Abstract
Epidemiologic data suggested that there was an obvious predominance of young adult patients with a slight female proneness in severe acute respiratory syndrome (SARS). The angiotensin-converting enzyme 2 (ACE2) was very recently identified as a functional receptor for SARS virus and is therefore a prime target for pathogenesis and pharmacological intervention. Rats of both genders at three distinct ages (young-adult, 3 months; middle-aged, 12 months; old, 24 months) were evaluated to determine the characteristic of ACE2 expression in lung and the effect of aging and gender on its expression. ACE2 was predominantly expressed in alveolar epithelium, bronchiolar epithelium, endothelium and smooth muscle cells of pulmonary vessels with similar content, whereas no obvious signal was detected in the bronchiolar smooth muscle cells. ACE2 expression is dramatically reduced with aging in both genders: young-adult vs. old P < 0.001 (by 78% in male and 67% in female, respectively) and middle-aged vs. old P < 0.001 (by 71% in male rats and 59% in female rats, respectively). The decrease of ACE2 content was relatively slight between young-adult and middle-aged groups (by 25% in male and 18% in female, respectively). Although there was no gender-related difference of ACE2 in young-adult and middle-aged groups, a significantly higher ACE2 content was detected in old female rats than male. In conclusion, the more elevated ACE2 in young adults as compared to aged groups may contribute to the predominance in SARS attacks in this age group.
Keywords: Angiotensin-converting enzyme 2, Aging, Severe acute respiratory syndrome, SARS-CoV
Introduction
The angiotensin-converting enzyme 2 (ACE2), the first known human homologue of angiotensin-converting enzyme (ACE), is an important regulator of the renin-angiotensin system (Donoghue et al., 2000). Since its discovery in 2000, ACE2 has been implicated in heart function (Crackower et al., 2002), hypertension and diabetes (Tikellis et al., 2003). Unexpectedly, it was very recently identified as a functional receptor for the coronavirus (CoV) that causes the severe acute respiratory syndrome (SARS), serving as the cellular entry point for the SARS virus (Li et al., 2003). The receptor is therefore a prime target for pathogenesis and pharmacological intervention.
SARS, an acute pulmonary syndrome characterized by an atypical pneumonia that results in progressive respiratory failure and death in close to 10% of infected individuals, is responsible for the first pandemic of the 21st century (Peiris et al., 2003). SARS-CoV spreads mainly via the respiratory route (Ksiazek et al., 2003, Drosten et al., 2003, Kuiken et al., 2003, Fouchier et al., 2003), and the lungs are the main targets (Ding et al., 2003). The spike protein of the coronavirus (a surface glycoprotein) mediates coronavirus entry into receptor-bearing cells, after which viral replication begins in the cytoplasm (Li et al., 2003). Interestingly, the epidemiologic data of SARS suggested that there was an obvious predominance of young adult patients in SARS attacks. The largest outbreak of SARS struck Beijing in spring 2003 and about 2521 cases of probable SARS occurred. Attack rates were highest in those 20–39 years of age (mean age 33), which account for 53.0% (Liang et al., 2004). The age-specific attack rates of SARS were supported by other outbreaks of SARS (Zhao et al., 2003, Lee et al., 2003, Hsu et al., 2003, Poutanen et al., 2003, Booth et al., 2003). Some of these data also showed a slight female proneness for SARS attacks. It seems likely that aging and gender might be implicated in SARS infection susceptibility. Thus, it can be hypothesized that age- or gender-related difference of ACE2 expression in lung tissue might contribute to different pathological processes in SARS-CoV attacks.
In this study rats of both genders at three distinct ages (3 months indicates young-adult, 12 months middle-age, 24 months old) are evaluated for the characteristic of ACE2 expression in lung tissue and the effect of aging and gender on its expression, which may be helpful for the elucidation of the pathogenesis and future treatment options for SARS.
Materials and methods
Animals
Healthy male and female Sprague Dawley rats were purchased from the Animal Experimental Center of Zhejiang Medical Research Institute. Upon arrival, rats were housed in a specific pathogen-free facility and kept on a 12-h light, 12-h dark cycle at 22 °C. Water and food were available ad libitum to all animals. Experiments were completed taking into account the entire age-scale encompassing the young adult, middle-aged and the old. Six groups of ten SD rats were used, at three ages of both genders: 3, 12 and 24 months to represent different stages in early, middle and later life of rats (Smith et al., 1995, Mendelson and Bergmann, 2000, Sniecinski and Liu, 2004, Kotz et al., 2005).
