Abstract
Background
Respiratory viruses frequently are recovered in the upper-respiratory tract during acute exacerbations of COPD (AECOPD), but their role as contributing pathogens remains unclear. The usefulness of procalcitonin and C-reactive protein as indicators of the presence or absence of viral infection in this setting also needs to be evaluated.
Methods
The study was of a prospective cohort of patients with COPD admitted to the ED for AECOPD. Reverse transcriptase-polymerase chain reaction (RT-PCR) for 14 respiratory viruses was performed on nasopharyngeal swabs collected at admission and after recovery in stable condition.
Results
Eighty-six patients (mean age, 72 years; male, 64%) were included. During AECOPD, upper-respiratory viral infections were detected in 44 (51%) patients: picornavirus in 22, metapneumovirus in seven, coronavirus in eight, influenza A/B in two, parainfluenza in two, and respiratory syncytial virus in three. A dual infection was present in three patients. After recovery, viruses were detected in only eight (11%) of 71 patients (P < .001 compared with AECOPD phase). In five of these patients, no virus had been identified during the initial exacerbation, thus suggesting a new viral infection acquired during follow-up. During AECOPD, procalcitonin and C-reactive protein levels did not differ significantly between patients with or without a proven viral infection.
Conclusions
Prevalence of upper-respiratory viral infection, as detected from nasopharyngeal swab by RT-PCR, is high in AECOPD and low after clinical recovery, suggesting that AECOPD frequently are triggered by viral infections initiated in the upper-respiratory tract. In our study, serum procalcitonin and C-reactive protein did not discriminate virus-associated exacerbations from others.
Trial registration
clinicaltrials.gov; Identifier: NCT00448604.
Abbreviations
- AECOPD
acute exacerbations of COPD
- CRP
C-reactive protein
- OR
odds ratio
- PCR
polymerase chain reaction
- PCT
procalcitonin
- PEF
peak expiratory flow
- RSV
respiratory syncytial virus
- RT-PCR
reverse transcriptase-polymerase chain reaction
- URT
upper-respiratory tract
Among the inflammatory processes triggering COPD exacerbations, respiratory tract viral infections are prime suspects. Although first localized in the upper-respiratory tract (URT), these infections could trigger a cascade of inflammatory events1, 2 that lead to clinically significant exacerbations. Current guidelines recommend the administration of antibiotics based mainly on clinical criteria,1 leading inevitably to an overuse of antibiotics, which is estimated to occur in approximately 55% of all acute exacerbations of COPD (AECOPD).3
The participation of viral infections in AECOPD has been confirmed in many studies.4, 5, 6, 7 The most recent reports using polymerase chain reaction (PCR)-based methods led to a positive detection of viral nucleic acids in 30% to 60% of AECOPD cases.5, 8, 9, 10, 11, 12 However, the role of recently identified viruses, such as coronavirus NL63 and HKU1 and human bocavirus as well as the new human rhinoviruses C, which are reported as the most frequent ones circulating in the community,13, 14 has not yet been fully established.
Biomarkers such as procalcitonin (PCT), when elevated, have been proposed as surrogate markers of bacterial disease and, when within normal values, often are considered in clinical practice as the signature of a viral infection. Although PCT has been studied for its potential to predict response to antibiotic therapy in respiratory tract infections,15, 16, 17 it has never been systematically correlated to virological investigations to assess its ability to discriminate between the bacterial and viral etiology of an exacerbation.
We aimed to describe the role of viruses, including the recently identified strains that, to our knowledge, have never been studied in this setting, as pathogens in COPD by documenting their presence during the acute phase of an exacerbation and their disappearance after recovery during a clinically stable phase in the same patients. We also aimed to explore the potential of biologic markers (C-reactive protein [CRP] and PCT) to distinguish between viral and nonviral infection in AECOPD.
Materials and Methods
Patients and Procedures
From June 2007 to December 2008, we prospectively recruited 86 adult patients admitted to our emergency department and hospitalized for severe AECOPD according to the Global Initiative for Chronic Obstructive Pulmonary Disease definition.1 Patients were identified by a review of admission codes for dyspnea and history of COPD. Exclusion criteria for this study were the presence of chronic lung diseases other than COPD (asthma, bronchiectasis, pulmonary fibrosis, pneumoconiosis), acute pulmonary embolism, acute pneumonia (based on chest radiographs), acute pulmonary edema, and orotracheal intubation requirement.
Peak expiratory flow (PEF) values; number of exacerbations during the previous year (as reported by family physicians, who were personally contacted), use of glucocorticosteroids and antibiotics, vaccination history, recent spirometric values (< 1 year), and presence of other comorbidities preceding hospitalization were recorded at study inclusion. Serum PCT (Kryptor PCT; Brahms Diagnostica Gmbh; Henningsdorf, Germany), CRP, blood cell count, blood gas analysis, sputum bacterial analysis, and blood chemistry were obtained at admission. If available, results of sputum bacterial culture, which were semiquantitative assays, were considered significant only in the presence of > 10 neutrophils per high power field and < 25 squamous epithelial cells per low power field to avoid contamination by oral flora. For the present study, we report only qualitative results. The study protocol was approved by the institutional research ethics committee, and written informed consent was obtained from all participants.
