Abstract
In the present study, glycyrrhizic acid (GA) the main component of Glycyrrhiza glabra was evaluated for its efficacy as antileishmanial agent and its mode of action explored. GA inhibits promastigotes and intracellular amastigotes in a dose dependent manner at an IC50 value of 34 ± 3.0 μM and 20 ± 4.2 μM respectively. GA was non-toxic against THP-1 macrophage host cell line. GA was found to inhibit recombinant Leishmania donovani HMG-CoA reductase (LdHMGR) enzyme at the half-maximum inhibitory concentration of 24 ± 4.3 μM indicating the sensitivity and specificity of GA towards the enzyme. However, GA could cause only 30% reduction in HMGR activity when measured in Leishmania promastigotes treated with 34 μM of GA. Interestingly western blot analysis revealed fivefold reduced HMGR expression in GLA treated promastigotes. To further study the mode of action of GA, we used transgenic parasites overexpressing LdHMGR. Results indicated that ∼2 fold resistance was exhibited by LdHMGR overexpressing promastigotes to GA with an IC50 value of 74 μM compared to the wild type parasite. This explained the specific binding of GA to LdHMGR enzyme. There was ∼2 fold depletion in ergosterol levels in wild type promastigotes compared to the HMGR overexpressors. This data was further validated by exogenous supplementation of GA treated cells with ergosterol and 40% reversal of growth inhibition was observed. The results obtained suggested that GA kills the parasite by affecting sterol biosynthetic pathway, especially by inhibiting the L. donovani HMGR and altering ergosterol levels. The finding from the current study shows that GA is a potential antileishmanial chemotherapeutic agent.
Keywords: Leishmania, Glycyrrhizic acid, HMGR, Ergosterol, HPLC
Graphical abstract
Highlights
-
•
Glycyrrhizic acid (GA) kills both promastigote and amastigote forms of Leishmania donovani (Ld).
-
•
It inhibits recombinant LdHMGR enzyme and native promastigote enzyme.
-
•
It depletes ergosterol levels in Leishmania promastigotes.
-
•
Ergosterol supplementation partially rescues GA inhibited promastigotes.
-
•
HMGR overexpressors are 2 fold resistant to GA.
1. Introduction
Leishmaniasis is a longstanding infectious disease that represents a major public health problem in various tropical and sub-tropical regions of the world (Trouiller et al., 2002). It is estimated that around one billion individuals suffer from one or more parasitic infections, with the greatest causes of morbidity being attributed to the trypanosomatid parasites. A variety of Leishmania organisms (approximately 20 species) are reported to cause disease which has afflicted about 12 million people in 98 countries of which Indian subcontinent, Sudan and Brazil are the major regions with more incidence of leishmaniasis (Balana-Fouce et al., 1998, Dorlo et al., 2012). The estimated annual number of new cases of leishmaniasis is 2 million, which is usually fatal. Currently, no vaccines are available against this disease, which necessitates the development of alternate strategies to limit its transmission and infection (Modabber, 2010).
The current treatment regimens for this disease include amphotericin B, miltefosine and paromomycin. However, they are not ideal because they are often associated with severe side effects (Murray, 2001). The emergence of resistance to currently used drugs exacerbates the need to identify novel drug targets and develop new drugs (Berman, 1988). Drugs like amphotericin B cause hepatotoxicity and nephrotoxicity whereas miltefosine causes severe teratogenicity (Pandey et al., 2009).
Plant extracts or plant-derived compounds have proven to be a valuable source of new medicinal agents (De Carvalho and Ferreira, 2001, Kayser and Kiderlen, 2001). WHO advocated the use of traditional medicine where appropriate health services are inaccessible (Ei Tahir et al., 1998, Weniger et al., 2001, Bhadra, 1993). Furthermore, the leads obtained from the search for natural products with antileishmanial activity give new impetus for obtaining valuable synthetic compounds (Carvalho et al., 2000).
Recently attention is being focused on the sterol metabolism of Leishmania as a potential molecular drug target for therapy. Parasites synthesize sterols and isoprenoids via mevalonate pathway. Ergosterol is the primary component of the Leishmania membrane and is functionally linked to maintenance of structural integrity and protection from biotic stress (Galea and Brown, 2009).
3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) is a key enzyme in this pathway. The mevalonate pathway is directly relevant to human health and inhibition of HMGR by statins controls sterol production and lowers blood pressure, and helps in the treatment of cardiovascular disease and inflammatory processes (Liao and Laufs, 2005). In L. donovani this pathway is currently being explored as a potential drug target.
Glycyrrhiza glabra, popularly known as liquorice, is one of the most important medicinal plants used for treatment of pulmonary diseases and inflammatory processes (Davis and Morris, 1991). The major bioactive components in liquorice root include glycyrrhizic acid (GA), licochalone A and Glycyrrhetinic acid that were reported to exert antileishmanial properties (Bhattacharjee et al., 2012, Fu et al., 2004, Ukil et al., 2005). GA was reported to exhibit potent antileishmanial immunomodulatory property with enhanced parasite clearance (Bhattacharjee et al., 2012). Besides, GA has shown broad spectrum of antimicrobial, antiprotozoal, antiallergic, antiviral, anti-cancer, antioxidant and anti-inflammatory activities with various pharmacological effects and multiple sites of action (Kumagai et al., 1967, Lin, 2003, Hibasami et al., 2005, Fujisawa et al., 2000). GA was reported to suppress the rise in fasting blood glucose and insulin levels and improve glucose tolerance (Takii et al., 2001).
GA inhibits the replication of the severe acute respiratory syndrome virus (SARS) (Hoever et al., 2005). GA is involved in the treatment of hepatitis C virus (HCV) infection where it inhibits HCV viral particles and its gene expression in a dose-dependent manner and has shown synergistic effect with interferon. GA affects various cellular signalling pathways such as casein kinase II, protein kinase C and transcription factors. The powerful anti-inflammatory capabilities of GA make it effective in the treatment of various types of viral hepatitis also (Ashfaq et al., 2011).
Several mechanisms were proposed for the mode of action of GA in L. donovani. Bhattacharjee et al., have reported prostaglandin E2 (PGE2), inhibition and NO generation as mechanism of action of GA, thus suggesting potential immunomodulatory role of GA against VL (Bhattacharjee et al., 2012). Recently, GA was reported to reduce the parasite load in sodium antimony gluconate (SAG) resistant L. donovani infected macrophages. When, GA was used in combination with SAG, intracellular antimony levels were greatly reduced by suppressing the cell surface expression of multidrug resistance-associated protein1 (MRP1) gene and ABC transporter MRPA (PGPA) transporters which are responsible for antimony efflux during SAG treatment (Bhattacharjee et al., 2015).
Earlier reports suggested GA to be very effective as a lipid-lowering agent in hamsters with diet induced hyperlipidemia and the effect was demonstrated to be via decreased HMGR activity and HMGR mRNA expression (Maurya and Srivastava, 2011). This led us to investigate its effect on L. donovani parasite HMGR activity and ergosterol levels. In view of the potential use of GA for the specific treatment of parasitic diseases like leishmaniasis, we have tried to elucidate its effect on sterol metabolism of the parasite. Our study reveals that it interferes with parasite sterol metabolism by inhibiting HMGR activity and lowering ergosterol levels.
