Abstract
Background
Limited understanding of the presentation and course of influenza A(H5N1) infection in humans hinders evidence-based management.
Methods
We reviewed the case records of patients admitted to the Persahabatan Hospital (RSP), Jakarta, Indonesia, with influenza A(H5N1) confirmed by real-time polymerase chain reaction.
Results
Twenty-two previously well patients, aged 3 to 47 years (median 24.5 years), were identified. All attended a clinic or hospital after a median of 2 days of illness (range 0–7). Times to first dose of oseltamivir (three died before receiving oseltamivir) were 2 to 12 days (median 7 days), administered mostly (n = 15) at RSP. Nineteen patients required mechanical ventilation. Deaths numbered 18 (case fatality = 82%) occurring within hours to 6 days of RSP admission, corresponding to 6 to 16 days of illness. Admission hyperglycemia (≥ 140 mg/dL), unrelated to steroids or known underlying diabetes mellitus, and elevated D-dimer levels (0.81–5.2 mg/L, upper limit of normal < 0.5 mg/L) were present in 14/21 (67%) and 20/21 (95%) patients, respectively. Fibrinogen concentrations were mostly low/normal at 129.9 to 517.9 mg/dL (median 241.1, normal 200–400 mg/dL), whereas C-reactive protein (9/11) and ferritin (6/8) levels were increased. Risk factors for death (univariate analysis) included: (1) increased D-dimers, (2) hyperglycema, (3) increased urea, (4) more extensive chest radiograph shadowing, and (5) lower admission oxygen saturation.
Conclusions
Early diagnosis and effective treatment of human influenza A(H5N1) infection remains challenging. Most patients were referred late with advanced disease. Oseltamivir had limited clinical impact. Elevated D-dimer levels, consistent with fibrinolysis, and hyperglycemia warrant more research to determine their underlying mechanisms and optimal treatment.
Abbreviations
- ALI
acute lung injury
- aPTT
activated partial thromboplastin time
- CXR
chest radiograph
- DIC
disseminated intravascular coagulation
- ISTH
International Society of Thrombosis and Hemostasis
- IVIG
IV human Ig
- LDH
lactate dehydrogenase
- PCR
polymerase chain reaction
- PT
prothrombin time
- RT
reverse transcriptase
- SOFA
Sepsis Organ-related Failure Assessment
- ULN
upper limit of normal
In 2005, Indonesia saw its first patients with highly pathogenic avian influenza A(H5N1).1 Total confirmed influenza A(H5N1) cases now number 141, accounting for just under one-third (32%) of the global total of 443.2 The circulating 2.1 clade in Indonesia is generally sensitive in vitro to oseltamivir carboxylate, intrinsically less sensitive than clade 1 viruses, and is mostly adamantine resistant.3, 4 Oseltamivir is the only treatment currently available in Indonesia.
Human influenza A(H5N1) mortality rates are high in Indonesia, around 81% (115/141). Independent prognostic factors for mortality in 127 Indonesian patients with confirmed influenza A(H5N1) were poverty, delayed oseltamivir treatment, and not being in a cluster.5 Clustered infections typically prompt screening of suspects and earlier treatment. Other smaller clinical series have also identified late presentation and suggest many other poor prognostic features, such as leukopenia, absolute lymphopenia (< 1,000/μL), neutropenia, thrombocytopenia, an elevated plasma lactate dehydrogenase (LDH), corticosteroid use, no oseltamivir or other antiviral treatment, bilateral shadowing on the chest radiograph (CXR), ARDS, viremia, high viral loads in respiratory samples, and high plasma cytokine concentrations.6, 7, 8, 9, 10, 11, 12
The main clinical features of influenza A(H5N1) are well described but biased because most of our knowledge derives almost entirely from severely ill, hospitalized patients. Mild disease and asymptomatic seroconversion have been reported in exposed family members and health-care workers,3, 13, 14 and, rarely, a predominantly gastrointestinal presentation has been identified.15, 16 Most patients are symptomatic within 7 days of exposure to diseased poultry, but up to 25% of patients do not report poultry/bird exposure.12
An understated aspect of influenza A(H5N1) is its early, nonspecific, febrile presentation that invites a broad differential diagnosis, such as dengue, typhoid fever, and the “flu,” including the 2009 swine-origin influenza.5, 12 Within the first 2 days of illness, Indonesian patients reported fever (100%), cough (32%), and rhinorrhea (17%), but only 9% reported dyspnea.5 At hospital admission, 88% and 84% reported cough and dyspnea, respectively, symptoms characteristic of a community-acquired pneumonia. Onset of dyspnea appears to prompt referral to a higher level of care for diagnosis and further management. The technical nature (ie, polymerase chain reaction [PCR]) of the laboratory diagnosis of influenza A(H5N1), particularly in a vast country like Indonesia, limits access to PCR diagnosis to those with severe illness or those known to have been exposed. Diagnostic difficulty at the early (primary care) or later stages (in hospital) of disease coupled with a low index of suspicion leads to diagnostic and antiviral treatment delays. Certainly, the rapid progression to respiratory failure, necessitating early ventilation, should alert clinicians to the possibility of influenza A(H5N1) infection.
This report summarizes the clinical and laboratory findings during the management of 22 influenza A(H5N1)-infected patients at our hospital in Jakarta, Indonesia. Our experience is similar to that reported by others, but we describe new findings that may bear upon clinical management and identify specific areas in need of more detailed investigation.