Sample preparation
The rats were killed by an intraperitoneal overdose of sodium pentobarbital. The lungs were rapidly removed and immediately frozen in liquid nitrogon. Frozen tissues were transferred to a − 80 °C freezer until processed. At least three tissue blocks were examined per case from different lung regions, in both immunohistochemical study and Western blotting. The results were the mean value of three measures. All animal procedures were in accordance with guidelines set by Animals Research Committee of School of Medicine, Zhejiang University.
Immunohistochemistry
Immunohistochemistry staining for ACE2 was performed as outlined below. Frozen sections of rat lung were cut at six-micrometer thickness, mounted on charged slides, fixed in cold acetone (Sigma), and immersed in 3% (vol/vol) hydrogen peroxide in PBS for 10 min to block endogenous peroxidase. A 1 : 2000 dilution of polyclonal rabbit anti-ACE2 (Millenium Pharmaceuticals) (Donoghue et al., 2000) was applied in 5%BSA/PBS for 1 h at room temperature. Specific staining was detected with the standard ABC (avidin–biotin complex) method. Briefly, slides were incubated for 20 min with the secondary antibody (Amersham) at a concentration of 1 / 250. The Vectastain ABC system (Vector Laboratories) was then applied for 20 min. After thorough washing, the final detection step was carried out with the use of 3,3′-diaminobenzidine (Sigma) as the chromogen. Sections were lightly counterstained with hematoxylin. For the negative control, the primary antibody was replaced with normal rabbit sera (Jingmei Biotech) or PBS. These control sections do not reveal any staining (Fig. 1C).
Fig. 1.
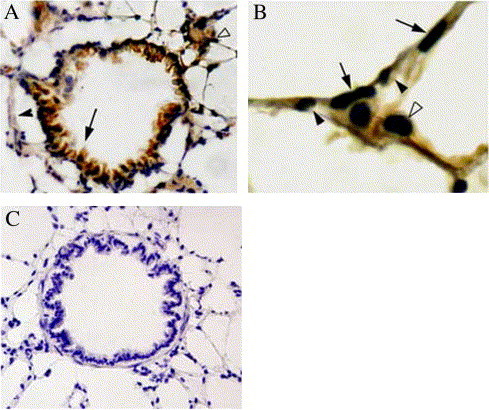
The immunohistochemical analysis of ACE2 expression in normal rat lung. A: Positive staining for ACE2 in alveolar epitheliums (empty arrowhead) and bronchiolar epitheliums (arrow), but no obvious signal in the bronchiolar smooth muscle cells (black arrowhead). B: ACE2 present in alveolar epithelial cells (type I black arrow and type II empty arrowhead) and capillary endothelium (black arrowhead) in larger magnification. C: Control section stained with normal rabbit serum instead of anti-ACE2 shows no staining in rat lung. Original magnification A and C, × 400; B × 800.
Western blotting
Rat lungs were quickly removed and minced with a scalpel blade, homogenized in buffer (10 mmol/L HEPES, 150 mmol/L NaCl, 1 mmol/L EGTA, 5 mmol/L MgCl2, and 0.02% NaN3, PH 7.4, containing 0.5 μg /mL pepstatin [Sigma], 0.25μg /mL leupeptin [Sigma], 50 μg/mL PMSF[Sigma]). The homogenates were then centrifuged at 1000 g (4 °C) for 30 min. Samples (50 μg of total protein) was loaded and run on a 10% sodium dodecyl sulfate denaturing gel, and proteins were transblotted onto nitrocellulose filters (Santa Cruz) through the use of a transfer tank at 200 mA for 90 min in 4 °C. At the end of the transfer, the filters were blocked with 10% nonfat skim milk powder in TBS containing 0.1% Tween-20 (TBST) for 1 h at room temperature with gentle rocking. The membrane was incubated with polyclonal rabbit anti-ACE2 (1 : 2000, Millenium Pharmaceuticals) (Donoghue et al., 2000) and anti-βactin (1 : 2000, sigma) at a overnight at 4 °C in 1% BSA/TBST. β-actin was used as loading control. The following day, the blot was washed in 1% TBS/Tween (3 × 15 min) and then incubated in goat–anti-rabbit HRP antibody (1 : 500, Zhongshan Biotech, China) for 1 h at room temperature and washed (3 × 15 min TBST). Signal was detected using the ECL plus kit (Amersham). Exposed films of bands representing ACE2 protein were quantified on an Automated Imaging System (Kodak). Rabbit normal sera were used as negative controls. A molecular weight marker (Invitrogen) was used for estimation of the ACE2 molecular weight.