2. Materials and methods
2.1. Chemicals
Glycyrrhizic acid, ergosterol and methanol solvent were purchased from Sigma-Aldrich (St. Louis, MO, USA). Atorvastatin was a kind gift from Dr. S. Suresh, NIPER, S.A.S. Nagar, India. Polyclonal anti-rat HMGR antibody was kindly gifted by Dr. Peter Edwards, UCLA Laboratory (Los Angeles, CA) (García-Pelayo et al., 2004). The anti-LdAceCS antibody was customized from Imgenex, Bhubaneshwar, India (Soumya et al., 2015).
2.2. Parasite and cell line maintenance
Wild type L. donovani (WT, MHOM/80/IN/Dd8) promastigotes were maintained at 24 °C in RPMI-1640 HEPES modified medium (Gibco/BRL, Life Technologies, Scotland, UK) containing 10% heat-inactivated foetal bovine serum (HI-FBS) supplemented with 0.2% sodium bicarbonate, 100 μg/mL penicillin, 100 μg/mL streptomycin and 100 μg/mL gentamycin. The pH of the media was maintained at 7.2. LdHMGR overexpressing transgenic promastigotes were maintained in the same medium with 60 μg/mL of hygromycin B. THP-1 monocyte cell line was maintained in RPMI-1640 medium containing 10% HI-FBS in a humidified atmosphere with 5% CO2 at 37 °C.
2.3. In vitro antipromastigote assay
Promastigotes of L. donovani strain were grown in RPMI-1640 medium as mentioned earlier (Dinesh et al., 2014). Two hundred microliters of culture with a cell density of 2 × 105 promastigotes with or without various GA concentrations (10 μM–100 μM) was seeded in 96-well microtiter plate. After 48 h of incubation at 24 °C, 20 µl of 5 mg/mL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) dye was added to each well. The plates were incubated for another 4 h at 37 °C and read with a BIOTEK microplate reader at 540 nm. The decrease of absorbance (which indicated inhibition) was expressed as the percentage of the absorbance of the control cultures and plotted against the drug concentrations. The 50% inhibitory concentrations (IC50) was calculated from the sigmoidal inhibition curves. Data were expressed as mean ± standard deviations from three independent experiments.
2.4. In vitro anti-amastigote assay
Antileishmanial screening against L. donovani amastigotes residing in differentiated THP-1 monocytes was adapted from that of Jain and co-workers (Jain et al., 2012). THP-1 monocytes were seeded at density of 2 × 105/well in 96-well plate and differentiated into macrophages by inducing with phorbol 12-myristate 13-acetate (PMA, 20 ng/mL). Macrophages were infected with 2 × 106 of L. donovani promastigotes at 37 °C in 5% CO2 for 24 h. Unadhered promastigotes were removed by washing with RPMI-1640 medium and the amastigote-infected macrophages were treated with GA at different concentrations (5, 10, 20, 50 and 100 μM) for 48 h. Infected macrophages were lysed by treating the cells with 20 μL of 0.05% sodium dodecyl sulfate (SDS) and amastigotes were reverted back into the promastigotes under suitable conditions. After 48 h, MTT assay was performed as described earlier. Miltefosine was used as the standard anti-leishmanial drug.
2.5. Cytotoxicity evaluation of glycyrrhizic acid
To estimate 50% inhibitory concentrations of GA on the viability of THP cell lines, MTT assay was performed as previously described (Mosmann, 1983). Briefly, THP cells (1 × 105 cells/100 μL) was seeded in 96-well microliter plates at 37 °C, 5% CO2. After 48 h of incubation varying concentrations (10–100 μM) of GA was added in fresh medium. MTT was added after 48 h of incubation followed by 4 h incubation at 37 °C. The cells were harvested, the violet crystals were dissolved by DMSO and finally the OD was measured at 540 nm using micro plate reader.
2.6. Growth curve of promastigotes in the presence and absence of GA
The effect of GA on growth of wild type promastigotes was determined in 25 cm2 flasks. Initially, 1 × 106 cells/mL were seeded at 24 °C in RPMI-1640 medium supplemented with 10% heat inactivated fetal bovine serum. Cell density was determined by Neubauer hemocytometer at every 24 h intervals for five days (120 h). Inhibitory concentration of GLA (34 μM) was added to cells at 48 h and the growth of GA treated promastigotes was compared with the untreated promastigotes.
2.7. Inhibition of recombinant L. donovani HMG-CoA reductase by glycyrrhizic acid
In order to express the recombinant L. donovani HMGR protein, E. coli BL21(DE3) cells were transformed with the construct pET30a-LdHMGR and then recombinant LdHMGR enzyme was purified by using affinity chromatography as reported earlier (Dinesh et al., 2014). LdHMGR enzyme activity measurements were carried out to determine the effect of GA on the activity of recombinant L. donovani HMGR enzyme in the presence of different concentrations of GA after 5 min incubation. The oxidation of NADPH was monitored at 340 nm. One unit (U) of HMGR was defined as the amount of enzyme required to catalyze the oxidation of 1 μmol of NADPH per min. Atorvastatin, the chemical inhibitor of HMGR was taken as the positive control. Various concentrations of atorvastatin ranging from 1 to 1000 nM were used in the assay.
2.8. Preparation of total cell lysates and HMGR activity in L. donovani promastigotes
Approximately, 1 × 108 cells were grown for 48 h and treated with IC50 concentration of GA (34 μM) and atorvastatin (19 μM) for 48 h at 24 °C. Atorvastatin was taken as the positive control for HMGR inhibition. The cells were harvested at 6000g for 10 min and washed with PBS. The cell pellet was resuspended in lysis buffer [50 mM KH2PO4 (pH 7.2), 1 mM DTT, 2 mM PMSF and 0.5 mg/mL leupeptin] and lysed by repeated freeze thaw cycles in liquid nitrogen and at 37 °C. The cell lysate was extracted after centrifugation at 13000g for 30 min at 4 °C. Cell lysate from untreated parasites was taken as control. HMGR activity was carried out as described earlier using an equal amount of untreated, GA treated and atorvastatin treated total cell lysate.
2.9. Protein immunoblotting
1 × 108 promastigotes were inoculated in 25 cm2 tissue culture flasks and treated with IC50 concentrations of GA for 48 h. The cells were lysed as described in section 2.7 and approximately 100 μg of protein samples were subjected to 10% SDS-PAGE and transferred onto nitrocellulose membrane by electroblotting (Bio-Rad) at 50 V, 350 mA for 3 h. The membrane was incubated with polyclonal anti-rat HMGR antibody (1:500) for overnight at 4 °C temperature followed by anti-rabbit IgG alkaline phosphatase conjugate as secondary antibody (Sigma,1:10,000) for 1 h. The specific protein bands were visualized by incubating with nitro blue tetrazolium (NBT) and 5-bromo-4-chloro3-indolyl phosphate disodium salt (BCIP) as substrates (Dinesh et al., 2014). Densitometric analysis of the blot was performed using Image J software. Acetyl CoA synthetase earlier reported from our lab was used as a negative control to show the specific effect of GA on HMGR (Soumya et al., 2015).
2.10. HPLC analysis of ergosterol levels in L. donovani promastigotes
A solution of 1 mg/mL of ergosterol served as the control. The injection volume was 50 μL. An Agilent technologies C18 column, 100 mm × 4.6 mm, 3.5 μm particles was used for RP-HPLC. To evaluate the effect of GA on ergosterol biosynthesis, promastigotes approximately 1 × 106 cells/mL were seeded in culture flask. After 48 h of incubation, the cells were treated with GA (at IC50 concentration) and further incubated for 48 h. The cells were harvested and the sample was prepared for HPLC as reported earlier (Ng et al., 2008). Appropriate controls without GA treatment was also taken.
