (24.1) | .44 |
| Charlson comorbidity score | 5.5 ± 3.2 | 4.8 ± 3.4 | .02 |
| Coronary artery disease, No. (%) | 28 (16.1) | 106 (15.2) | .78 |
| Congestive heart failure, No. (%) | 46 (26.4) | 151 (21.7) | .18 |
| Cerebrovascular disease, No. (%) | 19 (10.9) | 89 (12.8) | .50 |
| COPD, No. (%) | 89 (51.1) | 225 (32.3) | < .01 |
| Cirrhosis, No. (%) | 36 (20.7) | 114 (16.4) | .18 |
| Diabetes, No. (%) | 57 (32.8) | 255 (36.6) | .34 |
| Active malignancy, No. (%) | 21 (12.1) | 80 (11.5) | .83 |
| Chronic kidney disease, No. (%) | 38 (21.8) | 162 (23.3) | .69 |
| Surgical patient, No. (%) | 75 (43.1) | 291 (41.8) | .76 |
| Medical patient, No. (%) | 99 (56.9) | 405 (58.2) | .75 |
Values expressed as mean ± SD or No. (percent). NVHAP = nonventilated hospital-acquired pneumonia.
Table 2.
Clinical Outcomes
| Outcome | Cases With NVHAP n = 174 |
Control Subjects Without NVHAP n = 696 |
P Value |
|---|---|---|---|
| ICU admission, No. (%) | 98 (56.3) | 159 (22.8) | < .01 |
| Mechanical ventilation, No. (%) | 33 (19) | 27 (3.9) | < .01 |
| Hospital mortality, No. (%) | 27 (15.5) | 11 (1.6) | < .01 |
| Hospital LOS, d, rangea | 15.9 (9.8-26.3) | 4.4 (2.9-7.3) | < .01 |
| Readmission 30 d after hospital discharge, No. (%)b | 37 (25.2) | 145 (21.2) | .29 |
LOS = length of stay. See Table 1 legend for expansion of other abbreviation.
Median (interquartile range).
Only hospital survivors considered: cases, n = 147; control subjects, n = 685.
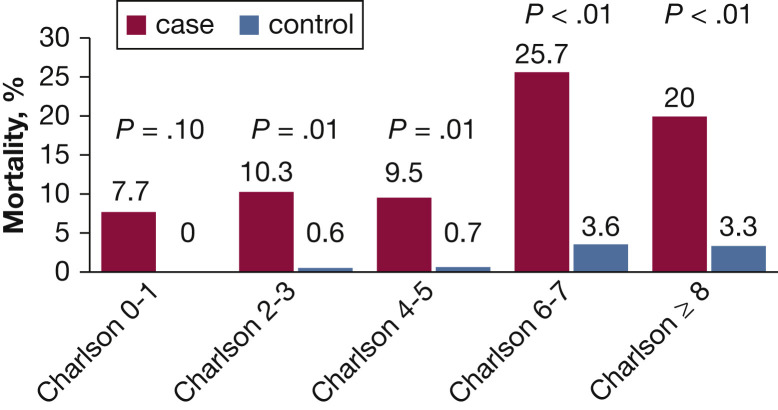
Adjusted odds ratios by conditional logistic regression for variables evaluated for their association with hospital mortality are presented in Table 3 . NVHAP with an adjusted OR of 8.4 (95% CI, 5.6-2.5) along with mechanical ventilation and increasing Charlson comorbidity scores were associated with a greater risk of hospital mortality. The Hosmer-Lemeshow test suggests that our model fit the data (P = .76). Mortality was greater for patients with NVHAP stratified by Charlson comorbidity index (Fig 2 ).
Table 3.
Conditional Logistic Regression Model of Hospital Mortality
| Variable | Adjusted OR | 95% CI | P Value |
|---|---|---|---|
| Hospital-acquired pneumonia | 8.4 | 5.6-12.5 | < .01 |
| Mechanical ventilation | 8.0 | 5.3-11.9 | < .01 |
| Charlson comorbidity index (1-point increments) | 1.2 | 1.1-1.2 | .01 |
Hosmer-Lemeshow goodness of fit, P = .76.