During hospitalization, patients were monitored daily by using a diary card to record respiratory symptoms and dyspnea severity according to the Medical Research Council dyspnea scale.18 PEF and oxygen saturation by pulse oximetry were measured daily under the supervision of a study nurse. Health-related quality of life was assessed using the St George Respiratory Questionnaire and the Maugeri Foundation Respiratory Failure Questionnaire. Treatment (antibiotics and oral glucocorticosteroids) was left to the discretion of the attending physician who was blinded to results of viral screening.
At a predetermined follow-up visit scheduled 4 months (mean ± SD, 123 ± 44 days) after the initial exacerbation, patients underwent a second assessment with virological testing, PEF measurement, and completion of the health-related quality-of-life questionnaires. Bacteriologic analyses were not repeated mainly because the large majority of patients was unable to produce sputum.
Virologic Samples
Nasopharyngeal swabs (pooled nasopharyngeal and pharyngeal swabs performed according to a standard operating procedure by the same study nurse) were obtained at admission (within 24 h) and at follow-up visit. Qualitative reverse transcriptase-PCR (RT-PCR) assays were performed on 200 μL of each specimen for the following 14 different respiratory viruses: influenza A and B; respiratory syncytial virus (RSV); parainfluenza 1, 2, and 3; human picornaviruses (human rhinovirus and enterovirus); human metapneumovirus; coronaviruses OC43, 229E, NL63, and HKU1; and bocavirus. All assays were conducted as previously described.19, 20, 21 Parainfluenza 1 and 3 were detected using a recently described assay.22 The picornavirus PCR assay was designed to detect all human enteroviruses and rhinoviruses, including human rhinovirus C.23 We did not discriminate rhinoviruses from enteroviruses.
Definitions
Rhinopharyngitis symptoms refers to the presence of nasal congestion, increased rhinorrhea, or sore throat. COPD severity was quantified according to the Global Initiative for Obstructive Pulmonary Disease guidelines1 based on recent spirometric testing (< 1 year). Using the diary card, time to recovery was calculated as the interval between enrollment in the study and the time when the patient considered having returned to his or her baseline clinical condition.
Statistical Analysis
Comparison between groups was performed using unpaired t tests, Mann-Whitney tests, χ2 tests, or Fisher exact tests when appropriate. Logistic regressions were performed to analyze the relationship between independent variables and the presence of viral nucleic acids, with adjustment for significant potential confounders. A Cox regression model was performed to predict mortality according to the presence of viruses. The results are presented as odds ratios (ORs) or hazard ratios with 95% CIs. All statistical analyses were performed using SPSS, version 15.0 for Windows, statistical software (SPSS, Inc; Chicago, IL).
Results
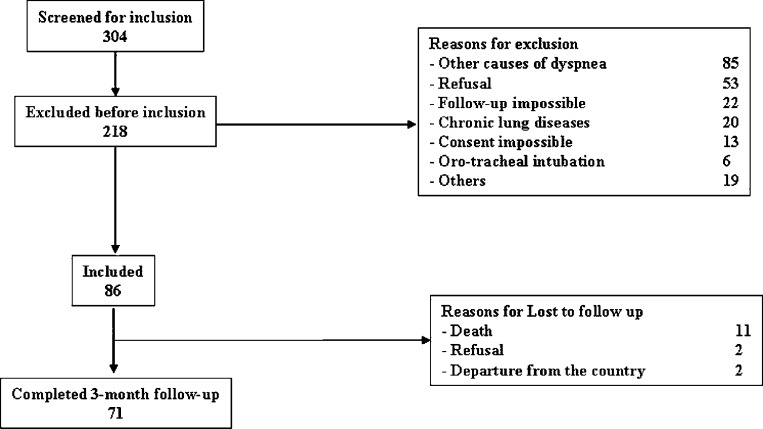
From June 2007 to December 2008, 304 patients were screened at admission to our ED, and 86 were enrolled in the study (Fig 1 ). Seventy-one patients completed the 4-month follow-up, and repeated viral samples were available for all. Baseline clinical conditions and outcomes according to the presence or absence of viruses are shown in Table 1 .
Figure 1.
Flowchart of patients included in the study. “Follow-up impossible” refers to patients transferred to another hospital.
Table 1.