2.11. Ergosterol reversion assay
This study was performed to examine the rescue of GA treated L. donovani promastigotes upon ergosterol supplementation. Briefly, promastigotes were seeded in 96-well plate for 48 h, treated with GA at its IC50 concentration. The cells were simultaneously supplemented with exogenous ergosterol in varying concentrations 50–200 mmol/L dissolved in methanol. The cells were supplemented with 200 mmol/L ergosterol as control. The percentage of promastigotes that were live upon rescue was analyzed by MTT assay. All assays were performed in triplicates and growth controls with the solvent and the solvent plus ergosterol were included to verify the lack of promastigote growth inhibitory effects.
2.12. Effect of glycyrrhizic acid on HMGR overexpressing L. donovani promastigotes
To confirm the specificity of GA towards L. donovani HMGR we used previously generated HMGR overexpressor promastigotes as described earlier (Singh et al., 2014). Briefly, late log phase wild type and overexpressing promastigotes were seeded in a 96-well flat bottom plate and incubated for 48 h at 24 °C. To examine the effect of GA, the parasites were incubated with range of concentrations of GA from 10 to 100 μM for 48 h. After 72 h of incubation, MTT (5 mg/mL, 20 μL per well) was added to each well and the plates were incubated for an additional 4 h at 37 °C. Then the cells were harvested and resultant pellet dissolved in DMSO. The final absorbance was measured at 540 nm.
2.13. Statistical analysis
One-way analysis of variance (ANOVA) was performed for comparison between groups. Statistical analysis of the data was done using Graph Pad Prism software version 5.0. Only values of p ≤ 0.05 were considered significant.
3. Results
3.1. Effect of glycyrrhizic acid on promastigotes, intracellular amastigotes and macrophages
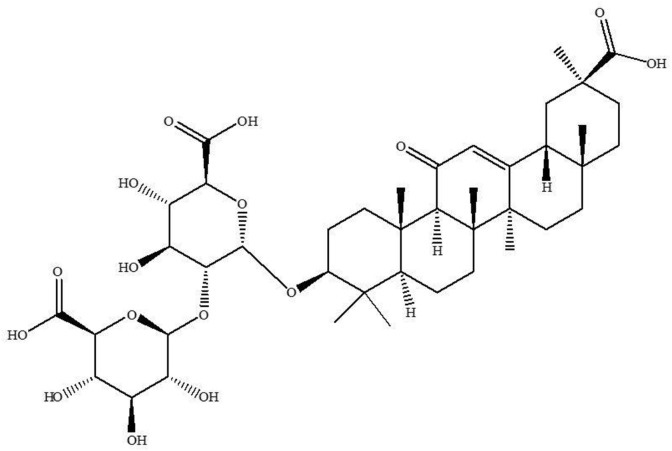
The chemical structure of GA is shown in Fig. 1 . To determine the dose-dependent effect of GA on L. donovani promastigotes, the cells were plated with various concentrations of investigating compound. The viability was assessed after 48 h exposure to the drug using MTT assay. In the presence of GA, a dose-dependent inhibition of the viability of L. donovani promastigotes was observed. The concentration of GA required to inhibit parasite viability by 50% was determined from the plot of drug concentrations against % of viability of promastigotes, assuming control (without drug) as 100% viable. The IC50 determined from the graph was 34 ± 3 μM. The cytotoxic property of the GA against macrophages was determined in 96-well microliter plate by MTT assay and the results were expressed as the concentration of GA that inhibited cell viability by 50% of the control. Macrophage cells were treated with varying concentrations of GA for 48 h, using standard drug miltefosine as the positive control. GA did not cause macrophage killing upto 100 μM concentrations. The reference drug miltefosine however, decreased the viability of macrophages up to 90% at 100 μM concentration (data not shown). GA was found to inhibit intracellular amastigotes in a dose dependent manner with an IC50 value of 20 ± 4.2 μM. A comparison of the IC50 and 50% cytotoxic concentration (CC50) values obtained for GA and miltefosine were represented in Table 1 . Selectivity index (SI) was calculated by dividing the CC50 value of THP cell line with IC50 value of amastigotes. The SI was calculated for GA and was found to be >5 whereas for miltefosine it was 11.5 (Table 1).
Fig. 1.
Molecular structure of Glycyrrhizic acid tested for activity against L. donovani.
Table 1.
Effect of GA on L. donovani promastigotes, amastigotes and THP-1 differentiated macrophages.
|
Inhibitors |
IC50 values (μM) |
Selectivity index (SI) | ||
|---|---|---|---|---|
| L. donovani promastigotes | L. donovani amastigotes | THP-1 differentiated macrophages | ||
| Glycyrrhizic acid | 34 ± 3 | 20 ± 4.2 | >100 | >5 |
| Miltefosine | 15.3 ± 2.1 | 3.8 ± 1.2 | 44.2 ± 5.3 | 11.5 |
3.2. Effect of GA on growth of L. donovani promastigotes
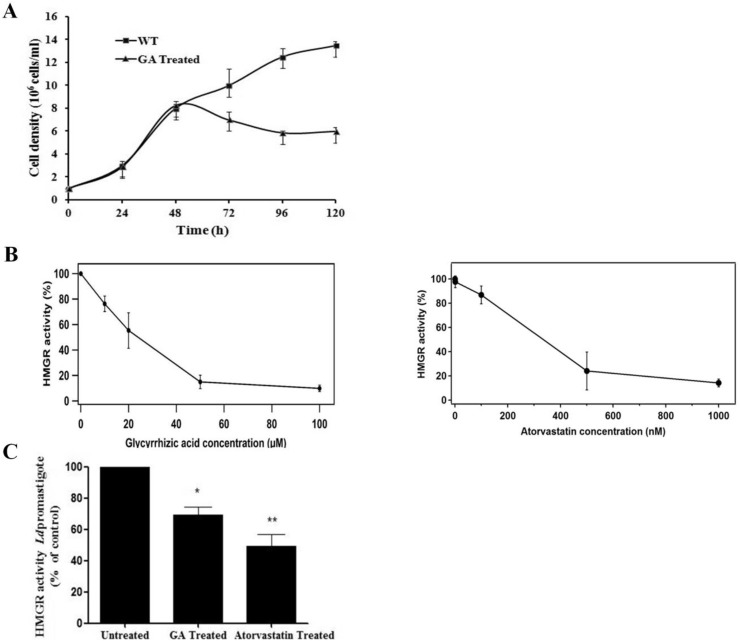
To determine the inhibitory effect of GA on growth of wild type promastigote parasite, the growth of L. donovani promastigote was monitored over a period of 120 h. Parasites were enumerated every 24 h by counting using hemocytometer. IC50 concentration of GA (34 μM) was added after 48 h. The result showed that GA affected the growth of L. donovani promastigotes. A decrease in growth rate of ∼1.4 fold after 24 h and ∼2.1 fold after 48 h of GA treated promastigotes compared to untreated wild type promastigotes was observed (Fig. 2 A). This data revealed that GA affected the proliferation of L. donovani promastigotes.
Fig. 2.