Figure 2.
Hospital mortality for patients with (cases) and without (control subjects) nonventilated hospital-acquired pneumonia stratified by Charlson comorbidity index.
Discussion
This study found that the occurrence of NVHAP was associated with adverse outcomes, including a greater risk of hospital mortality. Hospital resource use was also found to be greater for patients in whom NVHAP developed, as evidenced by greater ICU admission, need for mechanical ventilation, and longer length of stay. Thirty-day hospital readmission was not found to be different between patients with and those without NVHAP. We also found that a viral cause for NVHAP was common, accounting for 24.4% of all cases. The case-control study identified a strong association between hospital mortality and NVHAP.
Sopena and Sabrià12 examined 12 Spanish hospitals over 10 years and were able to prospectively identify only 186 patients with non-ICU HAP, representing less than 20 cases per year. Among the 165 patients with a complete data set, there were 60 with a microbiological cause established (36.4%). Seven immunocompromised patients with pneumonia due to Aspergillus species were included, and no cases of viral HAP were identified. Kollef et al13 examined 4,543 patients with pathogen-positive pneumonia admitted to 59 US hospitals between January 1, 2002 and December 31, 2003.13 NVHAP accounted for 18.4% of the patients with pneumonia, and again there were no cases of viral pneumonia identified in this study. More recently, the importance of viruses as a cause of HAP has been recognized because of the availability of molecular probes for the identification of respiratory viruses. A single-center study from South Korea identified 59 patients with severe HAP attributed to a respiratory virus over a 2-year period, accounting for 22.5% of all their cases of severe HAP.5 Over a 6-year period (August 2007 to September 2013), Andruska et al17 identified 9,624 patients with a discharge diagnosis of pneumonia from Barnes-Jewish Hospital. Although viral pneumonia accounted for only 2.7% of all pneumonia cases during this period, it was associated with the second highest rate of hospital readmission (8.3%) after pneumonia attributed to potentially antibiotic-resistant bacteria (11.4%).
More recently, with the routine application of commercially available viral multiplex testing, Crotty et al18 identified 284 patients with viral pneumonia at Barnes-Jewish Hospital from March 2013 to November 2014. The majority of these patients (51.8%) were immunocompromised, and 84 patients (29.6%) were found to have coinfections, with 48 having a bacterial coinfection (57.6%). Overall hospital mortality was high (23.2%), and readmissions were common within 30 days and 90 days of discharge (21.1% and 36.7%, respectively).
The clinical importance of NVHAP has been demonstrated by comparing outcomes with VAP. Esperatti et al14 examined patients in the ICU setting in whom either NVHAP or VAP developed.14 Despite a lower proportion of identified pathogens in the patients with NVHAP compared with those with VAP, the type of microbiological isolates and clinical outcomes were similar regardless of whether pneumonia was acquired during or without mechanical ventilation. This finding would suggest that patient-specific findings, such as severity of illness and immune function, may be more important factors predisposing to nosocomial pneumonia than previous intubation. Moreover, both types of patients should receive similar empirical antibiotic treatment and benefit from preventive measures that are preferentially directed at intubated individuals. Hospital-based quality-improvement initiatives have primarily focused on preventing the occurrence of VAP and not NVHAP.19, 20 However, it has been difficult to demonstrate attributable mortality from VAP because of the overall severity of illness in the “at risk” ventilated patient population.6 This may account for the inability of the majority of VAP prevention studies to demonstrate reductions in mortality. Our data suggest that NVHAP is associated with significant morbidity and mortality excess and that the prevention of NVHAP could potentially improve patient outcomes.