Baseline Characteristics, Clinical Features at Study Entry, and Outcomes in 86 Patients With COPD Exacerbation
| Variable | All Patients (N = 86; 100%) | Virus-Positive Group(n = 44; 51%) | Virus-Negative Group(n = 42; 49%) | P Value |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, y | 71 ± 9 | 70 ± 10 | 73 ± 8 | .16 |
| Male sex | 55 (64) | 28 (33) | 27 (67) | .22 |
| BMI, kg/m2 | 24.8 ± 6.2 | 24.8 ± 6.9 | 24.8 ± 5.4 | .99 |
| COPD severity according to GOLD staging | ||||
| Postbronchodilator FEV1, PPVa | .22 | |||
| > 80 | 2 (2) | 2 (4.5) | 0 (0) | |
| 80–50 | 25 (29) | 11 (25) | 14 (33.3) | |
| 50–30 | 43 (50) | 25 (57) | 18 (43) | |
| < 30 | 16 (19) | 6 (14) | 10 (24) | |
| Current smoker | 33 (38) | 19 (43) | 14 (33) | .30 |
| Influenza vaccinationb | 64 (75) | 32 (73) | 32 (76) | .71 |
| Number of prior COPD exacerbationsb | 1.1 ± 1.4 | 0.8 ± 1.0 | 1.4 ± 1.6 | .048 |
| Home oxygen therapy | 18 (21) | 6 (14) | 12 (43) | .09 |
| Systemic corticosteroids prior to admission | 20 (23) | 7 (16) | 13 (31) | .1 |
| Antibiotic treatment prior to admission | 26 (30) | 14 (32) | 12 (29) | .74 |
| Duration of symptoms prior to admission, d | 6.7 ± 5.2 | 4.8 ± 3.2 | 8.6 ± 5.9 | < .01 |
| Clinical features at study entry | ||||
| Increased dyspnea | 78 (91) | 40 (91) | 38 (91) | .26 |
| Increased cough | 65 (76) | 38 (86) | 27 (64) | .02 |
| Increased amount of sputum | 48 (56) | 29 (66) | 19 (45) | .15 |
| Purulent sputum | 53 (62) | 29 (66) | 24 (57) | .70 |
| Temperature, °C | 37.3 ± 0.9 | 37.5 ± 0.9 | 37.2 ± 0.9 | .14 |
| Sore throat | 25 (29) | 15 (34) | 10 (24) | .51 |
| Rhinopharyngitis | 51 (59) | 32 (73) | 19 (45) | < .01 |
| Bacterial infectionc | 20 (23) | 7 (16) | 13 (31) | .09 |
| Antibiotic treatment | 73 (85) | 42 (95) | 31 (74) | .05 |
| Duration, d | 8.1 ± 4.6 | 9.4 ± 4.3 | 6.8 ± 4.6 | < .01 |
| Systemic steroid use | 81 (94) | 42 (95) | 39 (93) | .36 |
| Duration, d | 19.8 ± 76 | 21.4 ± 82 | 18 ± 68 | .84 |
| ICU admission | 18 (21) | 9 (20) | 9 (21) | .91 |
| Outcomes and follow-up | ||||
| Time to clinical recovery, d | 8.7 ± 4.6 | 8.9 ± 4.4 | 8.7 ± 4.8 | .85 |
| Hospital stay, d | 10.6 ± 5.3 | 11.6 ± 6 | 9.5 ± 4.5 | .70 |
| Health-related quality-of-life questionnaire | ||||
| MRF-28 | 42.5 ± 26 | 37 ± 27.9 | 48.2 ± 22.9 | .45 |
| SGRQ | 52.5 ± 22 | 49.3 ± 22.1 | 56 ± 21.8 | .16 |
| Four-month mortality | 15 (17) | 6 (14) | 9 (21) | .34 |
Data are presented as No. (%) or mean ± SD. GOLD = Global Initiative for Obstructive Pulmonary Disease; MRF-28 = Maugeri Foundation Respiratory Failure Questionnaire; PPV = percent predicted value; SGRQ = St George Respiratory Questionnaire.
Performed in a stable state during previous year.
During the previous year.
Sputum analyses were available in 61 cases, and 39 met quality criteria of bacterial analysis.
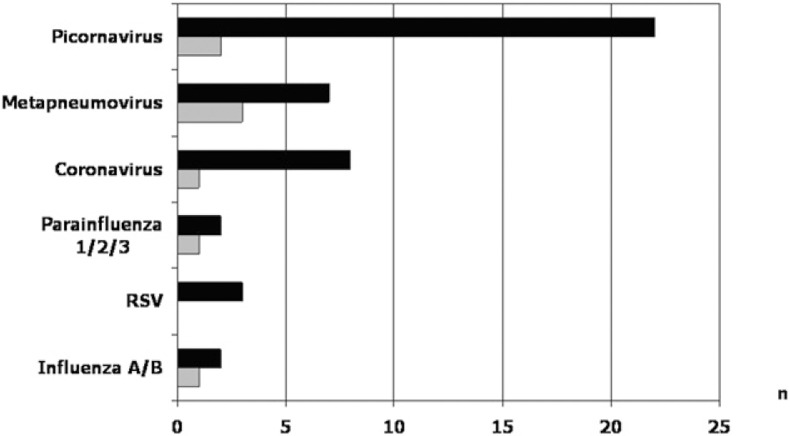
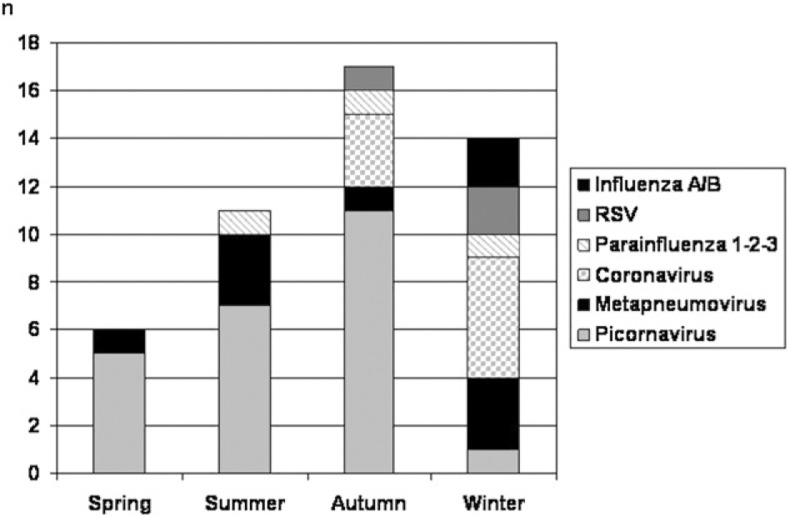
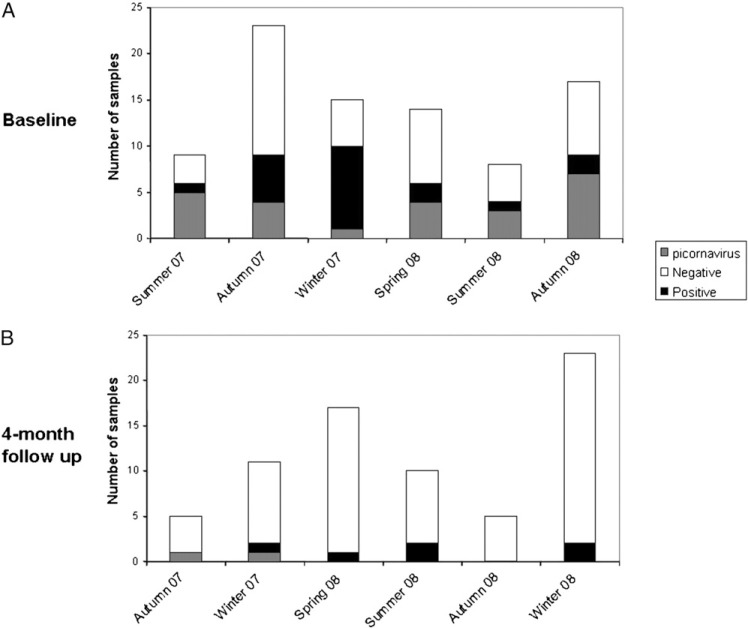