Effect of Glycyrrhizic acid on parasite growth and LdHMGR enzyme activity. A) Effect of GA on growth of L. donovani promastigote. The growth of L. donovani was monitored over a period of 120 h. GA (34 μM) was added after 48 h and parasites were enumerated every 24 h by counting using hemocytometer. B) Left panel: Effect of GA on recombinant LdHMGR enzyme activity. The enzyme was treated with different concentrations of GA (10–100 μM) for 5 min and subsequently HMGR activity was measured at 340 nm. Right panel: Effect of atorvastatin on recombinant HMGR enzyme activity. Concentrations ranging from 1 to 1000 nM were used. C) Effect of GA on HMGR activity in L. donovani promastigotes. Promastigotes were treated with the IC50 concentration of GA (34 μM) and atorvastatin (19 μM). HMGR activity was measured after 48 h of incubation with GA and atorvastatin. Data was expressed as mean ± standard deviations from three independent experiments. **p ≤ 0.01.
3.3. Inhibition of L. donovani HMG-CoA reductase enzyme by glycyrrhizic acid
To investigate the effect of GA on the catalytic efficiency of recombinant HMGR, the enzyme used for the assay was expressed and purified as reported earlier (Dinesh et al., 2014). GA was tested at the range of 10–100 μM concentration. The half-maximum inhibitory concentration was found to be 24 ± 4.3 μM. At higher concentrations 50 and 100 μM of GA only 15% residual HMGR activity was observed indicating that GA binds to HMGR causing its inhibition (Fig. 2B left panel). Atorvastatin was used a positive control for comparative analysis of GA as LdHMGR inhibitor (Fig. 2B right panel). At 1 μM approximately 86% inhibition was observed. The IC50 value was calculated to be 300 nM, which correlates with previously reported data (Dinesh et al., 2014). Atorvastatin is a more sensitive inhibitor of HMGR than GA as it inhibits the enzyme in lower micromolar range. HMGR activity was also measured in the promastigotes in the absence and presence of GA. The GA treated promastigotes exhibited ∼30% reduction in HMGR activity in comparison to untreated parasites. Promastigotes treated with 19 μM of atorvastatin exhibited 51% inhibition thus serving as a positive control and inhibitor of HMGR (Fig. 2C).
3.4. Effect of GA on HMGR protein expression by western blot analysis
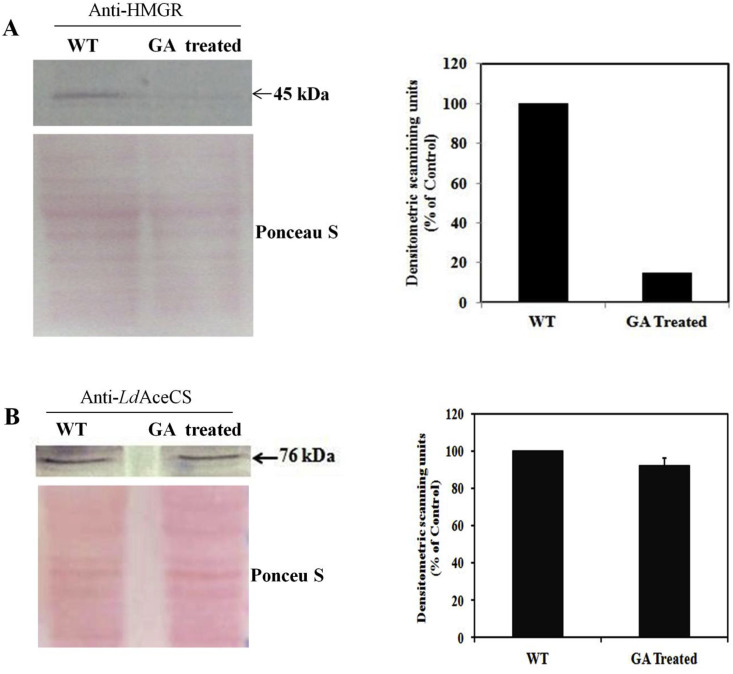
Effect of GA on HMGR protein expression was evaluated by western blotting of L. donovani cell extracts obtained from promastigotes treated with IC50 concentration of GA. The blot was stained with Ponceau S which served as loading control. Densitometric analysis of the blot revealed fivefold reduction in HMGR expression in GA treated promastigotes further confirming the effect of GA on HMGR (Fig. 3 A). To further prove the specificity of GA for HMGR, we randomly selected acetylCoA synthetase (AceCS) as control protein. There are no reports of AceCS being targeted by GA. Densitometric analysis revealed no change in expression of AceCS when promastigotes were treated with GA (Fig. 3B).
Fig. 3.
A) Effect of GA on HMGR protein expression by western blot. Left panel: The promastigotes were treated with the IC50 concentrations of GA. Ponceau S stained blot is shown as loading control. Right panel: Densitometric analysis of western blot. B) Western blot to show effect of GA on AceCS protein expression which serves as control. Left panel: Promastigotes were treated with the IC50 concentration of GA and its effect on AceCS protein expression was analyzed by western blot using anti-LdAceCS antibody (1:1000). Ponceau S stained blot is shown as loading control Right panel: Densitometric analysis of western blot.
3.5. Lowering of ergosterol levels in L. donovani promastigotes by glycyrrhizic acid
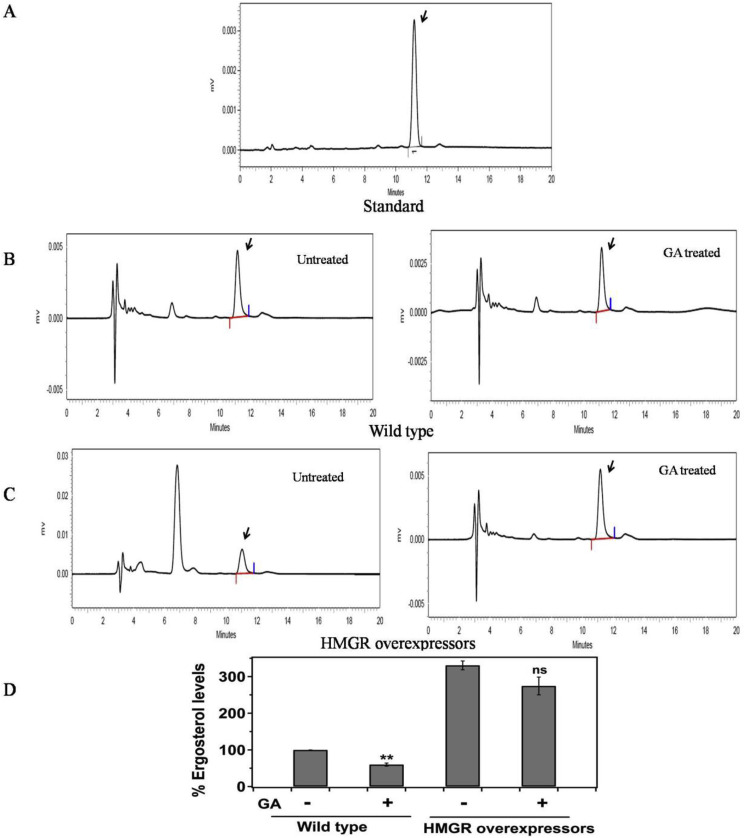
Standardized HPLC method was used for the isolation of neutral lipids of L. donovani promastigotes. The retention time of ergosterol extracted from promastigotes was found at 11.18 min which correlated with standard ergosterol peak retention time (Fig. 4 A). HPLC chromatograms of wild type control parasites and GA treated parasites (34 μM) are shown in Fig. 4B left and right panel respectively which revealed almost 43% reduction in ergosterol levels in GA treated wild type promastigotes. The % peak area for wild type control and GA treated promastigotes was 100% and 57% respectively. We had also estimated ergosterol levels in HMGR overexpressing untreated and GA treated L. donovani promastigotes (34 μM) as seen in Fig. 4C left and right panel respectively. The results showed that there was only ∼20% reduction in ergosterol levels in HMGR overexpressors upon treatment with GA in comparison to wild type where 43% reduction was observed. The % peak area for HMGR overexpressing control and GA treated promastigotes was found to be 330% and 274% respectively, calculated relatively from % peak area of wild type control which was considered 100%. It was observed that the ergosterol levels in HMGR overexpressors was ∼3 fold higher compared to wild type promastigotes. When the wild type and HMGR overexpressors were treated with the IC50 concentration of GA, wild type parasites exhibited ∼2 fold reduction in ergosterol levels as compared to the HMGR overexpressors (Fig. 4D).