Materials and Methods
All patients were admitted to the Respiratory Unit of the Persahabatan Hospital, a referral center for the diagnosis and treatment of human avian influenza. All patients with suspected influenza A(H5N1) infection had throat and nasal swabs (primary diagnostic respiratory samples) for analysis by real-time reverse transcriptase (RT)-PCR performed at the World Health Organization-approved reference laboratory of the National Institutes of Health Research and Development in Jakarta. This laboratory lacks a biosafety level 3 facility for viral culture. Rectal swabs, endotracheal aspirates, and pleural fluid were also obtained from a small number of patients as part of their care. All 22 patients in this report were RT-PCR-positive for influenza A(H5N1) by throat swab. Patients were managed intensively in an isolation ward equipped with ventilators.
Data were extracted retrospectively from patients' medical notes. We used standard definitions of acute lung injury (ALI) and ARDS (ie, a Pao 2:Fio 2ratio of < 300 and < 200, respectively; bilateral CXR infiltrates; and no evidence of left atrial hypertension.17 Lung-protective ventilation was used for ventilated patients, consistent with the ARDS Network guidelines.18 Presumptive evidence of a consumptive coagulopathy was defined as increased (greater than upper limit of normal [ULN]) D-dimer levels and prothrombin (PT) and activated partial thromboplastin times (aPTT), and a platelet count < 150,000/μL. Disseminated intravascular coagulation (DIC) was determined using the International Society of Thrombosis and Hemostasis (ISTH) score (platelet count, PT, fibrinogen and fibrinogen degradation).19 A score ≥ 5 is overt DIC and < 5 nonovert DIC.
Pleural fluid was defined as an exudate if the LDH concentration was > 2/3 ULN of serum LDH (ULN = 200 IU/L) or the pleural fluid-to-plasma ratios of: (1) total protein were > 0.5, or (2) LDH were > 0.6.20 The Sepsis Organ-related Failure Assessment (SOFA) was used to assess admission illness severity (higher scores indicate greater severity). SOFA scores (up to 4 per organ system) respiratory (Pao 2:Fio 2 ratio), liver (total bilirubin), and renal (creatinine) functions; level of consciousness (Glasgow Coma Score); mean arterial BP; and coagulation (platelet count).21 Ethical approval was not considered necessary for this retrospective study.
Results
Clinical and Laboratory Features at Admission
Of the 139 patients seen between January 2005 and May 2008 with suspected influenza A(H5N1) infection, 22 (16%) were confirmed by RT-PCR examination of throat swabs (Table 1 ). Other positive samples for influenza A(H5N1) were rectal swab (n = 2), endotracheal aspirate (n = 2), bronchial washings (n = 2), and pleural fluid (n = 2).
Table 1.
Demographic, Clinical, and Radiologic Characteristics of 22 Influenza A(H5N1) Infected Patients Who Survived or Died
| Characteristic | Survivors (n = 4) | Nonsurvivors (n = 18) | P Value |
|---|---|---|---|
| Median age (range), y | 16.5 (14–38) | 27 (3–47) | .67 |
| Sex, male to female ratio | 1:3 | 7:11 | 1 |
| Median days to first oseltamivir dose | 5 | 7 | .2 |
| Signs on admission | |||
| Temperature, °C | 38.5 | 37 | .094 |
| Heart rate, beats/min | 94 | 126 | .004 |
| Respiratory rate, breaths/min | 22 | 40 | .17 |
| Systolic BP, mm Hg | 130 | 110 | .03 |
| Diastolic BP, mm Hg | 87 | 70 | .042 |
| Reduced level of consciousness | 0 | 17 | .21 |
| Oxygen saturation (transcutaneous) % | 99 | 84 | .0069 |
| Chest radiograph | |||
| Unilateral < 50% | 2 | 0 | … |
| Unilateral ≥ 50% | 0 | 5 | … |
| Bilateral < 50% | 2 | 1 | … |
| Bilateral ≥ 50% | 0 | 12 | .001 |
| Pleural effusion | 2 | 15 | .2 |
Data are median, median (range) or No.
Eighteen patients or their family members gave a history of contact with chickens; 12 patients were able to date the contact for a median incubation period of 2 (1–8) days. All patients were previously well. None were known to have diabetes. The estimated prevalence of diabetes mellitus in Indonesia is 3.8% (8.4 million/220 million population).22 Counting the first day of reported illness as day 0, the median duration of illness before the first medical contact was 2 days (range 0–7) at a local clinic (n = 15 patients) or hospital (n = 7). The numbers of medical contacts before Persahabatan Hospital admission were: 1 (n = 8 patients), 2 (n = 11), 3 (n = 2), and 4 (n = 1). Before referral to our hospital, 14 patients were prescribed antibiotics; of these, 4 also received oseltamivir.
There were two family clusters: a mother and son and a mother and daughter. All survived except the mother of the son. The son became ill after his mother, and he was prescribed oral oseltamivir immediately after presenting to his general practitioner after 2 days of illness. He took two doses, did not improve, and was referred to our hospital. He was the earliest referral.