RT-PCR and PCR viral assays performed on the initial 86 nasopharyngeal specimens showed an overall positivity rate of 51% (n = 44). At follow-up visit 4 months after the index AECOPD, viruses were identified in only eight of 71 (11%) patients (P < .001). In five of these eight patients, no virus had been identified during the index exacerbation, thus suggesting a new viral infection acquired during the follow-up period. Human picornaviruses were the most frequently encountered viruses, followed by metapneumovirus and coronaviruses (Fig 2 ). Results suggestive of dual viral infection were found in three patients. Seasonal variations in distribution of implicated viruses and the seasonal variability of sampling are depicted in Figure 3, Figure 4 , respectively. The bulk of sampling at enrollment was performed during autumn, and therefore, the follow-up occurred frequently during the winter months.
Figure 2.
Viruses detected by polymerase chain reaction (PCR) in nasopharyngeal samples of 86 patients admitted for acute exacerbations of COPD (AECOPD) (black bars) and 71 patients who completed follow-up (white bars, stable period). RSV = respiratory syncytial virus.
Figure 3.
Seasonal variability of viruses detected by reverse transcriptase-polymerase chain reaction (RT-PCR) of nasopharyngeal swabs in patients admitted for AECOPD. See Figure 2 legend for expansion of abbreviations.
Figure 4.
Seasonal variability of samples at baseline during admission (A) and at 4-month follow-up (B).
Bacteriologic analyses of sputum samples were available in 61 cases (29% were unable to produce sputum), and 39 of 61 (64%) met microscopic criteria for bacteriological validity. Of these 39 cases, 20 (51%) had a positive culture for the following bacteria: Pseudomonas aeruginosa (n = 7), Haemophilus influenzae (n = 7), Escherichia coli (n = 2), Moraxella catarrhalis (n = 1), Streptococcus pneumoniae (n = 1), Staphylococcus aureus (n = 1), and Klebsiella pneumoniae (n = 1). Only seven of the 20 (8%) cases with a documented bacterial infection or colonization were coinfected with a virus. Of note, 49 (57%) patients were already receiving antibiotics.
Both virus-positive and virus-negative groups were comparable regarding baseline characteristics, except that patients with viral infection reported less frequent COPD exacerbations during the previous year and a shorter duration of symptoms before admission (Table 1). Symptoms of rhinopharyngitis were reported in 45% (n = 19) of cases with negative viral assays and in 73% (n = 32) of cases when a viral infection was documented. Probability of identifying a virus during AECOPD was three times higher in the presence of symptoms of rhinopharyngitis (Table 2 ). We found no statistically significant difference between patients with and without virus-associated AECOPD in terms of time to clinical recovery, length of hospital stay, or 4-month mortality (Table 1). Patients with respiratory viral infection were put on antibiotics more often and for longer periods (Table 1).
Table 2.