Statistical analysis
Data were expressed in mean ± SEM. The significance of aging difference among groups was determined by one-way ANOVA with SPSS version 10.0 software followed by Tukey's multiple comparison tests. For comparisons gender difference between two groups, Student's t-tests were used. P < 0.05 was considered significant.
Results
Localization of ACE2 in rat lung
Although it had been reported that ACE2 protein was expressed in bronchus and lung parenchyma as well as in the heart, kidney and gastrointestinal tract (Donoghue et al., 2000, Harmer et al., 2002, Hamming et al., 2004), the histocytochemical characteristics of ACE2 in lung tissue has not been elucidated adequately.
In immunohistochemical study, the major site of ACE2 protein was in alveolar type I epithelium, alveolar type II epithelium, bronchiolar epithelium, endothelium and smooth muscle cells of pulmonary vascular structure, whereas no obvious signal was detected in the bronchiolar smooth muscle cells. The ACE2 intensity of bronchiolar epithelium is similar to alveolar epithelium or endothelium of vascular structure (Fig. 1A, B), whereas Hamming et al. reported that the ACE2 content was not similar in bronchial epithelial and alveolar epithelium (Hamming et al., 2004).
Effect of age on ACE2 expression in rat lung
ACE2 expression in rat lung was quantified by Western blot analysis. As shown in Fig. 2 , an approximately 90 kDa immunoreactive band was present and its content significantly diminishes with age in rat lung of both genders. The ACE2 content is dramatically reduced in old rats of both genders: young-adult vs. old P < 0.001 (by 78% in male and 67% in female, respectively) and middle-aged vs. old P < 0.001 (by 71% in male rats and 59% in female rats, respectively), as compared to the 3-month and 12-month old groups (Fig. 2). Compared with old rats, there is only a weak decrease of ACE2 content between young-adult and middle-aged groups (by 25% in male and 18% in female, respectively).
Fig. 2.
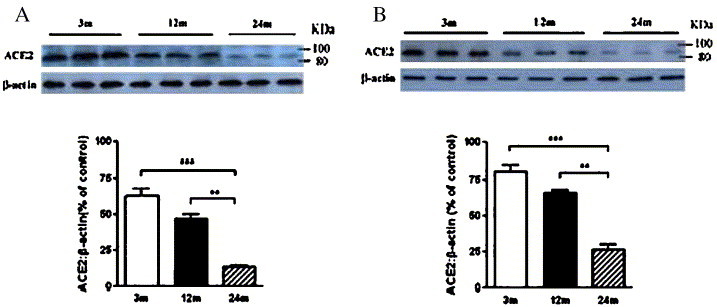
ACE2 protein content was decreased with age in rat lung tissue of both genders: male (A) and female (B). The amounts of ACE2 were determined by Western analysis as described in Methods section. β-actin was used as loading control. Values represent means ± SEM (n = 10). 3 m, young-adult group (3 months); 12 m, middle-aged group(12 months); 24 m, old group (24 months). The sizes of the molecular weight markers are shown to the right and indicate the 100 kDa and 86 kDa proteins. **, P < 0.01; ***, P < 0.001.
The age-associated change in ACE2 protein content was also roughly shown in immunohistochemical analysis. A significant decline in the number of cells stained for this enzyme and the intensity of immunostaining in rat lung tissue was observed in both genders during the aging process (Fig. 3 ).
Fig. 3.
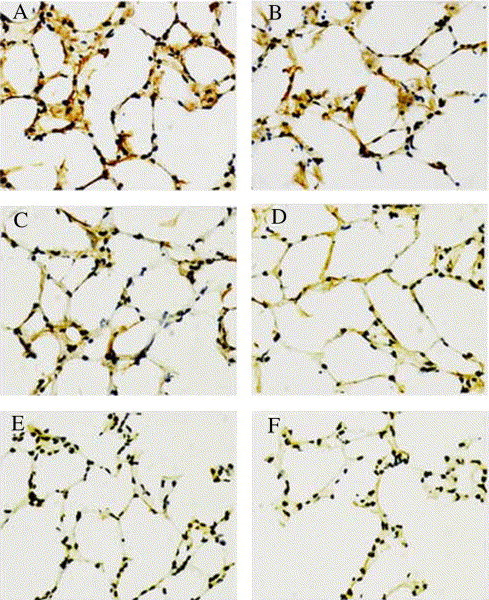
Immunohistochemical staining of ACE2 in rat lung of both genders at three distinct ages: female young-adult (A), male young-adult (B), female middle-aged (C), male middle-aged (D), female old (E) and male old (F). Original magnification: × 400.