Presentation to the Persahabatan Hospital ranged from 3 to 10 days (median 7) of illness. The time to the first dose of oseltamivir was similar: median 7 (range 2–10) because 18 patients received oseltamivir either at our hospital or on the day of transfer by the referring hospital, following consultation with our team. Three severely ill patients died of respiratory failure soon after admission without receiving oseltamivir. Clinically, 21 patients were diagnosed with pneumonia and 1 patient had a mild flulike illness.
All patients complained of fever and most complained of dyspnea (20/22) and a productive cough (19/22). Other symptoms were sore throat (n = 12), myalgia (11), vomiting (10), diarrhea (8), headache (8), drowsiness (7), runny nose (5), abdominal pain (2), dry cough (2), and spontaneous mucosal bleeding (1). No one reported sore or red eyes.
Sixteen of 22 patients had leukopenia (< 4,000/μL), 15 of 19 had absolute lymphopenia (< 1,000/μL), 3 of 19 had a neutropenia < 1,000/μL, and 13 of 22 were thrombocytopenic (< 150,000/μL), including 2 with severe (< 50,000/μL) thrombocytopenia (Table 2 ). Of the 21 patients with coagulation data, 20 had elevated D-dimer levels (all measured before IV human Ig [IVIG] administration) 1.6- to 10.4-fold, 17 had elevated aPTTs (maximum value 116 s [ULN = 38.1 s]), and 5 had elevated PTs (maximum value 15 s [ULN = 13.2 s]). On admission, five patients had evidence of a consumptive coagulopathy, but none had ISTH-defined overt DIC: ISTH scores were: 0 (n = 1), 2 (n = 13), 3 (n = 4), and 4 (n = 3).
Table 2.
Laboratory Features of 22 Influenza A(H5N1) Infected Patients Who Survived or Died
| Measures | Survivors (n = 4) | Nonsurvivors (n = 18) | P Value |
|---|---|---|---|
| Hematology | |||
| Hemoglobin, g/dL | 13.25 (8.7–14.3) | 13.1 (11–16.2) | .76 |
| Total WBC count,/μL | 3,250 (2,330–4,700) | 2,740 (1,470–8,090) | .55 |
| Neutrophil count,/μL | 1,900 (970–2,960) | 2,400 (540–6,900) | .61 |
| Lymphocyte count,/μL | 860 (600–2,100) | 640 (280–1,750) | .12 |
| Platelet count,/μL | 133,000 (92,000–250,000) | 128,500 (40,000–260,000) | .86 |
| D-dimer, mg/L | 1.35 (0–2.3) | 2.6 (1.2–5.2) | .015 |
| DIC score | 2 | 2 | .21 |
| Biochemistry | |||
| Urea, mg/dL | 18 (15–27) | 47 (10–158) | .017 |
| Creatinine, mg/dL | 0.65 (0.4–1) | 1.2 (0.6–6.7) | .011 |
| Glucose, mg/dL | 84.5 (70–89) | 202 (115–468) | .0023 |
| Blood gases, preventilation | |||
| pH | 7.44 (7.4–7.49) | 7.41 (7.1–7.59) | .49 |
| Pco2, mm Hg | 29.3 (26.4–38.6) | 40.9 (23.5–90.3) | .17 |
| Po2, mm Hg | 98.6 (78–204) | 43.5 (20.9–91.8) | .005 |
| Po2:Fio2 ratio | 239 | 44 | .017 |
| HCO3−, mmol/L | 21.3 (18.5–23.3) | 22.6 (12.2–62.9) | .55 |
| Sao2, % | 98.1 (96.8–99.9) | 79.8 (19.5–98) | .0033 |
| Median SOFA score | 2.5 | 5.5 | .0058 |
Data are median, median (range) or No. DIC = disseminated intravascular coagulation; HCO3– = bicarbonate; Sao2 = arterial oxygen saturation; SOFA = Sepsis Organ-related Failure Assessment.
Increased biochemical parameters were common: urea (10/21, ULN = 40 mg/dL), creatinine (8/21, 1.5 [male patients], 1.3 [female patients] mg/dL), aspartate aminotransferase (18/21, range 10–1,739 [median 174] IU/L), alanine aminotransferase (17/21, 9–421 [median 60] IU/L), LDH (8/8, 400–2,068 [median 1,194.5] IU/L) and creatine phosphokinase (9/9, 369–11,463 [median 2,308] IU/L). Total bilirubin values ranged from 0.19 to 2.7 (median 0.47) mg/dL; three patients had elevated values (ULN = 1.1 mg/dL). Fifteen of 20 had hypoalbuminemia (< 3.5 g/dL); all values were from 2 to 4.4 (median 2.75) g/dL. Hyperglycemia/impaired glucose tolerance (≥ 140 mg/dL) was detected in 14 of 21 patients; 10 had values ≥ 200 mg/dL. Seven of 21 patients had hyponatremia (serum Na < 135 mmol/L: 120–134 mmol/L). Fibrinogen, (range 130–517 mg/dL [ULN = 400 mg/dL]), ferritin (4.09–30,000 ng/mL [262 ng/mL female patients, 300 ng/mL male patients]), and C-reactive protein (0.88–128 mg/dL, [ULN = 0.3 mg/dL]) concentrations were elevated in 3/21, 6/8, and 9/11 patients, respectively.
The SOFA score (Table 3 ) ranged from 0 to 12 (median 5); the respiratory component was 0 to 4 (median 4). A higher median SOFA score was associated with death (5.5 vs 2.5, P = .0058).
Table 3.