Odds Ratio of Viral Infection in 86 Patients Admitted for COPD Exacerbations
| Univariate Analysis |
Multivariate Analysisa |
|||
|---|---|---|---|---|
| Viral Infection | OR | 95% CI | OR | 95% CI |
| Rhinopharyngitis | 3.23 | 1.13–7.94 | 3.01 | 1.11–8.69 |
| Increased cough | 3.54 | 1.45–8.65 | 1.79 | 0.52–6.13 |
| Increased dyspnea | 0.9 | 0.22–4.07 | 1.02 | 0.19–5.33 |
| Increased sputum volume | 2.34 | 0.98–5.59 | 1.98 | 0.63–6.27 |
| Increased sputum purulence | 1.48 | 0.53–4.15 | 0.93 | 0.29–3.03 |
OR = odds ratio.
Adjusted for rhinopharyngitis, cough, dyspnea, sputum volume, and sputum purulence.
During the 4-month follow-up period, 15 patients died (n = 6 in the virus-positive group; n = 9 in the virus-negative group). In a Cox regression model, a trend toward higher mortality was found for patients with viral infection (hazard ratio, 3.33; 95% CI, 0.98-11.11), but this association disappeared when FEV1 or age were added in the model.
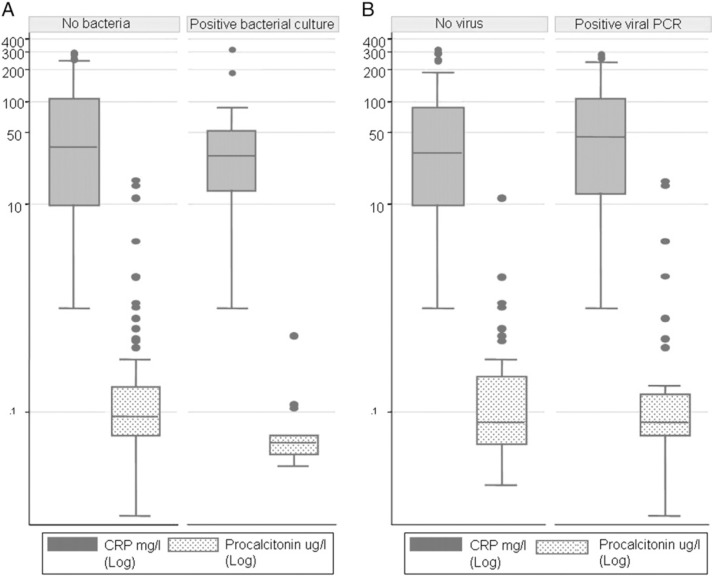
CRP and PCT values were obtained in 86 (100%) and 81 (94%) patients, respectively. Of the five cases without PCT values, two were in the virus-positive group and three in the virus-negative group. For all patients, median serum CRP level during AECOPD was 35 mg/L (range, 1-313 mg/L), and median PCT was 0.08 μg/L (range, 0.01-17.0 μg/L). In the presence of a documented bacterial infection, irrespective of detection of viruses in URT, CRP and PCT median values were 33 mg/L (range, 1-313 mg/L) and 0.06 μg/L (range, 0.03-0.55 μg/L), respectively. In the presence of viruses during AECOPD, median values for serum CRP and PCT were 45 mg/L (range, 1-283 mg/L) and 0.08 μg/L (range, 0.01-17.02 μg/L), respectively. These values did not differ significantly when compared to patients without bacterial infection (P = .08 and P = .14, respectively) and to patients without viral infection (P = .26 and P = .81, respectively) (Fig 5 ).
Figure 5.
Serum CRP (n = 86) and procalcitonin (PCT) (n = 81) in patients with AECOPD. Five values are missing for PCT (two in the virus-positive group; three in the virus-negative group). Patients with (n = 20) and without bacterial infection (n = 68) identified by semiquantitative bacterial analysis of sputum at admission for AECOPD (A). Patients with (n = 44) and without (n = 42) viral nucleic acids identified by RT-PCR of nasopharyngeal swabs at admission for AECOPD (B). Data are expressed as medians (interquartile range).
Discussion
Using a large panel of molecular assays, we identified respiratory viruses in the URT of 51% of patients with AECOPD. When sampling was repeated after clinical recovery in the same patients, virus detection rate dropped to 11%. The high rate of viral infection detected in nasopharyngeal swabs during AECOPD is in the upper range of that reported in the published literature.5, 8, 9, 10, 11, 12 The finding that viral infection rate is markedly lower once patients have recovered clinically supports the hypothesis of a pathogenic role of the viruses initially identified in AECOPD. One particular interest of our study resides in the fact that viral samples, unlike in most previous studies,5, 6, 9, 10 were not only collected during the acute exacerbation phase but also systematically collected several months after the initial exacerbation, with patients being their own controls. It is noteworthy that neither clinical features nor outcomes differed significantly between patients with or without viral infection, albeit for URT symptoms, more frequently in positive cases as already described.12
In our study, picornaviruses, metapneumovirus, and coronaviruses were the most common identified, which is consistent with recent studies.5, 10, 11, 12, 24 Of note, the RT-PCR assays used in our study did not discriminate between rhinoviruses and other enteroviruses. However, based on previous investigations, it can be surmised that rhinoviruses represented the large majority of these cases. This strategy also offered the advantage of screening for newly identified strains, such as rhinovirus C and other new respiratory enteroviruses.14, 23 RT-PCR assays have already shown their ability to improve diagnostic yield in different groups of patients,25 including those with COPD,5, 8, 9, 10, 11, 12, 26 but few investigations have used such a large panel of molecular assays. Using these new PCRs, we identified new viruses that had never been studied before in this setting, such as coronavirus OC43 and coronavirus HKU1. Taken together, these viruses correspond to an important proportion of 18% of all detected cases.