Fig. 4.
Effect of Glycyrrhizic acid on ergosterol levels in wild type and HMGR overexpressors by HPLC. Neutral lipids were extracted from L. donovani promastigotes and were subjected to RP-HPLC using C18 column. The wild type and HMGR overexpressing promastigotes were treated with IC50 concentration (34 μM) of GA for 48 h and then subjected to neutral lipid extraction. A) Represents the standard ergosterol peak with retention time at 11.18 min. B) Representation of chromatographic profiles of wild type promastigotes untreated (left panel) and GA treated (right panel). C) Representation of chromatographic profiles of HMGR overexpressing promastigotes, untreated (left panel) and GA treated (right panel). D) Representation of the comparison of ergosterol levels expressed as % peak area between wild type and HMGR overexpressing promastigotes in the absence and presence of GA. Data was expressed as mean ± standard deviations from three independent experiments. **p ≤ 0.01.
3.6. Reversal of glycyrrhizic acid mediated parasite inhibition by ergosterol supplementation in vitro
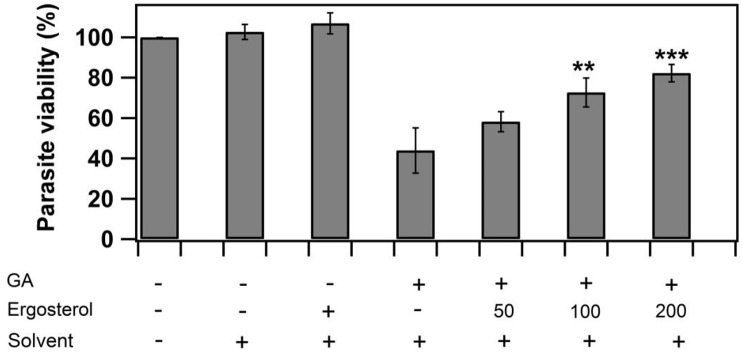
GA treated Leishmania promastigote cultures were exogenously supplemented with different concentrations of ergosterol to study the role of exogenous sterol to overcome GA mediated inhibition. Ergosterol rescued GA treated promastigotes at concentrations ranging from 50 to 200 mmol/L causing the reversal of GA mediated growth inhibition (Fig. 5 ). Promastigotes treated with IC50 value of GA showed 60% inhibition whereas in case of GA treated promastigotes supplemented with 200 mmol/L ergosterol showed only 20% inhibition i. e. 40% reversal of inhibition. These results indicate that GA inhibition occurred by interfering with ergosterol biosynthesis which is a key sterol component for the survival of the parasite. Solvent and solvent plus ergosterol were taken as controls and no background inhibition was observed.
Fig. 5.
Reversal of Glycyrrhizic acid mediated growth inhibition by exogenous supplementation of ergosterol. L. donovani promastigotes were treated with IC50 concentration of GA (34 μM) in the absence and presence of ergosterol for 48 h. Reversal was analyzed by performing MTT assay and change in OD measured at 540 nm using 96 well plate reader. Data was expressed as mean ± standard deviations from three independent experiments. **p ≤ 0.01 and ***p ≤ 0.001.
3.7. Effect of glycyrrhizic acid on L. donovani transfectants overexpressing HMGR
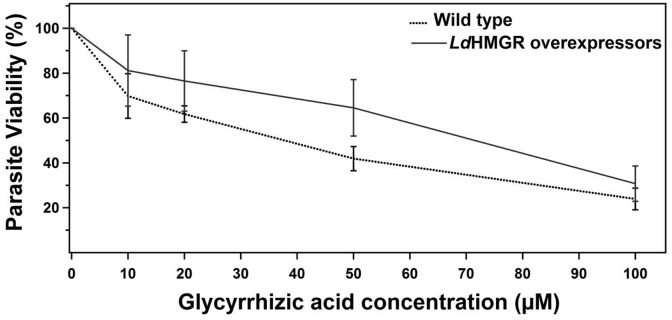
L. donovani promastigotes overexpressing HMGR was maintained in the RPMI-1640 medium with constant selection pressure of hygromycin B. The half-maximum inhibitory concentration (IC50) was 34 ± 3 μM and 74 ± 3.8 μM for wild type and LdHMGR overexpressing cells respectively. LdHMGR overexpressors exhibited ∼2 fold increase in IC50 of GA as compared to the wild-type promastigotes (Fig. 6 ). The result indicates that a higher concentration of drug is needed in case of LdHMGR overexpressing cells when compared to the wild type for obtaining similar therapeutic results. These results supports that HMGR overexpressing promastigotes showed resistance to GA, which depicts its prime role in inhibiting the sterol biosynthetic pathway.
Fig. 6.
Comparison of the effect of Glycyrrhizic acid on growth profiles of wild type and HMGR overexpressors. Promastigotes were incubated with varying concentrations of GA for 48 h and viability was measured using MTT assay. Data was expressed as mean ± standard deviation from three independent experiments.
4. Discussion
3-Hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase is an enzyme that catalyzes the conversion of HMG-CoA to mevalonate, a precursor of cholesterol in humans, and ergosterol in plants, fungi and protozoa (Henriksen et al., 2006, Macreadie et al., 2006). Ergosterol is reported to be essential for growth and replication of Leishmania and fungi (Kulkarni et al., 2013). Efficacy of ergosterol biosynthesis inhibitors as antiproliferative agents against Trypanosoma cruzi has been already reported (Urbina et al., 1995). HMGR is proposed as a potential drug target in L. donovani. Several natural and synthetic statins are reported to interfere with the growth of L. donovani by inhibiting HMGR (Dinesh et al., 2014). Besides several compounds from natural sources like cholestin, diosgenin and GA were reported to inhibit HMGR (Arguelles et al., 2010, Maurya and Srivastava, 2011).
Over the past few years it has been well documented that the plant source is being used for treating inflammation, asthma, bronchitis, tumor, fungal diseases and recently GA and its analogs were also reported as anti-filarial agents (Pandey et al., 2009, Kim et al., 2013, Kalani et al., 2013). Several mechanisms were proposed for GA mediated inhibition, which includes the anti-inflammatory property of GA by suppressing the TNF-α and anti-apoptotic effect via suppression of caspase-3, which have explained the hepatoprotective properties (Nasyrov et al., 1995, Wan et al., 2009).