The Admission SOFA Score and Its Components in 22 Influenza A(H5N1) Infected Indonesian Adult Patients
| SOFA Score | Respiratory Score | Platelet Count | Creatinine Score | GCS Score | Mean AP Score | Bilirubin Score | Died |
|---|---|---|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | N |
| 2 | 2 | 0 | 0 | 0 | 0 | 0 | N |
| 3 | 1 | 2 | 0 | 0 | 0 | 0 | N |
| 4 | 1 | 2 | 1 | 0 | 0 | 0 | Y |
| 4 | 3 | 1 | 0 | 0 | 0 | 0 | N |
| 4 | 4 | 0 | 0 | 0 | 0 | 0 | Y |
| 5 | 4 | 1 | 0 | 0 | 0 | 0 | Y |
| 5 | 4 | 1 | 0 | 0 | 0 | 0 | Y |
| 5 | 4 | 0 | 0 | 1 | 0 | 0 | Y |
| 5 | 4 | 0 | 0 | 1 | 0 | 0 | Y |
| 5 | 4 | 0 | 1 | 0 | 0 | 0 | Y |
| 6 | ND | 2 | 3 | 1 | ND | ND | Y |
| 6 | 4 | 2 | 0 | 0 | 0 | 0 | Y |
| 6 | 4 | 0 | 2 | 0 | 0 | 0 | Y |
| 6 | 4 | 0 | 1 | 1 | 0 | 0 | Y |
| 7 | 4 | 1 | 0 | 1 | 1 | 0 | Y |
| 7 | 4 | 3 | ND | 0 | 0 | ND | Y |
| 7 | 4 | 0 | 2 | 1 | 0 | 0 | Y |
| 7 | 4 | 1 | 2 | 0 | 0 | 0 | Y |
| 8 | 4 | 1 | 2 | 1 | 0 | 0 | Y |
| 9 | 4 | 3 | 2 | 0 | 0 | 0 | Y |
| 12 | 4 | 2 | 4 | 0 | 0 | 2 | Y |
When there were ND, the score was assumed to be 0. AP = arterial pressure; GCS = Glasgow Coma Score; N = no; ND = no data; Y = yes. See Table 2 for expansion of the other abbreviation.
Radiologic Features
Consolidation (21/22) was the most common CXR shadowing seen during the illness. At presentation, seven and 12 patients had unilateral or bilateral consolidation, respectively. Of these 19, six had a complete whiteout on one side but without tracheal deviation. Of the 21 patients with follow-up CXRs, 20 developed a worsening of their shadowing, sometimes within the same day. A total of 17 patients had features consistent with pleural effusions, but thoracic ultrasound was not done for confirmation.
Only one patient had a normal CXR. She was one of four survivors. Another survivor had right middle lobe consolidation, but the chest CT scan taken 1 day later showed more extensive disease (Figure 1, Figure 2 ). A repeat CT scan 11 days later (Fig 3 ), when the patient was clinically much improved, showed partial resolution of the consolidation and dilated bronchi. Four patients developed pneumothoraces (one bilateral, one with a pneumomediastinum) while ventilated. These were treated by chest drainage.
Figure 1.
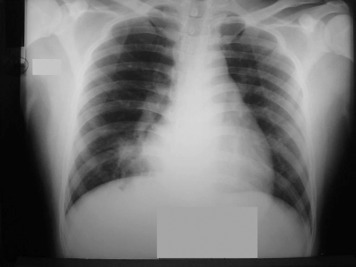
A posteroanterior chest radiograph on admission of a surviving patient showing middle lobe consolidation with air bronchograms.
Figure 2.
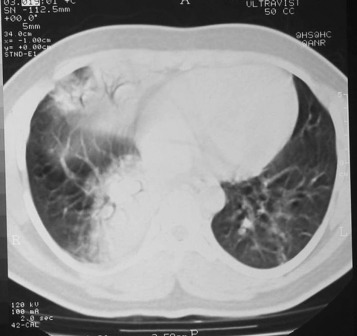
Chest CT scan of the surviving patient taken 1 day after the posteroanterior chest radiograph. The images have some breathing artifact. There is consolidation within the medial segment of the middle lobe and the medial basal segment of the right lower lobe with linear atelectasis in the lingula.
Figure 3.
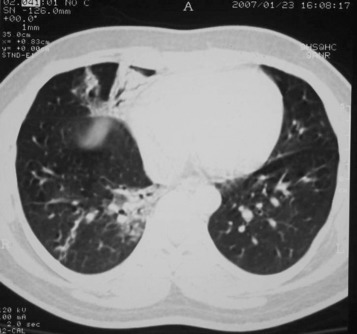
A repeat chest CT scan in the surviving patient taken 11 days after the earlier chest CT scan. There has been some improvement in the consolidation. There is now bronchial dilatation, a recognized finding in acute consolidation. There is also new linear atelectasis in the posterobasal segment of the right lower lobe.
Pleural Effusions
Pleural fluid was obtained from eight patients by thoracentesis (nine were tapped). All pleural fluid samples were exudates (Table 4 ). None was sent routinely for bacterial culture. Total and differential white cell counts varied widely; only three patients had lymphocyte-predominant fluid. There was no relationship between the blood and pleural fluid total white cell counts (P = .26, Spearman ρ). All pleural and blood samples were taken on the same day but not always simultaneously, making fluid blood glucose ratios unreliable (these have not been shown).