The clinical relevance of viral or bacterial infection identified through URT sampling (vs sputum samples), is indeed debated.27 However, in clinical settings, representative sputum during AECOPD often is difficult to obtain. Furthermore, bacterial colonization may contaminate and thus inhibit viral culture in sputum samples as well as RT-PCR, and RT-PCR assays using sputum face other technical limitations, such as lack of standardization and unknown reproducibility. In addition, potentially pathogenic viruses rarely found in lower airways, such as bocavirus or rhinovirus, would be overlooked if searched only in sputum. Many of these limitations can be overcome by nasopharyngeal sampling, which demands only very limited patient collaboration. Finally, the nasopharyngeal area is considered the most appropriate site for detecting viral replication, and lower respiratory tract replication in absence of URT infection is exceptional.28
To determine whether upper-respiratory viral infection is a causative agent of AECOPD is of major importance. Indeed, detection of viral nucleic acids in upper-respiratory secretions is not proof per se of a causal relationship, but there is substantial evidence2 supporting the role of respiratory viruses in promoting inflammatory processes leading to exacerbation. It is also established that viruses, such as RSV, may directly infect the lower airway in patients with COPD.29 The causal relationship between a viral infection initiated in the upper-respiratory tract and exacerbation has been demonstrated in a small experimental study in which four patients with stable COPD were infected with rhinoviruses.30 Thus, viruses detected in nasopharyngeal swab of patients with AECOPD do play a role in the development of respiratory symptoms either alone or as cofactors with other pathogens or nonbiologic agents, such as air pollution or tobacco smoke.2 Importantly, the concordance between nasopharyngeal swabs and lower-respiratory tract sampling has been found to be high, particularly for respiratory viruses.31, 32 Additionally, viruses detected in URT have been suspected to be causal agents in community-acquired pneumonia.33
Viral infection was cleared in most cases during the 4-month follow-up, and we did not observe prolonged viral shedding as reported in some studies.12 Prolonged shedding has been mainly described for RSV, and the small number of cases observed in our study precludes any further conclusions. We can hypothesize, however, that the seasonal pattern of RSV epidemics, varying substantially every year, might explain this discrepancy. Indeed, several studies, even in Switzerland, have reported a biennial activity of RSV that could underestimate its prevalence in our COPD population study compared with other studies.34, 35
The high rate of virus detection in URT during AECOPD also supports the idea that antibiotics may be overprescribed in these patients3 and that distinguishing between purely viral infections and mixed or bacterial infections is of major importance. The present study offered the advantage of an extensive molecular viral screening in a well-defined clinical entity (AECOPD) known to be associated with both bacterial and viral infections. Biologic markers, such as CRP, are elevated during COPD exacerbations,2, 36 but the association between viral infections and systemic inflammation biomarkers has been addressed only in a few studies until now.2, 12 We thus tested the performance of CRP and PCT for this indication. In AECOPD, PCT-based guidelines may lead to a decreased use of antibiotics.37 In practice, when PCT values are below a predefined limit, many clinicians consider that viruses, not bacteria, cause AECOPD. Our results did not show any correlation between either CRP or PCT values and sputum cultures or results of virological screening, and neither of these biomarkers could efficiently discriminate between these two types of infection. It is possible that a lack of power might explain these results, as our study was not properly powered to detect differences in PCT levels between groups. Furthermore, the fact that one-third of patients included was already on antibiotics at admission may have contributed to low PCT values; these results reflect, however, the real life of clinical practice, thus limiting the predictive value of PCT at hospital admission.
Our study has some limitations. It is quite possible that our study underestimated the rate of bacterial infection or coinfection in as much as sputum samples for bacterial analysis were available in only 45% of cases, and 57% of these patients had already received antibiotics. Conversely, our bacterial assays were only semiquantitative, and we did not perform bacterial detection at follow-up. Because bacterial colonization is common in patients with COPD, the possible identification of bacteria in AECOPD does not necessarily imply an etiologic role. This finding may have affected values of biomarkers and clinical outcome. Additionally, patients were not recruited at the very onset of AECOPD and may have passed the peak viral load, which could have underestimated the real prevalence of virus-related AECOPD. However, given the high sensitivity of RT-PCR and the fact that viral nucleic acid detection in URT is possible for as long as 8 to 11 days after AECOPD,30 we believe that the reported rate of viral infection is reliable. Finally, the lack of relation in our study between recovery time and detection of viruses also could be explained by the fact that patients were mainly referred by their physician and that it was difficult to determine precisely the exact start of the exacerbation because the thresholds for referral differ between physicians. This reflects the difficulties encountered in real-life management of patients with COPD.