As part of our drug discovery research, we were interested in natural product entities which could target sterol biosynthetic pathway and serve as antileishmanial agents and we came across GA. Literature search revealed antileishmanial activity of GA which proved to be efficacious causing enhanced release of cytokines (IL-12 and TNF-α) in Leishmania infected macrophages thus, indicating its immunomodulatory property (Bhattacharjee et al., 2012). These cytokines play an important role in controlling disease progression in visceral leishmaniasis (Descoteaux and Matlashewski, 1989, McSorley et al., 1996, Taylor and Murray, 1997). These findings prompted us to investigate the mechanism of antileishmanial action of GA in Leishmania promastigotes.
In this work, we have investigated for the first time the effect of GA on sterol metabolism of the parasite. The results of the present study showed in vitro growth suppression of L. donovani promastigotes and intracellular amastigotes by GA. Our results indicate that the GA exerts its antileishmanial action by significant reduction in the parasite viability at concentrations far below the cytotoxic concentration. It was earlier reported that 50 μg/mL of GA reduced the parasite burden up to 90% in L. donovani infected murine peritoneal macrophages and ∼10 μg/mL resulted in 50% inhibition (Bhattacharjee et al., 2012). The results showed that GA displayed antileishmanial activity at non-cytotoxic concentrations. The standard drug miltefosine exhibited ∼2 fold higher sensitivity against promastigotes and ∼5 fold higher sensitivity against amastigotes when compared to GA. However, the selectivity index was >5 for GA and almost comparable to miltefosine. The sensitivity of promastigotes, amastigotes and macrophages towards miltefosine was in agreement with the previously published data (Corral et al., 2014, Calogeropoulou et al., 2008, Dube et al., 2007).
Earlier studies showed that HMGR mRNA expression was markedly decreased in GA treated dyslipidemic hamsters (Maurya and Srivastava, 2011). Keeping this in view, effect of GA was studied on recombinant HMGR and native enzyme in promastigotes. At 100 μM concentration of GA, the recombinant LdHMGR activity was reduced by 90%. Interestingly, it was found that GA inhibits the recombinant enzyme at an IC50 value, which is close to the IC50 value obtained from cell based inhibition studies. This suggested that GA might specifically bind to LdHMGR enzyme. HMGR activity was also measured in GA treated L. donovani promastigotes where ∼30% reduction was observed. It was observed that the recombinant enzyme inhibition by GA was more compared to inhibition of native enzyme in L. donovani promastigotes (50% inhibition of recombinant HMGR at 24 μM of GA whereas only 30% inhibition of native HMGR in L. donovani promastigotes was observed at 34 μM of GA concentration). The differences in effect of GA on the recombinant enzyme activity and that of parasite enzyme activity may be due to the multi-targeted effect of GA (Bhattacharjee et al., 2012). Moreover, the effect of GA in promastigotes was measured on the whole cell lysate of parasite and not purified native HMGR enzyme. In both the native and recombinant HMGR inhibition studies, atorvastatin was used as chemical inhibitor of HMGR enzyme and acted as positive control. Fivefold lower HMGR expression was observed in immunoblot, which further indicated the specificity of GA for HMGR. Thus, the reduction of HMGR enzyme activity is associated with the reduction of HMGR protein levels. AceCS was selected as control protein however if GA has multitargeted effect, proteomics can provide more information regarding other targets. We also confirmed that GA treatment reduces the biosynthesis of ergosterol by inhibiting HMGR enzyme. Ergosterol levels in GA treated wild type promastigotes significantly decreased up to 40% whereas only 20% reduction was observed in GA treated HMGR overexpressors. This explains that it affects the sterol biosynthesis in the parasite and is one of the reasons for parasite killing. Moreover, when attempts were made to reverse the GA mediated growth inhibition with ergosterol, it could only partially revive the growth. This study clearly indicated that though GA acts by inhibiting LdHMGR enzyme and altering ergosterol levels, yet it is not the sole target for GA. It was well documented that GA has multiple mechanism of actions and multiple targets in the parasite. Recent report stated that it inhibits cox-2 enzyme leading to increase NO generation in L. donovani infected macrophages and thus arresting parasite survival (Bhattacharjee et al., 2012).
Further, in order to validate the effect of GA on HMGR enzyme, the effect of GA was tested on wild type and HMGR overexpressing transgenic parasites that were reported in our earlier studies (Singh et al., 2014). Our results indicated that HMGR overexpressing parasites showed ∼2 fold resistance towards GA mediated inhibition of promastigotes compared to non-transgenic parasites. Therefore, it is illustrated that episomal expression of HMGR in parasites protects them from GA induced lysis as is evident from the higher concentration of GA required to inhibit the transgenic parasites than the wild type parasites.
Interference with ergosterol biosynthesis results in disruption of parasite function and molecules involved in this pathway could serve as potential drug targets for antileishmanial chemotherapy (De Souza and Rodrigues, 2009). Interestingly, there are reports where Leishmania can survive altered sterol levels. However, changes in sterol profile have been linked to amphotericin B and fluconazole resistance (Mbongo et al., 1998, Roberts et al., 2003). The alteration of sterol levels could have important implications in drug response because ergosterol is required for action of some drugs. For example, amphotericin B, a polyene antifungal drug is also used for the treatment of leishmaniasis, which associates, with ergosterol for its action (Croft et al., 2006, Cohen, 1998).
In conclusion, the result of this work extends the investigation of GA as potential candidate for visceral leishmaniasis treatment. Overall mode of action of GA in vitro studies is via inhibition of HMGR enzyme (rate limiting enzyme of sterol biosynthetic pathway) and depletion of ergosterol levels, which culminates in parasite death. To the best of our knowledge, this is the first report, which conclusively proves that GA can be used as potential inhibitor of L. donovani HMGR, and is an interesting molecule as it possesses immunomodulatory properties too.
Acknowledgements
The authors are grateful for the financial support provided by the Ministry of Chemicals and Fertilizers, India[(2015-16)-nplc-Sushma Singh]. Special thanks to Director, NIPER for financial support. Special thanks to Mr. Neerupudi Kishore Babu for critical reading of the manuscript.