Effect of gender on ACE2 expression in rat lung
In order to see whether there was any gender-related difference in ACE2 expression during the aging process, we compared age-associated ACE2 content between male and female rats. The results showed that there was no significant difference in ACE2 content either between young male and female rats or between middle-aged male and female rats, but a significantly higher content was found in old female rats than male (P < 0.05) (Fig. 4 ). The content of ACE2 decreased by 67% and 78% in the lung of 24-month old female and male rats, respectively, as compared with 3-month old rats.
Fig. 4.
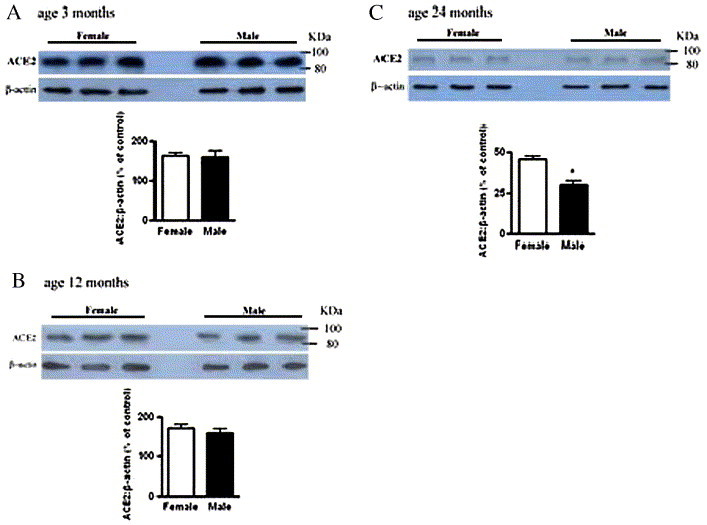
Effect of gender on ACE2 expression in rat lung tissue during aging process. A: young adult group (3 months); B: middle-aged group (12 months); C: old group (24 months). β-actin was used as loading control. Values represent means ± SEM (n = 10). The sizes of the molecular weight markers are shown to the right and indicate the 100 kDa and 86 kDa proteins. *, P < 0.05.
Discussion
In this study, the involvement of aging and gender in ACE2 protein expression in rat lung is explored for the first time. We assess three distinct ages (3, 12, and 24 months) and provide three major findings: 1) ACE2 is predominantly expressed in alveolar epithelium, bronchiolar epithelium, endothelium and smooth muscle cells of pulmonary vessels with similar content; whereas no obvious signal was detected in the bronchiolar smooth muscle cells. 2) There has been a significant age-specific decline of ACE2 expression in rat lung of both genders, especially during the later aging process. 3) Although the concentrations of ACE2 were similar between male and female rats in either young-adult group or middle-aged group, male rats seemed to experience more dramatic age-associated decrease in ACE2 expression than did female rats in lung tissue.
These data, although obtained from rats, may provide helpful clues to understanding the SARS pathogenesis, especially in lung tissue.
SARS is spread via the respiratory tract and is mainly a lower respiratory tract disease, causing pulmonary lesions and respiratory distress (Ding et al., 2003). Recent studies in an autopsy series using viral isolation, culture techniques, and in situ hybridization showed that SARS-CoV is present in alveolar epithelial cells and, to a lesser extent, macrophages and bronchial epithelial cells (To et al., 2004). The localization of ACE2 protein in human lung was detected very recently (Hamming et al., 2004). The results from the present study are consistent with the previous findings about SARS pathology and the coronavirus histological distribution. But in our study ACE2 content in alveolar epithelial cells and bronchial epithelial cells are similar, while Hamming et al. identified that ACE2 content was weak in bronchial epithelial. Interestingly, compared with the smooth muscle cells of pulmonary vessels, there was no obvious positive deposits are detected in the bronchial smooth muscle cells. High levels of ACE2 are seen in endothelial cells, but viral infection has not been demonstrated extensively in these cells of human tissues in previous studies (Ding et al., 2003). These differences might be a clue for disclosing the severe vasculitis without equivalent bronchiolar inflamma tion in SARS pathology. We could postulate that the efficiency of the virus replication is distinct in different respiratory cell types although the contents of ACE2 in these cell types are similar.