Conclusion
Prevalence of viruses, as detected from nasopharyngeal swabs by RT-PCR, is high in AECOPD and low after clinical recovery, suggesting that AECOPD are frequently triggered by viral infections initiated in the URT. In this study, current biologic markers such as CRP and PCT could not discriminate between viral and nonviral AECOPD. Rapid virological diagnostic procedures, therefore, are highly needed to accurately confirm a viral etiology in the presence of a COPD exacerbation and subsequently to apply appropriate treatment strategies.
Acknowledgments
Author contributions: Dr Kherad: contributed to the overall study design and protocol development; data management; oversight of data analysis; and the writing, review, and approval of the manuscript.
Dr Kaiser: contributed to the overall study design and protocol development; performance of the laboratory assays; and the writing, review, and approval of the manuscript.
Dr Bridevaux: contributed to the writing, review, and approval of the manuscript.
Dr Sarasin: contributed to the overall study design and protocol development and the writing, review, and approval of the manuscript.
Dr Thomas: contributed to the performance of the laboratory assays and review and approval of the manuscript.
Dr Janssens: contributed to the overall study design and protocol development and the writing, review, and approval of the manuscript.
Dr Rutschmann: contributed to the overall study design and protocol development, data management, oversight of data analysis, and review and approval of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: We thank Marie Metzger for her contribution as study nurse. We also thank Patricia Suter, Sandra van Belle, and Lara Turin for their excellent technical assistance and Rosemary Sudan for editorial assistance.
Footnotes
Funding/Support: This work was performed at Geneva's University Hospitals and Faculty of Medicine, University of Geneva, and was supported by the Pulmonary League of Geneva, Geneva's University Hospitals, and a grant of the Swiss National Science Foundation attributed to Dr Kaiser (3200B-101670).
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Calverley PM, Agusti A, Anzueto P. The global strategy for diagnosis, management and prevention of COPD (updated 2009) http://www.goldcopd.com/Guidelineitem.asp?/1=2&/2=1&intId=2003 Accessed May 2010.
- 2.Rohde G, Borg I, Wiethege A. Inflammatory response in acute viral exacerbations of COPD. Infection. 2008;36(5):427–433. doi: 10.1007/s15010-008-7327-5. [DOI] [PubMed] [Google Scholar]
- 3.Rosell A, Monsó E, Soler N. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch Intern Med. 2005;165(8):891–897. doi: 10.1001/archinte.165.8.891. [DOI] [PubMed] [Google Scholar]
- 4.Beckham JD, Cadena A, Lin J. Respiratory viral infections in patients with chronic, obstructive pulmonary disease. J Infect. 2005;50(4):322–330. doi: 10.1016/j.jinf.2004.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rohde G, Wiethege A, Borg I. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58(1):37–42. doi: 10.1136/thorax.58.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberg SB, Allen M, Wilson J, Atmar RL. Respiratory viral infections in adults with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162(1):167–173. doi: 10.1164/ajrccm.162.1.9911019. [DOI] [PubMed] [Google Scholar]
- 7.Borg I, Rohde G, Löseke S. Evaluation of a quantitative real-time PCR for the detection of respiratory syncytial virus in pulmonary diseases. Eur Respir J. 2003;21(6):944–951. doi: 10.1183/09031936.03.00088102. [DOI] [PubMed] [Google Scholar]
- 8.Camargo CA, Jr, Ginde AA, Clark S, Cartwright CP, Falsey AR, Niewoehner DE. Viral pathogens in acute exacerbations of chronic obstructive pulmonary disease. Intern Emerg Med. 2008;3(4):355–359. doi: 10.1007/s11739-008-0197-0. [DOI] [PubMed] [Google Scholar]
- 9.Cameron RJ, de Wit D, Welsh TN, Ferguson J, Grissell TV, Rye PJ. Virus infection in exacerbations of chronic obstructive pulmonary disease requiring ventilation. Intensive Care Med. 2006;32(7):1022–1029. doi: 10.1007/s00134-006-0202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutchinson AF, Ghimire AK, Thompson MA. A community-based, time-matched, case-control study of respiratory viruses and exacerbations of COPD. Respir Med. 2007;101(12):2472–2481. doi: 10.1016/j.rmed.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Papi A, Bellettato CM, Braccioni F. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173(10):1114–1121. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 12.Seemungal T, Harper-Owen R, Bhowmik A. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(9):1618–1623. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- 13.Mackay IM. Human rhinoviruses: the cold wars resume. J Clin Virol. 2008;42(4):297–320. doi: 10.1016/j.jcv.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tapparel C, Junier T, Gerlach D. New respiratory enterovirus and recombinant rhinoviruses among circulating picornaviruses. Emerg Infect Dis. 2009;15(5):719–726. doi: 10.3201/eid1505.081286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis. 2004;39(2):206–217. doi: 10.1086/421997. [DOI] [PubMed] [Google Scholar]