Table 4.
Pleural Fluid Characteristics in Eight Patients
| Appearance | Pleural Total Protein, g/L | Plasma Total Protein, g/L | Pleural: Plasma Ratio | Pleural LDH, IU/L | Serum LDH, IU/L | Pleural: Serum Ratio | Pleural Glucose, mg/dL | Pleural Total WBC Count,/μL | L, % | P, % |
|---|---|---|---|---|---|---|---|---|---|---|
| Straw colora | 4.1 | 6.4 | 0.64 | 1,870 | 1,175 | 1.6 | 155 | 247 | 70 | 30 |
| Straw colora | 4.6 | 5.2 | 0.88 | 1,541 | 1,319 | 1.2 | 151 | 1,510 | 85 | 15 |
| Straw color | 2.9 | 4.6 | 0.63 | 1,506 | 1,019 | 1.5 | 276 | 183 | 37 | 63 |
| Straw color | 2.7 | 4.5 | 0.6 | 1,008 | 1,298 | 0.8 | 78 | 900 | 49 | 51 |
| Straw color | 2.9 | 4.4 | 0.66 | 2,003 | ND | … | 134 | 950 | 47 | 53 |
| Serosanguineous | 3.3 | 5.3 | 0.62 | 1,323 | ND | … | 247 | 2,070 | 50 | 50 |
| Hemorrhagic | 3.6 | 5.3 | 0.68 | 1,708 | 660 | 2.6 | 139 | 569 | 92 | 8 |
| Hemorrhagic | 4.6 | 5.9 | 0.78 | 2,771 | 2,068 | 1.3 | 634 | 700 | 50 | 50 |
Some of the pleural and blood biochemical results were obtained several hours apart. Therefore, pleural-serum ratios should be interpreted in that light. L = lymphocytes; LDH = lactate dehydrogenase; P = polymorphs. See Table 3 legend for expansion of other abbreviation.
Influenza A(H5N1) positive.
Microbiologic Results
Two of nine patients had sputum-positive samples for Streptococcus pneumoniae and Pseudomonas aeruginosa. One survivor with suspected ventilator-associated pneumonia had three positive endotracheal aspirates for Klebsiella terrigans, Pseudomonas cepacia, and Acinetobacter baumannii. Of 17 blood cultures, 3 were positive: (1) coagulase-negative Staphylococcus, (2) coagulase-positive Staphylococcus (assumed to be Staphylococcus aureus), and (3) an α hemolytic Streptococcus.
Treatment and Clinical Course
Three patients died within hours of admission, one of whom was transferred already ventilated. The rest were treated with either standard or double dose (150 mg bid in an adult) oseltamivir. Antibiotics (third and fourth generation cephalosporins, azithromycin, levo/gatifloxacin, carbapenems, and aminoglycosides) were given to cover community- and hospital-acquired bacterial pneumonias. One patient only received methylprednisolone; one dose (62.5 mg IV) was given but then stopped after he had hematemesis. Eleven patients received 4 to 8 mg/kg daily of IVIG (Gammagard 10%, Baxter; Westlake Village, CA) for 4 days; three survived.
On admission, the median Pao 2:Fio 2 ratio was 52.6, range 21 to 327, interquartile range 34 to 80 (Table 2). Only three patients did not have ALI or ARDS; two of these patients survived. Nineteen (17 within 24 h) patients required mechanical ventilation within 3 days of admission. The tidal volume was set at ≤ 6 mL/kg and all patients required positive end-expiratory pressure of 10 to 15 cm water. One surviving patient developed a clinically suspected ventilator-associated pneumonia.
Patients (n = 10) with a blood glucose of ≥ 200 mg/dL were started on IV insulin. Two patients with diarrhea as part of their presenting illness were positive for influenza A(H5N1) virus by rectal swab. No patients had convulsions.
Patients (n = 18) died between 6 and 16 days after illness onset. Three died within hours of admission, the others between 2 and 6 days. All deaths were due to ARDS; in 15, hypotension necessitating inotropic support also contributed. In the four patients who survived, one was afebrile on admission with mild disease and the three others required ventilation for 7 to 9 days. Their times to fever clearance ranged from 3 to 6 days and their viruses (n = 3 had serial throat swabs taken) cleared by the second and fourth days of admission for times to viral clearance of 24 (n = 1) to 72 h (n = 2).
Survivors vs Nonsurvivors
By univariate analyses, 13 variables were associated significantly (P ≤ .05) with death. Owing to the small number of patients, a multivariate analysis was not done.
Discussion
The high death rate of patients with human influenza A(H5N1) at our tertiary referral hospital in Jakarta accords with the experience elsewhere in Indonesia. At presentation, measures of poor respiratory function were predictive of death, and we have identified hyperglycemia, elevated D-dimer levels, and elevated urea levels as possible poor prognostic features.
About two-thirds of our patients had hyperglycemia in the diagnostic range for impaired glucose tolerance or diabetes mellitus, and most of them were treated with insulin. None of our patients had been on corticosteroids or were known to have diabetes. Hyperglycemia on admission or developing after treatment with steroids has been observed previously in influenza A(H5N1)6, 11, 23 but has not been identified as a possible poor prognostic factor. By contrast, hyperglycemia was an independent risk factor for increased mortality in patients with severe acute respiratory syndrome whether they had diabetes or not.24 Suboptimal glucose control is associated with a poor prognosis in critically ill patients, often in association with bacterial sepsis,25 and important parallels may exist between sepsis and influenza A(H5N1). Our suggestive findings here on a limited number of patients emphasize the need for further research.