References
- Arguelles N., Sanchez-Sandoval E., Mendieta A., Villa-Tanaca L., Garduno-Siciliano L., Jimenez F., Cruz M., del C., Medina-Franco J.L., Charmorro-Cevallos G., Tamariz W. Design, synthesis, and docking of highly hypolipidemic agents: Schizosaccharomyces pombe as a new model for evaluating α-asarone-based HMG-CoA reductase inhibitors. Bioorg. Med. Chem. 2010;18:4238–4248. doi: 10.1016/j.bmc.2010.04.096. [DOI] [PubMed] [Google Scholar]
- Ashfaq U.A., Masoud M.S., Nawaz Z., Riazuddin S. Glycyrrhizin as antiviral agent against hepatitis C virus. J. Transl. Med. 2011;9:112. doi: 10.1186/1479-5876-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balana-Fouce R., Reguera R.M., Cubria J.C., Ordonez D. The pharmacology of leishmaniasis. Gen. Pharmacol. 1998;30:435–443. doi: 10.1016/s0306-3623(97)00268-1. [DOI] [PubMed] [Google Scholar]
- Berman J.D. Chemotherapy for leishmaniasis: biochemical mechanisms, clinical efficacy, and future strategies. Rev. Infect. Dis. 1988;10:560–586. doi: 10.1093/clinids/10.3.560. [DOI] [PubMed] [Google Scholar]
- Bhadra R. Antileishmanial agents. Drugs Future. 1993;18:451–463. [Google Scholar]
- Bhattacharjee S., Bhattacharjee A., Majumder S., Majumdar S.B., Majumdar S. Glycyrrhizic acid suppresses Cox-2-mediated anti-inflammatory responses during Leishmania donovani infection. J. Antimicrob. Chemother. 2012;67:1905–1914. doi: 10.1093/jac/dks159. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A., Majumder S., Majumdar S.B., Choudhuri S.K., Roy S., Majumdar S. Co-administration of glycyrrhizic acid with the antileishmanial drug sodium antimony gluconate (SAG) cures SAG-resistant visceral leishmaniasis. Int. J. Antimicrob. Agents. 2015;45:268–277. doi: 10.1016/j.ijantimicag.2014.10.023. [DOI] [PubMed] [Google Scholar]
- Calogeropoulou T., Angelou P., Detsi A., Fragiadaki I., Scoulica E. Design and synthesis of potent antileishmanial cycloalkylidene substituted ether phospholipid derivatives. J. Med. Chem. 2008;51:897–908. doi: 10.1021/jm701166b. [DOI] [PubMed] [Google Scholar]
- Carvalho P.B., Arribas M.A.G., Ferreira E.I. Leishmaniasis. What do we know about its chemotherapy? Rev. Bras. Ci. Farm. 2000;36:69–96. [Google Scholar]
- Cohen B.E. Amphotericin B toxicity and lethality: a tale of two channels. Int. J. Pharm. 1998;162:95–106. [Google Scholar]
- Corral M.J., Gonzalez-Sanchez E., Cuquerella M., Alunda J.M. In vitro synergistic effect of amphotericin B and allicin on Leishmania donovani and L. infantum. Antimicrob. Agents Chemother. 2014;58:1596–1602. doi: 10.1128/AAC.00710-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft S.L., Seifert K., Yardley V. Current scenario of drug development for leishmaniasis. Indian J. Med. Res. 2006;123:399–410. [PubMed] [Google Scholar]
- Davis E.A., Morris D.J. Medicinal uses of licorice through the millennia: the good and plenty of it. Mol. Cell Endocrinol. 1991;78:1–6. doi: 10.1016/0303-7207(91)90179-v. [DOI] [PubMed] [Google Scholar]
- De Carvalho P.B., Ferreira E.I. Leishmaniasis phytotherapy. Nature's leadership against an ancient disease. Fitoterapia. 2001;72:599–618. doi: 10.1016/s0367-326x(01)00301-x. [DOI] [PubMed] [Google Scholar]
- De Souza W., Rodrigues J.C.F. Sterol biosynthesis pathway as target for anti-trypanosomatid drugs. Interdiscip. Perspect. Infect. Dis. 2009;2009:1–19. doi: 10.1155/2009/642502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descoteaux A., Matlashewski G. c-fos and tumor necrosis factor gene expression in Leishmania donovani-infected macrophages. Mol. Cell Biol. 1989;9:5223–5227. doi: 10.1128/mcb.9.11.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinesh N., Pallerla D.S., Kaur P.K., Kishore Babu N., Singh S. Exploring Leishmania donovani 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) as a potential drug target by biochemical, biophysical and inhibition studies. Microb. Pathog. 2014;66:14–23. doi: 10.1016/j.micpath.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Dorlo T.P., Balasegaram M., Beijnen J.H., de Vries P.J. Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J. Antimicrob. Chemother. 2012;67:2576–2597. doi: 10.1093/jac/dks275. [DOI] [PubMed] [Google Scholar]
- Dube A., Singh N., Saxena A., Lakshmi V. Antileishmanial potential of a marine sponge, Haliclona exigua (Kirkpatrick) against experimental visceral leishmaniasis. Parasitol. Res. 2007;101:317–324. doi: 10.1007/s00436-007-0469-z. [DOI] [PubMed] [Google Scholar]
- Ei Tahir A., Ibrahim A.M., Satti G.M., Theander T.G., Kharazmi A., Khalid S.A. The potential antileishmanial activity of some Sudanese medicinal plants. Phytother. Res. 1998;12:576–579. [Google Scholar]
- Fu Y., Hsieh T.C., Guo J., Kunicki J., Lee M.Y., Darzynkiewicz Z., Wu J.M. Licochalcone-A, a novel flavonoid isolated from licorice root (Glycyrrhiza glabra), causes G2 and late-G1 arrests in androgen-independent PC-3 prostate cancer cells. Biochem. Biophys. Res. Commun. 2004;322:263–270. doi: 10.1016/j.bbrc.2004.07.094. [DOI] [PubMed] [Google Scholar]
- Fujisawa Y., Sakamoto M., Matsushita M., Fujita T., Nishioka K. Glycyrrhizin inhibits the lytic pathway of complement–possible mechanism of its anti-inflammatory effect on liver cells in viral hepatitis. Microbiol. Immunol. 2000;44:799–804. doi: 10.1111/j.1348-0421.2000.tb02566.x. [DOI] [PubMed] [Google Scholar]
- Galea A.M., Brown A.J. Special relationship between sterols and oxygen: were sterols an adaptation to aerobic life? Free Radic. Biol. Med. 2009;47:880–889. doi: 10.1016/j.freeradbiomed.2009.06.027. [DOI] [PubMed] [Google Scholar]
- García-Pelayo M.C., García-Peregrín E., Martínez-Cayuela M. Differential translational effects of myristic acid and eicosapentaenoic acid on 3-hydroxy-3-methylglutaryl-CoA reductase from Reuber H35 hepatoma cells. Exp. Biol. Med. (Maywood) 2004;229:781–786. doi: 10.1177/153537020422900810. [DOI] [PubMed] [Google Scholar]
- Henriksen J., Rowat A.C., Brief E., Hsueh Y.W., Thewalt J.L., Zuckermann M.J., Ipsen J.H. Universal behavior of membranes with sterols. Biophys. J. 2006;90:1639–1649. doi: 10.1529/biophysj.105.067652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibasami H., Iwase H., Yoshioka K., Takahashi H. Glycyrrhizin induces apoptosis in human stomach cancer KATO III and human promyelotic leukemia HL-60 cells. Int. J. Mol. Med. 2005;16:233–236. [PubMed] [Google Scholar]
- Hoever G., Baltina L., Michaelis M., Kondratenko R., Baltina L., Tolstikov G.A., Cinatl J. Antiviral activity of glycyrrhizic acid derivatives against SARS-coronavirus. J. Med. Chem. 2005;48:1256–1259. doi: 10.1021/jm0493008. [DOI] [PubMed] [Google Scholar]