Although SARS has attacked all age groups and both genders, the young adults seem to be more prone to SARS. The largest outbreak of SARS struck Beijing in spring 2003 and about 2521 cases of probable SARS occurred. Attack rates were highest in those 20–39 years of age (mean age 33), which account for 53.0% (Liang et al., 2004). The age-specific attack rates of SARS in Beijing support findings from other outbreaks of SARS. The mean age of affected patients is 28 years in Guangzhou (190 cases) (Zhao et al., 2003), 39 years in Hongkong (138 cases) (Lee et al., 2003) and 28 years in Singapore (20 cases) (Hsu et al., 2003), whereas it was 45 years (154 cases) in Canada (Poutanen et al., 2003, Booth et al., 2003). The patients enrolled in the former 4 reports are all Chinese ethic, which might be related to the slight discrepancy between the data from Asia and from Canada. But the total tendency is obvious that age might be an independent factor for the risk of susceptibility to SARS. Considering that ACE2 serves as the cellular entry point for the SARS virus, age-related difference of ACE2 expression might be related to the highest attack rates in the young adult. However, it is inconsistent with the high mortality in old patients in these reports. But the entry of virus into the alveolar pneumocytes in the lung is only at the dawn of our understanding of the pathogenesis of SARS, there are so many unknown factors taking part in the process.
The present study shows that male rats seem to experience more dramatic age-associated decline in ACE2 protein expression. Some reports showed the ratio of male to female in SARS patients is 0.58 : 1 (70 : 120) in Guangzhou, 0.8 : 1 (174 : 214) in Hongkong and 0.33 : 1 (5 : 15) in Singapore (Zhao et al., 2003, Lee et al., 2003, Hsu et al., 2003), but Liang et al. reported that male patients had similar rates as female patients in a large sample of 2521 cases of probable SARS in Beijing (Liang et al., 2004). The slight female predominance of SARS patients in previous reports might be related to the increased likelihood of exposure among nurses in early clinical work without enough personal protection equipment.
In summary, the study gives the profile of age- and gender-related ACE2 expression in rat lung, and suggests that the more elevated ACE2 in young adults as compared to aged groups may contribute to the predominance in SARS attacks in this age group, although these data are based on animal studies with limited numbers.
References
- Booth C.M., Matukas L.M., Tomlinson G.A., Rachlis A.R., Rose D.B., Dwosh H.A., Walmsley S.L., Mazzulli T., Avendano M., Derkach P., Ephtimios I.E., Kitai I., Mederski B.D., Shadowitz S.B., Gold W.L., Hawryluck L.A., Rea E., Chenkin J.S., Cescon D.W., Poutanen S.M., Detsky A.S. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. The Journal of the American Medical Association. 2003;289(21):2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- Crackower M.A., Sarao R., Oudit G.Y., Yagil C., Kozieradzki I., Scanga S.E., Oliveira-dos-Santos A.J., Costa J., Zhang L., Pei Y., Scholey J., Ferrario C.M., Manoukian A.S., Chappell M.C., Backx P.H., Yagil Y., Penninger J.M. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417(6891):822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- Ding Y., Wang H., Shen H., Li Z., Geng J., Han H., Cai J., Li X., Kang W., Weng D., Lu Y., Wu D., He L., Yao K. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. The Journal of Pathology. 2003;200(3):282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R., Breitbart R.E., Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circulation Research. 2000;87(5):E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. New England Journal of Medicine. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Fouchier R.A., Kuiken T., Schutten M., van Amerongen G., van Doornum G.J., van den Hoogen B.G., Peiris M., Lim W., Stohr K., Osterhaus A.D. Aetiology: Koch's postulates fulfilled for SARS virus. Nature. 2003;423(6937):240. doi: 10.1038/423240a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. Journal of Pathology. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer D., Gilbert M., Borman R., Clark K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Letters. 