There was evidence of a mild consumptive coagulopathy in five patients, consistent with the Chinese series11 (5/26) that also used a soft definition familiar to clinicians, but none of our patients met a more robust definition of overt DIC. In the Oner et al26 series, two of their eight patients had ISTH DIC scores of 8 (admission) and 5 (developing later). Published data suggest overt DIC affects a minority of patients with influenza A(H5N1) and when present does not usually cause spontaneous bleeding.11, 12, 26
D-dimer levels in our patients were elevated out of proportion to the PT and aPTT. Concentrations of fibrinogen (an acute-phase protein) were mostly low or normal despite intense inflammation, a hallmark of severe influenza A(H5N1),8 as evidenced by increased ferritin and CRP levels in most of our patients who had these acute-phase proteins measured. High D-dimer levels and low fibrinogen levels suggest influenza A(H5N1) associated fibrinolysis in the absence of DIC. However, we cannot exclude the possibility of clinically inapparent venous thromboembolic disease in some of our patients, as reported in patients with severe acute respiratory syndrome.27 Clinicians should be aware that IVIG increases the plasma viscosity that predisposes to arterial28 and venous29 thromboembolic disease resulting in elevated D-dimer levels.30, 31 An elevated urea level was another possible poor prognostic factor, reflecting a combination of hypercatabolism, dehydration, and renal impairment.
Eight patients (∼36%) had pleural effusions confirmed by thoracentesis, consistent with radiologic data from southern Vietnam.32 These are the first data confirming that influenza A(H5N1) causes exudative pleural effusions. Fluid values were similar between blood-stained and straw-colored pleural fluid, suggesting a necrotizing or hemorrhagic pneumonia and not traumatic taps. The LDH values were high, consistent with severe lung parenchymal damage. The total and differential white cell counts varied and are difficult to interpret in the absence of histopathologic and bacteriologic data.
Most patients presented late with respiratory failure and features of ARDS.6, 12 ARDS is common in influenza A(H5N1) but affects only a minority of patients with the 2009 pandemic influenza A(H1N1), which probably reflects the propensity for influenza A(H5N1) to bind to sialic acid receptors in the lower respiratory tract.33, 34 A high proportion (∼23%) developed pneumothoraces despite the use of low tidal volume ventilation. Pneumothoraces can also occur independent of ventilation,9 underlying the ease with which pneumothoraces can develop in influenza A(H5N1) infection. The mechanism is incompletely understood but may be related to the underlying destruction of lung architecture with pneumatocele and pseudocavity formation, and the effects of mechanical ventilation.32, 35, 36 The optimal ventilation settings in influenza A(H5N1) are currently unknown and need more research.
The duration between onset of illness and administration of oseltamivir has been described as a significant risk factor for a fatal outcome.5, 12 Most of our patients presented soon after illness onset, but treatment delay was in part related to the unavailability of reliable influenza A(H5N1) diagnostics below the tertiary care level and a low index of suspicion in the face of a nondescript febrile illness.5 By contrast, the apparent survivability of influenza A(H5N1) among patients diagnosed early incident to exposure to known human cases testifies to the importance of early diagnosis and treatment. Our findings affirm the urgent need for simple, affordable, and effective point-of-care diagnostics for this infection.
We identified many factors associated with death, but the numbers were too small to test for independence. However, clinicians should be aware that some of these may be clinically important. It seems prudent to ensure “optimal” control of hyperglycemia, even though defining this is problematic in critically ill patients.37, 38 The admission SOFA score may also be helpful to identify at-risk patients, but this has been developed for “Western” patients with sepsis who often have comorbidities, multiorgan failure, and shock.21 Whether the SOFA or a pneumonia scoring system in this predominantly respiratory illness is more relevant requires more research focused on affected populations.39
Four of our patients survived. Of these, only one had mild clinical disease and a normal CXR. Throat swab viral clearance was confirmed for three patients within 4 days, suggesting sufficient oseltamivir absorption and antiviral efficacy.40 By contrast, others have found that continuing influenza A(H5N1) replication despite oseltamivir treatment is associated with death.41 The efficacy of IVIG is unknown but may have contributed to survival in three of the patients. In China, convalescent serum administered to several influenza A(H5N1) patients also appeared beneficial.11 Combined immunomodulatory and antiviral drug therapies warrant further research.42
The primary limitation of our study was the small number of patients, thus restricting statistical power to discern more subtle effects. We also used a somewhat crude classification of the CXR findings by simply classifying the degree of consolidation as > 50% or not and whether consolidation was unilateral or bilateral. Such a system may be helpful to busy clinicians but lacks the finesse of other classifications.43 Not all patients were sampled daily for virus detection and no attempts were made to collect specimens to measure oseltamivir concentrations.
In summary, we have identified new clinical features of infection by influenza A(H5N1) linked to poor outcomes in our small series of patients. If confirmed in larger series, they may shed new light on the clinical consequences of human influenza A(H5N1) infection.
Acknowledgments
Author contributions: Dr Soepandi: contributed to clinical care, laboratory support, data collection, and reading and approving the final manuscript.