- Jain S.K., Sahu R., Walker L.A., Tekwani B.L. A parasite rescue and transformation assay for antileishmanial screening against intracellular Leishmania donovani amastigotes in THP1 human acute monocytic leukemia cell line. J. Vis. Exp. 2012;70:e4054. doi: 10.3791/4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalani K., Kushwaha V., Verma R., Murthy P.K., Srivastava S.K. Glycyrrhetinic acid and its analogs: a new class of antifilarial agents. Bioorg. Med. Chem. Lett. 2013;23:2566–2570. doi: 10.1016/j.bmcl.2013.02.115. [DOI] [PubMed] [Google Scholar]
- Kayser O., Kiderlen A.F. In vitro leishmanicidal activity of naturally occurring chalcones. Phytother. Res. 2001;15:148–152. doi: 10.1002/ptr.701. [DOI] [PubMed] [Google Scholar]
- Kim K.J., Choi J.S., Kim K.W., Jeong J.W. The anti-angiogenic activities of glycyrrhizic acid in tumor progression. Phytother. Res. 2013;27:841–846. doi: 10.1002/ptr.4800. [DOI] [PubMed] [Google Scholar]
- Kulkarni M.M., Reddy N., Gude T., McGwire B.S. Voriconazole suppresses the growth of Leishmania species in vitro. Parasitol. Res. 2013;112:2095–2099. doi: 10.1007/s00436-013-3274-x. [DOI] [PubMed] [Google Scholar]
- Kumagai A., Nanaboshi M., Asanuma Y., Yagura T., Nishino K. Effects of glycyrrhizin on thymolytic and immunosuppressive action of cortisone. Endocrinol. Jpn. 1967;14:39–42. doi: 10.1507/endocrj1954.14.39. [DOI] [PubMed] [Google Scholar]
- Liao J.K., Laufs U. Pleiotropic effects of statins. Annu. Rev. Pharmacol. Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.C. Mechanism of action of glycyrrhizic acid in inhibition of Epstein-Barr virus replication in vitro. Antivir. Res. 2003;59:41–47. doi: 10.1016/s0166-3542(03)00030-5. [DOI] [PubMed] [Google Scholar]
- Macreadie I.G., Johnson G., Schlosser T., Macreadie P.I. Growth inhibition of Candida species and Aspergillus fumigatus by statins. FEMS Microbiol. Lett. 2006;262:9–13. doi: 10.1111/j.1574-6968.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- Maurya S.K., Srivastava A.K. Glycyrrhizic acid attenuates the expression of HMG-CoA reductase mRNA in high fructose diet induced dyslipidemic hamsters. Prague Med. Rep. 2011;112:29–37. [PubMed] [Google Scholar]
- Mbongo N., Loiseau P.M., Billion M.A., Robert-Gero M. Mechanism of amphotericin B resistance in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 1998;42:352–357. doi: 10.1128/aac.42.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSorley S., Proudfoot L., O'Donnell C.A., Liew F.Y. Immunology of murine leishmaniasis. Clin. Derm. 1996;14:451–464. doi: 10.1016/0738-081x(96)00037-5. [DOI] [PubMed] [Google Scholar]
- Modabber F. Leishmaniasis vaccines: past, present and future. Int. J. Antimicrob. Agents. 2010;36:S58–S61. doi: 10.1016/j.ijantimicag.2010.06.024. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Murray H.W. Clinical and experimental advances in treatment of visceral leishmaniasis. Antimicrob. Agents Chemother. 2001;45:2185–2197. doi: 10.1128/AAC.45.8.2185-2197.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasyrov K.h.M., Chepurina L.S., Kireeva R.M. The hepatoprotective and cholagogic action of glycyrrhizic acid derivatives. Eksp. Klin. Farmakol. 1995;58:60–63. [PubMed] [Google Scholar]
- Ng H.E., Raj S.S., Wong S.H., Tey D., Tan H.M. Estimation of fungal growth using the ergosterol assay: a rapid tool in assessing the microbiological status of grains and feeds. Lett. Appl. Microbiol. 2008;46:113–118. doi: 10.1111/j.1472-765X.2007.02279.x. [DOI] [PubMed] [Google Scholar]
- Pandey K., Sinha P.K., Das V.R., Bimal S., Singh S.K., Das P. Pharmacotherapeutic options for visceral leishmaniasis-current scenario. Clin. Med. Pathol. 2009;2:1–4. [PMC free article] [PubMed] [Google Scholar]
- Roberts C.W., McLeod R., Rice D.W., Ginger M., Chance M.L., Goad L.J. Fatty acid and sterol metabolism: potential antimicrobial targets in apicomplexan and trypanosomatid parasitic protozoa. Mol. Biochem. Parasitol. 2003;126:129–142. doi: 10.1016/s0166-6851(02)00280-3. [DOI] [PubMed] [Google Scholar]
- Singh S., Dinesh N., Kaur P.K., Shamiulla B. Ketanserin, an antidepressant, exerts its antileishmanial action via inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) enzyme of Leishmania donovani. Parasitol. Res. 2014;113:2161–2168. doi: 10.1007/s00436-014-3868-y. [DOI] [PubMed] [Google Scholar]
- Soumya N., Kumar I.S., Shivaprasad S., Gorakh L.N., Dinesh N., Swamy K.K., Singh S. AMP-acetyl CoA synthetase from Leishmania donovani: identification and functional analysis of ‘PX4GK’motif. Int. J. Biol. Macromol. 2015;75:364–372. doi: 10.1016/j.ijbiomac.2015.01.042. [DOI] [PubMed] [Google Scholar]
- Takii H., Kometani T., Nishimura T., Nakae T., Okada S., Fushiki T. Antidiabetic effect of glycyrrhizin in genetically diabetic KK-Ay mice. Bio. Pharm. Bull. 2001;24:484–487. doi: 10.1248/bpb.24.484. [DOI] [PubMed] [Google Scholar]
- Taylor A.P., Murray H.W. Intracellular antimicrobial activity in the absence of interferon-gamma: effect of interleukin-12 in experimental visceral leishmaniasis in interferon-gamma gene-disrupted mice. J. Exp. Med. 1997;185:1231–1239. doi: 10.1084/jem.185.7.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouiller P., Olliaro P., Torreele E., Orbinski J., Laing R., Ford N. Drug development for neglected diseases: a deficient market and a public-health policy failure. Lancet. 2002;359:2188–2194. doi: 10.1016/S0140-6736(02)09096-7. [DOI] [PubMed] [Google Scholar]
- Ukil A., Biswas A., Das T., Das P.K. 18 Beta-glycyrrhetinic acid triggers curative Th1 response and nitric oxide up-regulation in experimental visceral leishmaniasis associated with the activation of NF-kappa B. J. Immunol. 2005;175:1161–1169. doi: 10.4049/jimmunol.175.2.1161. [DOI] [PubMed] [Google Scholar]
- Urbina J.A., Vivas J., Visbal G., Contreras L.M. Modification of the sterol composition of Trypanosoma (Schizotrypanum) cruzi epimastigotes by Δ 24(25)-sterol methyl transferase inhibitors and their combinations with ketoconazole. Mol. Biochem. Parasitol. 1995;73:199–210. doi: 10.1016/0166-6851(95)00117-j. [DOI] [PubMed] [Google Scholar]
- Wan X.Y., Luo M., Li X.D., He P. Hepatoprotective and anti-hepatocarcinogenic effects of glycyrrhizin and matrine. Chem. Biol. Interact. 2009;181:15–19. doi: 10.1016/j.cbi.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Weniger B., Robledo S., Arango G.J., Deharo E., Aragon R., Munoz V., Callapa J., Lobstein A., Anton R. Antiprotozoal activities of Colombian plants. J. Ethnopharmacol. 2001;78:193–200. doi: 10.1016/s0378-8741(01)00346-4. [DOI] [PubMed] [Google Scholar]