The emergence of MDR pathogens as a cause of HAP has also resulted in greater administration of inappropriate initial antimicrobial therapy, which is associated with excess patient mortality.21 MDR infection in NVHAP is increasingly common in many parts of the world, resulting in the delayed administration of appropriate antibiotic therapy.22, 23, 24 Moreover, attributable mortality from HAP may be greater than that associated with VAP because of the lower severity of illness existing at baseline in patients with HAP compared with those with VAP.25
Several limitations of our study should be recognized. First, the retrospective design did not allow for determination of the cause of mortality. Furthermore, it is possible that we did not identify all cases of NVHAP given that we used respiratory culture results and not International Classification of Diseases, Ninth Revision codes to screen for study entry. This was purposely done to obtain a patient cohort for whom the treating physicians had a high enough suspicion for pneumonia to obtain microbiological cultures. Second, the data are derived from a single center, and this necessarily limits the generalizability of our findings. As such, our results may not reflect what one might see at other institutions. For example, Barnes-Jewish Hospital has a regional referral pattern that includes community hospitals, regional long-term acute care hospitals, nursing homes, and chronic wound, dialysis, and infusion clinics. Patients transferred from these settings are more likely to be infected with potentially antibiotic-resistant bacteria. This may explain the relatively high rates of infection with potentially antibiotic-resistant gram-negative bacteria and S aureus. Third, given our sample size, we may have lacked power to identify all important confounders that could affect our mortality end point. Fourth, we did not use a protocol for obtaining specific types of culture samples in all patients, rather deferring this evaluation to the treating physicians. This may have contributed to sampling errors in identifying cases of NVHAP. Fifth, we limited the number of matching variables to maximize the number of patients with NVHAP in our study analysis. This may have contributed to unidentified differences in the case and control populations, such as severity of illness or admission diagnoses, which may have contributed to the outcome differences observed. Finally, we cannot exclude the possibility that bacterial coinfection was present among patients with a viral cause of NVHAP. Antibiotic administration may have limited the ability of conventional culture methods to identify antibiotic-susceptible bacteria in that setting.
In summary, our data suggest that the occurrence of NVHAP is associated with adverse patient outcomes and can be caused by both bacterial and viral pathogens. Interventional studies aimed at the prevention of NVHAP are required to determine whether the consequences of NVHAP can be avoided and patient outcomes improved.
Acknowledgments
Author contributions: S. T. M. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. S. T. M., B. C., N. H., and M. H. K. contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: M. H. K. was supported by the Barnes-Jewish Hospital Foundation. S. T. M. received a research grant from Sage Products, LLC. None declared (B. C. and N. H.)
Role of sponsors: The sponsors had no role in the design and conduct of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank William Shannon, PhD and Elena Deych, MS from the Medicine Biostatistics Consulting Center at Washington University School of Medicine for their assistance in conducting the statistical analysis.
Footnotes
FUNDING/SUPPORT: This research was supported by Sage Products LLC.
References
- 1.American Thoracic Society; Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 2.Chastre J., Fagon J.Y. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165(7):867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 3.Vincent J.L., Rello J., Marshall J. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 4.Denys G.A., Relich R.F. Antibiotic resistance in nosocomial respiratory infections. Clin Lab Med. 2014;34(2):257–270. doi: 10.1016/j.cll.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Hong H.L., Hong S.B., Ko G.B. Viral infection is not uncommon in adult patients with severe hospital-acquired pneumonia. PLoS One. 2014;9(4):e95865. doi: 10.1371/journal.pone.0095865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bekaert M., Timsit J.F., Vansteelandt S. Attributable mortality of ventilator-associated pneumonia: a reappraisal using causal analysis. Am J Respir Crit Care Med. 2011;184(10):1133–1139. doi: 10.1164/rccm.201105-0867OC. [DOI] [PubMed] [Google Scholar]