Dr Burhan: contributed to clinical care, laboratory support, data collection and management, statistical analysis, writing the first draft of the manuscript, and reading and approving the final manuscript.
Dr Mangunnegoro: contributed to clinical care, laboratory support, data collection, and reading and approving the final manuscript.
Dr Nawas: contributed to clinical care, laboratory support, data collection, and reading and approving the final manuscript.
Dr Aditama: contributed to clinical care, laboratory support, data collection, and reading and approving the final manuscript.
Dr Partakusuma: contributed to clinical care, laboratory support, data collection, and reading and approving the final manuscript.
Dr Isbaniah: contributed to data management, writing the first draft of the manuscript, and reading and approving the final manuscript.
Dr Ikhsan: contributed to clinical care, laboratory support, data collection, and reading and approving the final manuscript.
Dr Swidarmoko: contributed to clinical care, laboratory support, data collection, and reading and approving the final manuscript.
Dr Sutiyoso: contributed to clinical care, laboratory support, data collection, and reading and approving the final manuscript.
Dr Malik: contributed to PCR diagnostics and reading and approving the final manuscript.
Dr Benamore: contributed to reporting diagnostic radiology and reading and approving the final manuscript.
Dr Baird: contributed to writing the first draft of the manuscript and reading and approving the final manuscript.
Dr Taylor: contributed to statistical analysis, writing the first draft of the manuscript, and reading and approving the final manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: We thank Professor J. J. Farrar for his helpful comments on the manuscript. This work was undertaken as part of the South East Asia Infectious Disease Clinical Research Network.
Footnotes
Funding/Support: This study was supported by the South East Asia Infectious Disease Clinical Research Network (W. R. J. T. and J. K. B.).
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Sedyaningsih ER, Isfandari S, Setiawaty V. Epidemiology of cases of H5N1 virus infection in Indonesia, July 2005-June 2006. J Infect Dis. 2007;196(4):522–527. doi: 10.1086/519692. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization http://www.who.int/csr/disease/avian_influenza/country/cases_table_2009_11_20/en/index.htm Accessed November, 2009.
- 3.Kandun IN, Wibisono H, Sedyaningsih ER. Three Indonesian clusters of H5N1 virus infection in 2005. N Engl J Med. 2006;355(21):2186–2194. doi: 10.1056/NEJMoa060930. [DOI] [PubMed] [Google Scholar]
- 4.McKimm-Breschkin JL, Selleck PW, Usman TB, Johnson MA. Reduced sensitivity of influenza A (H5N1) to oseltamivir. Emerg Infect Dis. 2007;13(9):1354–1357. doi: 10.3201/eid1309.07-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kandun IN, Tresnaningsih E, Purba WH. Factors associated with case fatality of human H5N1 virus infections in Indonesia: a case series. Lancet. 2008;372(9640):744–749. doi: 10.1016/S0140-6736(08)61125-3. [DOI] [PubMed] [Google Scholar]
- 6.Beigel JH, Farrar J, Han AM, Writing Committee of the World Health Organization (WHO) Consultation on Human Influenza A/H5 Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005;353(13):1374–1385. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- 7.Chotpitayasunondh T, Ungchusak K, Hanshaoworakul W. Human disease from influenza A (H5N1), Thailand, 2004. Emerg Infect Dis. 2005;11(2):201–209. doi: 10.3201/eid1102.041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Jong MD, Simmons CP, Thanh TT. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12(10):1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liem NT, Tung CV, Hien ND. Clinical features of human influenza A (H5N1) infection in Vietnam: 2004–2006. Clin Infect Dis. 2009;48(12):1639–1646. doi: 10.1086/599031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuen KY, Chan PK, Peiris M. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351(9101):467–471. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- 11.Yu H, Gao Z, Feng Z. Clinical characteristics of 26 human cases of highly pathogenic avian influenza A (H5N1) virus infection in China. PLoS ONE. 2008;3(8):e2985. doi: 10.1371/journal.pone.0002985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdel-Ghafar AN, Chotpitayasunondh T, Gao Z, Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza A (H5N1) Virus Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med. 2008;358(3):261–273. doi: 10.1056/NEJMra0707279. [DOI] [PubMed] [Google Scholar]
- 13.Buxton Bridges C, Katz JM, Seto WH. Risk of influenza A (H5N1) infection among health care workers exposed to patients with influenza A (H5N1), Hong Kong. J Infect Dis. 2000;181(1):344–348. doi: 10.1086/315213. [DOI] [PubMed] [Google Scholar]
- 14.Chan PK. Outbreak of avian influenza A(H5N1) virus infection in Hong Kong in 1997. Clin Infect Dis. 2002;34(suppl 2):S58–S64. doi: 10.1086/338820. [DOI] [PubMed] [Google Scholar]
- 15.de Jong MD, Bach VC, Phan TQ. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N Engl J Med. 2005;352(7):686–691. doi: 10.1056/NEJMoa044307. [DOI] [PubMed] [Google Scholar]