2002;532(1–2):107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- Hsu L.Y., Lee C.C., Green J.A., Ang B., Paton N.I., Lee L., Villacian J.S., Lim P.L., Earnest A., Leo Y.S. Severe acute respiratory syndrome (SARS) in Singapore: clinical features of index patient and initial contacts. Emerging Infectious Diseases. 2003;9(6):713–717. doi: 10.3201/eid0906.030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotz C.M., Mullett M.A., Wang C. Diminished feeding responsiveness to orexin A (hypocretin 1) in aged rats is accompanied by decreased neuronal activation. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2005;289(2):R359–R366. doi: 10.1152/ajpregu.00717.2004. [DOI] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J. A novel coronavirus associated with severe acute respiratory syndrome. New England Journal of Medicine. 2003;348(20):1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Kuiken T., Fouchier R.A., Schutten M., Rimmelzwaan G.F., van Amerongen G., van Riel D., Laman J.D., de Jong T., van Doornum G., Lim W., Ling A.E., Chan P.K., Tam J.S., Zambon M.C., Gopal R., Drosten C., van der Werf S., Escriou N., Manuguerra J.C., Stohr K., Peiris J.S., Osterhaus A.D. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362(9380):263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M., Ahuja A., Yung M.Y., Leung C.B., To K.F., Lui S.F., Szeto C.C., Chung S., Sung J.J. A major outbreak of severe acute respiratory syndrome in Hong Kong. New England Journal of Medicine. 2003;348(20):1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W., Zhu Z., Guo J., Liu Z., Zhou W., Chin D.P., Schuchat A. Severe acute respiratory syndrome, Beijing, 2003. Emerging Infectious Diseases. 2004;10(1):25–31. doi: 10.3201/eid1001.030553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson W.B., Bergmann B.M. Age-dependent changes in recovery sleep after 48 hours of sleep deprivation in rats. Neurobiology of Aging. 2000;21(5):689–693. doi: 10.1016/s0197-4580(00)00154-8. [DOI] [PubMed] [Google Scholar]
- Peiris J.S., Yuen K.Y., Osterhaus A.D., Stohr K. The severe acute respiratory syndrome. New England Journal of Medicine. 2003;349(25):2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- Poutanen S.M., Low D.E., Henry B., Finkelstein S., Rose D., Green K., Tellier R., Draker R., Adachi D., Ayers M., Chan A.K., Skowronski D.M., Salit I., Simor A.E., Slutsky A.S., Doyle P.W., Krajden M., Petric M., Brunham R.C., McGeer A.J. Identification of severe acute respiratory syndrome in Canada. New England Journal of Medicine. 2003;348(20):1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- Smith C.B., Sun Y., Sokoloff L. Effects of aging on regional rates of cerebral protein synthesis in the Sprague–Dawley rat: examination of the influence of recycling of amino acids derived from protein degradation into the precursor pool. Neurochemistry International. 1995;27(4–5):407–416. doi: 10.1016/0197-0186(95)00022-z. [DOI] [PubMed] [Google Scholar]
- Sniecinski R., Liu H. Reduced efficacy of volatile anesthetic preconditioning with advanced age in isolated rat myocardium. Anesthesiology. 2004;100(3):589–597. doi: 10.1097/00000542-200403000-00019. [DOI] [PubMed] [Google Scholar]
- Tikellis C., Johnston C.I., Forbes J.M., Burns W.C., Burrell L.M., Risvanis J., Cooper M.E. Characterization of renal angiotensin-converting enzyme 2 in diabetic nephropathy. Hypertension. 2003;41(3):392–397. doi: 10.1161/01.HYP.0000060689.38912.CB. [DOI] [PubMed] [Google Scholar]
- To K.F., Tong J.H., Chan P.K., Au F.W., Chim S.S., Chan K.C., Cheung J.L., Liu E.Y., Tse G.M., Lo A.W., Lo Y.M., Ng H.K. Tissue and cellular tropism of the coronavirus associated with severe acute respiratory syndrome: an in-situ hybridization study of fatal cases. The Journal of Pathology. 2004;202(2):157–163. doi: 10.1002/path.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Zhang F., Xu M., Huang K., Zhong W., Cai W., Yin Z., Huang S., Deng Z., Wei M., Xiong J., Hawkey P.M. Description and clinical treatment of an early outbreak of severe acute respiratory syndrome (SARS) in Guangzhou, PR China. Journal of Medical Microbiology. 2003;52(Pt 8):715–720. doi: 10.1099/jmm.0.05320-0. [DOI] [PubMed] [Google Scholar]