- 16.Kristoffersen KB, Søgaard OS, Wejse C. Antibiotic treatment interruption of suspected lower respiratory tract infections based on a single procalcitonin measurement at hospital admission—a randomized trial. Clin Microbiol Infect. 2009;15(5):481–487. doi: 10.1111/j.1469-0691.2009.02709.x. [DOI] [PubMed] [Google Scholar]
- 17.Christ-Crain M, Jaccard-Stolz D, Bingisser R. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet. 2004;363(9409):600–607. doi: 10.1016/S0140-6736(04)15591-8. [DOI] [PubMed] [Google Scholar]
- 18.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garbino J, Soccal PM, Aubert JD. Respiratory viruses in bronchoalveolar lavage: a hospital-based cohort study in adults. Thorax. 2009;64(5):399–404. doi: 10.1136/thx.2008.105155. [DOI] [PubMed] [Google Scholar]
- 20.Regamey N, Kaiser L, Roiha HL, Swiss Paediatric Respiratory Research Group Viral etiology of acute respiratory infections with cough in infancy: a community-based birth cohort study. Pediatr Infect Dis J. 2008;27(2):100–105. doi: 10.1097/INF.0b013e31815922c8. [DOI] [PubMed] [Google Scholar]
- 21.Garbino J, Crespo S, Aubert JD. A prospective hospital-based study of the clinical impact of non-severe acute respiratory syndrome (Non-SARS)-related human coronavirus infection. Clin Infect Dis. 2006;43(8):1009–1015. doi: 10.1086/507898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cordey S, Thomas Y, Cherpillod P, van Belle S, Tapparel C, Kaiser L. Simultaneous detection of parainfluenza viruses 1 and 3 by real-time reverse transcription-polymerase chain reaction. J Virol Methods. 2009;156(1-2):166–168. doi: 10.1016/j.jviromet.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tapparel C, Cordey S, Van Belle S. New molecular detection tools adapted to emerging rhinoviruses and enteroviruses. J Clin Microbiol. 2009;47(6):1742–1749. doi: 10.1128/JCM.02339-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinello RA, Esper F, Weibel C, Ferguson D, Landry ML, Kahn JS. Human metapneumovirus and exacerbations of chronic obstructive pulmonary disease. J Infect. 2006;53(4):248–254. doi: 10.1016/j.jinf.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garbino J, Gerbase MW, Wunderli W. Lower respiratory viral illnesses: improved diagnosis by molecular methods and clinical impact. Am J Respir Crit Care Med. 2004;170(11):1197–1203. doi: 10.1164/rccm.200406-781OC. [DOI] [PubMed] [Google Scholar]
- 26.Ko FW, Ip M, Chan PK. Viral etiology of acute exacerbations of COPD in Hong Kong. Chest. 2007;132(3):900–908. doi: 10.1378/chest.07-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359(22):2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 28.Forman M, Valsamakis A. Specimen collection, transport, and processing: virology. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, editors. Manual of Clinical Microbiology. 9th ed. ASM Press; Washington, DC: 2007. pp. 1284–1296. [Google Scholar]
- 29.Papadopoulos NG, Bates PJ, Bardin PG. Rhinoviruses infect the lower airways. J Infect Dis. 2000;181(6):1875–1884. doi: 10.1086/315513. [DOI] [PubMed] [Google Scholar]
- 30.Mallia P, Message SD, Kebadze T, Parker HL, Kon OM, Johnston SL. An experimental model of rhinovirus induced chronic obstructive pulmonary disease exacerbations: a pilot study. Respir Res. 2006;7:116. doi: 10.1186/1465-9921-7-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lahti E, Peltola V, Waris M. Induced sputum in the diagnosis of childhood community-acquired pneumonia. Thorax. 2009;64(3):252–257. doi: 10.1136/thx.2008.099051. [DOI] [PubMed] [Google Scholar]
- 32.Xiang X, Qiu D, Chan KP, Chan SH, Hegele RG, Tan WC. Comparison of three methods for respiratory virus detection between induced sputum and nasopharyngeal aspirate specimens in acute asthma. J Virol Methods. 2002;101(1-2):127–133. doi: 10.1016/s0166-0934(01)00431-1. [DOI] [PubMed] [Google Scholar]
- 33.Jennings LC, Anderson TP, Beynon KA. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax. 2008;63(1):42–48. doi: 10.1136/thx.2006.075077. [DOI] [PubMed] [Google Scholar]
- 34.Terletskaia-Ladwig E, Enders G, Schalasta G, Enders M. Defining the timing of respiratory syncytial virus (RSV) outbreaks: an epidemiological study. BMC Infect Dis. 2005;5(1):20. doi: 10.1186/1471-2334-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duppenthaler A, Gorgievski-Hrisoho M, Frey U, Aebi C. Two-year periodicity of respiratory syncytial virus epidemics in Switzerland. Infection. 2003;31(2):75–80. doi: 10.1007/s15010-002-3124-8. [DOI] [PubMed] [Google Scholar]
- 36.Hurst JR, Donaldson GC, Perera WR. Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;174(8):867–874. doi: 10.1164/rccm.200604-506OC. [DOI] [PubMed] [Google Scholar]
- 37.Stolz D, Christ-Crain M, Bingisser R. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131(1):9–19. doi: 10.1378/chest.06-1500. [DOI] [PubMed] [Google Scholar]