- 16.Apisarnthanarak A, Kitphati R, Thongphubeth K. Atypical avian influenza (H5N1) Emerg Infect Dis. 2004;10(7):1321–1324. doi: 10.3201/eid1007.040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernard GR, Artigas A, Brigham KL. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 18.Cordingley JJ, Keogh BF. The pulmonary physician in critical care. 8: Ventilatory management of ALI/ARDS. Thorax. 2002;57(8):729–734. doi: 10.1136/thorax.57.8.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakhtiari K, Meijers JC, de Jonge E, Levi M. Prospective validation of the International Society of Thrombosis and Haemostasis scoring system for disseminated intravascular coagulation. Crit Care Med. 2004;32(12):2416–2421. doi: 10.1097/01.ccm.0000147769.07699.e3. [DOI] [PubMed] [Google Scholar]
- 20.Porcel JM, Light RW. Diagnostic approach to pleural effusion in adults. Am Fam Physician. 2006;73(7):1211–1220. [PubMed] [Google Scholar]
- 21.Vincent JL, de Mendonça A, Cantraine F. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26(11):1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 23.Tran TH, Nguyen TL, Nguyen TD, World Health Organization International Avian Influenza Investigative Team Avian influenza A (H5N1) in 10 patients in Vietnam. N Engl J Med. 2004;350(12):1179–1188. doi: 10.1056/NEJMoa040419. [DOI] [PubMed] [Google Scholar]
- 24.Yang JK, Feng Y, Yuan MY. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23(6):623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 25.van den Berghe G, Wouters P, Weekers F. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 26.Oner AF, Bay A, Arslan S. Avian influenza A (H5N1) infection in eastern Turkey in 2006. N Engl J Med. 2006;355(21):2179–2185. doi: 10.1056/NEJMoa060601. [DOI] [PubMed] [Google Scholar]
- 27.Lew TW, Kwek TK, Tai D. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290(3):374–380. doi: 10.1001/jama.290.3.374. [DOI] [PubMed] [Google Scholar]
- 28.Umapathi T, Kor AC, Venketasubramanian N. Large artery ischaemic stroke in severe acute respiratory syndrome (SARS) J Neurol. 2004;251(10):1227–1231. doi: 10.1007/s00415-004-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orbach H, Katz U, Sherer Y, Shoenfeld Y. Intravenous immunoglobulin: adverse effects and safe administration. Clin Rev Allergy Immunol. 2005;29(3):173–184. doi: 10.1385/CRIAI:29:3:173. [DOI] [PubMed] [Google Scholar]
- 30.Welsh P, Barber M, Langhorne P, Rumley A, Lowe GD, Stott DJ. Associations of inflammatory and haemostatic biomarkers with poor outcome in acute ischaemic stroke. Cerebrovasc Dis. 2009;27(3):247–253. doi: 10.1159/000196823. [DOI] [PubMed] [Google Scholar]
- 31.Prisco D, Grifoni E. The role of D-dimer testing in patients with suspected venous thromboembolism. Semin Thromb Hemost. 2009;35(1):50–59. doi: 10.1055/s-0029-1214148. [DOI] [PubMed] [Google Scholar]
- 32.Qureshi NR, Hien TT, Farrar J, Gleeson FV. The radiologic manifestations of H5N1 avian influenza. J Thorac Imaging. 2006;21(4):259–264. doi: 10.1097/01.rti.0000213573.94032.53. [DOI] [PubMed] [Google Scholar]
- 33.Chowell G, Bertozzi SM, Colchero MA. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med. 2009;361(7):674–679. doi: 10.1056/NEJMoa0904023. [DOI] [PubMed] [Google Scholar]
- 34.Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature. 2006;440(7083):435–436. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- 35.Boussarsar M, Thierry G, Jaber S, Roudot-Thoraval F, Lemaire F, Brochard L. Relationship between ventilatory settings and barotrauma in the acute respiratory distress syndrome. Intensive Care Med. 2002;28(4):406–413. doi: 10.1007/s00134-001-1178-1. [DOI] [PubMed] [Google Scholar]
- 36.Bay A, Etlik O, Oner AF. Radiological and clinical course of pneumonia in patients with avian influenza H5N1. Eur J Radiol. 2007;61(2):245–250. doi: 10.1016/j.ejrad.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Inzucchi SE, Siegel MD. Glucose control in the ICU—how tight is too tight? N Engl J Med. 2009;360(13):1346–1349. doi: 10.1056/NEJMe0901507. [DOI] [PubMed] [Google Scholar]
- 38.Finfer S, Chittock DR, Su SY, NICE-SUGAR Study Investigators Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 39.Yandiola PP, Capelastegui A, Quintana JM. Prospective comparison of severity scores for predicting clinically relevant outcomes for patients hospitalized with community-acquired pneumonia. Chest. 2009;135(6):1572–1579. doi: 10.1378/chest.08-2179. [DOI] [PubMed] [Google Scholar]
- 40.Taylor WR, Thinh BN, Anh GT. Oseltamivir is adequately absorbed following nasogastric administration to adult patients with severe H5N1 influenza. PLoS ONE. 2008;3(10):e3410. doi: 10.1371/journal.pone.0003410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Jong MD, Tran TT, Truong HK. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med. 2005;353(25):2667–2672. doi: 10.1056/NEJMoa054512. [DOI] [PubMed] [Google Scholar]
- 42.Simmons C, Farrar J. Insights into inflammation and influenza. N Engl J Med. 2008;359(15):1621–1623. doi: 10.1056/NEJMcibr0805865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hui DS, Wong KT, Antonio GE. Severe acute respiratory syndrome: correlation between clinical outcome and radiologic features. Radiology. 2004;233(2):579–585. doi: 10.1148/radiol.2332031649. [DOI] [PubMed] [Google Scholar]