- 7.Vallés J., Pobo A., García-Esquirol O. Excess ICU mortality attributable to ventilator-associated pneumonia: the role of early vs late onset. Intensive Care Med. 2007;33(8):1363–1368. doi: 10.1007/s00134-007-0721-0. [DOI] [PubMed] [Google Scholar]
- 8.Warren D.K., Shukla S.J., Olsen M.A. Outcome and attributable cost of ventilator-associated pneumonia among intensive care unit patients in a suburban medical center. Crit Care Med. 2003;31(5):1312–1317. doi: 10.1097/01.CCM.0000063087.93157.06. [DOI] [PubMed] [Google Scholar]
- 9.Roquilly A., Marret E., Abraham E., Asehnoune K. Pneumonia prevention to decrease mortality in intensive care unit: a systematic review and meta-analysis. Clin Infect Dis. 2015;60(1):64–75. doi: 10.1093/cid/ciu740. [DOI] [PubMed] [Google Scholar]
- 10.Branch-Elliman W., Wright S.B., Howell M.D. Determining the ideal strategy for ventilator-associated pneumonia prevention: cost-benefit analysis. Am J Respir Crit Care Med. 2015;192(1):57–63. doi: 10.1164/rccm.201412-2316OC. [DOI] [PubMed] [Google Scholar]
- 11.de Smet A.M., Kluytmans J.A., Cooper B.S. Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med. 2009;360(1):20–31. doi: 10.1056/NEJMoa0800394. [DOI] [PubMed] [Google Scholar]
- 12.Sopena N., Sabrià M. Multicenter study of hospital-acquired pneumonia in non-ICU patients. Chest. 2005;127(1):213–219. doi: 10.1378/chest.127.1.213. [DOI] [PubMed] [Google Scholar]
- 13.Kollef M.H., Shorr A., Tabak Y.P., Gupta V., Liu L.Z., Johannes R.S. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005;128(6):3854–3862. doi: 10.1378/chest.128.6.3854. [DOI] [PubMed] [Google Scholar]
- 14.Esperatti M., Ferrer M., Theessen A. Nosocomial pneumonia in the intensive care unit acquired by mechanically ventilated versus nonventilated patients. Am J Respir Crit Care Med. 2010;182(12):1533–1539. doi: 10.1164/rccm.201001-0094OC. [DOI] [PubMed] [Google Scholar]
- 15.Allou N., Allyn J., Snauwaert A. Postoperative pneumonia following cardiac surgery in non-ventilated patients versus mechanically ventilated patients: is there any difference? Crit Care. 2015;19:116. doi: 10.1186/s13054-015-0845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charlson M., Pompei P., Ales K., MacKenzie C. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Andruska A., Micek S.T., Shindo Y. Pneumonia pathogen characterization is an independent determinant of hospital readmission. Chest. 2015;148(1):103–111. doi: 10.1378/chest.14-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crotty M.P., Meyers S., Hampton N. Epidemiology, co-infections and outcomes of viral pneumonia in adults: an observational cohort study. Medicine. 2015;94(50):e2332. doi: 10.1097/MD.0000000000002332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Medicare & Medicaid Services. Resources: ventilator-associated pneumonia. https://partnershipforpatients.cms.gov/p4p_resources/tsp-ventilator-associatedpneumonia/toolventilator-associatedpneumoniavap.html. Accessed November 12, 2015.
- 20.Institute for Healthcare Improvement. How-to Guide: Prevent Ventilator-Associated Pneumonia. http://www.ihi.org/resources/pages/tools/howtoguidepreventvap.aspx. Accessed November 12, 2015.
- 21.Guillamet C.V., Kollef M.H. Update on ventilator-associated pneumonia. Curr Opin Crit Care. 2015;21(5):430–438. doi: 10.1097/MCC.0000000000000231. [DOI] [PubMed] [Google Scholar]
- 22.Chung D.R., Song J.H., Kim S.H. High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am J Respir Crit Care Med. 2011;184(12):1409–1417. doi: 10.1164/rccm.201102-0349OC. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y.S., Lee Y.T., Huang T.W. Acinetobacter baumannii nosocomial pneumonia: is the outcome more favorable in non-ventilated than ventilated patients? BMC Infect Dis. 2013;13:142. doi: 10.1186/1471-2334-13-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seligman R., Ramos-Lima L.F., Oliveira Vdo A., Sanvicente C., Sartori J., Pacheco E.F. Risk factors for infection with multidrug-resistant bacteria in non-ventilated patients with hospital-acquired pneumonia. J Bras Pneumol. 2013;39(3):339–348. doi: 10.1590/S1806-37132013000300011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguile-Makao M., Zahar J.R., Français A. Attributable mortality of ventilator-associated pneumonia: respective impact of main characteristics at ICU admission and VAP onset using conditional logistic regression and multi-state models. Intensive Care Med. 2010;36(5):781–789. doi: 10.1007/s00134-010-1824-6. [DOI] [PubMed] [Google Scholar]